Abstract
The size increase of a nanoscale material is commonly associated with the increased stability of its ordered phases. Here we give a counterexample to this trend by considering the formation of the defect-free L11 ordered phase in AgPt nanoparticles, and showing that it is better stabilized in small nanoparticles (up to 2.5 nm) than in larger ones, in which the ordered phase breaks in multiple domains or is interrupted by faults. The driving force for the L11 phase formation in small nanoparticles is the segregation of a monolayer silver shell (an Ag-skin) which prevents the element with higher surface energy (Pt) from occupying surface sites. With increasing particle size, the Ag-skin causes internal stress in the L11 domains which cannot thus exceed the critical size of ~2.5 nm. A multiscale modelling approach using full-DFT global optimization calculations and atomistic modelling is used to interpret the findings.
Subject terms: Nanoparticles, Structural properties, Metals and alloys
Increasing nanoparticle size usually increases the stability of ordered phases within them. In contrast, the authors show here that the L11 ordered phase only forms in AgPt nanoparticles smaller than 2.5 nm because the segregation of a monolayer Ag shell constrains the nanophase which becomes unstable at larger sizes.
Introduction
Increasing the size of a nanoscale material is commonly associated to a widening of the stability domain of its ordered phases at equilibrium1. Well-known examples are the melting point temperature increase with nanoparticle size2, which widens the stability range of the ordered solid phase, and the increase of the order−disorder transition temperature in nanoalloys, such as CoPt, to approach the bulk limit3,4. Here we show that this is not a general rule, since an increase of the system size can have the opposite effect: in small AgPt clusters, up to D ~ 2.5 nm, a well-ordered intermetallic phase is stabilized at equilibrium, but, above this size, either the ordered phase breaks down into less-ordered multidomains, or its regular arrangement is interrupted by faults. The small-size stable nanophase is characterized by a specific surface segregation effect, while stress accumulation contributes to the breaking of the ordered phase in larger objects.
The bulk AgPt system shows a very atypical phase diagram in the Ag-based family, with a large miscibility gap between Ag88Pt12at% and Ag2Pt98at% at low temperature5,6 and an ordered phase observed only for an extremely narrow composition range around Ag50Pt50at%7. This phase was observed by HAADF-STEM on bulk samples as made of small L11 ordered patches of few nanometers, irregularly alternating with disordered regions7. The L11 phase is quite difficult to obtain experimentally. Remarkably, an intermetallic compound has been obtained by annealing to 700 °C AgPt randomly intermixed nanoparticles8 (NPs). This compound was not an L11 intermetallic, but a new material with hexagonal and cubic close-packed sequences of alternating Ag and Pt planes. As regards AgPt nanoparticles, alloyed and core@shell Pt@Ag structures have been produced9–14, but without indications of L11 phases up to now. As we show in the following, the L11 phase can indeed be obtained in AgPt nanoparticles, as the result of a subtle interplay of different physical effects.
AgPt nanoparticles find application in catalysis and in fuel cells15–17, with the optimal chemical ordering depending on the reaction. In any case, a crucial point is the catalytic efficiency in terms of durability. This implies that the problem of structural evolution of the nanoparticles must be addressed when dealing with applications. This evolution may be either due to a non-equilibrium state of the as-synthesized structure or to the operando conditions. Therefore, assessing the thermodynamic stability of AgPt nanoparticles is very important for applications.
In the following we experimentally show that nanoparticles with L11 ordered domains are obtained for sizes up to ~2.5 nm. We demonstrate that in these nanoparticles the L11 domains are surrounded by an extremely thin (mostly monoatomic) Ag surface layer. This structure is denoted as L11@Ag-skin structure. For larger sizes, the ordered phase breaks into multiple L11 domains, or it is interrupted by faults. These results are rationalized by a combination of density functional theory (DFT) global optimization searches and of atomistic modelling. The calculations show that the L11 phase is indeed stabilized by Ag surface segregation in small nanoparticles, and that the breaking of the L11 phase in larger nanoparticles is due to the accumulation of stress in the structures.
Results
L11 phase formation
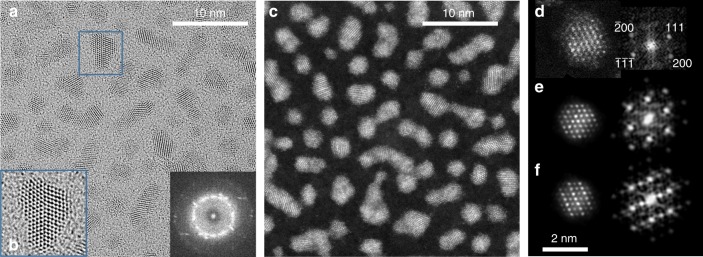
Ag and Pt atoms were codeposited at room temperature on the amorphous carbon grid, to achieve an average composition close to the equi-composition. For 2.3 × 1015 at cm−2, transmission electron microscopy (TEM) images show an assembly of isolated NPs with mean diameter D = 1.5 nm (see Supplementary Fig. 1). For 3.2 × 1015 at cm−2, static coalescence occurs due to the NPs close vicinity. Interdiffusion bridges form between neighbour particles leading to ramified objects, composed of 2–4 elementary particles with D = 1.9 nm on average. Grazing incidence small angle X-ray scattering (GISAXS) measurements confirm the average D and gives H/D = 0.76 (H is the average height, see Supplementary Fig. 2). H/D is lower than 1 because supported NPs look like truncated spheres due to the support-metal interaction. This value was taken into account for defining models used for simulated STEM-HAADF images. HRTEM images show an fcc structure with some incomplete surface planes with respect to the perfect truncated octahedron (TOh) morphology. Particles present many defects such as twins and stacking faults (Fig. 1a, b). On the fast Fourier transform (FFT, see the inset of Fig. 1a) of a large number of particles, d-spacings of 0.232 ± 0.005 and 0.201 ± 0.005 nm are obtained. They correspond to (111) and (200) interplanar distances of an fcc structure with lattice parameter a = 0.402 ± 0.005 nm, closer to the parameter of Ag (a = 0.4086 nm) than to that of Pt (a = 0.392 nm). A low magnification STEM-HAADF image of the as-prepared sample (Fig. 1c) shows NPs with homogeneous contrast. Figure 1d shows a high-resolution HAADF-STEM image along the [01] zone axis and its corresponding FFT. The lack of superstructure reflections in the FFT indicates that there is no order in the [111] direction. Simulated HAADF images of nanoparticles exhibiting a completely random solid solution and an L11 ordered structure are shown in Fig. 1e, f, respectively. The experimental image does not match the ordered phase but matches the random solid solution without Ag segregation (A1 phase). The absence of the ordered L11 phase may be due to slow diffusion at room temperature which prevents equilibration of chemical ordering, causing kinetic trapping into metastable disordered states. This is indeed the case, as demonstrated by our annealing experiments at 400 °C. For the 3.2 × 1015 at cm−2 sample, TEM images and GISAXS show that the NP shapes and sizes changed. D and H/D increase from 1.9 to 3.0 nm and from 0.76 to 0.82, respectively (see Supplementary Fig. 1 and Supplementary Table 1). This evolution is due to thermally activated diffusion and coalescence, leading to reorganization towards a morphology close to the quasi-spherical equilibrium shape of nanoparticles in vacuum4,18. The increase of H/D is indicative of some weakening of the nanoparticle/substrate interaction upon annealing. Atomic-resolution HRTEM images exhibit NPs with TOh structures but with stacking faults and twin boundaries (Supplementary Fig. 1). More important, and contrary to the as-prepared samples, STEM-HAADF images of the annealed samples exhibit particles with non-homogeneous contrast (Fig. 2a). Particles oriented along the [01] zone axis or equivalents show strong and weak bright contrasts appearing periodically along the [111] direction (Fig. 2b). On the corresponding FFT pattern, a superstructure reflection corresponding to the half order of the 111 reflection is observed. STEM-HAADF simulated images along the [01] zone axis of fully ordered L11 and random solid solution NPs are shown in Fig. 2c, d. The similarity between observed and simulated images confirms the presence of the L11 structure, with strong and weak bright contrasts corresponding to Pt and Ag (111) planes, respectively. The distance d111 = 0.230 ± 0.005 nm between the consecutive (111) planes corresponds to an fcc lattice parameter a = 0.400 ± 0.005 nm.
Fig. 1.
As-grown room temperature AgPt sample (flux 3.2 × 1015 at cm−2). a HRTEM images and in the inset, the total Fourier transform. b Zoom (×2) showing crystal twinning. c STEM-HAADF image with homogeneous contrast. High-resolution STEM-HAADF image and the corresponding FFT for d experimental NP along the [01] zone axis and the corresponding simulated images e for a random solid solution and f for an L11 ordered structure. HRTEM high resolution in TEM, STEM-HAADF high angle annular dark field in scanning transmission electron microscope, NP nanoparticle
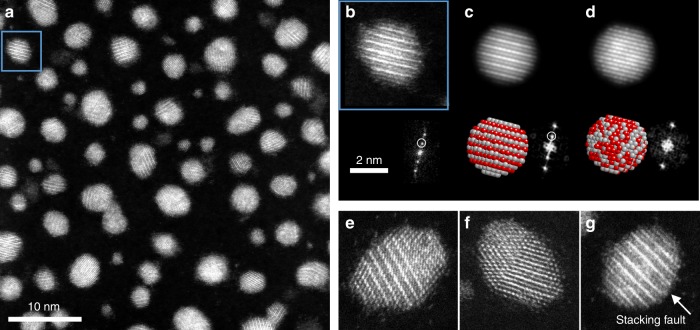
Fig. 2.
AgPt NPs after annealing at 400 °C. a STEM-HAADF image; b a zoom on one NP oriented along the [01] zone axis showing the L11 phase with alternating planes of Ag and Pt and its corresponding FFT showing the superstructure reflections. c, d Simulated STEM-HAADF images along the [01] zone axis for an L11 structure and a random solid solution respectively. e–g show multidomains, twin and a stacking fault for L11 NPs (Pt atoms are in red and Ag atoms in grey). The scale is the same for b–g. NP nanoparticle, FFT fast Fourier Ttransform, STEM-HAADF high angle annular dark field in scanning transmission electron microscope
To investigate the occurrence of the L11 phase in the annealed NPs and see whether this occurrence is size-dependent, we analysed a population of 500 NPs. We note that observing the L11 phase is possible only when the NP is oriented with a zone axis belonging to (111) planes. Assuming that the NPs are randomly oriented on the substrate, one finds that 13% of the NPs have the right orientation. The approximation of random orientations is crude, but it is useful for comparison with the observations, which are summarized as follows:
The L11 phase is observed in 24% of the particles (54 occurrences on 230 NPs with planes resolution), i.e. on the whole or on a part of the NP.
The smallest particles (D < 3 nm) are mostly monodomain while 85% of large particles (D > 3 nm) are multidomain (128 occurrences in 150 NPs), i.e. with antiphase boundaries or twins (Fig. 2e, f). The L11-phase domain size is between 2 and 3 nm.
Large monodomain particles showing the L11 phase (6 occurrences on 22 NPs) always exhibit at least one stacking fault corresponding generally to two consecutive pure Ag planes within the NP (cf. Fig. 2g).
Therefore, there is a clear size dependence, since the perfect alternation between pure Pt and Ag (111) planes is only observed in small particles or in small domains (<3 nm) inside larger particles. In these cases, the percentages exceed 13%, indicating that probably a large part of these NPs presents indeed the L11 phase.
Surface composition of the Ag-skin
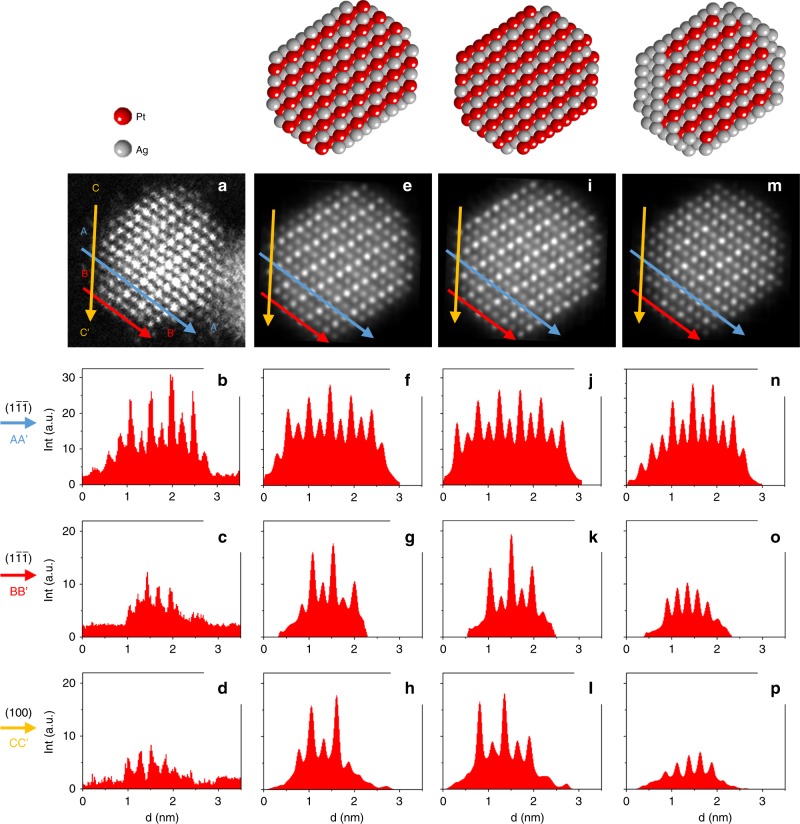
Since several studies report Ag surface segregation leading to Pt@Ag structures, we characterize the composition of the surface layer of our monodomain L11 NPs. The analysis of surface contrasts of a 2.8 nm NP is shown in Fig. 3. In order to have both the presence of {111} and {100} facets and the possibility to image the [111] direction, an NP along the [01] zone axis was analysed. The experimental image (Fig. 3a–d) was compared to simulated images for a 2.8 nm NP with an aspect ratio of 0.8 according to the GISAXS data. Three structural models were constructed from a fully ordered L11 domain: single-domain L11, beginning and finishing with pure Ag(111) or Pt(111) planes (model 1, Fig. 3e–h) and model 2, Fig. 3i–l, respectively, and L11@Ag-skin, with an Ag layer (mostly a monolayer) covering the L11 domain (model 3, Fig. 3m–p).
Fig. 3.
L11@Ag-skin nanoparticle. a–d High-resolution STEM-HAADF image of a 2.8 nm NP in the [01] zone axis with its corresponding intensity profile lines. e–h, i–l and m–p show the model 1, 2, and 3 in the [01] zone axis, respectively, with their corresponding intensity profile lines. The intensity profiles were done for three different locations: along the (1) plane close to the centre of the NP (AA′), along the (1) plane at the NP surface (BB′) and along the (100) plane at the NP surface (CC′). STEM-HAADF high angle annular dark field in scanning transmission electron microscope, NP nanoparticle
Intensity profiles parallel to (100) and (1) planes were considered. In these profiles, the global intensity variation from the centre to the surface is due to the reduction of the particle thickness in the peripheral region. The intensity variation of two consecutive peaks is attributed to the composition variation of [01] columns. Close to the NP centre and parallel to the (1 ) planes, the intensity variation (Fig. 3b, AA′ profile line) shows the alternation between bright Pt and darker Ag planes. At both line extremities (A and A′), the intensities correspond to a minimum which can be explained if the atomic columns at the NP surface are pure Ag. Comparison with the simulated profiles indicates that model 2 can be excluded (Fig. 3b, j). To discriminate between models 1 and 3, surface profile lines on the {1} and {100} facets (B to B′ and C to C′ lines, respectively) were collected. Model 1 shows alternating contrasts for both facets (Fig. 3g, h) contrary to model 3 (Fig. 3o, p) and to the experimental profiles (Fig. 3c, d), indicating that (1) and (100) surface planes are pure Ag. Moreover, to fully match the experimental profiles for this 2.8 nm NP, the model is composed of an L11 core with one monolayer Ag shell except on a {100} facet where there are three consecutive Ag planes (see model 3, Fig. 3).
Discussion
To rationalize the experimental results we adopt a multiscale modelling approach comprising DFT calculations for small sizes and atomistic modelling for larger sizes.
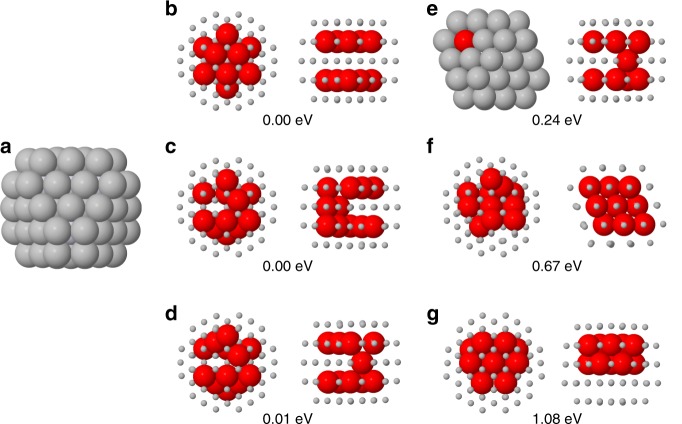
We checked the stability of the L11@Ag-skin phase by full-DFT global optimization of chemical ordering in the smallest nanoalloy supporting this phase, a TOh of 79 atoms (Fig. 4a). The perfect L11@Ag-skin phase is obtained for Ag67Pt12. The most significant structures for Ag67Pt12 are summarized in Fig. 4b–g. In all lowest-energy structures, all Pt atoms occupy subsurface positions. The best structures, very close in energy, are (b), (c) and (d): (b) is the perfect L11 phase with two Pt subsurface triangular leaflets, (c) and (d) are obtained from (b) by two and one atomic pair swaps, respectively. Structures with a Pt atom at the surface (e) or in the central site (f) are ~0.2 eV higher or more. A compact Pt aggregate (g) is ~1 eV higher than (b). These results show that the L11@Ag-skin phase (either perfect or with a few defects) is energetically preferred for small clusters. L11@Ag-skin structures with a single twin plane are close in energy to perfect L11@Ag-skin structures (see Supplementary Fig. 3). On the contrary, our DFT global optimization runs show that L11 structures without Ag-skin (as in models 1 and 2 of Fig. 2) turn out to be energetically unfavourable (see Supplementary Fig. 4). Therefore, the DFT results very well agree with the experiments in showing that the Ag-skin is crucial for stabilizing the L11 phase.
Fig. 4.
DFT results. a The truncated octahedron of 79 atoms. This structure has 19 internal sites, i.e. 18 subsurface sites plus the central site. b–g Structures of Ag67Pt12. Each cluster is shown in two views. Pt atoms are in red and Ag atoms in grey. Pt and Ag atoms are shown as large and small spheres, respectively, with the exception of the left view of (e), in which all atoms are shown as large spheres. In (b), the perfect L11@Ag-skin phase is shown. The numbers give the DFT energy differences from the lowest-energy homotops, which are the perfect L11@Ag-skin structure shown in (b) and the structure in (c). DFT density functional theory
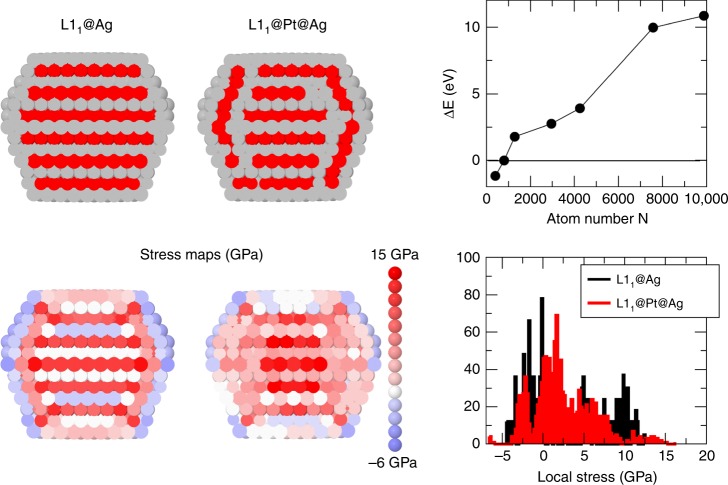
To determine the driving force for the L11 phase breaking, we developed an atomistic model to calculate the pressure pi on each atom i19. Positive and negative pi correspond to compressive and tensile stress, respectively. The equilibration of pi is a driving force for optimizing nanoalloy chemical ordering20. According to the model, the L11@Ag-skin phase is energetically favourable for sizes below 103 atoms, corresponding to D ~ 3 nm, in good agreement with the experiments. For larger sizes, more complex arrangements, as the L11@Pt@Ag-skin phase of Fig. 5, become more stable. In L11@Pt@Ag-skin, the subsurface layer is Pt-enriched, while the layer below is Ag-enriched. This is a kind of multidomain particle, with a central L11 core surrounded by thin L11 domains, with different orientations and three-layer thickness. To analyse stress equilibration, we use the average of the absolute value of pi, . Better stress equilibration corresponds to smaller 21. For the TOh of 1289 atoms of Fig. 5, we obtain and 3.31 GPa for the L11@Ag-skin and the L11@Pt@Ag phases, respectively. Finally, we checked the compression of Ag−Ag and the expansion of Pt−Pt distances in the inner part of the L11@Ag-skin particles by DFT calculations on cuboctahedral and truncated octahedral clusters of 147 and 201 atoms, respectively. We found a contraction of Ag−Ag distances up to 2.5% and an expansion of Pt−Pt distances up to 1.5%. Contractions and expansions are measured with respect to nearest-neighbour distances in the corresponding pure Pt and pure Ag clusters.
Fig. 5.
Theoretical predictions of L11 nanophases. L11@Ag core/shell and L11@Pt@Ag core/subshell/shell structures for the TOh1289 (Pt atoms in red, Ag atoms in grey) and their associated stress map in colour code from −6 GPa for tensile stress to 15 GPa for compressive stress. The graph on the bottom right illustrates the stress distribution inside the TOh1289 with the two structures and the graph on top shows the energy difference between the L11@Ag and the L11@Pt@Ag structures: positive sign means the L11@Pt@Ag is the stable structure
In summary, the L11 phase is stabilized at its extreme nanoscale limit in AgPt nanoalloys. Surface segregation of excess Ag atoms creates an Ag-skin which is crucial for this stabilization. Otherwise, Pt atoms would be exposed on the surface, which is quite unfavourable due to the larger surface energy of Pt. From this point of view, AgPt is different from FePt and CoPt4, in which the surface of L10-ordered nanoparticles is rather mixed or slightly Pt-enriched but without a pure skin segregation as in AgPt. As Ag atoms are larger than Pt atoms, the Ag-skin induces strong, size-dependent tensile/compressive stress in the alternating Pt/Ag (111) planes of the L11-ordered core, which becomes no longer energetically favourable with increasing size. This leads to the breaking of the L11 phase in multiple domains for D > 3 nm, a fact which also explains why this phase is very difficult to obtain in bulk samples.
Methods
Experiments
AgPt nanoalloys were prepared by atomic condensation on an amorphous carbon surface using two separate atoms sources operating simultaneously under ultrahigh vacuum conditions as already described22. The amorphous substrate was kept at room temperature during all atomic depositions. The atomic composition was adjusted with the atomic flux of each source and checked by energy dispersive X-ray (EDS) and Rutherford Backscattering Spectroscopies and was kept to 55 ± 3 at.% Ag. After the preparation, some samples were annealed up to 400 °C to promote atomic diffusion. The annealing was performed through different steps: at 200 °C for 60 min, at 300 °C for 60 min and at 400 °C for 60 min. Each step was reached with a 2.5 °C/min ramp. TEM and GISAXS4 were both performed to determine the NPs’ size and shape. The substrates used for all characterization methods are amorphous carbon layers prepared in the same preparation batch. They present the same thickness and roughness. The only difference comes from that for GISAXS and RBS; the carbon layer is deposited on a flat silica substrate and for TEM and EDX the carbon layer is self-supported on grids. For fitting the GISAXS experiments, IsGISAXS software was used with a truncated sphere model as the NPs’ shape23. High resolution in TEM mode (HRTEM) and High angle annular dark field in scanning transmission electron microscopy mode (STEM-HAADF) were performed, using a double aberration-corrected JEOL ARM200F microscope, to combine structural and chemical studies. STEM-HAADF images give a scattered intensity scale directly correlated to the atomic number of the elements composing the samples24. Since the HAADF contrast is both atomic number and thickness dependent, in order to quantitatively analyse these images, simulation of STEM-HAADF images were performed using the QSTEM software25. QSTEM simulations were performed for different chemical arrangements such as ordered or disordered phases, in the alloyed or core-shell configurations.
Density-functional theory (DFT) global optimization calculations
The global optimization of chemical ordering was achieved by interfacing our basin hopping code26 to the CP2K DFT code27. Some calculations (reported in Supplementary Note 2) have been checked also using the Quantum Espresso package28. The calculations were made by using the Perdew−Burke−Ernzerhof (PBE)29 exchange-correlation functional. The basin hopping searches were made starting from a random chemical ordering and then allowing swaps between Pt−Ag atom pairs. For each composition, 103 basin hopping steps were made.
Atomistic modelling and Monte Carlo simulations
To simulate larger clusters we used a semi-empirical potential in the second moment approximation (SMA)30 in which we added a Gaussian term for the mixed second neighbour interactions to stabilize the L11 phase against the L10 phase. This potential has been fitted to DFT-PBE calculations29 of the cohesive energies, lattice parameters and elastic constant of pure elements, and mixing enthalpies of the alloys for the mixed interactions. In order to reproduce the experimental order/disorder temperature of the L11 bulk phase (1200 K), we overestimate the formation enthalpy of the L11 phase (−0.213 eV per atom) as compared to the DFT-PBE calculation (−0.051 eV per atom). One of the L10 phase (0.063 eV per atom) is quite comparable to the DFT-PBE one which is equal to 0.041 eV per atom. The form of the potential is reported in Supplementary Note 3 and its parameters are given in Supplementary Table 2. Monte Carlo simulations were performed in the canonical ensemble. Elementary moves comprise both atomic exchanges of pairs of unlike atoms and atomic displacements. Atomic pressure was calculated as the trace of the atomic stress tensor19.
Supplementary information
Acknowledgements
We wish to express our gratitude to Dr. Eiji. Okunishi from Jeol Ltd., Tokyo, Japan for helpful discussions and access to the double corrected ARM200F microscope. The authors acknowledge financial support from the CNRS-CEA “METSA” French network (FR CNRS 3507) for the TEM experiments conducted on the MPQ-Paris Diderot platform. The authors would like to acknowledge support from the International Research Network—IRN “Nanoalloys” of CNRS.
Author contributions
J.P., C.A.-V. and P.A. performed the experimental part, A.F. and C.M. developed the atomistic model, D.R. and R.F. made the DFT calculations. The authors wrote the paper together.
Data availability
All data are available from the authors upon reasonable request.
Code availability
CP2K and Quantum Espresso codes are freely available at https://www.cp2k.org/ and https://www.quantum-espresso.org/, respectively. The BH and Monte Carlo codes are available upon request to ferrando@fisica.unige.it and mottet@cinam.univ-mrs.fr, respectively.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Talat Shahnaz Rahman and other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
C. Andreazza-Vignolle, Email: caroline.andreazza@univ-orleans.fr
P. Andreazza, Email: pascal.andreazza@univ-orleans.fr
C. Mottet, Email: mottet@cinam.univ-mrs.fr
R. Ferrando, Email: ferrando@fisica.unige.it
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09841-3.
References
- 1.Hill, T. L. Thermodynamics of Small Systems (Dover Publications, New York, 1994).
- 2.Borel P, Buffat JP. Size effect on the melting temperature of gold partlcles. Phys. Rev. A. 1976;13:2287–2298. doi: 10.1103/PhysRevA.13.2287. [DOI] [Google Scholar]
- 3.Alloyeau D, et al. Size and shape effects on the order/disorder phase transition in cobalt-platinum nanoparticles. Nat. Mater. 2009;8:940. doi: 10.1038/nmat2574. [DOI] [PubMed] [Google Scholar]
- 4.Andreazza P, Pierron-Bohnes V, Tournus F, Andreazza-Vignolle C, Dupuis V. Structure and order in cobalt/platinum-type nanoalloys: from thin films to supported clusters. Surf. Sci. Rep. 2015;70:188–258. doi: 10.1016/j.surfrep.2015.02.002. [DOI] [Google Scholar]
- 5.Okamoto H. Ag-Pt (silver-platinum) J. Phase Equilibria. 1997;18:485–485. doi: 10.1007/BF02647706. [DOI] [Google Scholar]
- 6.Durussel P, Feschotte P. A revision of the binary system Ag-Pt. J. Alloy. Compd. 1996;239:226–230. doi: 10.1016/0925-8388(96)02257-8. [DOI] [Google Scholar]
- 7.Hart GL, et al. Revisiting the revised Ag-Pt phase diagram. Acta Mater. 2017;124:325–332. doi: 10.1016/j.actamat.2016.10.053. [DOI] [Google Scholar]
- 8.Pan YT, Yan Y, Shao YT, Zuo JM, Yang H. Ag-Pt compositional intermetallics made from alloy nanoparticles. Nano Lett. 2016;16:6599–6603. doi: 10.1021/acs.nanolett.6b03302. [DOI] [PubMed] [Google Scholar]
- 9.Wojtysiak S, Solla-Gullón J, Dłużewski P, Kudelski A. Synthesis of core-shell silver-platinum nanoparticles, improving shell integrity. Colloids Surf. A: Physicochem. Eng. Asp. 2014;441:178–183. doi: 10.1016/j.colsurfa.2013.09.008. [DOI] [Google Scholar]
- 10.Hwang BJ, et al. Size and alloying extent dependent physiochemical properties of Pt−Ag/C nanoparticles synthesized by the ethylene glycol method. J. Phys. Chem. C. 2008;112:2370–2377. doi: 10.1021/jp077138p. [DOI] [Google Scholar]
- 11.Xu JB, Zhao TS, Liang ZX. Synthesis of active platinum-silver alloy electrocatalyst toward the formic acid oxidation reaction. J. Phys. Chem. C. 2008;112:17362–17367. doi: 10.1021/jp8063933. [DOI] [Google Scholar]
- 12.Peng Z, You H, Yang H. Composition-dependent formation of platinum silver nanowires. ACS Nano. 2010;4:1501–1510. doi: 10.1021/nn9016795. [DOI] [PubMed] [Google Scholar]
- 13.Cottancin E, et al. Optical properties of mixed clusters: comparative study of Ni/Ag and Pt/Ag clusters. Eur. Phys. J. D.—At., Mol., Opt. Plasma Phys. 2003;24:111–114. [Google Scholar]
- 14.Pan YT, Yan L, Shao YT, Zuo JM, Yang H. Regioselective atomic rearrangement of Ag-Pt octahedral catalysts by chemical vapor-assisted treatment. Nano Lett. 2016;16:7988–7992. doi: 10.1021/acs.nanolett.6b04411. [DOI] [PubMed] [Google Scholar]
- 15.Hwang SY, Zhang C, Yurchekfrodl E, Peng Z. Property of Pt-Ag alloy nanoparticle catalysts in carbon monoxide oxidation. J. Phys. Chem. C. 2014;118:28739–28745. doi: 10.1021/jp5101768. [DOI] [Google Scholar]
- 16.Esfandiari A, Kazemeini M, Bastani D. Synthesis, characterization and performance determination of an Ag@Pt/C electrocatalyst for the ORR in a PEM fuel cell. Int. J. Hydrog. Energy. 2016;41:20720–20730. doi: 10.1016/j.ijhydene.2016.09.097. [DOI] [Google Scholar]
- 17.Cao J, et al. Carbon-supported Ag@Pt core-shell nanoparticles with enhanced electrochemical activity for methanol oxidation and oxygen reduction reaction. J. Power Sources. 2015;277:155–160. doi: 10.1016/j.jpowsour.2014.12.017. [DOI] [Google Scholar]
- 18.Alloyeau D, et al. Ostwald ripening in nanoalloys: when thermodynamics drives a size-dependent particle composition. Phys. Rev. Lett. 2010;105:255901. doi: 10.1103/PhysRevLett.105.255901. [DOI] [PubMed] [Google Scholar]
- 19.Vitek V, Egami T. Atomic level stresses in solids and liquids. Phys. Status Solidi B. 1987;144:145–156. doi: 10.1002/pssb.2221440113. [DOI] [Google Scholar]
- 20.Ferrando, R. in Structure and Properties of Nanoalloys. Frontiers of Nanoscience Vol. 10 (Elsevier, Amsterdam, 2016).
- 21.Laasonen K, Panizon E, Bochicchio D, Ferrando R. Competition between icosahedral motifs in AgCu, AgNi, and AgCo nanoalloys: a combined atomistic-DFT study. J. Phys. Chem. C. 2013;117:26405–26413. doi: 10.1021/jp410379u. [DOI] [Google Scholar]
- 22.Penuelas J, Andreazza-Vignolle C, Andreazza P, Ouerghi A, Bouet N. Temperature effect on the ordering and morphology of CoPt nanoparticles. Surf. Sci. 2008;602:545–551. doi: 10.1016/j.susc.2007.11.002. [DOI] [Google Scholar]
- 23.Lazzari R. IsGISAXS: a program for grazing-incidence small-angle X-ray scattering analysis of supported islands. J. Appl. Crystallogr. 2002;35:406–421. doi: 10.1107/S0021889802006088. [DOI] [Google Scholar]
- 24.Alloyeau, D. Transmission electron microscopy: a multifunctional tool for the atomic-scale characterization of nanoalloys. In Nanoalloys—Synthesis, Structure and Properties (eds Alloyeau, D. et al.) 113–157 (Springer Verlag, Berlin, 2012).
- 25.Koch, C. T. Determination of Core Structure Periodicity and Point Defect Density along Dislocations. PhD thesis, Arizona State University (2002).
- 26.Rossi G, Ferrando R. Searching for low-energy structures of nanoparticles: a comparison of different methods and algorithms. J. Phys. Cond. Mat. 2009;21:084208. doi: 10.1088/0953-8984/21/8/084208. [DOI] [PubMed] [Google Scholar]
- 27.Lippert G, Hutter J, Parrinello M. A hybrid Gaussian and plane wave density functional scheme. Mol. Phys. 1997;92:477–488. doi: 10.1080/002689797170220. [DOI] [Google Scholar]
- 28.Giannozzi P, et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter. 2009;21:395502. doi: 10.1088/0953-8984/21/39/395502. [DOI] [PubMed] [Google Scholar]
- 29.Perdew J, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997;78:1396–1396. doi: 10.1103/PhysRevLett.78.1396. [DOI] [PubMed] [Google Scholar]
- 30.Rosato V, Guillopé M, Legrand B. Thermodynamical and structural properties of f.c.c. transition metals using a simple tight-binding model. Philos. Mag. A. 1989;59:321–336. doi: 10.1080/01418618908205062. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the authors upon reasonable request.
CP2K and Quantum Espresso codes are freely available at https://www.cp2k.org/ and https://www.quantum-espresso.org/, respectively. The BH and Monte Carlo codes are available upon request to ferrando@fisica.unige.it and mottet@cinam.univ-mrs.fr, respectively.