Abstract
The adult dentate gyrus continuously generates new neurons that endow the brain with increased plasticity, helping to cope with changing environmental and cognitive demands. The process leading to the birth of new neurons spans several precursor stages and is the result of a coordinated series of fate decisions, which are tightly controlled by extrinsic signals. Many of these signals act through modulation of cell cycle (CC) components, not only to drive proliferation, but also for linage commitment and differentiation. In this review, we provide a comprehensive overview on key CC components and regulators, with emphasis on G1 phase, and analyze their specific functions in precursor cells of the adult hippocampus. We explore their role for balancing quiescence versus self-renewal, which is essential to maintain a lifelong pool of neural stem cells while producing new neurons “on demand.” Finally, we discuss available evidence and controversies on the impact of CC/G1 length on proliferation versus differentiation decisions.
Keywords: dentate gyrus, neural stem cells, cyclins, cyclin-dependent kinases, proliferation, differentiation, fate determination, G1
Introduction
The presence of NSC capable of generating new neurons throughout life provides adult mammals with an exceptional level of brain plasticity. aNSC are multipotent, have the capacity to self-renew and generate progenitor cells which give rise to neurons that functionally integrate into pre-existing networks (Zhao et al., 2006; Suh et al., 2007; Toni et al., 2008; Zhao et al., 2008; Bonaguidi et al., 2011; Encinas et al., 2011; Mongiat and Schinder, 2011; Pilz et al., 2018). Under physiological conditions, adult neurogenesis is mainly restricted to two brain regions: the SVZ of the lateral ventricles and the SGZ of the DG (Doetsch, 2003; Alvarez-Buylla and Lim, 2004). Persuasive evidence suggests that adult-born neurons participate in specific brain functions, including learning and memory, mood regulation, and pheromone-related behaviors (Ming and Song, 2011). Adult neurogenesis is a dynamic, finely tuned process that involves repeated fate decisions, including the proliferation and differentiation of stem and progenitor cells, or survival and maturation of newborn neurons (Figure 1). Sustaining the balance between such fate decisions is critical for maintaining homeostasis in the system, as excessive proliferation may induce stem cell exhaustion followed by premature depletion of their pool (Kippin et al., 2005; Sierra et al., 2015). On the other hand, disordered generation of new neurons might disturb the function of the circuit in which they integrate and contribute to neurological disorders (Zhao et al., 2008; Ruan et al., 2014; Jessberger and Parent, 2015). Hence, every step of adult neurogenesis must be tightly regulated by a complex interplay of extrinsic and intrinsic genetic factors to facilitate proper circuit adaption to changing environmental demands (Zhao et al., 2008; Ming and Song, 2011; Faigle and Song, 2013; Opendak and Gould, 2015). In a wide array of cellular contexts, including neural cells, cell fate decisions are closely linked to the CC. In particular the G1 phase opens a time window through which cells can respond to mitogens and specification signals to execute their decision to divide, differentiate or exit the CC (Blomen and Boonstra, 2007; Salomoni and Calegari, 2010; Boward et al., 2016).
FIGURE 1.
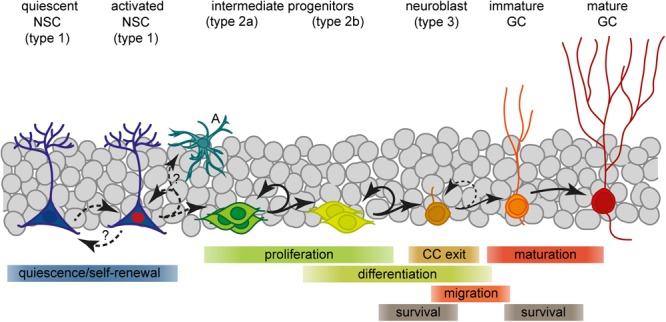
Scheme of the current view of lineage progression and fate decisions during adult hippocampal neurogenesis. A, astrocyte; GC, granule cell; NSC, neural stem cell.
We will herein discuss recent progress in our understanding of how the CC integrates extrinsic signals to regulate processes leading to the birth of a new neuron, with emphasis on the G1 phase. Since particularly the hippocampal newborn neurons play critical roles in learning and memory, with disturbances in their generation being associated to neurologic diseases and aging-related cognitive decline, we focus our review on the adult DG. Beyond that, we will discuss relevant findings obtained in the embryonic brain for which more comprehensive data exist, and draw comparisons to the SVZ, where applicable.
Neurogenesis in the Adult Dentate Gyrus
Neural stem cells residing in the hippocampal SGZ, a narrow area between the dentate granule cell layer and the hilus, are a source of lifelong neurogenesis. They generate intermediate progenitors (IPCs) which give rise to neuroblasts that exit the CC and convert into immature granule neurons (Figure 1; Kempermann et al., 2004; Ming and Song, 2011). These precursor stages can be distinguished by their specific morphologies, expression profiles, mitotic activity and their potential to generate distinct progeny. Prototypical aNSCs (also termed radial glia-like cell or type 1) have an apical process which extends radially through the granule cell layer to the molecular layer, and express glial and stem cell markers like GFAP, nestin, BLBP, and Sox2 (Seri et al., 2001; Filippov et al., 2003; Seri et al., 2004; Suh et al., 2007; Berg et al., 2018). Besides neurons, aNSCs can generate astrocytes and daughter NSCs, suggesting multipotency and self-renewal capacity (Suh et al., 2007; Bonaguidi et al., 2011; Encinas et al., 2011; Pilz et al., 2018). In contrast to embryonic neurogenesis, aNSCs are mostly quiescent with only a few progressing through the CC at any time (Lugert et al., 2010; Encinas et al., 2011). Upon activation, aNSCs predominantly divide asymmetrically to produce another NSC and a type 2 cell (Bonaguidi et al., 2011; Encinas et al., 2011). Type 2 progenitors represent an important stage of clonal expansion and linage choice: They are transit-amplifying, prevalently using a symmetric division mode (Encinas et al., 2011; Pilz et al., 2018), and comprise cell states that mark the transition from a glial/stem-like phenotype (type 2a) to a neuronal phenotype (type 2b; Filippov et al., 2003; Steiner et al., 2006). Phenotypically, all type 2 cells are characterized by an irregularly shaped cell body with short horizontal processes, expression of nestin and Tbr2/Eomes, but no longer GFAP (Filippov et al., 2003; Hodge et al., 2008; Bonaguidi et al., 2011; Encinas et al., 2011). Whereas type 2a cells still express BLBP and Sox2, type 2b cells instead show first signs of neuronal commitment, including the expression of NeuroD1, Prox1, and DCX (Steiner et al., 2006; Kempermann et al., 2015). Committed IPCs then give rise to slowly proliferating neuroblasts that, after migrating a short distance into the granule cell layer, exit the CC to become an early postmitotic neuron (Ming and Song, 2011). New neurons then pass through a continuous process of morphological and functional maturation to fully integrate into the hippocampal network (terminally differentiated stage; Zhao et al., 2006; Toni et al., 2008; Mongiat et al., 2009; Mongiat and Schinder, 2011). However, only a small subset of the newborn cells in the SGZ will eventually develop into a mature granule neuron (Biebl et al., 2000; Tashiro et al., 2006; Sierra et al., 2010; Encinas et al., 2011). The majority of these cells are eliminated through controlled cell death, and studies suggest that two critical periods exist for their survival (Sierra et al., 2010). The main critical period is settled around the transition from IPCs to neuroblasts, when cells exit the CC and most of them die. A later critical period exists at the immature neuron stage which is only survived by neurons that are properly integrated (Tashiro et al., 2006; Sierra et al., 2010). At this time, around 4 weeks of age, adult-born neurons are highly excitable whereas inhibitory input is not yet fully established, and exhibit enhanced synaptic plasticity (Zhao et al., 2006; Mongiat et al., 2009; Marín-Burgin et al., 2012). Owing to these unique features, the young granule neurons are considered to enhance hippocampal information processing. In fact, experimental and computational evidence suggest contributions of new neurons to learning, memory, pattern discrimination, and mood regulation (reviewed in Ming and Song, 2011; Toda and Gage, 2017). Whether or not adult neurogenesis is relevant also in humans is still an ongoing debate (Kempermann et al., 2018). In consideration of two recent reports that came to opposing conclusions regarding the neurogenic potential in adult humans (Boldrini et al., 2018; Sorrells et al., 2018), it definitely deserves further efforts to elucidate the significance of this process for human DG plasticity.
Trophic Factors and Morphogens Regulating Adult Hippocampal Neurogenesis
The adult SGZ is a specialized microenvironment that provides a wide range of extrinsic signals to preserve the self-renewing population of aNSCs and to ensure on-demand production of new granule cells. Besides the NPCs, the niche comprises a dense vascular network and several cell types, including astrocytes, endothelial cells, microglia and neurons (Massirer et al., 2011; Ming and Song, 2011; Faigle and Song, 2013; Licht and Keshet, 2015). NSCs in turn ensheath synapses and blood vessels and adhere to adjacent astrocytes with their fine processes to sense their local environment (Moss et al., 2016). As extensively reviewed by others (Suh et al., 2009; Faigle and Song, 2013; Choe et al., 2015; Toda and Gage, 2017), the effects of niche components on NSC/NPC are mediated by direct cell contacts and soluble molecules such as growth factors, morphogens, hormones, and neurotransmitters. These cues are concurrently acting on adult SGZ precursors not only to coordinate their self-renewal, expansion and fate decisions, but also to maintain NSCs in a quiescent state to prevent their premature exhaustion. Within this section we briefly recapitulate the pathways most vitally involved in CC control of NPCs and summarize their functions in adult hippocampal neurogenesis.
Brain-derived neurotrophic factor (BDNF) is known to promote adult neurogenesis through acting as an autocrine factor for dendritic maturation, functional integration and long-term survival of newborn granule cells (Bergami et al., 2008; Chan et al., 2008; Wang et al., 2015). BDNF and its receptor complex TrkB/p75NTR are also expressed by dividing SGZ progenitors (Chan et al., 2008; Li et al., 2008; Bernabeu and Longo, 2010). As shown in vitro and in vivo, increased levels of BDNF stimulate the proliferation of hippocampal NPCs, and enhance neurogenesis (Katoh-Semba et al., 2002; Li et al., 2008). It was further observed that NPC-specific deletion of TrkB impairs proliferation of DG progenitors, demonstrating that BDNF and TrkB are required to maintain basal levels of their proliferation (Li et al., 2008). Studies also suggest that increased BDNF signaling serves as mechanism through which exercise, environmental enrichment and antidepressant treatment improve hippocampal neurogenesis and cognition (Rossi et al., 2006; Li et al., 2008).
Fibroblast growth factor 2 (FGF-2) has been extensively studied in vitro where it is required for maintaining adult NPCs in a proliferative state (Gage et al., 1995; Gritti et al., 1996). In vivo studies revealed FGF-2 as potent modulator of proliferation and differentiation. For example, intraventricular administration of FGF-2 caused a strong increase in proliferation and neurogenesis in the SGZ (Jin et al., 2003; Rai et al., 2007). Moreover, the newborn neurons exhibited enhanced dendritic growth, indicating additional roles in neuronal differentiation and maturation (Rai et al., 2007; Werner et al., 2011). Increased astrocytic release of FGF-2 has recently been identified as requirement for the proliferative effects of acute stress (Kirby et al., 2013).
Insulin-like growth factor-1 (IGF-1) regulates various steps of adult SGZ neurogenesis, including proliferation, differentiation and maturation of neurons, perhaps in a dose-dependent manner (Aberg et al., 2003). IGF-1 directly stimulates proliferation and neurogenesis, both in vitro and in vivo (Aberg et al., 2000; Yuan et al., 2015). Peripheral administration of IGF-1 induces an increase of NPC proliferation through activation of their IGF-I receptors (Trejo et al., 2001; Aberg et al., 2003; Yuan et al., 2015). Moreover, the study of Trejo et al. (2001) showed that blocking brain uptake of IGF-1 completely abolishes the neurogenesis-promoting effect of voluntary exercise, suggesting that circulating IGF is an important determinant of exercise-induced changes in DG plasticity.
Vascular endothelial growth factor (VEGF) released from endothelial cells exerts direct mitogenic effects on hippocampal NPCs, as shown after intraventricular infusion of VEGF (Jin et al., 2002; Cao et al., 2004). VEGF activates quiescent aNSCs through an autocrine mechanism and VEGF signaling through VEGFR3 controls the response of aNSCs to voluntary exercise (Han et al., 2015). Congruently, blockade of VEGF signaling abolishes the neurogenic actions of running, environmental enrichment or antidepressant treatment (Cao et al., 2004; Warner-Schmidt and Duman, 2007). Altogether, previous investigations on the role of growth factors in the SGZ support a model in which they act as important mediators linking changes in environmental conditions with the processes of adult neurogenesis.
Morphogens play essential roles for neural patterning, proliferation and fate specification in the developing central nervous system. Many of these factors, like sonic hedgehog (Shh), bone morphogenetic proteins (BMPs), Wnts, and Notch continue to regulate adult NPCs. Their actions often span multiple steps of neurogenesis and differ depending on the specific cellular context. Moreover, many of these morphogen signaling cascades have been shown to cooperate with each other, adding an additional level of complexity to the control of adult neurogenesis (Shimizu et al., 2008; Antonelli et al., 2018; Armenteros et al., 2018).
Bone morphogenetic proteins released by granule neurons and NSCs are essential for maintaining the pool of undifferentiated aNSCs (Mira et al., 2010; Porlan et al., 2013). Beyond that, BMP4 signaling also decelerates the tempo of neurogenesis in later stages of the linage, by directing the transition between activation and quiescence in IPCs (Bond et al., 2014). This and other findings suggest that inhibition of BMP signaling likely represents a mechanism for rapid neuronal expansion in response to behavioral stimulation (Gobeske et al., 2009). Consistently it has been found that endogenous expression of the BMP antagonist Noggin releases NSCs from quiescence to support their proliferation, self-renewal and precursor production (Bonaguidi et al., 2008; Mira et al., 2010). Others discovered that augmented Noggin and BMP4 downregulation mediate the neurogenic and behavioral effects of antidepressants (Brooker et al., 2017). Besides that, BMPs have been shown to control glial fate decisions, having dual functions as promotor of astrogliogenesis and inhibitor of oligodendrogliogenesis (Cole et al., 2016). Accordingly, overexpression of BMP4 in the adult SGZ induces the generation of astrocytes from NSC at the expense of neurogenesis (Bonaguidi et al., 2005).
Notch signaling is reiteratively used to control cell fates during adult neurogenesis in a cell-type specific manner (Ables et al., 2011). It is well established that Notch effector genes Hes1 and Hes5 inhibit differentiation in the CNS by repressing proneural genes (Ohtsuka et al., 1999). Notch1 and Hes5 are highly expressed by aNSC, are absent from neuroblasts to become re-expressed in immature neurons (Stump et al., 2002; Breunig et al., 2007; Ehm et al., 2010; Lugert et al., 2010). Their ligands in turn are found on local astrocytes, IPCs and NSCs (Lavado et al., 2010; Lavado and Oliver, 2014). Inhibition of Notch signaling through manipulation of Notch1, Jagged1 or RBPJκ triggers the activation of quiescent NSC and the production of committed IPCs and neuroblasts, but ultimately exhausts aNSC resulting in premature depletion of their pool (Ables et al., 2010; Ehm et al., 2010; Imayoshi et al., 2010; Lugert et al., 2010; Lavado and Oliver, 2014). This demonstrates crucial roles of Notch signaling for maintaining a reserve of quiescent and undifferentiated NSC throughout life (Chapouton et al., 2010). More specifically, depletion of Jagged1-expressing committed IPCs revealed that Notch signaling serves as homeostatic feedback mechanism that links aNSC maintenance to neuronal differentiation of their progeny (Lavado et al., 2010). Later in the linage, Notch regulates the survival and dendritic morphology in the new, maturing neurons (Breunig et al., 2007).
Wnt ligands are well-established regulators of adult hippocampal neurogenesis (Varela-Nallar and Inestrosa, 2013). Secreted by local astrocytes and NPCs, Wnts act both as autocrine and as paracrine factors on the NSC niche (Lie et al., 2005; Wexler et al., 2009; Qu et al., 2010). Astrocyte-derived Wnt3 promotes neuroblast proliferation and neuronal differentiation via the canonical Wnt pathway (Lie et al., 2005). In IPCs, Wnt can directly induce the proneural gene NeuroD1 (Kuwabara et al., 2009). Accordingly, canonical Wnt signaling is required for neuronal differentiation in vivo, i.e., the transition of Sox2-expressing precursors into neuroblasts. In addition, Wnt signaling emerged as important pathway promoting multipotency and self-renewal of aNSCs (Mao et al., 2009). This is probably achieved through an autocrine signaling loop that has been identified in vitro (Wexler et al., 2009). Under conditions of low network activity, this pathway is repressed through tonic release of the inhibitor sFRP3 from granule cells, which keeps aNSCs quiescent (Jang et al., 2013). Neuronal activity decreases sFRP3 levels in the DG, resulting in aNSCs activation and accelerated maturation of new neurons, providing a mechanism to produce neurons on demand (Jang et al., 2013).
Sonic hedgehog is a member of the hedgehog family of secreted glycoproteins that acts through the patched-smoothened receptor complex to trigger the expression of GLI transcription factors (Ruiz I Altaba et al., 2002). Previous studies found that NSCs residing in the adult SGZ originate from Shh responsive cells in the ventral hippocampus and that Shh signaling is essential to establish their population (Han et al., 2008; Li et al., 2013). Although it is known that quiescent NSCs and their transit-amplifying progeny respond to Shh activity (Lai et al., 2003; Ahn and Joyner, 2005), the source of Shh in the adult DG is still obscure. Li et al. (2013) identified hilar mossy cells and neurons in the medial entorhinal cortex as principal sources of Shh in the postnatal DG. As they analyzed the mice at postnatal day 15, when the SGZ niche is almost fully established, it is conceivable that these neuronal sources sustain their activity far beyond postnatal development to provide Shh for adult SGZ precursors. Several recent reports emphasize the importance of Shh for balancing aNSC maintenance and proliferation (Lai et al., 2003; Machold et al., 2003; Bragina et al., 2010). Activation of Shh signaling elicits a strong, dose-dependent proliferative response, both in vitro and in vivo (Lai et al., 2003; Machold et al., 2003). Functional disruption of primary cilia, which are required for Shh signaling, increases CC exit in aNSCs and decreases the production of IPCs (Breunig et al., 2008; Amador-Arjona et al., 2011). A recent study showed that single copy deletion of patched1 results in deregulation at multiple steps of linage progression that accumulates with aging, including depletion of radial NSC, accumulation of IPCs, and a decrease in neurogenesis (Antonelli et al., 2018).
Basic Principles and Essential Players of the Mammalian Cell Cycle
The eukaryotic CC can be divided into two major periods composed of four consecutive phases: The interphase, in which cells typically spend most of their lifetime, comprising G1 phase, S phase, and G2 phase, and the M phase in which mitosis and cytokinesis occur (Figure 2). The G1 phase is the longest phase and the main period of cell growth. G1 prepares the cell for DNA replication and cell division before it can pass into the S phase, in which the cell duplicates its chromosomes. The S phase is followed by the G2 phase, in which the cell proceeds growing and prepares for entering the M phase that eventually results in the generation of two daughter cells that again enter G1. These cells may immediately commence a new round of the CC, as is the case for rapidly dividing progenitor cells. Other cells, like slowly dividing aNSCs or terminally differentiated neurons, may exit the CC to enter a temporary (quiescent) or permanent (post-mitotic/differentiated) G0 state.
FIGURE 2.
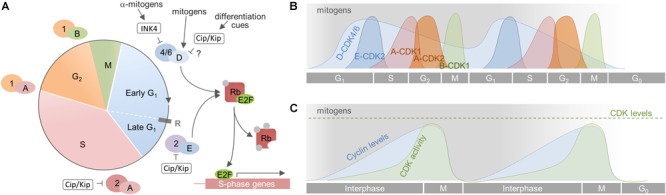
Cell cycle progression in mammalian cells. (A) The CC is divided into 4 phases (G1, S, G2, and M). The G1 phase is further divided in an early mitogen-dependent, and a late mitogen-independent phase, that are separated by the restriction point (R). Progression through the CC is driven by the sequential activation of CDKs by cyclins. The CC is initiated by mitogens [indicated by gray shading in (B) and (C)] that induce synthesis of D-cyclins, which in turn activate CDK4/6. This initiates phosphorylation of the tumor suppressor Rb that subsequently liberates E2F. This transcription factor transcribes genes necessary for the transition to S phase, including cyclin E which associates to CDK2 to fully inactivate Rb. All subsequent steps are independent from mitogen supply. (B) Activity dynamics of distinct cyclin-CDK complexes throughout the CC. Cyclin D-CDK4/6 complexes are active for as long as mitogens are present. Their upregulation induces expression of cyclin E and a subsequent autonomous sequence of cyclin expression and CDK activation. If mitogens are withdrawn, cyclin D-CDK4/6 complexes become inactive and the cell cannot transit through the next G1 Rather, and depending on the cellular context, it becomes quiescent, senescent or terminally differentiates (G0). (C) Cumulative cyclin expression and CDK activity throughout the CC. A, B, D, E – cyclins A, B, D, E; 1, 2, 4/6 – CDKs 1, 2, 4/6.
Unidirectional progression through the CC is driven by the coordinated activation and inactivation of cyclin-dependent kinases (CDKs) through association with cyclins and cyclin-dependent kinase inhibitors (CKIs; Figure 2). CDKs are serine/threonine kinases that require the binding of regulatory subunits, i.e., cyclins, and phosphorylation by CDK-activating kinases (CAKs) to exert their catalytic function (Sherr, 1995; Johnson and Walker, 1999; Lolli and Johnson, 2005; Malumbres, 2014). CDKs and CAKs are abundantly expressed and their levels remain fairly stable throughout the CC, consequently they are not rate-limiting for its progression (Lees, 1995; Morgan, 1995). Cyclin levels in turn fluctuate through synthesis and degradation and, in this way, periodically activate CDKs (Matsushime et al., 1994; King et al., 1996; Udvardy, 1996). The activated cyclin-CDK complexes phosphorylate multiple downstream targets, ensuring appropriate ordering of cell-cycle events (Swaffer et al., 2016). Depending on the activity of cyclin-CDK complexes in the G1 phase, cells will commit to divide or withdraw from CC (Blagosklonny and Pardee, 2000–2013, 2002). This decision depends on extracellular signals like mitogens and growth factors that induce the expression of D-cyclins (Matsushime et al., 1994; Sherr, 1994a; Ekholm and Reed, 2000). The D-cyclins assemble with CDK4 or CDK6 to drive progression through the G1 phase. Cyclin D-CDK complexes initiate the phosphorylation of Rb proteins and thus weaken their growth suppressive effect by releasing E2F transcription factors. E2Fs then start to transcribe various genes required for G1/S phase transition, including DNA polymerase α, cyclins E and A (Sherr, 1995; Lundberg and Weinberg, 1998; Julian and Blais, 2015). Assembly of cyclin E with CDK2 during later G1 further phosphorylates Rb, forming a positive feedback loop that fully activates the transcription of genes essential for DNA replication and onset of S phase (Lundberg and Weinberg, 1998; Johnson and Walker, 1999). Activation of cyclin E/CDK2 complexes demarks the point at which commitment occurs and cells do no longer require mitogens to complete division. This point, termed the restriction point, represents a point of no return and divides G1 into an early, mitogen-dependent phase and a late, mitogen-independent phase (Johnson and Walker, 1999; Blagosklonny and Pardee, 2000–2013, 2002). Soon thereafter, cyclin A interacts with CDK2 to turn off E2F-mediated transcription and drive the cell through S phase (Krek et al., 1994; Xu et al., 1994). In late S phase, cyclin A associates with CDK1 to facilitate transition to and progression through G2 and to prepare the cells entry into the M phase (Yam et al., 2002; Lindqvist et al., 2009). Finally, cyclin B builds an active complex with CDK1, termed M phase promoting factor, which coordinates the onset of M phase and the reorganization of cell structures required for mitosis and cytokinesis (Nigg, 2001; Gavet and Pines, 2010). As the newly born cells exit mitosis, cyclin B is degraded and the CC is reset, allowing the establishment of a new replication-competent state, i.e., G1 (King et al., 1996; Sherr and Roberts, 1999). Important to note, cyclins, CDKs and CKIs carry out important functions beyond CC regulation, either complexed or individually, such as transcription, stem cell self-renewal, differentiation, neuronal function, cell death, or metabolism (Lim and Kaldis, 2013; Pestell, 2013; Hydbring et al., 2016).
D-Cyclins
D-cyclins are the first cyclins induced when quiescent cells become stimulated to enter the CC (Matsushime et al., 1994; Sherr, 1994a,b). Unlike other cyclins that are periodically expressed during the CC, D-cyclins are induced by extracellular mitogens, including the above mentioned niche factors (Bottazzi and Assoian, 1997; Perry et al., 1998; Ekholm and Reed, 2000; Baek et al., 2003; Frederick and Wood, 2004; Campa et al., 2008; Shimizu et al., 2008; Chen et al., 2018), and hence are regarded as crucial direct link between the extracellular environment and the CC machinery (Matsushime et al., 1994). They comprise a family of three homologous proteins (cyclin D1, D2, and D3) that bind to and thereby activate either CDK4 or CDK6 (Sherr and Roberts, 1999, 2004). Extensive studies in knockout mice uncovered that D-cyclins are, to a great extent, functionally redundant but that each has unique tissue-specific functions (Wianny et al., 1998; Sherr and Roberts, 2004; Satyanarayana and Kaldis, 2009). Intriguingly, not all cells require D-cyclins/cyclin D-CDK complexes for proliferation and embryos lacking all three D-cyclins develop normally until midgestation (Kozar et al., 2004). D-cyclins drive cells through the G1 restriction point, after which mitogen stimulation is no longer required to complete the cycle. They are unstable proteins that become rapidly degraded via the ubiquitin/proteasome pathway if mitogens are withdrawn (Diehl et al., 1997; Ekholm and Reed, 2000). According to the classical model of G1 progression, active cyclin D/CDK complexes inactivate the Rb tumor suppressor through gradual hypo-phosphorylation to liberate E2F transcription factors required for CC progression (Mittnacht et al., 1994; Sherr and Roberts, 1999). This view has been recently challenged by a study demonstrating that cyclin D-CDK complexes do not inactivate, but instead activate Rb during early G1 through mono-phosphorylation (Narasimha et al., 2014). Hyper-phosphorylation of Rb through Cyclin E/CDK, which inactivates Rb to ensure E2F-dependent transcription in later G1, seems to be independent from that mono-phosphorylation. Instead, Narashima et al. (2014) propose that Rb-mono-phosphorylation by cyclin D-CDK complexes keeps cells in an “alert” state, priming them for CC entry and preventing CC exit. The debate was fueled again by subsequent work that suggests that cyclinD-CDK4/6 complexes control the timing of onset of E2F activity, indicating that D-cyclins indeed are the main regulators of G1 length, rather than being the initiator of CC entry (Dong et al., 2018). Despite this controversy on being drivers or modulators of G1 progression, D-cyclins are consistently regarded as crucial link between extracellular growth-stimulating signals and the CC machinery. In addition to their role in modulating Rb activity, cyclin D-CDK complexes have also kinase-independent CC functions in titrating away inhibitors p21cip1 and p27kip1 from CDK2-containing complexes to facilitate cyclin E-CDK activation and coordinated CC progression in later G1 (Sherr and Roberts, 1999; Kozar and Sicinski, 2005).
Cyclin-Dependent Kinases (CDKs)
To this day, eleven different CDKs have been related to the mammalian CC, out of which at least five (CDK1, 2, 3, 4, and 6) are directly involved in its progression (Satyanarayana and Kaldis, 2009; Malumbres, 2014). Contrasting many of the initial in vitro studies that showed specific requirements for each interphase CDK in cycle progression, mice lacking a single CDK survive, indicating profound functional redundancy among CDKs (Sherr and Roberts, 2004; Satyanarayana and Kaldis, 2009; Malumbres, 2014). Even mouse embryos lacking all interphase CDKs undergo organogenesis and develop until midgestation. This is possible because CDK1, by complexing with all necessary cyclins, can execute all crucial events required for cell division (Santamaria et al., 2007). Nevertheless, single-CDK knockout mice display more or less narrow, tissue-specific deficits demonstrating that CDKs have different roles and cannot fully compensate for each other (Sherr and Roberts, 2004; Kozar and Sicinski, 2005; Malumbres, 2014). In addition to cyclin binding, different mechanisms are engaged in the control of cyclin-CDK complexes, including phosphorylation through constitutively active CAKs and binding of CKIs (Lees, 1995; Morgan, 1995; Ekholm and Reed, 2000; Malumbres, 2014). Importantly, cyclin binding determines the timing of activation and contributes to substrate specificity of CDKs (Morgan, 1995; Satyanarayana and Kaldis, 2009).
CDK Inhibitors (CKIs)
CDK Inhibitors are generally assumed as negative CC regulators that constrain the activities of CDKs in response to anti-mitogenic factors or starvation. Thus, CKIs in addition to D-cyclins confer a second layer of CC control by extracellular signals. They comprise two families of inhibitory proteins that differ in structure and substrate specificity (Arellano and Moreno, 1997; Sherr and Roberts, 1999; Besson et al., 2008).
Members of the INK4 (INhibitors of CDK4) family (p16INK4a, p15INK4b, p18INK4c, and p19INK4d) act as brakes of G1 progression in response to mitogenic withdrawal, differentiation signals and other growth inhibiting cues (Koff et al., 1993; Kato et al., 1994; Polyak et al., 1994). They bind exclusively to and inhibit CDK4 and CDK6 to prevent their interaction with cyclin D (Guan et al., 1994; Hirai et al., 1995; Sherr and Roberts, 1999; Jeffrey et al., 2000; Pei and Xiong, 2005), but with different preferences. P18INK4c preferentially interacts with CDK6, whereas p16INK4a associates with both CDKs (Guan et al., 1994; Noh et al., 1999). INK4 proteins differ also in their regulation and function. P15INK4b is induced by growth-inhibitory factors such as TGFβ and contributes to their ability to induce growth arrest (Reynisdottir et al., 1995; Sherr and Roberts, 1999). P16INK4a is ubiquitously expressed at low levels in most tissues and accumulates as cells age. It becomes induced during cellular senescence, in response to oncogenic stimuli or inactivation of Rb (Serrano, 1997; Sherr and Roberts, 1999). P18INK4c and p19INK4d, even if sensitive to extrinsic signals, oscillate throughout the CC with maximum levels during S phase (Hirai et al., 1995; Roussel, 1999). Both are found in proliferating cells in which they facilitate CC exit during terminal differentiation (Hirai et al., 1995; Roussel, 1999). The INK4A locus is unique in that it transcribes another protein, p19ARF that is also associated with cellular senescence (Lowe and Sherr, 2003). Instead of binding CDKs, p19ARF confers its role through both, activation of p53 and inhibition of c-Myc (Quelle et al., 1995; Roussel, 1999; Qi et al., 2004).
In contrast, Cip/Kip proteins (p21cip1, p27kip1, and p57kip2) act more broadly by modulating the activities of cyclin D-, E- and A-dependent kinases. Cip/Kip proteins bind to preformed cyclin-CDK complexes and block their substrate access (Russo et al., 1996; Sherr and Roberts, 1999). They most effectively inhibit complexes containing CDK2 and thereby prevent S phase entry and transition (Sherr and Roberts, 1999). Discordance exists regarding their effects on cyclin D-CDK complexes. In addition to reports arguing in favor of a pan-CDK-inhibitory role of Cip/Kip proteins (Bagui et al., 2003; Pei and Xiong, 2005; Cerqueira et al., 2014), others suggest that they act as assembly and stabilization factors of cyclin D-CDK4/6 to facilitate cyclin D-dependent CC events (LaBaer et al., 1997; Cheng et al., 1999; Parry et al., 1999; Sherr and Roberts, 1999). Despite their general importance for restraining cell proliferation, each Cip/Kip protein has unique functions that distinguish it from the other family members. Expression and functional studies suggest that p21cip1 has a predominant role in DNA-damage-induced CC arrest, while p27kip1 inhibits cell growth and maintains cells in a quiescent state, and p57kip2 regulates growth and differentiation (Deng et al., 1995; Nakayama et al., 1996; Zhang et al., 1997; Besson et al., 2008). Unlike its siblings, p57kip2 expression is more restricted and under control of morphogen pathways such as Notch and BMP (Besson et al., 2008). A unique feature of p21cip1 and p57kip2 is their ability to inhibit PCNA (Zhang et al., 1993; Besson et al., 2008). This allows them to coordinately arrest the CC by both, preventing E2F-dependent transcription and inhibiting PCNA-dependent replication (Cayrol et al., 1998). During the past years Cip/Kip proteins have emerged as versatile factors with functions beyond cycle regulation, including direct transcriptional regulation (e.g., E2F and STAT3), fate determination and cell death control (Besson et al., 2008).
Rb Proteins and E2F
Rb and its family members p107 and p130, collectively known as “pocket proteins,” are placed at the core of the molecular machinery that regulates the G1 restriction point (Dick and Rubin, 2013). They impose a constitutive barrier on CC progression through sequestration of E2F transcription factors, for which each Rb protein displays distinct binding preferences (Hurford et al., 1997). Rb interacts specifically with “activator” E2Fs 1–3, whereas p107 and p130 interact with “repressor” E2Fs 4 and 5 (Mulligan and Jacks, 1998; Popov and Petrov, 2014). Importantly, growing evidence suggests that E2F activities are context-dependent, e.g., switching from activators in progenitors to repressors in differentiating cells (Chong et al., 2009). Another characteristic distinguishing Rb proteins is their allocation to different CC states: while Rb is constitutively expressed in all cells, acting as bona fide tumor suppressor, p107 predominates in cycling cells, and p130 is most prevalent in quiescent and differentiated cells (Mulligan and Jacks, 1998; Dick and Rubin, 2013). Moreover and in contrast to Rb, p107 and p130 confer growth suppression through another mechanism involving direct inhibition of CDK2-containing complexes (Lees et al., 1992; Woo et al., 1997). Beyond their direct role in CC progression, Rb proteins can interact with many other proteins to retain cells in G0/G1, such as histone deacetylases 1 and 2, histone methyltransferases, cyclins and complexes of the SWI/SNF nucleosome remodeling complex (Brehm et al., 1998; Mulligan and Jacks, 1998; Robertson et al., 2000; Strobeck et al., 2000). In addition to their CC regulatory function, Rb and E2Fs are capable of regulating genes and processes with much broader range of function, many of which directly instruct cell fate decisions (Julian and Blais, 2015). Accordingly, Rbs and E2Fs emerged as essential regulators of stem cell fate in a number of lineages (Sage, 2012; Julian and Blais, 2015). Rb deletion is sufficient to induce CC re-entry in various cell types, suggesting that it helps to maintain quiescence also in aNSCs (Sage et al., 2003; Sage, 2012). Furthermore Rb’s capacity to modulate chromatin structure has been proposed as important regulator of stem cell plasticity (Chinnam and Goodrich, 2011; Sage, 2012).
Specific Roles of Cell Cycle Components in Regulating Adult Hippocampal Neurogenesis
D-Cyclins
All three D-cyclins are dynamically expressed during neurulation (Wianny et al., 1998). Later in brain development, D3 becomes gradually lost, whereas D1 and D2 activities are maintained to regulate the generation of distinct progenitor populations (Sun et al., 1996; Bryja et al., 2004; Glickstein et al., 2007a; Komada et al., 2013). Recent studies suggest that, beside their role in proliferation, D-cyclins and D1 in particular may directly control stem cell fate decisions to induce neuronal differentiation (Lukaszewicz and Anderson, 2011; Pauklin and Vallier, 2013; Pauklin et al., 2016). Apparently, this is accomplished by two complementary mechanisms: D-cyclins cross-talk with the Activin/Nodal–Smad2/3 signaling pathway through CDK4/6 and also directly bind to transcription factors that suppress endoderm and activate neuroectoderm genes in stem cells (Pauklin and Vallier, 2013; Pauklin et al., 2016). Further, D1 has been identified as critical component of FGF-2 and Wnt signaling that inhibits astroglial differentiation of NSCs (Bizen et al., 2014).
During embryogenesis, D1 and D2 appear to have complementary roles in proliferation and differentiation of NPCs (Glickstein et al., 2007a,b, 2009; Lukaszewicz and Anderson, 2011). Initially it was shown that D1 and D2 define separate progenitor pools in the embryonic neocortex (Ross, 1996; Glickstein et al., 2007a). Whereas D1 is predominantly expressed in the VZ, D2 is localized mainly to the SVZ (Glickstein et al., 2007a). More detailed studies revealed that D1 and D2 co-label subsets of Pax6 expressing radial glia/apical precursors, which are the stem cells in the embryonic brain (Glickstein et al., 2009). As these cells commit to the neuronal linage, D1 is gradually downregulated and D2 becomes induced (Glickstein et al., 2009; Lange et al., 2009). The selective expression of D2 in Tbr2-positive IPCs/basal precursors and their loss upon genetic deletion of D2 suggest that D2 is selectively used for the expansion of the IPC pool (Glickstein et al., 2009). Withal, the knockout also diminishes the radial glia population. The same study revealed severe disturbances in CC dynamics in progenitors of D2 mutants, such as lengthening of G1, decreased in-cycle and increased cycle-exit fraction of IPCs, consistent with premature terminal differentiation. Interestingly, deletion of D2 not only altered G1 length but it also shortened the S phase. In contrast, CC parameters and numbers of Pax6 and Tbr2-positive precursors in the cortical neuroepithelium remained unchanged in D1 mutants (Chen et al., 2005; Glickstein et al., 2007a, 2009). This shows that D2, perhaps in conjunction with other CC proteins, can compensate for the loss of D1 but not vice versa (Carthon et al., 2005; Chen et al., 2005). Collectively, these findings show that D2 is more essential for cortical development than D1. They fit a concept in which D2 is required for expansive divisions of radial glia and IPCs whereas D1 drives asymmetric stem cell divisions to maintain their pool (Glickstein et al., 2009). Supporting the progenitor-specific requirement of D2 in brain formation, D2 mutant mice also display a disproportionate loss of parvalbuminergic interneurons in the neocortex and hippocampus, as well as loss of half of cerebellar granule neurons and virtually all cerebellar stellate interneurons (Huard et al., 1999; Glickstein et al., 2007b; Leto et al., 2011). Direct in vivo evidence for a CC-independent proneurogenic function of cyclin D1, as originally discovered in human pluripotent stem cells (Pauklin and Vallier, 2013; Pauklin et al., 2016), comes from loss- and gain-of-function experiments in the embryonic spinal cord, where D1 is expressed by differentiating NPCs and newly generated neurons (Lukaszewicz and Anderson, 2011). Knockdown of D1, but not that of D2, decreased neuronal fate specification and differentiation through activating the non-canonical Notch effector Hes6, whereas overexpression had the opposite effect. D2, on the other hand, promotes proliferation and maintains the undifferentiated progenitor pool, probably through regulation of the canonical Notch effector Hes5 (Lukaszewicz and Anderson, 2011). Strikingly, studies in the embryonic cortex have shown that asymmetrically inherited D2 may act as fate determinant in radial glia of the VZ (Tsunekawa et al., 2012). During division, these cells allocate D2 mRNA to their basal end-feet (Glickstein et al., 2007a; Tsunekawa et al., 2012). The daughter inheriting the basal process and thus D2 upon asymmetric mitosis will become another radial glia cell that maintains self-renewal capacity, while its sibling will differentiate into an IPC or a neuron (Tsunekawa et al., 2012). The molecular mechanisms are not fully understood, but the biased localization of D2 to one daughter might shorten her G1 phase. Alternatively, D2 might control cell fate independent from its direct action on CC progression. D2 might advance proliferation and inhibit neurogenesis through elimination of the CKI p27kip1, which promotes neuronal differentiation (Nguyen et al., 2006; Susaki et al., 2007; Tsunekawa et al., 2012). In opposition to neocortical development, both D-cyclins are expressed in the VZ of the early hippocampal primordium (Glickstein et al., 2007a). At perinatal ages, they are also found in the dentate anlage, but D1 predominates in the secondary and tertiary matrices, while more D2-positive neuroblasts are found in the dentate migratory stream. Postnatally, D1 and D2 are found in the tertiary matrix and the emerging SGZ. In the adult DG, D2 expression is restricted to the SGZ whereas D1 shows a more widespread, scattered expression throughout all layers of the DG (Kowalczyk et al., 2004; Glickstein et al., 2007a).
In accordance with the apparently distinct roles of cyclins D1 and D2 during development, absence of either cyclin is associated with strikingly different outcomes in the adult brain. As mentioned before, mice lacking D1 have essentially normal brain sizes with no selective deficits in particular regions, except a cerebellar hypoplasia (Chen et al., 2005; Pogoriler et al., 2006; Glickstein et al., 2007b, 2009). The gross architecture of D2 mutant brains is also normal, but these mice are microcephalic (about 25% reduced size), and especially the cortex, cerebellum, olfactory bulb and hippocampus are smaller in size (Kowalczyk et al., 2004; Glickstein et al., 2009; Ansorg et al., 2012). The lack of apparent deficits in D1 knockout mice may result from compensatory mechanisms, including the upregulation of other G1 cyclins, i.e., cyclin D2 and cyclin E (Carthon et al., 2005; Chen et al., 2005; Glickstein et al., 2007a).
Cyclin D1 is expressed in the adult SGZ. Some of the D1 cells proliferate as indicated by incorporation of the S phase label bromodeoxyuridine (Glickstein et al., 2007a). Studies evaluating the consequences of D1 deletion on hippocampal cell proliferation came to conflicting results, with one showing no effect on proliferation, and another reporting >40% reduction of BrdU incorporation into the adult SGZ (Kowalczyk et al., 2004; Ma et al., 2010). In support of a role of D1 in aNPC proliferation, Ma et al. (2010) found that cultured precursor cells of D1 knockout mice become arrested in G0/G1. In addition, mutated precursors displayed increased apoptosis and disturbed differentiation into astrocytes, while neuronal differentiation remained intact (Ma et al., 2010). Yet, the overall picture of D1 regulation of adult neurogenesis is far from clear and needs further investigation. For instance, none of the previous studies had looked on the specific requirements of D1 in distinct precursor types as has been published for the embryonic brain. Evaluating such roles is complicated through the fact that D2 becomes upregulated and might compensate in the SGZ of D1 mutant mice (Carthon et al., 2005; Glickstein et al., 2007a).
Available data on the role of cyclin D2 in adult hippocampal neurogenesis are more consistent. The SGZ of adult D2-deficient mice is virtually devoid of proliferating cells and neuroblasts (Kowalczyk et al., 2004; Ansorg et al., 2012). We showed that the requirement of D2 for adult neurogenesis builds up progressively during postnatal development. With full maturity of the DG/SGZ around 4 weeks of age, mutant mice are virtually devoid of newly born neurons in this region (Altman and Bayer, 1990; Ansorg et al., 2012; Nicola et al., 2015). Moreover, this impairment in adult neurogenesis cannot be overcome through exposure to physiological or pharmacological stimuli that normally increase adult hippocampal neurogenesis. For instance, numbers of cells incorporating BrdU remained negligible in response to environmental enrichment or fluoxetine treatment (Kowalczyk et al., 2004; Jedynak et al., 2012). So far it is not clear whether this deficit results from the inability of mutant SGZ precursors to divide or from a lack of precursors that can respond to such neurogenic stimuli (Ansorg et al., 2012). Interestingly, despite of their severe impairments in adult neurogenesis, D2KO mice are able to master hippocampus-dependent tasks, including contextual or trace fear conditioning, object recognition, and spatial learning in Barnes or water mazes (Jaholkowski et al., 2009; Jedynak et al., 2012; Ben Abdallah et al., 2013; Urbach et al., 2013). However, impairments became apparent in tasks requiring a certain degree of flexibility for integrating novel information into previously learned contexts (Garthe et al., 2014). Clinically relevant, loss-of-function mutations affecting the ccnD2 locus have been linked to brain pathologies like microcephaly and epilepsy (by loss of interneurons; Glickstein et al., 2009). In humans, de novo ccnD2 mutations stabilizing D2 have been identified as cause of the megalencephaly polymicrogyria-polydactyly hydrocephalus syndrome (Mirzaa et al., 2014).
In summary, available data suggest that D1 and D2 have discrete, non-overlapping functions in adult neurogenesis. Considering recent observations in human embryonic or pluripotent stem cells, available data suggest that the main function of D2 is controlling adult precursor proliferation, whereas D1 seems to be rather involved in neuronal differentiation (Lukaszewicz and Anderson, 2011; Pauklin and Vallier, 2013; Pauklin et al., 2016).
Cyclin-Dependent Kinases (CDKs)
All three G1 CDKs have been detected in apical and basal precursors of the embryonic VZ/SVZ (Telley et al., 2016). However, only combinatorial ablation of two G1 CDKs affected cortical progenitor proliferation in vivo, suggesting redundant or compensatory functions of CDKs during corticogenesis (Lim and Kaldis, 2013; Grison et al., 2018). Moreover, the effects of CDK2/4, CDK2/6, or CDK4/6 double-knockouts were progenitor type-dependent, indicating that discrete combinations of CDKs are used in distinct precursor populations (Lim and Kaldis, 2013; Grison et al., 2018).
CDK2 kinase activity is high during cerebral development but drops rapidly thereafter (Carey et al., 2002). This suggests important functions of this enzyme in cortex development, which has been corroborated by several in vitro studies. For instance, cultured cortical precursors increased CDK2 activity upon FGF-2 stimulation (Lukaszewicz et al., 2002; Li and Dicicco-Bloom, 2004). Also, inhibition of CDK2 activity through adenoviral delivery of double-negative CDK2 mutants induced complete growth arrest in isolated NSC from embryonic forebrain (Ferguson et al., 2000). Nevertheless, no developmental deficits or impaired proliferation were observed in the SVZ of CDK2 mutant mice (Jablonska et al., 2007; Grison et al., 2018). Even the concomitant loss of CDK6 had no effect on the proliferation rate of cortical precursors, suggesting functional compensation by CDK1, CDK4 or other CDKs. Indeed, a subsequent study found altered CC parameters and increased neurogenic divisions in NSCs of CDK2/CDK4 double knockout embryos (Lim and Kaldis, 2012). This prevented the expansion of the basal IPC pool culminating in a striking thinning of the embryonic cortex. Nevertheless, isolated embryonic precursors of these mice retained their capacity to divide due to compensatory upregulation of CDK1 (Lim and Kaldis, 2012). CDK2 appears also dispensable in the neurogenic niches of the adult brain. Functional deletion of CDKs had neither an effect on proliferation, nor on differentiation or survival of hippocampal NPCs, both under basal and under seizure conditions (Vandenbosch et al., 2007). Others showed that CDK2 is expendable for proliferation of NSCs and neuroblasts in the adult SVZ (Jablonska et al., 2007; Caillava et al., 2011). Instead, these loss-of-function studies revealed an involvement of CDK2 in the proliferation, lineage commitment and differentiation of oligodendrocyte precursors in the adult SVZ (Jablonska et al., 2007; Caillava et al., 2011).
Two recent studies indicate that CDK4 drives IPC expansion in the embryonic SVZ between E13.3 and E15.5 (Lim and Kaldis, 2013; Grison et al., 2018). This, however, became only apparent if CDK4 was depleted together with another G1 CDK, i.e., CDK2 or CDK6. Interestingly, any of these combinatorial depletions left the population of apical NSCs in the VZ intact (Lim and Kaldis, 2013; Grison et al., 2018). Yet, adult single mutants are microcephalic (Beukelaers et al., 2011), pointing toward important non-redundant functions of CDK4 at developmental time points not investigated in the before mentioned studies. CDK4 is also expressed in dividing precursors of the adult neurogenic niches (Beukelaers et al., 2011), but like during development the picture is far from clear. Initial functional evidence came from loss- and gain-of-function experiments that described an involvement of cyclin D-CDK4 in fate specification of embryonic and adult NSCs (Lange et al., 2009; Artegiani et al., 2011). They observed that overexpression of cyclin D1-CDK4 reduces G1 length and neurogenesis in the adult SGZ as well as in the embryonic cortex, while RNAi-mediated silencing of cyclin D1-CDK4 lengthens G1 and increases neuronal differentiation during corticogenesis. However, Calegari and his group manipulated CDK4 together with D1, so no clear conclusions on the role of either of them can be drawn from these studies (which, by the way, was not the their aim). Rather, they provided persuasive evidence for a link between G1 length and differentiation of NSC (Lange and Calegari, 2010; Salomoni and Calegari, 2010). More direct evidence was presented by subsequent in vitro studies (Roccio et al., 2013; Chirivella et al., 2017). Inhibition of CDK4 in precursors isolated from the adult SGZ or SVZ proportionally increased the number of cells in G1 and forced neuronal differentiation. Recently, activation of CDK4 has been identified as mechanism of the proliferative effect of insulin in aNSC (Chirivella et al., 2017). Unexpectedly, this study also implies that CDK4 activity in response to insulin can promote terminal differentiation of NSCs. Yet, CDK4 appears to be dispensable in vivo. Although CDK4 is expressed by virtually all Ki67-positive cells in the adult SGZ, its loss had no effect on CC parameters, proliferation or neurogenesis (Beukelaers et al., 2011).
Available evidence for a role of CDK6 in brain development is more or less indirect. A study in mice revealed that downregulation of CDK6 by Gli3 is required for the scheduled onset of cortical neurogenesis (Hasenpusch-Theil et al., 2018). In humans, mutations in the cdk6 locus have been linked to autosomal recessive primary microcephaly (Naveed et al., 2018), suggesting important functions in the expansion of cortical precursor cells. However, brains of CDK6 knockout mice exhibit no obvious phenotype despite smaller olfactory bulbs (Beukelaers et al., 2011), and even the concomitant deletion of CDK2 did not interfere with proliferation of cortical progenitors in mouse embryos (Grison et al., 2018).
In the adult SGZ, CDK6 displays essentially the same expression pattern as CDK4 (Beukelaers et al., 2011). Genetic inactivation of CDK6 reduced proliferation of SGZ precursors by >50%. Loss of CDK6 specifically prevents the expansion of neuronally committed precursors by lengthening G1 phase duration and premature CC exit, resulting in decreased neurogenesis (Beukelaers et al., 2011). The group of Caron et al. (2018) then asked for the mechanism of CDK action in adult SGZ precursors. By generating mice bearing a kinase-dead allele of CDK6 they demonstrated that the function of CDK6 in hippocampal neurogenesis relies essentially on its kinase activity. They also observed that p27cip1 is the main inhibitor of CDK6 activity in hippocampal progenitors.
Altogether, even if both early G1 phase CDKs are widely expressed in the adult neurogenic niches, available data imply that they are not functionally redundant. Whereas CDK4 appears to be the prevailing CDK during corticogenesis, CDK6 exerts important functions in the adult DG. It needs to be determined whether the distinct outcomes of manipulating CDK4 and CDK6 are caused by intrinsically different functions of CDK4 and CDK6 (e.g., as transcription factor or through site-specific mono-phosphorylation of Rb), by selective interactions with regulatory D-cyclins, CAK and CKIs, or other mechanisms (Grossel and Hinds, 2006; Bryja et al., 2008; Bockstaele et al., 2009; Kollmann et al., 2013). In support of the first, it has been shown that CDK4 and CDK6 phosphorylate Rb with different residue selectivity (Takaki et al., 2005; Satyanarayana and Kaldis, 2009). In addition to its role in proliferation, findings suggest that CDK6 prevents terminal differentiation in a variety of cell types. This function, which involves phosphorylation of fate-determining transcription factors, is not shared with CDK4 (Grossel and Hinds, 2006). For instance, expression of CDK6, but not CDK4, in primary astrocytes resulted in expression of progenitor cell markers and dedifferentiation (Ericson et al., 2003). Moreover, CDKs can directly inhibit pro-neuronal transcription factors as demonstrated in frog eggs (Ali et al., 2011). There, rising CDK levels quantitatively phosphorylate Ngn2 on multiple sites which inhibits neuronal differentiation.
CDK Inhibitors (CKIs)
INK4 family members are dynamically expressed during mouse development and adulthood (Zindy et al., 1997a,b; Canepa et al., 2007). P15INK4b and p16INK4a are first detected postnatally. Whereas p16INK4a becomes upregulated in all organs as mice age, p15INK4b is more restricted and lacking from brain (Zindy et al., 1997a,b). It is assumed that the ubiquitous increase in p16INK4a is important for preventing neoplastic transformation in later life (Serrano et al., 1996). On the contrary, p18INK4c and p19INK4d are steadily expressed during development and adulthood (Zindy et al., 1997a). Both are detectable also in embryonic and postnatal brains, albeit in disparate patterns (Zindy et al., 1997b).
In the developing cortex, p18INK4c is restricted to proliferating neuroblasts around the time when they lengthen G1 (Zindy et al., 1997b). Postnatally, p18INK4c is expressed by neuroblasts of the DG, but falls below detectable levels with reaching adulthood (Zindy et al., 1997b). Accordingly, loss of p18INK4c did neither impair proliferation nor neurogenesis in the adult SGZ (Caron et al., 2018).
P19INK4d, on the other hand, is highly expressed in adult brain (Zindy et al., 1997a,b). However, p19INK4d is restricted to postmitotic neurons, independent of developmental age (Zindy et al., 1997a,b). Here, it actively represses CC re-entry together with the Cip/Kip family member p27kip1 (Zindy et al., 1997b).
P16INK4a is the only INK4 family member displaying an essential role in adult neurogenesis. Supporting the expression data from embryonic brain, deletion of p16INK4a had no obvious effect on brain development nor on proliferation and self-renewal capacity of NSCs from young adult mice, both, in vivo and in vitro (Bachoo et al., 2002; Molofsky et al., 2006). In the aging SVZ, however, studies show that accumulation of p16INK4a contributes to the progressive decline of NSC function (Molofsky et al., 2006; Nishino et al., 2008). Deletion of p16INK4a partially reverses this phenotype by increasing both, the self-renewal capacity of NSCs and precursor proliferation in the SVZ of aged mice (Molofsky et al., 2006). In support of these findings, overexpression of p16INK4a in NSCs from the embryonic SVZ strongly impairs their self-renewal capacity (Nagao et al., 2008). On the contrary, studies found no evidence for an involvement of p16INK4a in the aging-related decline in adult hippocampal neurogenesis (Molofsky et al., 2006). Rather, as demonstrated in a very recent report, p16INK4a appears to prevent aNSC’s release from quiescence when neurogenic stimuli are present (Micheli et al., 2019). They showed that in the DG of middle-aged p16INK4a knockout mice, running highly increased aNSC numbers as well as IPCs through forcing their entry to CC, suggesting that p16INK4a plays a role in the maintenance of aNSCs after a neurogenic stimulus, to keep a reserve of their self-renewal capacity during aging (Micheli et al., 2019). Moreover, p16INK4a deletion counteracts the disruption of DG precursor proliferation after irradiation, demonstrating that p16INK4a expression is a mechanism mediating the radiation-induced loss of neurogenesis (Le et al., 2018). Another study showed that p16INK4a acts as a barrier to direct neuronal transdifferentiation and functions in the linage-restriction of astrocytes (Price et al., 2014). Altogether, in the light of INK4 family members being the main inhibitors of CDK4/6, their physiological significance in adult neurogenesis appears comparably low, with the exception of p16INK4a that emerged as important regulator of NSC self-renewal in the aging brain. However, whereas the aging-related rise in p16INK4a expression contributes to the progressive decline of NSC function and regenerative capacity in the SVZ, it helps to maintain the pool of quiescent NSCs in the DG through protecting them from excessive activation by neurogenic stimuli.
In accordance with their wider range of CDK inhibitory activity as compared to INK4 proteins, the Cip/Kip family members are vitally involved in a broad spectrum of cell fates, which is even extended through a range of CC-independent actions. The three Cip/Kip CKIs have distinct and overlapping functions, that either are surprisingly stable between different niches (e.g., for p27) or vary depending on time and context (e.g., for p21).
Contrary to p27kip1 and p57kip2, p21cip1 expression in the developing cortex is weak, suggesting it plays no major role during development (Van Lookeren Campagne and Gill, 1998; Tury et al., 2011). P21cip1 is expressed in the stem cell niches of the adult brain but, as seen for many other CC regulators, it has regionally distinct functions. In the adult SVZ, p21cip1 is required to balance quiescence, proliferation and differentiation of NSCs (Kippin et al., 2005; Marques-Torrejon et al., 2013; Porlan et al., 2013). Loss of p21cip1 results in CC shortening and cumulative hyperproliferation of aNSCs, leading to impaired long-term self-renewal and premature exhaustion of NSCs in aged mice (Kippin et al., 2005). The knockout had no effect on the proliferation of IPC, suggesting that the functions of p21cip1 are highly specific for aNSCs. The mechanisms involved in p21cip1-dependent regulation of NSCs were evaluated by two subsequent studies (Marques-Torrejon et al., 2013; Porlan et al., 2013). The first found that p21cip1 acts as transcriptional repressor of Sox2 in aNSCs, thereby preventing replicative stress and exhaustion of the NSC pool (Marques-Torrejon et al., 2013). Later, the same group reported that p21cip1 maintains aNSC in an undifferentiated, multipotent state through transcriptional repression Bmp2 (Porlan et al., 2013). Strikingly, p21cip1-dependent transcriptional regulation of both genes emerged to be independent from its role as CDK inhibitor and CC regulator. Rather, p21cip1 represses Sox2 through direct interaction with the Sox2 enhancer, whereas it modulates Bmp2 expression through association with E2F (Porlan et al., 2013). These data demonstrate that p21cip1 acts in distinct ways to link the relative quiescence of aNSCs to their longevity and potentiality (Porlan et al., 2013). P21cip1 is also expressed at high levels in the adult SGZ. There, it is expressed by IPCs, neuroblasts and immature neurons, but absent from radial glia-like NSC and mature granule cells (Pechnick et al., 2008, 2011). Accordingly, several studies show that committed IPCs or neuroblasts divide more actively in the DG of p21cip1 knockout mice, suggesting that p21cip1 restrains their proliferation (Pechnick et al., 2008, 2011; Zonis et al., 2013; Li and Wong, 2018). Another study, despite not reproducing the effects of p21cip1 deletion in native DG, identified p21cip1 as intrinsic suppressor of precursor proliferation after ischemic brain injury (Qiu et al., 2004). Given its importance for restricting adult neurogenesis, it is not surprising that p21cip1 is relevant also in neuropathological contexts. For instance, the studies of Pechnick et al. (2008, 2011) revealed that antidepressants exert their beneficial effects on hippocampal neurogenesis and behavior through down-regulation of p21cip1. Another report suggests that an increase of p21cip1 is responsible for the CC arrest of neuroblasts in response to acute systemic inflammation (Zonis et al., 2013). Together these studies again highlight the differences in the neurogenic niches of the adult brain, showing that p21cip1 serves to maintain a population of undifferentiated NSCs in the SVZ, whereas it acts as brake for proliferation of later stages of neuron development in the SGZ.
Several studies have demonstrated the importance of the Cip/Kip family members p27kip1 and p57kip2 as regulators of CC exit, differentiation and migration (Tury et al., 2012). Expression profiles and observations in mutant mice suggest that they regulate different sets of precursor cells in the embryonic cortex (Tury et al., 2012). Besides being co-expressed with p57kip2 in the cortical plate, P27kip1 is exclusively found in IPCs of the SVZ (Van Lookeren Campagne and Gill, 1998; Mairet-Coello et al., 2012). In this way, genetic manipulation assays revealed that p27kip1 promotes CC exit exclusively in IPCs (Goto et al., 2004; Tarui et al., 2005; Nguyen et al., 2006; Mairet-Coello et al., 2012; Clement et al., 2017). Moreover, they identified roles of p27kip1 in precursor differentiation and migration of cortical neurons (Nguyen et al., 2006; Tury et al., 2011; Hasan et al., 2013; Clement et al., 2017). These effects are independent from its CC activity, as neuronal differentiation is driven through stabilization of Ngn2 and migration is controlled through inhibition of RhoA signaling (Vernon et al., 2003; Nguyen et al., 2006).
In the adult SVZ, p27kip1 has very similar functions as during cortex development. As exemplified in p27kip1-deficient mice, p27kip1 specifically promotes CC exit of IPCs, while playing no role in NSCs (Doetsch et al., 2002; Gil-Perotin et al., 2011). Cells originating in the adult SVZ then migrate a long distance via the rostral migratory stream toward the olfactory bulb to replace resident granule neurons (Doetsch et al., 1997). P27kip1 is ubiquitously expressed in these areas to prevent the proliferation of newborn neurons (Li et al., 2009). The size of the rostral migratory stream is increased and olfactory bulb development is delayed in p27kip1 knockout mice, which may reflect a migration deficit (Li et al., 2009).
Two types of p27kip1-positive cells exist in the adult DG, strongly positive cells in the SGZ and weakly positive postmitotic neurons in the GCL and hilus (Qiu et al., 2009; Beukelaers et al., 2011; Andreu et al., 2015). Detailed immunophenotyping revealed that p27kip1 is expressed by virtually all type 1 NSCs, in the majority of IPCs, in neuroblasts and immature neurons (Qiu et al., 2009; Andreu et al., 2015). Deletion of p27kip1 increases proliferation of radial aNSC in vivo and in vitro, and Ki67 can be detected only in those few aNSCs that are p27kip1-negative, demonstrating a role of p27kip1 in aNSC quiescence (Qiu et al., 2009; Andreu et al., 2015; Horster et al., 2017). Activation of p27kip1 mutant NSCs has no effect on the total size of the aNSC pool, indicating that p27kip1 has no role in the choice between symmetric and asymmetric aNSC divisions (Andreu et al., 2015). Mechanistically, p27kip1 acts downstream of BMP4 to maintain aNSC quiescent (Andreu et al., 2015). Conversely, p27kip1 is low in transit-amplifying type 2a cells that accordingly are resistant to deletion of p27kip1 (Andreu et al., 2015). Later in the linage, p27kip1 deletion delays the CC exit of neuroblasts, resulting in a net increase in newborn neurons, just as in the aSVZ (Qiu et al., 2009; Andreu et al., 2015). Summing up, these studies demonstrate that p27kip1 acts as dual modulator of both aNSC quiescence and terminal CC exit of immature neurons. Recent studies also provided some insight into the role of p27kip1 in differentiation. P27kip1 increases upon neuronal differentiation in vitro and in vivo (Varodayan et al., 2009; Andreu et al., 2015; Horster et al., 2017). It becomes induced by proneural genes, such as NeuroD2 or Mash1, suggesting that p27kip1 is employed by neural determination factors to force differentiation and CC exit (Farah et al., 2000). Others showed that selective phosphorylation and stabilization of p27Kip1 by CDK5 is crucial to neuronal differentiation of NSCs and promotes neurite outgrowth as neurons differentiate (Zheng et al., 2010). The role of p27Kip1 in neuronal differentiation is further strengthened by observations in embryonic and pluripotent stem cells, in which 27Kip1 promotes neuronal differentiation through both stabilization of Ngn2 and repression of Sox2 (Nguyen et al., 2006; Li et al., 2012).
P57kip2 is expressed in cycling precursors of the embryonic VZ and SVZ (Ye et al., 2009; Mairet-Coello et al., 2012). Studies in mutant mice revealed that p57kip2 controls CC length and proliferation both in radial NSCs and IPCs (Tury et al., 2011; Mairet-Coello et al., 2012). Accordingly, it has been shown that p57kip2 knockout mice display macrocephaly due to embryonically increased proliferation of radial glia NSCs (Mairet-Coello et al., 2012). In promoting neuronal differentiation p57kip2 is even more effective than p27kip1 (Tury et al., 2011). And similar to p27kip1, p57kip2 regulates migration in the developing cortex, albeit at later stages (Nguyen et al., 2006; Tury et al., 2011). A feature that clearly distinguishes the two CKIs is the suppressive action of p57kip2 on astrogliogenic fate decisions (Tury et al., 2011). Noteworthy, all effects of p57kip2 on neurogenesis mentioned so far require their interaction with cyclin/CDK complexes.
Information on the role of p57kip2 in the adult DG is unfortunately sparse. Furutachi et al. (2013) show that conditional deletion of p57kip2 in aNSCs activates their proliferation and increases neurogenesis both in young and aged mice. Prolonged deletion, however, led to depletion of aNSCs and reduced neurogenesis (Furutachi et al., 2013). They further observed that the running-induced increase in aNSC proliferation, which is accompanied by a decreased expression of p57kip2 in wildtypes, is impaired in p57kip2 mutants. Their results suggest that p57kip2 maintains radial aNSCs in a quiescent state to preserve a pool of recruitable NSCs throughout life and that the reduction of p57kip2 is required for activation of aNSCs by a neurogenic stimulus such as running. In addition, suppression of p57kip2 directs NSCs isolated from the aSGZ toward the oligrodendrocyte linage while reducing astroglial characteristics, suggesting an involvement in linage commitment or maintenance of stem cell character (Jadasz et al., 2012).
Rb Proteins and E2F
In agreement with research reporting Rb functions in diverse cellular pathways of various cell types, recent studies identified distinct requirements for Rb/E2F in the brain, including cell division, differentiation and migration of precursor cells. During brain development, telencephalon-specific or heterozygous Rb mutants displayed increased and ectopic division of neuroblasts, demonstrating a requirement of Rb for CC exit after commitment to a neuronal fate (Lee et al., 1994; Callaghan et al., 1999; Ferguson et al., 2002). Ectopic division may be supported through the concomitant increase in FGF-2 levels in the VZ and cortical plate of the mutant mice (McClellan et al., 2009). Others identified severe deficits in the proliferation, maturation and subsequent tangential migration of specific interneuron subtypes in these mice, suggesting essential involvement of Rb in differentiation and migration of cortical precursor cells (Ferguson et al., 2005; Ghanem et al., 2012). Rb overexpression in cultured NSCs from newborn rats increased their differentiation – according to the specification factor added – into neurons, astroglia or oligodendrocytes (Jori et al., 2007). This suggests a requirement of Rb in determination of NSC fate in response to extracellular linage specification signals. A recent study examined DG development and adult hippocampal neurogenesis in conditional Rb knockout mice, essentially describing similar defects as observed in the embryonic cortex (Vandenbosch et al., 2016). Both, in the developing and adult DG they observed delayed CC exit and increased ectopic proliferation of neuronally committed progenitors and neuroblasts. However, the increased neuron birth was counteracted by an increase in cell death, which resulted in a long-term reduction of neurogenesis in the adult DG. Consistent with these findings, Rb-deletion in the aSVZ and rostral migratory stream was associated with increased progenitor proliferation and neurogenesis but impaired long-term survival (Naser et al., 2016). Together, these studies suggest conserved requirements for Rb in embryonic and adult neurogenic niches, i.e., exit from CC, restriction of proliferation and survival of newborn neurons (Vandenbosch et al., 2016). Consistent with prior findings in hematopoietic stem cells, none of the studies noticed consequences of the Rb knockout in the NSC population, suggesting that other Rb proteins are responsible for the control of self-renewal and quiescence in NSCs (Vanderluit et al., 2004; Walkley and Orkin, 2006; Naser et al., 2016; Vandenbosch et al., 2016).
Current knowledge on the role of p107 in neurogenesis relies only on studies conducted in the embryonic VZ and adult SVZ, in which it is highly expressed (Vanderluit et al., 2004, 2007). Contrary to Rb, p107 is expressed in uncommitted precursors and becomes downregulated as they commence to differentiate (Callaghan et al., 1999; Vanderluit et al., 2004). P107 knockout mice display increased proliferation of progenitors and slowly dividing NSCs in vivo that exhibit an increased self-renewal potential in in vitro (Vanderluit et al., 2004, 2007). Concomitantly, effectors of the Notch pathway including Notch1, Dll1, and Hes1 become upregulated in the mutated cells, suggesting that p107 controls the NPC population through suppression of Notch activity (Vanderluit et al., 2004, 2007). Indeed, p107 was shown to directly interact with regulatory sequences of Notch1 and Hes1, and accordingly the phenotype of p107 mutants could be rescued through deletion of Hes1 (Vanderluit et al., 2007). Nevertheless, p107 knockout mice ultimately display reduced neurogenesis due to decreased differentiation and increased apoptosis of uncommitted progenitor cells (Vanderluit et al., 2007). As reported by others, the p107/E2F pathway controls NPC division also through restricting the autocrine production of growth factors such as FGF-2 (McClellan et al., 2009). Together, these studies identified distinct mechanisms by which p107 regulates the expansion and neuronal commitment of stem and progenitor cells in the embryonic and adult brain.
Also, little is known about the role of p130, which is predominantly expressed in quiescent and terminally differentiated cells, during adult neurogenesis (Mulligan and Jacks, 1998). View lines of evidence suggest an involvement in NSC differentiation. For instance, p130 forms complexes with E2F4 and SIN3A to repress Sox2 transcription during iPSC differentiation (Li et al., 2012). Additionally, p130 overexpression in differentiating NSCs isolated from newborn rats causes a shift toward the cell type induced by differentiation cues, similar to the effects of Rb overexpression. No such effect was observed under proliferating conditions, suggesting that p130 and Rb reinforce the cellular responses of NSCs to extracellular fate specification signals (Jori et al., 2007).
Together, these studies exemplified diverse functions of distinct Rb proteins in the regulation of neural stem and progenitor cells, not only by controlling genes required for CC progression but also by regulating genes relevant for fate control and linage specification.
Control of Fate Decisions in G1
The commitment of cells to divide requires the presence of external mitogenic signals. It is during early G1 that cells are highly susceptible to such extrinsic cues and make critical decisions about their fate, including the commitment to proceed, pause or exit the CC (Matsushime et al., 1994; Sherr, 1994a; Pauklin and Vallier, 2013). In the presence of mitogens, D-cyclins become upregulated and initiate the cascade driving the cell through the restriction point to commence a new round of division. If mitogens are withdrawn or anti-mitogenic cues are present (e.g., BMPs), D-cyclins are lost and Cip/Kip proteins accumulate, forcing the cell into a reversible quiescent state, a hallmark of aNSCs (G0; Blomen and Boonstra, 2007; Andreu et al., 2015). Recent evidence suggests that aNSC quiescence is not merely an inactive state but rather is actively maintained (Wang et al., 2011; Morizur et al., 2018), at least partly by Cip/Kip proteins, whose deletion inevitably leads to excessive proliferation and premature depletion of aNSCs (Kippin et al., 2005; Cheung and Rando, 2013; Andreu et al., 2015). Exposure to differentiation cues is also accompanied by an increase in Cip/Kip CKIs, but this time forcing differentiation and terminal exit from CC (Lukaszewicz et al., 2002; Blomen and Boonstra, 2007; Horster et al., 2017). Of note, differentiation and proliferation are not mutually exclusive events, as is the case in neuronally committed progenitor cells. Cells rather continue to divide at slower pace and cease cycling toward the end of the differentiation process (Lukaszewicz et al., 2002; Coffman, 2003). Such a lengthening of the CC during neuronal differentiation has been initially described for the embryonic cortex (Takahashi et al., 1995). Subsequent studies applying sophisticated genetic tools to selectively manipulate important G1 regulators suggest that the length of G1 can directly influence proliferation/differentiation decisions in NPCs (Figure 3). For example, overexpression of cyclin D1-CDK4 complexes, or cyclin D1 and cyclin E alone were found to shorten G1, which was accompanied by a deceleration of neurogenesis and enhanced expansion of IPCs, both in embryonic and adult neurogenic niches (Lange et al., 2009; Pilaz et al., 2009; Artegiani et al., 2011; Bragado Alonso et al., 2019). Conversely, silencing of cyclin D-CDK4, deletion of CDK6 or pharmacological inhibition of CDK2 or CDK4 lengthen G1 and lead to premature neuronal differentiation (Calegari and Huttner, 2003; Lange et al., 2009; Beukelaers et al., 2011; Lim and Kaldis, 2012; Roccio et al., 2013). These observations have led to the hypothesis that CC lengthening may be a cause of differentiation, proposing that a longer G1 will increase the propensity of fate-determining signals to accumulate and drive differentiation (Calegari and Huttner, 2003; Salomoni and Calegari, 2010). However, for the adult DG there is also evidence not completely in line with this hypothesis, coming from two studies which examine the correlation between voluntary exercise-induced proliferation and CC length (Farioli-Vecchioli et al., 2014; Fischer et al., 2014). Whereas the study of Fischer et al. (2014) despite finding a tendency toward G1 shortening, reports no clear correlation between CC length and increased proliferation after a short period (5 days) of running, Farioli-Vecchioli et al. (2014) showed that the proliferative response after prolonged (12 days) running is accompanied by shortening of the CC, which was attributable to shortening of the S phase in neuronally committed progenitors. This is in line with others reporting that NPCs shorten S phase upon neuronal commitment both during development and in the adult DG (Arai et al., 2011; Brandt et al., 2012). Interestingly, Brandt and colleagues further observed that radial aNSCs, once activated, divide faster, also through shortening of their S phase, which is in agreement with the “disposable stem cell” model as put forward by Encinas et al. (2011), but in contrast to the “long-term repeated aNSC self-renewal” model proposed by Bonaguidi et al. (2011) and to the finding that expanding NPCs spend more time in S phase for DNA quality control (Arai et al., 2011). Taken together, the overall picture is more complex than previously assumed, and it still remains to be proven whether and how changes in CC dynamics translate into fate decisions. A possible explanation for the apparent discrepancies described before is that the G1 proteins manipulated to induce changes in CC length may in addition to their CC regulatory function directly influence cell fates. Indeed, several studies have provided evidence for direct roles of G1 proteins in differentiation, independent of their CC regulatory activity, through interaction with signaling pathways and transcription factors controlling fate decisions (Pauklin and Vallier, 2013; Pauklin et al., 2016). For cyclin D1, chromatin-wide location analyses identified hundreds of target genes, including Notch1 and Wnt3, suggesting direct roles in the maintenance and differentiation of stem cells (Bienvenu et al., 2010; Pauklin et al., 2016). P27Kip1 has been shown to promote differentiation through transcriptional repression of Sox2 and stabilization of the proneural protein Ngn2 (Vernon et al., 2003; Nguyen et al., 2006; Li et al., 2012). Ngn2 is moreover targeted by CDKs through direct phosphorylation at multiple sites (Ali et al., 2011). This results in quantitative inhibition of Ngn2’s ability to induce transcription of differentiation genes, thus providing a mechanism how CDK activity and perhaps also CC length can be sensed to coordinate neuronal differentiation.
FIGURE 3.

Simplified model on the coordination of G1 dynamics by extrinsic signals and their integration into fate decisions based on evidence from cyclin and CDK manipulation studies. (A) Fast proliferation is achieved in presence of high mitogen levels that stimulate the expression of D-cyclins, thus initiating the canonical CC cascade driving progression through G1. If cells pass the R-point (point of no return), mitogen stimulation is no longer required for commencing cell division. D-cyclin levels stay high until mitogens are withdrawn. (B) If specification signals come on board, CKIs increase on top and restrain but don’t block the activities of D-cyclins, resulting in a lengthening of G1. This, as proposed by the “cell cycle length theory,” provides sufficient time for specification signals to initiate the differentiation program before cells reach the R-point. (C) If mitogens decrease, D-cyclins become rapidly degraded and cells can not overcome the R-point. Adult NSCs then return into a reversible quiescent state (G0), whereas cells that initiated their differentiation program terminally exit the cell cycle to become an immature neuron (IN).
Concluding Remarks
Recently there has been a remarkable progress in our understanding about the control of adult hippocampal neurogenesis by the CC. However, despite accumulating evidence for multiple and diverse interactions between CC components and fate decisions of NPCs, we are far from drawing a comprehensive picture. Still, several CC proteins await investigation in adult NPCs or are subject to ongoing work. On the other hand, interpretation of existing data is frequently complicated because many G1 regulators, including D-cyclins and CDKs, (i) emerge as pleiotropic molecules regulating multiple cellular functions, often independent from their CC regulatory role and localized to different protein domains, and (ii) display functional redundancy and counter-regulate if the expression of related proteins is experimentally manipulated. Additional detailed analyses of expression patterns in combination with spatiotemporally controlled manipulation of genes, as well as of individual functional domains of CC regulators, may help to answer outstanding questions. We believe that a detailed understanding of the regulatory mechanisms underlying adult neurogenesis may open new avenues for developing therapies of neurodegenerative diseases. The first attempts to exploit this potential have already been made and have produced promising results.
Author Contributions
AU conceptualized and wrote the manuscript and prepared the figures. OW conceptualized and edited the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank C. Schmeer, S. Schmidt, and A. S. Zahra for their helpful discussions and/or critical comments on the manuscript, and F. Brand for assistance with literature search.
Abbreviations
- aNSC
adult neural stem cells
- CC
cell cycle
- DG
dentate gyrus
- IPC
intermediate or transit-amplifying progenitors
- NPC
neural precursor cells (undistinguished pool comprising NSC, IPC, and neuroblasts)
- NSC
neural stem cells
- SGZ
subgranular zone
- SVZ
subventricular zone
- VZ
ventricular zone
Footnotes
Funding. Funding has been provided by the ProExellenz initiative of the Thuringian Ministry for Research (TMWWDG, RegenerAging-FSU-I-03/14) and the Interdisciplinary Center for Clinical Research.
References
- Aberg M. A., Aberg N. D., Hedbacker H., Oscarsson J., Eriksson P. S. (2000). Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J. Neurosci. 20 2896–2903. 10.1523/jneurosci.20-08-02896.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg M. A., Aberg N. D., Palmer T. D., Alborn A. M., Carlsson-Skwirut C., Bang P., et al. (2003). IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol. Cell. Neurosci. 24 23–40. 10.1016/s1044-743128032900082-4 [DOI] [PubMed] [Google Scholar]
- Ables J. L., Breunig J. J., Eisch A. J., Rakic P. (2011). Not(ch) just development: notch signalling in the adult brain. Nat. Rev. Neurosci. 12 269–283. 10.1038/nrn3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ables J. L., Decarolis N. A., Johnson M. A., Rivera P. D., Gao Z., Cooper D. C., et al. (2010). Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J. Neurosci. 30 10484–10492. 10.1523/jneurosci.4721-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S., Joyner A.L. (2005). In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437 894–897. 10.1038/nature03994 [DOI] [PubMed] [Google Scholar]
- Ali F., Hindley C., Mcdowell G., Deibler R., Jones A., Kirschner M., et al. (2011). Cell cycle-regulated multi-site phosphorylation of Neurogenin 2 coordinates cell cycling with differentiation during neurogenesis. Development 138 4267–4277. 10.1242/dev.067900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J., Bayer S. A. (1990). Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J. Comp. Neurol. 301 325–342. 10.1002/cne.903010302 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Lim D. A. (2004). For the long run: maintaining germinal niches in the adult brain. Neuron 41 683–686. [DOI] [PubMed] [Google Scholar]
- Amador-Arjona A., Elliott J., Miller A., Ginbey A., Pazour G. J., Enikolopov G., et al. (2011). Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J. Neurosci. 31 9933–9944. 10.1523/jneurosci.1062-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu Z., Khan M. A., Gonzalez-Gomez P., Negueruela S., Hortiguela R., San Emeterio J., et al. (2015). The cyclin-dependent kinase inhibitor p27 kip1 regulates radial stem cell quiescence and neurogenesis in the adult hippocampus. Stem Cells 33 219–229. 10.1002/stem.1832 [DOI] [PubMed] [Google Scholar]
- Ansorg A., Witte O. W., Urbach A. (2012). Age-dependent kinetics of dentate gyrus neurogenesis in the absence of cyclin D2. BMC Neurosci. 13:46. 10.1186/1471-2202-13-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli F., Casciati A., Tanori M., Tanno B., Linares-Vidal M. V., Serra N., et al. (2018). Alterations in morphology and adult neurogenesis in the dentate gyrus of patched1 heterozygous mice. Front. Mol. Neurosci. 11:168. 10.3389/fnmol.2018.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y., Pulvers J. N., Haffner C., Schilling B., Nusslein I., Calegari F., et al. (2011). Neural stem and progenitor cells shorten S-phase on commitment to neuron production. Nat. Commun. 2:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano M., Moreno S. (1997). Regulation of CDK/cyclin complexes during the cell cycle. Int. J. Biochem. Cell Biol. 29 559–573. 10.1016/s1357-2725(96)00178-1 [DOI] [PubMed] [Google Scholar]
- Armenteros T., Andreu Z., Hortiguela R., Lie D. C., Mira H. (2018). BMP and WNT signalling cooperate through LEF1 in the neuronal specification of adult hippocampal neural stem and progenitor cells. Sci. Rep. 8:9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegiani B., Lindemann D., Calegari F. (2011). Overexpression of cdk4 and cyclinD1 triggers greater expansion of neural stem cells in the adult mouse brain. J. Exp. Med. 208 937–948. 10.1084/jem.20102167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoo R. M., Maher E. A., Ligon K. L., Sharpless N. E., Chan S. S., You M. J.et al. (2002). Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell 1 269–277. 10.1016/s1535-6108(02)00046-6 [DOI] [PubMed] [Google Scholar]
- Baek S. H., Kioussi C., Briata P., Wang D., Nguyen H. D., Ohgi K. A., et al. (2003). Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway. Proc. Natl. Acad. Sci. U.S.A. 100 3245–3250. 10.1073/pnas.0330217100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagui T. K., Mohapatra S., Haura E., Pledger W. J. (2003). P27Kip1 and p21Cip1 are not required for the formation of active D cyclin-cdk4 complexes. Mol. Cell. Biol. 23 7285–7290. 10.1128/mcb.23.20.7285-7290.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah N. M., Filipkowski R. K., Pruschy M., Jaholkowski P., Winkler J., Kaczmarek L., et al. (2013). Impaired long-term memory retention: common denominator for acutely or genetically reduced hippocampal neurogenesis in adult mice. Behav. Brain Res. 252 275–286. 10.1016/j.bbr.2013.05.034 [DOI] [PubMed] [Google Scholar]
- Berg D. A., Bond A. M., Ming G. L., Song H. (2018). Radial glial cells in the adult dentate gyrus: what are they and where do they come from? F1000Res. 7:277. 10.12688/f1000research.12684.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergami M., Rimondini R., Santi S., Blum R., Gotz M., Canossa M. (2008). Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl. Acad. Sci. U.S.A. 105 15570–15575. 10.1073/pnas.0803702105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu R. O., Longo F. M. (2010). The p75 neurotrophin receptor is expressed by adult mouse dentate progenitor cells and regulates neuronal and non-neuronal cell genesis. BMC Neurosci. 11:136. 10.1186/1471-2202-11-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A., Dowdy S. F., Roberts J. M. (2008). CDK inhibitors: cell cycle regulators and beyond. Dev. Cell 14 159–169. 10.1016/j.devcel.2008.01.013 [DOI] [PubMed] [Google Scholar]
- Beukelaers P., Vandenbosch R., Caron N., Nguyen L., Belachew S., Moonen G., et al. (2011). Cdk6-dependent regulation of G(1) length controls adult neurogenesis. Stem Cells 29 713–724. 10.1002/stem.616 [DOI] [PubMed] [Google Scholar]
- Biebl M., Cooper C. M., Winkler J., Kuhn H. G. (2000). Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci. Lett. 291 17–20. 10.1016/s0304-3940(00)01368-9 [DOI] [PubMed] [Google Scholar]
- Bienvenu F., Jirawatnotai S., Elias J. E., Meyer C. A., Mizeracka K., Marson A., et al. (2010). Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature 463 374–378. 10.1038/nature08684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizen N., Inoue T., Shimizu T., Tabu K., Kagawa T., Taga T. (2014). A growth-promoting signaling component cyclin D1 in neural stem cells has antiastrogliogenic function to execute self-renewal. Stem Cells 32 1602–1615. 10.1002/stem.1613 [DOI] [PubMed] [Google Scholar]
- Blagosklonny M. V., Pardee A. B. (2002). The restriction point of the cell cycle. Cell Cycle 1 103–110. [PubMed] [Google Scholar]
- Blagosklonny and Pardee (2000–2013). The Restriction Point of the Cell Cycle. Madame Curie Bioscience Database [Internet]. Austin, TX: Landes Bioscience. [Google Scholar]
- Blomen V. A., Boonstra J. (2007). Cell fate determination during G1 phase progression. Cell. Mol. Life Sci. 64 3084–3104. 10.1007/s00018-007-7271-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockstaele L., Bisteau X., Paternot S., Roger P. P. (2009). Differential regulation of cyclin-dependent kinase 4 (CDK4) and CDK6, evidence that CDK4 might not be activated by CDK7, and design of a CDK6 activating mutation. Mol. Cell. Biol. 29 4188–4200. 10.1128/mcb.01823-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M., Fulmore C. A., Tartt A. N., Simeon L. R., Pavlova I., Poposka V., et al. (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22 589–599.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi M. A., Mcguire T., Hu M., Kan L., Samanta J., Kessler J. A. (2005). LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development 132 5503–5514. 10.1242/dev.02166 [DOI] [PubMed] [Google Scholar]
- Bonaguidi M. A., Peng C. Y., Mcguire T., Falciglia G., Gobeske K. T., Czeisler C., et al. (2008). Noggin expands neural stem cells in the adult hippocampus. J. Neurosci. 28 9194–9204. 10.1523/jneurosci.3314-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi M.A., Wheeler M.A., Shapiro J.S., Stadel R.P., Sun G.J., Ming G.L., et al. (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A. M., Peng C. Y., Meyers E. A., Mcguire T., Ewaleifoh O., Kessler J. A. (2014). BMP signaling regulates the tempo of adult hippocampal progenitor maturation at multiple stages of the lineage. Stem Cells 32 2201–2214. 10.1002/stem.1688 [DOI] [PubMed] [Google Scholar]
- Bottazzi M. E., Assoian R. K. (1997). The extracellular matrix and mitogenic growth factors control G1 phase cyclins and cyclin-dependent kinase inhibitors. Trends Cell Biol. 7 348–352. 10.1016/s0962-8924(97)01114-8 [DOI] [PubMed] [Google Scholar]
- Boward B., Wu T., Dalton S. (2016). Concise review: control of cell fate through cell cycle and pluripotency networks. Stem Cells 34 1427–1436. 10.1002/stem.2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragado Alonso S., Reinert J. K., Marichal N., Massalini S., Berninger B., Kuner T., et al. (2019). An increase in neural stem cells and olfactory bulb adult neurogenesis improves discrimination of highly similar odorants. EMBO J. 38:e98791 10.15252/embj.201798791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragina O., Sergejeva S., Serg M., Zarkovsky T., Maloverjan A., Kogerman P., et al. (2010). Smoothened agonist augments proliferation and survival of neural cells. Neurosci. Lett. 482 81–85. 10.1016/j.neulet.2010.06.068 [DOI] [PubMed] [Google Scholar]
- Brandt M. D., Hubner M., Storch A. (2012). Brief report: adult hippocampal precursor cells shorten S-phase and total cell cycle length during neuronal differentiation. Stem Cells 30 2843–2847. 10.1002/stem.1244 [DOI] [PubMed] [Google Scholar]
- Brehm A., Miska E. A., Mccance D. J., Reid J. L., Bannister A. J., Kouzarides T. (1998). Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature 391 597–601. 10.1038/35404 [DOI] [PubMed] [Google Scholar]
- Breunig J. J., Sarkisian M. R., Arellano J. I., Morozov Y. M., Ayoub A. E., Sojitra S., et al. (2008). Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc. Natl. Acad. Sci. U.S.A. 105 13127–13132. 10.1073/pnas.0804558105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig J. J., Silbereis J., Vaccarino F. M., Sestan N., Rakic P. (2007). Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 104 20558–20563. 10.1073/pnas.0710156104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S. M., Gobeske K. T., Chen J., Peng C. Y., Kessler J. A. (2017). Hippocampal bone morphogenetic protein signaling mediates behavioral effects of antidepressant treatment. Mol. Psychiatry 22 910–919. 10.1038/mp.2016.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V., Pachernik J., Faldikova L., Krejci P., Pogue R., Nevriva I., et al. (2004). The role of p27(Kip1) in maintaining the levels of D-type cyclins in vivo. Biochim. Biophys. Acta 1691 105–116. 10.1016/j.bbamcr.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Bryja V., Pachernik J., Vondracek J., Soucek K., Cajanek L., Horvath V., et al. (2008). Lineage specific composition of cyclin D-CDK4/CDK6-p27 complexes reveals distinct functions of CDK4, CDK6 and individual D-type cyclins in differentiating cells of embryonic origin. Cell Prolif. 41 875–893. 10.1111/j.1365-2184.2008.00556.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillava C., Vandenbosch R., Jablonska B., Deboux C., Spigoni G., Gallo V., et al. (2011). Cdk2 loss accelerates precursor differentiation and remyelination in the adult central nervous system. J. Cell Biol. 193 397–407. 10.1083/jcb.201004146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari F., Huttner W. B. (2003). An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J. Cell Sci. 116 4947–4955. 10.1242/jcs.00825 [DOI] [PubMed] [Google Scholar]
- Callaghan D. A., Dong L., Callaghan S. M., Hou Y. X., Dagnino L., Slack R. S. (1999). Neural precursor cells differentiating in the absence of Rb exhibit delayed terminal mitosis and deregulated E2F 1 and 3 activity. Dev. Biol. 207 257–270. 10.1006/dbio.1998.9162 [DOI] [PubMed] [Google Scholar]
- Campa V. M., Gutierrez-Lanza R., Cerignoli F., Diaz-Trelles R., Nelson B., Tsuji T., et al. (2008). Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J. Cell Biol. 183 129–141. 10.1083/jcb.200806104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepa E. T., Scassa M. E., Ceruti J. M., Marazita M. C., Carcagno A. L., Sirkin P. F., et al. (2007). INK4 proteins, a family of mammalian CDK inhibitors with novel biological functions. IUBMB Life 59 419–426. 10.1080/15216540701488358 [DOI] [PubMed] [Google Scholar]
- Cao L., Jiao X., Zuzga D. S., Liu Y., Fong D. M., Young D., et al. (2004). VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 36 827–835. 10.1038/ng1395 [DOI] [PubMed] [Google Scholar]
- Carey R. G., Li B., Dicicco-Bloom E. (2002). Pituitary adenylate cyclase activating polypeptide anti-mitogenic signaling in cerebral cortical progenitors is regulated by p57Kip2-dependent CDK2 activity. J. Neurosci. 22 1583–1591. 10.1523/jneurosci.22-05-01583.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron N., Genin E. C., Marlier Q., Verteneuil S., Beukelaers P., Morel L., et al. (2018). Proliferation of hippocampal progenitors relies on p27-dependent regulation of Cdk6 kinase activity. Cell. Mol. Life Sci. 75 3817–3827. 10.1007/s00018-018-2832-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthon B. C., Neumann C. A., Das M., Pawlyk B., Li T., Geng Y., et al. (2005). Genetic replacement of cyclin D1 function in mouse development by cyclin D2. Mol. Cell. Biol. 25 1081–1088. 10.1128/mcb.25.3.1081-1088.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayrol C., Knibiehler M., Ducommun B. (1998). p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene 16 311–320. 10.1038/sj.onc.1201543 [DOI] [PubMed] [Google Scholar]
- Cerqueira A., Martin A., Symonds C. E., Odajima J., Dubus P., Barbacid M., et al. (2014). Genetic characterization of the role of the Cip/Kip family of proteins as cyclin-dependent kinase inhibitors and assembly factors. Mol. Cell. Biol. 34 1452–1459. 10.1128/mcb.01163-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. P., Cordeira J., Calderon G. A., Iyer L. K., Rios M. (2008). Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol. Cell. Neurosci. 39 372–383. 10.1016/j.mcn.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapouton P., Skupien P., Hesl B., Coolen M., Moore J. C., Madelaine R., et al. (2010). Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J. Neurosci. 30 7961–7974. 10.1523/jneurosci.6170-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Wang F., Liu Z., Zhao Y., Jiang Y., Chen L., et al. (2018). Brain-derived neurotrophic factor promotes proliferation and progesterone synthesis in bovine granulosa cells. J. Cell. Physiol. 234:8776-8787 [DOI] [PubMed] [Google Scholar]
- Chen Z., Duan R. S., Zhu Y., Folkesson R., Albanese C., Winblad B., et al. (2005). Increased cyclin E expression may obviate the role of cyclin D1 during brain development in cyclin D1 knockout mice. J. Neurochem. 92 1281–1284. 10.1111/j.1471-4159.2004.02934.x [DOI] [PubMed] [Google Scholar]
- Cheng M., Olivier P., Diehl J. A., Fero M., Roussel M. F., Roberts J. M., et al. (1999). The p21(Cip1) and p27(Kip1) CDK ’inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18 1571–1583. 10.1093/emboj/18.6.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung T. H., Rando T. A. (2013). Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 14 329–340. 10.1038/nrm3591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnam M., Goodrich D. W. (2011). RB1, development, and cancer. Curr. Top. Dev. Biol. 94 129–169. 10.1016/b978-0-12-380916-2.00005-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirivella L., Kirstein M., Ferron S. R., Domingo-Muelas A., Durupt F. C., Acosta-Umanzor C., et al. (2017). Cyclin-dependent kinase 4 regulates adult neural stem cell proliferation and differentiation in response to insulin. Stem Cells 35 2403–2416. 10.1002/stem.2694 [DOI] [PubMed] [Google Scholar]
- Choe Y., Pleasure S. J., Mira H. (2015). Control of adult neurogenesis by short-range morphogenic-signaling molecules. Cold Spring Harb. Perspect. Biol. 8:a018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J. L., Wenzel P. L., Saenz-Robles M. T., Nair V., Ferrey A., Hagan J. P., et al. (2009). E2f1-3 switch from activators in progenitor cells to repressors in differentiating cells. Nature 462 930–934. 10.1038/nature08677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement O., Hemming I. A., Gladwyn-Ng I. E., Qu Z., Li S. S., Piper M., et al. (2017). Rp58 and p27(kip1) coordinate cell cycle exit and neuronal migration within the embryonic mouse cerebral cortex. Neural Dev. 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman J. A. (2003). Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol. Int. 27 315–324. 10.1016/s1065-6995(03)00018-0 [DOI] [PubMed] [Google Scholar]
- Cole A. E., Murray S. S., Xiao J. (2016). Bone morphogenetic protein 4 signalling in neural stem and progenitor cells during development and after injury. Stem Cells Int. 2016:9260592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. (1995). Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82 675–684. 10.1016/0092-867428952990039-x [DOI] [PubMed] [Google Scholar]
- Dick F. A., Rubin S. M. (2013). Molecular mechanisms underlying RB protein function. Nat. Rev. Mol. Cell Biol. 14 297–306. 10.1038/nrm3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl J. A., Zindy F., Sherr C. J. (1997). Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 11 957–972. 10.1101/gad.11.8.957 [DOI] [PubMed] [Google Scholar]
- Doetsch F. (2003). A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 13 543–550. 10.1016/j.gde.2003.08.012 [DOI] [PubMed] [Google Scholar]
- Doetsch F., Garcia-Verdugo J. M., Alvarez-Buylla A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17 5046–5061. 10.1523/jneurosci.17-13-05046.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Verdugo J. M., Caille I., Alvarez-Buylla A., Chao M. V., Casaccia-Bonnefil P. (2002). Lack of the cell-cycle inhibitor p27Kip1 results in selective increase of transit-amplifying cells for adult neurogenesis. J. Neurosci. 22 2255–2264. 10.1523/jneurosci.22-06-02255.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong P., Zhang C., Parker B. T., You L., Mathey-Prevot B. (2018). Cyclin D/CDK4/6 activity controls G1 length in mammalian cells. PLoS One 13:e0185637. 10.1371/journal.pone.0185637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehm O., Goritz C., Covic M., Schaffner I., Schwarz T. J., Karaca E., et al. (2010). RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J. Neurosci. 30 13794–13807. 10.1523/jneurosci.1567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm S. V., Reed S. I. (2000). Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 12 676–684. 10.1016/s0955-0674(00)00151-4 [DOI] [PubMed] [Google Scholar]
- Encinas J. M., Michurina T. V., Peunova N., Park J. H., Tordo J., Peterson D. A., et al. (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8 566–579. 10.1016/j.stem.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson K. K., Krull D., Slomiany P., Grossel M. J. (2003). Expression of cyclin-dependent kinase 6, but not cyclin-dependent kinase 4, alters morphology of cultured mouse astrocytes. Mol. Cancer Res. 1 654–664. [PubMed] [Google Scholar]
- Faigle R., Song H. (2013). Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta 1830 2435–2448. 10.1016/j.bbagen.2012.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah M. H., Olson J. M., Sucic H. B., Hume R. I., Tapscott S. J., Turner D. L. (2000). Generation of neurons by transient expression of neural bHLH proteins in mammalian cells. Development 127 693–702. [DOI] [PubMed] [Google Scholar]
- Farioli-Vecchioli S., Mattera A., Micheli L., Ceccarelli M., Leonardi L., Saraulli D., et al. (2014). Running rescues defective adult neurogenesis by shortening the length of the cell cycle of neural stem and progenitor cells. Stem Cells 32 1968–1982. 10.1002/stem.1679 [DOI] [PubMed] [Google Scholar]
- Ferguson K. L., Callaghan S. M., O’hare M. J., Park D. S., Slack R. S. (2000). The Rb-CDK4/6 signaling pathway is critical in neural precursor cell cycle regulation. J. Biol. Chem. 275 33593–33600. 10.1074/jbc.m004879200 [DOI] [PubMed] [Google Scholar]
- Ferguson K. L., Mcclellan K. A., Vanderluit J. L., Mcintosh W. C., Schuurmans C., Polleux F., et al. (2005). A cell-autonomous requirement for the cell cycle regulatory protein, Rb, in neuronal migration. EMBO J. 24 4381–4391. 10.1038/sj.emboj.7600887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson K. L., Vanderluit J. L., Hebert J. M., Mcintosh W. C., Tibbo E., Maclaurin J. G., et al. (2002). Telencephalon-specific Rb knockouts reveal enhanced neurogenesis, survival and abnormal cortical development. EMBO J. 21 3337–3346. 10.1093/emboj/cdf338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V., Kronenberg G., Pivneva T., Reuter K., Steiner B., Wang L. P., et al. (2003). Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol. Cell. Neurosci. 23 373–382. 10.1016/s1044-7431(03)00060-5 [DOI] [PubMed] [Google Scholar]
- Fischer T. J., Walker T. L., Overall R. W., Brandt M. D., Kempermann G. (2014). Acute effects of wheel running on adult hippocampal precursor cells in mice are not caused by changes in cell cycle length or S phase length. Front. Neurosci. 8:314 10.3389/fnins.2014.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick T. J., Wood T. L. (2004). IGF-I and FGF-2 coordinately enhance cyclin D1 and cyclin E-cdk2 association and activity to promote G1 progression in oligodendrocyte progenitor cells. Mol. Cell. Neurosci. 25 480–492. 10.1016/j.mcn.2003.11.015 [DOI] [PubMed] [Google Scholar]
- Furutachi S., Matsumoto A., Nakayama K. I., Gotoh Y. (2013). p57 controls adult neural stem cell quiescence and modulates the pace of lifelong neurogenesis. EMBO J. 32 970–981. 10.1038/emboj.2013.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F. H., Coates P. W., Palmer T. D., Kuhn H. G., Fisher L. J., Suhonen J. O., et al. (1995). Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl. Acad. Sci. U.S.A. 92 11879–11883. 10.1073/pnas.92.25.11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A., Huang Z., Kaczmarek L., Filipkowski R. K., Kempermann G. (2014). Not all water mazes are created equal: cyclin D2 knockout mice with constitutively suppressed adult hippocampal neurogenesis do show specific spatial learning deficits. Genes Brain Behav. 13 357–364. 10.1111/gbb.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavet O., Pines J. (2010). Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J. Cell Biol. 189 247–259. 10.1083/jcb.200909144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem N., Andrusiak M. G., Svoboda D., Al Lafi S. M., Julian L. M., Mcclellan K. A., et al. (2012). The Rb/E2F pathway modulates neurogenesis through direct regulation of the Dlx1/Dlx2 bigene cluster. J. Neurosci. 32 8219–8230. 10.1523/jneurosci.1344-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Perotin S., Haines J. D., Kaur J., Marin-Husstege M., Spinetta M. J., Kim K. H., et al. (2011). Roles of p53 and p27(Kip1) in the regulation of neurogenesis in the murine adult subventricular zone. Eur. J. Neurosci. 34 1040–1052. 10.1111/j.1460-9568.2011.07836.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein S. B., Alexander S., Ross M. E. (2007a). Differences in cyclin D2 and D1 protein expression distinguish forebrain progenitor subsets. Cereb. Cortex 17 632–642. [DOI] [PubMed] [Google Scholar]
- Glickstein S. B., Monaghan J. A., Koeller H. B., Jones T. K., Ross M. E. (2009). Cyclin D2 is critical for intermediate progenitor cell proliferation in the embryonic cortex. J. Neurosci. 29 9614–9624. 10.1523/jneurosci.2284-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein S. B., Moore H., Slowinska B., Racchumi J., Suh M., Chuhma N., et al. (2007b). Selective cortical interneuron and GABA deficits in cyclin D2-null mice. Development 134 4083–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeske K. T., Das S., Bonaguidi M. A., Weiss C., Radulovic J., Disterhoft J. F., et al. (2009). BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One 4:e7506. 10.1371/journal.pone.0007506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Mitsuhashi T., Takahashi T. (2004). Altered patterns of neuron production in the p27 knockout mouse. Dev. Neurosci. 26 208–217. 10.1159/000082138 [DOI] [PubMed] [Google Scholar]
- Grison A., Gaiser C., Bieder A., Baranek C., Atanasoski S. (2018). Ablation of cdk4 and cdk6 affects proliferation of basal progenitor cells in the developing dorsal and ventral forebrain. Dev. Neurobiol. 78 660–670. 10.1002/dneu.22588 [DOI] [PubMed] [Google Scholar]
- Gritti A., Parati E. A., Cova L., Frolichsthal P., Galli R., Wanke E., et al. (1996). Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. 16 1091–1100. 10.1523/jneurosci.16-03-01091.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossel M. J., Hinds P. W. (2006). Beyond the cell cycle: a new role for Cdk6 in differentiation. J. Cell. Biochem. 97 485–493. 10.1002/jcb.20712 [DOI] [PubMed] [Google Scholar]
- Guan K. L., Jenkins C. W., Li Y., Nichols M. A., Wu X., O’keefe C. L., et al. (1994). Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function. Genes Dev. 8 2939–2952. 10.1101/gad.8.24.2939 [DOI] [PubMed] [Google Scholar]
- Han J., Calvo C. F., Kang T. H., Baker K. L., Park J. H., Parras C., et al. (2015). Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans. Cell Rep. 10 1158–1172. 10.1016/j.celrep.2015.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. G., Spassky N., Romaguera-Ros M., Garcia-Verdugo J. M., Aguilar A., Schneider-Maunoury S., et al. (2008). Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 11 277–284. 10.1038/nn2059 [DOI] [PubMed] [Google Scholar]
- Hasan S. M., Sheen A. D., Power A. M., Langevin L. M., Xiong J., Furlong M., et al. (2013). Mcl1 regulates the terminal mitosis of neural precursor cells in the mammalian brain through p27Kip1. Development 140 3118–3127. 10.1242/dev.090910 [DOI] [PubMed] [Google Scholar]
- Hasenpusch-Theil K., West S., Kelman A., Kozic Z., Horrocks S., Mcmahon A. P., et al. (2018). Gli3 controls the onset of cortical neurogenesis by regulating the radial glial cell cycle through Cdk6 expression. Development 145:dev163147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Roussel M. F., Kato J. Y., Ashmun R. A., Sherr C. J. (1995). Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol. Cell. Biol. 15 2672–2681. 10.1128/mcb.15.5.2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge R. D., Kowalczyk T. D., Wolf S. A., Encinas J. M., Rippey C., Enikolopov G., et al. (2008). Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J. Neurosci. 28 3707–3717. 10.1523/jneurosci.4280-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horster H., Garthe A., Walker T. L., Ichwan M., Steiner B., Khan M. A., et al. (2017). p27kip1 is required for functionally relevant adult hippocampal neurogenesis in mice. Stem Cells 35 787–799. 10.1002/stem.2536 [DOI] [PubMed] [Google Scholar]
- Huard J. M., Forster C. C., Carter M. L., Sicinski P., Ross M. E. (1999). Cerebellar histogenesis is disturbed in mice lacking cyclin D2. Development 126 1927–1935. [DOI] [PubMed] [Google Scholar]
- Hurford R. K., Jr., Cobrinik D., Lee M. H., Dyson N. (1997). pRB and p107/p130 are required for the regulated expression of different sets of E2F responsive genes. Genes Dev. 11 1447–1463. 10.1101/gad.11.11.1447 [DOI] [PubMed] [Google Scholar]
- Hydbring P., Malumbres M., Sicinski P. (2016). Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 17 280–292. 10.1038/nrm.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I., Sakamoto M., Yamaguchi M., Mori K., Kageyama R. (2010). Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 30 3489–3498. 10.1523/jneurosci.4987-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska B., Aguirre A., Vandenbosch R., Belachew S., Berthet C., Kaldis P., et al. (2007). Cdk2 is critical for proliferation and self-renewal of neural progenitor cells in the adult subventricular zone. J. Cell Biol. 179 1231–1245. 10.1083/jcb.200702031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadasz J. J., Rivera F. J., Taubert A., Kandasamy M., Sandner B., Weidner N., et al. (2012). p57kip2 regulates glial fate decision in adult neural stem cells. Development 139 3306–3315. 10.1242/dev.074518 [DOI] [PubMed] [Google Scholar]
- Jaholkowski P., Kiryk A., Jedynak P., Ben Abdallah N. M., Knapska E., Kowalczyk A., et al. (2009). New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn. Mem. 16 439–451. 10.1101/lm.1459709 [DOI] [PubMed] [Google Scholar]
- Jang M. H., Bonaguidi M. A., Kitabatake Y., Sun J., Song J., Kang E., et al. (2013). Secreted frizzled-related protein 3 regulates activity-dependent adult hippocampal neurogenesis. Cell Stem Cell 12 215–223. 10.1016/j.stem.2012.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynak P., Jaholkowski P., Wozniak G., Sandi C., Kaczmarek L., Filipkowski R. K. (2012). Lack of cyclin D2 impairing adult brain neurogenesis alters hippocampal-dependent behavioral tasks without reducing learning ability. Behav. Brain Res. 227 159–166. 10.1016/j.bbr.2011.11.007 [DOI] [PubMed] [Google Scholar]
- Jeffrey P. D., Tong L., Pavletich N. P. (2000). Structural basis of inhibition of CDK-cyclin complexes by INK4 inhibitors. Genes Dev. 14 3115–3125. 10.1101/gad.851100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S., Parent J. M. (2015). Epilepsy and adult neurogenesis. Cold Spring Harb. Perspect. Biol. 7:a020677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Sun Y., Xie L., Batteur S., Mao X. O., Smelick C., et al. (2003). Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell 2 175–183. 10.1046/j.1474-9728.2003.00046.x [DOI] [PubMed] [Google Scholar]
- Jin K., Zhu Y., Sun Y., Mao X. O., Xie L., Greenberg D. A. (2002). Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 99 11946–11950. 10.1073/pnas.182296499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. G., Walker C. L. (1999). Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. 39 295–312. 10.1146/annurev.pharmtox.39.1.295 [DOI] [PubMed] [Google Scholar]
- Jori F. P., Galderisi U., Napolitano M. A., Cipollaro M., Cascino A., Giordano A., et al. (2007). RB and RB2/P130 genes cooperate with extrinsic signals to promote differentiation of rat neural stem cells. Mol. Cell. Neurosci. 34 299–309. 10.1016/j.mcn.2006.11.009 [DOI] [PubMed] [Google Scholar]
- Julian L. M., Blais A. (2015). Transcriptional control of stem cell fate by E2Fs and pocket proteins. Front. Genet. 6:161. 10.3389/fgene.2015.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J. Y., Matsuoka M., Polyak K., Massague J., Sherr C. J. (1994). Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell 79 487–496. 10.1016/0092-8674(94)90257-7 [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R., Asano T., Ueda H., Morishita R., Takeuchi I. K., Inaguma Y., et al. (2002). Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 16 1328–1330. 10.1096/fj.02-0143fje [DOI] [PubMed] [Google Scholar]
- Kempermann G., Gage F. H., Aigner L., Song H., Curtis M. A., Thuret S., et al. (2018). Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell 23 25–30. 10.1016/j.stem.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Jessberger S., Steiner B., Kronenberg G. (2004). Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27 447–452. 10.1016/j.tins.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Song H., Gage F. H. (2015). Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 7:a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. W., Deshaies R. J., Peters J. M., Kirschner M. W. (1996). How proteolysis drives the cell cycle. Science 274 1652–1659. 10.1126/science.274.5293.1652 [DOI] [PubMed] [Google Scholar]
- Kippin T. E., Martens D. J., Van Der Kooy D. (2005). p21 loss compromises the relative quiescence of forebrain stem cell proliferation leading to exhaustion of their proliferation capacity. Genes Dev. 19 756–767. 10.1101/gad.1272305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E. D., Muroy S. E., Sun W. G., Covarrubias D., Leong M. J., Barchas L. A., et al. (2013). Acute stress enhances adult rat hippocampal neurogenesis and activation of newborn neurons via secreted astrocytic FGF2. eLife 2:e00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff A., Ohtsuki M., Polyak K., Roberts J. M., Massague J. (1993). Negative regulation of G1 in mammalian cells: inhibition of cyclin E-dependent kinase by TGF-beta. Science 260 536–539. 10.1126/science.8475385 [DOI] [PubMed] [Google Scholar]
- Kollmann K., Heller G., Schneckenleithner C., Warsch W., Scheicher R., Ott R. G., et al. (2013). A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell 24 167–181. 10.1016/j.ccr.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M., Iguchi T., Takeda T., Ishibashi M., Sato M. (2013). Smoothened controls cyclin D2 expression and regulates the generation of intermediate progenitors in the developing cortex. Neurosci. Lett. 547 87–91. 10.1016/j.neulet.2013.05.006 [DOI] [PubMed] [Google Scholar]
- Kowalczyk A., Filipkowski R. K., Rylski M., Wilczynski G. M., Konopacki F. A., Jaworski J., et al. (2004). The critical role of cyclin D2 in adult neurogenesis. J. Cell Biol. 167 209–213. 10.1083/jcb.200404181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar K., Ciemerych M. A., Rebel V. I., Shigematsu H., Zagozdzon A., Sicinska E., et al. (2004). Mouse development and cell proliferation in the absence of D-cyclins. Cell 118 477–491. 10.1016/j.cell.2004.07.025 [DOI] [PubMed] [Google Scholar]
- Kozar K., Sicinski P. (2005). Cell cycle progression without cyclin D-CDK4 and cyclin D-CDK6 complexes. Cell Cycle 4 388–391. 10.4161/cc.4.3.1551 [DOI] [PubMed] [Google Scholar]
- Krek W., Ewen M. E., Shirodkar S., Arany Z., Kaelin W. G., Jr.et al. (1994). Negative regulation of the growth-promoting transcription factor E2F-1 by a stably bound cyclin A-dependent protein kinase. Cell 78 161–172. 10.1016/0092-8674(94)90582-7 [DOI] [PubMed] [Google Scholar]
- Kuwabara T., Hsieh J., Muotri A., Yeo G., Warashina M., Lie D. C., et al. (2009). Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 12 1097–1105. 10.1038/nn.2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBaer J., Garrett M. D., Stevenson L. F., Slingerland J. M., Sandhu C., Chou H. S., et al. (1997). New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11 847–862. 10.1101/gad.11.7.847 [DOI] [PubMed] [Google Scholar]
- Lai K., Kaspar B. K., Gage F. H., Schaffer D. V. (2003). Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 6 21–27. 10.1038/nn983 [DOI] [PubMed] [Google Scholar]
- Lange C., Calegari F. (2010). Cdks and cyclins link G1 length and differentiation of embryonic, neural and hematopoietic stem cells. Cell Cycle 9 1893–1900. 10.4161/cc.9.10.11598 [DOI] [PubMed] [Google Scholar]
- Lange C., Huttner W. B., Calegari F. (2009). Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 5 320–331. 10.1016/j.stem.2009.05.026 [DOI] [PubMed] [Google Scholar]
- Lavado A., Lagutin O. V., Chow L. M., Baker S. J., Oliver G. (2010). Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 8:e1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A., Oliver G. (2014). Jagged1 is necessary for postnatal and adult neurogenesis in the dentate gyrus. Dev. Biol. 388 11–21. 10.1016/j.ydbio.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le O., Palacio L., Bernier G., Batinic-Haberle I., Hickson G., Beausejour C. (2018). INK4a/ARF expression impairs neurogenesis in the brain of irradiated mice. Stem Cell Rep. 10 1721–1733. 10.1016/j.stemcr.2018.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Hu N., Yuan S. S., Cox L. A., Bradley A., Lee W. H., et al. (1994). Dual roles of the retinoblastoma protein in cell cycle regulation and neuron differentiation. Genes Dev. 8 2008–2021. 10.1101/gad.8.17.2008 [DOI] [PubMed] [Google Scholar]
- Lees E. (1995). Cyclin dependent kinase regulation. Curr. Opin. Cell Biol. 7 773–780. [DOI] [PubMed] [Google Scholar]
- Lees E., Faha B., Dulic V., Reed S. I., Harlow E. (1992). Cyclin E/cdk2 and cyclin A/cdk2 kinases associate with p107 and E2F in a temporally distinct manner. Genes Dev. 6 1874–1885. 10.1101/gad.6.10.1874 [DOI] [PubMed] [Google Scholar]
- Leto K., Bartolini A., Di Gregorio A., Imperiale D., De Luca A., Parmigiani E., et al. (2011). Modulation of cell-cycle dynamics is required to regulate the number of cerebellar GABAergic interneurons and their rhythm of maturation. Development 138 3463–3472. 10.1242/dev.064378 [DOI] [PubMed] [Google Scholar]
- Li B., Dicicco-Bloom E. (2004). Basic fibroblast growth factor exhibits dual and rapid regulation of cyclin D1 and p27 to stimulate proliferation of rat cerebral cortical precursors. Dev. Neurosci. 26 197–207. 10.1159/000082137 [DOI] [PubMed] [Google Scholar]
- Li G., Fang L., Fernandez G., Pleasure S. J. (2013). The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron 78 658–672. 10.1016/j.neuron.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Collado M., Villasante A., Matheu A., Lynch C. J., Canamero M., et al. (2012). p27(Kip1) directly represses Sox2 during embryonic stem cell differentiation. Cell Stem Cell 11 845–852. 10.1016/j.stem.2012.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Tang X., Jablonska B., Aguirre A., Gallo V., Luskin M. B. (2009). p27(KIP1) regulates neurogenesis in the rostral migratory stream and olfactory bulb of the postnatal mouse. J. Neurosci. 29 2902–2914. 10.1523/jneurosci.4051-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Luikart B. W., Birnbaum S., Chen J., Kwon C. H., Kernie S. G., et al. (2008). TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59 399–412. 10.1016/j.neuron.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Q., Wong C. S. (2018). Effects of p21 on adult hippocampal neuronal development after irradiation. Cell Death Discov. 4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T., Keshet E. (2015). The vascular niche in adult neurogenesis. Mech. Dev. 138 (Pt. 1) 56–62. 10.1016/j.mod.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Lie D. C., Colamarino S. A., Song H. J., Desire L., Mira H., Consiglio A., et al. (2005). Wnt signalling regulates adult hippocampal neurogenesis. Nature 437 1370–1375. 10.1038/nature04108 [DOI] [PubMed] [Google Scholar]
- Lim S., Kaldis P. (2012). Loss of Cdk2 and Cdk4 induces a switch from proliferation to differentiation in neural stem cells. Stem Cells 30 1509–1520. 10.1002/stem.1114 [DOI] [PubMed] [Google Scholar]
- Lim S., Kaldis P. (2013). Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development 140 3079–3093. 10.1242/dev.091744 [DOI] [PubMed] [Google Scholar]
- Lindqvist A., Rodriguez-Bravo V., Medema R. H. (2009). The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell Biol. 185 193–202. 10.1083/jcb.200812045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolli G., Johnson L. N. (2005). CAK-Cyclin-dependent Activating Kinase: a key kinase in cell cycle control and a target for drugs? Cell Cycle 4 572–577. [PubMed] [Google Scholar]
- Lowe S. W., Sherr C. J. (2003). Tumor suppression by Ink4a-Arf: progress and puzzles. Curr. Opin. Genet. Dev. 13 77–83. 10.1016/s0959-437x(02)00013-8 [DOI] [PubMed] [Google Scholar]
- Lugert S., Basak O., Knuckles P., Haussler U., Fabel K., Gotz M., et al. (2010). Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6 445–456. 10.1016/j.stem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A., Savatier P., Cortay V., Kennedy H., Dehay C. (2002). Contrasting effects of basic fibroblast growth factor and neurotrophin 3 on cell cycle kinetics of mouse cortical stem cells. J. Neurosci. 22 6610–6622. 10.1523/jneurosci.22-15-06610.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A. I., Anderson D. J. (2011). Cyclin D1 promotes neurogenesis in the developing spinal cord in a cell cycle-independent manner. Proc. Natl. Acad. Sci. U.S.A. 108 11632–11637. 10.1073/pnas.1106230108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. S., Weinberg R. A. (1998). Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol. Cell. Biol. 18 753–761. 10.1128/mcb.18.2.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Yu Z., Qu W., Tang Y., Zhan Y., Ding C., et al. (2010). Proliferation and differentiation of neural stem cells are selectively regulated by knockout of cyclin D1. J. Mol. Neurosci. 42 35–43. 10.1007/s12031-010-9362-9 [DOI] [PubMed] [Google Scholar]
- Machold R., Hayashi S., Rutlin M., Muzumdar M. D., Nery S., Corbin J. G., et al. (2003). Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 39 937–950. 10.1016/s0896-6273(03)00561-0 [DOI] [PubMed] [Google Scholar]
- Mairet-Coello G., Tury A., Van Buskirk E., Robinson K., Genestine M., Dicicco-Bloom E. (2012). p57(KIP2) regulates radial glia and intermediate precursor cell cycle dynamics and lower layer neurogenesis in developing cerebral cortex. Development 139 475–487. 10.1242/dev.067314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M. (2014). Cyclin-dependent kinases. Genome Biol. 15:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Ge X., Frank C. L., Madison J. M., Koehler A. N., Doud M. K., et al. (2009). Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 136 1017–1031. 10.1016/j.cell.2008.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Burgin A., Mongiat L. A., Pardi M. B., Schinder A. F. (2012). Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 335 1238–1242. 10.1126/science.1214956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Torrejon M. A., Porlan E., Banito A., Gomez-Ibarlucea E., Lopez-Contreras A. J., Fernandez-Capetillo O., et al. (2013). Cyclin-dependent kinase inhibitor p21 controls adult neural stem cell expansion by regulating Sox2 gene expression. Cell Stem Cell 12 88–100. 10.1016/j.stem.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massirer K. B., Carromeu C., Griesi-Oliveira K., Muotri A. R. (2011). Maintenance and differentiation of neural stem cells. Wiley Interdiscip. Rev. Syst. Biol. Med. 3 107–114. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Quelle D. E., Shurtleff S. A., Shibuya M., Sherr C. J., Kato J. Y. (1994). D-type cyclin-dependent kinase activity in mammalian cells. Mol. Cell. Biol. 14 2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan K. A., Vanderluit J. L., Julian L. M., Andrusiak M. G., Dugal-Tessier D., Park D. S., et al. (2009). The p107/E2F pathway regulates fibroblast growth factor 2 responsiveness in neural precursor cells. Mol. Cell. Biol. 29 4701–4713. 10.1128/mcb.01767-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheli L., D’andrea G., Ceccarelli M., Ferri A., Scardigli R., Tirone F. (2019). p16Ink4a prevents the activation of aged quiescent dentate gyrus stem cells by physical exercise. Front. Cell. Neurosci. 13:10. 10.3389/fncel.2019.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G. L., Song H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70 687–702. 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H., Andreu Z., Suh H., Lie D. C., Jessberger S., Consiglio A., et al. (2010). Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7 78–89. 10.1016/j.stem.2010.04.016 [DOI] [PubMed] [Google Scholar]
- Mirzaa G., Parry D. A., Fry A. E., Giamanco K. A., Schwartzentruber J., Vanstone M., et al. (2014). De novo CCND2 mutations leading to stabilization of cyclin D2 cause megalencephaly-polymicrogyria-polydactyly-hydrocephalus syndrome. Nat. Genet. 46 510–515. 10.1038/ng.2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittnacht S., Lees J. A., Desai D., Harlow E., Morgan D. O., Weinberg R. A. (1994). Distinct sub-populations of the retinoblastoma protein show a distinct pattern of phosphorylation. EMBO J. 13 118–127. 10.1002/j.1460-2075.1994.tb06241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky A. V., Slutsky S. G., Joseph N. M., He S., Pardal R., Krishnamurthy J., et al. (2006). Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443 448–452. 10.1038/nature05091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat L. A., Esposito M. S., Lombardi G., Schinder A. F. (2009). Reliable activation of immature neurons in the adult hippocampus. PLoS One 4:e5320. 10.1371/journal.pone.0005320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat L. A., Schinder A. F. (2011). Adult neurogenesis and the plasticity of the dentate gyrus network. Eur. J. Neurosci. 33 1055–1061. 10.1111/j.1460-9568.2011.07603.x [DOI] [PubMed] [Google Scholar]
- Morgan D. O. (1995). Principles of CDK regulation. Nature 374 131–134. 10.1038/374131a0 [DOI] [PubMed] [Google Scholar]
- Morizur L., Chicheportiche A., Gauthier L. R., Daynac M., Boussin F. D., Mouthon M. A. (2018). Distinct molecular signatures of quiescent and activated adult neural stem cells reveal specific interactions with their microenvironment. Stem Cell Rep. 11 565–577. 10.1016/j.stemcr.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Gebara E., Bushong E. A., Sanchez-Pascual I., O’laoi R., El M’ghari I., et al. (2016). Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc. Natl. Acad. Sci. U.S.A. 113 E2536-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan G., Jacks T. (1998). The retinoblastoma gene family: cousins with overlapping interests. Trends Genet. 14 223–229. 10.1016/s0168-9525(98)01470-x [DOI] [PubMed] [Google Scholar]
- Nagao M., Campbell K., Burns K., Kuan C. Y., Trumpp A., Nakafuku M. (2008). Coordinated control of self-renewal and differentiation of neural stem cells by Myc and the p19ARF-p53 pathway. J. Cell Biol. 183 1243–1257. 10.1083/jcb.200807130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Ishida N., Shirane M., Inomata A., Inoue T., Shishido N., et al. (1996). Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85 707–720. 10.1016/s0092-8674(00)81237-4 [DOI] [PubMed] [Google Scholar]
- Narasimha A. M., Kaulich M., Shapiro G. S., Choi Y. J., Sicinski P., Dowdy S. F. (2014). Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. eLife 3:e02872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naser R., Vandenbosch R., Omais S., Hayek D., Jaafar C., Al Lafi S., et al. (2016). Role of the Retinoblastoma protein, Rb, during adult neurogenesis in the olfactory bulb. Sci. Rep. 6:20230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed M., Kazmi S. K., Amin M., Asif Z., Islam U., Shahid K., et al. (2018). Comprehensive review on the molecular genetics of autosomal recessive primary microcephaly (MCPH). Genet. Res. 100:e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L., Besson A., Heng J. I., Schuurmans C., Teboul L., Parras C., et al. (2006). p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 20 1511–1524. 10.1101/gad.377106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola Z., Fabel K., Kempermann G. (2015). Development of the adult neurogenic niche in the hippocampus of mice. Front. Neuroanat. 9:53 10.3389/fnana.2015.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. (2001). Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2 21–32. 10.1038/35048096 [DOI] [PubMed] [Google Scholar]
- Nishino J., Kim I., Chada K., Morrison S. J. (2008). Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell 135 227–239. 10.1016/j.cell.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh S. J., Li Y., Xiong Y., Guan K. L. (1999). Identification of functional elements of p18INK4C essential for binding and inhibition of cyclin-dependent kinase (CDK) 4 and CDK6. Cancer Res. 59 558–564. [PubMed] [Google Scholar]
- Ohtsuka T., Ishibashi M., Gradwohl G., Nakanishi S., Guillemot F., Kageyama R. (1999). Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 18 2196–2207. 10.1093/emboj/18.8.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M., Gould E. (2015). Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn. Sci. 19 151–161. 10.1016/j.tics.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Parry D., Mahony D., Wills K., Lees E. (1999). Cyclin D-CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol. Cell. Biol. 19 1775–1783. 10.1128/mcb.19.3.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauklin S., Madrigal P., Bertero A., Vallier L. (2016). Initiation of stem cell differentiation involves cell cycle-dependent regulation of developmental genes by Cyclin D. Genes Dev. 30 421–433. 10.1101/gad.271452.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauklin S., Vallier L. (2013). The cell-cycle state of stem cells determines cell fate propensity. Cell 155 135–147. 10.1016/j.cell.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechnick R. N., Zonis S., Wawrowsky K., Cosgayon R., Farrokhi C., Lacayo L., et al. (2011). Antidepressants stimulate hippocampal neurogenesis by inhibiting p21 expression in the subgranular zone of the hipppocampus. PLoS One 6:e27290. 10.1371/journal.pone.0027290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechnick R. N., Zonis S., Wawrowsky K., Pourmorady J., Chesnokova V. (2008). p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 105 1358–1363. 10.1073/pnas.0711030105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei X. H., Xiong Y. (2005). Biochemical and cellular mechanisms of mammalian CDK inhibitors: a few unresolved issues. Oncogene 24 2787–2795. 10.1038/sj.onc.1208611 [DOI] [PubMed] [Google Scholar]
- Perry J. E., Grossmann M. E., Tindall D. J. (1998). Epidermal growth factor induces cyclin D1 in a human prostate cancer cell line. Prostate 35 117–124. [DOI] [PubMed] [Google Scholar]
- Pestell R. G. (2013). New roles of cyclin D1. Am. J. Pathol. 183 3–9. 10.1016/j.ajpath.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaz L. J., Patti D., Marcy G., Ollier E., Pfister S., Douglas R. J., et al. (2009). Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc. Natl. Acad. Sci. U.S.A. 106 21924–21929. 10.1073/pnas.0909894106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz G. A., Bottes S., Betizeau M., Jorg D. J., Carta S., Simons B. D., et al. (2018). Live imaging of neurogenesis in the adult mouse hippocampus. Science 359 658–662. 10.1126/science.aao5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoriler J., Millen K., Utset M., Du W. (2006). Loss of cyclin D1 impairs cerebellar development and suppresses medulloblastoma formation. Development 133 3929–3937. 10.1242/dev.02556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K., Kato J. Y., Solomon M. J., Sherr C. J., Massague J., Roberts J. M., et al. (1994). p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 8 9–22. 10.1101/gad.8.1.9 [DOI] [PubMed] [Google Scholar]
- Popov B., Petrov N. (2014). pRb-E2F signaling in life of mesenchymal stem cells: cell cycle, cell fate, and cell differentiation. Genes Dis. 1 174–187. 10.1016/j.gendis.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porlan E., Morante-Redolat J. M., Marques-Torrejon M. A., Andreu-Agullo C., Carneiro C., Gomez-Ibarlucea E., et al. (2013). Transcriptional repression of Bmp2 by p21(Waf1/Cip1) links quiescence to neural stem cell maintenance. Nat. Neurosci. 16 1567–1575. 10.1038/nn.3545 [DOI] [PubMed] [Google Scholar]
- Price J. D., Park K. Y., Chen J., Salinas R. D., Cho M. J., Kriegstein A. R., et al. (2014). The Ink4a/Arf locus is a barrier to direct neuronal transdifferentiation. J. Neurosci. 34 12560–12567. 10.1523/jneurosci.3159-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y., Gregory M. A., Li Z., Brousal J. P., West K., Hann S. R. (2004). p19ARF directly and differentially controls the functions of c-Myc independently of p53. Nature 431 712–717. 10.1038/nature02958 [DOI] [PubMed] [Google Scholar]
- Qiu J., Takagi Y., Harada J., Rodrigues N., Moskowitz M. A., Scadden D. T., et al. (2004). Regenerative response in ischemic brain restricted by p21cip1/waf1. J. Exp. Med. 199 937–945. 10.1084/jem.20031385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Takagi Y., Harada J., Topalkara K., Wang Y., Sims J. R., et al. (2009). p27Kip1 constrains proliferation of neural progenitor cells in adult brain under homeostatic and ischemic conditions. Stem Cells 27 920–927. 10.1002/stem.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q., Sun G., Li W., Yang S., Ye P., Zhao C., et al. (2010). Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat. Cell Biol. 12 31–40. 10.1038/ncb2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle D. E., Zindy F., Ashmun R. A., Sherr C. J. (1995). Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 83 993–1000. 10.1016/0092-8674(95)90214-7 [DOI] [PubMed] [Google Scholar]
- Rai K. S., Hattiangady B., Shetty A. K. (2007). Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur. J. Neurosci. 26 1765–1779. 10.1111/j.1460-9568.2007.05820.x [DOI] [PubMed] [Google Scholar]
- Reynisdottir I., Polyak K., Iavarone A., Massague J. (1995). Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 9 1831–1845. 10.1101/gad.9.15.1831 [DOI] [PubMed] [Google Scholar]
- Robertson K. D., Ait-Si-Ali S., Yokochi T., Wade P. A., Jones P. L., Wolffe A. P. (2000). DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat. Genet. 25 338–342. 10.1038/77124 [DOI] [PubMed] [Google Scholar]
- Roccio M., Schmitter D., Knobloch M., Okawa Y., Sage D., Lutolf M. P. (2013). Predicting stem cell fate changes by differential cell cycle progression patterns. Development 140 459–470. 10.1242/dev.086215 [DOI] [PubMed] [Google Scholar]
- Ross M. E. (1996). Cell division and the nervous system: regulating the cycle from neural differentiation to death. Trends Neurosci. 19 62–68. 10.1016/0166-2236(97)90030-8 [DOI] [PubMed] [Google Scholar]
- Rossi C., Angelucci A., Costantin L., Braschi C., Mazzantini M., Babbini F., et al. (2006). Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 24 1850–1856. 10.1111/j.1460-9568.2006.05059.x [DOI] [PubMed] [Google Scholar]
- Roussel M. F. (1999). The INK4 family of cell cycle inhibitors in cancer. Oncogene 18 5311–5317. 10.1038/sj.onc.1202998 [DOI] [PubMed] [Google Scholar]
- Ruan L., Lau B. W., Wang J., Huang L., Zhuge Q., Wang B., et al. (2014). Neurogenesis in neurological and psychiatric diseases and brain injury: from bench to bedside. Prog. Neurobiol. 115 116–137. 10.1016/j.pneurobio.2013.12.006 [DOI] [PubMed] [Google Scholar]
- Ruiz I Altaba A., Palma V., Dahmane N. (2002). Hedgehog-Gli signalling and the growth of the brain. Nat. Rev. Neurosci. 3 24–33. [DOI] [PubMed] [Google Scholar]
- Russo A. A., Jeffrey P. D., Patten A. K., Massague J., Pavletich N. P. (1996). Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 382 325–331. [DOI] [PubMed] [Google Scholar]
- Sage J. (2012). The retinoblastoma tumor suppressor and stem cell biology. Genes Dev. 26 1409–1420. 10.1101/gad.193730.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage J., Miller A. L., Perez-Mancera P. A., Wysocki J. M., Jacks T. (2003). Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424 223–228. 10.1038/nature01764 [DOI] [PubMed] [Google Scholar]
- Salomoni P., Calegari F. (2010). Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 20 233–243. 10.1016/j.tcb.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Santamaria D., Barriere C., Cerqueira A., Hunt S., Tardy C., Newton K., et al. (2007). Cdk1 is sufficient to drive the mammalian cell cycle. Nature 448 811–815. 10.1038/nature06046 [DOI] [PubMed] [Google Scholar]
- Satyanarayana A., Kaldis P. (2009). Mammalian cell-cycle regulation: several Cdks, numerous cyclins and diverse compensatory mechanisms. Oncogene 28 2925–2939. 10.1038/onc.2009.170 [DOI] [PubMed] [Google Scholar]
- Seri B., Garcia-Verdugo J. M., Collado-Morente L., Mcewen B. S., Alvarez-Buylla A. (2004). Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 478 359–378. 10.1002/cne.20288 [DOI] [PubMed] [Google Scholar]
- Seri B., Garcia-Verdugo J. M., Mcewen B. S., Alvarez-Buylla A. (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 21 7153–7160. 10.1523/jneurosci.21-18-07153.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M. (1997). The tumor suppressor protein p16INK4a. Exp. Cell Res. 237 7–13. 10.1006/excr.1997.3824 [DOI] [PubMed] [Google Scholar]
- Serrano M., Lee H., Chin L., Cordon-Cardo C., Beach D., Depinho R. A. (1996). Role of the INK4a locus in tumor suppression and cell mortality. Cell 85 27–37. 10.1016/s0092-8674(00)81079-x [DOI] [PubMed] [Google Scholar]
- Sherr C. J. (1994a). G1 phase progression: cycling on cue. Cell 79 551–555. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. (1994b). Growth factor-regulated G1 cyclins. Stem Cells 12 47–55. [PubMed] [Google Scholar]
- Sherr C. J. (1995). Mammalian G1 cyclins and cell cycle progression. Proc. Assoc. Am. Physicians 107 181–186. [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. (1999). CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13 1501–1512. 10.1101/gad.13.12.1501 [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. (2004). Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18 2699–2711. 10.1101/gad.1256504 [DOI] [PubMed] [Google Scholar]
- Shimizu T., Kagawa T., Inoue T., Nonaka A., Takada S., Aburatani H., et al. (2008). Stabilized beta-catenin functions through TCF/LEF proteins and the Notch/RBP-Jkappa complex to promote proliferation and suppress differentiation of neural precursor cells. Mol. Cell. Biol. 28 7427–7441. 10.1128/mcb.01962-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Encinas J. M., Deudero J. J., Chancey J. H., Enikolopov G., Overstreet-Wadiche L. S., et al. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7 483–495. 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Martin-Suarez S., Valcarcel-Martin R., Pascual-Brazo J., Aelvoet S. A., Abiega O., et al. (2015). Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell Stem Cell 16 488–503. 10.1016/j.stem.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S. F., Paredes M. F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K. W., et al. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555 377–381. 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B., Klempin F., Wang L., Kott M., Kettenmann H., Kempermann G. (2006). Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia 54 805–814. 10.1002/glia.20407 [DOI] [PubMed] [Google Scholar]
- Strobeck M. W., Knudsen K. E., Fribourg A. F., Decristofaro M. F., Weissman B. E., Imbalzano A. N., et al. (2000). BRG-1 is required for RB-mediated cell cycle arrest. Proc. Natl. Acad. Sci. U.S.A. 97 7748–7753. 10.1073/pnas.97.14.7748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump G., Durrer A., Klein A. L., Lutolf S., Suter U., Taylor V. (2002). Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech. Dev. 114 153–159. 10.1016/s0925-4773(02)00043-6 [DOI] [PubMed] [Google Scholar]
- Suh H., Consiglio A., Ray J., Sawai T., D’amour K.A., Gage F.H. (2007). In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1 515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H., Deng W., Gage F. H. (2009). Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 25 253–275. [DOI] [PubMed] [Google Scholar]
- Sun W., Lee D. K., Lee C. C., Kim K. (1996). Differential expression of D-type G1 cyclins during mouse development and liver regeneration in vivo. Mol. Reprod. Dev. 43 414–420. [DOI] [PubMed] [Google Scholar]
- Susaki E., Nakayama K., Nakayama K. I. (2007). Cyclin D2 translocates p27 out of the nucleus and promotes its degradation at the G0-G1 transition. Mol. Cell. Biol. 27 4626–4640. 10.1128/mcb.00862-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaffer M. P., Jones A. W., Flynn H. R., Snijders A. P., Nurse P. (2016). CDK substrate phosphorylation and ordering the cell cycle. Cell 167 1750–1761.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Nowakowski R. S., Caviness V. S., Jr. (1995). The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J. Neurosci. 15 6046–6057. 10.1523/jneurosci.15-09-06046.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki T., Fukasawa K., Suzuki-Takahashi I., Semba K., Kitagawa M., Taya Y., et al. (2005). Preferences for phosphorylation sites in the retinoblastoma protein of D-type cyclin-dependent kinases, Cdk4 and Cdk6, in vitro. J. Biochem. 137 381–386. 10.1093/jb/mvi050 [DOI] [PubMed] [Google Scholar]
- Tarui T., Takahashi T., Nowakowski R. S., Hayes N. L., Bhide P. G., Caviness V. S. (2005). Overexpression of p27 Kip 1, probability of cell cycle exit, and laminar destination of neocortical neurons. Cereb. Cortex 15 1343–1355. 10.1093/cercor/bhi017 [DOI] [PubMed] [Google Scholar]
- Tashiro A., Sandler V. M., Toni N., Zhao C., Gage F. H. (2006). NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442 929–933. 10.1038/nature05028 [DOI] [PubMed] [Google Scholar]
- Telley L., Govindan S., Prados J., Stevant I., Nef S., Dermitzakis E., et al. (2016). Sequential transcriptional waves direct the differentiation of newborn neurons in the mouse neocortex. Science 351 1443–1446. 10.1126/science.aad8361 [DOI] [PubMed] [Google Scholar]
- Toda T., Gage F. H. (2017). Review: adult neurogenesis contributes to hippocampal plasticity. Cell Tissue Res. 373:693-709 [DOI] [PubMed] [Google Scholar]
- Toni N., Laplagne D. A., Zhao C., Lombardi G., Ribak C. E., Gage F. H., et al. (2008). Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 11 901–907. 10.1038/nn.2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo J. L., Carro E., Torres-Aleman I. (2001). Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 21 1628–1634. 10.1523/jneurosci.21-05-01628.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunekawa Y., Britto J. M., Takahashi M., Polleux F., Tan S. S., Osumi N. (2012). Cyclin D2 in the basal process of neural progenitors is linked to non-equivalent cell fates. EMBO J. 31 1879–1892. 10.1038/emboj.2012.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tury A., Mairet-Coello G., Dicicco-Bloom E. (2011). The cyclin-dependent kinase inhibitor p57Kip2 regulates cell cycle exit, differentiation, and migration of embryonic cerebral cortical precursors. Cereb. Cortex 21 1840–1856. 10.1093/cercor/bhq254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tury A., Mairet-Coello G., Dicicco-Bloom E. (2012). The multiple roles of the cyclin-dependent kinase inhibitory protein p57(KIP2) in cerebral cortical neurogenesis. Dev. Neurobiol. 72 821–842. 10.1002/dneu.20999 [DOI] [PubMed] [Google Scholar]
- Udvardy A. (1996). The role of controlled proteolysis in cell-cycle regulation. Eur. J. Biochem. 240 307–313. 10.1111/j.1432-1033.1996.0307h.x [DOI] [PubMed] [Google Scholar]
- Urbach A., Robakiewicz I., Baum E., Kaczmarek L., Witte O. W., Filipkowski R. K. (2013). Cyclin D2 knockout mice with depleted adult neurogenesis learn Barnes maze task. Behav. Neurosci. 127 1–8. 10.1037/a0031222 [DOI] [PubMed] [Google Scholar]
- Van Lookeren Campagne M., Gill R. (1998). Tumor-suppressor p53 is expressed in proliferating and newly formed neurons of the embryonic and postnatal rat brain: comparison with expression of the cell cycle regulators p21Waf1/Cip1, p27Kip1, p57Kip2, p16Ink4a, cyclin G1, and the proto-oncogene Bax. J. Comp. Neurol. 397 181–198. [DOI] [PubMed] [Google Scholar]
- Vandenbosch R., Borgs L., Beukelaers P., Foidart A., Nguyen L., Moonen G., et al. (2007). CDK2 is dispensable for adult hippocampal neurogenesis. Cell Cycle 6 3065–3069. 10.4161/cc.6.24.5048 [DOI] [PubMed] [Google Scholar]
- Vandenbosch R., Clark A., Fong B. C., Omais S., Jaafar C., Dugal-Tessier D., et al. (2016). RB regulates the production and the survival of newborn neurons in the embryonic and adult dentate gyrus. Hippocampus 26 1379–1392. 10.1002/hipo.22613 [DOI] [PubMed] [Google Scholar]
- Vanderluit J. L., Ferguson K. L., Nikoletopoulou V., Parker M., Ruzhynsky V., Alexson T., et al. (2004). p107 regulates neural precursor cells in the mammalian brain. J. Cell Biol. 166 853–863. 10.1083/jcb.200403156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderluit J. L., Wylie C. A., Mcclellan K. A., Ghanem N., Fortin A., Callaghan S., et al. (2007). The Retinoblastoma family member p107 regulates the rate of progenitor commitment to a neuronal fate. J. Cell Biol. 178 129–139. 10.1083/jcb.200703176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Nallar L., Inestrosa N. C. (2013). Wnt signaling in the regulation of adult hippocampal neurogenesis. Front. Cell. Neurosci. 7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan F. P., Zhu X. J., Cui X. N., Porter B. E. (2009). Seizures increase cell proliferation in the dentate gyrus by shortening progenitor cell-cycle length. Epilepsia 50 2638–2647. 10.1111/j.1528-1167.2009.02244.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon A. E., Devine C., Philpott A. (2003). The cdk inhibitor p27Xic1 is required for differentiation of primary neurones in Xenopus. Development 130 85–92. 10.1242/dev.00193 [DOI] [PubMed] [Google Scholar]
- Walkley C. R., Orkin S. H. (2006). Rb is dispensable for self-renewal and multilineage differentiation of adult hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 103 9057–9062. 10.1073/pnas.0603389103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Chang X., She L., Xu D., Huang W., Poo M. M. (2015). Autocrine action of BDNF on dendrite development of adult-born hippocampal neurons. J. Neurosci. 35 8384–8393. 10.1523/jneurosci.4682-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Z., Plane J. M., Jiang P., Zhou C. J., Deng W. (2011). Concise review: quiescent and active states of endogenous adult neural stem cells: identification and characterization. Stem Cells 29 907–912. 10.1002/stem.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt J. L., Duman R. S. (2007). VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc. Natl. Acad. Sci. U.S.A. 104 4647–4652. 10.1073/pnas.0610282104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Unsicker K., Von Bohlen Und Halbach O. (2011). Fibroblast growth factor-2 deficiency causes defects in adult hippocampal neurogenesis, which are not rescued by exogenous fibroblast growth factor-2. J. Neurosci. Res. 89 1605–1617. 10.1002/jnr.22680 [DOI] [PubMed] [Google Scholar]
- Wexler E. M., Paucer A., Kornblum H. I., Palmer T. D., Geschwind D. H. (2009). Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells 27 1130–1141. 10.1002/stem.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wianny F., Real F. X., Mummery C. L., Van Rooijen M., Lahti J., Samarut J., et al. (1998). G1-phase regulators, cyclin D1, cyclin D2, and cyclin D3: up-regulation at gastrulation and dynamic expression during neurulation. Dev. Dyn. 212 49–62. [DOI] [PubMed] [Google Scholar]
- Woo M. S., Sanchez I., Dynlacht B. D. (1997). p130 and p107 use a conserved domain to inhibit cellular cyclin-dependent kinase activity. Mol. Cell. Biol. 17 3566–3579. 10.1128/mcb.17.7.3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Sheppard K. A., Peng C. Y., Yee A. S., Piwnica-Worms H. (1994). Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol. Cell. Biol. 14 8420–8431. 10.1128/mcb.14.12.8420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam C. H., Fung T. K., Poon R. Y. (2002). Cyclin A in cell cycle control and cancer. Cell. Mol. Life Sci. 59 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., Mairet-Coello G., Pasoreck E., Dicicco-Bloom E. (2009). Patterns of p57Kip2 expression in embryonic rat brain suggest roles in progenitor cell cycle exit and neuronal differentiation. Dev. Neurobiol. 69 1–21. 10.1002/dneu.20680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Chen R., Wu L., Chen Q., Hu A., Zhang T., et al. (2015). The regulatory mechanism of neurogenesis by IGF-1 in adult mice. Mol. Neurobiol. 51 512–522. 10.1007/s12035-014-8717-6 [DOI] [PubMed] [Google Scholar]
- Zhang H., Xiong Y., Beach D. (1993). Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol. Biol. Cell 4 897–906. 10.1091/mbc.4.9.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Liegeois N. J., Wong C., Finegold M., Hou H., Thompson J. C., et al. (1997). Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature 387 151–158. 10.1038/387151a0 [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132 645–660. 10.1016/j.cell.2008.01.033 [DOI] [PubMed] [Google Scholar]
- Zhao C., Teng E. M., Summers R. G., Jr., Ming G. L., Gage F. H. (2006). Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 26 3–11. 10.1523/jneurosci.3648-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. L., Li B. S., Rudrabhatla P., Shukla V., Amin N. D., Maric D., et al. (2010). Phosphorylation of p27Kip1 at Thr187 by cyclin-dependent kinase 5 modulates neural stem cell differentiation. Mol. Biol. Cell 21 3601–3614. 10.1091/mbc.e10-01-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F., Quelle D. E., Roussel M. F., Sherr C. J. (1997a). Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene 15 203–211. [DOI] [PubMed] [Google Scholar]
- Zindy F., Soares H., Herzog K. H., Morgan J., Sherr C. J., Roussel M. F. (1997b). Expression of INK4 inhibitors of cyclin D-dependent kinases during mouse brain development. Cell Growth Differ. 8 1139–1150. [PubMed] [Google Scholar]
- Zonis S., Ljubimov V. A., Mahgerefteh M., Pechnick R. N., Wawrowsky K., Chesnokova V. (2013). p21Cip restrains hippocampal neurogenesis and protects neuronal progenitors from apoptosis during acute systemic inflammation. Hippocampus 23 1383–1394. 10.1002/hipo.22192 [DOI] [PMC free article] [PubMed] [Google Scholar]


