Abstract
Background
The modalities of therapy for obstructive sleep apnoea (OSA) include behavioural and lifestyle modifications, positional therapy, oral appliances, surgery and continuous positive airway pressure therapy (CPAP). Though CPAP has proven efficacy in treating OSA, adherence with CPAP therapy is suboptimal. Positional therapy (to keep people sleeping on their side) is less invasive and therefore expected to have better adherence. This review considered the efficacy of positional therapy compared to CPAP as well as positional therapy against no positional therapy. Devices designed for positional therapy include lumbar or abdominal binders, semi‐rigid backpacks, full‐length pillows, a tennis ball attached to the back of nightwear, and electrical sensors with alarms that indicate change in position.
Objectives
To compare the efficacy of positional therapy versus CPAP and positional therapy versus inactive control (sham intervention or no positional therapy intervention) in people with OSA.
Search methods
We identified studies from the Cochrane Airways' Specialised Register (including CENTRAL, MEDLINE, Embase, CINAHL, AHMED and PsycINFO), ClinicalTrials.gov, and the World Health Organization trials portal (ICTRP). It also contains results derived from handsearching of respiratory journals and abstract books of major annual meetings. We searched all databases from their inception to September 2018, with no restrictions on language of publication or publication type.
Selection criteria
We included randomised controlled trials comparing positional therapy with CPAP and positional therapy with inactive control.
Data collection and analysis
Two review authors independently selected studies and extracted the data. We used a random‐effects model in the meta‐analysis to estimate mean differences and confidence intervals. We assessed certainty of evidence using the GRADE approach.
Main results
We included eight studies. The studies randomised 323 participants into two types of interventions. The comparison between positional therapy and CPAP included 72 participants, while the comparison between positional therapy and inactive control included 251 participants. Three studies used supine vibration alarm devices, while five studies used physical positioning like specially designed pillows or semirigid backpacks.
Positional therapy versus CPAP
The three studies included for this comparison were randomised cross‐over trials. Two studies found that there was no difference in Epworth Sleepiness Scale (ESS) scores between CPAP and positional therapy. Two studies showed that CPAP produced a greater reduction in Apnoea‐Hypopnoea Index (AHI) with a mean difference (MD) of 6.4 events per hour (95% CI 3.00 to 9.79; low‐certainty evidence) compared to positional therapy. Subjective adherence, evaluated in one study, was found to be significantly greater with positional therapy (MD 2.5 hours per night, 95% CI 1.41 to 3.59; moderate‐certainty evidence).
In terms of secondary outcomes, one study each reported quality‐of‐life indices and quality‐of‐sleep indices with no significant difference between the two groups. One study reported cognitive outcomes using multiple parameters and found no difference between the groups. There were insufficient data to comment on other secondary outcomes like respiratory disturbance index (RDI), and frequency and duration of nocturnal desaturation. None of the studies clearly reported adverse effects.
Positional therapy versus inactive control
Three studies of positional therapy versus no intervention were randomised cross‐over trials, while two studies were parallel‐arm studies. Data from two studies showed that positional therapy significantly improved ESS scores (MD −1.58, 95% CI −2.89 to −0.29; moderate‐certainty evidence). Positional therapy showed a reduction in AHI compared with control (MD −7.38 events per hour, 95% CI −10.06 to −4.7; low‐certainty evidence). One study reported adherence. The number of participants who continued to use the device at two months was no different between the two groups (odds ratio (OR) 0.80, 95% CI 0.33 to 1.94; low‐certainty evidence). The same study reported adverse effects, the most common being pain in the back and chest, and sleep disturbance but there was no significant difference between the two groups in terms of device discontinuation (OR 1.25, 95% CI 0.5 to 3.03; low‐certainty evidence). One study each reported quality‐of‐life indices and quality‐of‐sleep indices, with no significant difference between the two groups. One study reported cognitive outcome, and found no difference between the groups. There was insufficient evidence to comment on other secondary outcomes (RDI, frequency and duration of nocturnal desaturation).
Authors' conclusions
The review found that CPAP has a greater effect on improving AHI compared with positional therapy in positional OSA, while positional therapy was better than inactive control for improving ESS and AHI. Positional therapy may have better adherence than CPAP. There were no significant differences for other clinically relevant outcomes such as quality of life or cognitive function. All the studies were of short duration. We are unable to comment on the long‐term effects of the therapies. This is important, as most of the quality‐of‐life outcomes will be evident only when the therapies are given over a longer period of time. The certainty of evidence was low to moderate.
Plain language summary
Are interventions to keep people sleeping on their side the best way to treat obstructive sleep apnoea?
What is obstructive sleep apnoea?
Obstructive sleep apnoea (OSA) is sleep disorder where the walls of the throat relax and narrow during sleep. This causes pauses in the breathing. The pauses can last for a few seconds to a few minutes and can happen many times in the night. This disrupts the person's sleep. Bed partners can be disturbed by associated loud snoring, chocking and snorting sounds. People living with OSA can be very tired in the day or even fall to sleep. This can be dangerous. In children, sleep apnoea can cause problems at school or hyperactivity.
What is positional OSA?
OSA that improves on changing position of the person while sleeping is known as positional sleep apnoea (POSA). People tend to have apneas when lying on their backs (supine) and the apneas may be reduced or go away when they lay on their side.
What is the standard in the treatment of OSA?
The standard treatment is a device called continuous positive airway pressure (CPAP) that provides a continuous jet of air to the airway as the person breathes, which helps to keep the throat from narrowing during sleep.
What is positional therapy?
Positional therapy is an intervention that helps to keep the person on their side during sleep. Examples include something on the person's back to stop them from rolling over (like a tennis ball), special pillows, or alarms that vibrate when the person rolls onto his or her back.
How is the severity of OSA estimated?
The severity of OSA is measured using a scale called Apnoea‐Hypopnea Index (AHI). AHI refers to number of times the breathing stops or becomes shallower per hour of sleep. AHI is measured by using a study done in sleep known as polysomnography.
The severity of OSA can be measured indirectly using a questionnaire called Epworth Sleepiness Scale (ESS). This assesses how sleepy a person is during the day.
What was the aim of this review?
We wanted to compare positional therapy with the CPAP therapy as well as with inactive control (no positional therapy or sham therapy).
Results
We found eight studies with 323 participants. The studies compared positional therapy with CPAP (72 participants) and positional therapy with inactive control (251 participants).
When studies compared positional therapy and CPAP, they found no difference in ESS between groups. CPAP therapy showed a greater improvement in AHI (6.4 fewer events per hour with CPAP) compared with positional therapy. In one small study, people adhered to positional therapy for 2.5 hours longer than they did for CPAP. No difference in quality of life or quality of sleep between the two groups was found.
In the comparison between positional therapy and inactive control, the studies found that positional therapy appeared to be better than control for ESS and AHI (ESS was 1.58 lower in positional therapy and AHI was 7.38 fewer events per hour with positional therapy). Another study noted adverse effects in 10% of participants. Common adverse effects were sleep disturbance and pain in the back and the chest. One study reported that there was no difference in quality of life and quality of sleep between positional therapy and inactive control.
All these studies lasted for a short time and included small number of participants.
Conclusions
1. Positional therapy was less effective than CPAP for reducing Apnoea‐Hypopnoea Index (AHI). People may use positional therapy for longer than CPAP in the night. In terms of other outcomes no differences were seen.
2. Positional therapy was shown to be better than inactive control for AHI and Epworth Sleepiness Scale (ESS).
Summary of findings
Background
Description of the condition
Obstructive sleep apnoea (OSA) is a common condition. Its prevalence varies between 9% to 38% and it is influenced by age and gender. The prevalence of severe OSA varies between 6% to 7% (Senaratna 2017). One study reports that the prevalence of OSA has increased by 14% to 55% over the last two decades, as estimated by an increase in Apnoea‐Hypopnoea Index (AHI) in laboratory‐based polysomnography (Peppard 2013). OSA is known to be a risk factor for road traffic accidents, and is associated with several systemic illnesses such as cardiovascular disease, cognitive impairment (Jackson 2018), and impaired recovery following stroke (Arzt 2005; Chobanian 2003; Dumitrascu 2012; Lal 2012; Marin 2005; Marshall 2008; Martinez‐Garcia 2005; Parra 2011; Peppard 2000; Shahar 2001; Yaggi 2005). OSA is two to four times more common among people with systemic hypertension, stroke or coronary artery disease than in the general population (Bassetti 1999; Harbison 2002; Wessendorf 2000).
OSA is characterised by repeated episodes of pharyngeal collapse leading to intermittent hypoxaemia (abnormally low oxygen level in the blood) and consequent sleep fragmentation. Repeated sleep disturbance at night may result in non‐refreshing sleep and excessive daytime sleepiness, especially in moderate to severe cases. Other clinical features of OSA include fatigue, insomnia, loud snoring, gasping, choking and breath holding during sleep. Studies suggest that OSA can induce oxidative stress and a state of subclinical inflammation (Chen 2015; Lavie 2015; Schulz 2000; Shamsuzzaman 2002; Vgontzas 1997).
The diagnosis of OSA is based on sleep‐related symptoms and polysomnography. OSA is confirmed if the number of apnoea, hypopnoea and respiratory event–related arousals on polysomnography is greater than either 15 per hour in asymptomatic people or five per hour in the presence of clinical features suggestive of OSA. The severity of OSA is graded by AHI score(Ruehland 2009). OSA is graded as mild, moderate and severe if the AHI is 5 to 14, 15 to 30, and greater than 30 respectively (Epstein 2009).
The prevalence of OSA that may improve on proper positioning is 50% to 60% (Cartwright 1984; Joosten 2014; Mador 2005; Oksenberg 2009). The prevalence of OSA that appears on sleeping on the back and disappears on sleeping in any position other than on the back is 25% to 30% (Joosten 2014). This type of OSA, which improves with change in sleeping position, is called positional OSA (POSA). POSA is variably defined. Cartwright 1984 defined POSA as, "50% or more reduction in Apnoea‐Hypopnoea Index (AHI) score while lying on his or her side (lateral recumbent position) than lying on the back". Mador 2005 defined POSA as "total AHI more than 5 and non‐supine AHI 5 or less with more than 50% reduction in AHI between supine and non‐supine postures". They found that 49.5% of mild, 19.4% of moderate and 6.5% of severe sleep apnoea participants had POSA (Mador 2005).
Description of the intervention
Treatment modalities for OSA include behavioural and lifestyle modifications, oral appliance devices, continuous positive airway pressure (CPAP) therapy and surgery (Giles 2006; Lim 2004; Shneerson 2001; Smith 2002; Sundaram 2005). Most of the guidelines given by various recognised professional bodies recommend the use of lifestyle modification and behavioural therapy in people with newly diagnosed mild OSA.
Positional therapy uses devices that help people to sleep on their side or in a non‐supine position. The American Academy of Sleep Medicine (AASM) task force on adult obstructive sleep apnoea recommends positional therapy as an effective secondary therapy for people with POSA (Epstein 2009). This recommendation was based on evidence of a moderate degree of clinical certainty, implying use of level II evidence or consensus of level III evidence (randomised controlled trials (RCTs) with high‐beta error or consensus of evidence from non‐randomised controlled studies). The European Respiratory Society task force on non‐CPAP therapies in OSA states that positional therapy can yield a moderate reduction in AHI score (Randerath 2011). With a grade C recommendation (evidence based on case studies or cohort studies or extrapolation of systematic reviews of cohort or case‐control studies with homogeneous results), the task force stated that positional therapy is inferior to CPAP but may be recommended for carefully selected patients (Randerath 2011).
Several devices are available for positional therapy. Devices that have been designed for this purpose include lumbar or abdominal binders, semi‐rigid backpacks, full‐length pillows, a tennis ball attached to the back of nightwear, and electrical sensors with alarms that indicate change in position. Other options include support devices, pillows and t‐shirts that can be used to progressively train people to sleep on their sides. These therapies may be an attractive option because of their cost‐effectiveness and possibly better patient adherence, especially in those with mild to moderate OSA, for whom adherence with CPAP therapy is generally poor (Ravesloot 2013; Ravesloot 2017; Sawyer 2011).
CPAP is the current gold standard of treatment for OSA. The AASM task force recommends positive airway pressure (PAP) as the treatment of choice for moderate and severe OSA, and an option for mild OSA. As a consensus recommendation, this group states that CPAP should be offered to all people with moderate to severe OSA (Epstein 2009). The National Institute for Health and Care Excellence (NICE) guidelines recommend CPAP as the best treatment option in cases of symptomatic moderate or severe OSA, where adherence is better for CPAP (Sarrell 2013). In mild OSA, NICE considers CPAP as a treatment option only if OSA is symptomatic enough to cause impairment in quality of life. For mild OSA, NICE recommends CPAP only after lifestyle advice and other treatment options have been unsuccessful or have been found to be inappropriate (NICE 2008).
How the intervention might work
Recurrent airway obstruction in OSA may be the culmination of interaction of several mechanisms like airway anatomical characteristics, pharyngeal critical closing pressure, action of airway dilator muscle, tracheal tug, ventilatory control instability and arousal threshold (Joosten 2014).
Sleeping on the sides may reduce pressure on the airway and lower the chance of sleep apnoea (Isono 2002). Change of body position from the side to the back results in shift in the directional effect of gravity on the structures of the upper airway. It is proposed that genioglossus activity, a critical muscle acting to compensate the collapsing forces acting on the airway when a person is lying on their back, may fall during sleep and contribute to airway collapse (Joosten 2014). Lying on the sides may counteract these influences and improve OSA.
Why it is important to do this review
Although CPAP therapy is considered to be the gold standard and the most effective currently available therapy for OSA, its clinical application can be compromised by poor adherence to the treatment (Kribbs 1993), as up to two‐thirds of people with OSA do not routinely use their CPAP machine (Sarrell 2013). Poor adherence is more common among people with mild to moderate OSA and largely asymptomatic disease and in people with other co‐morbidities. Furthermore, CPAP may not be a widely accessible choice in resource‐poor settings because of the cost involved. Therefore, as evidence continues to emerge, a focused systematic review should explore the role of positional therapy to make clear the benefits of CPAP treatment and the patient groups for which this approach is best suited.
Objectives
To compare the efficacy of positional therapy versus CPAP and positional therapy versus inactive control (sham intervention or no positional therapy intervention) in people with OSA.
Methods
Criteria for considering studies for this review
Types of studies
In the original protocol, we planned to include only parallel‐arm, randomised controlled trials (RCTs) that use positional therapy for OSA regardless of blinding, language or stage of publication, and we excluded cross‐over trials. However, the initial search results indicated that most of the relevant trials for this review were using cross‐over methodology. Hence, with the approval of the editorial board, we made a post‐hoc protocol change to include randomised cross‐over trials.
Types of participants
We included participants with OSA, irrespective of their age, disease severity or the diagnostic criteria used to diagnose the condition. We considered the AASM diagnostic criteria to be the gold standard for comparison purposes (Epstein 2009) .
Types of interventions
We used the following comparisons
Postional therapy versus CPAP
Positional therapy versus inactive control (sham intervention/no positional therapy intervention)
Our intervention was positional therapy to encourage people to sleep on their sides using devices like lumbar or abdominal binders, semi‐rigid backpacks, full‐length pillows, tennis ball attached to the back of nightwear, and electrical sensors with alarms that indicate change in position.
Our controls were:
continuous positive airway pressure (CPAP), provided and titrated according to standard methodology; and
inactive control, which included no intervention of any type or positional therapy device worn in off or inactive state.
Types of outcome measures
Primary outcomes
Epworth Sleepiness Scale (ESS)/symptoms of excessive daytime sleepiness (Kapur 2017)
Apnoea‐Hypopnoea Index (AHI) (Kapur 2017)
Adherence rate
Secondary outcomes
Quality of life (as assessed using appropriate scales such as Short Form‐36 (SF‐36) or Functional Outcomes Sleep Questionnaire (FOSQ))(Kapur 2017)
Sleep quality assessed by average duration of slow wave and REM sleep periods
Respiratory Disturbance Index (RDI) (Kapur 2017)
Frequency of desaturation episodes per hour of sleep
Average duration of oxygen desaturation
Cognitive dysfunction (as assessed using appropriate instruments such as the Psychomotor Vigilance Test or Hospital Anxiety Depression Scale)
Adverse effects (back discomfort and skin irritation due to application of the positional device; facial discomfort, nasal congestion, dry mouth and skin irritation due to CPAP therapy)
Search methods for identification of studies
Electronic searches
We searched for studies in the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org);
Weekly searches of MEDLINE Ovid SP 1946 to date;
Weekly searches of Embase Ovid SP 1974 to date;
Monthly searches of PsycINFO Ovid SP;
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature);
Monthly searches of AMED EBSCO (Allied and Complementary Medicine);
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We also searched the following trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/)
We searched the Cochrane Airways Trials Register and additional sources from inception to September 2018, with no restriction on language of publication.
Searching other resources
We searched primary studies and review articles for additional references.
We accessed additional information on ongoing trials from manufacturers' websites.
We checked for any errata or retractions from included studies that have been published.
We searched www.clinicaltrials.gov/ and WHO trial portal for ongoing trials. We contacted study authors regarding the publication status of the trials.
Data collection and analysis
Selection of studies
Two review authors (PRS and RA) independently reviewed the abstracts of the studies using the predefined inclusion criteria. We accessed full texts of selected studies and reviewed them at length. We assessed the screened studies for eligibility for inclusion using a pre‐designed eligibility form. A third review author (AG), who was not a member of the data extraction team, adjudicated any disagreements. We were careful to check for multiple publications of the same data. We documented our reasons for excluding studies.
We recorded the selection process in a PRISMA flow diagram (Moher 2009), and in a Characteristics of excluded studies table.
Data extraction and management
Two review authors (RA and JD) independently extracted data. RA and AG independently assessed the quality of studies. A third review author (PRS) adjudicated any disagreements. RA entered data into Review Manager 5 (RevMan 5) software for analysis (Review Manager 2014). The entries were double‐checked, and a second review author (PRS) checked the study characteristics for accuracy.
For study characteristics and outcome data, we used a data collection form that we piloted on one study in the review. Two review authors (RA and JD) independently extracted study characteristics from included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, country in which study was conducted, number of study centres, study setting, withdrawals and date of study
Participants: number of participants, mean age, age range, gender, severity of condition, diagnostic criteria, inclusion criteria and exclusion criteria
Interventions: intervention, comparison, concomitant medications and interventions
Outcomes: primary and secondary outcomes specified and collected and time points reported
Notes: funding for trial and notable conflicts of interest of study authors
Assessment of risk of bias in included studies
We assessed the risk of bias for all studies using the Cochrane tool for assessing risk of bias (Higgins 2017). Domains assessed include sequence generation and allocation concealment, blinding of outcome assessors, completeness of outcome data and selective outcome reporting. Other points considered include baseline imbalances, premature stopping of studies and commercial conflicts of interest of study authors. Two review authors (RA and AG) independently assessed risk of bias, with another review author (PRS) acting as adjudicator in case of disagreement. We recorded risk of bias in the 'Risk of bias' tables.
Assesment of bias in conducting the systematic review
We noted any deviation from the 'Risk of bias' assessment above in the Differences between protocol and review.
Measures of treatment effect
Outcome variables like AHI and oxygen saturation are continuous variables. We expressed data from each study as mean differences (MD) or standardised mean differences (SMD) with 95% confidence intervals (CI).
We expressed dichotomous data as odds ratios (OR).
We conducted meta‐analysis only when meaningful data, that were clinically and methodologically similar, were available.
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
We analysed data on an intention‐to‐treat basis. We contacted study authors for clarification on missing information.
Assessment of heterogeneity
We used the I² statistic (Higgins 2003), to measure heterogeneity among trials (Deeks 2017). In case of substantial heterogeneity, we planned to explore possible causes for it by prespecified subgroup analysis, when possible. If the heterogeneity could not be explained by subgroup analysis, we planned to do sensitivity analysis including and excluding the outlier studies.
Assessment of reporting biases
We were not able to prepare funnel plots as planned in the protocol because there were fewer than 10 studies available.
Data synthesis
We analysed data using Review Manager 2014 software.
When two or more appropriate studies were available we used RevMan 5 software to pool the results. We used means of outcomes to obtain a mean difference pooled from all studies. We combined studies using the random‐effects model. When clinical or methodological differences between the studies were significant we did not combine the results.
'Summary of findings' table
We used GRADEpro GDT to prepare 'Summary of findings' tables (GRADEpro GDT 2015). The outcomes included were ESS, AHI, adherence, adverse effects, quality of life, sleep quality and cognitive dysfunction.
We justified all decisions to downgrade the certainty of the evidence in the footnotes.
Subgroup analysis and investigation of heterogeneity
We had planned to carry out the following subgroup analyses to look for potential sources of heterogeneity.
Mild to moderate OSA versus severe OSA or asymptomatic OSA versus symptomatic OSA
Studies including only OSA participants without co‐morbidities such as stroke versus studies done on populations with co‐morbidities such as stroke survivors
Types of interventions used for positional therapy (e.g. mechanical restrainers vs electronic sensors with alarms with or without mechanical restrainers)
We planned to include the following outcomes in the subgroup analyses.
Epworth Sleepiness Scale/symptoms of excessive daytime sleepiness
Apnoea‐Hypopnoea Index (AHI)
Adherence rate
Quality of life (assessed using appropriate scales such as SF‐36 and FOSQ)
Cognitive dysfunction (assessed using appropriate instruments such as the Psychomotor Vigilance Test and the Hospital Anxiety Depression Scale)
Due to inadequate data, we only did one subgroup analysis of studies involving positional alarm device alone.
Sensitivity analysis
We did a sensitivity analysis for the robustness of the results excluding studies that did not use laboratory‐based polysomnography. We also did a sensitivity analysis for the effect of the post‐hoc amendment to the review protocol to include cross‐over trials.
Results
Description of studies
Results of the search
Our searches, conducted first in February 2012 and updated last in September 2018, found 446 references. After excluding duplicate publications and irrelevant reports, we identified 22 studies. Only two studies were eligible for inclusion according to the original protocol of this review, as we had excluded cross‐over trials. But a post‐hoc revision of the protocol, that considered cross‐over trials eligible for the review, enabled us to select eight studies for inclusion in the review. The selection process is shown in Figure 1. We also identified nine ongoing studies (see Characteristics of ongoing studies).
1.
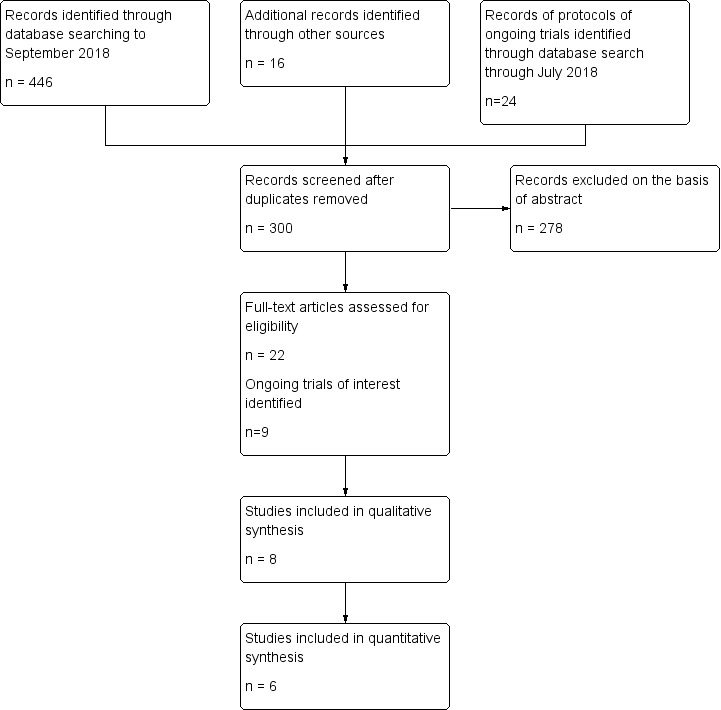
Study Flow diagram
Included studies
We included six randomised, cross‐over trials and two randomised, parallel‐group studies. The studies randomised a total of 323 participants. The comparison between positional therapy and CPAP included 72 participants, and the comparison between positional therapy and inactive control included 251 participants. An overview of the characteristics of the included studies is given in Table 3.
1. Study characteristics.
| Study, design | Participants | Device |
Age Mean, years (SD) |
AHI (events/h) Mean (SD) |
Supine AHI (events/h) Mean (SD) |
OSA type and definition of POSAa |
BMI Mean (SD) |
Men n (%) | Duration of intervention period (washout) |
|
Bignold 2011 Cross‐over |
16 | Vibrating alarm |
58.2 (13.9) | 24.1 (10.5) | 51.3 (23.3) | POSA Overall AHI ≥ 15; ≥ twice supine position; non‐supine AHI < 15 |
28.8 (2.5) | 13 (81.3%) | 1 week (1 week) |
|
Jackson 2015 Parallel |
86 | Tennis ball sleep position modification device | 48 (11.2)/ 51.2 (11.4) | 20.1(8.8)/ 21.8 (10) |
43.2(25.5)/ 39.7 (19.3)* |
POSA AHI > 10; supine AHI twice non‐supine AHI |
30.0 (5.3)/30.9 (7.7) | 37 (78.7%)/30 (76.9%) | 4 weeks |
|
Laub 2017 Parallel |
101 | Vibrating alarm | 50.3(12.9)/ 51.2 (13.3) |
16.9 (8.5)/19.9 (9.7) |
34.8 (17.5)/37.7 (15.5)b | POSA AHI supine ≥ twice AHI non‐supine; AHI supine ≥ 10; AHI non‐supine < 10 Daytime tiredness and/or disturbed sleep and/or snoring |
27.1/27.9 | 39 (75%)/38 (78%) | 6 months |
|
Van Maanen 2012 Cross‐over |
30 | Vibrating alarm |
48 (9.5) | 27.7 (SEM 2.4) |
59.7 (SEM 3.6) |
POSA AHI > 5; AHI supine twice or > in non‐ supine position |
27.7 (3.6) | 26 (86%) | 2 nights (1‐2 weeks apart) |
|
Permut 2010 Cross‐over |
38 | Zzoma® positional sleeper |
49 (12) | 13 (5) | 31 (19) | POSA AHI ≥ 5 with symptom of excessive daytime sleepiness or AHI ≥ 15 with 50% decrease in non‐supine position |
31 (5) | 25 (65.7%) | 2 nights (none) |
|
Jokic 1999 Cross‐over |
14 | Backpack with soft ball | 51 (9) | 17 (8) | 63.8 (SEM 41.3) |
POSA Supine AHI ≥ 2 times AHI in lateral position, and AHI in lateral position < 15; subjective daytime sleepiness |
30 (4) | 12 (85.7%) | 2 weeks (none) |
|
Skinner 2008 Cross‐over |
20 | Thoracic anti‐supine band | 55.9 (9.8) | 22.7 (12) | 59.6 (27.5) | POSA AHI > 5; supine AHI ≥ 2 times AHI in other positions; maximum AHI in all other non supine position ≤ 10 |
30.7 (5.1) | Not reported | 1 month (1 week washout) |
|
Svatikova 2011 Cross‐over |
18 | Sona® anti‐snoring pillow to prevent supine sleep | Median: 58 (IQR 54, 68) |
Median: 39 (IQR 21,54) | Median 49 (IQR 35,60) | AHI ≥ 5; not restricted to POSA |
Median 29 (IQR 28, 33) |
11 (61.1%) | 2 consecutive night (none) |
| AHI: apnoea hypopnoea index; BMI: body mass index; IQR: interquartile range; OSA: obstructive sleep apnoea; POSA: positional obstructive sleep apnoea; SD: standard deviation; SEM: standard error of mean | |||||||||
aCartwright criteria: POSA is defined as OSA with 50% or more reduction in AHI when the person is lying on his or her side rather than on the back. bCases and controls in parallel‐group study.
Positional therapy versus continuous positive airway pressure (CPAP)
We identified three trials with 72 participants (Jokic 1999; Permut 2010; Skinner 2008).
All of these studies were randomised cross‐over design with Skinner 2008 providing a washout period. Permut 2010 used a positional alarm, while Jokic 1999 and Skinner 2008 used the tennis ball technique or its variants.
The gender distribution is not available for Skinner 2008, but in other studies participants were predominantly male. The participants were mostly in their fourth and fifth decade of life and mean BMI of the participants in the individual studies ranged from 30.7 (Skinner 2008), to 31 (Permut 2010). The study participants were restricted to people with POSA. Two of the studies on POSA required the participants to be symptomatic as well (Permut 2010; Jokic 1999). Jokic 1999 and Permut 2010 used laboratory‐based polysomnography while Skinner 2008 used home‐based monitors. Permut 2010 reported the outcomes AHI, mean oxygen saturation, lowest oxygen saturation, percentage of total sleep time with pulse oxygen saturation less than 90%, sleep efficiency, spontaneous arousal index, sleep architecture and participant's preference for the intervention. Skinner 2008 reported AHI dichotomised as treatment success and treatment failure, defined as AHI of 10 or less. Skinner 2008 reported adequate adherence, defined as four or more hours per night on at least 70% of nights monitored. None of the studies reported adverse effects.
Positional therapy versus inactive control
Five trials (251 participants), compared positional therapy with inactive control (Bignold 2011; Jackson 2015; Laub 2017; Svatikova 2011; Van Maanen 2012). Bignold 2011, Van Maanen 2012 and Svatikova 2011 were cross‐over trials, while Laub 2017 and Jackson 2015 were parallel‐arm trials. Three studies used a vibration alarm (Bignold 2011; Laub 2017; Van Maanen 2012), while one used a technique similar to tennis ball (Jackson 2015), and the other a positional sleeping pillow (Svatikova 2011). Svatikova 2011 included participants with stroke and AHI of 5 or more. The participants were predominantly men in their fourth and fifth decade of life. The mean BMI of the participants in the studies ranged from 27.1 to 30.9.
Bignold 2011 studied AHI, snoring loudness and percentage of time in supine posture. Jackson 2015 reported supine sleep time, total sleep time, AHI, supine AHI, sleep efficiency, arousal index, body mass index, blood pressure, ESS, and quality‐of‐life scales, including Functional Outcome of Sleep Questionairre (FOSQ), Symptoms of Sleep Questionairre (SOSQ), and neuropsychological test battery including psychomotor vigilance test, response inhibition test, Trail Making Task A and B, digit symbol substitution ask, digits span test and controlled oral word association task. Van Maanen 2012 studied AHI, supine AHI, non‐supine AHI, total supine sleep time, sleep efficiency, oxygen saturation and arousal index. Svatikova 2011 assessed relative change in AHI, absolute difference in the mean oxygen saturation, and absolute difference in the time spent in the supine position. This study had a second part that assessed three‐month adherence. We excluded this part of the study from the analysis as the information on randomisation and allocation concealment was not clear.
Excluded studies
Of the thirteen excluded studies, four were non‐randomised trials (Afrashi 2015; Braver 1995; Cartwright 1991; Greer 2006), and two were systematic reviews (Ha 2014; Barnes 2017). One study included neonates only (Kurlak 1994), and another study included normal pregnant women (Zaremba 2015). We excluded Skinner 2004a and Skinner 2004b as these studies used interventions that did not meet our definition of positional therapy. Eijsvogel 2015 compared two methods of positional therapy against each other. We excluded Dieltjens 2015 and Benoist 2017 because they compared positional therapy with oral appliances. One study with an eligible population and intervention was available only as an abstract in conference proceedings and our attempts to contact the author for further details were unsuccessful (Magalang 2016).
Risk of bias in included studies
Full details of judgements can be found in Characteristics of included studies. For the graph showing the overall judgments see Figure 2.
2.
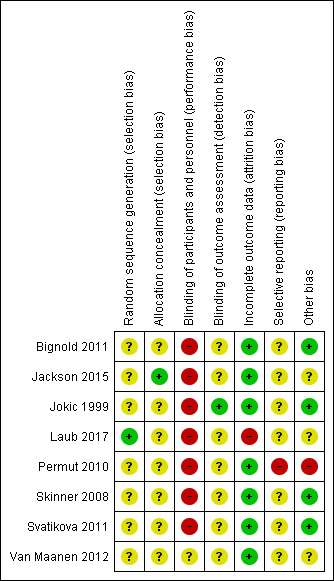
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
None of the studies were free from risk of bias for randomisation. Jackson 2015 used a third‐party, computer‐generated sequence, available on site in sealed envelopes that they opened sequentially. However, while the study had planned a 1:1 allocation, the numbers in the arms were dissimilar (37 and 49). We judged unclear risk for the study based on this issue. Laub 2017 did not mention the methods used for allocation concealment. None of the remaining seven studies clearly described the methods employed for random sequence generation and allocation concealment. Thus, they were all judged to be at unclear risk of bias.
Blinding
Blinding was not possible in the studies because of the nature of the intervention and control. However, some of the outcomes in the review such as AHI and oxygen desaturation are objectively measured physiological parameters and hence are at low risk of bias for lack of blinding for these outcomes. Few other outcome parameters such as cognitive outcomes were subjective measurements and are likely to be influenced by lack of blinding. Few studies employed blinding of outcome assessors.
Incomplete outcome data
Laub 2017 had a dropout of 26.6% in the first two months and 55.4% by the six‐month follow‐up. Most of the other studies had low or no attrition and we judged them to be at low risk of bias.
Selective reporting
We noted selective reporting of outcome data in one study (Permut 2010).
Other potential sources of bias
Svatikova 2011 tested for period effect and did not find it to be significant. Other studies did not mention period effect. Two studies had a one‐week wash‐out period between the two arms of the cross‐over trial (Bignold 2011; Skinner 2008). For studies conducted by Permut 2010 and Svatikova 2011, the absence of a wash‐out period was unlikely to affect the outcome parameters such as AHI, which are physiological variables that change instantaneously. Jokic 1999 studied quality of life and cognitive parameters that can be influenced by carry‐over of effect of treatment.
Included studies differ markedly in their inclusion criteria. Except for one (Svatikova 2011), all studies were conducted on participants with POSA (Bignold 2011; Jackson 2015; Jokic 1999;Laub 2017; Permut 2010; Skinner 2008; Van Maanen 2012). The definition of POSA was not uniform across studies and the studies also used different ways to measure AHI. Jokic 1999, Permut 2010 and Jackson 2015 used standard overnight multichannel polysomnography, while other studies used respiratory monitors. The studies often compared multiple parameters with no adjustment for random error (Van Maanen 2012), and one study did not specify one single primary outcome (Jackson 2015).
Effects of interventions
Summary of findings for the main comparison. Positional therapy compared to continuous positive airway pressure (CPAP) for obstructive sleep apnoea.
| Positional therapy compared to continuous positive airway pressure (CPAP) for obstructive sleep apnoea | ||||||
| Patient or population: adults with obstructive sleep apnoea Setting: interventions used at home Intervention: positional therapy Comparison: CPAP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
No. of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with CPAP | Risk with positional therapy | |||||
|
Epworth Sleepiness Scale (ESS) Follow‐up: 1 month |
The mean ESS was 10.4 | MD 1.2 higher
(1.91 lower to 4.31 higher) |
‐ | 20 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | Skinner 2008 was a home‐based study of single nights after 1 month of use in a cross‐over design. Lower ESS scores are better. MCID is estimated to be a fall of 2‐3 points |
|
Apnoea‐Hypopnoea Index (AHI) Follow‐up: 2 weeks to 1 month |
The mean AHI ranged from 3.4‐4.9 | MD 6.4 higher
(3 higher to 9.79 higher) |
‐ | 33 (2 RCT) | ⊕⊕⊝⊝ Lowc,d | Skinner 2008 was a home‐based study of single nights after 1 month of use in a cross‐over design. Jokic 1999 used overnight laboratory‐based PSG after 2 weeks in a cross‐over design. Lower AHI is better. MCID is considered as 5 events/ hour |
|
Self‐reported adherence time Follow‐up: 1 month |
The mean self‐reported adherence time was 4.9 hours/night |
MD 2.5 hours/night higher
(1.41 higher to 3.59 higher) |
‐ | 20 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Skinner 2008 was a home‐based study of single nights after 1 month of use in a cross‐over design. MCID is not established. |
|
Adverse effects Follow‐up: 1 month |
Skinner 2008 was a home‐based study of single nights after 1 month of use in a cross‐over design. They used the aggregate score of 19 self‐report questions graded as 0, no effect; 1, mild effect but did not disturb sleep; 2, sleep disturbed; 3, could not use device for assessing adverse events. They reported that this aggregate score was less for positional device compared to CPAP (MD: 3.6; 95% CI 3.4 to 5.8). | 20 (1 RCT) |
GRADE not applied | No details of the questionnaire are available to understand the nature of adverse events sought. | ||
|
Quality of life Assessed using SF‐36 or FOSQ Follow‐up:1 month |
SF‐36 physical: mean 44.6 SF‐36 mental mean: 49.7 FOSQ mean: 12.8 |
MD for SF‐36 physical 0.10 lower (6.79 lower to 6.59 higher); MD for SF‐36 mental was 0.60 higher (4.99 lower to 6.19 higher); MD for FOSQ was 0.40 lower (1.82 lower to 1.02 higher) | 20 (1 RCT) | ⊕⊕⊝⊝ Lowa,e | Skinner 2008 was a home‐based study of single nights after 1 month of use in a cross‐over design. They reported SF‐36 and FOSQ. There were no differences in either score between the groups. | |
|
Sleep quality Assessed by average duration of slow‐wave and REM sleep periods Follow‐up: 2 weeks |
Mean % of REM sleep: 26%; Mean % of slow wave sleep: 22% Mean sleep efficiency: 84% |
MD for % of REM sleep was 2% lower (8.22% lower to 4.22% higher); MD for % of slow‐wave sleep was 2% lower (9.12 lower to 5.12 higher); MD for sleep efficiency was 2% lower (8.4% lower to 5.4% higher) | 13 (1 RCT) | ⊕⊕⊝⊝ Lowa,e | Jokic 1999 used overnight laboratory‐based PSG after 2 weeks in a cross‐over design. They reported on proportion of sleep time spent in REM phase, in slow‐wave phase and also on sleep efficiency (total sleep time/total record time). They did not note any significant difference between positional therapy and CPAP. | |
|
Cognitive dysfunction Follow‐up: 1 month |
Jokic 1999 used overnight laboratory‐based PSG after 2 weeks in a cross‐over design. They studied cognitive outcomes using 6 tests with 34 subtests and noted no difference between the groups. | 13 (1 RCT) | GRADE not applied | The outcomes carry issues related to multiple comparisons. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CPAP: continuous positive airway pressure; FOSQ: Functional Outcomes Sleep Questionnaire; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; PSG: polysomnography; RCT: randomised controlled trial; REM: rapid eye movement; RR: risk ratio; SF‐36: short‐form 36 | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aMethods employed for randomisation and allocation concealment not explicitly stated in the study. Participant blinding not done. Downgraded for risk of bias. bThe confidence interval of the estimate is imprecise. Downgraded for imprecision. cNeither study mentioned the methods employed for randomisation and allocation concealment. Both had unblinded participants. Only one study reported outcome assessor blinding. Downgraded for risk of bias. dJokic 1999 used laboratory‐based polysomnography, while Skinner 2008 used home‐based monitors with reported kappa for agreement of 0.6 with polysomnography. Downgraded for imprecision of measuring techniques. eThe confidence interval of the estimate is imprecise. Downgraded for imprecision.
Summary of findings 2. Positional therapy compared to inactive control for obstructive sleep apnoea.
| Positional therapy compared to inactive control for obstructive sleep apnoea | ||||||
| Patient or population: adults with obstructive sleep apnoea Setting: intervention at home except in one study where intervention was for one night in the laboratory Intervention: positional therapy Comparison: no positional therapy or sham therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
No of participants (RCTs) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with positional therapy | |||||
|
Epworth Sleepiness Scale (ESS) Follow‐up: 4 weeks to 2 months |
The mean ESS ranged from 9.4‐10.9 | MD 1.58 lower (2.89 lower to 0.26 lower) | ‐ | 187 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | Laub 2017 was an open‐label study over 2 months with home polygraphy at 2 months. Jackson 2015 studied participants with hospital‐based PSG after 4 weeks of intervention. Lower ESS scores are better. MCID is estimated to be a fall of 2‐3 points |
|
Apnoea‐Hypopnoea Index (AHI) Follow‐up: 1 night to 2 months |
The mean AHI ranged from 16.8‐19.9 event/hour |
MD 7.38 event/hour lower (10.06 lower to 4.7 lower) |
‐ | 277 (4 RCTs) | ⊕⊕⊝⊝ Lowb,c | Laub 2017 was an open‐label study over 2 months with laboratory polygraphy at 2 months. Jackson 2015 studied participants with hospital‐based PSG after 4 weeks of intervention. The other 2 trials were cross‐over design, Bignold 2011 being conducted over 1 week with home PSG and Van Maanen 2012 over 1‐2 weeks with laboratory‐based PSG. Lower AHI is better. MCID for AHI is considered to be 5 |
|
Adherence Measured as number of participants who continued to use device at end of 2 months Follow‐up: 2 months |
Study population | OR 0.80 (0.33 to 1.94) | 101 (1 RCT) | ⊕⊕⊝⊝ Lowd | Laub 2017 was an open‐label study over 2 months with laboratory polygraphy at 2 months. They measured device use for minimum 4 h/night over 2 months. Rate of discontinuation of therapy used for this comparison, thus lower OR implies fewer dropouts and improved adherence. | |
| 755 per 1000 | 712 per 1000 (504 to 857) | |||||
|
Adverse effects Measured as number of participants who discontinued device at end of 2 months Follow‐up: 2 months |
255 per 1000 | 288 per 1000 | OR 1.25 (0.52 to 3.03) |
101 (1 RCT) | ⊕⊕⊝⊝ Lowd | Laub 2017 was an open‐label study over 2 months with laboratory polygraphy at 2 months. They reported that 15 participants dropped out of the SPT arm: 5 due to adverse effects (frequent awakening and poor sleep (2), pain in the thorax and unpleasant feeling (3); 2 for lack of effect; and remaining 8 for other reasons (lost to follow up 5, withdrawal 1, sleep problem improved 1 and did not understand trial 1) |
|
Quality of life Assessed using SF‐36 or FOSQ Follow‐up: 4 weeks |
Mean FOSQ: 3.3 | MD 0.2 higher (0.02 lower to 0.42 higher) | 86 (1 RCT) | ⊕⊕⊕⊝ Moderatee | Jackson 2015 studied participants with hospital‐based PSG after 4 weeks of intervention. They reported that FOSQ scores were significantly higher in positional therapy group (P < 0.01). Higher scores on FOSQ indicate improved quality of life. | |
|
Sleep quality Assessed by average duration of slow‐wave and REM sleep periods Follow‐up: 1 night |
Mean % of REM sleep: 19.2%; mean % of slow wave sleep: 19.7% | MD for % REM sleep 0.9% lower (5.06% lower to 3.26% higher); MD for % slow wave sleep 1.20% lower (5.22% lower to 2.82% higher) | 30 (1 RCT) | ⊕⊕⊝⊝ Lowe,f | Van Maanen 2012 conducted a cross‐over trial using hospital‐based PSG and reported that percentage of REM sleep and percentage of slow‐wave sleep were not significantly different between the groups. | |
|
Cognitive dysfunction Follow‐up: 4 weeks |
Mean motor reaction time in seconds is 193.5 | MD 12.4 seconds lower (23.10 lower to 1.70 lower) | 86 (1 RCT) | ⊕⊝⊝⊝ Very lowg |
Jackson 2015 studied participants with in‐hospital PSG after 4 weeks of intervention. They reported that results of motor vigilance test was not significantly different between the groups. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CPAP: continuous positive airway pressure; FOSQ: Functional Outcomes Sleep Questionnaire; MCID: minimal clinically important difference; MD: mean difference; OR: odds ratio; PSG: polysomnography; RCT: randomised controlled trial; REM: rapid eye movement; RR: risk ratio; SF‐36: short‐form 36; SPT: sleep position trainer | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aLaub 2017 has high risk of bias. It was an open‐label study and had a high attrition rate. It did not mention methods of allocation concealment. Downgraded for risk of bias. bBignold 2011 and Van Maanen 2012 did not explicitly state the randomisation and allocation concealment procedure. Jackson 2015 had discrepancy in the stated allocation procedure and the actual distribution of the participants in the study. Laub 2017 was an open‐label study. It did not state the procedure of allocation concealment and had significant loss to follow‐up. Downgraded for risk of bias. cLaub 2017 and Bignold 2011 used home‐based monitors, while the other two studies used laboratory‐based polysomnography with reported kappa for agreement 0.6. Downgraded for imprecision of measurement techniques. dLaub 2017 did not mention methods of allocation concealment. It was an open‐label study with a high attrition rate. Downgraded for risk of bias. eThe confidence interval of the estimate is imprecise. Downgraded for imprecision. fVan Maanen 2012 has moderate risk of bias, has no clear primary outcome and multiple comparisons. Downgraded for risk of bias. gThis study is at risk of bias, and has multiple comparisons. The study authors reported no difference between the groups for the outcomes on cognition. However, we noticed motor reaction time to be significantly different in favour of positional therapy. We downgraded this parameter for indirectness as its clinical significance in isolation is uncertain. Downgraded twice for risk of bias and once for indirectness.
Positional therapy versus continuous positive airway pressure (CPAP)
All the studies included in the comparison between positional therapy versus CPAP were cross‐over trials (3 studies; 72 participants; Jokic 1999; Permut 2010; Skinner 2008). Therefore, no studies would have been eligible for inclusion in this comparison according to the original protocol of this review. The results presented are as a result of the post‐hoc amendment to the review's protocol, which allowed the inclusion of cross‐over trials. Permut 2010 expressed their results as median and interquartile range (IQR) and hence we did not include them in the analysis. The remaining two studies contributed to the data synthesis.
Primary outcomes
Epworth Sleepiness Scale (ESS)
Two studies (34 participants; Jokic 1999; Skinner 2008), reported no difference in ESS scores between CPAP and positional therapy. Jokic 1999 reported the data as median (median difference −1.5, 95% CI −2.9 to 0.8; P = 0.2). Skinner 2008 reported a mean difference of 1.20 (95% CI −1.91 to 4.31; low‐certainty evidence; Analysis 1.1; Figure 3).
1.1. Analysis.
Comparison 1 Positional therapy versus CPAP, Outcome 1 Epworth Sleepiness Scale.
3.
Positional therapy versus CPAP: Epworth Sleepiness Scale
Apnoea‐Hypopnoea Index (AHI)
Two studies contributed data for this outcome (34 participants; Jokic 1999; Skinner 2008). Jokic 1999 excluded one participant from the analysis as they found on follow‐up that he had coexisting idiopathic hypersomnolence. The final analysis involved 33 participants. CPAP reduced AHI compared with positional therapy in both the studies. The mean difference was 6.4 events per hour (95% CI 3.00 to 9.79; low‐certainty evidence) in favour of CPAP (Analysis 1.2; Figure 4). Skinner 2008 reported that, of the participants with baseline AHI above 10, 72% on positional therapy achieved treatment success (defined as AHI less than 10), compared with 89% of participants with CPAP (P = 0.004). Senstivity analysis excluding Skinner 2008, which used respiratory monitoring instead of laboratory‐based polysomnography for assessing AHI did not show any change in the results.
1.2. Analysis.
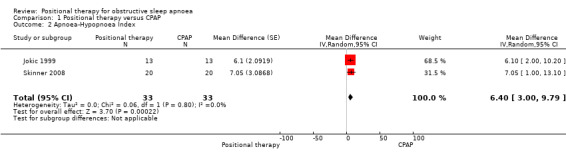
Comparison 1 Positional therapy versus CPAP, Outcome 2 Apnoea‐Hypopnoea Index.
4.
Positional therapy versus CPAP: Apnea‐Hypopnea Index
Adherence
Skinner 2008 (20 participants) reported subjective adherence to the intervention. Participants used positional therapy for more hours per night compared to CPAP (MD 2.5 hours per night, 95% CI 1.41 to 3.59; moderate‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1 Positional therapy versus CPAP, Outcome 3 Self‐reported adherence time.
Secondary outcomes
Skinner 2008 reported quality of life using Short Form 36 Health Survey (SF‐36), and Functional Outcomes of Sleep Questionnaire (FOSQ), and there was no significant difference between the two groups (MD for SF‐36 physical −0.10, 95% CI −6.79 to 6.59; MD for SF‐36 mental 0.60, 95% CI −4.99 to 6.19; MD for FOSQ −0.40, 95% CI −1.82 to 1.02; low‐certainty evidence; Analysis 1.4; Analysis 1.5; Analysis 1.6).
1.4. Analysis.
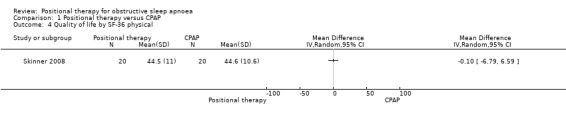
Comparison 1 Positional therapy versus CPAP, Outcome 4 Quality of life by SF‐36 physical.
1.5. Analysis.
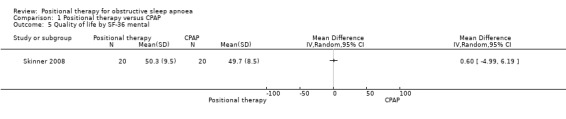
Comparison 1 Positional therapy versus CPAP, Outcome 5 Quality of life by SF‐36 mental.
1.6. Analysis.
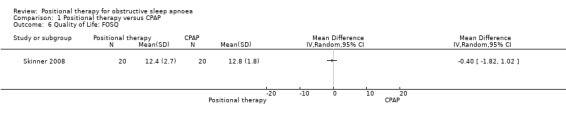
Comparison 1 Positional therapy versus CPAP, Outcome 6 Quality of Life: FOSQ.
Jokic 1999 reported sleep quality using percentage of REM sleep and percentage of slow‐wave sleep. There was no significant difference between the two groups (MD for percentage of REM sleep ‐2.00, 95% CI −8.22 to 4.22; low‐certainty evidence; Analysis 1.7; Figure 6; MD for percentage of slow‐wave sleep −2.00, 95% CI −9.12 to 5.12; low‐certainty evidence; Analysis 1.8). Jokic 1999 also reported cognitive outcomes using six tests with 34 subtests and reported no difference between the groups. We did not use these data in our quantitative analysis as there is a risk of bias associated with making multiple comparisons using different parameters.
1.7. Analysis.
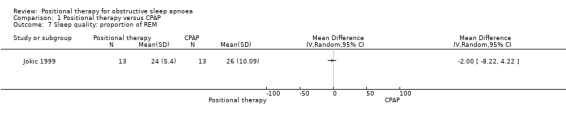
Comparison 1 Positional therapy versus CPAP, Outcome 7 Sleep quality: proportion of REM.
1.8. Analysis.
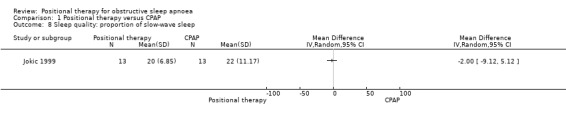
Comparison 1 Positional therapy versus CPAP, Outcome 8 Sleep quality: proportion of slow‐wave sleep.
Skinner 2008 used the aggregate score of 19 self‐reported questions for assessing adverse effects. The grading reported is as follows: 0, no effect; 1, mild effect but did not disturb sleep; 2, sleep disturbed; 3, could not use device. They reported that the aggregate score was less for the positional device compared to CPAP (MD 3.60, 95% CI 3.40 to 5.80). The study authors did not describe the exact questions asked, hence we cannot comment on the type and nature of the adverse effects. The other studies did not report adverse effects. There were insufficient data to comment on other secondary outcomes, including respiratory disturbance index (RDI), and frequency and duration of nocturnal desaturation. We were unable to do prespecified subgroup analysis due to lack of adequate data.
Positional therapy versus inactive control
Among the five studies (251 participants) included in the review for this comparison, two were parallel‐arm trials (187 participants; Jackson 2015; Laub 2017), while the other three were cross‐over trials (64 participants; Bignold 2011; Svatikova 2011; Van Maanen 2012). Based on the criteria stated in the original protocol of this review, only two studies would have been eligible for inclusion (187 participants; Jackson 2015; Laub 2017). We included three additional studies following the post‐hoc amendment to the protocol of the review. Four studies included only participants with POSA, while one (Svatikova 2011), included participants with ischaemic stroke. We did not include Svatikova 2011 in meta‐analysis as it reported outcomes as median and interquartile range.
Primary outcomes
Epworth sleepiness scale (ESS)
Data from two studies with 187 participants (Jackson 2015; Laub 2017), showed that positional therapy significantly improved ESS (MD ‐1.58, 95% CI ‐2.89 to ‐0.26; moderate‐certainty evidence; Analysis 2.1;Figure 5). The estimate is identical in the sensitivity analysis for the post‐hoc amendment to the review protocol as both the studies are parallel‐arm studies. However, sensitivity analysis excluding Laub 2017, who used a respiratory monitor for assessing sleep, reduced the size of the effect and widened the confidence interval so that the effect was no longer seen. This is less than the minimal clinically important difference (MCID) of between 2 to 3 as estimated by Patel 2017.
2.1. Analysis.
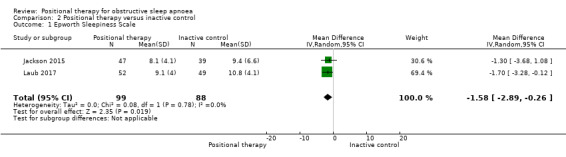
Comparison 2 Positional therapy versus inactive control, Outcome 1 Epworth Sleepiness Scale.
5.
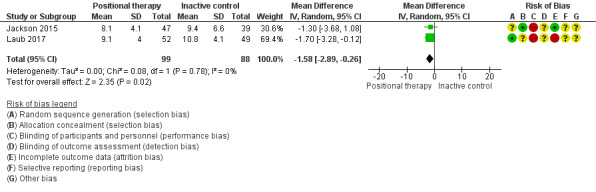
Forest plot of comparison: 2 Positional therapy versus inactive control, outcome: 2.1 Epworth Sleepiness Scale.
Apnoea hypopnoea index (AHI)
Data from four studies with 233 participants (Bignold 2011; Jackson 2015; Laub 2017; Van Maanen 2012), showed that positional therapy improved AHI compared to inactive control (MD −7.38 events per hour, 95% CI −10.06 to −4.70; low‐certainty evidence; Analysis 2.2; Figure 6). Studies using a vibration alarm positional device (3 studies; 147 participants; Bignold 2011; Laub 2017; Van Maanen 2012), showed a MD of −7.77 (95% CI −10.81 to −4.74; Analysis 2.2; Figure 8), while the study using a tennis ball technique positional device (1 study; 86 participants; Jackson 2015), showed MD of −6.00 (95% CI −11.72 to −0.28; low‐certainty evidence; Analysis 2.2; Figure 8). Sensitivity analysis excluding studies using respiratory monitors for assessing AHI (Bignold 2011; Laub 2017), did not change the results favouring positional therapy. We observed no subgroup difference. Sensitivity analysis for the post‐hoc amendment to the review protocol including only parallel‐arm studies resulted in lower estimate of MD in AHI in favour of positional therapy (MD −6.29 events per hour; 95% CI −9.36 to −3.21). Van Maanen 2012 reported that 23.3% of participants (7 of 30) had AHI less than 5 when tested with the vibration device switched on, the test being done after a median 1.5 months of follow‐up. In Laub 2017, 51.4% of the participants had AHI less than 10 and 40.5% had AHI less than 5 after two months' use of the vibration alarm. The differences in AHI are more than the MCID of 5 events per hour according to expert opinion (Kim 2017).
2.2. Analysis.
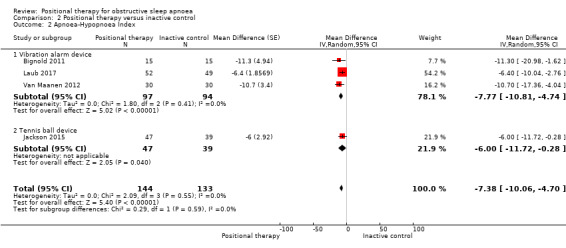
Comparison 2 Positional therapy versus inactive control, Outcome 2 Apnoea‐Hypopnoea Index.
6.
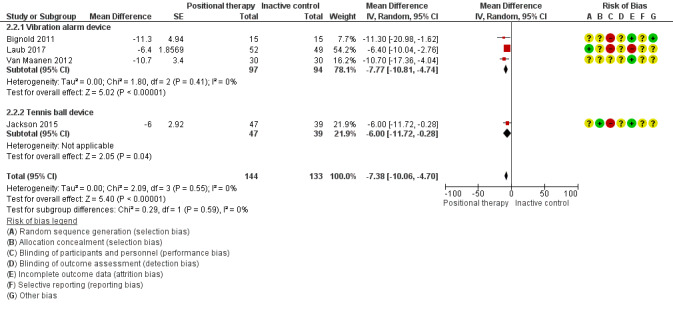
Forest plot of comparison: 2 Positional therapy versus inactive control, outcome: 2.2 Apnoea‐Hypopnoea Index.
Adherence
Laub 2017 (101 participants) reported adherence to therapy as number of participants who continued to use the device at two month. There was no clear difference between the groups (OR 0.80, 95% CI 0.33 to 1.94; low‐certainty evidence; Analysis 2.3). Sensitivity analysis for the post‐hoc amendment to the protocol yielded the same estimates, as the only study that contributed to analysis was parallel‐armed. However, the estimate is imprecise and includes the possibility of the opposite effect. Svatikova 2011 assessed adherence in the second phase of their study where they assigned the original 18 participants to two groups: nine participants to continue use of Sona® pillow and nine to use a pillow as they wished. In this section of the study (not included in the review as the randomisation of the second part of the study was not clearly stated), self‐reported adherence to the Sona pillow was reported as 4 of the 9 participants reporting using it on "most or all nights".
2.3. Analysis.
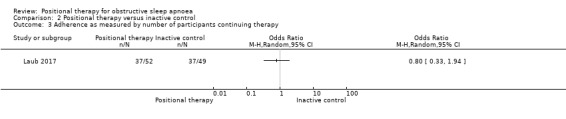
Comparison 2 Positional therapy versus inactive control, Outcome 3 Adherence as measured by number of participants continuing therapy.
Secondary outcomes
Laub 2017 noted adverse effects and the reasons for attrition. The reasons of attrition are as follows: improvement of the sleep problem (n = 1), lost to follow‐up (n = 5; 9.8%), failure to understand the trial (n = 1), lack of interest in continuing in the trial (n = 1), no effect from the study device (n = 2; 3.9%), sleep disturbance (n = 3; 5.9%), pain in back and thorax and unpleasant feeling in body (n = 2; 3.9%). Overall 10% of the participants reported adverse effects. However, there was no significant difference in the number of participants who discontinued use of the device at two months (OR 1.25, 95% CI 0.52 to 3.03; low‐certainty evidence; Analysis 2.3).
Jackson 2015 reported quality of life as measured by FOSQ. There was no significant difference between the two groups (MD 0.20, 95% CI −0.20 to 0.42; moderate‐certainty evidence; Analysis 2.4). They also reported cognitive outcomes as measured by motor reaction time. Although they reported no difference, our quantitative analysis showed a difference in favour of positional therapy (MD −12.5 seconds, 95% CI −23.1 to 1.7; very low‐certainty evidence; Analysis 2.5). Van Maanen 2012 reported quality of sleep as percentage of REM sleep and percentage of slow‐wave sleep. There was no difference between the two groups (MD for percentage REM sleep −0.9, 95% CI −5.06 to 3.26; MD for percentage slow‐wave sleep −1.2, 95% CI −5.22 to 2.82; low‐certainty evidence). Due to insufficient data, we could not analyse other specified secondary outcomes from the studies, like RDI, duration of oxygen desaturation, frequency of oxygen desaturation, quality of sleep and adverse effects. Sensitivity analysis for the post‐hoc amendment to the review protocol did not differ in the case of adverse effects, quality of life and cognitive performance. We could not perform sensitivity analysis on quality of sleep as the only study that provided data was a cross‐over study.
2.4. Analysis.
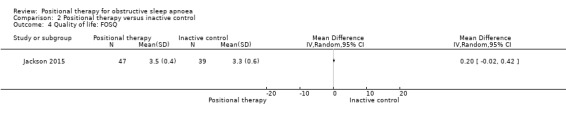
Comparison 2 Positional therapy versus inactive control, Outcome 4 Quality of life: FOSQ.
2.5. Analysis.
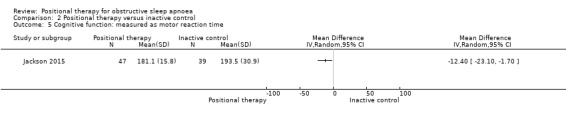
Comparison 2 Positional therapy versus inactive control, Outcome 5 Cognitive function: measured as motor reaction time.
We carried out a prespecified subgroup analysis based upon the type of positional device (alarm‐based positional trainer verus physical restraint). We did not note any subgroup difference (Analysis 2.2).
We could not perform the other prespecified subgroup analysis because there were not enough data.
Discussion
Summary of main results
In this review we compared the efficacy of positional therapy in the treatment of obstructive sleep apnoea with CPAP and with inactive control (sham or no intervention). We included eight studies, involving 323 participants. Three studies with 72 participants compared positional therapy with CPAP. Five studies with 251 participants compared positional therapy with inactive control.
The review revealed that CPAP is better than positional therapy in improving AHI in people with OSA. However, participants' self‐reported adherence was better with positional therapy than CPAP. Studies comparing positional therapy with inactive control therapy showed lower AHI and ESS in favour of positional therapy. The mean difference for AHI noted in the review for positional therapy versus inactive control is higher than the minimal clinically important difference (MCID) of 5 (Kim 2017), while that for the ESS is less than the MCID of 2 to 3 (Patel 2017).
Overall completeness and applicability of evidence
We judged the studies included in this review to be at significant risk of bias and therefore the results cannot be considered conclusive. The results of the review apply to people with POSA, who form a significant number of people with OSA. The criteria used for defining POSA were variable. Mador 2005's criteria that requires an AHI of less than 5 in the non‐supine position, effectively selects participants who would show significant response on the AHI, a diagnostic indicator of OSA. Studies employed different methods for measuring physiological parameters (laboratory‐based polysomnography versus home‐based monitoring device).
Only one study (Laub 2017), with a follow‐up of six months, found an improvement in daytime sleepiness. However, this study had a high risk of bias because of loss to follow‐up. The studies did not report any significant improvement in quality‐of‐sleep or quality‐of‐life parameters. This could be because they enrolled participants with mild to moderate OSA, a group that is likely to have minimal impairment of sleep quality or quality of life due to OSA. Furthermore, the studies were carried out over a short time span (the duration of use of each intervention in the included studies ranged from one night to four weeks with a median of 10.5 days), which may have made the impact on clinically relevant end points less evident. In studies of OSA using CPAP, daytime sleepiness tended to improve only after four weeks of treatment and cognitive symptoms improved only after many months of treatment (Canessa 2011; Lim 2007; Sanchez 2009).
Mador 2005 found that 49.5% of mild, 19.4% of moderate and 6.5% of severe sleep apnoea participants had POSA. By this definition, participants' AHI would be less than 5 per hour in a non‐supine position. In all these people, effectively instituted positional therapy is likely to normalise the diagnostic indicators of obstructive sleep apnoea. Within the current literature, a difference of 20 to 30 AHI is seen between supine and non‐supine positions (Jackson 2015; Skinner 2008). This indicates the scope for positional therapy in these people. The lack of benefit in the clinical end points may be due to shorter duration of intervention in the studies included in the review.
There have been attempts to define the clinical phenotype of POSA (Joosten 2012; Joosten 2014). The supine‐predominant type of POSA is the traditional definition of POSA, used in most of the studies reviewed here. It is defined as total AHI of 5 events per hour or more, with supine AHI twice the non‐supine AHI. The other type of POSA is the supine‐isolated type where non‐supine AHI is less than 5 and the ratio of supine and non‐supine AHI is 2:1. Supine‐predominant OSA forms about 60% of people with OSA, while supine‐isolated OSA forms about 30% of people with OSA. The supine‐isolated subset of people can potentially be treated with positional therapy as the sole agent. However, its clinical utility depends on how positional therapy devices fare in the clinically relevant end points like quality‐of‐sleep or quality‐of‐life parameters.
An important question is whether the clinical outcomes vary with respect to different types of positional therapy devices. In this review, studies used both physical restraint devices as well as sleep position training based on vibration alerts. The newer generation of positional therapy devices like the vibration alerts is thought to cause less sleep disruption compared to the 'tennis‐ball' paradigm of physical‐restraint type of positional therapy devices (Bignold 2009). This review was not designed to address this question. A prespecified subgroup analysis based upon the type of positional device (alarm‐based positional trainer verus physical restraint) did not show any subgroup difference. A study comparing vibrating alarm‐based sleep position trainer (SPT) with a 'tennis‐ball' positional device found that participants preferred SPT over the tennis‐ball positional device. However, this study was not able to demonstrate significant difference in quality‐of‐life or quality‐of‐sleep parameters (Eijsvogel 2015). As the study was of short duration, it is not clear that the better acceptability of SPT would translate to tangible benefits in the clinical end points in the long term. None of the studies reviewed here addressed this issue.
Quality of the evidence
Methodological quality of the included trials was low, which reduced our confidence in many of the pooled effect estimates. Blinding was often not possible because of the nature of the study and most of the studies did not specify outcome assessor blinding.
Only one study included all participants with OSA, while the remainder of the studies specified inclusion of people with POSA. The criteria used for defining POSA was variable. All the studies were of short duration (less than four weeks, with median of 10.5 days). Two included studies were for two nights (Permut 2010; Svatikova 2011). Three of the included trials did not study clinical outcome parameters (Bignold 2011; Permut 2010; Svatikova 2011). Three studies did not have a washout period between the two interventions (Jokic 1999; Permut 2010; Svatikova 2011). This was relevant with respect to Jokic 1999 as it had included clinical outcome parameters that might have a carry‐over effect. Only two studies included adherence as an outcome measure, a crucial factor in the success of interventions for OSA (Laub 2017; Skinner 2008). The included studies were all cross‐over trials except two, which were parallel‐arm studies (Jackson 2015; Laub 2017). We also judged the studies to have problems with precision of measurements, as some used home‐based monitors while others used laboratory‐based polysomnography. The estimates of ESS, sleep quality and quality of life (SF 36 and FOSQ) in the comparison positional therapy versus CPAP, and that of sleep quality and quality of life (SF 36 and FOSQ) in the comparison positional therapy and inactive control are imprecise.
In the comparison between positional therapy and CPAP, the certainty of estimate of mean difference of ESS is low due to high risk of bias (randomisation and allocation concealment not clearly stated, and participant blinding not done), and imprecision (wide confidence interval). The certainty of estimate of mean difference of AHI was low due to high risk of bias (methods of randomisation and allocation concealment not clearly stated and blinding not done), and imprecision of measuring techniques (one study used laboratory‐based polysomnography while the other study used home‐based monitors that had a kappa agreement of 0.6 with polysomnography). Estimates of quality of life assessed using SF‐36 or FOSQ and sleep quality assessed by duration of slow‐wave and REM sleep are low certainty due to high risk of bias (randomisation and allocation concealment not clearly stated, and participant blinding not done) and imprecision due to wide confidence of interval. Self‐reported adherence time has moderate‐certainty evidence as we downgraded it for risk of bias (randomisation and allocation concealment not clearly stated, and participant blinding not done).
In the comparison between positional therapy and inactive control, the estimate of mean difference of ESS has moderate‐certainty evidence as one of the studies had high risk of bias (open‐label study with high attrition rate, and incomplete mention of the methods of allocation concealment). The estimate of mean difference of AHI has low‐certainty evidence due to high risk of bias and imprecision of measuring techniques. The estimates of adherence measured by number of participants who continued to use positional therapy at the end of two months and adverse event rates measured by number of participants who discontinued at the end of two months have low‐certainty evidence due to high risk of bias (downgraded twice due to high attrition rate, lack of mention of methods of allocation concealment and the open‐label nature of the contributing study). Quality of life assessed using FOSQ has moderate‐certainty evidence due to imprecision of the estimate. Quality of sleep assessed by average duration of slow‐wave and REM sleep has low‐certainty evidence due to imprecision of the estimate and risk of bias of the contributing study. The estimate of cognitive dysfunction assessed using motor reaction has very low‐certainty evidence as the contributing study is at risk of bias, and the study has conducted multiple comparisons. The study authors reported no difference between the groups for the outcomes on cognition. We downgraded this parameter twice for risk of bias and once for indirectness, as its clinical significance in isolation is uncertain.
Potential biases in the review process
We decided in protocol of the review to exclude cross‐over randomised trials, however, we amended the protocol after publication. This is a post‐hoc deviation that carries a risk of bias. The review otherwise followed the standard protocol of Cochrane Reviews. The risk of bias involved in the observational nature of the review applies to the present review.
Agreements and disagreements with other studies or reviews
Two systematic reviews compared positional therapy in people with OSA. Ha 2014 reviewed positional therapy compared to CPAP in people with OSA. Barnes 2017 compared positional therapy with CPAP and with inactive control. Both the reviews gave results similar to that of the present review. However, our review differed from Ha 2014 and Barnes 2017 with respect to inclusion of certain studies. We did not include Cartwright 1991, as our communication with the author revealed that it was a quasi‐randomised trial. We did not include Eijsvogel 2015 and Dieltjens 2015, as the former compared two methods of positional therapy, while the latter compared positional therapy in addition to mandibular advancement device with mandibular advancement device alone. Neither of these studies satisfied our inclusion criteria. Unlike Ha 2014, we did not consider Permut 2010 for quantitative synthesis as it had provided relevant outcome parameters (e.g. AHI) as median and interquartile range (IQR). Ha 2014 had extrapolated mean and standard deviation from median and IQR. We did not consider this as a permissible standard, as the study would have presented the data as median and IQR because of non‐normal distribution of data, and to extrapolate mean and standard deviation assuming normality from it would be potentially erroneous. Ha 2014 did not include studies that compared positional therapy with inactive control.
Authors' conclusions
Implications for practice.
This review demonstrated that continuous positive airway pressure therapy (CPAP) is better than positional therapy in improving Apnoea‐Hypopnoea Index (AHI) in positional obstructive sleep apnoea (POSA). Self‐reported adherence appears to favour positional therapy over CPAP. Positional therapy is better than inactive control in improving AHI and Epworth Sleepiness Scale (ESS) in participants with POSA. The difference is higher than the minimal clinically important difference (MCID) for AHI, but lower than the MCID for ESS. As the duration of the studies was short, and clinically relevant endpoints such as quality of life were not adequately evaluated in the studies, we cannot make a conclusive statement with respect to the interventions.
Implications for research.
While CPAP therapy has been demonstrated as the most effective treatment for obstructive sleep apnoea (OSA) in terms of improving AHI, ongoing adherence is a matter of concern. Positional therapy, on the other hand appears to have better self‐reported adherence, but with modest effects compared to CPAP on measures like AHI. Future studies should aim to quantify adherence to positional therapy in a more objective way, and also establish its efficacy in POSA with respect to clinically relevant outcomes like quality‐of‐life parameters and clinical outcomes like vascular events. This requires long‐term studies with much larger sample sizes than are reported in this review. Positional therapy should also be studied in special groups of people like stroke patients, in whom CPAP may not be tolerated. The studies should focus on the clinical phenotype of the OSA and adopt uniform criteria for defining POSA.
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2019 | Amended | Typo in search startegy amended. This error arose as the result of routine spell‐check which converted an American spellling of hypopnea into an UK English spelling. This had no material effect on the review as the search string run in the databases was correct. |
Acknowledgements
We acknowledge the contributions from following colleagues.
Prathap Tharyan and his team, at South Asian Cochrane Network for giving us the intellectual impetus for writing this protocol.
Tamilarasu Kadiravan of Department of Medicine, JIPMER, Pondicherry provided timely practical advice.
Emma Dennett, Christopher Cates, Elizabeth Stovold and Emma Jackson from Cochrane Airways for their advice and support.
Jimmy Chong, Rebecca Fortescue and Denise Mitchell, the editors for this review, for their constructive and critical comments.
The Background and Methods section of this review are based on a standard template used by Cochrane Airways. This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AHMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the Register
Sleep apnoea search
1. exp Sleep Apnea Syndromes/
2. (sleep$ adj3 (apnoea$ or apnoea$)).mp.
3. (hypopnoea$ or hypopnoea$).mp.
4. OSA.mp.
5. SHS.mp.
6. OSAHS.mp.
7. or/1‐6
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategy to identify relevant trials from the Cochrane Airways Trials Register
#1 SLP:MISC2
#2 MeSH DESCRIPTOR Sleep Apnea, Obstructive
#3 sleep near3 (apnoea* or apnoea*)
#4 (hypopnoea* or hypopnea*)
#5 (OSA OR SHS OR OSAHS:TI,AB)
#6 (#1 OR #2 OR #3 OR #4 OR #5)
#7 MeSH DESCRIPTOR Posture Explode All
#8 MeSH DESCRIPTOR Patient Positioning
#9 postur*
#10 position*
#11 supine*
#12 lateral*
#13 #7 or #8 or #9 or #10 or #11 or #12
#14 #6 and #13
#15 (#14) AND (INREGISTER)
[Note: In search line #1, MISC1 refers to the field in the record where the reference has been coded for condition, in this case, obstructive sleep apnoea]
Data and analyses
Comparison 1. Positional therapy versus CPAP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Epworth Sleepiness Scale | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Apnoea‐Hypopnoea Index | 2 | 66 | Mean Difference (Random, 95% CI) | 6.40 [3.00, 9.79] |
| 3 Self‐reported adherence time | 1 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 4 Quality of life by SF‐36 physical | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Quality of life by SF‐36 mental | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Quality of Life: FOSQ | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Sleep quality: proportion of REM | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Sleep quality: proportion of slow‐wave sleep | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Positional therapy versus inactive control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Epworth Sleepiness Scale | 2 | 187 | Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.89, ‐0.26] |
| 2 Apnoea‐Hypopnoea Index | 4 | 277 | Mean Difference (Random, 95% CI) | ‐7.38 [‐10.06, ‐4.70] |
| 2.1 Vibration alarm device | 3 | 191 | Mean Difference (Random, 95% CI) | ‐7.77 [‐10.81, ‐4.74] |
| 2.2 Tennis ball device | 1 | 86 | Mean Difference (Random, 95% CI) | ‐6.0 [‐11.72, ‐0.28] |
| 3 Adherence as measured by number of participants continuing therapy | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Quality of life: FOSQ | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Cognitive function: measured as motor reaction time | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bignold 2011.
| Methods | Randomised controlled cross‐over trial | |
| Participants | POSA Participants randomised: 16 Gender: male:13; female: 2 Age (years): 58.2 ± 13.9 Inclusion criteria People with POSA
Exclusion criteria
|
|
| Interventions | Intervention: positional monitoring and supine alarm device in active mode Control: device in inactive mode | |
| Outcomes | AHI, snoring loudness, percentage of time in supine posture | |
| Notes | Setting: home‐based regimen Duration of treatment: 7 days; 1 week active vibration and 1 week inactive vibration in random order, separated by an intervening washout week No period effect as demonstrated using linear mixed‐model analysis with an auto regressive covariance structure (using supine time data among participants ‐ i.e. the control phase data) Funding: Flinders Medical Centre foundation grant; not industry sponsored Comment: study results are applicable only to POSA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The procedure for randomisation is not mentioned. High risk may be mitigated by the cross‐over design of the study. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not explicitly stated. The contribution of allocation concealment to the risk of bias is low by virtue of the design of the study |
| Blinding of participants and personnel (performance bias) Objective outcomes ( AHI) | High risk | Quote: "It is difficult to blind patients to active versus inactive treatment" (Protocol first paragraph page 378) Comment: likely unblinded. Participant blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear, as we could not access the protocol of the study |
| Other bias | Low risk | None identified |
Jackson 2015.
| Methods | Randomised controlled parallel‐arm trial | |
| Participants | POSA Participants randomised: 86 Inclusion criteria
Exclusion criteria
|
|
| Interventions | A 10‐point sleep guide to improve OSA with and without sleep position modification device. The sleep position modification device consisted of a band of stretch cotton worn around the chest, just below the nipple line and with straps over the shoulder to hold it in place. The band was secured at the front with buttons and the ball was contained in a pocket at the rear, over the thoracic spine. |
|
| Outcomes | Outcomes: supine sleep time, total sleep time, AHI, supine AHI, sleep efficiency, arousal index, BMI, blood pressure, ESS and quality‐of‐life scales e.g. FOSQ, SOSQ, and neuropsychological test battery like psychomotor vigilance test, response inhibition test, Trail Making Task A and B, digit symbol substitution task, digits span test and controlled word association task | |
| Notes | Setting: outpatient department Austin Health, USA
Duration of treatment: 4 weeks Adherence not assessed Funding: likely mixed funding "This work was supported by grants from the Institute for Breathing and Sleep, the Austin Health Medical Research Foundation and the Harold and Cora Brennen Benevolent Trust" Confict of interest declared: Dr Howard has received funding from ResMed foundation, Edansafe and Prevention Express |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomisation sequence at a 1:1 active:control ratio was computer generated by a third party and the investigators were provided with sealed envelopes", page 547 Comment: 1:1 allocation was planned, but numbers in the arms are very dissimilar (37 and 49) |
| Allocation concealment (selection bias) | Low risk | Quote: "A randomisation sequence at a 1:1 active:control ratio was computer generated by a third party and the investigators were provided with sealed envelopes. These were opened in order of enrolment", page 547 Comment: computer‐generated randomisation sequence was provided by a third party in sealed envelopes to be opened in the order of enrolment |
| Blinding of participants and personnel (performance bias) Objective outcomes ( AHI) | High risk | Not possible due to the nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome for which sample size was calculated is not reported explicitly |
| Other bias | Unclear risk | It is not clear whether the study adjusted for multiple comparisons. |
Jokic 1999.
| Methods | Randomised, single‐blind, cross‐over trial | |
| Participants | POSA Participants randomised: 14 (1 excluded after randomisation as he had idiopathic hypersomnolence) Gender: male: 12; female: 1 Age (years): 51 ± 9 Inclusion criteria People with POSA
Exclusion criteria
|
|
| Interventions | Intervention: backpack with soft ball to prevent supine sleep Control: CPAP | |
| Outcomes | AHI Other outcomes like SaO2, sleep architecture, mood scales | |
| Notes | Setting: home‐based regimen Duration of treatment: 4 weeks; 2 weeks of positional therapy; 2 weeks CPAP in random order CPAP machine withdrawn during positional therapy phase and vice versa Overnight sleep study at baseline, at end of every 2‐week period Funding: Grant from the Health Services Utilisation and Research Commission of Saskatchewan; not industry sponsored Comment: stringent criteria for POSA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The procedure for randomisation is not mentioned. High risk may be mitigated by the cross‐over design of the study. |
| Allocation concealment (selection bias) | Unclear risk | The procedure of allocation concealment is not explicitly stated. High risk may be mitigated by the cross‐over design of the study. |
| Blinding of participants and personnel (performance bias) Objective outcomes ( AHI) | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The researcher who scored the sleep studies and conducted the psychometric tests were blinded to the modality of treatment being used by the patient" page 773 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow up, but one excluded due to alternative diagnosis. Page 772 |
| Selective reporting (reporting bias) | Unclear risk | Unclear as we could not access the protocol of the study |
| Other bias | Low risk | None identified |
Laub 2017.
| Methods | Randomised controlled, parallel‐arm trial | |
| Participants | POSA Participants randomised: 101 Inclusion criteria
Exclusion criteria
|
|
| Interventions | SPT, a vibrating supine alert device worn across participant's chest | |
| Outcomes | Primary: short‐term efficacy and adherence of the SPT over a 2‐month period Secondary: long‐term efficacy and adherence of SPT for a 6‐month period |
|
| Notes | Funding: "Maribo Medicos A/S Denmark and Night Balance, the Netherlands provided the SPT devices free of charge. "The authors state that the study was an independent investigator‐initiated study Disclosure: "Philip Tonnesen has been a member of the steering committee for the annual sleep scientific meeting in Denmark arranged by Maribo Medico A/S" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers was used for randomisation |
| Allocation concealment (selection bias) | Unclear risk | The exact procedure adapted for allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) Objective outcomes ( AHI) | High risk | Open‐label trial; no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26.7% of the participants dropped out before the first follow‐up at 2 months |
| Selective reporting (reporting bias) | Unclear risk | Protocol (NCT022114424) mentions similar outcomes to the report |
| Other bias | Unclear risk | None identified, except for the disclosure of the potential conflict of interest |
Permut 2010.
| Methods | Randomised, cross‐over trial, non‐inferiority trial | |
| Participants | POSA Participants randomised: 38 Gender: 25 male, 13 female Age group: 49 ± 12 years Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intervention: Zzoma® positional sleeper. It consists of semirigid synthetic foam contained in a backpack‐type material worn using a Velcro elastic belt Control: CPAP Duration: 1 night each on intervention and on CPAP | |
| Outcomes |
|
|
| Notes | Setting:
Comment: study applicable to POSA as defined by the study Disclosure: "Financial support was provided by an Innovator Circle Grant from Abington Memorial Hospital, Abington, PA. Sleep Specialists, LLC, supplied the positional devices for the study and paid for a portion of the polysomnograms that were performed at Abington Memorial Hospital. Drs. Crocetti and Krachman have a financial interest in Sleep Specialists, LLC, makers of the Zzoma Positional Sleeper. The other authors have indicated no financial conflicts of interest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The procedure for randomisation is not mentioned. High risk may be mitigated by the cross‐over design of the study. |
| Allocation concealment (selection bias) | Unclear risk | The procedure of allocation concealment is not mentioned. High risk may be mitigated by the cross‐over design of the study. |
| Blinding of participants and personnel (performance bias) Objective outcomes ( AHI) | High risk | Not mentioned, but likely not blinded considering nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout rate. One participant lost after randomisation |
| Selective reporting (reporting bias) | High risk | Reporting is not according to the standard reporting of non‐inferiority trial. Non‐inferiority has not been demonstrated (as P = 0.16), but the study authors report this as the intervention showing 'equivalence' with that of the active control. The outcomes are reported as median and interquartile range, and as difference between baseline and following the intervention, and not between the intervention and the control. In most of the critical parameters, the study does not report the comparison of outcomes between the intervention and the control. |
| Other bias | High risk | The reporting of the study was not appropriate. The results from baseline are reported interchangeably with between‐group comparison. Non‐inferiority is not properly reported. The study is reported as a superiority trial. The reported result of the study is misleading. However, this issue is not a problem if the results are pooled for meta‐analysis. The reported outcome of the study independently should be considered high risk of bias due to selective outcome reporting and improper reporting vis‐a‐vis the design of the study. Overall poor‐quality study |
Skinner 2008.
| Methods | Randomised cross‐over trial | |
| Participants | Mild‐moderate position‐dependent obstructive sleep apnoea hypopnoea syndrome (OSAHS) Participants randomised: 20 Age: mean 55.9 years (SD 9.8) (range 37‐78) Gender: not mentioned Inclusion criteria: mild‐moderate severity POSA
Exclusion criteria
|
|
| Interventions | Intervention: thoracic anti‐supine band (TASB) Control: nasal CPAP | |
| Outcomes | Primary outcome measure
Secondary outcome
|
|
| Notes | Setting: outpatient study, used home‐based sleep monitoring
Duration of follow‐up: 1 month on each treatment process with a 1‐week washout
Drop out: none
Funding:
Other relevant issues: OSAHS diagnosed using a 9‐channel, level‐III portable digital recording device, the Embletta PDS (Flaga Medical Devices, Reykjavik, Iceland) Comment: study included only participants with AHI 5‐10 making the generalisability of the study poor |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to receive TASB or nasal CPAP for the first month". Comment: randomisation procedure not clearly stated; high risk may be mitigated by the cross‐over design of the study |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure not stated. High risk may be mitigated by the cross‐over design of the study. |
| Blinding of participants and personnel (performance bias) Objective outcomes ( AHI) | High risk | Not mentioned but likely not blinded considering nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear as we could not access the protocol of the study |
| Other bias | Low risk | None identified |
Svatikova 2011.
| Methods | Randomised cross‐over trial | |
| Participants | Acute ischaemic stroke or probable ischaemic stroke Participants included: 18 Diagnosis based on the WHO MONICA criteria ≥ 18 years Gender: male 11 (61%); female 7 (39%) Age: 54‐68 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intervention: positional device called Sona pillow‐ designed to prevent supine sleep Control: standard hospital pillow, participant positioned at liberty Duration: 2 consecutive nights‐ 1 night with intervention and 1 night without intervention | |
| Outcomes |
|
|
| Notes | Settings: inpatient neurology service, University of Michigan Duration of follow‐up: 3‐month follow‐up as the continuation for assessment of adherence Drop out: nil Funding: University of Michigan Clinical and translational science award | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation procedure not stated. High risk may be mitigated by the cross‐over design of the study. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure not stated. High risk may be mitigated by the cross‐over design of the study. |
| Blinding of participants and personnel (performance bias) Objective outcomes ( AHI) | High risk | Blinding not mentioned, but likely not blinded considering the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Unclear risk | Unclear as we could not access the protocol of the study |
| Other bias | Low risk | None identified |
Van Maanen 2012.
| Methods | Randomised cross‐over trial | |
| Participants | POSA Participants randomised: 30 Inclusion criteria
Exclusion criteria: no exclusion criteria given |
|
| Interventions | Vibrating device worn on the back of neck that senses supine position in the on and off position, on randomly assigned nights within 3 months of each other | |
| Outcomes |
|
|
| Notes | Settings: outpatient ENT services, Saint Lucas Andreas Hospital, Netherlands
Duration of follow‐up: 3 sleep studies over 3‐month period Adherence: overnight PSG studies in hospital, in 3 however device malfunctioned in 'on state' Drop out: nil Funding: Not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors have not stated the randomisation procedure in clear terms. There was a mere mention that the device was put on or off 'randomly'. We tried to contact the study author for clarification, but were unsuccessful |
| Allocation concealment (selection bias) | Unclear risk | The methods are not properly stated |
| Blinding of participants and personnel (performance bias) Objective outcomes ( AHI) | Unclear risk | Device worn on both nights, however due to nature of intervention participants cannot be blinded, but all reported outcomes are objective |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The data were reviewed manually for analysis by an experienced sleep investigator, blinded for the activity state of the device" page 323. Comment: outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Unclear risk | As no specific outcomes or comparisons were listed a priori, difficult to ascertain whether the listed outcomes are those planned or not |
| Other bias | Unclear risk | Statistically analysis is probably not appropriate. They have 3 group comparison, but used t‐tests individually. They have conceded that the distributions were not normal, and sometimes Wilcoxon rank sum test would be used. But it is not clear how and where it was used. It is also not clear whether paired t‐test was used |
AHI: Apnoea‐Hypopnoea Index; BMI: body mass index; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcome of Sleep Questionnaire; MOS: Medical Outcome Study; OSA: obstructive sleep apnoea; POSA: positional obstructive sleep apnoea; PSG: polysomnography; REM: rapid eye movement; SaO2: oxygen saturation; SD: standard deviation; SOSQ: Symptoms of Sleep Questionairre; SF‐36: Short Form Health Survey; SPT: sleep position trainer; WHO MONICA: World Health Organization Monitoring Trends and Determinants in Cardiovascular Disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afrashi 2015 | Non‐randomised trial of prone positioning in sleep apnoea |
| Barnes 2017 | Systematic review |
| Benoist 2017 | Compared positional therapy with oral appliance therapy |
| Braver 1995 | Non‐randomised study and participants are asymptomatic snorers and not people with POSA |
| Cartwright 1991 | Non‐randomised trial of tongue retaining device and posture alarm |
| Dieltjens 2015 | Compared positional device and mandibular advancement device with positional device alone |
| Eijsvogel 2015 | Compared 2 methods of positional intervention (position sensor with vibration and tennis ball technique) |
| Greer 2006 | Non randomised trial of wedge under the knee for OSA |
| Ha 2014 | Systematic review |
| Kurlak 1994 | Non randomised trial of wedge under the knee for OSA |
| Magalang 2016 | Available only as an abstract in conference proceedings |
| Skinner 2004a | Intervention was a shoulder‐head elevation pillow that does not satisfy the prespecified description of positional therapy in this review. |
| Skinner 2004b | Intervention was a cervicomandibular support device, not considered as a 'positional device' |
| Zaremba 2015 | Participants were not individuals with OSA, but asymptomatic post partum women |
| Zuberi 2004 | Non‐randomised study |
OSA: obstructive sleep apnoea
Characteristics of ongoing studies [ordered by study ID]
ISRCTN16170657.
| Trial name or title | Comparison of the NightBalance Lunoa to positive airway pressure (PAP) for the treatment of positional obstructive sleep apneas (POSA) |
| Methods | Randomised cross‐over trial |
| Participants | 150 (95 treatment‐naive and 55 PAP non‐adherent patients) All adults with diagnosis of POSA (AHI > 15 with sleepiness or co‐morbidities like atrial fibrillation, hypertension; supine AHI at least twice lateral AHI; Lateral AHI < 10; supine time > 30% and < 70%) who are either treatment‐naive or PAP non‐adherent (defined as current positive airway pressure user with < 3 h/night in the last 3 months per adherence download), and willing to use the positive airway pressure device per protocol |
| Interventions | Lunoa sleep position therapy vs APAP |
| Outcomes | Primary objective: AHI, adherence Secondary objective: ESS, SF‐36, Euroquol 5D (EQ5D), Pichot fatigue scale, patient comfort and satisfaction on Likert scale, adverse events, health economics and resource utilisation |
| Starting date | 1 August 2018 |
| Contact information | K. van der Geest research@nightbalance.com; Benoordenhoutseweg 46‐13 Den Haag 2596BC Netherlands |
| Notes |
NCT01699139.
| Trial name or title | Effect of positional device on the obstructive sleep apnoea in patients with ischaemic stroke |
| Methods | Randomised, cross‐over, single‐blinded (outcome assessor) efficacy trial |
| Participants | 13 participants with ischaemic stroke and mild‐moderate OSA |
| Interventions | ZZoma positional device vs lumbar corset |
| Outcomes | Primary: change in AHI from baseline Secondary: change in augmentation index, pulse wave velocity |
| Starting date | September 2012 |
| Contact information | jongyau2002@gmail.com, Dr. Chungyao Chen, Chang gung hospital, Keelung, Taiwan. Contacted on 26 January 2017 with no response |
| Notes |
NCT02553902.
| Trial name or title | Economic evaluation of treatment modalities for position dependent obstructive sleep apnoea |
| Methods | Prospective, multicentre, randomised, parallel‐arm, single‐blinded trial |
| Participants | 200 participants with moderate POSA |
| Interventions | SPT with mandibular advancement device versus CPAP |
| Outcomes | Primary: AHI Secondary: EQ5D, ESS, FOSQ, etc |
| Starting date | September 2015 |
| Contact information | a.beelen@olvg.nl; n.vries@slaz.nl; Professor de Vries OLVG west, Amsterdam, Netherlands |
| Notes |
NCT03061071.
| Trial name or title | POSAtive study: study for the treatment of positional sleep apnoea |
| Methods | Randomised cross‐over trial |
| Participants | 120 participants with POSA |
| Interventions | Night balance SPT vs automated adjusting PAP |
| Outcomes | Primary: adherence and AHI Secondary: ESS, FOSQ, SF‐36 etc |
| Starting date | November 2017 |
| Contact information | andrea@nightbalance.com; Dr. Richard B Berry, UF sleep health centre, Florida , USA |
| Notes |
NCT03125512.
| Trial name or title | Positional therapy versus CPAP for POSA |
| Methods | Randomised, cross‐over, clinical trial |
| Participants | 40 participants with POSA and ESS 10‐16 aged > 21 years |
| Interventions | Night shift positional device versus CPAP |
| Outcomes | Primary: ESS Secondary; FOSQ, SF‐36, AHI etc |
| Starting date | February 2017 |
| Contact information | nur_shameerah@cgh.com.sg; ying_juan_mok@cgh.com.sg; Dr. Yingjuan Mok, Changi General Hospital, Singapore |
| Notes |
NCT03336515.
| Trial name or title | The validity of a vibrating postural device for the treatment of positional obstructive sleep apnoea |
| Methods | Multicentre, randomised, parallel‐arm study |
| Participants | 112 participants with POSA |
| Interventions | 3 arms: A, general advice; B, positional device inactivated; C, positional device activated |
| Outcomes | Primary: AHI Secondary: supine sleep time, quality and quantity of sleep etc |
| Starting date | September 2015 |
| Contact information | Dr. Joaquin Duran‐Cantolla, Hospital Universitario Araba, Gasteiz/Vitoria, Araba, Spain, 01009 |
| Notes |
NCT03558659.
| Trial name or title | Positional therapy to treat obstructive sleep apneas in stroke patients |
| Methods | Randomised, parallel‐group, double‐blind (care provider, investigator) |
| Participants | 40 participants with acute ischaemic stroke treated at Sunnybrook Health Sciences Centre Exclusion criteria: unable to lie in a supine position; using PAP therapy or supplemental oxygen; unable to use the portable sleep‐monitoring device; physical impairment, aphasia, language barrier, facial/bulbar weakness or trauma restricting use of monitor; absence of care‐giver who can provide assistance; pregnant women |
| Interventions | Positional therapy belt produced by SlumberBUMP to avoid sleep in the supine position compared to no belt |
| Outcomes | Primary objective: AHI and oxygen desaturation at baseline vs 1 week/2week/3‐6 months Secondary objective: supine sleep time, sleep efficiency, National institute of Health Stroke scale (NIHSS), length of hospital stay, reaction time, Montreal Cognitive assessment, Centres for epidemiology scale‐Depression depression scale, SF‐12 quality of life, ESS, modified Rankin Scale and Barthel index |
| Starting date | September 2018 |
| Contact information | Dr. Mark I Boulos, Sunnybrook Health Sciences Centre, Toronto Ontario Canada M4N3M5. Ph: 4164804473; email: mark.boulos@utoronto.ca/mark.boulos@sunnybrook.ca |
| Notes |
NTR2826.
| Trial name or title | Use of Z‐cushion in patients with positional obstructive sleep apnoea syndrome |
| Methods | Randomised, parallel‐arm, placebo‐controlled trial |
| Participants | 44 participants with mild‐moderate obstructive sleep apnoea syndrome with POSA |
| Interventions | Z‐cushion vs no treatment |
| Outcomes | Primary: AHI Secondary: sleepiness, supine sleep time dips in oxygen saturation etc |
| Starting date | November 2011 |
| Contact information | e.lammers@gelre.nl; Dr. E. Lammers, Department of Pulmonary medicine, Zutphen Netherlands |
| Notes |
TCTR20150204001.
| Trial name or title | Innovation of positional therapy belt and its effectiveness as a treatment modality for positional sleep apnoea |
| Methods | Interventional |
| Participants |
Inclusion criteria
Positional Sleep Apnea is defined by supine RDI/non‐supine RDI ≥ 2 with overall RDI ≥ 5 Exclusion criteria:
|
| Interventions | Position therapy belt |
| Outcomes | Primary: RDI Secondary: AHI, supine time, sleep apnoea related symptoms |
| Starting date | 1 March 2015 |
| Contact information | narichac@hotmail.com Dr. Naricha Chirukalwasan, Phayatai plaza, Bangkok, Thailand |
| Notes | Contacted on 26 January 2017, study not finalised yet |
AHI: Apnoea‐Hypopnoea Index; CPAP: continuous positive airway pressure; ESS: Epworth Sleepiness Scale; FOSQ: Functional Outcome of Sleep Questionnaire; OSA: obstructive sleep apnoea; PAP: positive airway pressure; POSA: positional obstructive sleep apnoea; SF‐36: Short Form 36 health survey; SPT: sleep position trainer
Differences between protocol and review
The original protocol excluded cross‐over randomised trials. We amended the protocol post‐hoc to include cross‐over trials because we wanted to include relevant evidence and we accepted that the carry‐over effect was limited.
Contributions of authors
PRS wrote the protocol in collaboration with AG. Data extraction was done by RA, JD and PRS. Disagreement was adjudicated by PRS and AG. PRS wrote the final document. RA contributed to the text. Database searches were carried out by Elizabeth Stovold of Cochrane Airways.
Sources of support
Internal sources
Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
National Institute of Mental Health and Neurosciences, Bengaluru, India.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
PR Srijithesh: none known Rajeswari Aghoram: none known Amit Goel: none known Jayaraj Dhanya: none known
Edited (no change to conclusions)
References
References to studies included in this review
Bignold 2011 {published data only}
- Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine‐dependent obstructive sleep apnea with a new position recording and supine avoidance device. Journal of Clinical Sleep Medicine 2011;7(4):376‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2015 {published data only}
- Jackson M, Collins A, Berlowitz D, Howard M, O'Donoghue F, Barnes M. Efficacy of sleep position modification to treat positional obstructive sleep apnea. Sleep Medicine 2015;16(4):545‐52. [PUBMED: 25771294] [DOI] [PubMed] [Google Scholar]
Jokic 1999 {published data only}
- Jokic R, Klimaszewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest 1999;115(3):771‐81. [CENTRAL: 160858; CRS: 4900100000006299; EMBASE: 1999099776; PUBMED: 10084491] [DOI] [PubMed] [Google Scholar]
Laub 2017 {published data only}
- Laub RR, Tonnesen P, Jennum PJ. A sleep position trainer for positional sleep apnea: a randomized, controlled trial. Journal of Sleep Research 2017;26(5):641‐50. [PUBMED: 28370716] [DOI] [PubMed] [Google Scholar]
Permut 2010 {published data only}
- Permut I, Diaz‐Abad M, Chatila W, Crocetti J, Gaughan JP, D'Alonzo GE, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. Journal of Clinical Sleep Medicine 2010;6(3):238‐43. [CENTRAL: 749466; CRS: 4900100000024855; EMBASE: 2010364128; PUBMED: 20572416] [PMC free article] [PubMed] [Google Scholar]
Skinner 2008 {published data only}
- Skinner MA, Kingshott RN, Filsell S, Taylor DR. Efficacy of the 'tennis ball technique' versus nCPAP in the management of position‐dependent obstructive sleep apnoea syndrome. Respirology 2008;13(5):708‐15. [CENTRAL: 668305; CRS: 4900100000022899; EMBASE: 2008334874; PUBMED: 18713092] [DOI] [PubMed] [Google Scholar]
Svatikova 2011 {published data only}
- Svatikova A, Chervin RD, Wing JJ, Sanchez BN, Migda EM, Brown DL. Positional therapy in ischemic stroke patients with obstructive sleep apnea. Sleep Medicine 2011;12(3):262‐6. [CENTRAL: 787723; CRS: 4900100000026390; EMBASE: 2011122820; PUBMED: 21306949] [DOI] [PubMed] [Google Scholar]
Van Maanen 2012 {published data only}
- Maanen JP, Richard W, Kesteren ER, Ravesloot MJ, Laman DM, Hilgevoord AA, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. Journal of Sleep Research 2012;21(3):322‐9. [PUBMED: 22017727] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Afrashi 2015 {published data only}
- Afrashi A, Ucar ZZ. Effect of prone positioning in mild to moderate obstructive sleep apnea syndrome. Schlaf & Atmung [Sleep & Breathing] 2015;19(3):1027‐34. [PUBMED: 25618193] [DOI] [PubMed] [Google Scholar]
Barnes 2017 {published data only}
- Barnes H, Edwards BA, Joosten SA, Naughton MT, Hamilton GS, Dabscheck E. Positional modification techniques for supine obstructive sleep apnea: a systematic review and meta‐analysis. Sleep Medicine Reviews 2017;36:107‐15. [PUBMED: 28012784] [DOI] [PubMed] [Google Scholar]
Benoist 2017 {published data only}
- Benoist L, Ruiter M, Lange J, Vries N. A randomized, controlled trial of positional therapy versus oral appliance therapy for position‐dependent sleep apnea. Sleep Medicine 2017;34:109‐17. [PUBMED: 28522078] [DOI] [PubMed] [Google Scholar]
Braver 1995 {published data only}
- Braver HM, Block AJ, Perri MG. Treatment for snoring. Combined weight loss, sleeping on side, and nasal spray. Chest 1995;107(5):1283‐8. [CRS: 4900126000006536; PUBMED: 7750319] [DOI] [PubMed] [Google Scholar]
Cartwright 1991 {published data only}
- Cartwright R, Ristanovic R, Diaz F, Caldarelli D, Alder G. A comparative study of treatments for positional sleep apnea. Sleep 1991;14(6):546‐52. [PUBMED: 1798889] [DOI] [PubMed] [Google Scholar]
Dieltjens 2015 {published data only}
- Dieltjens M, Vroegop AV, Verbruggen AE, Wouters K, Willemen M, Backer WA, et al. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep and Breathing 2015;19(2):637‐44. [PUBMED: 25335642] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eijsvogel 2015 {published data only}
- Eijsvogel MM, Ubbink R, Dekker J, Oppersma E, DeJongh FH, Palen J, et al. Sleep position trainer versus tennis ball technique in positional obstructive sleep apnea syndrome. Journal of Clinical Sleep Medicine 2015;11(2):139‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greer 2006 {published data only}
- Greer SA, Straight LB, Schulman DA, Bliwise DL. Effect of supine knee position on obstructive sleep apnea. Sleep and Breathing 2006;10(2):98‐101. [CENTRAL: 592438; CRS: 4900100000021013; PUBMED: 16570204] [DOI] [PubMed] [Google Scholar]
Ha 2014 {published data only}
- Ha SC, Hirai HW, Tsoi KK. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta‐analysis of randomized trials. Sleep Medicine Reviews 2014;18(1):19‐24. [PUBMED: 23831268] [DOI] [PubMed] [Google Scholar]
Kurlak 1994 {published data only}
- Kurlak LO, Ruggins NR, Stephenson TJ. Effect of nursing position on incidence, type, and duration of clinically significant apnoea in preterm infants. Archives of Disease in Childhood 1994;71(1):F16‐9. [PUBMED: 8092863] [DOI] [PMC free article] [PubMed] [Google Scholar]
Magalang 2016 {published data only}
- Magalang U, Mindel J, Phillips G, Emmons K, Ribble D. A randomized controlled trial of a novel sleep surface for treatment of positional sleep apnea. European Respiratory Journal 2016;48:PA2387. [Google Scholar]
Skinner 2004a {published data only}
- Skinner MA, Kingshott RN, Jones DR, Homan SD, Taylor DR. Elevated posture for the management of obstructive sleep apnea. Sleep and Breathing 2004;8(4):193‐200. [PUBMED: 15611894] [DOI] [PubMed] [Google Scholar]
Skinner 2004b {published data only}
- Skinner MA, Kingshott RN, Jones DR, Taylor DR. Lack of efficacy for a cervicomandibular support collar in the management of obstructive sleep apnea. Chest 2004;125(1):118‐26. [PUBMED: 14718430] [DOI] [PubMed] [Google Scholar]
Zaremba 2015 {published data only}
- Zaremba S, Mueller N, Heisig AM, Shin CH, Jung S, Leffert LR, et al. Elevated upper body position improves pregnancy‐related OSA without impairing sleep quality or sleep architecture early after delivery. Chest 2015;148(4):936‐44. [PUBMED: 25905714] [DOI] [PubMed] [Google Scholar]
Zuberi 2004 {published data only}
- Zuberi NA, Rekab K, Nguyen HV. Sleep apnea avoidance pillow effects on obstructive sleep apnea syndrome and snoring. Sleep and Breathing 2004;8(4):201‐7. [PUBMED: 15611895] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN16170657 {published data only}
- ISRCTN16170657. Comparison of the NightBalance Lunoa to positive airway pressure (PAP) for the treatment of positional obstructive sleep apnea (POSA). www.isrctn.com/ISRCTN16170657 (first received May 2018). [ 10.1186/ISRCTN16170657; EU‐2018‐001]
NCT01699139 {published data only}
- Chen C. The effect of positional device on the obstructive sleep apnea in patients with ischemic stroke. clinicaltrials.gov/ct2/show/NCT01699139 (first received March 2015). [NSC101‐2314‐B‐182A‐111 ]
NCT02553902 {published data only}
- Benoist LBL, Gusthuis OLV. Economic evaluation of treatment modalities for position dependent obstructive sleep apnea. clinicaltrials.gov/ct2/show/NCT02553902 (First received July 2017). [NL52032.029.15 ]
NCT03061071 {published data only}
- Berry RB. The POSAtive Study: study for the treatment of positional obstructive sleep apnea. clinicaltrials.gov/ct2/show/NCT03061071 (First received July 2017). [US‐2017‐001]
NCT03125512 {published data only}
- Mok Y. Positional therapy versus CPAP for positional OSA. clinicaltrials.gov/ct2/show/NCT03125512 (First received July 2017). [OSA_RCT ]
NCT03336515 {published data only}
- NCT03336515. Validity of a vibrating postural device for the treatment of positional obstructive sleep apnea (postural) (POSTURAL). clinicaltrials.gov/ct2/show/NCT03336515 (First received March 2018). [2014142 ]
NCT03558659 {published data only}
- NCT03558659. Positional therapy to treat obstructive sleep apnea in stroke patients. clinicaltrials.gov/ct2/show/NCT03558659 (First received June 2018). [122‐2018 ]
NTR2826 {published data only}
- NTR2826. Use of the Z‐cushion in patients with positional obstructive sleep apnea syndrome. www.trialregister.nl/trial/2696 (First received March 2015). [ NL2696]
TCTR20150204001 {published data only}
- TCTR20150204001. Innovation of positional therapy belt and its effectiveness as a treatment modality for positional sleep apnea. clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1304 (first received 4 Feb 2015).
Additional references
Arzt 2005
- Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep‐disordered breathing and the occurrence of stroke. American Journal of Respiratory and Critical Care Medicine 2005;172(11):1447‐51. [PUBMED: 16141444] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bassetti 1999
- Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep 1999;22(2):217‐23. [PUBMED: 10201066] [DOI] [PubMed] [Google Scholar]
Bignold 2009
- Bignold JJ, Deans‐Costi G, Goldsworthy MR, Robertson CA, McEvoy D, Catcheside PG, et al. Poor long‐term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. Journal of Clinical Sleep Medicine 2009;5(5):428‐30. [PUBMED: 19961026] [PMC free article] [PubMed] [Google Scholar]
Canessa 2011
- Canessa N, Castronovo V, Cappa SF, Aloia MS, Marelli S, Falini A, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. American Journal of Respiratory and Critical Care Medicine 2011;183(10):1419‐26. [PUBMED: 21037021] [DOI] [PubMed] [Google Scholar]
Cartwright 1984
- Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep 1984;7(2):110‐4. [PUBMED: 6740055] [DOI] [PubMed] [Google Scholar]
Chen 2015
- Chen CY, Chen CL, Yu CC, Chen TT, Tseng ST, Ho CH. Association of inflammation and oxidative stress with obstructive sleep apnea in ischemic stroke patients. Sleep Medicine 2015;16(1):113‐8. [PUBMED: 25439077] [DOI] [PubMed] [Google Scholar]
Chobanian 2003
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003;289(19):2560‐72. [PUBMED: 12748199] [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dumitrascu 2012
- Dumitrascu R, Tiede H, Rosengarten B, Schulz R. Obstructive sleep apnea and stroke [Obstruktive schlaf‐apnoe und schlaganfall]. Pneumologie 2012;66(8):476‐9. [PUBMED: 22875731] [DOI] [PubMed] [Google Scholar]
Epstein 2009
- Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long‐term care of obstructive sleep apnea in adults. Journal of Clinical Sleep Medicine 2009;5(3):263‐76. [PUBMED: 19960649] [PMC free article] [PubMed] [Google Scholar]
Giles 2006
- Giles TL, Lasserson TJ, Smith B, White J, Wright JJ, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001106.pub3] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 8 April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Harbison 2002
- Harbison J, Ford GA, James OF, Gibson GJ. Sleep‐disordered breathing following acute stroke. QJM : Monthly Journal of the Association of Physicians 2002;95(11):741‐7. [PUBMED: 12391386] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Isono 2002
- Isono S, Tanaka A, Nishino T. Lateral position decreases collapsibility of the passive pharynx in patients with obstructive sleep apnea. Anesthesiology 2002;97(4):780‐5. [PUBMED: 12357140] [DOI] [PubMed] [Google Scholar]
Jackson 2018
- Jackson ML, McEvoy RD, Banks S, Barnes M. Neurobehavioral impairment and CPAP treatment response in mild‐moderate obstructive sleep apneas. Journal of Clinical Sleep Medicine 2018;14(1):47‐56. [PUBMED: 29198304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joosten 2012
- Joosten SA, Hamza K, Sands S, Turton A, Berger P, Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology 2012;17(1):99‐107. [PUBMED: 21848707] [DOI] [PubMed] [Google Scholar]
Joosten 2014
- Joosten SA, O'Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Medicine Reviews 2014;18(1):7‐17. [PUBMED: 23669094] [DOI] [PubMed] [Google Scholar]
Kapur 2017
- Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, Harrod CG. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. Journal of Clinical Sleep Medicine 2017;13(3):469‐503. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2017
- Kim J, Tran K, Seal K, Almeida F, Ross G, Messier MR, et al. Interventions for the treatment of obstructive sleep apnea in adults: a health technology assessment. Canadian Agency for Drugs and Technologies in Health; 2017 Mar; CADTH Optimal Use Report, No. 6.1b (accessed prior to 8 April 2019). [ISSN: 1927‐0127]
Kribbs 1993
- Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. American Review of Respiratory Disease 1993;147(4):887‐95. [PUBMED: 8466125] [DOI] [PubMed] [Google Scholar]
Lal 2012
- Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest 2012;141(6):1601‐10. [PUBMED: 22670023] [DOI] [PubMed] [Google Scholar]
Lavie 2015
- Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia‐‐revisited‐‐the bad ugly and good: implications to the heart and brain. Sleep Medicine Reviews 2015;20:27‐45. [PUBMED: 25155182] [DOI] [PubMed] [Google Scholar]
Lim 2004
- Lim J, Lasserson TJ, Fleetham J, Wright JJ. Oral appliances for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004435.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2007
- Lim W, Bardwell WA, Loredo JS, Kim EJ, Ancoli‐Israel S, Morgan EE, et al. Neuropsychological effects of 2‐week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized placebo‐controlled study. Journal of Clinical Sleep Medicine 2007;3(4):380‐6. [PUBMED: 17694727] [PMC free article] [PubMed] [Google Scholar]
Mador 2005
- Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest 2005;128(4):2130‐7. [PUBMED: 16236865] [DOI] [PubMed] [Google Scholar]
Marin 2005
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long‐term cardiovascular outcomes in men with obstructive sleep apnoea‐hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365(9464):1046‐53. [PUBMED: 15781100] [DOI] [PubMed] [Google Scholar]
Marshall 2008
- Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all‐cause mortality: the Busselton Health Study. Sleep 2008;31(8):1079‐85. [PUBMED: 18714779] [PMC free article] [PubMed] [Google Scholar]
Martinez‐Garcia 2005
- Martinez‐Garcia MA, Galiano‐Blancart R, Roman‐Sanchez P, Soler‐Cataluna JJ, Cabero‐Salt L, Salcedo‐Maiques E. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest 2005;128(4):2123‐9. [PUBMED: 16236864] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. NICE technology appraisal guidance 139. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. www.nice.org.uk/TA139 (accessed 10 January 2014).
Oksenberg 2009
- Oksenberg A, Arons E, Greenberg‐Dotan S, Nasser K, Radwan H. The significance of body posture on breathing abnormalities during sleep: data analysis of 2077 obstructive sleep apnea patients. Harefuah 2009;148(5):304‐9, 350‐1. [PUBMED: 19630360] [PubMed] [Google Scholar]
Parra 2011
- Parra O, Sanchez‐Armengol A, Bonnin M, Arboix A, Campos‐Rodriguez F, Perez‐Ronchel J, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. European Respiratory Journal 2011;37(5):1128‐36. [PUBMED: 20847081] [DOI] [PubMed] [Google Scholar]
Patel 2017
- Patel S, Kon SS, Nolan CM, Simonds AK, Morrell MJ, Man WD, et al. Minimum clinically important difference of the Epworth Sleepiness Scale. European Respiratory Journal 2017;50(Suppl 61):PA330. [DOI: 10.1183/1393003.congress-2017.PA330] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peppard 2000
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. New England Journal of Medicine 2000;342(19):1378‐84. [PUBMED: 10805822] [DOI] [PubMed] [Google Scholar]
Peppard 2013
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep‐disordered breathing in adults. American Journal of Epidemiology 2013;177(9):1006‐14. [PUBMED: 23589584] [DOI] [PMC free article] [PubMed] [Google Scholar]
Randerath 2011
- Randerath WJ, Verbraecken J, Andreas S, Bettega G, Boudewyns A, Hamans E, et al. Non‐CPAP therapies in obstructive sleep apnoea. European Respiratory Journal 2011;37(5):1000‐28. [PUBMED: 21406515] [DOI] [PubMed] [Google Scholar]
Ravesloot 2013
- Ravesloot MJ, Maanen JP, Dun L, Vries N. The undervalued potential of positional therapy in position‐dependent snoring and obstructive sleep apnea—a review of the literature. Sleep and Breathing 2013;17(1):39‐49. [PUBMED: 22441662] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ravesloot 2017
- Ravesloot MJL, White D, Heinzer R, Oksenberg A, Pepin JL. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta‐analysis. Journal of Clinical Sleep Medicine 2017;13(6):813‐24. [PUBMED: 28212691] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruehland 2009
- Ruehland Warren R, Rochford Peter D, O'Donoghue Fergal J, Pierce Robert J, Singh Parmjit, Thornton Andrew T. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 2009;32(2):150‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez 2009
- Sanchez AI, Martinez P, Miro E, Bardwell WA, Buela‐Casal G. CPAP and behavioral therapies in patients with obstructive sleep apnea: effects on daytime sleepiness, mood, and cognitive function. Sleep Medicine Reviews 2009;13(3):223‐33. [PUBMED: 19201228] [DOI] [PubMed] [Google Scholar]
Sarrell 2013
- Sarrell EM, Chomsky O, Shechter D. Treatment compliance with continuous positive airway pressure device among adults with obstructive sleep apnea (OSA): how many adhere to treatment?. Harefuah 2013;152(3):140‐4, 183‐4. [PUBMED: 23713371] [PubMed] [Google Scholar]
Sawyer 2011
- Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Medicine Reviews 2011;15(6):343‐56. [PUBMED: 21652236] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2000
- Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. American Journal of Respiratory and Critical Care Medicine 2000;162(2 Pt 1):566‐70. [PUBMED: 10934088] [DOI] [PubMed] [Google Scholar]
Senaratna 2017
- Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Medicine Reviews 2017;34:70‐81. [PUBMED: 27568340] [DOI] [PubMed] [Google Scholar]
Shahar 2001
- Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, et al. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study. American Journal of Respiratory and Critical Care Medicine 2001;163(1):19‐25. [PUBMED: 11208620] [DOI] [PubMed] [Google Scholar]
Shamsuzzaman 2002
- Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, et al. Elevated C‐reactive protein in patients with obstructive sleep apnea. Circulation 2002;105(21):2462‐4. [PUBMED: 12034649] [DOI] [PubMed] [Google Scholar]
Shneerson 2001
- Shneerson J, Wright JJ. Lifestyle modification for obstructive sleep apnoea. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002875] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2002
- Mason M, Welsh EJ, Smith I. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD003002.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sundaram 2005
- Sundaram S, Lim J, Lasserson TJ. Surgery for obstructive sleep apnoea in adults. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD001004.pub2] [DOI] [PubMed] [Google Scholar]
Vgontzas 1997
- Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. Journal of Clinical Endocrinology and Metabolism 1997;82(5):1313‐6. [PUBMED: 9141509] [DOI] [PubMed] [Google Scholar]
Wessendorf 2000
- Wessendorf TE, Teschler H, Wang YM, Konietzko N, Thilmann AF. Sleep‐disordered breathing among patients with first‐ever stroke. Journal of Neurology 2000;247(1):41‐7. [PUBMED: 10701896] [DOI] [PubMed] [Google Scholar]
Yaggi 2005
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. New England Journal of Medicine 2005;353(19):2034‐41. [PUBMED: 16282178] [DOI] [PubMed] [Google Scholar]


