Abstract
Background
Tyrosine kinase inhibitors (TKIs) are clinically effective in non-small cell lung cancer (NSCLC) patients harbouring epidermal growth factor receptor (EGFR) oncogene mutations. Genetic factors, other than EGFR sensitive mutations, that allow prognosis of TKI treatment remain undefined.
Methods
We retrospectively screened 423 consecutive patients with advanced NSCLC and EGFR 19del or 21L858R mutations. A total of 71 patients whose progression-free survivals (PFS) were shorter than 6 months or longer than 24 months were included and stratified into separate groups. Genetic background discrepancy was analysed in the two groups using next generation sequencing (NGS).
Findings
Sensitive EGFR mutations of 19del or 21L858R were detected by NGS in all patients; the 21L858R mutation was the major type. The most frequent accompanying somatic mutations were TP53, RB1, MAP2K. ALK fusion, MET amplification, and BRAF V600E were found only in the short PFS group. Concurrent pretreament T790 M mutation was found in both groups, but was proportionally higher in the short PFS group. In the short PFS group, patients had significantly more driver gene mutations than in long PFS group (P = 0·018). The numbers of concomitant somatic mutations, EGFR pathway-related mutations, and tumor mutation burden (TMB) were not significantly different between the two groups.
Interpretation
Co-occuring driver gene mutations were negative predictive factors of TKI therapy in EGFR-mutated patients. This study highlights the importance of exploring co-occuring genomic alterations before initiation of EGFR-TKIs.
Keywords: Non-small cell lung cancer, Epidermal growth factor receptor tyrosine kinase inhibitor, Predictive factor, Co-occurring mutations
Research in context.
Evidence before this study
We searched PubMed for the articles published up to October 2018 using the terms “non-small cell lung cancer (NSCLC)”, “epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI)”, “predictive factor”, and “co-occurring mutation”. We found that a few studies have investigated the associations between co-occurring somatic gene mutations and EGFR-TKI efficacy, with inconsistent results. Some studies indicated that concomitant somatic mutations were negative predictive factors of EGFR-TKI therapy, while the other studies indicated that the tumor-suppressor genes and oncogenes rather than the number of mutations were associated with poorer efficacy of EGFR-TKI. However, these relations have not been demonstrated in real world datasets.
Added value of this study
In this study, we collected two groups of EGFR positive patients with comparable clinical characteristics and different progression-free survival spans who were treated with first generation EGFR-TKIs. To the best of our knowledge, it is the first real-world cohort to demonstrate the relationship between co-occurring somatic gene mutations and EGFR-TKI efficacy. We investigated genetic discrepancy using next generation sequencing. We also compared the differences in mutation number, oncogenes, and tumor mutation burden between the two groups.
Implications of all the available evidence
Significantly more co-occurring oncogene mutations and higher T790 M to EGFR mutation abundance ratio were found in patients in the short PFS group. Our data confirmed the impact of co-occurring oncogenes in EGFR-TKI treatment. Thus, multiple oncogene mutations besides EGFR mutations could be tested prospectively to provide better predictions of EGFR-TKI efficacy and disease progression.
Alt-text: Unlabelled Box
1. Introduction
Tyrosine kinase inhibitors (TKIs) are clinically effective in non-small cell lung cancer (NSCLC) patients who have epidermal growth factor receptor (EGFR) oncogene mutations [1]. Among the variety of EGFR oncogenic mutations, in-frame microdeletions around the Leu-Arg-Glu-Ala (LREA) residues of exon 19, and the L858R substitution in exon 21 of EGFR comprise approximately 90% of all EGFR mutations detected in advanced NSCLC patients. The aforementioned mutations were described as sensitizing EGFR mutations and were reported to be predictive markers of tumor responses to EGFR-TKIs. During the last two decades, randomized trials have demonstrated a median progression-free survival (PFS) of 9·3–14·4 months in patients harbouring sensitizing EGFR mutations treated with first-generation EGFR-TKIs [[2], [3], [4], [5], [6]]. Despite the high efficacy of TKIs, some patients with EGFR-mutant lung cancer acquire resistance to the drug in <6 months, while others' PFS are longer than 24 months. As such, there may be factors other than EGFR mutations that contribute to disease progression. Although previous studies have investigated the association of concomitant genetic alterations with response and survival [[7], [8], [9], [10]], the results are conflicting.
We hypothesized that other molecular markers could contribute to the prediction of EGFR-TKI efficacy. To explore this hypothesis, we retrospectively screened our centre's patient database and performed next generation sequencing (NGS) of archival tissue from EGFR-mutant lung cancers patients. To better identify the differences between groups, we only selected patients with long or short PFS.
2. Materials and methods
2.1. Patient enrolment
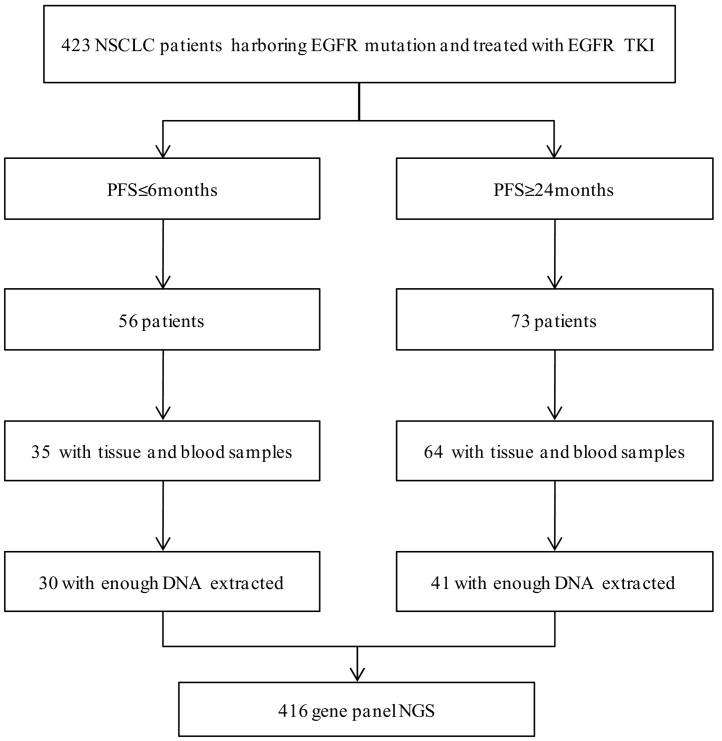
A total of 423 consecutive patients with advanced NSCLC (stage IIIB or IV) possessing 19del or 21L858R EGFR mutations who had received first generation EGFR-TKI therapy (gefitinib, erlotinib, or icotinib) at Peking Union Medical College Hospital between 2013 and 2018 were screened. Of these, 71 patients were included in the study.
All patients had been pathologically diagnosed with NSCLC. EGFR mutations were detected using an amplification refractory mutation system (ARMS). Patients were followed up regularly. Computed tomography (CT) of the thorax and abdomen were performed one month after the initiation of TKIs and every two months subsequently. Magnetic Resonance Imaging (MRI) was performed at baseline and every two months in patients with central nervous system (CNS) metastasis. Patients with symptomatic CNS metastasis or leptomeningeal metastasis were not included in the study. Objective response and progression of disease was assessed according to the response evaluation criteria in solid tumours (RECIST) 1·1 criteria [11]. Patients with PFS longer than 24 months or shorter than six months were included and stratified into two groups. PFS was calculated based on the start of EGFR-TKI therapy to disease progression or death. Overall survival (OS) was calculated from the start of EGFR-TKI therapy to death.
Demographic characters were reviewed based on patients' clinic record files. Age, sex, smoking status, clinical stage, Eastern Cooperative Oncology Group (ECOG) performance status (PS), and TKI treatment were included in analysis.
This study was approved by the Peking Union Medical College Hospital review board. Informed consent was obtained from all patients for use of tissue in genetic analysis. Pre-TKI treatment tumor tissue were collected and evaluated for NGS. Matched blood samples were collected as normal controls (Fig. 1). Genetic test using a panel of 416 cancer-related genes (Supplementary Table 1) were performed at Nanjing Shihe Jiyin Biotechnology Inc. (Jiangsu, China).
Fig. 1.
Flowchart of the study design.
NSCLC = non-small cell lung cancer. PFS = progression-free survival. EGFR = epidermal growth factor receptor. TKI = Tyrosine kinase inhibitors. NGS = next-generation sequencing.
3. Sequence analysis
3.1. Sample preparation
Tissue blocks with adequate tumor cellularity were selected by the pathologists who made the diagnosis. Formalin-fixed paraffin-embedded (FFPE) tissues from 10 to 15 unstained slides of 5-μm thick sections were prepared using PCR precautions. DNA was isolated and purified using the QIAamp DNA FFPE Kit (QIAGEN). Quantity of DNA was measured usingQubit 3·0 with a dsDNA HS Assay Kit (Life Technologies).
3.2. Library preparation and sequencing
Sequencing libraries were prepared using the KAPA Hyper Prep kit (KAPA Biosystems) with an optimised manufacturer's protocol. In brief, 1 μg of genomic DNA was sheared into fragments using the Covaris M220 instrument (Covaris), and then subjected to end-repairing, A-tailing, and ligation with indexed adapters sequentially, followed by size selection using Agencourt AMPure XP beads (Beckman Coulter). Finally, libraries were amplified by PCR and purified for target enrichment.
For target enrichment, indexed DNA libraries were pooled together for hybridization with customized biotinylated-DNA probes (GeneseeqOne, Nanjing Geneseeq Technology Inc.) targeting 416 cancer-relevant genes (exons and selected introns for fusion detection).
Enriched libraries were amplified and subjected for NGS on Illumina Hiseq4000 NGS platforms (Illumina) according to manufacturer's instructions. For blood samples, sequencing depth was at least 100× mean coverage by non-PCR duplicate read pairs. For tumor specimens, sequencing depth was 500× mean coverage by non-PCR duplicate read pairs.
3.3. Data processing
Trimmomatic was used for FASTQ file quality control (QC) [12]. Sequencingreads were mapped to the reference sequence Human Genome (hg19) using a Burrows-Wheeler Aligner (BWA-mem, v 0·7·12) [13]. Local realignment around indels and base quality score recalibration were applied using the Genome Analysis Toolkit (GATK 3·4·0) [14]. VarScan2 was employed for detection of somatic mutations [15]. We required VAF ≥2% (≥ 8 variant supporting reads) with somatic p-value = 0·1, minimum quality score = 20 and variant supporting reads mapped to both strands with strand bias no >10%. The mutation list was further filtered through an internally collected list (~2000 normal samples) of recurrent artifacts on the same sequencing platform. Mutations were also removed if they were present in >1% population frequency in the 1000 Genomes Project or 65,000 exomes project (ExAC). Annotation of mutations was performed using ANNOVAR [16]. Copy number variations (CNVs) were detected using ADTEx with default parameters. Genomic fusions were identified by FACTERAwith default parameters [17].
TMB was defined as the number of somatic, coding, base substitution, and indel mutations per megabase of genome examined. For our panel TMB calculation, all non-synonymous alterations and indels in the coding region of targeted genes were considered with the exception of known hotspot mutations in oncogenic drivers and truncations in tumor suppressors. The validation of TMB calculation using our panel is shown in Supplementary Fig. 1.
3.4. Statistical analysis
The Wilcoxon rank-sum test or χ2 test and Fisher's exact test was used to test differences in clinical and genetic parameters between patients with different PFS. Non-parametric tests were used to compare mutation number between groups. Survival curves were estimated using the Kaplan-Meier method. The statistical significance level was defined as two-sided P < 0·05. SPSS statistical software, version 19·0 (IBM Corp., Armonk, NY, USA) was used to perform statistical analysis. Forset plot was drawn by GraphPad Prism, version 8·0·2.
4. Results
4.1. Patient characteristics
Of the 71patients, 41 belonged to the long PFS group and 30 belonged to the short PFS group.The median PFS and OS in the two groups of patients were 36·3 months (95% CI 32·0–40·6) vs. 3·9 (95% CI 3·0–4·8) months, and 63·6 months (95% CI 57·4–69·8) vs. 19·4 months (95% CI 8·6–31·2) (Supplementary Fig. 2A and 2B). The median age of all the patients was 60 years(range, 36-78 years). The distributation of age, gender, smoking status, ECOG score, tumor stage, mutation types, and lines of TKIs were not significantly different between the two groups. Patients' clinical characteristics and EGFR mutations detected using ARMS sequencing are given in Table 1.
Table 1.
Baseline characteristics of patients in long and short PFS groups.
| Characteristic | No. of patients | Long PFS group | Short PFS group | P value |
|---|---|---|---|---|
| 71 | 41 | 30 | ||
| Age>65 years | 23 | 16 | 7 | 0·127 |
| Gender | 0·125 | |||
| Male | 23 | 10 | 13 | |
| Female | 48 | 31 | 17 | |
| Smoking status | 0·780 | |||
| Never smoker | 54 | 32 | 22 | |
| Ever smoker | 17 | 9 | 8 | |
| ECOG score | 0·304 | |||
| 0–1 | 67 | 40 | 27 | |
| ≥2 | 4 | 1 | 3 | |
| Clinical stage | 0·069 | |||
| IIIb | 5 | 5 | 0 | |
| IV | 66 | 36 | 30 | |
| EGFR mutations | 1·000 | |||
| 19del | 28 | 16 | 12 | |
| 21L858R | 43 | 25 | 18 | |
| TKI lines | 1·000 | |||
| 1st line | 59 | 34 | 25 | |
| ≥2nd line | 12 | 7 | 5 | |
| TKI treatments | 0·638 | |||
| Gefitinib | 53 | 29 | 24 | |
| Erlotinib | 13 | 9 | 4 | |
| Icotinib | 5 | 3 | 2 |
4.2. Mutations detected by NGS in all patients
Sensitive EGFR mutations of 19del or 21L858R were detected in all 71 patients. The EGFR mutation types detected by NGS were consistent with the result obtained through ARMS PCR, with the exception of two patients with co-occurring 19del and 21L858R mutations. The 21L858R mutation was the major EGFR mutation type in both groups (61% in the short PFS group vs. 60% in the long PFS group). The 19del mutations consisted of several different types. The most frequent 19del mutation type was del745/746-750 (41% in the short PFS group vs. 43% in the long PFS group), followed by del746insA, del747insS, S752fs, A750fs, and T751P. Complex EGFR mutations were found in 22 patients (31%), including 12 patients with complex exon20 T790M mutation and four patients with more than one EGFR-sensitive mutation of exons 19 or 21. The remaining five patients had co-occurring EGFR mutations of exon28 1142-1155del, exon20 R776H, exon18 E709K, exon18T710N, and exon18 L718 M. EGFR amplification was found in 18% patients. In the long PFS group, the most frequent accompanying somatic mutations were TP53 (51%), followed by MAP2K2 (15%), NKX2-1 (15%), CTNNB1 (15%), and RB1 (12%). In the short PFS group, the most frequent accompanying somatic mutations were TP53 (63%), followed by RB1 (17%), FAT1 (17%), and ABCB1 (13%) (Supplementary Fig. 3). We further did cluster analysis and gene ontology (GO) analysis of the 71 patients. The results were shown in Supplementary Fig. 4 and Supplementary Fig. 5.
4.3. Differences in mutations between two groups
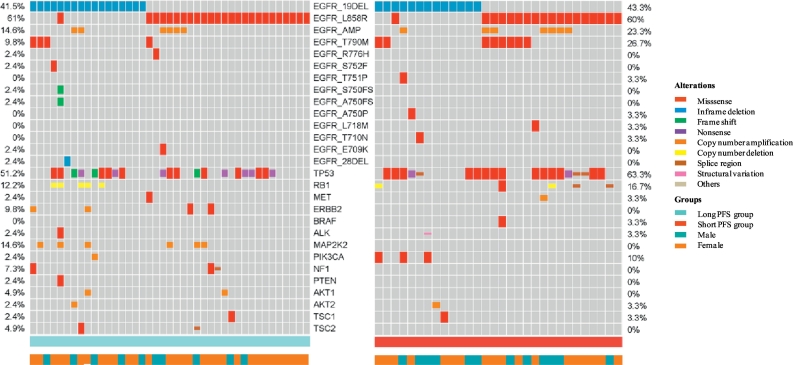
The percentage of 21L858R and 19del mutations were not significantly different between the two groups (P = 1·000). Patients in the long PFS group tended to have more exon19 deletion mutations of del745/746-750 (82% vs. 54% in the short PFS group, P = 0·121), fewer T790M mutations (10% vs. 27% in the short PFS group, P = 0·106), and fewer complex EGFR mutations (22% vs. 43% in the short PFS group; P = 0·071), but the differences were not statistically significant (Fig. 2).
Fig. 2.
Gene mutations in pretreated FFPE samples of patients in the two groups.
We further analysed T790M abundance in T790M-positive samples. Mean T790M frequency was 0·77% in the long PFS group and 9·15% in the short PFS group (P = 0·053). To minimise the impact of different proportions of cancer cells and genetic heterogeneity in each sample, we also compared the ratio of T790M alleles to EGFR sensitive mutation alleles. The result indicated that patients in the long PFS group had a relatively lower ratio (mean of 8·09% vs. 109·22% in the short PFS group; P = 0·037) (Supplementary Table 2).
TP53 mutations were the most frequent mutation in both groups, and the missense mutation occurred more often in the short PFS group (27% vs. 47% in the short PFS group, P = 0·131). The PIK3CA missense mutation was seen only in the short PFS group (P = 0·071), while the MAP2K2 mutations were seen only in the long PFS group (P = 0·036).
Co-occurring driver mutations [18] other than EGFR mutations were detected only in the short PFS group. Among them, one patient had ALK fusion gene, one patient had MET amplification,and one patient had BRAF V600E. Accompanying driver oncogene mutations were significantly prevalent in the short PFS group (10% vs. 33% in the short PFS group, P = 0·018).
Other genetic variants that might be associated with PFS were also analysed, including RB1, PIK3CA, EGFR amplification and BIM polymorphism, but no statistically significant differences could be found. The number of co-occurring somatic mutations detected by NGS were 7·63 ± 0·50 and 8·43 ± 0·65 (P = 0·314) in the long and short PFS groups, respectively. Among the somatic mutations, the mean number of EGFR pathway-related mutations were 1·51 ± 0·19 vs. 1·37 ± 0·18 (P = 0·652) in the two groups (Table 2).
Table 2.
Differences in genetic alterations between two groups detected using NGS.
| Long PFS group |
Short PFS group |
P |
|||
|---|---|---|---|---|---|
| No. | Percent (%) | No. | Percent (%) | ||
| 21L858R | 25 | 61% | 18 | 60% | 1·000 |
| 19 Del | 17 | 41% | 13 | 43% | 1·000 |
| Del746–750 | 14 | 82% | 7 | 54% | 0·121 |
| Complex EGFR mutations | 9 | 22% | 13 | 43% | 0·071 |
| T790M mutation | 4 | 10% | 8 | 27% | 0·106 |
| Co-occurring driver gene mutation | 4 | 10% | 10 | 33% | 0·018 |
| Number of mutations | 7.63 ± 0.50 | 8·43 ± 0·65 | 0·314 | ||
| EGFR pathway related mutations | 1.51 ± 0.19 | 1·37 ± 0·18 | 0·652 | ||
| Non EGFR pathway mutations | 4.83 ± 0.41 | 5·57 ± 0·59 | 0·337 | ||
| TP 53 mutations | 22 | 54% | 20 | 67% | 0·332 |
| TP53 missense mutation | 11 | 27% | 14 | 47% | 0·131 |
| RB1 mutations | 5 | 12% | 5 | 16% | 0·733 |
| PIK3CA missense mutation | 0 | – | 3 | 10% | 0·071 |
| MAP2K2 | 6 | 15% | 0 | – | 0·036 |
| EGFR amplification | 6 | 15% | 7 | 23% | 0·371 |
| BIM polymorphism | 4 | 10% | 4 | 13% | 0·714 |
The relationship between genetic heterogeneity and PFS were further analysed. In multivariate analysis, co-occurring driver mutations and MAP2K2 were independent predictive factors (Supplementary Fig. 6).
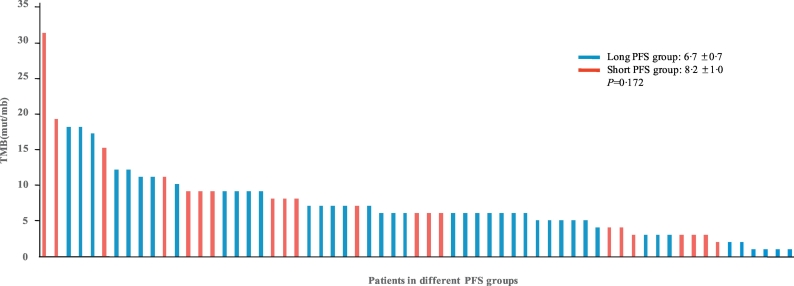
4.4. Tumor mutation burden (TMB) analysis
TMB for each patient was calculated. The mean TMB was 7·3 ± 5·2 mutations/Mb in the 71 patients. Mean TMBs for the two groups were 6·7 ± 0·7 for the long PFS group vs. 8·2 ± 1·0 for the short PFS group (P = 0·172). Patients with TMBs higher than eight mutations/Mb tended to fall into the short PFS group, where 29·3% of patients in the long PFS group vs. 50·0% of patients in the short PFS group had TMBs higher than eight mutations/Mb (P = 0·056) but the difference was not significant(Fig. 3).
Fig. 3.
Distribution of tumor mutation burden in the 71 patients.
TMB = tumor mutation burden. PFS = progression-free survival.
5. Discussion
EGFR mutations have long been recognized as the most important prognostic factor in advanced NSCLC patients in the era of EGFR-TKI. Despite the high efficacy of EGFR-TKI, patients harbouring EGFR mutations can have extremely short PFS, but means of identifying patients whose tumours do not respond well to these drugs are lacking. In this study, we collated two groups of advanced NSCLC patients harbouring EGFR mutations with different PFS spans and found the concurrent driver gene mutations to be more frequent in short PFS group. This finding indicates that the concurrent driver gene mutations might be a negative predictive factor. To the best of our knowledge, this is the first study comparing genetic differences between short and long PFS patients.
The recent wide spread use of NGS enables us a full view of genetic alteration in NSCLC. In several previous studies, concomitant somatic mutation and complex EGFR mutation have been reported to be predictive factors related to the PFS of patients treated with first generation TKI [7,9,19]. We profiled the genetic landscape of patients and explored for possible genomic alterations contributing to differences in TKI efficacy.
In our study, the clinical parameters in the two groups were not significantly different. Patients were mainly female and non-smoker, in accordance with previous reports. Mutation of exon 21 was the predominant mutation in both groups, which might because of the highly selection and limited number of patients. The high prevalence of complex EGFR mutations and high incidence of de novo T790M were observed in the study due to the selection of short PFS patients and the high sensitivity of the NGS method.
Acquired resistance of T790M was the most common resistance mutation in patients treated with first generation EGFR-TKIs. Additionally, previous studies suggested that the pre-existing T790M could present as a co-occurring mutation in a small population of EGFR positive NSCLC patients. The incidence of de novo T790M is 35%–60% in these patients [20,21]. Pretreatment T790M was reported to be a poor prognostic factor in NSCLC patients [[22], [23], [24]]. While using EGFR-TKI mono-therapy, shorter PFS was shown for patients with T790M than without [22]. The combined use of EGFR-TKI and bevacizumab might prolong PFS in the T790M-positive subgroup [25]. Our study also indicated that patients with short PFS tended to have more T790M mutations, although de novo T790M also existed in long PFS patients. The abundance of T790M was higher in the short PFS group and the T790M rate to EGFR was significantly higher in the short PFS group, which was also consistent with previous studies [26]. This may indicate that the abundance of T790M is important in predicting the efficacy of EGFR-TKIs, especially when using highly sensitive detection methods. And combination therapy with bevacizumab might be of choice in these patients [25].
Co-occurring driver gene mutations other than EGFR were found only in the short PFS group. These patients had ALK fusion, MET amplification, or BRAF V600E mutation aside from EGFR-sensitive mutations. Previous reports indicated that the co-occurring of driver mutations were rare [[27], [28], [29], [30], [31]] and were associated with resistance to TKI therapy [19,32]. In the current study, patients with co-occurring driver gene mutations were much more in the short PFS group than in the long PFS group (10% vs. 33%, P = 0·018), which suggests the co-occurrences of driver gene mutations might be the most important factor affecting clinical outcomes.
The reported incidence of concomitant ALK rearrangement and EGFR mutation was 1.3% to 1.6% [28,31]. And the coexistence of ALK rearrangement could be predictive of poor response in patients treated with EGFR-TKIs [33,34]. First line ALK-TKIs therapy might bring out a better outcome in these patients [32,35,36]. MET amplification is another known resistant mechanism of EGFR-TKI therapy. The baseline MET amplification in EGFR-mutant patients seems to be small (3.2%) [37]. These patients may response to MET inhibitors and crizotinib in previous reports [[38], [39], [40]]. Co-mutation of BRAF V600E was also rare. The experience of targeted therapy in these patients still needs to be defined. Due to the limited number of patients, all the experiences of treatment were from case reports. Moving forward, identifying the appropriate therapeutic strategy for co-occurring with ALK, MET, and BRAF mutations in EGFR-mutant NSCLC will require increasingly nuanced molecular screening strategies and well-designed clinical trials.
Several previous studies have investigated the association of concomitant genetic alterations with response and survival [[7], [8], [9], [10]]. These studies challenged the single driver oncogene view and revealed the potential function of co-occurring genetic alterations as co-drivers of tumor progression [10]. In the current study, the percentage of patients with co-occurring resistance mutations were higher in the short PFS group than in the long PFS group (P = 0·018). The result is consistent with previous studies and suggests that co-occurring driver gene mutations play an important role in tumor progression and drug resistance and might be a very important factor affecting clinical outcomes.
Co-existence of somatic gene mutations was also analysed. The results indicated that concomitant mutation of TP53 was the most frequent somatic mutation in advanced NSCLC [32,41], in line with previous reports [42]. The missense mutation of TP53 was more prevalent in the short PFS group, but the difference between the two groups was not significant.
RB1 is a tumor suppressor gene, whose mutations occurs in a variety of cancers and emerges as a cause of acquired resistance to EGFR antagonists [43]. Niederst et al. had described the association between RB1 loss and the transformation to SCLC in EGFR-mutant NSCLC [44]. Aurora B kinase inhibitor was reported to be efficacious in RB1 loss SCLC models [43]. But the impact of pretreatment RB1 in NSCLC was unknown. In previous report, the median interval between the initiation of EGFR-TKI to small cell lung cancer (SCLC) transformation was 15·8 months [45]. Thus, we speculated that the RB1 loss might be a relatively indolent mutation. In our study, RB1 were found in both groups. No different of frequency were seen between the two groups. Specified treatments for these patients need further clinical researches and investigations.
MAP2K2 amplification was found only in the long PFS group (P = 0·036), but we were unable to conclusively demonstrate its positive prognostic value due to the limited number of cases.
We further compared the occurance of concomitant mutations, the number of genes in the EGFR-related pathway and the number of total somatic gene mutations between the two groups, but found no significant differences, in contrast to previous studies [7]. This indicated that co-occurring somatic mutations that were not driver mutations were not strong predictive factors for patients treated by EGFR-TKIs.
In addition, our study analysed the differences of concomitant mutation number and TMB in the two groups. The result showed that the short PFS group had more patients whose TMB was higher than eight. However, neither the mean number of mutations nor the TMB level were significantly different between the two groups. There are several possible mechanisms for this. TMBs of patients developing T790M resistance were lower, and TMB was not affected in patients with SCLC transformation after developing TKI resistance [46]. This suggests an explicit resistance gene or mechanism serving as a single oncogene and leading to lower heterogeneity of lung cancer. In our study, the incidence of pretreatment-resistant mutations, including T790M, was high in the short PFS group; this may have contributed to the lower mean TMB. However, this hypothesis requires additional further study for confirmation.
There are several limitations in this study. First, since this was a retrospective analysis with all patients at advanced stages of disease, the number of patients and contributing tissue samples were limited. Only approximately 55% of the patients had sufficient DNA extracted for NGS. Second, we included patients receiving different lines of TKI treatment. The last, due to the insufficiency of remaining tissue, PD-L1 immunohistochemistry was not performed. As conflicting data are present in the literature on the prognostic role of TMB and concomitant somatic mutations in EGFR-positive NSCLC [7,41], additional prospective studies including more patients should be performed.
In conclusion, our study highlights the co-occurring driver gene mutations, which were significantly more frequent in short PFS group, may serve as important negative predictive factors for TKI therapy in patients with EGFR 19del or 21L858R mutations. A high T790M ratio to EGFR mutation ratio rather than existence of T790M predicted a shorter PFS. Co-occurring genomic alterations as well as EGFR sensitive mutations should be detected prior to treatment to identify subgroups of patients with less favourable outcomes of TKI therapy.
Acknowledgments
Acknowledgement
We thank the patients for providing their samples in this study. We acknowledge Xiaolei Li (Nanjing Geneseeq Technology Inc.) for technical support. We also thank Xiaoyan Liu (Department of Respiratory and Critical Care Medicine, Peking Union Medical College Hospital) and Hua Chen (Department of Rheumatology, Peking Union Medical College Hospital) for surpporting bioinformatic analysis.
Funding sources
The study was supported by National Natural Science Foundation of China (Grant No. 81702292) and CAMS Innovation Fund for Medical Sciences (Grant No. 2018-I2M-1-003).
Declaration of interests
The authors declare no potential conflicts of interest.
Author contributions
MW designed the study. MW and MC contributed to data analysis and preparation of the report. YB, YX, JZ, and LZ collected the samples assembled the clinical data. WZ conducted the statistical analysis. All authors read and approved the final manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.03.023.
Appendix A. Supplementary data
Supplementary material
References
- 1.Lynch T.J., Bell D.W., Sordella R. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non- small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.MaemondoM Inoue A., Kobayashi K. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Rosell R., Carcereny E., Gervais R. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C., Wu Y.L., Chen G. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open- label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 5.Mitsudomi T., Morita S., Yatabe Y. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 6.Yan X., Wang H., Li P. Efficacy of first-line treatment with epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) alone or in combination with chemotherapy for advanced non-small cell lung cancer (NSCLC) with low-abundance mutation. Lung Cancer. 2019;128:6–12. doi: 10.1016/j.lungcan.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Hong S., Gao F., Fu S. Concomitant genetic alterations with response to treatment and epidermal growth factor receptor tyrosine kinase inhibitors in patients with EGFR-mutant advanced non-small cell lung cancer. JAMA Oncol. 2018 doi: 10.1001/jamaoncol.2018.0049. [Epub ahead of print] PubMed PMID: 29596544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Y., Shi X., Zhao J. Mechanisms of primary resistance to EGFR targeted therapy in advanced lung adenocarcinomas. Lung Cancer. 2018;124:110–116. doi: 10.1016/j.lungcan.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z., Cheng Y., An T. Detection of EGFR mutations in plasma circulating tumour DNA as a selection criterion for first-line gefitinib treatment in patients with advanced lung adenocarcinoma (BENEFIT): a phase 2, single-arm, multicentre clinical trial. Lancet Respir Med. 2018;6(9):681–690. doi: 10.1016/S2213-2600(18)30264-9. Epub 2018 Jul 17.Erratum in: Lancet Respiratory Medicine 2018;6(9):e50. PubMed PMID: 30017884. [DOI] [PubMed] [Google Scholar]
- 10.Blakely C.M., Watkins T.B.K., Wu W. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet. 2017;49(12):1693–1704. doi: 10.1038/ng.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H., Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePristo M.A., Banks E., Poplin R. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koboldt D.C., Zhang Q., Larson D.E. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22(3):568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K., Li M., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-through put sequencing data. Nucleic Acids Res. 2010;38(16) doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman A.M., Bratman S.V., Stehr H. FACTERA: a practical method forthe discovery of genomic rearrangements at breakpoint resolution. Bioinformatics. 2014;30(23):3390–3393. doi: 10.1093/bioinformatics/btu549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Comprehensive Cancer Network. (NCCN) 2019. Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer, Version 1. [DOI] [PubMed] [Google Scholar]
- 19.Yu H.A., Suzawa K., Jordan E. Concurrent alterations in EGFR-mutant lung cancers associated with resistance to EGFR kinase inhibitors and characterization of MTOR as a mediator of resistance. Clin Cancer Res. 2018;24(13):3108–3118. doi: 10.1158/1078-0432.CCR-17-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe M., Kawaguchi T., Isa S. Ultra-sensitive detection of the pretreatment EGFR T790M mutation in non-small cell lung cancer patients with an EGFR-activating mutation using droplet digital PCR. Clin Cancer Res. 2015;21:3552–3560. doi: 10.1158/1078-0432.CCR-14-2151. [DOI] [PubMed] [Google Scholar]
- 21.Chen L.-Y., Molina-Vila M.A., Ruan S.-Y. Coexistence of EGFR T790M mutation and common activating mutations in pretreatment non-small cell lung cancer: a systematic review and meta-analysis. Lung Cancer. 2016;94:46–53. doi: 10.1016/j.lungcan.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Su K.Y., Chen H.Y., Li K.C. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30(4):433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- 23.Rosell R., Molina M.A., Costa C. Pretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutations. Clin Cancer Res. 2011;17(5):1160–1168. doi: 10.1158/1078-0432.CCR-10-2158. [DOI] [PubMed] [Google Scholar]
- 24.Costa C., Molina M.A., Drozdowskyj A. The impact of EGFR T790M mutations and BIM mRNA expression on outcome inpatients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in therandomized phase III EURTAC trial. Clin Cancer Res. 2014;20(7):2001–2010. doi: 10.1158/1078-0432.CCR-13-2233. [DOI] [PubMed] [Google Scholar]
- 25.Rosell R., Dafni U., Felip E. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFRmutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir Med. 2017;5(5):435–444. doi: 10.1016/S2213-2600(17)30129-7. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Cai W., Yang G. Comprehensive analysis of EGFR-mutant abundance and its effect on efficacy of EGFR TKIs in advanced NSCLC with EGFR mutations. J Thorac Oncol. 2017;12(9):1388–1397. doi: 10.1016/j.jtho.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Brega E., Brandao G. Non-small cell lung carcinoma biomarker testing: the pathologist's perspective. Front Oncol. 2014;4:182. doi: 10.3389/fonc.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J.J., Zhang X.C., Su J. Lung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylation. Clin Cancer Res. 2014;20(5):1383–1392. doi: 10.1158/1078-0432.CCR-13-0699. [DOI] [PubMed] [Google Scholar]
- 29.Lee J.K., Kim T.M., Koh Y. Differential sensitivities to tyrosine kinase inhibitors in NSCLC harboring EGFR mutation and ALK translocation. Lung Cancer. 2012;77(2):460–463. doi: 10.1016/j.lungcan.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Li S., Li L., Zhu Y. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer. 2014;110(11):2812–2820. doi: 10.1038/bjc.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulivi P., Chiadini E., Dazzi C. Nonsquamous, non-small-cell lung cancer patients who carry a double mutation of EGFR, EML4-ALK or KRAS: frequency, clinical-pathological characteristics, and response to therapy. Clin Lung Cancer. 2016;17:384–390. doi: 10.1016/j.cllc.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Lin L., Bivona T.G. Mechanisms of resistance to epidermal growth factor receptor inhibitors and novel therapeutic strategies to overcome resistance in NSCLC patients. Chemother Res Pract. 2012;2012:817297. doi: 10.1155/2012/817297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galetta D., Catino A., Misino A. Concomitant EGFR mutations/ALK rearrangements: beyond a simple dual target. Transl Lung Cancer Res. 2016;5:143–144. doi: 10.3978/j.issn.2218-6751.2016.01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo Russo G., Imbimbo M., Corrao G. Concomitant EML4-ALK rearrangement and EGFR mutation in non-small cell lung cancer patients: a literature review of 100 cases. Oncotarget. 2017;8(35):59889–59900. doi: 10.18632/oncotarget.17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Won J.K., Keam B., Koh J. Concomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitor. Ann Oncol. 2015;26:348–354. doi: 10.1093/annonc/mdu530. [DOI] [PubMed] [Google Scholar]
- 36.Lee T., Lee B., Choi Y.L. Non-small cell lung cancer with concomitant EGFR, KRAS, and ALK mutation: clinicopathologic features of 12 cases. J Pathol Transl Med. 2016;50:197–203. doi: 10.4132/jptm.2016.03.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai G.G.Y., Lim T.H., Lim J. Clonal MET amplification as a determinant of tyrosine kinase inhibitor resistance in epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol. 2019 doi: 10.1200/JCO.18.00177. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Li Y., Zhang R., Zhou Y. Combined use of crizotinib and gefitinib in advanced lung adenocarcinoma with leptomeningeal metastases harboring MET amplification after the development of gefitinib resistance: a case report and literature review. Clin Lung Cancer. 2018 doi: 10.1016/j.cllc.2018.12.004. https://www.ncbi.nlm.nih.gov/pubmed/30638795. pii: S1525-7304(18)30328-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Ahn M.J., Han J.Y., Sequist L.V. OA 09.03 TATTON Ph Ib expansion cohort: osimertinib plus savolitinib for pts with EGFR-mutant MET-amplified NSCLC after progression on prior EGFR-TKI. J Thorac Oncol. 2017;12(11) Supplement 2:S1768. [Google Scholar]
- 40.Gainor J.F., Niederst M.J., Lennerz J.K. Dramatic response to combination erlotinib and crizotinib in a patient with advanced, EGFR-mutant lung cancer harboring De novo MET amplification. J Thorac Oncol. 2016;11(7):e83–e85. doi: 10.1016/j.jtho.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 41.Jiao X.D., Qin B.D., You P., Cai J., Zang Y.S. The prognostic value of TP53 and its correlation with EGFR mutation in advanced non-small cell lung cancer, analysis based on cBioPortal data base. Lung Cancer. 2018;123:70–75. doi: 10.1016/j.lungcan.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y., Tong X., Yan J., Wu X., Shao Y.W., Fan Y. Short-term responders of non-small cell lung cancer patients to EGFR tyrosine kinase inhibitors display high prevalence of TP53 mutations and primary resistance mechanisms. Transl Oncol. 2018;11(6):1364–1369. doi: 10.1016/j.tranon.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oser M.G., Fonseca R., Chakraborty A.A. Cells lacking the RB1 tumor suppressor gene are hyperdependent on Aurora B kinase for survival. Cancer Discov. 2018 doi: 10.1158/2159-8290.CD-18-0389. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niederst M.J., Sequist L.V., Poirier J.T. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun. 2015;6:6377. doi: 10.1038/ncomms7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcoux N., Gettinger S.N., O'Kane G. EGFR-mutant adenocarcinomas that transform to small-cell lung cancer and other neuroendocrine carcinomas: clinical outcomes. J Clin Oncol. 2019;37(4):278–285. doi: 10.1200/JCO.18.01585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Offin M., Rizvi H., Tenet M. Tumor mutation burden and efficacy of EGFR-tyrosine kinase inhibitors in patients with EGFR-mutant lung cancers. Clin Cancer Res. 2018 doi: 10.1158/1078-0432.CCR-18-1102. [Epub ahead of print] PubMed PMID: 30045933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material