Abstract
Bone tumours around the elbow are rare. Even nowadays diagnostic dilemmas and delays are common. During recent decades the management and prognosis of patients with elbow bone tumours has improved significantly.
Benign tumours can be treated using minimally invasive procedures, whereas malignant ones require a multidisciplinary team approach based on an adjuvant therapeutic regimen of chemotherapy, radiotherapy and limb salvage procedures.
This article reviews the most commonly encountered elbow bone tumours and their management.
Cite this article: EFORT Open Rev 2019;4:133-142. DOI: 10.1302/2058-5241.4.180086
Keywords: benign, bone tumour, elbow, malignant
Introduction
Bone tumours around the elbow are rare and their incidence is approximately 1%.1 The literature regarding primary bone tumours of the elbow is sparse, with only two case series consisting of 75 patients and 25 patients respectively.2,3 During recent decades advances in the diagnosis, management and prognosis of patients with bone tumours around the elbow have been made. Early diagnosis and preoperative planning is essential and can dramatically change the treatment and prognosis of these patients.
Elbow tumours pose a diagnostic challenge for orthopaedic surgeons. Physical examination and a thorough history are the cornerstones of diagnosis. Patients usually present with persistent, unexplained, non-mechanical rest pain, soft tissue swelling, change in size of the mass, fever, night sweats and chills, which would warrant a higher level of suspicion for malignancy.4 Diagnostic imaging is an important component of the workup of a patient with a musculoskeletal tumour and should proceed in an organized fashion.
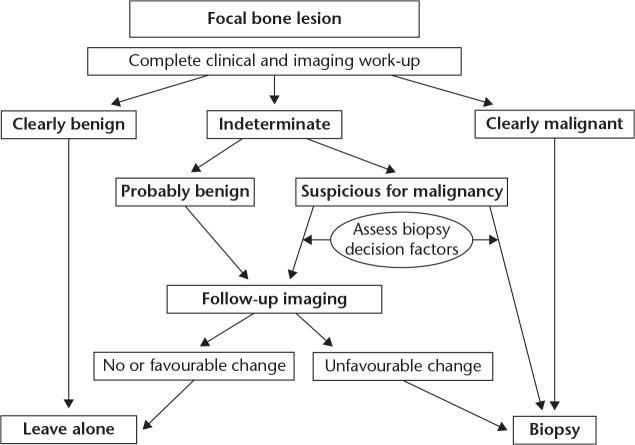
Patients with presence of bone lysis, cortical erosion, new bone formation, mineralization or periosteal reaction in plain radiographs of the elbow should have additional workup.5 Magnetic resonance imaging (MRI) is crucial in providing information regarding the location, size, tissue characteristics of the lesion and involvement of peripheral neurovascular structures. Other diagnostic modalities such as computerized tomography (CT) and bone scans are only performed in cases of lesions with particularly aggressive features. For bone lesions with such worrisome and aggressive imaging features, a histologic specimen should be obtained for diagnosis. A biopsy with a fine needle aspiration (FNA) or core needle biopsy, may be performed under CT or ultrasound guidance to confirm the diagnosis. However, these techniques may not yield sufficient tissue, thus an open biopsy with immunohistochemical stains and/or molecular studies may be required. An inappropriate or inaccurate biopsy may lead to poor outcome regarding limb salvage and even survivorship of the patient.6 Even nowadays delay in diagnosis is common, usually because of the rarity of these lesions, the atypical clinical presentation and the low index of suspicion, with misdiagnosis incidence up to 13%.2 Although these entities are rare, the treating physician must be aware of the possibility of a bone tumour in the elbow area. An algorithm for appropriate assessment of patients with a bone lesion is presented (Fig. 1).7
Fig. 1.
Algorithm for patients presenting with a bony lesion around the elbow.
A multidisciplinary team approach should include an orthopaedic oncologist, an interventional radiologist, a pathologist, an oncologist, a vascular surgeon, and a plastic surgeon. Nowadays, benign tumours around the elbow such as juxta-articular osteoid osteoma (ΟΟ) can be treated with minimally invasive techniques such as CT-guided percutaneous radiofrequency thermal ablation (RFA) or arthroscopic excision.8 Moreover, the management and prognosis of patients with malignant tumours, such as Ewing sarcoma and osteosarcoma, have improved thanks to the adjuvant chemotherapeutic protocols and improved radiation therapy techniques combined with ‘en bloc’ resection of the tumour and various limb salvage procedures and reconstructions with total elbow arthroplasties, megaprostheses, allografts, vascularized autografts, or allograft-prosthetic composite reconstructions.9 However, reconstruction of the elbow poses a unique challenge with limited options described in the literature. The elbow joint is a complex interplay between multiple joints which need to be stabilized for the optimal wrist and hand functional outcome and sometimes it is challenging to achieve ‘safe’ oncological margins.
Benign lesions are more common than malignant ones. They usually affect the proximal ulna and radius.2 The commonest benign tumours around the elbow joint are the osteoid osteoma, the giant cell tumour, the aneurysmal bone cyst and the fibrous dysplasia. Ewing sarcoma, osteosarcoma and chondrosarcoma of the elbow are the most common malignant tumours, and occur more frequently in older patients with the distal humerus more often affected.3 In a recent case series, these rare tumours continue to have significant morbidity and mortality, with recurrences which resulted in further surgery in over a quarter of the patients with a benign lesion, while the five-year mortality for the high grade malignancies was 68%.2 This article summarizes the current diagnosis and treatment of these tumours around the elbow and discusses some of the features that are unique to this anatomic area.
Benign bone tumours
Osteoid osteoma
Osteoid osteoma is not so sporadic in the elbow; however, its intra-articular location is rare.10–12 The typical age of presentation is between 7 and 30 years, but it may also be diagnosed in middle-aged and elderly patients. Symptoms at the elbow can last from weeks to years prior to diagnosis and meanwhile patients may usually be treated for other conditions. The average delay of diagnosis may be up to 2 years.13 Patients present with the characteristic clinical feature of pain mainly at night that usually subsides after administration of non-steroidal anti-inflammatory drugs (NSAIDs) or aspirin, along with swelling and tenderness. Some patients, though, may also present with non-specific clinical symptoms of joint effusion and synovitis and some degree of flexion contracture, instead of the characteristic nocturnal pain responsive to salicylates.14,15 In radiographic examination, osteoid osteoma presents as an intracortical radiolucent nidus surrounded by a rim of dense reactive bone. Thin-section (0.5 to 2.0 mm) CT with multiplanar reconstructions is the diagnostic gold standard to confirm the benign nature of the reactive bone and to identify the nidus of the lesion.16 Osteoid osteoma is usually smaller than 1.5–2.0 cm.10–12 Bone scintigraphy may show intense isotope uptake in these lesions. The role of MRI in the evaluation of osteoid osteoma is controversial. A constant finding on MRI scan is a marked bone marrow oedema corresponding to the highly vascularized mesenchymal tissue which is reported to be observed in all patients.16–18
Whereas some cases are self-limiting, surgical treatment options include intralesional curettage or radiofrequency ablation. The ‘en bloc’ resection and curettage of the lesion is the recommended treatment for juxta- or intra-articular osteoid osteoma of the elbow.15,19 Nowadays, complete excision of juxta-articular osteoid osteoma of the elbow may also be performed arthroscopically.20 Recently, Kamrani et al treated osteoid osteoma of the elbow through arthroscopic ablation in 10 patients.21 Arthroscopic excision of the lesion was performed at a mean of 23 months (range, 12–36 months) after initial symptoms. Postoperative elbow flexion–extension range of motion (ROM) (129° ± 5°) was statistically significantly higher than the preoperative (80° ± 12°). Moreover, the Mayo Elbow Performance Score and the visual analogue scale for the elbow and wrist were significantly higher compared with these before surgery (P < .001). The authors stated that arthroscopic ablation is a safe and efficient method of treatment for osteoid osteoma of the elbow, without the need for capsulectomy or intraoperative manipulation to treat the limitation of elbow ROM, and it has a relatively shorter rehabilitation time.21 Currently, percutaneous ablation under CT guidance, for lesion localization, with radiofrequency thermal ablation (RFA) is the most effective treatment option with a success rate of 87% to 100%.22 Radiofrequency thermal ablation has gained popularity as a cost-effective, minimally invasive method with lower morbidity and fewer complications compared to an open technique.23 However, this technique presents a high risk for bone necrosis and soft tissue damage, especially in tumours localized at the anterior aspect of the elbow joint and near (within approximately 1.5 cm) neurovascular structures.15,22,24 Moreover, local destruction without preserving the pathologic tissue for histological examination limits its indication in patients with unusual clinical presentation.25 Albisinni et al treated 27 patients (13 cases located in the humerus, 13 in the ulna and one in the radius) with intracapsular osteoid osteoma of the elbow by CT-guided percutaneous RFA.26 All patients were assessed in terms of function, successful eradication and complication rate. Twenty-five out of 27 patients (92.5%) presented with excellent functional results as their Mayo Elbow Performance Scores ranged from 90 to 100 points at final follow-up. Osteoid osteoma recurred in only one patient (3.7%) five months after the initial procedure and was successfully retreated using RFA. No major complications were observed and all patients were disease free at the final follow-up. However, the authors stated that this invasive treatment requires meticulous planning and technique application to minimize potential risks for the patient.26
Giant cell tumour
Another benign tumour but with aggressive behaviour encountered in the region of the elbow is the giant cell tumour (GCT). This tumour occurs mainly after skeletal maturity and has its peak incidence in the third and fourth decades of life.27 Most giant cell tumours are located within the epiphyses of long bones, they often extend to the articular subchondral bone or the cartilage, but they rarely invade the adjacent joint or its capsule. In skeletally immature patients they are often located into the metaphysis.28
Pain in the elbow is the most common symptom, while swelling and deformity are associated with large lesions. Histologically GCT is characterized by the presence of multinucleated giant cells (osteoclast-like cells), neoplastic stromal cells which are the predominant proliferating cell population and secondarily recruited mononuclear histiocytic cells.29 Giant cell tumours were initially classified by Enneking and later by Campanacci, based on radiographic appearance.30,31 Three stages were described – Stage I (latent), Stage II (active), Stage III (aggressive) – which correlate with tumour aggressiveness and risk of local recurrence. Radiographically, they usually appear as an eccentric epiphyseal or metaphyseal lytic lesion with cortical thinning and a ‘soap bubble’ appearance.32
Surgery with complete ablation to prevent recurrence and preserve the joint articulation remains the mainstay of treatment.33 Local recurrence has been found to be a risk factor for pulmonary metastasis, which occurs in approximately 2% to 9% of patients.34 The key in order to ensure an adequate curettage and complete excision of the tumour is to obtain sufficient exposure of the lesion with a large cortical ‘window’.35 Curettage of the bone cavity with high-speed burr or drill and the use of adjuvant cryosurgery (liquid nitrogen or a closed system of argon and helium) is recommended. The void is filled with bone graft or polymethyl-methacrylate (PMMA) bone cement. The use of internal fixation devices is controversial. Although early mobilization is facilitated with internal fixation, postoperative follow-up for tumour recurrence is more difficult.36–38 Giant cell tumour recurrence rates vary significantly between different centres, different methods (wide resection, curettage +/- burr +/- phenol, +/- PMMA) and the local presentation of the tumour, ranging from 0% to 65%, therefore close follow-up with serial imaging is mandatory with these benign aggressive tumours.39–41 In patients with highly aggressive lesions or local recurrence, where the tumour may invade through the cortex of the distal humerus to the surrounding soft tissue structures, curettage is unlikely to be effective and thus preserving the joint congruity of the elbow may not be possible. Nowadays, where it is not possible for the joint to be preserved, wide resection and total elbow arthroplasty using a custom-made prosthesis with good soft tissue coverage is a viable option, as it provides good pain relief and functional improvement with lower complication rates.42–44 In addition to skeletal reconstruction, of equal importance is to achieve good soft tissue coverage for both the implant and the elbow, as well as to preserve elbow function. Following tumour excision, hemi-articular and total elbow allografts have been used for reconstruction of these defects, but high complication rates were reported and thus these techniques are reserved as salvage procedures following failed total elbow arthroplasty.45
In light of current molecular biological understanding regarding the implication of the RANKL molecular pathway in the pathogenesis of GCT of bone, systemic targeted therapy has been advocated. In cases of locally advanced, unresectable, recurrent and/or metastatic CGT, the use of denosumab as a RANKL inhibitor has been introduced in order to facilitate surgery at a later stage, by making the tumour resectable or even appropriate for curettage.46 Many recent studies have shown significant clinical benefits regarding the use of denosumab in the treatment of GCT, leading to a surgical down-staging and demonstrating an objective response range from 86% to 100% of cases.47–50
Aneurysmal bone cyst
Another benign lesion of the elbow causing pain and swelling is the aneurysmal bone cyst (ABC). This tumour occurs mainly in patients under 20 years of age and may present either as a primary bone lesion (70% of cases), when no precursor bone lesion is identified or as a secondary bone lesion (30% of cases) when a pre-existing osseous lesion can be identified.51 In the Mayo Clinic’s experience, only eight examples of aneurysmal bone cysts were found in the elbow region.37 It is an osteolytic bone neoplasm characterized by several sponge-like blood or serum-filled, generally non-endothelialized spaces of various diameters.37 Regarding its aetiology, theories range from a post-traumatic reactive vascular malformation to a genetic predisposed bone tumour.52,53 The formation of an arteriovenous fistula within bone, caused by increased venous pressure and resultant dilation and rupture of the local vascular network has been the most common theory over the long term.54,55 However, studies have also demonstrated the clonal neoplastic nature of the cyst.56
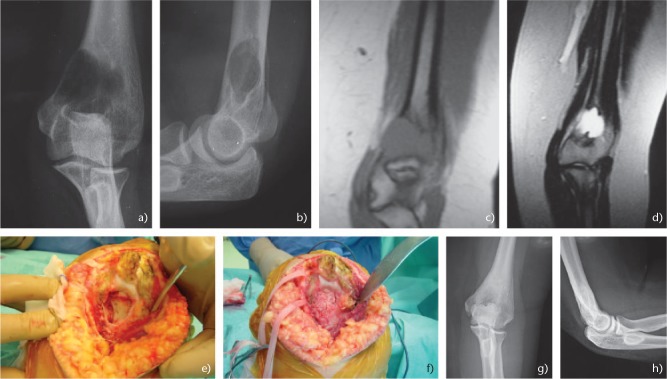
Patients usually present with pain, swelling, enlarging mass and even a pathologic fracture in the elbow area. The symptoms are usually presented for several weeks to months before the diagnosis. Neurologic symptoms may also develop secondary to pressure or tenting of the nerve structures in the elbow area. Radiographically, ABC usually presents as a metaphyseal eccentric lesion, that may elevate the periosteum and progressively cause erosion of the cortex. These tumours may be confused with malignancy, as imaging studies, even CT scan and MRI, do not always provide clear diagnostic criteria for the diagnosis. Nevertheless, the zone of rarefaction is usually well circumscribed, eccentric, and is associated with an obvious soft tissue extension (Fig. 2a–h).57 Differential diagnosis of ABC includes unicameral bone cyst, eosinophilic granuloma and giant cell tumour.58
Fig. 2.
(a) Anteroposterior and (b) lateral radiographs of the elbow showing an aneurysmal bone cyst of the distal humerus in 17-year-old female. (c) Τ1 and (d) T2 magnetic resonance images of the elbow. (e) Intraoperative images showing the gross destruction of the distal humerus. (f) The lesion was treated with curettage and bone grafting. (g) Anteroposterior and (h) lateral radiographs of the elbow 11 years postoperative showing a good incorporation of the graft and no sign of recurrence.
Curettage of the cyst remains the gold standard for treatment and it is usually curative. Local recurrence rates after curettage and polymethyl-methacrylate (PMMA) bone cement and curettage and bone grafting are reported at 17% and 37% respectively.54 Although wide resection of the lesion can lessen recurrence rates, these treatments require complicated reconstructive procedures and are not generally indicated in long bones.51,55 A plethora of new therapies has been proposed for the treatment of ABC which still remains controversial. New methods include embolization with sclerotherapy regiments based on an alcoholic solution of zein and intralesional implantation of demineralized bone particles with promising results; however, because of the serious side effects, they are mainly used in cases where the extent of the cyst makes the operative intervention hazardous.59,60 Modern sclerotherapy treatment utilizes polidocanol, which is regarded as a safe regiment with no serious side effects. Rastogi et al reported a healing rate of 97% in a case series of 72 patients treated with percutaneous intralesional injections of polidocanol, whereas Varshney et al reported that while sclerotherapy was equally effective to intralesional excision, it was accompanied by less morbidity.61,62 Therefore, due to the promising results, sclerotherapy is advocated in many centres as the treatment of choice. Reddy et al described the curopsy, a percutaneous limited curettage during biopsy, as another minimally invasive technique. The authors removed the lining membrane from various areas of the lesion and reported a healing rate of 81%. Although having an inferior success rate compared to curettage, the technique has a considerably faster recovery time, is safe, efficient and has good functional outcomes.63
Fibrous dysplasia
Fibrous dysplasia (FD) is a rare disease which typically occurs in spine, ribs, scull and diaphysis of long bones and accounts approximately for 5% to 7% of all benign bone tumours. However, in a large series of 75 patients FD was the most common (20% of cases) benign tumour encountered in the elbow.2 It commonly presents in adolescents and young adults, and may be either monostotic (70% to 80% of cases) or polyostotic.64,65 Most monostotic lesions are asymptomatic and are discovered when plain radiographs of the involved region are made for other reasons or because a pathological fracture has developed.66 At first presentation about 67% of patients may have pain at the site of the lesion and up to 20% of patients may have a pathological fracture at presentation.67 It may occasionally affect the structural integrity of the affected area and thus result in a bowing deformity.65 Histologically, FD is considered to be the result of excessive proliferation of fibrous tissue within the bone marrow, due to poorly differentiated mutated osteoblasts. The osteoblasts then produce a high amount of interleukin 6, resulting in significant osteoclastic activity, which consequently leads to the formation of lytic lesions within the fibrous tissue and surrounding normal bone.68 In radiographic examination FD usually has a characteristic radiolucent ‘ground-glass’ appearance with well-defined thick sclerotic borders. At times, calcified cartilaginous foci may also be evident within the lesion.65
When the diagnosis is confirmed by a biopsy, most lesions around the elbow can be treated non-operatively with immobilization in a cast. Non-steroidal anti-inflammatory drugs, opioids and bisphosphonates have been used to treat patients reporting bone pain, with the most favourable outcomes detected in individuals treated with bisphosphonates, mainly pamidronate.69,70 Although most lesions respond well to non-operative treatment, there are a few indications for surgery, including non-union after a pathologic fracture, persistent pain and severely progressive deformity. Intralesional curettage and bone allograft or vascularized bone graft, with or without internal fixation, have been used, whereas in cases with severe bone deformity corrective osteotomies and rigid internal fixation have satisfactory results and no major complications.67,71,72 Fibrous dysplasia has a good prognosis; however, malignant transformation can occur in up to 4% of patients.73
Malignant bone tumours
Ewing sarcoma
Management of Ewing sarcoma has improved remarkably within recent decades. Many theories have evolved regarding how Ewing sarcomas arise. While the origin of these tumours is still not definitively known, most cases of Ewing sarcoma (85%) are the result of a translocation between chromosomes 11 and 22, which fuses the EWS gene on chromosome 22 to the FLI1 gene on chromosome 11. Other translocations are at t(21;22) and t(7;22).74 Because a large percentage of Ewing sarcomas and primitive neuroectodermal tumours (PNET) have identical translocation, these two tumours have been grouped into a class of cancers entitled Ewing Sarcoma Family of Tumours (ESFT).43 Nowadays, immunohistochemical stains and molecular genetic testing are required for a definitive diagnosis.75 Patients usually experience extreme bone pain, intermittent fevers, anaemia, and other symptoms of inflammatory systemic illness. Ewing sarcoma may arise in any bone, including those in the region of the elbow. It is more frequent in children than adults. Radiographically Ewing sarcoma is a highly destructive moth-eaten radiolucent lesion without evidence of bone formation associated with periosteal elevation.74
The prognosis of patients with Ewing sarcoma has improved dramatically. Although 20% to 25% of patients with Ewing sarcoma are metastatic at presentation, overall survival in patients with lesions of the extremities now ranges between 40% and 75%.34
Osteosarcoma
Osteosarcoma is another malignant tumour occurring in the elbow joint, although it is not so frequent as in the distal femur, proximal tibia and proximal humerus.78 The prognosis for patients with non-metastatic osteosarcoma nowadays is significantly improved and 70% to 90% of these patients may be long-term survivors.44,79 Symptoms around the elbow joint may be present for weeks, months, or longer before osteosarcoma is diagnosed. The most common presenting symptom is pain, which is exaggerated with activity, and swelling. Patients may complain of a sprain or ‘growing’ pain. The patient often has a history of trauma. Systemic symptoms, such as fever and night sweats, are rare.1 Radiographically, osteosarcomas usually appear to be aggressive, with evidence of cortical erosion and reactive periosteal new bone formation. In the distal humerus, the classic ‘sunburst’ appearance may be evident.80 Nevertheless, the precise extent of the lesion may not be apparent on plain radiographs. Histologically, the majority of osteosarcomas are high-grade tumours. Approximately 8–15% of patients originally diagnosed with osteosarcoma have metastatic disease.81
Chondrosarcoma
Chondrosarcoma is the third most common primary malignant tumour of bone, though is remarkably rare in the elbow.2 It is a malignant bone tumour that develops from cartilaginous tissue but can also arise de novo in extra-skeletal tissue. There are a few case reports of chondrosarcoma arising from synovial chondromatosis of the elbow.82 Late diagnosis because of slow progression of the tumour and inadequate first treatment occurred frequently.82,83 Chondrosarcoma of the elbow has a poor prognosis and lung metastases occurred frequently at the time of diagnosis.83
Treatment of malignant bone tumours
Treatment of malignant bone tumours in the region of the elbow is more challenging than in other anatomic areas because of limited soft tissue envelope and neurovascular structures in close proximity to the tumour. For these anatomic considerations, in the past amputation was the treatment of choice. Nowadays, the majority of patients with Ewing sarcoma and osteosarcoma of the elbow can be treated with adjuvant chemotherapy, wide excision of the tumour and limb salvage procedures.34 The choice between amputation and limb-sparing resection must be made by an orthopaedic oncologist taking into account tumour location, size, extramedullary extension, distant metastatic disease and patient factors. Reconstructive options are limited and technically challenging and include endoprosthetic replacements, resection arthroplasty, interposition arthroplasty, arthrodesis, elbow osteoarticular allograft reconstruction, or allograft-prosthesis composite arthroplasty and vascularized fibular grafts.43,76,84 There is limited literature supporting the ability to achieve en bloc extra-articular excision of the tumour in the elbow area, with most case series describing trans-articular hemi-resection through the elbow joint.43,85–88
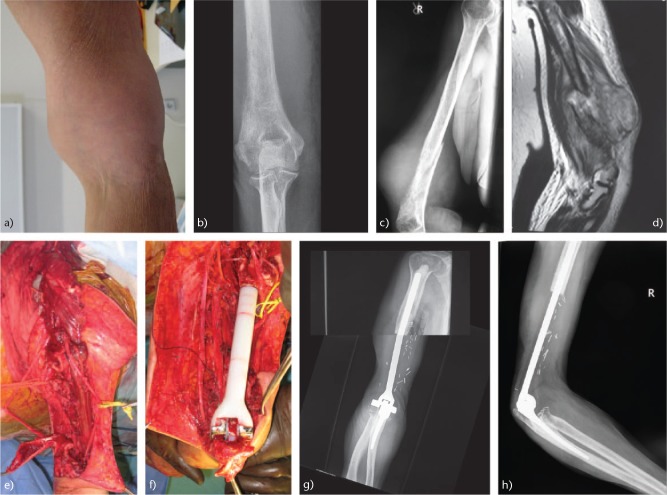
A total elbow arthroplasty in patients with large defects may result in instability with high rates of complications such as implant loosening and failure and postoperative infection. Endoprosthetic replacement using a constrained hinged megaprosthesis (Fig. 3a–h) cannot allow good function compared to that after a total elbow arthroplasty (TEA), in which the soft tissue ‘envelope’ is largely preserved.86 Infection is one of the major concerns in this group of immunocompromised patients. There is emerging evidence in the literature to support the finding that silver-coated megaprostheses can reduce postoperative infection, as silver has antimicrobial properties.89 Complex soft tissue reconstruction techniques such as pedicled myocutaneous latissimus dorsi rotation flap and reconstruction of the triceps may be necessary in these cases.77 In skeletally immature adolescent patients with Ewing sarcoma, an expandable elbow endoprosthesis may be used. Ayoub et al treated eight patients with Ewing sarcoma of the humerus with limb salvage with extensible endoprosthesis, with 90% five-year survival.90
Fig. 3.
(a) A rapidly enlarging mass in the right arm of a 69-year-old female. (b) Anteroposterior radiograph of the distal humerus showing a lytic lesion with permeation of lateral cortex. (c) High-grade sarcoma was diagnosed. Pathological fracture of the distal humerus. (d) T1-MRI image showing the tumour mass. (e) Intraoperative image of the right humerus after excision of the tumour with preservation of the neurovascular elements. (f) Elbow reconstruction using a custom-made cemented megaprosthesis (Link megaprostheses, Hamburg, Germany). (g) Anteroposterior and (h) lateral radiographs of the elbow 13 months postoperatively, showing the elbow endoprosthesis with no sign of local recurrence. Postoperatively, the patient had adjuvant chemotherapy. She died at 13 months due to lung metastatic disease.
Allograft elbow reconstructions, total or hemi-articular, although they provide certain advantages, are rarely undertaken due to the unpredictable outcomes and high complications rates.76,91,92 A vascularized fibular grafting including the fibular head can be used for reconstruction after excision of a malignant tumour in the proximal ulna. Kimura et al treated an eight-year-old girl with a Ewing sarcoma in of the proximal ulna using wide excision and reconstruction with a vascularized osteocutaneous fibular graft including the fibular head. Four years after surgery the patient was disease free with excellent elbow function and the upper extremity was growing without deformity.93 Recently, Graci et al presented the case of a 12-year-old girl with parosteal osteosarcoma of the right distal humerus treated with en bloc resection, intraoperative extracorporeal irradiation and implantation. The authors inserted a non-vascularized fibular autograft through the middle of the irradiated graft to obtain a greater stability. Ten years after surgery the patient had no recurrence with an excellent functional result.94
In cases where complete excision of the tumour is impossible, amputation is recommended. Radiation therapy is mandatory in cases of Ewing sarcoma with marginal resection or with poor response to chemotherapy with dose of 4500 to 6000 cGy, delivered over six to eight weeks.34,74
Conclusion
Bone tumours around the elbow are rare and pose a diagnostic challenge for orthopaedic surgeons. Delay in diagnosis is common because of atypical clinical presentation and the low index of suspicion. Treatment, even that of the benign varieties, remains challenging because of the interference of the tumour with neurovascular structures and inadequate soft tissue coverage. Nowadays, benign tumours can be treated using minimally invasive techniques, and malignant ones with limb salvage procedures. Various reconstruction options include endoprosthetic replacements, resection arthroplasty, interposition arthroplasty, arthrodesis, elbow osteoarticular allograft reconstructions, allograft-prosthesis composite arthroplasty and vascularized fibular grafts including the fibular head. Surgical options for reconstruction of the elbow joint remain technically challenging. Management strategies with a multidisciplinary team approach are mandatory and should be individualized and address the characteristics of the bone tumour while respecting the patient’s trajectory of illness.
Footnotes
ICMJE Conflict of interest statement: The author declares no conflict of interest relevant to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Morrey BF, Sanchez-Sotelo J. The elbow and its disorders. Philadelphia: Saunders/Elseiver, 2009. [Google Scholar]
- 2. Halai M, Gupta S, Spence S, Wallace D, Rymaszewski L, Mahendra A. Primary osseous tumours of the elbow: 60 years of registry experience. Shoulder Elbow 2015;7:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruguera JA, Newman RJ. Primary tumors of the elbow: a review of the Leeds Regional Bone Tumour Registry. Orthopedics 1998;21:551–553. [PubMed] [Google Scholar]
- 4. Simon MA, Finn HA. Diagnostic strategy for bone and soft-tissue tumors. J Bone Joint Surg Am 1993;75:622–631. [DOI] [PubMed] [Google Scholar]
- 5. Miller TT. Bone tumors and tumorlike conditions: analysis with conventional radiography. Radiology 2008;246:662–674. [DOI] [PubMed] [Google Scholar]
- 6. Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am 1982;64:1121–1127. [PubMed] [Google Scholar]
- 7. Wu JS, Hochman MG. Bone tumors: a practical guide to imaging. Anticancer Res 2012;32:6–8. [Google Scholar]
- 8. Soong M, Jupiter J, Rosenthal D. Radiofrequency ablation of osteoid osteoma in the upper extremity. J Hand Surg Am 2006;31:279–283. [DOI] [PubMed] [Google Scholar]
- 9. Link MP, Goorin AM, Miser AW, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med 1986;314:1600–1606. [DOI] [PubMed] [Google Scholar]
- 10. Moser RP, Jr, Kransdorf MJ, Brower AC, et al. Osteoid osteoma of the elbow: a review of six cases. Skeletal Radiol 1990;19:181–186. [DOI] [PubMed] [Google Scholar]
- 11. Lenoble E, Sergent A, Goutallier D. Preoperative, intraoperative, and immediate postoperative skeletal scintigraphy to locate and facilitate excision of an osteoid osteoma of the coronoid process. J Shoulder Elbow Surg 1994;3:323–326. [DOI] [PubMed] [Google Scholar]
- 12. Otsuka NY, Hastings DE, Fornasier VL. Osteoid osteoma of the elbow: a report of six cases. J Hand Surg Am 1992;17:458–461. [DOI] [PubMed] [Google Scholar]
- 13. Bednar MS, Weiland AJ, Light TR. Osteoid osteoma of the upper extremity. Hand Clin 1995;11:211–221. [PubMed] [Google Scholar]
- 14. Themistocleous GS, Chloros GD, Benetos IS, Efstathopoulos DG, Gerostathopoulos NE, Soucacos PN. Osteoid osteoma of the upper extremity: a diagnostic challenge. Chir Main 2006;25:69–76. [DOI] [PubMed] [Google Scholar]
- 15. Nourissat G, Kakuda C, Dumontier C. Arthroscopic excision of osteoid osteoma of the elbow. Arthroscopy 2007;23:799.e1–4. [DOI] [PubMed] [Google Scholar]
- 16. Glanzmann MC, Imhoff AB, Schwyzer HK. Osteoid osteoma of the shoulder and elbow: from diagnosis to minimally invasive removal. Int Orthop 2013;37:2403–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Kalle T, Langendörfer M, Fernandez FF, Winkler P. Combined dynamic contrast-enhancement and serial 3D-subtraction analysis in magnetic resonance imaging of osteoid osteomas. Eur Radiol 2009;19:2508–2517. [DOI] [PubMed] [Google Scholar]
- 18. Zampa V, Bargellini I, Ortori S, Faggioni L, Cioni R, Bartolozzi C. Osteoid osteoma in atypical locations: the added value of dynamic gadolinium-enhanced MR imaging. Eur J Radiol 2009;71:527–535. [DOI] [PubMed] [Google Scholar]
- 19. Zupanc O, Sarabon N, Strazar K. Arthroscopic removal of juxtaarticular osteoid osteoma of the elbow. Knee Surg Sports Traumatol Arthrosc 2007;15:1240–1243. [DOI] [PubMed] [Google Scholar]
- 20. Font Segura J, Barrera-Ochoa S, Gargallo-Margarit A, Correa-Vázquez E, Isart-Torruella A, Mir Bullo X. Osteoid osteoma of the distal humerus mimicking sequela of pediatric supracondylar fracture: arthroscopic resection-case report and a literature review. Case Rep Med 2013;2013:247328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamrani RS, Moradi A, Sharafat Vaziri A, Nabian MH, Ghane B. Arthroscopic ablation of an osteoid osteoma of the elbow: a case series with a minimum of 18 months’ follow-up. J Shoulder Elbow Surg 2017;26:e122–e127. [DOI] [PubMed] [Google Scholar]
- 22. Cantwell CP, Obyrne J, Eustace S. Current trends in treatment of osteoid osteoma with an emphasis on radiofrequency ablation. Eur Radiol 2004;14:607–617. [DOI] [PubMed] [Google Scholar]
- 23. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of osteoid osteomas with a percutaneously placed electrode: a new procedure. Radiology 1992;183:29–33. [DOI] [PubMed] [Google Scholar]
- 24. Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ. Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am 1998;80:815–821. [DOI] [PubMed] [Google Scholar]
- 25. Rosenthal DI, Hornicek FJ, Torriani M, Gebhardt MC, Mankin HJ. Osteoid osteoma: percutaneous treatment with radiofrequency energy. Radiology 2003;229:171–175. [DOI] [PubMed] [Google Scholar]
- 26. Albisinni U, Bazzocchi A, Bettelli G, Facchini G, Castiello E, Cavaciocchi M, et al. Treatment of osteoid osteoma of the elbow by radiofrequency thermal ablation. J Shoulder Elbow Surg 2014;23:e1–7. [DOI] [PubMed] [Google Scholar]
- 27. Carrasco CH, Murray JA. Giant cell tumors. Orthop Clin North Am 1989;20:395–405. [PubMed] [Google Scholar]
- 28. Hoeffel JC, Galloy MA, Grignon Y, et al. Giant cell tumor of bone in children and adolescents. Rev Rhum Engl Ed 1996;63:618–623. [PubMed] [Google Scholar]
- 29. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop 2006;30:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am 1987;69:106–114. [PubMed] [Google Scholar]
- 31. Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res 1986:9–24. [PubMed] [Google Scholar]
- 32. Yochum TR, Rowe LJ. Yochum and Rowe’s essentials of skeletal radiology. Philadelphia, PA: Lippincott/Williams & Wilkins, 2005. [Google Scholar]
- 33. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am 1987;69:106–114. [PubMed] [Google Scholar]
- 34. Wiesel SW. Operative techniques in orthopaedic surgery. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2011. [Google Scholar]
- 35. Khan MT, Gray JM, Carter SR, Grimer RJ, Tillman RM. Management of the giant-cell tumours of the distal radius. Ann R Coll Surg Engl 2004;86:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balke M, Schremper L, Gebert C, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol 2008;134:969–978. [DOI] [PubMed] [Google Scholar]
- 37. Dahlin DC. Bone tumors: general aspects and data on 6,221 cases. Springfield, IL: Thomas, 1978. [Google Scholar]
- 38. Hegde AS, Shenoy RM, Rai MP. Giant cell tumour of the distal humerus: a case report. J Orthop Surg (Hong Kong) 2014;22:427–429. [DOI] [PubMed] [Google Scholar]
- 39. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev 2010;36:1–7. [DOI] [PubMed] [Google Scholar]
- 40. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res 2011;469:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Turcotte RE, Wunder JS, Isler MH, et al. ; Canadian Sarcoma Group. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Relat Res 2002;397:248–258. [DOI] [PubMed] [Google Scholar]
- 42. Sait AS, Nithyanath M, Cherian VM. Giant cell tumour of the distal humerus treated with elbow arthroplasty: a case report. Int J Case Rep Imag 2012;5:37–40. [Google Scholar]
- 43. Sperling JW, Pritchard DJ, Morrey BF. Total elbow arthroplasty after resection of tumors at the elbow. Clin Orthop Relat Res 1999;367:256–261. [PubMed] [Google Scholar]
- 44. Tang X, Guo W, Yang R, Tang S, Yang Y. Custom-made prosthesis replacement for reconstruction of elbow after tumor resection. J Shoulder Elbow Surg 2009;18:796–803. [DOI] [PubMed] [Google Scholar]
- 45. Kharrazi FD, Busfield BT, Khorshad DS, Hornicek FJ, Mankin HJ. Osteoarticular and total elbow allograft reconstruction with severe bone loss. Clin Orthop Relat Res 2008;466:205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van der Heijden L, Dijkstra PD, van de Sande MA, et al. The clinical approach toward giant cell tumor of bone. Oncologist 2014;19:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res 2012;18:4415–4424. [DOI] [PubMed] [Google Scholar]
- 48. Chawla S, Henshaw R, Seeger L, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 2013;14:901–908. [DOI] [PubMed] [Google Scholar]
- 49. Müller DA, Beltrami G, Scoccianti G, Campanacci DA, Franchi A, Capanna R. Risks and benefits of combining denosumab and surgery in giant cell tumor of bone: a case series. World J Surg Oncol 2016;14:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 2010;11:275–280. [DOI] [PubMed] [Google Scholar]
- 51. Cottalorda J, Bourelle S. Modern concepts of primary aneurysmal bone cyst. Arch Orthop Trauma Surg 2007;127:105–114. [DOI] [PubMed] [Google Scholar]
- 52. Dabezies EJ, D’Ambrosia RD, Chuinard RG, Ferguson AB., Jr Aneurysmal bone cyst after fracture: a report of three cases. J Bone Joint Surg Am 1982;64:617–621. [PubMed] [Google Scholar]
- 53. Leithner A, Machacek F, Haas OA, Lang S, Ritschl P, Radl R, et al. Aneurysmal bone cyst: a hereditary disease? J Pediatr Orthop B 2004;13:214–217. [DOI] [PubMed] [Google Scholar]
- 54. Ozaki T, Hillmann A, Lindner N, Winkelmann W. Cementation of primary aneurysmal bone cysts. Clin Orthop Relat Res 1997;337:240–248. [DOI] [PubMed] [Google Scholar]
- 55. Ramírez AR, Stanton RP. Aneurysmal bone cyst in 29 children. J Pediatr Orthop 2002;22:533–539. [PubMed] [Google Scholar]
- 56. Leithner A, Windhager R, Kainberger F, Lang S. A case of aneurysmal bone cyst in father and son. Eur J Radiol 1998;29:28–30. [DOI] [PubMed] [Google Scholar]
- 57. Cory DA, Fritsch SA, Cohen MD, et al. Aneurysmal bone cysts: imaging findings and embolotherapy. AJR Am J Roentgenol 1989;153:369–373. [DOI] [PubMed] [Google Scholar]
- 58. Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol 2005;23:6756–6762. [DOI] [PubMed] [Google Scholar]
- 59. Docquier PL, Delloye C. Treatment of aneurysmal bone cysts by introduction of demineralized bone and autogenous bone marrow. J Bone Joint Surg Am 2005;87:2253–2258. [DOI] [PubMed] [Google Scholar]
- 60. Falappa P, Fassari FM, Fanelli A, et al. Aneurysmal bone cysts: treatment with direct percutaneous Ethibloc injection: long-term results. Cardiovasc Intervent Radiol 2002;25:282–290. [DOI] [PubMed] [Google Scholar]
- 61. Rastogi S, Varshney MK, Trikha V, Khan SA, Choudhury B, Safaya R. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol: a review of 72 cases with long-term follow-up. J Bone Joint Surg Br 2006;88:1212–1216. [DOI] [PubMed] [Google Scholar]
- 62. Varshney MK, Rastogi S, Khan SA, Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop Relat Res 2010;468:1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reddy KI, Sinnaeve F, Gaston CL, Grimer RJ, Carter SR. Aneurysmal bone cysts: do simple treatments work? Clin Orthop Relat Res 2014;472:1901–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chapurlat RD, Meunier PJ. Fibrous dysplasia of bone. Best Pract Res Clin Rheumatol 2000;14:385–398. [DOI] [PubMed] [Google Scholar]
- 65. Kumar R, Madewell JE, Lindell MM, Swischuk LE. Fibrous lesions of bones. Radiographics 1990;10:237–256. [DOI] [PubMed] [Google Scholar]
- 66. Chapurlat RD, Gensburger D, Jimenez-Andrade JM, Ghilardi JR, Kelly M, Mantyh P. Pathophysiology and medical treatment of pain in fibrous dysplasia of bone. Orphanet J Rare Dis 2012;7:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leet AI, Chebli C, Kushner H, et al. Fracture incidence in polyostotic fibrous dysplasia and the McCune–Albright syndrome. J Bone Miner Res 2004;19:571–577. [DOI] [PubMed] [Google Scholar]
- 68. Yamamoto T. Clinical approach to clarifying the mechanism of abnormal bone metabolism in McCune–Albright syndrome. J Bone Miner Metab 2006;24:7–10. [DOI] [PubMed] [Google Scholar]
- 69. Chapurlat R, Meunier PJ. The nonsurgical treatment of fibrous dysplasia. Rev Rhum Engl Ed 1999;66:1–3. [PubMed] [Google Scholar]
- 70. Parisi MS, Oliveri B, Mautalen CA. Effect of intravenous pamidronate on bone markers and local bone mineral density in fibrous dysplasia. Bone 2003;33:582–588. [DOI] [PubMed] [Google Scholar]
- 71. Keijser LC, Van Tienen TG, Schreuder HW, Lemmens JA, Pruszczynski M, Veth RP. Fibrous dysplasia of bone: management and outcome of 20 cases. J Surg Oncol 2001;76:157–166. [DOI] [PubMed] [Google Scholar]
- 72. Kokkalis ZT, Jain S, Sotereanos DG. Fibrous dysplasia around the elbow. J Shoulder Elbow Surg 2010;19:e6–11. [DOI] [PubMed] [Google Scholar]
- 73. Ruggieri P, Sim FH, Bond JR, Unni KK. Malignancies in fibrous dysplasia. Cancer 1994;73:1411–1424. [DOI] [PubMed] [Google Scholar]
- 74. Paulussen M, Bielack S, Jurgens H, Casali PG. Ewing’s sarcoma of the bone: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20:140–142. [DOI] [PubMed] [Google Scholar]
- 75. Desai SS, Jambhekar NA. Pathology of Ewing’s sarcoma/PNET: current opinion and emerging concepts. Indian J Orthop 2010;44:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Urbaniak JR, Black KE., Jr Cadaveric elbow allografts: a six-year experience. Clin Orthop Relat Res 1985;197:131–140. [PubMed] [Google Scholar]
- 77. Schwab JH, Healey JH, Athanasian EA. Wide en bloc extra-articular excision of the elbow for sarcoma with complex reconstruction. J Bone Joint Surg Br 2008;90:78–83. [DOI] [PubMed] [Google Scholar]
- 78. Schajowicz F, McGuire MH, Santini Araujo E, Muscolo DL, Gitelis S. Osteosarcomas arising on the surfaces of long bones. J Bone Joint Surg Am 1988;70:555–564. [PubMed] [Google Scholar]
- 79. Funovics PT, Schuh R, Adams SB, Jr, Sabeti-Aschraf M, Dominkus M, Kotz RI. Modular prosthetic reconstruction of major bone defects of the distal end of the humerus. J Bone Joint Surg Am 2011;93:1064–1074. [DOI] [PubMed] [Google Scholar]
- 80. Yoshikawa H, Shimizu K, Nakase T, Takaoka K. Periosteal sunburst spiculation in osteosarcoma: a possible role for bone morphogenetic protein. Clin Orthop Relat Res 1994;308:213–219. [PubMed] [Google Scholar]
- 81. Spanos PK, Payne WS, Ivins JC, Pritchard DJ. Pulmonary resection for metastatic osteogenic sarcoma. J Bone Joint Surg Am 1976;58:624–628. [PubMed] [Google Scholar]
- 82. Muramatsu K, Miyoshi T, Moriya A, Onaka H, Shigetomi M, Nakashima D, et al. Extremely rare synovial chondrosarcoma arising from the elbow joint: case report and review of the literature. J Shoulder Elbow Surg 2012;21:e7–11. [DOI] [PubMed] [Google Scholar]
- 83. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer 1991;67:155–162. [DOI] [PubMed] [Google Scholar]
- 84. Scaglioni MF, Chang EI, Gur E, et al. The role of the fibula head flap for joint reconstruction after osteoarticular resections. J Plast Reconstr Aesthet Surg 2014;67:617–623. [DOI] [PubMed] [Google Scholar]
- 85. Athwal GS, Chin PY, Adams RA, Morrey BF. Coonrad-Morrey total elbow arthroplasty for tumours of the distal humerus and elbow. J Bone Joint Surg Br 2005;87:1369–1374. [DOI] [PubMed] [Google Scholar]
- 86. Kulkarni A, Fiorenza F, Grimer RJ, Carter SR, Tillman RM. The results of endoprosthetic replacement for tumours of the distal humerus. J Bone Joint Surg Br 2003;85:240–243. [DOI] [PubMed] [Google Scholar]
- 87. Ross AC, Sneath RS, Scales JT. Endoprosthetic replacement of the humerus and elbow joint. J Bone Joint Surg Br 1987;69:652–655. [DOI] [PubMed] [Google Scholar]
- 88. Weber KL, Lin PP, Yasko AW. Complex segmental elbow reconstruction after tumor resection. Clin Orthop Relat Res 2003;415:31–44. [DOI] [PubMed] [Google Scholar]
- 89. Tow BP, Tan MH. Delayed diagnosis of Ewing’s sarcoma of the right humerus initially treated as chronic osteomyelitis: a case report. J Orthop Surg (Hong Kong) 2005;13:88–92. [DOI] [PubMed] [Google Scholar]
- 90. Ayoub KS, Fiorenza F, Grimer RJ, Tillman RM, Carter SR. Extensible endoprostheses of the humerus after resection of bone tumours. J Bone Joint Surg Br 1999;81:495–500. [DOI] [PubMed] [Google Scholar]
- 91. Capanna R, Campanacci D, Del Ben M, Masetti C, Donati D, eds. Total elbow replacement with osteoarticular allografts. Limb Salvage Current Trends: Procs 7th International Symposium, 1993. [Google Scholar]
- 92. Dean GS, Holliger EH, IV, Urbaniak JR. Elbow allograft for reconstruction of the elbow with massive bone loss: long term results. Clin Orthop Relat Res 1997;341:12–22. [PubMed] [Google Scholar]
- 93. Kimura K, Tatezaki S, Ishii T, Yonemoto T, Shigehara T, Takenouchi T. Hemiarthroplasty of the elbow with a vascularized fibular graft after excision of Ewing’s sarcoma of the proximal ulna: a case report. Jpn J Clin Oncol 2002;32:430–434. [DOI] [PubMed] [Google Scholar]
- 94. Graci C, Gaston CL, Grimer R, Jeys L, Ozkan K. Saving a child’s elbow joint: a novel reconstruction for a tumour of the distal humerus. Case Rep Orthop 2015;2015:404979. [DOI] [PMC free article] [PubMed] [Google Scholar]