Abstract
Lycopene and green tea consumption have been observationally associated with reduced prostate cancer risk, but the underlying mechanisms have not been fully elucidated. We investigated the effect of factorial randomisation to a 6‐month lycopene and green tea dietary advice or supplementation intervention on 159 serum metabolite measures in 128 men with raised PSA levels (but prostate cancer‐free), analysed by intention‐to‐treat. The causal effects of metabolites modified by the intervention on prostate cancer risk were then assessed by Mendelian randomisation, using summary statistics from 44,825 prostate cancer cases and 27,904 controls. The systemic effects of lycopene and green tea supplementation on serum metabolic profile were comparable to the effects of the respective dietary advice interventions (R 2 = 0.65 and 0.76 for lycopene and green tea respectively). Metabolites which were altered in response to lycopene supplementation were acetate [β (standard deviation difference vs. placebo): 0.69; 95% CI = 0.24, 1.15; p = 0.003], valine (β: −0.62; −1.03, −0.02; p = 0.004), pyruvate (β: −0.56; −0.95, −0.16; p = 0.006) and docosahexaenoic acid (β: −0.50; −085, −0.14; p = 0.006). Valine and diacylglycerol were lower in the lycopene dietary advice group (β: −0.65; −1.04, −0.26; p = 0.001 and β: −0.59; −1.01, −0.18; p = 0.006). A genetically instrumented SD increase in pyruvate increased the odds of prostate cancer by 1.29 (1.03, 1.62; p = 0.027). An intervention to increase lycopene intake altered the serum metabolome of men at risk of prostate cancer. Lycopene lowered levels of pyruvate, which our Mendelian randomisation analysis suggests may be causally related to reduced prostate cancer risk.
Keywords: prostate cancer, dietary intervention, lycopene, green tea, Mendelian randomisation
Short abstract
What's new?
Prostate cancer incidence varies by geographic region, suggesting that environmental factors, such as diet, play a role. Here, the authors investigated how green tea and lycopene intake affects prostate cancer risk. They conducted a 6‐month intervention on men with raised PSA levels but no cancer, testing levels of 159 serum metabolites by NMR. Lycopene supplementation, they found, reduced levels of circulating pyruvate, and Mendelian randomisation analysis suggests pyruvate may boost PC risk. These results suggest a possible mechanism of action by which consuming dietary lycopene may reduce prostate cancer risk.
Abbreviations
- 2LSR
2 least‐squares regression
- BMI
body mass index
- CI
confidence interval
- DHA
docosahexaenoic acid
- ECGC
epigallocatechin‐3‐gallate
- FA
fatty acid
- GCKR
glucokinase regulatory protein
- IV
instrumental variable
- MCT2
monocarboxylate transporter 2
- MR
Mendelian randomisation
- NMR
Nuclear magnetic resonance
- PCA
principle component analysis
- PCs
principle components
- PDPR
pyruvate dehydrogenase phosphatase regulatory
- PSA
prostate specific antigen
- PUFA
polyunsaturated fatty acids
- RCT
Randomised controlled trial
- SD
standard deviation
- SNP
single nucleotide polymorphism
Introduction
Prostate cancer is the second most common cancer diagnosed in males worldwide.1 The burden of the disease is not evenly distributed however, with “Western Countries” like the United States, Western Europe and Australia experiencing the highest incidences and Asia the lowest.1 Given this geographical variation, lifestyle factors are thought to influence prostate cancer risk2 and there has been growing interest in studying the impact of dietary changes on prostate cancer incidence.
Several dietary factors have been purported to modulate prostate cancer risk.3, 4 Green tea and lycopene, a bright‐red carotenoid found primarily in tomatoes, have received particular attention. This is largely because of their potent antioxidant activity in vitro, 5, 6 although other chemopreventative mechanisms have been suggested.7, 8 Epidemiological evidence that lycopene and green tea protect against prostate cancer is inconsistent however. Of the three published meta‐analyses that have considered the association of lycopene intake with prostate cancer, two report an inverse association,9, 10 while the other found insufficient evidence to either support or refute the use of lycopene for the prevention of prostate cancer.11 An initial meta‐analysis suggested that green tea consumption may have a protective effect, especially in Asian populations,12 but this finding was not supported in a more recent meta‐analysis.13
The results of observational studies of diet and cancer risk must be interpreted with caution as they are susceptible to confounding14 and measurement error in the reporting of dietary exposures, mainly due to recall and reporting bias.15 Such issues can be overcome using well‐designed and conducted randomised controlled trials (RCTs).
We recently reported the primary results of a randomised, placebo‐controlled factorial trial of lycopene and green tea in men at elevated risk of prostate cancer (ProDiet).16 Post‐intervention plasma lycopene and epigallocatechin‐3‐gallate (ECGC) (a bioactive component of green tea) levels were increased in the respective intervention arms, indicating high levels of adherence. In the present study, we investigated the metabolic effects of lycopene and green tea intake in men enrolled in the same trial, using nuclear magnetic resonance (NMR)‐based metabolic profiling. Where evidence of an effect of one of the interventions on metabolic measures was found, we used Mendelian randomisation (MR) to assess the causal role of these metabolic traits on prostate cancer risk. MR is a technique that uses genetic variation to proxy exposures of interest to circumvent issues of reverse causation and confounding that bias observational epidemiology.17, 18 Formal MR approaches have been used previously in this context, to capture causal relationships between metabolites and disease,19, 20, 21, 22 facilitated by strong genotypic effects on metabolites.23, 24, 25, 26, 27
Materials and Methods
Overview
The present report includes two separate but interlinked investigations (Fig. 1):
An intention‐to‐treat analysis of the ProDiet factorial RCT. This assessed the effects on serum metabolome of interventions to increase green tea and lycopene intake;
A two‐sample Mendelian randomisation analysis28 using summary statistics data from the PRACTICAL (Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome) consortium.29 This investigated the causal effects of those metabolic measures shown to be altered by the interventions on prostate cancer risk.
Figure 1.
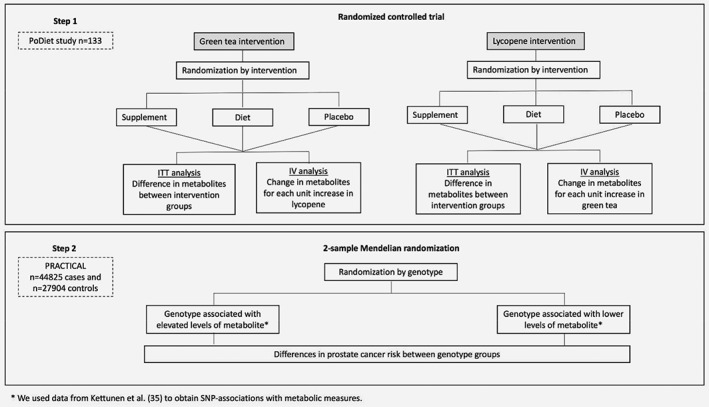
Analysis steps for investigating the effects of lycopene and green tea on serum metabolome of men at risk of prostate cancer, and the causal role of altered metabolic traits on prostate cancer risk. We conducted 2 analyses. In stage one, relationships between metabolic measures and lycopene or green tea randomisation arms were tested using an intention‐to‐treat analyses. In stage two, we used GWAS summary statistics from Kettunen et al to identify genetic variants that could be used as instrumental variables for the effects of metabolites on prostate cancer risk. Data on the association of these genetic variants with prostate cancer risk were obtained from the PRACTICAL consortium (44,825 prostate cancer cases and 27,904 controls of European ancestry). Data on the association of genetic variants with metabolite levels and with prostate cancer risk were combined to estimate the influence of metabolites on prostate cancer risk. ITT, intention‐to treat; IV, instrumental variable.
Effect of lycopene and green tea on serum metabolome
Study population
The ProDiet RCT (ISRCTN 95931417) included 133 men between the ages of 50 and 69 years with elevated prostate specific antigen (PSA) levels (results between 2.0 and 2.95 ng/mL or at least 3.0 ng/mL with a negative biopsy), who were identified as part of the community‐based PSA testing in the ProtecT (Prostate cancer testing and Treatment) study.30
Study design
Full details of the trial have been provided in the Supporting Information (available online). Briefly, ProDiet was a feasibility randomised‐controlled trial of dietary interventions for prostate cancer prevention. Men were randomised to a daily lycopene arm (active capsules or lycopene‐rich diet or placebo capsules) and a green tea arm (active capsules or green tea drink or placebo capsules) for 6 months16 in a 3 × 3 factorial design (Fig. 2).
Figure 2.
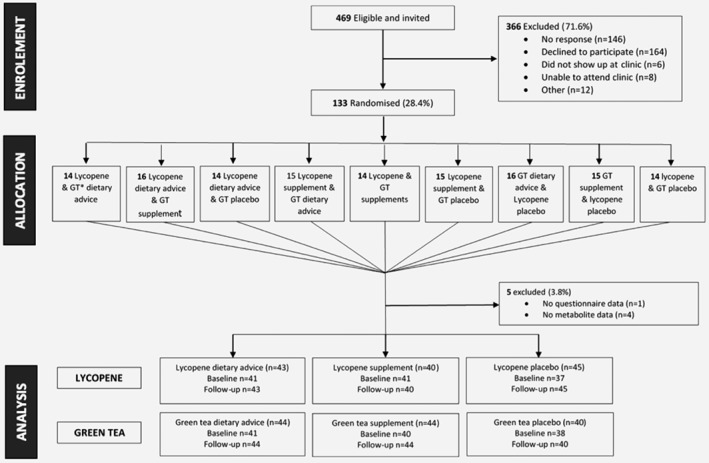
Flow of ProDiet participants through the study.Adapted from the main ProDiet study (Lane, AJ., unpublished), with thanks.
Data collection
At recruitment, trained nurses collected information on the men's weight, blood pressure, socio‐economic status and medical history.16, 31 Men were asked to complete a lifestyle questionnaire, which included questions on smoking, alcohol consumption and dietary intake of supplements or vitamins. Dietary intake was further assessed using a 117‐item food frequency questionnaire (FFQ), which was adapted from the UK arm of the EPIC study32 (see Supporting Information Methods). During the same clinic appointment, non‐fasted blood samples were drawn for baseline PSA, lycopene, EGCG and metabolic profiling, according to a standard protocol. Six‐months after randomisation, participants attended a follow‐up appointment, where repeat non‐fasted blood samples were taken. Samples were left at room temperature to clot and then centrifuged at 1640g for 20 min within 2 h of collection. They were kept at 5 °C during transportation to the laboratory, where they were aliquoted for storage at −80 °C within 36 h of collection. Serum lycopene levels were measured using reversed‐phase high‐performance liquid chromatography (HPLC).33 Plasma EGCG levels were analysed and quantified using HPLC‐mass spectroscopy (MS), as described by Stalmach et al.34
Measurement of metabolites
Metabolic profiling was performed using a high‐throughput serum nuclear magnetic resonance (NMR) metabolomics platform (Nightingale Health®, Helsinki, Finland), originally described by Soininen et al.35 A brief overview of the platform can be found in the Supporting Information methods.
Full details of the protocol and protocol, including information on quality control procedures, have been published elsewhere.36 In total, 159 metabolic traits were measured (see Supporting Information Table 8), including several amino acids (alanine, glutamine, glycine, histidine, isoleucine, leucine, valine, phenylalanine and tyrosine), glycolysis measures (glucose, lactate, pyruvate, citrate and glycerol), ketone bodies (acetate, acetoacetate and 3‐hydroxybutyrate), inflammatory markers (glycoprotein acetyls) and fatty acids (polyunsaturated, monounsaturated, linoleic, omega‐3, omega 6 and docosahexaenoic fatty acids), fatty acids traits (chain length, degree of unsaturation), as well as particle concentrations and lipid compositions of 14 lipoprotein subclasses (including low, intermediate, large and very large lipoprotein subclasses). In addition, fatty acids were also expressed as ratios (%) to total fatty acids. This set of metabolite traits are from multiple metabolic pathways, including those involved in carcinogenesis.
Statistical analysis
The effects of lycopene and green tea dietary interventions were examined in an intention‐to‐treat analysis, by comparing metabolic measures at 6‐months follow‐up for each intervention. To allow comparison of magnitudes of association across measures with different units, all metabolite concentrations were converted to standard deviation (z) scores. Linear regression was used to compare standardised (z‐scored) 6‐month metabolite measures across lycopene and green tea intervention groups, treating placebo as the reference category. As some metabolite concentrations had right skewed distributions, robust standard errors were estimated for all associations. In our primary analysis, no covariates were included in the models, as confounders were shown to be well balanced across the intervention arm (Supporting Information Tables 1 and 2). The overall match between the metabolic changes associated with supplement‐advice (vs. placebo) and the metabolic changes associated with dietary‐advice (vs. placebo) were assessed using linear regression, separately for both lycopene and green‐tea arms. The correspondence between respective supplement and dietary‐advice associations were assessed using the R2 statistic.
Given that many metabolic measures were analysed, the probability of finding evidence of association by chance (i.e., false positive) was high. Principle Component Analyses (PCA) was carried out on standardised metabolic measures data and used to set a significance threshold that takes into account both multiple testing and the correlation between metabolic traits,37 as discussed previously.19, 37, 38 This method assumes that the independence of the principle components (PCs) is equivalent to the degree of freedom of the original metabolic dataset, and that retaining a number of PCs that is enough to explain at least 95% of the variance will only result in a small chance of type 1 error. The first 14 principal components explained >95% of the variance in the metabolic measures data. We therefore set our significance threshold as p < 0.05/14 (=0.0036). p‐Values below this can be interpreted as providing strong evidence of an association of the respective intervention on metabolic trait levels.
Whilst randomisation aims to prevent bias in the allocation of participants to intervention arms in a RCT, this does not guarantee that groups will be comparable with respect to baseline measures, particularly in small feasibility trials. As a sensitivity analysis, we repeated the intention‐to‐treat analysis adjusting for pre‐intervention metabolic measures. All individuals with complete baseline and follow‐up metabolite data were included in the sensitivity analyses.
To establish the causal effect of lycopene and EGCG dose on metabolite measures at follow‐up, we employed instrumental variable (IV) analysis,39 using intervention status as an IV. The IV analysis was performed using a 2‐stage least squares (2SLS) regression method, implemented using the “ivreg2” function in Stata. F‐statistics and R2 values from the first‐stage regression between intervention arm and serum lycopene/EGCG levels were examined to check the instrumental variable assumption that the instrument is sufficiently associated with the exposure. Causal estimates for the instrumented effect of serum lycopene/EGCG levels on each follow‐up metabolite were obtained from the second‐stage regression. The regression coefficients were calculated in units of 1‐SD metabolite concentration per one‐unit increment in lycopene (μmol/L) or EGCG (nM). Associations were adjusted for baseline metabolic measures.40
Analyses were performed in Rstudio and Stata version 14.2.
Causal effect of altered metabolites on prostate cancer risk
Given evidence that the dietary interventions were associated with changes in some of the metabolic measures at follow‐up, we used MR to investigate whether these metabolites could have a causal role in mediating the effect of the dietary interventions on prostate cancer risk.
MR is a form of IV analysis that uses genetic variants as instruments to examine the causal effects of modifiable exposures on outcomes of interest.17, 41 This method depends on the existence of genetic variants that are robustly associated with metabolite levels (see Supporting Information materials).
We utilised the two‐sample MR approach, described in more detail in the Supporting Information Methods. Briefly, genetic variants robustly associated with the serum metabolites of interest were first identified using data from a recently published genome‐wide association study (GWAS) of 123 circulating metabolite levels.27 These genetic instruments were analysed in relation to prostate cancer risk in a series of 44,825 prostate cancer cases and 27,904 control subjects GWAS data from the PRACTICAL consortium.29 PRACTICAL samples were genotyped using an Illumina.
Custom Infinium genotyping array (OncoArray), details of which may be found on their website http://practical.icr.ac.uk/blog/?page_id=1244). Two types of sensitivity analyses were undertaken to assess potential horizontal pleiotropic effects: a weighted median approach and MR‐Egger regression.42
Results
One hundred and thirty‐three men were recruited and randomised into lycopene and green tea intervention groups (Fig. 2). One hundred and thirty‐two men attended the six‐month follow‐up appointment. Not all participants had sufficient blood available for metabolic profiling in the current study; metabolic measures were available for 119 men at baseline and 128 men at follow‐up.
Baseline characteristics
Supporting Information Tables 1 and 2 show the baseline characteristics of the men stratified by lycopene and green tea treatment groups, respectively. Men were a mean age of 64.5 years (SD 5.0), had a mean BMI of 27.0 kg/m2 and had a median baseline PSA level of 2.6 ng/mL. There were no apparent differences across intervention arms with respect to any of the sociodemographic or lifestyle variables considered. Two of the men, randomised to lycopene and green tea supplement arms had diabetes.
Among the 116 men with complete baseline metabolic measures data, there were no clear differences in metabolic traits between the lycopene randomisation arms pre‐intervention (Supporting Information Table 3). At baseline, there was evidence of by‐arm differences in glycine, phenylalanine, alanine, glycoprotein acetyls and a number of fatty acid metabolic measures in the green tea group (Supporting Information Table 3).
Difference in metabolite levels between intervention groups at six‐month follow‐up
Correlation between metabolic profiles from lycopene dietary advice and supplement arms
There was a strong correlation between the effects of supplements compared to the effects of the dietary advice interventions on 6‐month metabolic measures (slope = 1.07 ± 0.06; R 2 = 0.65) (Fig. 3), which remained when regression models were adjusted for baseline serum metabolite levels (Supporting Information Fig. 1). Forest plots comparing the metabolic profile of supplemental and dietary advice interventions provide further confirmation that they have broadly comparable effects on the measured metabolic traits (Fig. 3). There were consistent decreases in the glycolysis‐related metabolites glucose, lactate and pyruvate after lycopene supplement and dietary advice interventions, as well as decreases in branched‐chain amino acid measures isoleucine, leucine and valine.
Figure 3.
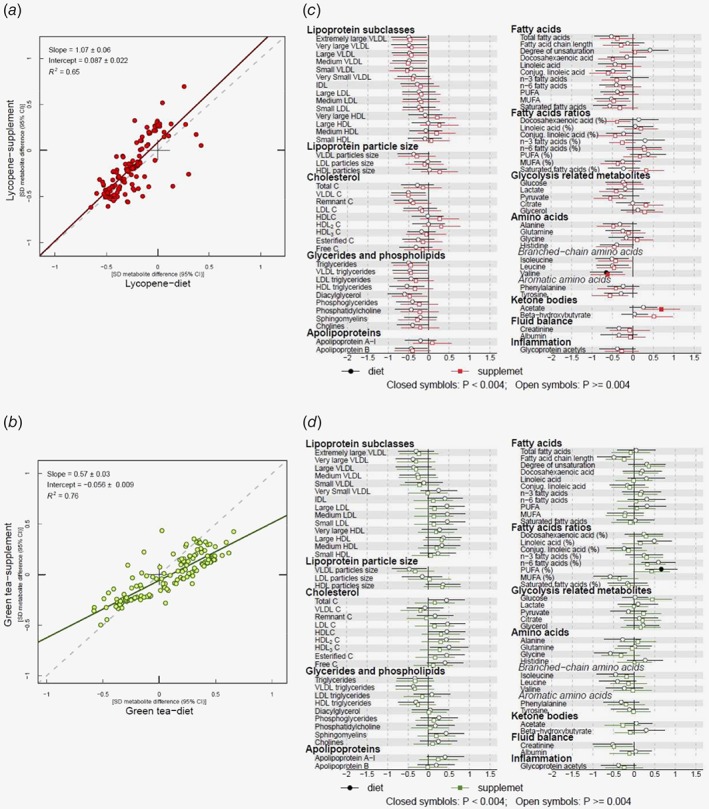
(a) Comparison of overall effects on serum metabolic traits between lycopene intervention arms vs. placebo models. Estimates of the standard deviation (SD) difference in metabolic trait concentration between lycopene dietary advice and placebo arms at follow‐up (x‐axis) plotted against the SD difference in metabolic trait concentration in the lycopene supplement arm vs. placebo (y‐axis). (b) Comparison of overall effects on serum metabolic traits between green tea intervention arms vs. placebo models. Corresponding results for green tea. Each dot on plots A and B represents an individual metabolic trait. A linear fit of the overall correspondence summarises the similarity in magnitude between diet and supplement associations (solid lines). A slope of 1 with an intercept of 0 (dashed lines), with all dots sitting on that line (R 2 = 1), would indicate that diet and supplement estimates had the same magnitude and direction. Corresponding results for green tea. (c) SD follow−up metabolic trait concentration difference between lycopene diet or supplement vs. placebo. (d) SD follow−up metabolic trait concentration difference between green tea diet (drink) or supplement vs. placebo. Circles indicate β‐regression coefficients for the dietary intervention arms. Squares indicate β‐regression coefficients for the supplement arms. Closed symbols denote values that reached the threshold for multiple testing (p ≤ 0.004). Association magnitudes are in units of 1‐SD metabolic measure concentration. Horizontal bars represent 95% confidence intervals. Abbreviations: C, cholesterol; HDL, high‐density lipoprotein; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; VLDL, very‐low‐density lipoprotein.
Lycopene effects
There was strong evidence of a reduction in valine in both supplement and dietary advice arms compared to placebo (β = −0.62; 95% CI = −1.03, −0.20; p = 0.004) and β = −0.65; 95% CI = −1.04, −0.26; p = 0.001 respectively, whereby β represents the standard deviation (SD) change in metabolic measures), as well as increased levels of acetate in the lycopene supplement group (β = 0.69; 95% CI = 0.24, 1.15; p = 0.003) (Table 1). There was some evidence that serum pyruvate and docosahexaenoic acid (DHA) were lower in the lycopene supplement group compared to placebo (β = −0.56; 95% CI = −0.95, −0.16; p = 0.006 and β = −0.50; 95% CI = −085, −0.14; p = 0.006 respectively), although effects did not reach our strict threshold for multiple testing (p = 0.004). Serum diacylglycerol levels were lower in the lycopene dietary advice group compared to placebo (β = −0.59; 95% CI = −1.01, −0.18; p = 0.006). See Supporting Information Table 4 for full results, including associations, expressed as magnitudes in absolute concentration units (e.g. mmol/L metabolite difference between diet or supplement vs. placebo).
Table 1.
Linear regression results for metabolic traits that were found to be altered by supplement or dietary advice interventions (n = 128)
| Metabolite | Intervention arm | Mean difference1 | Lower CI | Upper CI | p value |
|---|---|---|---|---|---|
| Lycopene | |||||
| Valine | Supplement | −0.62 | −1.03 | −0.2 | 0.0042 |
| Dietary advice | −0.65 | −1.04 | −0.26 | 0.0012 | |
| Acetate | Supplement | 0.69 | 0.24 | 1.15 | 0.0032 |
| Dietary advice | 0.26 | −0.08 | 0.59 | 0.129 | |
| Pyruvate | Supplement | −0.56 | −0.95 | −0.16 | 0.006 |
| Dietary advice | −0.30 | −0.75 | 0.15 | 0.196 | |
| Diacylglycerol | Supplement | −0.47 | −0.9 | −0.03 | 0.036 |
| Dietary advice | −0.59 | −1.01 | −0.18 | 0.006 | |
| DHA | Supplement | −0.5 | −0.85 | −0.14 | 0.006 |
| Dietary advice | −0.15 | −0.62 | 0.32 | 0.537 | |
| Green tea | |||||
| PUFA: FA | Supplement | 0.66 | 0.27 | 1.05 | 0.0012 |
| Dietary advice | 0.43 | −0.01 | 0.86 | 0.057 | |
| Cholesterol esters in small HDL | Supplement | 0.22 | −0.24 | 0.67 | 0.347 |
| Dietary advice | 0.62 | 0.19 | 1.04 | 0.005 | |
| Omega‐6: FA | Supplement | 0.32 | −0.12 | 0.76 | 0.148 |
| Dietary advice | 0.22 | −0.24 | 0.67 | 0.005 | |
| Glycine | Supplement | −0.32 | −0.79 | 0.14 | 0.172 |
| Dietary advice | −0.58 | −0.98 | −0.18 | 0.005 | |
Standardised mean difference (and 95% confidence interval [CI]) in metabolic trait concentration. Where there was evidence that one of the interventions altered follow‐up metabolic trait levels, results for the respective metabolic trait have been presented. For comparison, supplement and dietary advice results have been provided.
Metabolic measures that reached the principle component analysis based‐Bonferroni corrected threshold for multiple testing (p = 0.004).
Abbreviations: N, sample size; CI, confidence interval; DHA, docosahexaenoic acid; FA, fatty acid; HDL, high density lipoprotein; Omega‐6: FA, omega‐6 as a proportion of total FA; PUFA: FA, polyunsaturated fatty acids as a proportion of total FA. Omega‐6: FA and PUFA: FA are expressed as a % of total FA.
The trends for decreased valine, increased acetate and decreased pyruvate, were largely robust to adjustment for baseline metabolites (Supporting Information Table 5).
Correlation between metabolic profiles from green tea diet and supplement arms
Overall, the effects of green tea drinking and supplement interventions at follow‐up on serum metabolome were similar (slope = 0.57 ± 0.03, R 2 = 0.76) (Fig. 3).
Green tea effects
There was no strong evidence of an effect of green tea supplementation on serum metabolic profile. In the group advised to drink green tea, there was evidence of a reduction in the ratio of polyunsaturated fatty acids relative to total fatty acids (PUFA: FA) (vs. placebo), which survived correction for multiple testing (β = 0.66; 95% CI = 0.273, 1.049; p = 0.001) (Table 1). There was weaker evidence of a change in the proportions of omega‐6 and monounsaturated fatty acids relative to total fatty acids (β = 0.59; 95% CI = 0.19, 1.00; p = 0.005 and β = −0.58; 95% CI = −0.99, −0.17; p = 0.006, respectively). Post green‐tea drinking intervention levels of glycine were also lower compared to placebo (β = −0.58; 95% CI = −0.98, −0.18; p = 0.005) (full results in Supporting Information Table 6). The trends for increased PUFA: FA and reduced glycine, which were observed in the unadjusted green tea analyses, were not present in the adjusted analyses (Supporting Information Table 5).
IV estimates
Results of the lycopene IV regression were broadly consistent with those of the intention to treat analysis (Supporting Information Table 7). There was an increase in acetate (β = 2.13; p = 0.006) and decreases in pyruvate (β = −1.90; p = 0.009), valine (β = −1.79; p = 0.023), diacylglycerol (β = −1.81; p = 0.026) and DHA (p = 0.097). Alanine was also lower (β = −1.55; p = 0.015). The IV analysis provided no strong evidence that green tea altered circulating metabolite levels (Supporting Information Table 7).
Mendelian randomisation
Causal effects of metabolites on prostate cancer
Acetate, pyruvate, valine, diacylglycerol and DHA were taken forward for MR analysis because both the intention‐to‐treat and the IV analysis indicated they were modified in response to lycopene dietary intervention, although not all metabolic traits met our strict threshold for multiple testing. Glycine was also taken forward, since increased green tea intake was associated with altered glycine levels in the intention‐to‐treat analysis.
We identified 17 genetic variants associated with our metabolites of interest at genome‐wide significance (p < 5 × 10−8 for the allelic effect of each SNP on the exposure) in the MR Base GWAS database (http://www.mrbase.org/) (Table 2). The genetic variants comprised five sets of candidate genetic instruments corresponding to acetate, valine, pyruvate, DHA and glycine. Diacylglycerol was not available in the GWAS summary statistics from Kettunen et al., 27 therefore this metabolite could not be instrumented.
Table 2.
The association of individual SNPs with metabolites
| Phenotype | Chromosome | Position | SNP | Effect allele | Other allele | EAF | Beta | SE | p‐value1 | R2 | F‐stat | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetate | 6 | 12,042,473 | rs6933521 | C | T | 0.12 | −0.092 | 0.016 | 8.10E‐09 | 0.0017 | 44.3 | 24,742 |
| Pyruvate | 2 | 27,730,940 | rs1260326 | C | T | 0.64 | −0.081 | 0.010 | 5.47E‐16 | 0.0030 | 59.4 | 22,529 |
| Pyruvate | 16 | 69,979,271 | rs74249229 | T | C | 0.05 | −0.153 | 0.023 | 2.13E‐11 | 0.0022 | 23,561 | |
| Valine | 2 | 65,208,074 | rs10211524 | A | G | 0.41 | 0.086 | 0.009 | 5.24E‐20 | 0.0036 | 79.0 | 24,898 |
| Valine | 4 | 89,206,230 | rs9637599 | C | A | 0.47 | 0.114 | 0.009 | 1.67E‐35 | 0.0064 | 24,899 | |
| Valine | 11 | 116,661,826 | rs2072560 | C | T | 0.93 | 0.105 | 0.018 | 3.28E‐09 | 0.0014 | 24,895 | |
| Valine | 17 | 7,063,667 | rs7406661 | C | T | 0.24 | 0.079 | 0.013 | 5.35E‐10 | 0.0023 | 22,659 | |
| DHA | 11 | 116,651,115 | rs11604424 | T | C | 0.76 | −0.083 | 0.018 | 7.84e‐09 | 0.0025 | 41.7 | 13,495 |
| DHA | 19 | 19,667,254 | rs143988316 | T | C | 0.07 | −0.150 | 0.026 | 1.10e‐09 | 0.0029 | 13,494 | |
| DHA | 6 | 10,990,493 | rs2281591 | G | A | 0.13 | −0.108 | 0.003 | 3.66e‐09 | 0.0026 | 13,498 | |
| DHA | 15 | 58,726,744 | rs261334 | C | G | 0.77 | −0.110 | −0.020 | 1.44e‐13 | 0.0043 | 13,498 | |
| Glycine | 2 | 210,439,980 | rs147007805 | A | T | 0.07 | −0.140 | 0.024 | 8.05E‐09 | 0.0027 | 444.6 | 18,732 |
| Glycine | 2 | 211,540,507 | rs1047891 | A | C | 0.33 | 0.487 | 0.011 | 1.00E‐200 | 0.1055 | 18,730 | |
| Glycine | 3 | 125,909,669 | rs1992855 | C | T | 0.41 | 0.062 | 0.011 | 5.60E‐09 | 0.0019 | 18,733 | |
| Glycine | 8 | 9,181,395 | rs2169387 | G | A | 0.87 | −0.130 | 0.016 | 1.31E‐16 | 0.0039 | 18,729 | |
| Glycine | 9 | 5,934,989 | rs13298772 | C | T | 0.05 | 0.273 | 0.023 | 3.74E‐33 | 0.0075 | 18,732 | |
| Glycine | 16 | 81,065,282 | rs10083777 | T | C | 0.17 | −0.106 | 0.015 | 2.97E‐13 | 0.0032 | 18,732 |
Obtained from linear regression of exposure (metabolic trait) on instrument.
SNP, single nucleotide polymorphism; EAF, effect allele frequency; SE, standard error; N, sample size; DHA, docosahexaenoic acid.
The MR analysis provided some evidence that genetically raised pyruvate increased the odds of prostate cancer by 1.29 (95% CI: 1.03, 1.62; p = 0.027 (Bonferroni corrected p value = 0.05/4 = 0.0125)), using 2 SNPs (Table 3). There was no evidence that acetate, valine DHA, or glycine were causal in prostate cancer. Given evidence of a causal effect of pyruvate on prostate cancer risk, we further investigated functionality of the SNPs used to instrument pyruvate to identify potential pleiotropy. rs1260326 is located within GCKR, coding for the glucokinase regulatory protein which has a widespread effect on metabolite levels and is therefore likely to be highly pleiotropic (Supporting Information Fig. 2) rs74249229 is not consistently associated with other metabolites apart from pyruvate, alanine and lactate, which are all very closely related metabolites. However, as the variant is located within the PDPR (pyruvate dehydrogenase phosphatase regulatory) gene, this suggests the SNP primarily influences pyruvate, and therefore alanine and lactate through vertical (rather than horizontal) pleiotropy, suggesting that this SNP is likely to be a valid instrument for the MR analysis of pyruvate. Using only this SNP as an instrument, the causal effect of pyruvate on prostate cancer was found to be 1.31 (95% CI: 0.90, 1.93, p = 0.154), consistent with a positive causal effect of pyruvate on prostate cancer risk, albeit with a wider confidence interval.
Table 3.
Causal effect estimates of metabolites on prostate cancer using individual‐level data from the PRACTICAL consortium
| Metabolite | Number of SNPs | OR† | 95% CI | P‐value |
|---|---|---|---|---|
| Acetate | 1 | 0.89 | 0.63, 1.25 | 0.501 |
| Pyruvate | 2 | 1.29 | 1.03, 1.62 | 0.027 |
| Valine | 4 | 1.03 | 0.90, 1.18 | 0.647 |
| DHA | 4 | 0.97 | 0.85, 1.01 | 0.647 |
| Glycine | 6 | 0.99 | 0.92, 1.06 | 0.787 |
Mendelian randomisation estimates of odds ratios† [OR] (and associated 95% confidence intervals [CI]) of prostate cancer risk per 1 standard deviation [SD] increase in genetically instrumented metabolite levels. Results obtained using the inverse‐variance weighted (IVW) method. DHA, docosahexaenoic acid.
Discussion
In a sample of men with an elevated risk of prostate cancer, a 6‐month intervention to increase lycopene intake was found to modify circulating valine, acetate and pyruvate, compared to placebo, which were robust to adjustment for baseline metabolites. MR analysis provided some evidence that genetically predicted higher levels of pyruvate were associated with higher prostate cancer risk, supporting a causal role for this metabolite in prostate cancer aetiology. In this small/proof‐of‐concept trial, there was insufficient evidence to say whether supplementation with green tea affected the metabolome.
Metabolic reprogramming is a recognised hallmark of cancer.43 Elevated levels of important cellular metabolites (such as pyruvate) in the circulation may support and encourage the process of carcinogenesis, by fuelling metabolic pathways that are required to support cellular proliferation. Indeed, it has been demonstrated in vitro that cancer cells proliferate more rapidly in the presence of exogenous pyruvate through the fuelling of mitochondrial metabolism.44 Extracellular pyruvate may be a particularly important metabolite for prostate cancer cells because, unlike other cancer cell types, they do not rapidly metabolise glucose.45 This may mean their reliance on extracellular pyruvate as a source of acetyl‐CoA is crucial to tumour development. Consistent with this, the monocarboxylate transporter 2 (MCT2) which shows a high affinity for the transport of pyruvate46 is increased in expression in prostate cancer tissue.47, 48, 49
It was interesting to find a reduction in valine in the intention‐to‐treat analysis, even though we found no evidence of a causal link with PCa in the MR (using the four available SNPs). This is because there is an accumulating body of evidence to show that branched chain amino acids (BCAAs), including valine, leucine and isoleucine, may help support the high metabolic demands of tumour cells.50 For example, they can serve as indirect sources of nitrogen for nucleotide (and nonessential amino acid) biosynthesis and/or they can become further catabolised to yield acetyl‐CoA, which feeds into the tricarboxylic acids (TCA) cycle and can contribute to energy production.50 Further studies are needed to confirm our findings, and to establish whether a reduction in circulating BCAAs could have an impact on PCa risk, since few studies have examined the relation of BCAAs with PCa specifically.
Some studies suggest that certain tumours have acquired a dependency on acetate as a source of carbon to produce acetyl‐CoA.51, 52 In the current analysis, we observed an increase in acetate after lycopene dietary intervention however, which needs to be verified. If confirmed, this would suggest that any potential protective effects of lycopene intake on PCa are not acting through this metabolite.
Study strengths and limitations
Our study has several strengths. Firstly, adherence to the ProDiet study was high.16 Thus, any differences in metabolites across intervention groups at follow‐up are most likely the result of the intervention itself, since all other measured variables were comparable at baseline. Secondly, we obtained metabolite measures across multiple metabolic pathways including glycolysis, the citric acid cycle and amino acid metabolism, facilitated through high‐throughput NMR, which is highly reproducible.53 Thirdly, by utilising summary statistics from a large prostate cancer consortium and effect estimates from a previous GWAS of metabolites, we were able to conduct a two‐sample MR to appraise the causal role of altered metabolites in prostate cancer risk.
Several limitations of our study warrant mention. A major limitation of our study was that the ProDiet RCT was originally designed to test the feasibility of a dietary intervention; it was not therefore powered to detect an effect of the intervention on metabolite levels. The small sample size precludes us from ruling out additional effects of lycopene and green tea dietary interventions on the serum metabolome. Furthermore, whilst the RCT design is designed to minimise residual confounding by distributing any known or unknown confounding factors across randomisation arms, there was evidence of a difference in some metabolite measures in the green tea intervention groups at baseline (attributable to chance). To address this, we conducted a sensitivity analyses, in which models were adjusted for baseline metabolite levels. The results were largely consistent.
An important aspect in any metabolomics study is the analytical reproducibility of the platform and protocols used. NMR has been shown to provide excellent reproducibility and quantitative accuracy.53 Moreover, the technique requires minimal sample preparation, decreasing the chances of analytical variability. The reproducibility of data generated from the NMR platform used in the current analysis has been assessed previously.27 The coefficients of variation ([CV] (in percent)) for selected metabolic measures are provided in Supporting Information Table 9. For pyruvate, valine, acetate, DHA and glycine (i.e. metabolites taken forwards to MR), the CVs were 4.7, 3.9, 5.5, 2.7 and 7.7% respectively.
A further potential issue, which is common feature of many ‘omics’ studies, is the assessment of many traits in a relatively small number of samples. Not considering the possible effect of multiple testing can greatly increase the probability of false positives (Type I errors), whilst an overly conservative approach may result in low statistical power to detect true positive signals (47). We used a multiple testing correction based on PCA to reduce false positives, as has been done previously (46).
We were unable to fully rule out the possibility of pleiotropy in the association between pyruvate and prostate cancer due to the limited number of genetic instruments currently available for pyruvate.
Conclusions
In summary, our results suggest that in men with elevated prostate cancer risk, increasing dietary lycopene may result in changes in circulating levels of valine, acetate, pyruvate, diacylglycerol and docosahexaenoic acid. Our results provide some evidence that pyruvate may be causally related to prostate cancer risk and warrants investigation.
Supporting information
Appendix S1: Supporting Information
Appendix S2: Supporting Information
Acknowledgements
This work has been supported by Cancer Research UK (CRUK) (ref: C11046/A10052) and the UK National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme, HTA 96/20/99; ISRCTN20141297. Further information available at: http://www.bris.ac.uk/social-community-medicine/projects/protect/. RAB is funded by a Wellcome Trust 4‐year studentship (WT099874MA). RCR is funded by CRUK (grant number: C18281/A19169). EEV is funded by an RD Lawrence Fellowship from Diabetes UK (grant number: 17/0005587). RMM is supported by CRUK (C18281/A19169). RB, DDSF, RCR, EEV, CA, MAK and RMM work in a Unit that receives funds from the University of Bristol and the UK Medical Research Council (MC_UU_12013/1, MC_UU_12013/5). AL and CM work in a unit that receives National Institute for Health Research CTU Support Funding. PW is supported by the Academy of Finland (312476 and 312477) and the Novo Nordisk Foundation (15998). MAK was supported by the Sigrid Juselius Foundation, Finland.
References
- 1. Ferlay JSI, Ervik M, Dikshit R, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase no. 11, Lyon, France: International Agency for Research on Cancer (IARC), 2013. [Google Scholar]
- 2. Sonn GA, Aronson W, Litwin MS. Impact of diet on prostate cancer: a review. Prostate Cancer Prostatic Dis 2005;8:304–10. [DOI] [PubMed] [Google Scholar]
- 3. Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol 2005;23:8152–60. [DOI] [PubMed] [Google Scholar]
- 4. Stacewicz‐Sapuntzakis M, Borthakur G, Burns JL, et al. Correlations of dietary patterns with prostate health. Mol Nutr Food Res 2008;52:114–30. [DOI] [PubMed] [Google Scholar]
- 5. Anderson RF, Fisher LJ, Hara Y, et al. Green tea catechins partially protect DNA from (.)OH radical‐induced strand breaks and base damage through fast chemical repair of DNA radicals. Carcinogenesis 2001;22:1189–93. [DOI] [PubMed] [Google Scholar]
- 6. Di Mascio P, Kaiser S, Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys 1989;274:532–8. [DOI] [PubMed] [Google Scholar]
- 7. Holzapfel NP, Holzapfel BM, Champ S, et al. The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. Int J Mol Sci 2013;14:14620–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connors SK, Chornokur G, Kumar NB. New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutr Cancer 2012;64:4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Etminan M, Takkouche B, Caamano‐Isorna F. The role of tomato products and lycopene in the prevention of prostate cancer: a meta‐analysis of observational studies. Cancer Epidemiol Biomarkers Prev 2004;13:340–5. [PubMed] [Google Scholar]
- 10. Chen P, Zhang W, Wang X, et al. Lycopene and risk of prostate cancer: a systematic review and meta‐analysis. Medicine (Baltimore) 2015;94:e1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ilic D, Forbes KM, Hassed C. Lycopene for the prevention of prostate cancer. Cochrane Database Syst Rev 2011, (11). 10.1002/14651858. Art. No.: CD008007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng J, Yang B, Huang T, et al. Green tea and black tea consumption and prostate cancer risk: an exploratory meta‐analysis of observational studies. Nutr Cancer 2011;63:663–72. [DOI] [PubMed] [Google Scholar]
- 13. Lin YW, Hu ZH, Wang X, et al. Tea consumption and prostate cancer: an updated meta‐analysis. World J Surg Oncol 2014;12:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol 2007;166:646–55. [DOI] [PubMed] [Google Scholar]
- 15. Kipnis V, Midthune D, Freedman L, et al. Bias in dietary‐report instruments and its implications for nutritional epidemiology. Public Health Nutr 2002;5(6A):915–23. [DOI] [PubMed] [Google Scholar]
- 16. Lane AJ, Er V, Knl A, et al. ProDiet: a phase II randomized placebo‐controlled trial of green tea Catechins and lycopene in men at increased risk of prostate cancer. Cancer Prev Res 2018;11(11):1–10. [DOI] [PubMed] [Google Scholar]
- 17. Lawlor DA, Harbord RM, Sterne JA, et al. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008;27:1133–63. [DOI] [PubMed] [Google Scholar]
- 18. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23(R1):R89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wurtz P, Wang Q, Kangas AJ, et al. Metabolic signatures of adiposity in young adults: Mendelian randomization analysis and effects of weight change. PLoS Med 2014;11:e1001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holmes MV, Ala‐Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol 2017;14:577–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Q, Holmes MV, Davey Smith G, et al. Genetic support for a causal role of insulin resistance on circulating branched‐chain amino acids and inflammation. Diabetes Care 2017;40:1779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2013;45:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kettunen J, Tukiainen T, Sarin AP, et al. Genome‐wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet 2012;44:269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010;466:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Illig T, Gieger C, Zhai G, et al. A genome‐wide perspective of genetic variation in human metabolism. Nat Genet 2010;42:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petersen AK, Zeilinger S, Kastenmuller G, et al. Epigenetics meets metabolomics: an epigenome‐wide association study with blood serum metabolic traits. Hum Mol Genet 2014;23:534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kettunen J, Demirkan A, Wurtz P, et al. Genome‐wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun 2016;7:11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2‐sample instrumental variable estimators. Am J Epidemiol 2013;178:1177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The Institute of Cancer Research . Welcome to PRACTICAL, London: The Institute of Cancer Research, 2017. http://practical.icr.ac.uk/blog/. [Google Scholar]
- 30. Lane JA, Donovan JL, Davis M, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol 2014;15:1109–18. [DOI] [PubMed] [Google Scholar]
- 31. Lane A, Metcalfe C, Hamdy FC, et al. Prostate and Diet Study: Protocol v1:3 2010. https://core.ac.uk/download/pdf/73984079.pdf. In press. [Google Scholar]
- 32. Bingham SA, Welch AA, McTaggart A, et al. Nutritional methods in the European prospective investigation of cancer in Norfolk. Public Health Nutr 2001;4:847–58. [DOI] [PubMed] [Google Scholar]
- 33. Craft N. Carotenoid reversedd‐phase high‐performance liquid chromatography methods: reference compendium. Methods Enzymol 1992;213:185–205. [DOI] [PubMed] [Google Scholar]
- 34. Stalmach A, Troufflard S, Serafini M, et al. Absorption, metabolism and excretion of Choladi green tea flavan‐3‐ols by humans. Mol Nutr Food Res 2009;53(Suppl 1):S44–53. [DOI] [PubMed] [Google Scholar]
- 35. Soininen P, Kangas AJ, Wurtz P, et al. High‐throughput serum NMR metabonomics for cost‐effective holistic studies on systemic metabolism. Analyst 2009;134:1781–5. [DOI] [PubMed] [Google Scholar]
- 36. Soininen P, Kangas AJ, Wurtz P, et al. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet 2015;8:192–206. [DOI] [PubMed] [Google Scholar]
- 37. Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol 2008;32:361–9. [DOI] [PubMed] [Google Scholar]
- 38. Wang Q, Kangas AJ, Soininen P, et al. Sex hormone‐binding globulin associations with circulating lipids and metabolites and the risk for type 2 diabetes: observational and causal effect estimates. Int J Epidemiol 2015;44:623–37. [DOI] [PubMed] [Google Scholar]
- 39. Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol 2000;29:1102. [DOI] [PubMed] [Google Scholar]
- 40. Angrist JIG, Rubin D. Identification of causal effects using instrumental variables. J Am Stat Assoc 1996;91:444–55. [Google Scholar]
- 41. Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res 2007;16:309–30. [DOI] [PubMed] [Google Scholar]
- 42. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol 2015;44:512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 44. Diers AR, Broniowska KA, Chang CF, et al. Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition. Biochem J 2012;444:561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis 2006;9:230–4. [DOI] [PubMed] [Google Scholar]
- 46. Adeva‐Andany M, Lopez‐Ojen M, Funcasta‐Calderon R, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014;17:76–100. [DOI] [PubMed] [Google Scholar]
- 47. Pertega‐Gomes N, Baltazar F. Lactate transporters in the context of prostate cancer metabolism: what do we know? Int J Mol Sci 2014;15:18333–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pertega‐Gomes N, Vizcaino JR, Felisbino S, et al. Epigenetic and oncogenic regulation of SLC16A7 (MCT2) results in protein over‐expression, impacting on signalling and cellular phenotypes in prostate cancer. Oncotarget 2015;6:21675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pertega‐Gomes N, Vizcaino JR, Gouveia C, et al. Monocarboxylate transporter 2 (MCT2) as putative biomarker in prostate cancer. Prostate 2013;73:763–9. [DOI] [PubMed] [Google Scholar]
- 50. Ananieva EA, Wilkinson AC. Branched‐chain amino acid metabolism in cancer. Curr Opin Clin Nutr Metab Care 2018;21:64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kamphorst JJ, Chung MK, Fan J, et al. Quantitative analysis of acetyl‐CoA production in hypoxic cancer cells reveals substantial contribution from acetate. Cancer Metab 2014;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alderton GK. Metabolism: acetate nourishes stressed tumour cells. Nat Rev Cancer 2015;15:67. [DOI] [PubMed] [Google Scholar]
- 53. Markley JL, Bruschweiler R, Edison AS, et al. The future of NMR‐based metabolomics. Curr Opin Biotechnol 2017;43:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Appendix S2: Supporting Information


