Abstract
In this issue of Neuron, Stoeber et al. (2018) report a biosensor resolving the spatiotemporal organization of opioid receptor activation in living neurons. They delineate novel signaling mechanisms in endosomes and Golgi differentially engaged by opioid peptides and drugs.
Neuronal G-protein-coupled receptors (GPCRs) mediate the effects of a wide array of sensory stimuli, neurotransmitters, hormones, and exogenous drugs, including opioids. To battle the opioid epidemic (Volkow and McLellan, 2016), neuroscientists are currently pursuing two main research goals. First, discover novel non-opioid, analgesic treatments by resolving the neural mechanisms underlying pain perception. Second, eliminate the side effects of opioid analgesics by elucidating the mechanisms of action of opioids at the molecular, cellular, and neural circuit levels. The study by Stoeber and colleagues in this issue of Neuron (Stoeber et al., 2018) brings us closer to this second goal by shedding light on the subcellular localization of opioid receptors (ORs) that are activated and signal in response to opioid drugs.
Three main ORs mediate the effects of opioid ligands: the delta, kappa, and mu opioid receptors (DORs, KORs, MORs, respectively). In neural circuits, the enkephalins, dynorphins, and endorphins are endogenous opioid peptide agonists that act as neuromodulators by binding to ORs. Activated ORs then signal via a panoply of effectors that regulate neuronal function (Williams et al., 2013; Corder et al., 2018). ORs can then undergo internalization in endosomes followed by recycling to the plasma membrane (PM) or degradation in lysosomes. Interactions between endogenous opioid peptides and ORs are thought to fine-tune activity in PNS and CNS neural net works and to broadly influence behavior. An unresolved question in the opioid field is whether exogenous naturally occurring alkaloids (e.g., morphine) or synthetic/semi-synthetic drugs (e.g., fentanyl, etorphine) mimic the actions of endogenous peptides or, rather, have distinct signaling mechanisms that could contribute to their deleterious effects, in particular, drug abuse.
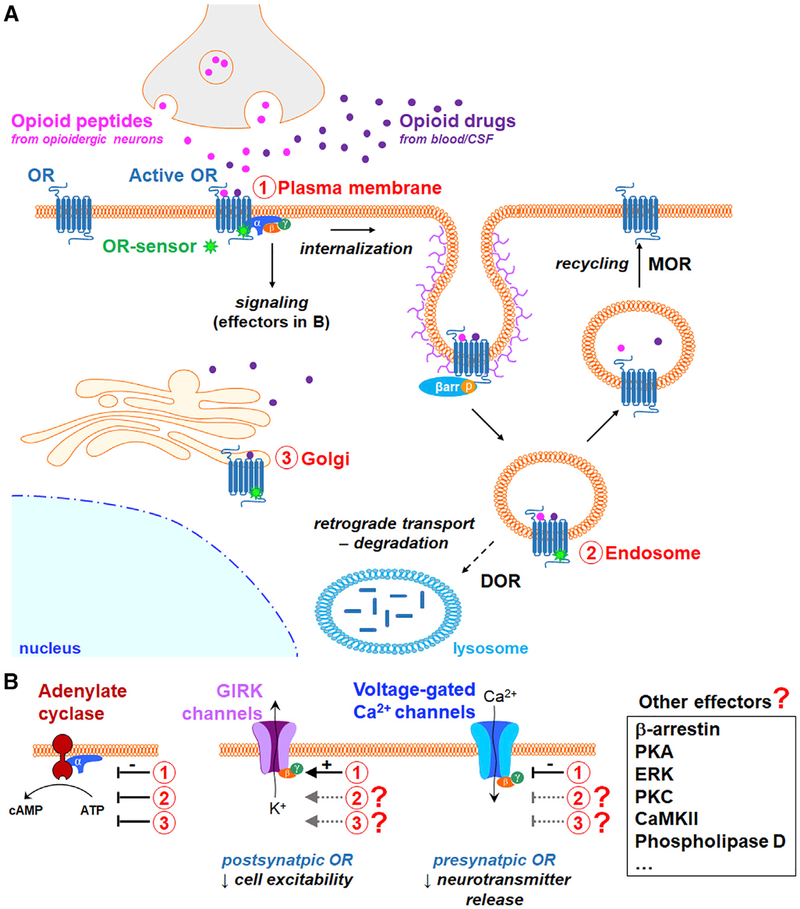
Despite a wealth of pharmacological studies, no major signaling differences could be identified; both exogenous opioid drugs and endogenous peptides were assumed to engage largely similar signaling pathways via PM ORs (Williams et al., 2013; Corder et al., 2018). With two transformative findings, Stoeber et al. (2018) propel the opioid field in a new direction. First, they show that signaling DORs and MORs are not only present at the PM of neurons, but also in intracellular endosomal vesicles and, unexpectedly, in the Golgi apparatus. Second, they found that while both agonist types activate ORs at the PM and endosomes, only opioid drugs activate ORs in Golgi, uncovering a fundamental difference between endogenous peptidergic- and exogenous drug-induced signaling.
The spatiotemporal organization of OR signaling in neurons has remained largely elusive. Previous real-time imaging studies of OR ligand binding and trafficking using fluorescent agonists or ORs did not specifically label ORs in active state. It thus remained unclear whether intracellular ORs, agonist bound or not, continue to signal following internalization. To address this, Stoeber et al. (2018) exploited single-domain antibody fragments called nanobodies that stabilize the MOR active state (Manglik et al., 2017). Building on a strategy that they used previously to study β1 and β2 adrenergic receptor signaling (Irannejad et al., 2013, 2017), Stoeber et al. (2018) fused such a nanobody to GFP (Nb33-GFP) to produce a genetically encoded fluores-cent conformational biosensor that binds to ORs in the ligand-bound active state (OR sensor). In HEK293 cells transfected to co-express the OR sensor and MOR, Stoeber et al. (2018) observed that DAMGO, a MOR-selective synthetic enkephalin analog, caused robust OR sensor labeling of the PM, where DAMGO-activated MORs are localized. Moreover, the OR sensor dynamically reported MOR activation and deactivation, as the patterns of OR sensor recruitment to the PM were reversed by washing out DAMGO or application of a competitive MOR antagonist. Stoeber et al. (2018) obtained similar results in cells expressing DOR and with DOR ligands, but not with the M2 muscarinic receptor and its agonist carbachol. These experiments establish that this novel OR sensor allows real-time and selective detection of ORs activated by agonists in a heterologous expression system.
Using this OR sensor, Stoeber et al. (2018) found that opioid agonists generate not one, but two spatially and temporally distinct waves of receptor activation, initially at the PM, followed by a secondary OR activation in endosomes after internalization (Figure 1A). Since reversal of OR activation in endosomes required antagonists able to cross the PM, this sustained endosomal OR activation is agonist dependent. Further, Stoeber et al. (2018) provided evidence that endosomal OR activity can regulate adenylate cyclase activity and cAMP levels. Collectively, these studies reveal that all DOR and MOR agonists that trigger receptor internalization also engage these two signaling waves regardless of their chemical or endogenous versus exogenous identity (i.e., endogenous peptides such as β-endorphin and met-enkephalin, synthetic and semi-synthetic exogenous peptides and drugs such as DAMGO and etorphine).
Figure 1.
Three Waves of Signaling by Activated Opioid Receptors at the Plasma Membrane, in Endosomes, and in Golgi Are Differentially Engaged by Opioid Peptides versus Opioid Drugs
GPCR signaling is cell context dependent and may be influenced by receptor expression levels. Next, Stoeber et al. (2018) investigated OR activation in cultured primary neurons from striatum, a brain region that contains neurons expressing ORs and is involved in opioid addiction. Consistent with their findings using non-neuronal cells, agonists generated OR sensor recruitment to the PM and in endosomes, in both the soma and neu-rites, and in neurons expressing epitope-tagged ORs. Stoeber et al. (2018) also investigated signaling of endogenous ORs and found that the application of the MOR peptide agonist dermorphin conjugated to a fluorescent molecule led to OR sensor signal at the PM as well as in endosomes where OR sensor and dermorphin colocalized. These results demonstrate the existence of active endogenous ORs in the PM and endosomes of neurons following agonist exposure.
So far, Stoeber et al. (2018) took advantage of their OR sensor to validate a hypothesis that had been proposed (i.e., ORs in endosomes may remain active). In contrast, with their last set of data, they formulate a new concept for the opioid field; opioid drugs have a distinct effect on OR activity compared to peptides because they have access to additional OR populations in intracellular organelles. Specifically, they show that drugs such as morphine generate a third wave of intracellular OR activation that regulates cAMP levels at the Golgi apparatus, including satellite organelles along the dendrites termed Golgi outposts. Stoeber et al. (2018) conclude that the distortion of intracellular OR activation by opioid drugs stems from their ability to efficiently diffuse across membranes compared to peptides with low membrane permeability.
Several outstanding questions arise from this study. First, what is the relevance of these novel loci of receptor signaling to opioid control of neuronal function and behavior, including by other GPCR effectors? Stoeber et al. (2018) focused their study of endosomal and Golgi OR signaling on the adenylate cyclase inhibition by Gαi/o proteins. It will be particularly exciting to establish the function of the adenylate cyclase inhibition mediated specifically by Golgi ORs. Additionally, there are many other intra-cellular signaling molecules regulated by ORs, including β-arrestins, kinases, or phospholipases (Corder et al., 2018). Essential for neurons are ion channel effectors, which critically contribute to opioid analgesia and reward. For example, activation of pre-synaptic ORs in axon terminals depresses neurotransmitter release by inhibiting voltage-gated calcium channels (VGCCs), while ORs active postsynaptically in somato-dendritic compartments activate G-protein-coupled inwardly rectifying potassium (GIRK or Kir3) channels, hyperpolarizing the membrane potential and reducing cell excitability (Figure 1B). In contrast to adenylate cyclase, OR modulation of VGCCs and GIRK channels is mediated by Gβγ subunits and is only effective for neurotransmission modulation if the ion channels are present at the PM. This arrangement raises a number of questions. Among all OR signaling effectors, including ion channels, which ones are modulated by ORs at the PM, in endosomes, and, more intriguingly, in the Golgi? Relatedly, what are the trafficking properties of Gβγ subunits that are released from activated Golgi ORs, and up to what intracellular distance can these Gβγ subunits regulate effectors; can they control effectors, such as ion channels, at the PM and, if so, exclusively in soma or dendrites or also in distant axon terminals? Answers to these questions might even depend on the type of neurons studied. For example, OR-mediated signaling may differ between small interneurons and long-range projection neurons. This opens the possibility that opioid drugs exhibit distinct spatiotemporal patterns of signaling and differentially contribute to opioid effects, such as analgesia versus reward, based on the morphological properties of the neurons mediating these behaviors.
Second, what signaling OR populations are recruited by endogenously released peptides? Stoeber et al. (2018) used cultured striatal neurons to investigate signaling of endogenously expressed ORs with application of endogenous peptides and drugs to the media. This approach may model how systemically administered opioid drugs broadly diffuse in the extracellular space from the blood and cerebrospinal fluid to access diverse pools of neuronal ORs, yet it is unclear that the concentrations and molecules used in vitro correspond to those found in vivo (e.g., morphine metabolites). In contrast, discrete neuronal populations release endogenous peptides, such as enkephalin, at precise sites in circuits, and the identification of ORs on which they act during volume transmission is challenging. It will thus be particularly interesting to reexamine enkephalinergic OR signaling in native neural circuits. Combining the OR sensor with enkephalinergic neuron-specific optogenetics would permit the gradual release of enkephalins from their native release sites in a light intensity-dependent manner (François et al., 2017). This may elucidate opioid peptidergic transmission principles by identifying the ORs that bind enkephalins and their locations in networks and within neurons.
Third, does endosomal signaling contribute to the differential effects of exogenous DOR and MOR agonists, including on pain, reward, and emotions (Corder et al., 2018)? For example, clinically used MOR analgesics are particularly potent for controlling perioperative pain, while pre-clinical studies suggest that DOR agonists might be more useful for controlling neuropathic tactile hyper-sensitivity. These distinct properties are thought to result primarily from the differential distribution of DOR and MOR in circuits (Corder et al., 2018; Erbs et al., 2015). However, while PM DORs and MORs are generally thought to use similar effectors in neurons, they are known to differ in their post-activation intracellular fate. While endosomal MORs are recycled to the PM following internalization, endosomal DORs are trafficked to the perinu-clear region and degraded in lysosomes (Figure 1A). Considering the findings by Stoeber et al. (2018), MOR agonists may primarily cause persistent endosomal signaling locally in the vicinity of the initially activated surface receptors, while DOR agonists may generate signaling in distant intracellular sites. For example, activated pre-synaptic DORs may be retrogradely trafficked from the axon terminal to the cell body and generate signaling all along the neurites. Such mechanisms could begin to explain the contribution of these two ORs to enkephalinergic neuromodulation. The development of DOR- and MOR-specific sensors could reveal the subcellular localizations of DORs and MORs responding to enkephalins, including in neurons that express both receptors in pain circuits (Wang et al., 2018).
In summary, answering the many questions that emerge from the novel findings by Stoeber et al. (2018) is likely to uncover multiple additional layers of complexity in OR signaling, which may represent novel opportunities for dissociating opioid anal-gesia from side effects, an urgent necessity in the battle against the opioid epidemic.
REFERENCES
- Corder G, Castro DC, Bruchas MR, and Scherrer G (2018). Endogenous and exogenous opioids in pain. Annu. Rev. Neurosci 41, 453–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, Koch M, Kessler P, Hentsch D, Birling MC, et al. (2015). A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct. Funct 220, 677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, et al. (2017). A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 93, 822–839.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J, Rasmussen SG, Sunahara RK, El-Samad H, Huang B, and von Zastrow M (2013). Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Pessino V, Mika D, Huang B, Wedegaertner PB, Conti M, and von Zastrow M (2017). Functional selectivity of GPCR-directed drug action through location bias. Nat. Chem. Biol 13, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manglik A, Kobilka BK, and Steyaert J (2017). Nanobodies to study G protein-coupled receptor structure and function. Annu. Rev. Pharmacol. Toxicol 57, 19–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber M, Jullié D, Lobingier BT, Laeremans T, Steyaert J, Schiller PW, Manglik A, and von Zastrow M (2018). A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 98, this issue, 963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, and McLellan AT (2016). Opioid abuse in chronic pain–misconceptions and mitigation strategies. N. Engl. J. Med 374, 1253–1263. [DOI] [PubMed] [Google Scholar]
- Wang D, Tawfik VL, Corder G, Low SA, Fran-çois A, Basbaum AI, and Scherrer G (2018). Functional divergence of delta and mu opioid receptor organization in CNS pain circuits. Neuron 98, 90–108.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, Koch T, Evans CJ, and Christie MJ (2013). Regulation of m-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev 65, 223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]