Abstract
Zeitgebers such as light, eating and physical activity provide input to the circadian clock. Chronic circadian misalignment is associated with significant adverse health effects. An improved understanding of the impact of the timing of zeitgebers on the stability of 24-hour rest-activity rhythm in free-living settings may identify behavioral and environmental intervention targets. 133 healthy adults, aged 21 to 60 years, wore a wrist actigraph for 7 consecutive days. We applied a non-parametric analysis to activity counts to derive rest-activity patterns. We administered a questionnaire through a smartphone app to collect self-reported timing of light exposure, eating episodes and physical activity. To assess the relationship between timing exposures (first and last exposure to outdoor light, first exposure to indoor light, last eating episode, first eating episode, morning physical activity proportion, evening physical activity proportion) and rest-activity or sleep outcomes (bedtimes, total sleep time, inter-daily stability, intra-daily variability, L5 and M10 midpoint), we first calculated Spearman correlations, using the false discovery rate method to control for multiple comparisons. From those significant associations, we then fit regression models adjusting for age, sex, race, household income, education level, study site, body mass index, as well as physical activity. Finally, we tested for interaction between chronotype and each timing-related exposure and stratified the analysis by morning type. All zeitgebers, except for evening physical activity proportion, were correlated with at least 4 of the 7 sleep and rest-activity outcomes. In adjusted analysis, later timing of first (after 6:30 to 7:45 AM versus earlier) and last exposure to indoor light (after 11:00 PM versus earlier) and first (after 7:45 to 9:45 AM versus earlier) and last eating episode (after 8:00 to 09:00 PM versus earlier) were associated with a shift of 0.60 to 1.39 hours to later bedtimes, M10 and L5 midpoints (i.e. timing of peak activities or inactivities). Later timing of first exposure to outdoor light (after 09:30 AM versus earlier) was also associated with 0.51 (95% CI: 0.19 to 0.83) hours longer total sleep time. Higher morning physical activity proportion (> 33%) was associated with 0.95 (95% CI: −1.38 to −0.53) hours earlier in-bed time and 0.69 (95% CI: −1.14 to −0.24) hours earlier out-of-bed time, 0.92 (95% CI: −1.41 to −0.42) hours earlier M10 and 0.96 (95% CI: −1.42 to −0.49) min earlier L5 midpoint. The results did not change substantially with further adjustment for total activity. There was a significant interaction between morning chronotype and first eating episode with rest-activity patterns (p<0.05), with first eating episode associating with timing of activities only in non-morning type adults. Timing of zeitgebers was associated with sleep and rest-activity patterns, including bedtimes, L5 and M10 midpoint. Future research should evaluate the impact of manipulating zeitgebers on both circadian rhythms and health outcomes.
Keywords: Actigraphy, Rest-activity patterns, Physical activity
Introduction
“Zeitgebers” refer to environmental and social cues that provide input to the circadian system and help to synchronize biological rhythms (Aschoff, Hoffmann et al., 1975). While light is the chief environmental cue (Pittendrigh, 1964), nonphotic stimuli, such as food intake and physical activity, can also influence circadian rhythms (Mistlberger & Skene, 2004; 2005). The importance of food exposure as a zeitgeber is supported by both animal and human experimental research (Stokkan, Yamazaki et al., 2001; Wehrens, Christou et al., 2017). For example, a rodent study demonstrated that the central biological clock in the suprachiasmatic nucleus can be entrained by diurnal cyclic exposure to food (Castillo, Hochstetler et al., 2004; Mendoza, Angeles-Castellanos et al., 2005). In humans, a laboratory-based study of 32 healthy women demonstrated that changes in meal timing affected circadian-related parameters such as temperature and cortisol, as well as glucose tolerance (Bandin, Scheer et al., 2015). The role of exercise as a zeitgeber is also supported by experimental research. Exercise or physical activity has different physiological effects according to its timing. Specifically, a laboratory study showed that evening, but not morning, exercise delayed the circadian rhythm by about 1 hour (as indicated by the rising phase of plasma melatonin) (Yamanaka, Hashimoto et al., 2015).
While these laboratory-based studies have helped to define the physiological impact of various environmental cues on biological rhythms, there is a gap in our understanding of how timing of energetic behaviors (e.g. meals, exercise) and light exposure may alter the stability of 24-hour activity rhythms and sleep in the free-living setting. Such research is needed given the large proportion of the population who experience curtailed sleep and/or “social jet-lag”— a phenomenon where marked changes in sleep and activity patterns on weekdays and weekends occur, resulting in chronic circadian misalignment and increased health risks, including obesity, cardio-metabolic disease and cancer (Arble, Bass et al., 2009; Gangwisch, 2009; Laing, Johnston et al., 2015; Robinson, Wu et al., 2013; Roenneberg, Allebrandt et al., 2012; Wittmann, Dinich et al., 2006).
In our study of 133 healthy adults, we examine associations between the timing of zeitgebers on the stability of 24-hour rest-activity rhythms and sleep in the free-living setting. We hypothesize that exposures to light, physical activity and eating episodes later (vs earlier) in the day are associated with more irregular and less robust rest-activity patterns and later timing of daily rest-activity patterns and sleep. Furthermore, given the growing appreciation of health effects of circadian misalignment, we speculate that misalignment of chronotype with actual diurnal patterns will modify the associations between zeitgebers and timing of rest-activity patterns and sleep.
Methods
Participants
Data from the Impact of Nocturnal Zeitgebers on Energy in TREC (INZEIT) study, supported by National Cancer Institute’s Transdisciplinary Research on Energetics and Cancer (TREC) was used for this study (Patterson, Colditz et al., 2013). Between November 2015 and March 2016, 133 adults aged 21–60 years were enrolled in three sites: Philadelphia, PA, San Diego, CA, and St, Louis, MO. Exclusion criteria were a report of a diagnosed sleep disorder, recent illnesses requiring bed rest, disability restricting movements, and regular travel across time zones, shift-work, pregnancy or breastfeeding. Study staff measured height and weight; age, race, ethnicity, sex, income and education were self-reported. Chronotype was assessed using the 19-item Horne-Ostberg Morningness Eveningness Questionnaire (Horne & Ostberg, 1976). Details of the study protocol have been described previously (Cespedes Feliciano, Quante et al., 2017; Mitchell, Quante et al., 2017). All participants provided informed consent. Institutional Review Boards approved the study protocol.
Actigraphy Protocol
Subjects were asked to wear an actigraph (GT3X; ActiGraph, Pensacola, FL) on their non-dominant wrist, for 7 consecutive days, only removing it when bathing or swimming. We recently validated the GT3X+ actigraph for detecting sleep and wakefulness versus polysomnography in adults and teens, showing high sensitivity to detect sleep periods, but low specificity to detect wake (Quante, Kaplan et al., 2018). Activity counts for the GT3X+ were collected in 1-minute epochs. Actigraphy data were processed at Brigham and Women’s Hospital (Boston, MA) using device-based software ActiLife (version 6.7). We discarded recording days (from 7:00 AM to 6:59 AM of the following day) if there were less than 10 hours of valid signal during wakefulness. Recordings with less than 4 valid days were also excluded.
Sleep Variables
For each 24-hour period, we manually identified a main rest interval as the primary sleep period based on self-completed logs and observation of a sharp decrease/increase in activity on the actigraph. Time in and out of bed was estimated using the start and end points of this interval. Then, we classified epochs as sleep or wake within this time employing the Cole-Kripke algorithm (Cole, Kripke et al., 1992). From these data, we calculated Total Sleep Time (TST) for each night.
Rest-activity Rhythm Analysis:
The most commonly used approach to modeling rest-activity patterns is the cosinor analysis, which fits the data with a sinusoidal shape. Non-parametric analyses of rest-activity rhythms were introduced to quantify rhythms independent from the assumption of sinusoidal shape, after it was observed that an asymmetrical square shape may be better suited to describe the pattern of the activity time series (Dowling, Hubbard et al., 2005; Van Someren, Kessler et al., 1997). This approach also provides indices on fragmentation and stability/instability in rest-activity rhythms. We elected to employ the non-parametric approach for our analysis, but we also briefly report the results of the cosinor approach in the supplement, which parallel the methods of our non-parametric analyses. A full description of the variables is available in a previous publication (Mitchell, Quante et al., 2017).
The code we used for the extraction of parametric and nonparametric variables has been made publicly available as the Matlab/Octave toolbox ActiCircadian, at https://sleepdata.org/community/tools/acticircadian.
PACO Smartphone Application
We used an open-source, customizable smartphone application, Personal Analytics Companion (PACO, www.pacoapp.com), to collect questionnaire data from the study subjects during the same 7-day period as the actigraphy recordings. The app prompted participants every day at 9 am, 1 pm and 7 pm to complete surveys, which included questions about timing of sleep, physical activity, light exposure, and meals. Each survey question had a range of response options that consisted of ordered time intervals (see table S1). For timing of exposure to sleep, light and meals, we computed the median timing category for each exposure across days. We treated these summary variables as ordered categorical variables, where larger numbers corresponded to later times. Response options that did not indicate a time frame, e.g. “I wasn’t outdoors”, were treated as missing values. For timing of exposure to physical activity, due to a large proportion of the participants choosing the response option “I did not do any moderate or vigorous activities”, we could not summarize activity across measurement days without considerable missing data. Thus, we summarized the variables across days for each participant by calculating the percentage of days that they were physically active in the morning (before 12 pm) and evening (after 7 pm). The final variables were: timing of last exposure to indoor light, timing of last exposure to outdoor light, timing of first exposure to indoor light, timing of last eating episode, timing of first eating episode, morning physical activity proportion, and evening physical activity proportion. Table S1 summarizes the exposures used in the analysis. Table S2 summarizes the outcomes.
Statistical Analyses
Analyses were restricted to participants with at least 5 days of PACO survey concurrent with actigraphy data (7 subjects were excluded, resulting in n=126). We calculated the Spearman correlation coefficients between 7 timing-related exposures (from surveys) and 7 actigraphy-estimated non-parametric rest-activity or sleep patterns. After controlling for multiple comparisons using the false discovery rate at 5% level (Benjamini & Hochberg, 1995), we included 25 significant associations for subsequent multiple regression analyses.
We dichotomized our exposures using cutoff values (reported in Table S3). These values were chosen based on the median of the exposure values over our population, with appropriate round-ups. Then, we modeled the relationship between each of the selected sleep and non-parametric rest-activity outcomes and each of the selected timing-related exposures. In our first model, we adjusted for age, sex, race, household income, education level, study site, BMI (body mass index), all considered as categorical variables, except for age and BMI. In our second model, we additionally adjusted for total physical activity, expressed as the mean counts per minute (CPM) per wear-time on the vertical axis from actigraphy.
We further explored for interaction between chronotype (morning type versus non-morning type) and the timing-related exposures. There is some evidence that the chronotype might influence the effect of the social jet-lag on individuals. For example, a study by and colleagues demonstrated that late chronotypes have the highest level of social jet-lag in day-working populations (Wittmann, Dinich et al., 2006), while Juda and colleagues uncovered a modulating effect of chronotype on sleep duration, social jetlag and sleep disturbances in shift workers (Juda, Vetter et al., 2013).
We performed an interaction test between six dichotomized exposures (last exposure to indoor light, first exposure to indoor light, last exposure to outdoor light, last eating episode, first eating episode, proportion of days with morning PA) and chronotype for two outcomes (L5 midpoint and M10 midpoint) based on our hypothesis, and adjusted for main effects of age, sex, race/ethnicity, household income, education level, study site and BMI. The effect modifier under analysis was “morning type” (yes vs. no). Stratified analyses were performed according to variables associated with a significant interaction term with morning type (p<0.05). As noted earlier, we also performed all analyses, including interaction tests and stratified models, on parametric rest-activity pattern variables (Table S4, S5 and Figure S1). We performed all analyses using SAS 9.3 (SAS Institute, Inc., Cary, NC).
Results
Approximately 37% of the subjects in our analytical sample (N=126) were men and 42% were of minority race/ethnicity (Table 1). Spearman’s correlation analysis showed that all timing-related exposures, except for evening physical activity proportion, were significantly associated with at least 4 of the 7 sleep and rest-activity outcomes. Specifically, timing of exposure to indoor and outdoor light and first and last eating episodes were positively correlated with actigraphy-based assessments of timing of in/out of bedtimes, and L5 and M10 midpoint (Table 2; p<0.001). Interestingly, IS and IV did not correlate with any of the zeitgebers.
Table 1.
Characteristics of the INZEIT study population (N=126).
| Overall (N=126) | Non-morning type (N=70) | Morning type (N=56) | P-value | |
|---|---|---|---|---|
| Age, mean (SD), median (Q1–Q3) | 35.4 (11.0), 32.0 (26.0–45.0) | 32.8 (10.2), 29.0 (24.0–41.0) | 38.6 (11.2), 37.5 (29.0–47.5) | 0.001 |
| Race, N (%) | ||||
| White | 73, 57.9 % | 38, 54.3 % | 35, 62.5 % | 0.35 |
| Other | 53, 42.1 % | 32, 45.7 % | 21, 37.5 % | |
| Hispanic, N (%) | 10, 7.9 % | 5, 7.1 % | 5, 8.9 % | 0.75 |
| Male, N (%) | 46, 36.5 % | 22, 31.4 % | 24, 42.9 % | 0.19 |
| BMI (kg/m2), mean (SD), median (Q1–Q3) | 27.6 (6.5), 26.4 (22.5 – 31.4) | 27.9 (6.7), 27.2 (22.3 – 31.8) | 27.3 (6.2), 25.8 (22.5 – 31.0) | 0.58 |
| Education, N (%) | ||||
| College or above | 77, 61.1 % | 47, 67.1 % | 30, 53.6 % | 0.12 |
| Less than full college | 49, 38.9 % | 23, 32.9 % | 26, 46.4 % | |
| Income, N (%) | ||||
| Less than 50k | 65, 51.6 % | 35, 50.0 % | 30, 53.6 % | 0.97 |
| At least 50K | 49, 38.9 % | 28, 40.0 % | 21, 37.5 % | |
| Not reported | 12, 9.5 % | 7, 10.0 % | 5, 8.9 % | |
| Chronotype, N (%) | ||||
| Definitely evening type | 0 (0%) | |||
| Moderately evening type | 11 (8.7%) | |||
| Neither type | 59 (46.8%) | |||
| Moderately morning type | 49 (38.9%) | |||
| Definitely morning type | 7 (5.6%) | |||
| Outcomes | ||||
| In-bed time (hours from midnight), mean (SD), median (Q1–Q3) | −0.5 (1.2), −0.5 (−1.3 – 0.2) | −0.0 (1.2), −0.1 (−0.9, 0.6) | −1.1 (1.1), −1.2 (−1.8, −0.5) | <0.001 |
| Out-of-bed time (hours from midnight), mean (SD), median (Q1–Q3) | 7.5 (1.3), 7.4 (6.7 8.2) | 8.1 (1.3), 8.0 (7.3 8.7) | 6.8 (0.9), 6.8 (6.2 7.5) | <0.001 |
| Total sleep time (hours), mean (SD), median (Q1–Q3) | 6.9 (0.9), 7.1 (6.3 – 7.5) | 7.1 (0.9), 7.1 (6.3, 7.6) | 6.8 (0.8), 7.0 (6.3, 7.4) | 0.07 |
| L5 midpoint (dec. hours), mean (SD), median (Q1–Q3) | 3.2 (1.4), 3.2 (2.3 – 4.0)* | 3.7 (1.3), 3.7 (2.9, 4.6)** | 2.5 (1.2), 2.5 (1.9, 3.4)*** | <0.001 |
| M10 midpoint (dec. hours), mean (SD), median (Q1–Q3) | 14.8 (1.5), 14.8 (13.9, 15.9)* | 15.4 (1.3), 15.5 (14.4, 16.3)** | 14.2 (1.5), 14.3 (13.3, 15.2)*** | <0.001 |
Mean (SD) and median (Q1–Q3) were presented for continuous measures, N and column % were presented for categorical measures. P values were based on parametric (ANOVA for continuous variables, chi-squared for categorical variables) and non-parametric tests (Wilcoxon rank sum test for continuous variables, Fisher exact test for categorical variables) where appropriate to compare distribution of these measures. Abbreviations: dec. hours=decimal hours, IS= inter-daily stability, IV=intra-daily variability, Q=quartile, SD= standard deviation.
N=118 (8 subjects had insufficient data to derive rest-activity rhythms)
N=65 (5 subjects had insufficient data to derive rest-activity rhythms)
N=53 (3 subjects had insufficient data to derive rest-activity rhythms)
Table 2.
Spearman correlations among timing exposures, sleep and non-parametric rest-activity outcomes.
| In-bed time (hours from midnight) | Out-of-bed time (hours from midnight) | Total sleep time (hours) | IS | IV | L5 midpoint (dec. hours) | M10 midpoint (dec. hours) | ||
|---|---|---|---|---|---|---|---|---|
| Timing of last exposure to Indoor light | r | 0.62 | 0.54 | −0.01 | −0.08 | 0.05 | 0.59 | 0.50 |
| p | <0.001 | <0.001 | 0.92 | 0.37 | 0.61 | <0.001 | <0.001 | |
| N | 125 | 125 | 125 | 117 | 117 | 117 | 117 | |
| Timing of first exposure to outdoor light | r | 0.46 | 0.46 | −0.03 | 0.09 | −0.02 | 0.49 | 0.29 |
| p | <0.001 | <0.001 | 0.76 | 0.35 | 0.85 | <0.001 | 0.001 | |
| N | 125 | 125 | 125 | 117 | 117 | 117 | 117 | |
| Timing of first exposure to indoor light | r | 0.52 | 0.70 | 0.27 | 0.01 | −0.07 | 0.69 | 0.50 |
| p | <0.001 | <0.001 | 0.003 | 0.89 | 0.48 | <0.001 | <0.001 | |
| N | 126 | 126 | 126 | 118 | 118 | 118 | 118 | |
| Timing of last eating episode | r | 0.55 | 0.56 | 0.05 | −0.04 | −0.04 | 0.57 | 0.43 |
| p | <0.001 | <0.001 | 0.57 | 0.68 | 0.64 | <0.001 | <0.001 | |
| N | 125 | 125 | 125 | 117 | 117 | 117 | 117 | |
| Timing of first eating episode | r | 0.37 | 0.42 | 0.07 | 0.07 | −0.08 | 0.40 | 0.34 |
| p | <0.001 | <0.001 | 0.42 | 0.49 | 0.39 | <0.001 | <0.001 | |
| N | 126 | 126 | 126 | 118 | 118 | 118 | 118 | |
| Morning PA proportion | r | −0.27 | −0.29 | −0.09 | −0.10 | 0.02 | −0.33 | −0.31 |
| p | 0.002 | 0.001 | 0.31 | 0.28 | 0.83 | <0.001 | 0.001 | |
| N | 126 | 126 | 126 | 118 | 118 | 118 | 118 | |
| Evening PA proportion | r | 0.11 | 0.11 | −0.19 | −0.02 | −0.07 | 0.13 | 0.18 |
| p | 0.25 | 0.27 | 0.05 | 0.84 | 0.51 | 0.21 | 0.07 | |
| N | 109 | 109 | 109 | 101 | 101 | 101 | 101 |
Abbreviations: dec. hours=decimal hours, IS= inter-daily stability, IV=intra-daily variability, PA=physical activity, p=p-value.
Table 3 reports the results of the regression analyses using the 25 associations selected in the correlation analysis as significant in univariate analysis. We observed significant positive associations between later exposure to indoor light (after 11:00 PM versus earlier) with more than one hour later in-and out-of-bed times, M10 midpoint, and L5 midpoint in model 1. Further adjustment for total activity in model 2 did not significantly attenuate the reported associations. Similarly, later timing of first exposure to indoor light (after 6:30 to 7:45 AM versus earlier), and first (after 7:45 to 09:45 AM versus earlier) and last (after 8:00 to 09:00 PM versus earlier) eating episode had significant associations with the same variables, for both models. In model 1, later time of first exposure to outdoor light (after 09:30 AM versus earlier) also associated with about 30 minutes longer total sleep time. Higher morning physical activity proportion (> 33%) was associated with about one hour earlier in-bed time and about 40 minutes earlier out-of-bed time, and about 1 hour earlier M10 midpoint and L5 midpoint in model 1. These results did not change substantially with controlling for total activity in model 2. Overall, the magnitude of association differed and was highest between the following exposures and outcomes (rank order): timing of first exposure to outdoor light and out-of-bed time, timing of first exposure to indoor light and L5 midpoint, timing of last exposure to indoor light and in-bed time, and timing of last exposures to indoor light and L5 midpoint. The magnitude of association was lowest between first exposure to outdoor light and sleep duration, and first eating episode with M10 midpoint. Very similar results to those found for M10 midpoint were observed for the cosinor rest-activity pattern acrophase (Table S4 and S5).
Table 3.
Regression results among 25 non-parametric rest-activity and sleep outcomes and timing exposures combinations selected by the correlation analysis. Beta values (confidence intervals).
| Outcomes | ||||||
|---|---|---|---|---|---|---|
| Exposures | In-bed time (hours from midnight) | Out-of-bed time (hours from midnight) | Total sleep time (hours) | L5 midpoint (dec. hours) | M10 midpoint (dec. hours) | |
|
Last exposure to
indoor light |
Model 1 | 1.32 (0.84,1.79) | 1.13 (0.64,1.62) | 1.32 (0.80,1.83) | 1.24 (0.68,1.79) | |
| Model 2 | 1.31 (0.86,1.77) | 1.13 (0.65,1.61) | 1.30 (0.80,1.80) | 1.23 (0.68,1.78) | ||
| First exposure to outdoor light | Model 1 | 0.92 (0.47,1.37) | 1.39 (0.97,1.81) | 0.51 (0.19,0.83) | 1.22 (0.74,1.70) | 0.81 (0.28,1.34) |
| Model 2 | 0.88 (0.43,1.32) | 1.35 (0.94,1.75) | 0.49 (0.18,0.81) | 1.16 (0.69,1.64) | 0.77 (0.23,1.31) | |
| First exposure to indoor light | Model 1 | 1.15 (0.70,1.60) | 1.15 (0.70,1.70) | 1.35 (0.90,1.82) | 0.91 (0.38,1.44) | |
| Model 2 | 1.09 (0.65,1.54) | 1.09 (0.64,1.54) | 1.31 (0.85,1.77) | 0.88 (0.35,1.41) | ||
| Last eating episode | Model 1 | 0.88 (0.42,1.33) | 1.14 (0.70,1.59) | 1.17 (0.69,1.65) | 1.05 (0.53,1.57) | |
| Model 2 | 0.89 (0.44,1.34) | 1.16 (0.73,1.58) | 1.20 (0.73,1.66) | 1.07 (0.56,1.59) | ||
| First eating episode | Model 1 | 0.60 (0.18,1.03) | 0.92 (0.51,1.33) | 0.69 (0.22,1.15) | 0.57 (0.07,1.06) | |
| Model 2 | 0.55 (0.13,0.97) | 0.87 (0.46,1.27) | 0.64 (0.19,1.10) | 0.54 (0.04,1.03) | ||
| Morning PA proportion | Model 1 | −0.95 (−1.38,−0.53) | −0.69 (−1.14,−0.24) | −0.96 (−1.42,−0.49) | −0.92 (−1.41,−0.42) | |
| Model 2 | −0.87 (−1.31,−0.43) | −0.56 (−1.02,−0.10) | −0.86 (−1.34,−0.39) | −0.86 (−1.37,−0.35) | ||
Linear regression models were performed to assess the association between 5 sleep and non-parametric rest-activity outcomes (Top Row) and 6 timing related exposures (left column). Betas (95% Confidence interval) are presented for each cell. Results from 2 models are presented for the first and second row for each exposure. Abbreviations: dec. hours= decimal hours, PA=physical activity
Model 1: age, sex, race/ethnicity, household income, education level, site, BMI (continuous)
Model 2: adjusted for the same covariates as in model 1, in addition to total physical activity (from wrist actigraphy)
We next explored whether any association was modified by chronotype. We observed a statistical interaction between morning type and dichotomized first eating episode for L5 midpoint and acrophase (p-interaction <0.05). In regression models stratified by chronotype, we observed an association between first eating episode with L5 midpoint (1.18 [95% CI: 0.58 to 1.77] hours) and acrophase (1.07 [95% CI: 0.50 to 1.64] hours) among non-morning types, but not morning types (Figure 1 and S1).
Figure 1.
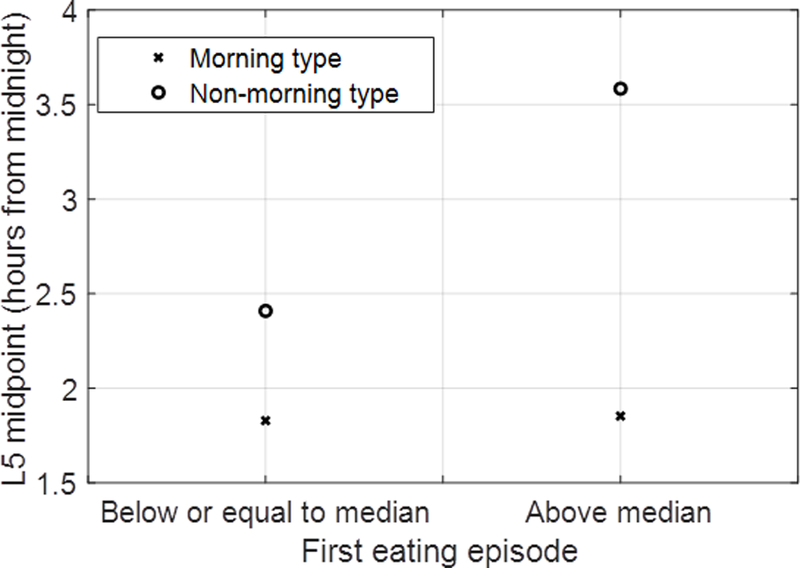
Interaction plot between morning type and first eating episode for L5 midpoint. The graph represents the relationship between L5 midpoint and first eating episode (dichotomized) adjusted for main effects of age, sex, race/ethnicity, household income, education level, study site and BMI.
Discussion
Our study offers a comprehensive analysis of associations between the timing of meals, physical activity and light exposure with 24-hour rest-activity patterns and sleep among adults in free-living settings. We had expected that exposures later (vs earlier) in the day to light, physical activity and eating episodes would be related to more irregular and less robust rest-activity patterns and later timing of daily rest-activity patterns and sleep. Confirming our hypotheses, we found that later light exposure (both in the morning and evening) and later timing of meals were associated with a shift of 0.60 to 1.39 hours to later bedtimes, and later timings of peak activity (0.57 to 1.35 hours) and inactivity (0.69 to 1.35 hours). Adults who perform more than one third of their total physical activity during morning hours also showed an earlier timing (approximately one hour) of their peak activity, inactivity and sleep compared with those who do not. Contrary to our hypothesis, we did not see an association of any of the zeitgebers with IS and IV. More fragmented and less stable 24-hour rhythms (lower IS and higher IV) have been related to cognitive deficits, obesity and overall mortality (Garaulet, Martinez-Nicolas et al., 2016; Luik, Zuurbier et al., 2015; Zuurbier, Luik et al., 2015). We studied a healthy population without shift-work: it may be that the associations with IS and IV in prior studies were due to confounding factors such as ill health rather than the influence of zeitgebers.
Our study is in line with laboratory findings, namely, that the timing of the three exposures of meals, physical activity and light plays a significant role in setting our circadian clock. Light, specifically outdoor light, was the most important zeitgeber determining sleep times and timings of peak activity and inactivity. Numerous studies have shown that light is the most effective environmental signal that sets the central biological clock in the hypothalamus (Arendt & Broadway, 1987; Khalsa, Jewett et al., 2003; Youngstedt, Kline et al., 2016). About 20 years ago, Duffy et al. used an inverted schedule of sleep timing, sedentary activity and social contact with or without bright light exposure to test the influence of light to re-set the human circadian system in healthy young men. In contrast to the behavioral events, timed light exposure was very powerful to re-set the human circadian system (Duffy, Kronauer et al., 1996). Thus, our study results reaffirm the role of light as a primary zeitgeber.
With respect to timing of meals, the last eating episode had a stronger association with sleep times and rest-activity patterns than the first eating episode. Gill et al. studied meal patterns and caloric intake in healthy adults using actigraphy and a smartphone app. The researchers found that 37.5% of the daily caloric intake was consumed after 6 PM (Gill & Panda, 2015). In fact, research in mice suggests that the phase shift depends on the caloric intake (Stephan, 1997). On the other hand, intermittent fasting had no significant influence on the circadian pattern of melatonin under controlled conditions of light exposure, meal composition, energy expenditure, and sleep-wake schedules (Almeneessier, Bahammam et al., 2017). Increased attention in future clinical studies on the influence of meal timing on our circadian system and investigations of its effect on physiological processes will help to expand our understanding and treatment of circadian misalignment disorders.
In our study, we did not measure melatonin but we found a significant and clinically meaningful delay of physical activity of about one hour for individuals who perform physical activity in the morning less than one third of the time. Our results are in accordance with those by Buxton et al., who showed that appropriately timed exercise can either phase advance or phase delay the human circadian system (Buxton, L’Hermite-Baleriaux et al., 1997; Buxton, Lee et al., 2003). In fact, a few studies have already attempted to treat circadian misalignment through exercise at specific times. The methodological problems of this approach have been summarized in a review by Atkinson and colleagues (Atkinson, Edwards et al., 2007). These include difficulties in controlling for confounding factors such as other zeitgebers including the timing of meals and light exposure, the characteristics of the exercise bout, and the athletic status of the participants. Atkinson et al. recognize that the substantial levels of physical activity needed to obtain phase shifts may not be attainable by many people. Moreover, these strategies have not yet been evaluated in large populations under field conditions.
Only non-morning type adults showed an association of the first eating episode with peak inactivity. This might indicate that non-morning type is more likely influenced by external stimuli. This agrees with previous literature, which showed greater social jet-lag and more sleep disturbances in non-morning types if their work hours interfered with their sleep timing (Juda, Vetter et al., 2013; Merikanto, Kronholm et al., 2012; Wittmann, Dinich et al., 2006). Since work hours usually start between 7 AM and 9 AM, late chronotypes show on average higher levels of social jet-lag. It might also be possible that chronotype drives the timing of exposure to light, eating episodes and physical activity. Non-morning types generally eat later than morning types, especially on non-work days (Fleig & Randler, 2009). Wehrens et al. investigated the effect of a 5-hour delay in meals on markers of the human master clock and multiple peripheral circadian rhythms in 10 healthy men under laboratory conditions and constant routines (Wehrens, Christou et al., 2017). The researchers did not see an effect of meal timing on plasma melatonin and cortisol or clock gene expression; however, plasma glucose rhythms were delayed. On the other hand, several animal studies could clearly show an entrainment of the circadian clock by scheduled feeding (Castillo, Hochstetler et al., 2004; Escobar, Salgado et al., 2011; Mendoza, Angeles-Castellanos et al., 2005). Therefore, it remains unclear if for example non-morning people can be phase-advanced through cyclic meals.
Our study has several strengths, including the enrollment of participants from three centers of different geographical regions, objective assessment of sleep, physical activity and rest-activity patterns via actigraphy, and momentary assessment of timing behaviors using a smartphone app. This study also has several limitations. The sample size is relatively small, and we collected our data cross-sectionally. The timing of behaviors was measured using a non-validated self-report approach. However, survey questions were informed by validated questionnaires regarding behavior and circadian timing (Bajaj, Rosner et al., 2011; Horne & Ostberg, 1976). The direction of our results suggests that early exercise may represent a zeitgeber. However, we cannot rule out that physical activity was performed outdoors and is thus an effect of more light exposure. The actigraphic parameters are not independent as they are all derived from activity counts. We also did not collect information on working hours, although working hours with concurrent behavioral light changes can entrain the circadian clock. Yet, shift work was an exclusion criterion and many participants had flexible working hours evidenced by a mean difference of about 20 min between time out of bed on weekdays and weekends. Also, summarizing data from multiple days might introduce some imprecisions, for example, days when participants were not outside were considered missing points in calculating first exposure to outdoor light. However, this occurred infrequently (~3% of all days) and we do not believe that excluding this information meaningfully influenced the derived summary variables or the associations we report. Finally, a large number of exposure and outcome associations were investigated and therefore the significant results need to be interpreted with caution due to multiple tests conducted.
Conclusion
In summary, we found that the timing of meals, physical activity and light exposure were associated with measures of activity and sleep timing (bedtimes, L5 midpoint and M10 midpoint). Future research should evaluate the impact of manipulating zeitgebers on both circadian rhythms and associated health outcomes (e.g. obesity, cardio-metabolic disease). Improved insights into the relationship of entrainment factors with rest-wake activity cycles may help inform future interventions aimed at improving energetic profiles. If efficacious, these interventions may serve as a non-pharmacological treatment to synchronize the human circadian rhythm (e.g. for jet-lag or shift-work problems).
Supplementary Material
Acknowledgments
This work was supported by the NCI Centers for Transdisciplinary Research on Energetics and Cancer (TREC) (U01 CA116850, U54 CA155496, U54 CA155626, U54 CA155435, U54 CA155850). Jonathan Mitchell was supported by NIH grant K01 HL123612 (NHLBI). Catherine Marinac was supported by the National Cancer Institute under award number 1F31 CA183125 and by an American Cancer Society Postdoctoral Fellowship under award number PF-17-231-01-CCE. Peter James was supported by National Cancer Institute grant K99 CA201542. Mirja Quante was supported by a scholarship from the Tuebinger Program for the Advancement of Women in Science. Sara Mariani was supported by NIH grant R24HL114473 (NHLBI).
Footnotes
Declaration of Interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Almeneessier AS, Bahammam AS, Sharif MM, Bahammam SA, Nashwan SZ, Pandi Perumal SR, Cardinali DP, Alzoghaibi M. (2017). The influence of intermittent fasting on the circadian pattern of melatonin while controlling for caloric intake, energy expenditure, light exposure, and sleep schedules: A preliminary report. Annals of thoracic medicine 12:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. (2009). Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17:2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt J, Broadway J. (1987). Light and melatonin as zeitgebers in man. Chronobiol Int 4:273–282. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Hoffmann K, Pohl H, Wever R. (1975). Re-entrainment of circadian rhythms after phase-shifts of the Zeitgeber. Chronobiologia 2:23–78. [PubMed] [Google Scholar]
- Atkinson G, Edwards B, Reilly T, Waterhouse J. (2007). Exercise as a synchroniser of human circadian rhythms: an update and discussion of the methodological problems. Eur J Appl Physiol 99:331–341. [DOI] [PubMed] [Google Scholar]
- Bajaj A, Rosner B, Lockley SW, Schernhammer ES. (2011). Validation of a light questionnaire with real-life photopic illuminance measurements: the Harvard Light Exposure Assessment questionnaire. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 20:1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandin C, Scheer FA, Luque AJ, Avila-Gandia V, Zamora S, Madrid JA, Gomez-Abellan P, Garaulet M. (2015). Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int J Obes (Lond) 39:828–833. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 57:289–300. [Google Scholar]
- Buxton OM, L’Hermite-Baleriaux M, Hirschfeld U, Cauter E. (1997). Acute and delayed effects of exercise on human melatonin secretion. J Biol Rhythms 12:568–574. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Lee CW, L’Hermite-Baleriaux M, Turek FW, Van Cauter E. (2003). Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Physiol Regul Integr Comp Physiol 284:R714–724. [DOI] [PubMed] [Google Scholar]
- Castillo MR, Hochstetler KJ, Tavernier RJ Jr., Greene DM, Bult-Ito A. (2004). Entrainment of the master circadian clock by scheduled feeding. Am J Physiol Regul Integr Comp Physiol 287:R551–555. [DOI] [PubMed] [Google Scholar]
- Cespedes Feliciano EM, Quante M, Weng J, Mitchell JA, James P, Marinac CR, Mariani S, Redline S, Kerr J, Godbole S, Manteiga A, Wang D, Hipp JA. (2017). Actigraphy-Derived Daily Rest-Activity Patterns and Body Mass Index in Community-Dwelling Adults. Sleep [DOI] [PMC free article] [PubMed]
- Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. (1992). Automatic sleep/wake identification from wrist activity. Sleep 15:461–469. [DOI] [PubMed] [Google Scholar]
- Cornelissen G (2014). Cosinor-based rhythmometry. Theor Biol Med Model 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJ. (2005). Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr 17:221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Kronauer RE, Czeisler CA. (1996). Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. J Physiol 495 ( Pt 1):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C, Salgado R, Rodriguez K, Blancas Vazquez AS, Angeles-Castellanos M, Buijs RM. (2011). Scheduled meals and scheduled palatable snacks synchronize circadian rhythms: consequences for ingestive behavior. Physiol Behav 104:555–561. [DOI] [PubMed] [Google Scholar]
- Fleig D, Randler C. (2009). Association between chronotype and diet in adolescents based on food logs. Eating behaviors 10:115–118. [DOI] [PubMed] [Google Scholar]
- Gangwisch JE. (2009). Epidemiological evidence for the links between sleep, circadian rhythms and metabolism. Obes Rev 10 Suppl 2:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Martinez-Nicolas A, Ruiz JR, Konstabel K, Labayen I, Gonzalez-Gross M, Marcos A, Molnar D, Widhalm K, Casajus JA, De Henauw S, Kafatos A, Breidenassel C, Sjostrom M, Castillo MJ, Moreno LA, Madrid JA, Ortega FB. (2016). Fragmentation of daily rhythms associates with obesity and cardiorespiratory fitness in adolescents: The HELENA study. Clin Nutr [DOI] [PubMed]
- Gill S, Panda S. (2015). A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4:97–110. [PubMed] [Google Scholar]
- Juda M, Vetter C, Roenneberg T. (2013). Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythms 28:141–151. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. (2003). A phase response curve to single bright light pulses in human subjects. J Physiol 549:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing EE, Johnston JD, Moller-Levet CS, Bucca G, Smith CP, Dijk DJ, Archer SN. (2015). Exploiting human and mouse transcriptomic data: Identification of circadian genes and pathways influencing health. Bioessays 37:544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Ikram MA, Tiemeier H. (2015). Associations of the 24-h activity rhythm and sleep with cognition: a population-based study of middle-aged and elderly persons. Sleep Med 16:850–855. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. (2005). A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. The European journal of neuroscience 22:2855–2862. [DOI] [PubMed] [Google Scholar]
- Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Lahti T, Partonen T. (2012). Relation of chronotype to sleep complaints in the general Finnish population. Chronobiol Int 29:311–317. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Skene DJ. (2004). Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev Camb Philos Soc 79:533–556. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Skene DJ. (2005). Nonphotic entrainment in humans? J Biol Rhythms 20:339–352. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Quante M, Godbole S, James P, Hipp JA, Marinac CR, Mariani S, Cespedes Feliciano EM, Glanz K, Laden F, Wang R, Weng J, Redline S, Kerr J. (2017). Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol Int 34:1042–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W, Tong YL, Lee JK, Halberg F. (1979). Methods for cosinor-rhythmometry. Chronobiologia 6:305–323. [PubMed] [Google Scholar]
- Patterson RE, Colditz GA, Hu FB, Schmitz KH, Ahima RS, Brownson RC, Carson KR, Chavarro JE, Chodosh LA, Gehlert S, Gill J, Glanz K, Haire-Joshu D, Herbst KL, Hoehner CM, Hovmand PS, Irwin ML, Jacobs LA, James AS, Jones LW, Kerr J, Kibel AS, King IB, Ligibel JA, Meyerhardt JA, Natarajan L, Neuhouser ML, Olefsky JM, Proctor EK, Redline S, Rock CL, Rosner B, Sarwer DB, Schwartz JS, Sears DD, Sesso HD, Stampfer MJ, Subramanian SV, Taveras EM, Tchou J, Thompson B, Troxel AB, Wessling-Resnick M, Wolin KY, Thornquist MD. (2013). The 2011–2016 Transdisciplinary Research on Energetics and Cancer (TREC) Initiative: Rationale and Design. Cancer Causes Control 24:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. (1964). The Entrainment of Circadian Oscillations by Skeleton Photoperiods. Science 144:565. [DOI] [PubMed] [Google Scholar]
- Quante M, Kaplan ER, Cailler M, Rueschman M, Wang R, Weng J, Taveras EM, Redline S. (2018). Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. Nature and science of sleep 10:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, Kalyana-Sundaram S, Wang R, Ning Y, Hodges L, Gursky A, Siddiqui J, Tomlins SA, Roychowdhury S, Pienta KJ, Kim SY, Roberts JS, Rae JM, Van Poznak CH, Hayes DF, Chugh R, Kunju LP, Talpaz M, Schott AF, Chinnaiyan AM. (2013). Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet 45:1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. (2012). Social jetlag and obesity. Current biology : CB 22:939–943. [DOI] [PubMed] [Google Scholar]
- Stephan FK. (1997). Calories affect zeitgeber properties of the feeding entrained circadian oscillator. Physiol Behav 62:995–1002. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. (2001). Entrainment of the circadian clock in the liver by feeding. Science 291:490–493. [DOI] [PubMed] [Google Scholar]
- Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. (1997). Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry 41:955–963. [DOI] [PubMed] [Google Scholar]
- Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD. (2017). Meal Timing Regulates the Human Circadian System. Curr Biol 27:1768–1775.e1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. (2006). Social jetlag: misalignment of biological and social time. Chronobiol Int 23:497–509. [DOI] [PubMed] [Google Scholar]
- Yamanaka Y, Hashimoto S, Takasu NN, Tanahashi Y, Nishide SY, Honma S, Honma KI. (2015). Moring and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am J Physiol Regul Integr Comp Physiol ajpregu 00127 02015. [DOI] [PubMed]
- Youngstedt SD, Kline CE, Elliott JA, Zielinski MR, Devlin TM, Moore TA. (2016). Circadian Phase-Shifting Effects of Bright Light, Exercise, and Bright Light + Exercise. Journal of circadian rhythms 14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuurbier LA, Luik AI, Hofman A, Franco OH, Van Someren EJ, Tiemeier H. (2015). Fragmentation and stability of circadian activity rhythms predict mortality: the Rotterdam study. Am J Epidemiol 181:54–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


