Abstract
Rumination syndrome (RS) is characterized by the repeated regurgitation of material during or soon after eating with the subsequent rechewing, reswallowing, or spitting out of the regurgitated material. Rumination syndrome is classified as both a “Functional Gastroduodenal Disorder” (by the Rome Foundation’s Functional Gastrointestinal Disorders: Disorders of Gut-Brain Interaction, 4th edition) and a “Feeding and Eating Disorder” (by the Diagnostic and Statistical Manual of Mental Disorders, 5th edition). Rumination syndrome is a disorder that is often inaccurately diagnosed or missed, resulting in patients experiencing protracted symptoms and not receiving treatment for long periods. There is a lack of clear consensus for RS diagnosis, mechanisms that maintain RS, and treatment. Guided by existing research and our clinical expertise, we synthesize available evidence and provide recommendations for clinical use. We present a case example and critically summarize the literature to date to (i) increase clinicians’ understanding of heterogeneous clinical presentations, (ii) suggest assessment strategies to facilitate accurate diagnosis, and (iii) provide a schematic for intervention options. Overall, we recommend clinicians recognize the heterogeneous features of RS when considering diagnosis, assess for RS symptoms by clinical history, and treat RS with targeted diaphragmatic breathing while using other methods as augmented intervention or alternative treatment.
INTRODUCTION
Rumination syndrome (RS) is characterized by repeated, effortless food regurgitation during or soon after eating, followed by rechewing, reswallowing, or spitting out of the regurgitant (1,2). Rumination syndrome is classified as both a “Functional Gastroduodenal Disorder” by the Rome Foundation’s Functional Gastrointestinal Disorders: Disorders of Gut-Brain Interaction, 4th edition (ROME-IV) (2,3) and a “Feeding and Eating Disorder” by Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5; “rumination disorder”) (1). Rumination syndrome can cause significant impairment, including medical complications (e.g., dental damage, weight loss, electrolyte disturbances) and psychosocial disturbances (e.g., avoidance of work or social eating) (1,2,4).
Rumination syndrome in an adult was first described in 1618 by an Italian anatomist (5,6), and the need for better detection and treatment of RS has been described over the past several decades (e.g., Blinder et al. (7)). However, systematic research on RS is continually scant and RS has historically been believed to be a rare condition. The prevalence of RS is “unclear” according to DSM-5 (1) and is reported between 0.8% and 10.6% in community samples (8–14). Prevalence data are also from self-report studies, most of which were unable to rule out organic disease. Because of a lack of awareness about RS, RS is often inaccurately diagnosed (15,16) or missed (17). Inaccurate or missed diagnosis often occurs when clinicians do not have patients elaborate on what they mean by terms such as “vomiting,” “reflux,” or “regurgitation.” As a result, patients report undergoing multiple medical diagnostic studies and medication trials (17–23) and experiencing protracted symptoms for long periods (4,17).
There is a lack of clear consensus for RS diagnosis, mechanisms that maintain RS, and treatment. Accordingly, a committee consisting of psychologists (H.B.M., A.S.J., and J.J.T.) and pediatric and adult gastroenterologists (C.D.L. and D.D.) convened to further address the existing knowledge of RS. Guided by existing research and our clinical expertise, we synthesize available evidence and provide recommendations for clinical use in the present report. We describe a case example (deidentified) and critically summarize the literature to date to (i) increase clinicians’ understanding of heterogeneous clinical presentations, (ii) suggest assessment strategies to facilitate accurate diagnosis, and (iii) provide a schematic for intervention options.
CASE EXAMPLE
We present a deidentified case example of a patient who provided informed consent to participate in a recent clinical trial of cognitive behavioral therapy for rumination disorder (CBT-RD). The study was approved by the Drexel University Institutional Review Board. We use the name “Zachary” as a pseudonym.
Zachary was a 28-year-old who identified as male and was evaluated and treated by a behavioral therapist (H.B.M.) using CBT-RD (24,25). He reported regurgitations without retching or gagging preceded by a sensation of pressure in his esophagus and abdomen (i.e., a visceral sensation that precedes the regurgitation known as a premonitory urge). Over the preceding month, he experienced regurgitations between 8 and 50 times daily. Regurgitations typically began within 30 minutes of eating and would last between 1.5 and 3 hours. An episode of regurgitation first included regurgitation of recognizable food material, but regurgitations became more acidic as the episode progressed. Less frequently (approximately 4 times per month), he experienced repeated regurgitation of acidic material in association with other stimuli not postprandially, primarily changes in visceral sensations with physical exertion.
Zachary described using several strategies in an attempt to prevent regurgitations. First, he tried “fighting it off” by forcing the regurgitant back down his esophagus before it reached his mouth. Second, he avoided foods that increased regurgitation frequency—primarily pasta, chicken, and most breakfast food items. Third, he avoided physical activity because he would experience premonitory urges with resultant regurgitations with small levels of physical exertion (e.g., walking up workplace stairs). Zachary described concerns about medical consequences of his RS, primarily weight loss; he had lost 50 pounds when his RS started 5 years prior and regained 20 pounds (body mass index 19.7 kg/m2 at evaluation) but wanted to regain 20–30 more pounds. He also described significant psychosocial interference, including reduced social interaction to avoid public regurgitation, frustration with his inability to increase his physical activity without regurgitating, and, at times, work avoidance.
When Zachary’s symptoms started 5 years prior after experiencing an episode of bronchitis, he sought evaluation and treatment from multiple providers and was given diagnoses of gastroesophageal reflux disease, anxiety, and dysautonomia. He tried multiple proton-pump inhibitors (PPIs), which decreased regurgitation acidity but not frequency, and fluoxetine with no effect on his regurgitations. Three years before evaluation with HBM, he was finally diagnosed with RS by a gastroenterologist who specialized in motility and provided with a handout on diaphragmatic breathing to prevent regurgitations. However, Zachary reported that he practiced diaphragmatic breathing with no change in RS symptoms. After Zachary started CBT-RD (24,25), he learned how to effectively implement diaphragmatic breathing on a schedule in relation to eating and in response to premonitory urges. He also learned other CBT-RD skills (e.g., behavioral exposure to foods associated with regurgitation) to prevent residual regurgitations he experienced after implementing diaphragmatic breathing. After 7 sessions, Zachary achieved a 95% reduction in daily regurgitations (to just 0–1/day), was no longer avoiding foods, and increased his physical activity.
Zachary’s case highlights several issues many patients with RS experience due to lack of consensus recommendations for RS. Misdiagnosis, years of protracted symptoms and medical care, and ineffective treatment are particularly common. To facilitate accurate diagnosis and treatment, we review and provide recommendations in 3 areas: (i) clinical features of RS, (ii) strategies for effective assessment, and (iii) suggested guidelines for intervention.
CLINICAL FEATURES
Rumination syndrome has shifted across diagnostic categories within each edition of ROME (2,27–29), was reclassified into a different category of disorders in DSM-5 (1), and is classified in multiple sections of the proposed International Classification of Diseases (30). In Table 1, we denote current criteria for RS under ROME-IV (2), DSM-5 (1), and 2 categories under the proposed International Classification of Diseases, 11th edition (30). Across classification systems, the core symptom of RS is effortless, repeated regurgitation of recently ingested food. Beyond this core feature, there is little clear consensus among diagnostic guidelines on RS features, largely because of a lack of research to inform diagnostic guidelines (B.T. Walsh, Personal Communication). In the following section, using existing data and our clinical expertise, we describe heterogeneous features that can maintain RS and synthesize existing diagnostic criteria.
Table 1.
Comparison of DSM-5, ROME-IV, and ICD-11 diagnostic information for RS with proposed diagnostic recommendations
|
ICD-11a |
|||||
|---|---|---|---|---|---|
| DSM-5 | ROME-IV | Feeding or Eating Disorders section | Functional Oesophageal or Gastroduodenal Disorders section | Diagnostic recommendations | |
| Duration of symptoms | Criteria met for past 1 mob | Onset 6 mo priorc Criteria met for past 2 mo (child)d; past 3 mo (adult)c |
Occurs over at least several weeks | None | Symptoms present for a sustained period (current criteria: 1–6 mo) |
| Operationalization of repeated regurgitation | None, but notes in “Diagnostic Features” that it should be “at least several times per wk, typically daily” | None | “At least several times per wk” | None | Persisting symptoms (frequency can vary; existing criteria examples are weekly or daily)e |
| Operationalization of regurgitation timing | “After feeding or eating” | “Recently ingested food” (adult)c “Soon after ingestion of a meal” (child)d The process tends to stop when the regurgitant becomes acidic,f but some people may continue to experience regurgitation after the regurgitant becomes acidic Does not occur during sleepd In supporting information (adult): |
None | “Recently ingested food” | Onset typically during or soon after eating Duration varies |
| • Within minutes of meal • Lasts 1–2 hr | |||||
| Regurgitation features | No involuntary retching No nausea No disgust Afterward: rechew, reswallow, or spit outb |
No preceding retching,c,d but in supporting information states “general lack of retching” Usually no preceding nauseaf Effortless or preceded by belching sensation The regurgitated material contains recognizable food and may taste pleasant,f but some people may find the regurgitated material to be sour or bitter Afterward: rechew, reswallow, or spit outc Some individuals describe that they do not have control over regurgitation |
Intentional Afterward: “rechewed and reswallowed, or deliberately spat out” |
Afterward: “spitting, or remastication and swallowing” | No involuntary retching Regurgitation can be intentional or unintentional (some patients describe both processes) Other sensations, including nausea, pain, discomfort, or belching, may represent premonitory urge before regurgitationg Material can be acidic or nonacidic and typically starts with material of recognizable taste (typical presentation) or not of recognizable taste in some cases (atypical presentation) Rechew, reswallow, or spit out afterward. |
| Rule outs | Anorexia nervosab Bulimia nervosab Binge eating disorderb Avoidant/restrictive food intake disorderb GI or other medical conditionsb If in the context of another mental disorder or neurodevelopmental disorder, symptoms are severe enough to warrant additional attentionb |
Anorexia nervosa Bulimia nervosa Eating disorderd GI conditions Symptoms cannot be fully explained by another medical conditiond |
Developmental age ≥ 2 yr Health condition that directly causes regurgitation or causes nausea/vomiting Adult rumination syndrome (ICD-11) |
Rumination-regurgitation disorder (ICD-11) | Diagnosis not appropriate if RS occurs in the presence of a full-threshold eating or feeding disorder (RS regurgitation can be comorbid but should not be diagnosed) Diagnosis appropriate if symptoms are severe enough beyond other mental disorders and neurodevelopmental disorders Other GI conditions can be comorbid but are not the primary cause of symptomsh |
| Clinical significance | Functional consequences: restrictive eating and avoidance of social eating Medical consequences: malnutrition and weight loss |
Functional consequences: avoidance of social eating Medical consequences: malnutrition and weight loss |
None | None | Functional OR medical consequences typically present |
DSM-5, Diagnostic and Statistical Manual of Mental Disorders, 5th edition; GI, gastrointestinal; ICD-11, proposed International Classification of Diseases, 11th edition; ROME-IV, Rome Foundation’s Functional Gastrointestinal Disorders: Disorders of Gut-Brain Interaction, 4th edition; RS, rumination syndrome.
Under the proposed ICD-11 criteria, RS is classified under multiple sections for billing purposes, including as “rumination-regurgitation disorder” in the “Feeding or Eating Disorders” section (aligned with DSM-5), as “adult rumination syndrome” in the “Functional Oesophageal or Gastroduodenal Disorders” section (aligned with ROME-IV), and within an other-specified pediatric category as “other functional digestive disorders of infants, neonates, and toddlers.” The other-specified pediatric category does not include a description of symptoms or criteria; thus, we only describe the former 2 sections.
Components of DSM-5 diagnostic criteria.
Components of ROME-IV adult RS diagnostic criteria.
Components of ROME-IV child RS diagnostic criteria.
ROME‐IV explicitly removed frequency requirement from the diagnostic criteria because of increased recognition that most patients who present for evaluation have regurgitations that occur regularly (e.g., after most meals) (68).
Components of ROME-IV adult “supportive criteria” for diagnosis.
ROME‐IV explicitly removed “painless” from the diagnostic criteria because of increased recognition that other visceral sensations and/or gastrointestinal symptoms could indicate premonitory urge (68).
DSM-5 working team explicitly changed language regarding comorbid conditions based on recommendations (69). DSM-IV criteria stated that regurgitation should not be “due to an associated gastrointestinal or other general medical condition (e.g., esophageal reflux)” (70); DSM-5 criteria now state that the regurgitation should “not be attributable to an associated gastrointestinal or other medical condition (e.g., gastroesophageal reflux, pyloric stenosis).
Heterogeneous features of RS maintenance
Habitual abdominal wall contraction is the most widely recognized and primary mechanism contributing to continued RS symptoms (i.e., primary maintenance pathway). However, we recommend clinicians consider the heterogeneous presentations of RS (31,32), including psychological and pathophysiological mechanisms that can secondarily maintain RS (2,22,31). We describe the basis for these features in the following sections and summarize them in our proposed model of RS maintenance in Figure 1.
Figure 1.
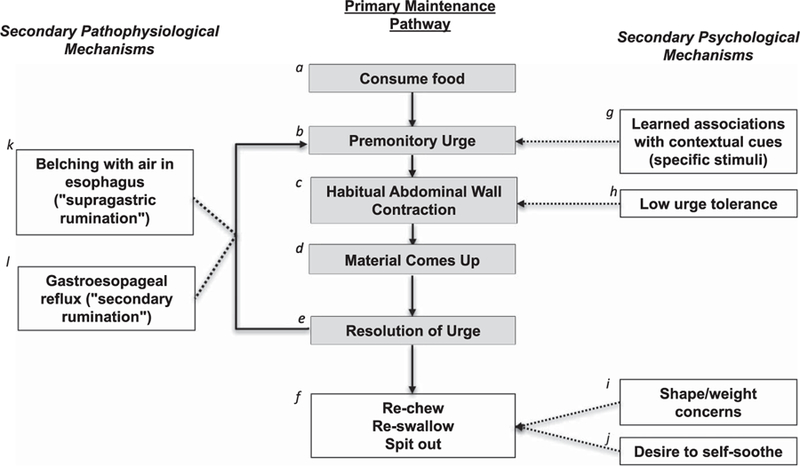
Proposed maintenance model for rumination syndrome (RS). Some patients may have secondary psychological and/or pathophysiological mechanisms that contribute to the negative reinforcement process occurring for the primary mechanism maintaining RS symptoms (habitual abdominal wall contraction); solid lines represent the primary negative reinforcement process, and dashed lines represent secondary maintenance mechanisms. aRumination syndrome is primarily maintained by a habit or reflex the body forms in relation to eating (and perhaps other stimuli). bPatients typically experience a sensation (e.g., pressure/discomfort in esophagus or abdomen) before the regurgitation called the premonitory urge. Similar to other habit-based behaviors (e.g., motor/vocal tics), the premonitory urge precedes the habitual behavior. cHabitual contraction of the abdominal wall is the most widely recognized habit or reflex as a conditioned response to eating. However, it is possible that other habitual or reflexive contractions occur (e.g., in the esophagus) but have not been studied. dThe material comes up in a manner that feels effortless (i.e., without retching). The abdominal wall contraction is believed to increase pressure in the abdomen, forcing stomach contents up into the esophagus and typically into the mouth. eA negative reinforcement process occurs—the premonitory urge temporarily resolves when the material comes up, reinforcing abdominal wall contraction in response to the stimulus (e.g., food). fAfter the regurgitant comes up into the mouth, individuals will re‐chew, re‐swallow, or spit out the regurgitant. Of note, some individuals report that they learn to have the regurgitant go back down before it enters the mouth. gSome individuals develop learned associations between abdominal wall contraction and specific contextual cues (e.g., specific foods, non–food stimuli-like specific activities, changes in visceral sensations). hSome individuals have difficulty tolerating premonitory urges, which contributes to the negative reinforcement process maintaining RS. For example, some individuals will purposefully allow the regurgitant to come up (i.e., instead of using diaphragmatic breathing as a competing response) to relieve the discomfort they experience associated with premonitory urges. iSome individuals have shape and weight concerns that negatively reinforce regurgitations. For example, some individuals will spit out the regurgitated material to attempt weight loss. jSome individuals have a desire to soothe themselves that positively reinforces regurgitations. For example, some individuals will rechew or reswallow the material because they enjoy tasting the regurgitated food or find the process soothing. This may also contribute to purposefully allowing the regurgitant to come up (i.e., instead of using diaphragmatic breathing as a competing response). kSome individuals experience belching that precedes or occurs simultaneously with abdominal wall contraction (called supragastric RS). lSome individuals have comorbid acid reflux events that lead to abdominal wall contraction (called secondary RS).
Primary maintenance pathway.
Several behavioral models of RS maintenance exist. Over the years, evidence has supported a 19th century physician’s self-experimentation (33) suggesting that regurgitations are a habit or reflex that develops through a conditioned response to stimuli. In relation to a stimulus (typically food), Barba and colleagues identified that intercostal muscles contract along with anterior abdominal muscle contraction (34). Individuals with RS (like Zachary) typically experience physical sensations before regurgitations similar to premonitory urges experienced before motor/vocal tics (35). Rumination syndrome has been described as a “habit” (36). It is hypothesized that when the material comes back up, the premonitory urge temporarily resolves, negatively reinforcing abdominal wall contraction. Of note, abdominal wall contraction etiology is unstudied, but some patients retrospectively report a stressor or trigger associated with RS onset [e.g., gastroenteritis (20,22), medical procedure (19), respiratory infection (as in Zachary’s case), psychosocial stressor (36,37), comorbid psychological symptoms (7,17,38), eating disorder history (39,40)].
Potential secondary psychological mechanisms.
Rumination syndrome may be secondarily maintained by features that either negatively or positively reinforce continued regurgitations. Johnson and Corrigan (19) originally proposed a behavioral model that included such mechanisms. With a lack of systematic research on these mechanisms, we propose our model of environmental, cognitive, and behavioral mechanisms that can contribute to RS maintenance (Figure 1) based on reports in the literature and our clinical expertise. First, some individuals report high regurgitation likelihood after eating particular foods (i.e., “learned associations with foods”) (6) or in response to another stimulus (e.g., changes in visceral sensations). As with Zachary’s case, patients can then avoid foods, situations, or sensations associated with regurgitation (25) but still not experience symptom relief. Second, some individuals with RS describe difficulty tolerating the premonitory urge and will allow regurgitations to occur to temporarily relieve their discomfort (24). Third, some individuals with RS report concerns about body shape/weight that could partially maintain symptoms (e.g., regurgitant expulsion for weight control) (25,26) but are not attributable to an eating disorder. However, some individuals with eating disorders can have comorbid RS symptoms (41). Fourth, RS can serve a positive function by alleviating psychological distress (e.g., anxiety) or providing a soothing/pleasant sensation (e.g., in infants and individuals with developmental disability (1,2) and those who seek out foods or times to ruminate (25)).
Potential secondary pathophysiological mechanisms.
Comorbidities may explain variation in pathophysiology that contributes to RS maintenance. Rumination syndrome can be comorbid with other reflux- and vomiting-based conditions, such as gastroparesis (42), gastroesophageal reflux disease (43), and self-induced vomiting (40). Preliminary studies suggest that some individuals display 3 primary pathophysiological RS variants: primary, secondary, and supragastric rumination (36,44). With “secondary rumination,” comorbid acid reflux occurs before the abdominal wall contracts and leads to RS regurgitation. With “supragastric rumination,” belching precedes RS regurgitation (32).
Diagnostic criteria
In Table 1, we synthesize diagnostic criteria. For example, for regurgitation timing, DSM-5 and ROME-IV describe that RS involves regurgitation “after feeding or eating” or of “recently ingested food,” respectively. In some cases, a temporal relationship may be clear and consistent (e.g., within 1 hour), but other patients (like Zachary) may also experience regurgitations not associated with eating events (e.g., some of his regurgitations occurred on waking or with physical exertion). ROME‐IV also has a supportive criterion (i.e., not necessary for diagnosis) that “the process tends to cease when the regurgitated material becomes acidic” but later notes that some people may continue to regurgitate after the regurgitant becomes acidic. Some patients, like Zachary, can experience regurgitation that turns acidic as regurgitations continue hours after food ingestion. Thus, our synthesized recommendation is that typically (not always) regurgitations occur during or soon after eating and that the duration of repeated regurgitations can vary. We synthesize other guidelines across diagnostic systems in Table 1.
STRATEGIES FOR EFFECTIVE ASSESSMENT
Effective assessment of RS is crucial to prevent the long periods patients report going without accurate diagnosis (17–23). In fact, when patients with RS finally receive accurate diagnosis, the diagnosis itself can be therapeutic.
Clinical assessment
No validated biomarker for RS exists. In our experience, existing self-report questionnaires (e.g., the ROME-IV diagnostic questionnaire (11)) can be used as a screening tool, but RS diagnosis is made based on clinical history (1,2). Semistructured interviews (45,46) have not been validated to capture the heterogeneous presentations of RS. We recommend assessing for RS when patients present with reflux, vomiting, or regurgitation, terms often used by patients with RS (4). In Table 2, we recommend questions clinicians use based on a research-based semistructured interview (46). When clinicians are not confident in the diagnosis, they can perform behavioral observation in-office (19,47) by having patients consume foods they report are associated with regurgitation. For example, behavioral assessment can be important with pediatric patients because parents may not be able to report on their child’s RS symptoms (48).
Table 2.
Example clinical assessment questions
| Feature of interest | Questions |
|---|---|
| Differentiate from vomiting (rumination does not include retching) | Has the material come back up into your mouth during or after eating in a way that felt different from being sick or throwing up (vomiting)? How did it feel different from being sick or throwing up (vomiting)? Do you experience retching when the material comes back up? |
| Determine onset (usual onset <1 hr after eating, but can onset in relation to non‐food stimuli) | How long after eating does the material first come back up? |
| Repetitive nature (sometimes may only occur once, but usually is repetitive) | How many times does the material come back up once it starts? |
| Determine if classic rumination presentation (food material) or atypical presentation (non–food material) | What does the material taste like? |
| Determine whether regurgitation is habit‐based by identifying the presence of a premonitory urge (note that some young patients typically are unable to describe this) | Do you experience a sensation that tells you the material is about to come back up? |
| Functional assessment | Have you rechewed it, reswallowed it, or spat it out? |
| • Spit out: if motivated by shape/ weight, assess for comorbid eating disorder (or refer for further evaluation) |
Medical assessment
Although RS diagnosis can be conferred by clinical history, many patients with RS (like Zachary) undergo extensive testing without avail. ROME‐IV and DSM-5 have different recommendations for medical testing. ROME‐IV (written by gastroenterologists) states that diagnostic testing to rule out organic causes is typically unnecessary (2), whereas DSM-5 (written by psychiatrists/ psychologists) states that “physical examinations and laboratory tests” should be conducted to rule out gastrointestinal conditions (1). On the basis of our clinical experience and recommendations made previously (e.g., Disney and Trudgill (49)), we recommend the use of clinical history alone unless the patient also presents with symptoms of another gastrointestinal condition that could be comorbid or underlie regurgitation. For example, some patients may describe sensation of food sticking in the esophagus; this could represent heightened attention to changes in visceral sensations associated with RS (e.g., the premonitory urge before regurgitation) or could represent structural causes (e.g., esophageal dysphagia). Future research is needed to examine the sensitivity, specificity, and incremental utility of high-resolution esophageal manometry with impedance, which recent research suggests can detect RS if postprandial gastric pressure exceeds 25–30 mm Hg (32,43,44).
TREATMENT
Table 3 provides a description of published reports on treatments that have been used with child, adolescent, and adult patients with RS. Studies on RS treatment have largely included case reports (n = 22), case series (n = 4), and retrospective chart reviews (n = 7), with just 2 randomized controlled trials (RCTs) and 3 open trials. We (H.B.M., A.S.J., C.D.L., D.D., and J.J.T.) make consensus recommendations informed by existing literature supplemented by our clinical experience. Overall, we recommend diaphragmatic breathing as a first-line approach because (1) there is a great deal of evidence to support its efficacy, (2) it is relatively straightforward to implement in an outpatient setting, and (3) it is low cost. Some patients with refractory or residual regurgitations may require more intervention than diaphragmatic breathing alone. In Figure 2, we synthesize our recommendations into a stepped care approach with the aim of conserving limited health care resources.
Table 3.
| Citation | Participant Characteristics | Treatment length | Intervention strategy |
Outcomed | |||
|---|---|---|---|---|---|---|---|
| Diaphragmatic breathing | Biofeedbackc | Self-monitoring | Other | ||||
| RCTs | |||||||
| Barba et al. (55) | 23 patients; age range = 17–79 yr; 75% female | 3 sessions over 10 d | X (at home 5 min before and after meals) | X (n = 12 w/test meal) | 120 mg simethicone placebo (n = 11) | Pre- to post-treatment: % M daily regurgitation frequency reduced by 74% ± 6% (biofeedback) vs 1% ± 14% (placebo; n = 2 improved) | |
| Pretreatment to 6-mo follow-up for n = 21 (including n = 9 from the placebo group who received biofeedback after): M daily regurgitation frequency reduced from 25 ± 3 to 0.7 ± 0.4 | |||||||
| Pauwels et al. (61) | 20 patients; age range = 18–21 yr; 65% female with RS and/or supragastric belching | 2 wk + 1-wk washout + 2 wk | 10 mg baclofen TID (n = 10) and placebo (n = 10) randomized to one first and then the other (for 2 wk periods) | Manometry-recorded regurgitations: median (range) frequency 8 (3–11) (baclofen) vs 13 (8–22) (placebo) | |||
| Self-reported in-lab regurgitations: median (range) frequency 4 (0–14) (baclofen) vs 6 (0–19) (placebo) | |||||||
| Self-reported subjective improvement rating: 63% (baclofen) vs 26% (placebo) reported improvement | |||||||
| Open trials | |||||||
| Barba et al. (34) | 28 patients | 3 sessions over 10 d | X (at home 5 min before and after meals) | X (w/test meal) | Pre- to post-treatment: daily regurgitation frequency reduced by 70% ± 11% | ||
| 6-mo follow-up for the subsample (n = 11): n = 5 remitted, n = 2 >95% reduction, n = 4 M 74% ± 8% reduction | |||||||
| Blondeau et al. (71) | 12 patients with RS or supragastric belching; age range = 18–89 yr; 67% female | 1 wk | Baclofen 10 mg TID | Manometry-recorded regurgitations in RS participants (n = 8): median (range) frequency 18 (10–14) to 4 (0–22) | |||
| Self-reported in-lab regurgitations: median (range) frequency 9 (0–11) to 1 (0–13) | |||||||
| Murray et al. (54) | 10 patients with RS; age range = 20–67 yr; 50% female | 6–8 sessions | X | X | Behavioral experiments, behavioral exposure, alternative self-soothing strategies, and cognitive strategies for urge management | Pre- to post-treatment for treatment completers (n = 8): M 87.7% ± 9.0% (range = 73.2%–98.3%) reduction in daily regurgitation frequency (P =.042; d = 0.88) | |
| Case reports | |||||||
| Birmingham, Firoz (72) | 21-yr-old female comorbid ED and reflux | Unk (inpatient) | Simethicone (for postprandial bloating), rabeprazole, and domperidone (to control reflux) | By discharge: reported “decrease” in rumination and attributed it to taking “deep breaths” (not prescribed by the treatment team and not indicated as diaphragmatic breathing) |
|||
| Brown (73) | 34-yr-old and 26-yr-old females | Unk (inpatient) | Propantheline bromide up to 60 mg BID (case 1); offered psychiatric care but refused (cases 1 and 2) | Case 1: no change in symptoms during inpatient treatment | |||
| Case 2: no change in symptoms during inpatient treatment | |||||||
| Bruni (74) | 17-yr-old female; comorbid ED | Unk | “Support and psychotherapy” | Ongoing treatment; no outcomes reported | |||
| Cooper et al. (75) | 25-yr-old female comorbid chronic nausea and diarrhea | 17 d (inpatient) | Removal of the gastric stimulator, placed the Mic-Key button in her jejunostomy tube, and confirmed that gastric biopsies were normal | By discharge: no change in symptoms during inpatient treatment | |||
| Referred for “relaxation behavioral techniques” with a psychologist; no outcomes reported | |||||||
| Dalton and Czyzewski (50) | 8-yr-old female; 14-yr-old male | 2 sessions (4–5 wk apart) | X | X | Distraction; aversion training (reswallow the regurgitant) | Case 1: | |
| • Pre- to post-treatment: regurgitations 15/hr to 0–3/2 hr (frequency Unk) • 2-yr follow-up: 1–2/d | |||||||
| Case 2: | |||||||
| • Pre- to post-treatment: regurgitations 1–2/d to 1–2/wk • 2-yr follow-up: “slight increase”/wk | |||||||
| Fernandez et al. (20) | 14-yr-old and 12-yr-old females with supragastric features | Unk (inpatient) | X (case 2) | Graded food hierarchy to increase the food size and consistency (case 2); case 1 treatment unclear | Case 1: able to tolerate swallowing food | ||
| Case 2: ongoing treatment; no outcomes reported | |||||||
| Fullerton et al. (37) | 13-yr-old female (no treatment with the second case in the report) | 2 mo (inpatient) | Staff monitoring during daytime/meals, bathroom access locked, and received individual, group, and family therapy | Pre- to post-treatment: regurgitations 3–4/ d to 1/wk | |||
| 4-mo follow-up: no regurgitations | |||||||
| Fox et al. (21) | 22-yr-old male | 6 mo (inpatient; 5 mo was medication treatment) | X | General education about RS | By discharge: no regurgitations for 1 wk; weight regain during the last 1 mo of stay after biofeedback | ||
| Gupta et al. (23) | 26-yr-old female w/ comorbid conversion disorder and pregnancy | <7 d (inpatient) | Reassurance, nasogastric tube, referral for outpatient psychotherapy | By discharge: no regurgitations for 2+d | |||
| Johnson et al. (76) | 5 patients; age range = 17–43 yr; 60% female; array of comorbid gastrointestinal disorders–rumination described as “refractory regurgitation” | 1 session w/ “regular” follow-up phone contact | X (n = 1) | Graded food hierarchy (n = 1); eating changes (n = 5; e.g., limit dietary intake, chew slower), sip water (n = 1), exercise increase (n = 4), weight reduction (n = 2), “relax while eating” (n = 3), and seek counseling for “stress management” (n = 2) | Case 1: no regurgitations up to 43-mo follow-up | ||
| Case 2: no regurgitations up to 9-mo follow-up, at which point she relapsed but reduced regurgitations again up to 43-mo follow-up | |||||||
| Case 3: no regurgitations up to 40-mo follow-up | |||||||
| Case 4: 80% reduction of regurgitations up to 24-mo follow-up | |||||||
| Case 5: no regurgitations up to 22-mo follow-up | |||||||
| Kanodia et al. (18) | 16-yr-old female w/comorbid nausea, vomiting, bloating, and constipation | 41 wk (Unk number of sessions) | Treatment with integrative medicine–herbal supplements and special diet. The patient reported previous use of diaphragmatic breathing and biofeedback treatment | Posttreatment: no regurgitations up to 69-wk follow-up | |||
| Larocca and Della-Fera (77) | 19-yr-old and 41-yr-old females, comorbid ED | Unk (inpatient) | Education about consequences of RS (n = 2); weight restoration (n = 2); reward contingencies (n = 1) | Case 1: no regurgitations posttreatment | |||
| Case 2: no regurgitations posttreatment | |||||||
| Murray et al. (52) | 27-yr-old female | 6 sessions | X (schedule after eating and in response to premonitory urge) | X | Behavioral exposure for learned associations with foods | Pre- to post-treatment: M (range) daily regurgitation frequency 7.5 (1–22) to 0.2 (0–2) 20-mo follow-up: M (range) daily regurgitation frequency since treatment 0.1 (0–3), only when eating foods with strong association with RS | |
| Reis (78) | 6-yr-old male and 5.5-yr-old female | No treatment (case 1); “dietary advice and reassurance” (case 2) | Case 1: no regurgitations up to 6-yr follow-up (approx.) | ||||
| Case 2: no regurgitations up to 13-yr follow-up (approx.) | |||||||
| Schroedl et al. (47) | 17-yr-old female | During 3 structured meals/d (inpatient) | X (unclear whether this was the “self-regulation strategy” during meals) | X (autonomic nervous system arousal while eating) | X (only during sessions) | Division of meals into trials with a graded increase in the number and duration of trials; graded food hierarchy to increase the amount and variety of foods; aversion training; distraction | By discharge: decreased regurgitations, but still experiencing symptoms 18-mo follow-up: reported gradual decrease in symptoms for 1 yr after discharge and no regurgitations for the past 6 mo |
| Shay et al. (79) | 31-yr-old male, comorbid GERD and heartburn | 8 sessions | X (w/ test meal for 5 sessions) | Self-reported subjective improvement: 80% overall improvement up to 6-mo follow-up | |||
| Smout, Breumelhof (80) | 22-yr-old female | 6 sessions | Operant conditioning with loud tone when gastric fundus pressure reached 15 mm Hg | Pre- to post-treatment: no changes in symptoms | |||
| Sokel et al. (81) | 9-yr-old male | 26 d (inpatient) | “Self-hypnotherapy” (relaxation exercises, mental imagery, and suggestions of symptom relief) through audio recordings before meals; behavioral reward plan to motivate prolongation of regurgitations; decreased parental and staff attention to regurgitations; individual therapy for “stress management techniques” | Pre- to post-treatment: no regurgitations 1-yr follow-up: no regurgitations | |||
| Thomas, Murray (26) | 27-yr-old female w/comorbid ED | 7 sessions | X (individualized schedule after eating and in response to premonitory urge) | X | Behavioral experiments for fear of weight gain | Pre- to post-treatment: M (range) daily regurgitation frequency 10 (0–42) to 1.1 (0–7) | |
| Over 23-wk follow-up period: M (range) daily regurgitation frequency 0.4 (0–6) | |||||||
| Wagaman et al. (51) | 6-yr-old female | 7 sessions | X | X | Social support from parents; behavioral reward plan to motivate the use of diaphragmatic breathing | Pre- to post-treatment: “significant reduction” in regurgitation frequency across time, with no regurgitations by the end of treatment. | |
| Weakley et al. (82) | 15-yr-old female; comorbid ED | 4 wk (inpatient) | Chewing gum after meal | Pre- to post-treatment: no regurgitations 1- and 2-yr follow-ups: no regurgitations | |||
| Williamson et al. (83) | 24-yr-old female, comorbid ED | 15 individual sessions and 18 group sessions | Contingency management of binge eating at night to target regurgitations indirectly; group sessions for ED behaviors | Pre- to post-treatment: regurgitations decreased from 8/wk to 0/wk 3-mo and 2-yr follow-ups: 0/wk | |||
| Case series | |||||||
| Fairburn and Cooper (39) | 7 patients; age range = unk; 100% female; in treatment of bulimia | Unk | Cognitive behavioral treatment of ED (nonspecific) | Pre- to post-treatment: n = 6 reported no regurgitations | |||
| Green et al. (84) | 5 patients; age range = 14–20 yr; 80% female; n = 1 with comorbid supragastric belching, headaches, and conversion disorder | 9–31 d (inpatient) | X (n = 5) | X (n = 1) | X (n = 5) | Distraction; massage therapy; aversion training; graded food hierarchy to increase food amount and variety for those going off enteral feeds; types of medication management unknown (e.g., promethazine for nausea for at least n = 1) | 1-yr after discharge: n = 5 decreased regurgitations enough to consume and retain enough calories for weight maintenance, but “many” still experienced regular symptoms |
| Levine et al. (85) | 9 patients; age range = 7–56 yr; 78% female; comorbid EDs | Unk | X | “Relax after meals” with taped instructions | Posttreatment: n = 1 decreased regurgitations | ||
| Oelschlager et al. (66) | 5 patients; age range = 18–61 yr; 83% female | NA | Nissen fundoplication (surgery) | Postsurgery: no regurgitations at 2-wk follow-up (n = 1), no regurgitations at 6-mo follow-up (n = 1), no regurgitations at 1-yr follow-up (n = 2), and unclear (n = 1) | |||
| Chart reviews | |||||||
| Amarnath et al. (22) | 12 patients; age range = 11–54 yr; 58% female (n = 1 treatment Unk) | Unk | (n = 7 possible) | X (n = 3; Unk approach) | No treatment (n = 1); progressive muscle relaxation (n = 3); “habit regulation program” (n = 7) | Posttreatment: | |
| n = 1 “minimal improvement” | |||||||
| n = 4 “improved” | |||||||
| n = 5 no regurgitations | |||||||
| Chial et al. (4) | 147 patients; age range = 5–20 yr (different treatments recommended) | M = 2.7 ± 0.5 sessions (for n = 46 who received behavioral treatment with data) | X | X (Unk approach) | Medication (n = 25) [H2 blockers (n = 9); PPIs (n = 3); prokinetics (n = 11); tricyclic antidepressant (n = 3); hyoscyamine (n = 1); antiemetic (n = 1)]; Relaxation training or “CBT” (n = Unk); Reassurance (n = 2) | Posttreatment (n = 48 available data): | |
| n = 10 no improvement | |||||||
| n = 32 “improved” (n = 2 reassurance/education only) | |||||||
| n = 16 no regurgitations | |||||||
| Khan et al. (86) | 12 patients; age range = 9–19 yr; 75% female | Unk | X (n = 7) | Nutrition support and medication (n = 6); pain management (n = 2); “relaxation” / “therapy” (n = 7) | Posttreatment (5–36 mo of follow-up): | ||
| n = 2 no improvement | |||||||
| n = 1 “improved” | |||||||
| n = 9 no regurgitations | |||||||
| O’Brien et al. (15) | 38 patients; age range = 13‐65yr; 76% female | Unk | Prokinetics (n = 6); antacid (n = 7); behavioral therapy (n = 3); psychotherapy (n = 2); behavioral and psychotherapy (n = 2) | Posttreatment (6–74 mo of follow-up available for n = 16): | |||
| n = 4 no improvement | |||||||
| n = 10 “improved” | |||||||
| n = 2 no regurgitations (but did not attribute it to treatment received) | |||||||
| Soykan et al. (53) | 10 patients; age range = 17–63 yr; 60% female | Followed for M 31.2 (range 6–72) mo | X (Unk whether this was the “breathing technique” used with biofeedback and 30–60 minutes after meals) | X (n = 5) | Medication (n = 9) [prokinetics (n = 7); PPIs (n = 3); leuprolide acetate (n = 1)]; biofeedback + “relaxation techniques” (n = 5); no treatment (n = 1) | Posttreatment: | |
| n = 4 no improvement | |||||||
| n = 5 improved | |||||||
| n = 2 no regurgitations | |||||||
| Tucker et al. (36) | 46 patients; age range = 18–68 yr; 74% female; n = 5 comorbid supragastric belching; n = 6 comorbid GERD | 1+ sessions (n = 23 had 1 session; other n = 23 Unk) | X (w/ test meal) | Previous to the treatment session: PPIs (n = 44), antiemetics (n = 31), endoscopic therapy (n = 2), botulinum toxin injection to distal esophagus and lower esophageal sphincter (n = 1), botulinum toxin injection to pylorus (n = 1), gastric neuromodulator implantation (n = 1), and Nissen fundoplication (n = 5) | Medications previous to the treatment session: reportedly no effect on regurgitation frequency | ||
| Posttreatment (3–11 mo of follow-up available for n = 35): | |||||||
| n = 2 no improvement | |||||||
| n = 13 partial improvement | |||||||
| n = 20 no regurgitations | |||||||
| Vijayvargiya et al. (17) | 57 patients w/comorbid rectal evacuation disorder; age range = 14–62 yr; 95% female | 1 session | X | Previous to the treatment session: antidepressants/anxiolytics (n = 8), antiemetics/antacid (n = 17), and laxatives/prokinetics (n = 9) | Medications previous to the treatment session: reportedly no effect on regurgitation frequency | ||
| Posttreatment: Unk (no outcomes reported for RS regurgitations) | |||||||
ED, eating disorder; GERD, gastroesophageal reflux disease; M, mean; NA, not applicable; RCT, randomized controlled trial; RS, rumination syndrome; Unk, unknown.
We chose not to include the treatment of RS in infancy because the treatment approach differs considerably (87,88).
We chose not to include samples solely composed of individuals with developmental disabilities because this has been reviewed previously (89).
Biofeedback involved abdominal wall and diaphragm activity, unless otherwise noted.
Because studies lack consistent measures of treatment response, we described treatment outcomes available but were not able to systematically synthesize outcome variables across reports.
Figure 2.

Recommended stepped care approach for treatment of rumination syndrome (RS). aIf the patient agrees with diagnosis, the clinician provides a brief education on abdominal wall contraction as a reflex or habit the body has formed in relation to eating (and perhaps other stimuli); abdominal wall contraction leads to an increase in gastric pressure that then forces contents back up. Of note, if the patient has a comorbid psychological disorder (e.g., generalized anxiety disorder), the patient should be treated separately for this comorbid disorder (e.g., referral to a mental health professional for behavioral treatment and/or psychiatric medication management). bThe clinician guides the patient through diaphragmatic breathing; the clinician may demonstrate diaphragmatic breathing and then ask the patient to try in either or both supine and upright positions. Chitkara et al. (67) provide instructions for clinicians to teach patients diaphragmatic breathing. cThe clinician can determine whether the patient is adequately engaging the diaphragm to have air fill the abdominal area (i.e., the abdomen will protrude on in-breath). dIf the patient is not able to demonstrate diaphragmatic breathing, the clinician may refer the patient to a specialist (e.g., behavioral health specialist) or another provider (e.g., paraprofessional) who can provide further diaphragmatic breathing instruction or biofeedback-guided diaphragmatic breathing. eWhen available, the specialist can provide biofeedback-guided diaphragmatic breathing. fThe specialist can instead or in addition to biofeedback provide further instruction and practice in diaphragmatic breathing. gOnce the patient demonstrates diaphragmatic breathing adequately, the clinician can give the patient brief instruction to breathe on a predetermined schedule after eating (e.g., for 2-minute intervals every 10 minutes after eating until a specified time point or until premonitory urges subside). If the patient is neither aware of the timing of their regurgitations (e.g., how longer after eating and how long regurgitations continue for) nor any premonitory urges (i.e., sensations preceding regurgitations that may signal that the regurgitation is about to occur), the clinician may consider having the patient self-monitor their regurgitations (i.e., tracking when they have regurgitations) to increase awareness before using diaphragmatic breathing. Some patients may benefit from diaphragmatic breathing instruction after just 1 session (e.g., 30% of patients in 1 chart review (17)). However, other patients may require more structured implementation guidance (like in Zachary’s case). hOn follow-up, the clinician determines whether the patient has achieved a significant reduction in the frequency of regurgitations (e.g., ≥70%–80% reduction). iIf the patient does not achieve a significant reduction in regurgitations after using diaphragmatic breathing on a schedule, the clinician can determine with the patient whether adjustments to the schedule could improve the effect (e.g., if the patient realizes that sometimes they start regurgitating toward the end of a meal they could start the schedule midway through eating). In addition, the patient can use diaphragmatic breathing whenever they experience a premonitory urge. jOnce the patient has achieved a significant reduction in their regurgitation frequency, some patients may still have residual regurgitations. The clinician can determine whether the patient is satisfied with the reduction or if they would like further treatment to reduce, and perhaps eliminate, residual regurgitations. kIf the patient is satisfied with the outcome, treatment is complete. lIf the patient is not satisfied with the outcome or has not achieved a significant reduction in regurgitation frequency, the clinician may refer the patient to a specialist (e.g., behavioral health specialist) to address secondary psychological mechanisms that may be maintaining residual regurgitations (Figure 1). Other behavioral interventions can target secondary psychological mechanisms. In cognitive behavioral therapy for rumination disorder (CBT-RD) (24,25), strategies include alternate self-soothing strategies, cognitive strategies to facilitate riding out premonitory urges, behavioral experiments to test fears (e.g., about using diaphragmatic breathing in public), and behavioral exposures (e.g., systematic exposure to stimuli associated with regurgitations). mIf the patient still does not achieve a significant reduction after seeing a specialist, the clinician may consider alternative treatment options. Some options may include adding an antispasmodic (e.g., baclofen) or referring for inpatient treatment. In addition, placing the patient on enteral feeding could allow the patient to be temporarily removed from food stimuli before slowly increasing oral nutrition with the use of behavioral strategies (e.g., diaphragmatic breathing) to prevent regurgitations.
Diaphragmatic breathing
Diaphragmatic breathing has the most support to date by reports and studies that clearly indicated decreases in regurgitations, including 4 case reports (26,50–52), 4 chart reviews (4,17,36,53), 2 open clinical trials (34,54), and 1 RCT (55). Initial research suggests that diaphragmatic breathing operates as a competing response to habitual abdominal wall contraction by relaxing the abdominal wall (34). However, more research is needed to compare diaphragmatic breathing with other competing responses (e.g., in tic disorders, competing responses that do not directly relate to the body area involved in the tic can still be efficacious) (56,57). Some patients may benefit from biofeedback-guided diaphragmatic breathing (with electromyography to decrease intercostal activity and anterior wall muscle activity while increasing diaphragm activity) (34,55). However, no research yet has compared diaphragmatic breathing instruction with vs without biofeedback. In addition, access to biofeedback specialists can be difficult, especially in nonpediatric medical settings. Given current evidence, we recommend diaphragmatic breathing as the first-line strategy for RS treatment (Figure 2). We recommend biofeedback conditionally—when available and when patients have difficulty with diaphragmatic breathing.
Other behavioral interventions
Various other behavioral strategies have been reported in case reports and chart reviews instead of diaphragmatic breathing [e.g., general relaxation (58), aversion training (7), and distraction (e.g., gum chewing) (58)], but there is no research to suggest that they are superior to diaphragmatic breathing. Instead, specific behavioral strategies have been used to augment treatment in comprehensive treatment protocols (e.g., CBT-RD developed by H.B.M. and J.J.T. (24,25) and pediatric inpatient protocol code-veloped by C.D.L. (59,60)). For example, in CBT-RD, interventions are selected to target secondary psychological mechanisms (Figure 1) maintaining regurgitations, including alternate self-soothing strategies, cognitive strategies to facilitate riding out premonitory urges, behavioral experiments to test fears (e.g., about using diaphragmatic breathing in public), and behavioral exposures (e.g., systematic exposure to stimuli associated with regurgitations) (24,26,52,54). Given current evidence, when patients continue to experience regurgitations after using diaphragmatic breathing, we suggest clinicians consider referring patients to specialists (e.g., behavioral health specialists) to learn strategies to augment diaphragmatic breathing.
Medical interventions
Baclofen.
The only RCT of a medication included crossover of baclofen (an antispasmodic) vs placebo pill for RS and/or supragastric belching (61). While on baclofen vs placebo, participants reported greater subjective overall improvement in symptoms. Although regurgitation frequency was lower while on baclofen vs placebo, the study only assessed within-subject differences postprandially in-lab with a resultant regurgitation median (range) that was overlapping [4 (0–14) baclofen vs 6 (0–19) placebo] (61). Future research is needed to compare and test the additive benefit of baclofen to behavioral interventions. Given current evidence, we recommend clinicians use baclofen only with patients who have not achieved a substantial reduction in regurgitation frequency after behavioral intervention.
Neuromodulators.
A Rome Foundation working team recently created recommendations for neuromodulator agents (e.g., tricyclic antidepressants) to treat disorders of gut‐brain interaction (62). However, no evidence yet supports the efficacy of neuromodulators in reducing RS regurgitations or in augmenting behavioral approaches. One chart review described that neuromodulators had no effect on regurgitation frequency self-reported by patients (n = 8) before trying behavioral strategies (17). In addition, research on another habit-based symptom (motor/vocal tics) has consistently shown that behavioral therapy produces similar outcomes regardless of psychiatric medication use (63–65). Given current evidence, we do not recommend neuromodulators as a standalone treatment of RS. We recommend neuromodulators to treat comorbid disorders of gut‐brain interaction that are characterized by visceral hypersensitivity (e.g., irritable bowel syndrome) and comorbid psychological disorders (e.g., generalized anxiety disorder). Patients with comorbid psychological disorders alternatively could be referred to a mental health provider for behavioral treatment.
Other medications.
Other medications have been tried to reduce RS regurgitations, including H2 blockers, PPIs, prokinetics, and antiemetics. In our experience, these medications have no effect on regurgitation frequency (e.g., one patient discontinued PPIs but continued diaphragmatic breathing and experienced no change in symptom frequency; Murray et al. (52)). Retrospective chart reviews also have shown that patients report no effect of such medications (17,37). Given current evidence, we recommend clinicians prescribe baclofen to treat RS (conditionally) and only prescribe other medications to treat comorbid conditions.
Surgery.
One case series (N = 5) reported Nissen fundoplication as effective in resolving regurgitations (66); however, retrospective chart evidence has revealed that effects of fundoplication may not provide long-term resolution of regurgitations (37). In addition, other techniques have been reported in chart reviews (e.g., botulinum toxin injections) with no reported effect. Given current evidence, we recommend clinicians do not use surgical approaches, which aligns with previous conclusions in the literature (2,32,58,61).
CONCLUSION
Rumination syndrome is understudied, and often, patients with RS go undetected and untreated. As highlighted with Zachary’s case, there are heterogeneous features of RS, diagnosis can often be delayed because of a lack of clinician knowledge to assess for RS, and patients can experience protracted symptoms without appropriate treatment. Through our synthesis of the literature and clinical consensus, we recommend clinicians recognize the heterogeneous features of RS when considering diagnosis, assess for RS symptoms by clinical history, and treat RS with targeted diaphragmatic breathing, reserving other methods for augmented or alternative treatment.
Acknowledgments
Financial support: No financial support for this review article.
Footnotes
Guarantor of the article: Helen B. Murray, MS.
CONFLICTS OF INTEREST
Potential competing interests: D. Drossman is the founder and Chair of the Rome Foundation.
REFERENCES
- 1.APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th edn. American Psychiatric Publishing: Arlington, VA, 2013. [Google Scholar]
- 2.Drossman D, Chang L, Chey WD, et al. (eds). ROME IV: Functional Gastrointestinal Disorders—Disorders of Gut-Brain Interaction, 4 edn. Rome Foundation: Raleigh, NC, 2016; No. 2. [Google Scholar]
- 3.Drossman DA, Hasler WL. Rome IV—functional GI disorders: Disorders of gut-brain interaction. Gastroenterology 2016;150:1257–61. [DOI] [PubMed] [Google Scholar]
- 4.Chial HJ, Camilleri M, Williams DE, et al. Rumination syndrome in children and adolescents: Diagnosis, treatment, and prognosis. Pediatrics 2003;111:158–62. [DOI] [PubMed] [Google Scholar]
- 5.Fabricius A Tractatus de gula, ventriculo et intestinis Padua, Italy, 1618. [Google Scholar]
- 6.Brockbank E Merycism or rumination in man. Br Med J 1907;1:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blinder BJ, Goodman SL, Goldstein R. Rumination: A critical review of diagnosis and treatment. In: The Eating Disorders, Blinder BJ, Chaitin BF, & Goldstein R. PMA Publishing Group: 1988;315–329. [Google Scholar]
- 8.Rajindrajith S, Devanarayana NM, Perera BJC. Rumination syndrome in children and adolescents: A school survey assessing prevalence and symptomatology. BMC Gastroenterol 2012;12:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson W, Irvine E, Pare P, et al. Functional gastrointestinal disorders in Canada: First population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci 2002;47:225–35. [DOI] [PubMed] [Google Scholar]
- 10.Drossman DA, Li Z, Andruzzi E, et al. US householder survey of functional gastrointestinal disorders. Dig Dis Sci 1993;38:1569–80. [DOI] [PubMed] [Google Scholar]
- 11.Palsson O, Whitehead W, van Tilburg M, et al. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology 2016;150:1481–91. [DOI] [PubMed] [Google Scholar]
- 12.Murray HB, Thomas JJ, Hinz A, et al. Prevalence in primary school youth of pica and rumination behavior: The understudied feeding disorders. Int J Eat Disord 2018;51:994–8. [DOI] [PubMed] [Google Scholar]
- 13.Koloski NA, Talley NJ, Boyce PM. Epidemiology and health care seeking in the functional GI disorders: A population-based study. Am J Gastroenterol 2002;97:2290–9. [DOI] [PubMed] [Google Scholar]
- 14.Hartmann AS, Poulain T, Vogel M, et al. Prevalence of pica and rumination behaviors in German children aged 7–14 and their associations with feeding, eating, and general psychopathology: A population-based study. Eur Child Adolesc Psychiatry 2018:27:1499–508. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien MD, Bruce BK, Camilleri M. The rumination syndrome: Clinical features rather than manometric diagnosis. Gastroenterology 1995;108: 1024–9. [DOI] [PubMed] [Google Scholar]
- 16.Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology 2016;150:1380–92. [DOI] [PubMed] [Google Scholar]
- 17.Vijayvargiya P, Iturrino J, Camilleri M, et al. Novel association of rectal evacuation disorder and rumination syndrome: Diagnosis, comorbidities, and treatment. United European Gastroenterol J 2014:2:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanodia AK, Kim I, Sturmberg JP. A personalized systems medicine approach to refractory rumination. J Eval Clin Pract 2011;17:515–9. [DOI] [PubMed] [Google Scholar]
- 19.Johnson WG, Corrigan SA. Behavioral assessment and treatment of postprandial regurgitation. J Clin Gastroenterol 1987;9:679–84. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez S, Aspirot A, Kerzner B, et al. Do some adolescents with rumination syndrome have “supragastric vomiting”? J Pediatr Gastroenterol Nutr 2010;50:103–5. [DOI] [PubMed] [Google Scholar]
- 21.Fox M, Young A, Anggiansah R, et al. A 22 year old man with persistent regurgitation and vomiting: Case outcome. Br Med J 2006;7559:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amarnath RP, Abell TL, Malagelada JR. The rumination syndrome in adults: A characteristic manometric pattern. Ann Intern Med 1986;105: 513–8. [DOI] [PubMed] [Google Scholar]
- 23.Gupta R, Kalla M, Gupta JB. Adult rumination syndrome: Differentiation from psychogenic intractable vomiting. Indian J Psychiatry 2012;54: 283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray HB, Thomas JJ. Cognitive-behavioral therapy for rumination disorder (CBT-RD). International Conference on Eating Disorders, Chicago, IL, April, 2018. [Google Scholar]
- 25.Murray HB, Thomas JJ. Rumination disorder in adults. In: Anderson L, Murray S, Kaye W (eds). Handbook of Complex and Atypical Eating Disorders Oxford University Press: New York, NY, 2017. [Google Scholar]
- 26.Thomas JJ, Murray HB. Cognitive-behavioral treatment of adult rumination behavior in the setting of disordered eating: A single case experimental design. Int J Eat Disord 2016;49:967–72. [DOI] [PubMed] [Google Scholar]
- 27.Drossman D, Corazziari E, Delvaux M, et al. (eds). ROME III: The Functional Gastrointestinal Disorders, 3rd edn. Degnon Associates: McLean, VA, 2006. [Google Scholar]
- 28.Drossman D, Corazziari E, Talley NJ, et al. (eds). ROME II: The Functional Gastrointestinal Disorders—Diagnosis, Pathophysiology and Treatment: A Multinational Consensus, 2 edn. Degnon Associates: McLean, VA, 2000. [Google Scholar]
- 29.Drossman D, Richter JE, Talley NJ, et al. (eds). The Functional Gastrointestinal Disorders: Diagnosis, Pathophysiology and Treatment, 1st edn. Little Brown & Co: Boston, MA, 1994. [Google Scholar]
- 30.World Health Organization. ICD-11 Maintenance Platform 2018. https://icd.who.int/dev11/l-m/en. Accessed June 17, 2018.
- 31.Mousa HM, Montgomery M, Alioto A. Adolescent rumination syndrome. Curr Gastroenterol Rep 2014;16:398. [DOI] [PubMed] [Google Scholar]
- 32.Absah I, Rishi A, Talley N, et al. Rumination syndrome: Pathophysiology, diagnosis, and treatment. Neurogastroenterol Motil 2017;29:1–8. [DOI] [PubMed] [Google Scholar]
- 33.Kanner L Historical notes on rumination in man. Med Life 1936;43: 27–60. [Google Scholar]
- 34.Barba E, Burri E, Accarino A, et al. Biofeedback-guided control of abdominothoracic muscular activity reduces regurgitation episodes in patients with rumination. Clin Gastroenterol Hepatol 2015;13: 100–106,e101. [DOI] [PubMed] [Google Scholar]
- 35.Reese HE, Scahill L, Peterson AL, et al. The premonitory urge to tic: Measurement, characteristics, and correlates in older adolescents and adults. Behav Ther 2014;45:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucker E, Knowles K, Wright J, et al. Rumination variations: Aetiology and classification of abnormal behavioural responses to digestive symptoms based on high-resolution manometry studies. Aliment Pharmacol Ther 2013;37:263–74. [DOI] [PubMed] [Google Scholar]
- 37.Fullerton DT, Neff S, Getto CJ. Persistent functional vomiting. Int J Eat Disord 1992;12:229–33. [Google Scholar]
- 38.Blinder BJ. Rumination: A benign disorder? Int J Eat Disord 1986;5:385–6. [Google Scholar]
- 39.Fairburn CG, Cooper PJ. Rumination in bulimia nervosa. BMJ 1984;288: 826–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckern M, Stevens W, Mitchell J. The relationship between rumination and eating disorders. Int J Eat Disord 1999;26:414–9. [DOI] [PubMed] [Google Scholar]
- 41.Delaney CB, Eddy KT, Hartmann AS, et al. Pica and rumination behavior among individuals seeking treatment for eating disorders or obesity. Int J Eat Disord 2015;48:238–48. [DOI] [PubMed] [Google Scholar]
- 42.Parkman HP, Yates K, Hasler WL, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology 2011;140:101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singendonk M, Oors J, Bredenoord A, et al. Objectively diagnosing rumination syndrome in children using esophageal pH-impedance and manometry. Neurogastroenterol Motil 2017;29. [DOI] [PubMed] [Google Scholar]
- 44.Kessing BF, Bredenoord AJ, Smout AJ. Objective manometric criteria for the rumination syndrome. Am J Gastroenterol 2014;109:52–9. [DOI] [PubMed] [Google Scholar]
- 45.Sysko R, Glasofer DR, Hildebrandt T, et al. The eating disorder assessment for DSM-5 (EDA-5): Development and validation of a structured interview for feeding and eating disorders. Int J Eat Disord 2015;48:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryant-Waugh R, Micali N, Cooke L, et al. Development of the Pica, ARFID, and Rumination Disorder Interview, a multi-informant, semi-structured interview of feeding disorders across the lifespan: A pilot study for ages 10‐22. Int J Eat Disord 2018. [Epub ahead of print October 12, 2018.] [DOI] [PMC free article] [PubMed]
- 47.Schroedl RL, Alioto A, Di Lorenzo C. Behavioral treatment for adolescent rumination syndrome: A case report. Clin Pract Pediatr Psychol 2013;1:89–93. [Google Scholar]
- 48.Robin SG, Keller C, Zwiener R, et al. Prevalence of pediatric functional gastrointestinal disorders utilizing the Rome IV criteria. J Pediatr 2018; 195:134–9. [DOI] [PubMed] [Google Scholar]
- 49.Disney B, Trudgill N. Managing a patient with rumination. Frontline Gastroenterol 2013;4:232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalton WT III, Czyzewski DI. Behavioral treatment of habitual rumination: Case reports. Dig Dis Sci 2009;54:1804–7. [DOI] [PubMed] [Google Scholar]
- 51.Wagaman JR, Williams DE, Camilleri M. Behavioral intervention for the treatment of rumination. J Pediatr Gastroenterol Nutr 1998;27:596–8. [DOI] [PubMed] [Google Scholar]
- 52.Murray HB, Juarascio A, Thomas JJ. Augmenting diaphragmatic breathing with behavioral exposure: Single case experimental design for rumination syndrome. Under review
- 53.Soykan I, Chen J, Kendall BJ, et al. The rumination syndrome (clinical and manometric profile, therapy, and long-term outcome). Dig Dis Sci 1997; 42:1866–72. [DOI] [PubMed] [Google Scholar]
- 54.Murray HB, Juarascio A, Call CC, et al. Feasibility, acceptability, and preliminary efficacy of cognitive-behavioral therapy for rumination disorder (CBT-RD). International Conference on Eating Disorders, Chicago, IL, March, 2019. [Google Scholar]
- 55.Barba E, Accarino A, Soldevilla A, et al. Randomized, placebo-controlled trial of biofeedback for the treatment of rumination. Am J Gastroenterol 2016;111:1007–13. [DOI] [PubMed] [Google Scholar]
- 56.Woods DW, Murray LK, Fuqua RW, et al. Comparing the effectiveness of similar and dissimilar competing responses in evaluating the habit reversal treatment for oral–digital habits in children. J Behav Ther Exp Psychiatry 1999;30:289–300. [DOI] [PubMed] [Google Scholar]
- 57.Sharenow EL, Fuqua RW, Miltenberger RG. The treatment of muscle tics with dissimilar competing response practice. J Appl Behav Anal 1989;22:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tack J, Blondeau K, Boecxstaens V, et al. Review article: The pathophysiology, differential diagnosis and management of rumination syndrome. Aliment Pharmacol Ther 2011;33:782–8. [DOI] [PubMed] [Google Scholar]
- 59.Alioto A, Di Lorenzo C, Parzanese MI. Interdisciplinary intervention in adolescents with rumination syndrome. In: Martin C, Dovey T (eds). Paediatric Gastrointestinal Disorders: A Psychosocial Perspective CRC Press: London, UK, 2014:151–69. [Google Scholar]
- 60.Alioto A, Di Lorenzo C. Long-term follow-up of adolescents treated for rumination syndrome in an inpatient setting. J Pediatr Gastroenterol Nutr 2018;66:21–25. [DOI] [PubMed] [Google Scholar]
- 61.Pauwels A, Broers C, Van Houtte B, et al. A randomized double-blind, placebo-controlled, cross-over study using baclofen in the treatment of rumination syndrome. Am J Gastroenterol 2018;113:97–104. [DOI] [PubMed] [Google Scholar]
- 62.Drossman DA, Tack J, Ford AC, et al. Neuromodulators for functional GI disorders (disorders of gut-brain interaction): A Rome foundation working team report. Gastroenterology 2018;154:1140–71. [DOI] [PubMed] [Google Scholar]
- 63.Wilhelm S, Peterson AL, Piacentini J, et al. Randomized trial of behavior therapy for adults with Tourette syndrome. Arch Gen Psychiatry 2012;69: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Connor KP, Laverdure A, Taillon A, et al. Cognitive behavioral management of Tourette’s syndrome and chronic tic disorder in medicated and unmedicated samples. Behav Res Ther 2009;47:1090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sukhodolsky DG, Woods DW, Piacentini J, et al. Moderators and predictors of response to behavior therapy for tics in Tourette syndrome. Neurology 2017;88:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oelschlager BK, Chan MM, Eubanks TR, et al. Effective treatment of ruminations with Nissen fundoplication. J Gastrointest Surg 2002;6: 638–44. [DOI] [PubMed] [Google Scholar]
- 67.Chitkara DK, Van Tilburg M, Whitehead WE, et al. Teaching diaphragmatic breathing for rumination syndrome. Am J Gastroenterol 2006;101:2449–52. [DOI] [PubMed] [Google Scholar]
- 68.Hyams JS, Di Lorenzo C, Saps M, et al. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2016;150:1456–68. [Google Scholar]
- 69.Bryant-Waugh R, Markham L, Kreipe RE, et al. Feeding and eating disorders in childhood. Int J Eat Disord 2010;43:98–111. [DOI] [PubMed] [Google Scholar]
- 70.APA. Diagnostic and Statistical Manual of Mental Disorders, 4th edn. American Psychiatric Association: Washington, DC, 2000. [Google Scholar]
- 71.Blondeau K, Boecxstaens V, Rommel N, et al. Baclofen improves symptoms and reduces postprandial flow events in patients with rumination and supragastric belching. Clin Gastroenterol Hepatol 2012; 10:379–84. [DOI] [PubMed] [Google Scholar]
- 72.Birmingham C, Firoz T. Rumination in eating disorders: Literature review. Eating and weight disorders-studies on anorexia, Bulimia Obes 2006;11:e85–e89. [DOI] [PubMed] [Google Scholar]
- 73.Brown WR. Rumination in the adult: A study of two cases. Gastroenterology 1968;54:933–9. [PubMed] [Google Scholar]
- 74.Bruni A Rumination syndrome in Ethiopia: A case study. Prim Care companion CNS Disord 2014;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper CJ, Said S, Nunez A, et al. Chronic vomiting and diarrhea in a young adult female. Am J Case Rep 2013;14:449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson WG, Corrigan SA, Crusco AH, et al. Behavioral assessment and treatment of postprandial regurgitation. J Clin Gastroenterol 1987;9:679–84. [DOI] [PubMed] [Google Scholar]
- 77.Larocca FE, Della-Fera MA. Rumination: Its significance in adults with bulimia nervosa. Psychosomatics 1986;27:209–12. [DOI] [PubMed] [Google Scholar]
- 78.Reis S Rumination in two developmentally normal children: Case report and review of the literature. J Fam Pract 1994;38:521–3. [PubMed] [Google Scholar]
- 79.Shay SS, Johnson LF, Wong RK, et al. Rumination, heartburn, and daytime gastroesphageal reflux: A case study with mechanisms denned and successfully treated with biofeedback therapy. J Clin Gastroenterol 1986;8:115–26. [PubMed] [Google Scholar]
- 80.Smout A, Breumelhof R. Voluntary induction of transient lower esophageal sphincter relaxations in an adult patient with the rumination syndrome. Am J Gastroenterol 1990;85:1621–5. [PubMed] [Google Scholar]
- 81.Sokel B, Devane S, Bentovim A, et al. Self hypnotherapeutic treatment of habitual reflex vomiting. Arch Dis Child 1990;65:626–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weakley MM, Petti TA, Karwisch G. Case study: Chewing gum treatment of rumination in an adolescent with an eating disorder. J Am Acad Child Adolesc Psychiatry 1997;36:1124–7. [DOI] [PubMed] [Google Scholar]
- 83.Williamson DA, Lawson OD, Bennett SM, et al. Behavioral treatment of night bingeing and rumination in an adult case of bulimia nervosa. J Behav Ther Exp Psychiatry 1989;20:73–7. [DOI] [PubMed] [Google Scholar]
- 84.Green AD, Alioto A, Mousa H, et al. Severe pediatric rumination syndrome: Successful interdisciplinary inpatient management. J Pediatr Gastroenterol Nutr 2011;52:414–8. [DOI] [PubMed] [Google Scholar]
- 85.Levine D, Wingate D, Pfeffer J, et al. Habitual rumination: A benign disorder. BMJ 1983;287:255–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khan S, Hyman PE, Cocjin J, et al. Rumination syndrome in adolescents. J Pediatr 2000;136:528–31. [DOI] [PubMed] [Google Scholar]
- 87.Fleisher DR. Functional vomiting disorders in infancy: Innocent vomiting, nervous vomiting, and infant rumination syndrome. J Pediatr 1994;125:S84–S94. [DOI] [PubMed] [Google Scholar]
- 88.Sauvage D, Leddet I, Hameury L, et al. Infantile rumination: Diagnosis and follow-up study of twenty cases. J Am Acad Child Psychiatry 1985;24: 197–203. [DOI] [PubMed] [Google Scholar]
- 89.Lang R, Mulloy A, Giesbers S, et al. Behavioral interventions for rumination and operant vomiting in individuals with intellectual disabilities: A systematic review. Res Dev Disabil 2011;32:2193–205. [DOI] [PubMed] [Google Scholar]


