Abstract
Accurate assessment of plasma corticosterone, the primary stress hormone in rodents, is an essential part of characterizing the stress response in experimental animals. To this end, both enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay (RIA) remain widely used. However, considerable assay-specific variability exists among commercially available corticosterone assays due to differing assay principles, detection methods, range, and sensitivity. While technical comparisons of commercially available corticosterone assays have previously been conducted, ability to detect acute stress-induced endocrine changes has not been compared among these methods to date. Using the forced swim test, a commonly utilized behavioral paradigm in rodents as a physiologically-relevant acute stress challenge, we compared four commercial corticosterone assays – three ELISA kits and one RIA kit – in their ability to detect corticosterone across a dynamic range of both baseline and acute swim stress-driven concentrations. While all methods yielded results that were consistent at measuring relative differences between samples, only two of the four assays evaluated detected a statistically significant increase in corticosterone in rats exposed to acute swim stress compared to rats at baseline. The ELISA kit from Enzo Lifesciences demonstrated the greatest percent increase in plasma corticosterone from baseline to acute stress conditions. The RIA kit from MP Biomedicals also detected a significant corticosterone increase and yielded higher concentrations of corticosterone both at baseline and in the acute stress condition relative to the other three methods. We conclude that choice of assay can impact interpretation of data due to differences in efficacy across a dynamic range of physiological concentrations of corticosterone.
Keywords: corticosterone, ELISA, radioimmunoassay, forced swim stress, rat, female
Introduction
Accurately assessing plasma corticosterone, the primary stress hormone in rodents, is an essential part of examining the stress response of experimental animals. Furthermore, being able to do so over the dynamic range of the corticosterone response is equally important as this enables researchers to make inferences about the physiological and/or behavioral paradigm that they are utilizing. Current methods of assessing corticosterone can yield results that vary considerably in absolute quantity. Importantly, while technical comparisons of commercially available corticosterone assays indicate overall correlations between these assays (Hofreiter et al., 1982; Kinn Rod et al., 2017), whether such assays demonstrate comparable abilities to detect acute stressor-induced corticosterone increases remains an important but unanswered question. The forced swim test, a common behavioral test in rodents, is extensively utilized as a stressor in rodent research. Notably, forced swim of various durations has been demonstrated to reliably increase plasma corticosterone concentrations in rats (Abel, 1993). In particular, the brevity of the test makes the forced swim paradigm a distinctly suitable acute stressor, and has been used in such a manner extensively (Bourke et al., 2013; Johnson et al., 2006; Neumann et al., 1998). Using the forced swim test as an acute stress challenge, we compared four commercially available corticosterone assays in their ability to detect corticosterone concentrations across a dynamic range with assessments at both baseline and following an acute swim stressor.
There are currently two primary methods of measuring plasma corticosterone: 1) enzyme-linked immunosorbent assay (ELISA), which quantifies the concentration of corticosterone present in a sample based on competing interactions of either endogenous or enzyme-linked-antigen with limited amounts of antibody and 2) radioimmunoassay (RIA) which is similarly based on competing interactions between antibody and endogenous or radiolabeled corticosterone. Although both methods remain widely in use, there are differences in both the potential confounds in various methods and a range of safety and practical considerations. While RIA is thought to be both sensitive and specific, performing RIA requires special equipment and handling radioactive substances. ELISAs in turn offer a more affordable and user-friendly option. However, a systematic comparison among ELISAs and RIAs with consideration of a dynamic physiological range of concentrations has not been performed to date and is needed considering the substantial methodological differences between assays.
Among most commercially available corticosterone assays, the underlying principles regarding the type of corticosterone being measured vary among manufacturers. Corticosterone, once released into the blood stream from the adrenal cortex, travels in blood bound to corticosteroid-binding globulin (CBG). In fact, 90% of total corticosterone is known to be bound to carrier proteins, including primarily CBG and, to a lesser extent, albumin (Breuner et al., 2013). While unbound or free cortisol is argued to be physiologically more relevant as a proxy to the stress response (Breuner et al., 2013), the majority of studies assessing serum or plasma corticosterone only report total corticosterone concentration; CBG levels are seldom measured. Furthermore, commercially available corticosterone assays also differ in sensitivity, specificity, range, detection chemistry, and sample handling procedures that may lead to variable results. In the present study, we aimed to compare the dynamic range and consistency of acute stress-induced increases in corticosterone using three commonly utilized rat corticosterone ELISA kits from the following vendors: Abcam (Cambridge, MA), Enzo Life Sciences (Farmingdale, NY), and R&D Systems (Minneapolis, MN). All three assays are examples of competitive ELISAs. However, they differ in terms of pretreatment of samples (displacement of CBG and other proteins that might affect total and free corticosterone), capture and detection of antigen, standard curve range, sensitivity, and specificity as reported by the manufacturers (see Tables 1 and 2). Furthermore, we compared these ELISA results to the results of a corticosterone RIA (MP Biomedicals, Santa Ana, CA), as RIAs have traditionally been used more widely than ELISAs. We report that the acute swim stress-induced increase in corticosterone concentration was detected as statistically significant by only two of the four assays evaluated, suggesting that the choice of assay influences the inferences that can be made concerning acute swim stress-induced physiological changes.
Table 1.
Details of each corticosterone assay as provided in the manufacturer’s manual.
| Abcam | Enzo | R&D | RIA | |
|---|---|---|---|---|
| Range of standard curve | 391–100,00 pg/ml | 32–20,000 pg/ml | 103–25,000 pg/mL | 25,000–1,000,000 pg/mL |
| Sensitivity | 8,000 pg/mL | 26.99 pg/mL | 28 pg/mL | 7,700 pg/mL |
| Dilution method | 25-fold | 30-fold | 50-fold | 200-fold |
| Type of kit | Competitive ELISA | RIA | ||
| Capture method | Direct (primary antibody) | Sandwich (primary (donkey anti-sheep lgG) and secondary antibodies) | Antibody | |
| Detection method | Indirect (biotin-streptavidin) | Indirect | 125I label | |
| Detection chemistry | Peroxidase and substrate | Alkaline phosphate and substrate | HRP and substrate | |
| Sample pretreatment | None | Incubated for > 5 min with provided steroid displacement reagent | Incubated for 15 min with 0.6 N Tricloroacetic acid | None |
| Free or total corticosterone measured | Free | Total | Total | Free |
Table 2. Specificity of corticosterone assays as listed by each assay manual.
Compounds tested by two or more of the assays are listed. Refer to the product manuals for a full list of compounds. “None” indicates cross-reactivity not detected as reported by each manufacturer. “N/A” indicates that the compound was not tested for cross-reactivity.
| Compound | % Cross Reactivity | |||
|---|---|---|---|---|
| Enzo | Abcam | R&D | RIA | |
| Corticosterone | 100 | 100 | N/A | 100 |
| Deoxycorticosterone | 28.6 | < 30 | 0.08 | 0.34 |
| Progesterone | 1.7 | < 2 | 0.1 | 0.02 |
| Testosterone | 0.13 | N/A | None | 0.1 |
| Aldosterone | 0.18 | < 2 | 0.31 | 0.03 |
| Cortisol | 0.05 | < 0.1 | N/A | 0.05 |
| Pregnenolone | < 0.03 | N/A | N/A | < 0.01 |
| β-Estradiol | < 0.03 | N/A | N/A | N/A |
| Estradiol-17α | N/A | N/A | N/A | < 0.01 |
| Estradiol-17β | N/A | N/A | N/A | < 0.01 |
| Estradiol | N/A | N/A | None | N/A |
| Cortisone | < 0.03 | None | None | N/A |
| Dexamethasone | N/A | N/A | 0.04 | < 0.01 |
| 17α-Hydroxyprogesterone | N/A | < 0.1 | N/A | < 0.01 |
| Hydrocortisone | N/A | None | 0.09 | N/A |
Methods
Animals
Adult female Wistar rats (n=7–8/group) were generated from breeding pairs purchased from Charles River Laboratories (Wilmington, MA), pair-housed in an AAALAC-approved facility under standard laboratory conditions, and maintained on a 14:10 reverse light:dark cycle (on at 00:00h and off at 14:00h) with standard rodent chow and water provided ad libitum. The Emory University Institutional Animal Care and Use Committee approved all animal use procedures.
Acute swim stress protocol
On postnatal day 94, rats were transported to a behavioral testing suite and acclimated for four hours. Eight rats were randomly selected to be exposed to a five-minute forced swim protocol, which has previously been demonstrated to robustly elevate plasma corticosterone and increase the expression of stress response-related genes in the brain (Bourke et al., 2013). All experimental procedures were completed at least two hours before the end of the light cycle to avoid the corticosterone awakening response which takes place at the beginning of the dark cycle. Briefly, rats were individually placed in a clear acrylic beaker (60 cm high * 22 cm in diameter) filled with 25 °C water for five minutes and were returned to their home cage at the end of the session. Seven littermates served as baseline home cage controls. Thirty minutes after initiation of the forced swim protocol, rats were rapidly decapitated and trunk blood was collected in an EDTA-treated glass tube (Becton, Dickinson and Company, Franklin Lakes, NJ). Although corticosterone has been reported to peak at 60–90 minutes in blood following forced swim onset (Qian et al., 2011), the thirty minute time point was chosen because this is when altered HPA axis function such as greater plasma corticosterone is evident in adult female rats with a background of chronic stress (Bourke et al., 2013). Anesthesia was not used prior to euthanasia due to previously demonstrated effects of commonly used inhalants such as isoflurane to increase plasma corticosterone levels (Bekhbat et al., 2016). Blood was centrifuged at 1800 rcf for 20 min at 4°C and plasma was collected, aliquoted, and stored at −80°C until further use. An aliquot from each rat was used in each assay such that exactly the same collected samples were run in all four assays. All samples underwent only one freeze-thaw cycle.
ELISA
Rat corticosterone ELISA kits were purchased from Abcam (#ab108821, Cambridge, MA), Enzo Life Sciences (#ADI-900–097, Farmingdale, NY), and R&D Systems (#KGE009, Minneapolis, MN), referred to as “Abcam,” “Enzo,” and “R&D” hereafter. A separate frozen sample aliquot from each subject was used for each assay, and the assays were performed in duplicate according to each manufacturer’s recommendations. While all reagents and materials were brought to room temperature prior to use, the samples were thawed immediately prior to use and kept on ice as binding of corticosterone to CBG declines with increasing temperature (Cameron et al., 2010). The dilution factor for each assay was optimized, and was 25-fold for Abcam, 30-fold for Enzo, and 50-fold for R&D. See Tables 1 and 2 for detailed information about each assay. The coefficient of variance among the duplicates was less than 15%. The plates were read on a Synergy HTX plate reader (Biotek, Winooski, VT), and its built-in four-parameter logistic regression software was used for plotting the standard curve and data extrapolation.
RIA
Circulating levels of corticosterone were measured in duplicate by radioimmunoassay (RIA) using a Corticosterone Double Antibody RIA kit (#07–120102, MP Biomedicals, Santa Ana, California). The dilution factor suggested by the manufacturer, 200-fold, was performed as directed. See Tables 1 and 2 for detailed information about the assay. The coefficient of variance among the duplicates equaled 15.8%. The tubes were read on a Wallac Wizard 1470 five detector automatic gamma counter (Global Medical Instrumentation, Ramsey, MN), using the built-in linear fitting and logarithmic plotting software for standard curve and data extrapolation.
Statistical Analysis
All statistical analyses were performed using GraphPad Prism 7.00 for Windows (GraphPad Software, La Jolla, CA), with the alpha value set to 0.05, unless otherwise noted. Corticosterone data were analyzed using a two-way repeated measures ANOVA with stress condition as a main factor (baseline or acute swim stress) and type of assay as repeated factor (Enzo, Abcam, R&D, or RIA). Sidak’s multiple comparison test was performed to compare assays within each stress condition. Planned comparisons between baseline and acute swim stress conditions within each assay were also conducted using Sidak’s multiple comparison test. Percent change in corticosterone was calculated as . Correlation between assays was calculated using two-tailed Pearson correlation using IBM® SPSS® Statistics (Version 24). Power analysis was conducted using G*Power 3.1.9.2 (Faul et al., 2007).
Results
The range of standard curve, sensitivity, and sample pre-treatment procedures for each assay are provided in Table 1. Information about the specificity of each assay as reported in the product manual is provided in Table 2. A two-way repeated measures ANOVA revealed a main effect of stress condition [F(1, 13)=9.94, p<0.01, η2=0.30], whereby acute stress significantly increased plasma corticosterone concentration (Figure 1). There was a significant main effect of type of assay [F(3, 39)=16.97, p<0.0001, η2=0.16]. No significant interaction between stress and assay was found [F(3, 39)=1.227, p>0.05]. Sidak’s multiple comparisons showed that at baseline, the RIA kit yielded significantly higher corticosterone concentrations compared to the Enzo assay [Mean ±SEM of 155±31.18 ng/mL vs. 55.45±15.5 ng/mL, p<0.05]. In the acute stress condition, the RIA kit again demonstrated significantly greater concentrations than the Enzo, Abcam, and R&D assays [Mean ±SEM of 301±28.24ng/mL vs. 179.7±40.38ng/mL (p<0.0001), 178.2±35.29ng/mL (p<0.0001), and 218.9±27.95ng/mL (p<0.01), respectively]. The three ELISA kits did not differ from each other in either baseline or acute stress conditions (p>0.05). Post-hoc comparisons between baseline and acute swim stress conditions within each assay revealed that swim stress led to a significant corticosterone increase as assessed by the Enzo assay [Mean ±SEM of 55.45±15.5 ng/mL vs 179.7±40.38ng/mL (p<0.05, d=1.448)] and the RIA kit [Mean ±SEM of 155±31.18 ng/mL vs 301±28.24ng/mL (p<0.01, d=1.797)] (Figure 1). Percent change in corticosterone from baseline to acute stress conditions was 224% for Enzo, 93.7% for Abcam, 86.7% for R&D, and 94.1% for RIA. Values for the same samples ran on the three ELISA kits correlated strongly with each other (r>0.93, p<0.01) (Table 3). The RIA samples also displayed significant correlation with those run on each of the ELISA kits, but the fit of the correlation was somewhat reduced (r>0.63, p<0.05) (Table 3).
Figure 1. Acute swim stress-induced corticosterone increases as measured by two out of four assays are statistically significant.
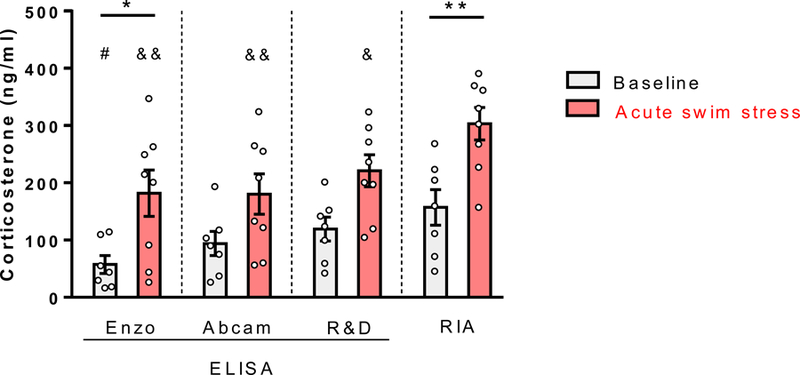
Swim stress led to a significant corticosterone increase as assessed by the Enzo assay [Mean ±SEM of 55.45±15.5 ng/mL vs 179.7±40.38ng/mL (p<0.05, d=1.448)] and the RIA kit [Mean ±SEM of 155±31.18 ng/mL vs 301±28.24ng/mL (p<0.01, d=1.797)]. Percent change in plasma corticosterone from baseline to acute stress condition was 224% for Enzo, 93.7% for Abcam, 86.7% for R&D, and 94.1% for RIA. * p < 0.05, ** p < 0.01compared to baseline within each assay. Mean ±SEM is shown for each assay/condition.
Table 3.
Pearson correlations between the four methods.
| Enzo | Abcam | R&D | RIA | |
|---|---|---|---|---|
| Enzo | 1 | .938** | .946** | .620* |
| Abcam | .938** | 1 | .970** | .635* |
| R&D | .946** | .970** | 1 | .724** |
| RIA | .620* | .635* | .724** | 1 |
indicate that the correlation is significant at the p < 0.05 and 0.01 levels, respectively (2-tailed).
Discussion
We demonstrate that all four corticosterone assays evaluated here yield consistent measurements of relative differences between samples, suggesting robust inter-assay congruence. Our results indicate for the first time that the choice of corticosterone assay influences whether the corticosterone surge following an acute challenge is deemed statistically significant. Only two of the four assays – Enzo and RIA – detected a statistically significant corticosterone increase in the plasma of rats exposed to acute swim stress compared to rats at baseline. Of the four methods evaluated, the ELISA kit from Enzo Lifesciences yielded the greatest percent increase in corticosterone from baseline to the acute stress condition.
One difference between the assays resided in each assay’s sample pre-treatment procedure which may impact the levels of corticosterone detected. Both Enzo and R&D assays, which measure total corticosterone, contain a pre-treatment step during which an included reagent was incubated with the samples for >5 and 15 minutes, respectively, to inhibit corticosterone binding to carrier proteins. Abcam and RIA, on the other hand, do not include an explicit pretreatment step and thus presumably measure free corticosterone plus any that may have become unbound during the several hours-long incubation at room temperature that is required for all ELISA and RIA kits. Despite this lack of pretreatment, the Abcam assay generated values similar to those of the other ELISA kits both at baseline and in the acute stress condition. It is possible that varying degrees of the effectiveness of CBG-uncoupling pretreatments partially drive the between-assay discrepancies in measuring identical corticosterone samples (Kinn Rod et al., 2017). Interestingly, the RIA kit demonstrated the highest concentrations of corticosterone both at baseline and in the acute stress condition. This may be due to the distinct detection method utilized by RIA compared to ELISAs. Another possibility is that normalizing the samples to room temperature prior to beginning the assay as instructed in the RIA manual may have contributed to the higher values detected by this method. While the binding of corticosteroids to CBG has been studied across physiological ranges of temperature (hypothermia-to-fever) (Carrell et al., 2011; Henley and Lightman, 2011), whether assay temperature (4°C versus room temperature) impacts experimental outcomes has not been systematically investigated (Schoech et al., 2013).
While strong correlation exists among measurement methods, the variables of sample pre-treatment, assay sensitivity, standard curve range, and exposure to ambient temperature specific to each assay, likely contribute to the variability between methods. Importantly, the findings presented here indicate that the choice of corticosterone assay can directly impact the conclusions that can be drawn regarding the endocrine response in a commonly used swim stress paradigm, and should therefore be an important consideration when designing behavioral and endocrine experiments.
Acknowledgements
The authors would like to thank Sabina Khantsis for her assistance with the RIA.
Funding source: This work was supported by the National Institutes of Health (NR014886 and MH110364 to GNN; T32-GM008602 to SAR).
Footnotes
Declaration of interest
The authors declare no conflicts of interest.
References
- Abel EL, 1993. Physiological correlates of the forced swim test in rats. Physiol Behav 54, 309–317. [DOI] [PubMed] [Google Scholar]
- Bekhbat M, Merrill L, Kelly SD, Lee VK, Neigh GN, 2016. Brief anesthesia by isoflurane alters plasma corticosterone levels distinctly in male and female rats: Implications for tissue collection methods. Behav Brain Res 305, 122–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN, 2013. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology 38, 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner CW, Delehanty B, Boonstra R, Fox C, 2013. Evaluating stress in natural populations of vertebrates: total CORT is not good enough. Functional Ecology 27, 24–36. [Google Scholar]
- Cameron A, Henley D, Carrell R, Zhou A, Clarke A, Lightman S, 2010. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J Clin Endocrinol Metab 95, 4689–4695. [DOI] [PubMed] [Google Scholar]
- Carrell R, Qi X, Zhou A, 2011. Serpins as hormone carriers: modulation of release. Methods Enzymol 501, 89–103. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A, 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Henley DE, Lightman SL, 2011. New insights into corticosteroid-binding globulin and glucocorticoid delivery. Neuroscience 180, 1–8. [DOI] [PubMed] [Google Scholar]
- Hofreiter BT, Allen JP, Mizera AC, Powers CD, Masi AM, 1982. High-performance liquid chromatography and radioimmunoassay of rat plasma corticosterone. Steroids 39, 547–555. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Fournier NM, Kalynchuk LE, 2006. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res 168, 280–288. [DOI] [PubMed] [Google Scholar]
- Kinn Rod AM, Harkestad N, Jellestad FK, Murison R, 2017. Comparison of commercial ELISA assays for quantification of corticosterone in serum. Sci Rep 7, 6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Johnstone HA, Hatzinger M, Liebsch G, Shipston M, Russell JA, Landgraf R, Douglas AJ, 1998. Attenuated neuroendocrine responses to emotional and physical stressors in pregnant rats involve adenohypophysial changes. J Physiol 508 ( Pt 1), 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Droste SK, Gutierrez-Mecinas M, Collins A, Kersante F, Reul JM, Linthorst AC, 2011. A rapid release of corticosteroid-binding globulin from the liver restrains the glucocorticoid hormone response to acute stress. Endocrinology 152, 3738–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoech SJ, Romero LM, Moore IT, Bonier F, Fox C, 2013. Constraints, concerns and considerations about the necessity of estimating free glucocorticoid concentrations for field endocrine studies. Functional Ecology 27, 1100–1106. [Google Scholar]


