Abstract
Objectives
The pathological bases for the cognitive and neuropsychiatric symptoms in normal pressure hydrocephalus (NPH) have not been elucidated. However, the symptoms may indicate dysfunction of subcortical regions. Previously, volume reductions of subcortical deep grey matter (SDGM) structures have been observed in NPH patients. The present study used automated segmentation methods to investigate whether SDGM structure volumes are associated with cognitive and neuropsychiatric measures.
Methods
Fourteen NPH patients and eight healthy controls were included in the study. Patients completed neuropsychological tests of general cognition, verbal learning and memory, verbal fluency and measures of apathy and depression pre‐ and postshunt surgery. Additionally, patients underwent 3 Tesla T1‐weighted magnetic resonance imaging at baseline and 6 months postoperatively. Controls were scanned once. SDGM structure volumes were estimated using automated segmentation (FSL FIRST). Since displacement of the caudate nuclei occurred for some patients due to ventriculomegaly, patient caudate volumes were also estimated using manual tracing. Group differences in SDGM structure volumes were investigated, as well as associations between volumes and cognitive and neuropsychiatric measures in patients.
Results
Volumes of the caudate, thalamus, putamen, pallidum, hippocampus and nucleus accumbens (NAcc) were significantly reduced in the NPH patients compared to controls. In the NPH group, smaller caudate and NAcc volumes were associated with poorer performance on neuropsychological tests and increased severity of neuropsychiatric symptoms, while reduced volume of the pallidum was associated with better performance on the MMSE and reduced apathy.
Conclusions
Striatal volume loss appears to be associated with cognitive and neuropsychiatric changes in NPH.
Keywords: apathy, cognition, neuroimaging, neuropsychology, normal pressure hydrocephalus
1. INTRODUCTION
Normal pressure hydrocephalus (NPH) is characterized by a build‐up of cerebrospinal fluid (CSF) in the brain which causes ventriculomegaly despite apparently normal CSF pressure at lumbar puncture. Symptoms include gait apraxia, urinary incontinence and cognitive decline.1 In addition, recent research suggests a high prevalence of apathy in NPH patients (65‐86%).2, 3, 4 NPH is treated via surgical CSF diversion which results in clinical and neuropsychological improvement for many patients.5
The pathological mechanisms underlying the cognitive and neuropsychiatric symptoms in NPH are not well established. The executive dysfunction in NPH may suggest impaired frontal lobe functioning,6 however, there is inconsistent evidence for frontal involvement from imaging studies of regional cerebral blood flow (rCBF) in NPH.6, 7 The pattern of cognitive decline is consistent with dysfunction of subcortical structures and may indicate disruption to the subfrontal white matter, limbic connections or connections between the frontal lobe and the subcortical deep grey matter (SDGM) structures.8 Indeed, impaired rCBF has been found in the periventricular white matter and in the basal ganglia.7, 9
The presence of apathy (impaired goal‐directed behaviour) in NPH is consistent with fronto‐subcortical pathology. Apathy can result from lesions affecting fronto‐subcortical circuitry,10 or from focal lesions of the basal ganglia,10 including lesions located in the caudate nucleus.11 Diminished caudate volume was previously observed in NPH patients using voxel‐based morphometry and hypothesized to contribute to the clinical symptoms of cognitive impairment and apathy.12 However, this association was not examined. We previously found evidence for an association between greater bicaudate ratio (greater ventriculomegaly) with increased levels of apathy and depression in NPH patients.13 Additionally, evidence for associations between apathy and rCBF in the anterior cingulate cortices and the right caudate nucleus has been reported.14
Subcortical volumes in NPH have not been extensively investigated using quantitative MRI techniques. The periventricular regions may be distorted due to ventriculomegaly. Atrophy may also occur over time due to ischaemia and subsequent cell death.15 The few studies that have examined SDGM volumes in NPH focused only on individual structures or did not relate volumetric data to clinical information.12, 16, 17 In the present study, we conducted volumetric assessments of a range of SDGM structures and investigated whether volumes are related to cognitive or neuropsychiatric measures. Subcortical volumes were estimated using automated segmentation techniques. However, in some of the patients there was displacement of the caudate nuclei due to the degree of ventriculomegaly. For this reason, we also used manual tracing techniques to estimate the volume of the caudate in all patients to supplement the analyses based on automated segmentation of the caudate.
2. METHODS
2.1. Ethics
Ethical approval was obtained from Cambridgeshire 2 Local Research Ethics Committee (LREC No: 06/Q0108/330) as part of a larger project entitled “Functional, Vascular and Structural Correlates of Reversible Dementia in Normal Pressure Hydrocephalus.”.
2.2. Participants
Sixteen patients with NPH were recruited between October 2007 and June 2009. Patients gave informed consent prior to enrolling in the study. Patients were assessed and scanned before and a mean of 6.4 months following shunt surgery. Patients were referred to be included in the study by a neurosurgeon (JDP) based on the presence of a clinical picture of gait disturbance, slowing of mentation and/or short‐term memory impairment.6 One patient was excluded from the following analyses as they had comorbid Alzheimer’s disease. One additional patient was excluded due to a complicated postoperative clinical course.
Healthy controls were recruited via verbal screening at the CSF multidisciplinary clinic for inclusion criteria, and via advertisement in the local area. Interested partners or spouses of study participants who met the inclusion criteria were also offered healthy volunteer scans. Some healthy volunteers self‐referred after becoming aware of the NPH imaging programme via the patient community. Exclusion criteria for the healthy control group were (i) a diagnosis of a neurological disorder, (ii) the presence of contraindications to MRI scanning and (iii) age <60. Eight healthy controls (HCs) were scanned at one time‐point.
2.3. Neuropsychological assessment
Patients completed a brief battery of neuropsychological tests, selected on the basis of their sensitivity to NPH,5 pre‐ and postoperatively. Global cognitive functioning was assessed using the Mini‐Mental State Examination (MMSE).18 Verbal learning and memory was assessed using the Hopkins Verbal Learning Test (HVLT).19 Phonemic and semantic fluency were assessed using the Controlled Oral Word Association Test.20 Apathy was assessed using the Cambridge‐developed State Apathy Evaluation scale (self‐rated; AES‐S),21 which measures state‐related changes in apathy. Depression was assessed using the short form of the Geriatric Depression Scale (GDS‐15).22 IQ was assessed using the National Adult Reading Test.23 To minimize practice effects, alternate versions of phonemic and semantic fluency categories and the HVLT word list were used at follow‐up.
2.4. MR imaging protocol
MR imaging was performed on a 3 T Siemens Tim Trio using a 12‐channel RF receive coil. The MR imaging protocol included a PDT2, FLAIR, MPRAGE and DTI. The T1‐weighted structural sequence (MPRAGE) was acquired with TR/TE of 2300/2.98 ms, with a resolution of 1x1x1mm.
2.5. Image analysis
2.5.1. Automated segmentation
Before processing, all images were manually reviewed for artefact. SDGM volumes were estimated for both groups (including at pre‐ and postshunt for NPH patients as the shunt artefact was above the level of the SDGM structures). Subcortical volumes (caudate, nucleus accumbens (NAcc), pallidum, putamen and thalamus) as well as volumes of the hippocampus and the amygdala were extracted from 3D T1‐weighted images using an automated subcortical registration and segmentation tool in FSL (FSL FIRST; www.fmrib.ox.ac.uk/fsl). Examples of subcortical segmentation are shown in Figure 1. All automated segmentations were thoroughly individually reviewed for accuracy. SDGM volumes were normalized for head size by multiplying by the volumetric scaling factors obtained using SIENAX,24 part of FSL.25 SIENAX starts by extracting brain and skull images from the single whole‐head input data.26 The brain image is then affine registered to MNI152 space (using the skull image to determine the registration scaling)27; primarily in order to obtain the volumetric scaling factor to be used as a normalization for head size. Next, tissue‐type segmentation with partial volume estimation is carried out,28 in order to calculate total volume of brain tissue. As laterality effects were not hypothesized, normalized right and left volumes of the SDGM structures were combined to yield a single total volume for each structure.
Figure 1.
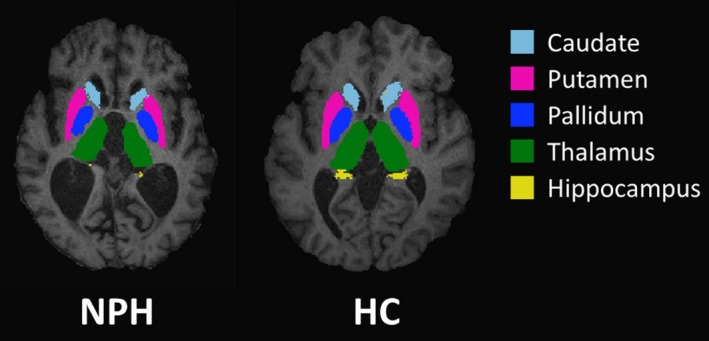
Representative FIRST segmentation of subcortical structures in a patient with normal pressure hydrocephalus (left) and a healthy control subject (right)
2.5.2. Caudate tracing
Manual tracing was performed on T1‐weighted images using ITK‐SNAP version 3.4.0 (www.itksnap.org). 29 Right and left caudate nuclei were traced on contiguous axial slices, as described by Looi et al,30 beginning with the most inferior slice where the caudate nucleus was visible, bounded by the frontal white matter, the anterior commissure and the internal capsule.30 Tracing proceeded superiorly from the head to the tail of the caudate until the tail was no longer distinguishable from the wall of the lateral ventricle. Caudate segmentation was then checked in the coronal and sagittal views where any errors were corrected. Finally, a 3D view of the segmented caudate nucleus was compiled and checked for abnormalities (Figure 2). Volumes were normalized for head size by multiplying by the volumetric scaling factors from SIENAX. Normalized right and left caudate volumes were combined to yield a single total volume.
Figure 2.
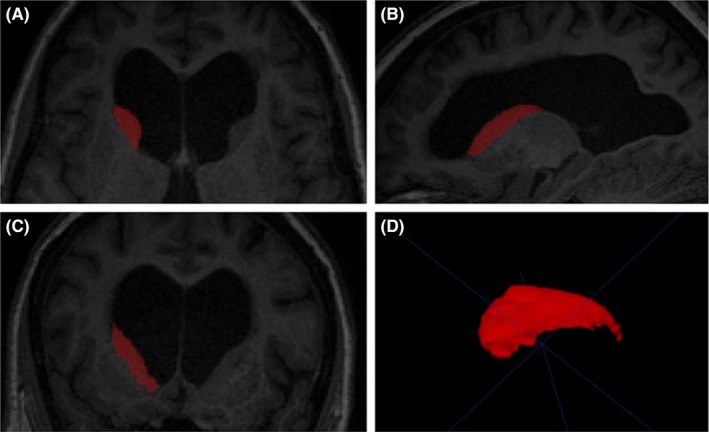
Segmented right caudate in (A) axial section, (B) sagittal section and (C) coronal section in a representative NPH patient; (D) 3D rendering of caudate volume
2.6. Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences (SPSS) software version 25 (IBM Corp, Armonk, NY). Independent samples t tests were conducted to test for group differences in age and SDGM volumes. Additionally, ANCOVAs were conducted to test for group differences in SDGM volumes while controlling for age and sex. Pearson’s r correlations were conducted to investigate associations between demographic data, volumetric data and neuropsychological test scores. However, where variables violated assumptions of normality, Spearman’s correlation coefficient (rs) was used. Paired samples t tests were conducted to test for pre‐ to postshunt differences in SDGM volumes and neuropsychological test scores.
Pearson’s r correlations investigated associations between caudate volumes obtained via automated segmentation and via manual tracing. Differences in the volumes obtained via the two methods were investigated using paired samples t tests. Independent samples t tests were conducted to investigate differences in volumes between patients included in or excluded from the automated segmentation analyses.
3. RESULTS
3.1. Demographics
The final patient group consisted of 14 patients (9 male, 5 female). Baseline mean (SD) age was 76.4 (3.9), range = 70‐84, IQ was 110.8 (10.4), MMSE was 24 (3.3), and years of education was 11.1 (2.2). Individual patient details are shown in Table 1. Two patients had comorbid depression (patients 6 and 9 in Table 1). Four HCs were male. The NPH group was significantly older than HCs (HC group mean (SD) age = 70.3 (6.6), range = 64‐80); (t (9.89) = 2.43, P = 0.036).
Table 1.
Normal pressure hydrocephalus patient demographics
| Patient No. | Age | Sex | IQ | Years of education | Time to retest | Aetiology | Gait disturb | Urinary symptoms | MMSE Pre | MMSE Post |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 84 | F | 122 | 11 | 7 | Idiopathic | + | + | 26 | 28 |
| 2 | 78 | F | 100 | 11 | 7 | Idiopathic | + | − | 18 | 21 |
| 3 | 75 | M | 103 | 11 | 8 | Idiopathic | + | + | 23 | 22 |
| 4 | 77 | M | 112 | 14 | 7 | Idiopathic | + | + | 26 | 27 |
| 5 | 78 | M | n/a | 13 | 5 | Idiopathic | + | + | n/a | 28 |
| 6 | 78 | M | n/a | 9 | 5 | Idiopathic | + | + | 20 | 15 |
| 7 | 72 | M | 87 | 10 | 7 | Query aqueduct stenosis | + | + | 18 | 26 |
| 8 | 82 | M | 118 | 9 | 7 | Secondary NPH | + | + | 24 | 25 |
| 9 | 71 | M | 116 | 16 | 5 | Idiopathic | + | + | 25 | 28 |
| 10 | 70 | F | 116 | 15 | 11 | Idiopathic | + | ‐ | 27 | 28 |
| 11 | 74 | F | 109 | 12 | 8 | Idiopathic | + | + | 26 | 26 |
| 12 | 77 | M | 115 | 9 | 5 | Idiopathic | + | + | 27 | 28 |
| 13 | 76 | M | 107 | 8 | <1 | Idiopathic | + | + | 26 | 22 |
| 14 | 78 | F | 124 | 12 | 8 | Idiopathic | + | n/a | 26 | 27 |
n/a, information not available.
3.2. Automated segmentation
The following analyses are based on automated subcortical segmentation. While this technique avoided observer bias, it reduced the sample size because of gross displacement caused by ventriculomegaly of all SDGM structures in three patients and displacement of the caudate nuclei in an additional three patients at pre‐shunt. At postshunt, there was displacement of all structures in two patients and displacement of the caudate nuclei in a further two patients. As a result of this, both pre‐ and postshunt volumetric data for each structure were available for a limited number of patients. Therefore, correlational analyses were conducted at two time‐points (pre‐ and postshunt) rather than on pre‐ to postshunt change scores.
Automated segmentation was successful in the HCs and all remaining patients so that analyses of SDGM volumetric data is based on 11 patients preoperatively and 12 postoperatively (apart from data for caudate volume which is based on eight patients preoperatively and 10 postoperatively).
3.2.1. Subcortical volumes
Mean volumes of the SDGM structures for both groups are shown in Table 2. Volumes of all SDGM structures except the amygdala were significantly smaller in the NPH group at both pre‐ and postshunt compared to controls. These remained significant after controlling for age and sex.
Table 2.
Group differences in SDGM volumes at pre‐ and postshunt
| SDGM structure |
Control Mean (SD) volume |
NPH Pre‐shunt Mean (SD) volume |
NPH Post‐shunt Mean (SD) volume |
|---|---|---|---|
| Caudate | 8627.6 (726.8) | 6720.4 (485.1)a | 6965.3 (822.6)a |
| Thalamus | 17590.6 (1865.1) | 13141.5 (883.4)a | 14112.4 (1287.1)a,b |
| Putamen | 11658.7 (1269.2) | 7150.3 (1771.8)a | 8737.2 (1410.2)a |
| Pallidum | 4139.9 (562.4) | 2903 (639)a | 3226.5 (646.5)a |
| Hippocampus | 9211.9 (1271.5) | 5356.7 (1142.8)a | 6302.9 (1051.6)a,b |
| Amygdala | 3148.5 (638.9) | 2631.4 (605.9) | 2889.4 (642.2) |
| Nucleus accumbens | 1010.6 (237.9) | 483.67 (100.9)a | 531.04 (182)a |
Volumes presented (including caudate volume) are taken from the automated segmentation analysis, and are in mm3
Significant volume difference compared to controls, P < 0.05
Significant volume difference compared to pre‐shunt, P < 0.05.
In the NPH group, volumes of the thalamus, t (10) = −2.85, P = 0.017; and hippocampus, t (10) = −3.02, P = 0.013, were significantly increased following shunt surgery.
3.2.2. Neuropsychological profile pre‐ and postshunt surgery
Neuropsychological test scores for the NPH patients are shown in Table 3. Performance on semantic fluency, HVLT immediate and HVLT learning was significantly improved following shunt surgery. In addition, there was a trend for improvement in all remaining test scores, except for GDS‐15.
Table 3.
Neuropsychological outcome following shunt surgery
| Test | N | Pre‐shunt Mean (SD) | Postshunt Mean (SD) | t | df | P |
|---|---|---|---|---|---|---|
| MMSE | 12 | 23.92 (3.40) | 24.58 (3.92) | −0.70 | 11 | 0.497 |
| AES‐S | 10 | 18.60 (7.95) | 15.60 (9.06) | 1.15 | 9 | 0.278 |
| GDS‐15 | 10 | 5.10 (2.51) | 5.10 (3.93) | 0.00 | 9 | 1.000 |
| Phonemic fluency | 12 | 29.75 (14.20) | 30.33 (16.96) | −0.19 | 11 | 0.853 |
| Semantic fluency | 11 | 10.00 (3.98) | 13.45 (3.83) | −2.40 | 10 | 0.038* |
| HVLT immediate | 12 | 3.83 (1.40) | 4.58 (1.68) | −3.00 | 11 | 0.012* |
| HVLT learning | 12 | 13.83 (4.86) | 17.50 (6.07) | −2.70 | 11 | 0.020* |
| HVLT delayed | 12 | 2.17 (2.76) | 3.08 (4.17) | −0.74 | 11 | 0.473 |
MMSE, Mini‐Mental State Examination; AES‐S, self‐rated State Apathy Evaluation; GDS‐15, Geriatric Depression Scale short form; HVLT, Hopkins Verbal Learning Test
P < 0.05.
3.2.3. Correlation of volumetric data with neuropsychology scores
The significance value for the Shapiro‐Wilk test was less than 0.05 for the following: pre‐shunt MMSE score, pre‐shunt semantic fluency score, pre‐shunt HVLT delayed score, postshunt MMSE score, postshunt AES‐S and postshunt HVLT delayed score, suggesting that these data are not normally distributed. As such, Spearman’s correlation coefficient (rs) was used to investigate associations with these variables.
Pre‐shunt
NAcc volume was significantly positively correlated with HVLT immediate (r = 0.70, P = 0.035). That is, greater volume was associated with better performance. There were no other significant correlations between SDGM volumes and cognitive test scores at pre‐shunt.
Postshunt
Caudate volume was significantly positively correlated with MMSE score (rs = 0.65, P = 0.040), and semantic fluency (r = 0.67, P = 0.035), and significantly negatively correlated with AES‐S (r = −0.83, P = 0.006). NAcc volume was significantly positively correlated with MMSE (rs = 0.78, P = 0.002), HVLT immediate (r = 0.58, P = 0.039), HVLT learning (r = 0.68, P = 0.011) and HVLT delayed (rs = 0.63, P = 0.022); and significantly negatively correlated with AES‐S (rs = 0.81, P = 0.001). For the above significant positive correlations, greater volumes were associated with better cognitive test performance and reduced apathy. Volume of the pallidum was significantly negatively correlated with MMSE score (rs = −0.55, P = 0.050) but significantly positively correlated with AES‐S (rs = 0.65, P = 0.023). That is, greater pallidum volume was associated with poorer performance on the MMSE and increased apathy.
3.3. Caudate tracing
Automated segmentation of the caudate failed for a number of the patients, reducing the sample size and meaning that pre‐ and postshunt correlations with clinical measures were based on different participant Ns. Therefore, manual caudate tracing was conducted to further examine the relationship between caudate volume and clinical scores in the patient group.
Manual tracing was conducted for all 14 patients at pre‐ and postshunt. Caudate volumes obtained via manual tracing and automated segmentation were significantly correlated at both pre‐ (r = 0.74, P = 0.035) and postshunt (r = 0.76, P = 0.011). Mean manually traced caudate volumes (pre‐shunt mean (SD) = 6288.7 (1386.2) mm3; postshunt mean (SD) = 5610 (901.5) mm3; N = 14) were smaller than those obtained via automated segmentation (pre‐shunt mean (SD) = 6720.4 (485.1), N = 8; postshunt mean (SD) = 6965.3 (822.6), N = 10). This difference was statistically significant for the postshunt volumes only, t (9) = 6.90, P < 0.001. Patients were split according to whether they were included in or excluded from (due to unsuccessful caudate segmentation) the automated segmentation analyses. The mean manually segmented caudate volumes did not differ significantly between the two groups at pre‐ (P > 0.05) or postshunt (P > 0.05). Therefore, the smaller volumes obtained via manual tracing are not due to the inclusion of additional participants.
3.3.1. Correlation of volumetric data with neuropsychology scores
Pre‐shunt
Manually traced caudate volumes were significantly positively correlated with MMSE score (r = 0.63, P = 0.022), phonemic fluency (r = 0.64, P = 0.019), semantic fluency (r = 0.78, P = 0.003) and HVLT delayed (r = 0.59, P = 0.035). That is, greater caudate volume was associated with better performance for all four tests.
Postshunt
At postshunt, manually traced caudate volumes were significantly positively correlated with MMSE score (r = 0.78, P = 0.001), meaning greater caudate volume was associated with better performance. Additionally, caudate volume was significantly negatively correlated with AES‐S (r = −0.69, P = 0.009) and GDS‐15 (r = −0.55, P = 0.042), meaning greater caudate volume was associated with reduced apathy and depression.
4. DISCUSSION
We conducted a volumetric assessment of subcortical structures in NPH patients using automated segmentation. Manual tracing was conducted to supplement the caudate volume data. Caudate volumes obtained via automated and manual segmentation were significantly correlated at both pre‐ and postshunt suggesting that automated segmentation was satisfactory for the majority of patients.
All of the SDGM structures apart from the amygdala were significantly reduced in the NPH patients compared to healthy controls, and remained so after adjusting for age and sex. We conducted correlations to investigate associations between SDGM structure volumes and neuropsychological test performance. Using the data derived from automated segmentation, only NAcc volume was significantly associated with HVLT immediate score at pre‐shunt; while at postshunt, larger caudate and NAcc volumes were associated with better performance on the MMSE, and reduced self‐rated apathy. Caudate volume was also positively associated with semantic fluency at postshunt; and NAcc with verbal learning and memory. In addition, greater volume of the pallidum at postshunt was associated with poorer performance on the MMSE and increased self‐rated apathy. The association between pallidum volume and scores is in the unexpected direction and should be clarified in a larger sample.
Since the analyses relating to caudate volume were based on small (and differing) Ns manual caudate tracing was also conducted. Larger manually traced caudate volumes were associated with better performance on the MMSE, verbal fluency and the HVLT delayed subtest at pre‐shunt. At postshunt, larger volumes were associated with better performance on the MMSE as well as reduced self‐rated apathy and depression. These results suggest that volume of the caudate and the NAcc in patients with NPH is associated with the cognitive and neuropsychiatric symptoms.
The caudate nucleus and the NAcc are two important structures within the striatum which play important roles in movement, cognition, learning and motivation or goal‐directed behaviour.31, 32, 33 The striatum is linked to the frontal cortex via parallel frontal‐subcortical circuits, lesions to which can cause executive dysfunction, personality changes and impaired motivation or apathy.10, 34 Reduced pre‐operative caudate volume in NPH patients was previously noted,12 and hypothesized to contribute to cognitive decline and apathy. The nature of the correlations in the present study indicated that reduced caudate and NAcc volume was associated with increased apathy, supporting the notion that dysfunction of these regions underlies apathy in NPH. Further, Kanemoto et al.14 reported evidence for an association between reduced apathy following shunt with improved rCBF in the anterior cingulate cortices and the right caudate nucleus. Therefore, volume changes of subcortical regions may be related to changes in rCBF. Taken together, these results provide evidence for the importance of striatal volume loss in the cognitive and behavioural symptoms in NPH and might suggest that at least some of the cognitive decline in patients could be due to subcortical dysfunction.
An important limitation of our study concerns the significant age difference between the patients and the controls in our sample. We cannot rule out the possibility that the smaller SDGM structure volumes seen in the patient group are due to the age difference compared to controls. However, the difference in volumes between the healthy controls and the NPH patients ranges from −16% to −52% which is substantially greater than would be expected according to published estimates of age‐related decline of subcortical structures.35 Therefore, while some of the group difference may be explained by the age difference, it is unlikely that the age difference can account for the majority of the group differences. While we have attempted to control for the age difference, a further study with more closely matched patients and controls will help to clarify this issue. Another limitation concerns the small sample sizes, and the fact that some of the sample sizes were further reduced due to the limitations of automated segmentation methods in this patient group. We attempted to overcome this issue by incorporating manual tracing to supplement the volumetric data obtained using automated segmentation. However, this study requires replication in the future using larger sample sizes. Finally, our results should be interpreted with caution, as we have not corrected for multiple comparisons since this is a small pilot study. Indeed, the unexpected direction of the correlations between the volume of the pallidum with test scores might suggest that some of our significant associations reflect a type I error. However, the associations relating to caudate and NAcc volume are consistent in terms of direction, and warrant further investigation.
In summary, while most of the SDGM structures investigated in the present study were significantly smaller in NPH patients compared to controls, striatal volume loss appears to be associated with the cognitive and neuropsychiatric symptomology in NPH. The results confirm our earlier hypothesis that reduced caudate volume is associated with increased apathy, and may implicate the striatal regions in cognitive and motivational impairments experienced by patients with NPH.
CONFLICTS OF INTERESTS
Barbara J. Sahakian consults for Cambridge Cognition, Peak (Brainbow), Mundipharma and has share options in Cambridge Cognition. John D. Pickard reports grants from NIHR Senior Investigator Award and grants from NIHR Cambridge Brain Injury HTC. All other authors have nothing to declare.
ACKNOWLEDGEMENTS
The authors would like to thank P. Simon Jones for his help and advice during the revision of this manuscript. The authors would also like to thank the administrative staff at the Departments of Psychiatry and Clinical Neurosciences, University of Cambridge, Addenbrooke’s Hospital for their assistance.
Peterson KA, Mole TB, Keong NCH, et al. Structural correlates of cognitive impairment in normal pressure hydrocephalus. Acta Neurol Scand. 2019;139:305–312. 10.1111/ane.13052
John D. Pickard and Barbara J. Sahakian these authors contributed equally to this work.
Funding information
The research was supported by the National Institute for Health Research (NIHR) Brain Injury MedTech in vitro Diagnostic Co‐operative based at the Cambridge University Hospitals NHS Foundation Trust and University of Cambridge. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Katie A. Peterson’s PhD was funded by a grant awarded to John D. Pickard from the NIHR Biomedical Research Centre. Katie A. Peterson also reports funding from St George’s University of London (FB MRC) (13120‐10). Tom B. Mole is a NIHR Academic Clinical Fellow in The Department of Psychiatry. Nicole C. H. Keong reports grants from Joint Royal College of Surgeons/Dunhill Medical Trust Fellowship, and from Tunku Abdul Rahman Project. Elise E. DeVito reports grants from Pinsent Darwin Fund, and from U.S. National Institutes of Health (ORWH, NIDA, NIAAA, OD). George Savulich is funded by Eton College and The Wallitt Foundation and is supported by the NIHR Cambridge Biomedical Research Centre (BRC) Mental Health theme. John D. Pickard reports grants from the NIHR Senior Investigator Award, the NIHR Brain Injury MedTech in vitro Diagnostic Co‐operative and Van Geest Grant for hydrocephalus research. Barbara J. Sahakian receives funding from the Wellcome Trust (Grant 089589/Z/09/Z), the MRC/Wellcome Trust Behavioural and Clinical Neuroscience Institute (joint award G00001354), the Human Brain Project, the NIHR Brain Injury MedTech in vitro Diagnostic Co‐operative, and the NIHR Cambridge Biomedical Research Centre (Mental Health and Neurodegeneration Themes).
REFERENCES
- 1. Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure: Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965;2:307–327. [DOI] [PubMed] [Google Scholar]
- 2. Kito Y, Kazui H, Kubo Y, et al. Neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. Behav Neurol. 2009;21:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanemoto H, Kazui H, Suzuki Y, et al. Effect of lumbo‐peritoneal shunt surgery on neuropsychiatric symptoms in patients with idiopathic normal pressure hydrocephalus. J Neurol Sci. 2016;361:206–212. [DOI] [PubMed] [Google Scholar]
- 4. Allali G, Laidet M, Armand S, Saj A, Krack P, Assal F. Apathy and higher level of gait control in normal pressure hydrocephalus. Int J Psychophysiol. 2017;119:127–131. [DOI] [PubMed] [Google Scholar]
- 5. Peterson KA, Savulich G, Jackson D, Killikelly C, Pickard JD, Sahakian BJ. The effect of shunt surgery on neuropsychological performance in normal pressure hydrocephalus: a systematic review and meta‐analysis. J Neurol. 2016;263(8):1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iddon JL, Pickard JD, Cross JJ, Griffiths PD, Czosnyka M, Sahakian BJ. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neurosurg Psychiatry. 1999;67(6):723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Owler BK, Momjian S, Czosnyka Z, et al. Normal pressure hydrocephalus and cerebral blood flow: a PET study of baseline values. J Cereb blood flow Metab. 2004;24:17–23. [DOI] [PubMed] [Google Scholar]
- 8. Keong N, Pena A, Price SJ, Czosnyka M, Czosnyka Z, Pickard JD. Imaging normal pressure hydrocephalus: theories, techniques, and challenges. Neurosurg Focus. 2016;41:E11. [DOI] [PubMed] [Google Scholar]
- 9. Ziegelitz D, Starck G, Kristiansen D, et al. Cerebral perfusion measured by dynamic susceptibility contrast MRI is reduced in patients with idiopathic normal pressure hydrocephalus. J Magn Reson imaging. 2014;39(6):1533–1542. [DOI] [PubMed] [Google Scholar]
- 10. Tekin S, Cummings JL. Frontal‐subcortical neuronal circuits and clinical neuropsychiatry: An update. J Psychosom Res. 2002;53(2):647–654. [DOI] [PubMed] [Google Scholar]
- 11. Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117(4):859–876. [DOI] [PubMed] [Google Scholar]
- 12. DeVito EE, Salmond CH, Owler BK, Sahakian BJ, Pickard JD. Caudate structural abnormalities in idiopathic normal pressure hydrocephalus. Acta Neurol Scand. 2007;116(5):328–332. [DOI] [PubMed] [Google Scholar]
- 13. Peterson KA, Housden CR, Killikelly C, et al. Apathy, ventriculomegaly and neurocognitive improvement following shunt surgery in normal pressure hydrocephalus. Br J Neurosurg. 2016;30(1):38–42. [DOI] [PubMed] [Google Scholar]
- 14. Kanemoto H, Kazui H, Suehiro T, et al. Association between apathy and regional cerebral blood flow in patients with idiopathic normal pressure hydrocephalus. Fluids and Barriers of the CNS. 2015;12(Suppl 1):O4. [Google Scholar]
- 15. Momjian S, Owler BK, Czosnyka Z, Czosnyka M, Pena A, Pickard JD. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain. 2004;127(5):965–972. [DOI] [PubMed] [Google Scholar]
- 16. Golomb J, de Leon MJ, George AE, et al. Hippocampal atrophy correlates with severe cognitive impairment in elderly patients with suspected normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 1994;57(5):590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Savolainen S, Laakso MP, Paljärvi L, et al. MR imaging of the hippocampus in normal pressure hydrocephalus: Correlations with cortical Alzheimer’s disease confirmed by pathologic analysis. Am J Neuroradiol. 2000;21(2):409–414. [PMC free article] [PubMed] [Google Scholar]
- 18. Cockrell JR, Folstein MF. Mini‐mental state examination (MMSE). Psychopharmacol Bull. 1988;24:689–692. [PubMed] [Google Scholar]
- 19. Brandt J. The hopkins verbal learning test: Development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5(2):125–142. [Google Scholar]
- 20. Benton AL, Hamsher K. Multilingual aphasia examination. Manual of instructions. Iowa City: AJA Associates; 1989. [Google Scholar]
- 21. Blackwell AD, Paterson NS, Barker RA, Robbins TW, Sahakian BJ. The effects of modafinil on mood and cognition in Huntington’s disease. Psychopharmacology. 2008;199(1):29–36. [DOI] [PubMed] [Google Scholar]
- 22. Sheikh JI, Yesavage JA. Geriatric depression scale (GDS) recent evidence and development of a shorter version. Clin Gerontol. 1986;5(1–2):165–173. [Google Scholar]
- 23. Nelson HE.The National Adult Reading Test (NART): Test Manual. Vol 124; 1982. [Google Scholar]
- 24. Smith SM, De Stefano N, Jenkinson M, Matthews PM. Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomogr. 2001;25(3):466–475. [DOI] [PubMed] [Google Scholar]
- 25. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(SUPPL. 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- 26. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156 [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. [DOI] [PubMed] [Google Scholar]
- 29. Yushkevich PA, Piven J, Hazlett HC, et al. User‐guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. NeuroImage. 2006;31(3):1116–1128. [DOI] [PubMed] [Google Scholar]
- 30. Looi J, Lindberg O, Liberg B, et al. Volumetrics of the caudate nucleus: reliability and validity of a new manual tracing protocol. Psychiatry Res Neuroimaging. 2008;163(3):279–288. [DOI] [PubMed] [Google Scholar]
- 31. Schultz W. The primate basal ganglia and the voluntary control of behaviour. J Conscious Stud. 1999;6:31–45. [Google Scholar]
- 32. Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. [DOI] [PubMed] [Google Scholar]
- 33. Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6(2):228–236. [DOI] [PubMed] [Google Scholar]
- 34. Mega MS, Cohenour RC. Akinetic mutism: disconnection of frontal‐subcortical circuits. Neuropsychiatry, Neuropsychol Behav Neurol. 1997;10(4):254–259. [PubMed] [Google Scholar]
- 35. Walhovd KB, Fjell AM, Reinvang I, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26(9):1261–1270. [DOI] [PubMed] [Google Scholar]


