Abstract
Background
Wide international variation in the prevalence of disabling low back pain (LBP) among working populations is not explained by known risk factors. It would be useful to know whether the drivers of this variation are specific to the spine or factors that predispose to musculoskeletal pain more generally.
Methods
Baseline information about musculoskeletal pain and risk factors was elicited from 11 710 participants aged 20–59 years, who were sampled from 45 occupational groups in 18 countries. Wider propensity to pain was characterized by the number of anatomical sites outside the low back that had been painful in the 12 months before baseline (‘pain propensity index’). After a mean interval of 14 months, 9055 participants (77.3%) provided follow‐up data on disabling LBP in the past month. Baseline risk factors for disabling LBP at follow‐up were assessed by random intercept Poisson regression.
Results
After allowance for other known and suspected risk factors, pain propensity showed the strongest association with disabling LBP (prevalence rate ratios up to 2.6, 95% CI: 2.2–3.1; population attributable fraction 39.8%). Across the 45 occupational groups, the prevalence of disabling LBP varied sevenfold (much more than within‐country differences between nurses and office workers), and correlated with mean pain propensity index (r = 0.58).
Conclusions
Within our study, major international variation in the prevalence of disabling LBP appeared to be driven largely by factors predisposing to musculoskeletal pain at multiple anatomical sites rather than by risk factors specific to the spine.
Significance
Our findings indicate that differences in general propensity to musculoskeletal pain are a major driver of large international variation in the prevalence of disabling low back pain among people of working age.
1. Introduction
Low back pain (LBP) is the leading cause of disability globally (Hoy et al., 2014), and a major contributor to incapacity for work among young and middle‐aged adults (Bevan et al., 2009). Risk factors for its incidence and/or persistence include activities such as heavy lifting that load the spine mechanically (Lötters et al., 2003), tendency to somatize (Pincus et al., 2002; Vargas‐Prada et al., 2013), low mood (Pincus et al., 2002; Ramond et al., 2011; Vargas‐Prada et al., 2013), psychosocial stressors in the workplace (Lang et al., 2012) and adverse beliefs about the prognosis of back disorders (Ramond et al., 2011). In Europe, the consistency of its association with mechanical loading has prompted legislation requiring employers to control manual handling in the workplace through appropriate design of tasks and equipment (European Agency for Safety and Health at Work, 1990). However, in randomized controlled trials, reductions in LBP from ergonomic interventions have been minimal (Driessen et al., 2010; Verbeek et al., 2012). Moreover, the descriptive epidemiology of LBP suggests that there are other more important determinants. For example, in Britain there was an eightfold increase in long‐term incapacity for work because of LBP between 1950 and the early 1990s – a change too large to be explained by known causes (Clinical Standards Advisory Group, 1994).
Given the established role of psychological factors in the occurrence of LBP, we hypothesized that trends in disability from back disorders could be a consequence of changes in health beliefs and expectations, and that culturally determined differences in health beliefs might lead also to large international variation in prevalence (Coggon, 2005). To test this theory, we initiated the CUPID (Cultural and Psychosocial Influences on Disability) study, in which information about musculoskeletal pain, associated disability and potential risk factors was collected from workers sampled from 47 occupational groups across 18 countries (Coggon et al., 2012). Analysis of cross‐sectional data at baseline confirmed that there were up to sevenfold differences between occupational groups in the 1‐month prevalence of disabling LBP, but the variation was not explained either by established risk factors or by knowledge and beliefs about LBP (Coggon et al., 2013a). It did, however, correlate with differences across occupational groups in the prevalence of disabling wrist/hand pain, suggesting that the two complaints might be driven importantly by one or more shared risk factors that are associated with a general propensity to experience and report musculoskeletal pain and associated disability (Coggon et al., 2013a). The existence of such propensity would accord with the observation that musculoskeletal pain often affects multiple anatomical sites, either simultaneously or closely in time (Natvig et al., 2001; IJzelenberg and Burdorf, 2004; Haukka et al., 2006; Coggon et al., 2013b), and that pain elsewhere predicts the future occurrence of LBP (Papageorgiou et al., 1996; Smith et al., 2004).
To explore the extent to which differences in general propensity to musculoskeletal pain might account for variation in the prevalence of LBP between occupational groups, we analysed longitudinal data from the CUPID study, looking at baseline risk factors for the 1‐month prevalence of disabling LBP at follow‐up, and taking as an index of pain propensity, the number of anatomical sites other than the low back that were reported as painful in the 12 months before baseline. We opted for a longitudinal design rather than a cross‐sectional analysis because it would avoid bias from simultaneous reporting of risk factors and outcomes.
2. Methods
The methods of the CUPID study have been reported in detail elsewhere (Coggon et al., 2012). Ethical approval for the investigation was provided by relevant ethics committees in each of the 18 participating countries.
2.1. Study sample
The 47 occupational groups that made up the initial study sample fell into three broad categories – nurses, office workers and ‘other workers’ mainly carrying out repetitive manual tasks. During 2006–11, men and women aged 20–59 years who were eligible for inclusion according to pre‐specified criteria, were identified (in most occupational groups from employers’ records) and invited to complete a baseline questionnaire, either by self‐administration, or in some occupational groups, at interview (overall response rate 70%).
2.2. Baseline questionnaire and specification of personal risk factors
The questionnaire was originally drafted in English, and then translated to local languages where necessary, with checks for accuracy by independent back‐translation. Among other things, it covered: sex; age; smoking habits (never smoked, ex‐smoker or current smoker); hours worked per week (< or ≥50 h per week); other psychosocial aspects of work, (incentives from piecework or bonuses; time pressure; lack of choice in what work was done, how and when; lack of support from colleagues or supervisor/manager; job dissatisfaction; and perceived job insecurity if off work for 3 months with illness); occupational lifting (whether an average working day entailed lifting weights ≥25 kg by hand); mental health; somatizing tendency; adverse beliefs about LBP (work‐relatedness, prognosis and effects of physical activity); and recent experience of musculoskeletal pain.
Mental health was assessed through questions taken from the Short Form‐36 (SF‐36) questionnaire (Ware and Sherbourne, 1992), and was graded to three levels (good, intermediate and poor) corresponding to approximate thirds of the distribution of scores in the full study sample. Somatizing tendency was determined through questions taken from the Brief Symptom Inventory (Derogatis and Melisoratos, 1983), and was graded according to the number of somatic symptoms from a total of five (faintness or dizziness, pains in the heart or chest, nausea or upset stomach, trouble getting breath, hot or cold spells) that had been at least moderately distressing during the past week. Questions on adverse beliefs were adapted from the Fear Avoidance Beliefs Questionnaire (Waddell et al., 1993). Participants were classed as having adverse believes about work‐relatedness if they completely agreed that back pain is commonly caused by work; about its relationship to physical activity if they completely agreed that for someone with back pain, physical activity should be avoided as it might cause harm, and that rest is needed to get better; and about its prognosis if they completely agreed that neglecting such problems can cause serious harm, and completely disagreed that such problems usually get better within 3 months.
The questions about musculoskeletal pain focused on 10 anatomical sites (low back; neck; and right and left shoulder, elbow, wrist/hand and knee), which were illustrated diagrammatically. For each site, participants were asked whether they had experienced pain during the past 12 months that had lasted for longer than a day. In addition, those who reported LBP were asked whether it had been present during the past month, and if so, whether during that time it had made it difficult or impossible to get dressed, do normal jobs around the house or cut toe nails (which we classed as disabling LBP).
2.3. Group‐level risk factors
In addition to the information obtained from questionnaires, the lead investigator in each country provided baseline information about group‐level factors (variables which took an identical value for all members of the same occupational group) that might impact on disability from musculoskeletal symptoms. These included: the unemployment rate in the community from which the occupational group came, whether it was necessary to pay for primary medical care, and the availability of: pay during sickness absence, financial support for ill‐health retirement, social security for long‐term unemployment and compensation for work‐related LBP.
2.4. Follow‐up
After an interval of approximately 14 months, participants in all but two of the occupational groups (manual workers in Costa Rica and office workers in South Africa) were asked to answer a shorter follow‐up questionnaire – as before by self‐administration or at interview. This included questions about experience of LBP for a day or longer in the past month, and again asked whether that pain had made it difficult or impossible to get dressed, do normal jobs around the house or cut toe nails (disabling LBP).
2.5. Statistical analysis
Statistical analysis was carried out with Stata v.12.1 software (Stata Corp LP 2012, Stata Statistical Software: Release 12.1; College Station TX, USA). For each participant, we derived a ‘pain propensity index’ defined by the number of anatomical sites other than the low back that were reported as having been painful in the 12 months before baseline. We used simple descriptive statistics to explore the relationship of this index to other personal characteristics at baseline.
We then applied Poisson regression (with robust standard errors) to examine the association of disabling LBP in the past month at follow‐up as an outcome variable with potential risk factors at baseline. To allow for possible clustering by occupational group, we used random intercept models. Associations were summarized by prevalence rate ratios (PRRs) with 95% confidence intervals (CIs). We first fitted a model that included personal risk factors, including pain propensity index.
Next, we explored the role of group‐level risk factors, analysing each in a separate Poisson regression model that also included all of the personal risk factors. As well as the group‐level measures that had been provided by local investigators, we also examined five group‐level variables that were derived from the individual questionnaires (the mean pain propensity index in the occupational group, and the group prevalence of knowing someone outside work with low back pain, and of adverse beliefs about LBP regarding its work‐relatedness, prognosis and the effects of physical activity). These were included to address the original hypothesis of the CUPID study that differences between occupational groups in the prevalence of musculoskeletal pain and associated disability might be importantly determined by differences in culturally determined health beliefs and expectations. Thus, for example, the group prevalence of knowing someone outside work with LBP was an indicator of the prominence of LBP as a symptom in the participant's community, which might influence how an individual perceived and responded to back symptoms when they occurred.
We then fitted a single model incorporating all of the personal and group‐level factors that had shown significant (p < 0.05) associations with disabling LBP in the earlier analyses, and from the PRRs obtained, we estimated population attributable fractions (PAFs) for each factor. These indicated the proportion of cases in the study population that would be eliminated if (after adjustment for other risk factors), the prevalence of disabling LBP in those with exposure to the risk factor were reduced to that in those unexposed. While they do not necessarily assume or imply that the risk factor caused disabling LBP to develop, persist or recur, they illustrate its potential importance as a driver of the prevalence of the symptom. Confidence intervals for PAFs were calculated by bootstrapping with 250 repetitions per estimate (Efron, 1979).
To explore the extent to which pain propensity and other risk factors might explain variation between occupational groups in the prevalence of disabling LBP, we compared the numbers of cases by occupational group with the numbers that would have been expected: (1) based only on the overall prevalence of disabling LBP in the full study sample; (2) calculated from a Poisson regression model that adjusted for pain propensity index (using predicted probabilities generated by Stata); and (3) calculated from the final Poisson regression model that included all statistically significant risk factors. The extent of variation was characterized by the geometric standard deviation of the ratios of observed to expected numbers. To set the results in context, we used random simulations to explore the expected distributions of geometric standard deviations under the assumption that each individual's probability of disabling LBP was that which would have been predicted from the relevant Poisson regression model given his/her exposure to risk factors. Thus, for the first and simplest analysis, the simulations assumed that each person had a probability of disabling LBP equal to the overall prevalence; for the second analysis, the simulations assumed that a person's probability of disabling LBP was that which would be expected from their pain propensity index, taking no other information into account; while the simulations for the third analysis, assumed that each person's probability of LBP was that predicted from the final Poisson regression model.
3. Results
Within the 45 occupational groups that contributed to the longitudinal component of the study, 11 992 participants answered the baseline questionnaire, including 11 710 who provided complete information about pain at anatomical sites other than the low back during the 12 months before baseline. Among the latter, 9055 (3083 men) answered the items about LBP in the follow‐up questionnaire, giving a usable response rate of 77.3%. The number of responders by occupational group ranged from 39 to 633, with follow‐up rates >70% in 36 of the 45 groups. Follow‐up was marginally higher in older participants, those with greater pain propensity, and those with disabling LBP in the month before baseline (Table 1).
Table 1.
Response rates at follow‐up according to demographic characteristics and report of pain at baseline
| Baseline characteristic | Number of participants who provided adequate information at baseline | Number (%) with usable follow‐up |
|---|---|---|
| Sex | ||
| Male | 4065 | 3083 (75.8%) |
| Female | 7645 | 5972 (78.1%) |
| Age (years) | ||
| 20–29 | 2817 | 2087 (74.1%) |
| 30–39 | 3784 | 2913 (77.0%) |
| 40–49 | 3275 | 2602 (79.5%) |
| 50–59 | 1834 | 1453 (79.2%) |
| Pain propensity score | ||
| 0 | 3598 | 2690 (74.8%) |
| 1 | 2582 | 1972 (76.4%) |
| 2 | 1973 | 1547 (78.4%) |
| 3 | 1522 | 1186 (77.9%) |
| 4 | 846 | 699 (82.6%) |
| 5 | 591 | 482 (81.6%) |
| 6 | 278 | 218 (78.4%) |
| 7 | 194 | 158 (81.4%) |
| 8 | 62 | 52 (83.9%) |
| 9 | 64 | 51 (79.7%) |
| Disabling LBP in past montha | ||
| No | 9046 | 7002 (77.4%) |
| Yes | 2529 | 1995 (78.9%) |
Data on disabling LBP in the past month at baseline were missing for 135 participants.
Among the 9055 participants who were suitable for analysis, the pain propensity index at baseline varied from 0 (2690 participants) to 9 (51 participants), with mean 1.9, median 1 and interquartile range 0–3. Mean values were higher in women than men, at older ages, in participants with poor mental health, and in those who reported distress from common somatic symptoms (Table 2). However, there was little difference in relation to smoking habits.
Table 2.
Relationship of pain propensity index to personal characteristics
| Characteristic | Pain propensity indexa | ||
|---|---|---|---|
| Mean (95% CI) | Median | Inter‐quartile range | |
| Sex | |||
| Male | 1.4 (1.3, 1.4) | 1 | 0–2 |
| Female | 2.2 (2.1, 2.2) | 2 | 1–3 |
| Age (years) | |||
| 20–29 | 1.5 (1.4, 1.5) | 1 | 0–2 |
| 30–39 | 1.8 (1.7, 1.8) | 1 | 0–3 |
| 40–49 | 2.1 (2.0, 2.2) | 2 | 0–3 |
| 50–59 | 2.4 (2.3, 2.5) | 2 | 1–4 |
| Smoking habits | |||
| Never smoked | 1.9 (1.8, 1.9) | 1 | 0–3 |
| Ex‐smoker | 2.0 (1.9, 2.1) | 2 | 0–3 |
| Current smoker | 1.8 (1.7, 1.9) | 1 | 0–3 |
| Mental health | |||
| Good | 1.6 (1.5, 1.7) | 1 | 0–2 |
| Intermediate | 1.9 (1.9, 2.0) | 1 | 0–3 |
| Poor | 2.2 (2.2, 2.3) | 2 | 0–3 |
| Somatising tendency (number of distressing somatic symptoms in past week) | |||
| 0 | 1.4 (1.4, 1.5) | 1 | 0–2 |
| 1 | 2.2 (2.1, 2.3) | 2 | 1–3 |
| ≥2 | 3.0 (2.9, 3.2) | 3 | 1–5 |
For definition of pain propensity index see text.
At follow‐up, 2003 participants (22%) reported disabling LBP in the past month, including 1663 (83%) who had also reported LBP in the 12 months before baseline, and 1027 (51%) with disabling LBP in the month before baseline. Table 3 summarizes the association of disabling LBP at follow‐up with personal risk factors assessed at baseline. With adjustment for occupational group by random intercept modelling, risk was notably higher in women than men (PRR: 1.4, 95% CI: 1.2–1.5), at older ages (PRR: 1.4, 95% CI: 1.2–1.6, for age 50–59 years vs. 20–29 years) and in participants with greater tendency to somatize (PRR: 1.4, 95% CI: 1.3–1.6, for report of ≥2 vs. 0 distressing somatic symptoms). However, after allowance for these and other covariates, pain propensity was by far the strongest risk factor. PRRs relative to a pain propensity index of zero increased progressively from 1.4 (95% CI: 1.2–1.5) for a value of 1, to 2.6 (95% CI: 2.2–3.1) for values ≥6.
Table 3.
Associations of disabling low back pain in past month at follow‐up with personal risk factors at baseline
| Risk factor | Number of subjects | Number with disabling LBP in past month at follow‐up | Association with disabling low back pain |
|---|---|---|---|
| Prevalence rate ratio (95% CI)d | |||
| Sex | |||
| Male | 3083 | 446 | 1 |
| Female | 5972 | 1557 | 1.4 (1.2, 1.5)c |
| Age (years) | |||
| 20–29 | 2087 | 365 | 1 |
| 30–39 | 2913 | 606 | 1.1 (1.0, 1.2) |
| 40–49 | 2602 | 649 | 1.3 (1.1, 1.4)c |
| 50–59 | 1453 | 383 | 1.4 (1.2, 1.6)c |
| Smoking status | |||
| Never smoked | 5850 | 1322 | 1 |
| Ex‐smoker | 1291 | 283 | 1.1 (1.0, 1.3)a |
| Current smoker | 1892 | 394 | 1.1 (1.0, 1.2) |
| Missing | 22 | 4 | |
| Lifting weights ≥25 kg | 3237 | 772 | 1.1 (1.0, 1.2) |
| Psychosocial aspects of work | |||
| Work for >50 h per week | 2039 | 343 | 1.0 (0.9, 1.1) |
| Time pressure at work | 6754 | 1586 | 1.1 (1.0, 1.2)a |
| Incentives at work | 2494 | 594 | 1.1 (1.0, 1.2)a |
| Lack of support at work | 2341 | 604 | 1.1 (0.9, 1.2) |
| Job dissatisfaction | 1759 | 395 | 1.0 (0.9, 1.1) |
| Lack of job control | 1811 | 408 | 1.0 (0.9, 1.1) |
| Job insecurity | 2665 | 658 | 1.1 (1.0, 1.2)a |
| Number of distressing somatic symptoms in past week | |||
| 0 | 5425 | 854 | 1 |
| 1 | 1973 | 529 | 1.3 (1.2, 1.5)c |
| 2+ | 1605 | 607 | 1.4 (1.3, 1.6)c |
| Missing | 52 | 13 | |
| Mental health | |||
| Good | 3596 | 658 | 1 |
| Intermediate | 2735 | 573 | 1.1 (1.0, 1.2) |
| Poor | 2690 | 766 | 1.3 (1.1, 1.4)c |
| Missing | 34 | 6 | |
| Adverse health beliefs about low back pain | |||
| Work‐relatedness | 3117 | 854 | 1.1 (1.0, 1.2)b |
| Physical activity | 1726 | 401 | 1.0 (0.9, 1.1) |
| Prognosis | 1262 | 332 | 1.1 (1.0, 1.3)a |
| Individual pain propensity index | |||
| 0 | 2690 | 301 | 1 |
| 1 | 1972 | 329 | 1.4 (1.2, 1.5)c |
| 2 | 1547 | 347 | 1.7 (1.5, 1.9)c |
| 3 | 1186 | 343 | 2.0 (1.8, 2.3)c |
| 4 | 699 | 230 | 2.1 (1.8, 2.4)c |
| 5 | 482 | 204 | 2.4 (2.0, 2.9)c |
| 6+ | 479 | 249 | 2.6 (2.2, 3.1)c |
p < 0.05.
p < 0.01.
p < 0.001.
Prevalence rate ratio with 95% confidence interval, derived from a single Poisson regression model that included all of the risk factors in the table.
Table 4 shows results from a series of regression models, each of which included a group‐level variable as well as the personal risk factors examined in Table 3. Only one of the group‐level risk factors was significantly associated with disabling LBP at follow‐up – lack of social security support for long‐term unemployment (PRR: 1.3, 95% CI: 1.0–1.6).
Table 4.
Associations of disabling low back pain in past month at follow‐up with group‐level risk factors at baseline
| Risk factorb | Number of occupational groups exposed | Level of exposure | Association with disabling low back pain in past month | |
|---|---|---|---|---|
| Mean | SD | Prevalence rate ratio (95% CI) | ||
| Group prevalence (%) of adverse beliefs about low back pain | ||||
| Work‐relatednessc | 45 | 32.9 | 19.9 | 1.1 (0.9, 1.2) |
| Physical activityc | 45 | 18.9 | 17.9 | 1.0 (0.9, 1.1) |
| Prognosisc | 45 | 12.5 | 8.5 | 1.0 (0.9, 1.1) |
| Group prevalence (%) of knowing someone outside work with low back painc | 45 | 59.9 | 14.0 | 1.0 (0.9, 1.1) |
| Availability of full sick pay in first 3 months absence | 24 | 0.9 (0.7, 1.1) | ||
| Availability of financial support for ill‐health retirement (sometimes or usually) | 26 | 0.9 (0.7, 1.1) | ||
| Lack of social security for long‐term unemployment | 19 | 1.3 (1.0, 1.6)a | ||
| Availability of compensation (any) for work‐related musculoskeletal disorders of back | 36 | 0.9 (0.7, 1.2) | ||
| Unemployment rate ≥10% | 11 | 1.3 (1.0, 1.7) | ||
| Payment for primary care (part or full) | 18 | 1.1 (0.9, 1.4) | ||
| Group mean propensity indexc | 45 | 1.8 | 0.7 | 1.1 (0.9, 1.2) |
p < 0.05.
Each risk factor was examined independently in a separate Poisson regression model with adjustment for all of the risk factors in Table 2.
Risk estimates for continuous variables are for an increase of one standard deviation.
To assess the potential importance of pain propensity and other risk factors at a population level, we entered all that were statistically significant into a single Poisson regression model, and calculated PAFs from the PRRs that were estimated. The largest PAF was for individual pain propensity (39.8%, 95% CI: 34.0–45.7%, for values >0), followed by female sex (20.3%), older age (16.3% for ages 30–59 vs. 20–29 years) and somatizing tendency (15.1% for ≥1 vs. 0 distressing symptoms). For the combination of individual pain propensity and/or somatizing tendency (32.7% of the study sample), the PAF was 54.9% (95% CI: 47.5–62.3%).
The prevalence of disabling LBP at follow‐up varied from 6% in Japanese sales workers to 46% in Nicaraguan nurses (Fig. 1). Within individual countries, nurses tended to have more disabling LBP than office workers, the mean ratio of prevalence rates across 12 countries being 1.6 (median 1.4, inter‐quartile range: 1.1–1.7). However, the differences were smaller than those between office workers in different countries, whose prevalence ranged from 7% in Pakistan, 10% in Sri Lanka, and 11% in the UK and Japan to >30% in Ecuador, Iran, Nicaragua and Brazil. Similarly, there was nearly fourfold variation between countries in the prevalence among nurses.
Figure 1.
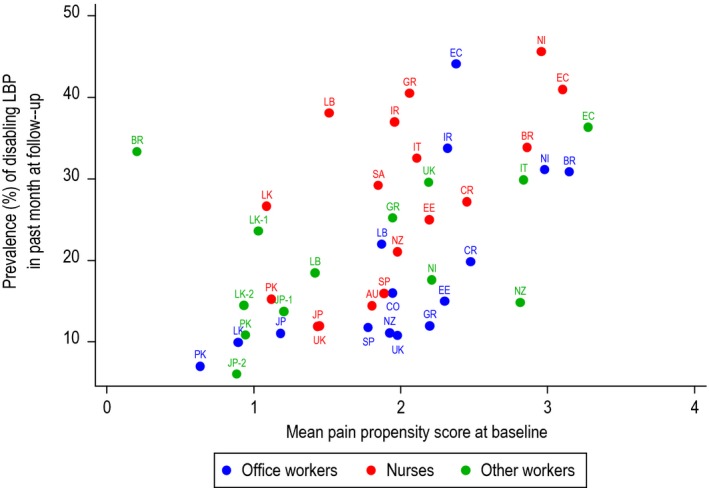
Prevalence of disabling low back pain and mean pain propensity score by occupational group. Key to countries: AU, Australia; BR, Brazil; CO, Colombia; CR, Costa Rica; EC, Ecuador; EE, Estonia; GR, Greece; IR, Iran; IT, Italy; JP, Japan; LB, Lebanon; LK, Sri Lanka; NI, Nicaragua; NZ, New Zealand; PK, Pakistan; SA, South Africa; SP, Spain; UK, United Kingdom.
Mean pain propensity indices by occupational group also varied markedly, ranging from 0.2 in Brazilian sugar cane cutters and 0.6 among office workers in Pakistan to 3.3 in manual workers in Ecuador. As illustrated in Fig. 1, there was a clear correlation across the 45 occupational groups between the prevalence of disabling LBP in the past month at follow‐up and the mean pain propensity index at baseline (Spearman correlation coefficient = 0.58).
When no account was taken of any risk factors, the dispersion of prevalence rates by occupational group was much greater than would have been expected by chance. Thus, the geometric mean of the ratio of observed to expected prevalence rates was 1.68, whereas a value less than 1.23 would have been expected at the 95% level. When account was taken of individual pain propensity, the dispersion of observed to expected ratios was reduced (geometric SD: 1.58), although still greater than the 95th centile value from randomized simulations (1.37). Adjustment also for other risk factors reduced the dispersion further (geometric SD: 1.49), such that it fell between the 75th and 95th centile of the expected distribution.
4. Discussion and conclusions
Our findings confirm large differences in the prevalence of disabling LBP between countries, even among workers carrying out similar occupational activities. These differences, which were substantially greater than those between nurses and office workers within the same country, appear to be driven largely by unidentified factors predisposing to musculoskeletal pain at multiple anatomical sites. After allowance for other known and suspected risk factors, including occupation, the strongest risk factor for future prevalent disabling LBP in individual participants was the number of other anatomical sites that had been painful in the year before baseline; while in occupational groups, the prevalence of disabling LBP at follow‐up correlated with the mean number of sites outside the low back that had earlier been reported as painful. This pattern of results suggests that much of the global burden of disability from LBP in working populations will not be eliminated by current ergonomic approaches to prevention which focus largely on mechanical loading of the spine, and indicates a need to understand better why workers in some countries are more prone to musculoskeletal pain in general.
Our analysis had the advantages of a large and geographically diverse study sample, with a longitudinal design and a fairly high response rate at follow‐up. The occupational groups studied were selected to allow comparison of workers carrying out similar occupational tasks in differing cultural environments, with participation restricted to men and women who initially were aged 20–59 years. Therefore, the study samples will not necessarily have been nationally representative, particularly in their exposure to occupational risk factors and their prevalence of musculoskeletal pain and disability. However, the associations of pain outcomes with risk factors can probably be generalized with greater confidence (although we included only one group of agricultural workers, whose relative risks of LBP may be exceptionally high (Driscoll et al., 2014)).
It is possible that in some occupational groups, a few potential participants were excluded because at the time of the baseline survey they were absent from work as a consequence of musculoskeletal disorders. Moreover, response rates at follow‐up were a little higher among participants who had more pain outside the low back at baseline. However, selective participation would cause serious bias only if the workers who completed follow‐up were substantially unrepresentative in the association of pain at other sites with later disabling LBP, and this seems unlikely.
Musculoskeletal pain is often persistent or recurrent, and 83% of the participants with disabling LBP at follow‐up had also suffered from LBP in the 12 months before baseline. However, we excluded LBP from our measure of pain propensity, and we have no reason to expect that earlier experience and report of pain at sites other than the low back would seriously bias report of disability from LBP at follow‐up. It might be that pain, particularly at multiple sites, lowers mood, rendering people more vulnerable to future symptoms and less able to cope with them when they occur. However, the association that we observed with pain propensity was apparent after adjustment for mental health.
Our data were collected by questionnaire, and we did not make a detailed assessment of ergonomic exposures. However, our regression analyses used random intercepts to allow for differences in the frequency of disabling LBP between occupational groups that were not explained by other risk factors in the models. Since each occupational group was selected to be fairly uniform in its occupational activities, this adjustment will have helped to account for effects of unmeasured ergonomic exposures. Furthermore, the risk factors in our final analysis accounted for most, if not all, of the variation between occupational groups in the prevalence of disabling LBP, beyond that which could be expected simply by chance. This suggests that we did not overlook any important risk factors acting independently of those in our model.
Variation between individuals in our measure of pain propensity could reflect differences either in their experience of pain, or in their inclination to report it, and since pain is an entirely subjective experience, there was no meaningful way of distinguishing between these two possibilities. Importantly, however, the outcome with which it was associated, was not report of LBP per se, but of disability for everyday activities because of LBP. There may have been some errors in recall of pain over the 12 months before baseline, but we have no reason to expect that it would be differential with respect to later report of disabling LBP at follow‐up, and any bias is therefore likely to have been towards the null.
Our reason for adopting a longitudinal design, in which risk factors were assessed at an earlier time‐point than the outcome (1‐month prevalence of disabling LBP), was that it guarded against the bias which can occur when risk factors and outcomes are assessed simultaneously. Nevertheless, it remains possible that baseline report of some risk factors was affected by the presence of disabling LBP in those participants who already had the symptom at that time.
As in earlier papers based on the CUPID study (Coggon et al., 2013a; Vargas‐Prada et al., 2013), we classed LBP as disabling if it made it difficult or impossible to get dressed, do normal jobs around the house or cut toe nails. This accords with the dictionary definition of ‘disabling’ as interfering with the way that someone can live their life, and was intended to distinguish symptoms that were more severe. The specification did not require disabling LBP to have been persistent, but 83% of the 2003 participants with disabling LBP in the past month at follow‐up had also reported LBP in the 12 months before baseline, indicating that in most cases the pain was in fact chronic or recurrent.
We took the prevalence of disabling LBP as our outcome (rather than its incidence) because the starting point for our investigation was unexplained variation in prevalence between occupational groups in different countries. The extent to which the observed associations reflected effects on the incidence of new episodes of LBP as opposed to the persistence or recurrence of pain that had already developed will be the subject of a future report. However, it is known from previous research that pain at other anatomical sites predicts the persistence of LBP (Mallen et al., 2007).
The association between LBP and earlier pain at other anatomical sites could have occurred through three mechanisms. First, pain elsewhere might make pain in the back more likely to develop or persist, perhaps through biomechanical effects of changes in posture or movement, or through altered central processing of pain. In practice any such effects are likely to be small, and we are not aware of evidence, for example, that upper limb fracture is importantly associated with LBP. Second, back pain could promote the occurrence or persistence of pain at other anatomical sites. For similar reasons, we think that is unlikely to be a major effect. Third, there could be one or more shared risk factors that predispose both to LBP and to pain at multiple other anatomical sites. This was our prior hypothesis, and seems the most likely explanation. The shared determinant(s) could be intrinsic psychological or physiological characteristics, or (currently unrecognized) external factors. Whatever their nature, our data suggest that they are important, and accounted for much of the variation in disabling LBP between our occupational groups.
Somatizing tendency is known to be strongly associated with multi‐site pain (Coggon et al., 2013b), and as expected, pain propensity was greater in participants who reported distress from common somatic symptoms (Table 2). Somatizing tendency is also a risk factor for LBP specifically (Pincus et al., 2002; Vargas‐Prada et al., 2013), but as for mental health, the association of disabling LBP with pain propensity was present after adjustment for tendency to somatize. It may be that among people who are predisposed to notice and worry about common somatic symptoms, some are particularly sensitive to musculoskeletal pain. When account was taken of both pain propensity and somatizing tendency, the PAF exceeded 50%.
The associations that we observed with other personal risk factors were largely as expected. Although the PRR for heavy lifting at work was relatively low (1.1), this may in part have been a consequence of the study design, such that there was more variation in occupational tasks between than within the occupational groups sampled. Thus, some of the effect of occupational lifting may have been obscured in the random intercept modelling that was used to allow for possible clustering by occupational group. However, while nurses tended to suffer more from disabling LBP than office workers, type of occupation accounted for less of the variation between occupational groups than mean pain propensity index.
Good ergonomics has clear benefits – it makes tasks more comfortable, and may enable people with musculoskeletal disorders to work productively when otherwise they could not. Moreover, it could be that trials to date have not tested the forms of ergonomic intervention that would be most effective in preventing LBP. However, our results reinforce the limitations of ergonomics alone as a means of preventing LBP in the workplace, and suggest that a focus also on modifying wider propensity to pain and tendency to somatize could be more productive.
As well as personal risk factors, we also explored the influence of characteristics relating to occupational groups. To reduce the possibility of spurious findings because some of the group‐level variables were mutually associated, we examined each independently, with adjustment only for personal risk factors. When analysed in this way, only one (lack of social security for long‐term unemployment) showed a statistically significant association with disabling LBP. If anything, this variable would have been expected to operate in the reverse direction, the financial threat of job loss acting as a disincentive to focusing on and worrying about pain. Thus, the association may have occurred simply by chance.
In conclusion, our analysis reaffirms wide international variation in the prevalence of disabling LBP, and indicates that, at least in the occupational groups studied, relatively little of this variation is attributable to causes specific to the low back – either physical or psychological. Rather the major driver appears to be factors that predispose to musculoskeletal pain more generally. An implication of this finding is that ergonomic interventions of the type that have been widely pursued in developed countries may have only limited impact on the global burden of disability from LBP, and that added potential for prevention may lie in understanding what determines general propensity to musculoskeletal pain, and how that propensity can be reduced to the low levels that currently occur in countries such as Pakistan, Japan and Sri Lanka.
Author contributions
David Coggon initiated and coordinated the CUPID study, led data collection in the UK, and wrote the first draft of the manuscript; Georgia Ntani carried out the statistical analysis; Keith Palmer provided input to the design of the CUPID study, and to the interpretation of findings; Vanda Felli led data collection in Brazil; Florencia Harari led data collection in Ecuador; Leonardo A Quintana coordinated data collection in Colombia; Sarah Felknor and Marianela Rojas led data collection in Costa Rica and Nicaragua; Anna Cattrell coordinated data collection in the UK; Sergio Vargas‐Prada coordinated data collection in Spain; Matteo Bonzini led data collection in Italy; Eleni Solidaki led data collection in Greece; Eda Merisalu led data collection in Estonia; Rima Habib led data collection in Lebanon; Farideh Sadeghian led data collection in Iran; Masood Kadir led data collection in Pakistan; Sudath Warnakulasuriya coordinated data collection in Sri Lanka; Ko Matsudaira led data collection in Japan; Busisiwe Nyantumbu led data collection in South Africa; Helen L Kelsall coordinated data collection in Australia; Helen Harcombe led data collection in New Zealand. In addition, all authors provided feedback on the initial draft manuscript, and agreed the final changes.
Acknowledgements
We thank the following who in various ways contributed to data collection for the CUPID study: Leila M M Sarquis and Maria H Marziale (Brazil); Raul Harari, Rocio Freire, Natalia Harari, Pietro Muñoz, Patricio Oyos, Gonzalo Albuja, María Belduma and Francisco Lara (Ecuador); Lope H Barrero and Magda V Monroy(Colombia); David Gimeno (Costa Rica and Nicaragua); Eduardo J. Salazar Vega, Patricia Monge, Melania Chaverri and Freddy Brenes (Costa Rica); Aurora Aragón, Alberto Berríos, Samaria Balladares, Martha Martínez and Alfredo José Jirón (Nicaragua); E Clare Harris (UK); Consol Serra, J Miguel Martinez, George Delclos, Fernando G Benavides, Catalina Torres, Ben and Marie Carmen Coggon, Cynthia Alcantara, Xavier Orpella, Josep Anton Gonzalez, Joan Bas, Pilar Peña, Elena Brunat, Vicente San José, Anna Sala March, Anna Marquez, Josefina Lorente, Cristina Oliva, Montse Vergara and Eduard Gaynés (Spain); Michele Carugno, Marco M Ferrario, Angela C Pesatori, Natale Battevi, Lorenzo Bordini, Marco Conti, Luciano Riboldi and Paul Maurice Conway (Italy); Manolis Kogevinas, Leda Chatzi and Panos Bitsios (Greece); Kristel Oha, Tiina Freimann and Tuuli Sirk (Estonia); Ali Sadeghian (Iran); Asad Ali Khan and Khalil Qureshi (Pakistan); Roshini J Peiris‐John, Nalini Sathiakumar and A Rajitha Wickremasinghe (Sri Lanka); Noriko Yoshimura, Masami Hirai, Tatsuya Isomura, Norimasa Kikuchi, Akiko Ishizuka and Takayuki Sawada (Japan); Malcolm Sim, Victor C W Hoe and Donna M Urquhart (Australia); Sarah Derrett, David McBride, Peter Herbison and Andrew Gray (New Zealand). Ken Cox collated the data set and prepared files for statistical analysis. We thank all of the organizations that allowed us to approach their employees; and all of the workers who kindly participated in the study.
Funding sources
David Coggon, Georgia Ntani and Keith T Palmer were supported by funding from the Medical Research Council and Arthritis Research UK. Monash University funded data collection in Australia. NHMRC (Australia) supported Helen Kelsall through a fellowship. Data collection in Central America and Colombia was supported by a research training grant to Southwest Center for Occupational and Environmental Health at the University of Texas Health Science Center from the NIH Fogarty International Center. The Deputy for Training and Research, Shahroud University of Medical Sciences provided financial support for data collection in Iran. Institute of Health Carlos III (ISCIII) funded data collection in Spain. The Health Research Council of New Zealand funded data collection in New Zealand. We are particularly grateful to the Colt Foundation, which funded data collection in Brazil, Ecuador, Costa Rica, Nicaragua, UK, Greece, Estonia, Lebanon, Pakistan and South Africa.
Conflicts of interest
None declared.
References
- Bevan, S. , Quadrello, T. , McGee, R. , Mahdon, M. , Vavrovsky, A. and Barham, L. (2009). Fit for work? Musculoskeletal disorders in the European workforce. The Work Foundation; http://www.fitforworkeurope.eu/Website-Documents/Fit%20for%20Work%20pan-European%20report.pdf. [Google Scholar]
- Clinical Standards Advisory Group (1994). Epidemiology Review: The Epidemiology and Cost of Back Pain. (London: HMSO; ). [Google Scholar]
- Coggon, D. (2005). Occupational medicine at a turning point. Occup Environ Med 62, 281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggon, D. , Ntani, G. , Palmer, K.T. , Felli, V.E. , Harari, R. et al. (2013a). Disabling musculoskeletal pain in working populations: Is it the job, the person or the culture? Pain 154, 856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggon, D. , Ntani, G. , Palmer, K.T. , Felli, V.E. , Harari, R. et al. (2013b). Patterns of multi‐site pain and associations with risk factors. Pain 154, 1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggon, D. , Ntani, G. , Palmer, K.T. , Felli, V.E. , Harari, R. et al. (2012). The CUPID (Cultural and Psychosocial Influences on Disability) Study: Methods of data collection and characteristics of study sample. PLoS ONE 7, e39820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis, L.R. , Melisoratos, N. (1983). The Brief Symptom Inventory: An introductory report. Psychol Med 13, 595–605. [PubMed] [Google Scholar]
- Driessen, M.T. , Proper, K.I. , van Tulder, M.W. , Anema, J.R. , Bongers, P.M. , van der Beek, A.J. (2010). The effectiveness of physical and organisational ergonomic interventions on low back pain and neck pain: A systematic review. Occup Environ Med 67, 277–285. [DOI] [PubMed] [Google Scholar]
- Driscoll, T. , Jacklyn, G. , Orchard, J. , Passmore, E. , Vos, T. , Freedman, G. , Lim, S. , Punnett, L. (2014). The global burden of occupationally related low back pain: Estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 73, 975–981. [DOI] [PubMed] [Google Scholar]
- Efron, B. (1979). Bootstrap methods: Another look at the jackknife. Ann Stat 7, 1–26. [Google Scholar]
- European Agency for Safety and Health at Work (1990). Directive 90/269/EEC – manual handling of loads. https://osha.europa.eu/en/legislation/directives/6
- Haukka, E. , Leino‐Arjas, P. , Solovieva, S. , Ranta, R. , Viikari‐Juntura, E. , Riihimäki, H. (2006). Co‐occurrence of musculoskeletal pain among female kitchen workers. Int Arch Occup Environ Health 80, 141–148. [DOI] [PubMed] [Google Scholar]
- Hoy, D. , March, L. , Brooks, P. , Woolf, A. , Bain, C. et al. (2014). The global burden of low back pain: Estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 73, 968–974. [DOI] [PubMed] [Google Scholar]
- IJzelenberg, W. , Burdorf, A. (2004). Impact of musculoskeletal co‐morbidity of neck and upper extremities on healthcare utilisation and sickness absence for low back pain. Occup Environ Med 61, 806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, J. , Ochsmann, E. , Kraus, T. , Lang, J.W. (2012). Psychosocial work stressors as antecedents of musculoskeletal problems: A systematic review and meta‐analysis of stability‐adjusted longitudinal studies. Soc Sci Med 75, 1163–1174. [DOI] [PubMed] [Google Scholar]
- Lötters, F. , Burdorf, A. , Kuiper, J. , Miedema, H. (2003). Model for the work‐relatedness of low back pain. Scand J Work Environ Health 29, 431–440. [DOI] [PubMed] [Google Scholar]
- Mallen, C.D. , Peat, G. , Thomas, E. , Dunn, K.M. , Croft, P.R. (2007). Prognostic factors for musculoskeletal pain in primary care: A systematic review. Br J Gen Pract 57, 655–661. [PMC free article] [PubMed] [Google Scholar]
- Natvig, B. , Bruusgaard, D. , Eriksen, W. (2001). Localised low back pain and low back pain as part of widespread musculoskeletal pain: Two different disorders? A cross‐sectional population study. J Rehabil Med 33, 21–25. [DOI] [PubMed] [Google Scholar]
- Papageorgiou, A.C. , Croft, P.R. , Thomas, E. , Ferry, S. , Jayson, M.I. , Silman, A.J. (1996). Influence of previous pain experience on the episode incidence of low back pain: Results from the South Manchester Back Pain Study. Pain 66, 181–185. [DOI] [PubMed] [Google Scholar]
- Pincus, T. , Burton, A.K. , Vogel, S. , Field, A.P. (2002). A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine 5, E109–E120. [DOI] [PubMed] [Google Scholar]
- Ramond, A. , Bouton, C. , Richard, I. , Roquelaure, Y. , Baulfreton, C. , Legrand, E. , Huez, J.F. (2011). Psychosocial risk factors for chronic low back pain in primary care – a systematic review. Fam Pract 28, 12–21. [DOI] [PubMed] [Google Scholar]
- Smith, B.H. , Elliott, A.M. , Hannaford, P.C. , Chambers, W.A. , Smith, W.C. (2004). Factors related to the onset and persistence of chronic back pain in the community: Results from a general population follow‐up study. Spine 29, 1032–1040. [DOI] [PubMed] [Google Scholar]
- Vargas‐Prada, S. , Serra, C. , Martinez, J. , Ntani, G. , Delclos, G.L. , Palmer, K.T. , Coggon, D. , Benavides, F.G. (2013). Psychological and culturally‐influenced risk factors for the incidence and persistence of low back pain and associated disability in Spanish workers: Findings from the CUPID study. Occup Environ Med 70, 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek, J. , Martimo, K.P. , Karppinen, J. , Kuijer, P.P. , Takala, E.P. , Viikara‐Juntura, E. (2012). Manual material handling advice and assistive devices for preventing and treating back pain in workers: A Cochrane systematic review. Occup Environ Med 69, 79–80. [DOI] [PubMed] [Google Scholar]
- Waddell, G. , Newton, M. , Henderson, I. , Somerville, D. , Main, C.J. (1993). A Fear‐Avoidance Beliefs Questionnaire (FABQ) and the role of fear‐avoidance beliefs in chronic low back pain and disability. Pain 52, 157–168. [DOI] [PubMed] [Google Scholar]
- Ware, J.E. , Sherbourne, C.D. (1992). The MOS 36‐item short‐form health survey (SF‐36). Med Care 30, 473–483. [PubMed] [Google Scholar]


