Summary
Given that FLT3 expression is highly restricted on lymphoid progenitors, it is possible that the established role of FLT3 in the regulation of B and T lymphopoiesis reflects its high expression and role in regulation of lymphoid‐primed multipotent progenitors (LMPPs) or common lymphoid progenitors (CLPs). We generated a Flt3 conditional knock‐out (Flt3 fl/fl) mouse model to address the direct role of FLT3 in regulation of lymphoid‐restricted progenitors, subsequent to turning on Rag1 expression, as well as potentially ontogeny‐specific roles in B and T lymphopoiesis. Our studies establish a prominent and direct role of FLT3, independently of the established role of FLT3 in regulation of LMPPs and CLPs, in regulation of fetal as well as adult early B cell progenitors, and the early thymic progenitors (ETPs) in adult mice but not in the fetus. Our findings highlight the potential benefit of targeting poor prognosis acute B‐cell progenitor leukaemia and ETP leukaemia with recurrent FLT3 mutations using clinical FLT3 inhibitors.
Keywords: conditional knock‐out mouse model, FLT3, haematopoiesis, lymphoid progenitors, lymphoid development
Haematopoiesis is characterized by a very high turnover of mature blood cells of multiple lineages as well as their progenitors, a process partly regulated by a large number of haematopoietic growth factors or cytokines (Metcalf, 2008). Signalling through multiple cell surface tyrosine kinase receptors, triggered through binding of their specific ligands, represents an important extrinsic regulation of distinct haematopoietic stem and progenitor cells both in human and mouse (Ullrich & Schlessinger, 1990; Scheijen & Griffin, 2002).
The FMS‐like tyrosine kinase 3 receptor (encoded by the Flt3 gene, also called Flk2) is a type III receptor tyrosine kinase (Matthews et al, 1991; Rosnet et al, 1991; Rosnet et al, 1993). Its ligand, FLT3 ligand (FLT3L) exists in a soluble as well as membrane‐bound form (Lyman et al, 1995; Lyman & Jacobsen, 1998). Studies in mice have established that FLT3 and FLT3L play an important role in lymphopoiesis (Lyman & Jacobsen, 1998; McKenna et al, 2000; Sitnicka et al, 2002; Tsapogas et al, 2017). Although not expressed on haematopoietic stem cells (HSCs), FLT3 expression is initiated on multipotent progenitors (MPPs) and sustained on common lymphoid progenitors (CLPs), but is only expressed on the very earliest B‐cell and T‐cell progenitors (Wasserman et al, 1995; Adolfsson et al, 2001, 2005; Sitnicka et al, 2002; Boyer et al, 2011; Buza‐Vidas et al, 2011; Luc et al, 2012). Lymphoid‐primed multipotent progenitors (LMPPs) express the highest levels of FLT3 (Adolfsson et al, 2005), and FLT3 plays a key role in LMPP and CLP maintenance (Sitnicka et al, 2002, 2007). As recently highlighted (Tsapogas et al, 2017), because no Flt3 conditional knockout mouse has been generated, it remains unclear to what degree the reductions observed in B lymphocyte and thymocyte progenitors in mice with germ line deletion of FLT3 or FLT3L (Mackarehtschian et al, 1995; Sitnicka et al, 2003, 2007), are secondary to loss of LMPPs and/or CLPs prior to becoming programmed for lymphoid‐restricted development, or also reflect a distinct role of FLT3 also in already lymphoid‐restricted progenitors. In fact, the expression of FLT3 in the B‐ and T‐lymphocyte lineages, is restricted to the earliest pre‐proB and early thymic progenitors (ETPs), respectively (Wasserman et al, 1995; Mansson et al, 2010; Luc et al, 2012), progenitors suggested largely to represent not fully lymphoid‐restricted progenitors (Rumfelt et al, 2006; Luc et al, 2012). Establishing to what degree FLT3 plays a direct role in regulating already lymphoid‐programmed progenitors, is of particular relevance for the high prevalence of two types of FLT3 driver mutations, internal tandem duplication (ITD) and recurrent FLT3 point‐mutations, both associated with a poor clinical outcome in acute leukaemia (Stirewalt & Radich, 2003; Tsapogas et al, 2017), including distinct ETP and B‐cell progenitor leukaemia (Armstrong et al, 2004; Neumann et al, 2013).
Furthermore, cytokine receptors and their ligands are thought to play distinct roles at different stages of development, and this has been also specifically suggested for FLT3 and FLT3L (Vosshenrich et al, 2003; Boiers et al, 2013; Beaudin et al, 2016). To more specifically address progenitor stage‐ and ontogeny‐specific roles of FLT3 in regulation of normal lymphopoiesis, we generated a Flt3 floxed/floxed conditional knock‐out (Flt3 fl/fl) mouse line, allowing us to specifically target FLT3 deletion in a temporal and spatial manner.
Methods and materials
Animals
The Flt3 floxed/floxed conditional knock‐out (Flt3 fl/fl) mouse line was generated using a DNA targeting construct in which the genomic fragment of the mouse Flt3 gene has exon 15 flanked by LoxP sites (flox) and with an Frt‐neomycin‐Frt cassette inserted into intron 15 of the mouse Flt3 gene. The IB10/C embryonic stem (ES) cell line (E14 subclone 129/Ola) was electroporated with the targeting construct and targeted clones selected using neomycin. Correctly‐targeted ES clones were introduced into C57BL6 blastocysts by injection into the blastocyst cavity. Injected blastocysts were then transplanted to the uterus of pseudo‐pregnant foster mothers. Offspring positive for the floxed Flt3 allele were then crossed with Flp‐deleter mice to remove the neomycin cassette. Screening of Flt3 fl/fl mice was carried out using 2 primers flanking the 5′ loxP site Primer 1: AGATGCCAGGACATCAGGAACCTG and Primer 2: ATCAGCCACACCAGACACAGAGATC. Flt3 fl/fl mice were then backcrossed for more than 5 generations with C57/Bl6 mice and subsequently crossed with different Cre‐recombinase mouse strains (all on a C57/Bl6 genetic background).
Vav1 cre/+ , Mx1 cre/+, Rag1 cre/+ mice have been previously described (Kuhn et al, 1995; McCormack et al, 2003; Stadtfeld & Graf, 2005). For each cross, non‐Cre expressing Flt3 fl/fl females were bred with Flt3 fl/fl males heterozygous for the Cre of interest to yield Cre + Flt3 fl/fl as well as Cre − Flt3 fl/fl control littermates. For timed pregnancies, mice were mated late afternoon and females were checked the following morning for the presence of a vaginal plug designated as embryonic day 0·5 (E0·5).
All mice were maintained under specific pathogen‐free conditions at Lund University Animal Facility. The Ethical Committee at Lund University approved all performed experiments.
Dissections and cell preparations
The fetal liver (FL) and fetal thymus were dissected and mechanically disrupted with a syringe. Bone marrow (BM) cells were extracted from femora and tibia using a mortar. Peritoneal cavity lavage was performed using 10 ml of phosphate‐buffered saline (PBS) (Thermo Fisher Scientific Inc, Logan, UT, USA) containing 5% of Fetal Bovine Serum (FBS) (Hyclone, Logan, UT, USA). Single‐cell suspensions were prepared in PBS containing 5% of FBS and filtered through a 70‐μm cell strainer (BD Biosciences, San Jose, CA, USA). Cells were counted with the Sysmex (KX‐21N) Haematology analyser (Sysmex Corporation Europe GmbH, Norderstedt, Germany).
Flow cytometry and fluorescence‐activated cell sorting (FACS)
Dissected fetal tissues and adult BM were treated with purified anti‐CD16/32 antibody (Fc‐block) and then stained with specific mouse monoclonal antibodies (mAb). mAbs used to stain cell surface markers are listed in Table SI. 7‐aminoactinomycinD (7‐AAD, Sigma‐Aldrich Company Ltd, St. Louis, MO, USA) was used to exclude dead cells from the analysis. Samples were analysed on an LSRII (BD Biosciences) and analysis was performed using FlowJo software (version 9.3; TreeStar, Ashland, OR, USA). For all the flow cytometry profiles shown, singlet viable cells were first gated as lineage negative and further gating is indicated with arrows.
Induction of Flt3 deletion
Mx1 cre/+ Flt3 fl/fl and Mx1 +/+ Flt3 fl/fl mice were injected at 7 weeks with 5 intraperitoneal injections of 300 μg of polyinositolic polycytidylic acid (pIpC) at two‐day intervals. Mice were analysed at 4 weeks post‐injection. Deletion efficiency was assessed by sorting 100 000 cells, extracting DNA and performing polymerase chain reaction (PCR) using the KAPA Mouse Genotyping Kit from KAPA Biosystems (Wilmington, MA, USA) with the following primers: Primer 1: AGATGCCAGGACATCAGGAACCTG, Primer 2: ATCAGCCACACCAGACACAGAGATC and Primer 3: CAGTCCCGAGGGGA TGATAC according to the manufacturer protocol.
Transplantation assay
Lethally irradiated (900 cGy) 12‐ to 16‐week‐old C57BL/6 CD45.1 wild type (WT) recipient mice were transplanted intravenously with 2 × 106 cells unfractionated BM cells from Mx1 cre/+ Flt3 fl/fl (CD45.2) or Mx1 +/+ Flt3 fl/fl (CD45.2) mice together with 2 × 106 unfractionated BM competitor cells from WT CD45.1 mice, or 2 × 106 unfractionated E14.5 FL cells from Rag1 cre/+ Flt3 fl/fl (CD45.2) or Rag1 +/+ Flt3 fl/fl (CD45.2) together with 2 × 106 unfractionated E14.5 FL competitor cells from WT CD45.1 mice. Four weeks after transplantation, mice transplanted with Mx1 cre/+ Flt3 fl/fl or Mx1 +/+ Flt3 fl/fl BM cells were injected with 5 intraperitoneal injections of 300 μg of pIpC at two‐day intervals and then analysed for reconstitution at 8 weeks post‐transplantation.
Statistics
Prism software (GraphPad Software Inc., La Jolla, CA, USA) was used for all statistical analysis. Statistical significances were determined using an unpaired Mann–Whitney test. The significance level was set at P < 0·05.
Results
Requirement for FLT3 during adult haematopoiesis
To investigate the requirement for FLT3 at different stages of development and distinct haematopoietic progenitor stages, we generated mice in which loxP sites had been inserted into the flanking introns of exon 15 of the Flt3 gene (Flt3 fl/fl). Exon 15 encodes for a kinase ATP binding domain required for signal transduction upon ligand binding after dimerization and auto‐phosphorylation of the FLT3 receptor (Ubersax & Ferrell, 2007). As such, excision of exon 15 using Cre/loxP recombination should result in a non‐functional FLT3 protein. It was however unclear to what degree this targeting strategy also would result in loss of FLT3 protein expression. Therefore, to first validate the impact of this targeting strategy on FLT3 expression and haematopoiesis, we crossed Flt3 fl/fl mice with Vav1 cre/+ mice, which efficiently targets Cre expression to the entire haematopoietic system, including HSCs, from an early stage of haematopoietic development following emergence of definitive HSCs (Ogilvy et al, 1999; Almarza et al, 2004; Stadtfeld & Graf, 2005). Whereas bone marrow cellularity was not affected (Figure S1A), adult Vav1 cre/+ Flt3 fl/fl mice demonstrated a complete loss of FLT3 expression on Lin−SCA‐1+KIT+ (LSK) cells as well as Lin−SCA‐1lowKITlowIL7R+ CLPs (Figure S1B). Moreover, in agreement with previous studies of conventional germ‐line Flt3 and Flt3l knockout mice (in which Flt3 or Flt3l expression is permanently disrupted in the entire mouse) (Mackarehtschian et al, 1995; McKenna et al, 2000; Sitnicka et al, 2003, 2007), pan‐haematopoietic loss of FLT3 expression from early fetal development did not affect numbers of LSKCD48−CD150+ long‐term (LT)‐HSCs (Figure S1C). Whereas LSKCD48−CD150− short‐term (ST)‐HSCs/MPPs and CD48+CD150+ MPPs were also unaffected in adult Vav1 cre/+ Flt3 fl/fl mice, distinct reductions were observed in CD48+CD150−MPPs which include the majority of LMPPs (Kiel et al, 2005; Mead et al, 2013), CLPs (Figure S1B–C), B‐lymphoid restricted progenitors (Figure S1D), and the earliest (double‐negative; DN) progenitors in the thymus, (Figure S1E).
As previous studies have suggested that FLT3 might have distinct roles in adult and fetal haematopoiesis (Vosshenrich et al, 2003; Boiers et al, 2013; Beaudin et al, 2016), we next specifically investigated the role of FLT3 in adult haematopoiesis by crossing Flt3 fl/fl with Mx1‐cre cre/+ mice in which Cre is only expressed upon induction with either interferon‐α or the interferon inducer pIpC (Kuhn et al, 1995). We treated adult (8‐week‐old) Mx1 cre/+ Flt3 fl/fl and Mx1 +/+ Flt3 fl/fl mice with intraperitoneal pIpC injections and analysed the impact 4 weeks later. As in Vav1 cre/+ Flt3 fl/fl mice, FLT3 expression was almost completely lost on Lin−SCA‐1+KIT+ and CLPs in adult Mx1 cre/+ Flt3 fl/fl mice following pIpC treatment (Fig 1A). No change was observed in total BM cellularity (Figure S2A), nor in the number of HSCs (Lin−SCA‐1+KIT+CD48−CD150+; Fig 1B), in agreement with the lack of FLT3 expression on mouse HSCs (Sitnicka et al, 2002; Buza‐Vidas et al, 2009; Beaudin et al, 2014, 2016). In contrast, CD48+CD150− MPPs, containing LMPPs normally expressing the highest levels of FLT3 (Adolfsson et al, 2005; Mead et al, 2013), were distinctly reduced (Fig 1B). Notably, despite of the loss of FLT3 expression, CLP numbers were unaffected in adult Mx1 cre/+ Flt3 fl/fl mice 4 weeks following pIpC treatment (Fig 1B), suggesting that the maintenance of adult CLPs is less dependent on FLT3 than LMPPs. Surprisingly, and in contrast to adult mice with constitutive knock‐out of FLT3 (Mackarehtschian et al, 1995), no changes were observed in distinct stages of B‐cell progenitors (ProB: Lin−B220+CD43+CD19+CD24+ CD93+; PreB: Lin−B220+CD43−CD19+IgM−; IgM+ B cells: Lin−B220+CD43−CD19+IgM+) in adult Mx1 cre/+ Flt3 fl/fl mice following pIpC treatment (Fig 1C). In contrast, while no significant change was observed in total thymus cellularity (Figure S2B), a clear reduction was observed in the ETP (Lin−CD4−CD8a−KIT+CD25−), Double Negative 2 (DN2; Lin−CD4−CD8a−KIT+CD25+) and Double Negative 3 (DN3; Lin−CD4−CD8a−KIT−CD25+) thymocytes in adult Mx1 cre/+ Flt3 fl/fl mice following pIpC treatment (Fig 1D), demonstrating a strict requirement for FLT3 function during steady‐state adult thymopoiesis.
Figure 1.
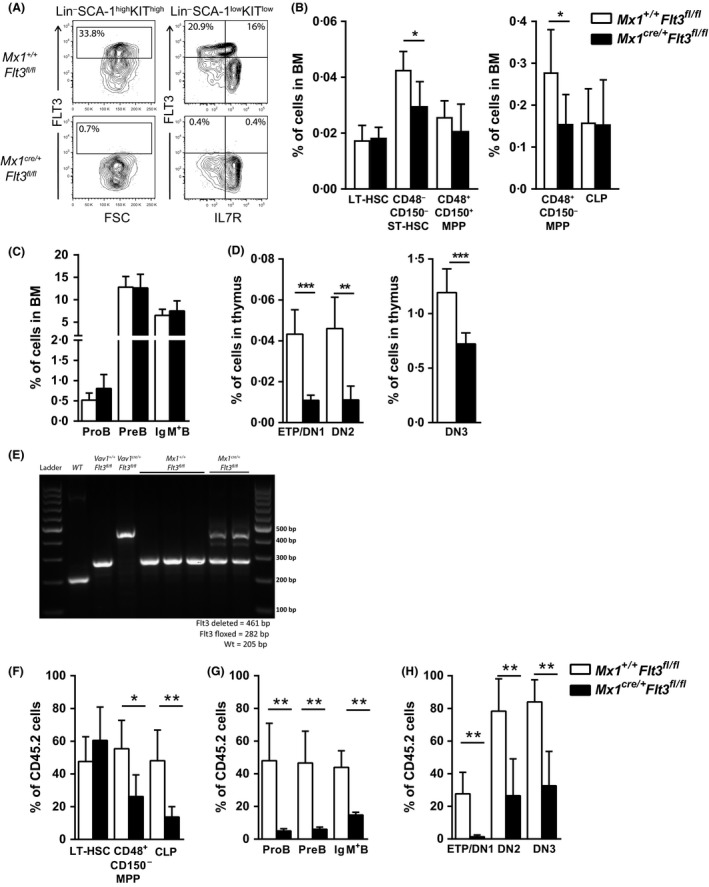
Role of FLT3 in steady‐state adult haematopoiesis. (A) Representative fluorescence‐activated cell sorting (FACS) profiles showing FLT3 surface expression on Lin−SCA‐1+KIT+ cells and Lin−SCA‐1lowKITlow cells in Mx1 +/+ Flt3 fl/fl compared to Mx1 cre/+ Flt3 fl/fl bone marrow (BM) (numbers represent mean percentages of 6–8 mice per genotype) 4 weeks after polyinositolic polycytidylic acid (pIpC) injection. In addition to isotype control and Fluorescence Minus One (FMO) controls, gates for FLT3 expression were set using long‐term haematopoietic stem cells (LT‐HSCs) as a negative internal reference population (IRP), to improve the reliable detection of FLT3 positive and negative cells, as HSCs have been established to lack cell surface FLT3 expression (Adolfsson et al, 2001). (B–C) Mean percentages (±SD of total BM cells) of (B) LT‐HSCs (Lin−SCA−1+KIT+CD48‐CD150+), CD48− CD150− short‐term (ST)‐HSCs (Lin−SCA‐1+KIT+CD48−CD150−), CD48+ CD150+ multipotent progenitors (MPPs) (Lin−SCA‐1+KIT+CD48+CD150+), CD48+ CD150− MPPs (Lin−SCA‐1+KIT+CD48+CD150−) and common lymphoid progenitors (CLPs) (Lin−SCA‐1lowKITlowIL‐7R+), (C) ProB cells (Lin−B220+ CD43+ CD19+ CD24+ CD93+), PreB cells (Lin−B220+ CD43− CD19+IgM−) and IgM+ B cells (Lin−B220+ CD43− CD19+IgM+) in 12‐week‐old Mx1 +/+ Flt3 fl/fl and Mx1 cre/+ Flt3 fl/fl mice (n = 6–8 mice per genotype in 3 experiments) 4 weeks after pIpC injection. (D) Mean percentages (±SD of total thymus cells) of early thymic progenitors (ETPs) (Lin− CD4− CD8a− KIT + CD25−), Double Negative 2 (DN2) (Lin− CD4− CD8a− KIT + CD25+) and Double Negative 3 (DN3) (Lin− CD4− CD8a− KIT − CD25+) cells in 12‐week‐old Mx1 +/+ Flt3 fl/fl and Mx1 cre/+ Flt3 fl/fl mice (n = 6–8 mice per genotype in 3 experiments) 4 weeks after pIpC injection. (E) Polymerase chain reaction analysis of recombination at the Flt3 locus in ProB cells in Mx1 +/+ Flt3 fl/fl and Mx1 cre/+ Flt3 fl/fl mice 4 weeks after pIpC injection. Also shown are Vav1 +/+ Flt3 fl/fl, Vav1 cre/+ Flt3 fl/fl and wild type (WT) controls. The upper band represents the deleted Flt3 allele (461 bp), the middle band the floxed Flt3 allele (282 bp) and the lower band the WT allele (205 bp). (F–H) Mean percentages (±SD) contribution of CD45.2 cells to (F) LT‐HSCs, CD48+ CD150− MPPs and CLPs, (G) ProB cells, PreB cells and IgM+ B cells in BM and (H) ETP, DN2 and DN3 cells in thymus of mice transplanted with 2 × 106 cells unfractionated BM cells from Mx1 +/+ Flt3 fl/fl (CD45.2) or Mx1 cre/+ Flt3 fl/fl (CD45.2) mice (n = 6 per genotype) together with 2 × 106 cells unfractionated BM competitor cells from WT CD45.1 mice, analysed 8 weeks post‐transplantation and 4 weeks after pIpC injection. *P < 0·05; **P < 0·01; ***P < 0·001.
Genomic PCR analysis of ProB cells purified from Mx1 cre/+ Flt3 fl/fl BM demonstrated that the majority of Mx1 cre/+ Flt3 fl/fl ProB cells had a remaining floxed Flt3 allele (Fig 1E) suggesting that non‐deleted (wild‐type) progenitors, such as CLPs, have a competitive advantage over Flt3‐deleted progenitors in sustaining adult early B cell progenitors. In agreement with this, upon transplantation of unfractionated adult BM cells from CD45.2 Mx1 +/+ Flt3 fl/fl or Mx1 cre/+ Flt3 fl/fl donor mice together with competitor CD45.1 BM cells into lethally‐irradiated adult CD45.1 recipients followed by pIpC treatment, we observed a consistently reduced contribution of Mx1 cre/+ Flt3 fl/fl cells to CD48+CD150− MPPs and CLPs, as well as B‐cell and T‐cell progenitors (Fig 1F–H, Figure S2C–E).
Thus, FLT3 plays an important role in sustaining multiple stages of lympho‐myeloid progenitors in adult haematopoiesis.
Requirement for FLT3 after initiation of lymphoid lineage programme
Previously reported reductions in the earliest B‐ and T‐lymphoid progenitors in conventional Flt3 knockout mice could potentially be secondary to reductions in high FLT3‐expressing LMPPs and/or CLPs rather than reflecting a specific and direct role of FLT3 in downstream B‐ and T‐cell committed progenitors. To more specifically investigate the potential direct role of FLT3 down‐stream of adult LMPPs and in already lymphoid‐programmed progenitors, we crossed Flt3 fl/fl and Rag1 cre/+ mice, to exclusively target loss of FLT3 function to cells already expressing high levels of Rag1 (McCormack et al, 2003). Importantly, and in agreement with only a fraction of adult LMPPs expressing Rag1 and at very low levels (Adolfsson et al, 2005; Mansson et al, 2007, 2010; Luc et al, 2012), we observed no change in FLT3 cell surface expression on LMPPs, whereas a reduced fraction of Lin−SCA‐1lowKITlowIL7R+ CLPs expressed FLT3 (Fig 2A). Despite this, not only long‐term (LT)‐HSCs, short‐term (ST)‐HSCs, CD48+CD150+ MPPs and CD48+CD150− MPPs, but also total CLP numbers were unaffected in the BM of adult Rag1 cre/+ Flt3 fl/fl mice (Fig 2B; Figure S3A), unlike in adult Vav1 cre/+ Flt3 fl/fl mice (Figure S1B–C). The proportion of CD48−CD150− ST‐HSCs, CD48+CD150+ MPPs and CD48+CD150− MPPs, expressing FLT3 were not altered (Fig 2C–D). Notably, although the total numbers of CLPs were unaffected, the FLT3+ fraction of CLPs in Rag1 cre/+ Flt3 fl/fl mice was significantly reduced with a corresponding increase in FLT3− CLPs (Fig 2C–D), compatible with a fraction of CLPs being sustained at normal levels although deleted for FLT3 expression. Notably, even if LMPP and CLP numbers in adult Rag1 cre/+ Flt3 fl/fl mice were unaffected, ProB, PreB and IgM+ B cells in the BM (Fig 2E) as well as ETPs in the thymus were significantly reduced (Fig 2F; Figure S3B), to a similar degree as in Vav1 cre/+ Flt3 fl/fl mice (Figure S1D–E), establishing a strict requirement for FLT3, independently of LMPPs and CLPs, and after initiation of lymphoid‐restricted gene expression in adult haematopoiesis.
Figure 2.
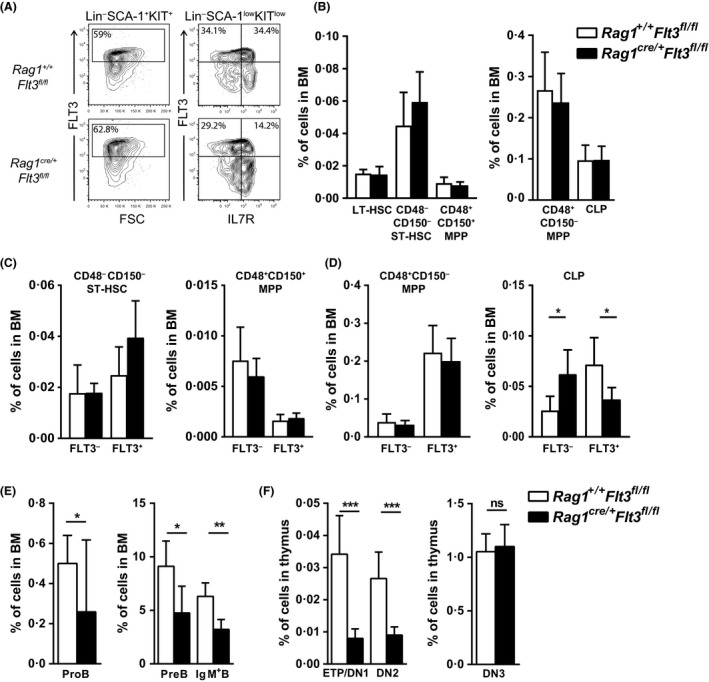
Role of FLT3 in adult lymphoid‐committed progenitors. (A) Representative fluorescence‐activated cell sorting (FACS) profiles showing FLT3 surface expression on Lin−SCA‐1+KIT+ cells and Lin−SCA‐1lowKITlow cells in Rag1 +/+ Flt3 fl/fl compared to Rag1 cre/+ Flt3 fl/fl bone marrow (BM) (numbers represent mean percentages of 7 mice per genotype). Gates were set using long‐term haematopoietic stem cells (LT‐HSCs) as a negative internal reference population (IRP). (B) Mean percentages (±SD of total BM cells) of CD48− CD150+ LT‐HSCs, CD48− CD150− short‐term (ST)‐HSCs, CD48+ CD150+ multipotent progenitors (MPPs), CD48+ CD150− MPPs and common lymphoid progenitors (CLPs) in 12‐week‐old Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl mice (n = 7 mice per genotype in 2 experiments). (C–D) Mean percentages (±SD of total BM cells) FLT3− and FLT3+ subsets of (C) CD48− CD150− ST‐HSCs, CD48+ CD150+ MPPs and (D) CD48+ CD150− MPPs and CLPs in 12‐week‐old Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl mice (n = 7 mice per genotype in 2 experiments). (E) Mean percentages (±SD of total BM cells) of ProB cells, PreB cells and IgM+ B cells in 12‐week‐old Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl mice (n = 7 mice per genotype in 2 experiments). (F) Mean percentages (±SD of total thymus cells) of early thymic progenitor (ETP), Double Negative 2 (DN2) and Double Negative 3 (DN3) cells in 12‐weekold adult thymus from Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl mice (n = 7 mice per genotype in 2 experiments). *P < 0·05; **P < 0·01; ***P < 0·001; ns, not significant.
Previous studies have suggested that the cytokine requirement might be distinct for fetal and adult lymphoid progenitors (Carvalho et al, 2001; Vosshenrich et al, 2003; Hesslein et al, 2006; Beaudin et al, 2016; Zriwil et al, 2016). Moreover, LMPPs in the FL express higher levels of lymphoid genes, including Rag1, when compared to their adult counterparts (Boiers et al, 2013). We therefore next investigated the impact of Flt3 deletion in the E14.5 FL of Rag1 cre/+ Flt3 fl/fl embryos. In agreement with their higher Rag1 expression (Boiers et al, 2013), we observed a slight reduction in LSK cells expressing FLT3 and a more distinct and significant reduction on Lin−SCA‐1lowKITlowIL‐7R+ CLPs (Fig 3A). Despite this, not only CD48−CD150+ LT‐HSCs, CD48−CD150− ST‐HSCs and CD48+CD150+ MPPs, but also CD48+CD150− MPPs and CLPs were unaffected in the E14.5 Rag1 cre/+ Flt3 fl/fl FL (Fig 3B). Whereas the FLT3+ and FLT3− fractions of the different LSK HSC and MPP fractions were unaffected in the Rag1 cre/+ Flt3 fl/fl FL, FLT3+ CLPs were reduced and FLT3− CLPs correspondingly increased (Fig 3C–D), similar to what was observed in the adult BM. Notably, the earliest ProB cell progenitors emerging in the FL at this stage, were reduced by almost 90%, demonstrating a direct and critical role of FLT3 in fetal B cell progenitors (Fig 3E, Figure S4A–B). In contrast, the earliest thymic progenitors in the E14.5 thymus were unaffected in Rag1 cre/+ Flt3 fl/fl embryos (Fig 3F, Figure S4C). Even when E14.5 CD45.2 Rag1 cre/+ Flt3 fl/fl FL cells were competitively transplanted into lethally‐irradiated adult wild‐type (WT) CD45.1 recipients, we observed no impact of Flt3 deficiency on the earliest LMPP and CLP progenitor compartments (Fig 3G), while early B‐ and T‐cell progenitors were distinctly reduced (Fig 3H–I, Figure S4D–F), again supporting a distinct role of FLT3 in early lymphoid progenitors subsequent to the initiation of lymphoid lineage programming.
Figure 3.
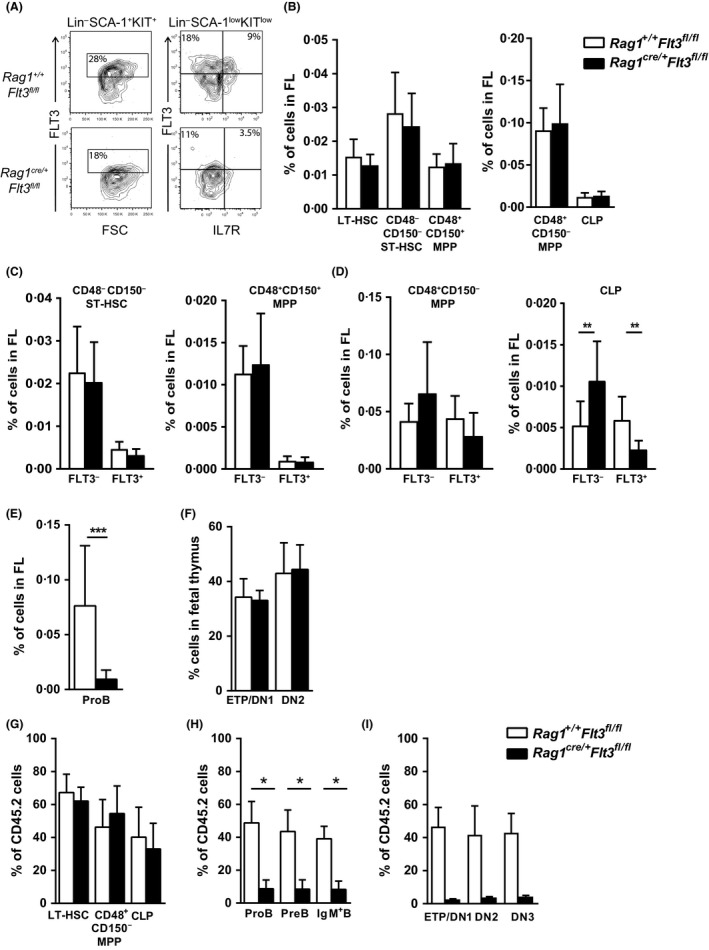
Role of FLT3 in fetal lymphoid‐committed progenitors. (A) Representative fluorescence‐activated cell sorting (FACS)profiles showing FLT3 surface expression on Lin−SCA‐1+KIT+ cells and Lin−SCA‐1lowKITlow cells in Rag1 +/+ Flt3 fl/fl compared to Rag1 cre/+ Flt3 fl/fl E14.5 fetal liver (FL) cells (numbers represent mean percentages of 8–11 embryos per genotype). (B) Mean percentages (±SD of total FL cells) of CD48− CD150+ long‐term haematopoietic stem cells (LT‐HSCs), CD48− CD150− short‐term (ST)‐HSCs, CD48+ CD150+ multipotent progenitors (MPPs), CD48+ CD150− MPPs and common lymphoid progenitors (CLPs) in E14.5 Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl embryos (n = 8–11 embryos per genotype in 2 experiments). (C–D) Mean percentages (±SD of total FL cells) FLT3− and FLT3+ subsets of (C) CD48− CD150− ST‐HSCs, CD48+ CD150+ MPPs and (D) CD48+ CD150− MPPs and CLPs in E14.5 Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl embryos (n = 8–11 embryos per genotype in 2 experiments). (E) Mean percentages (±SD of total FL cells) of ProB cells in E14.5 Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl embryos (n = 8–11 embryos per genotype in 2 experiments). (F) Mean percentages (±SD of total fetal thymus cells) of early thymic progenitor (ETP) and Double Negative 2 (DN2) cells in E14.5 Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl embryos (n = 4–7 embryos per genotype in 1 experiment). (G–I) Mean percentages (±SD) contribution of CD45.2 cells to (G) LT‐HSCs, CD48+ CD150− MPPs and CLPs, (H) ProB cells, PreB cells, and IgM+ B cells in bone marrow and (I) ETP, DN2 and Double Negative 3 (DN3) cells in thymus of lethally‐irradiated CD45.1 mice transplanted with 2 × 106 cells unfractionated E14.5 FL cells from Rag1 +/+ Flt3 fl/fl (CD45.2) or Rag1 cre/+ Flt3 fl/fl (CD45.2) mice (n = 5 per genotype) together with 2 × 106 cells unfractionated E14.5 FL competitor cells from wild type CD45.1 embryos, analysed 8 weeks post‐transplantation. *P < 0·05; **P < 0·01; ***P < 0·001.
Role of FLT3 in maintenance of distinct B cell subsets
As we observed a strong impact of lymphoid‐restricted deletion of FLT3 on B‐progenitor cell maintenance in the embryo as well as in adult haematopoiesis, we next investigated to what degree the generation and maintenance of the preferentially fetal‐derived mature B1a and Marginal Zone B (MZB) cells (Hardy & Hayakawa, 1991; Kantor et al, 1992; Yoshimoto et al, 2011) as well as conventional B2 cells which are also produced during adult haematopoiesis (Hao & Rajewsky, 2001) are dependent on intact FLT3 function. At steady state, follicular B2 cells (CD19+CD93−CD5−CD43−CD23+CD1d−) were unaffected in the spleen and peritoneal cavity (CD19+CD5−CD43−CD23+CD11b−) of adult Rag1 cre/+ Flt3 fl/fl mice, as were B1a (CD19+CD93−CD5+CD43+CD23−CD1d−) and MZB (CD19+CD93−CD23‐CD1d+) cells in the spleen and B1a (CD19+CD5+CD43+CD23−) cells in the peritoneal cavity (Fig 4A–D, Figure S3C). Fetal‐derived B1a cells and MZB cells are long‐lived and possess self‐renewal potential (Hao & Rajewsky, 2001), which could result in a compensatory expansion to correct any reductions due to loss of FLT3 function. To further assess the role of FLT3 in maintenance of B1a and MZB cells, we therefore analysed the spleen and peritoneal cavity of mice transplanted with unfractionated E14.5 FL cells from CD45.2 Rag1 cre/+ Flt3 fl/fl embryos, together with competitor E14.5 FL WT CD45.1 cells. At 8 weeks following transplantation, we observed an impairment in reconstitution of B1a, MZB as well as B2 cells in the spleen and peritoneal cavity of recipients of Rag1 cre/+ Flt3 fl/fl FL cells (Fig 4E–F), establishing an important role of FLT3 for the homeostasis of each of these distinct B cell populations from already lymphoid‐restricted progenitors.
Figure 4.
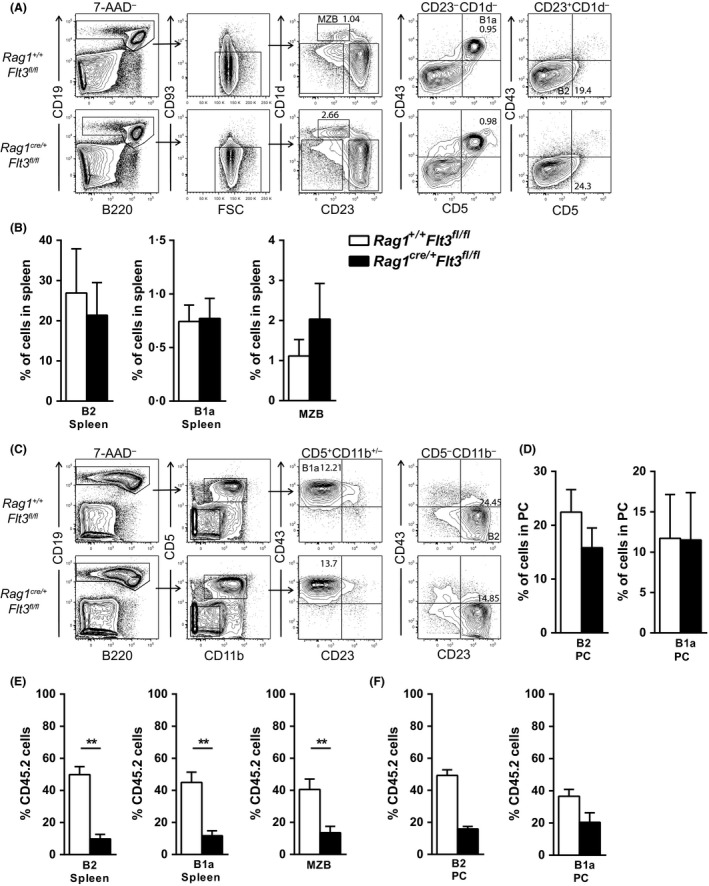
Role of FLT3 in generation of distinct subsets of B cells. (A) Representative fluorescence‐activated cell sorting (FACS)profiles of Follicular B2 cells (CD19+ CD93− CD5− CD43− CD23+ CD1d−), Marginal Zone B cells (MZB: CD19+ CD93− CD23‐CD1d+) and B1a cells (CD19+ CD93− CD5+ CD43+ CD23− CD1d−) in spleen in 12‐week‐old Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl mice (numbers in gates represent percentages of total spleen cells). (B) Mean percentages (±SD of total spleen cells) of follicular B2, MZB and B1a cells in 12‐week‐old Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl mice (n = 6 mice per genotype in 2 experiments). (C) Representative FACS profiles of B2 cells (CD19+ CD5− CD43− CD23+ CD11b−) and B1a cells (CD19+ CD5+ CD43+ CD23−) in the peritoneal cavity (PC) of 12‐week‐old Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl mice (numbers in gates represent percentages of total PC cells). (D) Mean percentages (±SD of total PC cells) B2 cells and B1a cells of 12‐week‐old Rag1 +/+ Flt3 fl/fl and Rag1 cre/+ Flt3 fl/fl mice (n = 6 mice per genotype in 2 experiments). (E–F) Mean percentages (±SD) contribution of CD45.2 cells to (E) follicular B2 cells, MZB cells and B1a cells in the spleen and to (F) B2 cells and B1a cells in the PC of CD45.1 wild type (WT) mice transplanted with 2 × 106 unfractionated E14.5 FL cells from Rag1 +/+ Flt3 fl/fl (CD45.2) or Rag1 cre/+ Flt3 fl/fl (CD45.2) mice (n = 2–5 per genotype) together with 2 × 106 cells unfractionated E14.5 FL WT CD45.1 competitor cells, analysed 8 weeks post‐transplantation.
Discussion
Despite the established role of FLT3 in B‐ and T‐lymphopoiesis (Mackarehtschian et al, 1995; McKenna et al, 2000; Sitnicka et al, 2003, 2007; Buza‐Vidas et al, 2007), and the involvement of recurrent FLT3 mutations in acute B‐ and T‐cell progenitor leukaemia (Carow et al, 1996; Gilliland & Griffin, 2002; Armstrong et al, 2004; Neumann et al, 2013), it has remained unclear whether lymphoid‐restricted progenitors are directly dependent on FLT3. This is particularly relevant because FLT3 expression is highest and critically important on the earliest adult lympho‐myeloid LMPPs, of which only a fraction express low levels of Rag1 (Adolfsson et al, 2005), whereas only the very earliest T‐ and B‐cell progenitors express FLT3 (Wasserman et al, 1995; Mansson et al, 2010; Luc et al, 2012). Herein, we developed a Flt3 conditional knockout mouse model to specifically investigate a potential requirement for FLT3 in progenitors already programmed for lymphopoiesis, as determined by high Rag1 expression, as well as potentially distinct roles in fetal and adult lymphopoiesis.
Our studies confirmed that while having no role in the regulation of HSCs, FLT3 is important for sustaining normal numbers of LMPPs in adult BM as well as ETPs in the adult thymus. In contrast, CLPs were only minimally affected when induced to loose FLT3 expression in adult mice, establishing that LMPPs are more dependent on intact FLT3 expression and function than CLPs in adult steady‐state haematopoiesis. Notably, B‐cell progenitor numbers were also unaffected upon inducible pan‐haematopoietic deletion of FLT3 in adult mice. Nevertheless, molecular analysis of B‐cell progenitors demonstrated that Flt3‐deleted adult progenitors, such as LMPPs or CLPs, have a considerable competitive disadvantage in producing B‐cell progenitors compared to the rare progenitors escaping Flt3 deletion in our model. In agreement with this observation, competitive Mx1Cre adult BM transplantation experiments demonstrated as soon as 4 weeks post‐transplantation that not only the generation of CD48+CD150−MPPs (representing predominantly LMPPs) and T‐cell progenitors, but also CLPs, B‐cell progenitors, and mature B cells were significantly impaired from FLT3‐deleted BM cells.
To address whether FLT3 plays a direct role in the regulation of adult lymphoid‐restricted progenitor maintenance, rather than in their generation from LMPPs, we specifically deleted Flt3 through Rag1 cre/+‐induced recombination (McCormack et al, 2003). As expected, due to their low levels of Rag1 expression, FLT3 expression was unaffected on LMPPs as were LMPP numbers in adult BM. Notably, whereas FLT3 expression was deleted in approximately 50% of CLPs, CLP number was also unaffected in the BM of adult Rag1 cre/+ Flt3 fl/fl mice. This allowed for the specific establishment of a distinct role of FLT3 in direct regulation of early B‐ and T‐cell progenitors independently of the role of FLT3 in regulation of earlier LMPPs and CLPs, because the earliest BM B‐cell progenitors and thymic T‐cell progenitors were distinctly reduced in adult Rag1 cre/+ Flt3 fl/fl mice, in the absence of an impact on LMPPs and CLPs. This is of considerable significance, given that FLT3 expression in the B and T cell lineages is restricted to the very earliest B‐ and T‐cell progenitors, pre‐proB cells (Wasserman et al, 1995) and ETPs (Luc et al, 2012), respectively. Importantly, these progenitors are likely to also be key cellular targets for recurrent FLT3 driver mutations in patients with acute B‐cell progenitor and ETP leukaemia (Carow et al, 1996; Armstrong et al, 2004; Neumann et al, 2013). Our findings therefore highlight the potential benefit of targeting these leukaemias with clinical FLT3 inhibitors (Annesley & Brown, 2014).
Also in the liver of Rag1 cre/+ Flt3 fl/fl embryos, we observed a dramatic reduction in early B‐cell progenitors in the absence of a significant reduction of fetal LMPPs and CLPs, demonstrating a distinct and prominent requirement for FLT3 in fetal B‐lymphoid progenitors, again independently of a role in earlier progenitors. Interestingly, distinct from adult haematopoiesis, E14.5 fetal thymic progenitor homeostasis was not affected in Rag1 cre/+ Flt3 fl/fl mice, despite FLT3 being expressed on fetal ETPs (Luis et al, 2016). However, at 4 weeks after competitive transplantation of Rag1 cre/+ Flt3 fl/fl FL cells into adult recipients, while CD48+CD150− MPPs and CLPs remained unaffected, an impaired generation of not only B cell progenitors but also early thymocyte progenitors could be readily detected from Rag1 cre/+ Flt3 fl/fl FL cells. The distinct thymocyte phenotype observed upon transplantation of E14.5 Rag1 cre/+ Flt3 fl/fl FL cells into adult recipients but not in the Rag1 cre/+ Flt3 fl/fl E14.5 fetal thymus, combined with a similar early thymocyte defect observed in unperturbed adult Rag1 cre/+ Flt3 fl/fl mice, suggest a distinct requirement for FLT3 signalling in adult but not fetal thymopoiesis, potentially explained by distinct differences in the fetal and adult thymic environment. In contrast, fetal and adult B‐lymphopoiesis are both dependent on intact FLT3 expression and function.
Despite its very restricted expression, our studies demonstrate that FLT3 plays an important and distinct role in the direct regulation of early B cell‐restricted progenitors in FL as well as in adult BM. In further agreement with its crucial role in B‐lymphopoiesis, our studies of Rag1 cre/+ Flt3 fl/fl mice establish a critical role for FLT3 in the maintenance of fetal‐derived B1a and MZB cells (Hardy & Hayakawa, 1991; Kantor et al, 1992; Yoshimoto et al, 2011) as well as conventional B2 cells (Hao & Rajewsky, 2001). Previous studies have shown that the important role of FLT3 in fetal B1 and B2 lymphopoiesis is even more evident in the absence of interleukin 7 (IL7). Specifically, in the absence of the IL7 ligand and FLT3 ligand, fetal and adult B lymphopoiesis is almost entirely lost, suggesting that thymic stromal lymphoprotein (TSLP), also acting through the IL7 receptor, is unable to rescue B cell development in the absence of FLT3 ligand and IL7 (Sitnicka et al, 2003; Jensen et al, 2008).
In conclusion, through lymphoid‐restricted and ontogeny‐specific deletion of FLT3 expression and function, we establish that the important role of FLT3 in fetal and adult B‐ and T‐lymphopoiesis is, in fact, not primarily explained by its role in regulation of LMPPs and CLPs, but rather by a direct and more prominent role in the regulation of the very earliest Rag1 expressing B‐ and T‐ cell progenitors, which are likely to be primary cellular targets for recurrent FLT3 mutations in clinically distinct B‐ and T‐cell progenitor cell leukaemia.
Author contributions
AZ, ES and SEWJ designed and conceptualized the overall research and analysed the data. CN designed and supervised the generation of the Flt3 conditional knockout targeting construct and targeting of ES cells. AZ performed the experiments. TAK performed B1 cell analysis experiments. LW provided expertise in the animal work. CB contributed to the design, analysis of experiments, data analysis and writing of the manuscript. JY contributed with expert advice and input on B cell development. AZ, ES and SEWJ wrote the manuscript. All authors read and approved the submitted manuscript.
Declaration of interest
The authors declare no competing financial interests.
Supporting information
Figure S1. Provides data validating the model.
Figure S2. Provides data that extends the findings in Fig 1.
Figure S3. Provides data that extends the findings in Figs 2, 3 and 4.
Figure S4. Provides data that extends the findings in Fig 3.
Table S1. Provides the list of antibodies used in the study.
Acknowledgements
The authors thank Professor Terence H Rabbitts for providing Rag1 cre/+ mice, Prof Thomas Graf for Vav1 cre/+ mice and Drs. Stephan Teglund and Johannes Wilbertz at the Karolinska Centre for Transgene Technologies for performing blastocyst injections. This work was supported by the Swedish Childhood Cancer Foundation (PR20130043, PR 20150063; ES and SEWJ), the Gunnar Nilsson Foundation (Project 522; ES), the ALF Clinical Research Award from Lund University Hospital (ALFSKANE 274081; ES), Hemato‐Linne (ES and SEWJ), Stem Therapy Program (ES), UK MRC G0801073 and MC_UU_12009/5 (SEWJ) and an International recruitment award from the Swedish Research Council (SEWJ). CB was supported by a postdoctoral fellow grant from the Swedish Childhood Cancer Foundation (PDS13/005) and the Swedish Research Council, Marie Sklodowska Curie Actions, Cofund, Project INCA (#2015‐00135, #600398). ES had an Associate Professor position supported by the Swedish Childhood Cancer Foundation (TFJ08).
Contributor Information
Ewa Sitnicka, Email: ewa.sitnicka@med.lu.se.
Sten E. W. Jacobsen, Email: sten.eirik.jacobsen@ki.se
References
- Adolfsson, J. , Borge, O.J. , Bryder, D. , Theilgaard‐Monch, K. , Astrand‐Grundstrom, I. , Sitnicka, E. , Sasaki, Y. & Jacobsen, S.E. (2001) Upregulation of Flt3 expression within the bone marrow Lin(‐)Sca1(+)c‐kit(+) stem cell compartment is accompanied by loss of self‐renewal capacity. Immunity, 15, 659–669. [DOI] [PubMed] [Google Scholar]
- Adolfsson, J. , Mansson, R. , Buza‐Vidas, N. , Hultquist, A. , Liuba, K. , Jensen, C.T. , Bryder, D. , Yang, L. , Borge, O.J. , Thoren, L.A. , Anderson, K. , Sitnicka, E. , Sasaki, Y. , Sigvardsson, M. & Jacobsen, S.E. (2005) Identification of Flt3+ lympho‐myeloid stem cells lacking erythro‐megakaryocytic potential a revised road map for adult blood lineage commitment. Cell, 121, 295–306. [DOI] [PubMed] [Google Scholar]
- Almarza, E. , Segovia, J.C. , Guenechea, G. , Gomez, S.G. , Ramirez, A. & Bueren, J.A. (2004) Regulatory elements of the vav gene drive transgene expression in hematopoietic stem cells from adult mice. Experimental Hematology, 32, 360–364. [DOI] [PubMed] [Google Scholar]
- Annesley, C.E. & Brown, P. (2014) The biology and targeting of FLT3 in pediatric leukemia. Frontiers in Oncology, 4, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, S.A. , Mabon, M.E. , Silverman, L.B. , Li, A. , Gribben, J.G. , Fox, E.A. , Sallan, S.E. & Korsmeyer, S.J. (2004) FLT3 mutations in childhood acute lymphoblastic leukemia. Blood, 103, 3544–3546. [DOI] [PubMed] [Google Scholar]
- Beaudin, A.E. , Boyer, S.W. & Forsberg, E.C. (2014) Flk2/Flt3 promotes both myeloid and lymphoid development by expanding non‐self‐renewing multipotent hematopoietic progenitor cells. Experimental Hematology, 42, e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin, A.E. , Boyer, S.W. , Perez‐Cunningham, J. , Hernandez, G.E. , Derderian, S.C. , Jujjavarapu, C. , Aaserude, E. , MacKenzie, T. & Forsberg, E.C. (2016) A transient developmental hematopoietic stem cell gives rise to innate‐like B and T cells. Cell Stem Cell, 19, 768–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiers, C. , Carrelha, J. , Lutteropp, M. , Luc, S. , Green, J.C. , Azzoni, E. , Woll, P.S. , Mead, A.J. , Hultquist, A. , Swiers, G. , Perdiguero, E.G. , Macaulay, I.C. , Melchiori, L. , Luis, T.C. , Kharazi, S. , Bouriez‐Jones, T. , Deng, Q. , Ponten, A. , Atkinson, D. , Jensen, C.T. , Sitnicka, E. , Geissmann, F. , Godin, I. , Sandberg, R. , de Bruijn, M.F. & Jacobsen, S.E. (2013) Lymphomyeloid contribution of an immune‐restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell, 13, 535–548. [DOI] [PubMed] [Google Scholar]
- Boyer, S.W. , Schroeder, A.V. , Smith‐Berdan, S. & Forsberg, E.C. (2011) All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3‐positive progenitor cells. Cell Stem Cell, 9, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buza‐Vidas, N. , Cheng, M. , Duarte, S. , Nozad, H. , Jacobsen, S.E. & Sitnicka, E. (2007) Crucial role of FLT3 ligand in immune reconstitution after bone marrow transplantation and high‐dose chemotherapy. Blood, 110, 424–432. [DOI] [PubMed] [Google Scholar]
- Buza‐Vidas, N. , Cheng, M. , Duarte, S. , Charoudeh, H.N. , Jacobsen, S.E. & Sitnicka, E. (2009) FLT3 receptor and ligand are dispensable for maintenance and posttransplantation expansion of mouse hematopoietic stem cells. Blood, 113, 3453–3460. [DOI] [PubMed] [Google Scholar]
- Buza‐Vidas, N. , Woll, P. , Hultquist, A. , Duarte, S. , Lutteropp, M. , Bouriez‐Jones, T. , Ferry, H. , Luc, S. & Jacobsen, S.E. (2011) FLT3 expression initiates in fully multipotent mouse hematopoietic progenitor cells. Blood, 118, 1544–1548. [DOI] [PubMed] [Google Scholar]
- Carow, C.E. , Levenstein, M. , Kaufmann, S.H. , Chen, J. , Amin, S. , Rockwell, P. , Witte, L. , Borowitz, M.J. , Civin, C.I. & Small, D. (1996) Expression of the hematopoietic growth factor receptor FLT3 (STK‐1/Flk2) in human leukemias. Blood, 87, 1089–1096. [PubMed] [Google Scholar]
- Carvalho, T.L. , Mota‐Santos, T. , Cumano, A. , Demengeot, J. & Vieira, P. (2001) Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(‐/)‐ mice. Journal of Experimental Medicine, 194, 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland, D.G. & Griffin, J.D. (2002) The roles of FLT3 in hematopoiesis and leukemia. Blood, 100, 1532–1542. [DOI] [PubMed] [Google Scholar]
- Hao, Z. & Rajewsky, K. (2001) Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. Journal of Experimental Medicine, 194, 1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, R.R. & Hayakawa, K. (1991) A developmental switch in B lymphopoiesis. Proceedings of the National Academy of Sciences of the United States of America, 88, 11550–11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslein, D.G. , Yang, S.Y. & Schatz, D.G. (2006) Origins of peripheral B cells in IL‐7 receptor‐deficient mice. Molecular Immunology, 43, 326–334. [DOI] [PubMed] [Google Scholar]
- Jensen, C.T. , Kharazi, S. , Boiers, C. , Cheng, M. , Lubking, A. , Sitnicka, E. & Jacobsen, S.E. (2008) FLT3 ligand and not TSLP is the key regulator of IL‐7‐independent B‐1 and B‐2 B lymphopoiesis. Blood, 112, 2297–2304. [DOI] [PubMed] [Google Scholar]
- Kantor, A.B. , Stall, A.M. , Adams, S. , Herzenberg, L.A. & Herzenberg, L.A. (1992) Differential development of progenitor activity for three B‐cell lineages. Proceedings of the National Academy of Sciences of the United States of America, 89, 3320–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel, M.J. , Yilmaz, O.H. , Iwashita, T. , Yilmaz, O.H. , Terhorst, C. & Morrison, S.J. (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell, 121, 1109–1121. [DOI] [PubMed] [Google Scholar]
- Kuhn, R. , Schwenk, F. , Aguet, M. & Rajewsky, K. (1995) Inducible gene targeting in mice. Science, 269, 1427–1429. [DOI] [PubMed] [Google Scholar]
- Luc, S. , Luis, T.C. , Boukarabila, H. , Macaulay, I.C. , Buza‐Vidas, N. , Bouriez‐Jones, T. , Lutteropp, M. , Woll, P.S. , Loughran, S.J. , Mead, A.J. , Hultquist, A. , Brown, J. , Mizukami, T. , Matsuoka, S. , Ferry, H. , Anderson, K. , Duarte, S. , Atkinson, D. , Soneji, S. , Domanski, A. , Farley, A. , Sanjuan‐Pla, A. , Carella, C. , Patient, R. , de Bruijn, M. , Enver, T. , Nerlov, C. , Blackburn, C. , Godin, I. & Jacobsen, S.E. (2012) The earliest thymic T cell progenitors sustain B cell and myeloid lineage potential. Nature Immunology, 13, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis, T.C. , Luc, S. , Mizukami, T. , Boukarabila, H. , Thongjuea, S. , Woll, P.S. , Azzoni, E. , Giustacchini, A. , Lutteropp, M. , Bouriez‐Jones, T. , Vaidya, H. , Mead, A.J. , Atkinson, D. , Boiers, C. , Carrelha, J. , Macaulay, I.C. , Patient, R. , Geissmann, F. , Nerlov, C. , Sandberg, R. , de Bruijn, M. , Blackburn, C.C. , Godin, I. & Jacobsen, S.E.W. (2016) Initial seeding of the embryonic thymus by immune‐restricted lympho‐myeloid progenitors. Nature Immunology, 17, 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman, S.D. & Jacobsen, S.E. (1998) c‐kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood, 91, 1101–1134. [PubMed] [Google Scholar]
- Lyman, S.D. , James, L. , Escobar, S. , Downey, H. , de Vries, P. , Brasel, K. , Stocking, K. , Beckmann, M.P. , Copeland, N.G. , Cleveland, L.S. , Jenkins, N.A. , Belmont, J.W. & Davison, B.L. (1995) Identification of soluble and membrane‐bound isoforms of the murine flt3 ligand generated by alternative splicing of mRNAs. Oncogene, 10, 149–157. [PubMed] [Google Scholar]
- Mackarehtschian, K. , Hardin, J.D. , Moore, K.A. , Boast, S. , Goff, S.P. & Lemischka, I.R. (1995) Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity, 3, 147–161. [DOI] [PubMed] [Google Scholar]
- Mansson, R. , Hultquist, A. , Luc, S. , Yang, L. , Anderson, K. , Kharazi, S. , Al‐Hashmi, S. , Liuba, K. , Thoren, L. , Adolfsson, J. , Buza‐Vidas, N. , Qian, H. , Soneji, S. , Enver, T. , Sigvardsson, M. & Jacobsen, S.E. (2007) Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity, 26, 407–419. [DOI] [PubMed] [Google Scholar]
- Mansson, R. , Zandi, S. , Welinder, E. , Tsapogas, P. , Sakaguchi, N. , Bryder, D. & Sigvardsson, M. (2010) Single‐cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood, 115, 2601–2609. [DOI] [PubMed] [Google Scholar]
- Matthews, W. , Jordan, C.T. , Wiegand, G.W. , Pardoll, D. & Lemischka, I.R. (1991) A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell‐enriched populations. Cell, 65, 1143–1152. [DOI] [PubMed] [Google Scholar]
- McCormack, M.P. , Forster, A. , Drynan, L. , Pannell, R. & Rabbitts, T.H. (2003) The LMO2 T‐cell oncogene is activated via chromosomal translocations or retroviral insertion during gene therapy but has no mandatory role in normal T‐cell development. Molecular and Cellular Biology, 23, 9003–9013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna, H.J. , Stocking, K.L. , Miller, R.E. , Brasel, K. , De Smedt, T. , Maraskovsky, E. , Maliszewski, C.R. , Lynch, D.H. , Smith, J. , Pulendran, B. , Roux, E.R. , Teepe, M. , Lyman, S.D. & Peschon, J.J. (2000) Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood, 95, 3489–3497. [PubMed] [Google Scholar]
- Mead, A.J. , Kharazi, S. , Atkinson, D. , Macaulay, I. , Pecquet, C. , Loughran, S. , Lutteropp, M. , Woll, P. , Chowdhury, O. , Luc, S. , Buza‐Vidas, N. , Ferry, H. , Clark, S.A. , Goardon, N. , Vyas, P. , Constantinescu, S.N. , Sitnicka, E. , Nerlov, C. & Jacobsen, S.E. (2013) FLT3‐ITDs instruct a myeloid differentiation and transformation bias in lymphomyeloid multipotent progenitors. Cell Reports, 3, 1766–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf, D. (2008) Hematopoietic cytokines. Blood, 111, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, M. , Coskun, E. , Fransecky, L. , Mochmann, L.H. , Bartram, I. , Sartangi, N.F. , Heesch, S. , Gokbuget, N. , Schwartz, S. , Brandts, C. , Schlee, C. , Haas, R. , Duhrsen, U. , Griesshammer, M. , Dohner, H. , Ehninger, G. , Burmeister, T. , Blau, O. , Thiel, E. , Hoelzer, D. , Hofmann, W.K. & Baldus, C.D. (2013) FLT3 mutations in early T‐cell precursor ALL characterize a stem cell like leukemia and imply the clinical use of tyrosine kinase inhibitors. PLoS ONE, 8, e53190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvy, S. , Metcalf, D. , Gibson, L. , Bath, M.L. , Harris, A.W. & Adams, J.M. (1999) Promoter elements of vav drive transgene expression in vivo throughout the hematopoietic compartment. Blood, 94, 1855–1863. [PubMed] [Google Scholar]
- Rosnet, O. , Marchetto, S. , deLapeyriere, O. & Birnbaum, D. (1991) Murine Flt3, a gene encoding a novel tyrosine kinase receptor of the PDGFR/CSF1R family. Oncogene, 6, 1641–1650. [PubMed] [Google Scholar]
- Rosnet, O. , Schiff, C. , Pebusque, M.J. , Marchetto, S. , Tonnelle, C. , Toiron, Y. , Birg, F. & Birnbaum, D. (1993) Human FLT3/FLK2 gene: CDNA cloning and expression in hematopoietic cells. Blood, 82, 1110–1119. [PubMed] [Google Scholar]
- Rumfelt, L.L. , Zhou, Y. , Rowley, B.M. , Shinton, S.A. & Hardy, R.R. (2006) Lineage specification and plasticity in CD19‐ early B cell precursors. Journal of Experimental Medicine, 203, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheijen, B. & Griffin, J.D. (2002) Tyrosine kinase oncogenes in normal hematopoiesis and hematological disease. Oncogene, 21, 3314–3333. [DOI] [PubMed] [Google Scholar]
- Sitnicka, E. , Bryder, D. , Theilgaard‐Monch, K. , Buza‐Vidas, N. , Adolfsson, J. & Jacobsen, S.E. (2002) Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity, 17, 463–472. [DOI] [PubMed] [Google Scholar]
- Sitnicka, E. , Brakebusch, C. , Martensson, I.L. , Svensson, M. , Agace, W.W. , Sigvardsson, M. , Buza‐Vidas, N. , Bryder, D. , Cilio, C.M. , Ahlenius, H. , Maraskovsky, E. , Peschon, J.J. & Jacobsen, S.E. (2003) Complementary signaling through flt3 and interleukin‐7 receptor alpha is indispensable for fetal and adult B cell genesis. Journal of Experimental Medicine, 198, 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitnicka, E. , Buza‐Vidas, N. , Ahlenius, H. , Cilio, C.M. , Gekas, C. , Nygren, J.M. , Mansson, R. , Cheng, M. , Jensen, C.T. , Svensson, M. , Leandersson, K. , Agace, W.W. , Sigvardsson, M. & Jacobsen, S.E. (2007) Critical role of FLT3 ligand in IL‐7 receptor independent T lymphopoiesis and regulation of lymphoid‐primed multipotent progenitors. Blood, 110, 2955–2964. [DOI] [PubMed] [Google Scholar]
- Stadtfeld, M. & Graf, T. (2005) Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non‐invasive lineage tracing. Development, 132, 203–213. [DOI] [PubMed] [Google Scholar]
- Stirewalt, D.L. & Radich, J.P. (2003) The role of FLT3 in haematopoietic malignancies. Nature Reviews Cancer, 3, 650–665. [DOI] [PubMed] [Google Scholar]
- Tsapogas, P. , Mooney, C.J. , Brown, G. & Rolink, A. (2017) The cytokine Flt3‐ligand in normal and malignant hematopoiesis. International Journal of Molecular Sciences, 18, 1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubersax, J.A. & Ferrell, J.E. Jr (2007) Mechanisms of specificity in protein phosphorylation. Nature Reviews Molecular Cell Biology, 8, 530–541. [DOI] [PubMed] [Google Scholar]
- Ullrich, A. & Schlessinger, J. (1990) Signal transduction by receptors with tyrosine kinase activity. Cell, 61, 203–212. [DOI] [PubMed] [Google Scholar]
- Vosshenrich, C.A. , Cumano, A. , Muller, W. , Di Santo, J.P. & Vieira, P. (2003) Thymic stromal‐derived lymphopoietin distinguishes fetal from adult B cell development. Nature Immunology, 4, 773–779. [DOI] [PubMed] [Google Scholar]
- Wasserman, R. , Li, Y.S. & Hardy, R.R. (1995) Differential expression of the blk and ret tyrosine kinases during B lineage development is dependent on Ig rearrangement. The Journal of Immunology, 155, 644–651. [PubMed] [Google Scholar]
- Yoshimoto, M. , Montecino‐Rodriguez, E. , Ferkowicz, M.J. , Porayette, P. , Shelley, W.C. , Conway, S.J. , Dorshkind, K. & Yoder, M.C. (2011) Embryonic day 9 yolk sac and intra‐embryonic hemogenic endothelium independently generate a B‐1 and marginal zone progenitor lacking B‐2 potential. Proceedings of the National Academy of Sciences of the United States of America, 108, 1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zriwil, A. , Boiers, C. , Wittmann, L. , Green, J.C. , Woll, P.S. , Jacobsen, S.E. & Sitnicka, E. (2016) Macrophage colony‐stimulating factor receptor marks and regulates a fetal myeloid‐primed B‐cell progenitor in mice. Blood, 128, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Provides data validating the model.
Figure S2. Provides data that extends the findings in Fig 1.
Figure S3. Provides data that extends the findings in Figs 2, 3 and 4.
Figure S4. Provides data that extends the findings in Fig 3.
Table S1. Provides the list of antibodies used in the study.


