Abstract
Purpose of review
Low HDL-cholesterol (HDL-C) levels are a strong predictor of cardiovascular disease risk and can be improved with regular exercise. However, raising HDL-C levels pharmacologically has not shown convincing clinical benefits. Thus, research has recently focused on identifying therapies that improve HDL function, with exercise representing such a potential therapy. The purpose of this review is to summarize the effects of exercise interventions on HDL function.
Recent findings
The effects of exercise and lifestyle interventions on the primary atheroprotective functions of HDL are reviewed, namely, cholesterol efflux, antioxidative, and anti-inflammatory properties. Differences in study design, study population, and assays are discussed to aid in the interpretation of the reviewed studies.
Summary
There is mixed evidence that regular aerobic exercise improves cholesterol efflux capacity, with recent research suggesting an exercise dose threshold needs to be exceeded to produce beneficial effects. There is preliminary evidence that exercise improves the antioxidative and anti-inflammatory properties of HDL. Although exercise represents a potential therapeutic approach to improve HDL function, the heterogeneity and/or lack of findings warrants more and larger studies to determine what HDL function(s) are most responsive to regular exercise and what dose of exercise elicits the greatest improvements in HDL functionality.
Keywords: cholesterol efflux, exercise training, inflammation, lifestyle intervention, oxidative stress
INTRODUCTION
Lifestyle modification, including adhering to a healthy diet and regular exercise, is considered the foundation for reducing cardiovascular disease (CVD) risk and a first-line intervention in the management of the blood lipid profile. It is well established that both acute and chronic aerobic exercise increase plasma HDL-cholesterol (HDL-C) levels in a dose–response manner, with exercise volume, rather than intensity, having a greater influence on HDL-C response to exercise [1,2]. Aerobic exercise training also increases the plasma concentration of large HDL particles [3]. However, the effects of regular exercise on the plasma HDL profile vary substantially across interventions and individuals [2–4]. Furthermore, despite strong epidemiological evidence of an inverse association between HDL-C and CVD risk, recent randomized, controlled drug trials have failed to improve CVD outcomes despite significantly increasing HDL-C levels. These findings suggest that HDL-C is not a therapeutic target and thus has led to a focus on identifying therapies that improve HDL functionality rather than HDL-C quantity. Exercise represents a potential therapy for the improvement of the atheroprotective functions of HDL.
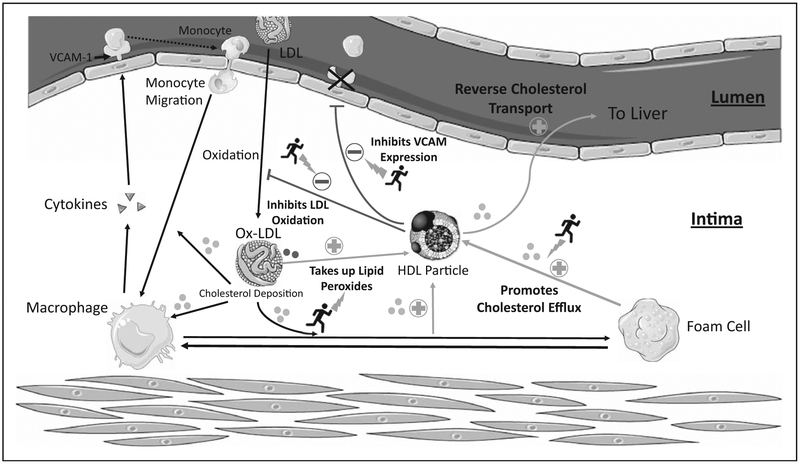
The atheroprotective properties of HDL include reverse cholesterol transport, inhibition of vascular inflammation, and reduction of oxidative stress (Fig. 1). Although exercise is well known to increase HDL-C levels, its effects on HDL functionality are less well understood. Several exercise interventions have provided evidence of improvements in certain aspects of HDL functionality, including cholesterol efflux capacity (CEC), antioxidative, and anti-inflammatory properties. Therefore, this review serves to summarize the key findings of the effects of exercise interventions on HDL function.
FIGURE 1.
HDL functions that may be influenced by exercise.
CHOLESTEROL EFFLUX CAPACITY
Reverse cholesterol transport is the pathway by which HDL accepts cholesterol in the periphery and transports it to the liver for excretion. Macrophage-specific cholesterol efflux, the initial step in the reverse cholesterol transport process, is considered one of the critical mechanisms by which HDL exhibits protection against atherosclerosis. Numerous animal and human studies have demonstrated a strong, inverse relationship between CEC and prevalent and incident CVD, independent of HDL-C levels. Conversely, the potential clinical utility of HDL’s other presumed atheroprotective functions is yet to be established and are limited by lack of reproducible and validated assays.
Assays that measure CEC quantify the movement of labeled cholesterol from cells to extracellular acceptors. Currently, there is no standardized method for measuring CEC in humans, as protocols differ by cholesterol label (e.g., radio-label or fluorescent-label), cell type (e.g., macrophage, monocyte, liver), and acceptor [e.g., isolated pure HDL, apolipoprotein B (apoB)-depleted plasma/serum]. Choice of cholesterol acceptor can have a significant impact on assessment of CEC and is the largest source of variation across studies [5]. Thus, evaluating the specific methodology utilized is critical when interpreting the effects of exercise on CEC in humans. Unless otherwise noted, most of the exercise studies discussed below used CEC assays that measured the global efflux of radiolabeled (3H) cholesterol from J774 macrophages to apoB-depleted plasma/serum (Table 1).
Table 1.
The effects of exercise interventions on cholesterol efflux capacity
| Reference | Population | Duration | Exercise intervention | HDL function assay | Effects of exercise training |
|---|---|---|---|---|---|
| Koba et al. [6] | ACS patients following PCI (n = 68) 57 CR 11 non-CR |
6 Months | CR: gymnastics & 30min supervised aerobic exercise on cycle Prescribed at home brisk walking for 30–60 min at 40–60% HRR or 12–13 RPE |
3H-labeled CEC (J774 macrophages) using apoB-depleted serum | CEC ↑ 10% |
| Furuyama et al. [7] | ACS patients following PCI (n = 84) 69 CR 15 non-CR |
5 Months | CR: gymnastics and 30min supervised aerobic exercise on cycle Prescribed at home brisk walking for 30–60 min at 40–60% HRR or 12–13 RPE |
3H-labeled CEC (J774 macrophages) using apoB-depleted serum | CEC ↑ 9% |
| Albaghdadi et al. [8] | PAD patients w/o intermittent claudication (n = 88) 33 treadmill training 29 strength training 26 control |
8 Months | 24 weeks of supervised treadmill exercise 3 times per week. Intensity at or near max leg symptoms Resistance training: 3 sets 8 reps of knee ext, leg press, leg curl |
3H-labeled CEC (J774 macrophages) using apoB-depleted serum | No change in CEC |
| Boyer et al. [9■] | Sedentary men with elevated waist circumference and atherogenic dyslipidemia (n = 145) 113 lifestyle modification 32 control | 12 Months | Personalized healthy eating strategy (−500 kcal/day goal) Physical activity counseling (goal of 160 min of aerobic physical activity per week: 50–80% HRmax) |
3H-labeled CEC (J774 macrophages and HepG2 cells) using apoB-depleted serum | J774 CEC ↑ 14% HepG2 CEC ↑ 3% ↑ post prandial CEC |
| Khan et al. [10■■] | Adults with metabolic syndrome (n = 53) 17 control 19 WL 17 WLEX |
3 Months | Caloric restriction (reduced 600 kcal/day – lost 8.4 kg) Exercise: 40 min cycle at 65% HRmax on alternate days. One session supervised, rest at home |
3H-labeled CEC (THP-1 cells) with HDL isolated by density gradient ultracentrifugation | CEC ↑ 25% in WLEX group |
| Wesnigk et al. [11■] | Obese adolescents (n = 16) 8 Lifestyle 8 Control |
10 months | Two hours of supervised play per day Two hours physical education at school per week Three 40-min supervised exercise sessions per week |
3H-labeled CEC (J774 macrophages) using apoB-depleted serum | CEC ↑ 10% |
| Sarzynski et al. [12■■] |
E-MECHANiC Sedentary, overweight/obese (n = 90) 33 General health 28 WL 29 Control STSTIDE-PD Sedentary, overweight/obese, with prediabetes (n = 106) 29 Low amount/moderate intensity 27 High amount/moderate intensity 24 High amount/vigorous intensity 26 Clinical lifestyle |
6 Months |
E-MECHANIC: Treadmill exercise at 65–85% VO2peak, 3–5 times per week Exercise dose General health: 8 KKW WL: 20KKW STRRIDE-PD: Cycle treadmill or elliptical exercise Exercise dose Low-mod: 10 KKW at 50% VO2reserve High-mod: 16 KKW at 50% VO2reserve High-vig: 16 KKW at 75% VO2reserve Clinical lifestyle: low-mod exercise + diet with goal of 7% WL |
3H-labeled and BODIPY-labeled CEC (J774 macrophages) using apoB-depleted serum HAE |
E-MECHANIC 3H non-ABCA1 CEC ↑ 6% in 20 KKW group STRRIDE-PD Global 3H CEC ↑ 6% in high/vigorous group No change in BODIPY CEC or HAE in any group of either study |
ABCA1, ATP binding cassette transporter A1; ACS, acute coronary syndrome; apoB, apolipoprotein B; CEC, cholesterol efflux capacity; CR, cardiac rehabilitation; HAE, HDL-apoA-I exchange; HRmax, maximal heart rate; HRR, heart rate reserve; KKW, kcal/kg body weight/week; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; RPE, rating of perceived exertion; WL, weight loss; WLEX, weight loss and exercise.
Studies examining the effects of exercise interventions on CEC have delivered mixed results and vary primarily by exercise dose, populations tested, and concomitant therapies (e.g., diet and medications) (Table 1). Although some studies provided supervised exercise sessions, others used lifestyle interventions centered on counseling and goal setting to reach intended activity levels, or a mix of both. In two independent studies of patients from the same hospital with acute coronary syndrome and post percutaneous coronary intervention that completed cardiac rehabilitation, 5 or 6 months of exercise training and lifestyle modification counseling significantly increased CEC by 9–10% [6,7]. The significant findings, however, were limited to patients who achieved partial or complete risk factor control of five targets including smoking cessation, weight control, control of DBP and SBP, lipid control, and glycemic control. Furthermore, the study by Koba et al. [6] observed an increase in CEC following cardiac rehabilitation in 57 patients compared with baseline, but no significant increase in comparison to 11 control patients who did not undergo cardiac rehabilitation. Similarly, Furuyama et al. [7] found the significant effects of cardiac rehabilitation on CEC in 69 patients were diminished after adjusting for baseline CEC level. In contrast, 6 months of supervised treadmill exercise (N = 33) or resistance training (N = 29) did not increase CEC in patients with peripheral artery disease [8]. However, peripheral artery disease patients may not have been able to exercise at a sufficient intensity to achieve beneficial changes in CEC. Thus, the inconsistent results across exercise training studies of CVD patients may be related to the diminished exercise tolerance in these individuals and thus less intensive training programs, as well as differences in age and comorbidities.
Recent lifestyle interventions that combine caloric restriction and regular exercise show mostly positive results for improving CEC. One year of caloric restriction and physical activity counseling significantly increased CEC in sedentary men with elevated waist circumference and atherogenic dyslipidemia (n = 113), whereas no change was observed in the control group (n = 32) [9■]. The lifestyle intervention increased CEC in both J774 macrophage (+14%) and HepG2 cells (+3.4%), as well as improved CEC at most time points during the postprandial state [9■]. Khan et al. [10■■] found that a 12-week combined weight loss and exercise intervention in men and postmenopausal women with metabolic syndrome significantly increased CEC (using THP-1 cells and isolated HDL) by 25% compared with baseline levels, whereas no change in HDL-C was observed. However, CEC changes in the combination group (N = 17) did not differ from the dietary weight loss only (N = 19) or control (N = 17) groups [10■■]. A 10-month lifestyle intervention in obese adolescents significantly increased CEC in the intervention group (+10%, n = 8) compared with the control group (−8%, n = 8) [11■].
A limitation of lifestyle intervention studies is that it is not possible to distinguish the respective contributions of diet and exercise to changes in HDL function. Furthermore, although each of the cited lifestyle interventions resulted in significant weight loss, none of the studies appear to have adjusted for weight loss or other confounding factors in their models. Thus, it is unclear whether the improvements in CEC observed were independent of weight loss in these studies. These limitations are exacerbated by relatively small sample sizes and a lack of objectively measured exercise, as most studies did not supervise and/or standardize all exercise sessions.
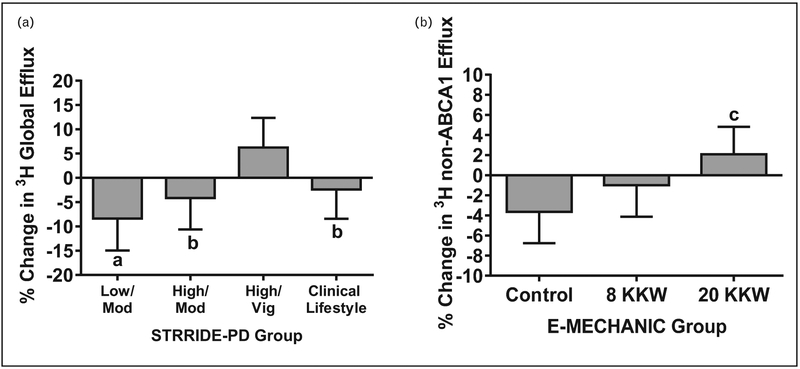
A recent study addressed many of the limitations of the current literature by having a large sample size from fully supervised and standardized exercise-only interventions, examining dose–response, and adjusting for body weight and confounding variables in the analyses [12■■]. The study investigated the effects of six different exercise interventions differing in exercise amount and/or intensity from two independent, randomized exercise trials on three different measures of CEC: radiolabeled and BODIPY-labeled CEC from J774 macrophages and the HDL-apolipoprotein A (apoA)-I exchange assay. The authors found that aerobic exercise training improved radiolabeled CEC only when a high amount of vigorous exercise was performed (Fig. 2) [12■■]. Specifically, in the STRRIDE-PD study of 106 prediabetic participants, 6 months of high amount and high intensity aerobic exercise training resulted in a significant increase in CEC (+6.2%) compared with the low amount/moderate intensity group (−8.4%), high amount/moderate intensity group (−4.2%), and clinical lifestyle (diet + exercise) group (−2.4%), whereas no significant within group changes in CEC were observed [12■■]. In the E-MECHANIC study, non-ATP binding cassette transporter A1-mediated radiolabeled CEC significantly increased (+5.7%) after 6 months of aerobic exercise training in the group assigned to the highest exercise dose (recommended amount of weekly physical activity for weight loss) compared with controls, whereas no changes were observed in the moderate exercise dose group (recommend amount of weekly physical activity for general health) [12■■]. In both cohorts, no significant effects of exercise training were observed for BODIPY-labeled CEC or HDL-apoA-I exchange, an indirect measure of CEC that measures the ability of apoA-I to exchange on and off HDL [12■■]. All results were independent of age, sex, race, BMI, and baseline CEC. These findings from six rigorous exercise interventions with high adherence suggest that an exercise dose threshold, particularly an exercise intensity threshold, needs to be exceeded to improve radiolabeled CEC.
FIGURE 2.
Adjusted mean (SEM) percentage change of radiolabeled global efflux in response to exercise training in STRRIDE-PD (a) and of non-ABCA1 efflux in E-MECHANIC (b). Values adjusted for age, sex, race, baseline BMI, and baseline value. ABCA1, ATP binding cassette transporter A1. aP=0.005 and bP<0.05 for difference from High-Vig. cP=0.01 for difference from control group. Modified from [12■■].
ANTIOXIDATIVE PROPERTIES
Oxidized lipids can exert widespread harmful effects on normal physiological function. Oxidized LDL lipids contribute to the development and progression of atherosclerosis. HDL particles have multiple antioxidative functions that protect against the effects of oxidized lipids (Fig. 1). HDL inhibits the oxidation of LDL and the subsequent atherosclerotic LDL by metabolizing lipid hydroperoxides and preventing their accumulation on LDL [13]. In addition, HDL can take up lipid peroxides, byproducts of lipid oxidation, and transport them to the liver for excretion [13,14].
The current literature on the effects of exercise on the ability of HDL to inhibit oxidation of LDL is limited to small studies of patients with metabolic syndrome or type 2 diabetes (Table 2). A 12-week educational program to reduce caloric intake and increase physical activity significantly decreased (−6.8%) the rate of LDL oxidation in the presence of HDL3c in normocholesterolemic [LDL-cholesterol (LDL-C) < 130mg/dl] metabolic syndrome patients (n = 18), but not in patients with elevated LDL-C (n = 15) [15■]. In 20 patients with metabolic syndrome, 3 months of supervised exercise training increased the LDL resistance to oxidation in the presence of both the HDL 2a and 3b subfractions by about 5% [16]. A 4-month supervised exercise training program improved the HDL3 protective effect against LDL oxidation by 15% in diabetic patients (n = 7), but not in healthy adults (n = 6) [17].
Table 2.
The effects of exercise interventions on the antioxidative and anti-inflammatory properties of HDL
| Reference | Population | Duration | Exercise intervention | HDL function assay | Effects of exercise training |
|---|---|---|---|---|---|
| Hansel et al.[15■] | MetS (n = 33) | 3 Months | Educational program Individually tailored diet program to reduce caloric intake Advised to achieve 150 min/week of moderate intensity endurance exercise |
Antioxidative activity of small, dense HDL3c on conjugated diene formation in reference LDL HDL isolated by DGU |
Whole population No change in antioxidative capacity of HDL3c Subgroup analysis Antioxidative capacity of HDL3c ↑ 6.8% in those w/LDL-cholesterol ≤130mg/dl |
| Casella-Filho et al. [16] | Adults from outpatient clinic (n =30) 20 MetS intervention group 10 healthy controls |
3 Months | Supervised cycle ergometer training 3 times/week for 45 min at heart rate associated with ventilatory threshold | Influence of HDL subfractions on resistance of LDL to oxidation HDL and LDL isolated by DGU |
LDL resistance to oxidation ↑ ~5% in both HDL2a & 3b |
| Ribeiro et al.[17] | Men and women (n = 32) 11 Healthy intervention group 11 Type 2 diabetes intervention 10 Type 2 diabetes controls |
4 months | Supervised cycle ergometer training 3 times/week for 40 min at intensity between anaerobic threshold and respiratory compensation point | HDL3 efficiency against in vitro LDL oxidation HDL and LDL isolated by DGU |
LDL resistance to oxidation ↑ 15% in people with diabetes No change in LDL oxidation in healthy adults |
| Tianen et al. [19■] | Sedentary, nonmenopausal, white women (n = 161) 79 Aerobic training 82 Control |
6 Months |
Unsupervised aerobic exercise for 50 min, 4 times per week, at an RPE of 13 to 16 (6–20 scale) Both the intervention and control groups attended lectures that covered topics of physical activity and general health |
OxHDL and OxLDL measured by level of conjugated dienes in isolated lipoproteins | OxHDL ↑ 5% No change in OxLDL |
| Sang et al.[20] | MetS patients (n = 39) 27 intervention group 12 control group |
10 weeks |
Supervised walk/run training Walk at 3.5 km/hw/speed increasing 0.3 km/h every 30 s until participant needed to run. Participants ran as long as they felt comfortable. Duration progressed from 30 to 60 min and intensity to 60–70% of HRmax Encouraged to train 5 times/week |
Ability of HDL3 to inhibit TNF-α induced VCAM-1 expression and monocyte adhesion in endothelial cells HDL isolated by sequential ultracentrifugation |
Ability of HDL3 to inhibit VCAM-1 expression ↑ ~20% Ability of HDL3 to inhibit monocyte adhesion ↑ ~33% |
| Roberts et al. [21] | Obese, middle-to-older aged men with MetS factors (n = 22) | 3 weeks |
Residential lifestyle intervention Diet: Prepared meals 15% fat, 20% protein, 65% carb Exercise: daily treadmill walking at 70–85% of HRmax for 45–60 min |
Ability of HDL to alter LDL-induced MCA in HAEC cells HDL isolated by FPLC |
Induction of MCA in presence of HDL ↓ ~18%: HDL inflammatory index decreased from proinflammatory (1.14) to anti-inflammatory (0.94) |
DGU, density gradient ultracentrifugation; FPLC, fast protein liquid chromatography; HAEC, human aortic endothelial cells; HRmax, maximal heart rate; MCA, monocyte chemotactic activity; MetS, metabolic syndrome; OxHDL, oxidized HDL lipids; OxLDL, oxidized LDL lipids; RPE, rating of perceived exertion.
The effect of acute and chronic exercise on the lipid peroxide transport function of HDL has also been demonstrated in two recent studies. Following an incremental maximal treadmill run to exhaustion in 24 elite and well trained endurance athletes, the concentration of oxidized HDL lipids significantly increased by 24% and remained elevated during 15 min of recovery, whereas oxidized LDL lipids decreased by 19%, resulting in a 55% increase in the ratio of oxidized HDL lipids to oxidized LDL lipids immediately after the treadmill run, which remained elevated (+71%) after 15 min of recovery [18■]. These findings suggest that exercise acutely elevates lipid peroxide transfer to HDL for reverse transport to the liver for clearance. In a 6-month randomized-controlled trial in sedentary women, the lipid peroxide transport function of HDL increased by 5% in the endurance exercise training (n = 79) group and decreased by 2% in the control group (n = 82), whereas no changes in oxidized LDL were observed [19■]. These limited studies provide evidence for the beneficial effects of both acute and chronic endurance exercise on the ability of HDL to transport lipid peroxides.
ANTI-INFLAMMATORY PROPERTIES
Few studies have examined the effects of exercise on the anti-inflammatory properties of HDL (Table 2). Sang et al. [20] found that 10 weeks of walk/run training in metabolic syndrome patients (n = 27) increased the ability of isolated HDL3 to protect endothelial cells from injury by decreasing TNF-α-induced VCAM-1 expression and monocyte adhesion. A 3-week residential diet and exercise intervention in 22 overweight or obese men with metabolic syndrome factors shifted the properties of HDL from being proinflammatory to anti-inflammatory according to the HDL inflammatory index [21]. In summary, there is limited, but promising evidence that short-term exercise training or lifestyle modification improves the anti-inflammatory properties of HDL.
MEDIATORS OF HDL FUNCTION CHANGES WITH EXERCISE
The exact mechanisms by which exercise impacts HDL function are not well defined. It is thought that the molecular composition of HDL particles, particularly the protein and lipid components, mediate the biological functions of HDL [22,23]. Several studies found that the strongest predictors of exercise-induced change in CEC were concomitant changes in HDL-C and/or apoA-I levels [7,9■,12■■]. The study by Sarzynski et al. [12■■] also found that change in CEC was positively correlated with changes in total HDL particle concentration and mean HDL size and negatively with change in proportion of calories from fat. Casella-Filho et al. [16] showed that the exercise-induced increase in HDL antioxidative capacity in metabolic syndrome patients was related to a decrease in the cholesterol and triglyceride content and increase of cholesterol esters in HDL3b. Khan et al. [10■■] found that a weight loss and exercise intervention in metabolic syndrome patients increased CEC and normalized the HDL lipidome toward that of healthy individuals. Significantly, the authors found numerous HDL lipid species that associated with CEC at baseline [10■■], but did not directly test whether change in the lipid species associated with change in CEC. These limited data suggest that HDL function and composition are interdependent and that exercise-induced changes in HDL composition may mediate concomitant exercise-induced changes in HDL function.
CONCLUSION
The effects of regular exercise on HDL function are variable and dependent upon several factors, including exercise dose and participant characteristics. A considerable number of studies have examined the effects of exercise or lifestyle interventions on CEC with mixed results. However, results from rigorously controlled, large exercise training interventions show that regular prolonged vigorous exercise improves CEC. Thus, more intensive and structured exercise programs may be required to improve CEC, whereas exercise interventions may be less effective at improving CEC in older, diseased individuals compared with younger, healthier individuals. In addition, limited studies with small sample sizes of patients with metabolic disorders provide preliminary evidence that exercise training also improves the antioxidative and anti-inflammatory properties of HDL. The lack of studies related to these properties of HDL may partially result from the lower throughput and reproducibility of cell-based assays, as well as the lack of clinical validation for the current HDL-based antioxidative and anti-inflammatory assays. Importantly, improvements in HDL function following exercise training are often found in the absence of significant changes in HDL-C, providing evidence for a discordance between HDL-C levels and HDL function. Furthermore, although exercise still represents a viable therapeutic option to improve HDL functionality, not all of the currently proposed atheroprotective properties of HDL have been evaluated in response to exercise. Thus, future studies using standardized methodologies in diverse populations are needed to further determine the effects of acute and chronic exercise on the entire repertoire of athero-protective properties of HDL.
KEY POINTS.
Exercise represents a potential therapy for the improvement of not just HDL-C, but HDL function as well.
Prolonged, high intensity exercise training may improve the cholesterol efflux capacity of HDL.
There is some evidence for the beneficial effects of exercise training on the antioxidative and anti-inflammatory properties of HDL.
Overall, the effects of exercise on HDL function are still largely unclear.
Acknowledgments
Financial support and sponsorship
The work was supported by the National Institute of General Medical Sciences of the NIH under award numbers P20 GM103499, P20 GM103641, and T32GM081740.
Footnotes
Conflicts of interest
M.A.S. is a consultant for Genetic Direction, LLC. For the remaining authors no conflicts were declared.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Durstine JL, Grandjean PW, Davis PG, et al. Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med 2001; 31:1033–1062. [DOI] [PubMed] [Google Scholar]
- 2.Kodama S, Tanaka S, Saito K, et al. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: a meta-analysis. Arch Intern Med 2007; 167:999–1008. [DOI] [PubMed] [Google Scholar]
- 3.Sarzynski MA, Burton J, Rankinen T, et al. The effects of exercise on the lipoprotein subclass profile: a meta-analysis of 10 interventions. Atherosclerosis 2015; 243:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leon AS, Gaskill SE, Rice T, et al. Variability in the response of HDL cholesterol to exercise training in the heritage family study. Int J Sports Med 2002; 23:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Rohatgi A High-density lipoprotein function measurement in human studies: focus on cholesterol efflux capacity. Prog Cardiovasc Dis 2015; 58:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koba S, Ayaori M, Uto-Kondo H, et al. Beneficial effects of exercise-based cardiac rehabilitation on high-density lipoprotein-mediated cholesterol efflux capacity in patients with acute coronary syndrome. J Atheroscler Thromb 2016; 23:865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuyama F, Koba S, Yokota Y, et al. Effects of cardiac rehabilitation on high-density lipoprotein-mediated cholesterol efflux capacity and paraoxonase-1 activity in patients with acute coronary syndrome. J Atheroscler Thromb 2018; 25:153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albaghdadi MS, Wang Z, Gao Y, et al. High-density lipoprotein subfractions and cholesterol efflux capacity are not affected by supervised exercise but are associated with baseline interleukin-6 in patients with peripheral artery disease. Front Cardiovasc Med 2017; 4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ■.Boyer M, Mitchell PL, Poirier DP, et al. Impact of a 1-year lifestyle modification program on cholesterol efflux capacities in men with abdominal obesity and dyslipidemia. Am J Physiol Endocrinol Metab 2018; 315:E460–E468.The study had a large sample size that completed a 1-year lifestyle intervention and found that the intervention increased cholesterol efflux capacity in two different cell types.
- 10. ■■.Khan AA, Mundra PA, Straznicky NE, et al. Weight loss and exercise alter the high density lipoprotein lipidome and improve high-density lipoprotein functionality in metabolic syndrome. Arterioscler Thromb Vasc Biol 2018; 38:438–447.The study found that a weight loss and exercise intervention shifted the HDL lipidome toward that of healthy individuals, which was combined with improvements in cholesterol efflux capacity. This is the first study to examine changes in the untargeted HDL lipidome and HDL function in the same individuals in response to an exercise intervention.
- 11. ■.Wesnigk J, Bruyndonckx L, Hoymans VY, et al. Impact of lifestyle intervention on HDL-induced eNOS activation and cholesterol efflux capacity in obese adolescent. Cardiol Res Pract 2016; 2016:2820432.The study is novel in that it is among the only studies to examine the effects of a lifestyle intervention on HDL function in adolescents.
- 12. ■■.Sarzynski MA, Ruiz-Ramie JJ, Barber JL, et al. Effects of increasing exercise intensity and dose on multiple measures of HDL (high-density lipoprotein) function. Arterioscler Thromb Vasc Biol 2018; 38:943–952.The study is the first to examine the effects of different doses of exercise training on multiple measures of cholesterol efflux capacity functionality. The study found that radiolabeled cholesterol efflux capacity only improved with a high dose of exercise training, particularly high-intensity exercise.
- 13.Soran H, Schofield JD, Durrington PN. Antioxidant properties of HDL. Front Pharmacol 2015; 6:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahotupa M, Suomela JP, Vuorimaa T, et al. Lipoprotein-specific transport of circulating lipid peroxides. Ann Med 2010; 42:521–529. [DOI] [PubMed] [Google Scholar]
- 15. ■.Hansel B, Bonnefont-Rousselot D, Orsoni A, et al. Lifestyle intervention enhances high-density lipoprotein function among patients with metabolic syndrome only at normal low-density lipoprotein cholesterol plasma levels. J Clin Lipidol 2016; 10:1172–1181.The study found that a lifestyle intervention, including physical activity, improved HDL antioxidative function in metabolic syndrome patients with low LDL-cholesterol.
- 16.Casella-Filho A, Chagas AC, Maranhao RC, et al. Effect of exercise training on plasma levels and functional properties of high-density lipoprotein cholesterol in the metabolic syndrome. Am J Cardiol 2011; 107:1168–1172. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro IC, Iborra RT, Neves MQ, et al. HDL atheroprotection by aerobic exercise training in type 2 diabetes mellitus. Med Sci Sports Exerc 2008; 40:779–786. [DOI] [PubMed] [Google Scholar]
- 18. ■.Valimaki IA, Vuorimaa T, Ahotupa M, et al. Strenuous physical exercise accelerates the lipid peroxide clearing transport by HDL. Eur J Appl Physiol 2016; 116:1683–1691.One of the only studies to examine the effects of acute exercise on HDL function. Found that acute exercise increased the concentration of oxidized HDL lipids in trained endurance athletes.
- 19. ■.Tiainen S, Luoto R, Ahotupa M, et al. 6-mo aerobic exercise intervention enhances the lipid peroxide transport function of HDL. Free Radic Res 2016; 50:1279–1285.Found that 6 months of endurance exercise training increased the concentration of oxidized HDL lipids in nonmenopausal women.
- 20.Sang H, Yao S, Zhang L, et al. Walk-run training improves the anti-inflammation properties of high-density lipoprotein in patients with metabolic syndrome. J Clin Endocrinol Metab 2015; 100:870–879. [DOI] [PubMed] [Google Scholar]
- 21.Roberts CK, Ng C, Hama S, et al. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in over-weight/obese men with cardiovascular risk factors. J Appl Physiol 2006; 101:1727–1732. [DOI] [PubMed] [Google Scholar]
- 22.Shah AS, Tan L, Long JL, et al. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res 2013; 54:2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kontush A, Lhomme M, Chapman MJ. Unraveling the complexities of the HDL lipidome. J Lipid Res 2013; 54:2950–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]