Abstract
Objective:
Habitual use of emotion regulation strategies may influence physical health. We examined whether the tendencies to employ cognitive reappraisal and suppression were associated with health biomarkers, and whether stress and sleep quality mediated these associations.
Design & main outcome measures:
Using data from the Biomarkers substudy (n = 1255) of the national Midlife in the U.S. Study, we tested the hypothesis that there would be indirect, but not direct, associations of cognitive reappraisal and suppression to biomarker indicators of multisystem physiological dysregulation, that is, allostatic load (AL). We computed the proportion of biomarkers in the highest risk quartile within seven biological systems, and summed these scores to compute AL. Associations with the biological systems were also examined separately.
Results:
Neither reappraisal nor suppression was directly associated with AL or biomarker function in the seven biological systems. Suppression was indirectly associated with higher AL and greater dysregulation in the inflammatory, metabolic, and hypothalamic-pituitary-adrenal systems via its relations to stress and sleep, p < 0.05. Reappraisal was indirectly associated with lower AL and less metabolic and inflammatory dysregulation, ps<0.05.
Conclusions:
Suppression and reappraisal may have different downstream health effects via stress, sleep, and biomarker expression, suggesting malleable emotion regulation strategies may be an important intervention target.
Keywords: Emotion regulation, stress, sleep, biomarkers, allostatic load
A large literature demonstrates that individuals who report frequent experiences of negative emotions/affect, such as depression, anxiety, and stress, are at greater risk for developing a cadre of chronic health problems, ranging from the common cold to cardiovascular disease and Type 2 diabetes (Bower et al., 2007; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002; Miller, Chen, & Cole, 2009; Miyamoto et al., 2013; Salovey, Rothman, Detweiler, & Steward, 2000; Steptoe, O’Donnell, Badrick, Kumari, & Marmot, 2008). In contrast, the effective regulation of negative emotions has been associated with lower disease risk (e.g., Kubzansky, Park, Peterson, Vokonas, & Sparrow, 2011; Potijk, Janszky, Reijneveld, & Falkstedt, 2016). Two common strategies used to regulate negative emotions 一 cognitive reappraisal and emotion suppression 一 differ in their effectiveness, with reappraisal generally being more effective in downregulating the subjective and physiological experience of negative emotion than suppression (Gross, 2002; John & Gross, 2004). Preliminary work also suggests habitually using suppression contributes to worse physiological health (Appleton, Buka, Loucks, Gilman, & Kubzansky, 2013; Appleton, Loucks, Buka, & Kubzansky, 2014; Chapman, Fiscella, Kawachi, Duberstein, & Muennig, 2013; Otto, Sin, Almeida, & Sloan, 2018), but little is known about the psychological or behavioral mechanisms underlying the associations between emotion regulation and physical health outcomes. The current study used a national sample of middle-aged adults to examine psychological and behavioral mediators of the relations between habitual use of specific strategies used to regulate negative emotions 一cognitive reappraisal and emotion suppression 一 and disease biomarkers.
Cognitive reappraisal is characterized by reinterpreting situations to modulate emotional responses, whereas emotion suppression is characterized by restricting the outward expression of an emotion (Gross, 1998, 1999). An individual might employ cognitive reappraisal by reframing anxiety about a physical symptom as motivation to seek quick medical attention. In contrast, an individual may engage in emotion suppression by containing the expression of their anxiety in order to keep their family from worrying. Although individuals can engage in either strategy on its own or together depending on the situation, cognitive reappraisal and emotion suppression do tend to be used habitually (Aldao, Sheppes, & Gross, 2015; Ehring, Tuschen-Caffier, Schnülle, Fischer, & Gross, 2010; Gross & John, 2003).
Reappraisal is generally more effective at reducing negative emotions and their acute physiological effects than suppression, regardless of whether the strategy is employed spontaneously or in controlled experimental settings (Egloff, Schmukle, Burns, & Schwerdtfeger, 2006; Ehring et al., 2010; Gross, 2002; Gross & John, 2003; Gross & Levenson, 1993, 1997; Haga, Kraft, & Corby, 2007; John & Gross, 2004; Webb, Miles, & Sheeran, 2012). Moreover, experimental evidence suggests that suppression elicits greater acute sympathetic activation, such as elevated heart rate and blood pressure, compared to reappraisal (Gross, 2002). Since these strategies tend to be used habitually, individual differences in the use of them may influence physiological processes over time (e.g., Aldao et al., 2015; Ehring et al., 2010). Indeed, the one study that has examined the association between emotion regulation strategies and biological health found that more routine use of suppression was associated with higher levels of circulating C-reactive protein (CRP; Appleton et al., 2013), a marker of inflammation implicated in the pathogenesis of several chronic diseases (e.g., cardiovascular disease; Ridker, 2003). In contrast, greater use of reappraisal was associated with lower levels of CRP (Appleton et al., 2013).
The mechanisms underlying the relations between cognitive reappraisal and suppression as emotion regulation strategies and physical health remain unstudied, but two possible mediators that are known to be associated with both emotion regulation and physical health outcomes are perceived psychological stress and sleep quality. Because the physiological stress response is activated in response to a negative appraisal of a situation (Lazarus & Folkman, 1984), use of cognitive reappraisal to reinterpret the situation has been associated with lower levels of both subjective and physiological measures of stress and negative emotions (Gaab et al., 2003; Jamieson, Nock, & Mendes, 2012; Mauss, Cook, Cheng, & Gross, 2007; Pakenham, 2005). In contrast, suppression is associated with a greater psychological and physiological stress response (Egloff et al., 2006; Levitt, Brown, Orsillo, & Barlow, 2004; Moore, Zoellner, & Mollenholt, 2008).
Cognitive reappraisal and suppression may also influence physical health outcomes through their effects on sleep, directly and/or indirectly via their effects on perceived stress. For instance, suppression is less effective than reappraisal at downregulating the stress response and related affective and cognitive states, such as rumination (Gross & John, 2003; John & Gross, 2004), which can have adverse effects on sleep quality (Garde, Albertsen, Persson, Hansen, & Rugulies, 2011; Kahn, Sheppes, & Sadeh, 2013; Martin & Dahlen, 2005; Mezick et al., 2009; Racine et al., 2013; Vandekerckhove et al., 2012). Poor sleep, including short sleep duration, poor sleep continuity, and poor subjective sleep quality, is strongly linked to a myriad of negative physical health outcomes (Carroll, Irwin, Merkin, & Seeman, 2015; Chen, Redline, Shields, Williams, & Williams, 2014; Irwin, Cole, & Nicassio, 2006; McEwen & Karatsoreos, 2015; Miller et al., 2009; Morris et al., 2018; Okun, 2011; Okun et al., 2011), raising the possibility that sleep serves as an important pathway through which emotion regulation could impact physical health.
The aim of the current study was to examine associations between the habitual use of specific emotion regulation strategies (cognitive reappraisal and emotion suppression) with biomarkers of disease risk using a multisystem approach. This approach recognizes that physiological stressors, such as negative affect, perceived stress, and poor sleep, lead to a greater burden and deterioration across multiple regulatory systems, termed allostatic load (McEwen, 1998, 2006). Thus, there are multiple routes to disease, as these multiple physiological systems interact with one another and result in a cumulative burden (e.g., McEwen, 1998). Our hypothesis was that greater use of suppression as an emotional regulation strategy and/or infrequent use of cognitive reappraisal would be associated with greater allostatic load, and that perceived stress and poor sleep, both of which are associated with greater allostatic load (Chen et al., 2014; McEwen, 1998, 2006; McEwen & Stellar, 1993; Morris et al., 2018), would serve as mediators of these associations. We also conducted sensitivity analyses to test whether this mediational model was robust to alternative ways of coding the biomarker data.
Methods
The current study is a secondary analysis of data from The Midlife in the United States (MIDUS) study, a longitudinal study of a national (U.S.) sample of adults aged 25–74 at baseline. MIDUS is aimed at investigating the role of behavioral, psychological, and social factors underlying age-related physical and mental health outcomes. As a subcomponent of MIDUS, a subset of participants (n = 1255) completed the Biomarkers Project, in which participants provided comprehensive biological assessments as a way to integrate behavioral and psychosocial factors with biology (Dienberg Love, Seeman, Weinstein, & Ryff, 2010). We included the measures described below because they best captured our constructs of interest.
Full details on the MIDUS biomarker protocol are available elsewhere (Dienberg Love et al., 2010; Gruenewald et al., 2012). Data and codebooks are also available at http://www.midus.wisc.edu/. In summary, MIDUS participants were originally recruited in 1995–1996 using a national sample obtained through random-digit dialing procedures. To be as inclusive as possible, all living participants in the first MIDUS survey who could safely travel to the clinic were considered eligible for participation in the Biomarkers Project. They were recruited to participate using mailings and follow-up phone calls. Data were collected between 2002 and 2006 at one of three MIDUS-affiliated General Clinical Research Centers (University of Wisconsin-Madison; University of California, Los Angeles; Georgetown University). Using a standardized protocol that was consistent across the three sites, participants completed a detailed medical history interview, self-administered questionnaires, and the collection of blood, urine, and saliva specimens during a 2-day visit. Participants were remunerated $200 for participating and travel expenses were covered. The Biomarkers Project protocol was approved by the institutional review boards at each General Clinical Research Center, and all participants provided informed written consent.
Participants
Participants were aged 34–84 (M = 54.52, SD = 11.71) and 54.8% were female. Most self-identified as White (91.4%); the other racial/ethnic identities represented were: Hispanic (3.6%), Black (2.6%), American Indian (1.2%), and Asian/Pacific Islander (0.29%). Three quarters (71.6%) were married; 10.6% were divorced; 10.1% were never married. See Table 1 for full participant characteristics.
Table 1.
Participant characteristics.
| % or M (SD) | |
|---|---|
| Sociodemographic characteristics | |
| Age (M) | 54.52 (11.71) |
| Gender (% female) | 54.8% |
| Race (% white) | 91.4% |
| Marital status (% married) | 71.6% |
| Education (% with bachelor’s degree or higher) | 42.1% |
| Health indicators | |
| BMI (M) | 28.5 (6.1) |
| Smoking status (% current smoker) | 13.8% |
| Number of chronic health conditions (M) | 3.1 (2.4) |
| Prescription medication use (% using any) | 64.6% |
| Lifetime depression (% clinically diagnosed) | 19.8% |
| Model predictors | |
| Suppression (M) | 3.98 (1.32) |
| Reappraisal (M) | 5.05 (1.09) |
| PSS (M) | 22.24 (6.34) |
| PSQI (M) | 6.23 (3.68) |
Abbreviations: BMI: body mass index; PSQI: Pittsburgh Sleep Quality Index; PSS: Perceived Stress Scale.
Measures
Allostatic load
A total of 23 biomarkers representing seven physiological regulatory systems were measured and included in the allostatic load score. They included biomarkers of the: (1) sympathetic nervous system (SNS): urinary norepinephrine and epinephrine; (2) parasympathetic nervous system (PNS): standard deviation of R-R intervals (a measure of heart rate variability), low frequency, and high frequency spectral power; (3) hypothalamic-pituitary-adrenal (HPA) system: urinary cortisol1 and serum dehydroepiandrosterone sulfate (DHEA-S); (4) inflammatory/ immune system: CRP, Interleukin-6 (IL-6), e-Selectin, intracellular adhesion molecule-1 (ICAM-1), and fibrinogen; (5) cardiovascular system: systolic blood pressure, pulse pressure, and heart rate; (6) glucose metabolism: fasting blood glucose, glycosylated hemoglobin, and the homeostasis model of assessment of insulin resistance (HOMA-IR); and (7) lipid metabolism: triglycerides, high density lipoprotein (HDL), low density lipoprotein (LDL), body mass index (BMI), and waist-hip ratio.
All biomarkers were collected during an in-person medical exam at one of three General Clinical Research Centers. Biomarkers were obtained from a fasting blood draw, 12-h urine collection (7:00 pm to 7:00 am), electrocardiography, and a clinical assessment that included medication history. Full measurement methods have been reported in detail elsewhere (Dienberg Love et al., 2010; Gruenewald et al., 2012). Table 2 summarizes the biomarker collection methods and cut-off scores used to compute allostatic load. Outliers and individuals with biologically implausible data were identified by the MIDUS research group and coded as missing/inappropriate data prior to the public release of the data.
Table 2.
Biological subsystems with component biomarker indicators and descriptive statistics.
| Biological system | Component biomarkers | Collection method | High-risk cut-pointa | |
|---|---|---|---|---|
| Sympathetic nervous systemb | 1. Epinephrine (μg/g creatine) | Urine | ≥2.47 | |
| 2. Norepinephrine (μg/g creatine) | Urine | ≥32.97 | ||
| Hypothalamic pituitary axis | 1. Cortisol (μg/g creatine) | Urine | ≥20.00 | |
| 2. DHEA-S (μg/dl) | Blood | ≤50.00 | ||
| Parasympathetic nervous systemc | 1. Root mean square of successive differences of beat-to-beat intervals (RMSSD) | Clinician assessment | ≤12.14 | |
| 2. Low frequency spectral power | Clinician assessment | ≤114.95 | ||
| 3. High frequency spectral power | Clinician assessment | ≤58.80 | ||
| Inflammation | 1. IL-6 (pg/ml) | Blood | ≥3.48 | |
| 2. Fibrinogen (mg/dl) | Blood | ≥400.00 | ||
| 3. CRP (mg/l) | Blood | ≥3.65 | ||
| 4. sE-Selectin (ng/MI) | Blood | ≥51.90 | ||
| 5. slCAM-1 (ng/MI) | Blood | ≥335.78 | ||
| Cardiovascular system | 1. Resting systolic blood pressure (mm Hg) | Clinician assessment | ≥144.00 | |
| 2. Resting heart rate (bpm) | Clinician assessment | ≥79.00 | ||
| 3. Pulse pressure (SBP - DBP) | Clinician assessment | ≥65.00 | ||
| Glucose metabolism | 1. Glycosylated hemoglobin (HbA1c) | Blood | ≥6.24 | |
| 2. Fasting glucose (mg/dl) | Blood | ≥105.00 | ||
| 3. Insulin resistance (HOMA-IR) | Blood | ≥4.36 | ||
| Lipid metabolism | 1. Body mass index (BMI) | Clinician assessment | ≥33.05 | |
| 2. Waist-to-hip ratio | Clinician assessment | ≥0.97 | ||
| 3. Triglycerides (mg/dl) | Blood | ≥156.00 | ||
| 4. HDL cholesterol (mg/dl) | Blood | ≤42.00 | ||
| 5. LDL Cholesterol (mg/dl) | Blood | ≥127.59 | ||
Abbreviations: HDL: high density lipoprotein; LDL: low density lipoprotein.
These values represent the cut-off for the highest quartile of scores in the MIDUS sample with the exception of the parasympathetic nervous system, DHEA, and LDL cholesterol, for which the value represents the cut-off for the lowest quartile. Participants falling beyond this value were classified as high-risk on that biomarker.
In a sensitivity analysis, heart rate was included as part of the sympathetic nervous system.
In a sensitivity analysis, RMSSD was omitted from this system to reduce redundancy.
Consistent with prior allostatic load computations using MIDUS data (e.g., Bei, Seeman, Carroll, & Wiley, 2017; Brooks et al., 2014; Gruenewald et al., 2012), participants were first assigned a score of 1 or 0 on each biomarker, depending on whether they were in the riskiest quartile of the sample (1 = high-risk; 0 = low-risk). The riskiest quartile represented the top 25% of scores for all biomarkers except DHEA, HDL, and the PNS biomarkers, for which they represented the lowest 25% of scores (see Table 2 for cut-offs). These scores were consistent with the cut-offs identified by the National Health and Nutrition Examination Survey (NHANES), as well as clinically meaningful thresholds where they are available (Gruenewald et al., 2012; Reading, 2015).
Scores were adjusted for medication use; participants taking a medication to treat a condition affecting that biomarker were assigned a score of 1. Because biological systems differed in the number of biomarker indicators, system scores were calculated as the proportion of relevant biomarkers classified as high risk. An allostatic load score was computed as the sum of these proportional system scores, with a range from 0 to 7. Participants needed to have a score on at least six of seven biological systems to compute allostatic load; scores for 13 participants without these data were coded as missing. If participants were only missing parasympathetic system data (n = 94), allo-static load scores were imputed using a regression-based estimation method developed by the MIDUS researchers (Ryff et al., 2011); if participants were only missing data on one other system (n = 13), they received a score of zero for that system.
Because this measure of allostatic load presumes that all biological systems are equally dysregulated and this may not always be the case (Wiley, Gruenewald, Karlamangla, & Seeman, 2016), we also examined the associations between emotion regulation strategies and each biological system separately.
Emotion regulation
A shortened four-item version of the Emotion Regulation Questionnaire (ERQ) was used to assess participants’ tendency to utilize cognitive reappraisal and emotion suppression (Gross & John, 2003). All items used a 7-point scale ranging from (1) strongly disagree to (7) strongly agree. Reappraisal was assessed with two items: (1) ‘I control my emotions by changing the way I think about the situation I’m in,’ and (2) ‘When I’m faced with a stressful situation, I make myself think about it in a way that helps me stay calm,’ r = 0.38, p < 0.001. Tendency to utilize emotion suppression was also measured with two items: (1) ‘When I am feeling negative emotions (such as sadness or anger), I make sure not to express them’ and (2) ‘I keep my emotions to myself,’ r = 0.54, p < 0.001.
While the correlations between the reappraisal and suppression items were lower than expected, both the cognitive reappraisal and emotion suppression subscales of the ERQ have demonstrated adequate reliability (all a >.79) and test-retest reliability (α = 0.69) in prior work (e.g., Gross & John, 2003).
Self-reported global sleep quality
Self-reported global sleep quality was measured with the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). The PSQI is a widely used and reliable measure of global sleep quality and sleep disturbances over the past month. The 19 items are grouped into seven component scores that reflect the frequency of sleep problems in the following areas: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medication, and daytime dysfunction. A global sleep score ranging from 0 to 21 can be obtained by summing the seven components after weighting them on a scale ranging from 0 to 3 (α = 0.74). For each component as well as the global score, higher scores indicate worse sleep quality (Buysse et al., 1989).
Perceived psychological stress
The Perceived Stress Scale (PSS) is a 10-item measure that assesses the degree to which participants perceive situations in their lives as stressful (Cohen, Kamarck, & Mermelstein, 1983). Each item (e.g., ‘In the past month, how often have you been upset because of something that happened unexpectedly?’) used a 5-point scale ranging from (1) never to (5) very often and items were reverse-coded as needed so that higher scores indicated greater perceived stress (α = 0.87).
Participant characteristics
Participant characteristics known to influence emotion regulation, stress, sleep, and biomarkers, such as age, gender, and race/ ethnicity were self-reported as part of the survey. Participants also listed all medications they were currently using. These medications were coded according to their target condition and used in the computation of allostatic load scores.
Data analysis strategy
All analyses were conducted in Stata, version 14 (Stata Corp, College Station, TX). Participants with missing data on the emotion regulation scales (n = 6), perceived stress scale (n = 7), or PSQI (n = 83) were excluded from analyses. Path models were used to test whether the regular use of suppression and/or cognitive reappraisal as an emotion regulation strategy were directly associated with allostatic load, and/or indirectly associated with it through (i.e., mediated by) perceived stress and global sleep quality. We first tested a model using allostatic load as the dependent variable (Model 1; see Figure 1). We then tested a model in which each biological system was included separately (Model 2; see Figure 2). Lastly, we conducted a series of sensitivity analyses to examine whether the models were robust to changes in the coding and classification of biomarkers. This was particularly important given that the MIDUS biomarkers have been interpreted, classified, scored, and used in a variety of ways in prior studies (e.g., Carroll et al., 2015; Friedman, 2011; Gruenewald et al., 2012; Wiley et al., 2016).
Figure 1.
Model 1: Associations between emotion regulation strategies and allostatic load via perceived stress and global sleep quality (note: higher global sleep quality values indicate worse sleep).
Notes: *p<0.05; **p <0.01; ***p <0.001.
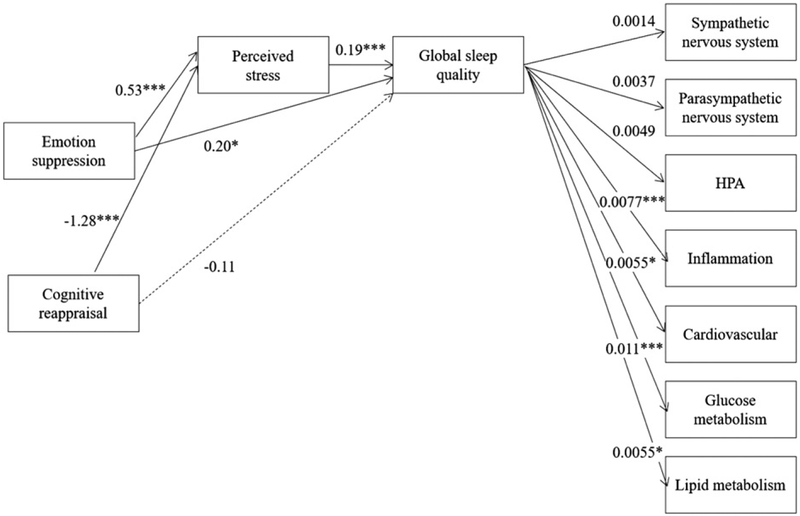
Figure 2.
Model 2: Associations between emotion regulation strategies and biomarkers representing seven biological systems via perceived stress and global sleep quality (note: higher global sleep quality values indicate worse sleep).
Notes: *p<0.05; **p <0.01; ***p <0.001. All direct associations between emotion regulation strategies and biological systems were non-significant. Lines representing these associations were omitted for readability.
To test for mediation, we examined the statistical significance of the indirect paths between emotion regulation and biomarkers through stress and sleep. Consistent with prior research using MIDUS data to investigate allostatic load outcomes (e.g., Gruenewald et al., 2012), gender (male or female), race (white or nonwhite), and age (continuous) were included as covariates in all paths, but adjusting for these participant characteristics did not change the pattern of results. Models were tested using unstandardized variables, but we report the standardized coefficients to facilitate comparison across scales that use different metrics.
Results
Bivariate (Pearson r) correlations between emotion regulation strategies, perceived stress, global sleep quality, and the proportion of high-risk biomarkers in each subsystem can be found in Table 3. There were significant differences in each of these constructs across age, gender, and race/ethnicity (Table 4). Women reported more frequent use of reappraisal, less use of suppression, greater perceived stress, and poorer global sleep quality than men, ps < 0.05. Compared to nonwhites, whites reported less use of reappraisal and suppression, less stress, and better global sleep quality, ps < 0.05. Use of suppression, but not reappraisal, increased with age, whereas stress and global sleep quality decreased, ps < 0.05.
Table 3.
Bivariate (Pearson r) correlations using standardized variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Cognitive reappraisal | |||||||||||
| 2. Emotion suppression | 0.16*** | ||||||||||
| 3. Perceived stress | −0.18*** | 0.069* | |||||||||
| 4. Global sleep quality | −0.049 | 0.088** | 0.37*** | ||||||||
| Biomarker subsystems | |||||||||||
| 5. SNS | 0.024 | 0.009 | −0.066* | 0.001 | |||||||
| 6. PNS | 0.006 | −0.037 | −0.070* | 0.002 | 0.075* | ||||||
| 7. HPA | 0.023 | −0.050 | −0.038 | 0.030 | 0.18*** | 0.10*** | |||||
| 8. Inflammatory | 0.039 | 0.008 | 0.096*** | 0.19*** | 0.12*** | 0.082** | 0.069* | ||||
| 9. Cardiovascular | 0.011 | 0.031 | 0.021 | 0.055 | 0.11*** | 0.25*** | 0.025 | 0.11*** | |||
| 10. Glucose metabolism | 0.049 | 0.064* | 0.043 | 0.13*** | 0.014 | 0.086** | −0.013 | 0.26*** | 0.088** | ||
| 11. Lipid metabolism | −0.055 | 0.048 | 0.069* | 0.092** | −0.10*** | 0.082** | −0.13*** | 0.28*** | 0.21*** | 0.32*** | |
Biomarker subsystems calculated as proportion of ‘high-risk’ biomarkers within each system (see Analysis strategy). Abbreviations: HPA, hypothalamic pituitary axis; PNS, parasympathetic nervous system; SNS, sympathetic nervous system.
p<0.05;
p <0.01;
p <0.001
Table 4.
Standardized regression coefficients representing associations between participant characteristics, emotion regulation strategies, perceived stress, and sleep disturbance.
| Cognitive reappraisal | Emotion suppression | Perceived stress | Global sleep quality | |
|---|---|---|---|---|
| Gender (ref: male) | 0.10*** | −0.15*** | 0.070* | 0.12*** |
| Race (ref: nonwhite) | −0.14*** | −0.10*** | −0.20*** | −0.19*** |
| Age | 0.031 | 0.074** | −0.19*** | −0.081** |
p < 0.05;
p < 0.01;
p < 0.001.
With the exceptions of the inflammatory and lipid subsystems, the proportion of biomarkers qualifying as high-risk increased with age, ps<0.05. Gender and race/ethnicity had less consistent associations, with women and whites (relative to men and nonwhites respectively) having higher scores on some biomarkers and lower scores on others (Table 5). The analyses reported below adjusted for age, gender, and race/ ethnicity in each path, although results did not differ substantively between adjusted and unadjusted models.
Table 5.
Standardized regression coefficients representing associations between participant characteristics and biomarkers.
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Allostatic load | SNS | PNS | HPA | Inflammatory | Cardiovascular | Glucose metabolism | Lipid metabolism | |
| Gender (female vs. male) | 0.051 | 0.12*** | −0.0054 | 0.21*** | 0.10*** | 0.091*** | −0.066* | −0.32*** |
| Race (non-white vs. white) | −0.044 | 0.082** | 0.071* | 0.11*** | −0.24*** | −0.051 | −0.20*** | 0.0027 |
| Age | 0.37*** | 0.21*** | 0.28*** | 0.32*** | 0.034 | 0.25*** | 0.12*** | −0.033 |
Abbreviations: HPA, hypothalamic pituitary axis; PNS, parasympathetic nervous system; SNS, sympathetic nervous system.
p < 0.05;
p < 0.01;
p < 0.001.
Model 1: Perceived stress and global sleep quality as mediators between emotion regulation and allostatic load
We first tested a model using allostatic load as the dependent variable (Figure 1). Emotion regulation was associated with perceived stress, such that greater use of suppression was associated with greater perceived stress, β = 0.53, p < 0.001, 95% CI (0.26, 0.80), whereas greater use of cognitive reappraisal was associated with lower perceived stress, β = −1.34, p < 0.001, 95% CI (−1.67, −1.02).
Emotion regulation was also associated with global sleep quality. Greater use of emotion suppression was both directly, β = 0.20, p = 0.010, 95% CI (0.047, 0.35), and indirectly (through perceived stress), β = 0.10, p < 0.001, 95% CI (0.047, 0.15), associated with poorer global sleep quality (higher sleep scores indicate worse sleep). Greater use of cognitive reappraisal was not directly associated with global sleep quality, but was indirectly associated with it through its negative association with perceived stress, indirect effect: β = −0.26, p < 0.001, 95% CI (−0.33, −0.18).
Use of cognitive reappraisal and suppression were not directly associated with allo-static load, ps > 0.33, but were indirectly associated with it through global sleep quality and perceived stress (i.e., sleep and perceived stress mediated the associations; Table 6). Greater use of suppression was indirectly associated with higher allostatic load, β = 0.017, p < 0.001, 95% CI (0.0077, 0.027), whereas greater use of reappraisal was indirectly associated with lower allostatic load, β = −0.028, p< 0.001, 95% CI (−0.044, −0.013).
Table 6.
Standardized regression coefficients representing indirect effects of emotion regulation strategies through perceived stress and subjective sleep on allostatic load (Model 1) and bio-marker subsystems (Model 2).
| Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Allostatic load | SNS | PNS | HPA | Inflammatory | Cardiovascular | Glucose metabolism | Lipid metabolism | |
| Suppression | 0.017*** | 0.00027 | 0.0022 | 0.0022* | 0.0035** | 0.0023* | 0.032* | 0.027** |
| Reappraisal | −0.028*** | −0.00015 | −0.0040 | −0.0036 | −0.0056** | −0.0035 | −0.0039 | −0.0046* |
Abbreviations: HPA: hypothalamic pituitary axis; PNS: parasympathetic nervous system; SNS: sympathetic nervous system.
p < 0.05;
p < 0.01;
p < 0.001.
Model 2: Perceived stress and global sleep quality as mediators between emotion regulation and biomarker subsystems
We next tested a model in which the seven biological systems were modeled as separate dependent variables (i.e., Model 2). Poorer global sleep quality was associated with high-risk biomarkers representing both glucose and lipid metabolism, as well as inflammation and the cardiovascular system, ps < 0.05 (Figure 2). Global sleep quality was not associated with the biomarker profiles of the sympathetic, parasympathetic, or HPA subsystems.
Consistent with our findings relating emotion regulation strategies to allostatic load (Model 1), we observed no direct associations between emotion regulation strategies and the biomarker subsystems (Figure 2). However, both use of suppression and cognitive reappraisal were indirectly associated with the proportion of high-risk bio-markers of the lipid metabolic and inflammatory systems, ps < 0.05 (Table 6). Suppression, but not reappraisal, was also indirectly associated with the proportion of high-risk biomarkers of the HPA, cardiovascular, and glucose metabolic systems, ps < 0.05. Neither emotion regulation strategy was directly or indirectly associated with the sympathetic or parasympathetic nervous system biomarkers, ps > 0.05 (Table 6). Thus, suppression and reappraisal were indirectly associated with overall allostatic load through stress and subjective sleep, but these associations varied across biological subsystems.
Sensitivity analyses
Given the lack of consensus surrounding the categorization and coding of biomarkers, we tested several additional models to examine whether the observed indirect effects of emotion regulation were robust to changes in the categorization and coding of the biomarker indicators. In the first set of sensitivity analyses, a series of separate models were tested with only one biological subsystem included as the dependent variable. In a second set of sensitivity analyses, we standardized biomarkers (to mean = 0 and SD = 1) and created mean scores for each subsystem using these standardized values rather than the at-risk cut-off values. Doing so avoided use of arbitrary, clinically irrelevant, and sample-dependent risk cut-off scores. We then tested Models 1 and 2 with these alternative dependent variables. In a third set of analyses, we reclassified the biomarkers such that heart rate was included with the sympathetic nervous system and RMSSD was removed from the parasympathetic nervous system to reduce redundancy. Again, we tested Models 1 and 2 with these alternative dependent variables.
Across these alternative models, there were no changes in the pattern of results. In all models, there were no direct effects of emotion regulation. There was an indirect effect of both suppression and reappraisal on inflammatory and lipid biomarkers, as well as an indirect effect of suppression on HPA, cardiovascular, and glucose metabolism biomarkers.
Discussion
Our findings support an indirect pathway between specific emotion regulation strategies (i.e., cognitive reappraisal and suppression) and biomarkers of disease, through their relations to perceived stress and global sleep quality. Specifically, the tendency to employ emotion suppression as a regulation strategy is indirectly associated with greater allostatic load (and HPA, inflammatory, cardiovascular, and metabolic dysregulation in particular), through its adverse effects on both perceived stress and global sleep quality. On the other hand, cognitive reappraisal is indirectly associated with lower allostatic load (and inflammation and lipid metabolism in particular) through its beneficial relations to perceived stress and global sleep quality. The connections uncovered among habitual reappraisal and suppression and these biomarkers contribute to a growing body of evidence suggesting that suppression may be a less adaptive way of regulating emotions than reappraisal, and that these strategies, when employed over time, may have lasting physical consequences.
Our results are consistent with recent evidence that cognitive reappraisal is associated with lower levels of systemic inflammation, as measured by CRP, and emotion suppression with higher levels of CRP (Appleton et al., 2013; Gruenewald et al., 2012; Irwin et al., 2006; Miller et al., 2009; Moore et al., 2008). They extend this work by including a broader set of inflammatory biomarkers, as well as measures that reflect a diverse set of biological systems, making it the first study to examine the associations between cognitive reappraisal and suppression and allostatic load. Our findings suggest these emotion regulation strategies may indirectly (via perceived stress and sleep quality) contribute to a greater allostatic burden and deterioration across several regulatory systems beyond inflammation. Given that these multiple physiological systems interact with one another, produce a cumulative burden, and contribute to multiple disease pathways (McEwen, 1998, 2006), the physical health implications of reappraisal and suppression may extend well beyond the inflammatory system. Suppression was associated with riskier biomarkers profiles for five out of seven biological subsystems, suggesting it may be a particularly consequential strategy for a broad set of adverse health outcomes.
The indirect pathways we observed are consistent with the evidence that psychological processes related to emotion regulation, particularly perceived stress, are associated with sleep quality (e.g., Garde et al., 2011; Kahn et al., 2013), and that both stress and poor sleep quality are associated with greater allostatic load (Chen et al., 2014; McEwen, 1998, 2006; McEwen & Karatsoreos, 2015; McEwen & Stellar, 1993; Morris et al., 2018). The current study integrated these prior lines of work in a model that more fully captures the interrelations between cognitive reappraisal/suppression, perceived stress, sleep disturbances, and allostatic load. It also extends prior work on allostatic load to identify the biological subsystems that seem to be most influenced by this emotion regulation pathway.
Sleep disturbances are prevalent (Morin, LeBlanc, Daley, Gregoire, & Merette, 2006) and have many health implications (Patel et al., 2004). No work has examined whether poor sleep disrupts the habitual and spontaneous use of reappraisal and suppression; however poor sleep adversely influences emotional reactivity, negative affect, executive functioning, and use of cognitive reappraisal in experimental settings (Gruber & Cassoff, 2014; Mauss, Troy, & LeBourgeois, 2013; Prather, Bogdan, & Hariri, 2013; Walker, 2009; Yoo, Gujar, Hu, Jolesz, & Walker, 2007). This suggests there may be recursive associations between emotion regulation and sleep that exacerbate the adverse effects of habitual reappraisal and suppression on allo-static load. Given the lack of evidence on how sleep influences the habitual use of specific emotion regulation strategies, we focused on suppression and reappraisal as mechanisms in this study, but future work ought to explore these bidirectional associations further.
The current findings suggest targeting specific emotion regulation strategies may be an effective means of reducing perceived stress and improving sleep, thereby influencing more distal physiological health outcomes. Reliance on strategies like cognitive reappraisal and suppression reflects learned strategies acquired through early socialization and experiences (John & Gross, 2004). As such, they may be amenable to change. In fact, cognitive behavioral therapy, a common technique employed in clinical psychology practice, often targets reappraisal techniques and evidence suggests these efforts successfully reduce stress, depression, and anxiety levels, in part through their effects on emotion regulation strategies (Aldao, Jazaieri, Goldin, & Gross, 2014; Gaab et al., 2003; Gratz, Weiss, & Tull, 2015). Other approaches, such as mindfulness (Farb, Anderson, Irving, & Segal, 2014) and compassion cultivation training (Jazaieri et al., 2014), have likewise been shown to reduce the use of suppression. Even simply encouraging individuals to expect that they will be able to successfully regulate their emotions can increase their ability to do so (Bigman, Mauss, Gross, & Tamir, 2016; Kassel, Bornovalova, & Mehta, 2007). Thus, encouraging the use of cognitive reappraisal and discouraging the habitual use of suppression to regulate one’s emotions may have important benefits for both perceived stress and sleep quality, ultimately improving physiological health. Future work ought to examine whether individuals can change which emotion regulation strategies they habitually employ, whether sleep interventions can initiate such changes (given the bidirectional associations between the two), and whether these efforts can change health outcomes.
Some limitations should be considered when interpreting the current findings, including the study’s cross-sectional design, which prevents the examination of causal pathways. Although the MIDUS study is longitudinal, cognitive reappraisal, suppression, sleep hygiene, perceived stress, and biomarkers were only assessed in the Biomarkers Project (MIDUS II), making it impossible to examine our research question longitudinally. Our findings are consistent with previous experimental evidence that has demonstrated a causal link between use of cognitive reappraisal and suppression and short-term psychological and physical health outcomes. However, future experimental and longitudinal research is necessary to further elucidate these associations, and to determine whether the statistical mediation demonstrated using the current data is an accurate reflection of the temporal associations in the real world. This work would also help to determine whether intervening on cognitive reappraisal and suppression has the potential to influence downstream behavioral and health outcomes.
Because this was a secondary data analysis of a national survey, there may be factors known to correlate with cognitive reappraisal, suppression, and/or sleep, such as health behaviors (e.g., alcohol consumption or eating behaviors), social support, and psychiatric disorders (or use of psychiatric medications), that were not examined as part of the modeled pathways. Future work should examine these possibilities. Given the large sample size, which was determined by the MIDUS project researchers rather than a priori to specifically examine our research question, Type 1 errors (i.e., false positives) are possible. Future work using sample sizes determined by a priori power calculations is needed to assess this possibility.
There may also be methodological differences in biomarker acquisition between this and previous studies that prevent direct comparisons and may explain some inconsistencies in results (e.g., Takase, Akima, Uehata, Ohsuzu, & Kurita, 2004; Tobaldini et al., 2013). For instance, given that participants were required to travel to participate in this study, circadian patterns in cortisol levels may have been disrupted. Additionally, the literature is not conclusive as to whether high and/ or low levels of basal cortisol are desirable (Seeman et al., 2010), and this study’s cut-off system may have failed to capture the full range of high-risk levels. Alternatively, these findings may reflect a stronger underlying association between the inflammatory and metabolic subsystems and allostatic load. There may be additional alternative explanations that warrant testing in future studies.
Some associations between cognitive reappraisal, suppression, sleep, and bio-markers may have also been attenuated due to the older age of the current sample or the reliance on retrospective reports of emotion regulation strategies and self-reported subjective sleep quality, rather than an objective measure or a measure that captured a wider variety of sleep disturbances (e.g., acute vs. chronic sleep deprivation). Moreover, emotional suppression and reappraisal were each assessed with two items. Brief measures are often necessary on long national surveys, but may not capture the construct as well as longer measures. Future work is needed to replicate these findings with more nuanced measures of emotion regulation and sleep.
Lastly, there are some limits to the generalizability of the current findings. In order to participate, participants needed to be healthy enough to travel to a MIDUS research center, introducing the potential for bias. Compared to the broader MIDUS sample, Biomarkers Project participants had higher levels of formal education, were more likely to have health insurance, and less likely to be a current smoker (Dienberg Love et al., 2010). However, most demographic (e.g., age, income, marital status) and health characteristics (e.g., BMI, subjective physical health, number of chronic health conditions) did not differ between the two samples (Dienberg Love et al., 2010), suggesting the sample was generally representative of the bigger MIDUS sample. However, the overall differences between MIDUS participants and the general public should be noted. The proportion of white participants in MIDUS was higher than the proportion in the U.S. population (91% vs. 77%; US Census, 2010). Median household income was also slightly higher ($57,500 vs. $55,3220), as was the proportion of individuals with a bachelor’s degree or higher (42% vs. 30%; US Census, 2010). To the extent that the observed associations may be influenced by such sociodemographic characteristics, the current findings may not generalize to populations of lower socioeconomic status. In addition, perceived stress (as measured by the PSS) was higher than what was observed in other similarly-aged participant populations (Cohen & Janicki-Deverts, 2012). Future research should examine whether these associations are observed in more diverse and representative populations.
These limitations are offset by several strengths, including utilization of a large, national sample of Americans and a survey protocol that included particularly high-quality assessments of the study’s key constructs, including the use of validated psychosocial scales, a rigorous and validated measure of subjective sleep behavior, and a comprehensive assessment of biomarkers. In addition, although several studies have used the MIDUS biomarkers data set to examine psychosocial predictors of physiological health, this is the first study to examine the role of emotion regulation. Moreover, by examining a more complex model of risk factors, as well as system-specific biological effects, these findings may facilitate greater precision in our understanding of the interrelation between the psychological, behavioral, and biological risk factors for chronic disease.
Behavioral practices are a primary determinant of health (Ford, Bergmann, Boeing, Li, & Capewell, 2012), and stress and poor sleep remain key risk factors for several acute and chronic health conditions. Given that specific emotion regulatory strategies, including cognitive reappraisal and suppression, can be induced or discouraged experimentally (Ehring et al., 2010; Gross & Levenson, 1993, 1997) and their habitual use may be malleable or learned (Gaab et al., 2003; John & Gross, 2004), this work may inform novel health interventions that target emotion regulatory strategies as a means of changing health outcomes indirectly via beneficial effects on perceived stress and sleep.
Footnotes
Salivary cortisol was also collected during an experimental protocol that included both a cognitive and orthostatic challenge, but it was intended to measure acute stress, rather than the chronic inflammation associated with allostatic load. Thus, the measure of 24-h urinary cortisol was used instead.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aldao A, Jazaieri H, Goldin PR, & Gross JJ (2014). Adaptive and maladaptive emotion regulation strategies: Interactive effects during CBT for social anxiety disorder. Journal of Anxiety Disorders, 28, 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldao A, Sheppes G, & Gross JJ (2015). Emotion regulation flexibility. Cognitive Therapy and Research, 39, 263–278. [Google Scholar]
- Appleton AA, Buka SL, Loucks EB, Gilman SE, & Kubzansky LD (2013). Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychology, 32, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton AA, Loucks EB, Buka SL, & Kubzansky LD (2014). Divergent associations of antecedent- and response-focused emotion regulation strategies with midlife cardiovascular disease risk. Annals of Behavioral Medicine, 48, 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei B, Seeman TE, Carroll JE, & Wiley JF (2017). Sleep and physiological dysregulation: A closer look at sleep intraindividual variability. Sleep, 40, zsx109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigman YE, Mauss IB, Gross JJ, & Tamir M (2016). Yes I can: Expected success promotes actual success in emotion regulation. Cognition and Emotion, 30, 1380–1387. [DOI] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, & Cole SW (2007). Inflammatory responses to psychological stress in fatigued breast cancer survivors: Relationship to glucocorticoids. Brain, Behavior, and Immunity, 21, 251–258. [DOI] [PubMed] [Google Scholar]
- Brooks KP, Gruenewald T, Karlamangla A, Hu P, Koretz B, & Seeman TE (2014). Social relationships and allostatic load in the MIDUS study. Health Psychology, 33, 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, & Kupfer DJ (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. [DOI] [PubMed] [Google Scholar]
- Carroll JE, Irwin MR, Merkin SS, & Seeman TE (2015). Sleep and multisystem biological risk: A population-based study. PLoS One, 10, e0118467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Fiscella K, Kawachi I, Duberstein P, & Muennig P (2013). Emotion suppression and mortality risk over a 12-year follow-up. Journal of Psychosomatic Research, 75, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Redline S, Shields AE, Williams DR, & Williams MA (2014). Associations of allo-static load with sleep apnea, insomnia, short sleep duration, and other sleep disturbances: findings from the National Health and Nutrition Examination Survey 2005 to 2008. Annals of Epidemiology, 24, 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, & Janicki-Deverts D (2012). Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 20091. Journal of Applied Social Psychology, 42, 1320–1334. [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Dienberg Love G, Seeman TE, Weinstein M, & Ryff CD (2010). Bioindicators in the MIDUS National Study: Protocol, measures, sample, and comparative context. Journal of Aging and Health, 22, 1059–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff B, Schmukle SC, Burns LR, & Schwerdtfeger A (2006). Spontaneous emotion regulation during evaluated speaking tasks: associations with negative affect, anxiety expression, memory, and physiological responding. Emotion, 6, 356–366. [DOI] [PubMed] [Google Scholar]
- Ehring T, Tuschen-Caffier B, Schnülle J, Fischer S, & Gross JJ (2010). Emotion regulation and vulnerability to depression: Spontaneous versus instructed use of emotion suppression and reappraisal. Emotion, 10, 563–572. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Anderson AK, Irving JA, & Segal ZV (2014). Mindfulness interventions and emotion regulation. Handbook of emotion regulation (2nd ed., pp. 548–567). New York, NY, US: Guilford Press. [Google Scholar]
- Ford ES, Bergmann MM, Boeing H, Li C, & Capewell S (2012). Healthy lifestyle behaviors and all-cause mortality among adults in the United States. Preventive Medicine, 55, 23–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM (2011). Sleep quality, social well-being, gender, and inflammation: An integrative analysis in a national sample. Annals of the New York Academy of Sciences, 1231, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J, Blättler N, Menzi T, Pabst B, Stoyer S, & Ehlert U (2003). Randomized controlled evaluation of the effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects. Psychoneuroendocrinology, 28, 767–779. [DOI] [PubMed] [Google Scholar]
- Garde AH, Albertsen K, Persson R, Hansen AM, & Rugulies R (2011). Bi-directional associations between psychological arousal, cortisol, and sleep. Behavioral Sleep Medicine, 10, 28–40. [DOI] [PubMed] [Google Scholar]
- Gratz KL, Weiss NH, & Tull MT (2015). Examining emotion regulation as an outcome, mechanism, or target of psychological treatments. Current Opinion in Psychology, 3, 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (1998). Sharpening the focus: Emotion regulation, arousal, and social competence. Psychological Inquiry, 9, 287–290. [Google Scholar]
- Gross JJ (1999). Emotion regulation: Past, present, future. Cognition & Emotion, 13, 551–573. [Google Scholar]
- Gross JJ (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39, 281–291. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–362. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Levenson RW (1993). Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology, 64, 970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, & Levenson RW (1997). Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology, 106, 95. [DOI] [PubMed] [Google Scholar]
- Gruber R, & Cassoff J (2014). The interplay between sleep and emotion regulation: Conceptual framework empirical evidence and future directions. Current Psychiatry Reports, 16, 1–9. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, & Seeman TE (2012). History of socioeconomic disadvantage and allostatic load in later life. Social Science & Medicine (1982), 74, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SM, Kraft P, & Corby E-K (2007). Emotion regulation: Antecedents and well-being outcomes of cognitive reappraisal and expressive suppression in cross-cultural samples. Journal of Happiness Studies, 10, 271–291. [Google Scholar]
- Irwin MR, Cole JC, & Nicassio PM (2006). Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+ years of age. Health Psychology, 25, 3–14. [DOI] [PubMed] [Google Scholar]
- Jamieson JP, Nock MK, & Mendes WB (2012). Mind over matter: reappraising arousal improves cardiovascular and cognitive responses to stress. Journal of Experimental Psychology: General, 141, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazaieri H, McGonigal K, Jinpa T, Doty JR, Gross JJ, & Goldin PR (2014). A randomized controlled trial of compassion cultivation training: Effects on mindfulness, affect, and emotion regulation. Motivation and Emotion, 38, 23–35. [Google Scholar]
- John OP, & Gross JJ (2004). Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality, 72, 1301–1334. [DOI] [PubMed] [Google Scholar]
- Kahn M, Sheppes G, & Sadeh A (2013). Sleep and emotions: Bidirectional links and underlying mechanisms. International Journal of Psychophysiology, 89, 218–228. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Bornovalova M, & Mehta N (2007). Generalized expectancies for negative mood regulation predict change in anxiety and depression among college students. Behaviour Research and Therapy, 45, 939–950. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, & Glaser R (2002). Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology, 53, 83–107. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Park N, Peterson C, Vokonas P, & Sparrow D (2011). Healthy psychological functioning and incident coronary heart disease: The importance of self-regulation. Archives of General Psychiatry, 68, 400–408. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping. New York, NY: Springer. [Google Scholar]
- Levitt JT, Brown TA, Orsillo SM, & Barlow DH (2004). The effects of acceptance versus suppression of emotion on subjective and psychophysiological response to carbon dioxide challenge in patients with panic disorder. Behavior Therapy, 35, 747–766. [Google Scholar]
- Martin RC, & Dahlen ER (2005). Cognitive emotion regulation in the prediction of depression, anxiety, stress, and anger. Personality and Individual Differences, 39, 1249–1260. [Google Scholar]
- Mauss IB, Cook CL, Cheng JYJ, & Gross JJ (2007). Individual differences in cognitive reappraisal: Experiential and physiological responses to an anger provocation. International Journal of Psychophysiology, 66, 116–124. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Troy AS, & LeBourgeois MK (2013). Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cognition and Emotion, 27, 567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Protective and damaging effects of stress mediators. New England Journal ofMedicine, 338, 171–179. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2006). Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism, 55, S20–S23. [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Karatsoreos IN (2015). Sleep deprivation and circadian disruption. Sleep Medicine Clinics, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153, 2093–2101. [PubMed] [Google Scholar]
- Mezick EJ, Matthews KA, Hall M, Kamarck TW, Buysse DJ, Owens JF, & Reis SE (2009). Intra-individual variability in sleep duration and fragmentation: Associations with stress. Psychoneuroendocrinology, 34, 1346–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E, & Cole SW (2009). Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology, 60, 501–524. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Boylan JM, Coe CL, Curhan KB, Levine CS, Markus HR, … Ryff CD (2013). Negative emotions predict elevated interleukin-6 in the United States but not in Japan. Brain, Behavior, and Immunity, 34, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SA, Zoellner LA, & Mollenholt N (2008). Are expressive suppression and cognitive reappraisal associated with stress-related symptoms? Behaviour Research and Therapy, 46, 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, LeBlanc M, Daley M, Gregoire J, & Merette C (2006). Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Medicine, 7, 123–130. [DOI] [PubMed] [Google Scholar]
- Morris G, Stubbs B, Köhler CA, Walder K, Slyepchenko A, Berk M, & Carvalho AF (2018). The putative role of oxidative stress and inflammation in the pathophysiology of sleep dys-function across neuropsychiatric disorders: Focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep Medicine Reviews. 41, 255–265. [DOI] [PubMed] [Google Scholar]
- Okun ML (2011). Biological consequences of disturbed sleep: Important mediators of health? Japanese Psychological Research, 53, 163–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Reynolds CF III, Buysse DJ, Monk TH, Mazumdar S, Begley A, & Hall M (2011). Sleep variability, health-related practices and inflammatory markers in a community dwelling sample of older adults. Psychosomatic Medicine, 73, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto LR, Sin NL, Almeida DM, & Sloan RP (2018). Trait emotion regulation strategies and diurnal cortisol profiles in healthy adults. Health Psychology, 37, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakenham KI (2005). Relations between coping and positive and negative outcomes in carers of persons with multiple sclerosis (MS). Journal of Clinical Psychology in Medical Settings, 12, 25–38. [Google Scholar]
- Patel SR, Ayas NT, Malhotra MR, White DP, Schernhammer ES, Speizer FE, … Hu FB (2004). A prospective study of sleep duration and mortality risk in women. Sleep, 27, 440–444. [DOI] [PubMed] [Google Scholar]
- Potijk MR, Janszky I, Reijneveld SA, & Falkstedt D (2016). Risk of coronary heart disease in men with poor emotional control: A prospective study. Psychosomatic Medicine, 78, 60–67. [DOI] [PubMed] [Google Scholar]
- Prather AA, Bogdan R, & Hariri PAR (2013). Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosomatic Medicine, 75, 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine C, Kalra K, Ceide M, Williams NJ, Zizi F, Mendlowicz MV, & Jean-Louis G (2013). Sleep duration, insomnia symptoms, and emotion regulation among black women. Journal of Sleep Disorders & Therapy, 2, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading SR (2015). Relationship between psychosocial stress and allostatic load: Findings from the MIDUS study (Doctoral dissertation, UCLA).
- Ridker PM (2003). Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation, 107, 363–369. [DOI] [PubMed] [Google Scholar]
- Ryff C, Almeida DM, Ayanian JS, Carr DS, Cleary PD, Coe C, & Williams D (2011). National Survey of Midlife Development in the United States (MIDUS II) 2004–2006. ICPSR 4652. Field Report for MIDUS 2 Longitudinal Sample. [Google Scholar]
- Salovey P, Rothman AJ, Detweiler JB, & Steward WT (2000). Emotional states and physical health. American Journal of Psychology, 55, 110. [DOI] [PubMed] [Google Scholar]
- Seeman T, Gruenewald T, Karlamangla A, Sidney S, Liu K, McEwen B, & Schwartz J (2010). Modeling multisystem biological risk in young adults: The coronary artery risk development in young adults study. American Journal of Human Biology, 22, 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, O’Donnell K, Badrick E, Kumari M, & Marmot M (2008). Neuroendocrine and inflammatory factors associated with positive affect in healthy men and women the Whitehall II Study. American Journal of Epidemiology, 167, 96–102. [DOI] [PubMed] [Google Scholar]
- Takase B, Akima T, Uehata A, Ohsuzu F, & Kurita A (2004). Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clinical Cardiology, 27, 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaldini E, Cogliati C, Fiorelli EM, Nunziata V, Wu MA, Prado M, … Montano N (2013). One night on-call: Sleep deprivation affects cardiac autonomic control and inflammation in physicians. European Journal of Internal Medicine, 24, 664–670. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove M, Kestemont J, Weiss R, Schotte C, Exadaktylos V, Haex B, … Gross JJ (2012). Experiential versus analytical emotion regulation and sleep: Breaking the link between negative events and sleep disturbance. Emotion, 12, 1415–1421. [DOI] [PubMed] [Google Scholar]
- Walker MP (2009). The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences, 1156, 168–197. [DOI] [PubMed] [Google Scholar]
- Webb TL, Miles E, & Sheeran P (2012). Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychological Bulletin, 138, 775–808. [DOI] [PubMed] [Google Scholar]
- Wiley JF, Gruenewald TL, Karlamangla AS, & Seeman TE (2016). Modeling multisystem physiological dysregulation. Psychosomatic Medicine, 78, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-S, Gujar N, Hu P, Jolesz FA, & Walker MP (2007). The human emotional brain without sleep - A prefrontal amygdala disconnect. Current Biology, 17, R877–R878. [DOI] [PubMed] [Google Scholar]