Summary
Larvae of Schistosoma (schistosomula) are highly susceptible to host immune responses and are attractive prophylactic vaccine targets, although cellular immune responses against schistosomula antigens in endemic human populations are not well characterized. We collected blood and stool from 54 Schistosoma mansoni‐infected Ugandans, isolated peripheral blood mononuclear cells and stimulated them for 24 hours with schistosome adult worm and soluble egg antigens (AWA and SEA), along with schistosomula recombinant proteins rSmKK7, Lymphocyte Antigen 6 isoforms (rSmLy6A and rSmLy6B), tetraspanin isoforms (rSmTSP6 and rSmTSP7). Cytokines, chemokines and growth factors were measured in the culture supernatants using a multiplex luminex assay, and infection intensity was determined before and at 1 year after praziquantel (PZQ) treatment using the Kato‐Katz method. Cellular responses were grouped and the relationship between groups of correlated cellular responses and infection intensity before and after PZQ treatment was investigated. AWA and SEA induced mainly Th2 responses. In contrast, rSmLy6B, rSmTSP6 and rSmTSP7 induced Th1/pro‐inflammatory responses. While recombinant antigens rSmKK7 and rSmLy6A did not induce a Th1/pro‐inflammatory response, they had an association with pre‐treatment infection intensity after adjusting for age and sex. Testing more schistosomula antigens using this approach could provide immune‐epidemiology identifiers necessary for prioritizing next generation schistosomiasis vaccine candidates.
Keywords: cytokines, Schistosoma mansoni, schistosomula, Th1/pro‐inflammatory, vaccine
1. INTRODUCTION
There are currently 206.4 million people suffering from schistosomiasis worldwide1 with the majority of infected individuals living in Africa. National control programmes in high‐risk countries such as Uganda treat infected school‐age children and adults through mass drug administration (MDA), using the drug praziquantel (PZQ). Although the MDA programmes have a high coverage in the targeted areas, national coverage still remains relatively low. For instance, in 2015 just over a third of the estimated 13.2 million people who required treatment in Uganda were reported to have received it.1 In addition, MDA programmes have no effect on recurrent reinfections in hotspots of transmission.2 More unsettlingly, there are reports of reduced efficacy of PZQ with multiple rounds of MDA.3, 4 This suggests that MDA alone is insufficient to control morbidity and prevent schistosomiasis transmission. Therefore, an integrated approach with other interventions such as vaccines is required.5, 6
A few schistosomiasis vaccines, such as Sh28GST, Sm‐TSP‐2 and Sm‐14 are currently in early human clinical trials,7, 8 although the trial data are not yet available. Therefore, novel schistosome vaccine antigens are still needed for the vaccine development pipeline. Schistosome larvae within the vertebrate host (known as schistosomula) are susceptible to host immune responses in animal models, and thus antigens from this stage are thought to be potential vaccine candidates.9, 10, 11 The schistosomula develop when infective free‐swimming fresh‐water larvae (cercariae) burrow through the host skin and lose their bifurcated tails in a process called transformation.12 Additional structural changes, such as the loss of the cercarial glycocalyx coat during the transformation process, expose the developing schistosomula to host‐mediated immune responses. As the skin‐stage schistosomula develop into the lung‐stage schistosomula and adult worms, they acquire host antigens13 masking themselves from host immune effector mechanisms.14 By targeting antigens from the early schistosomula, it might be possible to attack this stage and prevent infection of the host. Potential vaccine candidates expressed in the newly transformed schistosomula include SmKK7 (Smp_194830),15, 16 SmLy6A (Smp_019350) and SmLy6B (Smp_105220)13, 17 and the tetraspanins SmTSP6 (Smp_059530) and SmTSP7 (Smp_099770).18, 19
SmKK7 is secreted by both the cercariae and the schistosomula16; it has also been reported to be found in the nervous system of adult S. mansoni and to be homologous to a component in scorpion venom, acting as a potassium ion channel blocker. Schistosoma mansoni lymphocyte antigens, rSmLy6A and rSmLy6B (also known as SmCD59a and SmCD59b), are members of the three‐finger protein domain (TFPD) superfamily. Although they are homologous to the TFPD‐containing human CD59, which protects human cells from complement fixation, rSmLy6A and rSmLy6B do not inhibit host complement fixation and as such their function remains unknown.20 rSmLy6A and rSmLy6B are highly expressed by the schistosomula, and as probable GPI‐anchored proteins on the schistosome tegument, they likely interact directly with host immune cells.21 Schistosoma mansoni transmembrane proteins, tetraspanins rSmTSP6 and rSmTSP7, similarly to other tetraspanin family members, are thought to be involved in cell membrane biology.19, 22 As all of the proteins described above are at the interface between the parasite and the host immune system, and thus may be novel vaccine antigens, we assessed the cellular responses to these antigens in individuals residing in an S. mansoni endemic area.
2. MATERIALS AND METHODS
2.1. Ethics statement
Ethical approval for the study was obtained from the Makerere University School of Biomedical Sciences Higher Degrees Research and Ethics Committee (reference number SBS 300) and the Uganda National Council for Science and Technology (reference number HS 1040). A signed informed consent form was obtained from participants, or parents or legal guardians of children below 18 years of age, for enrolment in the study.
2.2. Recruitment of study participants
We examined immune responses in peripheral blood mononuclear cells (PBMCs) of participants from TheSchistoVac study (http://www.theschistovac.eu) which aimed to develop safe candidates for a prophylactic schistosomiasis vaccine. The design of TheSchistoVac study is described elsewhere.23 Briefly, a cohort of 372 people from an S. mansoni endemic fishing village, Namoni, in Mayuge District, Eastern Uganda, were recruited in September 2011; infected individuals were treated with two doses of PZQ (40 mg/kg) 1 week apart and followed up at 5 weeks and at 1 year after PZQ treatment. Venous blood was drawn before treatment and peripheral blood mononuclear cells (PBMCs) isolated by density gradient centrifugation using Lymphoprep™ (STEMCELL Technologies Inc, Cambridge, MA, USA) and cryopreserved in liquid nitrogen. Here, we report pre‐treatment cellular immune responses in 54 participants from the cohort described above.
2.3. Microscopic examination of stool for ova
Stool samples were collected before treatment (to determine pre‐treatment infection intensity), 5 weeks after treatment (to examine effectiveness of the treatment) and at 1 year after PZQ treatment (to determine reinfection). Three stool samples were collected on three consecutive days from study participants. Two thick smears were made from each of the stool samples, processed using the Kato‐Katz method24 and examined under a light microscope to determine S. mansoni egg count.
2.4. Schistosoma mansoni antigens
TheSchistoVac consortium provided the antigens used in this study. The antigens included S. mansoni adult worm antigen (AWA)25 and S. mansoni soluble egg antigen (SEA)25 as well as the S. mansoni schistosomula‐enriched recombinant antigens rSmKK7 (smp_194830), rSmLy6A (smp_019350), rSmLy6B (smp_105220), rSmTSP6 (smp_059530) and rSmTSP7 (smp_099770).19 The antigens used in the present study are summarized in Table 1. These antigens were identified as highly expressed products in the schistosomula life cycle stage after screening the Schistosoma transcriptome using a DNA microarray, as previously described.17, 19 Specifically, a >5‐fold increase in expression when comparing normalized expression averages from snail (egg, miracidia, mother sporocyst and daughter sporocyst) to schistosomula (3‐, 24‐hours, 3‐ and 6‐day schistosomula) life stages was observed.17 Recombinant antigens were expressed in Escherichia coli and purified using methods as previously described for rSmKK7,14 rSmLy6A and rSmLy6B.15 For rSmTSP6 and rSmTSP7, 84 and 78 amino acids were expressed, representing the extracellular loop 2 of these proteins, respectively, as defined by TMHMM2.0 software.26 About 106‐189 amino acid of rSmTSP6 and 108‐185 amino acid of rSmTSP7 were expressed using the same vector, expression parameters and purification methods as rSmLy6A and B.27 Endotoxin contamination of the recombinant antigens was determined by Limulus amebocyte lysate (LAL) assay using the Pierce LAL chromogenic endotoxin quantitation kit (Thermo Fisher, Pittsburgh, PA, USA). The endotoxin levels were 7.56 endotoxin units (EU)/mg for rSmKK7, undetectable (<0.4 EU/mg) for rSmLy6A, 0.892 EU/mg for rSmLy6B, 0.626 EU/mg for rSmTSP6 and 0.42 EU/mg for rSmTSP7. The limit of detection of the LAL assay was 0.4 EU/mg.
Table 1.
The list of recombinant antigens used in the present study
| Name | Alternative names | Schistosoma mansoni GeneDB identificationa | Predictions from protein data | Protein size (amino acids)d | References |
|---|---|---|---|---|---|
| SmKK7 | Smp_194830 |
Signal peptide predictedb
No transmembrane regions predictedc |
79 | 15, 16 | |
| SmLy6a | SmCD59a, SmCD59.1 | Smp_019350 | One transmembrane regions predictedc | 126 | 13, 20 |
| SmLy6b | SmCD59b, SmCD59.2 | Smp_105220 | One transmembrane regions predictedc | 124 | 13, 20, 21 |
| SmTSP6 | Smp_059530 | Four transmembrane regions predictedc | 196 | 18, 19 | |
| SmTSP7 | Smp_099770 | Four transmembrane regions predictedc | 225 | 18, 19 |
GeneDB is an annotation database for pathogens.58
Protein data retrieved from GeneDB.
Determined using SignalP.
Determined using TMHMM.
2.5. PBMC stimulation
Peripheral blood mononuclear cells were thawed, rested for 6 hours and stimulated for 24 hours with a panel of S. mansoni antigens in RPMI 1640 Medium (Thermo Fischer): AWA (10 μg/mL), SEA (10 μg/mL), rSmKK7 (2 μg/mL), rSmLy6A (2 μg/mL), rSmLy6B (2 μg/mL), rSmTSP6 (2 μg/mL) and rSmTSP7 (2 μg/mL). Due to a limited number of PBMCs available per individuals and a large number of antigens tested, each antigen was tested in singlicate. After 24 hours, the supernatants were collected and stored at −80°C. Medium without stimulus was used as a negative control.
2.6. Multiplex luminex assay
Supernatants were analysed for cytokines (IFN‐γ, L‐1β, IL‐1ra, IL‐2, IL‐4, IL‐5, IL‐6, IL‐7, IL‐9, IL‐10, IL‐12, IL‐13, IL‐15, IL‐17 and TNF), chemokines (Eotaxin, IL‐8, IP‐10, MCP‐1, MIP1a, MIP1b, RANTES) and growth factors (bFGF, GCSF, GMCSF, PDGFbb, VEGF) using a commercial Bio‐Plex Luminex assay (Bio‐Rad, Hercules, CA, USA) according to the manufacturers’ recommendations, and acquired using Bio‐Plex 200 System (Bio‐Rad). Samples below the limit of detection were assigned values corresponding to half of the lowest standard value and those above the highest limit of detection were given the value of the highest standard. Cytokines that were wholly below the lower limit of the assay (IL‐4 and IL‐7) and chemokines wholly above the upper limit of the assay (MIP1α, MIP1β and RANTES) were excluded from analysis. Samples from unstimulated PBMCs that produced TNF levels >100 pg/mL were also excluded from analysis, as these were considered unreliable28, 29; high IL‐1β and IL‐1ra levels were observed in the same samples.
2.7. Statistical data analysis
Statistical analysis was carried out using STATA version 13 (StataCorp, College Station, TX, USA) and graphs drawn using GraphPad Prism version 6.0g (GraphPad Software Inc., San Diego, CA, USA). Cytokine, chemokine and growth factor levels were not normally distributed and were, therefore, transformed using Box‐Cox transformation.30, 31 The paired Student's t test was used to compare responses between antigen stimulated and unstimulated PBMCs. Because the mean cytokine/chemokine/growth factor levels of more than two antigen‐stimulated PBMCs was compared to that of the unstimulated PBMCs, the error rate due to multiple testing was adjusted by considering a Bonferroni correction, taking into account the number of comparisons being made. In this instance, a P‐value of 0.007 (= 0.05/7, the number of antigens in the study) for each cytokine/chemokine/growth factor was taken to be statistically significant. Responses showing significant variation from the unstimulated PBMCs were further analysed. Unsupervised hierarchical clustering of the immune responses using Spearman correlation as the measure of similarity was performed using GENE‐E (Broad Institute Inc., Cambridge, MA, USA). The association between clusters (groups of correlated immune parameters) and infection intensity before treatment and following treatment was investigated using the global test version 5.29.1. The global test was based on the logistic regression model and was implemented in R version 3.3.2 (The R Foundation, Vienna, Austria). For this analysis, the post‐treatment infection intensity of the participants was classified into two groups: light, and moderate to heavy infection intensity, rather than the WHO categories (light/moderate/heavy infection intensity), as when stratified by the three WHO categories there were insufficient numbers within the moderate and heavy infection intensity groups for statistical analysis.
3. RESULTS
3.1. Characteristics of the study population
Table 2 shows the demographics of the study population. The age range of the study participants was 6‐40 years and 28 (51.9%) were female. All study participants were infected with S. mansoni with most individuals having heavy infection intensity (76%) at baseline. The median infection intensity was 1412 (interquartile range 443‐2567) eggs per gram of stool at baseline. Five weeks after PZQ treatment, all the participants had zero eggs, indicative of treatment success (data not shown). At follow‐up (1 year after PZQ treatment), all participants were re‐infected with most (57.0%) having a light infection intensity.
Table 2.
Characteristics of study population (N = 54)
| Age, median (range) | 12 (6‐40) |
| Females, n (%) | 28 (52) |
| Pre‐treatment infection intensity | |
| Light (1‐99 epg), n (%) | 7 (13) |
| Moderate (100‐399 epg), n (%) | 6 (11) |
| Heavy (400+ epg), n (%) | 41 (76) |
| 1‐year follow‐up infection intensity | |
| Light (1‐99 epg), n (%) | 31 (57) |
| Moderate to heavy (100+ epg), n (%) | 23 (43) |
epg, eggs per gram.
3.2. Schistosoma mansoni adult worm and egg antigens induce Th2 responses
Whole parasite preparations of S. mansoni, particularly the egg antigens, are known to induce Th2 responses.27, 28, 29 We sought to validate our protocol by examining immune responses to crude parasite preparations of AWA and SEA. As expected, and confirming our analysis approach, the adult worm and soluble egg antigens induced the production of significant levels of IL‐5 and IL‐13 compared to medium (Figure 1). None of the schistosomula antigens induced significant Th2 cytokine production, with the exception of rSmTSP7 induced IL‐13.
Figure 1.
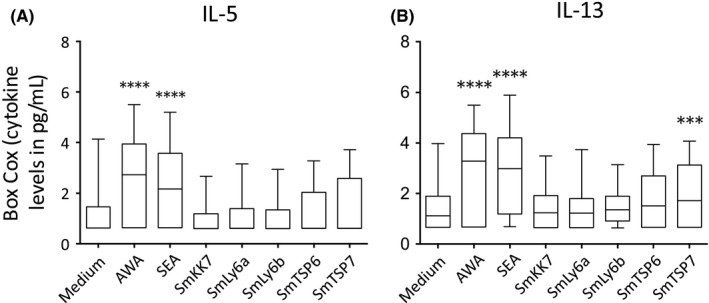
Box‐Cox transformed Th2 cytokine levels in response to stimulation of PBMCs from Schistosoma mansoni‐infected participants (n = 54) before PZQ treatment with AWA, SEA and schistosomula antigens compared with medium (A) IL‐5 (B) IL‐13. Box and whisker plots show median, interquartile range, maximum and minimum of cytokine levels. A paired Student's t test was used to test differences between medium and antigens. *P < 0.05, **P < 0.007, ***P < 0.001, ****P < 0.0001
3.3. Recombinant rSmLy6B, rSmTSP6 and rSmTSP7 induce a Th1/Pro‐inflammatory cytokine profile
Recombinant schistosomula antigens rSmLy6B, rSmTSP6 and rSmTSP7 induced significant and robust pre‐treatment cytokine responses characterized by Th1 (IFN‐γ), pro‐inflammatory (IL‐1β, IL‐2, IL‐6, IL‐9, IL‐12, IL‐15, IL‐17 and TNF) (Figure 2) and regulatory responses (IL‐1ra and IL‐10) compared to medium (Figure 3). Moreover, rSmLy6B, rSmTSP6 and rSmTSP7 induced high levels of chemokines (Figure S1) and growth factors (Figure S2). Interestingly, rSmKK7 and rSmLy6A in contrast were associated with much lower responses and suppressed production of IL‐17, IL‐1ra, bFGF, GMCSF, GCSF compared to medium (Figures 2 and 3, Figures S1 and S2).
Figure 2.
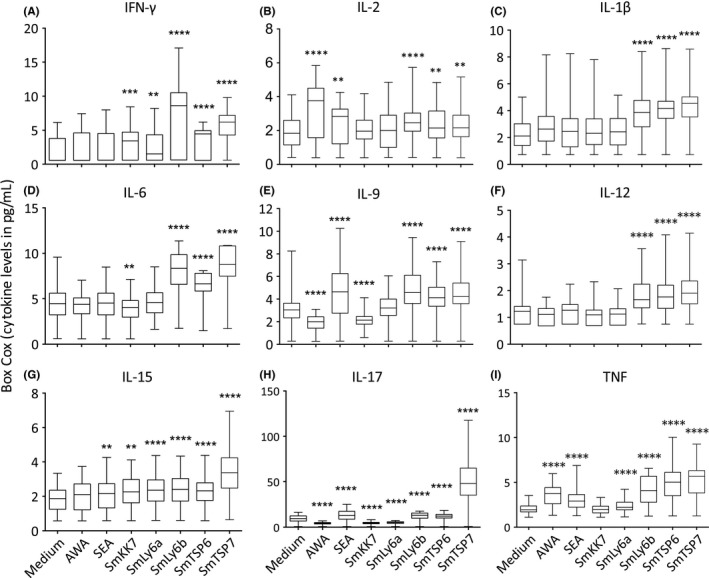
Box‐Cox transformed Th1 (A, B) and pro‐inflammatory (C‐I) cytokine levels in response to stimulation of PBMCs from Schistosoma mansoni‐infected participants (n = 54) before PZQ treatment with AWA, SEA and schistosomula antigens compared with medium. Box and whisker plots show median, interquartile range, maximum and minimum of cytokine levels. A paired Student's t test was used to test differences between medium and antigens. *P < 0.05, **P < 0.007, ***P < 0.001, ****P < 0001
Figure 3.
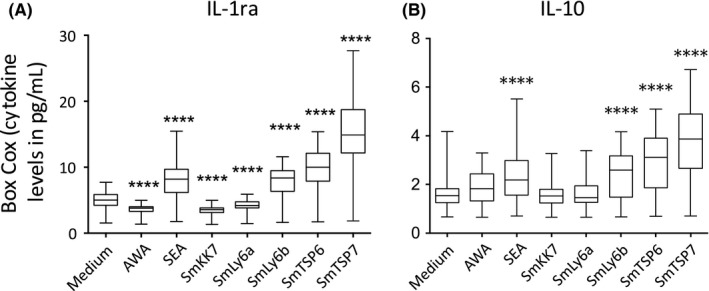
Box‐Cox transformed regulatory cytokine levels in response to stimulation of PBMCs from Schistosoma mansoni‐infected participants (n = 54) before PZQ treatment with AWA, SEA and schistosomula antigens compared with medium (A) IL‐1ra and (B) IL‐10. Box and whisker plots show median, interquartile range, maximum and minimum of cytokine levels. A paired Student's t test was used to test differences between medium and antigens. *P < 0.05, **P < 0.007, ***P < 0.001, ****P < 0.0001
3.4. Cytokine, chemokine and growth factor responses to schistosomula antigens cluster depending on the antigen
To provide a more global assessment of responses to schistosomula antigens in S. mansoni‐infected individuals, cytokine responses were grouped together using Spearman correlation as a similarity measure, to reduce the number of variables for statistical analyses.
Cytokine responses directed against schistosomula recombinant proteins grouped into six clusters: two clusters composed of individual cytokines (an IFN‐γ only cluster A and IL‐15 only cluster B); two clusters composed of Th1/pro‐inflammatory/regulatory response responses to rSmTSP6 and rSmTSP7 (clusters C and F); a cluster composed mainly of Th1/pro‐inflammatory/regulatory response mainly to rSmLy6B (cluster D); and a pro‐inflammatory cluster in response to rSmKK7, SmLy6A and SmLy6B (cluster E) (Figure 4).
Figure 4.
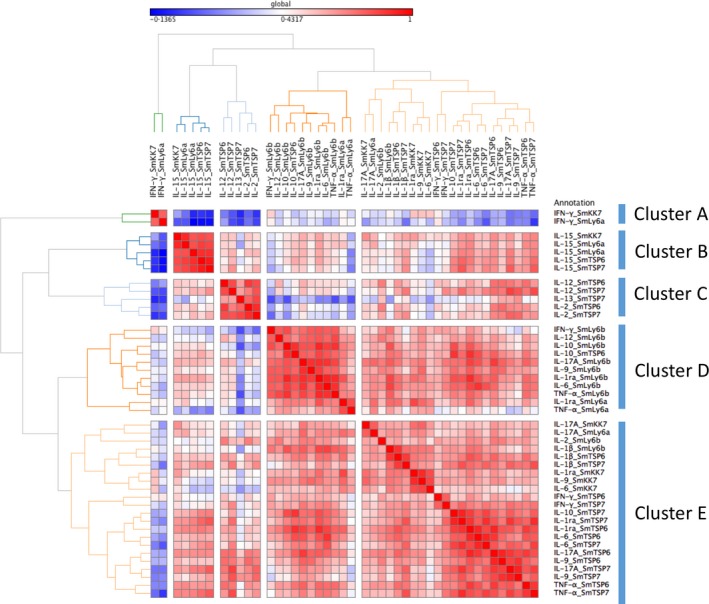
Unsupervised hierarchical clustering of cytokine responses from PBMCs of Schistosoma mansoni‐infected participants (n = 54) before PZQ treatment stimulated with S. mansoni schistosomula antigens. Red and blue colours indicate strong positive and negative correlations respectively
Similarly, chemokine responses to the schistosomula antigens clustered around individual chemokines (clusters A and C) and an eotaxin/IL‐8 cluster in response to all antigens tested (Figure S3A).
Finally, growth factor responses clustered around responses to rSmKK7 and rSmLy6A (cluster A), responses to rSmLy6B, rSmTSP6 and rSmTSP7 (cluster B) and a single growth factor cluster for PDGFFbb (cluster C) (Figure S3B).
3.5. Responses to rSmKK7 and rSmLy6A are positively associated with pre‐treatment infection intensity
To explore whether clusters of pre‐treatment cellular responses were associated with infection intensity before or 1 year post‐treatment, the global test was performed.32 This test examines the association between clusters (eg, groups of correlated immune parameters) and an outcome (eg, pre‐treatment or post‐treatment infection intensity). After adjusting for age and sex, a significant positive association between pre‐treatment infection intensity and responses to rSmKK7 and rSmLy6a was found for the IFN‐γ cytokine cluster (Table 3, P = 0.026), for the eotaxin chemokine cluster (Table S1, P = 0.020)) and a combination of growth factors (Table S2, P = 0.015). No significant associations were found between clusters of immune response and 1‐year post‐treatment infection intensity (Table 3, Tables S1 and S2).
Table 3.
Association between clusters of cytokine responses to schistosomula antigens and pre‐treatment infection intensity
| Clustera | Pre‐treatment infection intensityb | 1‐Year post‐treatment infection intensityb | ||
|---|---|---|---|---|
| Crude | Adjustedc | Crude | Adjustedc | |
| A | 0.010 d | 0.026 d | 0.060 | 0.131 |
| B | 0.663 | 0.746 | 0.550 | 0.853 |
| C | 0.189 | 0.312 | 0.178 | 0.363 |
| D | 0.496 | 0.134 | 0.262 | 0.481 |
| E | 0.307 | 0.140 | 0.810 | 0.946 |
| F | 0.578 | 0.427 | 0.912 | 0.866 |
Clusters are shown in Figure 4.
Global test P‐value.
Adjusted for age and sex.
Positive direction of association.
Bold text represents a significant difference.
4. DISCUSSION
The schistosomula are vulnerable to host immune responses after transformation from the cercariae stage.33, 34 As a result, antigens of the early schistosomula have been suggested as potential vaccine candidates.21, 35 Here we measured cytokine, chemokine and growth factor responses to a selection of S. mansoni schistosomula‐enriched antigens in endemic subjects with high infection intensity, and who become re‐infected 1 year after successful treatment.
To validate our study, we first looked at immune responses to the crude parasite antigen preparations AWA and SEA. In line with previous studies amongst individuals from high endemicity areas,36, 37, 38 we found predominant Th2 cytokine responses characterized by high IL‐5 and IL‐13.
Few schistosomula antigens have been tested in S. mansoni endemic populations. A non‐inclusive list of these antigens includes Sm29 (also known as SmLy6D)39 and Sm1440, 41 that have both been tested in Brazil. We found that three of the recombinant schistosomula antigens tested (rSmLy6B, rSmTSP6 and rSmTSP7) induced strong Th1/pro‐inflammatory and regulatory responses while two others (rSmKK7, and rSmLy6A) induced low responses in a Ugandan S. mansoni endemic population. Th1 and pro‐inflammatory cytokines have been implicated in antibody‐independent killing of schistosomula by stimulating macrophages to produce nitric oxide that mediates the killing of schistosomula in animal models.42, 43 Although the observed cytokine responses are consistent with a previous study in which a recombinant schistosomulum antigen, rSm14, induced significant levels of Th1/pro‐inflammatory cytokines at 72 hours after stimulation,40 our study shows that these responses can be measured earlier at 24 hours. An early response to the schistosomulum is necessary due to its transient nature in the tissues including the skin.
The generation of Th1 responses is associated with protective immunity to S. mansoni.44 For instance, the schistosomula antigen SmLy6D (Sm29) induces Th1 and pro‐inflammatory cytokines (IFN‐γ, TNF and IL‐12) that protect vaccinated mice against challenge infection.45 Furthermore, vaccinating mice with a chimera of two recombinant schistosomula antigens SmTSP2 and SmLy6D (Sm29) formulated in CpG‐Alum also protects vaccinated mice against infection.46 Whether the schistosomula antigens used in the present study, SmLy6B, SmTSP6 and SmTSP7, are protective in animal models of schistosomiasis remains to be determined. In a study in Brazil, Sm14‐specific Th1 responses were produced by PBMCs of people who were resistant to reinfection with schistosomiasis.40 This suggests that high antigen‐specific Th1 responses may play a protective role in human resistance to reinfection. However, with no clear resistant group in our cohort 1‐year post‐treatment, we instead looked at the association between cytokine responses and infection intensity before and after treatment with PZQ. We found no association between infection intensity at either pre‐ or post‐treatment and that Th1/pro‐inflammatory cytokines to rSmLy6B, rSmTSP6 and rSmTSP7 suggesting that in our cohort these responses may not be protective in humans and other schistosomula antigenic targets should be explored.
Recombinant SmLy6B, rSmTSP6 and rSmTSP7 induced production of regulatory cytokines IL‐10 and IL‐1ra. This is consistent with work done with another schistosomula tegument antigen, SmLy6D (Sm29), that induced IL‐10 production in PBMCs of S. mansoni‐infected individuals.41 In fact, the expression of SmLy6D (Sm29) is similar to that of rSmLy6B16, 19 and rSmTSP6 and rSmTSP7.19 The concomitant regulatory responses induced by the recombinant antigens in the present study may be needed to balance effector responses to limit immunopathology. This suggests that antigens rSmLy6B, rSmTSP6 and rSmTSP7 that induce regulated Th1 responses might be good targets to consider further as prospective human vaccine candidates in combination with adjuvants to induce a stronger Th1 response. In the present study, rSmKK7 did not significantly produce IL‐10. However, IL‐10 has been shown to be elevated in patients stimulated with cercarial excreted‐secreted (ES) products47 of which SmKK7 is a component.16 This inconsistency suggests that other constituents of cercarial ES material could be the major stimulants of IL‐10 production. In fact, glycosylated components of cercarial ES may play a role in the production of IL‐10.47 In addition, the anomaly in IL‐10 production could be caused by a difference in the stimulant used. Turner and colleagues used native cercarial ES material released from transforming cercariae,47 while the SmKK7 used in the present study is a purified recombinant protein without glycosylated moieties.
Interestingly, rSmTSP7 induced IL‐13 production in stimulated PBMCs of infected participants, whereas the other schistosomula antigens did not. In addition, the same rSmTSP7 induced Th1/pro‐inflammatory cytokine production. These findings imply a mixed Th1/Th2 response to recombinant schistosomula antigen SmTSP7. As much as murine studies have suggested that Th1 responses against migrating larvae may be more host protective than Th2 responses,46, 48, 49 a mixed Th1/Th2 response in mice vaccinated with radiation‐attenuated cercariae indicates that induction and balance of both Th1 and Th2 are required for vaccine‐induced protection and not a highly polarized Th1 or Th2.50 Unregulated and highly polarized immune responses are responsible for the immunopathology associated with schistosomiasis in mice51 and humans.52 This property of rSmTSP7 to induce a regulated mixed Th1/Th2 response positions it as a potential vaccine candidate to investigate further.
Recombinant rSmKK7 and rSmLy6A induced low responses and suppressed the production of IL‐17 and IL‐1ra compared to PBMCs cultured in medium alone. rSmKK7 shares homology with a venom protein BmKK7 of the Asian scorpion Buthus martensi Karsch (BmK) that blocks potassium channels.16, 53 As potassium channels are vital in the regulation of T cell activation after antigenic stimulation,54 it is possible that SmKK7 affects potassium channels, thereby modulating T cell activation and suppressing cytokine production by T cells.16 It remains to be experimentally determined if indeed SmKK7 modulates human T cell activation. Furthermore, Th1/pro‐inflammatory immune responses to rSmKK7 and rSmLy6A were positively associated with pre‐treatment infection intensity. However, the suitability of these antigens as vaccine candidates is debatable because they elicited low responses and were not associated with protection against S. mansoni reinfection.
The cytokine responses to the recombinant antigens observed in this study likely reflect the differences in the expression of the gene products of the schistosomula antigens, and their localisation within the S. mansoni organism. SmLy6B, SmTSP6 and SmTSP7 are localized in the tegument and their gene products are highly expressed during the 3‐, 24‐hour, 3‐ and 6‐day schistosomula as well as adult life stages17, 19; SmLy6A, also a tegument antigen, is only highly expressed in the 6‐day schistosomula, while SmKK7 is a secreted molecule.16
Because of the small number of participants in the present analysis, a larger study may be warranted to investigate the association between cytokine responses to the schistosomula antigens and infection intensity 1 year after PZQ treatment. Nonetheless, a large number of cytokines were evaluated per participant. While this creates a problem of multiple comparisons, the approach taken in the present study using unsupervised hierarchical clustering allows for large numbers of cytokines/ growth factors/chemokines to be tested as a group rather than as individual parameters. The responses to the crude antigens AWA and SEA were not represented in the cluster analysis of response to recombinant schistosomula antigens. The global test in the present study utilised the groups of cytokines/growth factors/chemokines to find a relationship with either pre‐treatment or 1‐year post‐treatment infection intensity. The global test allowed for a global analysis of responses which may give broader and generalized insights. The global test has been used elsewhere to study clusters of microarray gene data and how the gene clusters relate to two types of leukaemia.32 In addition, the global test adjusts for multiple comparisons since few groups are tested.32 In essence, the global test may be useful for studies of cohorts with many independent measurements.
The rate of exposure to the Schistosoma through water contact is associated with resistance to reinfection with schistosomiasis.55, 56, 57 It follows that exposure history during follow‐up is an important variable in the understanding of reinfection with schistosomiasis. The participants in the present study might have had varied rates of exposure during follow‐up, which was not assessed in the present study and thus affecting immune responses to schistosomula antigens. To control exposure to infection in future, immunogenicity studies of vaccine antigens, a controlled human infection model (CHIM) for schistosomiasis, could be explored. Hitherto the hookworm was the only human helminth to be studied in CHIM for its potential as a vaccine (NCT01940757). The first CHIM for schistosomiasis is ongoing with European volunteers using a single sex (male cercariae) infection to assess safety and optimal dose of such controlled infections (NCT02755324). Based on the findings from this trial, future CHIM for schistosomiasis could investigate human responses to promising Schistosoma antigens including schistosomula antigens in endemic populations.
In conclusion, our findings show that the schistosomula antigens rSmLy6B, rSmTSP6 and rSmTSP7 induce a predominantly Th1/pro‐inflammatory response. The recombinant antigens SmLy6B, SmTSP6 and SmTSP7 might be good targets to investigate further as vaccine antigens against S. mansoni infection.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
We thank the participants of Namoni for taking part in this study. We appreciate the work done by the field team from the Vector Control Division, Uganda Ministry of Health and Kenya Medical Research Institute, Nairobi. We acknowledge the contribution of Prof. Alison M. Elliott of the MRC/UVRI and LSHTM Uganda Research Unit and Prof. Jelle J. Goeman of Leiden University Medical Centre during analysis of the data. ME was supported by a Wellcome Trust Uganda PhD Fellowship in Infection and Immunity funded by a Wellcome Trust Strategic Award (Grant no. 084344) and through the DELTAS Africa Initiative (Grant no. 107743). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (Grant no. 107743) and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. ME also received support from TheSchistoVac (Grant no. 242107) under the European Community's Seventh Framework Programme (FP7‐Health‐2009‐4.3.1‐1).
Egesa M, Lubyayi L, Tukahebwa EM, et al. Schistosoma mansoni schistosomula antigens induce Th1/Pro‐inflammatory cytokine responses. Parasite Immunol. 2018;40:e12592 10.1111/pim.12592
REFERENCES
- 1. WHO . Schistosomiasis and soil‐transmitted helminthiases number of people treated in 2016. Wkly Epidemiol Rec. 2017;92(49):749‐760. [PubMed] [Google Scholar]
- 2. Barbosa LM, Reis EA, Dos Santos CR, et al. Repeated praziquantel treatments remodel the genetic and spatial landscape of schistosomiasis risk and transmission. Int J Parasitol. 2016;46(5–6):343‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crellen T, Walker M, Lamberton PH, et al. Reduced efficacy of praziquantel against Schistosoma mansoni is associated with multiple rounds of mass drug administration. Clin Infect Dis. 2016;63(9):1151‐1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lamberton PHL, Hogan SC, Kabatereine NB, Fenwick A, Webster JP. In vitro praziquantel test capable of detecting reduced in vivo efficacy in Schistosoma mansoni human infections. Am J Trop Med Hyg. 2010;83(6):1340‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mo AX, Agosti JM, Walson JL, Hall BF, Gordon L. Schistosomiasis elimination strategies and potential role of a vaccine in achieving global health goals. Am J Trop Med Hyg. 2014;90(1):54‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ross AG, Olveda RM, Chy D, et al. Can mass drug administration lead to the sustainable control of schistosomiasis? J Infect Dis. 2015;211(2):283‐289. [DOI] [PubMed] [Google Scholar]
- 7. Fonseca CT, Oliveira SC, Alves CC. Eliminating schistosomes through vaccination: what are the best immune weapons? Front Immunol. 2015;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merrifield M, Hotez PJ, Beaumier CM, et al. Advancing a vaccine to prevent human schistosomiasis. Vaccine. 2016;34(26):2988‐2991. [DOI] [PubMed] [Google Scholar]
- 9. Gobert GN, Tran MH, Moertel L, et al. Transcriptional changes in Schistosoma mansoni during early schistosomula development and in the presence of erythrocytes. PLoS Negl Trop Dis. 2010;4(2):e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eberl M, Langermans JA, Vervenne RA, et al. Antibodies to glycans dominate the host response to schistosome larvae and eggs: is their role protective or subversive? J Infect Dis. 2001;183(8):1238‐1247. [DOI] [PubMed] [Google Scholar]
- 11. Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol. 2010;8(11):814‐826. [DOI] [PubMed] [Google Scholar]
- 12. Paveley RA, Aynsley SA, Cook PC, Turner JD, Mountford AP. Fluorescent imaging of antigen released by a skin‐invading helminth reveals differential uptake and activation profiles by antigen presenting cells. PLoS Negl Trop Dis. 2009;3(10):e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castro‐Borges W, Dowle A, Curwen RS, Thomas‐Oates J, Wilson RA. Enzymatic shaving of the tegument surface of live schistosomes for proteomic analysis: a rational approach to select vaccine candidates. PLoS Negl Trop Dis. 2011;5(3):e993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El Ridi R, Tallima H. Schistosoma mansoni ex vivo lung‐stage larvae excretory‐secretory antigens as vaccine candidates against schistosomiasis. Vaccine. 2009;27(5):666‐673. [DOI] [PubMed] [Google Scholar]
- 15. Reimers N, Homann A, Hoschler B, et al. Drug‐induced exposure of Schistosoma mansoni antigens SmCD59a and SmKK7. PLoS Negl Trop Dis. 2015;9(3):e0003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Curwen RS, Ashton PD, Sundaralingam S, Wilson RA. Identification of novel proteases and immunomodulators in the secretions of schistosome cercariae that facilitate host entry. Mol Cell Proteomics. 2006;5(5):835‐844. [DOI] [PubMed] [Google Scholar]
- 17. Chalmers IW, Fitzsimmons CM, Brown M, et al. Human IgG1 responses to surface localised Schistosoma mansoni Ly6 family members drop following praziquantel treatment. PLoS Negl Trop Dis. 2015;9(7):e0003920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parker‐Manuel SJ, Ivens AC, Dillon GP, Wilson RA. Gene expression patterns in larval Schistosoma mansoni associated with infection of the mammalian host. PLoS Negl Trop Dis. 2011;5(8):e1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fitzpatrick JM, Peak E, Perally S, et al. Anti‐schistosomal intervention targets identified by lifecycle transcriptomic analyses. PLoS Negl Trop Dis. 2009;3(11):e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Farias LP, Krautz‐Peterson G, Tararam CA, et al. On the three‐finger protein domain fold and CD59‐like proteins in Schistosoma mansoni . PLoS Negl Trop Dis. 2013;7(10):e2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farias LP, Tararam CA, Miyasato PA, et al. Screening the Schistosoma mansoni transcriptome for genes differentially expressed in the schistosomulum stage in search for vaccine candidates. Parasitol Res. 2011;108(1):123‐135. [DOI] [PubMed] [Google Scholar]
- 22. Tran MH, Freitas TC, Cooper L, et al. Suppression of mRNAs encoding tegument tetraspanins from Schistosoma mansoni results in impaired tegument turnover. PLoS Pathog. 2010;6(4):e1000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farnell EJ, Tyagi N, Ryan S, et al. Known allergen structures predict Schistosoma mansoni IgE‐binding antigens in human infection. Front Immunol. 2015;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick‐smear technique in Schistosomiasis mansoni . Rev Inst Med Trop Sao Paulo. 1972;14(6):397‐400. [PubMed] [Google Scholar]
- 25. Naus CW, Booth M, Jones FM, et al. The relationship between age, sex, egg‐count and specific antibody responses against Schistosoma mansoni antigens in a Ugandan fishing community. Trop Med Int Heal. 2003;8(6):561‐568. [DOI] [PubMed] [Google Scholar]
- 26. Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305(3):567‐580. [DOI] [PubMed] [Google Scholar]
- 27. Yoshino TP, Brown M, Wu X‐J, et al. Excreted/secreted Schistosoma mansoni venom allergen‐like 9 (SmVAL9) modulates host extracellular matrix remodelling gene expression. Int J Parasitol. 2014;44(8):551‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walker D, Jason J, Wallace K, et al. Spontaneous cytokine production and its effect on induced production. Clin Diagn Lab Immunol. 2002;9(5):1049‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Molina MA, Gamboa EM, Tello PC, et al. Spontaneous inflammatory cytokine gene expression in normal human peripheral blood mononuclear cells. Lymphat Res Biol. 2006;4(1):34‐40. [DOI] [PubMed] [Google Scholar]
- 30. Osborne JW. Improving your data transformations: applying the Box‐Cox transformation. Pract Assess Res Eval. 2010;15(12):1‐9. [Google Scholar]
- 31. Olivier J Norberg M. Positively skewed data: revisiting the box‐cox power transformation. Int J Psychol Res. 2010;3(1):68. [Google Scholar]
- 32. Goeman JJ, van de Geer SA, de Kort F, van Houwelingen HC. A global test for groups of genes: testing association with a clinical outcome. Bioinformatics. 2004;20(1):93‐99. [DOI] [PubMed] [Google Scholar]
- 33. Wilson RA, Coulson PS. Immune effector mechanisms against schistosomiasis: looking for a chink in the parasite's armour. Trends Parasitol. 2009;25:423‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou Y, Zhang H, Sun XJ, et al. Murine CD8+T cell cytotoxicity against schistosomula induced by inoculation of schistosomal 22.6/26GST coupled Sepharose 4B beads. Vaccine. 2012;30(14):2440‐2447. [DOI] [PubMed] [Google Scholar]
- 35. McWilliam H, Driguez P, Piedrafita D, McManus D, Meeusen E. Discovery of novel Schistosoma japonicum antigens using a targeted protein microarray approach. Parasit Vectors. 2014;7(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joseph S, Jones FM, Kimani G, et al. Cytokine production in whole blood cultures from a fishing community in an area of high endemicity for Schistosoma mansoni in Uganda: the differential effect of parasite worm and egg antigens. Infect Immun. 2004;72(2):728‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walter K, Fulford AJ, McBeath R, et al. Increased human IgE induced by killing Schistosoma mansoni in vivo is associated with pretreatment Th2 cytokine responsiveness to worm antigens. J Immunol. 2006;177(8):5490‐5498. [DOI] [PubMed] [Google Scholar]
- 38. Roberts M, Butterworth AE, Kimani G, et al. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993;61(12):4984‐4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cardoso FC, Pacífico RNA, Mortara RA, Oliveira SC. Human antibody responses of patients living in endemic areas for schistosomiasis to the tegumental protein Sm29 identified through genomic studies. Clin Exp Immunol. 2006;144(3):382‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brito CF, Caldas IR, Coura Filho P, Correa‐Oliveira R, Oliveira SC. CD4+ T cells of schistosomiasis naturally resistant individuals living in an endemic area produce interferon‐gamma and tumour necrosis factor‐alpha in response to the recombinant 14KDA Schistosoma mansoni fatty acid‐binding protein. Scand J Immunol. 2000;51(6):595‐601. [DOI] [PubMed] [Google Scholar]
- 41. Santini‐Oliveira M, Coler RN, Parra J, et al. Schistosomiasis vaccine candidate Sm14/GLA‐SE: phase 1 safety and immunogenicity clinical trial in healthy, male adults. Vaccine. 2016;34(4):586‐594. [DOI] [PubMed] [Google Scholar]
- 42. Wynn TA, Oswald IP, Eltoum IA, et al. Elevated expression of Th1 cytokines and nitric oxide synthase in the lungs of vaccinated mice after challenge infection with Schistosoma mansoni . J Immunol. 1994;153(11):5200‐5209. [PubMed] [Google Scholar]
- 43. Oswald IP, Eltoum I, Wynn TA, et al. Endothelial cells are activated by cytokine treatment to kill an intravascular parasite, Schistosoma mansoni, through the production of nitric oxide. Proc Natl Acad Sci USA. 1994;91(3):999‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mountford AP, Coulson PS, Pemberton RM, Smythies LE, Wilson RA. The generation of interferon‐gamma‐producing T lymphocytes in skin‐draining lymph nodes, and their recruitment to the lungs, is associated with protective immunity to Schistosoma mansoni . Immunology. 1992;75(2):250‐256. [PMC free article] [PubMed] [Google Scholar]
- 45. Cardoso FC, Macedo GC, Gava E, et al. Schistosoma mansoni tegument protein Sm29 is able to induce a Th1‐type of immune response and protection against parasite infection. PLoS Negl Trop Dis. 2008;2(10):e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pinheiro CS, Ribeiro AP, Cardoso FC, et al. A multivalent chimeric vaccine composed of Schistosoma mansoni SmTSP‐2 and Sm29 was able to induce protection against infection in mice. Parasite Immunol. 2014;36(7):303‐312. [DOI] [PubMed] [Google Scholar]
- 47. Turner JD, Meurs L, Dool P, et al. Schistosome infection is associated with enhanced whole‐blood IL‐10 secretion in response to cercarial excretory/secretory products. Parasite Immunol. 2013;35(5–6):147‐156. [DOI] [PubMed] [Google Scholar]
- 48. Hogg KG, Kumkate S, Anderson S, Mountford AP. Interleukin‐12 p40 secretion by cutaneous CD11c+ and F4/80+ cells is a major feature of the innate immune response in mice that develop Th1‐mediated protective immunity to Schistosoma mansoni . Infect Immun. 2003;71(6):3563‐3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Teixeira de Melo T, Michel de Araujo J, Do Valle Duraes F, et al. Immunization with newly transformed Schistosoma mansoni schistosomula tegument elicits tegument damage, reduction in egg and parasite burden. Parasite Immunol. 2010;32(11–12):749‐759. [DOI] [PubMed] [Google Scholar]
- 50. Hoffmann KF, James SL, Cheever AW, Wynn TA. Studies with double cytokine‐deficient mice reveal that highly polarized Th1‐ and Th2‐type cytokine and antibody responses contribute equally to vaccine‐induced immunity to Schistosoma mansoni . J Immunol. 1999;163(2):927‐938. [PubMed] [Google Scholar]
- 51. Hoffmann KF, Cheever AW, Wynn TA. IL‐10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol Am Assoc Immnol. 2000;164(12):6406‐6416. [DOI] [PubMed] [Google Scholar]
- 52. Mbow M, Larkin BM, Meurs L, et al. T‐helper 17 cells are associated with pathology in human schistosomiasis. J Infect Dis. 2013;207(1):186‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xu J, Zhang X, Guo Z, et al. Short‐chain peptides identification of scorpion Buthus martensi Karsch venom by employing high orthogonal 2D‐HPLC system and tandem mass spectrometry. Proteomics. 2012;12(19‐20):3076‐3084. [DOI] [PubMed] [Google Scholar]
- 54. Panyi G, Varga Z, Gaspar R. Ion channels and lymphocyte activation. Immunol Lett. 2004;92(1‐2):55‐66. [DOI] [PubMed] [Google Scholar]
- 55. Black CL, Mwinzi PN, Muok EM, et al. Influence of exposure history on the immunology and development of resistance to human Schistosomiasis mansoni . PLoS Negl Trop Dis. 2010;4(3):e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Etard JF, Audibert M, Dabo A. Age‐acquired resistance and predisposition to reinfection with Schistosoma haematobium after treatment with praziquantel in Mali. Am J Trop Med Hyg. 1995;52(6):549‐558. [DOI] [PubMed] [Google Scholar]
- 57. Mbanefo EC, Huy NT, Wadagni AA, Eneanya CI, Nwaorgu O, Hirayama K. Host determinants of reinfection with schistosomes in humans: a systematic review and meta‐analysis. PLoS Negl Trop Dis. 2014;8(9):e3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Logan‐Klumpler FJ, De Silva N, Boehme U, et al. GeneDB—an annotation database for pathogens. Nucleic Acids Res. 2012;40(Database issue):D98‐D108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


