Abstract
Dextromethorphan, a wildly used over-the-counter antitussive drug, is reported to have anti-inflammatory effects. Previously, we and others have demonstrated that dextromethorphan at micromolar doses displays potent hepatoprotective effects and enhances mice survival in a sepsis model. Moreover, we also observed potent anti-inflammatory and neuroprotective effects of subpicomolar concentrations of dextromethorphan in rodent primary neuron-glial cultures. The purpose of this study was to provide a proof of principle that ultralow dose dextromethorphan displays anti-inflammatory and cytoprotective effects in animal studies. Here, we report that subpico- and micromolar doses of dextromethorphan showed comparable efficacy in protecting mice from lipopolysaccharide/ D-galactosamine (LPS/GalN)-induced hepatotoxicity and mortality. Mice were given injections of dextromethorphan from 30 min before and 2, 4 hours after an injection of LPS/GalN (20 μg/600 mg/kg). Our results showed that dextromethorphan at subpicomolar doses promoted survival rate in LPS/GalN-injected mice. Ultralow dose dextromethorphan also significantly reduced serum alanine aminotransferase activity, TNF-α level and liver cell damage of endotoxemia mice. Mechanistic studies using primary liver Kupffer cell cultures revealed that subpicomolar concentrations of dextromethorphan reduced the NADPH oxidase-generated superoxide free radicals from Kupffer cells, which in turn reduced the elevation of its downstream reactive oxygen species (iROS) to relieve the oxidative stress and decreased TNF-α production in Kupffer cells. Taken together, these findings suggest a novel therapeutic concept of using ultralow doses of dextromethorphan for the intervention of sepsis or septic shock.
Keywords: Dextromethorphan, Ultra-low dose, Sepsis, Anti-inflammation, NADPH Oxidase
Introduction
Sepsis is a severe, overdrive immune response to infection, including bacteria, fungi, and viruses, but bacteria are the most common causes of sepsis. In clinics, the progression of sepsis starts from inflammation to sepsis, severe sepsis, septic shock, and to multiple organ failures, which is one of the most life-threatening illnesses among intensive care patients worldwide. Moreover, the incidence of severe sepsis and septic shock is increasing in the older or low and middle-income populations leading to increased admissions to intensive care units [1, 2]. The clinical appearance of sepsis induced by infections is highly variable. Pneumonia, abdominal infection, kidney infection, and bloodstream infections are the common sources in sepsis patients [3–5]. Infection by microorganisms triggers production of inflammatory mediators and symptoms, such as fever or hypothermia, tachycardia, tachypnea, the increase or decrease of white blood cells, and development of the cytokine storm, all of which can contribute to the development of sepsis. Sepsis often triggers multi-organ failure, including lungs, kidneys, and liver [6].
Injection of lipopolysaccharide/ D-galactosamine (LPS/GalN) has been widely used as an acute animal model for sepsis [7]. One of the main target organs affected in this model is the liver. Liver dysfunction is an early sign of sepsis, which occurs about 4 hours after mice received a single injection of lipopolysaccharide/ D-galactosamine (LPS/GalN) [8]. Accumulating evidence has shown that liver damage and dysfunction in sepsis could directly contribute to disease progression and death [3, 9, 10]. Clinical and experimental studies suggested that early liver dysfunction in patients with sepsis exhibited significantly increased mortality and poor prognosis [9]. Since an overwhelming immune response is critical in the development of sepsis, combating the inflammation might reduce liver injury and lower the mortality rate of sepsis [10].
Dextromethorphan (DM), a dextrorotatory enantiomer of the opioid agonist levorphanol, is a wildly used over-the-counter antitussive drug [11]. Our earlier reports indicate that DM displays potent anti-inflammatory and neuroprotective effects in rodent primary neuron-glial cultures and animals [8, 12]. Mechanistic studies reveal that inhibition of microglial NADPH oxidase (NOX2)-generated superoxide plays a key role in mediating DM-elicited anti-inflammatory properties [8]. We also discovered potent anti-inflammatory and hepatoprotective effects of DM in LPS/D-GalN-injected acute endotoxic mice [8] and group A streptococcal-induced sepsis mouse models [13]. Further studies in midbrain neuron-glial cell cultures reveal a bimodal dose response, both micromolar and subpicomolar concentrations of DM protect mesencephalic dopaminergic neurons from inflammation-triggered damage [14]. This ultralow action is not only a scientific curiosity, but also offers great potential therapeutic advantages due to lower toxicity or side effects in clinical uses. These results prompted us to further study the anti-inflammatory effect of ultralow dose DM in lipopolysaccharide/ D-galactoasamine (LPS/GalN)–induced sepsis in mice. We report here that ultralow doses of DM exert potent anti-inflammatory, hepatoprotective effects against LPS/GalN-induced damage and reduce mice mortality comparable to that of regular doses.
MATERIALS AND METHODS
Reagents
Lipopolysaccharide (LPS, from Escherichia coli 0111:B4), D-galactosamine (GalN), dextromethorphan (DM) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in normal saline. Cell culture ingredients were obtained from Life Technologies (Grand Island, NY, USA).2´7-Dichlorofluorescin diacetate (DCFH-DA) was obtained from Calbiochem (La Jolla, CA, USA). WST-1 was purchased from Dojindo Laboratories (Gaithersburg, MD, USA). TNF-α enzyme-linked immunosorbent assay (ELISA) kit was obtained from R&D Systems (Minneapolis, MN, USA).
Animals
All mouse experiment protocols were approved by the Animal Care and Use Committee at the National Institute of Environmental Health Sciences and were performed in accordance with the National Institutes of Health guidelines. Six week old male CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and maintained at our institute’s laboratory animal center for 2 weeks prior to experiments.
Treatments and sample collection
Mice were fasted for 12 hours and then injected with LPS/GalN at the dose of 20 μg/600 mg/kg via intraperitoneal (i.p.) route. DM was injected subcutaneously (s.c.) to mice with doses ranging from 10 mg/kg to 10 pg/kg at 30 minutes before, and 2 and 4 hours after LPS/GalN injection. Control mice received the same volume of normal saline. Mice were sacrificed, blood and liver were collected for further analysis. LPS/GalN has been primarily used as a mouse model for acute sepsis. About 40 % of mice injected with LPS/GalN died within 12 hours. In addition, the cytokines and liver enzyme changes peaked at early hours after LPS/GalN injection. Thus, most measurements were performed at 6.5 hr or earlier timepoints after toxin injection.
Evaluation of hepatotoxicity
The activity of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and sorbitol dehydrogenase (SDH) were used as an indicator of hepatocellular damage. Blood was collected from the retro-orbital vein of the eye under anesthetization, stored at 4 °C overnight, and then centrifuged at 1,500 Xg at 4 °C for 5 minutes. Serum was collected and stored at −70 °C prior to analysis. We determined the levels of ALT by using a detection kit from Sigma-Aldrich (St. Louis, MO, USA). AST detection kit was purchased from Beckman Coulter (Melville, NY, USA). SDH detection kit was purchased from Sekisui Diagnostics (Framingham, MA, USA). Both AST and SDH were measured using the Olympus AU400e clinical analyzer Beckman Coulter (Irving, TX, USA). A portion of liver was fixed in 4% neutral formaldehyde, processed, and stained with hematoxylin and eosin (H&E) to examine liver injury by morphological changes.
TNF-α assay
The levels of TNF- α in the serum, and Kupffer cell cultures were determined with a TNF- α ELISA kit following the manufacturer’s instructions.
Kupffer cell culture
Kupffer cells were isolated from CD-1 mice by collagenase digestion and differential centrifugation using Percoll (Pharmacia, Uppsala, Sweden) as described previously with slight modifications [15]. Briefly, after pentobarbital anesthesia, the liver was perfused with Ca2+- and Mg2+-free Hanks’ balanced salt solution (HBSS) at 37 °C for 5 minutes at a flow rate of 13 ml/min. Additional perfusion with HBSS containing 0.05% collagenase IV (Sigma, St. Louis, MO, USA) was performed at 37 °C for 5 minutes to dissociate the liver tissue. The liver was then excised and cut into small pieces in collagenase containing buffer. To remove parenchymal cells, the liver-collagenase mixture was collected and filtered through Nylon gauze mesh and centrifuged at 50 Xg for 3 minutes. The nonparenchymal cell fraction was spun at 450 Xg for 10 min at 4 °C. Cells were centrifuged on a density cushion of 50% of Percoll at 1000 Xg for 15 min and the Kupffer cell fraction was collected and washed. The viability of cells determined by trypan blue exclusion was > 90%. Cells were seeded in 24-well culture plates and cultured in RPMI 1640 media supplemented with 10% fetal bovine serum and antibiotics (100 U/ml penicillin G and 100 μg/ml streptomycin sulfate) at 37 °C with 5% CO2. Non-adherent cells were removed after 2 hours by replacing media and cells were cultured for 24 h before experiments.
Measurement of extracellular superoxide and intracellular reactive oxygen species (iROS) production
Extracellular superoxide production was determined by WST-1 assay as reported previously [16]. In this study, Kupffer cells (1 × 105 cells/well) were plated in 96-well plate, cultured in medium with 10% FBS for 2 hours. After incubation, cells were washed twice with HBSS and pre-treated with DM (10−5, 10−10, 10−13, or 10−14 M) for 30 minutes. Subsequently, 50 μl of SOD (50 U/ml), 50 μl of WST-1 (1 mM), and 50 μl of vehicle or LPS (10 ng/ml) in HBSS were added into each well. These cultures were then read immediately using a spectrophotometer with absorbance at wavelength of 550 nm. The amount of superoxide was calculated by subtracting the absorbance of the SOD added group, and the vehicle-treated group was set as 100%.
The levels of intracellular ROS were determined by DCFH oxidation, as described previously [17]. DCFH-DA (1 μM) was added into the cultures and incubated at 37 °C for 30 minutes. The cells were pre-treated with or without DM for 30 minutes. Subsequently, LPS was added and incubated for an additional 90 minutes. After incubation, the fluorescence was read with excitation at 485 nm and emissions at 530 nm using a fluorescence plate reader.
Statistics
The data was expressed as the mean ± SEM and statistical significance was assessed with an analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons. P < 0.05 was considered statistically significant.
Results
Ultralow doses of DM ameliorate LPS/GalN-induced hepatotoxicity
A wide range of dose-response studies was performed to determine the efficacy of the protective effect of DM in sepsis-mediated hepatotoxicity. We treated LPS/GalN-injected mice with DM ranging from 10 pg/kg to 10 mg/kg with serial10-fold increments. The experimental design and dosing procedures were described in Figure 1A. Mice were pretreated with DM 30 minutes before receiving an injection of LPS/GalN (20 μg/600 mg/kg). Supplemental doses of DM were given 2 and 4 hours after initial LPS/GalN injection. At 6.5 hours after LPS/GalN injection, serum was collected for the measurement of alanine aminotransferase (ALT), an acute liver injury indicator. As shown in Figure 1B, injection of LPS/GalN greatly increases the serum ALT level compared to the saline-treated group, indicating serious liver injuries. The most striking finding was that DM in a range of 100 pg/kg – 10 ng/kg, which is more than one million-fold less than the regular doses, showed similar efficacy to that at 10 mg/kg dosage in the reduction of serum ALT levels. It is interesting to note the dose-response curve displays a bimodal pattern; doses of DM between 100 ng/kg to 1 mg/kg showed no significant protective effect (Fig. 1B). Based on this finding, we have chosen a dose of 1ng/kg to further study the protective effect of ultralow dose DM on the other liver toxicity parameters. Comparable to the effect on ALT, serum levels of aspartate aminotransferase (AST) and sorbitol dehydrogenase (SDH) at 6.5 hours after LPS/GalN injection were significantly attenuated with the treatment of DM (1 ng/kg) (Figure 1C and 1D).
Figure 1.
Serum enzyme levels in LPS/GalN-injected mice treated with various doses of DM. (A) The time points that mice were injected with LPS/GalN (20 μg/600 mg/kg, i.p.) and treated with DM. (B) Liver injury was evaluated by serum ALT activity at 6.5 hours after LPS/GalN injection. The serum ALT of LPS/GalN alone group was set as 100%.
Data show mean ± SEM of 10–20 mice from at least 3 independent experiments. *P < 0.05, Bonferroni t-test compared to LPS/GalN group. The serum (C) AST and (D) SDH levels at 6.5 hours after LPS/GalN injection. Data show mean ± SEM of 10 mice per group from at least 3 independent experiments. *P < 0.05, post hoc analysis by Bonferroni t-test.
Histological analysis of liver damage in LPS/GalN-injected mice treated with an ultralow dose of DM
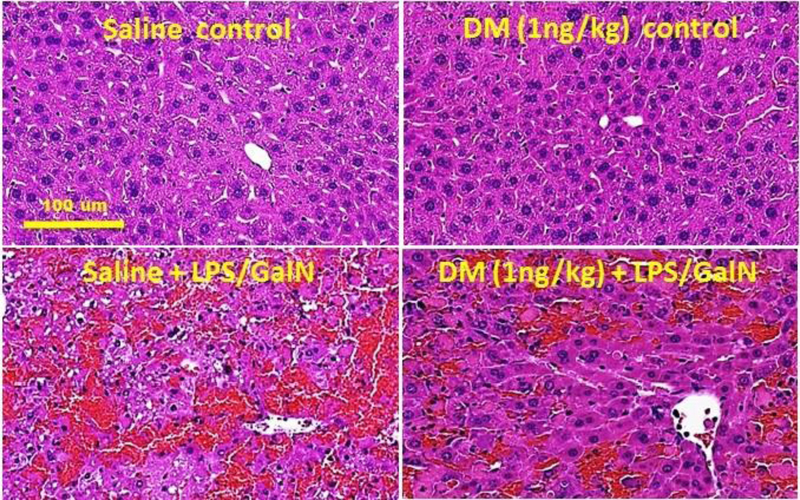
The protective effect of an ultralow dose of DM (1 ng/kg) on LPS/GalN-induced liver injury was assessed by histopathological analysis. As shown in Figure 2, severe liver damage shown as hemorrhaging, cell necrosis, and neutrophil infiltration was observed 6.5 hours after LPS/GalN injection. The liver damage is significantly ameliorated in mice treated with 1ng/kg of DM. These results are consistent with biochemical measurements of liver enzyme markers (Fig. 1) demonstrating potent hepatoprotective effects of DM at an ultralow dose against LPS/GalN-induced hepatotoxicity.
Figure 2.
Representative histological analysis of liver damage in LPS/GalN-injected mice treated with different doses of DM. Sections of livers from mice injected with saline, DM (1 ng/kg) alone, LPS/GalN alone, or DM + LPS/GalN. N=5, Scale bar: 100 μm.
Ultralow dose of DM reduced TNF-α production in serum
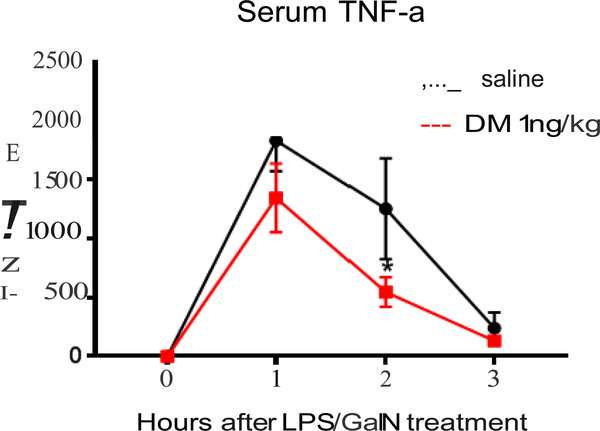
Inflammatory cytokines, such as TNF-α, have been reported to play important roles in acute liver injury induced by LPS/GalN injection in rodent models [18, 19]. To determine whether the hepatoprotective effect of DM is related to the inhibition of TNF-α release, mice were pre-treated with DM with a dose of 1 ng/for 30 minutes before LPS/GalN injection, then the serum was collected at 1,2 and 3 hours after LPS/GalN injection. As shown in Fig. 3, pre-treatment of ultralow DM reduced about 20% of TNF-α at one hour and more than 50% at two hours after LPS/GalN injection. Since liver Kupffer cells are known to be the major source of TNF-α production in LPS/GalN-injected mice, these results suggest that the reduction of serum TNF-α levels might be closely related to the hepatoprotective effects of the ultralow dose of DM.
Figure 3.
Effects of femtomolar doses of DM on TNF-α production in the serum. Mice were pre-treated with or without 1 ng/kg of DM 30 minutes before LPS/GalN (20 μg/600 mg/kg, i.p.). The level of TNF-α was determined at 1, 2, and 3 hours post-injection by TNF-α ELISA kit. Data show mean ± SEM of total 10 mice from at least 3 independent experiments. *P < 0.05, post hoc analysis by Bonferroni t-test, indicates significant different from the LPS/GalN alone group.
Ultralow dose of DM reduced pro-inflammatory factors production in LPS-stimulated Kupffer cells
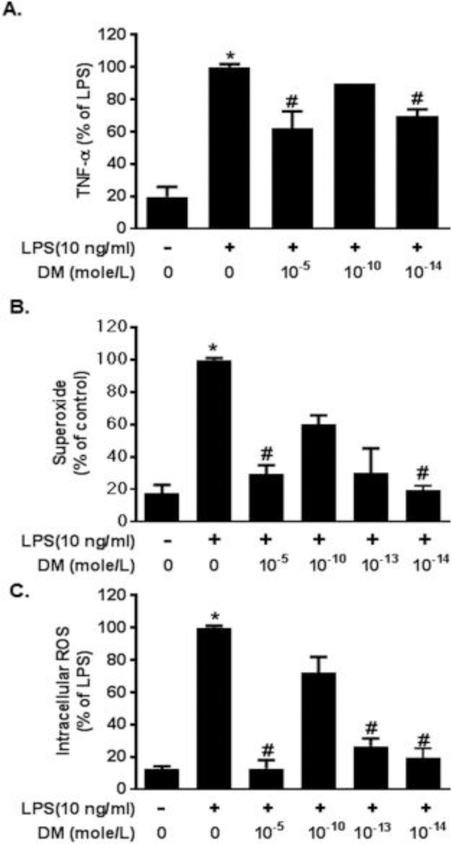
To further decipher the molecular mechanism underlying the hepatoprotective effects of ultralow DM, we isolated Kupffer cells, the major macrophages in the liver, and determined the anti-inflammatory effects of DM at various concentrations on the release of various inflammatory mediators such as TNF-α, superoxide radicals, and intracellular reactive oxygen species(iROS) after LPS treatment. In this series of experiments, only LPS was used, because combined LPS/GalN was too toxic for the cells in culture. First, we measured the effect of subpicomolar concentration of DM on TNF-α production in Kupffer cells treated with LPS. Isolated Kupffer cells were pretreated with three concentrations of DM (10−5,10−10 or 10−14 M) for 30 minutes before adding LPS (10 ng/ml). Six hours after LPS treatment, culture supernatant TNF-α levels were measured. Results showed that the TNF-α level was significantly reduced by 10−5 M and 10−14 M, but not by10−10 M of DM (Figure 4A). These results indicated that DM can suppress TNF-α production in Kupffer cells with either higher (10−5 M) or ultralow (10−14 M) concentrations. Second, we determined the level of superoxide and intracellular ROS (iROS) production in Kupffer cells. Kupffer cells were pretreated with various concentrations of DM for 30 minutes. The production of superoxide and iROS was measured at 30 minutes following the addition of 10 ng/ml LPS. Similar to the bimodal dose-response shown in the in vivo study (Fig.1), both 10−5 M and 10−13 or10−14 M concentrations of DM completely prevented LPS-induced increase in the productions of both superoxide (Fig. 4B) and iROS (Fig. 4C). By contrast, DM in 10−10 M was much less potent in inhibiting the production of these superoxide free radicals (Fig. 4B, C). Taken together, these results indicate that one millionth of a regular dose of DM can exert potent anti-inflammatory and hepatoprotective effects both in mice and Kupffer cell cultures.
Figure 4.
Ultralow concentrations of DM reduced pro-inflammatory factors production in LPS-stimulated Kupffer cells. Kupffer cells ere pre-treated with DM (10−5, 10−10, or 10−14 M) or vehicle for 30 minutes and then incubated with LPS (10 ng/ml). (A) TNF-α level.
Supernatant were collected from Kupffer cells with different treatments and determined TNF-α production by TNF-α ELISA kit. Femtomolar concentrations of DM reduced (B) superoxide and (C) iROS expression in LPS-stimulated Kupffer cells. Kupffer cells are pre-treated with DM (10−5, 10−10, 10−13, or 10−14 M) or vehicle for 30 minutes and then incubated with LPS (10 ng/ml). After incubation, the cultures were subjected to superoxide and iROS assays. Data show mean ± SEM from 3 independent experiments with 3 replicates per experiment. *P < 0.05, post hoc analysis by Bonferroni t-test, indicates significant different from control group. # P < 0.05, post hoc analysis by Bonferroni t-test, indicates significant different from LPS alone group.
Ultralow doses of DM reduced LPS/GalN-induced mortality
The acute liver injury induced by LPS/GalN injection can result in high mortality in the short term [3, 20]. To evaluate whether ultralow doses of DM could increase the survival rate of LPS/GalN-injected mice, we chose 10 mg/kg, 1 μg/kg and 100 pg/kg DM to determine the survival rate in each group within 12 hours duration after injection. The survival rate of LPS/GalN vehicle-group is 57.8%, pretreatment with DM 10 mg/kg and 100 pg/kg significantly increased the survival rate to 87.2% and 70.0% respectively, but 1 μg/kg of DM had no protective effect (55.6%) (Table 1.). These results clearly indicate that the ultralow dose as well as the regular dose of DM can effectively protect mice from LPS/GalN-induced mortality.
Table 1.
Survival rates of LPS/GalN-injected mice treated with DM or vehicle
| Dextromethorphan | Total animal number | Survival animal number | Survival rate (%) |
|---|---|---|---|
| 0 | 64 | 37 | 57.8 |
| 10 mg/kg | 47 | 41 | 87.2* |
| 1 μg/kg | 27 | 15 | 55.6 |
| 100 pg/kg | 80 | 56 | 70.0* |
Mice were pretreated DM with indicated doses at 30 minutes before LPS/GalN injection. The survival rate was recorded at 12 hours post-injection.
P < 0.05 compared to LPS/GalN alone group.
Discussion
This report is the first in vivo study demonstrating potent protection of mice from LPS/GalN-induced acute hepatotoxicity and mortality by ultralow doses of DM. We showed that ultralow doses of DM greatly reduced LPS/GalN-induced increases in cytokine levels. The potent anti-inflammatory effects of ultralow DM play a key role in hepatoprotection as evidenced by reduced levels of ALT, AST, SDH, and neutrophil infiltration. Mechanistic studies revealed that both micromolar and subpicomolar DM inhibited LPS-induced Kupffer cell activation and subsequently reduced production of inflammatory factors such as TNF-α, superoxide and reactive oxygen species. Taken together, the finding of ultralow DM provides a novel strategy for developing anti-inflammatory therapy due to less side effects/toxicities.
We tested a wide range of doses of DM and found a very interesting W-shape bimodal curve in serum ALT level (Fig.1B). DM protects LPS/GalN-induced mortality and liver damage only at micromolar and subpicomolar doses, but not at nanomolar or picomolar doses. This bimodal dose-response phenomenon has been previously described [14], but has not been mechanistically investigated in great depth. In addition to DM, we and others reported other agents exhibiting subpicomolar-acting neuroprotective or anti-inflammatory properties, such as dynorphin [21, 22], dynorphin-related opioid peptide (Gly-GLy-Phe; GGF) [23], NAP (davunetide, an eight amino acid peptide; NAPVSIPQ) [24, 25], and diphenyleneiodonium (DPI) [26, 27]. However, most of these reports are based on in vitro studies. One study using minipump infusion with ultralow dose of diphenyleneiodonium showed potent anti-inflammatory and neuroprotectic effects in neuroinflammation-mediated neurodegeneration mouse model [26]. Thus, it is critical to document whether this bimodal dose-response also occur in animal or human studies.
Our previous studies indicate that one of the major sites of action mediating the anti-inflammatory effects of micromolar concentrations of DM is NADPH oxidase (NOX2) in brain microglia or liver Kupffer cells [8]. Inhibition of NOX2 reduces production of superoxide and its related iROS, which leads to the decreased expression of a variety of proinflammatory genes such as TNF-α and then the subsequent production of these cytokines [8, 14]. The critical role of NOX2 in mediating DM-elicited anti-inflammatory and protective effects was shown by the failure of DM to reduce LPS-induced TNF-α production in Kupffer cells from NOX2-deficient (PHOX−/−) mice [8].
To determine whether NOX2 is also involved in the anti-inflammatory and protective effects of ultralow DM, we compared the potency of both micromolar and subpicomolar DM on LPS-induced production of superoxide and iROS using cultured Kupffer cells. Interestingly, we observed equipotent effects by both concentrations of DM in this bimodal dose-response. It is intriguing to observe DM in the middle part of the dose-response curve (10−10 M) was much less effective in inhibiting LPS-induced increases in both superoxide and iROS production. Presently, the mechanisms of the weaker protective effect DM at nanomolar and picomolar concentrations are still unknown. One potential explanation is that DM binds to multiple sites of NOX2 with different affinities; a high-affinity site (Ki around subpicomolar) and a low-affinity site (Ki around micromolar). DM at nanomolar concentrations exert weaker potency, which could be attributed to the fact that DM at this concentration over-saturates the high-affinity site of NOX2 and reduces the binding of DM to this high-affinity site. This speculation is an analogy to so called “substrate inhibition” [28]. A more direct approach to test this possibility is to perform binding assay using label DM on microglial cell membranes which contains the catalytic subunit of NOX2 (pg91phox). Unfortunately, the specific activity of labeled DM is not high enough to permit the binding experiment at sub-picomolar concentrations.
Roles of a variety of pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8, IL-10 and IFN-γ and reactive oxygen species like superoxide and iROS, in the pathogenesis of sepsis have been intensively studied [10, 29, 30]. These studies have provided evidence to develop immunotherapies to abrogate these immune factors to protect mice from sepsis-mediated lethality. However, the efficacy of immunotherapy in sepsis aimed to abrogate certain immune factors, such as TNF-α, remains controversial in clinical trials [10, 30, 31]. Our study offers a novel approach by using ultralow DM, which selectively targets NOX2. Moreover, findings from this study are particularly significant from both pharmacological and therapeutic viewpoints: 1) Our study demonstrates that ultralow DM exhibits high anti-inflammatory and hepatoprotective efficacies not only in cell cultures, but also in animal study. This is the first proof of principle suggesting a potential use of ultralow DM in various inflammation-related therapies. 2) DM has been widely used as an antitussive drug with an excellent record of safety; however, micromolar DM is known to act on many sites such as NMDA receptor or sigma 1 receptor and produce complex pharmacological actions [32]. Ultralow concentrations of DM are likely to be selective in targeting NOX2 since very few molecules/receptors exhibit subpicomolar affinity in binding to DM. This unique selectivity not only makes DM even safer, but also offers great advantages in designing other ultralow therapies for other diseases. 3) Clinically, antibiotics and supportive care (including oxygen and large amounts of intravenous fluids) are the most common practice in the treatment of infection-related septic patients. Since LPS or other endotoxins from bacteria are the main cause of sepsis, it is conceivable to design clinical trials by a combined use of antibiotics and ultralow DM for either prevention or therapy in sepsis-prone patients.
In summary, this study demonstrated that ultralow doses of DM elicited potent anti-inflammatory/hepatoprotective effects through the inhibition of overactivated NOX2 activity in Kupffer cells. These results provide new insights into the potential therapeutic applications of DM in sepsis patients.
Highlights.
This study provides the anti-inflammatory and hepatoprotective effect of Dextromethorphan (DM) at ultra-low dose.
There are no potential side effects of ultra-low dose of DM.
The ultralow dose of DM inhibits NOX2-generated superoxide production from Kupffer cells in the liver.
Acknowledgements
We thank Mr. Ralph Wilson from the Molecular Pathology Core at NIEHS for the help of liver injury index measurement.
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, USA; as well as grants from the National Natural Science Foundation of China (81270144 and 81570844 to J.F.), and the National Key Technology R&D Program (2015BAI12B11 to J.F.).
Footnotes
Conflicts of interests
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nasa P, Juneja D, Singh O, Severe sepsis and septic shock in the elderly: An overview, World J Crit Care Med 1(1) (2012) 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dondorp AM, Haniffa R, Critical care and severe sepsis in resource poor settings, Trans R Soc Trop Med Hyg 108(8) (2014) 453–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Minemura M, Tajiri K, Shimizu Y, Liver involvement in systemic infection, World J Hepatol 6(9) (2014) 632–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Angus DC, van der Poll T, Severe sepsis and septic shock, N Engl J Med 369(9) (2013) 840–51. [DOI] [PubMed] [Google Scholar]
- [5].Wang T, Derhovanessian A, De Cruz S, Belperio JA, Deng JC, Hoo GS, Subsequent infections in survivors of sepsis: epidemiology and outcomes, J Intensive Care Med 29(2) (2014) 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lelubre C, Vincent JL, Mechanisms and treatment of organ failure in sepsis, Nat Rev Nephrol 14(7) (2018) 417–427. [DOI] [PubMed] [Google Scholar]
- [7].Buras JA, Holzmann B, Sitkovsky M, Animal models of sepsis: setting the stage, Nat Rev Drug Discov 4(10) (2005) 854–65. [DOI] [PubMed] [Google Scholar]
- [8].Li G, Liu Y, Tzeng NS, Cui G, Block ML, Wilson B, Qin L, Wang T, Liu B, Liu J, Hong JS, Protective effect of dextromethorphan against endotoxic shock in mice, Biochemical pharmacology 69(2) (2005) 233–40. [DOI] [PubMed] [Google Scholar]
- [9].Kramer L, Jordan B, Druml W, Bauer P, Metnitz PG, Austrian ASG Epidemiologic Study on Intensive Care, Incidence and prognosis of early hepatic dysfunction in critically ill patients--a prospective multicenter study, Crit Care Med 35(4) (2007) 1099–104. [DOI] [PubMed] [Google Scholar]
- [10].van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG, The immunopathology of sepsis and potential therapeutic targets, Nat Rev Immunol 17(7) (2017) 407–420. [DOI] [PubMed] [Google Scholar]
- [11].Ang ET, Wong PT, Moochhala S, Ng YK, Cytokine changes in the horizontal diagonal band of Broca in the septum after running and stroke: a correlation to glial activation, Neuroscience 129(2) (2004) 337–47. [DOI] [PubMed] [Google Scholar]
- [12].Liu Y, Qin L, Li G, Zhang W, An L, Liu B, Hong JS, Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation, The Journal of pharmacology and experimental therapeutics 305(1) (2003) 212–8. [DOI] [PubMed] [Google Scholar]
- [13].Li MH, Luo YH, Lin CF, Chang YT, Lu SL, Kuo CF, Hong JS, Lin YS, Dextromethorphan efficiently increases bactericidal activity, attenuates inflammatory responses, and prevents group a streptococcal sepsis, Antimicrobial agents and chemotherapy 55(3) (2011) 967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li G, Cui G, Tzeng NS, Wei SJ, Wang T, Block ML, Hong JS, Femtomolar concentrations of dextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 19(6) (2005) 489–96. [DOI] [PubMed] [Google Scholar]
- [15].Valatas V, Xidakis C, Roumpaki H, Kolios G, Kouroumalis EA, Isolation of rat Kupffer cells: a combined methodology for highly purified primary cultures, Cell Biol Int 27(1) (2003) 67–73. [DOI] [PubMed] [Google Scholar]
- [16].Liu B, Du L, Kong LY, Hudson PM, Wilson BC, Chang RC, Abel HH, Hong JS, Reduction by naloxone of lipopolysaccharide-induced neurotoxicity in mouse cortical neuron-glia co-cultures, Neuroscience 97(4) (2000) 749–56. [DOI] [PubMed] [Google Scholar]
- [17].Liu J, Shen HM, Ong CN, Role of intracellular thiol depletion, mitochondrial dysfunction and reactive oxygen species in Salvia miltiorrhiza-induced apoptosis in human hepatoma HepG2 cells, Life Sci 69(16) (2001) 1833–50. [DOI] [PubMed] [Google Scholar]
- [18].Hesse DG, Tracey KJ, Fong Y, Manogue KR, Palladino MA Jr., Cerami A, Shires GT, Lowry SF, Cytokine appearance in human endotoxemia and primate bacteremia, Surg Gynecol Obstet 166(2) (1988) 147–53. [PubMed] [Google Scholar]
- [19].Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A, Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models, Am J Pathol 146(5) (1995) 1220–34. [PMC free article] [PubMed] [Google Scholar]
- [20].Yan J, Li S, Li S, The role of the liver in sepsis, Int Rev Immunol 33(6) (2014) 498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu B, Qin L, Yang SN, Wilson BC, Liu Y, Hong JS, Femtomolar concentrations of dynorphins protect rat mesencephalic dopaminergic neurons against inflammatory damage, J Pharmacol Exp Ther 298(3) (2001) 1133–41. [PubMed] [Google Scholar]
- [22].Baskin DS, Hosobuchi Y, Loh HH, Lee NM, Dynorphin(1–13) improves survival in cats with focal cerebral ischaemia, Nature 312(5994) (1984) 551–2. [DOI] [PubMed] [Google Scholar]
- [23].Qin L, Block ML, Liu Y, Bienstock RJ, Pei Z, Zhang W, Wu X, Wilson B, Burka T, Hong JS, Microglial NADPH oxidase is a novel target for femtomolar neuroprotection against oxidative stress, FASEB journal : official publication of the Federation of American Societies for Experimental Biology 19(6) (2005) 550–7. [DOI] [PubMed] [Google Scholar]
- [24].Leker RR, Teichner A, Grigoriadis N, Ovadia H, Brenneman DE, Fridkin M, Giladi E, Romano J, Gozes I, NAP, a femtomolar-acting peptide, protects the brain against ischemic injury by reducing apoptotic death, Stroke 33(4) (2002) 1085–92. [DOI] [PubMed] [Google Scholar]
- [25].Zemlyak I, Manley N, Sapolsky R, Gozes I, NAP protects hippocampal neurons against multiple toxins, Peptides 28(10) (2007) 2004–8. [DOI] [PubMed] [Google Scholar]
- [26].Wang Q, Qian L, Chen SH, Chu CH, Wilson B, Oyarzabal E, Ali S, Robinson B, Rao D, Hong JS, Post-treatment with an ultra-low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson’s disease models, Brain 138(Pt 5) (2015) 1247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang Q, Chu CH, Oyarzabal E, Jiang L, Chen SH, Wilson B, Qian L, Hong JS, Subpicomolar diphenyleneiodonium inhibits microglial NADPH oxidase with high specificity and shows great potential as a therapeutic agent for neurodegenerative diseases, Glia 62(12) (2014) 2034–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reed MC, Lieb A, Nijhout HF, The biological significance of substrate inhibition: a mechanism with diverse functions, Bioessays 32(5) (2010) 422–9. [DOI] [PubMed] [Google Scholar]
- [29].Schulte W, Bernhagen J, Bucala R, Cytokines in sepsis: potent immunoregulators and potential therapeutic targets--an updated view, Mediators Inflamm 2013 (2013) 165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Deutschman CS, Tracey KJ, Sepsis: current dogma and new perspectives, Immunity 40(4) (2014) 463–75. [DOI] [PubMed] [Google Scholar]
- [31].Vincent JL, Sun Q, Dubois MJ, Clinical trials of immunomodulatory therapies in severe sepsis and septic shock, Clin Infect Dis 34(8) (2002) 1084–93. [DOI] [PubMed] [Google Scholar]
- [32].Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR, Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta(R)) clinical use, Pharmacol Ther 164 (2016) 170–82. [DOI] [PubMed] [Google Scholar]