Abstract
Theory of mind (i.e., the ability to infer others’ mental states) – a fundamental social cognitive ability – declines with increasing age. Prior investigations have focused on identifying task-evoked differences in neural activation that underlie these performance declines. However, these declines could also be related to dysregulation of the baseline, or ‘intrinsic’, functional connectivity of the brain. If so, age differences in intrinsic connectivity may provide novel insight into the mechanisms that contribute to poorer theory of mind in older adults. To examine this possibility, we assessed younger and older adults’ theory of mind while they underwent task-based fMRI, as well as the intrinsic functional connectivity measured during resting-state within the (task-defined) theory of mind network. Older adults exhibited poorer theory of mind behavioral performance and weaker intrinsic connectivity within this network compared to younger adults. Intrinsic connectivity between the right temporoparietal junction and the right temporal pole mediated age differences in theory of mind. Specifically, older adults had weaker intrinsic connectivity between right temporoparietal junction and right temporal pole that explained their poorer theory of mind behavioral performance. These findings broaden our understanding of aging and social cognition and reveal more specific mechanisms of how aging impacts theory of mind.
Keywords: aging, theory of mind, default mode network, resting-state, functional magnetic resonance imaging, functional connectivity
Social interactions are a fundamental human need (Baumeister & Leary, 1995). An inability to develop and maintain social relationships has myriad negative repercussions, including increased loneliness (Bailey, Henry, & Von Hippel, 2008; Cacioppo, Capitanio, & Cacioppo, 2014). Loneliness is particularly prevalent among older adults (OA; individuals over the age of 60), and is associated with pernicious outcomes (Perissinotto, Stijacic Cenzer, & Covinsky, 2012). For example, OA who experience loneliness have higher mortality rates (Luo, Hawkley, Waite, & Cacioppo, 2012), poorer mental and physical health (Cornwell & Waite, 2009), and increased stress (Adam, Hawkley, Kudielka, & Cacioppo, 2006). Loneliness may also exacerbate cognitive decline in pathological aging (e.g., Alzheimer’s disease). For example, one longitudinal study on aging found that more loneliness among OA was associated with both increased risk for developing Alzheimer’s disease and greater cognitive decline (Wilson et al., 2007). One reason why OA might be especially susceptible to loneliness and its negative outcomes is that healthy and pathological aging are associated with declines in the basic social cognitive functions (e.g., understanding others’ mental states) that allow OA to develop and maintain social relationships (Bora, Walterfang, & Velakoulis, 2015; Moran, 2013). Despite these well-documented findings, relatively few neuroimaging studies have attempted to elucidate the mechanisms underlying age differences in social cognition (but see Castle et al., 2012; Moran, Jolly, & Mitchell, 2012). The goal of the current work was to provide novel insight into a mechanism for age deficits in a fundamental social cognitive ability: theory of mind (i.e., understanding others’ mental states; Frith & Frith, 2001; Premack & Woodruff, 1978).
Extant work examining the mechanisms underlying age deficits in social cognition has focused on identifying the brain regions that are differentially engaged by younger adults (YA; 18-35 years old) and OA when they perform specific social cognitive tasks. While this work has been important for identifying the specific brain regions that are impacted by age, it rests on the assumption that age deficits in social cognition stem from differences in the extent to which certain brain regions are engaged during social cognitive tasks. However, an alternate possibility is that aging may also disrupt how brain regions communicate even when no explicit tasks are being performed (i.e., at a baseline state of the brain). This question has the important theoretical contribution that it will provide insight into the previously observed age differences in task-evoked neural activation by raising the possibility that these differences could be due, at least in part, to baseline dysfunction in brain connectivity. Baseline communication between brain regions can be quantified by measuring intrinsic functional connectivity, which is the coactivation between pairs of brain regions or brain networks during resting-state (i.e., when participants are engaged in undirected thought; Greicius, Krasnow, Reiss, & Menon, 2003; Meindl et al., 2009). Resting-state is proposed to reflect a baseline state of the brain because the patterns of intrinsic connectivity at rest are highly present across a variety of brain states (Cole, Bassett, Power, Braver, & Petersen, 2014). Because intrinsic connectivity is related to task-evoked activations (Cole, Ito, Bassett, & Schultz, 2016), examining whether age differences in intrinsic connectivity predict age deficits in theory of mind is a natural extension of prior work that has characterized age differences in task-evoked brain activation (Moran et al., 2012).
Several studies have demonstrated that aging is associated with weaker intrinsic connectivity within numerous resting-state brain networks, notably the Default Mode Network (DMN; Andrews-Hanna et al., 2007; Bertolero, Yeo, & D’Esposito, 2015; Campbell, Grigg, Saverino, Churchill, & Grady, 2013; Damoiseaux et al., 2008; Geerligs, Renken, Saliasi, Maurits, & Lorist, 2015; Hampson, Driesen, Skudlarski, Gore, & Constable, 2006; Meunier, Achard, Morcom, & Bullmore, 2009; Onoda & Yamaguchi, 2013). The DMN is notable because its weaker intrinsic connectivity in healthy and, to a greater extent, pathological aging may be related to the susceptibility of regions within this network to exhibit amyloid-β deposition associated with Alzheimer’s disease (Hafkemeijer, van der Grond, & Rombouts, 2012). Weaker intrinsic connectivity may reflect relative dysfunction because cohesive networks – that is, networks with strong internal connectivity that are only weakly connected to other networks – are a hallmark feature of the underlying functional architecture of the brain (Sporns & Betzel, 2016). Because distinct networks support different cognitive functions (Stevens & Spreng, 2014), stronger intrinsic connectivity within particular brain networks is associated with their better functional specialization (Bertolero et al., 2015). For example, weaker connectivity within major brain networks predicted several age deficits in cognition (e.g., executive function, processing speed, and working memory; Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Hampson et al., 2006; for a review, see Ferreira & Busatto, 2013).
The current work focused on the within-network connectivity of the DMN. We chose to focus on the DMN because it was the most theoretically-relevant candidate network for this investigation given that it has a common neural basis with social cognition and, in particular, theory of mind (Mars et al., 2012; Schilbach, Eickhoff, Rotarska-Jagiela, Fink, & Vogeley, 2008; Spreng & Grady, 2010). Specifically, the DMN includes the medial prefrontal cortex (mPFC), precuneus, bilateral TPJ, and bilateral temporal poles (TP), which have all been shown to play prominent roles in theory of mind (Molenberghs, Johnson, Henry, & Mattingley, 2016; Moran et al., 2012; Schurz, Radua, Aichhorn, Richlan, & Perner, 2014). If age deficits in theory of mind are related, at least in part, to weakened intrinsic connectivity then age differences should emerge within the DMN. Thus, the first goal of this work was to examine whether age-related declines in theory of mind arise because of OAs’ overall weaker intrinsic connectivity in the DMN.
Because brain networks are complex (Sporns, 2013), the intrinsic connectivity of resting-state networks can be described at multiple levels. Network neuroscience is an integrative approach to describe networks of the brain and their components, such as brain regions (nodes) and connections between regions (edges; Sporns, 2013). Edges are commonly quantified by the correlation (i.e., functional connectivity) in brain activity between two nodes at rest. Much work on how aging affects intrinsic connectivity has examined relatively global network connectivity (i.e., networks comprising many brain regions and thus edges). Yet, aging may affect some specific edges within networks more than others. Supporting this possibility, OA versus YA have weaker intrinsic connectivity of a DMN subsystem including right temporoparietal junction (rTPJ), but not weaker connectivity within a DMN subsystem including medial temporal lobe structures (Campbell et al., 2013). Further supporting this possibility, the connectivity of only certain edges within the DMN affects cognition (e.g., recall and prospection; Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010). Together, these findings suggest that beyond effects of global network function, edges within networks may specifically predict age differences in cognition. Thus, the second goal of this work was to examine how multiple levels of intrinsic connectivity relate to age differences in social cognition.
To address these goals in the current work, we examined whether the intrinsic connectivity of the DMN or more specific components of the DMN related to age differences in theory of mind. YA and OA completed a resting-state scan and a task-based scan widely used to localize brain regions involved in theory of mind (Saxe & Kanwisher, 2003; Zaitchik, 1990). Although the task-evoked neural activations related to theory of mind are known (Molenberghs et al., 2016; Schurz et al., 2014), the critical benefit of using the localizer was that we were able to define in the same subjects the specific network of regions and edges between them involved in theory of mind. That is, we were able to examine age differences in intrinsic connectivity at three levels: the DMN overall, the task-defined theory of mind network nested within the DMN, and the edges within the task-defined theory of mind network. Moreover, we were then able to use participants’ behavioral performance on the localizer task to elucidate at what level of the network would emergent age differences in connectivity predict the target behavior. In sum, investigating age differences in intrinsic connectivity at multiple levels of the DMN that predict theory of mind is a well-suited context for the two goals of the study.
We expected to replicate two principal findings from the literature. First, we expected OA would have poorer theory of mind compared to YA. Second, we expected the intrinsic connectivity within the DMN to be weaker in OA than YA. Replicating these finding would lay the groundwork for a novel examination of how intrinsic connectivity relates to age differences in theory of mind. If overall intrinsic connectivity of the DMN (Hypothesis 1A) or theory of mind network (Hypothesis 1B) predicts theory of mind, we expected weaker connectivity in OA versus YA to relate to poorer theory of mind behavioral performance. However, if the intrinsic connectivity of specific edges isolated from the localizer predicts theory of mind, we expected weaker connectivity in those specific edges in OA versus YA to relate to poorer theory of mind (Hypothesis 1C).
Method
Participants
Forty young adults (YA; 18-33 years old, 25 female, Mage = 21.58, SD = 2.81; years of education: M = 21.58, SD = 2.81) and 35 older adults (OA; 61-86 years old, 22 female, Mage = 16.96, SD = 2.19; years of education: M = 15.24, SD = 1.88) who were right-handed, White, not Hispanic, and had no recent history of neurological problems gave informed consent to participate. We recruited YA from Indiana University in Bloomington and OA from the Bloomington, Indiana community via newspaper and electronic advertisements. The study was approved by the Indiana University Institutional Review Board. Relative to YA, OA had lower executive function on a shortened version of the operation span task (Oswald, McAbee, Redick, & Hambrick, 2015; for the predictive ability of the operation span task for executive function, see Engle, 2002), higher vocabulary scores (Shipley, 1986), and slower processing speed (Hedden et al., 2002). Neither OA nor YA were cognitively impaired, as evidenced by a score of 26 or higher on the Mini-Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975). See Supplementary Table 1 for descriptive and inferential statistics.
Behavioral Testing Session
Participants completed the study in the lab across two testing sessions that were approximately one week apart. The first testing session was a behavioral testing session that lasted approximately two hours. During this session, participants completed initial screening for eligibility to undergo MRI, measures of cognitive function (e.g., the MMSE, short operation span task), and other behavioral measures unrelated to the current research.
MRI Testing Session
The second testing session was conducted approximately one week following the behavioral testing session. During this session, participants underwent MRI for approximately one hour. Relevant here, participants completed a 15-minute resting-state scan and a theory of mind localizer task. Two unrelated tasks were also administered during session in a counterbalanced order with the theory of mind localizer. The resting-state scan was conducted before all task-based scans.
Theory of mind localizer.
To localize brain regions involved in theory of mind, participants completed the false belief task (Saxe & Kanwisher, 2003; Zaitchik, 1990), which has been used in past aging-related neuroimaging work (Moran et al., 2012). During this task, participants responded if statements about stories referring to either a person’s beliefs (false belief condition) or to physical representations (false photo condition) were true or false. Both conditions required that participants make an inference. However, only the false belief condition required an inference about another person’s mental state (i.e., theory of mind). For example, the story “When Lisa left Jacob, he was deep asleep on the beach. A few minutes later, a wave woke him. Seeing Lisa was gone, Jacob decided to go swimming” followed by the statement “Lisa now believes that Jacob is sleeping” was a false belief trial. In contrast, the story “When the picture was taken of the house, it was one story tall. Since then, the renovators added an additional story and a garage” followed by the statement “In the picture, the house is two stories tall and has a garage” was a false photo trial.
Twelve stories of each trial type were presented across two runs lasting 5 minutes and 28 seconds each, with six of each trial type in each run. The order of false belief and false photo trials was determined via a random number generator. Run order was counterbalanced between participants. Each trial began with a story presented for 10s. The story was followed by a variable delay of 0-6s in the form of a fixation cross at the center of the display. Finally, a statement that was true or false was presented for 6s. In each run, there were three 0s delays, three 2s delays, three 4s delays, and three 6s delays (Mdelay = 3 s, SD = 2.34), with 8s of fixation at the beginning of the run and 10s of fixation at the end, for a total of 128s of fixation and 192s of stimulus presentation. Four dummy scans were included at the start of each run to allow for stabilization of the scanner signal. Dummy scans were excluded from analyses.
Resting-state scan.
Resting-state functional data was collected over one run that lasted 15 minutes and took place prior to the anatomical and task-based scans. The duration of this scan was determined based on work showing that it produced reliable estimates of functional connectivity within and across participants (Shah, Cramer, Ferguson, Birn, & Anderson, 2016). Participants were instructed to remain still, stay awake, and keep their eyes open. No stimuli were presented during this scan, and the projector was off.
Data Acquisition and Analysis
Whole-brain imaging was performed on a Siemens 3.0T Prisma MRI Scanner using a 20-channel phase arrayed head coil at the Indiana University Imaging Research Facility in Bloomington, Indiana. Stimuli were presented using a back projector (Sony WUXGA VPLFH30) and behavioral data were collected on a Dell laptop running Windows 7. The scanner was synced to the data collection equipment via scanner TTL. Anatomical images were acquired with a high-resolution 3-D magnetization prepared rapid gradient echo sequence (sagittal rotation; 160 slices, TE = 2.7ms, TR = 1800ms, TI = 900ms, flip angle = 9 degrees, 1.0mm isotropic voxels; with no fat suppression).
Theory of mind localizer.
Functional theory of mind localizer images were collected over two runs of 160 time points each (320 total). Functional scans were collected using an echoplanar image (EPI) sequence sensitive to blood oxygen level dependent contrast (T2*; 54 slices with 2.2mm thickness and no gap, TE = 30ms, TR = 2000ms, flip angle = 52 degrees, FOV = 242mm, in-plane matrix size = 110 × 110, A/P phase encoding direction). Slices were collected in an interleaved order (multi-band acceleration factor = 2). These slices provided partial-brain coverage (i.e., the entire cortex with partial cerebellum, but not brainstem). At the beginning of each functional run, the scanner acquired and discarded four dummy scans.
Preprocessing of functional data was conducted in SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK; www.fil.ion.ucl.ac.uk/spm). Images were realigned to correct for motion, normalized to the Montreal Neurological Institute (MNI) template, and smoothed using an 8mm FWHM isotropic Gaussian kernel. We used a larger smoothing kernel (8mm) that is typical for aging samples in fMRI research to address potential biases associated with spatial registration to a template across age groups (Cassidy, Lee, & Krendl, 2016; Castle et al., 2012; Krendl et al., 2016; Zebrowitz, Ward, Boshyan, Gutchess, & Hadjikhani, 2018; Zebrowitz, Ward, Boshyan, Gutchess, & Hadjikhani, 2016). Data were resampled to 3mm-isotropic voxels. Within each trial, the story preceded a statement that participants judged to be true or false. We thus examined the neural response to the story and statement separately, and chose to focus on the neural response during the statement because that was period during the trial when the behavioral choice was made. A general linear model with four conditions (Story/False belief, Story/False photo, Statement/False belief, Statement/False photo) and covariates of no interest (a session mean, a linear trend, and six movement parameters derived from realignment corrections) computed parameter estimates (β) and t-contrast images (containing weighted parameter estimates) for each comparison at each voxel and for each participant. We localized neural activity by examining a main effect of Statement from a 2 (Age: YA, OA) x 2 (Statement: false belief, false photo) whole-brain ANOVA. The whole-brain ANOVA was conducted using an alpha level of p < .05 corrected for multiple comparisons (controlling family-wise error rate; FWE-correction, k = 20). The main effect of Statement yielded activations consistent with past work on theory of mind (e.g., TPJ, mPFC; see Supplementary Table 2 for a complete list of activations). Activations were verified to be specific to theory of mind by examining the Statement/False belief > Statement/False photo t-contrast (see Supplementary Table 3).
Resting-state preprocessing.
Resting-state images were collected over one run of 450 time points (15 minutes) using the same scan parameters as the theory of mind localizer. No dummy scans were acquired. Using SPM12, images were realigned to correct for motion, slicetime corrected, normalized to the MNI template, and smoothed using an 8mm FWHM isotropic Gaussian kernel.
Connectivity matrix generation.
The resting-state preprocessed data were then submitted to the CONN functional connectivity toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012) to test for motion artifacts and create resting-state connectivity matrices for each participant. The resting-state scans were analyzed using custom artifact detection software (http://www.nitrc.org/projects/artifact_detect) on a participant-by-participant basis to detect outlier time points. Volumes were excluded if the signal for that time point fell three standard deviations outside the mean global signal for the entire run or if the scan-to-scan head motion exceeded .5mm in any direction. Outlier time points were excluded from analysis via the use of participant-specific regressors of no interest. Other nuisance regressors included motion regressors from realignment, white matter, CSF, and other variables of no interest using a PCA-based approach as described by Whitfield-Gabrieli and Nieto-Castanon (2012). It is typical in aging studies to find greater motion for OA than YA; indeed, OA (M = 60.74, SD = 63.31) had more outlier scans compared to YA (M = 31.85, SD = 20.56), t(73) = 2.73, p = .008, d = .63. When removing these outlier scans, OA retained an average of 12.98 minutes (SD = 2.11; approximately 87% of the total 15 minutes) and YA retained an average of 13.94 minutes (SD = .69; approximately 93% of the total 15 minutes) of resting-state data. Although OA had more outlier scans, prior work has indicated that stable correlations can be computed from resting-state functional scans using approximately five minutes of data remaining after common data cleaning practices (Power et al., 2014; Van Dijk et al., 2010). Because all participants met this threshold, no participants were excluded from analyses based on the quantity of outliers. The residual time series was band-pass filtered in the .008-.08 Hz range.
Whole-brain functional connectivity matrices were then calculated across the time series using Fisher’s z coefficients between 114 cortical regions of interest (ROIs) isolated using the Yeo 17 network split-label parcellation based on the MNI template (Yeo et al., 2011) used in prior aging research (Cao et al., 2014), and an additional 14 subcortical ROIs isolated using the maximum likelihood subcortical FSL Harvard-Oxford Atlas (Desikan et al., 2006). The values were subsequently transformed from Fisher’s z to Pearson’s r correlation coefficients for ease of interpretation. In sum, this procedure generated a matrix of correlations between all pairs of nodes (i.e., edges) in the parcellation (128×128 nodes) for each participant. The subject-specific connectivity matrices were the data from which we derived the connectivity of the DMN overall and the connectivity of the specific edges related to theory of mind using the methods described below.
DMN connectivity – modularity calculation.
To reflect network-level connectivity, we calculated a network-specific modularity index of the DMN (QDMN) for each subject (for similar approaches, see Baum et al., 2017; Betzel et al., 2014). The modularity of the DMN reflects the extent to which it is strongly internally connected to itself and weakly externally connected to the other resting-state networks. Because negative correlations are difficult to interpret in this context (for a discussion, see Rubinov & Sporns, 2010), we thresholded negative edges at zero to examine only positive edges. Specifically, we calculated modularity as the sum of the edges within the DMN (i.e., the correlations between pairs of DMN nodes) divided by the sum of all edges in the network (i.e., external connections such as the correlations between pairs of DMN nodes with nodes from other networks, and between pairs of nodes from other networks).
Theory of mind network construction and modularity calculation.
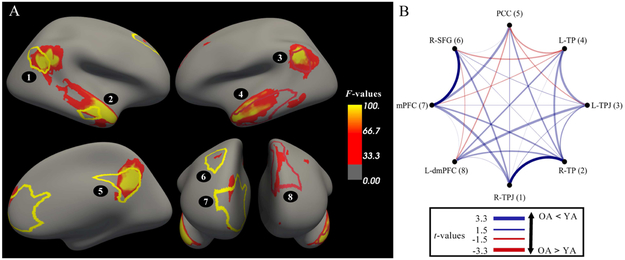
We used the theory of mind localizer to determine the network of brain regions that were involved when engaging in theory of mind. From the localizer, we defined the main effect by the theory of mind contrast [false belief > false photo] collapsed across age (see Supplementary Tables 2 and 3). We registered the peak coordinates to the resting-state parcellation, which resulted in eight unique nodes belonging to the DMN as defined by Yeo et al. (2011; see Figure 3A). Cerebellum peaks were excluded because the functional images provided only partial coverage of the cerebellum. We then calculated, following the procedure for the DMN, the network-specific modularity of the theory of mind network (QTOM).
Figure 3.
Edgewise connectivity results. (A) Activations from the main effect of Belief from the theory of mind localizer projected to a cortical surface. The whole-brain ANOVA was conducted using an alpha level of p < .05 corrected for multiple comparisons (controlling family-wise error rate; FWE-correction, k = 20). The corresponding node definitions from Yeo et al. (2011) are overlaid. (B) The circle graph depicts the age differences (as t-values; legend not drawn to scale) in the edgewise connectivity between the nodes associated with theory of mind. The opaque (vs. transparent) lines represent edges where significant age differences emerged. R = right, L = left. TPJ = temporoparietal junction, TP = temporal pole, PCC = posterior cingulate cortex, SFG = superior frontal gyrus, mPFC = medial prefrontal cortex, dmPFC = dorsal medial prefrontal cortex.
Theory of mind edgewise analysis.
The connectivity between specific nodes (edgewise connectivity) may have also yielded age differences related to theory of mind. We were interested in edges between nodes that were specifically implicated in theory of mind. For this analysis, we subset the 8×8 matrix reflecting the connectivity for the edge between each pair of these nodes associated with theory of mind from each participant’s complete connectivity matrix. To assess age differences, we conducted a t-test between OA and YA at each edge. Then, to create a null distribution of age differences attributed to chance, YA and OA were randomly assigned to one of two groups while preserving group sizes (N1 = 35, N2 = 40) across 10,000 iterations. We conducted t-tests between the random groups for each iteration. We calculated the probability (p-value) that the t-value from the observed age group comparison was more extreme than in the randomized group permutations. Finally, we accounted for multiple comparisons across the eight nodes using a Bonferroni correction with alpha (p = .05), resulting in a Bonferroni-corrected p-value of .0014.
Results
Establishing Age Differences in Theory of Mind
The false belief task used here to measure theory of mind (Moran et al., 2012; Saxe & Kanwisher, 2003; Zaitchik, 1990) included two conditions: a mental inference condition requiring theory of mind (false belief condition) and a physical inference condition that did not require theory of mind (false photo condition). People are typically more accurate at false belief versus false photo inferences (Wang & Su, 2013; Zaitchik, 1990). Thus, the extent to which people engage in theory of mind (false belief condition) beyond their general ability to make inferences (false photo condition) represents better theory of mind performance. In past work, YA had better theory of mind performance because they were more accurate on false belief trials than false photo trials while OA were not. This yielded a significant, moderate effect of age group on theory of mind performance (Cohen’s d = .57; Moran et al., 2012). In the current work, to quantify theory of mind performance for each participant in all analyses, we thus subtracted percent accuracy in the false photo condition from percent accuracy in the false belief condition. Although the age difference did not meet traditional criteria for statistical significance (MOA = .01, SD = .13; MYA = .07, SD = .12; t(73) = 1.80, p = .08, d = .48, 95% CI [.02, .94]; see Supplementary Figure 1), the direction and size of the effect was consistent with past work showing relatively poorer theory of mind in older adults (d = .57; Moran et al., 2012; Moran, 2013).
Age Differences in Intrinsic Connectivity
We expected that the overall connectivity of the DMN (Hypothesis 1A) or theory of mind network (Hypothesis 1B) or more specific edgewise connectivity in the theory of mind network (Hypothesis 1C) would relate to age differences in theory of mind. To address these possibilities, we first examined the extent of age differences in overall DMN connectivity, the overall connectivity of the specific network of brain regions in the DMN isolated from the theory of mind localizer, and the connectivity within specific edges of that theory of mind network. We then tested whether the intrinsic connectivity at each level of the network predicted age differences in theory of mind performance.
Hypothesis 1A: DMN connectivity mediates age differences in theory of mind.
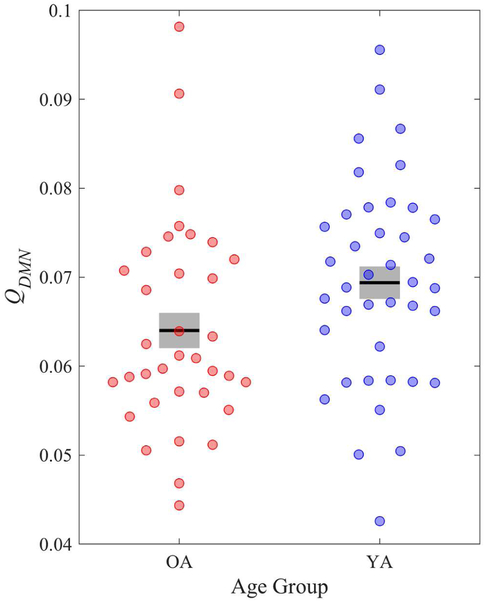
To characterize the intrinsic connectivity of the DMN, we calculated a network-specific modularity index (Q) for each participant, given a predefined network structure (Yeo et al., 2011). QDMN quantifies the extent to which the DMN is strongly internally connected with itself and weakly externally connected to other resting-state networks. Simply put, QDMN reflects the cohesiveness of the DMN. Prior work has used network-specific modularity to characterize how brain networks develop over the lifespan and to predict behaviors (Baum et al., 2017; Betzel et al., 2014; Xia et al., 2018). Replicating past work showing weaker DMN connectivity with age, OA (M = .08, SD = .02) had lower DMN modularity versus YA (M = .09, SD = .01), t(73) = 2.56, p = .01, d = .59, 95% CI [.13, 1.05] (see Figure 1). However, DMN modularity did not predict theory of mind performance across all participants, r(73) = .06, p = .62; or separately by age group: YA, r(38) = −.08, p = .65; OA, r(33) = .07, p = .70.
Figure 1.
Modularity (Q) of the DMN by age group. Gray bars represent +/− 1 standard error from the mean (black bar).
Hypothesis 1B: Connectivity of a task-defined theory of mind network mediates age differences in theory of mind.
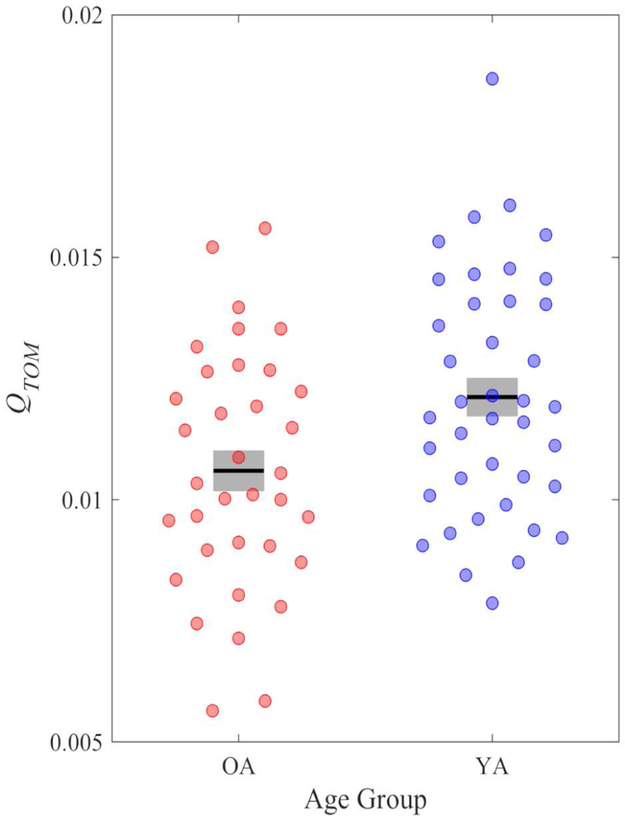
We also tested the possibility that the network-level connectivity of the task-defined theory of mind network demonstrated age differences related to theory of mind. Therefore, we calculated the modularity of the theory of mind network (QTOM) using the same procedure as for the DMN. We found that OA (M = .011, SD = .002) had significantly lower modularity of the theory of mind network compared to YA (M = .012, SD = .002), t(73) = 2.65, p = .01, d = .60, 95% CI [.14, 1.06] (see Figure 2); but modularity of this network did not predict theory of mind behavioral performance across all participants, r(73) = .10, p = .40; or separately by age group: YA, r(38) = −.06, p = .70; OA, r(33) = .15, p = .40.
Figure 2.
Modularity (Q) of the task-defined theory of mind network by age group. Gray bars represent +/− 1 standard error from the mean (black bar).
Support for Hypothesis 1C: Edgewise connectivity mediates age differences in theory of mind.
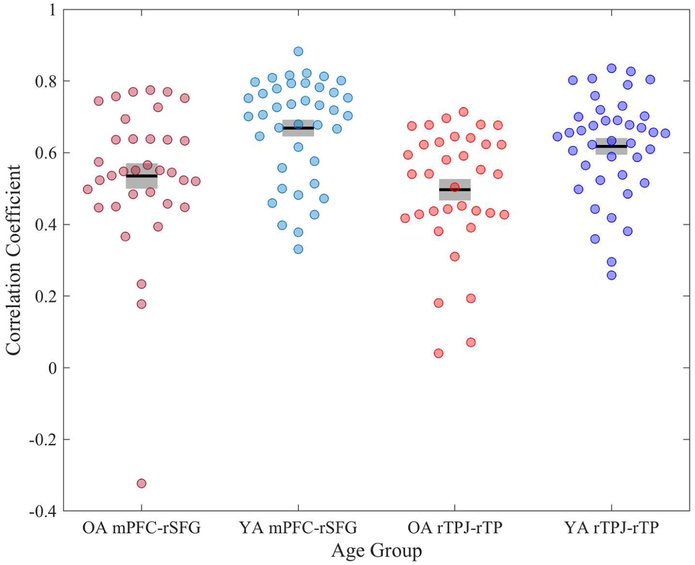
We next examined whether intrinsic connectivity of specific edges associated with theory of mind related to age differences in theory of mind. To identify nodes comprising these edges, we defined the main effect by the theory of mind contrast [false belief > false photo] collapsed across age (see Supplementary Tables 2 and 3). Eight regions emerged – all within the DMN, as expected (see Figure 3A). For each participant, we extracted the correlation coefficients between each pair of these nodes (i.e., edgewise connectivity) and compared these values between YA and OA, using a Bonferroni-corrected p-value of .0014. Two edges within the DMN were significantly weaker in OA than YA (Figure 3B, 4): (1) rTPJ with rTP, t(73) = 3.30, p = .0009, d = .76, 95% CI [.29, 1.23]; and (2) mPFC with right superior frontal gyrus (rSFG), t(73) = 3.27, p = .0006, d = .76, 95% CI [.29, 1.28]. No edges were significantly stronger in OA versus YA.
Figure 4.
Connectivity values (i.e., correlation of activation at rest between the two nodes) by group for each of the two edges that demonstrated significant age differences. Gray bars represent +/− 1 standard error from the mean (black bar).
Next, we used linear regression analysis to determine whether the connectivity of either or both edges predicted theory of mind. Both edges were simultaneously included in the model. QDMN was not included because it, by definition, included both edges and also did not correlate with theory of mind (see above). The overall model was significant and accounted for 9% of the variance in theory of mind, F(2,74) = 3.52, p = .04. Stronger rTPJ-rTP connectivity predicted better theory of mind (β = .27, p = .02, 95% CI [.03, .38]; see Figure 5A), but mPFC-rSFG connectivity was non-significant (β = .09, p = .47, 95% CI [−.10, .21]). One OA participant’s edgewise connectivity between mPFC-rSFG was identified as a potential outlier (r = −.32). Excluding this participant did not change the results – OA had weaker mPFC-rSFG connectivity than YA (MOA = .56, SD = .15; MYA = .67, SD = .14; t(72) = 3.21, p = .0011, d = .76, 95% CI [.29, 1.23]). Additionally, the mPFC-rSFG edge, included simultaneously with the rTPJ-rTPJ edge, did not predict theory of mind when excluding this potential outlier, β = .02, p = .87, 95% CI [−.18, .22].
Figure 5.
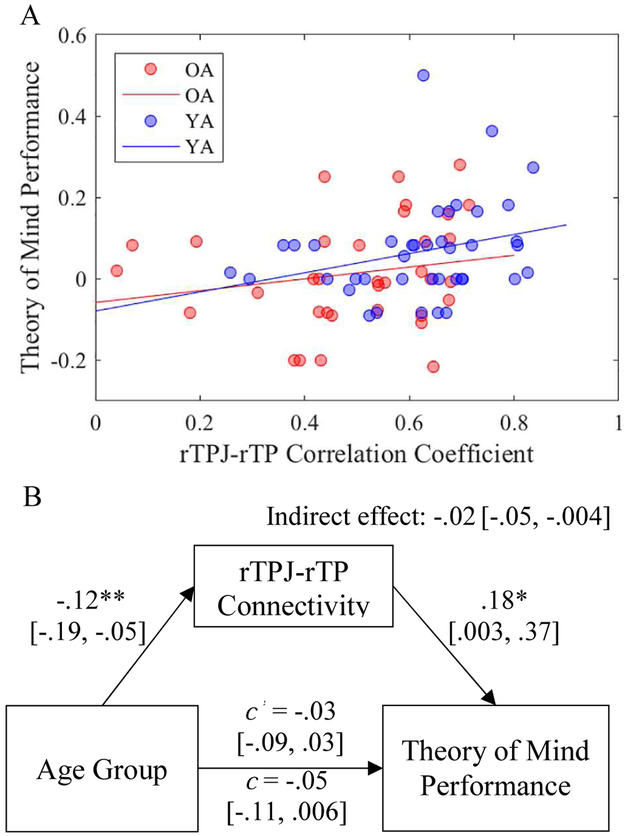
Relating edgewise connectivity to theory of mind performance. (A) Scatter plot of the relationship between theory of mind performance and the intrinsic connectivity between the right temporoparietal junction (rTPJ) and right temporal pole (rTP) for OA (red) and YA (blue). (B) Results from a mediation analysis demonstrating that the intrinsic connectivity between the rTPJ-rTP explained the effect of age group (0 = YA, 1 = OA) on theory of mind performance. *p ≤ .05, **p ≤ .01. Path c represents the total effect, whereas path c ‘ represents the direct effect while controlling for the mediator. Coefficients are unstandardized. Values in brackets are 95% confidence intervals.
The data suggest intrinsic rTPJ-rTP connectivity is weaker in OA versus YA and positively predicts theory of mind. To test if a relationship between age group and theory of mind could be explained, at least in part, by the intrinsic connectivity of the edge (Hypothesis 3b), we conducted a mediational analysis using PROCESS for SPSS (Hayes, 2013) with 10,000 bootstrap samples for bias-corrected bootstrap confidence intervals (for a depiction of the model, see Figure 5B). There are strong limitations of using mediational analysis in cross-sectional data to make causal claims about the effect of aging (Raz & Lindenberger, 2011). However, mediation can still be useful to inform whether one variable may explain the relationship between two others without assumptions of time or causality (Salthouse, 2011). Thus, we use mediation in the current work to examine the extent to which intrinsic connectivity may be responsible for age differences in theory of mind. The following coefficients are unstandardized. Age did not directly predict theory of mind performance, b = −.03, SE = .03, p = .33, 95% CI [−.09, .03]; however, a significant direct effect is not required to test an indirect path (see Hayes, 2009; Shrout & Bolger, 2002). Indeed, age (YA = 0, and OA = 1) negatively predicted rTPJ-rTPJ connectivity, b = −.12, SE = .04, p = .002, 95% CI [−.19, −.05], and rTPJ-rTP edgewise connectivity positively predicted theory of mind, b = .18, SE = .09, p = .05, 95% CI [.003, .37]. Critically, the indirect effect of rTPJ-rTP connectivity was significant, b = −.02, SE = .01, 95% CI [−.05, −.004].
To rule out the possibility that the rTPJ-rTP edge more broadly mediated age differences in behavior, and not just in theory of mind, we tested whether the intrinsic connectivity between rTPJ-rTP mediated age differences in a measure of executive function (short operation span task – partial score; Oswald et al., 2015). Executive function was a relevant behavior for this analysis given that prior work indicates that the impact of age on theory of mind may depend on executive function among OA (Moran, 2013). OA (M = 18.49, SD = 8.15) had poorer executive function compared to YA (M = 25.28, SD = 4.78), t(73) = 4.46, p < .001, d = 1.02, 95% CI [.55, 1.51]. However, rTPJ-rTP intrinsic connectivity did not mediate age differences in executive function (b = −.78, SE = .70, 95% CI [−2.21, .58]). Thus, rTPJ-rTP intrinsic connectivity does not broadly explain age differences in behavior.
Discussion
The current study showed that the extent of intrinsic functional connectivity within the DMN contributes to age differences in theory of mind. Specifically, OAs’ weaker connectivity between rTPJ and rTP, a specific edge within the DMN, predicted theory of mind. Although we identified weaker intrinsic network-level connectivity in OA, these overall differences did not relate to theory of mind. Thus, the current data suggest that age differences in theory of mind may arise, at least in part, from weakening in the underlying baseline connectivity of brain regions implicated in theory of mind.
A key strength of the current work is that we examined how age-related weakening of intrinsic connectivity of the DMN at multiple levels affected theory of mind. Prior work has demonstrated that aging is associated with weaker intrinsic DMN connectivity (e.g., Damoiseaux et al., 2008) and weaker intrinsic connectivity of specific components of the DMN (e.g., Campbell et al., 2013). However, this work critically connects these differences to declines in theory of mind associated with age. The novel approach of defining a specific network by the theory of mind localizer - in which all nodes overlapped with the DMN – allowed us to demonstrate that more localized connectivity within the DMN related to age differences in theory of mind, whereas more global connectivity within the DMN did not. Taken together, these findings suggest that intrinsic connectivity, rather than task-evoked activation alone, underlies age differences in theory of mind. Future work should disentangle the relationship between intrinsic connectivity and task-evoked neural activations and if these mechanisms have separable contributions to age differences in theory of mind. While outside the scope of the current paper, another important consideration is that networks other than the DMN may be relevant to other social cognitive behaviors. We had chosen to focus on the DMN given its common neural basis with theory of mind task activations, but weaker intrinsic connectivity among OA is evident within multiple other resting-state networks, as are changes in the connectivity between networks (Betzel et al., 2014; Damoiseaux, 2017).
Although the DMN and specific theory of mind network within the DMN exhibited weaker connectivity in OA, particular edges were especially affected (consistent with other work; Campbell et al., 2013). Among the eight regions identified by the theory of mind localizer, two edges (rTPJ-rTP, mPFC-rSFG) demonstrated significant age differences. These findings converge with prior work on subsystems of the DMN that differentiated the functional roles of a subsystem including TPJ and TP from a ‘core’ subsystem that included mPFC (Andrews-Hanna et al., 2010). While the former appears to activate largely when making mental state inferences about others, the latter is often implicated in self-referential thought (e.g., recalling autobiographical memories; Andrews-Hanna, Smallwood, & Spreng, 2014). In fact, only rTPJ-rTP connectivity predicted age differences in theory of mind. RTPJ engagement plays a fundamental (Saxe & Wexler, 2005) and causal (Young, Camprodon, Hauser, Pascual-Leone, & Saxe, 2010) role in enabling theory of mind. And, importantly, OA engage rTPJ less than YA during theory of mind (Cassidy, Hughes, & Krendl, under review).
Given its critical role in theory of mind and its lesser engagement with age, it is, perhaps, not surprising that edgewise connectivity from rTPJ was related to age differences in theory of mind in the current work. Recent work has debated whether the rTPJ has a task-dependent, joint or separable role in theory of mind or cognitive processes involved with paying attention to contextual information (Krall et al., 2016; Lavoie, Vistoli, Sutliff, Jackson, & Achim, 2016; Schuwerk, Schurz, Müller, Rupprecht, & Sommer, 2017). In our work, we found that rTPJ-rTP connectivity during resting-state – which is task-unconstrained – predicted theory of mind performance, but not executive function (a measure of general cognitive function that includes attentional control). However, it is important to note that our rTPJ-edgewise connectivity finding could have been driven, at least in part, by the fact that our network analysis was constrained by the theory of mind localizer. An examination of the intrinsic connectivity of the rTPJ outside of the task-defined theory of mind network (e.g., with networks involved in attentional control; Schuwerk et al., 2017) may inform the role of the rTPJ in broader cognitive processes. Nevertheless, the fact that our results show that the edgewise connectivity between rTPJ and rTP mediated the effect of age on theory of mind performance importantly demonstrates that dysregulation in intrinsic connectivity is associated with age-related deficits in social cognition. That the behaviorally-relevant edge was between rTPJ and rTP is consistent with the TP’s role in theory of mind (Molenberghs et al., 2016) and accessing social knowledge more broadly (Olson, McCoy, Klobusicky, & Ross, 2013). Interestingly, TP engagement improves OAs’ ability to recognize faces (Ross, McCoy, Coslett, Olson, & Wolk, 2011), raising the possibility that modulating neural connections with TP improves aspects of OAs’ social behavior, and specifically, how they remember others. Speculatively, stronger rTPJ-rTPJ edgewise connectivity may allow them to access social knowledge in order to more efficiently process, understand, and remember others’ mental states. Future work should assess these intriguing possibilities.
Although mPFC-rSFG connectivity was weaker with age, it did not relate to theory of mind performance. Prior work (Moran et al., 2012) showed less mPFC activity among OA versus YA during the same theory of mind localizer used here. However, that work did not show if or how the extent of mPFC activity corresponded to task behavior. Other work using a connectivity-based approach among healthy adults; however, found that individuals who had more spontaneous thoughts about theory of mind during a resting-state scan had stronger intrinsic connectivity among frontal lobe regions including mPFC and rSFG (Marchetti et al., 2015). Because we used a task-based measure of theory of mind performance (i.e., deliberate engagement) to relate to intrinsic connectivity, a more specific interpretation of our findings may thus be that age differences in the intrinsic connectivity between mPFC-rSFG are unrelated to deliberate theory of mind, but that mPFC-rSFG connectivity may have a relationship with spontaneous theory of mind – consistent with the subsystems interpretation above (Andrews-Hanna et al., 2014). Because mPFC supports, but is not be essential to, theory of mind (Otti et al., 2015), these possibilities may provide important nuances for understanding age differences in theory of mind and related social cognitive behaviors.
A limitation of this work is that, although yielding an effect size comparable to that of prior work (Moran et al., 2012), OA were not significantly impaired on theory of mind compared to YA. However, consistent with prior work (Moran et al., 2012; Moran, 2013), OA did have lower accuracy than YA in the false belief condition (which required theory of mind) but not the false photo trials (which did not require theory of mind). As such, age differences emerged as poorer performance in mental state inferences rather than OAs’ ability to make inferences more broadly. Stronger effects might be expected among cohorts of older adults who are lower functioning than the sample in the present study (Moran, 2013). Indeed, older adults who are willing and eligible to participate in fMRI studies of healthy aging are often relatively high functioning (i.e., able to attend several hours of lab testing, a score of 26 or higher on the MMSE – see Method) and are physically (e.g., no major neurological disease or complications) and socially active members of the community. Such maintained activity may constrain age-related decline on social tasks that are oft-used in everyday life, like theory of mind. Conversely, OA experiencing pathological aging demonstrate greater declines in theory of mind (Charles & Carstensen, 2010) and more severely decreased intrinsic functional connectivity in the DMN (Hafkemeijer et al., 2012). Our findings may provide initial evidence for a relationship between these specific changes and insight as to the mechanism by which some OA are more vulnerable than others to the pathological neurocognitive and social effects of aging (e.g., increased loneliness; Cacioppo et al., 2014).
Finally, it is important to note that while the current work has the critical benefit of measuring and linking task-evoked, resting-state, and behavioral data in the same participants, there are limitations to inferring age differences in a cross-sectional sample (Raz & Lindenberger, 2011; but see Salthouse, 2011). Relatedly, more reliable estimates of the relationship between intrinsic connectivity and age or behavior are derived from larger samples (Biswal et al., 2010; e.g., Betzel et al., 2014; Xia et al., 2018). It will thus be important for future work to expand on the foundational work provided by the current study using larger samples of participants. An exciting avenue for future research that may address these points is the development of large-scale neuroimaging datasets – such as the Cambridge Centre for Ageing Neuroscience project (http://www.cam-can.org/) and the Lifespan Human Connectome Project in Aging (Bookheimer et al., 2019) – which can provide rich neural and behavior data within larger and more diverse cross-sectional and longitudinal samples than might be typically feasible.
The current work makes significant methodological and theoretical contributions to the literature on aging and theory of mind. Methodologically, the intrinsic connectivity of brain regions elicited from task-based fMRI may be a rich source by which to gain an understanding of how aging impacts social cognition. Not all social cognitive abilities decline with age (Scheibe & Blanchard-Fields, 2009), just as not all connectivity – even within the DMN (Campbell et al., 2013) – is weaker in OA. It could be that edgewise connectivity preserved with age is central to eliciting age-equivalent social behaviors. Given the connection between mental state understanding and maintaining social relationships (Bailey et al., 2008; Frith & Frith, 2001), the present work has important implications for addressing age-specific concerns such as loneliness and its myriad negative outcomes (Adam et al., 2006; Cornwell & Waite, 2009; Luo et al., 2012). For example, our findings suggest that improving OAs’ theory of mind may require understanding how brain regions communicate beyond when the participant is actively engaged in a specific behavior. The findings of the current work broaden our understanding of aging andsocial cognition and reveal more specific mechanisms of how aging impacts theory of mind.
Supplementary Material
Acknowledgments.
The authors wish to thank the Indiana University Imaging Research Facility, Rachel Brown, Lindsey Fisher, Jaclyn Lisnek, and Lauren Lu for assistance with data collection. This research was supported in part by NIMH grant T32MH103213 to C.H. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Data and Code Availability. The data and code that support the findings of this study are available from the corresponding author upon request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Hawkley LC, Kudielka BM, & Cacioppo JT (2006). Day-to-day dynamics of experience-cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Sciences, 103(45), 17058–17063. 10.1073/pnas.0605053103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65(4), 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316(1), 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, & Buckner RL (2007). Disruption of large-scale brain systems in advanced aging. Neuron, 56(5), 924–935. 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey PE, Henry JD, & Von Hippel W (2008). Empathy and social functioning in late adulthood. Aging & Mental Health, 12(4), 499–503. 10.1080/13607860802224243 [DOI] [PubMed] [Google Scholar]
- Baum GL, Ciric R, Roalf DR, Betzel RF, Moore TM, Shinohara RT, … Satterthwaite TD (2017). Modular segregation of structural brain networks supports the development of executive function in youth. Current Biology, 27(11), 1561–1572.e8. 10.1016/j.cub.2017.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, & Leary MR (1995). The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117(3), 497–529. [PubMed] [Google Scholar]
- Bertolero MA, Yeo BTT, & D’Esposito M (2015). The modular and integrative functional architecture of the human brain. Proceedings of the National Academy of Sciences of the United States of America, 112(49), E6798–807. 10.1073/pnas.1510619112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Byrge L, He Y, Goñi J, Zuo X-N, & Sporns O (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. NeuroImage, 102, 345–357. 10.1016/j.neuroimage.2014.07.067 [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, … Milham MP (2010). Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America, 107(10), 4734–4739. 10.1073/pnas.0911855107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Salat DH, Terpstra M, Ances BM, Barch DM, Buckner RL, … Yacoub E (2019). The Lifespan Human Connectome Project in Aging: An overview. NeuroImage, 185, 335–348. 10.1016/J.NEUROIMAGE.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Walterfang M, & Velakoulis D (2015). Theory of mind in behavioural-variant frontotemporal dementia and Alzheimer’s disease: a meta-analysis. J Neurol Neurosurg Psychiatry, 86(7), 714–719. 10.1136/JNNP-2014-309445 [DOI] [PubMed] [Google Scholar]
- Cacioppo S, Capitanio JP, & Cacioppo JT (2014). Toward a neurology of loneliness. Psychological Bulletin, 140(6), 1464–1504. 10.1037/a0037618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Grigg O, Saverino C, Churchill N, & Grady CL (2013). Age differences in the intrinsic functional connectivity of default network subsystems. Frontiers in Aging Neuroscience, 5, 73 10.3389/fnagi.2013.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wang JH, Dai ZJ, Cao XY, Jiang LL, Fan FM, … He Y (2014). Topological organization of the human brain functional connectome across the lifespan. Developmental Cognitive Neuroscience, 7(16), 76–93. 10.1016/j.dcn.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy BS, Hughes C, & Krendl AC (n.d.). Age differences related to theory of mind emerge in person perception. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy BS, Lee EJ, & Krendl AC (2016). Age and executive ability impact the neural correlates of race perception. Social Cognitive and Affective Neuroscience, 11(11), 1752–1761. 10.1093/scan/nsw081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E, Eisenberger NI, Seeman TE, Moons WG, Boggero IA, Grinblatt MS, & Taylor SE (2012). Neural and behavioral bases of age differences in perceptions of trust. Proceedings of the National Academy of Sciences of the United States of America, 109(51), 20848–20852. 10.1073/pnas.1218518109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, & Carstensen LL (2010). Social and emotional aging. Annual Review of Psychology, 61(1), 383–409. 10.1146/annurev.psych.093008.100448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, & Petersen SE (2014). Intrinsic and task-evoked network architectures of the human brain. Neuron, 83(1), 238–251. 10.1016/j.neuron.2014.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Ito T, Bassett DS, & Schultz DH (2016). Activity flow over resting-state networks shapes cognitive task activations. Nature Neuroscience, 19(12), 1718–1726. 10.1038/nn.4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell EY, & Waite LJ (2009). Social disconnectedness, perceived isolation, and health among older adults. Journal of Health and Social Behavior, 50(1), 31–48. 10.1177/002214650905000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS (2017). Effects of aging on functional and structural brain connectivity. NeuroImage, 160, 32–40. 10.1016/J.NEUROIMAGE.2017.01.077 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, … Rombouts SARB (2008). Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex, 18(8), 1856–1864. 10.1093/cercor/bhm207 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Engle RW (2002). Working memory capacity as executive attention. Current Directions in Psychological Science, 11(1), 19–23. 10.1111/1467-8721.00160 [DOI] [Google Scholar]
- Ferreira LK, & Busatto GF (2013). Resting-state functional connectivity in normal brain aging. Neuroscience & Biobehavioral Reviews, 37(3), 384–400. 10.1016/J.NEUBIOREV.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). "Mini-Mental State" A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. [DOI] [PubMed] [Google Scholar]
- Frith U, & Frith C (2001). The biological basis of social interaction. Current Directions in Psychological Science, 10(5), 151–155. [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, & Lorist MM (2015). A brain-wide study of age-related changes in functional connectivity. Cerebral Cortex, 25(7), 1987–1999. 10.1093/cercor/bhu012 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, & Menon V (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 100(1), 253–258. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkemeijer A, van der Grond J, & Rombouts SARB (2012). Imaging the default mode network in aging and dementia. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1822(3), 431–441. 10.1016/J.BBADIS.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, & Constable RT (2006). Brain connectivity related to working memory performance. The Journal of Neuroscience, 20(51), 13338–13343. 10.1523/JNEUROSCI.3408-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76(4), 408–420. 10.1080/03637750903310360 [DOI] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: The Guilford Press; 10.5539/ass.v11n9p207 [DOI] [Google Scholar]
- Hedden T, Park DC, Nisbett R, Ji L-J, Jing Q, & Jiao S (2002). Cultural variation in verbal versus spatial neuropsychological function across the life span. Neuropsychology, 16(1), 65–73. 10.1037/0894-4105.16.1.65 [DOI] [PubMed] [Google Scholar]
- Krall SC, Volz LJ, Oberwelland E, Grefkes C, Fink GR, & Konrad K (2016). The right temporoparietal junction in attention and social interaction: A transcranial magnetic stimulation study. Human Brain Mapping, 37(2), 796–807. 10.1002/hbm.23068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendl AC, Kensinger EA, Wang X, Han S, Brammer M, & David A (2016). Does older adults’ cognitive function disrupt the malleability of their attitudes toward outgroup members?: An fMRI investigation. PLOS ONE, 11(4), e0152698 10.1371/journal.pone.0152698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie M-A, Vistoli D, Sutliff S, Jackson PL, & Achim AM (2016). Social representations and contextual adjustments as two distinct components of the Theory of Mind brain network: Evidence from the REMICS task. Cortex, 81, 176–191. 10.1016/J.CORTEX.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Luo Y, Hawkley LC, Waite LJ, & Cacioppo JT (2012). Loneliness, health, and mortality in old age: A national longitudinal study. Social Science & Medicine, 74(6), 907–914. 10.1016/J.SOCSCIMED.2011.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti A, Baglio F, Costantini I, Dipasquale O, Savazzi F, Nemni R, … Castelli I (2015). Theory of mind and the whole brain functional connectivity: Behavioral and neural evidences with the Amsterdam Resting State Questionnaire. Frontiers in Psychology, 6, 1855 10.3389/fpsyg.2015.01855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, & Rushworth MFS (2012). On the relationship between the “default mode network” and the “social brain.” Frontiers in Human Neuroscience, 6(June), 1–9. 10.3389/fnhum.2012.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl T, Teipel S, Elmouden R, Mueller S, Koch W, Dietrich O, … Glaser C (2009). Test-retest reproducibility of the default-mode network in healthy individuals. Human Brain Mapping, 31(2), 237–46. 10.1002/hbm.20860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D, Achard S, Morcom A, & Bullmore E (2009). Age-related changes in modular organization of human brain functional networks. NeuroImage, 44(3), 715–723. 10.1016/j.neuroimage.2008.09.062 [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Johnson H, Henry JD, & Mattingley JB (2016). Understanding the minds of others: A neuroimaging meta-analysis. Neuroscience & Biobehavioral Reviews, 65, 276–291. 10.1016/j.neubiorev.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Moran JM (2013, January 15). Lifespan development: The effects of typical aging on theory of mind Behavioural Brain Research. Elsevier; 10.1016/j.bbr.2012.09.020 [DOI] [PubMed] [Google Scholar]
- Moran JM, Jolly E, & Mitchell JP (2012). Social-cognitive deficits in normal aging. Journal of Neuroscience, 32(16), 5553–5561. 10.1523/JNEUROSCI.5511-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, & Ross LA (2013). Social cognition and the anterior temporal lobes: A review and theoretical framework. Social Cognitive and Affective Neuroscience, 8(2), 123–133. 10.1093/scan/nss119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda K, & Yamaguchi S (2013). Small-worldness and modularity of the resting-state functional brain network decrease with aging. Neuroscience Letters, 556, 104–108. 10.1016/J.NEULET.2013.10.023 [DOI] [PubMed] [Google Scholar]
- Oswald FL, McAbee ST, Redick TS, & Hambrick DZ (2015). The development of a short domain-general measure of working memory capacity. Behavior Research Methods, 47(4), 1343–1355. 10.3758/s13428-014-0543-2 [DOI] [PubMed] [Google Scholar]
- Otti A, Wohlschlaeger AM, Noll-Hussong M, Blakemore S, Brito S, & McCrory E (2015). Is the medial prefrontal cortex necessary for theory of mind? PLOS ONE, 10(8), e0135912 10.1371/journal.pone.0135912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perissinotto CM, Stijacic Cenzer I, & Covinsky KE (2012). Loneliness in older persons. Archives of Internal Medicine, 172(14), 1078–1083. 10.1001/archinternmed.2012.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, & Petersen SE (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. 10.1016/J.NEUROIMAGE.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premack D, & Woodruff G (1978). Does the chimpanzee have a theory of mind? Behavioral and Brain Sciences, 1(04), 515 10.1017/S0140525X00076512 [DOI] [Google Scholar]
- Raz N, & Lindenberger U (2011). Only time will tell: Cross-sectional studies offer no solution to the age-brain-cognition triangle: Comment on Salthouse (2011). Psychological Bulletin, 137(5), 790–795. 10.1037/a0024503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross LA, McCoy D, Coslett HB, Olson IR, & Wolk DA (2011). Improved proper name recall in aging after electrical stimulation of the anterior temporal lobes. Frontiers in Aging Neuroscience, 3, 16 10.3389/fnagi.2011.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, & Sporns O (2010). Complex network measures of brain connectivity: Uses and interpretations. Neuroimage, 52(3), 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Salthouse TA (2011). All data collection and analysis methods have limitations: Reply to Rabbitt (2011) and Raz and Lindenberger (2011). Psychological Bulletin, 137(5), 796–799. 10.1037/a0024843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, & Kanwisher N (2003). People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind.” NeuroImage, 19(4), 1835–1842. [DOI] [PubMed] [Google Scholar]
- Saxe R, & Wexler A (2005). Making sense of another mind: The role of the right temporo-parietal junction. Neuropsychologia, 43(10), 1391–1399. 10.1016/J.NEUROPSYCHOLOGIA.2005.02.013 [DOI] [PubMed] [Google Scholar]
- Scheibe S, & Blanchard-Fields F (2009). Effects of regulating emotions on cognitive performance: What is costly for young adults is not so costly for older adults. Psychology and Aging, 24(1), 217–223. 10.1037/a0013807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, & Vogeley K (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Consciousness and Cognition, 17(2), 457–467. 10.1016/j.concog.2008.03.013 [DOI] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, & Perner J (2014). Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews, 42, 9–34. 10.1016/j.neubiorev.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Schuwerk T, Schurz M, Müller F, Rupprecht R, & Sommer M (2017). The rTPJ’s overarching cognitive function in networks for attention and theory of mind. Social Cognitive and Affective Neuroscience, 12(1), 157–168. 10.1093/scan/nsw163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah LM, Cramer JA, Ferguson MA, Birn RM, & Anderson JS (2016). Reliability and reproducibility of individual differences in functional connectivity acquired during task and resting state. Brain and Behavior, 6(5), e00456 10.1002/brb3.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley W (1986). Shipley Institute of Living Scale. Western Psychological Services. [Google Scholar]
- Shrout PE, & Bolger N (2002). Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods, 7(4), 422–445. [PubMed] [Google Scholar]
- Sporns O (2013). Structure and function of complex brain networks. Dialogues in Clinical Neuroscience, 15(3), 247–262. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24174898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, & Betzel RF (2016). Modular brain networks. Annu Rev Psychol, 67, 613–640. 10.1146/annurev-psych-122414-033634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, & Grady CL (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience, 22(6), 1112–1123. 10.1162/jocn.2009.21282 [DOI] [PubMed] [Google Scholar]
- Stevens WD, & Spreng RN (2014). Resting-state functional connectivity MRI reveals active processes central to cognition. Wiley Interdisciplinary Reviews: Cognitive Science, 5(2), 233–245. 10.1002/wcs.1275 [DOI] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, & Buckner RL (2010). Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. Journal of Neurophysiology, 103(1), 297–321. 10.1152/jn.00783.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, & Su Y (2013). Age-related differences in the performance of theory of mind in older adults: A dissociation of cognitive and affective components. Psychology and Aging, 28(1), 284–291. 10.1037/a0030876 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, … Bennett DA (2007). Loneliness and risk of alzheimer Disease. Archives of General Psychiatry, 64(2), 234 10.1001/archpsyc.64.2.234 [DOI] [PubMed] [Google Scholar]
- Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, … Satterthwaite TD (2018). Linked dimensions of psychopathology and connectivity in functional brain networks. Nature Communications, 9(1), 3003 10.1038/s41467-018-05317-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Camprodon JA, Hauser M, Pascual-Leone A, & Saxe R (2010). Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proceedings of the National Academy of Sciences, 107(15), 6753–6758. 10.1073/pnas.0914826107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitchik D (1990). When representations conflict with reality: the preschooler’s problem with false beliefs and "false" photographs. Cognition, 35(1), 41–68. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Ward N, Boshyan J, Gutchess A, & Hadjikhani N (2018). Older adults’ neural activation in the reward circuit is sensitive to face trustworthiness. Cognitive, Affective, & Behavioral Neuroscience, 18(1), 21–34. 10.3758/s13415-017-0549-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz L, Ward N, Boshyan J, Gutchess A, & Hadjikhani N (2016). Dedifferentiated face processing in older adults is linked to lower resting state metabolic activity in fusiform face area. Brain Research, 1644, 22–31. 10.1016/j.brainres.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.