Abstract
Background
Progressive muscle weakness is a main symptom of most hereditary and acquired muscle diseases. Creatine improves muscle performance in healthy individuals. This is an update of our 2007 Cochrane review that evaluated creatine treatment in muscle disorders. Previous updates were in 2009 and 2011.
Objectives
To evaluate the efficacy of creatine compared to placebo for the treatment of muscle weakness in muscle diseases.
Search methods
On 11 September 2012, we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL (2012, Issue 9 in The Cochrane Library), MEDLINE (January 1966 to September 2012) and EMBASE (January 1980 to September 2012) for randomised controlled trials (RCTs) of creatine used to treat muscle diseases.
Selection criteria
RCTs or quasi‐RCTs of creatine treatment compared to placebo in hereditary muscle diseases or idiopathic inflammatory myopathies.
Data collection and analysis
Two authors independently applied the selection criteria, assessed trial quality and extracted data. We obtained missing data from investigators.
Main results
A total of 14 trials, including 364 randomised participants, met the selection criteria. The risk of bias was low in most studies. Only one trial had a high risk of selection, performance and detection bias. No new studies were identified at this update.
Meta‐analysis of six trials in muscular dystrophies including 192 participants revealed a significant increase in muscle strength in the creatine group compared to placebo, with a mean difference of 8.47%; (95% confidence intervals (CI) 3.55 to 13.38). Pooled data of four trials including 115 participants showed that a significantly higher number of participants felt better during creatine treatment compared to placebo with a risk ratio of 4.51 (95% CI 2.33 to 8.74). One trial in 37 participants with idiopathic inflammatory myopathies also showed a significant improvement in functional performance. No trial reported any clinically relevant adverse event.
In metabolic myopathies, meta‐analyses of three cross‐over trials including 33 participants revealed no significant difference in muscle strength. One trial reported a significant deterioration of activities of daily living (mean difference 0.54 on a 1 to 10 scale; 95% CI 0.14 to 0.93) and an increase in muscle pain during high‐dose creatine treatment in McArdle disease.
Authors' conclusions
High quality evidence from RCTs shows that short‐ and medium‐term creatine treatment increases muscle strength in muscular dystrophies. There is also evidence that creatine improves functional performance in muscular dystrophy and idiopathic inflammatory myopathy. Creatine is well tolerated in these people. High quality but limited evidence from RCTs does not show significant improvement in muscle strength in metabolic myopathies. High‐dose creatine treatment impaired activities of daily living and increased muscle pain in McArdle disease.
Plain language summary
Creatine for treating muscle disorders
Hereditary muscle diseases usually lead to a progressive muscle weakness. Treatment is mainly symptomatic because curative therapies are lacking. Creatine, a popular nutritional supplement among athletes, improves muscle performance in healthy individuals. This is an update of our review evaluating creatine treatment in muscle disorders that was first published in 2007. At this update we identified no new studies but we had previously found 14 randomised controlled trials with 364 participants which met our defined selection criteria. The methodological quality of these studies was high, with only one exception. Analysis of pooled results showed a significant increase in muscle strength in muscular dystrophies and an improvement in activities of daily living in muscular dystrophies and inflammatory myopathies during creatine treatment compared to placebo. Significant adverse events occurred only in people with glycogen storage disease type V presenting as an increase in muscle pain episodes and impairment in activities of daily living.
Summary of findings
Summary of findings for the main comparison. Creatine for treating muscular dystrophies.
| Creatine for treating muscular dystrophies | ||||||
| Patient or population: patients with muscular dystrophies Settings: outpatient treatment Intervention: creatine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | creatine | |||||
| Maximum voluntary contraction in dystrophinopathies % | The mean maximum voluntary contraction in dystrophinopathies in the control groups was 30.8 N (mean value of combined testing)1 | The mean maximum voluntary contraction in dystrophinopathies in the intervention groups was 9.2 higher (3.52 to 14.88 higher) | 121 (3 studies) | ⊕⊕⊕⊕ high | ||
| Maximum voluntary contraction in myotonic dystrophy type 1 % | The mean maximum voluntary contraction in myotonic dystrophy type 1 in the control groups was 104.4 Nm (knee extension)2 | The mean maximum voluntary contraction in myotonic dystrophy type 1 in the intervention groups was 4.39 higher (8.61 lower to 17.39 higher) | 76 (2 studies) | ⊕⊕⊕⊝ moderate3 | ||
| Maximum voluntary contraction in myotonic dystrophy type 2 % | The mean maximum voluntary contraction in myotonic dystrophy type 2 in the control groups was 52.4 kPa (grip strength)4 | The mean maximum voluntary contraction in myotonic dystrophy type 2 in the intervention groups was 8.8 higher (6.12 lower to 23.72 higher) | 10 (1 study) | ⊕⊕⊕⊕ high | ||
| Adverse events | See comment | See comment | 261 (8 studies) | ⊕⊕⊕⊕ high | Differences in side effect profiles of creatine and placebo treatment were not detected. No trial reported any clinically relevant adverse event. | |
| Global assessment of improvement felt better | 98 per 1000 | 442 per 1000 (228 to 857) | RR 4.51 (2.33 to 8.74) | 183 (4 studies) | ⊕⊕⊕⊕ high5 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Mean value of combined quantitative muscle testing (QMT) of elbow flexors and extensors, knee flexors and extensors, and grip at the end of placebo treatment (Escolar 2005). 2 Measurement at the end of placebo treatment (Tarnopolsky 2004b). 3 Quality of evidence was decreased due to inconsistency: one trial reported an increase in MVC (MD 8.4%, 95% CI ‐7.7 to 24.5; Walter 2002) and one trial showed a decrease in MVC (MD ‐3.1%, 95% CI ‐25 to 19.0; Tarnopolsky 2004b). 4 Measurement at the end of placebo treatment (Schneider‐Gold 2003). 5 One single blinded study was included (Banerjee 2010) but result would also be significant and similar if this study would not be considered (RR 4.71, 95% CI 2.22 to 10.00).
Summary of findings 2. Creatine for treating metabolic myopathies.
| Creatine for treating metabolic myopathies | ||||||
| Patient or population: patients with metabolic myopathies Settings: outpatient treatment Intervention: creatine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | creatine | |||||
| Maximum voluntary contraction in mitochondrial diseases % | The mean maximum voluntary contraction in mitochondrial diseases in the control groups was 693.43 N (foot plantar flexion)1 | The mean maximum voluntary contraction in mitochondrial diseases in the intervention groups was 3.65 lower (11.17 lower to 3.86 higher) | 38 (2 studies) | ⊕⊕⊕⊕ high | ||
| Maximum voluntary contraction in glycogen storage disease type V % | The mean maximum voluntary contraction in glycogen storage disease type v in the control groups was 942.71 N (foot plantar flexion) | The mean maximum voluntary contraction in glycogen storage disease type V in the intervention groups was 1.69 lower (6.48 lower to 3.09 higher) | 28 (1 study) | ⊕⊕⊕⊕ high | ||
| Impairment of activities of daily living (ADL) in glycogen storage disease type V Visual numeric scale. Scale from: 1 to 10. | The mean impairment of activities of daily living (ADL) in glycogen storage disease type v in the control groups was 2.82,3 | The mean impairment of activities of daily living (ADL) in glycogen storage disease type V in the intervention groups was 0.54 higher (0.14 to 0.93 higher)3 | 19 (1 study) | ⊕⊕⊕⊕ high | ||
| Adverse events in mitochondrial diseases | See comment | See comment | 38 (3 studies) | Two patients reported muscle cramps during creatine treatment. | ||
| Adverse events in glycogen storage disease type V | See comment | See comment | 28 (2 studies) | Significant increase in muscle pain episodes in one trial. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Data obtained from the study by Kornblum et al. (Kornblum 2005). 2 Additional data from the study by Vorgerd et al. (Vorgerd 2002). 3 Higher values indicate more impairment.
Summary of findings 3. Creatine for treating idiopathic inflammatory myopathies.
| Creatine for treating idiopathic inflammatory myopathies | ||||||
| Patient or population: patients with idiopathic inflammatory myopathies Settings: outpatient treatment Intervention: creatine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | creatine | |||||
| Aggregate functional performance time (AFPT), intention‐to‐treat analysis % | The mean aggregate functional performance time (AFPT), intention‐to‐treat analysis in the control groups was 28 s | The mean aggregate functional performance time (AFPT), intention‐to‐treat analysis in the intervention groups was 8.4 lower (15.4 to 1.49 lower) | 37 (1 study) | ⊕⊕⊕⊕ high | ||
| AFPT, valid compliant completer analysis % | The mean AFPT, valid compliant completer analysis in the control groups was 28 s | The mean AFPT, valid compliant completer analysis in the intervention groups was 11.6 lower (19.59 to 3.6 lower) | 29 (1 study) | ⊕⊕⊕⊕ high | ||
| Adverse events | See comment | See comment | 37 (1 study) | See comment | No clinically relevant adverse events were associated with creatine treatment. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Myopathies affect primarily skeletal muscle and can cause a wide variety of complaints including progressive muscle weakness and wasting, myalgias, early fatigue and painful muscle cramps. The main hereditary myopathies are classified into muscular dystrophies (for example, X‐linked dystrophinopathies (Duchenne and Becker muscular dystrophy), facioscapulohumeral muscular dystrophy (FSHD), limb girdle muscular dystrophies (LGMD), congenital muscular dystrophies (CMD)), myotonic dystrophies (type 1 myotonic dystrophy or DM1 (Curschmann‐Steinert disease) and type 2 myotonic dystrophy (DM2) or proximal myotonic myopathy (PROMM)), ion channel disorders (for example, periodic paralysis, congenital myotonias), metabolic myopathies (lipid and glycogen storage diseases, mitochondrial myopathies), congenital myopathies, distal myopathies and myofibrillar myopathies (MFM). Important progress has been made in the discovery of the molecular causes and pathogenesis of these hereditary myopathies. However, curative therapies such as gene or protein replacement, splice‐modulation therapy, stem cell therapy or specific drug treatment are still not available or at the experimental stage with the exception of glycogenosis type II (Pompe disease) in which an enzyme replacement therapy have been available since 2006. Treatment is mainly symptom‐oriented and includes regular physical therapy, aerobic exercise training, orthopaedic corrections, artificial ventilation in respiratory insufficiency and pharmacologic interventions, for example corticosteroids in Duchenne muscular dystrophy (DMD). Idiopathic inflammatory myopathies (IIM) are a heterogeneous group of acquired muscular disorders characterised by inflammation of the skeletal muscle not caused by a pathogen. The most common IIM of autoimmune origin are dermatomyositis (DM), polymyositis (PM) and sporadic inclusion body myositis (s‐IBM). DM and PM are usually responsive to immunosuppressive therapy but side effects are frequent.
Creatine is a naturally occurring compound that is found in meat and fish in high concentration. It is produced endogenously in the liver and the kidneys (Guthmiller 1994). Creatine is transported freely in the blood and is mainly taken up into muscle, testes and brain (Guimbal 1993; Wyss 2000). Creatine has a role in high energy phosphoryl group transfer during skeletal muscle contraction via the creatine kinase reaction. It also plays a role in transferring energy from the mitochondria to the cytosol in tissues with a high energy requirement such as brain and skeletal muscle (Wyss 1992). Finally, creatine may play a role in the regulation of muscle protein metabolism (Parise 2001). Creatine has been proposed as a potential pharmacological treatment for several neuromuscular diseases.
In healthy humans, creatine monohydrate supplementation has been shown to result in an increase in muscle total creatine and phosphocreatine (PCr) concentration in skeletal muscle (Harris 1992; Hultman 1996) and brain (Dechent 1999). An increase in muscle creatine concentration in skeletal muscle is seen with a dose of about 0.3 g/kg/d for six days or 0.04 g/kg/d for 30 days (Hultman 1996). The magnitude of the increase in skeletal muscle appears greatest in persons with low endogenous stores (Harris 1992). Both prospective and retrospective studies in humans have not found evidence for long‐ or short‐term significant side effects from creatine monohydrate supplementation taken in recommended doses (Mihic 2000; Poortmans 1999; Poortmans 2000; Poortmans 2005). The possibility of idiosyncratic reactions from creatine monohydrate must be considered but they are rare (Terjung 2000).
People with neuromuscular disorders can have lower skeletal muscle total creatine and PCr concentrations than control subjects (Matthews 1990; Matthews 1991; Park 1994; Park 1995; Tarnopolsky 1999a; Tarnopolsky 2001; Banerjee 2010). This may be a function of lower creatine transporter content or an impairment of energy charge (a measure of the relative amounts of adenosine triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP) in the cell) in these groups (Tarnopolsky 1999a; Tarnopolsky 2001). Therefore, the potential for creatine monohydrate supplementation to enhance performance may be greater in people who have reduced endogenous stores, such as those with neuromuscular disease. The dosing and magnitude of the response from creatine monohydrate supplementation in people with secondary defects in the creatine transporter has not been fully elucidated.
Creatine monohydrate supplementation has been shown to enhance force output in healthy volunteers by approximately 10% (Casey 1996; Tarnopolsky 2000; Terjung 2000). Another reported consequence of creatine monohydrate supplementation is an increase in fat‐free mass of about one kilogram (Harris 1992; Hultman 1996; Mihic 2000; Safdar 2008). This seems to be an effect of fluid retention in myocytes due to osmotic potential of high intracellular creatine abundance and an increased expression of genes and proteins related to muscle hypertrophy (Deldicque 2008; Safdar 2008). In animal models of neurodegenerative disease, creatine monohydrate supplementation has been shown to increase brain energy charge, reduce oxidative stress and attenuate neuronal degeneration (Andreassen 2001; Dedeoglu 2003; Ferrante 2000; Hersch 2006; Klivenyi 1999; Klivenyi 2004; Malcon 2000; Matthews 1998; Zhu 2004). Improved calcium handling resulting in lower intra‐cellular calcium concentrations and enhanced cell survival have been shown in cultured dystrophin‐deficient mdx cells, a mouse model of dystrophinopathy (Pulido 1998). Improvement in muscle function in mdx mice has also been noted following creatine supplementation (Passaquin 2002).
In theory, creatine monohydrate supplementation may benefit people with muscle disorders by non‐specifically enhancing muscle force output (temporal energy buffering) or the accretion of fat‐free mass, assuming this is muscle, or more directly by enhancing energy shuttling, reducing intra‐cellular calcium accumulation and apoptosis (Sullivan 2000), preventing oxidative stress (primary and secondary effect), activating glycolysis and glycogenolysis, and attenuating cell death (Gualano 2010; Klivenyi 1999; Klivenyi 2004; Passaquin 2002; Pulido 1998). Together, these considerations support the initiation of trials in people with myopathies that result in a reduction in energy charge.
The first prospective randomised study reporting the use of creatine monohydrate in a neurometabolic condition was published in 1997 (Tarnopolsky 1997). This study reported an increase in high intensity power output of hand grip and dorsi‐flexion, with no increase in endurance performance (Tarnopolsky 1997). Based upon these results, the apparent safety and efficacy in enhancing power output in athletes (Casey 1996; Poortmans 1999; Tarnopolsky 2000; Terjung 2000), and the theoretical benefits to people with muscle disorders (Klivenyi 1999; Park 1995; Passaquin 2002; Pulido 1998; Tarnopolsky 1999a; Tarnopolsky 2001; Wyss 1998), several human trials were initiated. There was significant interest in the results of an open (N = 81 participants) and single blinded (N = 21) study that found an increase in high intensity power output with creatine monohydrate supplementation in a heterogenous group of people with neuromuscular disorders (Tarnopolsky 1999b). Because this study was not randomised and the subgroups were not defined a priori this pilot study provided a catalyst for future studies.
This is an update of our systematic Cochrane review (Kley 2007) evaluating benefits of creatine treatment in muscle disorders. It was previously updated in 2008 and 2011 (Kley 2011).
Objectives
To evaluate the efficacy of oral creatine supplementation in muscle diseases.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all randomised controlled trials (RCTs) or quasi‐randomised controlled trials of creatine treatment in hereditary muscle diseases and idiopathic inflammatory myopathies.
Types of participants
We included participants of all ages with a proven diagnosis of hereditary muscle disorder (including muscular dystrophies and metabolic myopathies) or idiopathic inflammatory myopathy. Criteria for diagnosis depended on the type of muscle disease. They included clinical features, electromyographic characteristics, muscle biopsy findings and genetic results. People with motor neuron disease or (spino‐)cerebellar degeneration (for example, Friedreich's ataxia) were excluded.
Types of interventions
We considered any treatment with oral creatine supplementation of at least 0.04 g/kg body weight/day. If the loading dose was at least 0.3 g/kg/d for two days, a maintenance dose of at least 0.03 g/kg/d was accepted. Cross‐over trials with washout periods shorter than 28 days required statistical examination of carryover effects to be included. These inclusion criteria took into account the knowledge about the clinical pharmacology of creatine (Harris 1992; Hultman 1996; Persky 2003; Rawson 2004).
Types of outcome measures
The duration of supplementation lasted between three weeks and six months. Due to studies in healthy persons, we assumed that the maximum intramuscular concentration of creatine was reached in all trials. We did not categorise the trials into short‐term and long‐term supplementation because of lack of evidence on differences in efficacy and accepted all time points of measurements.
Primary outcomes
Changes in muscle strength measured by quantitative muscle testing.
Secondary outcomes
Changes in activities of daily living (ADL) assessed by questionnaires or functional tests.
Changes in muscle strength measured by manual muscle testing.
Changes in muscle energy parameters assessed by 31 phosphorous resonance spectroscopy.
Percentage changes in muscle mass or a surrogate for muscle mass.
Adverse events during supplementation.
Search methods for identification of studies
Electronic searches
On 11 September 2012, we searched the Cochrane Neuromuscular Disease Group Specialized Register, CENTRAL (2012, Issue 9 in The Cochrane Library), MEDLINE (January 2010 to September 2012) and EMBASE (January 1980 to September 2012) for RCTs on the use of creatine for treating muscle diseases. We reviewed the bibliographies of the RCTs identified and contacted the authors and known experts in the field to identify additional published or unpublished data.
The detailed search strategies are in the appendices: CENTRAL Appendix 1, MEDLINE Appendix 2 and EMBASE Appendix 3.
We also performed searches of trials registries (ClinicalTrials.gov, http://clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP, http:/apps.who.int/trialsearch/)) for ongoing trials (search date 20 February 2013, search term "creatine").
Data collection and analysis
Selection of studies
Two review authors screened the titles and abstracts of the articles identified by the database searches. We obtained the full text of all potentially relevant studies for independent assessment by two authors. The review authors resolved any differences regarding which studies to include by consensus reached after discussion.
Data extraction and management
Two review authors independently extracted the data from the original reports using a data extraction form. If possible, we obtained missing data from the trial authors.
Assessment of risk of bias in included studies
For each trial fulfilling the inclusion requirements, two review authors independently assessed risk of bias (Higgins 2011). The review authors contacted authors of studies when the need for additional information arose. They resolved differences in assessment by consensus.
Data synthesis
We performed fixed‐effect meta‐analyses using the generic inverse variance (GIV) and inverse variance weighting facilities in Review Manager (RevMan, current version 5.2 (RevMan 2012)), the Cochrane statistical package. Results were expressed as mean differences (MD) and 95% confidence intervals (CI). We analysed primary and secondary outcomes under consideration whenever the data allowed. Due to its long ordinal scale, we analysed Medical Research Council (MRC) sum scores as continuous data. We used P values described as < 0.05 as 0.05 in calculation of standard errors. This is a conservative approach because derived standard errors are generally larger than those used by the authors in the analysis.
We did not scale the treatment effects and standard errors to a specified follow‐up period because we have supposed a nearly constant treatment effect shortly after the beginning of creatine supplementation instead of a constant rate of change over time. This assumption relies on many studies in healthy people. For example, the maximum increase in muscle force seems to correlate with the achievement of the upper limit of intramuscular creatine concentration, which can be reached after a few days of creatine ingestion depending on its dosage (Persky 2003). However, most of these studies are short‐term trials. Studies on the effects of prolonged creatine supplementation are rare and the results inconsistent.
For the primary outcome measure 'muscle strength measured by quantitative muscle testing', we calculated changes after treatment phases as percentage of baseline values to make measurements of different muscle groups comparable. In the case of cross‐over trials, differences between creatine and placebo phases were converted into percentage of baseline values of the placebo phase. In the absence of baseline data we used the available values of the placebo phase. One trial reported only differences between treatment periods (Walter 2002). In this case, we decided to use the baseline data of Tarnopolsky 2004b for participants affected by the same muscle disease, since the mean age of the participants and the muscle groups examined were similar. In the case of measurements of more than one muscle group, we accepted sum scores (Escolar 2005) or chose a muscle group measured in other trials of the same muscle disorder. In case of bilateral measurements, we chose the right side (Schneider‐Gold 2003).
For the secondary outcome measure 'Changes in activities of daily living (ADL) assessed by questionnaires or functional tests', participants' global assessment of improvement was converted into dichotomous data (improvement yes/no). We calculated mean differences (MD) and confidence intervals (CIs) of aggregate functional performance time (AFPT, trial of Chung 2007) using t‐tests.
For the secondary outcome measure 'muscle strength measured by manual muscle testing', we calculated percentage changes of MRC sum scores. We replaced missing change‐from‐baseline standard deviations in the trial from Tarnopolsky 2004b by standard deviations from Walter 2002 (see above for explanation). On the same grounds, we used correlation coefficients derived from data of the trial from Escolar 2005 for imputing change‐from‐baseline standard deviations for the trial from Tarnopolsky 2004a.
Where meta‐analysis was possible for a disease intervention, we constructed a 'Summary of findings' table for each appropriate group of diseases. The pre‐defined outcomes were our primary outcome, change in muscle strength measured by quantitative muscle testing, the changes in activities of daily living (ADL) assessed by questionnaires or functional tests, the patient global assessment, and adverse events.
Subgroup analysis and investigation of heterogeneity
Due to detected heterogeneity across the trials, we separated data of dystrophic and metabolic myopathies. We classified inflammatory myopathies in a third group. This decision took into account pathophysiological and genetic differences between these groups of myopathies. We also performed a subgroup analysis regarding the type of treated myopathy.
Results
Description of studies
See Tables: Characteristics of included studies
In the searches for this update there were 900 references from MEDLINE, 794 from EMBASE, 177 from CENTRAL and 86 from the Cochrane Neuromuscular Disease Group Register. We identified no new trials for inclusion from these most recent searches. Our searches of trials registries revealed records for 204 trials in ClinicalTrials.gov and for 71 trials in ICTRP. We detected one ongoing creatine trial in juvenile dermatomyositis (see Characteristics of ongoing studies) that may be included in a future update of this review.
Our previous and recent searches revealed a total of 28 papers that were selected for closer scrutiny. We identified 15 RCTs that met the inclusion criteria for this review. One trial (Sejersen 2000) was only published in abstract form and we could not obtain full details; we therefore excluded it (see Characteristics of excluded studies). The remaining 14 trials fulfilling the selection criteria included a total number of 364 randomised participants. One three‐arm trial compared creatine and glutamine treatment with placebo (Escolar 2005). The other 13 trials compared creatine treatment with placebo. A total of 277 participants received creatine and 275 participants received placebo due to the fact that 10 of the 14 trials used a cross‐over design. The duration of supplementation periods lasted between three weeks (Tarnopolsky 1997) and six months (Escolar 2005; Chung 2007). The doses of creatine varied from 3 to 20 g daily.
Four trials examined the effect of creatine in people with dystrophinopathies (Banerjee 2010; Escolar 2005; Louis 2003; Tarnopolsky 2004a). One RCT compared creatine with placebo in various types of muscular dystrophies (Walter 2000). Three trials studied the efficacy of creatine in myotonic dystrophies, two in people with type 1 myotonic dystrophy (Tarnopolsky 2004b; Walter 2002) and one in persons with type 2 myotonic dystrophy (Schneider‐Gold 2003). Three trials compared creatine with placebo in people with mitochondrial cytopathy (Klopstock 2000; Kornblum 2005; Tarnopolsky 1997) and two trials evaluated the effect of creatine in glycogen storage disease type V (McArdle disease) (Vorgerd 2000; Vorgerd 2002). One trial tested the hypothesis that creatine with exercise is more effective than exercise with placebo in people with polymyositis or dermatomyositis (Chung 2007).
We excluded 14 studies that did not fulfill the inclusion criteria (see Characteristics of excluded studies). Twelve of these studies were case reports or non‐controlled trials. One controlled study was not randomised and one trial used a combination therapy (creatine, coenzyme Q10 and lipoic acid).
Risk of bias in included studies
See Characteristics of included studies (risk of bias tables) and Figure 1.
1.
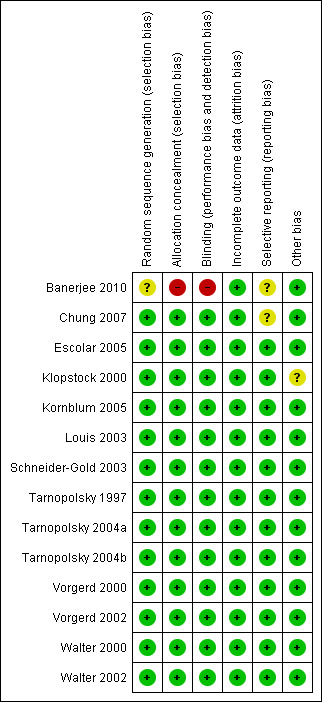
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We based our 'Risk of bias' assessments on information described in the text and additional information obtained from study authors. We assessed sequence generation and allocation concealment as adequate in 13 of 14 trials. One study was only single‐blinded (Banerjee 2010). In the other trials, participants and observers were blinded. Incomplete outcome data were adequately addressed. In one cross‐over trial (Walter 2000), the washout period lasted only three weeks and carry‐over effects were detected. Therefore, authors included only data from the group of participants who received placebo in the first and creatine in the second period in the final statistical evaluation. In the other nine cross‐over trials, the washout period was sufficient except for two participants in one trial (Klopstock 2000) who had a washout period of 24 and 26 days. We assessed the diagnostic criteria as adequate in all trials. In the majority of cases, diagnosis of hereditary muscle disease was confirmed by genetic analysis. Significant differences in baseline values between study groups were not detected.
Effects of interventions
See: Table 1; Table 2; Table 3
Primary outcome measure:
Changes in muscle strength measured by quantitative muscle testing
Muscular dystrophies
This outcome was available for six of eight studies (Escolar 2005; Louis 2003; Schneider‐Gold 2003; Tarnopolsky 2004a; Tarnopolsky 2004b; Walter 2002). Overall, 138 participants received creatine and 139 placebo. The follow‐up periods ranged from eight weeks to six months (see Characteristics of included studies). Five trials measured the isometric maximum voluntary contraction (MVC) of various muscle groups (see Characteristics of included studies). One trial (Schneider‐Gold 2003) used a hand‐held dynamometer to calculate a compound strength score of proximal arm and leg muscles as well as a grip balloon dynamometer to evaluate the maximum grip strength.
Analysis of pooled data demonstrated a statistically significant improvement in maximum voluntary contraction after creatine treatment as compared to placebo, with a mean difference (MD) of 8.47% (95% CI 3.55 to 13.38) (see Analysis 1.1; Figure 2). In studies on dystrophinopathies, the standard errors were smaller in the cross‐over trials (Louis 2003; Tarnopolsky 2004a) than in the parallel group trial (Escolar 2005). A change of standard errors dependent on the follow‐up period was not found.
1.1. Analysis.
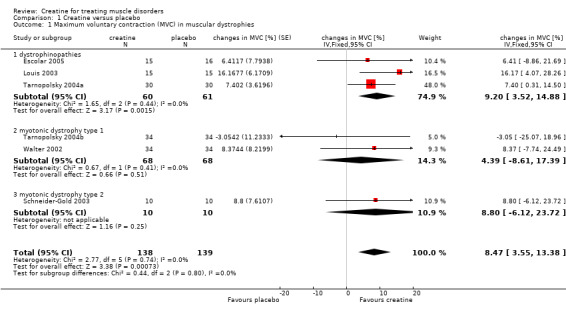
Comparison 1 Creatine versus placebo, Outcome 1 Maximum voluntary contraction (MVC) in muscular dystrophies.
2.
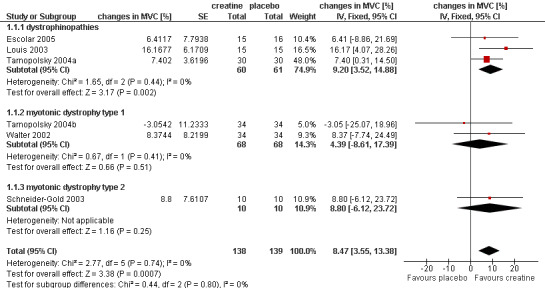
Forest plot of comparison: 1 Creatine versus placebo, outcome: 1.1 Maximum voluntary contraction (MVC) in muscular dystrophies.
Metabolic myopathies
Four of five studies performed an isometric MVC measurement (Klopstock 2000; Kornblum 2005; Tarnopolsky 1997; Vorgerd 2002). The length of follow‐up ranged from three (Tarnopolsky 1997) to six weeks (Kornblum 2005). The trial by Klopstock et al. (Klopstock 2000) in 16 participants with mitochondrial diseases found no significant treatment effect on maximum voluntary muscle torques of biceps and quadriceps muscle. The meta‐analysis of the other three cross‐over trials including 33 participants also showed no significant change in MVC after creatine treatment compared to placebo (Kornblum 2005; Tarnopolsky 1997; Vorgerd 2002) (see Analysis 1.2; Figure 3).
1.2. Analysis.
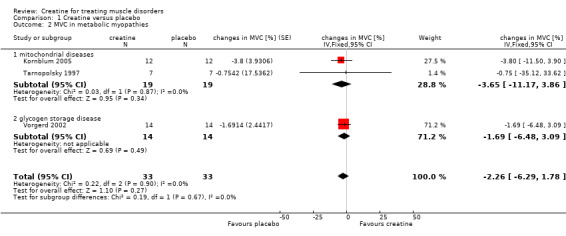
Comparison 1 Creatine versus placebo, Outcome 2 MVC in metabolic myopathies.
3.
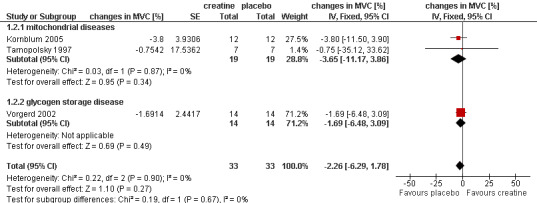
Forest plot of comparison: 1 Creatine versus placebo, outcome: 1.2 MVC in metabolic myopathies.
Inflammatory myopathies
A quantitative muscle testing was not performed in the study by Chung et al. (Chung 2007).
Secondary outcome measures:
Changes in activities of daily living assessed by questionnaires or functional tests
Muscular dystrophies
Seven trials evaluated this outcome in a variety of ways (Banerjee 2010; Escolar 2005; Schneider‐Gold 2003; Tarnopolsky 2004a; Tarnopolsky 2004b; Walter 2000; Walter 2002).
Two trials in participants with Duchenne muscular dystrophy (Escolar 2005; Tarnopolsky 2004a) and one trial in myotonic dystrophy type I (Tarnopolsky 2004b) performed functional tests. In Escolar 2005, there was a significant lesser deterioration of time to climb 4 stairs and of time to run 10 metres after six months of creatine treatment (14 participants) compared to placebo (14 participants) (mean difference of changes between groups: time to climb 4 stairs ‐3.4 s (95% CI ‐6.18 to ‐0.56), time to walk 10 metres ‐1.0 s (95% CI ‐1.60 to ‐0.34)). Moreover, post hoc analysis in boys younger than seven years (26 participants) revealed significant improved scores on time to stand from supine position in the creatine group when compared with placebo group. Differences in the trials by Tarnopolsky et al. (Tarnopolsky 2004a; Tarnopolsky 2004b) were not significant.
In four trials, participants (Schneider‐Gold 2003; Walter 2000; Walter 2002) or parents of participants (Banerjee 2010) were asked for global assessment of improvement during treatment periods. One trial in Duchenne muscular dystrophy reported that this statement considered changes in activity levels, falls and time needed for motor tasks (Banerjee 2010). Overall 91 participants received creatine and 92 placebo. Analysis of pooled data revealed that an improvement was reported significantly more frequently after creatine treatment compared to placebo, with a risk ratio (RR) of 4.51 (95% CI 2.33 to 8.74) (see Analysis 1.3; Figure 4). The result was also significant and similar when the single‐blinded study with a higher risk of bias (Banerjee 2010) was not included (RR 4.71; 95% CI 2.22 to 10.00, 156 participants). Three trials (Schneider‐Gold 2003; Walter 2000; Walter 2002) used the Neuromuscular Symptom Score (NSS) (Soueidan 1993) to assess the subjective impairment of participants in 14 activities of daily living (ADL) but analysis of pooled data did not show a significant change in NSS after creatine supplementation compared to placebo (see Analysis 1.4; Figure 5). Two trials used visual analogue scales (VAS) for assessment of daily activities (Schneider‐Gold 2003; Tarnopolsky 2004b). In 20 people with type 2 myotonic dystrophy (Schneider‐Gold 2003), VAS revealed greater absolute but not percentage improvement in the creatine group compared to placebo (difference of 20.0 points (scale from 0 to 100), P < 0.05). The trial by Tarnopolsky et al. (Tarnopolsky 2004b) in 34 participants with type 1 myotonic dystrophy reported no significant differences between creatine treatment and placebo.
1.3. Analysis.
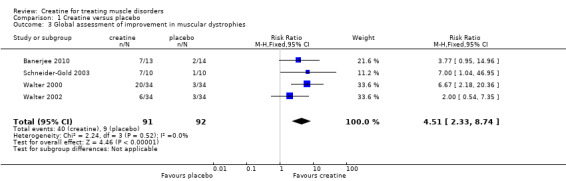
Comparison 1 Creatine versus placebo, Outcome 3 Global assessment of improvement in muscular dystrophies.
4.
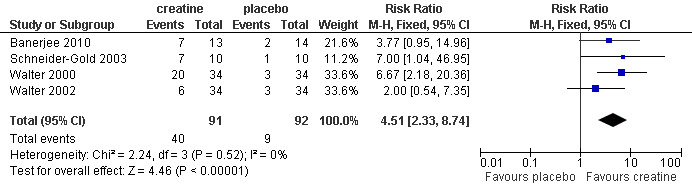
Forest plot of comparison: 1 Creatine versus placebo, outcome: 1.3 Global assessment of improvement in muscular dystrophies.
1.4. Analysis.
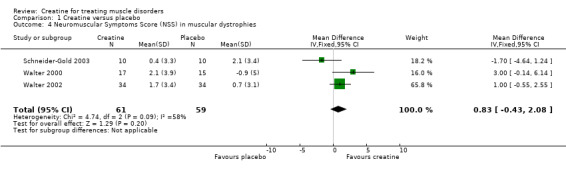
Comparison 1 Creatine versus placebo, Outcome 4 Neuromuscular Symptoms Score (NSS) in muscular dystrophies.
5.
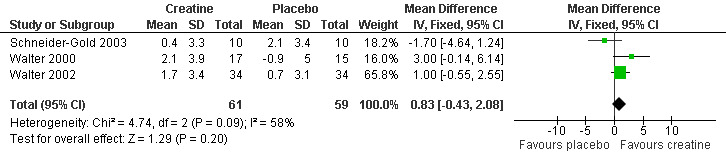
Forest plot of comparison: 1 Creatine versus placebo, outcome: 1.4 Neuromuscular Symptoms Score (NSS) in muscular dystrophies.
Metabolic myopathies
Three studies in mitochondriopathies (Klopstock 2000; Kornblum 2005; Tarnopolsky 1997) and one trial in glycogenosis type V (Vorgerd 2002) reported changes in ADL.
In mitochondriopathies, two trials used a VAS for patients own assessment of six ADL (Tarnopolsky 1997) and general activity (Klopstock 2000). Tarnopolsky et al. also performed a two‐minute walk test (Tarnopolsky 1997). In Klopstock 2000, measurements included the Hammersmith motor ability score (Scott 1982), NSS and function time tests. One trial utilised the modified Boston score (testing of activities as climbing a stair, squat and sitting up, elevation of arms) (Kornblum 2005). There was no significant change after creatine treatment compared to placebo in any of these tests.
In the cross‐over trial in glycogenosis type V (Vorgerd 2002), participants daily assessed impairment of ADL on a visual numeric scale (scale of 1 to 10, higher values indicated more impairment). Statistical analysis of data from 17 participants revealed a significant deterioration of ADL during creatine treatment compared to placebo (MD of 0.54 on visual numeric scale, 95% CI 0.14 to 0.93). Participants' global assessment of study periods showed a significant difference in assignment favouring placebo period (eleven individuals felt better in placebo period, three in creatine phase and three persons did not perceived a clear‐cut difference).
Inflammatory myopathies
Aggregate functional performance time (AFPT) was the primary outcome in Chung 2007. AFPT aggregates four functional assessments measured in seconds: 1. 50‐foot walk, 2. get up and go test involving a 50‐foot walk, 3. stair ascent test (19 steps), 4. stair descent test (19 steps). Intention‐to‐treat (ITT) analysis (37 participants) and valid compliant completer (VCC) analysis (29 participants) revealed a significant improvement of AFPT at six months of creatine supplementation compared to placebo, with a MD of ‐8.4% (95% CI ‐15.40 to ‐1.49; ITT analysis) and ‐11.6% (95% CI ‐19.59 to ‐3.60; VCC analysis). Differences at three months were not significant.
In the same trial, a functional index in myositis (Josefson 1996) was used to evaluate endurance of activities. The differences in changes between creatine and placebo group were not significant (Chung 2007).
Changes in muscle strength measured by manual muscle testing
Muscular dystrophies
Six trials reported Medical Research Council (MRC) sum scores (Escolar 2005; Schneider‐Gold 2003; Tarnopolsky 2004a; Tarnopolsky 2004b; Walter 2000; Walter 2002). Overall 138 participants received creatine and 137 placebo. Analysis of pooled data showed a significant increase in MRC sum score after creatine treatment compared to placebo. The MD was 1.10% (95% CI 0.37 to 1.82) (see Analysis 1.5; Figure 6). One trial in Duchenne muscular dystrophy also performed manual muscle testing of 34 muscle groups using a 1 to 10 scale (Banerjee 2010). The adjusted mean MMT score in the creatine group including 13 participants (6.2, 95% CI 5.9 to 6.5) was significantly higher compared to the placebo group including 14 participants (5.8, 95% CI 5.5 to 6.1).
1.5. Analysis.

Comparison 1 Creatine versus placebo, Outcome 5 Percentage change in MRC sum score in muscular dystrophies.
6.
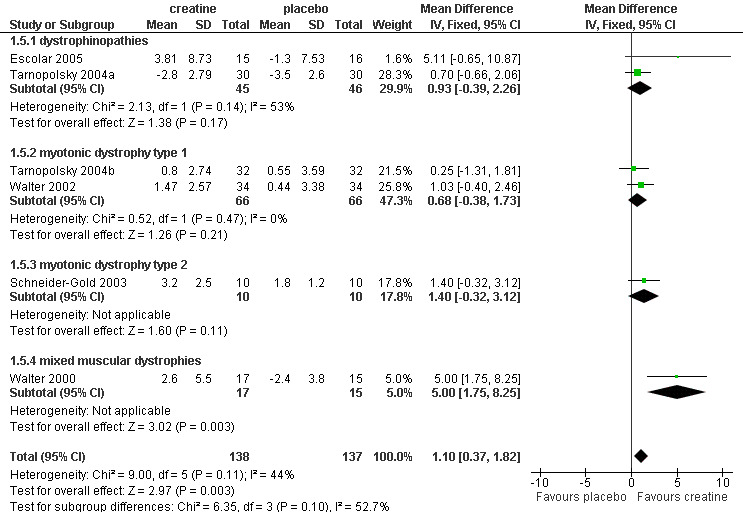
Forest plot of comparison: 1 Creatine versus placebo, outcome: 1.5 Percentage change in MRC sum score in muscular dystrophies.
Metabolic myopathies
Only one study (Klopstock 2000), with 16 participants, reported MRC sum scores. The improvement following creatine treatment was not significant.
Inflammatory myopathies
Only selected muscle groups were assessed by manual muscle testing (Chung 2007). MRC sum scores were not reported. Shoulder abduction and hip flexion improved significantly after three and six months of creatine treatment in 19 participants compared to placebo (18 participants). Significant improvement of elbow flexion and knee extension compared to placebo was only detected after three months of creatine supplementation.
Muscle energy parameters assessed by 31 phosphorous resonance spectroscopy
Muscular dystrophies
Three trials measured the ratio of PCr to beta‐ATP. Louis 2003 examined six participants with dystrophinopathies. After creatine supplementation, the investigators observed an increase of PCr/beta‐ATP ratio of about 8% whereas the ratio remained almost unchanged in the placebo group. The difference was described as not significant in the text but a P value of 0.04 was presented in a table (level of statistical significance was set at P < 0.05). In a second trial in Duchenne muscular dystrophy (Banerjee 2010), differences between creatine and placebo group were not significant. In a cross‐over trial in 34 participants with type 1 myotonic dystrophy, the PCr/beta‐ATP ratios were not significantly different in the creatine and placebo periods (Tarnopolsky 2004b).
In the study by Banerjee et al. (Banerjee 2010), analysis of covariance (ANCOVA) taking into account the age and stature showed that the adjusted mean ratio of PCr to Pi in the creatine group (4.7, 95% CI 3.9 to 5.6, 13 participants) was significantly higher compared to the placebo group (3.3, 95% CI 2.5 to 4.2, 14 participants).
Metabolic myopathies
This outcome was published in two studies in participants with glycogen storage disease type V including seven (Vorgerd 2000) and 14 (Vorgerd 2002) participants as well as in one trial in 13 participants with mitochondriopathies (Kornblum 2005). The treatment periods lasted five (Vorgerd 2000; Vorgerd 2002) and six weeks (Kornblum 2005). Analysis of pooled data showed no significant increase in PCr/beta‐ATP ratio after creatine treatment as compared to placebo (see Analysis 1.6).
1.6. Analysis.
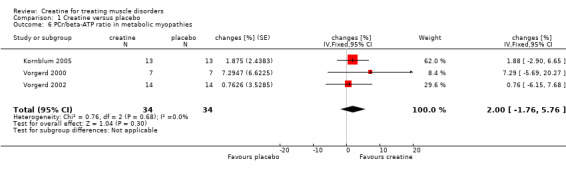
Comparison 1 Creatine versus placebo, Outcome 6 PCr/beta‐ATP ratio in metabolic myopathies.
All three studies used the same standardised calf muscle ergometric test to assess the effects of treatment on skeletal muscle bioenergetics. In the first study by Vorgerd et al. (Vorgerd 2000), the PCr depletion increased during aerobic and ischaemic exercise. Moreover they reported an increase in accumulation of inorganic phosphate during ischaemic exercise. However, the force‐time integral during Ischaemic exercise was significantly higher with creatine. In the other two trials (Kornblum 2005; Vorgerd 2002), there were neither significant changes in PCr consumption nor in increases in Pi and ADP values during aerobic and ischaemic exercise. Meta‐analysis demonstrated a reduction of ATP consumption during aerobic exercise in the creatine phase as compared to placebo with a MD of ‐1.75 %PCri (95% CI ‐2.98 to ‐0.51) (see Analysis 1.7). This indicates a higher resynthesis of ATP by phosphoryl group transfer from PCr to ADP via creatine kinase reaction. This might be interpreted as a favourable increase in exercise tolerance. Changes in ATP consumption during ischaemic exercise did not reach statistical significance (see Analysis 1.8). Moreover meta‐analysis revealed no significant improvement in time constants (tau) for oxidative PCr recovery from aerobic (see Analysis 1.9) and ischaemic (see Analysis 1.10) exercise.
1.7. Analysis.
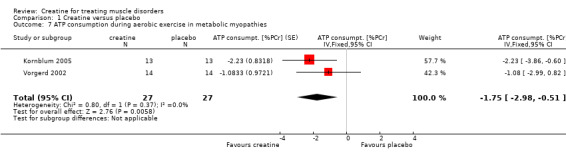
Comparison 1 Creatine versus placebo, Outcome 7 ATP consumption during aerobic exercise in metabolic myopathies.
1.8. Analysis.
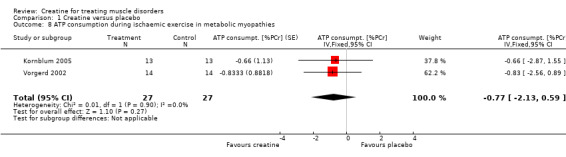
Comparison 1 Creatine versus placebo, Outcome 8 ATP consumption during ischaemic exercise in metabolic myopathies.
1.9. Analysis.
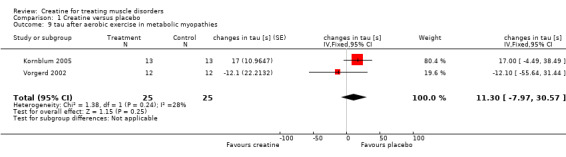
Comparison 1 Creatine versus placebo, Outcome 9 tau after aerobic exercise in metabolic myopathies.
1.10. Analysis.
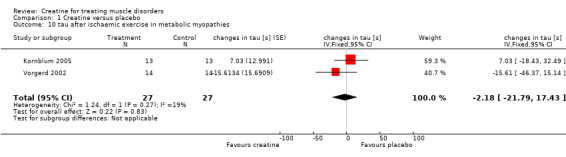
Comparison 1 Creatine versus placebo, Outcome 10 tau after ischaemic exercise in metabolic myopathies.
Inflammatory myopathies
The PCr/beta‐nucleoside triphosphate (NTP) ratio was evaluated in a subset of 24 participants (Chung 2007). Fourteen participants received creatine and ten placebo. A significant increase in mean PCr/beta‐NTP was only detected in the creatine group (5.4% at three months, 3.4% at six months).
Percentage changes in muscle mass or a surrogate for muscle mass
Muscular dystrophies
This outcome was available for three cross‐over studies including 98 participants. Walter 2002 assessed the lean body mass (LBM) from reactance and resistance measurements with bioelectrical impedance analysis. In two trials by Tarnopolsky et al. (Tarnopolsky 2004a; Tarnopolsky 2004b), participants underwent a whole‐body dual‐energy X‐ray absorptiometry (DEXA) scan for determination of fat‐free mass (FFM). Meta‐analysis demonstrated a significant increase in LBM during creatine treatment compared to placebo. The MD was 0.63 kg (95% CI 0.02 to 1.25) (see Analysis 1.11).
1.11. Analysis.
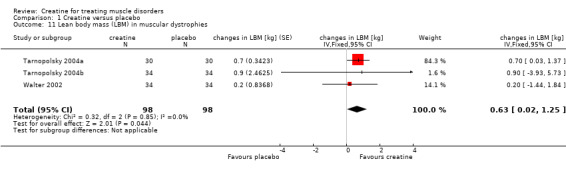
Comparison 1 Creatine versus placebo, Outcome 11 Lean body mass (LBM) in muscular dystrophies.
Metabolic myopathies
This outcome was not published for any of the trials.
Inflammatory myopathies
This was not an outcome measure in Chung 2007.
Adverse events during supplementation
Muscular dystrophies
In one trial in 34 participants with type 1 myotonic dystrophy, the serum creatinine concentration increased with creatine supplementation (Tarnopolsky 2004b). However, an effect of creatine on serum creatinine clearance was not found in three studies, which included 79 participants (Louis 2003; Tarnopolsky 2004a; Tarnopolsky 2004b). Side effect profiles were similar to placebo treatment without significant differences (Escolar 2005; Tarnopolsky 2004b). No trial reported any clinically relevant adverse events.
Metabolic myopathies
In the first trial (nine participants) on glycogen storage disease type V, one participant reported slight headache and dizziness during the first four days of the creatine phase (Vorgerd 2000). In the second trial, which involved 17 participants, a significant increase in muscle pain episodes occurred (Vorgerd 2002). Studies on mitochondrial myopathy participants reported muscle cramps in two participants (Klopstock 2000) and in another trial, flatulence during creatine intake in three of 15 participants (during placebo treatment in one of 13 participants) (Kornblum 2005). In one cross‐over trial (7 participants), participants did not complain of any side effects (Tarnopolsky 1997).
Inflammatory myopathies
No clinically relevant adverse events were associated with creatine treatment (Chung 2007).
Discussion
The data analysis reveals that there is high quality evidence that short‐ to intermediate‐term supplementation with creatine monohydrate in people with muscular dystrophy resulted in a modest, but significant, increase in maximal isometric force of approximately 8.5% using quantitative muscle testing, with a less robust improvement noted using MRC manual muscle testing scores (1.1%) (Table 1). These findings were associated with a significant increase in fat free mass of 0.63 kg. Meta‐analysis of changes in activities of daily living was complicated by the fact that a variety of tests were used to evaluate this outcome. Pooled data of participants' global assessment of improvement showed that 44% felt better during creatine treatment compared to less than 10% in the placebo groups. Improvement in functional tests and participants' own assessment of activities of daily living also indicate that creatine increases functional performance in muscular dystrophies. Changes in Neuromuscular Symptom Score were not significant but it may be that this score is not very sensitive to detect effects of creatine supplementation.
Most of the clinically relevant effects were seen in dystrophinopathy and type 2 myotonic dystrophy, but not consistently in type 1 myotonic dystrophy. This latter finding suggests that there may be clinical heterogeneity in the response and findings in one type of dystrophy, for example dystrophinopathy, cannot be applied directly to another, for example facioscapulohumeral dystrophy (FSHD). Although no specific trial of creatine in FSHD has been performed, 12 of the 32 muscular dystrophy patients studied by (Walter 2000) had FSHD and another Cochrane review has extracted the FSHD data (Rose 2004). These data seemed to show no benefit for creatine in FSHD but the numbers were small and the data were not analysed in the same way as for this review.
The different lengths of follow‐up period among the trials included in this meta‐analysis should be kept in mind. Even though a significant heterogeneity was not detected, changes in treatment effects depending on the duration of supplementation could not be excluded. It may be possible that there is a decline in response with longer intake. It would be useful to study the effects of long‐term creatine supplementation and it may be interesting to compare continuous long‐term ingestion with a cyclic intake.
There is also evidence that creatine improves functional performance in dermatomyositis and polymyositis. In metabolic myopathies (including mitochondrial myopathies) there was high quality evidence, but from only three diverse studies, that creatine has no effect on increasing strength by any measurement (Table 2), although ATP consumption during exercise was slightly attenuated. There is also evidence that creatine improved functional performance in idiopathic inflammatory myopathy (Table 3).The discrepancy between the fairly consistent effects noted in muscular dystrophy and possible effects in idiopathic inflammatory myopathy compared with metabolic myopathies is not entirely clear. However, it is possible that having fewer participants led to a type II statistical error (failing to detect an effect when in truth there is one).
Overall, the finding of an increased fat free mass and maximal strength are consistent with the balance of data in young healthy individuals (Casey 1996; Harris 1992; Hultman 1996; Mihic 2000; Tarnopolsky 2000) and older adults (Brose 2003; Burke 2003; Chilibeck 2004; Chrusch 2001). Quantitative muscle testing seems to be more sensitive to detect moderate changes in strength than manual muscle testing. This conclusion is in accordance with findings in Duchenne muscular dystrophy: In a 6‐month trial by Fenichel et al. (Fenichel 2001), QMT revealed a significant deterioration in strength of about ‐11% in the placebo group whereas MMT showed a non‐significant change of about ‐2%. Similar changes have been reported in the trial by Escolar et al. (Escolar 2005). The increase in muscle creatine and phosphocreatine documented using magnetic resonance spectroscopy appears to be somewhat less in people with muscular dystrophy and metabolic myopathies as compared to healthy individuals (Brose 2003; Casey 1996; Harris 1992; Hultman 1996). However, the participant numbers were quite small and studies using direct measurements from muscle biopsies have not been completed.
Long‐term studies (greater than one year) are not currently available to access the safety of creatine monohydrate supplementation in people with muscle disorders. However, in studies up to six months in duration there did not appear to be any significant side effects except for a significant increase in muscle pain and impairment in ADL in people with glycogenosis type V (Vorgerd 2002). The reason for this exacerbation of pain remains to be elucidated. Most studies in healthy participants did not report any significant increase in symptoms over placebo (Greenwood 2003; Kreider 2003; Mihic 2000; Poortmans 1999; Poortmans 2000; Shao 2006; Terjung 2000). An increase in serum creatinine of approximately 10% to 15 % is likely indicative of increased rate of delivery of creatinine to the nephron with no evidence of alterations in creatinine clearance rate (Brose 2003; Poortmans 1999; Poortmans 2005). These safety results are very similar to those reporting few side effects in trials in people with neurological disorders (amyotrophic lateral sclerosis, Charcot‐Marie‐Tooth disease and Huntington's disease) (Chetlin 2004; Groeneveld 2005; Hersch 2006; Shefner 2004; Verbessem 2003).
Given that a reduction in total muscle mass and strength are characteristic hallmarks of muscular dystrophy, even a slight improvement in these variables seen with creatine supplementation could potentially be of functional significance. For example, corticosteroids are a mainstay of therapy in dystrophinopathy in spite of the known side effects, due to the fact that modest increases in strength appear to translate into longer‐term improvements in functional status and outcome (Manzur 2004). Preliminary evidence suggests that creatine monohydrate supplementation increased strength and fat‐free mass in boys with dystrophinopathy who were already taking corticosteroids (Tarnopolsky 2004a). Whether or not a protective effect of creatine monohydrate with respect to bone mass (Chilibeck 2005; Roy 2002; Tarnopolsky 2004a), or type II muscle fibre atrophy (Roy 2002), will be realised in clinical trials in participants with muscular dystrophy taking corticosteroids remains possible, but unproven. To date, the functional implications of the short‐term increases in strength and fat free mass following creatine supplementation have not been subjected to longer‐term trials. There is a need for longer‐term studies of the efficacy and side effects of creatine in muscle diseases.
Authors' conclusions
Implications for practice.
There is high quality evidence from RCTs that short‐ and intermediate‐term creatine treatment can significantly improve muscle strength in people with muscular dystrophies.There is also evidence that creatine improved functional performance in muscular dystrophy and idiopathic inflammatory myopathy. Creatine was well tolerated in these people. Limited but high quality evidence from RCTs did not show significant improvement in muscle strength in metabolic myopathies. High‐dose creatine treatment impaired ADL and increased muscle pain in glycogenosis type V.
Implications for research.
There is a need for long‐term studies of creatine in muscle diseases. Particular attention should be given to the specific type of myopathy. Diseases such as FSHD or myofibrillar myopathies have not yet been specifically targeted for evaluation. There is also a need for more studies in the metabolic myopathies due to the fact that dose dependent paradoxical effects were seen in McArdle disease and no studies have been conducted in other metabolic disorders such as fatty acid oxidation defects. There is a need for standardisation of outcome measures, particularly with regard to activities of daily living. Regarding strength measurement, quantitative muscle testing should be chosen in preference to manual muscle testing.
What's new
| Date | Event | Description |
|---|---|---|
| 20 February 2013 | New search has been performed | Searches of trial registries added. Minor revisions to text including description of one ongoing trial. |
| 28 September 2012 | New citation required but conclusions have not changed | A review of the updated search results revealed no new relevant trials. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 22 December 2010 | New citation required and conclusions have changed | Searches updated to October 2010. One new trial identified and added. Meta‐analysis of global assessment of improvement added. SoF tables added. Changes in ADL moved to the secondary outcome measures. Discussion and conclusions updated. |
| 17 August 2010 | New search has been performed | Searches updated to July 2009. One new study identified and included. Added 'Changes in activities of daily living' as second primary outcome measure. Meta‐analyses extended. Risk of bias tables added. Discussion and conclusions updated. |
| 3 November 2008 | Amended | Converted to RevMan 5 |
| 15 May 2008 | Amended | Converted to new review format. |
Acknowledgements
Editorial support from the Cochrane Neuromuscular Disease Group for an earlier update was funded by the TREAT NMD European Union Grant 036825 and for this update from by the MRC Centre for Neuromuscular Diseases and the Muscular Dystrophy campaign. We particularly thank the staff at the editorial base of the Cochrane Neuromuscular Disease Group. We also thank authors of trials for providing unpublished data.
Appendices
Appendix 1. CENTRAL search strategy
#1creatine #2MeSH descriptor Neuromuscular Diseases explode all trees #3MeSH descriptor Muscular Diseases explode all trees #4MeSH descriptor Myositis explode all trees #5("neuromuscular disease*" or "neuromuscular disorder*" or "muscular disease*" or "muscular disorder*" or "muscle disease*") #6"myotoni* dystroph*" or "myotoni* disorder*" or "muscular dystroph*" or myopath* or "myotonia congenita" or "paramyotonia congenita" #7"periodic paralys*" or "central core disease*" or "mitochondrial cytopath*" #8"glycogen storage disease" or "glycogen storage disorder" or "fatty oxidation disorder" or polymyositis or dermatomyositis or "inclusion body myositis" #9(#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10(#1 AND #9)
Appendix 2. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to August Week 5 2012> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (336136) 2 controlled clinical trial.pt. (85111) 3 randomized.ab. (239114) 4 placebo.ab. (134375) 5 drug therapy.fs. (1566004) 6 randomly.ab. (171899) 7 trial.ab. (247764) 8 groups.ab. (1125860) 9 or/1‐8 (2913768) 10 exp animals/ not humans.sh. (3778623) 11 9 not 10 (2475023) 12 Creatine/ (7617) 13 creatine.tw. (28948) 14 12 or 13 (32644) 15 exp Neuromuscular Disease/ (233769) 16 exp Muscular Disease/ or exp Myositis/ (124360) 17 (neuromuscular disease$ or neuromuscular disorder$ or muscular disease$ or muscular disorder$ or muscle disease$).tw. (9569) 18 (myotoni$ dystroph$ or myotoni$ disorder$ or muscular dystroph$ or myopath$ or myotonia congenita or paramyotonia congenita).tw. (35774) 19 (periodic paralys$ or central core disease$ or mitochondrial cytopath$).mp. (2384) 20 (glycogen storage disease$ or glycogen storage disorder$ or fatty oxidation disorder$ or inflammatory myopath$ or polymyositis or dermatomyositis or inclusion body myositis or endocrine myopath$).mp. (16300) 21 or/15‐20 (303716) 22 11 and 14 and 21 (900)
Appendix 3. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2012 Week 36> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (34922) 2 double‐blind procedure.sh. (110736) 3 single‐blind procedure.sh. (16360) 4 randomized controlled trial.sh. (328650) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (895595) 6 trial.ti. (134856) 7 clinical trial/ (871161) 8 or/1‐7 (1494644) 9 (animal/ or nonhuman/ or animal experiment/) and human/ (1205207) 10 animal/ or nonanimal/ or animal experiment/ (3308636) 11 10 not 9 (2740220) 12 8 not 11 (1406577) 13 limit 12 to embase (1090148) 14 CREATINE/ (9796) 15 14 or creatine.tw. (37379) 16 exp muscle disease/ (306916) 17 (neuromuscular disease$ or neuromuscular disorder$ or muscular disease$ or muscular disorder$ or muscle disease$).tw. (11977) 18 (myotoni$ dystroph$ or myotoni$ disorder$ or muscular dystroph$ or myopath$ or myotonia congenita or paramyotonia congenita).mp. (57327) 19 (periodic paralys$ or central core disease$ or mitochondrial cytopath$).mp. (3372) 20 (glycogen storage disease$ or glycogen storage disorder$ or fatty oxidation disorder$ or polymyositis or dermatomyositis or inclusion body myositis).mp. (21455) 21 or/16‐20 (322472) 22 13 and 15 and 21 (797) 23 remove duplicates from 22 (794)
Data and analyses
Comparison 1. Creatine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximum voluntary contraction (MVC) in muscular dystrophies | 6 | 277 | changes in MVC [%] (Fixed, 95% CI) | 8.47 [3.55, 13.38] |
| 1.1 dystrophinopathies | 3 | 121 | changes in MVC [%] (Fixed, 95% CI) | 9.20 [3.52, 14.88] |
| 1.2 myotonic dystrophy type 1 | 2 | 136 | changes in MVC [%] (Fixed, 95% CI) | 4.39 [‐8.61, 17.39] |
| 1.3 myotonic dystrophy type 2 | 1 | 20 | changes in MVC [%] (Fixed, 95% CI) | 8.8 [‐6.12, 23.72] |
| 2 MVC in metabolic myopathies | 3 | 66 | changes in MVC [%] (Fixed, 95% CI) | ‐2.26 [‐6.29, 1.78] |
| 2.1 mitochondrial diseases | 2 | 38 | changes in MVC [%] (Fixed, 95% CI) | ‐3.65 [‐11.17, 3.86] |
| 2.2 glycogen storage disease | 1 | 28 | changes in MVC [%] (Fixed, 95% CI) | ‐1.69 [‐6.48, 3.09] |
| 3 Global assessment of improvement in muscular dystrophies | 4 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.51 [2.33, 8.74] |
| 4 Neuromuscular Symptoms Score (NSS) in muscular dystrophies | 3 | 120 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [‐0.43, 2.08] |
| 5 Percentage change in MRC sum score in muscular dystrophies | 6 | 275 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [0.37, 1.82] |
| 5.1 dystrophinopathies | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [‐0.39, 2.26] |
| 5.2 myotonic dystrophy type 1 | 2 | 132 | Mean Difference (IV, Fixed, 95% CI) | 0.68 [‐0.38, 1.73] |
| 5.3 myotonic dystrophy type 2 | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐0.32, 3.12] |
| 5.4 mixed muscular dystrophies | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [1.75, 8.25] |
| 6 PCr/beta‐ATP ratio in metabolic myopathies | 3 | 68 | changes [%] (Fixed, 95% CI) | 2.00 [‐1.76, 5.76] |
| 7 ATP consumption during aerobic exercise in metabolic myopathies | 2 | 54 | ATP consumpt. [%PCr] (Fixed, 95% CI) | ‐1.75 [‐2.98, ‐0.51] |
| 8 ATP consumption during ischaemic exercise in metabolic myopathies | 2 | 54 | ATP consumpt. [%PCr] (Fixed, 95% CI) | ‐0.77 [‐2.13, 0.59] |
| 9 tau after aerobic exercise in metabolic myopathies | 2 | 50 | changes in tau [s] (Fixed, 95% CI) | 11.30 [‐7.97, 30.57] |
| 10 tau after ischaemic exercise in metabolic myopathies | 2 | 54 | changes in tau [s] (Fixed, 95% CI) | ‐2.18 [‐21.79, 17.43] |
| 11 Lean body mass (LBM) in muscular dystrophies | 3 | 196 | changes in LBM [kg] (Fixed, 95% CI) | 0.63 [0.02, 1.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Banerjee 2010.
| Methods | Randomised, single‐blind, placebo‐controlled trial | |
| Participants | 33 steroid‐naive DMD boys (age range, 3 to 12 years) | |
| Interventions | Creatine 5 g per day versus placebo (500 mg vitamin C) for 8 weeks | |
| Outcomes | Manual muscle testing Vignos' functional scale Parents' overall response to intervention (change in the activity levels, falls, time taken for daily motor tasks) P‐31 MRS (right calf muscle) Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | High risk | One experimenter who was involved in recruitment, randomisation and treatment of participants was aware of the treatment group allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were blinded but one experimenter was not blinded (see above). Creatine and placebo (vitamin C) may be different in taste |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were not balanced in numbers across intervention groups (5 dropouts in creatine group, 1 dropout in placebo group) but reasons were unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Chung 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | 37 participants with polymyositis (22 participants) or dermatomyositis (15 participants), mean age 59 years (creatine group) and 50 years (placebo group) | |
| Interventions | Creatine (loading dosage of 20 g/day for 8 days followed by a maintenance dosage of 3 g/day) versus placebo, 6 months duration | |
| Outcomes | Aggregate functional performance time (AFPT) Functional index in myositis Manual muscle testing using the Medical Research Council (MRC) extended scale Serum creatine kinase (CK) levels Health status using the Nottingham Health Profile (NHP) Short Form McGill Pain Questionnaire Hospital Anxiety and Depression Scales Chalder fatigue score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was organised by hospital pharmacies using random number lists |
| Allocation concealment (selection bias) | Low risk | Creatine and placebo were prepared by the hospital pharmacies and were provided in identical containers. Sequences were concealed until the study had ended and the statistical analysis had been completed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, supervising clinicians, and assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome. Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Escolar 2005.
| Methods | Randomised, double‐blind, placebo‐controlled, three‐arm multicentre trial | |
| Participants | 50 steroid‐naive DMD boys aged 4 to 10 years recruited at 10 Cooperative International Neuromuscular Research Group (CINRG) Centres | |
| Interventions | Creatine 5 g daily (15 participants) or glutamine 0.6 mg/kg daily (19 participants) or placebo (16 participants) 6 months duration | |
| Outcomes | Quantitative muscle testing (QMT) of bilateral elbow flexors and extensors, knee flexors and extensors, and grip Combined QMT arm and leg scores Manual muscle testing score of 34 muscle groups Functional test Pulmonary function testing | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An adaptive, biased‐coin (urn) randomisation technique was applied |
| Allocation concealment (selection bias) | Low risk | Central allocation by the Biostatistics Centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Creatine and placebo were given as powders of identical colour, texture and taste in predetermined proportions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes described on http://clinicaltrials.gov that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Klopstock 2000.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 16 participants with mitochondrial cytopathies (mean age 46.4 ± SD 17.2 years) | |
| Interventions | Creatine 20 g per day versus placebo (microcrystalline cellulose) 4‐week treatment phases Washout period of at least 29 days in all participants except two (24 and 26 days) | |
| Outcomes | Maximum isometric strength of biceps and quadriceps muscle Isokinetic biceps and quadriceps contractions with 15% of MVC until muscular exhaustion Manual muscle testing using MRC scale, Hammersmith motor ability score (HMAS), neuromuscular symptom score (NSS) Function time test, function ranking test, ataxia score Aerobic cycle ergometry with pre‐ and post‐lactate measurements Neuro‐orthoptic examination Subjective muscle weakness and general activity using a visual analogue scale | |
| Notes | Two participants had muscle cramps during the creatine period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy did the randomisation and distributed the study agents. Opaque, sealed envelopes and sealed drug containers of identical appearance were sequentially numbered and opened sequentially |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Creatine and placebo were undistinguishable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Unclear risk | Washout period may be too short in two participants |
Kornblum 2005.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 15 participants with mitochondrial cytopathies (mean age 49 ± 9 years) | |
| Interventions | Creatine 0.15 g/kg daily versus placebo Each treatment phase lasted 6 weeks 4‐week washout period | |
| Outcomes | Isometric maximum voluntary contraction of the calf muscles Tensiometric hand grip test P‐31 MRS during calf muscle ergometric test (aerobic and ischaemic exercise at 30% of MVC) Skeletal muscle function assessed by modified Boston score Subjective endurance performance and general physical condition using individual rating scales Creatine and creatinine plasma levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used |
| Allocation concealment (selection bias) | Low risk | Central allocation by hospital pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Capsules used for administration of creatine and placebo were identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Louis 2003.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 15 participants with muscular dystrophy (mean age 10.8 ± 2.8 years):
|
|
| Interventions | Creatine 3 g daily versus placebo (maltodextrin) 3‐month treatment phases Washout period of 2 months | |
| Outcomes | Maximal voluntary contraction of isometric elbow flexion Fatigue resistance at 75% MVC, total joint stiffness at 50% MVC Intramuscular PCr/beta‐ATP ratio at rest measured by P‐31 MRS Bone mineral density determined by dual energy X‐ray absorptiometry Body mass Plasma creatine level, serum CK activity Hepatic and renal function assessed by blood and urine studies | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin tossing was used for sequence generation |
| Allocation concealment (selection bias) | Low risk | Sealed drug containers of identical appearance were sequentially numbered and opened sequentially |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the study protocol have been reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Schneider‐Gold 2003.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | 20 participants with PROMM (mean age 57.7 ± 8.7 years) | |
| Interventions | Creatine 10 g per day (10 participants) versus placebo (10 participants), 3 months duration | |
| Outcomes | Maximum grip strength Compound strength score of proximal arm and leg muscles calculated from hand‐held dynamometry MRC, NSS Subjective assessment of activity in daily life using a visual analogue scale | |
| Notes | In the creatine group, myalgia improved in two participants and chest pain resolved in another patient | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used |
| Allocation concealment (selection bias) | Low risk | Central allocation by hospital pharmacy. Creatine and placebo were filled in coded boxes of identical appearance. Code numbers were broken only at the final data evaluation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Creatine and placebo were administered in an identical powder form |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tarnopolsky 1997.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 7 participants with mitochondrial cytopathies (mean age 42 ± 14 years) | |
| Interventions | Creatine (10 g daily for 2 weeks followed by 4 g per day for 1 week) versus placebo 5‐week washout period | |
| Outcomes | Evoked and voluntary isometric contraction strength of the dorsiflexors Nonischemic, isometric, dorsiflexion torque (NIDFT, 2 min) Ischaemic isometric handgrip strength (1 min) Basal and postischemic exercise lactate Aerobic cycle ergometry with pre‐ and post‐lactate measurements Activities of daily living assessed by a visual analogue scale Lean body mass measured by DEXA scanning | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pieces of labelled paper were put into envelopes and shuffled |
| Allocation concealment (selection bias) | Low risk | An independent person who was not a co‐author allocated sequentially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Creatine and placebo were administered in an identical and indistinguishable powder form |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tarnopolsky 2004a.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 31 participants with DMD (mean age 10 ± 3 years) | |
| Interventions | Creatine 0.1 g/kg daily versus placebo 4‐month treatment phases 6‐week washout period | |
| Outcomes | Maximal isometric handgrip strength Manual muscle testing Pulmonary function testing Fat‐free mass, body fat, bone mineral content and density measured by DEXA scanning Functional tests and Activity Scale for Kids questionnaire Serum CK activity, gamma glutamyl transferase activity, serum and urine creatinine concentration DNA oxidative damage assessed by urinary 8‐hydroxy‐2‐deoxy‐guanosine Bone breakdown measured by N‐telopeptide excretion in urine | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pieces of labelled paper were put into envelopes and shuffled in blocks |
| Allocation concealment (selection bias) | Low risk | An independent person who was not a co‐author allocated sequentially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Creatine and placebo were indistinguishable (flavoured tablets) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Tarnopolsky 2004b.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 42 participants with DM1 (mean age 41 ± 10 years) | |
| Interventions | Creatine 5 g per day versus placebo 4‐month treatment phases 6‐week washout period | |
| Outcomes | Maximum isometric strength (handgrip, foot dorsiflexion, knee extension) Manual muscle testing Pulmonary function assessed by spirometry PCr/beta‐ATP ratio measured by P‐31 MRS Functional tasks Assessment of activities of daily living using a visual analogue scale Body mass Fat‐free mass, body fat, bone mineral content and density measured by DEXA scanning Side effects using a questionnaire Serum activities of CK, aspartate aminotransferase, gamma glutamyl transferase Serum bilirubin concentration, serum and urine creatinine concentration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pieces of labelled paper were put into envelopes and shuffled in blocks |
| Allocation concealment (selection bias) | Low risk | An independent person who was not a co‐author allocated sequentially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Creatine and placebo were indistinguishable (flavoured tablets) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups. Reasons for missing data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes that are of interest in the review have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Vorgerd 2000.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 9 participants with glycogen storage disease type V (mean age 39.1 ± 17.9 years) | |
| Interventions | Creatine 0.15 g/kg daily for 1 week followed by 0.06 g/kg daily for 4 weeks versus placebo 4‐week washout period | |
| Outcomes | Ergometer exercise test Fatigue Severity Scale, muscle cramp scale P‐31 MRS and S‐EMG during calf muscle ergometric test (aerobic and ischaemic exercise with 30% of MVC) Serum creatine level and CK activity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy did the randomisation and distributed the study agents. Drug containers of identical appearance were sequentially numbered and opened sequentially |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Capsules used for administration of creatine and placebo were identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Vorgerd 2002.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 19 participants with glycogen storage disease type V (mean age 33.8 ± 11.8) | |
| Interventions | Creatine 0.15 g/kg daily versus placebo Each treatment phase lasted 5 weeks 4‐week washout period | |
| Outcomes | Isometric maximum voluntary contraction of the calf muscles P‐31 MRS and S‐EMG during calf muscle ergometric test (aerobic and ischaemic exercise with 30% of MVC) Exercise intolerance and adverse effects using a diary Serum creatine level and CK activity Body mass index | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy did the randomisation and distributed the study agents. Drug containers of identical appearance were sequentially numbered and opened sequentially |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Capsules used for administration of creatine and placebo were identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Walter 2000.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 36 participants with muscular dystrophies (mean age 26 ± 16 years):
|
|
| Interventions | Creatine (adults 10 g/day, children 5 g/day) versus placebo, 8‐weeks treatment phases, washout period of 3 weeks | |
| Outcomes | MRC score NSS vital capacity, subjective assessment of improvement |
|
| Notes | Only the placebo‐creatine sequence was included in the final evaluation because of carryover effects in the creatine‐placebo sequence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy did the randomisation and distributed the study agents. Opaque, sealed envelopes and sealed drug containers of identical appearance were sequentially numbered and opened sequentially |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel. Creatine and placebo were administered in identical powder form |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Low risk | Small carryover effects have been detected but this point was considered in the final evaluation (only data of placebo ‐ creatine sequence was included). The study appears to be free of other sources of bias |
Walter 2002.
| Methods | Randomised, double‐blind, placebo‐controlled cross‐over trial | |
| Participants | 34 participants with DM1 (mean age 44 ± 13 years) | |
| Interventions | Creatine (10.6 g daily for 10 days followed by 5.3 g daily for 46 days) versus placebo, 6‐week washout period | |
| Outcomes | Maximum isometric strength of biceps and quadriceps muscle MRC, NSS Body weight and lean body mass Spirometry Patients´ global assessment of improvement | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacy did the randomisation and distributed the study agents. Opaque, sealed envelopes and sealed drug containers of identical appearance were sequentially numbered and opened sequentially |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of participants and study personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes have been reported in the pre‐specified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
BMD: Becker muscular dystrophy CK: creatine kinase DMD: Duchenne muscular dystrophy DM1: myotonic dystrophy type 1 DM2 or PROMM: myotonic dystrophy type II or proximal myotonic dystrophy FSHD: facioscapulohumeral muscular dystrophy LGMD: limb girdle muscular dystrophy NSS: neuromuscular symptoms score QMT: quantitative muscle testing SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barisic 2002 | Case report (1 participant with MELAS) |
| Beurey 1967 | Case report (1 participant with polymyositis) |
| Borchert 1999 | Non‐controlled trial (4 people with mitochondrial encephalomyopathies) |
| Duperrat 1972 | Case report (1 participant with dermatomyositis) |
| Felber 2000 | Case report (1 participant with DMD) |
| Freddi 1979 | Non‐controlled trial (9 participants with myotonic dystrophy type 1) |
| Hagenfeldt 1994 | Case report (1 participant with MELAS) |
| Komura 2003 | Non‐controlled trial (2 people with mitochondrial encephalomyopathy) |
| Matsumura 2004 | Open, non‐controlled trial (22 muscular dystrophy participants) |
| Rodriguez 2007 | Combination therapy (creatine monohydrate, coenzyme Q10, lipoic acid; 16 participants with mitochondrial cytopathies) |
| Sejersen 2000 | Trial on 38 participants with DMD, published in abstract form. Necessary details could not be obtained |
| Stout 2001 | Case study (1 participant with myasthenia gravis) |
| Tarnopolsky 1999 | Study 1 (81 participants with neuromuscular disease): open, non‐controlled trial; study 2 (n = 21): single‐blinded, non‐randomised trial |
| Tarnopolsky 2004 | Case report (1 participant with mitochondriopathy) |
DMD: Duchenne muscular dystrophy; MELAS: mitochondrial encephalomyopathy, lactic acidosis, and stroke‐like episodes
Characteristics of ongoing studies [ordered by study ID]
NCT01217320.
| Trial name or title | Creatine supplementation in pediatric rheumatology |
| Methods | Randomised, double‐blind, placebo‐controlled trial |
| Participants | Estimated enrollment of 40 participants (age 6 to 18 years) with juvenile systemic lupus erythematosus or juvenile dermatomyositis |
| Interventions | Creatine 5 g per day versus placebo (dextrose 5 g/day) for 12 weeks |
| Outcomes | Quality of life, muscle function, kidney function parameters |
| Starting date | January 2011 |
| Contact information | Bruno Gualano, PhD, University of Sao Paulo, Brazil, gualano@usp.br |
| Notes |
Differences between protocol and review
We added 'Changes in activities of daily living' as second primary outcome measure because of its clinical relevance and deleted 'Electrophysiological parameters of surface‐EMG' as secondary outcome measure because of its low relevance. We decided to accept all time points of measurements for meta‐analysis because there is no evidence that the effect of short‐ and medium‐term creatine treatment changes in a function of time. We included searches of ClinicalTrial.gov and ICTRP in our search strategy for identification of studies and adapted the assessment of methodological quality and risks of bias to the new Cochrane methodology. The review also includes 'Summary of findings' tables.
Contributions of authors
All authors searched for relevant trials. RAK and MV assessed methodological quality, extracted the data and performed meta‐analysis. All three authors wrote the text of the review and agreed the final version.
Sources of support
Internal sources
Neuromuscular Center Ruhrgebiet/Kliniken Bergmannsheil, Ruhr‐University Bochum, Germany.
Hamilton Health Sciences Corporation, Dept. of Rehabilitation, Canada.
External sources
No sources of support supplied
Declarations of interest
RAK was involved in one and MV in two double blinded trials with creatine supplementation in McArdle disease. Furthermore, MV was co‐author in a trial that examined the effect of creatine in participants with single mitochondrial DNA deletions.
MAT has coordinated a single blinded trial that studied efficacy of creatine in participants with neuromuscular disorders and three double blinded trials that compared creatine with placebo in participants with mitochondrial cytopathies, Duchenne muscular dystrophy and myotonic dystrophy type 1. He was on the scientific advisory board of a sports nutrition company, EAS (2001‐2005). Neither trial was industry funded. MAT is the Chief Scientific Officer for Life Science Nutritionals. This company makes gummie omega 3 supplements, vitamin D and multivitamins for children. It does not manufacture any product containing creatine and does not have plans to do so in the foreseeable future.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Banerjee 2010 {published data only}
- Banerjee B, Sharma U, Balasubramanian K, Kalaivani M, Kalra V, Jagannathan NR. Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: a randomized, placebo‐controlled 31P MRS study. Magnetic Resonance Imaging 2010;28(5):698‐707. [PUBMED: 20395096] [DOI] [PubMed] [Google Scholar]
Chung 2007 {published and unpublished data}
- Chung YL, Alexanderson H, Pipitone N, Morrison C, Dastmalchi M, Stahl‐Hallengren C, et al. Creatine supplements in patients with idiopathic inflammatory myopathies who are clinically weak after conventional pharmacologic treatment: Six‐month, double‐blind, randomized, placebo‐controlled trial. Arthritis and Rheumatism 2007;57(4):694‐702. [PUBMED: 17471547] [DOI] [PubMed] [Google Scholar]
Escolar 2005 {published data only}
- Escolar DM, Buyse G, Henricson E, Leshner R, Florence J, Mayhew J, et al. CINRG randomized controlled trial of creatine and glutamine in Duchenne muscular dystrophy. Annals of Neurology 2005;58(1):151‐5. [PUBMED: 15984021] [DOI] [PubMed] [Google Scholar]
Klopstock 2000 {published and unpublished data}
- Klopstock T, Querner V, Schmidt F, Gekeler F, Walter M, Hartard M, et al. A placebo‐controlled crossover trial of creatine in mitochondrial diseases. Neurology 2000;55(11):1748‐51. [PUBMED: 11113239] [DOI] [PubMed] [Google Scholar]
Kornblum 2005 {published and unpublished data}
- Kornblum C, Schröder R, Müller K, Vorgerd M, Eggers J, Bogdanow M, et al. Creatine has no beneficial effect on skeletal muscle energy metabolism in patients with single mitochondrial DNA deletions: a placebo‐controlled, double‐blind 31P‐MRS cross‐over study. European Journal of Neurology 2005;12(4):300‐9. [PUBMED: 15804248] [DOI] [PubMed] [Google Scholar]
Louis 2003 {published and unpublished data}
- Louis M, Lebacq J, Poortmans JR, Belpaire‐Dethiou MC, Devogelaer JP, et al. Beneficial effects of creatine supplementation in dystrophic patients. Muscle and Nerve 2003;27(5):604‐10. [PUBMED: 12707981] [DOI] [PubMed] [Google Scholar]
Schneider‐Gold 2003 {published and unpublished data}
- Schneider‐Gold C, Beck M, Wessig C, George A, Kele H, Reiners K, et al. Creatine monohydrate in DM2/PROMM: a double‐blind placebo‐controlled clinical study. Proximal myotonic myopathy. Neurology 2003;60(3):500‐2. [PUBMED: 12578937] [DOI] [PubMed] [Google Scholar]
Tarnopolsky 1997 {published and unpublished data}
- Tarnopolsky MA, Roy BD, MacDonald JR. A randomized, controlled trial of creatine monohydrate in patients with mitochondrial cytopathies. Muscle and Nerve 1997;20(12):1502‐9. [PUBMED: 9390662] [DOI] [PubMed] [Google Scholar]
Tarnopolsky 2004a {published and unpublished data}
- Tarnopolsky MA, Mahoney DJ, Vajsar J, Rodriguez C, Doherty TJ, Roy BD, et al. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology 2004;62(10):1771‐7. [PUBMED: 15159476] [DOI] [PubMed] [Google Scholar]
Tarnopolsky 2004b {published and unpublished data}
- Tarnopolsky M, Mahoney D, Thompson T, Naylor H, Doherty TJ. Creatine monohydrate supplementation does not increase muscle strength, lean body mass, or muscle phosphocreatine in patients with myotonic dystrophy type 1. Muscle and Nerve 2004;29(1):51‐8. [PUBMED: 14694498] [DOI] [PubMed] [Google Scholar]
Vorgerd 2000 {published and unpublished data}
- Vorgerd M, Grehl T, Jager M, Muller K, Freitag G, Patzold T, et al. Creatine therapy in myophosphorylase deficiency (McArdle disease): a placebo‐controlled cross‐over trial. Archives of Neurology 2000;57(7):956‐63. [PUBMED: 10891977] [DOI] [PubMed] [Google Scholar]
Vorgerd 2002 {published and unpublished data}
- Vorgerd M, Zange J, Kley R, Grehl T, Husing A, Jager M, et al. Effect of high‐dose creatine therapy on symptoms of exercise intolerance in McArdle disease: double‐blind, placebo‐controlled cross‐over study. Archives of Neurology 2002;59(1):97‐101. [PUBMED: 11790236] [DOI] [PubMed] [Google Scholar]
Walter 2000 {published and unpublished data}
- Walter MC, Lochmuller H, Reilich P, Klopstock T, Huber R, Hartard M, et al. Creatine monohydrate in muscular dystrophies: A double‐blind, placebo‐controlled clinical study. Neurology 2000;54(9):1848‐50. [PUBMED: 10802796] [DOI] [PubMed] [Google Scholar]
Walter 2002 {published and unpublished data}
- Walter MC, Reilich P, Lochmuller H, Kohnen R, Schlotter B, Hautmann H, et al. Creatine monohydrate in myotonic dystrophy: a double‐blind, placebo‐controlled clinical study. Journal of Neurology 2002;249(12):1717‐22. [PUBMED: 12529796] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barisic 2002 {published data only}
- Barisic N, Bernert G, Ipsiroglu O, Stromberger C, Muller T, Gruber S, et al. Effects of oral creatine supplementation in a patient with MELAS phenotype and associated nephropathy. Neuropediatrics 2002;33(3):157‐61. [PUBMED: 1220074] [DOI] [PubMed] [Google Scholar]
Beurey 1967 {published data only}
- Beurey J, Duc M, Weber M, Gurecki H, Perello R. Trial therapy of acute polymyositis with phosphocreatine. [Essai de traitement de polymyosite aigue par phosphocreatine.]. Bulletin de la Societe Francaise de Dermatologie et de Syphiligraphie 1967;74(2):276‐80. [PubMed] [Google Scholar]
Borchert 1999 {published data only}
- Borchert A, Wilichowski E, Hanefeld F. Supplementation with creatine monohydrate in children with mitochondrial encephalomyopathies. Muscle and Nerve 1999;22(9):1299‐300. [DOI] [PubMed] [Google Scholar]
Duperrat 1972 {published data only}
- Duperrat B, Puissant A, Mallard D, Jourdain JC, Thomas D. Dermatomyositis with cardiac involvement and trial treatment with phosphocreatine [Dermatomyosite avec atteinte cardiaque et essai de traitement par la phosphocréatine]. Bulletin de la Societe Francaise de Dermatologie et de Syphiligraphie 1972;79(3):208‐10. [PubMed] [Google Scholar]
Felber 2000 {published data only}
- Felber S, Skladal D, Wyss M, Kremser C, Koller A, Sperl W. Oral creatine supplementation in Duchenne muscular dystrophy: a clinical and 31P magnetic resonance spectroscopy study. Neurological Research 2000;22(2):145‐50. [DOI] [PubMed] [Google Scholar]
Freddi 1979 {published data only}
- Freddi A, Paci A, DeCiantis R, Lancia G, DeSantis L. Phosphocreatinine in myotonic dystrophy (Steinert´s disease). Lancet 1979;1(8131):1400‐1. [DOI] [PubMed] [Google Scholar]
Hagenfeldt 1994 {published data only}
- Hagenfeldt L, Döbeln U, Solders G, Kaijser L. Creatine treatment in MELAS. Muscle and Nerve 1994;17(10):1236‐7. [PubMed] [Google Scholar]
Komura 2003 {published data only}
- Komura K, Hobbiebrunken E, Wilichowski EK, Hanefeld FA. Effectiveness of creatine monohydrate in mitochondrial encephalomyopathies. Pediatric Neurology 2003;28(1):53‐8. [DOI] [PubMed] [Google Scholar]
Matsumura 2004 {published data only}
- Matusmura T, Yokoe M, Nakamori M, Hattori N, Saito T, Nozaki S, et al. A clinical trial of creatine monohydrate in muscular dystrophy patients. Rinsho Shinkeigaku 2004;44(10):661‐6. [PubMed] [Google Scholar]
Rodriguez 2007 {published data only}
- Rodriguez MC, MacDonald JR, Mahoney DJ, Parise G, Beal MF, Tarnopolsky MA. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle and Nerve 2007;35(2):235‐42. [DOI] [PubMed] [Google Scholar]
Sejersen 2000 {published and unpublished data}
- Sejersen T, Hovmoller M, Hok L, Nordmark‐Andersson E, Winberg P, Nylander E, et al. Lack of detectable effect of creatine supplementation on skeletal muscle or cardiac function in Duchenne muscular dystrophy. Neuromuscular Disorders 2000;10:375. [Google Scholar]
Stout 2001 {published data only}
- Stout JR, Eckerson JM, May E, Coulter C, Bradley‐Popovich GE. Effects of resistance exercise and creatine supplementation on myasthenia gravis: a case study. Medicine and Science in Sports and Exercise 2001;33(6):869‐72. [DOI] [PubMed] [Google Scholar]
Tarnopolsky 1999 {published data only}
- Tarnopolsky M, Martin J. Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology 1999;52(4):854‐7. [DOI] [PubMed] [Google Scholar]
Tarnopolsky 2004 {published data only}
- Tarnopolsky MA, Simon DK, Roy BD, Chorneyko K, Lowther SA, Johns DR, et al. Attenuation of free radical production and paracrystalline inclusions by creatine supplementation in a patient with a novel cytochrome b mutation. Muscle and Nerve 2004;29(4):537‐47. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01217320 {published data only}
- NCT01217320. Creatine supplementation in pediatric rheumatology. http://www.clinicaltrials.gov/ct2/show/NCT01217320 (accessed 26 April 2013).
Additional references
Andreassen 2001
- Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, et al. Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington's disease. Neurobiology of Disease 2001;8(3):479‐91. [DOI] [PubMed] [Google Scholar]
Brose 2003
- Brose A, Parise G, Tarnopolsky MA. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 2003;58(1):11‐9. [DOI] [PubMed] [Google Scholar]
Burke 2003
- Burke DG, Chilibeck PD, Parise G, Candow DG, Mahoney D, Tarnopolsky M. Effect of creatine and weight training on muscle creatine and performance in vegetarians. Medicine and Science in Sports and Exercise 2003;35(11):1946‐55. [DOI] [PubMed] [Google Scholar]
Casey 1996
- Casey A, Constantin‐Teodosiu D, Howell S, Hultman E, Greenhaff PL. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. American Journal of Physiology 1996;271(1 Pt 1):E31‐7. [DOI] [PubMed] [Google Scholar]
Chetlin 2004
- Chetlin RD, Gutmann L, Tarnopolsky MA, Ullrich IH, Yeater RA. Resistance training exercise and creatine in patients with Charcot‐Marie‐Tooth disease. Muscle and Nerve 2004;30(1):69‐76. [DOI] [PubMed] [Google Scholar]
Chilibeck 2004
- Chilibeck PD, Stride D, Farthing JP, Burke DG. Effect of creatine ingestion after exercise on muscle thickness in males and females. Medicine and Science in Sports and Exercise 2004;36(10):1781‐8. [DOI] [PubMed] [Google Scholar]
Chilibeck 2005
- Chilibeck PD, Chrusch MJ, Chad KE, Shawn Davison K, Burke DG. Creatine monohydrate and resistance training increase bone mineral content and density in older men. Journal of Nutrition, Health and Aging 2005;9(5):352‐3. [PubMed] [Google Scholar]
Chrusch 2001
- Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Medicine and Science in Sports and Exercise 2001;33(12):2111‐7. [DOI] [PubMed] [Google Scholar]
Dechent 1999
- Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J. Increase of total creatine in human brain after oral supplementation of creatine‐monohydrate. American Journal of Physiology 1999;277(3 Pt 2):R698‐704. [DOI] [PubMed] [Google Scholar]
Dedeoglu 2003
- Dedeoglu A, Kubilus JK, Yang L, Ferrante KL, Hersch SM, Beal MF, et al. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington's disease transgenic mice. Journal of Neurochemistry 2003;85(6):1359‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deldicque 2008
- Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. Journal of Applied Physiology 2008;104(2):371‐8. [DOI] [PubMed] [Google Scholar]
Fenichel 2001
- Fenichel GM, Griggs RC, Kissel J, Kramer TI, Mendell JR, Moxley RT, et al. A randomized efficacy and safety trial of oxandrolone in the treatment of Duchenne dystrophy. Neurology 2001;56(8):1075‐9. [PUBMED: 11320181] [DOI] [PubMed] [Google Scholar]
Ferrante 2000
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. Journal of Neuroscience 2000;20(12):4389‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenwood 2003
- Greenwood M, Kreider RB, Melton C, Rasmussen C, Lancaster S, Cantler E, et al. Creatine supplementation during college football training does not increase the incidence of cramping or injury. Molecular and Cellular Biochemistry 2003;244(1‐2):83‐8. [PubMed] [Google Scholar]
Groeneveld 2005
- Groeneveld GJ, Beijer C, Veldink JH, Kalmijn S, Wokke JH, Berg LH. Few adverse effects of long‐term creatine supplementation in a placebo‐controlled trial. International Journal of Sports Medicine 2005;26(4):307‐313. [DOI] [PubMed] [Google Scholar]
Gualano 2010
- Gualano B, Artioli GG, Poortmans JR, Lancha Junior AH. Exploring the therapeutic role of creatine supplementation. Amino Acids 2010;38(1):31‐44. [DOI] [PubMed] [Google Scholar]
Guimbal 1993
- Guimbal C, Kilimann MW. A Na(+)‐dependent creatine transporter in rabbit brain, muscle, heart, and kidney. cDNA cloning and functional expression. Journal of Biological Chemistry 1993;268(12):8418‐21. [PubMed] [Google Scholar]
Guthmiller 1994
- Guthmiller P, Pilsum JF, Boen JR, McGuire DM. Cloning and sequencing of rat kidney L‐arginine: glycine amidinotransferase. Studies on the mechanism of regulation by growth hormone and creatine. Journal of Biological Chemistry 1994;269(26):17556‐60. [PubMed] [Google Scholar]
Harris 1992
- Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clinical Science 1992;83(3):367‐74. [DOI] [PubMed] [Google Scholar]
Hersch 2006
- Hersch SM, Gevorkian S, Marder K, Moskowitz C, Feigin A, Cox M, et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2'dG. Neurology 2006;66(2):250‐2. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hultman 1996
- Hultman E, Soderlund K, Timmons JA, Cederlad G, Greenhaff PL. Muscle creatine loading in men. Journal of Applied Physiology 1996;81(1):232‐7. [DOI] [PubMed] [Google Scholar]
Josefson 1996
- Josefson A, Romanus E, Carlsson J. A functional index in myositis. Journal of Rheumatology 1996;23(8):1380‐4. [PubMed] [Google Scholar]
Klivenyi 1999
- Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nature Medicine 1999;5(3):347‐50. [DOI] [PubMed] [Google Scholar]
Klivenyi 2004
- Klivenyi P, Calingasan NY, Starkov A, Stavrovskaya IG, Kristal BS, Yang L, et al. Neuroprotective mechanisms of creatine occur in the absence of mitochondrial creatine kinase. Neurobiology of Disease 2004;15(3):610‐7. [DOI] [PubMed] [Google Scholar]
Kreider 2003
- Kreider RB, Melton C, Rasmussen CJ, Greenwood M, Lancaster S, Cantler EC, et al. Long‐term creatine supplementation does not significantly affect clinical markers of health in athletes. Molecular and Cellular Biochemistry 2003;244(1‐2):95‐104. [PubMed] [Google Scholar]
Malcon 2000
- Malcon C, Kaddurah‐Daouk R, Beal MF. Neuroprotective effects of creatine administration against NMDA and malonate toxicity. Brain Research 2000;860(1‐2):195‐8. [DOI] [PubMed] [Google Scholar]
Manzur 2004
- Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003725.pub3] [DOI] [PubMed] [Google Scholar]
Matthews 1990
- Matthews PM, Arnold DL. Phosphorus magnetic resonance spectroscopy of brain in mitochondrial cytopathies. Annals of Neurology 1990;28(6):839‐40. [DOI] [PubMed] [Google Scholar]
Matthews 1991
- Matthews PM, Allaire C, Shoubridge EA, Karpati G, Carpenter S, Arnold DL. In vivo muscle magnetic resonance spectroscopy in the clinical investigation of mitochondrial disease. Neurology 1991;41(1):114‐20. [DOI] [PubMed] [Google Scholar]
Matthews 1998
- Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah‐Daouk R, et al. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington´s disease. Journal of Neuroscience 1998;18(1):156‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mihic 2000
- Mihic S, MacDonald JR, McKenzie S, Tarnopolsky MA. Acute creatine loading increases fat‐free mass, but does not affect blood pressure, plasma creatinine, or CK activity in men and women. Medicine and Science in Sports and Exercise 2000;32(2):291‐6. [DOI] [PubMed] [Google Scholar]
Parise 2001
- Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed‐muscle protein synthesis. Journal of Applied Physiology 2001;91(3):1041‐7. [DOI] [PubMed] [Google Scholar]
Park 1994
- Park JH, Vital TL, Ryder NM, Hernanz‐Schulman M, Partain CL, Price RR, et al. Magnetic resonance imaging and P‐31 magnetic resonance spectroscopy provide unique quantitative data useful in the longitudinal management of patients with dermatomyositis. Arthritis and Rheumatism 1994;37(5):736‐46. [DOI] [PubMed] [Google Scholar]
Park 1995
- Park JH, Olsen NJ, King L, Jr, Vital T, Buse R, Kari S, et al. Use of magnetic resonance imaging and P‐31 magnetic resonance spectroscopy to detect and quantify muscle dysfunction in the amyopathic and myopathic variants of dermatomyositis. Arthritis and Rheumatism 1995;38(1):68‐77. [DOI] [PubMed] [Google Scholar]
Passaquin 2002
- Passaquin AC, Renard M, Kay L, Challet C, Mokhtarian A, Wallimann T, et al. Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromuscular Disorders 2002;12(2):172‐82. [DOI] [PubMed] [Google Scholar]
Persky 2003
- Persky AM, Muller M, Derendorf H, Grant M, Brazeau GA, Hochhaus G. Single‐ and multiple‐dose pharmacokinetics of oral creatine. Journal of Clinical Pharmacology 2003;43(1):29‐37. [DOI] [PubMed] [Google Scholar]
Poortmans 1999
- Poortmans JR, Francaux M. Long‐term oral creatine supplementation does not impair renal function in healthy athletes. Medicine and Science in Sports and Exercise 1999;31(8):1108‐10. [DOI] [PubMed] [Google Scholar]
Poortmans 2000
- Poortmans JR, Francaux M. Adverse effects of creatine supplementation: fact or fiction?. Sports Medicine 2000;30(3):155‐70. [PUBMED: 10999421] [DOI] [PubMed] [Google Scholar]
Poortmans 2005
- Poortmans JR, Kumps A, Duez P, Fofonka A, Carpentier A, Francaux M. Effect of oral creatine supplementation on urinary methylamine, formaldehyde, and formate. Medicine and Science in Sports and Exercise 2005;37(10):1717‐20. [DOI] [PubMed] [Google Scholar]
Pulido 1998
- Pulido SM, Passaquin AC, Leijendekker WJ, Challet C, Wallimann T, Ruegg UT. Creatine supplementation improves intracellular Ca2+ handling and survival in mdx skeletal muscle cells. FEBS Letters 1998;439(3):357‐62. [DOI] [PubMed] [Google Scholar]
Rawson 2004
- Rawson ES, Persky AM, Price TB, Clarkson PM. Effects of repeated creatine supplementation on muscle, plasma, and urine creatine levels. Journal of Strength and Conditioning Research 2004;18(1):162‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rose 2004
- Rose MR, Tawil R. Drug treatment for facioscapulohumeral muscular dystrophy. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD002276.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 2002
- Roy BD, Bourgeois JM, Mahoney DJ, Tarnopolsky MA. Dietary supplementation with creatine monohydrate prevents corticosteroid‐induced attenuation of growth in young rats. Canadian Journal of Physiology and Pharmacology 2002;80(10):1008‐14. [DOI] [PubMed] [Google Scholar]
Safdar 2008
- Safdar A, Yardley NJ, Snow R, Melov S, Tarnopolsky MA. Global and targeted gene expression and protein content in skeletal muscle of young men following short‐term creatine monohydrate supplementation. Physiological Genomics 2008;32:219‐28. [DOI] [PubMed] [Google Scholar]
Scott 1982
- Scott OM, Hyde SA, Goddard C, Dubowitz V. Quantitation of muscle function in children: a prospective study in duchenne muscular dystrophy. Muscle and Nerve 1982;5(4):291‐301. [DOI] [PubMed] [Google Scholar]
Shao 2006
- Shao A, Hathcock JN. Risk assessment for creatine monohydrate. Regulatory Toxicology and Pharmacology 2006;45(3):242‐51. [DOI] [PubMed] [Google Scholar]
Shefner 2004
- Shefner JM, Cudkowicz ME, Schoenfeld D, Conrad T, Taft J, Chilton M, et al. A clinical trial of creatine in ALS. Neurology 2004;63(9):1656‐61. [PUBMED: 15534251] [DOI] [PubMed] [Google Scholar]
Soueidan 1993
- Soueidan SA, Dalakas MC. Treatment of inclusion‐body myositis with high‐dose intravenous immunglobulin. Neurology 1993;43(5):876‐9. [DOI] [PubMed] [Google Scholar]
Sullivan 2000
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against traumatic brain injury. Annals of Neurology 2000;48(5):723‐9. [PubMed] [Google Scholar]
Tarnopolsky 1999a
- Tarnopolsky MA, Parise G. Direct measurement of high‐energy phosphate compounds in patients with neuromuscular disease. Muscle and Nerve 1999;22(9):1228‐33. [DOI] [PubMed] [Google Scholar]
Tarnopolsky 1999b
- Tarnopolsky M, Martin J. Creatine monohydrate increases strength in patients with neuromuscular disease. Neurology 1999;52(4):854‐7. [DOI] [PubMed] [Google Scholar]
Tarnopolsky 2000
- Tarnopolsky MA, MacLennan DP. Creatine monohydrate supplementation enhances high‐intensity exercise performance in males and females. International Journal of Sport Nutrition and Exercise Metabolism 2000;10(4):452‐63. [DOI] [PubMed] [Google Scholar]
Tarnopolsky 2001
- Tarnopolsky MA, Parshad A, Walzei B, Schlattner U, Wallimann T. Creatine transporter and mitochondrial creatine kinase protein content in myopathies. Muscle & Nerve 2001;24(5):682‐8. [DOI] [PubMed] [Google Scholar]
Terjung 2000
- Terjung RL, Clarkson P, Eichner ER, Greenhaff PL, Hespel PJ, Israel RG, et al. American College of Sports Medicine round table. The physiological and health effects of oral creatine supplementation. Medicine and Science in Sports and Exercise 2000;32(3):706‐17. [DOI] [PubMed] [Google Scholar]
Verbessem 2003
- Verbessem P, Lemiere J, Eijnde BO, Swinnen S, Vanhees L, Leemputte M, et al. Creatine supplementation in Huntington's disease: a placebo‐controlled pilot trial. Neurology 2003;61(7):925‐30. [DOI] [PubMed] [Google Scholar]
Wyss 1992
- Wyss M, Smeitink J, Wevers RA, Wallimann T. Mitochondrial creatine kinase: a key enzyme of aerobic energy metabolism. Biochimica et Biophysica Acta 1992;1102(2):119‐66. [DOI] [PubMed] [Google Scholar]
Wyss 1998
- Wyss M, Felber S, Skladal D, Koller A, Kremser C, Sperl W. The therapeutic potential of oral creatine supplementation in muscle disease. Medical Hypotheses 1998;51(4):333‐6. [PUBMED: 9824841] [DOI] [PubMed] [Google Scholar]
Wyss 2000
- Wyss M, Kaddurah‐Daouk R. Creatine and creatinine metabolism. Physiological Reviews 2000;80(3):1107‐213. [PUBMED: 10893433] [DOI] [PubMed] [Google Scholar]
Zhu 2004
- Zhu S, Li M, Figueroa BE, Liu A, Stavrovskaya IG, Pasinelli P, et al. Prophylactic creatine administration mediates neuroprotection in cerebral ischemia in mice. Journal of Neuroscience 2004;24(26):5909‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kley 2004
- Kley RA, Vorgerd M, Tarnopolsky MA. Creatine for treating muscle disorders. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004760] [DOI] [PubMed] [Google Scholar]
Kley 2007
- Kley RA, Vorgerd M, Tarnopolsky MA. Creatine for treating muscle disorders. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004760.pub2] [DOI] [PubMed] [Google Scholar]
Kley 2011
- Kley RA, Tarnopolsky MA, Vorgerd M. Creatine for treating muscle disorders. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD004760.pub3] [DOI] [PubMed] [Google Scholar]


