Abstract
Background
Neuroleptic‐induced akathisia is one of the most common and distressing early‐onset adverse effects of antipsychotic drugs, being associated with poor compliance with treatment, and thus, ultimately, to an increase risk of relapse. This review assesses the role of benzodiazepines in the pharmacological treatment of this problem.
Objectives
To determine the effects of benzodiazepines versus placebo for people with neuroleptic‐induced acute akathisia.
Search methods
Biological Abstracts (January 1982‐March 1999), The Cochrane Library (Issue 3 1999), The Cochrane Schizophrenia Group's Register (May 2001), EMBASE (January 1980‐March 1999), LILACS (January 1982‐March 1999), MEDLINE (January 1964‐March 1999), PsycLIT (January 1974‐March 1999), and SCISEARCH were searched. Further references were sought from published trials and their authors.
Selection criteria
All randomised clinical trials comparing benzodiazepines with placebo for people with antipsychotic‐induced acute akathisia.
Data collection and analysis
Two reviewers, working independently, selected, quality assessed and extracted data. These data were then analysed on an intention‐to‐treat basis. For homogeneous dichotomous data the fixed effects relative risk (RR), the 95% confidence intervals (CI) and, where appropriate, the number needed to treat (NNT) were calculated on an intention‐to‐treat basis. For continuous data, reviewers calculated weighted mean differences.
Main results
Two small (total N=27) randomised controlled trials were included. By seven to 14 days, there was a reduction in symptoms for those patients receiving clonazepam compared with placebo (2 RCTs, N=26, RR 0.09 CI 0.01 to 0.6, NNT 1.2 CI 0.9 to 1.5). No significant difference was found for adverse events (2 RCTs, N=26, RR 3.00 CI 0.2 to 62) or the need for anticholinergic medication (2 RCTs, N=26, RR 1.56 CI 0.9 to 2.7). No one left the two studies early. Data on mental, social and family outcomes could not be pooled and there was little or no data on user satisfaction, deaths, violence, criminal behaviour and costs.
Authors' conclusions
Over a short follow‐up period, the use of benzodiazepines may reduce the symptoms of antipsychotic‐induced acute akathisia. This review highlights the need for well designed, conducted and reported clinical trials to address the claims of open studies.
Plain language summary
Benzodiazepines for neuroleptic‐induced acute akathisia
Akathisia is a common and distressing adverse effect of many antipsychotic drugs. It is characterised by restlessness and mental unease, which can be intense. It is associated with patterns of restless, including rocking, walking on the spot when standing, shuffling, or swinging one leg on the other when sitting. People may constantly pace up and down in an attempt to relieve the sense of unrest. Several strategies have been used to decrease akathisia, and this review is one in a series over viewing the effects of drug treatments. Evidence for the use of benzodiazepines is so limited that no firm treatment recommendations are possible, although there may be some effects that are worthy of further investigation.
Background
The management of schizophrenia and related disorders was revolutionised in the 1950s by the introduction of antipsychotic (or neuroleptic) medication. These medications are effective in the control of florid symptoms of psychoses such as hallucinations, thought disorder (impaired communication) and delusions. In addition to their therapeutic action in acute psychotic episodes, maintenance therapy with antipsychotic drugs is associated with a reduced risk of relapse (Schooler 1993). However, neuroleptic medications have been associated with a range of adverse effects for people taking these medications. These adverse effects can lead to poor compliance with neuroleptic treatment, and thus, ultimately, to an increased risk of relapse (Barnes 1993). Some of the most troublesome adverse effects associated with antipsychotic medication involve abnormal involuntary movements.
Shortly after the introduction of antipsychotic drugs, akathisia was recognised as one of the most common and distressing early‐onset adverse effects. This movement disorder is characterised by a subjective report of inner restlessness, mental unease, or dysphoria, which can be intense (Marder 1991, Halstead 1994). Associated with this experience are patterns of restless, including rocking from foot to foot and walking on the spot when standing, shuffling, rocking back and forth, or swinging one leg on the other when sitting (Braude 1983). In severe cases, patients constantly pace up and down in an attempt to relieve the sense of unrest. Estimates of the prevalence of akathisia in neuroleptic‐treated people ranges between 20% and 75%, occurring more frequently in the first three months of treatment (Ayd 1961, Grebb 1995). It is usually related not only to acute administration of a neuroleptic, but also to a rapid dosage increase (Barnes 1992). Akathisia may be difficult to distinguish from psychotic agitation or anxiety, especially if the person describes a subjective experience of akathisia in terms of being controlled by an outside force (Grebb 1995). If the akathisia is mistaken for psychosis, the antipsychotic drug dose may be increased leading to a worsening of the condition.
Drugs that influence neurotransmitter functions, such as anticholinergics, beta‐blockers, benzodiazepines, have been proposed as treatments for neuroleptic‐induced acute akathisia. However, there is no clear evidence of the effectiveness of these drugs in akathisia. This review attempts to systematically evaluate the use of benzodiazepines for neuroleptic‐induced acute akathisia.
Technical background While the pathophysiology of neuroleptic‐induced acute akathisia remains unknown, antagonism of mesocortical and mesolimbic dopaminergic pathways is a plausible if not completely satisfactory hypothesis. The notion that dopaminergic blockade underlies the emergence of akathisia is supported by the PET studies of Farde and co‐investigators (Farde 1992a, Farde 1992b). In one study these investigators examined striatal dopamine D2 receptor occupancy in patients who had responded to antipsychotic medication. In those who exhibited extrapyramidal side‐effects (parkinsonism or akathisia) the D2 receptor occupancy ranged from 77‐89%, while the range for those without such symptoms was 74‐80%. These findings link D2 occupancy to extrapyramidal side effects.
The involvement of serotonergic mechanisms in the pathophysiology of akathisia is supported by the reported efficacy of ritanserin, a selective 5‐HT2 antagonist and the lower liability for akathisia with newer antipsychotic drugs with relatively potent 5‐HT2‐receptor blockade. Further, the occasional occurrence of akathisia during treatment with SSRI antidepressants, which potentate 5‐HT neurotransmission, is now well recognised.
Objectives
To determine whether benzodiazepines are clinically effective for the treatment of neuroleptic‐induced acute akathisia.
A secondary objective was to examine a possible differential therapeutic effect for such medication according to psychiatric diagnosis (schizophrenia and other related disorders, mood disorders and other disorders).
Methods
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials were scrutinised. Where a trial was described as 'double‐blind' and the means of allocation not made explicit, but it was implied that the study was randomised, these could be included only where the participant's demographic details were similar in each group. Quasi‐randomised studies, such as those allocating treatment depending on the day of the week, were excluded.
Types of participants
People with neuroleptic‐induced acute akathisia, diagnosed by any criteria, irrespective of gender, age or psychiatric diagnosis.
Types of interventions
1. Adjunctive benzodiazepines: any dose, pattern or means of administration. The following benzodiazepines were considered: alprazolam, bromazepam, chlordiazepoxide, clobazam, clonazepam, clorazepate, diazepam, flunitrazepam, flurazepam, loprazolam, lormetazepam, midazepam, midazolam, nitrazepam, oxazepam , temazepam, lorazepam, triazolam, halazepan, cyprazepam, fosazepam, doxefazepam, nordazepam, girisopam, tofisopam, pinasepam, meclonazepam, prazepam, ketazolam, oxazolam, adinazolam, mexazolam, estazolam, metaclazepam, triflubazam, cinolazepam, cloxazolam and haloxazepam.
2. Placebo.
Benzodiazepines compared to other active drugs, such as anticholinergics and centrally‐acting beta‐blockers, were not considered in this review.
Types of outcome measures
1. Akathisia symptoms 1.1 Number of people failing to demonstrate a complete remission (that is, not showing a 100% reduction in symptoms). 1.2 Number of people failing to achieve at least 50% reduction in symptoms. 1.3 Number of people who dropped out due to lack of efficacy. 1.4 Mean difference in severity of symptoms at endpoint. 1.5 Mean changes in severity of akathisia symptoms between baseline and endpoint (see Methods section).
2. General mental state changes 2.1 Deterioration in general psychiatric symptoms (such as delusions and hallucinations). 2.2 Mean difference in severity of symptoms at endpoint. 2.3 Mean changes in severity of symptoms between baseline and endpoint (see Methods section).
3. Acceptability and tolerability of treatment 3.1 Number of people who left the study early for any reason. 3.2 Number of people who left early because of adverse events.
4. Adverse effects 4.1 Number of people who presented at least one adverse event. 4.2 Number of people whose adverse effects were 'severe'. 4.3 Mean difference in severity of adverse effects at endpoint. 4.3 Mean changes in severity of adverse effects between baseline and endpoint (see Methods section).
Three time periods for reporting of outcomes were pre‐stated: short term (less than 6 weeks), medium term (between 6 weeks and 6 months) and long term (over 6 months).
Search methods for identification of studies
Please see Collaborative Review Group search strategy.
1. Electronic searching 1.1 Biological Abstracts (January 1982 to March 1999) was searched using the Cochrane Schizophrenia Group's search strategy for randomised controlled trials combined with the phrase:
[and AKATHISI* or ACATHISI*]
1.2 The Cochrane Library (Issue 3, 1999) was searched using the phrase:
[(akathisia‐drug induced in ME) or AKATHISI* or ACATHISI*]
1.3 The Cochrane Schizophrenia Group's Register (May 2001) was searched using the phrase:
[AKATHISI* or ACATHISI*]
1.4 EMBASE (January 1980 to March 1999) was searched using the Cochrane Schizophrenia Group's search strategy for randomised controlled trials combined with the phrase:
[and (akathisia‐drug induced in thesaurus ‐all subheadings) or AKATHISI* or ACATHISI*]
1.5 LILACS (January 1982 to March 1999) was searched using the Cochrane Schizophrenia Group's search strategy for randomised controlled trials combined with the phrase: [and (akathisia‐drug induced in thesaurus ‐all subheadings) or AKATHISI* or ACATHISI* or (Mh acatisia or Mh acatisia induzida por drogas)] .
1.6 MEDLINE (January 1966 to March 1999) was searched using the Cochrane Schizophrenia Group's search strategy for randomised controlled trials combined with the phrase:
[and (akathisiadrug‐induced in thesaurus ‐all subheadings) or AKATHISI* or ACATHISI*]
1.7 PsycLIT (January 1974 to March 1999) was searched using the Cochrane Schizophrenia Group's search strategy for randomised controlled trials combined with the phrase:
[and (explode akathisia‐drug induced in DE) or AKATHISI* or ACATHISI*]
1.8 SCISEARCH ‐ Science Citation Index Each of the included studies was sought as a citation on the SCISEARCH database. Reports of articles that had cited these studies were inspected in order to identify further trials.
2. Reference searching and personal contact The reference lists in all identified studies were also inspected for more relevant studies.
3. Personal contact. The first author of each included study was contacted in order to obtain further information regarding their published study or unpublished trials in akathisia.
Data collection and analysis
1. Selection of trials One reviewer (ARL) evaluated the abstract of each reference identified by the search in order to see if the study was likely to be relevant to the review. If any abstract referred to a study in which there was a possibility that treatment had been randomised, a full copy of the study report was obtained. Two reviewers (ARL, KSW), working independently, then decided if the acquired studies met the review's inclusion criteria. An inter‐rater reliability study between reviewers was performed by means of the weighted Kappa coefficient as a measure of agreement for inclusion criteria.
2. Quality assessment Two methods were used to assess the quality of the trials included in this review: the Cochrane Handbook quality criteria (Mulrow 1997) and the Jadad Scale (Jadad 1996). The Cochrane Handbook quality criteria are based on the adequacy of the concealment of treatment allocation. Trials that use well‐concealed randomisation techniques for group allocation have a reduced potential for bias (Khan 1996, Schulz 1995). The methods of randomisation with a low potential of bias include central computer generated randomisation, random number tables or coin tossing (criterion A); moderate potential of bias was assumed for trials where the allocation procedure was unclear or not reported (criterion B). The use of chart file number or date of birth to decide group allocation are examples of quasi‐randomisation and are open to manipulation (criterion C). Only trials meeting criteria A or B were included in this review. The Jadad Scale examines a broader range of quality parameters, allocating higher scores to trials that: (i) are stated to be randomised (ii) use a randomisation procedure that is adequately concealed (iii) state that double‐blind methodology was used (iv) describe an adequate double‐blind procedure; and, (v) use an intention‐to‐treat‐analysis. The range of the Jadad score is 0 to 5, with higher scores indicating higher quality (Jadad 1996). A cut‐off of 2 points was used in the Jadad scale to check the assessment made by the Cochrane Handbook criteria. However, these scores will not be used as inclusion criteria in this review.
3. Data extraction Two reviewers (ARL, KSW) independently extracted data from the included trials. Any disagreement was discussed, the decisions documented and, where necessary, the authors of the trials were contacted for clarification.
4. Data analysis 4.1 Binary data For binary outcomes (remission, clinical improvement and leaving the study early) relative risks (RR) and its 95% confidence interval (CI) were estimated. A DerSimonian‐Laird estimate of RR from the individual trials was used to estimate the pooled risk ratio for all strata under the assumption of a random effects model. This model takes into account any difference between studies (even if there is no statistically significant heterogeneity) and gives the same result as the fixed effects model when there is no between‐study variance (studies are homogeneous). By convention, RRs smaller than 1 indicates that an event is less likely to occur in the active treatment group than in the placebo group. A chi‐square statistic was given with associate probability of the pooled RR being equal to 1.0. When overall results were significant the number need to harm (NNH) or the number need to treat (NNT) were calculated on the inverse of the pooled absolute risk difference.
4.2 Continuous data 4.2.1 Valid scales Continuous data from rating scales were included only if the measuring instrument had been described in a peer‐reviewed journal and the instrument was either a self report questionnaire or completed by an independent rater or relative (not the therapist).
4.2.2 Skewed data Continuous data on clinical and social outcomes are not often normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, the following standards are applied to all data before inclusion: (i) standard deviations and means were reported in the paper or were obtainable from the authors; (ii) when a scale starts from a finite number (such as 0), the standard deviation, when multiplied by 2, was less than the mean (as otherwise the mean was unlikely to be an appropriate measure of the centre of the distribution) (Altman 1996). Endpoint scores on scales often have a finite start and end point, and this rule can be applied to them. Change data is more problematic, and this rule cannot be applied with confidence. Change data were therefore only presented if no endpoint data were available.
4.2.3 Summary statistic Data that met the two standards were analysed by the estimation of the weight mean difference (WMD) between groups.
4.3. Intention to treat analysis Data were excluded from studies where more than 50% of participants in any group were lost to follow up (this did not include the outcome of 'leaving the study early'). In studies where the proportion of dropouts was less than 50%, those people leaving early were considered to have had no change in their symptoms. The impact of including studies with high attrition rates (25‐50%) was analysed in a sensitivity analysis. If inclusion of data from this latter group resulted in a substantive change in the estimate of effect their data were not added to trials with less attrition, but presented separately.
4.4. Crossover studies Only the first segment of crossover trials was used in order to exclude the potential additive effect in the subsequent segments of these trials (Armitage 1991).
5. Test for heterogeneity A Chi‐square test was used, as well as visual inspection of graphs, to investigate the possibility of heterogeneity. A significance level less than 0.10 was interpreted as evidence of heterogeneity, and the studies responsible for this were not added to the main body of homogeneous trials, but summated and presented separately. Reasons for heterogeneity were explored by re‐inspecting the original studies. In order to restrict the number of planned subgroup analyses, only three reasons for heterogeneity were pre‐specified: (i) that response differs according to the quality of the trial; (ii) that response differs according to lengths of follow up; and (iii) that response differs according to psychiatric diagnosis.
6. Assessing the presence of publication bias Selective publication results from the tendency to publish only statistically significant finding or those supporting the hypothesis. A funnel plot, plotting the estimate of effect for each trial by its total sample size, was examined visually in order to estimate potential selection bias (publication and selection biases) (Egger 1997). The logarithm of the estimate of effect (relative risk) was used to avoid the naturally asymmetric distribution of this measure.
7. Sensitivity analyses The effect of including studies with high attrition rates was analysed in a sensitivity analysis. Reviewers hoped to investigate whether there were differences in outcome for people with : i. schizophrenia; ii. mood disorders; and, iii. other psychiatric diagnoses.
8. General Where possible, reviewers entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for the experimental intervention.
Results
Description of studies
1. Excluded Studies Most of the studies identified were not controlled trials. They were open clinical studies where outcomes in the same individuals were compared before and after the use of benzodiazepines. Studies by Gagrat 1978 and Horiguchi 1992 met all eligibility criteria except that they made between benzodiazepines and treatment with other active drugs.
2. Awaiting assessment There are no studies awaiting assessment.
3. Ongoing studies The reviewers know of no ongoing studies.
4. Included Studies Two very small (total N=26) studies were included. See 'Characteristics of included studies' for descriptions of each study.
4.1 Methods Both studies had randomised allocation to treatment groups. Follow up was not longer than two weeks.
4.2 Participants Both studies included focused on people who were 'acutely ill', suffering from antipsychotic‐induced akathisia. Each trial also included people of both sexes, with ages ranging between 18‐65 years old, with a diagnosis of a psychotic illness such as schizophrenia and schizoaffective disorders.
4.3 Interventions 4.3.1 Benzodiazepine: The two studies both used clonazepam (oral 0 ‐ 2.5mg / day). 4.3.2 Placebo. The trials compared clonazepam to an oral placebo. 4.3.3 Background medication: In Pujalte 1994, the authors mentioned the use of antiparkinsonian medication in five of six patients in the intervention group and three of six in the placebo group. In Kucther 1989, antiparkinsonian medication was prescribed in six of seven patients in the intervention group and four of seven patients in the placebo group.
4.4 Outcomes Very limited data were available and little of clinical utility. Scales were used to objectively rate 'remission' and these data were possible to include. The mean scores were not possible to include, however, as only inexact p values were reported. 4.4.1 Extrapyramidal Symptom Rating Scale ‐ESRS (Chouinard 1980). The ESRS rates parkinsonian symptoms (nine items), neurological side‐effects (eight items), automatic side‐effects (11 items) and other side‐effects (19 items). Each item is scored on a four‐point scale, 0‐3, where 0 means not or doubtfully present. The possible score ranges from 0 to 144. This scale was used in Kutcher 1989 but
4.4.2 Barnes Rating Scale for Drug‐Induced Akathisia (Barnes 1989). The scale comprises items rating the observable, restless movements that characterise akathisia, a subjective awareness of restlessness, and any distress associated with the condition. These items are rated from 0 ‐ normal to 3 ‐ severe. In addition, there is an item for rating global severity (from 0 ‐ absent to 5 ‐ severe). A low score indicates low levels of akathisia. This scale was used in Pujalte 1994 but mean scores were not possible to include in this review as only inexact p values were reported.
4.4.3 Missing outcomes None of the studies evaluated hospital/service outcomes, satisfaction with care and economic outcomes.
Risk of bias in included studies
1. Randomisation The process of random allocation to the intervention groups was not explicit in any of the studies.
2. Blinding at outcome The two included studies stated that blind evaluation of outcome was undertaken. 3. Follow‐up One study actively excluded people from the analysis (Kutcher 1989): 1 out 15 people. The excluded person had decided not to take his medication during the study. It was unclear from which treatment group this individual was excluded.
4. Quality of reporting of outcomes There was a tendency for studies to present their findings in tables and p‐values (requests for the raw data from authors have so far failed). It was common for p‐values to be used as a measure of association between intervention and outcomes, instead of showing the strength of the association. Although p‐values are influenced by the strength of the association, they also depend on the sample size of the groups. It is possible to pool p‐values from different studies, but it is necessary to know their exact value. In the reviewed studies it was not possible to pool then as they were reported in a "p< 0.05" or "p > 0.05" style.
Effects of interventions
1. The search, data selection and data extraction One thousand and eight citations were found using the search strategy. Eleven citations were related to benzodiazepines but only seven referred to controlled clinical trials (all of them published in journals). Six different studies were identified from these citations and two were included in the review.
2. COMPARISON: BENZODIAZEPINES versus PLACEBO
2.1 Akathisia symptoms by 7‐14 days For the outcomes of 'clinical efficacy', Kutcher 1989 presented graphs of reduction in the Extrapyramidal Symptom Rating Scale scores and stated that there was difference between the two groups. Pujalte 1994 presented tables and graphs of the correlations between clinical improvement and daily doses of clonazepam. In the latter study, the authors found that the difference between baseline and day 14 scores was greater among those receiving clonazepam. For the outcome of 'complete remission', pooled data showed no significant difference between the benzodiazepine and control groups (2 RCTs, N=26, RR 0.86 CI 0.6 to 1.2 ), but such differences were apparent when pooled data were examined using the outcome criterion of 'at least 50% remission' (2 RCTs, N=26, RR 0.09 CI 0.01 to 0.6). 2.2 General mental state changes Neither trial addressed this item.
2.3 Adverse effects by 7‐14 days 2.3.1 At least one adverse effect Pujalte 1994 stated that one patient in the benzodiazepine group presented mild drowsiness while Kutcher 1989 did not report any adverse effects. Pooled data showed no significant difference between the benzodiazepine and control groups (2 RCTs, N=26, RR 3.0 CI 0.2 to 62).
2.3.2 Needing anticholinergic drugs by 7‐14 days There was no difference between those allocated clonazepam compared with people given placebo for needing anticholinergic drugs (2 RCTs, N=26, RR 1.56 CI 0.9 to 2.7).
2.4 Acceptability and tolerability of treatment by 7‐14 days The two small studies both suggest that clonazepam and placebo are well tolerated for the first couple of weeks.
2.5 Hospital and service outcomes, satisfaction with care, economic outcomes Neither study addressed these issues.
Discussion
1. Applicability of findings 1.1 Setting The studies included in this review involved small numbers of participants from a narrow range of cultural settings. 1.2. Diagnosis Most participants had a DSM‐III R diagnosis. As such, the homogeneity of the patient group can be assumed. However, the restriction to the diagnosis of DSM‐III R psychotic illness excludes many people seen in routine practice that can develop antipsychotic‐induced acute akathisia after receiving antipsychotic medication for other psychiatric diagnoses. For example, many people will receive antipsychotic medication for presumed schizophrenia‐like disorders in the absence of DSM‐III R psychotic symptoms. Similarly, many will have co‐existing substance abuse disorders or other co‐morbid mental disorders, such as depression. The results of the review cannot be assumed to be externally valid and applicable to the large numbers of people in routine practice that, although being in the DSM‐III R classificatory system, still requires antipsychotic medication.
1.3 Interventions This is essentially a review of the effectiveness of clonazepam as compared with placebo and it is not possible to generalise the findings to other active drug treatments. The included trials were 'short term' (14 days) and therefore no conclusions can be drawn regarding the long‐term effects of benzodiazepines.
2. COMPARISON: BENZODIAZEPINES versus PLACEBO
2.1 Akathisia symptoms by 7‐14 days The benzodiazepine clonazepam, was more effective than placebo in achieving 'partial remission' by seven to 14 days (NNT 1.2 CI 0.9 to 1.5). The studies defined partial remission as a 50% reduction in the akathisia subscale of the ESRS or the Barnes Akathisia Rating Scale. Neither of these scales is commonly used in routine clinical practice. They both include a restricted range of items relating to signs and symptoms of akathisia (including objective and subjective items). Each of these individual items has equal weight. The validity of using a 50% reduction in these scales must be viewed with caution. It is not clear whether a 50% reduction in scores represents an externally valid and clinically important improvement in akathisia symptoms for people with antipsychotic‐induced acute akathisia.
2.2 Adverse effects by 7‐14 days 2.2.1 At least one adverse effect No differences were observed between benzodiazepines and placebo for those side effects considered 'common' with benzodiazepines (such as sedation and drowsiness). This is reassuring but data are too limited to be convincing.
2.2.2 Needing anticholinergic drugs by 7‐14 days Although there was no difference between those allocated clonazepam compared with people given placebo for needing anticholinergic drugs, it would be interesting to see if greater numbers in studies of the future would continue to implicate benzodiazepines as promoting the use of anticholinergic drugs (RR 1.56 CI 0.9 to 2.7).
2.3 Acceptability and tolerability of treatment by 7‐14 days When the acceptability of treatment was assessed indirectly by the number of people leaving the studies early, benzodiazepines and placebo seemed to be equally acceptable to those with antipsychotic induced acute akathisia. Only one out 27 people left the studies early, reflecting good compliance with treatment. It was not clear if this patient dropped out before or after the randomisation procedure. The authors were contacted but did not reply. It is important to point out that compliance only refers to a follow‐up period of two weeks.
2.4 Other outcomes As no trial specified relapse as an outcome, we are unable to comment on how benzodiazepines affect these important aspects of care. Neither was information reported on quality of life, social functioning, and employment status or cost effectiveness.
3. Sensitivity analyses. As no trial focused on individuals with psychiatric diagnoses other than schizophrenia, such as mood disorder, we are unable to assess whether these individuals differed in their treatment response.
Authors' conclusions
Implications for practice.
1. For people with neuroleptic‐induced acute akathisia Benzodiazepines may improve symptoms associated with antipsychotic‐induced acute akathisia, although caution is required due to the limited data available, the short duration of follow up and the potential for tolerance to these drugs in the medium to long term.
2. Clinicians The question of whether benzodiazepines are really more effective than placebo remains to be proven. Benzodiazepines appear to have some advantage over placebo in terms of early alleviation of symptoms but the results of such small studies should be seen as hypotheses generating rather than hypotheses proving. Compared with placebo, they seem to be equally acceptable to those with antipsychotic‐induced acute akathisia, perhaps due to their low liability for adverse events, at least in the short term. At present, there are no available trials on long‐term effects.
3. Managers or policy makers There is a paucity of data regarding the clinical implications of using benzodiazepines in antipsychotic‐induced acute akathisia, and no data related to service utilisation, hospitalisation or functioning in the community.
Implications for research.
1. General 1.1 Reporting could be much better Allocation concealment is a fundamental part of trial methodology. If readers are to be reassured that selection bias was minimised, the randomisation process should be clearly described. The included studies mentioned the use of double‐blind evaluation of the outcomes, but again, no details were made explicit. It is also essential to know from which treatment group patients withdrew, in order to evaluate exclusion bias. Kutcher 1989 excluded one out 15 people from the analyses due to protocol non‐compliance but it was not clear whether this exclusion occurred before or after randomisation, or from which group the patient was excluded.
Authors should present measures of association between intervention and outcome, for example, relative risks, odds‐ratios, and risk and mean differences, as well as the raw numbers. It is strongly suggested that authors report confidence intervals and statistical power for the comparisons presented in the papers. If p‐values are used, the exact value should be reported.
1.2 Outcomes Future studies should also consider hospital and service outcomes, satisfaction with care and economic outcomes. Concrete outcomes of disturbance such as 'disturbed episode', 'use of special nursing observation' or 'avoiding relapses' for those in the community would also be of interest.
2. Specific Akathisia is a most distressing movement disorder that is highly prevalent, both in the developed and developing world. This review highlights the need for well designed, conducted and reported clinical trials to address the claims of open studies as regards the effects of the benzodiazepine group of drugs for akathisia.
What's new
| Date | Event | Description |
|---|---|---|
| 23 April 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 1, 2000 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 11 August 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The reviewers would like to thank Kristian Wahlbeck, Leanne Roberts, and Clive Adams for advice during the production of the protocol and Evandro da Silva Freire Coutinho for advice during the production of this review.
Data and analyses
Comparison 1. BENZODIAZEPINES versus PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Akathisia | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 not in full remission by 7‐14 days (100% reduction in symptoms) | 2 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.59, 1.26] |
| 1.2 not in partial remission by 7‐14 days (50% reduction in symptoms) | 2 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.58] |
| 2 Adverse effects: 1. At least one adverse event by 7‐14 days | 2 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.15, 61.74] |
| 3 Adverse effects: 2. Needing anticholinergic medication by 7‐14 days | 2 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.90, 2.71] |
| 4 Leaving the study early by 7‐14 days ‐ any reason | 2 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
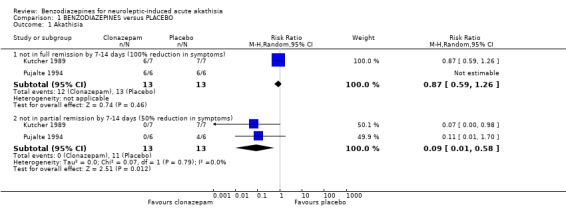
Comparison 1 BENZODIAZEPINES versus PLACEBO, Outcome 1 Akathisia.
1.2. Analysis.
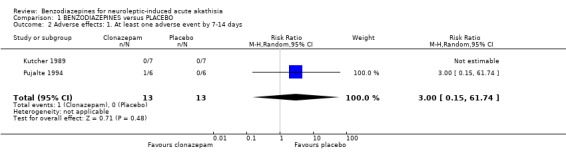
Comparison 1 BENZODIAZEPINES versus PLACEBO, Outcome 2 Adverse effects: 1. At least one adverse event by 7‐14 days.
1.3. Analysis.
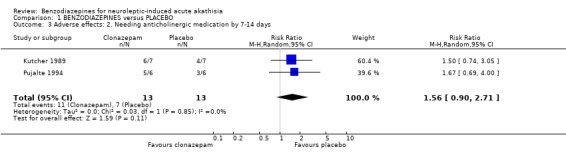
Comparison 1 BENZODIAZEPINES versus PLACEBO, Outcome 3 Adverse effects: 2. Needing anticholinergic medication by 7‐14 days.
1.4. Analysis.
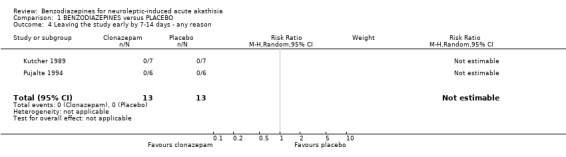
Comparison 1 BENZODIAZEPINES versus PLACEBO, Outcome 4 Leaving the study early by 7‐14 days ‐ any reason.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kutcher 1989.
| Methods | Allocation: "random allocation" ‐ no futher details. Blindness: double ‐ no futher details. Duration: 1 week. | |
| Participants | Diagnosis: psychotic illness (no futher details) plus neuroleptic‐induced akathisa (EPRS = 3‐6). N=15. Age: mean 18.5 years. Sex: female 5, male 10. History: "acutely ill", duration of illness, number of previous hospitalisations, duration of current hospitalisation ‐ not specified. | |
| Interventions | 1. Clonazepam: dose 1mg/day. N=8. 2. Placebo. N=7. | |
| Outcomes | Akathisia: EPRS, use of antiparkinsonian medication.
Adverse effects.
Leaving the study early. Unable to use ‐ Akathisia: EPRS score (inexact p‐value) |
|
| Notes | Authors contacted for details on 17th March 2000. Intention to treat analysis not undertaken. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pujalte 1994.
| Methods | Allocation: "random allocation" ‐ no further details. Blindness: double ‐ placebo identical appearance to clonazepam solution. Duration: 2 weeks. | |
| Participants | Diagnosis: schizophrenia and schizoaffective disorders (DSM‐III‐R) plus neuroleptic‐induced akathisa (Barnes Rating Scale for Drug‐Induced Akathisia). N=12. Age: mean ˜34 years. Sex: female 4, male 8. History: "acutely ill", duration of illness not specified. | |
| Interventions | 1. Clonazepam: dose 0.5‐2.5mg/day. N=6. 2. Placebo. N=6. | |
| Outcomes | Akathisia: Barnes Rating Scale for Drug‐Induced Akathisia, use of antiparkinsonian medicaton.
Adverse effects. Unable to use ‐ Akathisia: Barnes Rating Scale for Drug‐Induced Akathisia score (inexact p‐value). |
|
| Notes | Authors contacted for details on 17th March 2000. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
EPRS ‐ Extra‐pyramidal symptom rating scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adler 1985 | Allocation: randomised. Participants: those with psychotic disorder, suffering from neuroleptic‐induced akathisia. Interventions: propranolol versus lorazepam, not versus placebo. |
| Gagrat 1978 | Allocation: randomised. Participants: those with psychotics disorders, suffering from neuroleptic‐induced akathisia. Interventions: benzodiazepine versus diphenhydramine, not versus placebo. |
| Hermesh 1988 | Allocation: not randomised, not controlled clinical trial. |
| Horiguchi 1992 | Allocation: randomised. Participants: people with schizophrenia, suffering from neuroleptic‐induced akathisia. Interventions: benzodiazepine versus anticholinergics, not versus placebo. |
Contributions of authors
Adriano Lima ‐ protocol production, searching, data extraction, assimilation and report writing. Corresponding author.
Karla Soares‐Weiser ‐ protocol production, searching and data extraction.
Josué Bacaltchuk ‐ protocol production.
Thomas Barnes ‐ protocol production and report writing.
Sources of support
Internal sources
Federal University of São Paulo, Brazil.
Cochrane Schizophrenia Group, UK.
FAPESP ‐ State of São Paulo, Brazil.
Imperial College of Science, Technology and Medicine, UK.
External sources
No sources of support supplied
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Kutcher 1989 {published data only}
- Kutcher S, Williamson, Mackenzie S, Marton P, Ehrlich M. Successfull clonazepam treatment of neuroleptic‐induced akathisia in older adolescents and young adults: a double‐blind, placebo controlled study. Journal of Clinical Psychopharmacology 1989;9(6):403‐6. [PubMed] [Google Scholar]
Pujalte 1994 {published data only}
- Pujalte D, Bottai T, Hue B, Alric R, Pouget R, Blayac JP, Petit P. A double‐blind comparision of clonazepam and placebo in the treatment of neuroleptic‐induced akathisia. Clinical Neuropharmacology 1994;17(3):236‐42. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adler 1985 {published data only}
- Adler LA, Angrist B, Peselow E, Corwin J, Rotrosen J. Efficacy of propranolol in neuroleptic‐induced akathisia. Journal of Clinical Psychopharmacology 1985;5(3):164‐6. [PubMed] [Google Scholar]
Gagrat 1978 {unpublished data only}
- Gagrat D, Hamilton J, Belmaker RH. Intravenous clonazepam in the treatment of neuroleptic‐induced acute dystonia and akathisia. American Journal of Psychiatry 1978;135(10):1232‐3. [DOI] [PubMed] [Google Scholar]
Hermesh 1988 {published data only}
- Hermesh H, Molcho A, Munitz H. Successful propranolol therapy for neuroleptic‐induced akathisia resistant to anticholinergic and benzodiazepinic drugs. Clinical Neuropharmacology 1988;11(4):369‐72. [DOI] [PubMed] [Google Scholar]
Horiguchi 1992 {published data only}
- Horiguchi J, Nishimatsu O. Usefulness of antiparkinsonian drugs during neuroleptic treatment and the effect of clonazepam on akathisia and parkinsonism ocurred after antiparkinsonian drug withdrawl: a double‐blind study. Japanese Journal of Psychiatry and Neurology 1992;46(3):733‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armitage 1991
- Armitage P. [Should we cross‐off the cross‐over?]. British Journal of Clinical Pharmacology 1991;32:1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayd 1961
- Ayd FJ. A survey of drug‐induced extrapyramidal reactions. JAMA 1961;175:1054‐60. [DOI] [PubMed] [Google Scholar]
Ayd 1984
- Ayd FJ. High potency neuroleptics and akathisia. Journal of Clinical Psychopharmacology 1984;4:237. [DOI] [PubMed] [Google Scholar]
Barnes 1989
- Barnes TRE. A rating scale for drug induced akathisia. British Journal of Psychiatry 1989;154:672‐6. [DOI] [PubMed] [Google Scholar]
Barnes 1993
- Barnes TRE, Edwards JG. The side effects of antipsychotic drugs. I. CNS and neuromuscular effects. In: Barnes TRE editor(s). Antipsychotic drugs and their side effects. London: Harcourt Brace & Company, 1993. [Google Scholar]
Barton 1990
- Barton A, Bowie J, Edmier K. Low plasma iron status and akathisia. Journal Neurology Neurosurgery Psychiatry 1990;53:671‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braude 1983
- Braude WM, Barnes TRE, Gore S. Clinical characteristics of akathisia: a systematic investigation of acute psychiatric inpatient admissions. British Journal of Psychiatry 1983;143:139‐50. [DOI] [PubMed] [Google Scholar]
Brown 1987
- Brown KW, Glen SE, White T. Low plasma iron status and akathisia. Lancet 1987;1:1234‐6. [DOI] [PubMed] [Google Scholar]
Chatellier 1996
- Chatellier G, Zapletal E, Lemaitre D, Menard J, Degoulet P. The number needed to treat: a clinically useful nomogram in its proper context. BMJ 1996;312:426‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chouinard 1980
- Chouinard G, Ross‐Chouinard A, Annable L, Jones B. Extrapyramidal rating scale. Can Journal of Neurological Sciences 1980;1:233‐40. [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD. Cochrane Collaboration Handbook. The Cochrane Library 2000, Issue 4. [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farde 1992a
- Farde L, Norstrom AL, Halldin C. PET studies of dopamine receptors in relation to antipsychotic drug treatment. Clinical Journal of Neuropharmacology 1992;15(Suppl 1 Pt A):468A‐469A. [DOI] [PubMed] [Google Scholar]
Farde 1992b
- Farde L. Journal Clinical of Neuropharmachology (Proceedings of the 18th Collegium Internationale Neuro ‐ Psychopharmacologicum Congress 1992, 28th June ‐2nd July, Nice, France) 1992;15(Suppl 1 Pt ‐ B):1B‐653B. [PubMed] [Google Scholar]
Grebb 1995
- Grebb JA. Medication induced movement disorders. In: Kaplan HI, Sadock BJ editor(s). Comprehensive textbook of psychiatry. New York: Williams & Wilkins, 1995. [Google Scholar]
Halstead 1994
- Halstead SM, Barnes TR, Speller JC. Akathisia: prevalence and associated dysphoria in a inpatient population with chronic schizophrenia. British Journal of Psychiatry 1994;164(2):177‐83. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad A, Moore A, Carrol D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay H. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Khan 1996
- Khan KS, Daya S, Jadad AR. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156:661‐6. [PubMed] [Google Scholar]
Lipinski 1989
- Lipinski JF Jr, Mallya G, Zimmerman P, Pope HG Jr. Fluoxetine‐induced akathisia: clinical and theoretical implications. Journal of Clinical Psychiatry 1989;50(9):339‐42. [PubMed] [Google Scholar]
Marder 1991
- Marder SR, Midha KK, Putten T, Aravagiri M, Hawes EM, Hubbard JW, McKay G, Mintz J. Plasma levels of fluphenazine in patients receiving fluphenazine decanoate. British Journal of Psychiatry 1991;158:658‐65. [DOI] [PubMed] [Google Scholar]
Schooler 1993
- Schooler NR, Keith SJ. The clinical research base for the treatment of schizophrenia. Psychopharmacology Bulletin 1993;29:431‐46. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]


