Abstract
Background
Hypercholesterolaemia, characterised by raised blood cholesterol levels, is not a disease itself but a metabolic derangement that often contributes to many diseases, notably cardiovascular disease. In most cases, elevated cholesterol levels are associated with high‐fat diet, especially saturated fat, coupled with an inactive lifestyle. Less commonly, raised cholesterol may be related to an inherited disorder, familial hypercholesterolaemia. This systematic review is only concerned with acquired hypercholesterolaemia.
Objectives
To assess the effects of low‐fat diets for acquired hypercholesterolaemia and to investigate the incidence of adverse effects from low‐fat dietary interventions. We planned to compare the relative effectiveness of low‐fat diets with calorie‐restricted diets for acquired hypercholesterolaemia. We also wanted to look into the relative effectiveness of low‐fat diets and pharmacological interventions for acquired hypercholesterolaemia.
Search methods
Studies were obtained from computerised searches of The Cochrane Library, MEDLINE, EMBASE and databases of ongoing trials. Date of last search was February 2010.
Selection criteria
Otherwise healthy adults (equal to or greater than 18 years) with acquired (not familial) hypercholesterolaemia. We defined hypercholesterolaemia as either total cholesterol greater than 5.2 mmol/L, LDL‐cholesterol greater than 3.0 mmol/L, HDL‐cholesterol less than 1.0 mmol/L or a combination thereof, although investigators' definitions were also accepted. We wanted to include any low‐fat dietary intervention, like low‐fat and low‐saturated fat diets, intended to lower serum total and LDL‐cholesterol or to raise HDL‐cholesterol. A low‐fat diet was considered as a fat calorie intake less than 20% of the total calories. The minimum duration of the intervention had to be six months. We excluded studies in unhealthy people.
Data collection and analysis
Two authors were planned to independently assess risk of bias and extract data.
Main results
No study met our inclusion criteria.
Authors' conclusions
Well designed, adequately powered randomised controlled trials investigating patient‐relevant outcomes of low‐fat diets for otherwise healthy people with hypercholesterolaemia are required.
Keywords: Adult; Humans; Diet, Fat‐Restricted; Diet, Fat‐Restricted/adverse effects; Diet, Fat‐Restricted/methods; Hypercholesterolemia; Hypercholesterolemia/diet therapy
Low‐fat diets for acquired hypercholesterolaemia
There is currently no firm evidence of the long‐term (at least six months) effects of low‐fat diets for otherwise healthy people with acquired, that is not familial hypercholesterolaemia (high cholesterol levels in the blood). Various low‐fat diets have been investigated in people with long‐term illnesses, however, a high quality trial of at least six months duration in otherwise healthy people with high blood cholesterol is needed.
Background
Description of the condition
Hypercholesterolaemia, characterised by raised blood cholesterol levels, is not a disease itself but a metabolic derangement that can be secondary and often contributes to many diseases, most notably cardiovascular disease (CVD). In most cases, elevated cholesterol levels are associated with a diet high in fat, especially saturated fat, coupled with an inactive lifestyle. Less commonly, raised cholesterol may be related to an inherited disorder, familial hypercholesterolaemia. This systematic review is only concerned with acquired hypercholesterolaemia.
Hypercholesterolaemia is often associated with raised levels of low‐density lipoprotein (LDL), a type of lipoprotein that transports cholesterol and triglycerides from the liver to peripheral tissues. LDL also regulates cholesterol synthesis at these sites. Hypercholesterolaemia is likely to also be associated with low levels of high‐density lipoproteins (HDL), another type of lipoprotein which enable lipids like cholesterol and triglycerides to be transported within the blood stream. HDL can remove cholesterol from atheroma within arteries and transport it back to the liver for excretion or re‐utilization. Hypercholesterolaemia can increase a person's risk of cardiovascular disease via the intermediate step of plaque creation along artery walls, or atherosclerosis. Plaques can eventually obstruct or even block the flow of blood to the brain, heart, and other organs, which in turn can lead for example to stroke or heart attack. Atherosclerosis is a chronic inflammatory response in the walls of the arteries, which largely is due to the accumulation of white blood cells called macrophages. Atherosclerosis is promoted by LDL, when there is inadequate removal of fats and cholesterol from the macrophages by functional HDL, leading to the formation of multiple plaques within the arteries.
Atherosclerosis, though typically asymptomatic for years, eventually produces two main problems. The first problem is when plaques rupture, and clots form inside the artery lumen over the ruptures. The clots heal and usually shrink but leave behind stenosis of the artery, or worse, complete closure, and therefore, an insufficient blood supply to the tissues and organ it feeds. The second problem is when aneurysm results, due to the excessive enlargement process of the compensating artery. These complications of advanced atherosclerosis are chronic, slowly progressive and cumulative. Most commonly, soft plaque suddenly ruptures causing the formation of a thrombus that will rapidly slow or stop blood flow, leading to an infarction, hence leading to heart attack or stroke.
The link between hypercholesterolaemia and cardiovascular disease is equivocal with extrapolations from cardiovascular risk factor statistics to atherosclerosis deemed questionable in some cases (Stehbens 2001). It has been postulated that atherosclerosis is an inflammatory process which may explain inconsistencies between risk factors and disease prevalence and severity. Some of the cholesterol‐independent effects of statins involve improved endothelial function, stability of atherosclerotic plaques, attenuation of oxidative stress and inflammation, as well as inhibition of the thrombogenic response (Athyros 2009). Evidence suggests that in high CVD risk patient groups pleiotropic effects of statins (see below) may play a role in the reduction of morbidity and mortality. However, this concept requires proof in appropriately designed trials that will include clinically relevant end points in order to set specific targets in new CVD‐related bio markers, in addition to lipid levels, that should be used to fully assess the statin contribution to CVD treatment (Athyros 2009). In contrast, other works cite moderately good correlation between dietary saturated fatty acids and coronary heart disease (CHD) when populations in different parts of the world are compared, although this may not be true within the same cultural community or for individuals (Oliver 1982). Total energy, essential fatty acids (EFA), dietary fibre, alcohol and salt also contribute to the relationship of diet to CHD. Saturated fatty acids exert their pathogenic role mostly through altering the homoeostasis of lipoprotein metabolism, leading to an increase in cholesterol‐rich low‐density lipoproteins, and influencing adversely the balance between the accumulation in and clearance of cholesterol esters from the arterial wall. Polyunsaturated fatty acids (PUFA) alter lipoprotein metabolism directly by decreasing synthesis and increasing catabolism and excretion and indirectly by being substitutes for saturated fatty acids, which are therefore consumed in smaller quantities (Oliver 1982). In summary, normal or raised levels of HDL seem to protect against cardiovascular diseases, whereas low HDL‐cholesterol levels seem to increase the risk for heart disease, although other dietary and environmental factors and inter‐individual variations also affect the rate of atherosclerotic disease progression.
Prevalence and burden
For over 50 years physicians have been aware that the aetiology of atherosclerosis is usually hypercholesterolaemia (Pollak 1953). The prevalence of hypercholesterolaemia in US adults is estimated to be between 37% and 55% (Ford 2003). According to US data the leading cause (over 60%) of all mortalities in 2002 were due to cardiovascular disease, equating to over 700,000 deaths annually (Callow 2005). A study of 7640 patients estimated total disease‐related costs, related to objective hypercholesterolaemia to be a mean of EUR 2498 to 4898 (USD 3229 to 6332) per patient over six months, comprising direct (44%) and indirect (56%) costs (Muller‐Nordhorn 2008). Other disease‐related burdens like early retirement were responsible for 42% of costs, followed by hospital visits (19%), medication (15%), workdays lost (14%), physician visits (5%), outpatient therapy (2%), and rehabilitation (2%).
Diagnosis and treatment targets
Diagnosis and treatment of hypercholesterolaemia is based on blood measurements or estimations (Friedwald formula estimates LDL, Niedbala 1985) of at least three different fractions of cholesterol; total cholesterol (TC), low density lipoprotein cholesterol (LDL) and high density lipoprotein cholesterol (HDL). The following Table 1 demonstrates how treatment targets for TC, LDL and HDL differ slightly in European (De Backer 2004) and Australian (Tonkin 2005) guidelines.
Table 1.
Primary treatment targets for blood lipids
| Guidelines | Total cholesterol | HDL‐C | LDL‐C | |||
| ESC (De Backer 2004) | General | < 5.0 mmol/L (< 190 mg/dL) |
Women | > 1.2 mmol/L (> 45 mg/dL) |
General | < 3.0 mmol/L (< 115 mg/dL) |
| High riska | < 4.5 mmol/L (< 175 mg/dL) |
Men | > 1.0 mmol/L (> 40 mg/dL) |
High risk | < 2.5 mmol/L (< 100 mg/dL) |
|
| NHF (Tonkin 2005) | General | < 5.5 mmol/L (< 210 mg/dL) |
> 1.0 mmol/L (> 40 mg/dL) |
General | < 3.0 mmol/L (< 115 mg/dL) |
|
| High riska | < 4.0 mmol/L (< 155 mg/dL) |
> 1.0 mmol/L (> 40 mg/dL) |
High riska | < 2.5 mmol/L (< 100 mg/dL) |
||
a High risk indicates that the patient has other risk factors for cardiovascular disease which may include age, smoking, metabolic syndrome.
ESC: European Society of Cardiology; HDL‐C: high‐density lipoprotein cholesterol; LDL‐C: low‐density lipoprotein cholesterol; NHF: National Heart Foundation (Australia)
Description of the intervention
It is recommended that first line treatment for hypercholesterolaemia entails dietary and 'lifestyle' modification. Examples of lifestyle interventions are diets that place daily limits on total energy, fat, saturated fat, cholesterol (usually less than 200 mg per day); increased consumption of soluble fibre and inclusion of two grams of plant sterols/stanols per day in addition to a program of physical activity (De Backer 2004; National Cholesterol Education Program 2002; Tonkin 2005; Varady 2005). As excess energy intake will lead to weight gain via fat storage all low‐fat diets by definition require total energy restriction. Most low‐fat diets also restrict the proportion (percentage) of calories obtained from fat and in some cases restrictions are placed on proportion of calories obtained from saturated fat also (low saturated fat diets). Furthermore, it is widely accepted that patients should be advised to increase their intake of essential fatty acids of marine origin, n‐3 eicosapentanoic acid (EPA) and docosahexanoic acid (DHA) due to their potential protective effect against cardiovascular disease (WHO 2003).
A meta‐analysis of studies revealed that replacement of 60% of calories from saturated fats with a combination of polyunsaturated fats and monounsaturated fats resulted in falls in total cholesterol of 0.8 mmol (10% to 15%), with this reduction being primarily LDL‐cholesterol (Clarke 1997). Moreover, studies measuring the effects of plant sterols and stanols on cholesterol have demonstrated that the mean LDL reduction was approximately 12% (Noakes 2002) and on average, an increase in soluble fibre of 510 grams per day is accompanied by an approximately 5% reduction in LDL‐cholesterol (National Cholesterol Education Program 2002). Moreover, a systematic review reported small but potentially important reductions in cardiovascular risk (rate ratio 0.84, 95% confidence interval (CI) 0.72 to 0.99) with reduction or modification of dietary fat intake, seen particularly in trials of longer duration (Hooper 2000).
The combined effects of step 1 and 2 dietary interventions (see below) can potentially result in a decrease in total cholesterol in the order of up to 30% (Yu‐Poth 1999). However, if after a period of 3 to 6 months lifestyle modification is deemed ineffective or has not effected desired treatment goals (see Table 1) then lipid‐lowering medications may be prescribed. The first‐line pharmacotherapy for hypercholesterolaemia is a HMG‐CoA (3‐hydroxy‐3‐methyl‐glutaryl coenzyme A) reductase inhibitor or 'statin'. Statins lower cholesterol by inhibiting the enzyme HMG‐CoA reductase, which is the rate‐limiting enzyme of the mevalonate pathway of cholesterol synthesis. Inhibition of this enzyme in the liver stimulates LDL receptors, resulting in an increased clearance of LDL from the bloodstream and a decrease in blood cholesterol levels. The first results can be seen after one week of use and the effect is maximal after 4 to 6 weeks. If possible, it is preferable to avoid statin therapy as there are in some cases side‐effects, cost‐implications, compliance is poor (Liberopoulos 2008) and targeting of statins at low‐risk populations is associated with major uncertainties (Ward 2007). Dietary interventions may look as follows:
Step 1 diet of the American Heart Association or its equivalent (less than 30% of total energy intake as fat, with 8% to 10% as saturated fat; ratio of polyunsaturated to saturated fatty acid greater than 1.0; cholesterol intake less than 300 mg/day; and energy intake to achieve desirable body weight).
Step 2 diet of the American Heart Association or its equivalent (less than 30% of total energy intake as fat, with 7% or less as saturated fat; ratio of polyunsaturated to saturated fatty acid greater than 1.4; cholesterol intake less than 200 mg/day; and energy intake to achieve desirable body weight).
How the intervention might work
Blood cholesterol is related to dietary intake of saturated fat, so if saturated fat intake is restricted blood cholesterol levels should fall. A meta‐analysis by Clarke et al (Clarke 1997) reported that for each 1% increase in energy from saturated fats, serum LDL‐cholesterol will increase between 0.33 mmol/L and 0.45 mmol/L. Several large, well‐designed, randomised controlled drug trials have established that effectively treating hypercholesterolaemia may retard or prevent cardiovascular disease (Pederson 1998; WOSCOPS 1996), however pleiotropic effects of statins suggest that alternate mechanisms (than only lowering plasma cholesterol) such as, stabilising plaques and attenuating oxidative stress and inflammation, may also have a role (Athyros 2009). The Scandinavian Simvastatin Survival Study reported that cholesterol lowering with simvastatin reduced the incidence of carotid bruits and cerebrovascular events as well as new‐onset or worsening of angina pectoris and intermittent claudication (Pederson 1998). A Cochrane systematic review has suggested small but potentially important reductions in cardiovascular risk in dietary intervention trials longer than two years are possible in those at high risk of cardiovascular disease (especially where statins are unavailable or rationed). Moreover, lower risk population groups, should continue to include permanent reduction of dietary saturated fat and partial replacement by un‐saturates (Hooper 2000). We therefore wish to examine if via the intermediate step, of reducing cholesterol levels by low‐fat diet, there may be positive effects on cardiovascular outcomes.
Adverse effects of the intervention
Adverse effects are rare but an excessive rate of weight loss can result in muscle wasting and deficiencies in vitamins and minerals as well as possible psychological side‐effects.
Why it is important to do this review
The review is important as natural or non‐pharmacological solutions to most chronic diseases and metabolic derangements, hypercholesterolaemia included, are preferred as side‐effects of pharmacological therapy present health risks. Overweight, obesity, cardiovascular, renal disease and diabetes have reached global epidemic proportions and are related to hypercholesterolaemia and significantly raised risk of morbidity and mortality. Therefore, the importance of treating hypercholesterolaemia is great and potential benefits exist.
A Cochrane systematic review of dietary advice for reducing cardiovascular risk reported modest reductions in TC and LDL‐cholesterol (Brunner 2007). Another systematic review evaluated various dietary and pharmacological interventions for reducing mortality (Struder 2005). A 1999 meta‐analysis evaluated the effects of the National Cholesterol Education Program's step 1 and step 2 dietary interventions on major cardiovascular disease risk factors (National Cholesterol Education Program 2002). This analysis found that plasma TC, LDL‐cholesterol, triacylglycerol, and TC: HDL‐cholesterol decreased by 0.63 mmol/L (10%), 0.49 mmol/L (12%), 0.17 mmol/L (8%), and 0.50 mmol/L (10%), respectively, in step 1 intervention studies (P < 0.01 for all). As no recent systematic review has looked at isolated effects of low‐fat (step 1) diets, for lowering cholesterol profiles and other effects, this is the primary focus of our work.
Objectives
To assess the effects of low‐fat diets for acquired hypercholesterolaemia.
To compare the relative effectiveness of low‐fat diets with calorie‐restricted diets for acquired hypercholesterolaemia.
To compare the relative effectiveness of low‐fat diets and pharmacological interventions for acquired hypercholesterolaemia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials.
Types of participants
Otherwise healthy adults (equal to or greater than 18 years) with acquired (not familial) hypercholesterolaemia. The preferred definition of hypercholesterolaemia was either total cholesterol greater than 5.2 mmol/L, LDL‐cholesterol greater than 3.0 mmol/L, HDL‐cholesterol less than 1.0 mmol/L or a combination thereof, although other definitions were accepted. Studies of familial hypercholesterolaemia were excluded. Changes in diagnostic criteria could produce significant variability in the clinical characteristics of the patients included as well as in results obtained. These differences were planned to be considered and explored in a sensitivity analysis.
Types of interventions
The minimum duration of the intervention had to be six months.
Intervention
Any low‐fat dietary intervention (e.g. low‐fat and low‐saturated fat diets) intended to lower serum total or LDL‐cholesterol or to raise HDL‐cholesterol.
Control
Usual care, calorie‐restricted diets or pharmacological interventions.
Types of outcome measures
Primary outcomes
blood lipids (serum total cholesterol, LDL‐ and HDL‐cholesterol);
cardiovascular events (e.g. myocardial infarction, stroke);
death from any cause.
Secondary outcomes
adverse effects;
health‐related quality of life;
healthcare costs;
serum triglyceride concentration (fasting and non‐fasting).
Covariates, effect modifiers and confounders
gender;
age.
Timing of outcome measurement
Minimum duration of outcome assessment had to be six months, meaningful time points were one year, two years and five years. We planned to clearly separate length of follow‐up from treatment duration. We also wanted to consider length of follow‐up under randomised and non‐randomised conditions.
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of trials:
The Cochrane Library (Issue 1, 2010);
MEDLINE (until February 2010);
EMBASE (until February 2010).
We also searched databases of ongoing trials:
Current Controlled Trials (http://www.controlled‐trials.com ‐ with links to other databases of ongoing trials)
UK National Research Register (http://www.update‐software.com/National/)
USA ‐ CenterWatch Clinical Trials Listing Service (http://www.CenterWatch.com/)
USA ‐ National Institutes of Health (http://clinicalstudies.info.nih.gov/)
For detailed search strategies please see under Appendix 1.
Additional key words of relevance could have been detected during the electronic or other searches. If this was the case, electronic search strategies would have been modified to incorporate these terms. We planned to include studies published in any language.
Searching other resources
We planned to identify additional studies by searching the reference lists of included trials and (systematic) reviews, meta‐analyses and health technology assessment reports noticed.
Data collection and analysis
Selection of studies
A flow‐chart of the number of studies (Figure 1) identified and excluded at each stage was prepared in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement (Liberati 2009). The flow‐chart was applied by two authors (EB and JW) and inter‐rater agreement was 100% (Cohen 1960), differences in opinion would have been resolved by a third author (NS) by consensus. We did not plan to blind trials for trial selection and risk of bias assessment.
Figure 1.
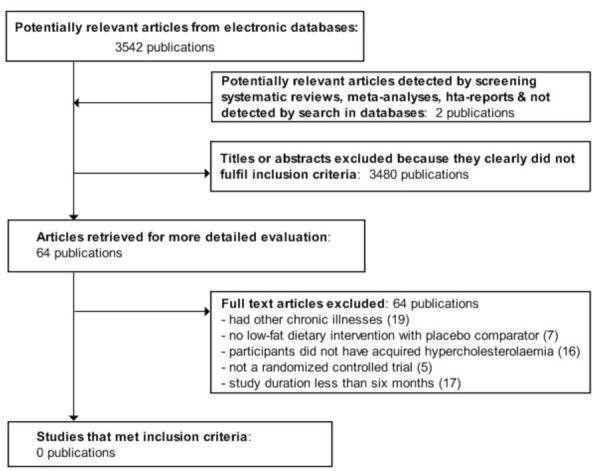
Adapted PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow chart of study selection
Data extraction and management
Data extraction tables were planned to be used according to the template provided by the Cochrane Metabolic and Endocrine Disorders Review Group. Both data extraction and entry was to be done in duplicate by two authors, LM and EB. Differences in data extraction would have been resolved by consulting a third person (NS) and referring back to the original paper. We wanted to contact authors of identified papers about duplicate studies, missing information in their trials, e.g. methods of randomisation and allocation concealment, separate information for certain patient subgroups, information about complications etc.
Assessment of risk of bias in included studies
We planned to perform risk of bias assessment in duplicate by two authors BM and CE using the Cochrane Collaboration's risk of bias tool (Higgins 2008). We wanted to report inter‐rater agreement using Cohen's kappa (Cohen 1960) on key quality issues (like concealment of allocation) and differences in opinion would have been resolved by consulting a third author (NS).
Measures of treatment effect
Dichotomous data
Dichotomous outcomes (for example stroke yes/no) would have been expressed as odds ratios (OR) or relative risks (RR) with 95% confidence intervals (CI). Odds ratios were planned to be reported in analysed studies if event rates were less than 10%.
Continuous data
Continuous outcomes (for example LDL‐cholesterol) were planned to be expressed, if possible, as mean differences with 95% CI. We intended to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Time‐to‐event data
Time‐to‐event outcomes (for example time until death) were planned to be expressed as hazard ratios (HR) with 95% CI.
Unit of analysis issues
We planned to exclude data from cluster‐randomised trials.
Dealing with missing data
Relevant missing data would have been obtained from authors, if feasible. Evaluation of important numerical data such as screened, eligible and randomised patients as well as intention‐to‐treat and per‐protocol population would have been carefully performed. Attrition rates like drop‐outs, misses to follow‐up and withdrawn study participants were planned to be investigated. Issues of last‐observation‐carried‐forward (LOCF) would have been critically appraised and compared to specification of primary outcome parameters and power calculation.
In the case of duplicate publications and companion papers of a primary study, we intended to try to maximise yield of information by simultaneous evaluation of all available data. In cases of doubt, the original publication (usually the oldest version) would have obtained priority.
Assessment of heterogeneity
We planned to investigate heterogeneity irrespective of whether it was statistically significant or not. If appropriate, causes of heterogeneity may be subdivided into five domains:
the patient (for example rate of drug metabolism, risk factors, co‐morbidity, compliance);
the disorder (for example stage of development, duration);
the intervention (for example doses, routes, timing, study design factors);
the comparison (for example different control interventions, co‐medication);
the outcome (for example timing of measurement, method of measurement).
In the event of substantial clinical or methodological or statistical heterogeneity, we did not intend to combine study results by means of meta‐analysis. Heterogeneity would have been identified by visual inspection of the forest plots, by using a standard Chi2 test and a significance level of α = 0.1, in view of the low power of such tests. Heterogeneity would have been specifically examined with the I2 statistic (Higgins 2002), where I2 values of 50% and more indicate a substantial level of heterogeneity (Higgins 2003). In case heterogeneity was detected, we planned to attempt to determine potential reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
Funnel plots would have been used to assess for the potential existence of small study bias. There are a number of explanations for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to study size, poor methodological design of small studies and publication bias (Sterne 2001). Therefore, we would have carefully interpreted findings (Lau 2006).
Data synthesis
Data were planned to be summarised statistically if available, sufficiently similar and of sufficient quality. Statistical analysis would have been performed according to the statistical guidelines referenced in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Method of meta‐analysis
We planned to use the Mantel‐Haenzel fixed‐effect model, reporting odds ratios and 95% confidence intervals. In order to contrast absolute and relative measures, we also wanted to express results as number‐needed‐to‐treat (to benefit or to harm) (NNTB, NNTH). NNTB and NNTH depend among other things upon follow‐up time and baseline risk. We therefore planned to adjust for follow‐up time by annualising NNTH and NNTB as described previously (Mayne 2006). For discrete data we planned to adjust for baseline risk by analysing change in event rates, for continuous data if groups were not matched at baseline we intended to only report descriptively.
Expression of results
We aimed to express results in a way that is easily comprehensible to the reader. We wanted to use a combination of underlying event rates, expressing results as NNTB and NNTH adjusted for follow‐up time and baseline risk.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were planned to determine whether there were any systematic differences between groups of patients. Examples of possible subgroup analyses are: stratification by age, sex, different comparison interventions, different follow‐up duration. In each subgroup analysis the groups were planned to be clearly defined. We wanted to avoid subgroup analyses if there was no statistically significant treatment effect in one of our primary outcomes.
The following subgroup analyses were planned:
analyses of patients with and without co‐morbid conditions, e.g. diabetes, cardiovascular disease, obesity.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect size:
use of non‐validated measurement scales or tools;
repeating the analysis excluding unpublished studies;
repeating the analysis taking account of risk of bias, as specified above;
repeating the analysis excluding any very long or large studies to establish how much they dominate the results;
repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country.
The robustness of the results would have been tested by repeating the analysis using different measures of effects size (relative risk, odds ratio etc.) and different statistical models (fixed‐effect model and random‐effects model).
Results
Description of studies
The search identified 3544 publications, 64 articles were excluded after full text evaluation (Figure 1). For description of excluded studies, please see table Characteristics of excluded studies.
Risk of bias in included studies
No study met our inclusion criteria.
Effects of interventions
No study met our inclusion criteria.
Discussion
No study met our inclusion criteria.
Sub‐optimal management of hypercholesterolaemia has potentially huge global health and economic burdens. Often those newly diagnosed with hypercholesterolaemia will be advised to follow a low‐fat diet. Our initial search for studies of low‐fat diets to manage isolated hypercholesterolaemia, in otherwise healthy people, revealed no studies that met our inclusion criteria. The main reasons for exclusion were that studies were often in either mixed populations (those with and without hypercholesterolaemia, or those with and without cardiovascular disease) so the effect of isolated hypercholesterolaemia could not be quantified. Other studies were excluded because they were not of insufficient duration (less than six months) to reasonably expect a cholesterol lowering effect. Other excluded studies were either combined interventions, for example drugs plus low‐fat diets or were studies undertaken in individuals concurrently taking cholesterol lowering medication, so the effects of low‐fat diets could not be isolated. We were therefore unable to draw conclusions whether the isolated effects of low‐fat diets are effective in managing isolated hypercholesterolaemia. As uncontrolled hypercholesterolaemia has significant global health and economic implications, adequately designed randomised, controlled trials are required to guide clinical practice.
Authors' conclusions
Many physicians and allied health practitioners advise people with hypercholesterolaemia to undertake a low‐fat diet. Additionally many people may 'self‐treat' by placing themselves on low‐fat diets. In both these scenarios the treatment goal can be to manage or lower patients' blood cholesterol levels. Our review has illustrated that no firm published evidence exists to support the widely held opinion that low‐fat diets will in fact lower blood cholesterol levels.
This review provides significant implications for research, as no randomised, controlled study of low‐fat dietary intervention versus usual care, of at least six months duration in healthy people, has been published to date.
Appendices
Appendix 1. Search strategies
| Search terms |
| Unless otherwise stated, search terms are free text terms; MeSH = Medical subject heading (Medline medical index term); exp = exploded MeSH; the dollar sign ($) stands for any character(s); the question mark (?) substitutes one or no characters; tw = text word; pt = publication type; sh = MeSH; adj = adjacent. The Cochrane Library, Issue 1.2010 #1 MeSH descriptor Hypercholesterolemia explode all trees #2 MeSH descriptor Hyperlipidemias explode all trees #3 MeSH descriptor Cholesterol, HDL explode all trees #4 MeSH descriptor Cholesterol, LDL explode all trees #5 hypercholester* in All Text #6 hyperlipid?emia* in All Text #7 hyperlip?emia* in All Text #8 (high in All Text and density in All Text and lipoprotein in All Text and cholesterol* in All Text) #9 HDL‐C in All Text #10 (low in All Text and density in All Text and lipoprotein in All Text and cholesterol* in All Text) #11 LDL‐C in All Text #12 (total in All Text and cholesterol* in All Text) #13 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12) #14 MeSH descriptor Diet therapy explode all trees #15 MeSH descriptor Diet, Fat‐Restricted explode all trees #16 (diet* in All Text near/6 fat* in All Text) #17 (#14 or #15 or #16) #18 (#13 and #17) #19 MeSH descriptor Adult explode all trees #20 adult* in All Text #21 (#19 or #20) #22 (#18 and #21) MEDLINE (until February 2010) 1. exp Hypercholesterolemia/ 2. exp Hyperlipidemias/ 3. exp Cholesterol, HDL/ 4. exp Cholesterol, LDL/ 5. Hypercholester$.ab,ti,ot. 6. hyperlipid?emia$.ab,ti,ot. 7. hyperlip?emia$.ab,ti,ot. 8. high density lipoprotein cholesterol$.ab,ti,ot. 9. HDL‐C.ab,ti,ot. 10. low density lipoprotein cholesterol$.ab,ti,ot. 11. LDL‐C.ab,ti,ot. 12. total cholesterol$.ab,ti,ot. 13. or/1‐12 14. exp Diet Therapy/ 15. exp Diet, Fat‐Restricted/ 16. (diet$ adj6 fat$).ab,ti,ot. 17. or/14‐16 18. 13 and 17 19. randomized controlled trial.pt. 20. controlled clinical trial.pt. 21. randomized.ab. 22. placebo.ab. 23. drug therapy.fs. 24. randomly.ab. 25. trial.ab. 26. groups.ab. 27. or/19‐26 28. Meta‐analysis.pt. 29. exp Technology Assessment, Biomedical/ 30. exp Meta‐analysis/ 31. exp Meta‐analysis as topic/ 32. hta.tw,ot. 33. (health technology adj6 assessment$).tw,ot. 34. (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 35. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systemat$)).tw,ot. 36. or/28‐35 37. 27 or 36 38. 18 and 37 39. limit 38 to "all adult (19 plus years)" EMBASE: (until February 2010) 1. exp Hypercholesterolemia/ 2. exp Hyperlipidemia/ 3. exp High Density Lipoprotein Cholesterol/ 4. exp Low Density Lipoprotein Cholesterol/ 5. hypercholester$.ab,ti,ot. 6. hyperlipid?emia$.ab,ti,ot. 7. hyperlip?emia$.ab,ti,ot. 8. high density lipoprotein cholesterol$.ab,ti,ot. 9. HDL‐C.ab,ti,ot. 10. low‐density lipoprotein cholesterol$.ab,ti,ot. 11. LDL‐C.ab,ti,ot. 12. exp Cholesterol Blood Level/ 13. total cholesterol$.ab,ti,ot. 14. or/1‐13 15. exp Diet Therapy/ 16. exp Low Fat Diet/ 17. (low fat diet$ or restricted fat diet$).ab,ti,ot. 18. or/15‐17 19. 14 and 18 20. random$.tw. 21. (crossover$ or cross over$).tw. 22. (double adj blind$).tw. 23. (single adj blind$).tw. 24. (assign$ or allocat$ or volunteer$).tw. 25. Crossover Procedure/ 26. Double Blind Procedure/ 27. Randomized Controlled Trial/ 28. Controlled Clinical Trial/ 29. Single Blind Procedure/ 30. Randomization/ 31. or/20‐30 32. exp meta analysis/ 33. (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 34. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 35. exp Literature/ 36. exp Biomedical Technology Assessment/ 37. hta.tw,ot. 38. (health technology adj6 assessment$).tw,ot. 39. or/32‐38 40. 31 or 39 41. 19 and 40 42. limit 41 to (adult <18 to 64 years> or aged <65+ years>) |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aquilani 1999 | Participants had other chronic disease at baseline |
| Avogaro 1983 | Not low fat dietary intervention (fibrate trial) |
| Baumann 1982 | Not low‐fat diet versus placebo (niacin) |
| Birley 1997 | Participants did not have acquired hyperlipidaemia |
| Blacket 1979 | Participants did not have acquired hyperlipidaemia |
| Bowen 1996 | Not a randomised controlled trial |
| Boyd 1996 | Participants did not have acquired hyperlipidaemia |
| Bravo‐Herrera 2004 | Study less than six months duration |
| Brehm 2003 | Participants had other chronic disease at baseline |
| Brown 1984 | Participants had other chronic disease at baseline |
| Carmena 1984 | Participants did not have acquired hyperlipidaemia |
| Cortese 1983 | Study less than six months duration |
| Davidson 1998 | Study less than six months duration |
| de Bont 1981 | Participants had other chronic disease at baseline |
| Dengel 1994 | Not a randomised controlled trial |
| Due 2008 | Not low fat dietary intervention (post weight‐loss trial) |
| Fagerberg 1998 | Participants had other chronic disease at baseline |
| Fernandez 2002 | Study less than six months duration |
| Fleming 2002 | Participants did not have acquired hyperlipidaemia |
| Gonzalez 1996 | Study less than six months duration |
| Gordon 1982 | Participants did not have acquired hyperlipidaemia |
| Gosselin 1996 | Participants had other chronic disease at baseline |
| Hannah 1997 | Study less than six months duration |
| Haugaard 2009 | Study less than six months duration |
| Hjermann 1980 | Participants had other chronic disease at baseline |
| Hutchinson 1983 | Participants had other chronic disease at baseline |
| Hyman 1998 | No dietary intervention, no measure of dietary effects |
| Jenkins 1993 | Study less than six months duration |
| Jenkins 1999 | Study less than six months duration |
| Jenkins 2009 | Participants had other chronic disease at baseline |
| Judd 1988 | Study less than six months duration |
| Kuo 1976 | Participants did not have acquired hyperlipidaemia |
| Kuo 1978 | Participants did not have acquired hyperlipidaemia |
| Lantz 2003 | Study less than six months duration |
| Lewis 1981 | Study less than six months duration |
| Liu 1984 | Study less than six months duration |
| MacMahon 1998 | Participants did not have acquired hyperlipidaemia |
| Marckmann 1998 | Participants did not have acquired hyperlipidaemia |
| Mellies 1987 | Not dietary intervention, no measure of dietary effects |
| Miida 1998 | Study less than six months duration |
| Mishra 1994 | Not a randomised, controlled trial |
| Muller 2000 | Participants had other chronic disease at baseline |
| Nessim 1983 | Participants did not have acquired hyperlipidaemia |
| Nicklas 2003 | Participants had other chronic disease at baseline |
| Niebauer 1995 | Participants had other chronic disease at baseline |
| Niebauer 1997 | Participants had other chronic disease at baseline |
| O'Bryne 1998 | Study less than six months duration |
| O'Byrne 1997 | Not a randomised controlled trial |
| Oh 1985 | Participants did not have acquired hyperlipidaemia |
| Paisey 1995 | Participants had other chronic disease at baseline |
| Reuter 1984 | Participants did not have acquired hyperlipidaemia |
| Schaefer 1981 | Study less than six months duration |
| Shai 2008 | Participants had other chronic disease at baseline |
| Shikany 2005 | Study less than six months duration |
| Shorey 1976 | Participants did not have acquired hyperlipidaemia |
| Tapsell 2004 | Participants had other chronic disease at baseline |
| Turner 1981 | Participants had other chronic disease at baseline |
| Vidgren 1999 | Not dietary intervention (statin trial) |
| Wass 1981 | Participants had other chronic disease at baseline |
| Williams 1994 | Not exclusive dietary intervention (secondary intervention was employed) |
| Wolfe 1995 | Not a randomised controlled trial |
| Wood 1991 | Participants did not have acquired hyperlipidaemia |
| Yancy 2004 | Participants had other chronic disease at baseline |
| Zambon 1999 | Participants did not have acquired hyperlipidaemia |
Contributions of authors
NEIL SMART: Edited the protocol and verified included/excluded studies.
ELIE BOULOS: Assisted with writing the protocol.
NIGEL KWOK: Assisted with writing the protocol.
BELINA J MARSHALL: Provided dietary advice with regard to the background and protocol, classified studies to be included/excluded, assisted with report writing.
NADINE BAKER: Provided dietary advice with regard to the background and protocol, classified studies to be included/excluded, assisted with report writing.
MAXINE DALEY: Provided dietary advice with regard to the background and protocol, classified studies to be included/excluded, assisted with report writing.
Sources of support
Internal sources
-
PHCRED, Australia.
Primary Healthcare Research Education and Development
External sources
No sources of support supplied
Declarations of interest
None known.
New
References
References to studies excluded from this review
- Aquilani R, Tramarin R, Pedretti RF, Bertolotti G, Sommaruga M, Mariani P, et al. Despite good compliance, very low fat diet alone does not achieve recommended cholesterol goals in outpatients with coronary heart disease. European Heart Journal 1999;20(14):1020‐9. [DOI] [PubMed] [Google Scholar]
- Avogaro P, Bittolo Bon G, Belussi F, Pontoglio E, Cazzolato G. Variations in lipids and proteins of lipoproteins by fenofibrate in some hyperlipoproteinaemic states. Atherosclerosis 1983;47(1):95‐100. [DOI] [PubMed] [Google Scholar]
- Baumann J, Martschick R. Therapy of hyperlipidemia with xanthinol nicotinate as opposed to low fat diet. Medizinische Welt 1982;33(4):139‐41. [PubMed] [Google Scholar]
- Birley AJ, MacLennan R, Wahlqvist M, Gerns L, Pangan T, Martin NG. MN blood group affects response of serum LDL cholesterol level to a low fat diet. Clinical Genetics 1997;51(5):291‐5. [DOI] [PubMed] [Google Scholar]
- Blacket RB, Leelarthaepin B, McGilchrist CA, Palmer AJ, Woodhill JM. The synergistic effect of weight loss and changes in dietary lipids on the serum cholesterol of obese men with hypercholesterolaemia: implications for prevention of coronary heart disease. Australian and New Zealand Journal of Medicine 1979;9(5):521‐529. [DOI] [PubMed] [Google Scholar]
- Bowen D, Clifford CK, Coates R, Evans M, Feng Z, Fouad M, et al. The Women's Health Trial Feasibility Study in Minority Populations: design and baseline descriptions. Annals of Epidemiology 1996;6(6):507‐9. [DOI] [PubMed] [Google Scholar]
- Boyd NF, Martin LJ, Beaton M, Cousins M, Kriukov V. Long‐term effects of participation in a randomized trial of a low‐fat, high‐carbohydrate diet. Cancer Epidemiology, Biomarkers and Prevention 1996;5(3):217‐22. [PubMed] [Google Scholar]
- Bravo‐Herrera MD, López‐Miranda J, Marín C, Gómez P, Gómez MJ, Moreno JA, et al. Tissue factor expression is decreased in monocytes obtained from blood during Mediterranean or high carbohydrate diets. Nutrition, metabolism and cardiovascular diseases June 2004;14(3):128‐32. [DOI] [PubMed] [Google Scholar]
- Brehm BJ, Seeley RJ, Daniels SR, D'Alessio DA. A randomized trial comparing a very low carbohydrate diet and a calorie‐restricted low fat diet on body weight and cardiovascular risk factors in healthy women. Journal of Clinical Endocrinology and Metabolism 2003;88(4):1617‐23. [DOI] [PubMed] [Google Scholar]
- Brown GD, Whyte L, Gee MI, Crockford PM, Grace M, Oberle K, et al. Effects of two "lipid‐lowering" diets on plasma lipid levels of patients with peripheral vascular disease. Journal of the American Dietetic Association 1984;84(5):546‐50. [PubMed] [Google Scholar]
- Carmena R, Ascaso JF, Tebar J, Soriano J. Changes in plasma high‐density lipoproteins after body weight reduction in obese women. International Journal of Obesity 1984;8(2):135‐40. [PubMed] [Google Scholar]
- Cortese C, Levy Y, Janus ED, Turner PR, Rao SN, Miller NE, et al. Modes of action of lipid‐lowering diets in man: studies of apolipoprotein B kinetics in relation to fat consumption and dietary fatty acid composition. European Journal of Clinical Investigation 1983;13(1):79‐85. [DOI] [PubMed] [Google Scholar]
- Davidson MH, Synecki C, Maki KC, Drennen KB. Effects of dietary inulin in serum lipids in men and women with hypercholesterolaemia. Nutrition research 1998;3:503‐17. [Google Scholar]
- Bont AJ, Baker IA, Leger AS, Sweetnam PM, Wragg KG, Stephens SM, et al. A randomised controlled trial of the effect of low fat diet advice on dietary response in insulin independent diabetic women. Diabetologia 1981;21(6):529‐33. [DOI] [PubMed] [Google Scholar]
- Dengel DR, Hagberg JM, Coon PJ, Drinkwater DT, Goldberg AP. Comparable effects of diet and exercise on body composition and lipoproteins in older men. Medicine and science in sports and exercise 1994;26(11):1307‐15. [PubMed] [Google Scholar]
- Due A, Larsen TM, Mu H, Hermansen K, Stender S, Astrup A. Comparison of 3 ad libitum diets for weight‐loss maintenance, risk of cardiovascular disease, and diabetes: A 6‐mo randomized, controlled trial. American Journal of Clinical Nutrition 2008;88(5):1232‐41. [DOI] [PubMed] [Google Scholar]
- Fagerberg B, Wikstrand J, Berglund G, Samuelsson O, Agewall S. Mortality rates in treated hypertensive men with additional risk factors are high but can be reduced. A randomized intervention study. American Journal of Hypertension 1998;11(1 Pt 1):14‐22. [DOI] [PubMed] [Google Scholar]
- Fernández De La Puebla RA, Carmona J, Fuentes F, Marín C, Gómez P, López‐Miranda J, et al. Response degree of cholesterol LDL to feeding in males with hypercholesterolemia according to baseline value. Medicina clínica (Barcelona) 2002;118(19):737‐40. [PubMed] [Google Scholar]
- Fleming RM. The effect of high‐, moderate‐, and low‐fat diets on weight loss and cardiovascular disease risk factors. Preventive cardiology 2002;5(3):110‐8. [DOI] [PubMed] [Google Scholar]
- Gonzalez MM, Cabello M, Tinahones F, Burgos D, Lillo J, Soriguer F, et al. Hyperlipoproteinemia in renal transplant patients: Effect of hypocaloric diet, exercise and HMG‐CoA reductase inhibition [HIPERLIPOPROTEINEMIA EN EL TRASPLANTE RENAL: RESPUESTA A DIETA LA HIPOCALORICA, EJERCICIO FISICO Y LOVASTATINA]. Nefrologia 1996;16(4):359‐64. [Google Scholar]
- Gordon DJ, Salz KM, Roggenkamp KJ, Franklin FA Jr. Dietary determinants of plasma cholesterol change in the recruitment phase of the Lipid Research Clinics Coronary Primary Prevention Trial. Arteriosclerosis 1982;2(6):537‐48. [DOI] [PubMed] [Google Scholar]
- Gosselin P, Verreault R, Gaudreault C, Guillemette J, Niebauer J, Hambrecht R, et al. Dietary treatment of mild to moderate hypercholesterolemia. Effectiveness of different interventions. Canadian Family Physician 1996;42(1):2160‐7. [PMC free article] [PubMed] [Google Scholar]
- Hannah JS, Jablonski KA, Howard BV. The relationship between weight and response to cholesterol‐lowering diets in women. International Journal of Obesity 1997;21(6):445‐50. [DOI] [PubMed] [Google Scholar]
- Haugaard SB, Vaag A, Mu H, Madsbad S. Skeletal muscle structural lipids improve during weight‐maintenance after a very low calorie dietary intervention. Lipids in Health and Disease 2009;8(34):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjermann I, Leren P, Norman N, Helgeland A, Holme I. Serum insulin response to oral glucose load during a dietary intervention trial in healthy coronary high risk men: the Oslo study. Scandinavian Journal of Clinical and Laboratory Investigation 1980;40(1):89‐94. [DOI] [PubMed] [Google Scholar]
- Hutchinson K, Oberle K, Crockford P, Grace M, Whyte L, Gee M, et al. Effects of dietary manipulation on vascular status of patients with peripheral vascular disease. JAMA 1983;249(24):3326‐30. [DOI] [PubMed] [Google Scholar]
- Hyman DJ, Ho KS, Dunn JK, Simons‐Morton D. Dietary intervention for cholesterol reduction in public clinic patients. American Journal of Preventive Medicine 1998;15(2):139‐45. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Wolever TM, Rao AV, Hegele RA, Mitchell SJ, Ransom TP, et al. Effect on blood lipids of very high intakes of fiber in diets low in saturated fat and cholesterol. New England Journal of Medicine 1993;329(1):21‐6. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Mehling CC, Parker T, Rao AV, Agarwal S, et al. Combined effect of vegetable protein (soy) and soluble fiber added to a standard cholesterol‐lowering diet. Metabolism 1999;48(6):809‐16. [DOI] [PubMed] [Google Scholar]
- Jenkins D, Wong JMW, Kendall CWC, Esfahani A, Ng VWY, Leong TCK, et al. The effect of a plant based low carbohydrate ("Eco‐Atkins") diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Archives of Internal Medicine 2009;169(11):1046‐54. [DOI] [PubMed] [Google Scholar]
- Judd JT, Oh SY, Hennig B, Dupont J, Marshall MW. Effects of low fat diets differing in degree of fat unsaturation on plasma lipids, lipoproteins, and apolipoproteins in adult men. Journal of the American College of Nutrition 1988;7(3):223‐34. [DOI] [PubMed] [Google Scholar]
- Kuo PT, Fan WC, Kostis JB, Hayase K. Combined para‐aminosalicylic acid and dietary therapy in long‐term control of hypercholesterolemia and hypertriglyceridemia (Types IIa and IIb hyperlipoproteinemia). Circulation 1976;53(2):338‐41. [DOI] [PubMed] [Google Scholar]
- Luepker RV, Smith LK, Rothchild SS, Gillis A, Kochman L, Warbasse JR. Management of hypercholesterolemia: evaluation of practical clinical approaches in healthy young adults. American Journal Cardiology 1978;41(3):590‐6. [DOI] [PubMed] [Google Scholar]
- Lantz H, Peltonen M, Agren L, Torgerson JS. Intermittent versus on‐demand use of a very low calorie diet: a randomized 2‐year clinical trial. Journal of Internal Medicine 2003;253(4):463‐71. [DOI] [PubMed] [Google Scholar]
- Lewis B, Hammett F, Katan M, Kay RM, Merkx I, Nobels A, et al. Towards an improved lipid‐lowering diet: additive effects of changes in nutrient intake. Lancet 1981;2(8259):1310‐3. [DOI] [PubMed] [Google Scholar]
- Liu G, Coulston A, Hollenbeck C, Reaven G. The effect of sucrose content in high and low carbohydrate diets on plasma glucose, insulin, and lipid responses in hypertriglyceridemic humans. Journal of Clinical Endocrinology and Metabolism 1984;59(4):636‐42. [DOI] [PubMed] [Google Scholar]
- MacMahon S, Sharpe N, Gamble G, Hart H, Scott J, Simes J, et al. Effects of lowering average of below‐average cholesterol levels on the progression of carotid atherosclerosis: results of the LIPID Atherosclerosis Substudy. LIPID Trial Research Group. Circulation 1998;97(18):1784‐90. [DOI] [PubMed] [Google Scholar]
- Marckmann P, Toubro S, Astrup A. Sustained improvement in blood lipids, coagulation, and fibrinolysis after major weight loss in obese subjects. European Journal of Clinical Nutrition 1998;52(5):329‐33. [DOI] [PubMed] [Google Scholar]
- Mellies MJ, Stein EA, Khoury P, Lamkin G, Glueck CJ. Effects of fenofibrate on lipids, lipoproteins, and apolipoproteins in 33 subjects with primary hypercholesterolemia. Atherosclerosis 1987;63(1):57‐64. [DOI] [PubMed] [Google Scholar]
- Miida T, Yamaguchi T, Tsuda T, Okada M. High prebeta1‐HDL levels in hypercholesterolemia are maintained by probucol but reduced by a low‐cholesterol diet. Atherosclerosis 1998;138(1):129‐34. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Sharma AK, Salila M, Srivastava AK, Bal S, Ramesh V. Efficacy of low fat diet in the treatment of benign breast disease. National Medical Journal of India 1994;7(2):60‐2. [PubMed] [Google Scholar]
- Müller MJ, Wiechmann M, Helms C, Wulff C, Kolenda KD. Nutrient intake with low‐fat diets in rehabilitation of patients with coronary heart disease. Zeitschrift fur Kardiologie 2000;89(5):454‐63. [DOI] [PubMed] [Google Scholar]
- Nessim SA, Chin HP, Alaupovic P, Blankenhorn DH. Combined therapy of niacin, colestipol, and fat‐controlled diet in men with coronary bypass. Effect on blood lipids and apolipoproteins. Arteriosclerosis 1983;3(6):568‐73. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Dennis KE, Berman DM, Sorkin J, Ryan AS, Goldberg AP. Lifestyle intervention of hypocaloric dieting and walking reduces abdominal obesity and improves coronary heart disease risk factors in obese, postmenopausal, African‐American and Caucasian women. The journals of gerontology. Series A, Biological sciences and medical sciences 2003;58(2):181‐9. [DOI] [PubMed] [Google Scholar]
- Niebauer J, Hambrecht R, Schlierf G, Marburger C, Kälberer B, Kübler W, et al. Five years of physical exercise and low fat diet: effects on progression of coronary artery disease. Journal of Cardiopulmonary Rehabilitation 1995;15(1):47‐64. [DOI] [PubMed] [Google Scholar]
- Niebauer J. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation1997; Vol. 96, issue 8:2534‐41. [DOI] [PubMed]
- O'Bryne DJ, O'Keefe SF, Shireman RB. Low‐fat, monounsaturate‐rich diets reduce susceptibility of low density lipoproteins to peroxidation ex vivo. Lipids 1998;33(2):149‐57. [DOI] [PubMed] [Google Scholar]
- O'Byrne DJ, Knauft DA, Shireman RB. Low fat‐monounsaturated rich diets containing high‐oleic peanuts improve serum lipoprotein profiles. Lipids 1997;32(7):687‐95. [DOI] [PubMed] [Google Scholar]
- Oh SY, Monaco PA. Effect of dietary cholesterol and degree of fat unsaturation on plasma lipid levels, lipoprotein composition, and fecal steroid excretion in normal young adult men. American Journal of Clinical Nutrition 1985;42(3):399‐413. [DOI] [PubMed] [Google Scholar]
- Paisey RB, Harvey P, Rice S, Belka I, Bower L, Dunn M, et al. Short‐term results of an open trial of very low calorie diet or intensive conventional diet in Type 2 diabetes. Practical Diabetes International 1995;12(6):263‐7. [Google Scholar]
- Reuter W, Heybey U. Effects of a basic metabolic diet rich in polyene fatty acids on the pattern of glycerophospholipid fatty acids in patients with hyperlipoproteinemia. Zeitschrift fur Die Gesamte Innere Medizin und Ihre Grenzgebiete 1984;39(6):100‐2. [PubMed] [Google Scholar]
- Schaefer EJ, Levy RI, Ernst ND, Sant FD, Brewer HB Jr. The effects of low cholesterol, high polyunsaturated fat, and low fat diets on plasma lipid and lipoprotein cholesterol levels in normal and hypercholesterolemic subjects. American Journal of Clinical Nutrition 1981;34(9):1758‐63. [DOI] [PubMed] [Google Scholar]
- Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low‐carbohydrate, Mediterranean, or low‐fat diet. New England Journal of Medicine 2008;359(3):229‐41. [DOI] [PubMed] [Google Scholar]
- Shikany JM Goudie A. Comparison of a low‐fat/low‐glycemic index diet to a low‐fat only diet in the treatment of adults with hypercholesterolemia. Nutrition Research 2005;25(11):971‐81. [Google Scholar]
- Shorey RL, Sewell B, O'Brien M. Efficacy of diet and exercise in the reduction of serum cholesterol and triglyceride in free‐living adult males. American Journal of Clinical Nutrition 1976;29(5):512‐21. [DOI] [PubMed] [Google Scholar]
- Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Baré M, et al. Including walnuts in a low‐fat/modified‐fat diet improves HDL cholesterol‐to‐total cholesterol ratios in patients with type 2 diabetes. Diabetes Care 2004;27(12):2777‐83. [DOI] [PubMed] [Google Scholar]
- Turner JD, Le NA, Brown WV. Effect of changing dietary fat saturation on low‐density lipoprotein metabolism in man. American Journal of Physiology 1981;241(1):E57‐63. [DOI] [PubMed] [Google Scholar]
- Vidgren HM, Agren JJ, Valve RS, Karhunen LJ, Rissanen AM, Uusitupa MI. The effect of orlistat on the fatty acid composition of serum lipid fractions in obese subjects. Clinical Pharmacology and Therapeutics 1999;66(3):315‐22. [DOI] [PubMed] [Google Scholar]
- Wass VJ, Jarrett RJ, Meilton V, Start MK, Mattock M, Ogg CS, et al. Effect of a long‐term fat‐modified diet on serum lipoprotein levels of cholesterol and triglyceride in patients on home haemodialysis. Clinical Science 1981;60(1):81‐6. [DOI] [PubMed] [Google Scholar]
- Williams PT, Krauss RM, Stefanick ML, Vranizan KM, Wood PD. Effects of low‐fat diet, calorie restriction, and running on lipoprotein subfraction concentrations in moderately overweight men. Metabolism 1994;43(5):655‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BM, Nisker JA, Hutton LC, Rutt BK. Novel low‐dosage hormonal replacement therapy complements dietary treatment of moderately hypercholesterolemic postmenopausal women. Clinical and investigative medicine. Médecine clinique et experimentale 1995;18(5):362‐9. [PubMed] [Google Scholar]
- Wood PD, Stefanick ML, Williams PT, Haskell WL. The effects on plasma lipoproteins of a prudent weight‐reducing diet, with or without exercise, in overweight men and women. New England Journal of Medicine 1991;325(7):461‐6. [DOI] [PubMed] [Google Scholar]
- Yancy WS, Olsen MK, Guyton JR. A low‐carbohydrate, ketogenic diet versus a low‐fat diet to treat obesity and hyperlipidemia. A randomized, controlled trial. Annals of Internal Medicine 2004;140:769‐77. [DOI] [PubMed] [Google Scholar]
- Zambon A, Sartore G, Passera D, Francini‐Pesenti F, Bassi A, Basso C, et al. Effects of hypocaloric dietary treatment enriched in oleic acid on LDL and HDL subclass distribution in mildly obese women. Journal of Internal Medicine 1999;246(2):191‐201. [DOI] [PubMed] [Google Scholar]
Additional references
- Athyros, V. G.Kakafika, A. I.Tziomalos, K.Karagiannis, A.Mikhailidis, D. P. Pleiotropic effects of statins ‐ clinical evidence. Current Pharmaceutical Design 15;5:479‐89. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Rees K, Ward K, Burke M, Thorogood M. Dietary advice for reducing cardiovascular risk. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
- Callow, AD. Cardiovascular disease 2005 ‐ the global picture. Vascular Pharmacology 2005;45(5):302‐7. [DOI] [PubMed] [Google Scholar]
- Clarke R, Frost C, Collins R, Appleby P, Peto R. Dietary lipids and blood cholesterol: quantitative meta‐analysis of metabolic ward studies. BMJ 1997;314(7074):112‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement 1960;20:37‐46. [Google Scholar]
- Backer G, Ambrosioni E, Borch‐Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force Of European and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of eight societies and by invited experts). Archives des Maladies du Coeur et des Vaisseaux 2004;97(10):1019‐30. [PubMed] [Google Scholar]
- Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation 2003;107(17):2185‐9. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
- Hooper L, Summerbell CD, Higgins JPT, Thompson RL, Clements G, Capps N, et al. Reduced or modified dietary fat for prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2000 [updated 2001;(3)], Issue 2. [DOI: 10.1002/14651858.CD002137] [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic and Meta‐Analyses of Studies That Evaluate Interventions: Explanation and Elaboration. PLoS Med 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberopoulos EN, Florentin M, Mikhailidis DP, Elisaf MS. Compliance with lipid‐lowering therapy and its impact on cardiovascular morbidity and mortality. Expert Opinion on Drug Safety 2008;7(6):717‐25. [DOI] [PubMed] [Google Scholar]
- Mayne TJ, Whalen E, Vu A. Annualized was found better than absolute risk reduction in the calculation of number needed to treat in chronic conditions. Journal of Clinical Epidemiology 2006;59:217‐23. [DOI] [PubMed] [Google Scholar]
- Muller‐Nordhorn J, Englert H, Wegscheider K, Berger H, Sonntag F, Voller H, et al. Productivity loss as a major component of disease‐related costs in patients with hypercholesterolemia in Germany. Clinical Research in Cardiology 2008;97(3):152‐9. [DOI] [PubMed] [Google Scholar]
- National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143‐421. [PubMed] [Google Scholar]
- Niedbala, R. S.Schray, K. J.Foery, R.Clement, G. Estimation of low‐density lipoprotein by the Friedewald formula and by electrophoresis compared. Clinical Chemistry 1985;31(10):1762‐3. [PubMed] [Google Scholar]
- Noakes M, Clifton P, Ntanios F, Shrapnel W, Record I, McInerney J. An increase in dietary carotenoids when consuming plant sterols or stanols is effective in maintaining plasma carotenoid concentrations. American Journal of Clinical Nutrition 2002;75(1):79‐86. [DOI] [PubMed] [Google Scholar]
- Oliver MF. Diet and coronary heart disease. Clinical Nutrition 1982;36(6):413‐27. [PubMed] [Google Scholar]
- Pedersen TR, Kjekshus J, Pyorala K, Olsson AG, Cook TJ, Musliner TA, et al. Effect of simvastatin on ischemic signs and symptoms in the Scandinavian simvastatin survival study (4S). American Journal of Cardiology 1998;81(3):333‐5. [DOI] [PubMed] [Google Scholar]
- Pollak OJ. Reduction of blood cholesterol in man. Circulation 1953;7(5):702‐6. [DOI] [PubMed] [Google Scholar]
- Stehbens WE. Coronary heart disease, hypercholesterolemia, and atherosclerosis. I. False premises. Experimental and Molecular Pathology 2001;70(2):103‐19. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
- Struder M, Briel M, Leimenstoll B, Glass TR, Bucher HC. Effect of different antilipidemic agents and diets on mortality: a systematic review. Archives of Internal Medicine 2005;165(7):725‐30. [DOI] [PubMed] [Google Scholar]
- Tonkin A, Barter P, Best J, Boyden A, Furler J, Hossack K, et al. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: position statement on lipid management ‐ 2005. Heart, Lung and Circulation 2005;14(4):275‐91. [DOI] [PubMed] [Google Scholar]
- Varady KA, Jones PJH. Combination diet and exercise interventions for the treatment of dyslipidemia: an effective preliminary strategy to lower cholesterol levels?. Journal of Nutrition 2005;135(8):1829‐35. [DOI] [PubMed] [Google Scholar]
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technology Assessment (Winchester, England) 2007;11(14):1‐160 (iii‐iv). [DOI] [PubMed] [Google Scholar]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases. WHO Technical Report Series 9162003; Vol. http://www.who.int/hpr/NPH/docs/who_fao_expert_report.pdf. [PubMed]
- WOSCOPS. West of Scotland Coronary Prevention Study: identification of high‐risk groups and comparison with other cardiovascular intervention trials. Lancet 1996;348(9038):1339‐42. [PubMed] [Google Scholar]
- Yu‐Poth S, Zhao G, Etherton T, Naglak M, Jonnalagadda S, Kris‐Etherton PM. Effects of the National Cholesterol Education Program's Step I and Step II dietary intervention programs on cardiovascular disease risk factors: a meta‐analysis. American Journal of Clinical Nutrition 1999;69(4):632‐46. [DOI] [PubMed] [Google Scholar]


