Abstract
Direct‐acting antiviral (DAA) therapies have revolutionized the treatment of chronic hepatitis C virus infection, achieving sustained virological response (SVR) rates of >90% even in patients with advanced liver cirrhosis. Having observed an unusual case of repeated DAA therapy failures in a patient with a transjugular intrahepatic portosystemic shunt (TIPS), we assessed a possible association between prior TIPS placement and DAA failure. A structured search of our clinical database revealed 10 patients who had received DAA therapy after TIPS placement. At the time of therapy, most patients (8; 80%) presented with a Child‐Pugh score B, and the following DAA regimens were used: sofosbuvir/ledipasvir ± ribavirin (5 patients), sofosbuvir/daclatasvir ± ribavirin (3), sofosbuvir/velpatasvir (2), and sofosbuvir/velpatasvir/voxilaprevir (1). In total, 5 patients (50%) achieved an SVR, whereas a virological relapse occurred in the other half of the cases, including 2 patients with multiple relapses. In this patient cohort, SVR rates were unusually low for all regimens: sofosbuvir/ledipasvir ± ribavirin, 3/5 (60%); sofosbuvir/daclatasvir ± ribavirin, 2/3 (66%); sofosbuvir/velpatasvir, 0/2 (0%); and sofosbuvir/velpatasvir/voxilaprevir, 0/1 (0%), and patients with a TIPS made up a relevant proportion of DAA failures in patients with cirrhosis at our center: sofosbuvir/ledipasvir, 2/18 (11%); sofosbuvir/daclatasvir, 1/4 (25%); sofosbuvir/velpatasvir, 2/3 (66%); and sofosbuvir/velpatasvir/voxilaprevir, 1/1 (100%). Conclusion: We observed a high rate of virological relapse in patients with a TIPS who received DAA treatment and therefore postulate that TIPS placement may be a possible risk factor for DAA failure due to the profound hemodynamic changes evoked by the intervention. Longer treatment duration or addition of ribavirin might be warranted in these patients.
Abbreviations
- CPS
Child‐Pugh score
- DAA
direct‐acting antiviral
- GT
genotype
- HCV
hepatitis C virus
- LTFU
lost to follow‐up
- MELD
Model for End‐Stage Liver Disease
- RAS
resistance‐associated substitutions
- SVR
sustained virological response
- TIPS
transjugular intrahepatic portosystemic shunt
Since the advent of direct‐acting antiviral (DAA) therapies for the treatment of chronic hepatitis C virus (HCV) infection, sustained virological response (SVR) rates have steadily increased from initially 60% in interferon‐based DAA treatment regimens1 to >90% for currently used DAA regimens, even in patients with liver cirrhosis.2 However, several factors are associated with increased rates of treatment failure, including previous DAA treatment experience, advanced liver cirrhosis,2 the presence of resistance‐associated substitutions (RAS),3 and conditions that have an impact on the bioavailability of the drugs, such as prior gastric surgery.4, 5
In patients with decompensated liver cirrhosis, the implantation of a transjugular intrahepatic portosystemic shunt (TIPS) has become the treatment of choice for portal‐hypertensive complications of end‐stage liver disease, such as esophageal varices or refractory ascites.6 Although the primary aim of this intervention is to decrease portal pressure, it has also a profound effect on hepatic hemodynamics, including an increase in hepatic arterial perfusion7 and changes on tissue stiffness.8 An unusual case of repeated DAA therapy failures in a patient who had previously been treated with a TIPS led us to analyze our clinical database for a possible association between TIPS and DAA failure in a structured way.
Patients and Methods
Patients with HCV‐associated liver cirrhosis receiving a TIPS between January 2009 and January 2018 at the Medical Center Hamburg‐Eppendorf were identified by chart review. Standard laboratory results, including calculation of the Child‐Pugh score (CPS), the Model for End‐Stage Liver Disease (MELD) score, and flow rates on Doppler ultrasound prior to the first DAA therapy, were retrospectively assessed in patients who underwent DAA therapy after TIPS placement. The proportion of patients with a TIPS who experienced a virological relapse in comparison with the overall SVR rate was calculated by retrospective analysis of the treatment response of all patients who received sofosbuvir/ledipasvir, sofosbuvir/daclatasvir, sofosbuvir/velpatasvir, and sofosbuvir/velpatasvir/voxilaprevir between January 2014 and February 2018 at our center. SVR was defined as documented negative HCV‐polymerase chain reaction 12 weeks after the end of treatment, whereas a virological relapse was defined as the renewed detection of viral RNA after HCV treatment (and after intermittent complete viral control of HCV viremia). Patients who had initiated an antiviral DAA therapy but did not have a documented control of viremia at least 12 weeks after the end of treatment were defined as lost to follow‐up (LTFU). The study protocol was approved by the local ethics committee in accordance with the Declaration of Helsinki.
Statistical Analysis
Categorical data are given in percentages. Mean or median values with the corresponding SD or interquartile range were calculated for continuous data using Excel 2016 (Microsoft, Inc., Redmond, WA) and SPSS Statistics version 25 (IBM Corp., Armonk, NY). SVR rates were calculated using a per‐protocol analysis. Comparison of flow velocities for the group of patients with relapse or SVR was carried out using a Mann‐Whitney U test; P < 0.05 was considered statistically significant.
Results
Patient Cohort
A total of 74 patients with HCV‐associated liver cirrhosis and TIPS were identified by review of our clinical database. Of these 74 patients, 38 patients (51%) remained without HCV therapy, 16 patients (22%) were treated before TIPS placement, and 7 patients (9%) received treatment after liver transplantation. DAA treatment was initiated in 13 patients after TIPS placement, including 2 patients who underwent liver transplantation during therapy and 1 patient who was LTFU, leaving 10 cases of DAA therapy after TIPS placement for detailed analysis (Fig. 1).
Figure 1.
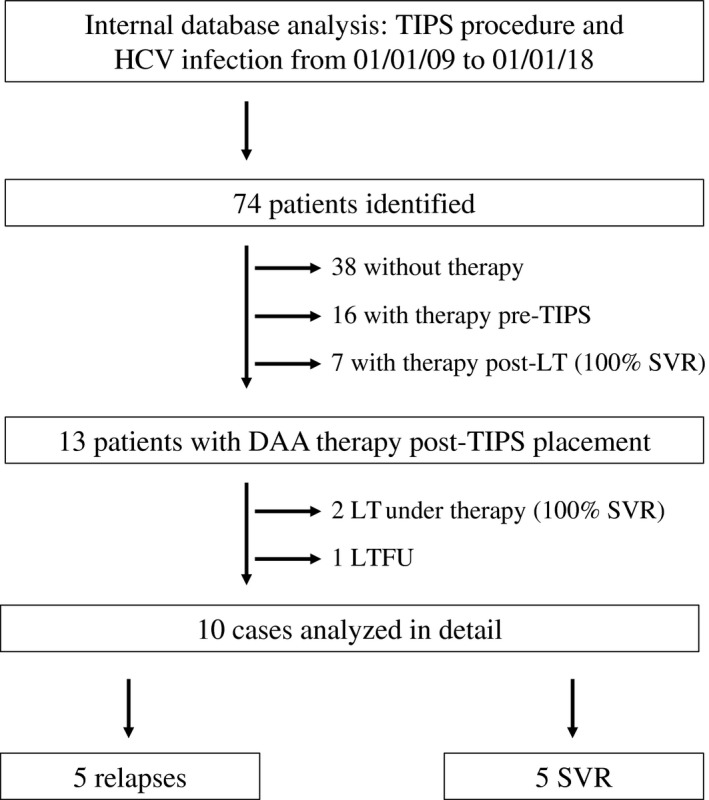
Flow chart of the database analysis to identify patients with DAA treatment post‐TIPS. Interestingly, all 7 patients receiving DAA treatment after liver transplantation and the 2 patients undergoing liver transplantation while being treated cleared the virus, whereas only 50% of patients with a TIPS reached an SVR. Abbreviation: LT, liver transplantation.
General Characteristics
Most patients in this cohort were male adults (8/10; 80%), and the mean age was 52.8 ± 11.7 years. At the time of DAA therapy, 2 patients presented with CPS A, whereas most patients were CPS B (8/10; 80%) (Table 1). The most prevalent genotype (GT) was GT 1a (5/10; 50%), followed by GT 3 (3/10; 30%) and GT 1b (2/10; 20%). Further cofactors with a potential impact on treatment response were present in 5 patients (50%), including HCV‐associated membranoproliferative glomerulonephritis (2/10; 20%), baseline RAS (2/10; 20%), and coinfection with the human immunodeficiency virus (1/10; 10%).
Table 1.
General Characteristics and Treatment Responses of 10 Patients who Received DAA Therapy Post‐Tips
| General Characteristics | Viral Parameters | First DAA Therapy | Second DAA Therapy | Third DAA Therapy | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | Sex | Indication for TIPS | IFN experienced | Cofactors | GT | RAS | CPS (points) | MELD | Albumin (g/L) | INR | Viral load (IU/mL) | DAA therapy | Outcome | CPS (points) | MELD | Viral load (IU/mL) | DAA therapy | Outcome | CPS (points) | MELD | Viral load (IU/mL) | DAA therapy | Outcome |
| 1 | 62 | Male | Variceal bleeding | No | None | 1a | NS3: Q80K, D168D/E; NS5A: L31V, Y93N | B (7) | 13 | 22 | 1.46 | 4,000,000 | SOF/SMV/RBV (16 weeks) | Relapse | B (7) | 12 | 370,000 | SOF/LDV (24 weeks) | Relapse | B (7) | 9 | 83,800,000 | SOF/VEL/VOX (12 weeks) | Relapse |
| 2 | 50 | Male | Refractory ascites | Yes | MPGN | 1a | None | B (7) | 9 | 20 | 1 | 100,000 | SOF/RBV/IFN (12 weeks) | Relapse | B (8) | 10 | 222,000 | SOF/LDV/RBV (24 weeks) | Relapse | |||||
| 3 | 44 | Male | Refractory ascites | No | MPGN | 3 | N/A | B (9) | 9 | 12 | 0.97 | 500,000 | SOF/DAC (24 weeks) | SVR | ||||||||||
| 4 | 61 | Female | Refractory ascites | Yes | None | 3 | N/A | B (7) | 9 | 27 | 1.06 | 500,000 | SOF/DAC (24 weeks) | Relapse | ||||||||||
| 5 | 46 | Male | Variceal bleeding | No | None | 3 | N/A | B (7) | 10 | 24 | 1.34 | 236,800 | SOF/DAC/RBV (24 weeks) | SVR | ||||||||||
| 6 | 40 | Male | Refractory ascites | No | None | 1a | N/A | A (6) | 6 | 32 | 0.99 | 190,000 | SOF/LDV (12 weeks) | SVR | ||||||||||
| 7 | 53 | Male | Variceal bleeding | Yes | HIV coinfection | 1a | N/A | B (7) | 12 | 30 | 1.23 | 367,000 | SOF/LDV (24 weeks) | SVR | ||||||||||
| 8 | 76 | Female | Variceal bleeding | No | None | 1b | N/A | A (6) | 9 | 28 | 1.1 | 320,000 | SOF/LDV/RBV (12 weeks) | SVR | ||||||||||
| 9 | 58 | Male | Refractory ascites | No | None | 1b | N/A | B (7) | 9 | 26 | 1.3 | 149,000 | SOF/VEL (12 weeks) | Relapse | ||||||||||
| 10 | 38 | Male | Variceal bleeding | No | Surgery under therapy | 1a | NS5A: Q30R, L31L/M | B (7) | 9 | 27 | 1.2 | 347,000 | SOF/VEL (12 weeks) | Relapse | ||||||||||
At the time of treatment, most patients presented with Child‐Pugh B liver cirrhosis and low MELD scores ranging from 6 to 13 points.
Abbreviations: DAC, daclatasvir; HIV, human immunodeficiency virus; IFN, interferon; LDV, ledipasvir; MPGN, membranoproliferative glomerulonephritis; N/A, not applicable; RBV, ribavirin; SOF, sofosbuvir; SMV, simeprevir; VEL, velpatasvir; VOX, voxilaprevir.
Treatment Responses in Patients with a Tips
Treatment was well tolerated and completed in all patients with a TIPS. However, 5 patients (50%) had a virological relapse after DAA therapy. In 2 patients, a virological relapse occurred after two or even three consecutive DAA treatment courses. Both virological responses or relapses were observed in patients receiving the combination sofosbuvir/ledipasvir ± ribavirin (three responses, two relapses; 60% SVR) or sofosbuvir/daclatasvir ± ribavirin (two responses, one relapse; 66% SVR), whereas all patients with a TIPS who had received sofosbuvir/velpatasvir (2; 0% SVR) or sofosbuvir/velpatasvir/voxilaprevir (1; 0% SVR) showed a relapse.
Virological Relapse After Tips Placement Compared to General SVR Rates
The high percentage of virological relapses in patients with HCV and TIPS became even more evident when compared to the “real life” SVR data of patients treated with DAA at our center. In patients with a documented 12‐week follow‐up, SVR rates were high for sofosbuvir/ledipasvir in all patients (366/390; 94%) and also in the subgroup of patients with liver cirrhosis (69/87; 79%). The same was true for sofosbuvir/daclatasvir (all patients, 24/30 [80% SVR]; patients with cirrhosis, 16/20 [80% SVR]), sofosbuvir/velpatasvir (all patients, 91/94 [97% SVR]; patients with cirrhosis, 21/24 [88% SVR]), and sofosbuvir/velpatasvir/voxilaprevir (all patients, 12/13 [92% SVR]; patients with cirrhosis, 6/7 [86% SVR]), resulting in an overall high SVR rate of 112/138 (81%) in patients with liver cirrhosis receiving one of the above‐mentioned DAA regimens. Therefore, patients with a TIPS made up a relevant proportion of patients with cirrhosis that experienced a virological relapse (6/26; 23%); this was true even for the recently approved “next‐generation” DAA regimens (sofosbuvir/ledipasvir, 2/18 [11%]; sofosbuvir/daclatasvir, 1/4 [25%]; sofosbuvir/velpatasvir, 2/3 [66%]; and sofosbuvir/velpatasvir/voxilaprevir, 1/1 [100%]). Additionally, even in the difficult to treat subgroup of patients with advanced liver cirrhosis CPS B (32) or C (2), the overall SVR rate was substantially higher (27/34; 79%) in patients without a TIPS compared to patients undergoing HCV treatment after TIPS placement, especially when comparing sofosbuvir/velpatasvir (4/4, 100% SVR in patients without a TIPS versus 0/2, 0% SVR in patients with a TIPS) and sofosbuvir/velpatasvir/voxilaprevir (3/3, 100% SVR in patients without a TIPS versus 0/1, 0% SVR in patients with a TIPS).
Flow Velocities with Doppler Ultrasound are Comparable Between Groups
Doppler ultrasound results of individual patients achieving an SVR or experiencing a relapse were similar. Accordingly, portal venous flow was antegrade toward the liver, whereas the intrahepatic portal venous flow was retrogradely directed toward the TIPS in all patients. In patients with a DAA treatment relapse, the median portal venous flow velocity was lower (22.9 versus 37.0 cm/second), albeit not statistically significant (P = 0.310). Furthermore, the intra‐TIPS flow velocity was comparable between the groups, with a median flow velocity of 105.0 cm/second in patients with relapse and 100.0 cm/second in patients achieving an SVR (Table 2).
Table 2.
Flow Velocities on Doppler Ultrasound Prior to the First DAA Therapy
| Patient | Portal Venous Flow (cm/second) | Intra‐TIPS Flow (cm/second) | Intrahepatic Portal Venous Flow (cm/second) | Hepatic Arterial Flow (cm/second) | Outcome |
|---|---|---|---|---|---|
| 1 | 48.8 | 105.0 | −21.5 | 47.1 | Relapse |
| 2 | 22.9 | 106.7 | −28.4 | 62.4 | Relapse |
| 3 | 25.0 | 100.0 | −10.5 | N/A | SVR |
| 4 | 37.0 | 98.1 | −13.2 | 44.1 | Relapse |
| 5 | 37.0 | 93.7 | −12.1 | N/A | SVR |
| 6 | 35.0 | 132.0 | −25.7 | N/A | SVR |
| 7 | 38.2 | 116.0 | −10.9 | N/A | SVR |
| 8 | 46.0 | 92.9 | −13.8 | 79.7 | SVR |
| 9 | 21.7 | 74.0 | −13.6 | N/A | Relapse |
| 10 | 22.0 | 126.7 | Retrograde | N/A | Relapse |
| Median flow velocities according to outcome (range) | |||||
| Patients with relapse | 22.9 (21.9‐42.9) | 105.0 (86.1‐116.7) | −17.6 (−26.7 to −13.3) | ||
| Patients achieving SVR | 37.0 (30.0‐42.1) | 100.0 (93.3‐124.0) | −12.1 (−19.8 to −10.7) |
Before DAA treatment, flow velocities of the hepatic vessels and intra‐TIPS flow rates were similar among individual patients. Portal venous flow was orthograde, whereas intrahepatic portal flow was retrogradely directed toward the TIPS in all patients. Median flow velocities were not significantly different between the groups (Mann‐Whitney U test; P = 0.310). The upper portion of the table shows individual patient data, whereas the lower part shows median flow velocities with the corresponding range.
Discussion
Here, we present the HCV DAA treatment results of a small cohort of patients with cirrhosis previously treated with TIPS and in whom an unusually high rate of DAA treatment failure was observed. Even though the reasons for treatment failure are manifold, we hypothesize that TIPS placement could be a possible contributing factor. From a pathophysiological point of view, a TIPS placement reduces portal pressure by shunting blood from the portal to the hepatic vein, leading to sustained changes in hepatic perfusion and biomechanical properties, such as tissue stiffness,8 including an intrahepatic retrograde flow toward the TIPS as also seen in this cohort. All our patients received a sofosbuvir‐based treatment regimen, a prodrug that is activated intrahepatically. Additionally, ledipasvir, velpatasvir, and voxilaprevir are metabolized by enzymes of the cytochrome family and subject to biliary excretion. Therefore, the changes in hepatic perfusion caused by a TIPS placement might also have an impact on the pharmacokinetic and pharmacodynamic properties of these drugs. In this context, higher rates of treatment failures after a gastric bypass and therefore possible changes in the bioavailability of the drugs have been reported.5 Another possible explanation could be that, due to the changes in hepatic hemodynamics, parts of the liver tissue are no longer adequately perfused, leading to reservoirs where the virus is safely harbored.
However, this retrospective study has several limitations, first and foremost due to the limited sample size, which made further statistical validation not feasible, including matching of patients with versus without a TIPS according to HCV genotype, Child‐Pugh and MELD score, and type and duration of treatment regimen used. Furthermore, although not documented in the files, compliance issues in patients with a TIPS because of episodes of hepatic encephalopathy cannot be completely excluded due to the retrospective study design. Additionally, other possible reasons for a relapse were present in some patients, including RAS, and 1 patient with relapse received a treatment that would not be considered optimal today (sofosbuvir/daclatasvir), even though 2 other patients cleared the virus with this regimen. Even though most patients failed to highly potent DAA regimens (sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and sofosbuvir/velpatasvir/voxilaprevir), it could be argued that the additional use of ribavirin in all cases as recommended by current guidelines in patients with Child‐Pugh B2 liver cirrhosis might have prevented some of the relapses. Furthermore, the only regimen that has been specifically approved for the treatment of patients with a virological relapse (sofosbuvir/velpatasvir/voxilaprevir)9 was used off‐label in 1 patient of this current cohort because it contains a protease inhibitor, which is not recommended to be used in patients with Child‐Pugh B liver cirrhosis.
Although larger studies will have to evaluate whether longer or more intensified treatment regimens are warranted in these patients, our preliminary data suggest that treatment should be carried out under optimized conditions (e.g., taking RAS baseline testing into account, addition of ribavirin or extension of treatment duration) or should even postponed until after liver transplantation if feasible. In this context, a detailed evaluation of DAA treatment results in patients with decompensated liver cirrhosis and large portosystemic shunts also merits further research because altered hepatic hemodynamics are also conceivable under these circumstances.
In summary, we present a case series of HCV DAA therapy in a cohort of patients with cirrhosis who have previously been treated with a TIPS. Although the DAA therapy was safely tolerated by all patients, we observed a strikingly high rate of virological relapses. Therefore, we postulate that TIPS placement may pose a possible risk factor for DAA failure due to the hemodynamic changes and alterations in liver perfusion evoked by the intervention.
Supporting information
Supported by the Dr. Liselotte and Dr. Karl‐Robert Brauns Foundation (Dr. Liselotte Brauns Research Award for Internal Medicine 2018 to F.P. and J.K.), the German Research Agency (SFB 841 to H.I., A.W.L., and J.S.z.W.), and the German Center for Infection Research (DZIF 05.809 to A.W.L. and J.S.z.W.).
Potential conflict of interest: Dr. Kluwe consults for Novartis. Dr. Pischke consults for MSD and AbbVie. Dr. Schulze sur Wiesch is on the speakers’ bureau for and received grants from Gilead and is on the speakers’ bureau for MSD and AbbVie. The other authors have nothing to report.
References
- 1. Wehmeyer MH, Eissing F, Jordan S, Roder C, Hennigs A, Degen O, et al. Safety and efficacy of protease inhibitor based combination therapy in a single‐center “real‐life” cohort of 110 patients with chronic hepatitis C genotype 1 infection. BMC Gastroenterol 2014;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018;69:461‐511. [DOI] [PubMed] [Google Scholar]
- 3. Dietz J, Susser S, Vermehren J, Peiffer KH, Grammatikos G, Berger A, et al.; European HCV Resistance Study Group . Patterns of resistance‐associated substitutions in patients with chronic HCV infection following treatment with direct‐acting antivirals. Gastroenterology 2018;154:976‐988.e974. [DOI] [PubMed] [Google Scholar]
- 4. Darwich AS, Henderson K, Burgin A, Ward N, Whittam J, Ammori BJ, et al. Trends in oral drug bioavailability following bariatric surgery: examining the variable extent of impact on exposure of different drug classes. Br J Clin Pharmacol 2012;74:774‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hupa KL, Deterding K, Port K, Kimmann M, Manns MP, Wedemeyer H, et al. Stomach reduction or gastric bypass as risk factor for treatment failure after DAA therapy for hepatitis C? J Hepatol 2018;68:851‐853. [Letter to the editor] [DOI] [PubMed] [Google Scholar]
- 6. Gerbes AL, Gulberg V, Sauerbruch T, Wiest R, Appenrodt B, Bahr MJ, et al. German S 3‐guideline “ascites, spontaneous bacterial peritonitis, hepatorenal syndrome”. [in German] Z Gastroenterol 2011;49:749‐779. [DOI] [PubMed] [Google Scholar]
- 7. Gulberg V, Haag K, Rossle M, Gerbes AL. Hepatic arterial buffer response in patients with advanced cirrhosis. Hepatology 2002;35:630‐634. [DOI] [PubMed] [Google Scholar]
- 8. Piecha F, Paech D, Sollors J, Seitz HK, Rossle M, Rausch V, et al. Rapid change of liver stiffness after variceal ligation and TIPS implantation. Am J Physiol Gastrointest Liver Physiol 2018;314:G179‐G187. [DOI] [PubMed] [Google Scholar]
- 9. Bourliere M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, et al. POLARIS‐1 and POLARIS‐4 Investigators. Sofosbuvir, velpatasvir, and voxilaprevir for previously treated HCV infection. N Engl J Med 2017;376:2134‐2146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


