Abstract
People with decompensated cirrhosis are often prescribed a complex regimen of therapeutic and prophylactic medications. In other chronic diseases, polypharmacy increases the risk of medication misadventure and medication‐related problems (MRPs), with associated increased morbidity, mortality, and health care costs. This study examined MRPs in a cohort of ambulatory patients with a history of decompensated cirrhosis who were enrolled in a randomized controlled trial of a pharmacist‐led, patient‐oriented medication education intervention and assessed the association between MRPs and patient outcomes. A total of 375 MRPs were identified among 57 intervention patients (median, 6.0; interquartile range, 3.5‐8.0 per patient; maximum 17). Nonadherence (31.5%) and indication issues (29.1%) were the most prevalent MRP types. The risk of potential harm associated with MRPs was low in 18.9% of instances, medium in 33.1%, and high in 48.0%, as categorized by a clinician panel using a risk matrix tool. Patients had a greater incidence rate of high‐risk MRPs if they had a higher Child‐Pugh score (incidence rate ratio [IRR], 1.31; 95% confidence interval [CI], 1.09‐1.56); greater comorbidity burden (IRR, 1.15; 95% CI, 1.02‐1.29); and were taking more medications (IRR, 1.12; 95% CI, 1.04‐1.22). A total of 221 MRPs (58.9%) were resolved following pharmacist intervention. A greater proportion of high‐risk MRPs were resolved compared to those of low and medium risk (68.9% versus 49.7%; P < 0.001). During the 12‐month follow‐up period, intervention patients had a lower incidence rate of unplanned admissions compared to usual care (IRR, 0.52; 95% CI, 0.30‐0.92). Conclusion: High‐risk MRPs are prevalent among adults with decompensated cirrhosis. Pharmacist intervention facilitated identification and resolution of high‐risk MRPs and was associated with reduced incidence rate of unplanned hospital admissions in this group.
Abbreviations
- ADE
adverse drug event
- aOR
adjusted odds ratio
- ARIA
Accessibility/Remoteness Index of Australia
- CI
confidence interval
- CLD
chronic liver disease
- HE
hepatic encephalopathy
- IQR
interquartile range
- IRR
incidence rate ratio
- IRSD
Index for Relative Socioeconomic Disadvantage
- MELD
Model for End‐Stage Liver Disease
- MRP
medication‐related problem
- NSAID
nonsteroidal anti‐inflammatory drug
- OR
odds ratio
Pharmacotherapy has a key role in the management of many people with chronic liver disease (CLD). In patients with decompensated cirrhosis who are ineligible for liver transplantation, optimal medication management is important to reduce and manage decompensation events and reduce or delay unplanned hospital admissions. Pharmacotherapy for specific disease etiologies, such as chronic hepatitis B or C, may also lead to improvement in liver function and survival.1, 2 However, medication‐related problems (MRPs), such as nonadherence, mismanagement related to poor patient understanding, and suboptimal monitoring, have been linked with early hospital readmission and substantial resource burden in this group.3, 4 Despite being a source of potentially preventable harm, the prevalence of MRPs and the factors that contribute to them have not been explored in ambulatory patients with decompensated cirrhosis.
Broadly defined, MRPs are any event or circumstance involving medications that can interfere with an optimum outcome of care.5 A summary of common MRP types is provided in Table 1; they include nonadherence, adverse drug reactions, drug interactions, and issues surrounding medication selection and/or dose. MRPs are diverse and multifactorial problems that may arise due to factors related to the patient, their disease and therapy, the health care system, or social and economic variables. In Australia, there is a high prevalence of MRPs in community‐based patients with chronic diseases.6, 7, 8 It was estimated that 230,000 Australians had a medication‐related hospitalization annually in 2011‐2012, at a cost of A$1.2 billion.9
Table 1.
MRP Categories and Subtypes, Adapted From Hepler and Strand5
| Category | Subclassification | Definition |
|---|---|---|
| Adverse drug reaction | Side effect of a drug, including sensitivities, intolerances, and allergies. | |
| Drug interactions |
Drug–drug Drug–disease |
Actual or potential problem associated with a combination of medications and/or a medical condition. |
| Incorrect dosage |
Subtherapeutic Supratherapeutic |
A medical condition that is being treated with drug therapy; however, the dose may be too low or too high. |
| Incorrect drug choice | A medical condition that is being treated with a suboptimal medication when an alternative is available. | |
| Nonadherence |
Intentional Unintentional Financial Sociological |
The patient is prescribed a drug for a medical condition but is not taking it due to psychological, sociological, or economic reasons. |
| Unnecessary drug use | The patient is taking a medication in the absence of a current indication. | |
| Untreated indications | A medical condition that requires drug therapy but is not being treated with medication. |
Harm from an MRP often occurs in the form of an adverse drug event (ADE), defined as “an injury resulting from the use of a drug.”10 People with decompensated cirrhosis may be at increased risk of ADEs associated with MRPs because of disease‐driven pharmacokinetic and pharmacodynamic changes.11 For example, reduced clearance of beta blockers increases the probability of dizziness and hypotension,11 and ADE‐associated morbidity has been linked with nephrotoxic medications, such as nonsteroidal anti‐inflammatory drugs (NSAIDs).12 Use of potentially inappropriate medicines, such as opioids and benzodiazepines, can precipitate hepatic encephalopathy, a substantial potentially preventable burden on patients, caregivers, and the health care system.13
In existing models of outpatient practice in Australia, ambulatory patients with decompensated cirrhosis are managed by hepatologists in dedicated hepatology clinics. In other chronic diseases, there is increasing evidence that pharmacist‐led medication review in a multidisciplinary outpatient setting effectively identifies and facilitates resolution of MRPs and improves outcomes for patients with complex chronic diseases.6, 14, 15, 16, 17, 18, 19 The aims of this study were to: (1) investigate the prevalence and types of MRPs in a cohort of Australian patients with a history of decompensated cirrhosis; (2) determine the association between MRPs and patient outcomes; and (3) measure the resolution rate of MRPs following pharmacist intervention in a multidisciplinary hepatology outpatient center.
Patients and Methods
Study Cohort
The study cohort comprised patients who were enrolled in a randomized controlled trial20 investigating the effectiveness of a pharmacist‐led patient‐orientated medication intervention. Ambulatory patients with cirrhosis who had experienced a decompensation event (ascites, hepatic encephalopathy [HE], or variceal bleed) within the preceding 2 years were recruited from general hepatology clinics at a tertiary hospital and randomly allocated to pharmacist intervention or usual care treatment arms (Fig. 1). In the standard model of care, patients received education and clinical review by a hepatologist (or gastroenterology trainee) in a dedicated hepatology clinic. In the intervention arm, patients received additional review by the pharmacist to obtain a complete reconciled list of current medications and identify MRPs. The pharmacist collaborated with the treating hepatologists and primary care clinicians to facilitate resolution of MRPs following each review. Usual care patients were not interviewed by the pharmacist and did not receive medication reconciliation. As a consequence, MRPs were not examined in the usual care group. All patients were followed to study close‐out at 12 months.
Figure 1.
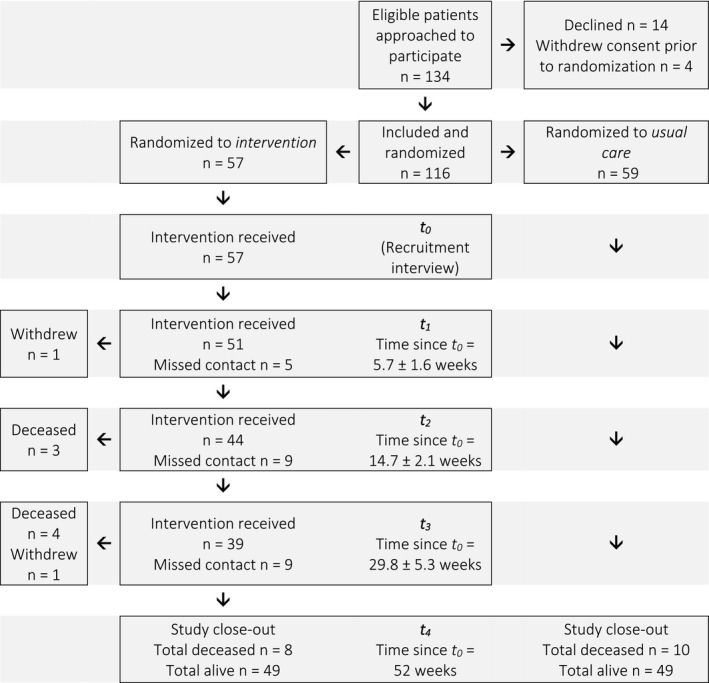
Flow diagram of patient recruitment and follow‐up timelines. Reasons for missed contact among intervention patients included overseas travel (3 patients), current inpatient (5 patients), transfer of care to another facility (1 patient), and failure to attend appointment/answer phone for other reasons (9 patients).
Data Collection and Analysis
Intervention participants were interviewed by the pharmacist on up to four occasions over a 6‐month period and followed to study close‐out at 12 months. Interviews were conducted face‐to‐face if the patient was scheduled for routine hepatologist review or by telephone if no appointment was scheduled. Patients were not excluded or removed from the study if they failed to attend a scheduled appointment or respond to telephone contact.20 At each interview, the pharmacist constructed a reconciled list of current pharmacotherapy using information from several sources (including the patient, general practitioner, pharmacy, own medications, caregiver). MRPs identified by the pharmacist were documented in patients’ medical records and brought to the attention of relevant health providers for review and appropriate action.
For both intervention and usual care groups, medical history and clinical/demographic variables, including routine pathology and medical imaging, were obtained from medical records. Liver disease severity was classified using the Child‐Pugh and Model for End‐Stage Liver Disease (MELD) scores. Comorbidity burden was measured using the Charlson Comorbidity Index.21 Sociodemographic items included patient‐reported measures of education level and employment status and area‐based measures, namely the Index for Relative Socioeconomic Disadvantage22 and the Accessibility/Remoteness Index of Australia23 for classification of geographic remoteness in terms of accessibility to services.
Patient outcome data were collected at 52 weeks from medical records and through data linkage provided by the Department of Health (in accordance with the Principles on Open Public Sector Information and the Freedom of Information Act 1982) to examine patients’ use of health care services at other hospital sites. All hospital admissions, emergency department presentations, and day procedures were independently reviewed by K.H. and E.P. Encounters were categorized as elective or unplanned, medication or nonmedication related, and preventable or nonpreventable (refer to Supporting Information File 1 for definitions and examples). Where discrepancies arose, medical records were jointly reviewed and a discussion was held to facilitate consensus. Death and hospitalization outcomes were censored at 365 days following recruitment.
Assessment of MRPs
MRPs were counted once (at the time of identification). For example, if an MRP identified at t0 was still present at t3, it was not counted again. Identified MRPs were categorized using modified Hepler and Strand classifications5, 6 into the most appropriate type (Table 1). For example, nonadherence with a diuretic was classified as nonadherence rather than an untreated indication (ascites) because nonadherence was the primary issue identified. The clinical significance of MRPs was assessed by a clinician panel using a risk matrix tool (refer to Supporting Information File 2 for definitions and examples). The matrix assigned a risk score for measures of severity, likelihood, and duration of time until potential harm may occur due to the MRP in order to assign an overall composite risk of low, medium, or high. The clinician panel consisted of a hepatologist, specialist in internal medicine, clinical pharmacist, and hepatology fellow. MRPs were de‐identified, randomized, and independently assessed by at least two members of the panel. Consensus of individual rankings was used to determine the final measure of potential harm. Where there was disagreement between individual rankings, a roundtable panel discussion was held to facilitate consensus.
MRPs were considered resolved if the action recommended by the pharmacist was taken or if another appropriate course of action resulted in resolution of the problem. MRPs that remained unresolved at follow‐up were also reviewed by the panel to determine the relevance and clinical consequences.
Statistical Analysis
Data were analyzed using IBM SPSS version 20.0. Normally distributed variables are presented as mean ± SD. Nonparametric data are presented as median (interquartile range [IQR; Tukey’s hinges]), and differences between groups were analyzed using the Mann‐Whitney U test. Categorical variables are presented as count (%) and compared using Pearson’s chi‐squared or Fisher’s exact test as appropriate.
A generalized linear model with negative binomial distribution and log link was used to examine factors associated with the incidence rate of high‐risk MRPs and unplanned hospital admissions. MRP count was offset by number of intervention encounters (minimum, 1; maximum, 4) as patients with greater exposure to the intervention had more opportunities for MRPs to be identified. Incidence rate ratios (IRRs) and 95% confidence intervals (CIs) are reported.
Binary logistic regression was used to calculate the odds of having an unplanned hospital admission and liver‐related mortality. The rate of MRP identification (total MRP count divided by number of intervention contacts) was used as a continuous variable in logistic regression models. The Child‐Pugh score was treated as a continuous variable (possible range, 5‐15). Variables with unadjusted P ≤ 0.200 and those of potential clinical significance (e.g., age, sex) were systematically assessed using stepwise conditional backward regression to determine significant factors for inclusion in the final multivariable model. Odds ratios (ORs), adjusted odds ratios (aORs), and 95% CI are reported. All tests were two‐tailed, and significance was set at α = 0.05.
Results
Fifty‐nine patients were randomized to usual care, and 57 received the intervention (Fig. 1). Baseline characteristics of participants are presented in Table 2. Usual care participants had a nonsignificantly lower Child‐Pugh score (P = 0.070) and higher level of self‐reported education (P = 0.036). Intervention patients appeared to be taking a greater number of medications at baseline (P = 0.006) as active medication reconciliation by the pharmacist identified therapies that may not have been routinely documented or disclosed by patients (non‐CLD medications, vitamins and supplements, over‐the‐counter therapies). The majority of medications taken by both groups were non‐CLD therapies.
Table 2.
Clinical and Demographic Details of Study Participants
| Intervention n = 57 | Usual care n = 59 | P | ||
|---|---|---|---|---|
| Age (mean ± SD) | 58.1 ± 10.0 | 58.9 ± 10.7 | 0.660* | |
| Male sex | 39 (68.4%) | 37 (62.7%) | 0.518 | |
| Medication management † | Self‐managed | 34 (59.6%) | 44 (77.2%) | 0.144 ‡ |
| Professional caregiver, professionally packed dosage administration aid | 9 (15.8%) | 4 (7.0%) | ||
| Partner, family, or other caregiver helps | 14 (24.6%) | 9 (15.8%) | ||
| Current alcohol consumption | 10 (17.5%) | 13 (22.0%) | 0.544 | |
| Etiology | Alcoholic liver disease | 22 (38.6%) | 34 (57.6%) | 0.165 ‡ |
| Hepatitis C | 21 (36.8%) | 17 (28.8%) | ||
| Nonalcoholic fatty liver disease | 8 (14.0%) | 6 (10.2%) | ||
| Other | 6 (10.5%) | 2 (3.4%) | ||
| MELD § (median [IQR]) | 14.5 (10.5‐18.0) | 12.5 (10.0‐16.0) | 0.157 || | |
| Child‐Pugh § | Score (median [IQR]) | 8.0 (7.0‐9.0) | 7.5 (6.0‐9.0) | 0.070 || |
| A | 8 (14.3%) | 18 (31.0%) | 0.089 | |
| B | 36 (64.3%) | 32 (55.2%) | ||
| C | 12 (21.4%) | 8 (13.8%) | ||
| Ascites at t0 (including suppressed by diuretics) | 45 (78.9%) | 46 (78.0%) | 0.898 | |
| Encephalopathy at t0 (including suppressed by medication) | 23 (40.0%) | 17 (28.8%) | 0.191 | |
| Variceal bleeding (in the preceding 2 years) | 7 (12.3%) | 11 (17.2%) | 0.449 | |
| Hepatocellular carcinoma | 4 (7.0%) | 11 (18.6%) | 0.095 ‡ | |
| Number of medications (median [IQR]) † | Total | 10.0 (6.5‐12.0) | 8.0 (6.0‐9.5) | 0.006 || |
| CLD | 3.0 (2.0‐4.0) | 2.0 (1.0‐3.0) | 0.014 || | |
| Non‐CLD | 7.0 (4.0‐9.0) | 6.0 (3.5‐7.0) | 0.061 || | |
| Charlson Comorbidity Index (median [IQR]) | 4.0 (3.0‐5.0) | 4.0 (3.0‐9.0) | 0.688 || | |
| Highest level of education ¶ | Nil, primary, middle school | 26 (53.1%) | 18 (32.7%) | 0.036 |
| Completed high school and/or additional education | 23 (46.9%) | 37 (67.3%) | ||
| Employment status # | Employed | 11 (21.6%) | 8 (14.3%) | 0.325 |
| Government welfare | 37 (72.5%) | 45 (80.4%) | 0.340 | |
| No active income | 4 (7.8%) | 4 (7.1%) | 1.000 ‡ | |
| ARIA | Living in “highly accessible” areas | 53 (93.0%) | 49 (83.1%) | 0.153 ‡ |
| Living in “accessible” to “remote” areas | 4 (7.0%) | 10 (16.9%) | ||
| IRSD | Living in “most disadvantaged” areas | 18 (31.6%) | 20 (33.9%) | 0.790 |
| Living in areas of “low” to “moderate” disadvantage | 39 (68.4%) | 39 (66.1%) |
Data presented are counts (proportions) and differences between groups as assessed using Pearson’s chi‐squared test unless otherwise denoted.
*Independent samples t test;
†excluding 2 usual care patients who did not disclose their medications at recruitment. Professionally packaged dose administration aids included Webster‐Pak and multidose medication sachet systems;
‡Fisher’s exact test;
§excluding 1 intervention patient and 1 usual care patient with no pathology for >6 months due to nonadherence;
||Mann‐Whitney U test;
¶excluding 4 usual care and 8 intervention patients who did not report this information. “Additional education” included a trade qualification, certificate, diploma, or university degree;
#excluding 3 usual care and 6 intervention patients who did not report this information. Two patients reported concurrent part‐time employment and government welfare support and are represented twice. “Employed” includes full‐time, part‐time, casual, and self‐employment. “Government welfare” includes disability support, aged pension, caregiver’s pension, total permanent disability, and Newstart allowance.
Abbreviations: ARIA, Accessibility/Remoteness Index of Australia; IRSD, Index for Relative Socioeconomic Disadvantage.
MRPs
All intervention patients received at least one interview with the clinical pharmacist; 7.0% received two, 24.6% received three, and 59.6% received four interviews. A total of 375 MRPs were documented during pharmacist interviews with intervention patients throughout the study period. All patients had two or more MRPs identified throughout the study (median, 6.0; IQR, 3.5‐8.0 per patient; maximum, 17). The risk of potential harm associated with MRPs was considered low in 18.9% of instances, medium in 33.1%, and high in 48.0%. Approximately half of the MRPs (53.6%) were identified at the baseline encounter. Fifty‐five patients (96.5%) had at least one MRP identified at baseline, including at least one high‐risk MRP in 30 patients (52.6%).
The types of MRPs identified during the study period and the proportion associated with high risk for potential harm are described in Table 3; nonadherence (31.5%) and indication issues (29.1%) were the most prevalent. Most nonadherence was intentional (65.3% of instances). Almost two thirds of intervention patients (63.6%) prescribed lactulose during the study period were nonadherent on at least one occasion, and nonadherence rates were higher than 20% for diuretics, propranolol, and spontaneous bacterial peritonitis prophylaxis (Supporting Fig. S1A). Lactulose and diuretics were associated with a substantial proportion of high‐risk nonadherence (Supporting Fig. S1B). Nonadherence occurred most commonly with prescribed vitamins/supplements, but 90.3% of instances were considered low or medium risk by the panel due to lack of current indication.
Table 3.
Prevalence and Examples of MRPs Identified During the Study Period
| n (%) Patients With ≥ 1 MRP* | n (%) Instances of MRPs | n (%) Instances of High‐Risk MRPs | Examples of High‐Risk MRPs | ||
|---|---|---|---|---|---|
| Nonadherence | 38 (66.7%) | 118 (31.5%) | 57 (48.3%) | ||
| Intentional | 28 (49.1%) | 77 (65.3%) | 39 (50.6%) | Nonadherence with diuretics in a patient with large volume ascites due to urinary urgency | |
| Unintentional | 14 (24.6%) | 22 (18.6%) | 9 (40.9%) | Nonadherence with spontaneous bacterial peritonitis prophylaxis as the patient assumed antibiotics would cease after course completed | |
| Other † | 12 (21.1%) | 19 (16.1%) | 9 (47.4%) | Financial circumstance impacting adherence with lactulose in a patient with HE | |
| Adverse drug reaction | 18 (31.6%) | 21 (5.6%) | 12 (57.1%) | Irritability and mood disturbances (on a background of depression and anxiety) while taking prednisolone prescribed for alcoholic hepatitis | |
| Drug interactions | 19 (33.3%) | 24 (6.4%) | 21 (87.5%) | ||
| Drug–drug | 5 (8.8%) | 5 (20.8%) | 3 (60.0%) | High‐dose tramadol and sertraline coadministration causing tremors, agitation, and sweating | |
| Drug–disease | 16 (28.1%) | 19 (79.2%) | 18 (94.7%) | Use of NSAIDs by a patient with a history of ascites and renal impairment | |
| Indication | 47 (82.5%) | 109 (29.1%) | 34 (31.2%) | ||
| Wrong drug | 14 (24.6%) | 16 (14.7%) | 12 (75.0%) | Opioid‐naive patient prescribed a fentanyl patch for chronic pain by general practitioner | |
| Unnecessary drug | 15 (26.3%) | 21 (19.3%) | 3 (14.3%) | Ongoing insulin use by a patient with hypoglycemia (previously started for elevated blood sugar levels while taking prednisolone) | |
| Untreated indication | 40 (70.2%) | 72 (67.9%) | 19 (26.4%) | Constipation in a patient at risk of encephalopathy not prescribed lactulose or an alternative aperient | |
| Suboptimal dose | 31 (54.4%) | 62 (16.5%) | 41 (66.1%) | ||
| Dose too high | 19 (33.3%) | 30 (48.4%) | 18 (60.0%) | Significant diarrhea associated with high lactulose dose in a patient with a history of encephalopathy | |
| Dose too low | 23 (40.4%) | 32 (51.6%) | 23 (71.9%) | Patient with moderate volume ascites intended to increase diuretics following prior review; however, dose change not made in Webster‐Pak | |
| Monitoring issues | 30 (52.6%) | 41 (10.9%) | 15 (36.6%) | Pathology not requested for a patient restarted on diuretics for ascites, following recent hyponatremia and acute kidney injury |
*Patients may have had an MRP in ≥1 subtype;
†nonadherence due to financial or social circumstance.
Factors Associated With Incidence Rate of MRPs
In general, patients had a higher incidence rate of high‐risk MRPs identified over the course of the study if they were younger (P = 0.042); had a higher Child‐Pugh score (P = 0.004), MELD score (P = 0.050), or Charlson Comorbidity Index (P = 0.024); or were taking more medications (P = 0.005) (Supporting Table S1). There was no effect of marital status, sociodemographic status, level of self‐reported education, receiving professional or nonprofessional support to manage medications, or current alcohol consumption on the incidence rate of high‐risk MRPs.
MRP Resolution
A total of 221 MRPs (58.9%) were resolved during the study period following pharmacist intervention. Time point of identification did not affect probability of resolution before study close‐out (P > 0.050). A greater proportion of high‐risk MRPs were resolved compared to those of low and medium risk (68.9% versus 49.7%; P < 0.001). The panel reviewed the 154 unresolved MRPs (median, 1.0; IQR, 0.0‐2.0 per patient) to determine their clinical significance. Failure to resolve 32 high‐risk MRPs was considered clinically significant by the panel. These MRPs were predominantly issues related to persisting nonadherence (40.6%) and indication (21.3%). Twelve unresolved MRPs (including n = 3 high risk) were in patients who died prior to resolution; none were associated with the cause of death.
Patient Outcomes
Hospitalization
There were 74 unplanned admissions among intervention patients and 93 among usual care participants during the 12‐month follow‐up period (annual unplanned admission rate, 1.3 versus 1.6; P = 0.477; Fig. 2). Among the 51 medication‐related admissions, 64.7% were considered preventable, including n = 9 admissions for untreated/undertreated ascites (n = 2 related to diuretic nonadherence); n = 10 related to suboptimal patient use of or adherence with lactulose; n = 8 admissions were considered preventable with improved monitoring of electrolytes (including n = 7 diuretic‐related admissions); n = 2 were associated with cardiology medicines (apixaban and digoxin); n = 1 due to nonadherence with respiratory inhalers; and n = 3 related to use of other potentially inappropriate medicines (opioids, benzodiazepines, NSAIDs, high‐dose pregabalin).
Figure 2.
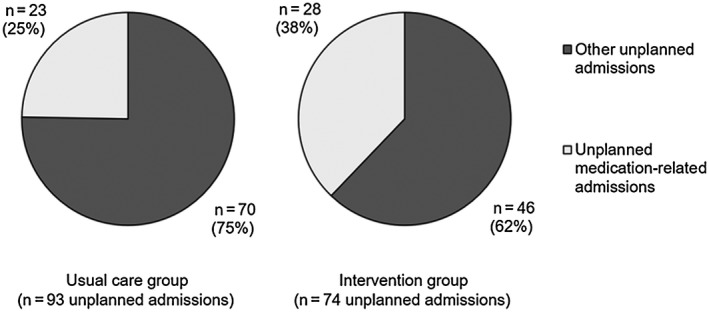
Proportion of unplanned admissions among intervention and usual care patients during the follow‐up period.
Factors associated with the incidence rate of unplanned admissions are summarized in Table 4. Following adjustment for Child‐Pugh score, number of medications, history of variceal bleeding, and alcoholic liver disease, intervention patients had a significantly lower incidence rate of unplanned admissions compared to usual care patients (IRR, 0.52; 95% CI, 0.30‐0.92; P = 0.025). Among the intervention group, patients who had one or more unplanned admissions had a higher incidence rate of high‐risk MRPs compared to those who did not have an admission (IRR, 2.48; 95% CI, 1.29‐4.77; P = 0.006). However, following adjustment for Child‐Pugh score in a logistic regression model, the incidence rate of high‐risk MRPs was not independently associated with hospital admissions (P = 0.158).
Table 4.
Factors Associated With the Incidence Rate of Unplanned Admissions
| Unadjusted IRR (95% CI) | Adjusted* IRR (95% CI) | P | ||
|---|---|---|---|---|
| Randomization | Intervention | 0.82 (0.51‐1.33) | 0.52 (0.30‐0.92) | 0.025 |
| Age | 0.98 (0.96‐1.01) | 1.00 (0.98‐1.03) | 0.907 | |
| Sex | Male | 0.75 (0.46‐1.22) | 1.08 (0.60‐1.95) | 0.805 |
| Alcoholic liver disease | 0.68 (0.42‐1.10) | 0.53 (0.30‐0.91) | 0.023 | |
| MELD score † | 1.07 (1.02‐1.12) | – | – | |
| Child‐Pugh score † | 1.44 (1.24‐1.67) | 1.57 (1.32‐1.86) | <0.001 | |
| Variceal bleeding (in the preceding 2 years) | 2.01 (1.09‐3.69) | 3.02 (1.52‐5.99) | 0.002 | |
| Hepatocellular carcinoma | 1.98 (1.03‐3.81) | 1.85 (0.87‐3.91) | 0.109 | |
| Charlson Comorbidity Index | 1.04 (0.97‐1.12) | 1.03 (94‐1.12) | 0.551 | |
| Number of medicines at t0 | Total | 1.08 (1.02‐1.14) | 1.08 (1.01‐1.16) | 0.028 |
| CLD | 1.25 (1.07‐1.47) | – ‡ | – | |
| Non‐CLD | 1.06 (0.99‐1.13) | – | – |
*The final model included randomization, Child‐Pugh score, number of medications, history of variceal bleeding, and alcoholic liver disease;
†Child‐Pugh score was entered as a continuous variable (possible range, 5‐15) in the model;
‡– indicates factor not included in the model.
Mortality
Eight intervention patients (14.0%) and 10 usual care patients (16.9%) died during the study follow‐up period, including one non‐liver‐related death in the usual care group (P = 0.665). Among the intervention group, patients who died had a significantly greater incidence rate of high‐risk MRPs than those who did not die (IRR, 5.04; 95% CI, 2.04‐12.46; P < 0.001). Of the 26 high‐risk MRPs identified in these patients, 34.6% were indication issues, 38.5% were dose related, 11.5% were drug interactions, 7.7% were related to nonadherence, 3.8% were monitoring issues, and 3.8% were adverse drug reactions. These included n = 4 instances of untreated/undertreated HE; n = 7 untreated/undertreated ascites; n = 4 inappropriate benzodiazepine/opioid/anticholinergic use; n = 3 instances of renal impairment requiring change to therapy; n = 1 nonadherence with spontaneous bacterial peritonitis prophylaxis; and n = 7 miscellaneous MRPs. Most (88.5%) were resolved prior to death. In a logistic regression model, every 1‐unit increase in the rate of high‐risk MRPs identified was associated with more than 3‐fold higher odds of mortality (aOR, 3.84; 95% CI, 1.41‐10.50; P = 0.009) following adjustment for the presence of hepatocellular carcinoma (aOR, 86.30; 95% CI, 4.79‐1.55 × 103; P = 0.003) (Table 5). This effect was independent of the Child‐Pugh score (or MELD score) and number of medications on multivariable analysis.
Table 5.
Unadjusted and Adjusted Odds of Liver‐Related Mortality Among Intervention Patients Within 12 Months of Recruitment
| Clinical and Demographic Variables | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | aOR* (95% CI) | P | ||
| Age | 1.01 (0.94‐1.09) | 0.741 | 1.06 (0.96‐1.17) | 0.215 | |
| Sex | Male | 0.74 (0.16‐3.48) | 0.698 | 1.00 (0.11‐8.83) | 1.000 |
| Current alcohol consumption | 1.71 (0.29‐10.04) | 0.553 | 0.61 (0.04‐10.76) | 0.738 | |
| MELD score | 1.11 (0.97‐1.27) | 0.147 | 1.08 (0.91‐1.28) | 0.372 | |
| Child‐Pugh score | 1.52 (0.95‐2.41) | 0.079 | 1.36 (0.72‐2.58) | 0.350 | |
| Variceal bleeding (in the preceding 2 years) | 1.02 (0.11‐9.84) | 0.984 | 1.03 (0.04‐26.48) | 0.986 | |
| Hepatocellular carcinoma | 28.80 (2.50‐331.55) | 0.007 | 86.30 (4.79‐1.56 × 103) | 0.003 | |
| Number of medicines at baseline | Total | 1.26 (1.03‐1.53) | 0.026 | 1.27 (0.94‐1.70) | 0.116 |
| CLD | 2.06 (1.14‐3.71) | 0.016 | 2.01 (0.92‐4.38) | 0.081 | |
| Non‐CLD | 1.17 (0.96‐1.42) | 0.127 | 1.15 (0.87‐1.51) | 0.341 | |
| Number of high‐risk MRPs per contact | 2.46 (1.12‐5.38) | 0.025 | 3.84 (1.41‐10.50) | 0.009 | |
| Charlson Comorbidity Index | 1.47 (1.12‐1.94) | 0.006 | 1.25 (0.81‐1.93) | 0.309 | |
| Education | Nil to middle school | 6.60 (0.73‐59.68) | 0.093 | 3.56 (0.31‐41.03) | 0.309 |
| Living in “most disadvantaged” areas | 4.62 (0.96‐22.09) | 0.056 | 3.71 (0.51‐27.07) | 0.197 | |
| Living in “accessible” to “remote” areas | 7.83 (0.93‐66.33) | 0.059 | 10.45 (0.68‐161.51) | 0.093 | |
All patients who died were unemployed and on government welfare.
*Adjusted for number of high‐risk MRPs per contact and presence of hepatocellular carcinoma.
Discussion
To our knowledge, this is the first study to explore the prevalence and types of MRPs in people with a history of decompensated cirrhosis. In this cohort of Australian outpatients, we found a high prevalence of polypharmacy and MRPs, with more than 95% of patients having at least one MRP and 50% having high‐risk MRPs at recruitment. Patients who had more contacts with the pharmacist over the study period had more opportunity for MRPs to be identified.
The MRPs identified in this study were heterogeneous in type and severity. The most prevalent MRP types were nonadherence and indication issues, which is similar to findings in other studies of community‐based Australians with chronic diseases.6, 7, 24 Medication nonadherence in people with cirrhosis may be influenced by patients’ perceptions surrounding the severity of their liver disease (symptoms, timeline of progression, development of complications) and the perceived helpfulness and harms of treatment (previous therapy failure, side effects, complexity of therapy, long‐term benefits of treatment).25 For example, nonadherence with lactulose and diuretics is often attributed to the prohibitive medication side‐effect profile that can affect patients’ quality of life and freedom to participate in work and leisure activities. Indeed, lactulose and diuretics were associated with more than one third of all instances of high‐risk nonadherence in the present study. Agrawal and colleagues4 reported nonadherence with lactulose and diuretics to be associated with 36% and 55% of potentially preventable 30‐day readmissions, respectively. We similarly found that nonadherence and monitoring issues with these medicines contributed to more than half of potentially preventable medication‐related admissions in our group. However, approximately one third of nonadherence in this study was not “intentional.” When discussing nonadherence with patients, it is important that clinicians are aware of unintentional, financial, and social barriers that may impair adherence and offer patient‐oriented solutions.
There were several medication‐related admissions in the intervention group despite pharmacist intervention. This is likely reflective of the complex and frequently changing regimen of medications consumed by patients with decompensated cirrhosis. We found that intervention patients who had unplanned hospital admissions and those who died had a higher incidence rate of high‐risk MRPs. This is important because people with more severe liver disease are often prescribed more medications, and thus sicker patients have more opportunities to experience MRPs and ADEs. Pharmacist intervention, which proactively sought to identify and resolve MRPs, was associated with a significant reduction in the incidence rate of unplanned admissions compared to usual care, but not reduced mortality.
Management of patients with decompensated cirrhosis may be complex due to the systemic nature of the disease with multiorgan impairment and cirrhosis‐associated immune dysfunction. Medicines that are indicated for comorbidities (e.g., cardiovascular disease) may have relative contraindications in people with cirrhosis due to the risk of exacerbating hemodynamic disturbances and renal impairment or precipitating HE. A large proportion of medications consumed by patients in the current study were non‐CLD therapies prescribed by a general practitioner or other specialist. Unlike other patient groups,26, 27 a comprehensive list of potentially inappropriate medicines is not readily available to guide prescribing in decompensated cirrhosis. Development of this list could be of benefit to assist non‐hepatology health care providers in the management of comorbidities. Ambulatory care multidisciplinary case management, such as in a chronic disease model of care, has been proposed to improve patient outcomes.28, 29, 30 However, outside of the post‐liver transplant setting,31 there is a paucity of appropriately powered clinical trials to inform development and implementation in CLD. Our findings supporting pharmacist‐led medication review to identify and aid resolution of MRPs in the ambulatory setting will be useful for future development and translation of chronic disease management models for people with decompensated cirrhosis.
In other multidisciplinary models of outpatient care, pharmacist suggestions are generally well received by prescribing clinicians. Between 60% and 70% of MRPs were reported to be resolved in other studies of pharmacist‐led interventions.6, 14, 15 In the present study, more than two thirds of high‐risk MRPs were resolved within 12 months of recruitment. Variability in the resolution of MRPs may have occurred for several reasons. Patients were recruited from seven concurrent clinics led by different hepatologists and may have been reviewed by medical staff with different levels of experience (consultant physician, basic physician trainee, or advanced gastroenterology trainee). Similarly, some patients engaged more readily with the education and medication management intervention and therefore may have been more likely to act upon pharmacist recommendations, particularly with respect to medication adherence. Furthermore, the heterogeneity of the recruited population meant some patients may have deteriorated rapidly and died prior to MRP resolution or treatment priorities changed in relation to their disease management. This reflects real‐world patient management.
This study had several strengths and limitations. This was a small prospective study in which MRPs were explored in a real‐world clinic environment. The single clinical pharmacist was experienced in the management of patients with cirrhosis, and a comprehensive protocol was used to facilitate patient interviews. Although some studies screen for outcome variables and target recruitment of patients at highest likelihood of improvement (e.g., adherence studies), we recruited all patients with a history of decompensated cirrhosis who were interested in participating, irrespective of whether they had MRPs at baseline.
MRPs were classified using well‐documented categories within the literature. It has been suggested that prospective studies may identify more MRPs than other methods of detection (with a focus on active issues) compared to retrospective studies or chart reviews, which can have variable accuracy.32 Furthermore, our study methodology restricted classification of MRPs into only one category. For example, a patient with constipation may have been categorized as having an untreated indication (constipation), adverse drug reaction (precipitated by amitriptyline), or nonadherence (not taking lactulose as directed). When this occurred, clinician panel consensus was used to determine the final classification. Clinician panel consensus was also used to categorize potential harm associated with MRPs using a risk matrix tool. It is possible that this tool may have overcategorized or undercategorized harm in some instances. However, similar risk classification tools have been used in other studies,33, 34 and the clinician panel considered final categorization appropriate in all instances.
Systematic assessment of variables was conducted to determine factors associated with patient outcomes; however, findings must be interpreted within the context of the small number of patients. There was significant heterogeneity among the recruited patients, as was expected considering the group of interest. Usual care patients had a higher level of self‐reported education; however, education level was not associated with the incidence rate of high‐risk MRPs among intervention patients or unplanned hospitalization or mortality. While the small study size and potential sample bias need to be considered, it is unlikely that this difference between the study groups is of consequence to our findings. Although all patients had experienced a decompensating event in the 2 years preceding recruitment, disease severity within the study cohort was variable and several patients had Child‐Pugh A cirrhosis at recruitment. Similarly, medication and comorbidity burden varied greatly as did patient/caregiver engagement with the study intervention. Therefore, applicability of our findings to patients with cirrhosis at other centers will be dependent on the patient demographic.
MRPs are prevalent in ambulatory patients with decompensated cirrhosis, and a subset of high‐risk problems is associated with patient harm. Pharmacist intervention identified and facilitated resolution of many high‐risk MRPs and was associated with a reduced incidence rate of unplanned admissions. These findings have implications for evolving outpatient management of people with end‐stage liver disease.
Supporting information
Acknowledgment
We thank Mrs. Valery Logan and Dr. Antara Karmakar for their technical assistance during the study and biostatistician Dr. Justin Scott for reviewing the data and analyses presented in this manuscript.
Supported by a University of Queensland Research Scholarship (to K.H.) and an Australian National Health and Medical Research Council Career Development Fellowship (no. 1083090 to P.V.).
Potential conflict of interest: Nothing to report.
See Article on Page 603.
References
Author names in bold designate shared co‐first authorship.
- 1. Guan R, Lui HF. Treatment of hepatitis B in decompensated liver cirrhosis. Int J Hepatol 2011;2011:918017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. El‐Sherif O, Jiang ZG, Tapper EB, Huang KC, Zhong A, Osinusi A, et al. Basline factors associated with improvements in decompensated cirrhosis after direct‐acting antiviral therapy for hepatitis C virus infection. Gastroenterology 2018;154:2111‐2121. [DOI] [PubMed] [Google Scholar]
- 3. Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol 2012;107:247‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agrawal K, Kumar P, Markert R, Agrawal S. Risk factors for 30‐day readmissions of individuals with decompensated cirrhosis. South Med J 2015;108:682‐687. [DOI] [PubMed] [Google Scholar]
- 5. Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm 1990;47:533‐543. [PubMed] [Google Scholar]
- 6. Benson H, Lucas C, Kmet W, Benrimoj SI, Williams K. Pharmacists in general practice: a focus on drug‐related problems. Int J Clin Pharm 2018;40:566‐572. [DOI] [PubMed] [Google Scholar]
- 7. Rao D, Gilbert A, Strand LM, Cipolle RJ. Drug therapy problems found in ambulatory patient populations in Minnesota and South Australia. Pharm World Sci 2007;29:647‐654. [DOI] [PubMed] [Google Scholar]
- 8. Roughead EE, Barratt JD, Gilbert AL. Medication‐related problems commonly occurring in an Australian community setting. Pharmacoepidemiol Drug Saf 2004;13:83‐87. [DOI] [PubMed] [Google Scholar]
- 9. Roughead L, Semple S, Rosenfeld E. Literature Review: Medication Safety in Australia. Sydney, NSW: Australian Commission on Safety and Quality in Health Care; 2013. [Google Scholar]
- 10. Gurwitz JH, Field TS, Avorn J, McCormick D, Jain S, Eckler M, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med 2000;109:87‐94. [DOI] [PubMed] [Google Scholar]
- 11. Lewis JH, Stine JG. Review article: Prescribing medications in patients with cirrhosis—a practical guide. Aliment Pharmacol Ther 2013;37:1132‐1156. [DOI] [PubMed] [Google Scholar]
- 12. Elia C, Graupera I, Barreto R, Sola E, Moreira R, Huelin P, et al. Severe acute kidney injury associated with non‐steroidal anti‐inflammatory drugs in cirrhosis: a case‐control study. J Hepatol 2015;63:593‐600. [DOI] [PubMed] [Google Scholar]
- 13. Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In‐hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol 2012;10(1034–1041):e1031. [DOI] [PubMed] [Google Scholar]
- 14. Cheen MHH, Goon CP, Ong WC, Lim PS, Wan CN, Leong MY, et al. Evaluation of a care transition program with pharmacist‐provided home‐based medication review for elderly Singaporeans at high risk of readmissions. Int J Qual Health Care 2017;29:200‐205. [DOI] [PubMed] [Google Scholar]
- 15. Basheti IA, Tadros OK, Aburuz S. Value of a community‐based medication management review service in Jordan: a prospective randomized controlled study. Pharmacotherapy 2016;36:1075‐1086. [DOI] [PubMed] [Google Scholar]
- 16. Basaraba JE, Picard M, George‐Phillips K, Mysak T. Pharmacists as care providers for stroke patients: a systematic review. Can J Neurol Sci 2018;45:49‐55. [DOI] [PubMed] [Google Scholar]
- 17. Gastelurrutia P, Benrimoj SI, Espejo J, Tuneu L, Mangues MA, Bayes‐Genis A. Negative clinical outcomes associated with drug‐related problems in heart failure (HF) outpatients: impact of a pharmacist in a multidisciplinary HF clinic. J Card Fail 2011;17:217‐223. [DOI] [PubMed] [Google Scholar]
- 18. Walter C, Mellor JD, Rice C, Kirsa S, Ball D, Duffy M, et al. Impact of a specialist clinical cancer pharmacist at a multidisciplinary lung cancer clinic. Asia Pac J Clin Oncol 2016;12:e367‐e374. [DOI] [PubMed] [Google Scholar]
- 19. Nkansah N, Mostovetsky O, Yu C, Chheng T, Beney J, Bond CM, et al. Effect of outpatient pharmacists’ non‐dispensing roles on patient outcomes and prescribing patterns. Cochrane Database Syst Rev 2010;CD000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayward KL, Martin JH, Cottrell WN, Karmakar A, Horsfall LU, Patel PJ, et al. Patient‐oriented education and medication management intervention for people with decompensated cirrhosis: study protocol for a randomized controlled trial. Trials 2017;18:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 22. Australian Bureau of Statistics . Census of population and housing: socio‐economic indexes for areas (SEIFA), Australia, 2011. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/2033.0.55.0012011?OpenDocument. Published March 28, 2013. Accessed February 2015.
- 23. Australian Institute of Health and Welfare . Rural, regional and remote health: a guide to remoteness classifications. http://www.aihw.gov.au/publication-detail/?id=6442467589. Published March 19, 2004. Accessed February 2015.
- 24. Gilbert AL, Roughead EE, Beilby J, Mott K, Barratt JD. Collaborative medication management services: improving patient care. Med J Aust 2002;177:189‐192. [DOI] [PubMed] [Google Scholar]
- 25. Hayward KL, Valery PC, Martin JH, Karmakar A, Patel PJ, Horsfall LU, et al. Medication beliefs predict medication adherence in ambulatory patients with decompensated cirrhosis. World J Gastroenterol 2017;23:7321‐7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society . updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;2015(63):2227‐2246. [DOI] [PubMed] [Google Scholar]
- 27. McLeod PJ, Huang AR, Tamblyn RM, Gayton DC. Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ 1997;156:385‐391. [PMC free article] [PubMed] [Google Scholar]
- 28. Thomson MJ, Lok AS, Tapper EB. Optimizing medication management for patients with cirrhosis: evidence‐based strategies and their outcomes. Liver Int 2018;38:1882‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clin Gastroenterol Hepatol 2013;11:217‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wigg AJ, Chinnaratha MA, Wundke R, Volk ML. A chronic disease management model for chronic liver failure. Hepatology 2015;61:725‐728. [DOI] [PubMed] [Google Scholar]
- 31. Russo MW, Levi DM, Pierce R, Casingal V, Eskind L, deLemos A, et al. A prospective study of a protocol that reduces readmission after liver transplantation. Liver Transpl 2016;22:765‐772. [DOI] [PubMed] [Google Scholar]
- 32. Manias E. Detection of medication‐related problems in hospital practice: a review. Br J Clin Pharmacol 2013;76:7‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stevenson J, Parekh N, Ali K, Timeyin J, Bremner S, Van Der Cammen T, et al. Protocol for a prospective (P) study to develop a model to stratify the risk (RI) of medication (M) related harm in hospitalized elderly (E) patients in the UK (the PRIME study). BMC Geriatr 2016;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fertleman M, Barnett N, Patel T. Improving medication management for patients: the effect of a pharmacist on post‐admission ward rounds. Qual Saf Health Care 2005;14:207‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


