Abstract
Alcohol use disorder, substance abuse, and depression are illnesses that deteriorate the quality of life (QOL) of patients with chronic liver disease (CLD). Screening and behavioral health programs integrated into routine practice can mitigate the deleterious effects of such illnesses but have not been adopted in hepatology practices. We implemented a behavioral health program based on the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model and assessed its acceptability and effectiveness in improving QOL. This was a quality improvement study. Patients with CLD and a scheduled outpatient visit in the hepatology clinic were screened while waiting for their appointment. All patients who screened positive for any of the three illnesses were offered a brief intervention (BI) at the point of care and at 3 months by a trained social worker. The BI used the principles of motivational interviewing and cognitive behavioral therapy. Severity of illness was assessed at baseline, 3 months, and 6 months. Participants completed an acceptability survey at 6 months. We screened 303 patients; 61.7% were positive for any of the three illnesses assessed. Among the positive patients, depression was most common (48.4%), alcohol and substance abuse were each 26%. For the 95 patients who underwent BI, QOL improved from baseline to 3 and 6 months (P < 0.001) and patients with depression improved the most. Depression was the only independent predictor of change in QOL over time. Of the enrolled patients, 82% agreed BIs improved their overall care and 87% indicated a desire to continue with the behavioral program. Conclusion: An outpatient behavioral health program based on the SBIRT model is acceptable to patients with CLD and may help improve QOL over time.
Abbreviations
- ANOVA
analysis of variance
- AUDIT
Alcohol Use Disorders Identification Test
- BI
Brief Intervention
- CLD
chronic liver disease
- CLDQ
Chronic Liver Disease Questionnaire
- DAST‐10
Drug Abuse Screen Test
- PHQ
Patient Health Questionnaire
- QOL
quality of life
- SBIRT
Screening, Brief Intervention, and Referral to Treatment
Behavioral health interventions integrated into routine clinical practice for chronic diseases have been shown to achieve the triple aim of improved health outcome, better quality of care, and reduced health care costs.1, 2 The American Hospital Association recommends that behavioral health services be integrated throughout the health care delivery system.3 However, evidence to support integrated behavioral health for patients with chronic liver disease (CLD) is limited.4
CLD is the fourth and seventh leading cause of death among adults ages 45‐54 years and 55‐64 years, respectively, and is projected to peak in 2021.5 Compared to other chronic diseases, CLD is associated with higher health care use and worsening mortality over time.6 Furthermore, CLD is uniquely associated with complex psychosocial comorbidities, including alcohol use disorder (increasingly among the young population)7, 8 and substance abuse, both of which occur more frequently and at a greater severity among patients with CLD than the general population.9 Depression remains under diagnosed and untreated in CLD10 and is associated with worse clinical outcomes and higher mortality.11 Therefore, there is a critical need for a behavioral health program targeting these three illnesses together, using screening tools and tailored brief interventions.
The objective of our project was to implement the Screening, Brief Intervention, and Referral to Treatment12 (SBIRT)‐based behavioral health program in an ambulatory hepatology setting, targeting alcohol use disorder, substance abuse, and depression. SBIRT is a proactive comprehensive public health model designed to offer universal screening, brief intervention, and referral to treatment for individuals with alcohol or substance abuse disorders. The primary aim was to assess its acceptability to patients and explore the effectiveness of this program in improving quality of Life (QOL) and reducing the targeted illnesses over time. We also evaluated if patients with or without depression responded differently.
Participants and Methods
This was designed as a pragmatic quality improvement study to integrate a behavioral health program within a routine ambulatory hepatology practice at an academic medical center. Figure 1 shows the behavioral health approach we utilized, based on the three key elements of SBIRT Model‐universal screening, brief intervention and referrals. All patients with diagnosed CLD and a scheduled appointment in the outpatient hepatology office were eligible. All eligible patients underwent screening for alcohol use disorder, substance abuse, and depression at the time of check‐in for their clinical visit, using validated screening questions (Supporting Material Appendix 1). Patients were asked to complete a screening form given to them by the front desk staff and were informed that this was part of a quality improvement study. A single question for alcohol use disorder was adapted from the National Institute of Alcohol Abuse and Alcoholism asking about their use of alcohol in the past 30 days. Any man drinking more than five drinks in a day or woman taking more than four drinks in a day on two to three occasions was considered positive. A single question assessed substance abuse in the past year with responses of never or ≥1 times; any use of ≥1 times was considered positive. Patient Health Questionnaire 9 (PHQ‐9) was used to screen for depression; a score >3 was considered positive. Patients who screened positive for one or more of the three assessments were offered a brief intervention (BI).
Figure 1.
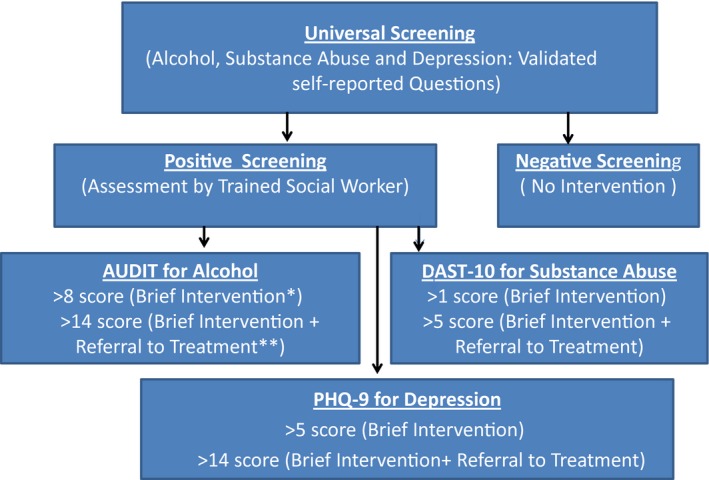
Standard Clinical/Behavioral Health Integration Protocol (based on the SBIRT model). *Brief intervention: offered by the social worker at baseline and 3 months. **Referral to treatment: 1) to behavioral health specialist (for alcohol and substance abuse); 2) to psychiatry (for depression).
The BI used the principles of motivational interviewing and cognitive behavioral therapy and targeted six elements: feedback on behavior and consequences, responsibility to change, advice, menu of options to bring about change, empathy, and self‐efficacy for change (the FRAMES elements).13 The BI was delivered by a social worker who was trained in behavioral therapies. The social worker identified ambivalence, taught motivation and self‐efficacy techniques, and coached the patient to build a commitment to change. For depression, the social worker offered cognitive behavioral therapy and facilitated conversations from negative to positive thoughts leading to changes in attitudes and behaviors.14, 15 BI lasted about 15‐20 minutes, and a repeat BI occurred at 3 months. Based on severity of illness and patient needs, some patients received referrals for counseling or psychiatric evaluation. Intervention fidelity was maintained by keeping an algorithm of BIs to be followed for each patient interaction.
The SBIRT Model in Hepatology
During the development of the program, we considered known barriers to behavioral health integration, such as insurance coverage for behavioral health services, access, limited number of behavioral health providers for liver disease, patients navigating care from multiple clinicians, mental health services, substance abuse counselors, and above all the stigma associated with seeking additional behavioral health services. We developed an integrated program within the hepatology clinic based on an established SBIRT model. The SBIRT approach has been shown to be effective in a variety of clinical settings, such as primary care offices and emergency rooms.16 It allows identifying individual patient needs through a universal screening approach, followed by tailored brief interventions and referrals for complex cases. It has been shown to reduce health care costs by $366 per month per member,17 reduce emergency department use,18 and reduce rates of substance use by 67.7% and heavy alcohol use by 38.6% from baseline to 6 months, with significant improvements in general health and well‐being.19
Outcome Measures
The primary outcomes of the study were patient acceptability and change in QOL from baseline to 3 months. Secondary outcomes included change in illness severity scores over time and sustainability of change in QOL at 6 months.
QOL was assessed using the Chronic Liver Disease Questionnaire (CLDQ). The Alcohol Use Disorders Identification Test (AUDIT), Drug Abuse Screen Test (DAST‐10), and PHQ‐9 were used to evaluate illness severity (alcohol use disorder, substance abuse, and depression, respectively). These were assessed only if the patient was positive for that specific illness.
All study instruments were completed at baseline, 3 months, and 6 months. At 6 months, participants also completed an end of study acceptability survey.
Study Instruments
CLDQ
This is a validated tool to measure liver disease‐specific QOL.20 It assesses six subdomains: abdominal symptoms, systemic symptoms (e.g., pain, muscle cramps, itching), worry, fatigue, physical function, and emotional function. It is a self‐administered tool with 29 items. The range of options is rated on a 7‐point Likert scale. Subdomain scores and an overall score can be calculated using the mean of responses to questions for each domain and a mean for all responses, respectively. Higher scores imply a better QOL.
AUDIT
This is a 10‐item questionnaire developed by the World Health Organization to screen for harmful alcohol use.21 The Likert scale options are added together to obtain a total score. A total score of 0‐7 means low risk, 8‐15 medium risk, 16‐19 high risk, and 20‐40 addiction likely.22 It has been used in a variety of settings, such as primary care and mental health facilities where patients do not present for alcohol problems directly. It has high internal consistency and validity in a variety of populations.17
DAST‐10
This is a 10‐item self‐reported instrument used for evaluation of substance use in the past year.23 Any score ≥3 is considered positive for drug and substance use. It has been shown to be reliable and valid in independent samples of psychiatric patients.24
PHQ‐9
This is a commonly used tool to assess severity of depression in clinical settings and has nine questions.25 Each question is rated on a 4‐point scale, with total score ranging from 0 to 27. Higher scores reflect greater severity of depression. Scores from 0‐4 equate to no depression, 5‐9 mild, 10‐14 moderate, 15‐19 moderate–severe, and >20 reflects severe depression.26
Average time to respond to screening questions was 2‐3 minutes, whereas average time for all the other instruments was 5‐6 minutes each. All questionnaires were collected using paper form.
End of Study Acceptability Survey
This survey comprised four questions with 5‐point Likert scale response options (definitely yes to definitely no) assessing 1) if the BIs improved their overall care, 2) desire to continue with BIs within the hepatology practice, 3) if the BI at the point of care overcame the barrier of making appointments for behavioral health services separately, and 4) if screening for these illnesses at the time of check‐in was useful to identify individual patient needs. Agreement >60% to these questions was considered the benchmark for acceptability. Definitely yes and probably yes were grouped as “agree,” while definitely no and probably no were grouped as “disagree” for analyses.
Statistical Methods
Descriptive statistics were used for summarizing continuous variables (mean [SD, range]) using SAS version 9.0 (SAS Institute, Cary, NC). A univariate and multivariate repeated measures analysis of variance (ANOVA) determined the independent effects of risk factors (alcohol, substance use, or either of the two with depression; depression alone) on the overall CLDQ scores.
The AUDIT, DAST‐10, and PHQ‐9 scores underwent nonparametric analyses based on failures to meet goodness‐of‐fit tests for normal distributions by the Kologorov‐Smirnov test. When paired differences (3 months to baseline; 6 months to baseline) distributed symmetrically, the Wilcoxon test was applied; when paired differences were asymmetric, the sign test was applied. All statistical tests were considered significant at a two‐sided 5% level. Pairwise comparisons were done only with significance of the omnibus ANOVA test. End of study acceptability results are summarized.
Results
During 6 months of recruitment (from September 2015 to February 2016), 303 patients were approached at the time of routine check‐in at a single outpatient hepatology clinic, 3 days per week (Fig. 2). Ten patients declined to complete the screening questionnaire, 11 had incomplete responses, and 95 (31.3%) screened negative. Among the positive patients (n = 187), 49 (26%) screened positive for alcohol, 48 (25.6%) for substance abuse, and 90 (48.4%) for depression. Of all the positives, 18 declined participation and 169 (90%) were willing to participate.
Figure 2.
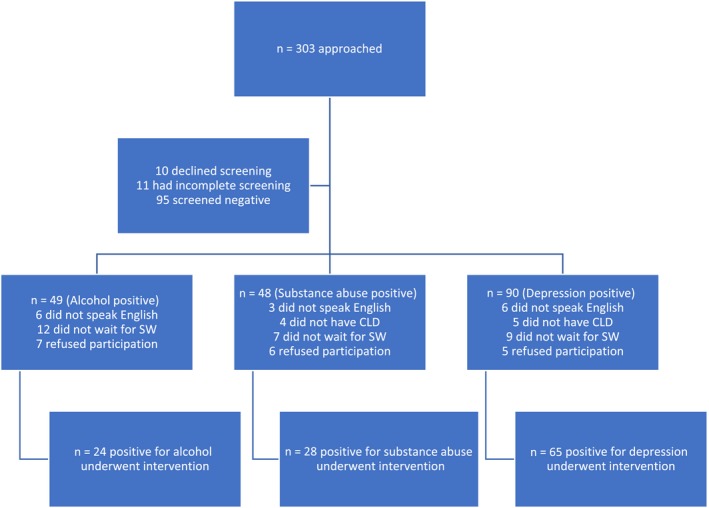
Diagram showing disposition of screened patients. Abbreviations: CLD, Chronic Liver Disease; SW, social worker.
Of those willing to participate (n = 169), we could not enroll 23 (13.6%) patients due to a language barrier (interpreters were not available at the time of enrollment), 38 (22.5%) had to leave early for work and said they would have participated if given earlier notice, and 13 (7.6%) did not have confirmed CLD. A total of 95 patients (56.3%) were enrolled in the study.
The 95 patients who screened positive and underwent the BI (Fig. 3) had an average age of 53 years (SD, 10.4 years; range, 24 to 84 years), 56% were men, 58% were educated to high school or less, 64% had a primary diagnosis of hepatitis C virus and 68.4% were positive for alcohol. Additional demographic data are displayed in Table 1. A total of 82 (86.3%) patients completed the 3‐month assessments, and 84 (88.4%) completed the 6‐month assessments.
Figure 3.
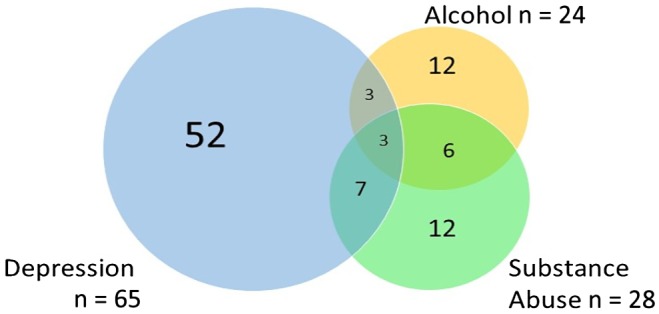
Venn diagram showing the overlap of the three problems assessed.
Table 1.
Patient Demographics
| Demographic Information (n = 95) | |
|---|---|
| Age (mean, SD, range) | 53, 10.4, 24‐84 |
| Sex (males) | 56% |
| Race (White, African American, other) | 42%, 48%, 6% |
| Ethnicity (non‐Hispanic) | 83% |
| Marital status (single, married, divorced, widowed) | 40%, 30%, 19%, 11% |
| Employment status | 20%, 12%, 38%, 13%, 17% |
| (FT, PT, unemployed, disability, retired) | |
| Education (less than HS, HS, some college, college, PG) | 20%, 38%, 24%, 12%, 3% |
| Psychiatric comorbidity (yes) | 50% |
| Primary liver diagnosis | |
| HCV | 64% |
| Alcohol‐induced liver disease | 17% |
| HCC | 11% |
| NASH | 8% |
| Primary health concern | |
| Liver disease | 61% |
| Family | 25% |
| Housing, work, finances | 14% |
Abbreviations: FT, fulltime; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HS, high school; NASH, nonalcoholic steatohepatitis; PG, postgraduate; PT, parttime.
End of study acceptability survey results revealed that 82% of the participants agreed that the behavioral health intervention improved their overall care; 87% indicated a desire to continue with the BIs within the hepatology practice, and 70% agreed that BI at the point of care in the hepatology office overcame the barrier of making an additional appointment to seek behavioral care separately. In addition, 89% of patients agreed to the statement that screening at the time of check‐in is a good way to identify individual behavioral health needs of patients with liver disease.
The mean QOL score as assessed by the overall CLDQ for the enrolled patients at baseline was 4.17 (SD, 1.28; range, 1.34‐6.9) (Table 2). For paired data, the overall CLDQ score improved by a mean of 0.74 (SD, 0.97) points at 3 months and 1.1 (SD, 1.05) points at 6 months (both P < 0.0001). Changes in subdomain scores for abdominal symptoms, systemic symptoms, worry, and physical function demonstrated similar improvement (P < 0.0001) from baseline to 3 and 6 months. The subdomain scores for emotion (P = 0.14) and fatigue (P = 0.116) did not significantly change at 3 months (Table 3).
Table 2.
Summary Statistics for the Overall and Subdomain Cldq Scores at Each Time Point
| Symptom Domain | Baseline (n = 95) | 3 Months (n = 82) | 6 Months (n = 84) |
|---|---|---|---|
| Overall | 4.17 ± 1.28 (1.34‐6.9) | 4.93 ± 1.06 (2.13‐6.76) | 5.28 ± 1.01 (2.54‐6.95) |
| Abdominal | 4.68 ± 1.97 (1‐7) | 5.61 ± 1.79 (1‐7) | 5.90 ± 1.70 (1‐7) |
| Fatigue | 3.44 ± 1.63 (1‐7) | 3.75 ± 1.70 (1‐7) | 4.26 ± 1.47 (1.2‐7) |
| Emotion | 3.89 ± 1.55 (1.25‐7) | 4.32 ± 1.34 (1.3‐7) | 4.73 ± 1.26 (1.375‐7) |
| Worry | 4.02 ± 1.86 (1‐7) | 5.31 ± 1.41 (1.4‐7) | 5.72 ± 1.28 (2.6‐7) |
| Activity | 4.50 ± 1.65 (1‐7) | 5.49 ± 1.15 (2.33‐7) | 5.89 ± 1.18 (2.0‐7) |
| Systemic | 4.47 ± 1.25 (1.2‐6.8) | 5.11 ± 1.07 (2.3‐6.9) | 5.21 ± 1.08 (2.2‐7) |
Entries are mean ± SD (range).
Table 3.
Change in Cldq Scores for Paired Data
| BL‐3 Months (n = 82) | P Value | BL‐6 Months (n = 84) | P Value | |
|---|---|---|---|---|
| Overall | –0.738 ± 0.9673 | <0.0001 | –1.111 ± 1.0527 | <0.0001 |
| Abdominal | –0.845 ± 1.5328 | <0.0001 | –1.186 ± 1.8277 | <0.0001 |
| Fatigue | –0.241 ± 1.3745 | 0.12 | –0.758 ± 1.5475 | <0.0001 |
| Emotion | –0.419 ± 1.1466 | 0.14 | –0.848 ± 1.2572 | <0.0001 |
| Worry | –1.280 ± 1.6488 | <0.0001 | –1.725 ± 1.7255 | <0.0001 |
| Activity | –1.030 ± 1.2382 | <0.0001 | –1.391 ± 1.8850 | <0.0001 |
| Systemic | –0.612 ± 1.2279 | <0.0001 | –0.760 ± 1.4812 | <0.0001 |
Entries are mean ± SD of paired differences.
Abbreviation: BL, baseline.
ANOVA tests (to determine the independent contributions of the illnesses assessed to the overall CLDQ scores) showed alcohol use disorder (P = 0.0007), substance abuse (P = 0.014), and depression (P < 0.0001) each had a significant univariate impact on baseline overall CLDQ scores but only depression had a significant independent effect, after controlling for other factors (P < 0.0001). Similarly, at 3 and 6 months all had a significant univariate impact but only depression had a significant independent effect (P < 0.001).
When comparing change in overall CLDQ scores over time separately (baseline to 3 months, baseline to 6 months) within each of the three subgroups (alcohol use disorder, substance abuse, and depression), no illness impacted the baseline to 3‐month score difference; however, depression at baseline impacted the baseline to 6‐month CLDQ score difference (P = 0.04). This shows that depression alone could influence the overall QOL of patients with CLD.
We further compared change in CLDQ scores in patients with depression (n = 65) to those without depression (n = 30) (Fig. 4). The baseline overall CLDQ scores in the depression subgroup (mean, 3.7; SD, 1.09) was significantly lower than those without depression (mean, 5.18; SD, 1.06). The differences persisted at 3 and 6 months (P < 0.001). Mean overall CLDQ scores for patients with depression improved from 3.7 to 5.0 over 6 months (P = 0.0401), while those for patients without depression started at 5.2 and ended at 5.9 (P > 0.05). The improvement in CLDQ scores was clinically and statistically significant for the depression subgroup. This indicates that patients with depression benefitted the most from this behavioral health program and BI.
Figure 4.
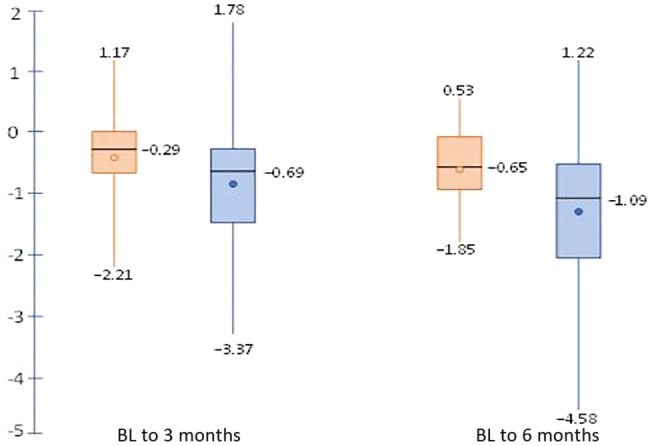
Change in QOL for depressed versus nondepressed subgroups. Negative values represent increases in score. Orange denotes patients without depression; blue denotes patients with depression. Circles are mean changes. Horizontal lines are median changes, and whiskers denote minimum and maximum changes.
Changes in illness severity scores over time are shown in (Fig. 5). AUDIT scores decreased by a mean of 3.5 from baseline to 3 months (P = 0.0018, sign test) and by a mean of 4.2 points at 6 months (P = 0.0048, Wilcoxon test). This indicates a decline in alcohol consumption in patients participating in this program. DAST‐10 scores at 3 months did not differ significantly from those at baseline (P = 0.41, Wilcoxon test) but improved at 6 months (P = 0.038, Wilcoxon test). PHQ‐9 scores improved by 1.9 points at 3 months and by 3.7 points at 6 months (both P < 0.0001). This suggests an improvement in depression both clinically and statistically from baseline to 3 and 6 months. Possibly, the improvement in depression contributed to improvement in the CLDQ scores.
Figure 5.
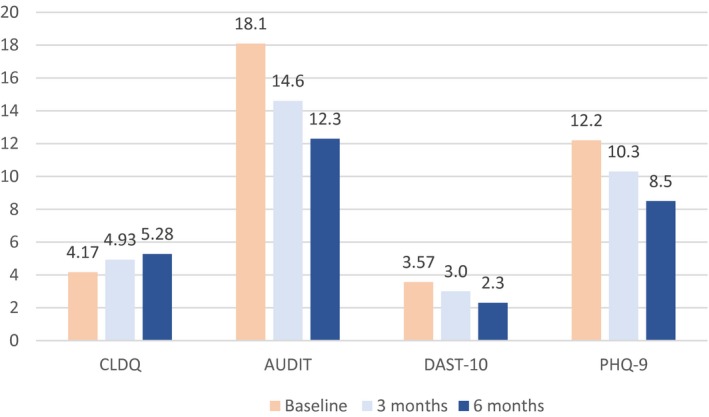
Mean scores at baseline and at 3 and 6 months for all study instruments. CLDQ scores measure quality of life, with higher values better. AUDIT, DAST‐10, and PHQ‐9 scores measure disease severity, with lower values better. CLDQ improved at 3 months and at 6 months (each P < 0.0001). AUDIT improved at 3 months (P = 0.0018) and 6 months (P = 0.0048). DAST‐10 improved at 6 months (P = 0.038). PHQ‐9 improved at 3 months and at 6 months (each P < 0.0001).
Using the standardized cut‐off scores to assess severity of illness for AUDIT, DAST‐10, and PHQ‐9, the number of patients in each of the severe categories decreased from 46% to 30% for AUDIT >15, 32% to 7.4% for DAST‐10 >5, and 25% to 5.1% for PHQ‐9 >14 from baseline to 6 months (Fig. 6).
Figure 6.
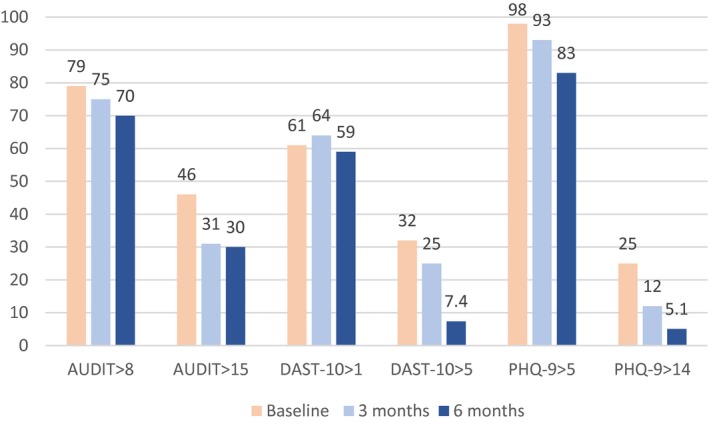
Percentage of patients with moderate and high illness severity scores. Moderate disease defined as AUDIT >8, DAST‐10 >1, or PHQ‐9 >5. Severe disease defined as AUDIT >15, DAST‐10 >5, or PHQ‐9 >14. The percentage of patients with severe disease improved for each disease modality between baseline and 6 months (P < 0.05).
Discussion
Recognition of modifiable risk factors, such as alcohol, substance use, and depression, through screening at the point of care helped identify the target population that needs behavioral health services. In this study of a behavioral health program for patients with CLD, we show that the SBIRT model is highly acceptable to patients and may have contributed to an improvement in outcomes, including QOL, depression severity, alcohol use disorder, and substance abuse. The improvement in QOL scores over time in patients with depression was significantly better than those without depression. It could possibly be that depression, if identified and addressed promptly, can contribute to improvement in overall QOL of patients with CLD. A recent review27 highlights the feasibility and accuracy of universal screening for alcohol misuse in acute admissions coupled with stratification of illness severity drive the tailored intervention. We found similar findings, but our study adds the knowledge about feasibility in an outpatient ambulatory setting, targeting three illnesses together (alcohol use disorder, substance abuse, and depression), and possible impacts on improving overall QOL. These novel data inform the management and future study of behavioral health care in CLD in multiple ways.
Behavioral Health is a Major Unmet Need Among Patients With CLD
Integrating behavioral health services within care of patients with noncommunicable diseases, such as heart failure, diabetes, and cancer, has become a national priority.28 However, CLD is not included or targeted as a national priority. Research shows that deploying and offering behavioral health services at the point of care reduces the stigma against seeking behavioral health care and improves the patient experience.29 It also improves access to and timeliness of treatment of behavioral illnesses. Our program overcame barriers for patients with CLD seeking routine care in a hepatology outpatient clinic; these included access, time, and resources required for an additional visit to a behavioral health provider.
Depression Should be Assessed and Managed During CLD Care
Depression and CLD are known to have some common underlying biological mechanisms.30 Depression is known to be more strongly associated with reduced QOL than to liver disease severity.31 Patients with and without depression are known to respond differently to the same treatments for liver disease.32, 33 Our results are in line with these findings and showed that patients with depression at baseline benefitted most from the behavioral health program. Our finding of almost 48.4% of patients with CLD with depression is higher than has been reported (30%).34 This could be the result of our reliance on a self‐reported assessment of depression symptoms rather than using International Classification of Diseases diagnostic codes or a psychiatric diagnosis.
Behavioral Health Programs Can and Should be Part of Hepatology Clinical Practice
There are three key implications of behavioral health programs in hepatology clinical practice. First, screening of all patients with CLD can help identify individual needs, which can help to individualize care, making it more patient centered. Second, behavioral health service at the point of care overcomes several reported barriers, such as access and stigma, and immediately addresses the needs of patients. Third, this intervention specifically helps patients with depression with or without alcohol use disorder or substance abuse. The sustainability of such a program must be based on allowing reimbursements to social work or behavioral staff in hepatology clinics and adding this to the pay for performance measures for hepatology practice. Fourth, risk stratification should be used to tailor brief interventions and referrals in various health care delivery settings.
There are several limitations to this study. First, the study is a single‐center uncontrolled study with a limited number of providers, thereby restricting generalizability. Second, absence of a control group precludes comparative assessment of the efficacy of any treatment. Third, in an uncontrolled study, evaluating change in QOL cannot be separated from clinical treatment effects, self‐management, or trajectory of liver disease. Other variables, such as timing of BI, expertise of the social worker, and provider interaction, all have a substantial effect on response to BI. Fourth, we did not include homeless and otherwise marginalized patients who have a high prevalence of depression and needs that are distinct from patients with CLD matriculated into ongoing care. Fifth, 22.5% positively screened patients were willing to participate but could not participate due to limited time and no prior notice. This means that if there were ways to conduct screening before the office visit coupled with an advanced notice to patients that they should plan for an additional 20 minutes for behavioral health care we could have possibly enrolled these patients.
Challenges that we faced during implementation included limited time between consecutive patients, space availability during clinic hours, and a single social worker offering BI for patients seen by multiple providers. There are some possible solutions to these challenges. Telehealth or phone‐based behavioral health interventions could overcome barriers, such as time, access, and office space. Universal screening conducted through the patient portal or automated phone‐based screening before the clinical appointment could help prepare patients to spend additional time during their routine clinical visit.
In summary, the results of our study support that a SBIRT‐based approach of screening and BI within a hepatology practice is acceptable to patients with CLD and may help improve the overall QOL of patients with CLD. Routine screening will help identify the needs of patients promptly and help structure tailored BIs. Ultimately, the SBIRT‐based approach may improve morbidity and mortality of patients with CLD.
Further research with a special focus on health outcomes, recruitment and retention rates, and implementation challenges is needed to solidify the findings of this program. In addition, future research should explore which components of the intervention contributed to the improvement in QOL, e.g., the additional attention received, the brief intervention, or merely the screening.
Supporting information
Acknowledgment
We thank Dr. Elliot Tapper for feedback on the manuscript.
Supported by an Albert Einstein Society Innovative Award (to M.V.).
Potential conflict of interest: Dr. Horrow is employed by Merck. The other authors have nothing to report.
References
- 1. Woltmann E, Grogan‐Kaylor A, Perron B, Georges H, Kilbourne AM, Bauer MS. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta‐analysis. Am J Psychiatry 2012;169:790‐804. [DOI] [PubMed] [Google Scholar]
- 2. Collins PY, Insel TR, Chockalingam A, Daar A, Maddox YT. Grand challenges in global mental health: integration in research, policy, and practice. PLoS Medicine 2013;10:e1001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Hospital Association . Integrating behavioral health across the continuum of care. https://www.aha.org/system/files/2018-01/integrating-behavioral-health-across-continuum-care-2014.pdf. Published February 2014. Accessed Sep 6, 2018.
- 4. Bonner JE, Barritt AS, Fried MW, Evon DM. Time to rethink antiviral treatment for hepatitis C in patients with coexisting mental health/substance abuse issues. Dig Dis Sci 2012;57:1469‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver‐related mortality in the United States. Gastroenterology 2013;145:375‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asrani SK, Kouznetsova M, Ogola G, Taylor T, Masica A, Pope B, et al. Increasing healthcare burden of chronic liver disease compared to other chronic diseases, 2004‐2013. Gastroenterology 2018;155:719‐729.e4. [DOI] [PubMed] [Google Scholar]
- 7. Lucey MR. Alcohol‐associated cirrhosis. Clin Liver Dis 2019;23:115‐126. [DOI] [PubMed] [Google Scholar]
- 8. Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999‐2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS, et al. Changing trends in etiology‐based annual mortality from chronic liver disease, from 2007 through 2016. Gastroenterology 2018;155:1154‐1163.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bianchi G, Marchesini G, Nicolino F, Graziani R, Sgarbi D, Loguercio C, et al. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis 2005;37:593‐600. [DOI] [PubMed] [Google Scholar]
- 11. Russ TC, Kivimäki M, Morling JR, Starr JM, Stamatakis E, Batty GD. Association between psychological distress and liver disease mortality: a meta‐analysis of individual study participants. Gastroenterology 2015;148:958‐966.e4. [DOI] [PubMed] [Google Scholar]
- 12. Rahm AK, Boggs JM, Martin C, Price DW, Beck A, Backer TE, et al. Facilitators and barriers to implementing screening, brief intervention, and referral to treatment (SBIRT) in primary care in integrated health care settings. Subst Abus 2015;36:281‐288. [DOI] [PubMed] [Google Scholar]
- 13. McQueen J, Howe TE, Allan L, Mains D, Hardy V. Brief interventions for heavy alcohol users admitted to general hospital wards. Cochrane Database Syst Rev 2011. Aug 10:CD005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li M, Fitzgerald P, Rodin G. Evidence‐based treatment of depression in patients with cancer. J Clin Oncol 2012;30:1187‐1196. [DOI] [PubMed] [Google Scholar]
- 15. Sudak DM. Cognitive behavioral therapy for depression. Psychiatr Clin North Am 2012;35:99‐110. [DOI] [PubMed] [Google Scholar]
- 16. Agerwala SM, McCance‐Katz EF. Integrating screening, brief intervention, and referral to treatment (SBIRT) into clinical practice settings: a brief review. J Psychoactive Drugs 2012;44:307‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Estee S, Wickizer T, He L, Shah MF, Mancuso D. Evaluation of the Washington state screening, brief intervention, and referral to treatment project: cost outcomes for Medicaid patients screened in hospital emergency departments. Med Care 2010;48:18‐24. [DOI] [PubMed] [Google Scholar]
- 18. Bray J, Cowell A, Hinde J. A systematic review and meta‐analysis of health care utilization outcomes in alcohol screening and brief intervention trials. Med Care 2011;49:287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend 2009;99:280‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee EH, Cheong JY, Cho SW, Hahm KB, Kim HY, Park JJ, et al. Development and psychometric evaluation of a chronic liver disease‐specific quality of life questionnaire. J Gastroenterol Hepatol 2008;23:231‐238. [DOI] [PubMed] [Google Scholar]
- 21. Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res 2007;31:185‐199. [DOI] [PubMed] [Google Scholar]
- 22. Babor TF, Higgins‐Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorder Identification Test: Guidelines for Use in Primary Care. 2nd ed Geneva, Switzerland: World Health Organization; 2001. whqlibdoc.who.int/hq/2001/WHO_MSD_MSB_01.6a.pdf. [Google Scholar]
- 23. Yudko E, Lozhkina O, Fouts A. A comprehensive review of the psychometric properties of the drug abuse screening test. J Subst Abuse Treat 2007;32:189‐198. [DOI] [PubMed] [Google Scholar]
- 24. Maisto SA, Carey MP, Carey KB, Gordon CM, Gleason JR. Use of the AUDIT and the DAST‐10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess 2000. Jun;12:186‐192. [DOI] [PubMed] [Google Scholar]
- 25. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the patient health questionnaire (PHQ): a diagnostic meta‐analysis. J Gen Intern Med 2007;22:1596‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kroenke K, Spitzer RL. The PHQ‐9: a new depression diagnostic and severity measure. Psychiat Ann 2002;32:509‐515. [Google Scholar]
- 27. Mellinger JL. Spotlight on impactful research: universal screening for alcohol misuse in acute admissions is feasible and identifies patients at high risk for liver disease. Clin Liver Dis 2018;12:83‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ngo VK, Rubinstein A, Ganju V, Kanellis P, Loza N, Rabadan‐Diehl C, et al. Grand challenges: integrating mental health care into the non‐communicable disease agenda. PLoS Medicine 2013;10:e1001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mastellos N, Gunn L, Harris M, Majeed A, Car J, Pappas Y. Assessing patients' experience of integrated care: a survey of patient views in the North West London Integrated Care Pilot. Int J Integr Care 2014;14:e015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang X, Liu X, Yu Y. Depression and chronic liver diseases: are there shared underlying mechanisms? Front Mol Neurosci 2017;10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Silva LD, Cunha CC, Cunha LR, Araújo RF, Barcelos VM, Menta PL, et al. Depression rather than liver impairment reduces quality of life in patients with hepatitis C. Braz J Psychiatry 2015;37:21‐30. [DOI] [PubMed] [Google Scholar]
- 32. Tomeno W, Kawashima K, Yoneda M, Saito S, Ogawa Y, Honda Y, et al. Non‐alcoholic fatty liver disease comorbid with major depressive disorder: the pathological features and poor therapeutic efficacy. J Gastroenterol Hepatol 2015;30:1009‐1014. [DOI] [PubMed] [Google Scholar]
- 33. Rogal SS, Mankaney G, Udawatta V, Chinman M, Good CB, Zickmund S, et al. Pre‐transplant depression is associated with length of hospitalization, discharge disposition, and survival after liver transplantation. PLoS One 2016;11:e0165517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mullish BH, Kabir MS, Thursz MR, Dhar A. Review article: depression and the use of antidepressants in patients with chronic liver disease or liver transplantation. Aliment Pharmacol Ther 2014;40:880‐892. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


