Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common form of chronic liver disease worldwide, and its aggressive form of nonalcoholic steatohepatitis (NASH) is becoming a leading cause for end‐stage liver disease and liver transplantation in the United States. In patients with NASH, the presence of advanced fibrosis is considered the most important prognostic factor in predicting liver‐related morbidity and mortality. Unfortunately, there are no US Food and Drug Administration (FDA)–approved medications to treat patients with NASH‐induced advanced fibrosis. However, the field of drug development to treat NASH and fibrosis has witnessed major advances over the past 5 years with several medications in phase III trials. Results from some of these trials are expected in 2019 with potential FDA approval in 2020. Clinicians who treat patients with NAFLD are likely to face several challenges over the next few years related to identifying patients with advanced fibrosis who may derive most benefit from pharmacologic treatment, the requirement for liver biopsy to assess histologic severity and response to treatment, and the urgent need to validate noninvasive tests to replace liver biopsy—to determine treatment initiation, response, futility, and the need for combination therapy with multiple drugs. Conclusion: In this review, we aim to dissect each of these challenges and attempt to provide suggested solutions while fully realizing that knowledge gaps still exist where future research is likely to provide urgently needed answers.
Abbreviations
- ALT
alanine aminotransferase
- ELF
enhanced liver fibrosis
- FDA
US Food and Drug Administration
- FIB‐4
Fibrosis‐4 index
- MRE
magnetic resonance elastography
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NFS
NAFLD fibrosis score
- OCA
obeticholic acid
- SEL
selonsertib
- T2D
type 2 diabetes
- VCTE
vibration‐controlled transient elastography
Nonalcoholic fatty liver disease (NAFLD) is now considered a global epidemic affecting 25% of the population worldwide.1 Nonalcoholic steatohepatitis (NASH) is considered the advanced form of NAFLD and the driving force behind the development of fibrosis and eventually cirrhosis and its complications. In adults in the United States, NASH cirrhosis is the leading cause of liver transplantation in women,2 the second leading cause of liver transplantation in men, and contributes significantly to the increased incidence of hepatocellular carcinoma.3 Patients with NASH and advanced fibrosis defined as the presence of bridging fibrosis (F3) or cirrhosis (F4) have the highest rates of liver‐related morbidity and mortality,4, 5, 6 making them the group with the highest unmet need for treatment.
Luckily, over the past few years, there has been great interest from all major stakeholders in developing effective treatments for these patients. In the near future, more than one drug that can improve fibrosis may be approved, paving the way to being able to provide valuable treatment. Two drugs in late‐stage development are the farnesoid X receptor (FXR) agonist obeticholic acid (OCA) and the apoptosis signal–regulating kinase 1 inhibitor selonsertib (SEL). There are two large phase III clinical trials, REGENERATE evaluating OCA in patients with NASH and stage 2‐3 fibrosis and STELLAR 3 evaluating SEL in patients with NASH and stage 3 fibrosis, which are on target to have results in the first half of this year.7 In fact, a recent press release revealed positive preliminary results from the REGENERATE trial on improvement in liver fibrosis, but full results are not available yet. The RESOLVE‐IT trial is testing the efficacy of elafibranor, a dual peroxisome proliferator–activated receptor α/δ agonist, on histological improvement defined as NASH resolution without worsening of fibrosis. In addition, the AURORA phase III trial is enrolling patients with NASH fibrosis to be treated with cenicriviroc, a chemokine receptor type 2 and type 5 dual inhibitor with antifibrotic efficacy. Other compounds have shown efficacy at different levels of disease development and progression, including those with metabolic, anti‐inflammatory, and antifibrotic effects providing a robust pipeline for drug development.8, 9
For patients to qualify for these medications, they will most likely need to have stage 3 or 4 fibrosis, and the primary treatment endpoint will be improvement of fibrosis by at least one stage. However, several challenges are likely to arise immediately after US Food and Drug Administration (FDA) approval of medications intended to treat fibrosis, including choosing the best approach for identifying patients with NASH‐associated advanced fibrosis, the duration of treatment, methods to assess response to treatment, and finally, decision‐making tools to determine futility and the need to add on or switch treatments, especially when additional medications become available. The objective of this review is to outline key clinical challenges that physicians face in managing patients with NASH. A summary of these challenges is shown in Fig. 1.
Figure 1.
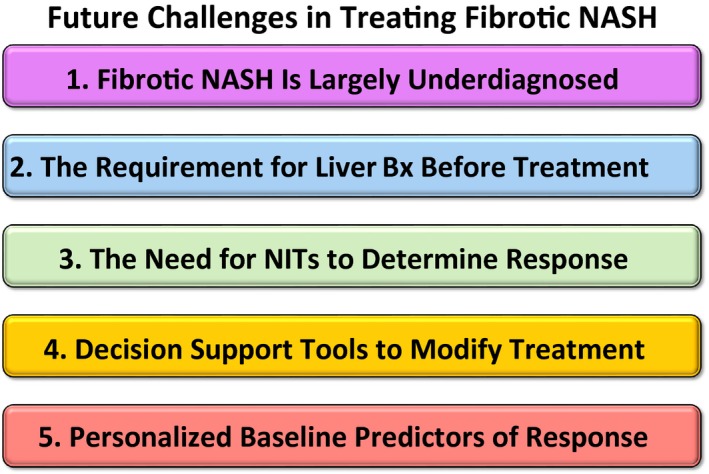
Summary of future challenges in the management of fibrotic NASH treatment.
Challenge #1: Most Patients With NASH‐Associated Advanced Fibrosis and Cirrhosis Are Not Identified
Recent data demonstrated that approximately 4.5 million people might have advanced fibrosis related to NASH in the United States.10 Unfortunately, studies by our group and others have clearly shown that most patients with end‐stage liver disease secondary to NASH had no previous diagnosis of liver disease.11, 12 This is related to the lack of clear guidance on whom to screen for NASH and how to do it in high‐risk individuals. The most recent European Association for the Study of the Liver guidelines recommend screening for fatty liver in patients at risk, including those who meet the criteria for metabolic syndrome (MetS), by obtaining their alanine aminotransferase (ALT) level and a liver ultrasound.13 Several studies have shown the high burden of NAFLD and advanced fibrosis in patients with type 2 diabetes (T2D), making them a high target for screening.14 However, multiple issues surround the proposition to screen for NAFLD in high‐risk groups, including the lack of cost‐effectiveness studies.
ALT‐Related Limitations
The definition of elevated ALT is controversial for several reasons. It has become clear recently that many laboratories set the cutoff value too high to detect chronic liver diseases such as NAFLD.15 Moreover, many primary care providers think that an elevated ALT is defined by an ALT value that is above 2 or even 3 times the upper limit of normal.16 Here it is worth emphasizing the following points:
Abnormal ALT is defined as any value above sex‐specific cutoffs (19 for females and 30 for males).17 Clinicians might identify patients with suspected NAFLD more consistently by using absolute values rather than relying on laboratory‐specified reference ranges, although the feasibility and cost‐effectiveness of this approach requires further research; and
Abnormal ALT is abnormal, as shown in previous NAFLD studies.18 It does not have to be 2 or 3 times the upper limit of normal.
Liver Ultrasound–Related Limitations
Liver ultrasound has limited sensitivity to detect NAFLD when less than 20% of hepatocytes have steatosis on liver biopsy.19 Furthermore, ultrasound is not specific, because the diagnosis of NAFLD is based primarily on increased echogenicity of the liver, which could be due to fibrosis or other infiltrative processes. However, given the fact that many patients with NAFLD, even with advanced disease, may have normal ALT,20 the addition of ultrasound to ALT as a screening modality might be justified in high‐risk individuals such as those with T2D.
Identifying Patients With Advanced Fibrosis Who Will Benefit From Pharmacologic Treatment
A key aspect of any screening strategy for NAFLD will be to subsequently find patients at highest risk for liver‐related outcomes (i.e., those with advanced fibrosis [F3‐F4]). Although liver biopsy, and more recently advanced imaging modalities such as vibration‐controlled transient elastography (VCTE) and magnetic resonance elastography (MRE), can identify these individuals, their cost‐effectiveness needs additional research and their availability is limited, especially in the primary care setting. Therefore, primary care providers need an inexpensive, readily available method to decide which patients should be referred to a specialist for consideration of pharmacologic treatment. The Fibrosis‐4 (FIB‐4) index and the NAFLD fibrosis score (NFS) rely on readily available clinical variables, such as liver enzymes and platelet counts, to identify patients at risk for having F3‐F4.21, 22 They both meet the criteria of being simple tools that have acceptable accuracy to detect F3‐F4 with extensive validation in multiple cohorts and endorsement by the American Association for the Study of Liver Diseases.23
Suggested Solution to Challenge #1
As the disease burden and health‐related costs have increased, new cost‐effectiveness studies to evaluate the value of screening patients at risk for NAFLD are urgently needed. This is timely, as new NAFLD economic studies have recently emerged,24, 25 new medications are likely to be approved soon, and new accurate noninvasive tests have been developed. Until such studies are done, a “due diligence” approach and increased awareness in primary care and other clinical settings are warranted. Screening for NAFLD/NASH in patients with T2D and MetS using both ALT and ultrasound can be considered. Identifying patients at high risk for advanced fibrosis can be done by simple fibrosis scores such as FIB‐4 index and NFS. Patients with features suggestive of advanced disease should be referred to a specialist. This approach is summarized in Fig. 2.
Figure 2.
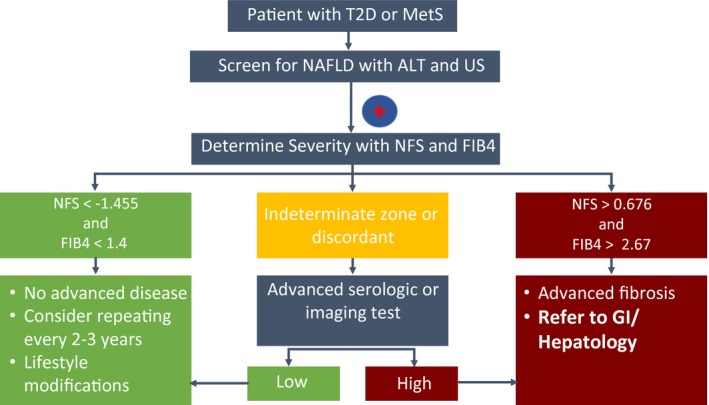
Primary care physicians’ algorithm for the identification of patients with NAFLD and advanced fibrosis. Screening for NAFLD with ALT and ultrasonography should be considered in high‐risk patients, such as those with metabolic syndrome and type 2 diabetes or MetS. In patients with NAFLD, the presence of advanced fibrosis can be determined by the NFS or FIB‐4 index. For patients with indeterminate or discordant results, an advanced serologic or imaging test can be ordered, such as the ELF test and VCTE, respectively.
Challenge # 2: Do We Need to Obtain a Liver Biopsy on All Patients Before Starting Treatment?
Liver biopsy is an invasive procedure with potential for serious complications, including but not limited to bleeding (hemobilia, subcapsular hematoma, hemoperitoneum), pneumothorax, and even death.26 Historically, biopsy was the only reliable method to determine the stage of fibrosis. However, over the last decade, several developments in serologic and radiologic tests for fibrosis have rendered a liver biopsy less necessary to identify patients with advanced fibrosis.27, 28 Imaging studies such as VCTE or MRE and serologic tests such as the enhanced liver fibrosis (ELF) can potentially determine which patients have advanced disease, although some limitations still exist as reviewed by Wong et al.28 In fact, a recent study that assessed the efficacy of combination therapy for NASH‐induced advanced fibrosis (NCT03449446) demonstrated that patients who have liver stiffness measurement (LSM) greater than or equal to 14.0 kPa and ELF score greater than or equal to 9.8 had very high likelihood of having F3‐F4 on liver biopsy (data in preparation for publication), which supports the use of noninvasive scores to determine the need for treatment with agents that have antifibrotic activity. Another recent study has shown that using a composite score of controlled attenuation parameter (measured using VCTE), liver stiffness (also measured by VCTE), and aspartate aminotransferase can accurately predict patients with a fibrosis score greater than or equal to 2 and a NAFLD activity score greater than or equal to 4 (a histological score to determine whether patients have NASH before entry in NASH clinical trials).29 This score might need to be modified to identify patients with F3‐F4.
Suggested Solution to Challenge #2
Several algorithms that include imaging studies (e.g., VCTE) and serologic tests (e.g., ELF) can be used by specialists to eliminate the need for liver biopsy when deciding on the need for pharmacotherapy. Longitudinal studies to assess whether changes in such biomarkers and algorithms correlate with treatment response are needed and should become available prior to drug approval.
Challenge #3: How Do We Monitor Response to Treatment?
The two drugs that could potentially make it to FDA approval first (OCA and SEL) have a primary outcome that includes the improvement in liver fibrosis by one stage based on liver histology obtained by biopsy after 1 year of treatment. Given that repeat biopsy is the primary method to assess response and that response rate in terms of improvement in liver fibrosis by one stage occurs at best in approximately 45% of patients based on phase II data, how will this play out in real‐world settings when payers may require some evidence that the medication is having a positive effect before allowing continuation? This could lead insurance providers to require a liver biopsy after 1 year of treatment to determine eligibility for longer duration. However, this will expose patients to additional risk related to biopsy complications, especially when using relatively safe medications. Therefore, noninvasive methods to assess response at the 1‐year mark are needed.
Although noninvasive tests such as ELF and VCTE have shown acceptable accuracy in predicting advanced fibrosis in NASH patients, their utility to predict treatment response needs further validation. Furthermore, what constitutes a clinically meaningful response using these tests after 1 year of treatment remains highly controversial. Fortunately, longitudinal data including correlation among VCTE, blood biomarkers and histology are being collected in current phase III trials. These studies will need to focus on longitudinal analyses of noninvasive assessment of treatment response if the drugs prove to be effective.
Suggested Solution to Challenge #3
One reasonable approach to noninvasively determine response after 1 year of treatment will be to use a combination of a biomarker (or set of biomarkers) and an imaging test.30 Although simple fibrosis scores such as FIB‐4 index and NFS are helpful in case identification of patients with advanced fibrosis, they are less likely to be responsive to treatment effects. A combination of tests such as ELF to assess “biochemical response,” and VCTE or MRE to assess “radiological response,” might be the best approach.
For example, if both VCTE and ELF indicate improvement, then the drug should be continued. If both indicate no response or worsening of disease severity, then the drug could be discontinued. If the tests show discordant results, then a liver biopsy might be helpful. Figure 3 provides a simplified algorithm that can be used by practicing clinicians to determine treatment response. Further data from the current ongoing treatment programs are emerging and need to support these proposed solutions.
Figure 3.
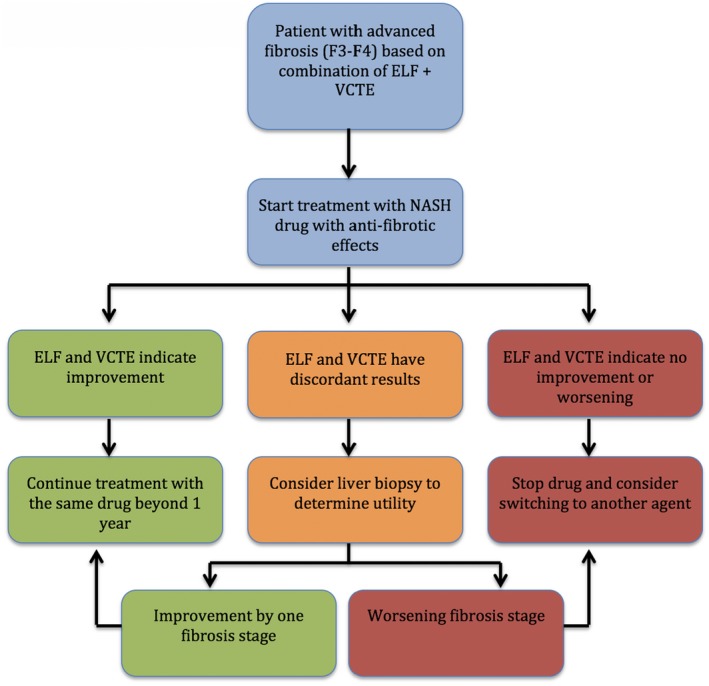
Proposed algorithm to determine treatment response to fibrotic NASH medications. We use the ELF test as an example of serologic tests and the VCTE as an example of imaging studies. More data are needed before this algorithm can be adopted in clinical practice.
Challenge #4: Treatment Modification/Escalation: Add‐on Therapy Versus Switching
It is likely that in clinical practice certain patients will experience initial improvement with a drug but possibly no further improvement following continuation of therapy. For example, a patient may show improvement in both VCTE and ELF at year 1 of treatment but no further improvement at year 2. One may argue that preventing progression is a successful strategy by itself; stopping the drug may increase the risk for worsening disease. However, most experts will agree that further improvement is preferable. If we have multiple approved drugs with different mechanisms of action, then the next logical step could be to add on a second agent to increase the chances of response. However, one question will be whether switching to another agent is sufficient for continuation of improvement. This is largely unknown at this point, but lessons from diabetes have taught us that an add‐on strategy might be the best approach.8 The initial management of individuals with new‐onset T2D typically starts with lifestyle modifications and metformin monotherapy, but when this approach fails to achieve the glycemic target, combination therapy with another agent is usually started (dual therapy).31 If the hemoglobin A1c (A1C) target is not achieved after 3 months of dual therapy, a third agent could be added as part of triple therapy. Patients who present with very high A1C at diagnosis should be started on dual therapy from the beginning.
Another question will be how to determine response to adding a second agent. It is plausible that the same tests used to determine response to the initial agent should be used to assess the response to add‐on therapy.
Suggested Solution to Challenge #4
For patients with partial response to a single agent based on serologic and radiologic tests, adding a second agent and using the same tests to assess further response to combination therapy might be proven to be the best strategy. What constitutes an acceptable response based on noninvasive tests in comparison to histologic improvement in fibrosis remains to be determined. Given the fact that fibrosis is scored on a 5‐point scale (F0‐F4), it is reasonable to suggest that a reduction in biomarker level or liver stiffness by 20% from baseline constitutes a meaningful response. Using this logic, a decrease in LSM by VCTE by 20% from baseline or to less than the cutoff for F3 (approximately 9 kPa) could be considered a sufficient response after 1 year of treatment. A biomarker response can be defined as a 2‐point reduction in ELF score as an example. Assessing treatment response with noninvasive biomarkers (such as with VCTE+ELF) should be done within phase III trials and published prior to a drug’s approval. The same algorithm provided in Fig. 2 could be used to determine the efficacy of combination therapy.
Challenge #5: If We Have Two Approved Antifibrotics, How Do We Decide Which One to Use First in Individual Patients?
It is a likely scenario over the next few years that we will have more than one drug approved for the treatment of NASH‐related advanced fibrosis, but no head‐to‐head data comparing the available agents. This begs the question of how clinicians will choose which agent to use as initial treatment. Adverse effects will definitely play a role in the decision‐making process, along with the presence of comorbidities. For example, clinicians may raise a concern regarding the use of OCA in a patient with extensive coronary artery disease, given its effects on lipid profiles,32 although this could be mitigated by increasing the statin dose. Using baseline factors that may predict response to certain mechanism(s) of action of a drug is an intriguing idea. For example, OCA is an FXR agonist that blocks the synthesis of bile acids. Could measuring baseline serum bile acids and C4 levels predict which patients with NASH fibrosis are more likely to respond to OCA? Similarly, could measuring baseline cytokeratin 18 fragments, a biomarker of hepatocyte apoptosis,33 predict response to SEL? These data are likely to emerge quickly with the conclusion of the phase III clinical trials that indeed measured these factors at baseline and in response to treatment. For example, The HepQuant SHUNT test is a minimally invasive test that measures hepatocyte function and inflow to the liver from the simultaneous clearances of cholate from systemic and portal circulations.34, 35 It is being tested in ongoing NASH treatment clinical trials to assess its correlation with other baseline tests of liver disease severity and its longitudinal changes with improvement or worsening of disease severity.
Suggested Solution to Challenge #5
Measuring baseline predictors that may determine the most relevant pathophysiologic mechanism for disease progression may prove to be useful in deciding which therapeutic agent should be tried first. Using the same biomarkers to assess for target engagement and response to therapy is an attractive option that needs further validation in a prospective manner.
In conclusion, the landscape of pharmacologic treatment for NASH‐related advanced fibrosis is on the verge of witnessing major changes.36 This requires professional societies to provide clear guidance to practicing clinicians on how to best use these treatment options. In an effort to start this conversation in our hepatology community, the following proposal may help with the development of a NASH management algorithm:
Screening for NAFLD in at‐risk populations will likely be recommended and should be followed.
In patients at risk for advanced fibrosis, determining the presence of advanced fibrosis by using the combination of biomarkers and imaging tests will likely be useful. Liver biopsy should be reserved for patients with equivocal results.
If advanced fibrosis is present, treatment will likely be initiated with a single agent that has antifibrotic activity such as OCA or SEL.
Re‐assessment of response to treatment with a single agent in 1 year and then on a yearly basis. If the patient has biomarker and imaging response, continue treatment with single agent.
If there is no biomarker or imaging response, treatment with the initial single agent is futile, so stop and switch to another agent.
If there is a partial biomarker and imaging response, the treatment was partially effective and adding another agent should be considered.
This article is based on prediction of the future NASH treatment landscape, taking into consideration the data available to date. Further research is needed and we are fully aware of the controversy that these suggestions may stimulate among hepatologists. We welcome the debate that will unfold over the next few years, anticipating that it will be full of exciting developments for patients with this common and potentially progressive disease. In the expectation that multiple drug approvals for treatment of NASH fibrosis are approaching, such a dialog should be welcomed to promote further research.
Potential conflict of interest: Dr. Alkhouri is on the speakers’ bureau and received grants from Gilead and Intercept; he received grants from Allergan and Genfit. Dr. Lawitz is on the speakers’ bureau and received grants from Gilead and Intercept; he received grants from Allergan and Genfit. Dr. Noureddin received grants from Intercept, Gilead, Allergan, and Genfit.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol 2018;113:1649‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White DL, Thrift AP, Kanwal F, Davila J, El‐Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 2017;152:812‐820.e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Angulo P, Kleiner DE, Dam‐Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology 2015;61:1547‐1554. [DOI] [PubMed] [Google Scholar]
- 6. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta‐analysis of paired‐biopsy studies. Clin Gastroenterol Hepatol 2015;13:643‐654.e641‐649; quiz e639‐e640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology 2018;67:549‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alkhouri N, Poordad F, Lawitz E. Management of nonalcoholic fatty liver disease: lessons learned from type 2 diabetes. Hepatol Commun 2018;2:778‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Noureddin M, Zhang A, Loomba R. Promising therapies for treatment of nonalcoholic steatohepatitis. Expert Opin Emerg Drugs 2016;21:343‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, et al. Prevalence of nonalcoholic steatohepatitis‐associated cirrhosis in the United States: an analysis of National Health and Nutrition Examination Survey Data. Am J Gastroenterol 2017;112:581‐587. [DOI] [PubMed] [Google Scholar]
- 11. Nagpal SJ, Kabbany MN, Mohamad B, Lopez R, Zein NN, Alkhouri N. Portal hypertension complications are frequently the first presentation of NAFLD in patients undergoing liver transplantation evaluation. Dig Dis Sci 2016;61:2102‐2107. [DOI] [PubMed] [Google Scholar]
- 12. Bertot LC, Jeffrey GP, Wallace M, MacQuillan G, Garas G, Ching HL, et al. Nonalcoholic fatty liver disease‐related cirrhosis is commonly unrecognized and associated with hepatocellular carcinoma. Hepatol Commun 2017;1:53‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. European Association for the Study of the Liver; European Association for the Study of Diabetes; European Association for the Study of Obesity . EASL‐EASD‐EASO Clinical Practice Guidelines for the management of non‐alcoholic fatty liver disease. J Hepatol 2016;64:1388‐1402. [DOI] [PubMed] [Google Scholar]
- 14. Cusi K, Sanyal AJ, Zhang S, Hartman ML, Bue‐Valleskey JM, Hoogwerf BJ, et al. Non‐alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab 2017;19:1630‐1634. [DOI] [PubMed] [Google Scholar]
- 15. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 2017;112:18‐35. [DOI] [PubMed] [Google Scholar]
- 16. Blais P, Husain N, Kramer JR, Kowalkowski M, El‐Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol 2015;110:10‐14. [DOI] [PubMed] [Google Scholar]
- 17. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1‐10. [DOI] [PubMed] [Google Scholar]
- 18. Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286‐1292. [DOI] [PubMed] [Google Scholar]
- 19. Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol 2009;51:1061‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Portillo‐Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab 2015;100:2231‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 22. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317‐1325. [DOI] [PubMed] [Google Scholar]
- 23. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 24. Allen AM, Van Houten HK, Sangaralingham LR, Talwalkar JA, McCoy RG. Healthcare cost and utilization in nonalcoholic fatty liver disease: real‐world data from a large U.S. claims database. Hepatology 2018;68:2230‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Younossi ZM, Tampi R, Priyadarshini M, Nader F, Younossi IM, Racila A. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology 2019;69:564‐572. [DOI] [PubMed] [Google Scholar]
- 26. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver D . Liver biopsy. Hepatology 2009;49:1017‐1044. [DOI] [PubMed] [Google Scholar]
- 27. Singh S, Muir AJ, Dieterich DT, Falck‐Ytter YT. American Gastroenterological Association Institute technical review on the role of elastography in chronic liver diseases. Gastroenterology 2017;152:1544‐1577. [DOI] [PubMed] [Google Scholar]
- 28. Wong VW, Adams LA, de Ledinghen V, Wong GL, Sookoian S. Noninvasive biomarkers in NAFLD and NASH—current progress and future promise. Nat Rev Gastroenterol Hepatol 2018;15:461‐478. [DOI] [PubMed] [Google Scholar]
- 29. Sasso M, Chan WK, Harrison SA, Czernichow S, Allison MED, Tsochatzis EA, et al. Fibroscan‐based score (FS3) to identify NASH patients with NAS≥4 and F≥ 2: development in a NAFLD UK cohort—external validation in a Malaysian NAFLD cohort, a US Screening cohort and a French bariatric surgery cohort. Hepatology 2018;68(S1):140. [Google Scholar]
- 30. Alkhouri N, McCullough A. Noninvasive diagnosis of NASH and liver fibrosis within the spectrum of NAFLD. Gastroenterol Hepatol 2012;8:661‐668. [PMC free article] [PubMed] [Google Scholar]
- 31. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract 2018;24:91‐120. [DOI] [PubMed] [Google Scholar]
- 32. Neuschwander‐Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non‐cirrhotic, non‐alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo‐controlled trial. Lancet 2015;385:956‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin‐18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 2009;50:1072‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Everson GT, Hoefs JC, Niemann CU, Olthoff KM, Dupuis R, Lauriski S, et al. Functional elements associated with hepatic regeneration in living donors after right hepatic lobectomy. Liver Transpl 2013;19:292‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Helmke S, Colmenero J, Everson GT. Noninvasive assessment of liver function. Curr Opin Gastroenterol 2015;31:199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Konerman MA, Jones JC, Harrison SA. Pharmacotherapy for NASH: current and emerging. J Hepatol 2018;68:362‐375. [DOI] [PubMed] [Google Scholar]


