Abstract
Background
The common cold is one of the most common illnesses in humans and constitutes an economic burden both in terms of productivity and expenditure for treatment. There is no proven cure for the common cold and symptomatic relief is the mainstay of treatment. The use of intranasal ipratropium bromide (IB) has been addressed in several studies and might prove an effective treatment for the common cold.
Objectives
To determine the effect of IB versus placebo or no treatment on severity of rhinorrhoea and nasal congestion in children and adults with the common cold. Subjective overall improvement was another primary outcome and side effects (for example, dry mucous membranes, epistaxis and systemic anticholinergic effects) were reported as a secondary outcome.
Search methods
In this updated review we searched CENTRAL 2013, Issue 3, MEDLINE (1950 to March week 4, 2013), MEDLINE in‐process and other non‐indexed citations (8 April 2013), EMBASE (1974 to April 2013), AMED (1985 to April 2013), Biosis (1974 to February 2011) and LILACS (1985 to April 2013).
Selection criteria
Randomised controlled trials (RCTs) comparing IB to placebo or no treatment in children and adults with the common cold.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality. We used a standardised form to extract relevant data and we contacted trial authors for additional information.
Main results
Seven trials with a total of 2144 participants were included. Four studies (1959 participants) addressed subjective change in severity of rhinorrhoea. All studies were consistent in reporting statistically significant changes in favour of IB. Nasal congestion was reported in four studies and was found to have no significant change between the two groups. Two studies found a positive response in the IB group for the global assessment of overall improvement. Side effects were more frequent in the IB group, odds ratio (OR) 2.09 (95% confidence interval (CI) 1.40 to 3.11). Commonly encountered side effects included nasal dryness, blood tinged mucus and epistaxis. The overall risk of bias in the included studies was moderate.
Authors' conclusions
For people with the common cold, the existing evidence, which has some limitations, suggests that IB is likely to be effective in ameliorating rhinorrhoea. IB had no effect on nasal congestion and its use was associated with more side effects compared to placebo or no treatment although these appeared to be well tolerated and self limiting. There is a need for larger, high‐quality trials to determine the effectiveness of IB in relieving common cold symptoms.
Plain language summary
A spray containing ipratropium bromide administered into the nose to treat common cold symptoms
The common cold is caused by a range of viruses and bacteria. It is the most common illness affecting humans. It causes a runny and stuffy nose, sore throat and sneezing. There is no proven cure for the cold and only symptom relief is available. The aim of this review was to investigate the use of a nasal spray containing ipratropium bromide (IB), which may improve cold symptoms. This review has found that IB may improve the runny nose but has no effect on nasal stuffiness.
We identified seven trials that included 2144 participants. There were more side effects with IB, such as dryness of the nose, mucus with streaks of blood and bleeding from the nose. Limitations in this review included two studies with missing participants and four studies with unclear blinding of the participants, personnel or outcome assessors. These limitations resulted in the majority of studies having an unclear risk of bias, which raises the concern of overestimation of the overall effect of IB. We concluded from this review that IB may be effective in improving the runny nose with some side effects that are well tolerated. There is a need for more high‐quality studies to determine the effectiveness of IB in relieving common cold symptoms.
Background
Description of the condition
The common cold is a benign, self limiting syndrome that manifests clinically with multiple symptoms caused by over 200 different respiratory viruses. The most common are the rhinoviruses (Heikkinen 2003). Symptoms include an acute onset of a number of symptoms at variable degrees: nasal discharge, congestion, sneezing, sore throat, cough, low‐grade fever, headache and malaise (Tyrrell 1993). It is one of the most common illnesses in humans, in which the incidence in adults is two to four episodes per year, while in preschool children it ranges between five to seven episodes per year. It constitutes an economic burden both in terms of productivity and expenditure for treatment (Fendrick 2003). People with the common cold tend to seek relief of the most vexing symptoms: rhinorrhoea and nasal congestion.
A number of systematic reviews have explored the evidence behind commonly used interventions. In one review (Arroll 2013) antibiotics were found to have more side effects than benefit and the authors concluded that there was insufficient evidence to recommend their routine use in adults with the common cold. A review of Chinese medicinal herbs concluded that evidence did not support using any Chinese herbal preparation(s) either, in light of the high risk of bias in 15 of the 17 identified trials (Zhang 2009). Non‐steroidal anti‐inflammatory drugs (NSAIDs) were found in another review to be effective in relieving discomfort or pain caused by the common cold, but not rhinorrhoea (Kim 2009). Vitamin C showed no consistent differences from the placebo group in the duration or severity of colds (Hemilä 2013). The most recent Cochrane Review showed that zinc administered within 24 hours of the onset of symptoms reduced the duration and severity of the common cold in healthy people (Singh 2011). However, the optimum dose, formulation and duration are yet to be determined. Until an effective cure is found, symptomatic relief remains the accepted clinical approach.
Description of the intervention
Ipratropium bromide (IB) is a quaternary ammonium derivative of atropine that is minimally absorbed across biological membranes. It therefore has limited systemic anticholinergic side effects when applied locally to the nasal mucosa. Clinical effects are predominately due to its competitive antagonism of muscarinic receptors (Gaffey 1988).
How the intervention might work
Nasal secretions produced during the common cold are partly from glands under parasympathetic control (Gaffey 1988). The parasympathetic nervous system regulates the mucous and seromucous glands of the nasal mucosa that play a role in the bothersome symptoms in the early stages of the common cold. Therefore IB, an anticholinergic agent, has been proposed to alleviate rhinorrhoea that is mainly due to reflex‐mediated glandular secretions (Østberg 1997).
Why it is important to do this review
There is no proven cure for the cold and symptomatic relief is the mainstay of treatment. A number of studies have shown the efficacy of intranasal IB but no systematic review has been produced. This review aims to provide physicians and consumers with sound evidence upon which to base their treatment decisions. This is an update of a Cochrane Review (AlBalawi 2011).
Objectives
To determine the effect of IB versus placebo or no treatment on severity of rhinorrhoea and nasal congestion in children and adults with the common cold. Subjective overall improvement was another primary outcome and side effects (for example, dry mucous membranes, epistaxis and systemic anticholinergic effects) were reported as a secondary outcome.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing intranasal IB with placebo or no treatment. We included abstracts presented in conferences only if the trial authors were able to provide study details enabling assessment of risk of bias and data extraction.
Types of participants
Children five years and older and adults older than 18 years of age. Efficacy and safety have not been established for children below five years of age and so we excluded this group. We included participants with recent symptoms suggestive of the common cold that were self diagnosed by runny or stuffy nose (or both), sneezing with or without symptoms of malaise, headache and cough (Tyrrell 1993). We also considered naturally occurring as well as experimental rhinovirus infections, since they are the most common causative viruses of the common cold. We excluded those who suffered from allergic rhinitis, perennial non‐allergic rhinitis, other concurrent respiratory infections, asthma, sinusitis, other chronic diseases, influenza or myalgia.
Types of interventions
We compared any dose of intranasal IB as a single active agent with a control that was either placebo or no treatment. We also included trials permitting co‐interventions as long as they were equally balanced in both groups.
Types of outcome measures
Primary outcomes
Change in severity of symptoms of rhinorrhoea and congestion assessed subjectively by validated scales; for example, visual analogue scales (VAS) (Stewart 1992).
Global assessment of overall improvement from use of the intervention by a standardised questionnaire to participants at the end of the study. These could include the Global Rating of Change: respondents' evaluation of changes in one or more domains of health‐related quality of life indicating whether they are better, about the same or worse (Patrick 1993). Another method is the Global Measure, which involves an assessment of the overall quality of the health status of the patient at a given point in time (Guyatt 1991).
Secondary outcomes
Assessment of type and frequency of adverse events (for example, epistaxis, dry mucous membranes, systemic anticholinergic effects).
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 3, part of The Cochrane Library, (www.thecochranelibrary.com) (accessed 9 April 2013), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (1950 to March week 4, 2013), MEDLINE in‐process and other non‐indexed citations (8 April 2013), EMBASE (1974 to April 2013), AMED (1985 to April 2013), Biosis (1974 to February 2011) and LILACS (1985 to April 2013).
We used the following search terms to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy for EMBASE (see Appendix 1), AMED (see Appendix 2), Biosis (See Appendix 3) and LILACS (see Appendix 4).
1 Common Cold/ 2 common cold*.tw. 3 coryza.tw. 4 ((nasopharyng* or rhinopharyng*) adj2 acute).tw. 5 Rhinovirus/ 6 exp Adenoviridae/ 7 adenoviridae infections/ or adenovirus infections, human/ 8 adenovir*.tw. 9 rhinovir*.tw. 10 respiratory syncytial viruses/ or respiratory syncytial virus, human/ 11 Respiratory Syncytial Virus Infections/ 12 (respiratory syncytial virus* or rsv).tw. 13 Coronavirus/ 14 Coronavirus Infections/ 15 coronavirus*.tw. 16 Respiratory Tract Infections/ 17 (respiratory infection* or respiratory tract infection*).tw. 18 or/1‐17 19 Ipratropium/ 20 ipratropium.tw,nm. 21 exp Cholinergic Antagonists/ 22 anticholinergic*.tw,nm. 23 atrovent*.tw,nm. 24 or/19‐23 25 18 and 24
Searching other resources
We also searched the following database for ongoing trials: www.clinicaltrials.gov. We scanned references of included studies to identify any further relevant trials. We contacted experts in the field and authors of included studies to inquire about other available or ongoing trials. We also contacted a related pharmaceutical company for any published or unpublished data on their products. We imposed no language or publication restrictions.
Data collection and analysis
Selection of studies
Two review authors (ZHB, SSO) independently screened all titles and abstracts retrieved by the searches and assessed whether they should be included or excluded. We obtained original articles for trials that were found to be relevant and for abstracts that were insufficient to judge whether the study should be included or not. We resolved any conflict through discussion and by consulting the third author (KA).
Data extraction and management
The same review authors (ZHB, SSO) independently extracted data by using standard data extraction forms. All studies were in English. We resolved disagreements by consulting the third review author (KA).
Assessment of risk of bias in included studies
Two authors (ZHB, SSO) independently assessed the included trials for methodological quality using The Cochrane Collaboration's tool for assessing 'Risk of bias' (Higgins 2011). This set of criteria is based on evidence of associations between overestimate of effect and high risk of bias. The components of this tool include: sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting. The categories are as follows:
low risk of bias;
high risk of bias; and
uncertain risk of bias.
We resolved disagreements by discussion and consensus between the two assessing review authors and by consulting the third review author (KA) when needed.
Measures of treatment effect
Primary outcome measures included the change in severity of symptoms of rhinorrhoea and nasal congestion and global overall improvement. We planned to express results as a risk ratio (RR) with 95% confidence intervals (CI) for dichotomous outcomes. We also planned to use the mean difference (MD) where continuous scales of measurement were used, or the standardised mean difference (SMD) if different scales were used.
Unit of analysis issues
We carried out analyses according to intention‐to‐treat (ITT). Where inappropriate analysis was used, we dealt with it as a 'Risk of bias' issue and considered it in terms of sensitivity analysis.
Dealing with missing data
We contacted trial authors for missing information which would be included in the review if provided. We gave careful consideration to the reasons for data that remained missing. Where there was a large amount of missing data, we dealt with this as a 'Risk of bias' issue and considered it in terms of sensitivity analysis.
Assessment of heterogeneity
Heterogeneity is defined as significant if the Chi² test P < 0.1 or the I² statistic > 50%. We used a fixed‐effect model to pool data where appropriate. However, we used a random‐effects model where heterogeneity was identified. Expected sources of heterogeneity were: age, treatment setting, dosage, mode of drug delivery (for example, metered dose nasal spray, nasal drops) and variation in outcome assessments (for example, subjective, objective or visual analogue scale) and quality of trials. We reported rates of adverse events and compared their likelihood using odds ratios (ORs).
Assessment of reporting biases
If appropriate, we had planned to use a funnel plot to assess publication bias (Egger 1997). However, since our review included fewer than 10 studies, we made no attempt to investigate the risk of publication bias via a funnel plot.
Subgroup analysis and investigation of heterogeneity
It was proposed to undertake subgroup analysis based on different age groups; paediatrics (five years to 17 years) and adults (18 years or older), and different dosages (for example, low and high dose). However, this was not found to be appropriate as the data provided in the studies included a mixed population ranging from 12 to 70 years of age. Neither did we attempt subgroup analysis for different dosages, as the primary outcomes were presented in a narrative form, given the missing data and heterogenous outcome parameters used among the studies.
An additional subgroup analysis was proposed to be undertaken based on the use of placebo control versus no treatment and on spontaneous common cold infection versus experimentally induced. This was not found to be appropriate given the inability to pool the results as described previously.
There was only one study with experimental cold infection (Gaffey 1988) and there were issues with the diagnostic criteria which are described in detail under Potential biases in the review process. We therefore elected not to perform a subgroup analysis as it might be misleading.
Sensitivity analysis
A sensitivity analysis including high‐quality studies was not conducted because it was not possible to pool the results.
Results
Description of studies
Results of the search
Our search generated 426 results. After screening, we considered nine to be potentially eligible and from these we excluded two due to the reasons mentioned in the Characteristics of excluded studies table. Seven RCTs comparing IB and placebo for the common cold fulfilled the inclusion criteria for this review. No relevant ongoing trials were found upon searching www.clinicaltrials.gov.
The updated search for this review run in April of 2013 did not identify any new relevant studies. The results and conclusions are therefore unchanged.
Included studies
We included seven RCTs, all of which were in English. The included trials involved a total of 2144 participants that were randomised to IB or placebo, and/or no treatment, for the common cold. Included participants ranged between 12 and 70 years of age.
Inclusion criteria
Inclusion criteria were fairly consistent among trials in the diagnosis of the common cold (self diagnosed). Among these seven trials, only one included participants with an experimental infection by an inoculum of rhinovirus type 39 (Gaffey 1988). In this particular study, clinical colds were defined by a modification of the criteria established by Jackson 1958. However, all participants with an infection were included in the analysis, regardless of symptom development.
Exclusion criteria
Common features to exclude participants were fever, allergic rhinitis, sinusitis and asthma, as well as chronic rhinitis. Further details are provided in the Characteristics of included studies table.
Intervention
For the intervention group, IB was used in various doses. The majority of studies used a dose of 84 micrograms per nostril (Dockhorn 1992; Eccles 2007; Hayden 1996). Two other studies used a lower dose of 40 micrograms per nostril (Borum 1981; Gaffey 1988) and one study used a very high dose of 200 micrograms per nostril (Østberg 1997). There was one study that compared three different doses of IB; 42, 84 and 168 micrograms per nostril (Diamond 1995). Dosing frequency also varied between three times a day in four studies (Diamond 1995; Eccles 2007; Gaffey 1988; Hayden 1996) and four times a day in the remaining studies.
Excluded studies
We excluded two studies. Kim 2005 included a paediatric group of children less than five years old and Pitkäranta 1998 because the intervention group used IB + xylometazoline.
Risk of bias in included studies
Risks of bias are illustrated in Figure 1 and Figure 2.
1.
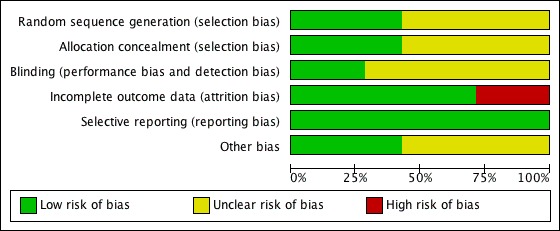
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
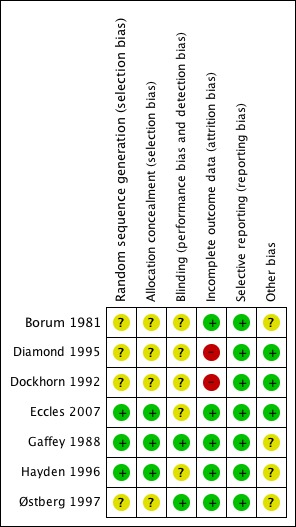
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The allocation process was adequately concealed in only three studies (Eccles 2007; Gaffey 1988; Hayden 1996). For the remaining studies, there was insufficient information to make a conclusive judgement.
Blinding
Two studies were blinded (Gaffey 1988; Østberg 1997). It was unclear in the rest of the studies whether blinding was maintained throughout the study period. It would have been relevant to blind the investigators, as a number of the outcomes were subjective.
Incomplete outcome data
The majority of studies either had no missing data or adequately addressed this. However, there were two studies that did not address missing outcome data and this was considered a potential risk of bias. Dockhorn 1992 enrolled 321 participants; of these, four were not included in the intention‐to‐treat (ITT) analysis and 20 were excluded from the full‐efficacy analysis with no details as to why that was done. Diamond 1995 had 12 participants missing from the analysis with no further information provided.
Selective reporting
All studies included the primary and secondary outcomes described in their introduction and methods.
Other potential sources of bias
Four studies had unclear sources of bias related to their funding source (Borum 1981; Gaffey 1988; Hayden 1996; Østberg 1997). Interestingly, all four were supported by the same pharmaceutical company; Boehringer Ingelheim. Support was either by a grant or supply of the intervention. In one of those studies (Hayden 1996) two of the trial authors worked at Boehringer Ingelheim Pharmaceuticals with no declaration of conflicts of interest. These trials did not provide any further detail of whether their source of funding had any role in the design, data analysis or approval of publishing the study. In the calculations performed, two decimals were taken and rounded to the closest whole number.
Effects of interventions
Different scales, measurements and parameters were used to report the primary outcomes. Therefore, pooling of data using meta‐analysis techniques was not performed. A narrative description of these outcomes is provided below. The only consistent outcome reported in all seven studies was side effects and for this we undertook a meta‐analysis.
Primary outcomes
Change in severity of symptoms of rhinorrhoea
Four studies (including 1959 participants) addressed subjective change in the severity of rhinorrhoea (Diamond 1995; Dockhorn 1992; Eccles 2007; Hayden 1996).
Diamond 1995 assessed this change using a 0 to 10‐point visual analogue scale (VAS). A score of 0 represented no symptoms and a score of 10 represented an unbearable level of discomfort necessitating additional treatment and withdrawal from the trial. Intermediate degrees of discomfort were defined as very mild (doubtful, trivial or just noticeable), mild (present but not uncomfortable), moderate (present and somewhat uncomfortable or annoying) and severe (present and definitely uncomfortable or annoying). Recordings were reported in 943 participants at baseline, hourly for six hours on day 1, as well as for three hours on day 2 of the study. The baseline mean for the VAS scores was estimated to be 6.5 from figure 2 in the study for all five groups; IB 0.12%, IB 0.06%, IB 0.03%, placebo; and the no treatment group. The mean improvement in severity of rhinorrhoea by VAS was reported to be: 3.35, 3.07, 2.92, 2.48 and 1.77, respectively. These changes were all statistically significant with a P < 0.001 when compared to no treatment and P < 0.004 when compared to placebo. We calculated the change from baseline to be 51.5%, 47.2%, 44.9%, 38.2% and 27.2%, respectively (Table 2). The authors concluded that IB was effective in the amelioration of rhinorrhoea and that the magnitude of improvement was dose‐related.
1. Change in severity of rhinorrhoea*.
| Study | Change in severity of rhinorrhoea | IB 0.12%/nostril (168 µg) | IB 0.06%/nostril (84 µg) | IB 0.03%/nostril (42 µg) | Placebo | No treatment |
| Diamond 1995 | Calculated change in the mean of the VAS from baseline for days 1 and 2 | 51.5% | 47.2% | 44.9% | 38.2% | 27.2% |
| Dockhorn 1992 | Improvement between the 2 groups was 22% more in the IB group for days 1 and 2 averaged | N/A | √ | N/A | √ | N/A |
| Eccles 2007 | Calculated change in the mean of the VAS from baseline for day 1 | N/A | 36.4% | N/A | 20.5% | N/A |
| Hayden 1996 | Calculated change in the mean of the VAS from baseline for days 1 and 2 | N/A | 48.2% | N/A | 36.3% | N/A |
IB: ipratropium bromide N/A: not applicable µg: microgram VAS: visual analogue scale
* This table is only to provide tabular presentation of the text under Effects of interventions. It is not meant for comparison among the four studies, as methods used for assessment varied among them.
Dockhorn 1992 used the same VAS described in Diamond 1995. A total of 301 participants marked the rate of severity of their symptoms at baseline and then repeated it on day 1 and 2 at one, two and three hours after administration of IB. Baselines were measured but not reported in the article; also it was unclear if means or other statistical measures were used. Other missing data were the N value for each group. We contacted the trial author to provide this information but no reply was received. We were therefore unable to provide the calculated change in severity. The authors provided comparison in improvement between both groups, which was 19% more in the IB group on day 1 (P < 0.02), 23% more on day 2, and 22% more for the two days averaged (P < 0.02) in favour of IB (Table 2). They concluded that IB significantly reduced rhinorrhoea.
In Eccles 2007, this outcome was reported in 305 participants. Rhinorrhoea severity was scored on a four‐point scale: 0 indicating absent symptoms (no sign/symptom evident); one, mild symptoms (sign/symptom clearly present but minimal awareness and easily tolerated); two, moderate symptoms (definite awareness of sign/symptom that is bothersome but tolerable); three, severe symptoms (sign/symptom that is hard to tolerate; causes interference with activities of daily living and/or sleeping). These scores were recorded in a diary immediately before and three hours after each dose, and then an overall score was made after each 24‐hour period. Baseline scores were recorded in all participants but not presented in the study. We contacted trial authors and they provided the missing information which was a mean of 2.2 for both groups. End value scores were reported and this was estimated from figure 3 of their study. The mean and standard error of the mean (SEM) for the IB group was 1.4 ± 0.05 and 1.75 ± 0.05 for the placebo group (P < 0.0001). We calculated the change in severity of rhinorrhoea to be 0.8 (36.4%) for the IB group and 0.45 (20.5%) for the placebo (Table 2).
Finally, Hayden 1996 reported this outcome for 410 participants. A VAS was used to evaluate symptom severity. This scale ranged from 0 to 10 and included five descriptions of severity: very mild (doubtful, trivial or just noticeable), mild (present but not uncomfortable), moderate (present and somewhat uncomfortable or annoying), severe (present and definitely uncomfortable or annoying) and unbearable (necessitating additional treatment). Baseline scores were recorded and were similar in all three groups with a mean score of 6.5 ± 0.9 (SD). Participants then provided hourly evaluations of the severity of their rhinorrhoea for six hours on day 1, and three hours on day 2 of the study. The hourly improvement from baseline for the first three hours of treatment on study days 1 and 2 combined averaged 3.13 for IB, 2.36 for placebo and 1.76 for the no treatment group. The P value was statistically significant (P < 0.001) for IB compared with placebo and IB compared with no treatment. We calculated percentages for the average improvement from baseline which was 48.2% for the IB group and 36.3% for the placebo group (Table 2).
Change in severity of symptoms of nasal congestion
Four studies addressed subjective change in severity of nasal congestion (Dockhorn 1992; Eccles 2007; Hayden 1996; Østberg 1997).
In Dockhorn 1992, participants were asked to rate the severity of their nasal congestion by using the same VAS described earlier for the severity of rhinorrhoea. No further data were provided in regards to the sample size for each group, the mean for baseline scores or the change in severity. We contacted the trial author but no reply was received. We concluded that there was no significant difference between the two treatment groups.
In Eccles 2007, 304 participants reported this outcome by using the same subjective four‐point scale described earlier for rhinorrhoea severity. Baseline scores were recorded in all participants but not presented in the study. We contacted the trial authors and they provided the missing data which were a mean of 2.2 for both groups. End value scores were reported and this was estimated from figure 4 of their study. The mean ± SEM for both groups was similar at 1.84 ± 0.02. We calculated the change from baseline, which was 0.4 (18.2%) for both groups.
In Hayden 1996, 410 participants performed daily VAS assessments of nasal congestion on the evening of each of the study days. The VAS score was the same one the authors described for rhinorrhoea above. There was no baseline recording and the authors concluded that resolution of nasal congestion did not differ in the IB and control groups (P > 0.2).
Lastly, Østberg 1997 reported nasal blockage in 50 participants. They gave a self assessment score every hour for nasal blockage and a mean symptom index of blockage was calculated for each day as described in Toft 1982. Baseline measures were not recorded and so calculation of change in severity was not possible. The review authors concluded that there was no significant change between the IB and placebo group.
Global assessment of overall improvement
This was only described in two studies (Eccles 2007; Hayden 1996).
In Eccles 2007, the general impression of test treatment was scored on a categorical five‐point rating scale (1 = poor; 2 = fair; 3 = good; 4 = very good; 5 = excellent) after the first 24‐hour period and at the end of the trial. Scores were 2.3 and 2.5 for the IB group after 24 hours and seven days, respectively. For the placebo group, these were 2.1 and 2.3, respectively. After 24 hours, 42.2% of the IB group had a general impression that the test treatment was good, or very good. On the other hand, 29.7% of the placebo group had an impression that it was good, very good or excellent.
In Hayden 1996, this outcome was reported in 410 participants. On each of the two testing days, participants were asked a single global question: "Compared to when you came into the clinic this morning, how was your cold over today's testing period?" One of four categorical responses (much better, better, no difference or worse) was recorded. On study day 5, patients were asked, "Overall, how do you think being in this study helped your cold?" and "Overall, how was this treatment in the relief of your symptoms?".
On study day 1, 87% of IB, 73% of placebo and 57% of the untreated patients rated their condition as much better or better (P = 0.004 for IB compared with placebo; and P < 0.001 for IB compared with no treatment). On day 2, 74% of IB, 61% of placebo and 58% of the untreated group indicated that their condition was much better or better (P = 0.015 for IB compared to placebo; P = 0.004 for IB compared with no treatment). During the last global assessment on day 5, 81% of IB, 65% of placebo and 18% of the untreated group indicated that they felt much better or better (P = 0.003 for IB compared to placebo; P < 0.001 for IB compared with no treatment). For the second question on day 5, 88% of IB compared with 74% of placebo indicated that their treatment had been very useful or useful (P = 0.002).
Secondary outcomes
Adverse events
This was the only outcome that was reported in all seven studies with a total number of 2144 participants. See Figure 3 for details.
3.
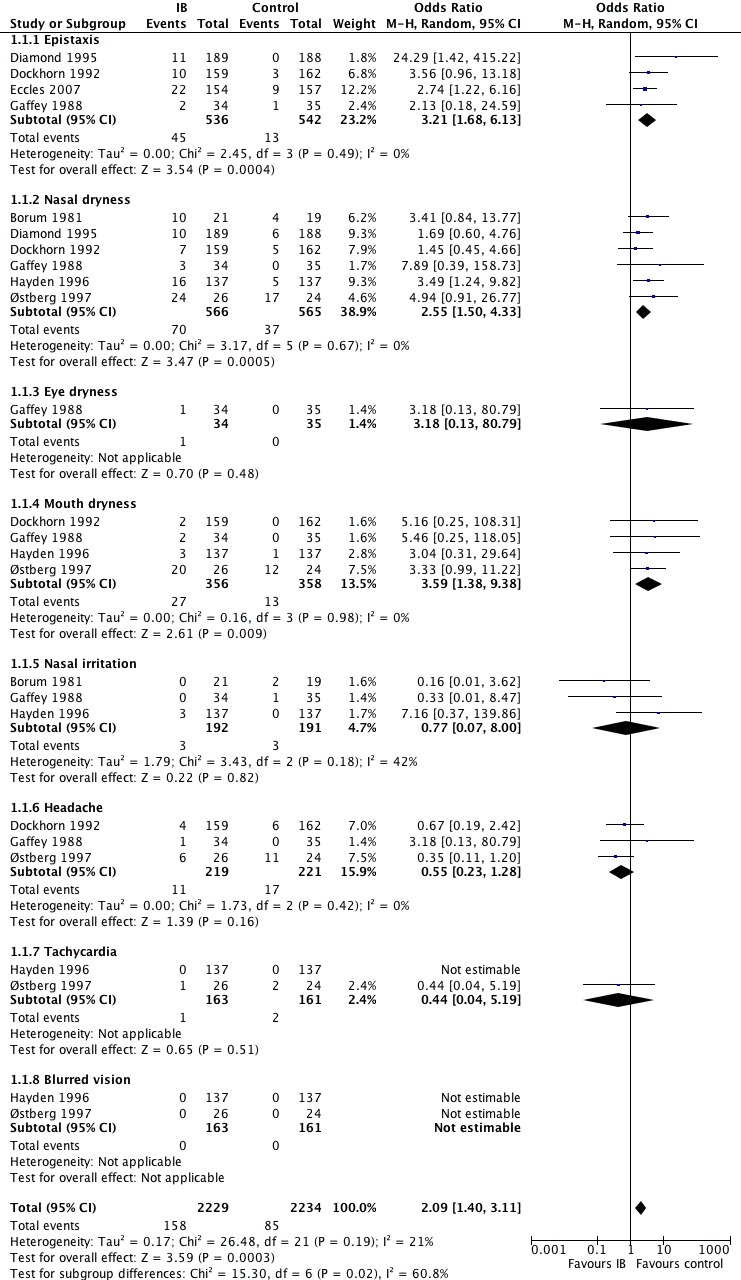
Forest plot of comparison: 1 IB versus placebo, outcome: 1.1 Adverse effects.
Nasal dryness was reported in all seven studies (Analysis 1.1.2). Epistaxis was reported in only four studies (Diamond 1995; Dockhorn 1992; Eccles 2007; Hayden 1996) (Analysis 1.1.1). Four studies reported nasal irritation/burning (Borum 1981; Eccles 2007; Gaffey 1988; Hayden 1996) (Analysis 1.1.5). In Eccles 2007, nasal irritation/burning was described to be more common in the IB group but the number of events were not provided. We contacted the trial author and he replied but was unable to provide the missing information. Headache was another common side effect in four studies (Diamond 1995; Dockhorn 1992; Gaffey 1988; Østberg 1997) (Analysis 1.1.6). We were only able to include the three latter studies in the analysis (Figure 3). Diamond 1995 reported headache to be more frequent in the intervention group but the number of events was not reported. It was therefore not possible to include it in the analysis. We contacted the trial author but no reply was received. Other reported adverse effects included blurred vision (Analysis 1.1.8), tachycardia (Analysis 1.1.7), mouth and eye dryness (Analysis 1.1.4; Analysis 1.1.3), as well as blood‐tinged mucus. Overall, side effects were more common in the intervention group (odds ratio (OR) 2.09; 95% confidence interval (CI) 1.40 to 3.11) (Analysis 1.1).
1.1. Analysis.
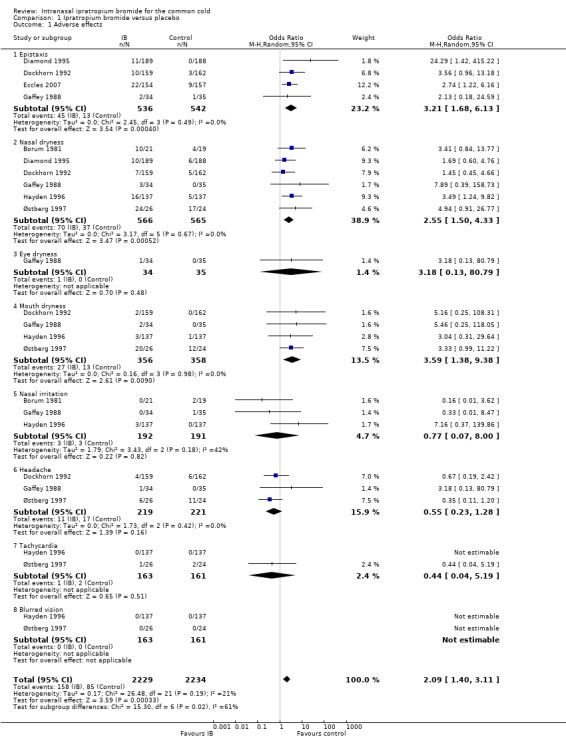
Comparison 1 Ipratropium bromide versus placebo, Outcome 1 Adverse effects.
Discussion
Summary of main results
The most bothersome symptoms associated with the common cold are rhinorrhoea and nasal congestion. The findings of this review showed a consistent trend of intranasal ipratropium (IB) effectiveness in ameliorating rhinorrhoea across the studies. We assessed this outcome using subjective measures, as it was thought to be clinically relevant. Many studies reported other surrogate outcomes such as number of paper tissues used or number of nose blowings (or both) (Borum 1981; Eccles 2007; Gaffey 1988; Østberg 1997). Another reported objective measure was weight of nasal discharge. Both outcomes were found to correlate with the subjective change in rhinorrhoea in Eccles 2007 and were found to favour IB in improving rhinorrhoea. Nasal congestion, on the other hand, was shown to be similar in both the intervention and placebo group, with no significant change.
Global assessment of the effectiveness of IB in improving cold symptoms was in favour of the IB group in one of the two included studies, which was statistically significant (Hayden 1996). On the other hand, Eccles 2007 showed no difference between groups based on the global assessment of effectiveness.
Overall the intervention was well tolerated despite its side effects. Reported withdrawals secondary to side effects were small; 7% in Eccles 2007. A small number of participants were lost to follow‐up in Diamond 1995 and Dockhorn 1992 (1.3% and 6.2%, respectively). No details of the exact reasons were reported.
Different dosing as well as frequency of administrating IB were found across the studies. Three times a day dosing appeared to be sufficient to demonstrate an effect in four of the studies (Diamond 1995; Eccles 2007; Gaffey 1988; Hayden 1996). Four times a day dosing was the frequency of IB administration in the remainder of the studies. This may be associated with fewer side effects. We were unable to perform a subgroup analysis for side effects for different dosages, as reported adverse outcomes were inconsistent among the studies. Common reported side effects of IB included blood‐tinged mucus and epistaxis, as well as mucous membrane dryness. These were self managed by participants and there were no serious adverse events necessitating seeking of medical advice.
Overall completeness and applicability of evidence
All included studies reported the efficacy of the intervention in participants with the common cold compared to a control group (placebo or no treatment). All studies excluded asthmatic patients with a history of allergic rhinitis that might hamper the ability to generalise its overall conclusion.
Only one study (Østberg 1997) utilised a very high IB dosing regimen that is not currently commercially available. All included studies reported at least one outcome relevant to our review and only two studies addressed all our primary and secondary outcomes (Eccles 2007; Hayden 1996). IB is commercially available as a nasal spray in two doses: 0.03% and 0.06%, in which two sprays are equivalent to 42 and 84 micrograms, respectively. Dosing is recommended as two sprays per nostril three or four times a day.
Quality of the evidence
Our review included seven RCTs with a total of 2144 participants. Key methodological limitations included incomplete outcome data in two studies (Diamond 1995; Dockhorn 1992). Details are found under Risk of bias in included studies. Randomisation and allocation concealment were unclear in four studies (Borum 1981; Dockhorn 1992; Diamond 1995; Østberg 1997). Blinding was clearly described in two studies (Gaffey 1988; Østberg 1997), but it was unclear whether participants, personnel or outcome assessors were blinded in the remainder of the studies. In two studies, the extent of involvement of their funding provider, Boehringer Ingelheim Pharmaceutical Company, was not addressed. This would have been relevant given that the company manufactures IB nasal spray (Gaffey 1988; Hayden 1996). In two other studies, the same company provided the intervention and placebo vehicle (Borum 1981; Østberg 1997). The impact of this doubtful issue of financial support on the overall results and conclusions of our review was uncertain. All seven studies showed consistent results favouring IB over placebo for rhinorrhoea. They also all reported side effects to be more frequent in the IB group but that they were generally well tolerated.
Potential biases in the review process
It is likely that all relevant studies were identified, as the search was comprehensive (see Search methods for identification of studies). We also contacted Boehringer Ingelheim Pharmaceuticals to inquire about ongoing and unpublished trials, as well as the authors of included studies to inquire about any ongoing trials. A limitation we came across was the frequent occurrence of missing data as described previously (see Effects of interventions). We contacted trial authors but only one replied (Eccles 2007) and provided the missing data. Another limitation was that most of the authors' correspondence was by mail. Attempts to search for their email addresses through search engines, their educational institutions and other publications were unsuccessful.
We did consider experimental viral infections and one study met the inclusion criteria (Gaffey 1988). In this study, the authors included in their analysis all participants, whom after virus inoculation were determined to be infected by isolation of the virus and presence of homotypic neutralisation antibody titre from paired specimens on day 1 and three weeks later. However, among those infected participants, only 50% of the IB group had clinical colds, compared to 76% of the placebo group. This could overestimate the effect in the IB group given that half of them did not have clinical symptoms. However, this study did not report on any of our primary outcomes and only side effects were considered in the analysis. None of the studies addressed any conflict of interest.
Other potential sources of bias were in reporting side effects. We did not pre‐specify which outcomes we would report and so we took into consideration all reported outcomes. All seven studies reported side effects but were not consistent in the components they reported. We expected all studies to report on epistaxis or blood‐tinged mucus (or both). However, this was not reported in two studies (Borum 1981; Østberg 1997). It is a surprise that it was not reported in Østberg 1997 in particular, given that it utilised a very high dose of IB (200 micrograms/nostril). This study had no dropouts or withdrawals.
Agreements and disagreements with other studies or reviews
The results of this review are in agreement with Graf's systematic review (Graf 2009). The authors concluded that IB was effective in relieving rhinorrhoea in the common cold, and that it was well tolerated with mild to moderate side effects.
Authors' conclusions
Implications for practice.
This review suggests that the use of intranasal ipratropium (IB) in the common cold may be effective in relieving rhinorrhoea. Patients may be informed that if they have runny nose as their predominant symptom, they are likely to benefit more. However, IB has no effect on nasal congestion and its use is associated with more adverse effects. Common side effects include epistaxis, blood‐tinged mucus and mucous membrane dryness. Generally, these side effects are well tolerated. Reasonable dosing of IB includes 0.03% or 0.6% nasal sprays by two sprays per nostril, three to four times per day for the first couple of days of a cold.
Implications for research.
This review suggests that IB nasal spray may be effective in ameliorating rhinorrhoea in the common cold. Some of the studies in the review were outdated and there is a need for larger, high‐quality trials to determine effectiveness and to address the current gap in the literature. It would be important to ensure adequate blinding in future trials given the subjective outcomes being assessed. Different dosages should also be included to determine the most suitable dose: 42, 84 and 168 micrograms/nostril are suggested. Use of standardised visual analogue scales (VAS) to assess change in severity of symptoms from baseline would be more relevant clinically and would improve the applicability of the results.
Nasal congestion remains another bothersome symptom that is not altered by IB. Xylometazoline, an alpha blocker, has been shown in a systematic review to be effective in improving nasal congestion (Eccles 2010). A number of trials have proposed a combination of IB with xylometazoline to resolve both those symptoms in one vehicle (Eccles 2007; Pitkäranta 1998). This seems to be a convenient and practical solution for symptomatic relief. A systematic review comparing IB and xylometazoline combination therapy to placebo would be needed to determine their efficacy and tolerability in the common cold. It would be important for primary outcomes to take into account subjective measures of improvement in both rhinorrhoea and nasal congestion, as well as overall improvement.
What's new
| Date | Event | Description |
|---|---|---|
| 9 April 2013 | New citation required but conclusions have not changed | No new trials were included or excluded in this update. Our conclusions remain unchanged. |
| 9 April 2013 | New search has been performed | Searches updated. |
Acknowledgements
Special thanks to Clare Dooley for her assistance in registering this review's title. We would also like to thank the ARI Group's Trials Search Co‐ordinator, Sarah Thorning, for her valuable additions to the electronic search section. Finally, we wish to thank the following people for commenting on the draft protocol: Felix Ram, Emin Ünüvar, Owen Hendley, Max Bulsara and Susan Smith; and the following people for commenting on the draft review: Hanan Khalil, Lorraine Johnson, Emin Ünüvar, Owen Hendley, Mark Jones and Susan Smith.
Appendices
Appendix 1. Embase.com search strategy
30. #22 AND #29 29. ((nose OR nasal*) NEAR/5 (spray* OR drop* OR irrigat* OR atomis* OR nebulis* OR aerosol* OR vapour* OR vapor*)):ab,ti 28. intranasal*:ab,ti OR endonasal*:ab,ti 27. 'intranasal drug administration'/de 26. #22 AND #25 25. #23 OR #24 24. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR ((singl* OR doubl*) NEAR/2 (blind* OR mask*)):ab,ti 23. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 22. #16 AND #21 21. #17 OR #18 OR #19 OR #20 20. anticholinergic*:ab,ti OR atrovent*:ab,ti 19. 'cholinergic receptor blocking agent'/exp 18. ipratropium*:ab,ti 17. 'ipratropium bromide'/de 16. #1 OR #2 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 15. (acute NEAR/2 (nasopharyng* OR rhinopharyng*)):ab,ti 14. (respiratory NEAR/2 infection*):ab,ti 13. 'respiratory tract infection'/de OR 'rhinopharyngitis'/de OR 'upper respiratory tract infection'/de OR 'nose infection'/de 12. coronavirus*:ab,ti 11. 'coronavirus'/de 10. rsv:ab,ti 9. ('respiratory syncytial' NEAR/2 virus*):ab,ti 8. 'rhinovirus infection'/de 7. 'respiratory syncytial pneumovirus'/de 6. adenovir*:ab,ti OR rhinovir*:ab,ti 5. 'adenovirus'/de OR 'adenovirus infection'/de 4. 'human rhinovirus'/de 2. coryza:ab,ti OR 'common cold':ab,ti OR 'common colds':ab,ti 1. 'common cold'/exp
Appendix 2. AMED search strategy
1 common cold/ 2 common cold*.tw. 3 coryza.tw. 4 ((nasopharyng* or rhinopharyng*) adj3 acute).tw. 5 (rhinovir* or adenovir*).tw. 6 (respiratory syncytial virus* or rsv).tw. 7 coronavirus*.tw. 8 respiratory tract infections/ 9 (respiratory tract infection* or respiratory infection*).tw. 10 or/1‐9 11 ipratropium.ti,ab. 12 anticholinergic*.ti,ab. 13 (cholinergic adj2 antagonist*).ti,ab. 14 or/11‐13 15 10 and 14
Appendix 3. Biosis Previews search strategy
Topic=(common cold* or coryza* or rhinovir* or adenovir* or nasopharyngit* or rhinopharyngit* or respiratory syncytial virus* or rsv or coronavir* or respiratory tract infection* or upper respiratory infection*) AND Topic=(ipratropium or anticholinergic* or "cholinergic antagonist*" or atrovent)
Refined by: Topic=(random* or placebo* or allocat* or assign* or single blind* or double blind*)
Appendix 4. LILACS search strategy
| Database: | LILACS |
| Search on: | (mh:"Common Cold" OR "common cold" OR "common colds" OR "Resfriado Común" OR "Resfriado Comum" OR coryza OR mh:nasopharyngitis OR nasofaringitis OR nasofaringite OR nasopharyngitis OR rhinopharyngitis OR mh:rhinovirus OR rhinovirus* OR mh:adenoviridae OR adenovir* OR mh:"Adenoviridae Infections" OR mh:"Adenovirus Infections, Human" OR mh:"Respiratory Syncytial Viruses" OR "respiratory syncytial viruses" OR "respiratory syncytial virus" OR "Virus Sincitiales Respiratorios" OR "Vírus Sinciciais Respiratórios" OR mh:"Respiratory Syncytial Virus, Human" OR "Virus Sincitial Respiratorio" OR "Vírus Sincicial Respiratório Humano" OR mh:"Respiratory Syncytial Virus Infections" OR "Infecciones por Virus Sincitial Respiratorio" OR "Infecções por Vírus Respiratório Sincicial" OR rsv OR mh:coronavirus OR coronavirus* OR mh:"Coronavirus Infections" OR "Infecciones por Coronavirus" OR "Infecções por Coronavirus" OR mh:"Respiratory Tract Infections" OR "respiratory infection" OR "respiratory infections" OR "respiratory tract infection" OR "respiratory tract infections" OR "Infecciones del Sistema Respiratorio" OR "Infecções Respiratórias" OR "Infecciones del Tracto Respiratorio Superior" OR "Infecciones de las Vías Respiratorias Superiores" OR "Infecções do Trato Respiratório Superior" OR "Infecções das Vias Respiratórias Superiores" OR "Infecções do Sistema Respiratório Superior") AND (mh:ipratropium OR ipratropium OR ipratropio OR mh:"Cholinergic Antagonists" OR mh:d27.505.519.625.120.200* OR mh:d27.505.696.577.120.200* OR "Antagonistas Colinérgicos" OR "Antagonistas Colinérgicos" OR anticholinergic* OR atrovent* OR "Antagonistas de la Acetilcolina" OR "Agentes Anticolinérgicos" OR "Agentes Bloqueadores Colinérgicos" OR "Antagonistas da Acetilcolina" OR "Agentes Anticolinérgicos" OR "Agentes Bloqueadores Colinérgicos") AND db:("LILACS") |
Data and analyses
Comparison 1. Ipratropium bromide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects | 7 | 4463 | Odds Ratio (M‐H, Random, 95% CI) | 2.09 [1.40, 3.11] |
| 1.1 Epistaxis | 4 | 1078 | Odds Ratio (M‐H, Random, 95% CI) | 3.21 [1.68, 6.13] |
| 1.2 Nasal dryness | 6 | 1131 | Odds Ratio (M‐H, Random, 95% CI) | 2.55 [1.50, 4.33] |
| 1.3 Eye dryness | 1 | 69 | Odds Ratio (M‐H, Random, 95% CI) | 3.18 [0.13, 80.79] |
| 1.4 Mouth dryness | 4 | 714 | Odds Ratio (M‐H, Random, 95% CI) | 3.59 [1.38, 9.38] |
| 1.5 Nasal irritation | 3 | 383 | Odds Ratio (M‐H, Random, 95% CI) | 0.77 [0.07, 8.00] |
| 1.6 Headache | 3 | 440 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.23, 1.28] |
| 1.7 Tachycardia | 2 | 324 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.04, 5.19] |
| 1.8 Blurred vision | 2 | 324 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Borum 1981.
| Methods | Randomised controlled trial | |
| Participants | Number randomised: 41 (1 was excluded because he lost his completed symptom card), 21 in treatment group and 19 in placebo Setting: out‐patient, Denmark Study period: October to November 1979 Age: treatment group mean age 23.5 (21 to 38), placebo group mean age 24.6 (21 to 28) Sex: 12 men and 9 women in the treatment group,10 men and 9 women in the placebo group Diagnostic criteria: participants felt confident that they had caught a cold and had sudden onset of rhinorrhoea and attacks of sneezing and/or nasal blockage < 24 hours Inclusion criteria: medical students who feel confident that they had caught a cold and have sudden onset of rising rhinorrhoea and attacks of sneezing and/or nasal blockage for less than 24 hours, had no history of allergic, chronic or recurrent airway disease or frequent complications to common colds (sinuitis or bronchitis), were able to produce enough macroscopic non‐purulent secretion for microscopic examination (at least 1.0 ml), had no eosinophilia in the secretion (more than 10% of all leukocytes) and gave informed consent to participate Exclusion criteria: any participant who did not fulfil the inclusion criteria Aetiology: natural |
|
| Interventions | Intervention: ipratropium was given from a Freon‐propelled pressurised canister used for inhalation therapy, it was equipped with a nasal adapter. The dose was 1 puff of 20 micrograms in the upper and 1 puff of 20 micrograms in the lower part of each nostril 4/day for 7 days Control: the placebo aerosol contained Freon alone. The dose was 1 puff of 20 micrograms in the upper and 1 puff of 20 micrograms in the lower part of each nostril 4/day for 7 days Note: a vasoconstrictor spray (xylometazoline) was taken 5 min before each medication of both groups of subjects in order to ensure adequate mucosal distribution |
|
| Outcomes | Primary: number of paper handkerchiefs used per day Other outcomes reported: number of sneezes per day, type of nasal secretion and side effects |
|
| Notes | Funding source: unclear, Boehringer Ingelheim supplied the Atrovent and placebo aerosols Conflict of interest: not stated Calculated sample size: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Subjects were allocated at random to the ipratropium group or to the placebo group" Comment: insufficient information to judge |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not addressed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "one was rejected later because he lost his completed symptom card" Comment: no further missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods appear clearly in the results section |
| Other bias | Unclear risk | Atrovent and placebo aerosols were provided by Boehringer Ingelheim; no further details provided in regards to their involvement in the study or not |
Diamond 1995.
| Methods | Randomised controlled trial (5‐arm study, 3 groups received IB treatment with different concentrations and the other 2 were control groups who received placebo or no treatment) | |
| Participants | Number randomised: 955 (only 943 completed the study), 568 in treatment groups (188, 189 and 191 in groups receiving IB 0.12%, 0.06% and 0.03%), 375 in control group (188 vehicle and 187 no treatment) Setting: out‐patient. The study was conducted at 6 geographically diverse sites in the United States Study period: 30 October 1990 to 29 March 1991 Age: range 12 to 70 years, mean age is 27.5 for all groups Sex: 492 male and 463 female Diagnostic criteria: subjective reporting of a moderate or greater degree of rhinorrhoea present for no more than 36 hours Inclusion criteria: (1) swollen, erythematous nasal mucosal membranes on visual examination; (2) subjective reporting of a moderate or greater degree of rhinorrhoea present for no more than 36 hours; and (3) production of 1.5 g or more of nasal discharge during a 1‐hour baseline measurement period Exclusion criteria: participants were excluded from the study if they had significant cardiovascular, renal, hepatic, endocrine, metabolic, neurologic, pulmonary or other systemic disease or if they had a history of asthma or other chronic respiratory disease, perennial rhinitis, nasal polyps, glaucoma or unresolved prostatic hypertrophy. Participants with allergic rhinitis were excluded if their allergen was in season or ubiquitous in their environment (for example, animal dander). A positive Streptococcus culture, the presence of rales or rhonchi indicative of a lower respiratory tract infection, an oral temperature of higher than 102°F, or a history of frequent complications such as sinusitis or bronchitis arising from an upper respiratory tract infection. Also excluded were lactating women, with positive results of a pregnancy test, of childbearing potential who were not using a medically approved method of contraception, persons judged incapable of accurately maintaining a symptom record form and individuals who could not tolerate anticholinergic drugs or who were sensitive to the preservative present in the IB nasal spray vehicle; benzalkonium chloride Aetiology: natural |
|
| Interventions | Treatment: each participant got a nasal spray which delivered 0.07 ml per actuation. Three concentrations of IB nasal spray were used: 0.03%, 0.06% and 0.12%. The dose of each concentration was 2 sprays (yielding 42, 84 or 168 micrograms) in each nostril 3 times daily for 4 days Control: 2 groups; 1 received a vehicle (a buffered saline solution) nasal spray that delivered 0.07 ml/actuation, dose was 2 sprays 3/day for 4 days. The other group received no treatment |
|
| Outcomes | 2 primary efficacy endpoints were selected to detect changes in rhinorrhoea: nasal discharge weights and in‐clinic VAS scores Secondary efficacy endpoints were the daily at‐home VAS scores for rhinorrhoea, sneezing and nasal congestion Other outcomes reported: adverse events |
|
| Notes | Funding source: not stated Conflict of interest: not stated Calculated sample size: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were randomly assigned to receive either no treatment, vehicle treatment, or treatment with IB nasal spray." Comment: insufficient information to judge |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk |
Quote: "The study was double‐blind with respect to IB and vehicle" Comment: insufficient information to judge |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 patients were not included in data analysis and no further information provided |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods were mentioned in the results section |
| Other bias | Low risk | |
Dockhorn 1992.
| Methods | Randomised controlled trial | |
| Participants | Number randomised: 321 participants, 159 in the treatment group and 162 in the placebo group (317 were included in ITT analysis and 301 in full efficacy analysis) Setting: out‐patient, multicentre, USA Study period: not stated Age: mean age 29 Sex: 144 male and 177 female Diagnostic criteria: moderate rhinorrhoea for less than 36 hours Inclusion criteria: moderate rhinorrhoea for less than 36 hours and swollen, erythematous nasal mucosa on examination Exclusion criteria: significant cardiovascular, renal, hepatic, endocrine, metabolic, neurologic, pulmonary or other systemic disease. History of asthma or other chronic respiratory disease, perennial rhinitis, nasal polyps, glaucoma, or unresolved prostatic hypertrophy. Participants with allergic rhinitis were excluded if their allergen was in season or ubiquitous in their environment (for example, animal dander). A positive Streptococcus culture, the presence of rales or rhonchi indicative of a lower respiratory tract infection, an oral temperature of higher than 102°F, or a history of frequent complications such as sinusitis or bronchitis arising from an upper respiratory tract infection. Lactating women, women with positive results of a pregnancy test, of childbearing potential who were not using a medically approved method of contraception. Persons judged incapable of accurately maintaining a symptom record form, and individuals who could not tolerate anticholinergic drugs or who were sensitive to the preservative present in the IB nasal spray vehicle, benzalkonium chloride Aetiology: natural |
|
| Interventions | Treatment: nasal spray, each delivered 42 micrograms of IB; at each dose 2 sprays were delivered to each nostril for a total of 84 micrograms per nostril per dose, taken 4/day for 4 days Control: placebo nasal spray 4/day for 4 days |
|
| Outcomes | Primary endpoints to evaluate change in rhinorrhoea: average weight of nasal discharge over 3 hours on days 1 and 2 of drug administration, change from baseline in patients' evaluation of rhinorrhoea symptoms using VAS over 3 hours on days 1 and 2 and for both days' average versus baseline and change from baseline in rhinorrhoea symptoms as evaluated by participants using VAS, averaged during days 1 and 2 and separately averaged during days 3 and 4 Secondary: VAS for sneezing and nasal congestion, averaged separately for days 1 and 2, then days 3 and 4 Other outcomes reported: adverse events and nasal examination changes |
|
| Notes | Funding source: not stated Conflict of interest: not state Calculated sample size: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "before randomizations to either drug or placebo..." Comment: insufficient information to judge |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk |
Quote: "The study was a double‐blind..." Comment: insufficient information to judge |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "321 participants were enrolled (159 received IB and 162 received placebo). Of these, 317 were included in the intent‐to‐treat analysis, and 301 were included in the full efficacy analysis." Comment: no explanation for missing data and dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes sated in the methods appear clearly in the results section |
| Other bias | Low risk | |
Eccles 2007.
| Methods | Randomised controlled trial (5‐arm study, 2 groups received combination of IB and xylometazoline with different concentrations; the other 3 groups received: IB alone, xylometazoline alone or no treatment) | |
| Participants | Number randomised 786 (number completed the study 703)
Intervention group receiving IB: 154, control group receiving no treatment: 157 Setting: out‐patient, multicentre based in the United Kingdom, Denmark, Finland, Norway and Sweden Study period: not stated Age: 18 or older, mean age of intervention group 28.9 (18 to 59) and mean age of control group 30 (18 to 70) Sex: 85 female and 69 male in the intervention group, 73 female and 84 male in the control group Diagnostic criteria: history of common cold symptoms of 36 hours Inclusion criteria: age 18 years or older, history of common cold symptoms of 36 hours, a rhinorrhoea score of at least 2 (moderate) and a nasal congestion score of at least 2 (moderate) Exclusion criteria: evidence or history of hypersensitivity to study medications, significant cardiovascular or endocrine disorders, perennial allergic rhinitis, nasal polyps or significant nasal abnormalities. Narrow‐angle glaucoma, prostatic hypertrophy, sinusitis, bronchitis, pregnancy or lactation, inadequate contraception over trial period in fertile females. Rhinitis medicamentosa, use of nasal decongestants or antisecretory medicines within the last week, use of monoamine oxidase inhibitors within the previous month, use of tricyclic antidepressants. Rhinitis that required medical attention, and/or extensive physical activity during treatment Aetiology: natural |
|
| Interventions | Intervention group: ipratropium, 0.6 mg/ml Control group: placebo solution For all groups, the test treatments were formulated as preservative‐free aqueous solutions in nasal multiple‐dose spray devices consisting of a glass bottle (Fiolax brown type I), a non‐vented pump and an actuator. The nasal spray had a declared volume of 10 ml and delivered a dose of 140 microlitre. Participants took 1 spray in each nostril 3/day |
|
| Outcomes | The primary efficacy variables were subjective ratings of severity of rhinorrhoea and nasal congestion after the first 24‐hour period Secondary efficacy variables for rhinorrhoea: tissue use over the first 24 hours Other outcomes: general impression score of test treatment after the first 24‐hour period and at the end of the study and adverse events |
|
| Notes | Funding source: not stated Conflict of interest: not stated Calculated sample size: yes. The sample size was based on the 2 independent primary end points, rhinorrhoea and nasal congestion, during the first 24 hours. Participants per group: 150 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "patients were allocated to treatment according to a randomised sequence prepared by the Nycomed Company" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote: "Patients were allocated to treatment according to a randomisation sequence prepared by the Nycomed Co. The randomisation key was kept in sealed envelopes, only to be opened in case of emergency." Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk |
Quote: "The test formulations were in identical spray bottles to blind the study." Comment: insufficient information regarding blinding the investigators and analysts |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "Eighty‐three patients did not complete the study; 55 patients withdrew due to adverse events as listed previously, 8 patients withdrew because of lack of effect of their medication, 11 patients did not return diaries, 4 patients were excluded as noncompliant, and 5 patients withdrew without giving any reason. There were no serious adverse events." Comment: incomplete data and dropouts addressed |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods are mentioned in the results section |
| Other bias | Low risk | |
Gaffey 1988.
| Methods | Randomised controlled trial | |
| Participants | Number randomised: 69 subjects, 34 in the intervention group and 35 in the placebo group Setting: hotel rooms, Virginia, USA Study period: not stated Age: not reported (young adults) Sex: male Diagnostic criteria: volunteers whom after virus inoculation had clinical colds defined by a modification of the criteria established by (Jackson 1958) Inclusion criteria: healthy young adult male volunteers with titers of neutralising antibody to rhinovirus type 39 in serum of < 1:2 Exclusion criteria: upper respiratory tract infection or fever of unknown origin within 1 week of the study; concurrent use of oral or intranasal medication; and histories of atopy, sinusitis, asthma, chronic rhinitis and chronic medical illness Aetiology: experimental cold |
|
| Interventions | Treatment: ipratropium formulated in a buffered saline solution to a final concentration of 0.03%. Each spray delivers 20 micrograms, dose was 2 sprays per nostril 3/day. The total ipratropium doses were 80 micrograms per treatment Control: a vehicle solution served as placebo Both formulations were self administered under observation as 2 sprays per nostril 3 times daily for 5 days beginning 24 hours after virus challenge |
|
| Outcomes | Developing infection and clinical cold, rhinorrhoea subjective score, weight of nasal discharge, number of paper tissue used and adverse effects | |
| Notes | Funding source: supported in part by grants from Boehringer Ingelheim Pharmaceuticals, Inc. Conflict of interest: not stated Calculated sample size: yes (but the number calculated was not stated) Quote: "The sample size was calculated from nasal mucus weight data from an earlier study testing a similar drug to provide statistical power of 80% for detecting a reduction of 40% in mucus weights at an alpha value of 0.05." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Volunteers were randomly assigned to either treatment or placebo groups by a table of random numbers" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote: "Both formulations were supplied in consecutively numbered, identical‐appearing metered‐spray devices" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote: "Staff members responsible for recording clinical symptoms, collecting clinical samples, and weighing mucus were blind as to the treatment status of the volunteers." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes addressed in the introduction appear clearly in the results |
| Other bias | Unclear risk |
Quote: "This work was supported in part by grants from Boehringer Ingelheim Pharmaceuticals, Inc" Comment: it is unclear if the funding source did or did not have any role in developing the protocol, approving the final results or even the results. There is insufficient detail to judge whether it is or is not a source of bias |
Hayden 1996.
| Methods | Randomised controlled trial | |
| Participants | Number randomised: 411 (only one was excluded from ITT; this was an untreated patient who was disqualified because of a history of frequent complications during upper respiratory infections)
IB group: 137, control group: 137, no treatment group: 137 Setting: out‐patient (3 university student health services), USA Study period: 1 November 1993 to 29 March 1994 Age: 12 to 79 years, mean age of all groups is 22 years Sex: male and female Diagnostic criteria: history of rhinorrhoea associated with a common cold for no more than 36 hours Inclusion criteria: the rhinorrhoea had to be scored as at least moderate in severity (a score of 5 or more on a visual analogue scale) and the severity had to be confirmed by the recovery of at least 1.5 g of nasal discharge over a 1‐hour baseline observation period Exclusion criteria: participants with a history of asthma or chronic respiratory disease, allergic or perennial rhinitis, nasal polyps, seasonal allergic rhinitis with allergen in season, or frequent complications associated with upper respiratory infections (such as sinusitis or bronchitis). Pregnant and lactating women. Participants with a positive result on a streptococcal antigen screening test (Q‐Test‐Strep, Becton‐Dickinson, Cockeysville, Maryland), signs of lower respiratory tract disease, or an oral temperature greater than 102°F were also excluded from participating Aetiology: natural |
|
| Interventions | Treatment: IB nasal spray 0.06% in a buffered salt solution (2 sprays per nostril, administered with a metered pump spray bottle designed to deliver a total dose of 84 micrograms per nostril); 3 to 4/day for 4 days Control: nasal spray, which contained the same excipients as the IB spray but did not contain IB; administered 3 to 4/day for 4 days Third group: no treatment group |
|
| Outcomes | Primary end point for efficacy: patient's global assessment of overall improvement obtained on study days 1, 2 and 5 Secondary measures of efficacy included: quantitation of nasal discharge weights, subjective assessments of rhinorrhoea severity on the first 2 study days and assessment of the severity of cold symptoms made using the visual analogue scale on the evening of each of the first 4 study days Other outcomes reported: adverse events |
|
| Notes | Funding source: yes, supported in part by grants from Boehringer Ingelheim Pharmaceuticals, Inc" Conflict of interest: not stated Calculated sample size: yes Quote: "The sample size for this trial was determined on the basis of global assessments that required the patients to choose one of four ordinal categories (much better, better, no difference, or worse) to describe the amelioration or lack of amelioration of their cold. By comparing the percentage of patients in the ipratropium group who chose either "much better" or "better" with the percentage of patients in the control group who chose these responses, we determined that a sample size of 130 patients per group would be sufficient to detect 20% differences in proportions ranging from 20% versus 40% to 70% versus 90% between the control and ipratropium groups, respectively, with a 90% power or greater (alpha = 0.05). The same sample size had a similar power to detect 20% differences in the mean visual analogue scale assessments of severity of rhinorrhoea (alpha = 0.05)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Patients who met the entrance criteria were randomly assigned by a computer‐generated randomizations sequence to one of three groups..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote: "The ipratropium and control spray bottles were identical in appearance. To minimize unintentional bias in assigning patients to receive no treatment, empty bottles were used as place holders for these patients. The bottles were placed in sequence with the filled bottles and were labelled "Do Not Dispense to Patient." Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk |
Quote: "The treatments were administered under double‐blind conditions; the untreated group was not blinded." Comment: participants blinded, but insufficient information to judge about investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "only one was excluded from ITT; this was an untreated patient who was disqualified because of a history of frequent complications during upper respiratory infections." "Two ipratropium recipients were lost to follow‐up (one on study day 2 and one on study day 5), but all patients completed the first 2 days of the trial and were included in the analysis." Comment: no other missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes addressed in the methods appear clearly in the results |
| Other bias | Unclear risk | It was supported in part by a grant from Boehringer Ingelheim pharmaceuticals; also 2 of the authors are affiliated with the company that manufactures the intervention studied. No further detail provided on what roles they had in approving the protocol, analysing data or approving the final draft |
Østberg 1997.
| Methods | Randomised controlled trial | |
| Participants | Number enrolled: 50 participants, 26 in treatment group and 24 in control group Setting: out‐patient, Denmark Study period: not stated Age: mean 30.7 (range 20 to 55) Sex: 33 women and 17 men Diagnostic criteria: subjects felt confident that they had caught a cold and had suffered from rhinorrhoea and attacks of sneezing for less than 24 h (throat symptoms less than 36 h) Inclusion criteria: were able to produce at least 0.2 ml of nasal secretion during a 15‐min observation period, showed obvious signs of rhinitis during observation period (red nose, sneezing, discharge, blockage, rhinolalia) and gave informed consent to participate Exclusion criteria: allergic disease, chronic or recurrent airway disease, or frequent complications to common colds (otitis media, sinusitis, bronchitis). Use of other medications Aetiology: natural |
|
| Interventions | Intervention: IB as a micronised powder, was propelled by CFC gas (Freon) from a pressurised canister, equipped with a nasal adaptor. The dose was 2 puffs each of 100 micrograms IB in each nostril 4/day for 3 days Control: placebo (no further details) |
|
| Outcomes | Number of sneezes and nose blowing, weight of nasal discharge, physical characteristics of secretions and side effects | |
| Notes | Funding source: unclear, coded aerosols were provided by Boehringer Ingelheim Conflict of interest: not stated Calculated sample size: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "The patients were allocated at random to treatment with IB or placebo" Comment: insufficient information to judge |
| Allocation concealment (selection bias) | Unclear risk | Insufficient data |
| Blinding (performance bias and detection bias) All outcomes | Low risk |
Quote: "The trial was performed as a double‐blind, placebo‐controlled, randomised study", "Coded aerosols were provided by Boehringer Ingelheim", "..was carried out by two of the investigators before the code was broken." Comment: probably done and maintained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Quote: "All patients who entered the study completed the treatment and were included in the assessment of effects and side effects." Comment: no missing data |
| Selective reporting (reporting bias) | Low risk | All outcomes addressed in the methods are present in the results section |
| Other bias | Unclear risk |
Quote: "Coded aerosols were provided by Boehringer Ingelheim" Comment: no further details provided in regards to their involvement in the study or not |
CFC: chlorofluorocarbon Co.: company °F: degrees Fahrenheit h: hour IB: ipratropium bromide Inc.: incorporation ITT: intention‐to‐treat min: minute ml: millilitre USA: United States of America VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kim 2005 | Included a paediatric group of < 5 years, which was part of our exclusion criteria |
| Pitkäranta 1998 | The intervention group included the combination of IB + xylometazoline |
IB: ipratropium bromide
Differences between protocol and review
For the search methods, the strategy has been modified to include two more databases for the primary search run in 2010: LILACS and Biosis, as well as the MEDLINE in‐process and other non‐indexed citations. For this 2013 update, we no longer had access to Biosis and so this was excluded from the search run in April 2013.
Contributions of authors
AlBalawi ZH: protocol development, selection of studies, quality assessment, data extraction, data analysis, writing the manuscript. AlFaleh K: protocol development, critical revision of the content, final editing and approval of the protocol and review manuscripts. Othman SS: selection of studies, quality assessment, data extraction, writing the manuscript.
Sources of support
Internal sources
None provided, Not specified.
External sources
None provided, Not specified.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Borum 1981 {published data only}
- Borum P, Olsen L, Winther B, Mygind N. Ipratropium nasal spray: a new treatment for rhinorrhea in the common cold. American Review of Respiratory Disease 1981;123:418‐20. [DOI] [PubMed] [Google Scholar]
Diamond 1995 {published data only}
- Diamond L, Dockhorn R, Grossman J, Kisicki J, Posner M, Zinny M, et al. A dose‐response study of the efficacy and safety of ipratropium bromide nasal spray in the treatment of the common cold. Journal of Allergy and Clinical Immunology 1995;95:1139‐46. [DOI] [PubMed] [Google Scholar]
Dockhorn 1992 {published data only}
- Dockhorn R, Grossman J, Posner M, Zinny M, Tinkleman D. A double‐blind, placebo‐controlled study of the safety and efficacy of ipratropium bromide nasal spray versus placebo in patients with the common cold. Journal of Allergy and Clinical Immunology 1992;90:1076‐82. [DOI] [PubMed] [Google Scholar]
Eccles 2007 {published data only}
- Eccles R, Pedersen A, Regberg D, Tulento H, Borum P, Stjärne P. Efficacy and safety of topical combinations of ipratropium and xylometazoline for the treatment of symptoms of runny nose and nasal congestion associated with acute upper respiratory tract infection. American Journal of Rhinology 2007;21:40‐5. [DOI] [PubMed] [Google Scholar]
Gaffey 1988 {published data only}
- Gaffey M, Hayden F, Boyd J, Gwaltney J. Ipratropium bromide treatment of experimental rhinovirus infection. Antimicrobial Agents and Chemotherapy 1988;32:1644‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayden 1996 {published data only}
- Hayden F, Diamond L, Wood P, Korts D, Wecker M. Effectiveness and safety of intranasal ipratropium bromide in common colds. Annals of Internal Medicine 1996;125:89‐97. [DOI] [PubMed] [Google Scholar]
Østberg 1997 {published data only}
- Østberg B, Winther B, Borum P, Mygind N. Common cold and high‐dose ipratropium bromide: use of anticholinergic medication as an indicator of reflex‐mediated hypersecretion. Rhinology 1997;35(2):58‐62. [PubMed] [Google Scholar]
References to studies excluded from this review
Kim 2005 {published data only}
- Kim KT, Kerwin E, Landwehr L, Bernstein JA, Bruner D, Harris D, et al. Use of 0.06% ipratropium bromide nasal spray in children aged 2 to 5 years with rhinorrhea due to a common cold or allergies. Annals of Allergy Asthma and Immunology 2005;94(1):73‐9. [DOI] [PubMed] [Google Scholar]
Pitkäranta 1998 {published data only}
- Pitkäranta A, Wecker MT, Korts DC, Hayden FG. Combined intranasal ipratropium bromide and oxymetazoline in experimental rhinovirus infection. American Journal of Rhinology 1998;12(2):125‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Arroll 2013
- Arroll B, Kenealy T. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD000247.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eccles 2010
- Eccles R, Martensson K, Chen S. Effects of intranasal xylometazoline, alone or in combination with ipratropium, in patients with common cold. Current Medical Research & Opinion 2010;26(4):889‐99. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fendrick 2003
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non‐influenza‐related viral respiratory tract infection in the United States. Archives of Internal Medicine 2003;163(4):487‐94. [DOI] [PubMed] [Google Scholar]
Graf 2009
- Graf P, Eccles R, Chen S. Efficacy and safety of intranasal xylometazoline and ipratropium in patients with common cold. Expert Opinion on Pharmacotherapy 2009;10(5):889‐908. [DOI] [PubMed] [Google Scholar]
Guyatt 1991
Heikkinen 2003
- Heikkinen T, Jarvinen A. The common cold. Lancet 2003;361(9351):51‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemilä 2013
- Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD000980.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Jackson 1958
- Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. 1. The common cold as a clinical entity. Archives of Internal Medicine 1958;101:267‐8. [DOI] [PubMed] [Google Scholar]
Kim 2009
- Kim SY, Chang Y‐J, Cho HM, Hwang Y‐W, Moon YS. Non‐steroidal anti‐inflammatory drugs for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD006362.pub2] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Patrick 1993
- Patrick DL, Erickson P. Health Status and Health Policy. Quality of Life in Health Care Evaluation and Resource Allocation. New York and Oxford: Oxford University Press, 1993. [Google Scholar]
Singh 2011
- Singh M, Das RR. Zinc for the common cold. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD001364.pub3] [DOI] [PubMed] [Google Scholar]
Stewart 1992
- Stewart AL, Ware JE Jr. Measuring Functioning and Well‐being: the Medical Outcomes Study Approach. Durham and London: Duke University Press, 1992. [Google Scholar]
Toft 1982
- Toft A, Wihl J‐A, Toxman J, Mygind N. Double‐blind comparison between beclomethasone dipropionate as aerosol and as powder in patients with nasal polyposis. Clinical Allergy 1982;12:391‐401. [DOI] [PubMed] [Google Scholar]
Tyrrell 1993
- Tyrrell DA, Cohen S, Schlarb JE. Signs and symptoms in common colds. Epidemiology and Infection 1993;111(1):143‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2009
- Zhang X, Wu T, Zhang J, Yan Q, Xie L, Liu GJ. Chinese medicinal herbs for the common cold. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004782.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
AlBalawi 2011
- AlBalawi ZH, Othman SS, AlFaleh K. Intranasal ipratropium bromide for the common cold. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD008231.pub2] [DOI] [PubMed] [Google Scholar]


