Abstract
Background
Many strategies are in use with the intention of preventing or minimising delayed onset muscle soreness and fatigue after exercise. Cold‐water immersion, in water temperatures of less than 15°C, is currently one of the most popular interventional strategies used after exercise.
Objectives
To determine the effects of cold‐water immersion in the management of muscle soreness after exercise.
Search methods
In February 2010, we searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register, the Cochrane Central Register of Controlled Trials (The Cochrane Library (2010, Issue 1), MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health (CINAHL), British Nursing Index and archive (BNI), and the Physiotherapy Evidence Database (PEDro). We also searched the reference lists of articles, handsearched journals and conference proceedings and contacted experts.
In November 2011, we updated the searches of CENTRAL (2011, Issue 4), MEDLINE (up to November Week 3 2011), EMBASE (to 2011 Week 46) and CINAHL (to 28 November 2011) to check for more recent publications.
Selection criteria
Randomised and quasi‐randomised trials comparing the effect of using cold‐water immersion after exercise with: passive intervention (rest/no intervention), contrast immersion, warm‐water immersion, active recovery, compression, or a different duration/dosage of cold‐water immersion. Primary outcomes were pain (muscle soreness) or tenderness (pain on palpation), and subjective recovery (return to previous activities without signs or symptoms).
Data collection and analysis
Three authors independently evaluated study quality and extracted data. Some of the data were obtained following author correspondence or extracted from graphs in the trial reports. Where possible, data were pooled using the fixed‐effect model.
Main results
Seventeen small trials were included, involving a total of 366 participants. Study quality was low. The temperature, duration and frequency of cold‐water immersion varied between the different trials as did the exercises and settings. The majority of studies failed to report active surveillance of pre‐defined adverse events.
Fourteen studies compared cold‐water immersion with passive intervention. Pooled results for muscle soreness showed statistically significant effects in favour of cold‐water immersion after exercise at 24 hour (standardised mean difference (SMD) ‐0.55, 95% CI ‐0.84 to ‐0.27; 10 trials), 48 hour (SMD ‐0.66, 95% CI ‐0.97 to ‐0.35; 8 trials), 72 hour (SMD ‐0.93; 95% CI ‐1.36 to ‐0.51; 4 trials) and 96 hour (SMD ‐0.58; 95% CI ‐1.00 to ‐0.16; 5 trials) follow‐ups. These results were heterogeneous. Exploratory subgroup analyses showed that studies using cross‐over designs or running based exercises showed significantly larger effects in favour of cold‐water immersion. Pooled results from two studies found cold‐water immersion groups had significantly lower ratings of fatigue (MD ‐1.70; 95% CI ‐2.49 to ‐0.90; 10 units scale, best to worst), and potentially improved ratings of physical recovery (MD 0.97; 95% CI ‐0.10 to 2.05; 10 units scale, worst to best) immediately after the end of cold‐water immersion.
Five studies compared cold‐water with contrast immersion. Pooled data for pain showed no evidence of differences between the two groups at four follow‐up times (immediately, 24, 48 and 72 hours after treatment). Similar findings for pooled analyses at 24, 48 and 72 hour follow‐ups applied to the four studies comparing cold‐water with warm‐water immersion. Single trials only compared cold‐water immersion with respectively active recovery, compression and a second dose of cold‐water immersion at 24 hours.
Authors' conclusions
There was some evidence that cold‐water immersion reduces delayed onset muscle soreness after exercise compared with passive interventions involving rest or no intervention. There was insufficient evidence to conclude on other outcomes or for other comparisons. The majority of trials did not undertake active surveillance of pre‐defined adverse events. High quality, well reported research in this area is required.
Keywords: Humans, Immersion, Cryotherapy, Cryotherapy/methods, Exercise, Exercise/physiology, Muscular Diseases, Muscular Diseases/prevention & control, Muscular Diseases/therapy, Pain, Pain/prevention & control, Pain Management, Pain Management/methods, Randomized Controlled Trials as Topic
Plain language summary
Cold‐water immersion for preventing and treating muscle soreness after exercise
Delayed onset muscle soreness commonly results after sports and exercise activity. Cold‐water immersion (CWI), which involves people immersing themselves in water at temperatures of less than 15°C, is sometimes used to manage muscle soreness after exercise and to speed up recovery time.
Our review included 17 small trials, involving a total of 366 participants. Study quality was low. Fourteen trials compared cold‐water immersion applied after exercise with 'passive' treatment involving rest or no treatment. The temperature, duration and frequency of cold‐water immersion varied between the different trials as did the exercises and settings. There was some evidence that cold‐water immersion reduces muscle soreness at 24, 48, 72 and even at 96 hours after exercise compared with 'passive' treatment. Limited evidence from four trials indicated that participants considered that cold‐water immersion improved recovery/reduced fatigue immediately afterwards. Most of the trials did not consider complications relating to cold‐water immersion and so we cannot say whether these are a problem. There were only limited data available for other comparisons of cold‐water immersion versus warm or contrasting (alternative warm/cold) water immersion, light jogging, and compression stockings. None of these showed important differences between the interventions being compared.
While the evidence shows that cold‐water immersion reduces delayed onset muscle soreness after exercise, the optimum method of cold‐water immersion and its safety are not clear.
Background
The benefits of maintaining an active lifestyle have been well documented, and daily exercise can reduce the risk of serious health problems such as obesity and cardiovascular disease (Van Dam 2008). Depending on intensity levels, exercise can induce various degrees of fatigue to the musculoskeletal, nervous and metabolic systems. Exercise is also associated with microscopic tears in muscle tissue, commonly known as exercise induced muscle damage (EIMD), which may lead to delayed onset muscle soreness (DOMS).
Description of the condition
DOMS commonly peaks between 24 and 48 hours (in some reports up to 72 hours) after exercise and is characterised by muscle shortening, increased passive stiffness, swelling, decreases in strength and power, localised soreness and altered proprioception (Cleak 1992; Proske 2001). DOMS is more likely to occur with unaccustomed exercise or intense exercise involving eccentric muscle activity (i.e. when the muscle is forcibly stretched when active) (Cheung 2003). Although the physiological mechanism underpinning DOMS has not been fully elucidated, it may relate to primary mechanical damage that occurs to muscle cells during exercise. Microscopic disruption of the small muscle fibre units (known as sarcomeres) (Proske 2001) is also related to a number of inflammatory events, and the release of intracellular enzymes such as creatine kinase (CK) (Chatzinikolaou 2010). This response is thought to contribute to the characteristic pain, swelling and decreased muscle function associated with DOMS.
DOMS usually resolves clinically after approximately four to five days, and therefore it is generally regarded as being less severe than other muscle traumas such as strains or complete tears. Notwithstanding this, there are currently a number of prophylactic strategies to prevent or minimise the impact of DOMS thereby allowing athletes to recover faster following exercise. The most common approach is ensuring adequate rest between exercise bouts; however, this is often complemented with other intervention strategies. These strategies include cool down, stretching, massage, non‐steroidal anti‐inflammatory drugs, hydrotherapy and compression garments. Currently, there is little scientific evidence to support their use. Indeed, a recent Cochrane review found that stretching has no clinically important effect in reducing muscle soreness (Herbert 2011).
Description of the intervention
Recently, cold‐water immersion (CWI) has emerged as one of the most popular interventions to prevent DOMS and promote recovery after exercise. Having started in elite level sport, it is becoming increasingly popular amongst amateur athletes. Immediately after exercise, individuals immerse themselves into cold‐water baths which vary from custom built temperature controlled spas, to large containers filled with water and ice. In practice there are large variations in the CWI protocols that are employed in terms of: the duration of immersion; water temperature; and the volume of body parts immersed. Sellwood 2007b reported that, anecdotally, contemporary practice among “high‐level sports in Australia” was three cycles, each comprising a one‐minute immersion in ice water (at approximately 5°C water temperature) followed by one minute out, started immediately after exercise. However, some clinical studies have employed longer durations of application of up to 15 minutes (Banfi 2008; Vaile 2008d), and higher water temperatures of up to 15°C (Vaile 2008e).
How the intervention might work
Despite positive anecdotal reports, the exact physiological rationale for using CWI has not been elucidated. Cold therapy (cryotherapy) has traditionally been reserved for acute soft tissue injuries (e.g. sprains, strains and muscle contusions) to reduce pain, swelling, metabolism and inflammation associated with secondary injury (Knight 2000). It is proposed that cooling tissue immediately after exercise could have the same anti‐inflammatory effect, thereby reducing the potential for DOMS. Another common concept is that CWI causes vasoconstriction (decreased blood vessel diameter) in the immersed musculature, which stimulates blood flow and nutrient and waste transportation through the body after exercise. Additionally CWI may decrease nerve transmission speed (Wilcock 2006) and alter receptor threshold, cumulating in decreased pain perception. There may also be a psychological mechanism, whereby the body simply feels more 'awake' with a reduced sensation of fatigue after exercise (Cochrane 2004).
Why it is important to do this review
Common clinical practices must have a clear rationale and high quality evidence to support their use. Currently, the practice of CWI is based on anecdotal evidence and there are no clear guidelines of an optimal or clinically effective treatment protocol. It is also important to consider that CWI induces a degree of shock on the body; therefore, the potential for short and long term side effects must be fully elucidated. Continued disparity in the rationale for using CWI, coupled with vague guidelines for its use, mean that athletes could risk employing more extreme temperatures or longer immersion times before determining actual benefit or risk.
Objectives
To determine the effects of cold‐water immersion (CWI) in the management (prevention and treatment) of muscle soreness after exercise. The following comparisons were made:
Cold‐water immersion versus no cold‐water immersion or placebo
Cold‐water immersion versus contrast water immersion (alternate cold and warm‐water immersion)
Cold‐water immersion versus warm‐water immersion
Cold‐water immersion versus other interventions (including non water based interventions)
Different durations or dosages of cold‐water immersion
Methods
Criteria for considering studies for this review
Types of studies
Any randomised and quasi‐randomised (e.g. using date of birth, record number or alternation to allocate participants into groups) trials evaluating cold‐water immersion (cryotherapy) for preventing and treating muscle soreness after exercise.
Types of participants
We focused on participants using cold‐water immersion (CWI) after exercise. No restrictions were placed on age group, gender, type or level of exercise. Trials that included participants with localised injury, whether acute (e.g. sprains, strains, contusions), or due to repetitive strain (e.g. tendinopathy) were excluded. We anticipated that people with vascular problems, such as Raynaud's disease, who are contraindicated to cryotherapy would have been excluded from trials.
Types of interventions
One group in the trial must have comprised participants treated with CWI after exercise. CWI was defined as immersion in water at less than 15°C (Low 2000). No restrictions were made on the duration or frequency of immersions. Interventions described as 'plunge' or 'dip' immersions were included. Immersion depth could be to any level of the body, including isolated immersion of a single body part (e.g. arm or leg), provided that the immersed body part (muscle) had undertaken prior exercise.
Comparisons were made to interventions designed to prevent or treat delayed onset muscle soreness including: passive interventions (rest, placebo or no intervention), warm‐water immersion (immersion in water at more than 15°C), contrast water immersion (alternating immersions in hot and cold water); cool down, stretching, massage, and compression garments. Studies comparing different durations or dosages of CWI were included. Trials where the same CWI protocol was used in both arms as a co‐intervention were not included. Comparisons with pharmacological interventions were not included.
Types of outcome measures
We collected data for the following follow‐up times: immediately and 24, 48, 72 and 96 hours post intervention.
Primary outcomes
Pain (muscle soreness) or tenderness (pain on palpation)
Subjective recovery (return to previous activities without signs or symptoms)
Secondary outcomes
Objective measures of muscle strength or power
Functional assessment measures
Swelling
Range of movement
Biochemical markers (e.g. lactate dehydrogenase (LDH), creatine kinase (CK), creatine phosphokinase (CPK) or isoform muscle creatine kinase (CKmm), myoglobin, skeletal troponin I, interleukin‐6 (IL‐6), C‐reactive protein (CRP), tumour necrosis factor alpha (TNF‐alpha).
Complications or adverse effects as reported by the individual trials
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (to February 2010), the Cochrane Central Register of Controlled Trials (The Cochrane Library 2010, Issue 1), MEDLINE (1950 to February 2010), EMBASE (1988 to February 2010), Cumulative Index to Nursing and Allied Health (CINAHL) (1982 to February 2010), British Nursing Index and archive (BNI) (1985 to February 2010), and the Physiotherapy Evidence Database (PEDro) (1929 to February 2010).
A search update was performed to check the more recent literature in November 2011. These were run from January 2010 onwards for the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (to November 2011), CENTRAL (The Cochrane Library 2011, Issue 4), MEDLINE (2010 to November Week 3 2011), EMBASE (2010 to 2011 Week 46) and CINAHL (2010 to 28 November 2011).
In MEDLINE, the subject‐specific search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (Lefebvre 2009). This strategy was modified for use in other databases. In EMBASE, the subject‐specific search was combined with the Scottish Intercollegiate Guidelines Network (SIGN) search filter for randomised controlled trials (seeAppendix 1 for search strategies).
We also searched Current Controlled Trials and the WHO International Clinical Trials Registry for ongoing and recently completed trials (February 2010).
No language restrictions were applied.
Searching other resources
We searched the reference list of articles, and the table of contents of the following journals not registered as being handsearched by The Cochrane Collaboration (February 2010):
Australian Journal of Science and Medicine in Sport (1998 to February 2010)
British Journal of Sports Medicine (1964 to February 2010)
Clinical Journal of Sport Medicine (1991 to February 2010)
International Journal of Sports Medicine (2005 to February 2010)
Journal of Applied Physiology (1948 to February 2010)
Journal of Sports Medicine and Physical Fitness (1998 to February 2010)
Journal of Sports Sciences (1985 to 1987; 1990 to 1991; 1994; 1996; 2000 to February 2010)
Medicine and Science in Sports and Exercise (1980 to February 2010)
Physical Therapy in Sport (2000 to 2002; 2007 to February 2010)
We also searched the conference proceedings of the following organisations:
American College of Sports Medicine (1986 to February 2010) (in Medicine and Science in Sports and Exercise)
American Physical Therapy Association (1980 to February 2010) (in Physical Therapy)
British Association of Sport and Exercise Medicine (BASEM) (1964 to February 2010) (in British Journal of Sports Medicine)
British Association of Sport and Exercise Sciences (BASES) (1964 to February 2010) (in Journal of Sports Sciences)
World Confederation for Physical Therapy (2003, 2007) (CD ROM)
Between February 2010 and March 2010, we contacted experts in the field (identified by personal contacts, lead authors in published studies, World Wide Web searching) for relevant data in terms of published, unpublished or ongoing studies.
Data collection and analysis
Selection of studies
Two authors (CB, SMcD) independently selected trials for inclusion. The titles and abstracts of publications obtained by the search strategy were screened. All trials classified as relevant by either of the authors were retrieved. Based on the information within the full reports, we used a standardised form to select the trials eligible for inclusion in the review. Where possible, translation of non‐English language studies was undertaken. If necessary, we contacted primary authors for clarification of study characteristics. Disagreement between the authors was resolved by consensus, or third party adjudication (GDB, TH).
Data extraction and management
Data were extracted independently by two review authors (CB, SMcD) using a customised form tested prior to use. This was used to extract relevant data on methodological issues, eligibility criteria, interventions (including detailed characteristics of the exercise protocols and cold‐water immersions employed), comparisons and outcome measures. Any disagreement was resolved by consensus, or third party adjudication (GDB, TH). Primary authors were contacted to clarify any omitted data or study characteristics. To perform intention‐to‐treat analysis, data were extracted according to the original allocation groups, and losses to follow‐up were noted where possible. There was no blinding to study author, institution or journal at this stage.
Assessment of risk of bias in included studies
The risk of bias was assessed by two authors independently (CB, GDB), using the tool described (and the criteria outlined) in the Cochrane Handbook (Higgins 2009). To minimise bias in the interpretation of this scale, two review authors (CMB, GDB) initially assessed a small sample of unrelated studies (not included in the current review); disparities in risk of bias judgements were reviewed and discussed, prior to evaluating any of the included studies.
Each study was graded for risk of bias in each of the following domains; sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting. Two other sources of bias were considered based on the following questions: a) Was the exercise protocol clear and consistent between groups? b) Were no co‐interventions used, or if present, were they standardised across groups? For each study, the domains were described as reported in the published study report (or if appropriate based on information from related protocols, published comments, or after discussion with the relevant authors) and judged by the review authors as to their risk of bias. They were assigned 'high risk' or 'low risk'. If insufficient detail of what happened in the study was reported, or if what happened in the study was known, but the risk of bias was unknown; or if an entry was not relevant to the study at hand (for example when the outcome being assessed was not measured in the study) then the risk of bias was deemed 'unclear' for that domain. Disagreements between authors regarding the risk of bias for domains was resolved by consensus.
Differences in the details of the treatment intervention (e.g. duration of immersion, frequency of immersion, water temperature), and characteristics of participants (professional or highly trained athletes, amateur, sedentary) was regarded as a potential source of bias and addressed in the subgroup analysis.
Measures of treatment effect
For each study, risk ratios and 95% confidence intervals were calculated for dichotomous outcomes, and mean differences and 95% confidence intervals were calculated for continuous outcomes. For continuous outcomes that were pooled on different scales, standardised mean differences were used. We had planned to preferentially extract data based on changes from baseline (mean change scores); however, the majority of studies reported follow‐up scores.
Unit of analysis issues
Some studies included multiple observations of the same outcome; therefore we extracted data at clinically relevant time points. When available this was: immediately after the intervention, and then at 24 hour intervals following exercise up to 96 hours. In studies using a randomised cross‐over design, we aimed to undertake paired analysis when sufficient data were available; otherwise, data were analysed as if these studies used a parallel group design (Deeks 2008). Studies using within‐participant designs where contralateral limbs acted as controls were excluded. Had any study used a cluster randomised design, for example by sports teams, data would have been adjusted for clustering.
Dealing with missing data
If necessary, original investigators were contacted and requests made for any missing data. Depending on the nature of the data, assumptions were made on whether the data were missing at random or missing not at random. Data missing at random were ignored, and we focused on the available data only. If data were deemed to be missing not at random, replacement values were not imputed; sensitivity analyses were not undertaken.
When standard deviations were missing from continuous data, studies were scanned for any other statistics (confidence intervals, standard errors, T values, P values, F values) that allow for their calculation. There were no outcomes where the majority of data were unavailable because of missing standard deviations.
Assessment of heterogeneity
Initially, the clinical diversity across studies was qualitatively assessed. If two or more studies were deemed to be clinically homogeneous in terms of participants’ characteristics, intervention, comparison, and outcomes, then data were assessed for statistical heterogeneity using RevMan. The chi‐squared (Chi²) test in conjunction with the I² statistic was used. The significance for Chi² was set at P < 0.1 (Deeks 2008a). The I² statistic was used to quantify inconsistency using the following formula I² = [(Q‐df)/Q] x 100%, where Q is the Chi² statistic and df its degrees of freedom. I² values greater than 50% were considered to represent substantial heterogeneity (Higgins 2003).
Assessment of reporting biases
Should sufficient trials become available in future, we plan to use funnel plots to assess for publication bias based on the effect estimates (horizontal scale) against standard error (on a reversed scale, vertical) using Review Manager, with continuous data represented as standardised mean differences, and dichotomous data represented as risk ratios on a logarithmic scale.
Data synthesis
In the event that there was no evidence of heterogeneity of effect (P > 0.1), a fixed‐effect model was used for meta‐analysis. In cases where there was evidence of statistical heterogeneity, we checked the results using a random‐effects model. We also considered the causes for the heterogeneity in terms of the clinical characteristics and the magnitude and direction of effects.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analysis according to the details of the treatment intervention (duration of immersion, frequency of immersion, water temperature), the type of exercise (normal sporting activity, laboratory controlled muscle damage), exercise intensity (duration, environmental temperature), and the participants' characteristics (professional or highly trained athletes, amateur, sedentary). An additional subgroup analysis was planned according to methodological quality (high risk versus low risk of bias) (Deeks 2008b). This was to take the form of stratified analyses (high risk versus low risk) or in a meta‐regression. A 'low risk study' was a study scoring low risk in each of the risk of bias domains (excluding blinding of participants and personnel).
Sensitivity analysis
In the event that no studies could be classified as low risk, we considered but did not perform sensitivity analysis based on individual risk of bias domains. We also planned to undertake additional sensitivity analysis should any other study peculiarities become apparent during the review process.
Results
Description of studies
Results of the search
The searches undertaken in February 2010 identified 58 potentially eligible studies (from 65 reports). No new studies were found after contacting experts in the area. A total of 17 studies were included, 38 were excluded and a further three await classification.
Subsequently, the search was updated to November 2011 to check for more recent publications. This resulted in the identification of four more potentially eligible trials (Ascensao 2011; Pournot 2011; Stacey 2010; Vaile 2011), which have been placed in Studies awaiting classification; and another publication (Rowsell 2011) of an already included trial (Rowsell 2009).
Included studies
The 17 included studies were all published between 1998 and 2009 from centres in Australia (Cassar 2010; Halson 2008; Ingram 2009; King 2009; Montgomery 2008; Rowsell 2009; Sellwood 2007a; Vaile 2008c), Japan (Yanagisawa 2003a; Yanagisawa 2003b), United Kingdom (Bailey 2007; Eston 1999; Goodall 2008; Jakeman 2009) or other areas of Europe (Buchheit 2009; Skurvydas 2006), and the USA (Kuligowski 1998). All were published in English, and in peer reviewed journals. The majority were identified in MEDLINE, EMBASE or CENTRAL, with one (Cassar 2010) identified from searching conference proceedings.
All 17 studies were randomised controlled trials. Ten were parallel group trials (Bailey 2007; Eston 1999; Goodall 2008; Jakeman 2009; Kuligowski 1998; Montgomery 2008; Rowsell 2009; Sellwood 2007a; Yanagisawa 2003a; Yanagisawa 2003b). Six studies used a randomised cross‐over design (Buchheit 2009; Cassar 2010; Halson 2008; Ingram 2009; King 2009; Skurvydas 2006) with the time between intervention arms being three (Halson 2008), five (King 2009), seven (Buchheit 2009; Cassar 2010) and 14 (Ingram 2009) days, with one study using an eight month period (Skurvydas 2006). One study (Vaile 2008c) used a mixed design whereby randomisation was undertaken for the three active treatment groups but not the passive treatment group; data were not used from the passive treatment group in this review. In Buchheit 2009, data for some outcomes were extracted from another report (Peiffer 2010) based on the same experiment and participants.
In total, there were 366 trial participants of which 19% were female; the individual studies reported mean ages between 16 and 29 years. Studies were generally small, with 72% using 20 participants or less. The largest study used 54 participants (Kuligowski 1998). All participants were male in 12 studies (Bailey 2007; Buchheit 2009; Cassar 2010; Goodall 2008; Halson 2008; Ingram 2009; Montgomery 2008; Rowsell 2009; Skurvydas 2006; Vaile 2008c; Yanagisawa 2003a; Yanagisawa 2003b). Three studies involved all female participants (Eston 1999; Jakeman 2009; King 2009), and for the remainder, the male:female ratio was 1:1 (Kuligowski 1998) or 1:2.6 (Sellwood 2007a). Details of included studies can be found in the Characteristics of included studies.
Details of exercise
Exercise type, duration and intensity varied across studies. In nine studies, the exercise was designed to produce delayed onset muscle soreness (DOMS) under laboratory controlled conditions; all involved multiple repetitions (50 to 100 repetitions) of resistance to lengthening (eccentric) (Goodall 2008; Jakeman 2009; Kuligowski 1998; Sellwood 2007a; Skurvydas 2006; Vaile 2008c), or alternative lengthening and shortening exercises (Eston 1999; Yanagisawa 2003a; Yanagisawa 2003b). Five studies (Eston 1999; Kuligowski 1998; Sellwood 2007a; Yanagisawa 2003a; Yanagisawa 2003b) targeted a single muscle group, with other studies targeting a number of related muscle groups (lower limb muscles) using a resistance (leg press) machine (Vaile 2008c) or plyometric exercises (repetitive jumping) (Goodall 2008; Jakeman 2009; Skurvydas 2006). Participants subjected to DOMS were described as untrained (Yanagisawa 2003a; Yanagisawa 2003b); healthy (Eston 1999; Jakeman 2009; Kuligowski 1998; Sellwood 2007a), or active (Goodall 2008; Skurvydas 2006). It is likely that the majority were unaccustomed to this type of exercise; only one study specified that participants had a resistance training history (Vaile 2008c).
The remaining eight studies employed running or cycling based exercise. A single bout of cycling was used in two studies; one was a short duration sprint (Buchheit 2009), and one was a 40 minute steady cycle (75% V02 max) plus a time trial (Halson 2008). Both were undertaken by trained cyclists using controlled laboratory conditions and consistent environmental conditions during cycling (34°C to 35°C ambient temperature, 40% relative humidity). In a further study (Cassar 2010), a 30 minute cycling session was undertaken once per day, over a five day period, by trained cyclists at 70% V02 max.
Three studies (Bailey 2007; Ingram 2009; King 2009) involved running based with active or trained participants exercising for over an hour (maximum 90 minutes). In Bailey 2007, running was intermittent and based on an average intensity of 75% V02 max. In the other two studies (Ingram 2009; King 2009), the nature and intensity of the exercise was designed to simulate the running demands of a team sport, with one (Ingram 2009) finishing with an additional shuttle run to exhaustion.
In two studies (Montgomery 2008; Rowsell 2009), exercise involved competing in a team sport (basketball, soccer); in both cases trained/high performance participants played one game per day, over a three (Montgomery 2008) or four (Rowsell 2009) day tournament.
Details of cold‐water immersion
All studies employed some form of CWI intervention after exercise. The most popular water immersion temperature was between 10°C and 15°C, which was used by just over 75% of studies; the remainder used lower temperatures of 9°C (King 2009) or 5°C (Sellwood 2007a; Yanagisawa 2003a; Yanagisawa 2003b). In 10 studies (Bailey 2007; Buchheit 2009; Eston 1999; Goodall 2008; Jakeman 2009; Kuligowski 1998; Montgomery 2008; Vaile 2008c; Yanagisawa 2003a; Yanagisawa 2003b), treatment involved continuous immersion for between 5 and 24 minutes; the average treatment duration across these studies was 12.6 minutes. The remaining studies (Halson 2008; Ingram 2009; King 2009; Rowsell 2009; Sellwood 2007a; Skurvydas 2006) undertook CWI in sets where participants got out of the water at pre‐determined time points; treatment therefore consisted of: three (Halson 2008; Sellwood 2007a) to five (Rowsell 2009) sets of one minute immersions, two sets of five minute immersions (Ingram 2009; King 2009), or two sets of 15 minute immersions (Skurvydas 2006). Three studies (Bailey 2007; Kuligowski 1998; Rowsell 2009) reported that the water was periodically agitated during immersion.
In the majority of studies, CWI was undertaken to approximately the level of the waist (Bailey 2007; Cassar 2010; Goodall 2008; Ingram 2009; Jakeman 2009; King 2009; Sellwood 2007a; Skurvydas 2006), sternum (Buchheit 2009; Halson 2008; Montgomery 2008; Rowsell 2009) or shoulder (Vaile 2008c). In the remaining four studies, CWI was confined to the arm (Eston 1999; Kuligowski 1998) or lower leg muscles (Yanagisawa 2003a; Yanagisawa 2003b). The timing of initiating CWI after exercise was generally consistent across studies; initiated immediately after (Bailey 2007; Cassar 2010; Eston 1999; Goodall 2008; Ingram 2009; King 2009; Kuligowski 1998; Sellwood 2007a; Skurvydas 2006; Vaile 2008c; Yanagisawa 2003a; Yanagisawa 2003b), or within approximately 10 minutes (Jakeman 2009; Montgomery 2008) or 20 minutes (Halson 2008; Rowsell 2009) after finishing treatment. Seven studies undertook additional CWI interventions after completing a single exercise session: Eston 1999 (12, 24, 36, 48, 60 and 72 hours); Goodall 2008 (24, 48 and 72 hours); Ingram 2009 (24 hours); Kuligowski 1998 (24, 48 and 72 hours); Skurvydas 2006 (4, 8 and 24 hours); Vaile 2008c (24, 48 and 72 hours); and one CWI group of Yanagisawa 2003a (24 hours).
Details of comparisons
The 17 included studies were divided into five different groups: studies comparing CWI with passive intervention (no CWI or rest); studies comparing CWI with contrast immersion; studies comparing CWI with warm‐water immersion; studies comparing CWI with active recovery; studies comparing CWI with compression garments; and studies comparing two different dosages of CWI. Six studies (Ingram 2009; King 2009; Kuligowski 1998; Montgomery 2008; Vaile 2008c; Yanagisawa 2003a) used more than one relevant treatment comparison and therefore appear in two different sections.
Cold‐water immersion versus passive (no intervention/rest)
This was the most common comparison, which was made by 14 studies (Bailey 2007; Buchheit 2009; Cassar 2010; Eston 1999; Goodall 2008; Halson 2008; Ingram 2009; Jakeman 2009; King 2009; Kuligowski 1998; Montgomery 2008; Skurvydas 2006; Yanagisawa 2003a; Yanagisawa 2003b). CWI was compared with a passive intervention defined as either seated rest (Bailey 2007; Buchheit 2009; Cassar 2010; Goodall 2008; Halson 2008; Ingram 2009; Jakeman 2009; King 2009; Skurvydas 2006) or no intervention (Eston 1999; Kuligowski 1998; Yanagisawa 2003a; Yanagisawa 2003b).
Cold‐water immersion versus contrast immersion
Five studies made this comparison (Cassar 2010; Ingram 2009; Kuligowski 1998; King 2009; Vaile 2008c). Contrast immersion involved alternate immersions in cold (between 8°C and 15°C) and warm‐water (38°C to 45°C); one study (King 2009) used a hot shower. The overall duration of contrast treatment varied across groups: 12 minutes (2 minutes cold: 2 minutes hot x 3 sets (Ingram 2009); 14 minutes (1 minute cold: 1 minute hot x 7 sets) (Cassar 2010; Vaile 2008c); 15 minutes (1 minute cold: 2 minute hot x 5 sets) King 2009; or 24 minutes (3 minutes hot: 1 minute cold x 6 sets) (Kuligowski 1998).
Cold‐water immersion versus warm‐water immersion
Four studies used this comparison (Kuligowski 1998; Rowsell 2009; Sellwood 2007a; Vaile 2008c) but the details of warm‐water immersion across studies. Three (Kuligowski 1998; Rowsell 2009; Vaile 2008c) used immersion in water between 34°C and 40°C, and one (Sellwood 2007a) used water temperatures of 24°C. The total duration of warm‐water immersion was: 3 (Sellwood 2007a), 5 (Rowsell 2009), 14 (Vaile 2008c) and 24 minutes (Kuligowski 1998).
Cold‐water immersion versus active recovery
One study (King 2009) compared CWI with an active recovery intervention, which involved 15 minutes of jogging at a predetermined and controlled speed; the exercise in this study was a single bout of netball related running.
Cold‐water immersion versus compression
One study (Montgomery 2008) compared CWI with compression therapy over the course of a three day (one game per day) basketball tournament. Participants in the compression group wore full length compression garments (18 mm/Hg) post game and at night (18 hours), over the tournament; CWI involved a single immersion after each game.
Cold‐water immersion versus cold‐water immersion (different dosage)
One study (Yanagisawa 2003a) specifically compared two different treatment dosages of CWI. Both groups completed a CWI treatment immediately after exercise, and one group undertook an additional treatment 24 hours later.
Primary outcomes
Studies were selected to include at least one primary outcome. Pain was the most commonly reported outcome and 15 studies assessed muscle soreness using a Likert scale or a visual analogue scale (VAS). A five point scale was used in two trials (Yanagisawa 2003a; Yanagisawa 2003b), 10 or 11 point or 10 cm scales were used in 11 trials (Bailey 2007; Cassar 2010; Halson 2008; Ingram 2009; Jakeman 2009; King 2009; Montgomery 2008; Rowsell 2009; Sellwood 2007a; Skurvydas 2006; Vaile 2008c), a 12 cm VAS was used in Kuligowski 1998 and a 20 cm VAS was used in Goodall 2008). The written descriptors used at each end of the scale were specified in all but two studies (Ingram 2009; Rowsell 2009). Seven studies specified that pain was measured during self palpation of muscle (Bailey 2007) or during a functional movement associated with the exercised body part(s) (Goodall 2008; Jakeman 2009; Kuligowski 1998; Sellwood 2007a; Vaile 2008c; Yanagisawa 2003b). We anticipated that the remainder of studies were based on muscle soreness measured at rest.
Two studies (Eston 1999; Sellwood 2007a) measured pain on pressure (tenderness) using a hand held algometer device; one recorded the force in Newtons (N) at which discomfort turned to pain (Eston 1999) and the other measured pain levels on a VAS (10 cm) during application of a standard pressure of 6 lb/cm² (Sellwood 2007a). Only one study (Buchheit 2009) did not measure any component of pain.
Five studies (Buchheit 2009; Cassar 2010; Halson 2008; Montgomery 2008; Rowsell 2009) measured subjective recovery based on a VAS (10 points or 10 cm). In each study, the participants rated different correlates of subjective recovery which we put into the following subcategories: general fatigue (Cassar 2010; Halson 2008; Montgomery 2008; Rowsell 2009), general recovery (Buchheit 2009); or separate ratings of physical and mental recovery (Halson 2008; Rowsell 2009).
Secondary outcomes
There were a range of secondary outcomes reported. Strength was assessed by 10 studies. The majority used a dynamometer or isokinetic dynamometer (Bailey 2007; Buchheit 2009; Eston 1999; Goodall 2008; Jakeman 2009; Kuligowski 1998; Sellwood 2007a), with others using a cable tensiometer (Ingram 2009), force platform (Vaile 2008c), or an unspecified force measuring device (Skurvydas 2006). Two focused on concentric muscle strength (Buchheit 2009; Jakeman 2009) with the remainder measuring isometric strength at a specific mid‐range joint angle.
Eight studies reported on power. In five studies, power was assessed by measuring vertical jump performance (centimetres (cm)) either with (King 2009; Rowsell 2009; Skurvydas 2006) or without a counter movement (Bailey 2007; Montgomery 2008). Sellwood 2007a used a related measure based on hopping distance (cm). Using a specialist device, Vaile 2008c measured the power produced in Watts (W) during a weighted squat jump, and Buchheit 2009 measured power output on a cycle ergometer.
Functional performance outcomes were reported by seven studies. Five were based on the time (in seconds) participants took to complete various exercise tasks; these were put into subcategories according to their nature or distance in metres (m) as follows: cycle sprint (Buchheit 2009;Cassar 2010), single running sprint (10 to 20 m) (Bailey 2007; King 2009), multiple running sprint (20 m x 10 to 12 sets) (Ingram 2009; Montgomery 2008; Rowsell 2009), and agility based or sports specific running (King 2009; Montgomery 2008).
Range of movement (ROM) was reported by four studies. Four studies measured active range of movement using a hand held goniometer; we focused on joint movements which tended to lengthen the exercised muscle group (Goodall 2008; Kuligowski 1998; Yanagisawa 2003a). Three reported total range of movement in degrees (Goodall 2008; Kuligowski 1998; Yanagisawa 2003a) and one (Montgomery 2008) reported range of movement at a range of joints based on the sit and reach flexibility test (cm).
Five studies reported swelling using lower limb girth measured with a tape measure (Eston 1999; Goodall 2008; Montgomery 2008; Sellwood 2007a; Vaile 2008c), with values reported in centimetres of limb girth.
Biochemical markers were reported in 12 studies (Bailey 2007; Cassar 2010; Eston 1999; Goodall 2008; Halson 2008; Ingram 2009; Jakeman 2009; Rowsell 2009; Sellwood 2007a; Skurvydas 2006; Vaile 2008c; Yanagisawa 2003a); laboratory procedures were well described with a number of different biomarker molecules assessed. These outcomes were divided into two subcategories: biomarkers of inflammation (IL‐1b; IL‐6; IL‐10; CRP) and muscle damage (creatine kinase (CK); lactate dehydrogenase (LDH); myoglobin).
The majority of studies did not monitor adverse events or complications relating to the interventions. Two studies (Buchheit 2009; Halson 2008) measured core temperature changes associated with CWI; in both studies, participants' mean core temperature (extracted from graphs) did not drop below 37°C.
Missing data
In five included studies (Eston 1999; Halson 2008; Ingram 2009; Kuligowski 1998; Montgomery 2008), the information provided in the trial report was sufficient for analysis of between group differences using Review Manager. In three studies (Bailey 2007; Eston 1999; Jakeman 2009), group dispersion was based on standard errors, with values converted to standard deviations by two independent review authors. In 12 included studies, additional data were requested from study authors; six (Bailey 2007; Cassar 2010; Goodall 2008; Jakeman 2009; King 2009; Skurvydas 2006) were successfully contacted, with each providing the requested information. In five studies (Buchheit 2009; Rowsell 2009; Vaile 2008c; Yanagisawa 2003a; Yanagisawa 2003b), we were able to extract data from graphs; this was undertaken by two independent review authors. In one study (Sellwood 2007a) only median and interquartile range figures were available.
Follow‐up
All studies undertook multiple follow‐up observations for each outcome. Eleven studies (Bailey 2007; Buchheit 2009; Eston 1999; Goodall 2008; Halson 2008; Ingram 2009; Jakeman 2009; King 2009; Vaile 2008c; Yanagisawa 2003a; Yanagisawa 2003b) reported multiple follow‐ups in the first few hours succeeding the exercise (e.g. pre‐exercise, post exercise, pre intervention and post intervention); in such cases, we considered outcomes reported immediately after completing the treatment intervention, to be most clinically relevant. In three studies (Skurvydas 2006; Yanagisawa 2003a; Yanagisawa 2003b) the timing of the first follow‐up could not be configured from the trial report and data were extracted from the next clearly defined follow‐up time. In such cases (Skurvydas 2006; Yanagisawa 2003a; Yanagisawa 2003b), additional follow‐ups were reported within four hours of completing the intervention, which, for the purposes of comparison, was also defined as an 'immediate' follow‐up. In all but two studies (Buchheit 2009; Halson 2008) follow‐ups were undertaken at 24 hours, with further follow‐ups repeated at 48 (Bailey 2007; Eston 1999; Goodall 2008; Ingram 2009; Jakeman 2009; Kuligowski 1998; Sellwood 2007a; Skurvydas 2006; Vaile 2008c; Yanagisawa 2003a; Yanagisawa 2003b), 72 (Eston 1999; Goodall 2008; Jakeman 2009; Sellwood 2007a; Skurvydas 2006; Vaile 2008c) or 96 hours (Goodall 2008; Jakeman 2009; Kuligowski 1998; Yanagisawa 2003a; Yanagisawa 2003b).
In three studies, competitive sports tournaments (Montgomery 2008; Rowsell 2009) or multiple cycling exercises (Cassar 2010) were undertaken over three to five days (one game or cycling bout per day); outcomes were generally reported before and after each game or cycling bout, and repeated for up to 24 hours after the last day of exercise. In all these studies, we considered the 'exercise' to be the entire tournament or days of exercise, and therefore only extracted outcome data collected in the post exercise time period (e.g. after tournament completion or after the last day of exercise).
Excluded studies
After appraisal of the full study reports, we excluded 38 studies. The majority were excluded because they did not use a relevant CWI intervention (n = 19). In a further two studies, the CWI was relevant but used in conjunction with another therapeutic intervention that was not controlled for. Two others were excluded because CWI did not involve the exercised body‐part. Ten studies were excluded as they did not report a primary outcome measure (rating of perceived exertion and rating of thermal discomfort were not considered to be measures of subjective recovery). Two trials did not report any form of randomisation, and two used a contralateral body part as a control. One study did not use a relevant time point for follow‐up. Detailed reasons for exclusion can be found in the Characteristics of excluded studies.
Studies awaiting classification
Two studies (Fowles 2003; Smith 2008) did not provide sufficient detail to determine their relevance for inclusion, despite contacting the corresponding authors. A further four studies (Ascensao 2011; Pournot 2011; Stacey 2010; Vaile 2011) were identified subsequently to the full search date. Further details of these trials are given in Characteristics of studies awaiting classification.
Risk of bias in included studies
Full details of the quality assessment are given in the Characteristics of included studies. All corresponding authors were contacted, and asked to provide any methodological details which were unclear or missing in the original trial reports. Our requests for information were open ended to avoid any bias resulting from leading questions. Just over 55% of authors responded. Unless an author specifically stated that they did not understand our question, we avoided making multiple requests for information. Risk of bias judgement was made by two independent authors, based on information from trial reports and author correspondence; the results are summarised in Figure 1 and Figure 2. The significance of the variations in risk of bias is unclear as studies could not be subgrouped by high and low risk of bias.
1.
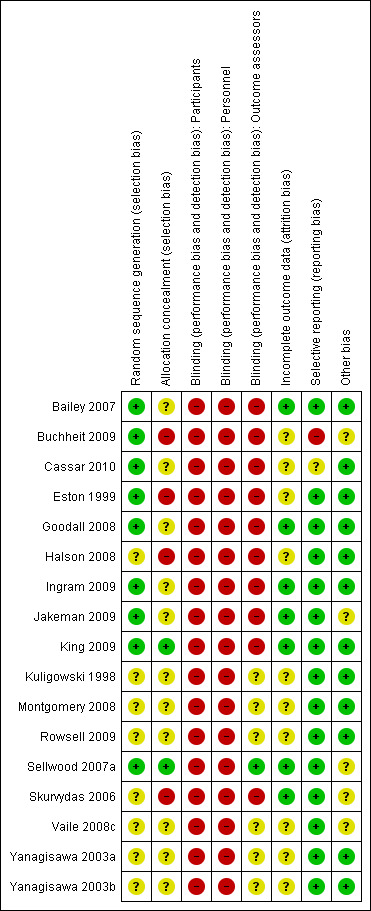
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.
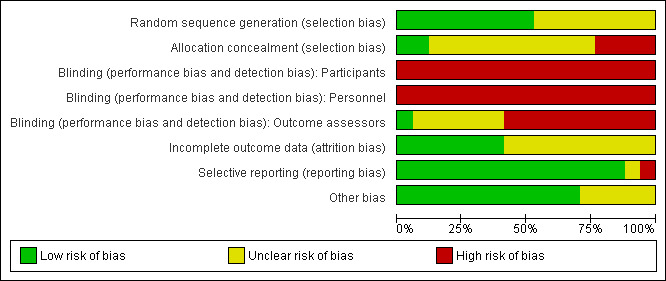
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Randomisation procedure was described in just nine studies. Sellwood 2007a used a random numbers table; with the remainder clarified after personal correspondence and were based on random numbers table (Bailey 2007; Cassar 2010; Goodall 2008), computer (Ingram 2009) or predetermined sequence generation (Jakeman 2009), coin toss (Buchheit 2009; Eston 1999), or hat draw (King 2009). Allocation concealment was adequately described in just one study (Sellwood 2007a) through the use of opaque sealed envelopes, and is likely in another (King 2009) based on personal correspondence. In the remaining studies, there was no clear indication that the investigators would be unable to predict the prospective group, or in the case of cross‐overs, the order of treatments to which participants would be allocated.
Blinding
None of the studies utilised blinding of participants or care givers. One study (Sellwood 2007a) did not inform participants as to which intervention (5°C or 24°C water temperature) was deemed to be therapeutic; however, this was not considered to be true blinding. Only one study (Sellwood 2007a) used blinded outcome assessment. This study also states that participants were requested not to reveal their treatment group to the assessors; however, they did not appear to test the success of their blinding strategy.
Incomplete outcome data
This area of methodology was poorly described. Only one study (Sellwood 2007a) specifically indicated intention‐to‐treat analysis (with imputation in the event of missing data); however, their results seemed to indicate that there were no missing data or violation from the protocol. After correspondence, six trials (Bailey 2007; Goodall 2008; Ingram 2009; Jakeman 2009; King 2009; Skurvydas 2006) confirmed no losses to follow‐up or violation from the study protocol. Six studies (Eston 1999; Kuligowski 1998; Montgomery 2008; Vaile 2008c; Yanagisawa 2003a; Yanagisawa 2003b) provided no information on drop outs, exclusion, missing data or approach to analysis. Four others (Buchheit 2009;Cassar 2010; Halson 2008;Rowsell 2009) were difficult to interpret in this regard. Buchheit 2009 reported that one participant was dismissed because of incomplete outcomes. Halson 2008 recruited 11 participants who each completed both intervention arms; however, biochemical outcomes were missing for six participants as they were unwilling to provide blood samples. Rowsell 2009 was the only study not to specify the numbers allocated to each study arm; furthermore, just under one third of participants were excluded from the analysis due to injuries sustained at an unspecified time during the exercise protocol (competitive soccer tournament over four days).
Selective reporting
None of the studies made any reference to a published protocol. Buchheit 2009 did not report on two measured outcomes (strength and power) within the trial report, however, these data were available from a secondary publication (Peiffer 2010) based on the same study population and experiment. All studies described outcomes and follow‐up times with corresponding results presented by intervention group. In five cases (Bailey 2007; Goodall 2008; Jakeman 2009; King 2009; Skurvydas 2006), additional group summary data were provided by corresponding authors in order to calculate effect size. Data were extracted from graphs in five studies (Buchheit 2009; Rowsell 2009; Vaile 2008c; Yanagisawa 2003a; Yanagisawa 2003b); in one of the studies (Yanagisawa 2003a), summary data from two time points (24 hours, 48 hours) could not be extracted for two outcomes (pain and biomarkers).
Other potential sources of bias
All studies provided in‐depth descriptions of the exercise protocols based on exercise type, duration, and intensity. Seven studies (Bailey 2007; Cassar 2010; Goodall 2008; Ingram 2009; Kuligowski 1998; Yanagisawa 2003a; Yanagisawa 2003b) stated that participants were informed to refrain from using co‐interventions for the duration of the study; in five cases these interventions were specified: massage (Bailey 2007; Kuligowski 1998), medications/supplements (Bailey 2007; Goodall 2008; Kuligowski 1998), exercise (Goodall 2008;Kuligowski 1998; Yanagisawa 2003a; Yanagisawa 2003b), stretching (Kuligowski 1998), or other physical modalities (Kuligowski 1998; Yanagisawa 2003a; Yanagisawa 2003b). Five studies (Bailey 2007; Halson 2008; King 2009; Montgomery 2008; Rowsell 2009) standardised specific co‐interventions across groups: water consumption (Bailey 2007; Halson 2008; King 2009), carbohydrate/food ingestion (Halson 2008; King 2009; Montgomery 2008; Rowsell 2009) and stretching (Montgomery 2008; Rowsell 2009), with two monitoring adherence using participant diaries (Halson 2008; King 2009). Five studies did not provide any details on co‐interventions (Buchheit 2009; Jakeman 2009; Sellwood 2007a; Skurvydas 2006; Vaile 2008c).
Effects of interventions
The 17 included studies were divided into five different groups based on comparison. Within each comparison, results are presented in subcategories based on follow‐up time (immediate, 24, 48, 72, 96 hours). Although there were no complications or side effects reported within any of the individual studies, it was unclear whether any study actively monitored specific adverse effects as part of their outcomes.
Cold‐water immersion versus passive (no intervention/rest)
Primary outcomes
Pain (muscle soreness: VAS, various scales or scores; highest values = worst pain)
Twelve out of the 14 studies in this comparison presented data on muscle soreness based on various visual analogue scores or scales. Pooled results are presented in five subcategories based on follow‐up time (seeAnalysis 1.1; Figure 3). There were no significant differences between groups at immediate follow‐up (SMD ‐0.07, 95% CI ‐0.43 to 0.28; 7 trials). At all four subsequent times, pooled results showed significantly lower levels of pain in the cold‐water immersion group (24 hours: SMD ‐0.55, 95% CI ‐0.84 to ‐0.27, 10 trials); (48 hours: SMD ‐0.66, 95% CI ‐0.97 to ‐0.35; 8 trials); (72 hours: SMD ‐0.93, 95% CI ‐1.36 to ‐0.51; 4 trials); (96 hours: SMD ‐0.58; 95% CI ‐1.00 to ‐0.16; 5 trials). However, there was significant heterogeneity in all four analyses. While increasing the 95% confidence intervals, the findings in favour of CWI were upheld when applying the random‐effects model (seeAnalysis 1.2). In the 24, 48 and 72 hours analyses, cross‐over trials have been combined with parallel group trials. Subgroup analysis by study design show statistically significant differences between the pooled results of cross‐over trials and parallel group trials at all three follow‐up times. For 24 hours, seeAnalysis 1.3 (test for subgroup differences: Chi² = 9.66, df = 1 (P = 0.002), I² = 89.7%); for 48 hours, seeAnalysis 1.4 (test for subgroup differences: Chi² = 6.28, df = 1 (P = 0.01), I² = 84.1%); and for 72 hours (test for subgroup differences: Chi² = 14.34, df = 1 (P = 0.0002), I² = 93.0%). It is clear from all three analyses that the findings in favour of CWI from the three cross‐over trials (Ingram 2009; King 2009; Skurvydas 2006) are considerably stronger than those for the parallel group trials.
1.1. Analysis.
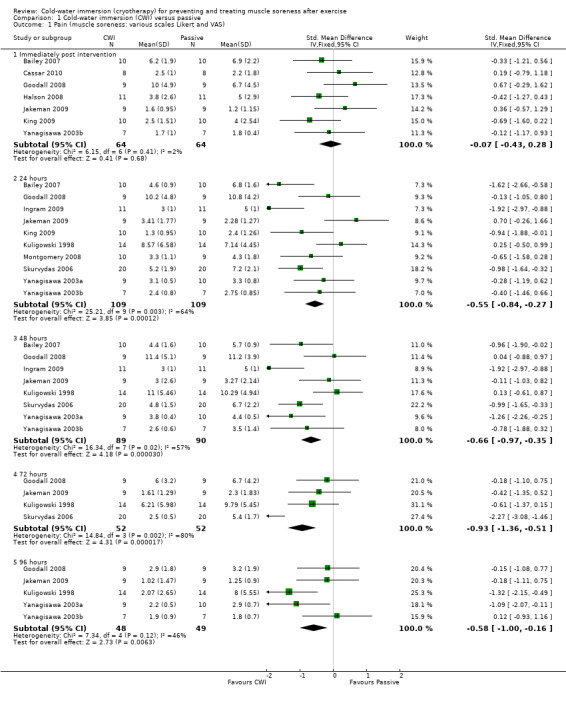
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 1 Pain (muscle soreness: various scales Likert and VAS).
3.
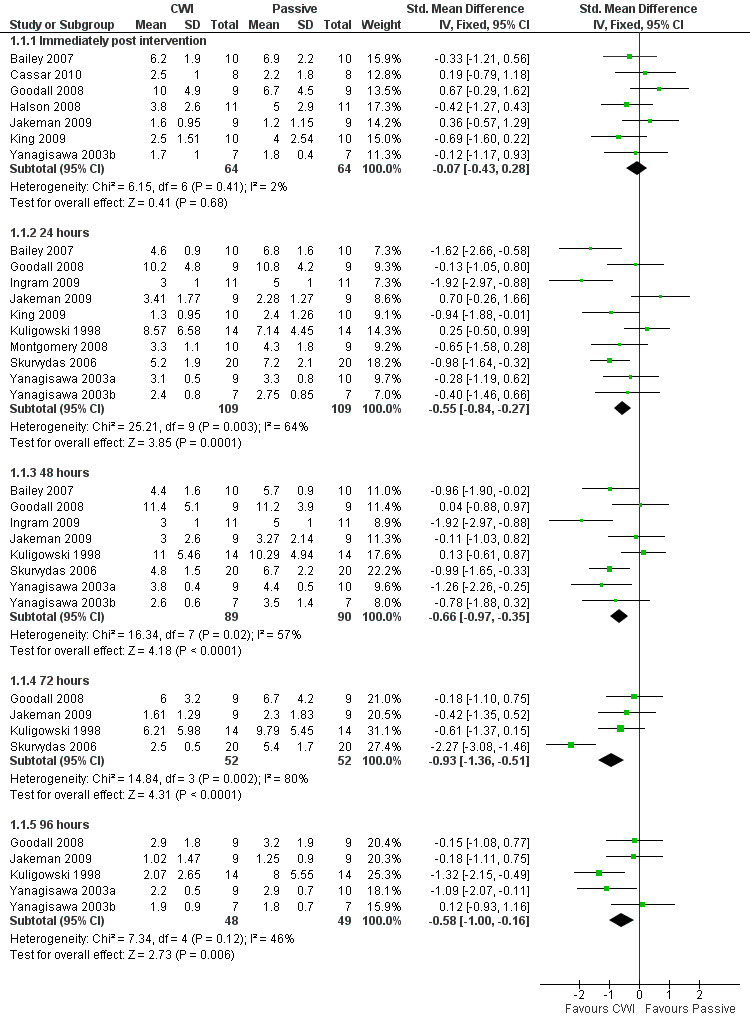
Forest plot of comparison: 1 Cold water immersion (CWI) versus passive, outcome: 1.1 Pain (muscle soreness: various scales Likert and VAS)
1.2. Analysis.
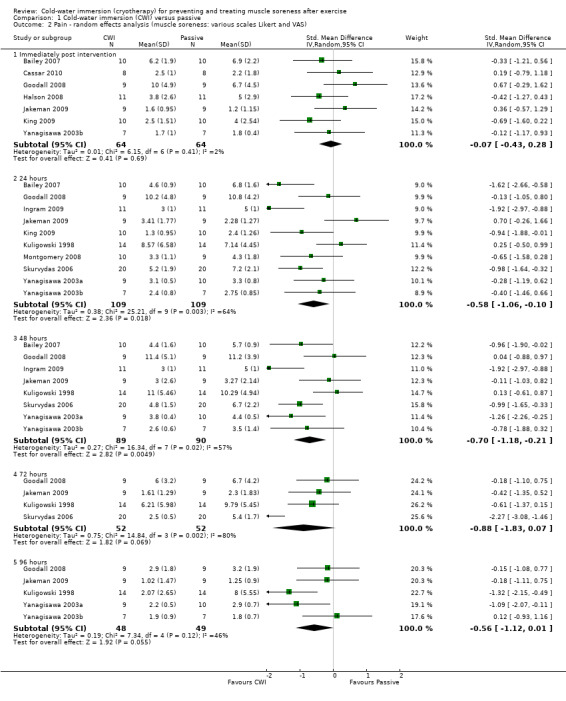
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 2 Pain ‐ random effects analysis (muscle soreness: various scales Likert and VAS).
1.3. Analysis.
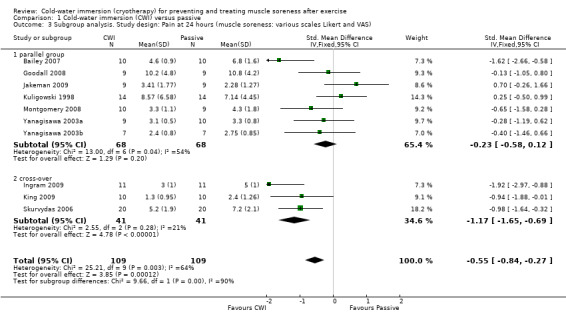
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 3 Subgroup analysis. Study design: Pain at 24 hours (muscle soreness: various scales Likert and VAS).
1.4. Analysis.
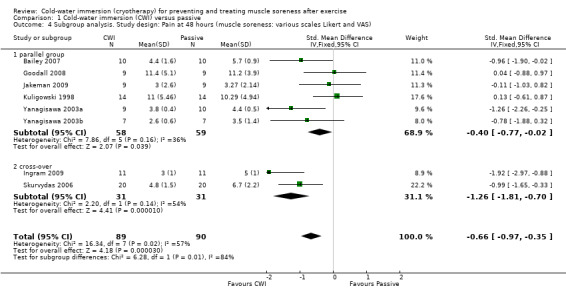
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 4 Subgroup analysis. Study design: Pain at 48 hours (muscle soreness: various scales Likert and VAS).
We had planned to perform subgroup analyses according to the specific details of the treatment intervention: e.g. duration of CWI, frequency of CWI, and water temperature. It was only possible to perform subgroup analysis by frequency of immersion (single immersion versus multiple immersions) which showed no statistically significant differences at 24 hours, seeAnalysis 1.6 (test for subgroup differences: Chi² = 0.10, df = 1 (P = 0.75), I² = 0%); and 48 hours, seeAnalysis 1.7 (test for subgroup differences: Chi² = 0.21, df = 1 (P = 0.64), I² = 0%). (Of note is that Yanagisawa 2003a, which compared single versus double CWI (also at 24 hours after exercise) also found no difference between these two groups in muscle soreness at 24 and 48 hour follow‐up.)
1.6. Analysis.
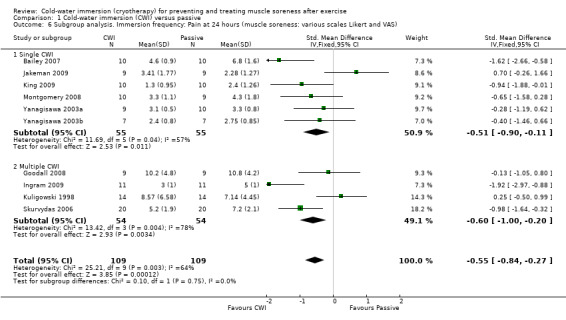
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 6 Subgroup analysis. Immersion frequency: Pain at 24 hours (muscle soreness: various scales Likert and VAS).
1.7. Analysis.
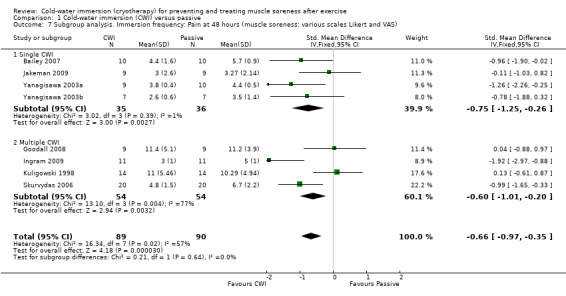
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 7 Subgroup analysis. Immersion frequency: Pain at 48 hours (muscle soreness: various scales Likert and VAS).
Further subgroup analysis based on the type of exercise undertaken showed significant differences at 24 hours, seeAnalysis 1.8 (test for subgroup differences: Chi² = 10.82, df = 1 (P = 0.001), I² = 90.8%); and 48 hours, seeAnalysis 1.9 (test for subgroup differences: Chi² = 5.19, df = 1 (P = 0.02), I² = 80.7%). These suggest that effects were stronger when CWI was used after running based exercises (Bailey 2007; Ingram 2009; King 2009; Montgomery 2008) rather than after resistance exercises under laboratory controlled conditions (Goodall 2008; Jakeman 2009; Kuligowski 1998; Skurvydas 2006; Yanagisawa 2003a; Yanagisawa 2003b). However, it should be noted that there was clear heterogeneity in each subgroup as well as the numbers of participants in each subgroup being small.
1.8. Analysis.
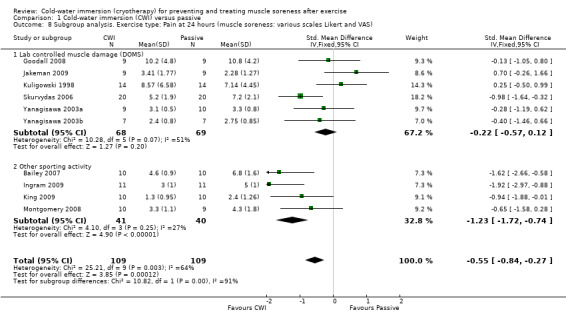
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 8 Subgroup analysis. Exercise type: Pain at 24 hours (muscle soreness: various scales Likert and VAS).
1.9. Analysis.
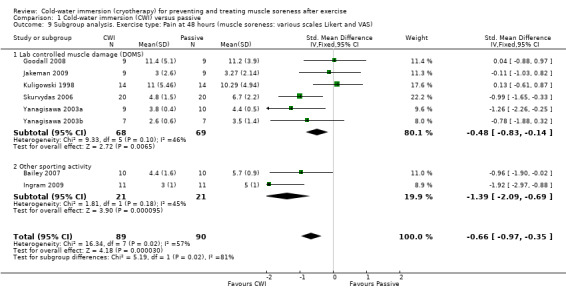
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 9 Subgroup analysis. Exercise type: Pain at 48 hours (muscle soreness: various scales Likert and VAS).
The majority of participants in the DOMS studies were untrained, and those in the running/cycling studies were athletic or were specifically trained (Ingram 2009; King 2009; Montgomery 2008; Rowsell 2009); we therefore did not perform the planned subgroup analysis on trained versus untrained participants, as it was likely to replicate the subgroup analysis by type of exercise.
Given that all studies were at high risk of bias, we were unable to perform our planned subgroup analysis based on risk of bias.
Pain (Tenderness: algometer)
The one study (Eston 1999) reporting this outcome found no differences between groups at 24, 48 and 72 hour follow‐ups (seeAnalysis 1.10).
1.10. Analysis.
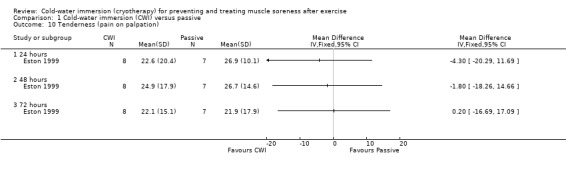
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 10 Tenderness (pain on palpation).
Subjective recovery (10 point or 10 cm VAS)
Four studies measured different components of subjective recovery at immediate (Buchheit 2009; Cassar 2010; Halson 2008) or 24 hour (Montgomery 2008) follow‐up (seeAnalysis 1.11). Two studies (Buchheit 2009; Halson 2008) found that cold‐water immersion tended to result in an immediate improvement in ratings of physical recovery after cycling in hot and humid conditions (MD 0.97 units, 95% CI ‐0.10 to 2.05). However, the results of the two trials are heterogeneous, possibly in part reflecting that Buchheit 2009 involved a short duration cycle (1 km) and Halson 2008, a longer duration cycle (20 minutes). At the same follow‐up (immediate), Halson 2008 found no significant difference in mental recovery (MD 0.60 units, 95% CI ‐0.86 to 2.06).
1.11. Analysis.
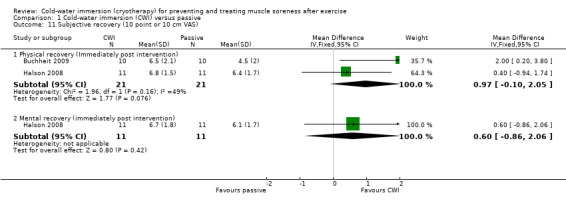
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 11 Subjective recovery (10 point or 10 cm VAS).
Three studies reported subjective rating of fatigue (seeAnalysis 1.12). At immediate follow‐up, the pooled results from two studies (Cassar 2010; Halson 2008) found significantly lower ratings of fatigue in the cold‐water immersion group (MD ‐1.70 units, 95% CI ‐2.49 to ‐0.90). Montgomery 2008 reported lower, but not significantly lower, levels of fatigue in favour of cold‐water immersion, 24 hours after competing in a three day basketball tournament (three interventions undertaken after each match: MD ‐0.70 units, 95% CI ‐1.88 to 0.48).
1.12. Analysis.
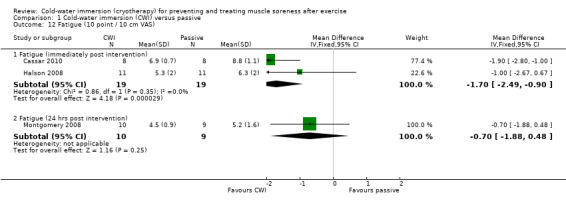
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 12 Fatigue (10 point / 10 cm VAS).
Secondary outcomes
Strength
Studies were placed in two analysis according to the type of continuous data extracted (final outcome value or percentage of baseline (pre‐intervention) value) and subgrouped by follow‐up time.
Strength (Final outcome values: Newton metres (Nm), N or kilograms (kg))
Maximal strength was reported by five studies (Bailey 2007; Buchheit 2009; Eston 1999; Jakeman 2009; Kuligowski 1998) based on final outcome values, and reported over a range of time points post intervention. There was some variation in the measurement device, contraction type and body part tested. Pooled results are displayed in Analysis 1.13. These tended to favour the passive group but showed no significant differences between groups aside from that at immediate follow‐up. However, the lack of robustness of the significant finding for immediate follow‐up using the fixed‐effect model (MD ‐19.92 Nm, 95% CI ‐33.24 to ‐6.59) is demonstrated by the non‐significant effect when using the random‐effects model (MD ‐11.50 Nm, 95% CI ‐44.17 to 21.17; analysis not shown), reflecting the highly significant heterogeneity.
1.13. Analysis.
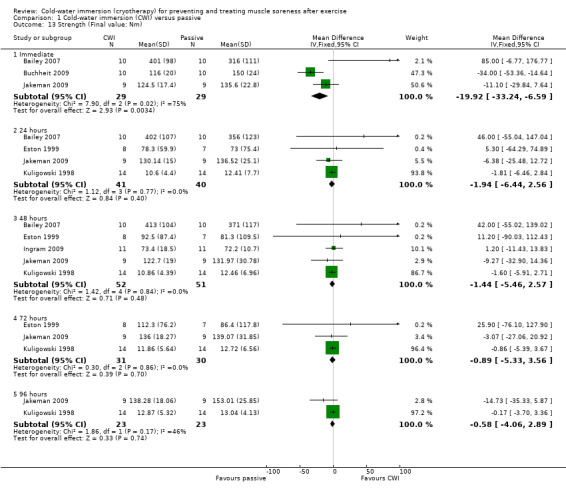
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 13 Strength (Final value: Nm).
Strength (% of baseline)
Two studies (Goodall 2008; Skurvydas 2006) reported isometric quadriceps strength at various follow‐up times, based on the % of the baseline value (baseline = 100%) (seeAnalysis 1.14). Goodall 2008, which used an isokinetic dynamometer, found no significant differences between groups. The cross‐over study (Skurvydas 2006), which did not specify what recording device had been used, found significant differences in favour of cold‐water immersion at 24 hours (MD 22.70%, 95% CI 10.66 to 34.74), 48 hours (MD 27.20%, 95% CI 15.73 to 38.67) and 72 hours (MD 25.20%, 95% CI 13.07 to 37.33) follow‐up.
1.14. Analysis.
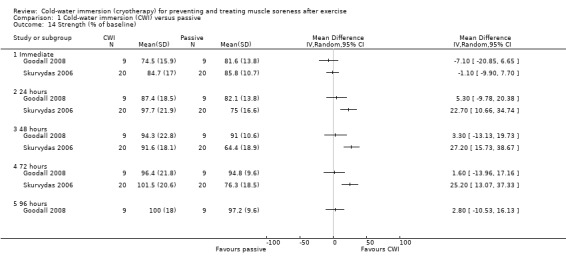
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 14 Strength (% of baseline).
Power
Six studies assessed lower limb power, again results were placed in sub‐categories according to the type of continuous data extracted [Final outcome value or percentage (%) decrement in power performance], measurement device, and follow‐up time.
Power (jumping performance: final outcome data in centimetres)
At 24 hour follow‐up, pooled results from three studies (Bailey 2007; Montgomery 2008; Rowsell 2009) measuring maximal jump height (using a timing mat or similar measuring device) (seeAnalysis 1.15) had low heterogeneity (I² = 0%), and found no difference between groups (MD 0.26 cm, 95% CI ‐2.04 to 2.56). Only Montgomery 2008 undertook additional follow‐up, but found no differences between groups at 48 hours. Skurvydas 2006, a cross‐over trial, also measured jump height, and although final outcome values were presented as flight time in centimetres, these were estimated from force plate data. Skurvydas 2006 found no differences between groups immediately post intervention, but jump height was significantly higher in the cold‐water immersion group at 24 hours (MD 5.40 cm, 95% CI 2.12 to 8.68), 48 hours (MD 9.60 cm, 95% CI 6.40 to 12.80) and 72 hours (MD 6.60 cm, 95% CI 3.16 to 10.04) follow‐up.
1.15. Analysis.
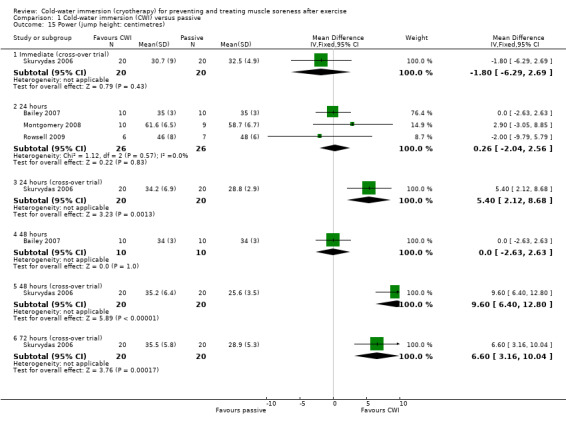
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 15 Power (jump height: centimetres).
Power (jumping performance: percentage decrement)
King 2009 assessed the percentage decrement in jumping performance during five consecutive jumps; this was significantly lower and in favour of cold‐water immersion at 24 hours (MD ‐3.70%, 95% CI ‐7.17 to ‐0.23) (seeAnalysis 1.16).
1.16. Analysis.
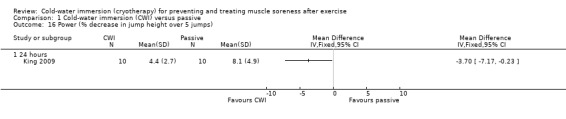
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 16 Power (% decrease in jump height over 5 jumps).
Power (cycling ergometer: Watts)
Buchheit 2009 found no significant difference between groups in peak power output during a 1 km cycling test (seeAnalysis 1.17).
1.17. Analysis.
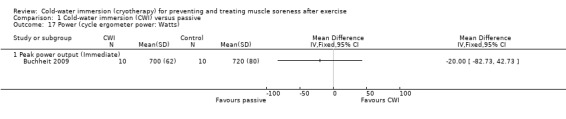
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 17 Power (cycle ergometer power: Watts).
Functional performance
Seven studies (Bailey 2007; Buchheit 2009; Cassar 2010; Ingram 2009; King 2009; Montgomery 2008; Rowsell 2009) reported this outcome. Of these, five were based on the time taken to complete a specific performance task over a range of follow‐up times (seeAnalysis 1.18). Results, which are presented as subcategories based on the nature of the performance task and follow‐up time showed no significant differences between group for the various measures of functional performance. The largest effect was reported by Cassar 2010, where there was less percentage decrease in performance (from baseline) in the time to volitional fatigue during a cycling task in the cold‐water immersion group (MD ‐13.10%, 95% CI ‐27.13 to 0.93) at immediate follow‐up (seeAnalysis 1.19).
1.18. Analysis.
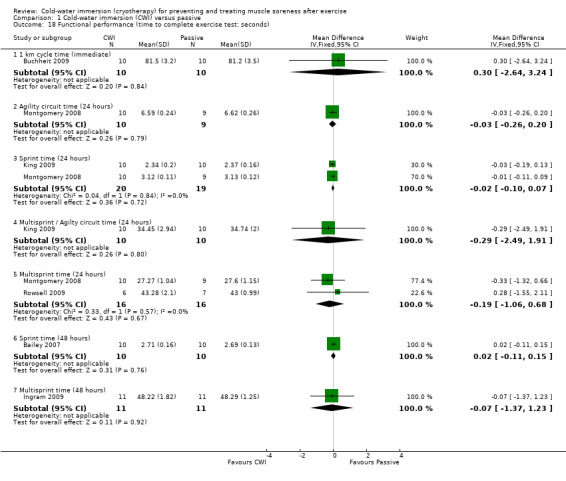
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 18 Functional performance (time to complete exercise test: seconds).
1.19. Analysis.
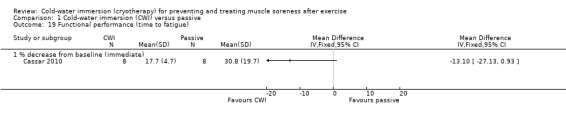
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 19 Functional performance (time to fatigue).
Range of movement
Four studies measured range of movement, three (Goodall 2008; Kuligowski 1998; Yanagisawa 2003a) of which measured active joint range (in degrees) using a hand held goniometer, based on a movement which lengthened the exercised (primary) muscle group. Studies tested range of movement at various body parts: knee (Goodall 2008), elbow (Kuligowski 1998), ankle (Yanagisawa 2003a), or lumbar flexion (Montgomery 2008), which was based on a sit and reach flexibility test (centimetres). All studies presented final value outcomes. Out of the four trials, only Yanagisawa 2003a reported significant between‐group differences, finding higher levels of ankle range of movement in favour of the cold‐water immersion group at immediate, 24, 48 and 96 hours follow‐up (seeAnalysis 1.20).
1.20. Analysis.
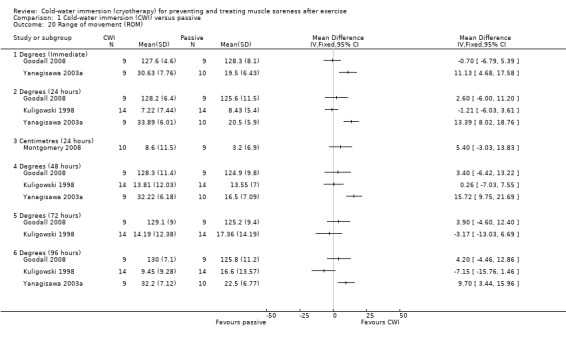
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 20 Range of movement (ROM).
Swelling
Two studies (Eston 1999; Goodall 2008) measured thigh girth using a measuring tape, presenting final value outcomes in centimetres. The pooled results showed no significant differences between groups at 24, 48 or 72 hours follow‐up (seeAnalysis 1.21).
1.21. Analysis.
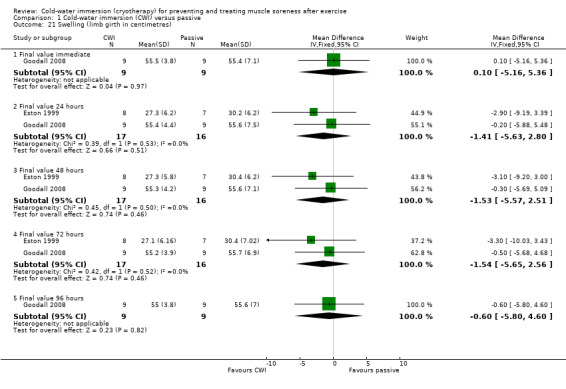
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 21 Swelling (limb girth in centimetres).
Biomarker (muscle damage)
Creatine kinase (CK) was the most commonly reported biomarker. Pooled results found no significant difference between groups at any follow‐up: immediate, 24, 48, 72 and 96 hours follow‐up (seeAnalysis 1.22). Removal of Skurvydas 2006 from the 24 and 48 hour plots, reduces the heterogeneity (respectively, I² = 82% and 84%) to zero.
1.22. Analysis.
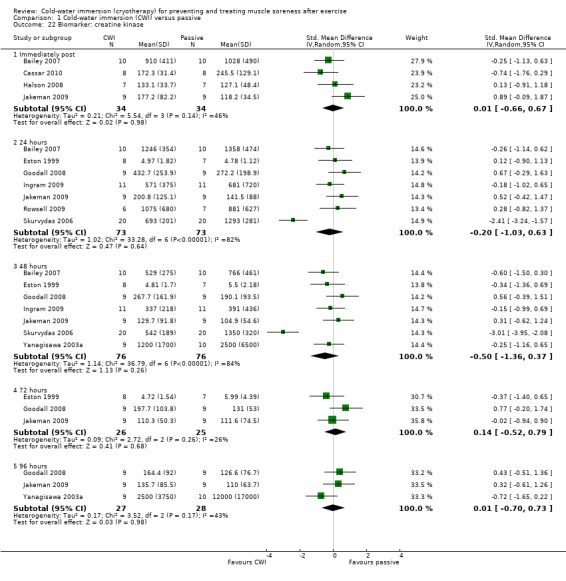
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 22 Biomarker: creatine kinase.
Results from two studies found no differences in lactate dehydrogenase (LDH) at immediate (Cassar 2010) or at 24, 48 and 96 hours follow‐up (Yanagisawa 2003a) (seeAnalysis 1.23). Data from Yanagisawa 2003a are only presented for 96 hours as we were unable to extract accurate data from graphs at the earlier follow‐up points.
1.23. Analysis.
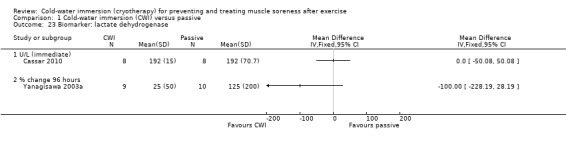
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 23 Biomarker: lactate dehydrogenase.
Two studies (Bailey 2007; Rowsell 2009) reported on myoglobin levels. While tending to favour cold‐water immersion, none of the results for myoglobin levels at immediate, 24 and 48 hours follow‐up were statistically significant (seeAnalysis 1.24).
1.24. Analysis.
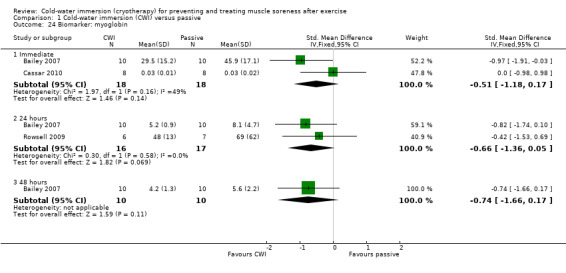
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 24 Biomarker: myoglobin.
Biomarker (inflammatory)
There were similar levels of interleukin‐6 (IL‐6) (Halson 2008) and C‐reactive protein (CRP) (Cassar 2010; Halson 2008) between groups in the immediate follow‐up (seeAnalysis 1.25 and Analysis 1.26). Ingram 2009 reported similar patterns with CRP based on follow‐up at 24 and 48 hours.
1.25. Analysis.
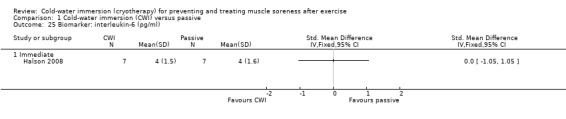
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 25 Biomarker: interleukin‐6 (pg/ml).
1.26. Analysis.
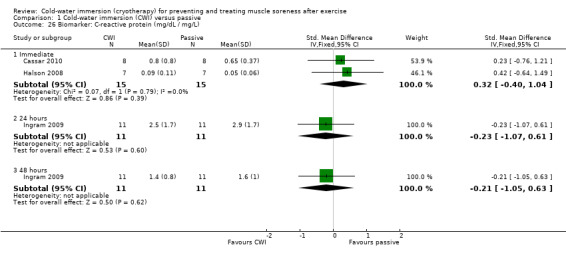
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 26 Biomarker: C‐reactive protein (mg/dL / mg/L).
Cold‐water immersion versus contrast immersion
Primary outcomes
Pain (muscle soreness: VAS, 10 points or 10 or 12 cm VAS)
Five studies reported this outcome based on a 10 point or 10 or 12 cm visual analogue scale with either unbearable (Kuligowski 1998) or extremely sore (Ingram 2009; King 2009; Vaile 2008c) used as the 'worst' pain descriptor. None of the results at five follow‐up times showed a significant difference between the two groups (seeAnalysis 2.1; random‐effects applied due to significant heterogeneity). Only Ingram 2009 showed a significant effect in favour of cold‐water immersion at 24 and 48 hours; it should be noted that results of this trial were reported to no decimal places.
2.1. Analysis.
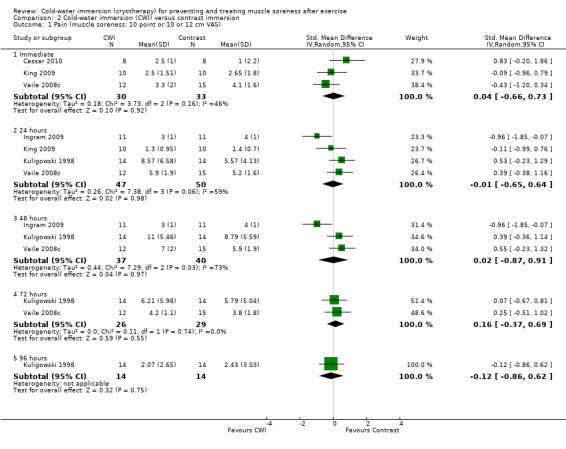
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 1 Pain (muscle soreness: 10 point or 10 or 12 cm VAS).
Subjective recovery (10 point visual analogue scale)
One study (Cassar 2010) found no differences between groups at immediate follow‐up (seeAnalysis 2.2).
2.2. Analysis.
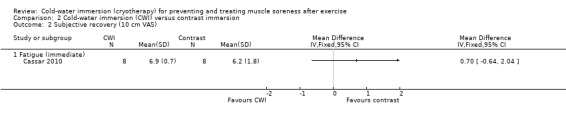
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 2 Subjective recovery (10 cm VAS).
Secondary outcomes
Strength
Three studies (Ingram 2009; Kuligowski 1998; Vaile 2008c) reported strength. The details of the measuring device, and joint movements tested were different across studies. None of the pooled or individual trial results showed significant differences between the two groups at the five follow‐up times (seeAnalysis 2.3).
2.3. Analysis.
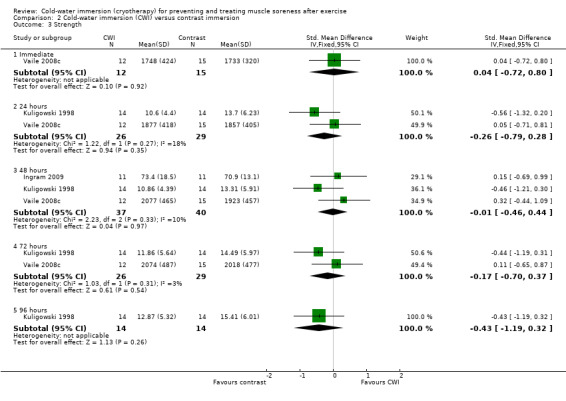
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 3 Strength.
Power
Two studies measured power (King 2009; Vaile 2008c). Vaile 2008c found no between group differences in jump squat performance at any follow‐up (immediate, 24, 48, 72 hours) (seeAnalysis 2.4). King 2009 found no significant difference between groups in the % decrement in jumping performance (over five consecutive jumps) at 24 hours (MD ‐3.70%, 95% CI ‐7.67 to 0.27; seeAnalysis 2.5).
2.4. Analysis.
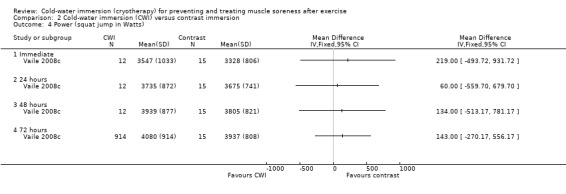
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 4 Power (squat jump in Watts).
2.5. Analysis.
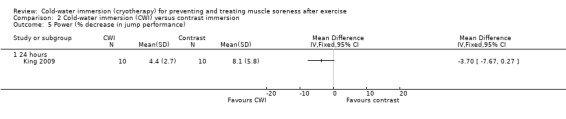
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 5 Power (% decrease in jump performance).
Functional performance
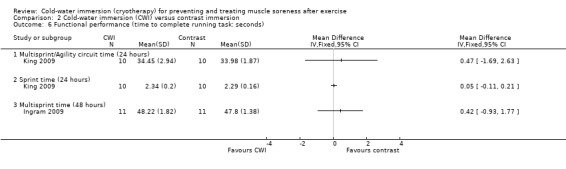
Time to complete running task in seconds
King 2009 recorded similar between‐group running agility times (MD 0.47 seconds, 95% CI ‐1.69 to 2.63) and running sprint times (MD 0.05 seconds, 95% CI ‐0.11 to 0.21) at 24 hours (seeAnalysis 2.6). Ingram 2009 also found that multi‐sprint times were similar between groups at 48 hours (MD 0.42 seconds, 95% CI ‐0.93 to 1.77; seeAnalysis 2.6).
2.6. Analysis.
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 6 Functional performance (time to complete running task: seconds).
Time to fatigue
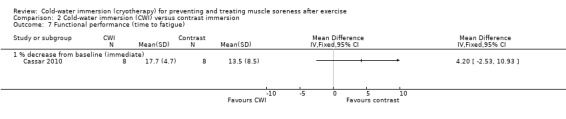
Cassar 2010 found no significant difference between the groups in the percentage decrement in performance (MD 4.20% less decrement in performance, 95% CI ‐2.53 to 10.93) at immediate follow‐up (seeAnalysis 2.7).
2.7. Analysis.
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 7 Functional performance (time to fatigue).
Swelling
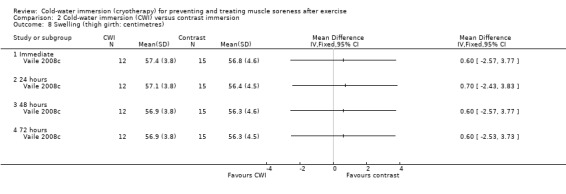
This was reported by one study (Vaile 2008c) with no between‐group differences found (seeAnalysis 2.8).
2.8. Analysis.
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 8 Swelling (thigh girth: centimetres).
Range of movement
This was reported by one study (Kuligowski 1998) with no between‐group differences found (seeAnalysis 2.9).
2.9. Analysis.
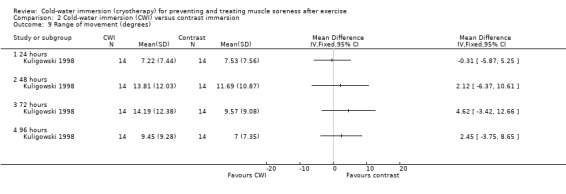
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 9 Range of movement (degrees).
Biomarker (muscle damage)
Data for these outcomes are shown in Analysis 2.10 (creatine kinase), Analysis 2.11 (myoglobin) and Analysis 2.12 (lactate dehydrogenase). Reporting for immediate follow‐up only, Cassar 2010 found significantly lower levels of creatine kinase but higher levels of myoglobin and lactate dehydrogenase in the CWI group. Ingram 2009 found no difference between the two groups in creatine kinase at 24 and 48 hour follow‐ups. The results for Vaile 2008c favoured CWI for all three biomarkers, but the differences between the two groups were only statistically significant for lactate dehydrogenase at 24, 48 and 72 hours (see Analysis 2.12).
2.10. Analysis.
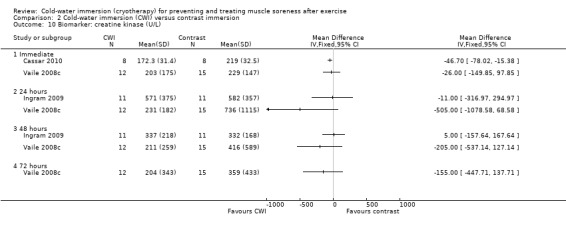
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 10 Biomarker: creatine kinase (U/L).
2.11. Analysis.
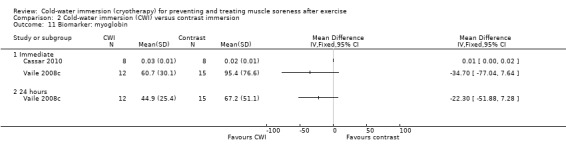
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 11 Biomarker: myoglobin.
2.12. Analysis.
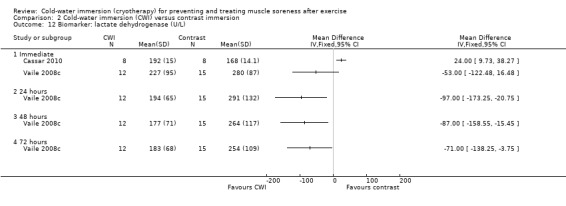
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 12 Biomarker: lactate dehydrogenase (U/L).
Biomarker (inflammatory)
Two trials (Cassar 2010; Vaile 2008c) found no significant differences between groups in inflammatory biomarkers (C‐reactive protein and interleukin‐6) at various follow‐ups (seeAnalysis 2.13 and Analysis 2.14).
2.13. Analysis.
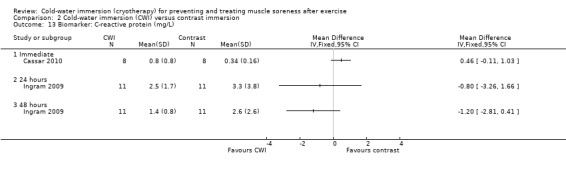
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 13 Biomarker: C‐reactive protein (mg/L).
2.14. Analysis.
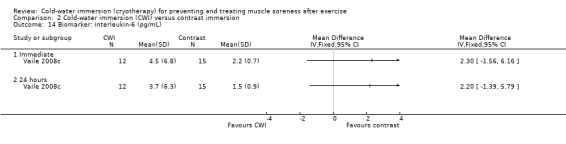
Comparison 2 Cold‐water immersion (CWI) versus contrast immersion, Outcome 14 Biomarker: interleukin‐6 (pg/mL).
Cold‐water immersion versus warm‐water immersion
Four studies made this comparison (Kuligowski 1998; Rowsell 2009; Sellwood 2007a; Vaile 2008c).
Primary outcomes
Pain (muscle soreness: 10 point or 10 or 12 cm VAS)
There were no significant between‐group differences in muscle soreness at immediate (Vaile 2008c), 24 hours (Kuligowski 1998; Rowsell 2009; Vaile 2008c), 48 hours (Kuligowski 1998; Vaile 2008c) or 72 hours (Kuligowski 1998; Vaile 2008c) follow‐up (seeAnalysis 3.1). One study (Kuligowski 1998) found significantly lower levels of pain in favour of CWI at 96 hours (MD ‐4.64 points, 95% CI ‐7.72 to ‐1.56 points on a 12 point scale) (seeAnalysis 3.1).
3.1. Analysis.
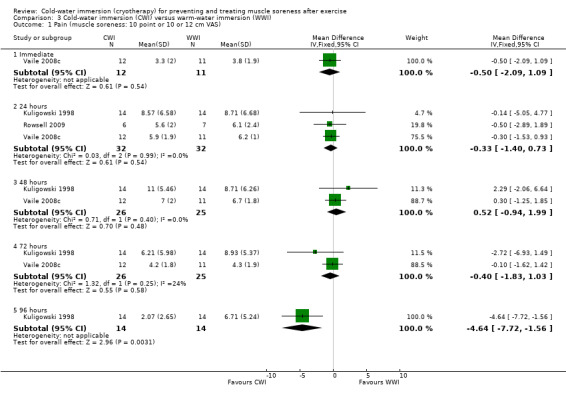
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 1 Pain (muscle soreness: 10 point or 10 or 12 cm VAS).
Sellwood 2007a recorded various pain outcomes based on: muscle soreness during five different activities (sit to stand, passive stretch, hopping running, isometric contraction) and muscle tenderness at two regions of the thigh at 24, 48 and 72 hours follow‐up. The only significant between‐group differences were higher levels of muscle soreness in the cold‐water immersion group during sit to stand at 24 hours; this amounted to a between‐group difference in medians of 6 mm based on a 100 mm VAS.
Subjective recovery
Rowsell 2009 measured two different components of subjective recovery (seeAnalysis 3.2). At 24 hours, they found no difference between groups using a 10 point VAS for levels of fatigue (MD ‐0.80 points, 95% CI ‐3.25 to 1.65). However, also at 24 hours, six out of seven participants of the warm‐water immersion group felt that the intervention had no benefit for recovery, compared to one out of seven participants of the cold‐water immersion group (risk ratio 0.09, 95% CI 0.01 to 1.30) (seeAnalysis 3.3).
3.2. Analysis.
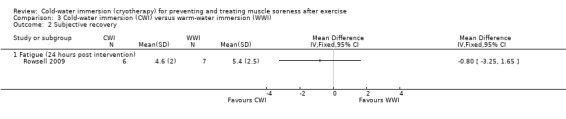
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 2 Subjective recovery.
3.3. Analysis.
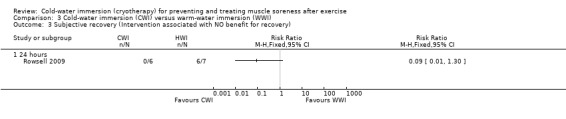
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 3 Subjective recovery (Intervention associated with NO benefit for recovery).
Secondary outcomes
Strength
Individual and pooled results from Kuligowski 1998 and Vaile 2008c showed no significant differences between the two groups in strength at immediate (Vaile 2008c), 24 hour (both trials), 48 hour (both trials), 72 hour (both trials) and 96 hour (Kuligowski 1998) follow‐ups (seeAnalysis 3.4). Sellwood 2007a also reported no significant differences between the two groups in strength at 24, 48 and 72 hours.
3.4. Analysis.
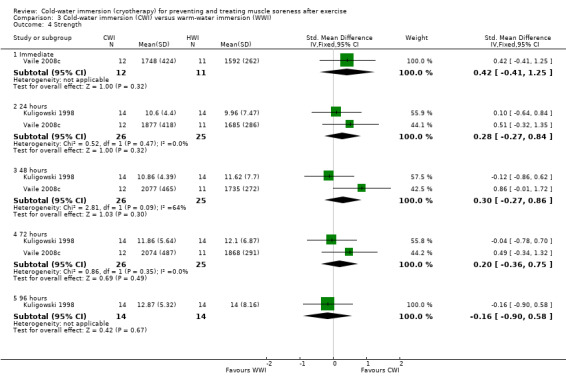
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 4 Strength.
Power
There were no significant differences between groups in terms of counter movement jump height at 24 hours (Rowsell 2009), or peak force produced during a weighted squat jump test at immediate or 24, 48 or 72 hour follow‐ups (Vaile 2008c) (seeAnalysis 3.5). Based on single leg hop distance, Sellwood 2007a also found no significant difference between groups at 24, 48 or 72 hours.
3.5. Analysis.
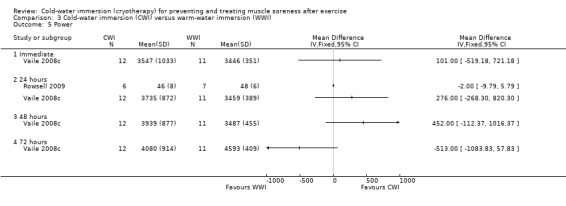
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 5 Power.
Functional performance
One study (Rowsell 2009) found no differences between the two groups in multi‐sprint times at 24 hour follow‐up (MD 0.28 seconds, 95% CI ‐1.55 to 2.11) (seeAnalysis 3.6).
3.6. Analysis.
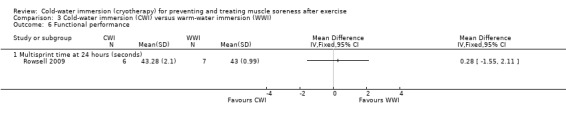
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 6 Functional performance.
Range of movement
Kuligowski 1998 found no differences between the two groups in active elbow range of movement at any follow‐up (24, 48, 72, 96 hours) (seeAnalysis 3.7).
3.7. Analysis.
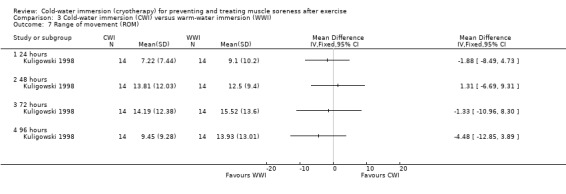
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 7 Range of movement (ROM).
Swelling
Similarly both Vaile 2008c and Sellwood 2007a found no between‐group differences in thigh girth at any follow‐up (immediate, 24, 48, 72 hours) (seeAnalysis 3.8).
3.8. Analysis.
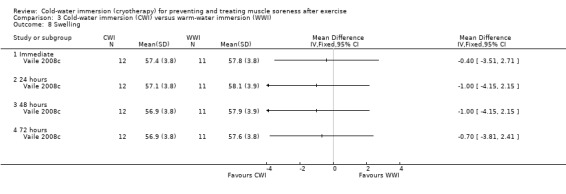
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 8 Swelling.
Biomarker (muscle damage)
Three studies (Rowsell 2009; Sellwood 2007a; Vaile 2008c) found similar levels of creatine kinase and myoglobin across groups (seeAnalysis 3.9, Analysis 3.11). Lactate dehydrogenase (LDH) levels were also similar at immediate (Vaile 2008c) and 24 hours (Rowsell 2009). However, further follow‐ups by Vaile 2008c found significantly lower levels of LDH after cold‐water immersion at 24, 48 and 72 hours (seeAnalysis 3.10).
3.9. Analysis.
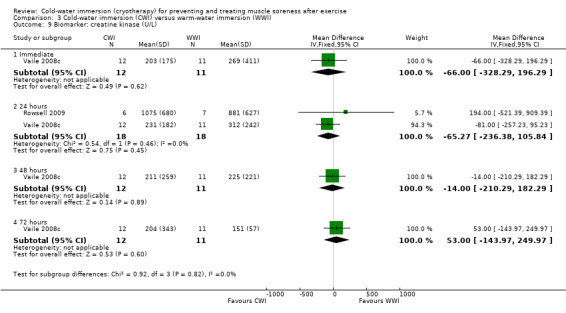
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 9 Biomarker: creatine kinase (U/L).
3.11. Analysis.
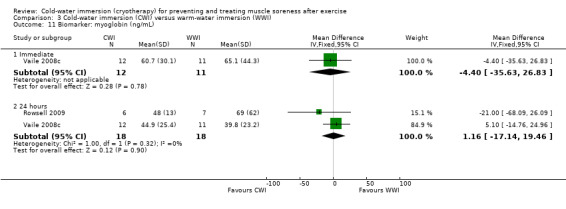
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 11 Biomarker: myoglobin (ng/mL).
3.10. Analysis.
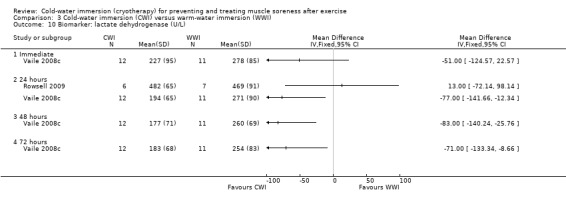
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 10 Biomarker: lactate dehydrogenase (U/L).
Biomarker (Inflammatory)
Interleukin‐6 levels were similar in the two groups at immediate (Vaile 2008c) and 24 hour follow‐up (Rowsell 2009; Vaile 2008c) (seeAnalysis 3.12). At 24 hours, Rowsell 2009 found that interleukin‐10 levels were similar across groups (seeAnalysis 3.13), but found a tendency towards lower levels of interleukin‐1b in the CWI group (seeAnalysis 3.14).
3.12. Analysis.
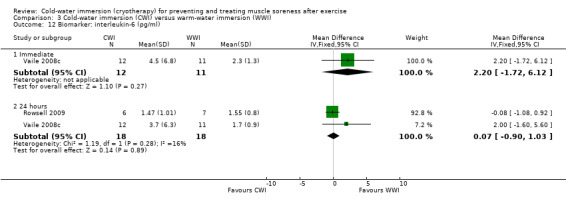
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 12 Biomarker: interleukin‐6 (pg/ml).
3.13. Analysis.
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 13 Biomarker: interleukin‐10 (pg/ml).
3.14. Analysis.
Comparison 3 Cold‐water immersion (CWI) versus warm‐water immersion (WWI), Outcome 14 Biomarker: interleukin‐1b (pg/ml).
Cold‐water immersion versus active recovery
King 2009, in a cross‐over trial involving 10 netballers, found significantly lower levels of muscle soreness in favour of CWI at immediate follow‐up (MD ‐2.65 units, 95% CI ‐4.05 to ‐1.25). Although pain levels were still lower in this group at 24 hours (MD ‐0.90 units, 95% CI ‐2.00 to 0.20), the difference between the two groups was not statistically significant (seeAnalysis 4.1).
4.1. Analysis.
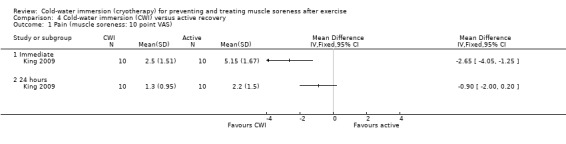
Comparison 4 Cold‐water immersion (CWI) versus active recovery, Outcome 1 Pain (muscle soreness: 10 point VAS).
King 2009 found a significantly smaller decrement in power (measured by the percentage decrement in jump height over five repetitions) in the CWI group at 24 hours (MD ‐3.30%, 95% CI ‐6.14% to ‐0.46%) (seeAnalysis 4.2). Also at 24 hour follow‐up, King 2009 found no differences between groups in terms sprint time (MD ‐0.03 seconds, 95% CI ‐0.21 to 0.15) and time taken to complete a multi‐sprint test with an agility component (MD ‐1.12 seconds, 95% CI ‐3.86 to 1.62) (seeAnalysis 4.3).
4.2. Analysis.
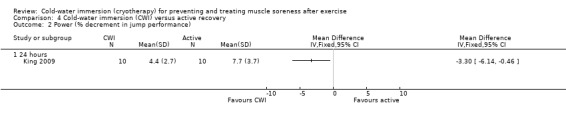
Comparison 4 Cold‐water immersion (CWI) versus active recovery, Outcome 2 Power (% decrement in jump performance).
4.3. Analysis.
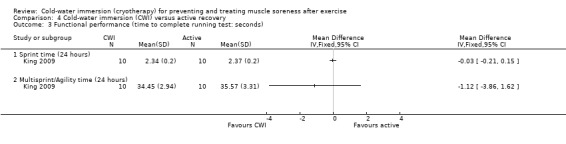
Comparison 4 Cold‐water immersion (CWI) versus active recovery, Outcome 3 Functional performance (time to complete running test: seconds).
Cold‐water immersion versus compression
Only Montgomery 2008 compared CWI with compression therapy (tights). There were no between group differences at 24 hours after completing a three match soccer tournament for any outcome: pain (muscle soreness) (seeAnalysis 5.1), subjective recovery (rating of fatigue) (seeAnalysis 5.2), power (vertical jump) (seeAnalysis 5.3), range of movement (sit and reach) (seeAnalysis 5.4) and two different functional performance tests (time to complete multi‐sprint test and agility test) (seeAnalysis 5.5).
5.1. Analysis.
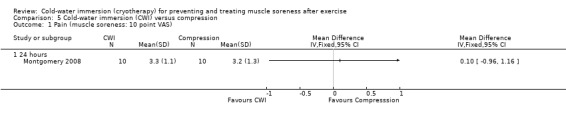
Comparison 5 Cold‐water immersion (CWI) versus compression, Outcome 1 Pain (muscle soreness: 10 point VAS).
5.2. Analysis.
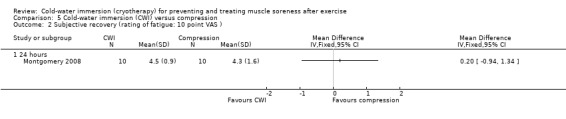
Comparison 5 Cold‐water immersion (CWI) versus compression, Outcome 2 Subjective recovery (rating of fatigue: 10 point VAS ).
5.3. Analysis.
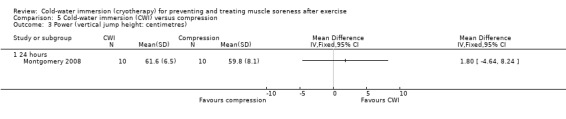
Comparison 5 Cold‐water immersion (CWI) versus compression, Outcome 3 Power (vertical jump height: centimetres).
5.4. Analysis.
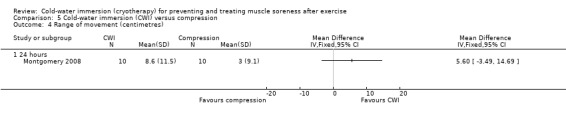
Comparison 5 Cold‐water immersion (CWI) versus compression, Outcome 4 Range of movement (centimetres).
5.5. Analysis.
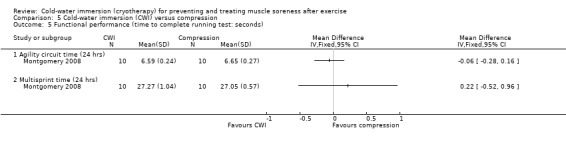
Comparison 5 Cold‐water immersion (CWI) versus compression, Outcome 5 Functional performance (time to complete running test: seconds).
Cold‐water immersion versus multiple cold‐water immersion
Only one study (Yanagisawa 2003a) specifically compared two different treatment dosages of CWI. Both groups undertook CWI immediately after exercise; however, one group used an additional treatment 24 hours later. Summary data were reported from range of movement; however, data were extracted from graphs for the remaining outcomes.
Pain was significantly lower in the single immersion group at 96 hours (MD ‐0.80 units, 95% CI ‐1.36 to ‐0.24 points on a 5 point VAS), but there were no differences between groups in the earlier follow‐ups (24 and 48 hours) (seeAnalysis 6.1). Range of movement (ankle dorsiflexion) was slightly higher in the single immersion group at all follow‐ups; however, no differences were significant (seeAnalysis 6.2). Values for both biomarker outcomes (creatine kinase and lactate dehydrogenase) were very similar across groups at 24 and 48 hour follow‐ups (we were unable to differentiate between groups on the graph). While both levels of both biomarkers were lower in the single immersion group after 96 hours, there were no statistically significant differences between groups (seeAnalysis 6.3 and Analysis 6.4).
6.1. Analysis.
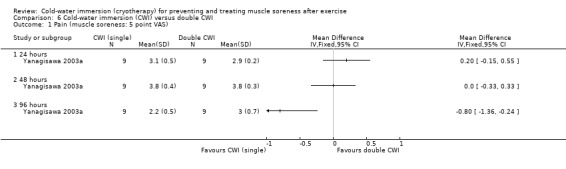
Comparison 6 Cold‐water immersion (CWI) versus double CWI, Outcome 1 Pain (muscle soreness: 5 point VAS).
6.2. Analysis.
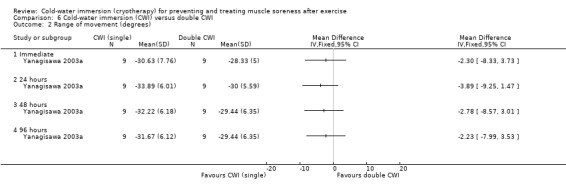
Comparison 6 Cold‐water immersion (CWI) versus double CWI, Outcome 2 Range of movement (degrees).
6.3. Analysis.
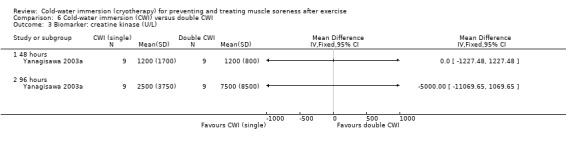
Comparison 6 Cold‐water immersion (CWI) versus double CWI, Outcome 3 Biomarker: creatine kinase (U/L).
6.4. Analysis.
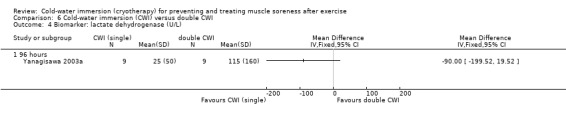
Comparison 6 Cold‐water immersion (CWI) versus double CWI, Outcome 4 Biomarker: lactate dehydrogenase (U/L).
Discussion
Summary of main results
This review examined the effectiveness of cold‐water immersion (CWI) for preventing and treating muscle soreness after exercise. Seventeen studies, involving a total of 366 (mostly young male) participants, were included. For the purposes of the review, we considered exercise to be laboratory induced delayed onset muscle soreness (DOMS) models, running, cycling or team sport activities. We included studies using randomised controlled or randomised cross‐over designs. CWI was generally undertaken immediately after exercise, but the temperatures, durations and frequency of CWI varied across studies. Studies were divided into five clearly distinguishable comparison groups; where available, data were extracted at five short term follow‐up points: immediate, 24, 48, 72 and 96 hours. Muscle soreness and subjective recovery were the primary outcomes. In many cases it was not possible to draw definite well founded conclusions from the comparisons, due to lack of statistical significance and poor methodological quality. The largest volume of data were extracted for the comparison CWI versus passive intervention (specified as rest or no CWI), which was based on 14 studies.
Cold‐water immersion versus passive (no intervention/rest)
Cold‐water immersion resulted in significantly greater improvements in muscle soreness. Pooling of muscle soreness data found a significant effect in favour of CWI at four time points (24, 48, 72 and 96 hours); these findings were upheld when a random‐effects model was applied. Pooled results from cross‐over trials were more favourable for CWI than pooled results from parallel group trials. Subjective recovery and fatigue data were also in favour of CWI; however, these were based on results from just four studies, and mainly at immediate follow‐up. Pooled results from two studies found cold‐water immersion groups had significantly lower ratings of fatigue, and potentially improved ratings of physical recovery.
The Minimal Important Difference (MID) has been defined as “the smallest difference in score in the domain of interest that patients perceive as important, either beneficial or harmful, and which would lead the clinician to consider a change in the patient’s management” (Guyatt 2002). The MID for pain reduction in musculoskeletal conditions of the shoulder has been estimated at 1.4 cm, based on a 10 cm visual analogue scale (Tashjian 2009). One study (Sellwood 2007a) in the current review detailed a sample size calculation which was based on a MID of 25% pain reduction after CWI. The significant findings for muscle soreness reported in favour of CWI (over no intervention/rest) in the current review for 24, 48 and 72 hours all include this MID as shown in Analysis 1.27, where the results for all the trials have been adjusted to fit the same 10 cm VAS (24 hours: MD ‐1.27 cm, 95% CI ‐1.70 to ‐0.84; 48 hours: MD ‐1.58 cm, 95% CI ‐2.07 to ‐1.10; 72 hours: MD ‐2.16 cm, 95% CI ‐2.79 to ‐1.53). While Analysis 1.27 should be considered an exploratory analysis, it illustrates that the statistical significant differences are likely to be clinical relevant also. The clinical relevance of such differences may be dependent also on numerous factors such as the time of the outcome, the capacity for natural recovery after DOMS, and the time and costs associated with CWI. We suggest that a 13% to 22% difference in muscle soreness would be important for many athletes, particularly those in an elite sporting environment.
1.27. Analysis.
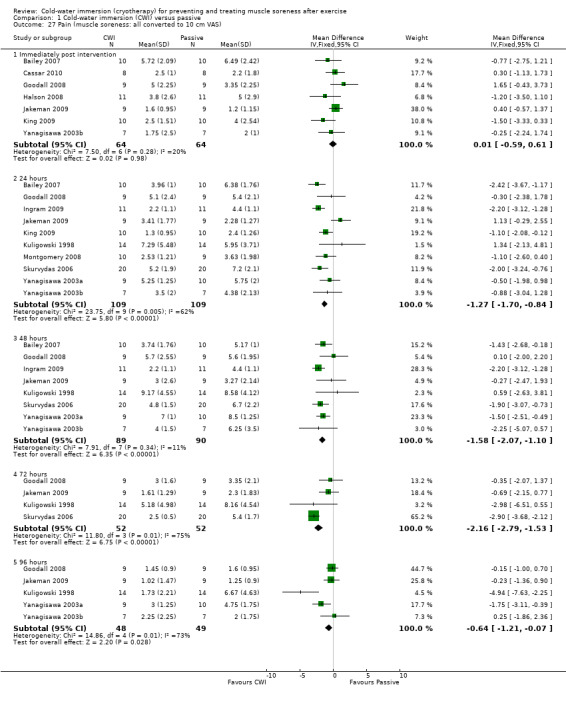
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 27 Pain (muscle soreness: all converted to 10 cm VAS).
Due to clinical and methodological diversity, it was not possible to perform extensive pooling of secondary outcome data from studies comparing CWI and passive. In particular, there were variable methods used for reporting similar outcomes across studies. In terms of isokinetic strength, there was conflicting evidence from three studies at the immediate follow‐up (Bailey 2007; Buchheit 2009; Jakeman 2009); this is likely to relate to differences in the type of exercise, body part and measurement device employed. A single study (Skurvydas 2006) reported significant differences in favour of CWI for isometric strength at all follow‐ups, but provided no details on the measurement device. Pooled data from three studies (Bailey 2007; Montgomery 2008; Rowsell 2009) found no differences in jump height (muscular power) at each follow‐up point. This conflicted with the results of a single study (Skurvydas 2006) which reported effects in favour of CWI; this study was not pooled based on differences in exercise type, and measurement device. No differences were reported based on muscular power measured on a cycle ergometer; and although one study (King 2009) found significantly less decrement in jumping performance in favour of CWI, this may not be a clinically important change (MD of 3%). Three out of four studies found no differences in range of movement, with one study (Yanagisawa 2003a) reporting significant differences in favour of CWI at four follow‐up points. There were no differences between CWI and rest in terms of swelling, functional performance and a range of biomarker outcomes.
Cold‐water immersion versus warm‐water immersion or contrast immersion or active recovery or compression
Despite a large number of treatment comparisons, there was little evidence for a superior treatment intervention. Pooled analyses found no differences in terms of muscle soreness between CWI versus contrast immersion, or CWI versus warm‐water immersion. In accordance, a single study (Sellwood 2007a), which had a low risk of bias, reported no differences in muscle soreness between CWI versus warm‐water immersion. Kuligowski 1998 found a large effect (MD 4.6 points on a 12 cm VAS) in favour of CWI over warm‐water immersion, at 96 hour follow‐up.
There were very few differences in any of the reported secondary outcomes, when CWI was compared with contrast immersion, warm‐water immersion, active recovery or compression tights. One study (King 2009) found a significant effect in favour of active recovery based on a single secondary outcome; but this result was of questionable clinical relevance (MD of 3.3% in muscular power).
Overall completeness and applicability of evidence
We located 17 studies with a total of 366 participants; substantially fewer participants were available for pooling. The majority of participants were young (mean ages between 16 and 29 years), and only 19% were female. Although this probably reflects the current CWI user population in terms of age, we had expected larger numbers of female participants within this review.
We could not find any reports of adverse events or complications in the included studies. One study (Halson 2008) specified that short duration immersion (three minutes) in 11.5°C water temperatures, after exercise in hot conditions, does not alter core temperature to dangerous or hypothermic levels. However, the remainder of studies did not undertake active surveillance for pre‐defined adverse events; subsequently the short and long term safety of CWI and comparison interventions remains unknown.
The type of exercise undertaken prior to CWI varied across studies. Eight studies involved trained athletes who used CWI after familiar bouts of cycling or running based exercise. The remaining nine studies used resistance exercises under laboratory controlled conditions to produce DOMS, largely within an untrained population. A subgroup analysis indicated that CWI had a stronger effect on muscle soreness after running based exercises, and was less effective when used after resistance exercises (DOMS models). The significance of these results is difficult to ascertain based on the high risk of bias, the heterogeneity of the trials within each subgroup and the small number of studies and participants within each subgroup. A key difference is that resistance exercises under laboratory controlled conditions are associated with high levels of muscle damage and pain, particularly in an untrained population. In contrast, trained athletes undertaking running based exercises have lower risk of muscle damage and generally have a higher capacity for recovery after exercise.
Trained or elite athletes are most likely to use CWI regularly. Although DOMS is most prevalent in novice athletes, we acknowledge that it may also occur in elite sport: e.g. at the beginning of the sporting season (when returning to training following a period of reduced activity), or after the introduction of a new movement or training method. Rehabilitative eccentric training (e.g. in the management of tendinopathy) may also be associated with DOMS; however, our findings are less applicable to this setting.
It is thought that CWI can reduce muscle soreness through a number of mechanisms including: intracellular‐intravascular fluid shifts, reduction of muscle oedema, increased cardiac output (without increasing energy expenditure), enhanced blood flow, nutrient and waste transportation, with an additional psychological benefit (Wilcock 2006). The potential for inducing each of these effects may relate to the type of CWI undertaken. The details of CWI varied in the current review, with water temperatures and immersion time ranging between 5°C and 15°C, and from 3 to 24 minutes respectively. It is not clear whether such variations in treatment parameters affect clinical outcomes. Interestingly, our subgroup analysis found no differences between single and multiple cold‐water immersions based on muscle soreness outcome. Further research will ascertain if different dosing schedules are more effective or if there is a specific time frame after exercise when CWI is most effective.
We must also consider that other mechanisms of effect for CWI may exist. Immersion in ice or cold‐water is current best practice for managing exertional hyperthermia (McDermott 2009; Smith 2005). CWI may also be of value in cases of mild hyperthermia, as reducing core temperature after exercise should minimise perceived fatigue, enhancing subsequent exercise performance. Three studies (Buchheit 2009; Halson 2008; Rowsell 2009) in the current review, used CWI after exercise in hot conditions (35°C and 40% humidity); however, none reported large effects. We noted that their maximum duration of CWI was five minutes and suggest that longer periods may be required to achieve a clinically relevant effect.
CWI is generally used to enhance recovery after exercise. Reducing muscle soreness is considered to be an important component of recovery, and as such was the most reported primary outcome in this review. Restoration of muscle strength and power, range of movement, and functional performance are also considered to be important; these were included as secondary outcomes. However, we found few significant differences in these outcomes. We acknowledge that our range of secondary outcomes was not exhaustive and the effect of CWI on full system recovery was not fully addressed. There is evidence also from other systematic reviews (Bleakley 2010) that in certain clinical circumstances, CWI can induce significant biochemical effects including oxidative stress and a potential increase in free radical species formation.
Quality of the evidence
The study quality in this review was low. The majority of studies had a high risk of bias making the validity of most of the results uncertain. The sample size of included studies was also consistently small, raising questions as to the power of individual trials.
We were unable to meaningfully subgroup studies into high and low quality. Just over half of the studies used adequate sequence generation; failure to do this can overestimate treatment effects (Kunz 2007). Concealment of group allocation is also important in this regard (Pildal 2007), particularly when dealing with subjective outcomes (Wood 2008), but was undertaken in just one study (Sellwood 2007a). Blinding was also poorly performed. We acknowledge that, based on the nature of CWI and the comparison interventions, stringent blinding of participants and caregivers is virtually impossible. However, blinding of assessors for most of the outcomes is feasible but was reported in just one study (Sellwood 2007a). The primary outcomes in this review were patient reported. However, a lack of assessor blinding is likely to implicate the secondary outcomes which were mainly objective measures (e.g. strength, swelling, range of movement). Few studies adequately described their approach to analysis; in many cases there was no detail on drop‐outs, exclusions, missing data or follow‐up points, and we were unable to consider intention‐to‐treat analysis. Previous Cochrane reviews (Bennett 2005; Herbert 2011) dealing with DOMS studies also found that often missing data were inadequately dealt with, and that few studies indicated intention‐to‐treat analysis.
Muscle soreness resulting from exercise is usually short lived and self limiting. Cross‐over designs are therefore popular in this area of research, with seven included in the current review (however, the cross‐over component of Vaile 2008c was not relevant to this review). Cross‐over designs can risk certain carry‐over effects between treatment periods, which are not present in parallel group designs. Perhaps the most likely source of carry‐over is insufficient recovery from the first exercise bout, particularly when studies induce DOMS on an untrained population. This carry‐over was minimised in the current review as only one of the cross‐over studies in this review used a laboratory based DOMS model and they allowed several months between treatment periods. The remainder of cross‐over studies used a shorter time (between 3 and 14 days) between treatment periods; however, it is possible that these times were also appropriate as they used trained individuals completing familiar cycling or running exercises, who were likely to recover faster.
Potential biases in the review process
In this review, we undertook an extensive search of electronic databases and grey literature sources; 17 studies were included, one of which was unpublished. Although we think this was an adequate selection, it is difficult to fully exclude publication bias, study identification bias and study selection bias. We also acknowledge that additional research studies have been undertaken and published since our searches were completed in February 2010. An exploratory search update carried out in November 2011 resulted in the identification of four potentially eligible trials. Based on information provided in the abstracts, two trials (Ascensao 2011; Vaile 2011), which involved a total of 30 participants, are likely to be included in a future update, while the other two trials (Pournot 2011; Stacey 2010), involving a total of 50 participants, may be excluded. Given this, we anticipate that the results of the current review are unlikely to be substantially affected by the inclusion of data from these trials.
Missing data within this review include, in particular, missing outcomes and summary data. As none of the included studies had a registered protocol, bias from selective reporting of results was difficult to fully ascertain. Although we made a concerted attempt to retrieve missing summary data, we were unable to contact some of the authors. To minimise the missing summary data, some of the data presented in the review graphs were estimated from graphs published in the trial reports. While clearly unsatisfactory, this was the best we could do in the absence of information from the investigators. To minimise error, estimations were undertaken by two independent reviewers, with inconsistencies checked through reviewer consensus and a third party.
Six of the included studies used a randomised cross‐over design. We were unable to perform any paired analysis, and data were analysed as if these studies used a parallel group design. Cross‐over studies were also combined with parallel group trials in the same meta‐analysis. Although this approach gives rise to bias through unit of analysis error, this is expected to be conservative as cross‐over studies analysed in this way tend to be under‐weighted (Deeks 2008). In contrast, our subgroup analysis showed that cross‐over designs had a more positive effect on the primary outcome (muscle soreness), compared with parallel group trials. The reasons for this are not clear; however, it may indicate that parallel studies represent the best methodological design for future research in this area.
Three studies used a unique approach based on three to four days of exercising with daily interventions undertaken after each exercise bout. This produced multiple outcome data over a number of days. Within this review, we only focused on outcomes reported after the final exercise session. This provided an indication of the cumulative effect of using CWI throughout the course of the entire three to four day tournament. As we did not extract data after each match, we may have overlooked important intermittent effects.
Finally, we consider that the practical relevance of some of the secondary outcome measures should be questioned and their results interpreted cautiously. Although there were few significant differences reported, many different types of tests were used and the clinical importance of each may be difficult to assess. In some cases (e.g. functional outcomes based on the time taken to complete an exercise test), the clinical significance of the results may depend on the type of exercise, or level of competition. Although we considered a large number of biochemical outcome markers, some were not included as they were not considered to be applicable to our review objectives. These decisions were made based on the current evidence base but may change in light of new evidence in this area.
Agreements and disagreements with other studies or reviews
Recent narrative reviews (Barnett 2006; Cheung 2003) of the best methods of recovery after exercise report little to no evidence for the use of massage, active recovery, contrast water immersion therapy, cryotherapy (including CWI), hyperbaric oxygen therapy, non‐steroidal anti‐inflammatory drugs, compression garments, stretching, electromyostimulation, combination modalities, homeopathy, ultrasound and electrical current modalities.
Two Cochrane reviews have examined respectively the effectiveness of stretching (Herbert 2011) and hyperbaric oxygen therapy (Bennett 2005) for treating muscle soreness after exercise. Both reviews found little evidence to support the use of each of the respective interventions. Similarly a systematic review (Hing 2008) of randomised controlled studies reported no evidence to support the use of contrast water immersion for aiding recovery after exercise, with the majority of included studies reported as being of limited methodological quality.
Cryotherapy remains a popular intervention for treating muscle strains, joint sprains and contusions. The rationale is somewhat similar to the post exercise environment, and various forms of cooling (including CWI) are used to relieve pain relief, decrease local tissue metabolism and inflammation, and redirect blood flow in the acute phases after injury. A systematic review of randomised controlled trials (Bleakley 2004) found limited evidence that various cooling interventions can induce analgesia after acute soft tissue injury and orthopaedic surgery, and found little to no effect on other important clinical outcomes.
Authors' conclusions
Implications for practice.
Muscle soreness commonly results after sports and exercise activity. A priority for clinicians and athletes is to encourage full recovery between exercise bouts using an effective intervention programme. This review provides some evidence that cold‐water immersion reduces delayed onset muscle soreness after exercise when compared with passive interventions involving rest or no intervention. However, it is not possible to draw definitive conclusions on pain or recovery because of the poor methodological quality, and small sample sizes. The majority of reviewed trials failed to undertake active surveillance of pre‐defined adverse events relating to cold‐water immersion. Given these findings and the self‐limiting nature of most forms of muscle soreness resulting from exercise, there is currently only limited evidence for using cold‐water immersion after exercise. There are no data available to determine the optimal method of cold‐water immersion.
Implications for research.
High quality, well reported research in this area is required. In particular, future studies should: incorporate a randomised controlled parallel group design with adequate sequence generation and allocation concealment; use appropriate sample sizes with power to detect expected differences; use cold‐water immersion protocols based on a defined physiological rationale in accordance with the type of exercise and climatic environment; use effective and explicit blinding of outcome with appropriate and full reporting of data; and undertake active surveillance of pre‐defined adverse events.
Notes
The search update in November 2011 to assess recent literature was initiated and carried out by the editorial base of the Cochrane Bone, Joint and Muscle Trauma Group. Study selection was performed by Helen Handoll.
Acknowledgements
We acknowledge the contribution of Philip Glasgow to the protocol and early drafts of the review.
We would like to thank Dr Nigel Hanchard, Dr Helen Handoll, Dr Vicki Livingstone, Dr Paddy Pearce, Dr Janet Wale and Mr Matthew Wright for valuable comments on the protocol and review. In particular, we acknowledge Dr Joanne Elliott for her help with the search strategy, and Mrs Lindsey Elstub for her help during editorial processing.
Appendices
Appendix 1. Search strategies
Note: Search strategies for CENTRAL, MEDLINE, EMBASE and CINAHL include line numbers for the search update carried out in November 2011:
Cochrane Central Register of Controlled Trials (Wiley InterScience interface)
#1 MeSH descriptor Cryotherapy, this term only (36) #2 MeSH descriptor Cold Temperature, this term only (990) #3 MeSH descriptor Immersion, this term only (203) #4 (#2 AND #3) (52) #5 (#1 OR #4) (417) #6 ((cold or ice or contrast) NEAR/3 (immers* or bath or therapy)):ti,ab,kw (901) #7 (cryotherapy):ti,ab,kw (727) #8 (#5 OR #6 OR #7) (1558) #9 MeSH descriptor Exercise, this term only (7811) #10 MeSH descriptor Muscle, Skeletal, this term only (4004) #11 MeSH descriptor Athletic Injuries, this term only (0) #12 MeSH descriptor Soft Tissue Injuries, this term only (0) #13 MeSH descriptor Creatine Kinase explode all trees (1111) #14 MeSH descriptor Physical Exertion, this term only (3090) #15 MeSH descriptor Muscle Fatigue, this term only (480) #16 MeSH descriptor Muscle Cramp, this term only (127) #17 MeSH descriptor Spasm, this term only (171) #18 MeSH descriptor Muscle Rigidity, this term only (58) #19 MeSH descriptor Sprains and Strains, this term only (0) #20 MeSH descriptor Muscle Weakness, this term only (194) #21 (sore* NEAR/3 musc*):ti,ab,kw (328) #22 (DOMS):ti,ab,kw (100) #23 (exercise induced and (muscle* NEAR/2 (damage* or injur*))):ti,ab,kw (124) #24 MeSH descriptor Lactic Acid, this term only (1497) #25 (lactate* or lactic):ti,ab,kw (5373) #26 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) (18950) #27 (#8 AND #26), from 2010 to 2011 (12)
MEDLINE (Ovid interface)
1 Cryotherapy/ (3223) 2 Cold Temperature/ (38292) 3 Immersion/ (4157) 4 2 and 3 (682) 5 1 or 4 (3896) 6 ((cold or ice or contrast) adj3 (immers$ or bath or therapy)).tw. (3113) 7 cryotherapy.tw. (4562) 8 5 or 6 or 7 (9925) 9 Exercise/ (57099) 10 Muscle, Skeletal/ (89754) 11 Athletic Injuries/ (18407) 12 Soft Tissue Injuries/ (2989) 13 exp Creatine Kinase/ (22424) 14 Physical Exertion/ (51283) 15 Muscle Fatigue/ (4927) 16 Muscle Cramp/ or Spasm/ or Muscle Rigidity/ or "Sprains and Strains"/ or Muscle Weakness/ (16965) 17 (sore$ adj3 musc$).tw. (964) 18 DOMS.tw. (303) 19 (exercise induced and (muscle$ adj2 (damage$ or injur$))).tw. (488) 20 Lactic Acid/ (27006) 21 (lactate$ or lactic).tw. (95395) 22 or/9‐21 (336771) 23 and/8,22 (557) 24 randomized controlled trial.pt. (323380) 25 controlled clinical trial.pt. (84102) 26 randomized.ab. (238622) 27 placebo.ab. (134849) 28 randomly.ab. (174807) 29 trial.ab. (247461) 30 groups.ab. (1147391) 31 29 or 27 or 24 or 26 or 30 or 25 or 28 (1671645) 32 animals/ not (humans/ and animals/) (3631995) 33 31 not 32 (1371077) 34 33 and 23 (157) 35 limit 34 to ed=20100101‐20120101 (40)
EMBASE (Ovid Interface)
1 Cryotherapy/ (9985) 2 Low temperature procedures/ or Cold treatment/ or Cooling/ or Cooling water/ or Cold/ (30464) 3 ((cold or ice or contrast) adj3 (immers$ or bath or therapy)).tw. (3510) 4 cryotherapy.tw. (5525) 5 or/1‐4 (45294) 6 Exercise/ or Muscle exercise/ or Exercise recovery/ (145790) 7 exp Skeletal Muscle/ (174363) 8 Muscle Injury/ (6215) 9 Soft tissue injury/ or Sport injury/ (25459) 10 Creatine Kinase/ or Creatine kinase blood level/ (29906) 11 Muscle Fatigue/ (7631) 12 Muscle Stiffness/ or Muscle Cramp/ or Muscle Spasm/ or Muscle Tightness/ or Muscle Rigidity/ or Muscle Strain/ or Muscle Weakness/ (43431) 13 (sore$ adj3 musc$).tw. (1064) 14 DOMS.tw. (359) 15 (exercise induced and (muscle$ adj2 (damage$ or injur$))).tw. (521) 16 Lactic Acid/ (37432) 17 (lactate$ or lactic).tw. (100509) 18 or/6‐17 (493490) 19 and/5,18 (2675) 20 Clinical trial/ (821137) 21 Randomized controlled trial/ (292701) 22 Randomization/ (54986) 23 Single blind procedure/ (14442) 24 Double blind procedure/ (101701) 25 Crossover procedure/ (31195) 26 Placebo/ (187497) 27 randomi?ed controlled trial$.tw. (66241) 28 rct.tw. (7997) 29 random allocation.tw. (1065) 30 randomly allocated.tw. (15806) 31 allocated randomly.tw. (1718) 32 (allocated adj2 random).tw. (688) 33 single blind$.tw. (11219) 34 double blind$.tw. (119142) 35 ((treble or triple) adj blind$).tw. (250) 36 placebo$.tw. (161437) 37 Prospective study/ (176527) 38 or/20‐37 (1156411) 39 Case study/ (13795) 40 case report.tw. (210181) 41 Abstract report/ or Letter/ (799539) 42 or/39‐41 (1019415) 43 38 not 42 (1122896) 44 limit 43 to human (1023188) 45 19 and 44 (330) 46 (2010$ or 2011$).em. (2360483) 47 45 and 46 (75)
CINAHL (EBSCO Interface)
S1 (MH "Cryotherapy") (994) S2 "water immersion" OR "ice" OR "cold" OR "cooling" (10204) S3 S1 or S2 (10896) S4 Muscle or injury or sport or soft tissue or stiffness or cramp or spasm or recovery or exercise or performance (260896) S5 S3 and S4 (2266) S6 (MH "Clinical Trials+") (132628) S7 (MH "Evaluation Research+") (17559) S8 (MH "Comparative Studies") (64058) S9 (MH "Crossover Design") (8666) S10 PT Clinical Trial (68624) S11 (MH "Random Assignment") (31138) S12 S6 or S7 or S8 or S9 or S10 or S11 (217079) S13 TX ((clinical or controlled or comparative or placebo or prospective or randomi?ed) and (trial or study (372280) S14 TX (random* and (allocat* or allot* or assign* or basis* or divid* or order*)) (54007) S15 TX ((singl* or doubl* or trebl* or tripl*) and (blind* or mask (588697) S16 TX ( crossover* or 'cross over' ) or TX cross n1 over (10971) S17 TX ((allocat* or allot* or assign* or divid*) and (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)) (67272) S18 S13 or S14 or S15 or S16 or S17 (890995) S19 S12 or S18 (945591) S20 S5 and S19 (1164) S21 (MH “Animals”) (40857) S22 S20 Not S21 (138) S23 2010 or 2011 (702465) S24 S22 and S23 (224)
PEDro
Using the 'Advanced search' option, each term in the ‘Abstract & Title’ field was combined with each term in the ‘Subdiscipline’ field in separate searches.
Abstract & Title: cryotherapy/ cold/ immersion/ hydrotherapy/"contrast therapy"/ ice/ water/CWI Subdiscipline: musculoskeletal/ orthopaedics/ sports/ Method: clinical trial Match all search terms (AND)
BNI (Ovid Interface)
1 Cryotherapy.mp. 2 immersion.mp. 3 ((cold or ice or contrast) adj3 (immers$ or bath or therapy)).tw. 4 exercise/ or muscle exercise/ or exercise recovery/ 5 exp "Musculoskeletal System and Disorders"/ 6 soft tissue injury.mp. 7 creatine kinase.mp. 8 muscle.mp. 9 lactic acid.mp. 10 athletic.mp. 11 physical exertion.mp. 12 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13 cold.mp. 14 body temperature/ or low temperature.mp. 15 cooling.mp. 16 1 or 2 or 3 or 13 or 14 or 15 17 12 and 16
Data and analyses
Comparison 1. Cold‐water immersion (CWI) versus passive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (muscle soreness: various scales Likert and VAS) | 12 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Immediately post intervention | 7 | 128 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.43, 0.28] |
| 1.2 24 hours | 10 | 218 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.84, ‐0.27] |
| 1.3 48 hours | 8 | 179 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| 1.4 72 hours | 4 | 104 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.36, ‐0.51] |
| 1.5 96 hours | 5 | 97 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [1.00, ‐0.16] |
| 2 Pain ‐ random effects analysis (muscle soreness: various scales Likert and VAS) | 12 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Immediately post intervention | 7 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.43, 0.28] |
| 2.2 24 hours | 10 | 218 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.06, ‐0.10] |
| 2.3 48 hours | 8 | 179 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.18, ‐0.21] |
| 2.4 72 hours | 4 | 104 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐1.83, 0.07] |
| 2.5 96 hours | 5 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐1.12, 0.01] |
| 3 Subgroup analysis. Study design: Pain at 24 hours (muscle soreness: various scales Likert and VAS) | 10 | 218 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.84, ‐0.27] |
| 3.1 parallel group | 7 | 136 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.58, 0.12] |
| 3.2 cross‐over | 3 | 82 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐1.65, ‐0.69] |
| 4 Subgroup analysis. Study design: Pain at 48 hours (muscle soreness: various scales Likert and VAS) | 8 | 179 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| 4.1 parallel group | 6 | 117 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.77, ‐0.02] |
| 4.2 cross‐over | 2 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.26 [‐1.81, ‐0.70] |
| 5 Subgroup analysis. Study design: Pain at 72 hours (muscle soreness: various scales Likert and VAS) | 4 | 104 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.93 [‐1.36, ‐0.51] |
| 5.1 parallel groups | 3 | 64 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.93, 0.07] |
| 5.2 cross‐over | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐2.27 [‐3.08, ‐1.46] |
| 6 Subgroup analysis. Immersion frequency: Pain at 24 hours (muscle soreness: various scales Likert and VAS) | 10 | 218 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.84, ‐0.27] |
| 6.1 Single CWI | 6 | 110 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.90, ‐0.11] |
| 6.2 Multiple CWI | 4 | 108 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.00, ‐0.20] |
| 7 Subgroup analysis. Immersion frequency: Pain at 48 hours (muscle soreness: various scales Likert and VAS) | 8 | 179 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| 7.1 Single CWI | 4 | 71 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐1.25, ‐0.26] |
| 7.2 Multiple CWI | 4 | 108 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.01, ‐0.20] |
| 8 Subgroup analysis. Exercise type: Pain at 24 hours (muscle soreness: various scales Likert and VAS) | 10 | 218 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐0.84, ‐0.27] |
| 8.1 Lab controlled muscle damage (DOMS) | 6 | 137 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.57, 0.12] |
| 8.2 Other sporting activity | 4 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.23 [‐1.72, ‐0.74] |
| 9 Subgroup analysis. Exercise type: Pain at 48 hours (muscle soreness: various scales Likert and VAS) | 8 | 179 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐0.97, ‐0.35] |
| 9.1 Lab controlled muscle damage (DOMS) | 6 | 137 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.83, ‐0.14] |
| 9.2 Other sporting activity | 2 | 42 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐1.39 [‐2.09, ‐0.69] |
| 10 Tenderness (pain on palpation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Subjective recovery (10 point or 10 cm VAS) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Physical recovery (Immediately post intervention) | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.97 [‐0.10, 2.05] |
| 11.2 Mental recovery (immediately post intervention) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.86, 2.06] |
| 12 Fatigue (10 point / 10 cm VAS) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Fatigue (immediately post intervention) | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.49, ‐0.90] |
| 12.2 Fatigue (24 hrs post intervention) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.88, 0.48] |
| 13 Strength (Final value: Nm) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Immediate | 3 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐19.92 [‐33.24, ‐6.59] |
| 13.2 24 hours | 4 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐6.44, 2.56] |
| 13.3 48 hours | 5 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐1.44 [‐5.46, 2.57] |
| 13.4 72 hours | 3 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐5.33, 3.56] |
| 13.5 96 hours | 2 | 46 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐4.06, 2.89] |
| 14 Strength (% of baseline) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 14.1 Immediate | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 24 hours | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 48 hours | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.4 72 hours | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.5 96 hours | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Power (jump height: centimetres) | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 Immediate (cross‐over trial) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐6.29, 2.69] |
| 15.2 24 hours | 3 | 52 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐2.04, 2.56] |
| 15.3 24 hours (cross‐over trial) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 5.40 [2.12, 8.68] |
| 15.4 48 hours | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.63, 2.63] |
| 15.5 48 hours (cross‐over trial) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 9.60 [6.40, 12.80] |
| 15.6 72 hours (cross‐over trial) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 6.60 [3.16, 10.04] |
| 16 Power (% decrease in jump height over 5 jumps) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Power (cycle ergometer power: Watts) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Peak power output (Immediate) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Functional performance (time to complete exercise test: seconds) | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 1 km cycle time (immediate) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐2.64, 3.24] |
| 18.2 Agility circuit time (24 hours) | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.26, 0.20] |
| 18.3 Sprint time (24 hours) | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.10, 0.07] |
| 18.4 Multisprint / Agilty circuit time (24 hours) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐2.49, 1.91] |
| 18.5 Multisprint time (24 hours) | 2 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐1.06, 0.68] |
| 18.6 Sprint time (48 hours) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.11, 0.15] |
| 18.7 Multisprint time (48 hours) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐1.37, 1.23] |
| 19 Functional performance (time to fatigue) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1 % decrease from baseline (immediate) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Range of movement (ROM) | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Degrees (Immediate) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Degrees (24 hours) | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.3 Centimetres (24 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.4 Degrees (48 hours) | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.5 Degrees (72 hours) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.6 Degrees (96 hours) | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 Swelling (limb girth in centimetres) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21.1 Final value immediate | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐5.16, 5.36] |
| 21.2 Final value 24 hours | 2 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐5.63, 2.80] |
| 21.3 Final value 48 hours | 2 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.53 [‐5.57, 2.51] |
| 21.4 Final value 72 hours | 2 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐1.54 [‐5.65, 2.56] |
| 21.5 Final value 96 hours | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐5.80, 4.60] |
| 22 Biomarker: creatine kinase | 10 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 Immediately post | 4 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.66, 0.67] |
| 22.2 24 hours | 7 | 146 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.03, 0.63] |
| 22.3 48 hours | 7 | 152 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.36, 0.37] |
| 22.4 72 hours | 3 | 51 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.52, 0.79] |
| 22.5 96 hours | 3 | 55 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.70, 0.73] |
| 23 Biomarker: lactate dehydrogenase | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 23.1 U/L (immediate) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 % change 96 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 Biomarker: myoglobin | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 24.1 Immediate | 2 | 36 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐1.18, 0.17] |
| 24.2 24 hours | 2 | 33 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.36, 0.05] |
| 24.3 48 hours | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐1.66, 0.17] |
| 25 Biomarker: interleukin‐6 (pg/ml) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 25.1 Immediate | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 26 Biomarker: C‐reactive protein (mg/dL / mg/L) | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 26.1 Immediate | 2 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.40, 1.04] |
| 26.2 24 hours | 1 | 22 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐1.07, 0.61] |
| 26.3 48 hours | 1 | 22 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐1.05, 0.63] |
| 27 Pain (muscle soreness: all converted to 10 cm VAS) | 12 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 27.1 Immediately post intervention | 7 | 128 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.59, 0.61] |
| 27.2 24 hours | 10 | 218 | Mean Difference (IV, Fixed, 95% CI) | ‐1.27 [‐1.70, ‐0.84] |
| 27.3 48 hours | 8 | 179 | Mean Difference (IV, Fixed, 95% CI) | ‐1.58 [‐2.07, ‐1.10] |
| 27.4 72 hours | 4 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐2.16 [‐2.79, ‐1.53] |
| 27.5 96 hours | 5 | 97 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.21, ‐0.07] |
1.5. Analysis.
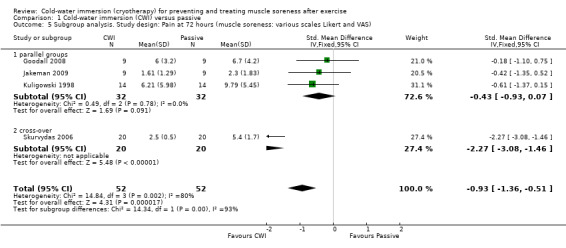
Comparison 1 Cold‐water immersion (CWI) versus passive, Outcome 5 Subgroup analysis. Study design: Pain at 72 hours (muscle soreness: various scales Likert and VAS).
Comparison 2. Cold‐water immersion (CWI) versus contrast immersion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (muscle soreness: 10 point or 10 or 12 cm VAS) | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Immediate | 3 | 63 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.66, 0.73] |
| 1.2 24 hours | 4 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.65, 0.64] |
| 1.3 48 hours | 3 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.87, 0.91] |
| 1.4 72 hours | 2 | 55 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.37, 0.69] |
| 1.5 96 hours | 1 | 28 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.86, 0.62] |
| 2 Subjective recovery (10 cm VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Fatigue (immediate) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Strength | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Immediate | 1 | 27 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.72, 0.80] |
| 3.2 24 hours | 2 | 55 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.79, 0.28] |
| 3.3 48 hours | 3 | 77 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.46, 0.44] |
| 3.4 72 hours | 2 | 55 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.70, 0.37] |
| 3.5 96 hours | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐1.19, 0.32] |
| 4 Power (squat jump in Watts) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Immediate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Power (% decrease in jump performance) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Functional performance (time to complete running task: seconds) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Multisprint/Agility circuit time (24 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Sprint time (24 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Multisprint time (48 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Functional performance (time to fatigue) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 % decrease from baseline (immediate) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Swelling (thigh girth: centimetres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Immediate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Range of movement (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 96 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Biomarker: creatine kinase (U/L) | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Immediate | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 24 hours | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 48 hours | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Biomarker: myoglobin | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Immediate | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Biomarker: lactate dehydrogenase (U/L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Immediate | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Biomarker: C‐reactive protein (mg/L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Immediate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Biomarker: interleukin‐6 (pg/mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Immediate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Cold‐water immersion (CWI) versus warm‐water immersion (WWI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (muscle soreness: 10 point or 10 or 12 cm VAS) | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Immediate | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.09, 1.09] |
| 1.2 24 hours | 3 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐0.33 [‐1.40, 0.73] |
| 1.3 48 hours | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.94, 1.99] |
| 1.4 72 hours | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.83, 1.03] |
| 1.5 96 hours | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐4.64 [‐7.72, ‐1.56] |
| 2 Subjective recovery | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Fatigue (24 hours post intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Subjective recovery (Intervention associated with NO benefit for recovery) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 24 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Strength | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Immediate | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.41, 1.25] |
| 4.2 24 hours | 2 | 51 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.27, 0.84] |
| 4.3 48 hours | 2 | 51 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.27, 0.86] |
| 4.4 72 hours | 2 | 51 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.36, 0.75] |
| 4.5 96 hours | 1 | 28 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.90, 0.58] |
| 5 Power | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Immediate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 24 hours | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Functional performance | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Multisprint time at 24 hours (seconds) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Range of movement (ROM) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 96 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Swelling | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Immediate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Biomarker: creatine kinase (U/L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Immediate | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐66.0 [‐328.29, 196.29] |
| 9.2 24 hours | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | ‐65.27 [‐236.38, 105.84] |
| 9.3 48 hours | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐210.29, 182.29] |
| 9.4 72 hours | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 53.0 [‐143.97, 249.97] |
| 10 Biomarker: lactate dehydrogenase (U/L) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Immediate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 24 hours | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.4 72 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Biomarker: myoglobin (ng/mL) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Immediate | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐4.40 [‐35.63, 26.83] |
| 11.2 24 hours | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | 1.16 [‐17.14, 19.46] |
| 12 Biomarker: interleukin‐6 (pg/ml) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 Immediate | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 2.2 [‐1.72, 6.12] |
| 12.2 24 hours | 2 | 36 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.90, 1.03] |
| 13 Biomarker: interleukin‐10 (pg/ml) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Biomarker: interleukin‐1b (pg/ml) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Cold‐water immersion (CWI) versus active recovery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (muscle soreness: 10 point VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Immediate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Power (% decrement in jump performance) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Functional performance (time to complete running test: seconds) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Sprint time (24 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Multisprint/Agility time (24 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Cold‐water immersion (CWI) versus compression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (muscle soreness: 10 point VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Subjective recovery (rating of fatigue: 10 point VAS ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Power (vertical jump height: centimetres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Range of movement (centimetres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Functional performance (time to complete running test: seconds) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Agility circuit time (24 hrs) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Multisprint time (24 hrs) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Cold‐water immersion (CWI) versus double CWI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (muscle soreness: 5 point VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 96 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Range of movement (degrees) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Immediate | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 96 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Biomarker: creatine kinase (U/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 96 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Biomarker: lactate dehydrogenase (U/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 96 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bailey 2007.
| Methods | Randomised controlled trial (matched by several anthropometric and physiological characteristics) | |
| Participants | N = 20 males, mean age 22.3 (SD 3.3) | |
| Interventions | CWI (n = 10): Seated immersion to iliac crest in 10°C water for 10 minutes, repeated water agitation Passive (n = 10): Seated rest for 10 minutes |
|
| Outcomes |
Pain
General whole body soreness (visual analogue scale, 1 "not sore" to 10 "very very sore") (follow‐up: immediately, 24, 48 hours) Strength Maximal isometric knee extension / flexion (isokinetic dynamometer, % change from baseline) (follow‐up: 24, and 48 post exercise) Power Vertical jump height (no counter movement) (electronic timing mat, peak height in m) (follow‐up: immediately, 1, 24, and 48 hours) Functional assessment Sprint performance (15m sprint, photoelectric recording, seconds) (follow‐up: 48 hours post exercise) Biomarker Muscle damage: Myoglobin (nmol/L), CK (U/L) (Follow‐up: immediately, 24 and 48 hours) |
|
| Exercise type / intensity | 90 minutes of intermittent running (average exercise intensity equal to 75% VO2 max) | |
| Time between completing exercise and initiating intervention | Immediately | |
| Participants' fitness level | Active males, habitually undertaking a variety of sports, weekly number of exercise sessions: passive group: 4 (SD 1); CWI group: 5 (SD 2) VO2 max (ml/kg/min) CWI: 55.2 (SD 4.8); Passive: 56.2 (SD 5.3) Refrained from exercise for at least 2 days prior to the study Unfamiliar with 90 minute intermittent running protocol |
|
| Notes | Data for pain, strength, power, functional assessment and biomarkers obtained after personal correspondence (converted from SE to SD) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated (Methods, cryotherapy treatment, pg. 1164) Random numbers table used for sequence generation (personal correspondence) |
| Allocation concealment (selection bias) | Unclear risk | No details in manuscript No details on implementation, personal correspondence stated random numbers table only |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | No details in manuscript No blinding of outcome assessors (personal correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No details on drop outs, exclusions, missing data or approach to analysis (per protocol / ITT) within the manuscript No drops outs or protocol deviations (personal correspondence) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Timing of outcome measures stated in methods for two out of five outcomes (pain and repeated sprint) No data for power and repeated sprint by intervention group (whole sample data only) within the manuscript, however this was provided after author correspondence |
| Other bias | Low risk | Exercise protocol clearly described and referenced Participants refrained from massage and anti‐inflammatory drugs for the duration of the investigation; water consumption standardised |
Buchheit 2009.
| Methods | Randomised cross‐over (7 days between interventions) | |
| Participants | N = 10 male cyclists, mean age: 29 (SD 6) | |
| Interventions | CWI (n = 10): Seated immersion to mid‐sternal level in 14°C water for five minutes. Total recovery 20 minutes, resting in seated position when not in water bath. Passive (n = 10): Seated rest for 20 minutes |
|
| Outcomes |
Subjective recovery
Rating of recovery, visual analogue scale (0 'feeling not recovered' to 10 'feeling fully recovered') Strength Concentric knee extension (Isokinetic dynamometer, Nm) Power Peak power output during exercise test (1 km time trial, cycle ergometer, Watts) Functional assessment Time taken to complete exercise test (1 km time trial, cycle ergometer, seconds) [Follow‐up for all outcomes: immediate] |
|
| Exercise type / intensity | Supramaximal cycling trial (1 km) undertaken at 35°C, 40% humidity | |
| Time between completing exercise and initiating intervention | 7.5 minutes | |
| Participants' fitness level | Trained cyclists, mean VO2 max 56.5 (SD 5.0) ml/kg/min | |
| Notes | Strength and power outcomes were extracted from graphs in a second publication (Peiffer 2010) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States 'randomly assigned' (pg. H422) Coin toss (personal correspondence) |
| Allocation concealment (selection bias) | High risk | No details in manuscript No details on implementation, personal correspondence stated random numbers table only |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | No details in manuscript "No" (personal correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details on drop out or missing data within the manuscript N = 11 participants at the start of study, one was dismissed due to incomplete outcome, no details of how missing value was addressed (e.g. intention to treat issues) (personal correspondence) |
| Selective reporting (reporting bias) | High risk | Data from this trial is presented in a secondary publication (seePeiffer 2010) |
| Other bias | Unclear risk | Exercise protocol clearly described No details on co‐interventions (immediate follow‐up only so may be less associated with bias) |
Cassar 2010.
| Methods | Randomised cross‐over study (7 days between conditions) | |
| Participants | N = 8 male cyclists, aged 22 to 35 | |
| Interventions | CWI (n = 8): Immersion to waist in water at 8 to 12°C for 14 minutes Contrast (n = 8): Immersion to waist in water at 8°C for 1 minute followed by immersion at 40 to 45°C for 1 minute; x 7 sets Passive (n = 8): Rest |
|
| Outcomes |
Subjective recovery
Rating of fatigue, visual analogue scale (0 to 10 cm) Pain Rating of soreness, visual analogue scale (0 to 10 cm) Functional assessment Time to fatigue during an exercise test (time maintaining 80 revolutions per minute, cycle ergometer, seconds) Biomarkers Muscle damage [CK (U/L), LDH (U/L), Myoglobin (mg/L) Inflammation [CRP (mg/L)] [Follow‐up for all outcomes: immediate] |
|
| Exercise type / intensity | 30 minutes cycling (70% VO2 max) | |
| Time between completing exercise and initiating intervention | Immediate | |
| Participants' fitness level | Trained cyclists | |
| Notes | All data provided after personal correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned (abstract) Random numbers table (personal correspondence) |
| Allocation concealment (selection bias) | Unclear risk | Randomisation order was single blind (personal correspondence) |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. Personal correspondence |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. Personal correspondence |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | Personal correspondence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants dropped out of the study (personal correspondence) |
| Selective reporting (reporting bias) | Unclear risk | Published as an abstract only |
| Other bias | Low risk | Exercise protocol clearly described No co‐interventions (personal correspondence) |
Eston 1999.
| Methods | Randomised controlled trial | |
| Participants | N = 15 females, mean age 22 (SE 2) | |
| Interventions | CWI (n = 8): Exercised arm fully submerged in water at 15°C for 15 minutes Passive (n = 7): No treatment |
|
| Outcomes |
Pain (tenderness)
One site on the biceps (pressure when discomfort changed to pain; algometer, N) Strength Maximum isometric elbow flexion (2.36 rad) (dynamometer, N) Swelling Thigh circumference (tape measure, cm) Biomarker Muscle damage (CK, IU/L) [Follow‐up for all measures: immediately, 24, 48, and 72 hours after exercise] |
|
| Exercise type / intensity | 8 sets of 5 maximal elbow flexions (eccentric and concentric), isokinetic dynamometer, 60 seconds rest between each set | |
| Time between completing exercise and initiating intervention | Immediately and every 12 hours for the following 3 days | |
| Participants' fitness level | ||
| Notes | SE converted to SD for: tenderness, strength, swelling and CK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated at random to either a cryotherapy or control group (Methods, pg. 232) Coin toss (author correspondence) |
| Allocation concealment (selection bias) | High risk | No details in manuscript "No" (author correspondence) |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | ‐ No details in manuscript ‐ No (author correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ‐ No details in manuscript |
| Selective reporting (reporting bias) | Low risk | ‐ No published protocol available ‐ Outcomes and follow‐ups stated in methods ‐ Means and SD/SE presented by intervention group for all outcomes, at all follow‐ups |
| Other bias | Low risk | ‐ Exercise protocol well described ‐ No evidence of co‐interventions |
Goodall 2008.
| Methods | Randomised controlled trial | |
| Participants | n = 18 males, mean age 24 (SD 5) | |
| Interventions | CWI (n = 9): Seated immersion up to iliac crest in water of 15°C for 12 minutes Passive (n = 9): Seated rest |
|
| Outcomes |
Pain
Muscle soreness (visual analogue scale; 0 to 200 mm, 'no pain' to 'extremely painful') rating during standing AND during unweighted squat Strength Maximal voluntary contraction: isometric knee extension at 70 degrees (isokinetic dynamometer, % change from baseline) Swelling Thigh circumference (tape measure, cm) ROM Active knee flexion (goniometer, degrees) Biomarker Muscle damage (CK, IU/L) [Follow‐up for all outcomes: immediately, 24, 48, 72 and 96 hours after exercise] |
|
| Exercise type / intensity | 5 sets of 20 drop jumps on a concrete based floor, 10 seconds rest between each jump, and 2 minutes rest between each set | |
| Time between completing exercise and initiating intervention | Immediately and every 24 hours for the following 3 days | |
| Participants' fitness level | Physically active males not familiar with eccentric based training; refrained from resistance training or exercise that may induce eccentric muscle damage/soreness for 3 weeks prior to, and for the duration of the study | |
| Notes | Means and SD obtained after personal correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | States equally but randomly allocated (pg 236) Participants randomised using a random numbers table (personal correspondence) |
| Allocation concealment (selection bias) | Unclear risk | No details in manuscript |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | No details in manuscript "No" (personal correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No details on drop outs, exclusions, missing data or approach to analysis in manuscript n = 9 participants were randomised to each group and a full data set was obtained at each follow‐up (personal correspondence) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented (graphically) by intervention group for all outcomes, at all follow‐ups. |
| Other bias | Low risk | Exercise protocol clearly described Participants refrained from exercise and resistance training for three weeks prior to the study, and refrained from exercise, resistance training, NSAIDs and nutritional supplements during the study. |
Halson 2008.
| Methods | Randomised cross‐over (3 days between interventions ‐ personal correspondence) | |
| Participants | N = 11 male cyclists unfamiliar with CWI, mean age 23.8 (SD 1.6) | |
| Interventions | CWI (n = 11): Immersion to mesosternum in water at 11.5°C for 60 seconds x 3, 120 seconds between immersion Passive (n = 11): Seated rest, room maintained at 24.2°C and 45.6 relative humidity |
|
| Outcomes |
Subjective recovery
‐ Rating of recovery (visual analogue scale, 1 to 10); physical / mental
‐ Rating of fatigue (visual analogue scale, 1 to 10) Pain ‐ Leg soreness (visual analogue scale, 1 to 10) [Immediately after intervention] Biomarker Muscle damage (CK,U/L) Inflammation [CRP (mg/dL), IGF‐1 (ng/mL), IL‐6 pg/mL)] Stress hormones [prolactin (ng/mL), GH (mIU/L), Cortisol (nM), testosterone (ng/dL), adrenaline (pg/mL), noradrenaline (ng/mL)] (Follow‐up: Immediately post exercise, and immediately post intervention) Adverse effects Core temperature before, during and immediately after immersion (disposable rectal thermometer, ºC) |
|
| Exercise type / intensity | 40 minutes cycling (20 minutes at 75% VO2 max; time trial of approximately 20 minutes) in an environmental chamber maintained at 34.3°C and 41.2% relative humidity | |
| Time between completing exercise and initiating intervention | 20 minutes | |
| Participants' fitness level | Endurance trained cyclists, VO2 peak: 71.3 (SD 1.2), abstaining from intense exercise for 24 hours prior to each testing session | |
| Notes | Biomarkers were not recorded for N = 4 / 11 (unable to obtain blood sample) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random and counterbalanced order (pg. 333, 2nd paragraph) Participants pair‐matched according to peak power obtained from a cycling VO2 max tests (personal correspondence) |
| Allocation concealment (selection bias) | High risk | No details in manuscript No measures undertaken to conceal allocation (personal correspondence) |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. Confirmed with author contact |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. Confirmed with author contact |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | No details in manuscript No blinding (personal correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants completed each intervention arm (personal correspondence) n = 4 participants had missing data for all biochemical outcomes with reasons (unwilling to provide blood sample due to forthcoming racing event) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented by intervention group for all outcomes, at all follow‐ups |
| Other bias | Low risk | Exercise intervention clearly described Participants provided with instructions for fluid and carbohydrate consumption during the study, and food diaries and training diaries were completed during the study (pg. 333) |
Ingram 2009.
| Methods | Randomised cross‐over (14 days between interventions) | |
| Participants | N = 11 male athletes, mean age 27.5 (SD 6) | |
| Interventions | CWI (n = 11): Seated immersion to umbilicus in water at 10°C for 5 minutes x 2 (2.5 minutes standing in room temperature between immersions) Contrast (n = 11): Seated immersion to umbilicus in water at 10°C for 2 minutes, followed by seated immersion to umbilicus in water at 40°C for 2 minutes, x 3 sets (30 second transfer time) Passive (n = 11): Seated rest |
|
| Outcomes |
Pain
Muscle soreness (10 point Likert scale) (Follow‐up: immediately, 24 and 48 hours post exercise) Strength Isometric strength at quads, hamstring, hips (cable tensiometer, kgf) (Follow‐up: 48 hours post exercise) Functional assessment Time to complete exercise (10 x 20 m sprints, seconds) (Follow‐up: 48 hours post exercise) Biomarker Muscle damage (CK, U/L) Inflammation (CRP, mg/L) [Follow‐up: 24 and 48 hours post exercise] |
|
| Exercise type / intensity | 80 minutes of simulated team sports exercise, followed by a 20 m shuttle run test to exhaustion | |
| Time between completing exercise and initiating intervention | Immediately (?) and after 24 hours | |
| Participants' fitness level | Athletes with team games experience | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomly assigned, counterbalanced design' pg. 418) computer generated program (personal correspondence) |
| Allocation concealment (selection bias) | Unclear risk | No details in manuscript Likely with computer generated program Not confirmed after personal correspondence |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | No details in manuscript "No" (personal correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Manuscript shows n=11 in results (Tables 1 and 2) All participants completed each intervention arm and that there were no missing data (personal correspondence) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD/SE presented by intervention group for all outcomes, at all follow‐ups |
| Other bias | Low risk | Exercise protocol well described Manuscript states "no additional exercise or recovery procedures undertaken within 48 hours post exercise" (pg. 418) |
Jakeman 2009.
| Methods | Randomised controlled trial | |
| Participants | N = 18 healthy females, mean age 19.9 (SE 0.97) | |
| Interventions | CWI (n = 9): Seated immersion to iliac crest in 10°C water, for 10 minutes Passive (n = 9): Seated rest |
|
| Outcomes |
Pain
Leg soreness during unweighted squat (10 cm visual analogue scale, 0 "no pain" to 10 "worst pain ever") Strength Concentric knee extension (isokinetic dynamometry at 45°/s, Nm) Biomarker Muscle damage (CK, U/L) (Follow‐up: 1 (immediate), 24, 48, 72 and 96 hours after exercise) |
|
| Exercise type / intensity | 10 counter movement jumps x 10, adopting a 90 degree knee angle on each landing | |
| Time between completing exercise and initiating intervention | Within 10 minutes | |
| Participants' fitness level | Not engaged in any specific plyometric or lower limb training 6 weeks prior to testing | |
| Notes | Data for pain, strength and biomarkers obtained after personal correspondence (converted from SE to SD) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomly allocated to a control or treatment group' (Methods, participants and design, pg. 457) participants randomised using a predetermined sequence generation (personal correspondence) |
| Allocation concealment (selection bias) | Unclear risk | No details in manuscript Likely with predetermined sequence generation Not confirmed after personal correspondence |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | No details in manuscript No (personal correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No details in manuscript All participants received intervention as allocation and there was no missing data (personal correspondence) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD/SE presented (graphically) by intervention group for all outcomes, at all follow‐ups |
| Other bias | Unclear risk | Exercise protocol is clearly described Few details on the control intervention within the manuscript |
King 2009.
| Methods | Randomised cross‐over (5 days between interventions) | |
| Participants | n = 10 female netballers, mean age 19.5 (SD 1.5) | |
| Interventions | CWI (n = 10): Immersion to iliac crest level in water at 9.3°C (+/‐1.6°C) for 5 minutes x 2 (2.5 minutes seated at air temperature between immersions) Passive (n = 10): Seated rest for 15 minutes Active (n = 10): Low intensity jogging at predetermined speed for 15 minutes Contrast (n = 10): Immersion to iliac crest level in water at 9.7°C (SD 1.4°C) for 1 minutes followed by a warm shower in water at 39.1°C (SD 2°C) for 2 minute, x 5 sets |
|
| Outcomes |
Muscle soreness (Likert scale; 0 = normal, 10 = extreme soreness) (Follow‐up: immediately, 24 hours after intervention) Power Countermovement jump (5 jumps in 20 seconds, % decrement during 5 reps (follow‐up: 24 hrs) Functional assessment ‐ Exercise test (10 m sprint, seconds) ‐ Time to complete exercise (netball/agility related circuit, seconds) (Follow‐up: 24 hours after intervention) |
|
| Exercise type / intensity | Netball related circuit (4 x 15 minutes of intermittent sprinting, jumping, agility, striding) at environmental temperature of 16.9 (SD 2.1°C) | |
| Time between completing exercise and initiating intervention | Immediate (after post exercise outcomes) | |
| Participants' fitness level | Trained netballers, competing at local and regional representative matches (1 to 2 per week), and training (˜3 times per week) | |
| Notes | Data on muscle soreness and subjective recovery obtained after personal correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'randomly assigned counterbalanced order' (pg. 1796) drawn from a hat (personal correspondence) |
| Allocation concealment (selection bias) | Low risk | No details Participants were unaware of which recovery intervention would be used until it was time to complete the recovery. Allocation was completed by the chief investigator alone. This information was then stored in the investigators office and only used for reference by the chief investigator (personal correspondence) |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | No details No (personal correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results Table 1, and Figures 2 and 3 state n=10, so probably no drop outs, however, no other details on exclusions or missing data No missing data, all participants completed as randomised (personal correspondence) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented by intervention group for all outcomes, at all follow‐ups |
| Other bias | Low risk | Exercise protocol clearly described Water consumption was standardised during the testing procedures; participants recorded all food and drink activity in the 24 hours leading up to the first testing session and replicated for the all testing sessions, participants requested to present in a rested state |
Kuligowski 1998.
| Methods | Randomised controlled trial | |
| Participants | N = 56 pain free participants; 28 male, mean age 21.1 (SD 3); 28 female, mean age 20.1 (SD 2.1) | |
| Interventions | CWI (n = 14): Arm immersion in agitated water at 12.8°C to deltoid level for 24 minutes Passive (n = 14): no treatment Contrast Immersion (n = 14): Arm immersion in agitated water at 38.9°C to deltoid level for 3 minutes, repeated in water at 12.8°C for 1 minute, x 6 sets (24 minutes) arm‐water immersion (n = 14): Arm immersion in agitated water at 38.9°C to deltoid level for 24 minutes |
|
| Outcomes |
Pain
Soreness during active elbow motion (12 cm graphic pain rating scale: dull ache, to unbearable pain) Strength Isometric elbow flexion (dynamometer, kg) ROM Active elbow flexion (hand held goniometer, degrees) (Follow‐up: 24, 48, 96 hours post exercise) |
|
| Exercise type / intensity | Eccentric biceps loading, 10 reps at 1 repetition maximum +2.27 kg x 5 sets, 1 minute rest between sets | |
| Time between completing exercise and initiating intervention | Immediately and at 24, 48 and 72 hours | |
| Participants' fitness level | No upper extremity weight training in the previous 9 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly assigned subjects to ...' (pg. 224) |
| Allocation concealment (selection bias) | Unclear risk | No details in manuscript |
| Blinding (performance bias and detection bias) Participants | High risk | |
| Blinding (performance bias and detection bias) Personnel | High risk | |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | No details in manuscript |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details on drop outs, exclusions, missing data or approach to analysis (per protocol / ITT) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented by intervention group for all outcomes, at all follow‐ups |
| Other bias | Low risk | Exercise protocol clearly described Participants were instructed not to use therapeutic modalities, massage, stretching or medications, and refrain from strenuous activity during the course of the study (pg 223) |
Montgomery 2008.
| Methods | Randomised controlled trial (matched by playing position and anthropometrics) | |
| Participants | N = 29 male basketball players, mean age 19.1 (SD: 2.1) | |
| Interventions | All groups: Carbohydrate and stretching CWI group (n = 10): Immersion to mesosternale level in 11°C water, 5 x 1 minute. Between immersions participants rested passively for 2 minutes in ambient air (23°C) Compression (n = 10): Full length compression garments (18 mm/Hg) post game and overnight (18 hours) Control (n = 9): Carbohydrate and stretching only, post game carbohydrate snack (1 g/kg body mass) and 600 ml of fluid in sports drink followed by a standardised programme of 10 stretches (bilateral leg and lower back, each stretch held for 15 seconds, 2 sets) |
|
| Outcomes |
Subjective recovery
Rating of general fatigue (visual analogue scale, 1 "not at all" to 10 "extremely tired") Pain Leg soreness (visual analogue scale, 1 "normal" to 10 "extremely sore") Power Vertical jump (cm) Functional assessment Time to complete exercise test (20 sprint; repeated sprint; agility test; all seconds) Swelling Limb girth (cm, calf and thigh) ROM Sit and reach flexibility test (cm) [Follow‐up: 24 hours (after finishing the 3 day tournament)] |
|
| Exercise type / intensity | 3 day basketball tournament (48 minute game each day), comprising a total of 144 minutes of basketball in mean temperature of 23.2°C | |
| Time between completing exercise and initiating intervention | ˜10 minutes (all groups undertook control after each game estimated to take 10 minutes) | |
| Participants' fitness level | Regularly competing in state competitions; training load of 8 to 10 hours per week | |
| Notes | Our analysis only included outcomes recorded at the end of the tournament, ˜24 hrs after the final game ["pre tournament tests were repeated on the morning of the 4th day" (pg. 1137)]. Outcomes (subjective recovery, pain, muscle power) recorded after each game were not extracted or included in our analysis. Measurement device for vertical jump not clear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomised controlled trial' (Methods, experimental design, pg. 1137) players matched for positional and anthropometric characteristics and assigned to one of three treatment groups (Methods, experimental procedures, pg. 1137) |
| Allocation concealment (selection bias) | Unclear risk | No details in manuscript |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | No details in manuscript |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details on drop outs, exclusions, missing data or approach to analysis (per protocol / ITT) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented by intervention group for all outcomes, at all follow‐ups |
| Other bias | Low risk | Cumulative game time is expressed for participants within each treatment group, and is similar (also used as a covariate in statistical analysis) Participants undertook standardised carbohydrate ingestion and stretching between games |
Rowsell 2009.
| Methods | Randomised controlled design (pair‐matched according to playing position) | |
| Participants | N = 20 male soccer players, mean age: 15.9 (SD: 0.6) | |
| Interventions | CWI (n = 6): Immersion to mesosternale level in 10°C water, 1 minute x 5, manual water agitation throughout. Between immersions participants rested for 60 seconds WWI (n = 7): Immersion to mesosternale level in 34°C water, 5 x 1 minute, manual water agitation throughout. Between immersions participants rested for 60 seconds Both groups: Standardardised warm up and cool down, carbohydrate, protein and fluid ingestion |
|
| Outcomes |
Subjective recovery
Rating of fatigue (visual analogue scale, 1 to 10)
Rating or recovery (visual analogue scale, 1 to 10 ): physical / mental
Do you believe that the treatment enhanced recovery from the previous match and improved subsequent physical performance (Yes/No) Pain Leg soreness (visual analogue scale, 1 to 10) Power Countermovement jump (jump height ‐ standing reach height, cm) Functional assessment Time to complete exercise test (repeated sprint (12 x 20 m), seconds) Biochemical Inflammatory: IL‐1b (pg/ml); IL‐6 (pg/ml); IL‐10 (pg/ml) Muscle damage: myoglobin (ng/ml), LDH (U/L), CK (U/L) (Follow‐up for all outcomes: ˜ 22 hours after tournament) |
|
| Exercise type / intensity | Soccer tournament, 4 matches in 4 days, similar tactical strategies adopted in each match. Ambient temperature during matches ranged from 25 to 36°C, relative humidity 25 to 58%. | |
| Time between completing exercise and initiating intervention | 20 minutes after each match | |
| Participants' fitness level | High performance male junior soccer players | |
| Notes | Data on muscle soreness and subjective recovery (fatigue) extracted from graph. Other data on subjective recovery were not available (rating of physical and mental recovery) N = 7 players sustained injuries throughout the tournament and were not included in the final analysis Our analysis only included outcomes recorded at the end of the tournament. Outcomes undertaken before each of the games were not extracted or included in our analysis. An additional report of the trial (Rowsell 2011) was located in November 2011 ‐ this will be assessed in the update of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomly allocated' (Methods, participants, pg. 566), "players matched for playing position" (pg. 566) |
| Allocation concealment (selection bias) | Unclear risk | "randomly allocated" (Methods, participants, pg. 566), no further details in manuscript |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | No details in manuscript |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of participants randomised to each group is not clear n = 7 were excluded from the analysis with reasons (all 7 sustained a soft tissue injury and were unable to complete the study) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented by intervention group for all outcomes, at all follow‐ups (except physical and mental recovery which stated no between group difference) |
| Other bias | Low risk | Players in each group aged matched according to playing position so likely to undertake similar exercise intensity; no measure of cumulative game time during the tournament Standardised carbohydrate ingestion and stretching between games |
Sellwood 2007a.
| Methods | Randomised controlled trial | |
| Participants | N = 40 healthy adults, mean age 21.3 (SD 4.3), 11 male / 29 female | |
| Interventions | Cold‐water immersion (n = 20): Immersion in water at 5°C, 1 minute x 3 sets. Warm‐water immersion (n = 20): Immersion in water at 24°C, 1 minute x 3 sets Both groups: Immersion to anterior superior iliac spine, participants rested out of the bath for 60 seconds between sets |
|
| Outcomes |
Pain
Pain during: sit‐stand, passive stretch, hopping, running, isometric contraction (100 mm visual analogue scale, "no pain" to "worst pain possible") Tenderness Pain (100 mm visual analogue scale) when pressure applied at two points on the thigh, pressure standardised at 6 lb/cm2 using an algometer) Strength Isometric knee extension (isokinetic dynamometer, 60° flexion, Nm) Power Hop for distance (tape measure, m) Swelling Thigh circumference (use of tape measure?, mm) Biomarker Muscle damage (CK, IU/L) (Follow‐up: 24, 48 and 72 hours after exercise) |
|
| Exercise type / intensity | 5 sets of 10 repetitions of eccentric quadriceps exercise at 120% of 1 repetition maximum, leg extension machine, 1 minute rest between sets | |
| Time between completing exercise and initiating intervention | Immediate | |
| Participants' fitness level | No eccentric quadriceps exercise within past 3 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Generated using a random numbers table" (Methods, randomisation and masking, pg. 393) |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered opaque sealed envelopes held at a central location" (Methods, randomisation and masking, pg. 393) |
| Blinding (performance bias and detection bias) Participants | High risk | Difficult due to nature of intervention, however participants were not informed as to which intervention was considered therapeutic (Methods, randomisation and masking, pg. 393) |
| Blinding (performance bias and detection bias) Personnel | High risk | No details |
| Blinding (performance bias and detection bias) Outcome assessors | Low risk | "Investigator responsible for outcome assessments was blinded to group allocation, and participants advised not to reveal their allocation" (Methods, randomisation and masking, pg. 393) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data analysis (pg. 395) states that analysis was based on ITT, with imputation using Last Observation Carried Forward (LOCF) in the event of missing data drop outs/missing data is not reported in text however Table 5 which states that n = 20 were followed up in each group; therefore likely that imputation (LOCF) was not required |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD (or median and IQ range) presented by intervention group for all outcomes, at all follow‐ups |
| Other bias | Unclear risk | Exercise protocol clearly described No details on co‐interventions |
Skurvydas 2006.
| Methods | Randomised cross‐over (9 to 10 months between interventions) | |
| Participants | N = 20 healthy untrained men, aged 20.4 (SD 1.7) | |
| Interventions | CWI (n = 20): Immersion of legs in water at 15°C (SD 1°C) for 15 minutes x 2. 10 minutes between each immersion Passive (n = 20): Seated rest |
|
| Outcomes |
Pain
Muscle soreness (0 to 10 points) (follow‐up: 24, 48, 72 hours post exercise) Strength Isometric knee extension (90 degree knee angle, force measuring device, % of baseline) (follow‐up: immediate (4 hours), 24, 48, 72 hours post exercise) Power Counter movement jump (Kistler force plate, height of jump in cm) (follow‐up: immediate (4 hours), 24, 48, 72 hours post exercise) Biomarker Muscle damage (CK, IU/L) (Follow‐up: 24 hours and 48 hours post exercise) |
|
| Exercise type / intensity | 100 counter movement jumps from a 0.75 m height | |
| Time between completing exercise and initiating intervention | Immediately and at 4, 8, and 24 hours | |
| Participants' fitness level | Physically active but did not take part in any formal exercise or sports programme | |
| Notes | Data on pain, strength, power and CK were obtained after personal correspondence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The choice of their participation in the first and second experiments was made at random" (pg. 142) |
| Allocation concealment (selection bias) | High risk | No details in manuscript "No" (personal correspondence) |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | High risk | No details in manuscript "No" (personal correspondence) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No details in manuscript All 20 participants completed each treatment arm and data were available for all 20 participants (personal correspondence) |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented by intervention group for all outcomes, at all follow‐ups |
| Other bias | Unclear risk | Exercise protocol well described No details on co‐interventions |
Vaile 2008c.
| Methods | Randomised cross‐over (8 months between interventions) (see Notes) | |
| Participants | N = 38 males | |
| Interventions | CWI (n = 12): Whole body immersion (excluding head and neck) in water at 15°C WWI (n = 11): Whole body immersion (excluding head and neck) in water at 38°C Contrast (n = 15): Whole body immersion (excluding head and neck) in water at 15°C for 1 minute, followed by whole body immersion (excluding head and neck) in water at 38°C for 1 minute, x 7 sets (Passive (n = 38): Seated with minimal movement (see Notes)) All groups: Interventions were 14 minute durations |
|
| Outcomes |
Pain
(visual analogue scale, 0 'normal ' to 10 'extremely sore') Strength Isometric squat (force platform, peak force, N) Power Weighted squat jump (peak force, W) Swelling Thigh girth (measuring tape, cm) Biomarker Muscle damage [CK (U/L), LDH (U/L), myoglobin (ng/mL)] Inflammation (IL‐6, pg/mL) [Follow‐up: immediately, 24, 48, 72 hours post exercise] |
|
| Exercise type / intensity | 10 eccentric repetitions on a leg press machine x 7 sets, 3 minutes rest between sets | |
| Time between completing exercise and initiating intervention | Immediately and at 24, 48 and 72 hours | |
| Participants' fitness level | Strength trained | |
| Notes | Data on pain were extracted from graph * This was a cross‐over of hydrotherapy versus passive (it is unclear whether this was randomised). Only the non‐cross‐over data from the three hydrotherapy groups are presented in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned..." (pg. 448) |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented by intervention group for all outcomes, at all follow‐ups |
| Other bias | Unclear risk | Exercise protocol was well described No details on co‐interventions |
Yanagisawa 2003a.
| Methods | Randomised controlled trial | |
| Participants | N = 28 healthy participants, mean age 23.8 (SD 1.8) | |
| Interventions | CWI (n = 9): Immersion to the head of the fibula in water at 5°C for 15 minutes Double CWI (n = 9): Immersion to the head of the fibula in water at 5°C for 15 minutes Control (n = 10): no intervention (?) |
|
| Outcomes |
Pain
Calf soreness (visual analogue scale, 1 (normal) to 5 (very, very sore) (follow‐up: 24, 48 and 96 and 168 hours post exercise) ROM Ankle joint dorsiflexion (goniometer, degrees) (follow‐up: immediate (20 minutes), 24, 48 and 96 and 168 hours post exercise) Biomarker Muscle damage [CK, LDH (IU/L)] (Follow‐up: 24, 48 and 96 and 168 hours post exercise) |
|
| Exercise type / intensity | 5 sets of 20 resisted calf raises at 30% of participants maximal voluntary contraction, 1 minute rest between sets | |
| Time between completing exercise and initiating intervention | Immediate (double CWI group repeated intervention at 24 hours) | |
| Participants' fitness level | Untrained | |
| Notes | No details on control intervention Data for pain and biomarkers were extracted from graphs. We were unable to extract data for biomarkers at 24 and 48 hours. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects randomly allocated" (Methods, subjects, pg 54) |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented by intervention group for all outcomes, at all follow‐up |
| Other bias | Low risk | Exercise intervention clearly reported Participants refrained from physical exercise, other forms of physical therapy, or drinking (alcohol?) for the duration of the study |
Yanagisawa 2003b.
| Methods | Randomised controlled trial | |
| Participants | N = 14 healthy males, mean age 23.8 (SD 2.3) | |
| Interventions | CWI group (n = 7): Immersion to the head of the fibula in water at 5°C for 15 minutes Control (n = 7): no intervention (?) |
|
| Outcomes |
Pain
Calf soreness during walking (visual analogue scale, 1 (normal) to 5 (very, very sore) [Follow‐up: immediate (30 min), 24, 48 and 96 and 168 hours post exercise] |
|
| Exercise type / intensity | 5 sets of 12 resisted calf raises at 30% of participants maximal voluntary contraction, 1 minute rest between sets | |
| Time between completing exercise and initiating intervention | Immediate | |
| Participants' fitness level | Untrained | |
| Notes | No details on control intervention; data on pain extracted from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned" (Abstract, pg. 1517) |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) Participants | High risk | No blinding. |
| Blinding (performance bias and detection bias) Personnel | High risk | No blinding. |
| Blinding (performance bias and detection bias) Outcome assessors | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details |
| Selective reporting (reporting bias) | Low risk | No published protocol available Outcomes and follow‐ups stated in methods Means and SD presented by intervention group for all outcomes, at all follow‐up |
| Other bias | Low risk | Exercise protocol well described Participants refrained from physical exercise, and private therapeutic treatment for the duration of the study |
CK = creatine kinase CPK = creatine phosphokinase IL = interleukin LDH = lactate dehydrogenase SD = standard deviation SE = standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Banfi 2008a | Intervention was CWI combined with active cycle intervention |
| Chen 2006 | Unclear if intervention is CWI (from abstract). Unable to source full text, unable to contact authors |
| Clements 2002 | No relevant outcomes |
| Coffey 2004 | No CWI intervention |
| Connolly 2003 | No CWI intervention |
| Crowe 2007 | No primary outcomes |
| Dawson 2005 | No CWI intervention |
| Gill 2006 | No CWI intervention |
| Goosey‐Tolfrey 2008 | CWI did not include muscles/body parts primarily involved in the exercise protocol (isolated wrist immersion only) |
| Gurovich 2006 | No CWI (from abstract) |
| Hayashi 2004 | CWI at 20°C |
| Heyman 2009 | No primary outcomes |
| Higgins 2010 | No relevant follow‐up times (abstract unclear however exclusion confirmed after full text version sourced from authors) |
| Hudson 1999 | CWI at 33°C |
| Khomenok 2008 | CWI did not include muscles/body parts primarily involved in the exercise protocol (isolated wrist immersion only) |
| Kinugasa 2009 | Intervention was CWI combined with active cycle intervention |
| Kokkinidis 1998 | No CWI intervention |
| Lane 2004 | No primary outcomes |
| Lapier 1995 | No CWI (abstract unclear, confirmed after correspondence with authors) |
| Nosaka 1996 | No CWI (abstract unclear, confirmed after correspondence with authors and review of full text) |
| Paddon‐Jones 1997 | Participants randomised by body site (one arm CWI; other arm control) |
| Peiffer 2009a | No primary outcomes |
| Peiffer 2009b | No randomisation; states 'counterbalanced' only |
| Peiffer 2010b | No randomisation; states 'counterbalanced' only |
| Peñailillo 2006 | No CWI (from abstract) |
| Price 2004 | No CWI intervention |
| Schniepp 2002 | No primary outcomes |
| Sipaviciene 2007 | Not clear if intervention is CWI or cold pack (English abstract only, full text in Lithuanian), no response from authors |
| Taylor 2008 | No relevant outcomes |
| Tessitore 2007 | No CWI intervention |
| Udermann 1997 | No CWI (abstract unclear, confirmed after correspondence with primary author and review of full text) |
| Vaile 2007 | No CWI intervention |
| Vaile 2008a | No primary outcomes |
| Vaile 2008b | No primary outcomes |
| Verducci 2000 | No CWI intervention |
| Webborn 2005 | No post exercise CWI intervention |
| Yamane 2006 | No randomisation; each participants had an untreated limb and cooled limb |
| Yeargin 2006 | No primary outcome |
CWI = cold‐water immersion
Characteristics of studies awaiting assessment [ordered by study ID]
Ascensao 2011.
| Methods | Randomised controlled trial |
| Participants | 20 male soccer players |
| Interventions | CWI: 10 min cold water immersion at 10ºC Warm water immersion: 10 min thermoneutral water immersion at 35ºC |
| Outcomes |
Pain
DOMS Strength Maximal isometric quadriceps strength Functional assessment Jump and sprint abilities Biomarker Muscle damage [creatine kinase, myoglobin] Inflammation (C‐reactive protein) [Follow‐up: immediately, 24 and 48 hours post exercise] |
| Notes | Outside initial search limit. |
Fowles 2003.
| Methods | Randomised cross‐over trial |
| Participants | 16 active participants (9 male, 7 female) |
| Interventions | CWI group: immersion to waist level at 8ºC Control group: light stretching exercises |
| Outcomes |
Pain
Soreness rating Power Vertical jump Functional assessment Treadmill run to fatigue (time) Intense 6 km interval run (time to complete) (Follow‐up: 48 hours after exercise) |
| Notes | Exercise was treadmill run to fatigue followed by 6 km run (mean time to completion was 43.5 minutes) Abstract only |
Pournot 2011.
| Methods | Randomised controlled trial |
| Participants | 41 elite athletes (all male) |
| Interventions | CWI: at 10°C Warm water immersion: at 36°C Contrast: 10 to 42°C Passive recovery |
| Outcomes |
Strength Maximal isometric voluntary contraction (MVC) of the knee extensor muscles Power Maximal vertical counter‐movement jump Functional assessment Maximal 30‐s rowing test Biomarker Muscle damage [creatine kinase, lactate dehydrogenase] Inflammation (IL‐6, pg/mL) [Follow‐up: immediately and 24 hours post exercise] |
| Notes | Outside initial search limit. May not include primary outcomes. |
Smith 2008.
| Methods | Randomised controlled trial |
| Participants | 31 participants |
| Interventions | Cryotherapy Thermotherapy |
| Outcomes |
Strength Upper arm 1 repetition maximum |
| Notes | Exercise was a program of high volume, medium intensity resistance exercises Abstract only |
Stacey 2010.
| Methods | Randomised controlled trial (probably cross‐over) |
| Participants | 9 active males (3 cycling bouts) |
| Interventions | CWI: cold tub with water at 10°C Active recovery: cycling |
| Outcomes |
Pain
Self‐assessment Subjective measures Perceived exertion and lower extremity sensations Biomarker Blood samples (lactate, IL‐6, total leukocyte, neutrophil, and lymphocyte cell counts) [Follow‐up: immediately, post cycling bouts] |
| Notes | Outside initial search limit. Study design may not be appropriate for inclusion. |
Vaile 2011.
| Methods | Randomised cross‐over trial (7 days between interventions) |
| Participants | 10 endurance trained male athletes |
| Interventions | CWI group: Body immersion (excluding head and neck) in water at 15°C for 15 minutes Control group: Active recovery, cycling at 40% of maximum power for 15 minutes |
| Outcomes |
Functional assessment Total work (kJ) during cycling performance test |
| Notes | Outside initial search limit |
CWI = cold‐water immersion
Differences between protocol and review
The order of the two primary outcomes was reversed, with muscle soreness becoming the lead item.
We presented outcome data split into 24 hour instead of 12 hour intervals.
Contributions of authors
Chris Bleakley developed the research idea, and wrote the original protocol. Evie Gardner provided statistical support.
Selection of studies was carried out by Suzanne McDonough and Chris Bleakley.
Data extraction and interpretation were carried out by Chris Bleakley, Ty Hopkins, Gareth Davison and Philip Glasgow.
Risk of bias assessment and data analysis were carried by Chris Bleakley, David Baxter and Suzanne McDonough.
All authors commented on drafts and approved the final version. Chris Bleakley wrote the final review and is the guarantor.
Sources of support
Internal sources
-
Health and Rehabilitation Sciences Research Institute, University of Ulster, UK.
Provided computer hardware, Internet, library access and e‐mail facilities
External sources
-
Research and Development Office (HSC R&D Office), Belfast, UK.
Provided salaried non‐clinical time, travel and subsistence for the lead author
Declarations of interest
None known.
New
References
References to studies included in this review
Bailey 2007 {published data only}
- Bailey DM. personal communication November 11 2010.
- Bailey DM, Erith SJ, Griffin PJ, Dowson A, Brewer DS, Gant N, et al. Influence of cold water immersion on indices of muscle damage following prolonged intermittent shuttle running. Journal of Sports Sciences 2007;25(11):1163‐70. [DOI] [PubMed] [Google Scholar]
Buchheit 2009 {published data only}
- Buchheit M. personal communication 18 October 2010.
- Buchheit M, Peiffer JJ, Abbiss CR, Laursen PB. Effect of cold water immersion on postexercise parasympathetic reactivation. American Journal of Physiology ‐ Heart and Circulatory Physiology 2009;296(2):H421‐7. [DOI] [PubMed] [Google Scholar]
- Peiffer JJ, Abbiss CR, Watson G, Nosaka K, Laursen PB. Effect of cold water immersion on repeated 1‐km cycling performance in the heat. Journal of Science and Medicine in Sport 2010;13(1):112‐6. [DOI] [PubMed] [Google Scholar]
Cassar 2010 {published data only}
- Cassar S, Kidgell D, Pearce A. The effect of hydrotherapy recovery on central fatigue: A preliminary examination using transcranial magnetic stimulation. Journal of Science and Medicine in Sport 2010;12(e1‐1232):Abstract no. 110. [Google Scholar]
- Cassar S, Pearce A. personal communication December 6 2010.
Eston 1999 {published data only}
- Eston R. personal communication 4 October 2010.
- Eston R, Peters D. Effects of cold water immersion on the symptoms of exercise‐induced muscle damage. Journal of Sports Sciences 1999;17(3):231‐8. [DOI] [PubMed] [Google Scholar]
Goodall 2008 {published data only}
- Goodall S. personal communication 4 October 2010.
- Goodall S, Howatson G. The effects of multiple cold water immersions on indices of muscle damage. Journal of Sports Science and Medicine 2008;7:235‐41. [PMC free article] [PubMed] [Google Scholar]
- Howatson G, Goodall S, Someren KA. The influence of cold water immersions on adaptation following a single bout of damaging exercise. European Journal of Applied Physiology 2009;105(4):615‐21. [DOI] [PubMed] [Google Scholar]
Halson 2008 {published data only}
- Halson SL. personal communication 12 October 2010.
- Halson SL, Quod MJ, Martin DT, Gardner AS, Ebert TR, Laursen PB. Physiological responses to cold water immersion following cycling in the heat. International Journal of Sports Physiology and Performance 2008;3(3):331‐46. [DOI] [PubMed] [Google Scholar]
Ingram 2009 {published data only}
- Ingram J. personal communication 11 October 2010.
- Ingram J, Dawson B, Goodman C, Wallman K, Beilby J. Effect of water immersion methods on post‐exercise recovery from simulated team sport exercise. Journal of Science and Medicine in Sport 2009;12(3):417‐21. [DOI] [PubMed] [Google Scholar]
Jakeman 2009 {published data only}
- Jakeman JR, Eston R. personal communication 3 November 2010.
- Jakeman JR, Macrae R, Eston R. A single 10‐min bout of cold water immersion therapy after strenuous plyometric exercise has no beneficial effect on recovery from the symptoms of exercise induced muscle damage. Ergonomics 2009;52(4):456‐60. [DOI] [PubMed] [Google Scholar]
King 2009 {published data only}
- King M. personal communication 9 November 2010.
- King M, Duffield R. The effects of recovery interventions on consecutive days of intermittent sprint exercise. Journal of Strength and Conditioning Research 2009;23(6):1795‐802. [DOI] [PubMed] [Google Scholar]
Kuligowski 1998 {published data only}
- Kuligowski LA, Lephart SM, Giannantonio FP, Blanc RO. Effect of whirlpool therapy on the signs and symptoms of delayed‐onset muscle soreness. Journal of Athletic Training 1998;33(3):222‐8. [PMC free article] [PubMed] [Google Scholar]
Montgomery 2008 {published data only}
- Montgomery PG, Pyne DB, Hopkins WG, Dorman JC, Cook K, Minahan CL. The effect of recovery strategies on physical performance and cumulative fatigue in competitive basketball. Journal of Sports Sciences 2008;26(11):1135‐45. [DOI] [PubMed] [Google Scholar]
Rowsell 2009 {published data only}
- Rowsell G, Coutts A, Reaburn P. Effect of cold water immersion on match running performance in high‐level junior soccer players during a four‐day tournament. Journal of Science and Medicine in Sport 2007;10(October):24. [Google Scholar]
- Rowsell GJ, Coutts AJ, Reaburn P, Hill‐Haas S. Effect of post‐match cold‐water immersion on subsequent match running performance in junior soccer players during tournament play. Journal of Sports Sciences 2011;29(1):1‐6. [DOI] [PubMed] [Google Scholar]
- Rowsell GJ, Coutts AJ, Reaburn P, Hill‐Haas S. Effects of cold‐water immersion on physical performance between successive matches in high‐performance junior male soccer players. Journal of Sports Sciences 2009;27(6):565‐73. [DOI] [PubMed] [Google Scholar]
Sellwood 2007a {published data only}
- Sellwood KL, Brukner P, Williams D, Nicol A, Hinman R. Ice water immersion and delayed‐onset muscle soreness: a randomised controlled trial. British Journal of Sports Medicine 2007;41(6):392‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skurvydas 2006 {published data only}
- Skurvydas A. personal communication 13 October 2010.
- Skurvydas A, Sipaviciene S, Krutulyte G, Gailiuniene A, Stasiulis A, Mamkus G, et al. Cooling leg muscles affects dynamics of indirect indicators of skeletal muscle damage. Journal of Back and Musculoskeletal Rehabilitation 2006;19(4):141‐51. [Google Scholar]
Vaile 2008c {published data only}
- Vaile J, Halson S, Gill N, Dawson B. Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. European Journal of Applied Physiology 2008;102(4):447‐55. [DOI] [PubMed] [Google Scholar]
Yanagisawa 2003a {published data only}
- Yanagisawa O, Niitsu M, Yoshioka H, Goto K, Kudo H, Itai Y. The use of magnetic resonance imaging to evaluate the effects of cooling on skeletal muscle after strenuous exercise. European Journal of Applied Physiology 2003;89(1):53‐62. [DOI] [PubMed] [Google Scholar]
Yanagisawa 2003b {published data only}
- Yanagisawa O, Niitsu M, Takahashi H, Goto K, Ital Y. Evaluations of cooling exercised muscle with MR imaging and 31P MR spectroscopy. Medicine and Science in Sports and Exercise 2003;35(9):1517‐23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Banfi 2008a {published data only}
- Banfi G, D'Eril GM, Barassi A, Lippi G. N‐terminal proB‐type natriuretic peptide (NT‐proBNP) concentrations in elite rugby players at rest and after active and passive recovery following strenuous training sessions. Clinical Chemisty and Laboratory Medicine 2008;46(2):247‐9. [DOI] [PubMed] [Google Scholar]
- Banfi G, Melegati G. Effect on sport hemolysis of cold water leg immersion in athletes after training sessions. Laboratory Hematology 2008;14(2):15‐8. [DOI] [PubMed] [Google Scholar]
- Banfi G, Melegati G, Valentini P. Effects of cold‐water immersion of legs after training session on serum creatine kinase concentrations in rugby players. British Journal of Sports Medicine 2007;41(5):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen TC, Hsieh SS. The effects of stretching and cryotherapy on delayed onset muscle soreness. Medicine and Science in Sports and Exercise 1996;28(5):181 (abstract number 1077). [Google Scholar]
Clements 2002 {published data only}
- Clements JM, Casa DJ, Knight J, McClung JM, Blake AS, Meenen PM, et al. Ice‐water immersion and cold‐water immersion provide similar cooling rates in runners with exercise‐induced hyperthermia. Journal of Athletic Training 2002;37(2):146‐50. [PMC free article] [PubMed] [Google Scholar]
Coffey 2004 {published data only}
- Coffey V, Leveritt M, Gill N. Effect of recovery modality on 4‐hour repeated treadmill running performance and changes in physiological variables. Journal of Science and Medicine in Sport 2004;7(1):1‐10. [DOI] [PubMed] [Google Scholar]
Connolly 2003 {published data only}
- Connolly DAJ, Brennan KM, Lauzon CD. Effects of active versus passive recovery on power output during repeated bouts of short term, high intensity exercise. Journal of Sports Science and Medicine 2003;2:47‐51. [PMC free article] [PubMed] [Google Scholar]
Crowe 2007 {published data only}
- Crowe MJ, O'Connor D, Rudd D. Cold water recovery reduces anaerobic performance. International Journal of Sports Medicine 2007;28(12):994‐8. [DOI] [PubMed] [Google Scholar]
Dawson 2005 {published data only}
- Dawson B, Cow S, Modra S, Bishop D, Stewart G. Effects of immediate post‐game recovery procedures on muscle soreness, power and flexibility levels over the next 48 hours. Journal of Science and Medicine in Sport 2005;8(2):210‐21. [DOI] [PubMed] [Google Scholar]
Gill 2006 {published data only}
- Gill ND, Beaven CM, Cook C. Effectiveness of post‐match recovery strategies in rugby players. British Journal of Sports Medicine 2006;40(3):260‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goosey‐Tolfrey 2008 {published data only}
- Goosey‐Tolfrey V, Swainson M, Boyd C, Atkinson G, Tolfrey K. The effectiveness of hand cooling at reducing exercise‐induced hyperthermia and improving distance‐race performance in wheelchair and able‐bodied athletes. Journal of Applied Physiology 2008;105(1):37‐43. [DOI] [PubMed] [Google Scholar]
Gurovich 2006 {published data only}
- Gurovich AN, Penailillo L, Reyes A, Plaza P. What is the best treatment to control Delayed Onset Muscle Soreness: Ultrasound, cryotherapy or both on a combined therapy?. Medicine and Science in Sports and Exercise 2006;38(5):689. [Google Scholar]
Hayashi 2004 {published data only}
- Hayashi K, Honda Y, Ogawa T, Wada H, Kondo N, Nishiyasu T. Effects of brief leg cooling after moderate exercise on cardiorespiratory responses to subsequent exercise in the heat. European Journal of Applied Physiology 2004;92(4‐5):414‐20. [DOI] [PubMed] [Google Scholar]
Heyman 2009 {published data only}
- Heyman E, Geus B, Mertens I, Meeusen R. Effects of four recovery methods on repeated maximal rock climbing performance. Medicine and Science in Sports and Exercise 2009;41(6):1303‐10. [DOI] [PubMed] [Google Scholar]
Higgins 2010 {published data only}
Hudson 1999 {published data only}
- Hudson OD, Loy SF, Vincent WJ, Yaspelkis BB. Blood lactate concentration and rated perceived exertion following active recovery in water. Sports Medicine, Training and Rehabilitation 1999;9(1):41‐50. [Google Scholar]
Khomenok 2008 {published data only}
- Khomenok GA, Hadid A, Preiss‐Bloom O, Yanovich R, Erlich T, Ron‐Tal O, et al. Hand immersion in cold water alleviating physiological strain and increasing tolerance to uncompensable heat stress. European Journal of Applied Physiology 2008;104(2):303‐9. [DOI] [PubMed] [Google Scholar]
Kinugasa 2009 {published data only}
- Kinugasa T, Kilding AE. A comparison of post‐match recovery strategies in youth soccer players. Journal of Strength and Conditioning Research 2009;23(5):1402‐7. [DOI] [PubMed] [Google Scholar]
Kokkinidis 1998 {published data only}
- Kokkinidis E, Tsamourtas A, Buckenmeyer P, Machairidou M. The effect of static stretching and cryotherapy on the recovery of delayed onset muscle soreness. Exercise and Society Journal of Sports Science 1998;19:45‐53. [Google Scholar]
Lane 2004 {published data only}
- Lane KN, Wenger HA. Effect of selected recovery conditions on performance of repeated bouts of intermittent cycling separated by 24 hours. Journal of Strength and Conditioning Research 2004;18(4):855‐60. [DOI] [PubMed] [Google Scholar]
Lapier 1995 {published data only}
- Lapier TK, Sheely SL, Moore R, Hubert CL, Brower C, Barnhard ML, Creelman J. The effects of massage or cryotherapy on isometric torque and perceived pain in muscles with contraction‐induced delayed onset muscle soreness. 12th International Congress World Confederation of Physical Therapy. 1995; Vol. 30:86.
Nosaka 1996 {published data only}
- Sakamoto K, Nosaka K, Clarkson P. Effect of reduced muscle temperature on eccentric exercise‐induced muscle damage. Medicine and Science in Sports and Exercise 1996;28(5):188. [Google Scholar]
Paddon‐Jones 1997 {published data only}
- Paddon‐Jones DJ, Quigley BM. Effect of cryotherapy on muscle soreness and strength following eccentric exercise. International Journal of Sports Medicine 1997;18(8):588‐93. [DOI] [PubMed] [Google Scholar]
Peiffer 2009a {published data only}
- Peake J, Peiffer JJ, Abbiss CR, Nosaka K, Okutsu M, Laursen PB, et al. Body temperature and its effect on leukocyte mobilization, cytokines and markers of neutrophil activation during and after exercise. European Journal of Applied Physiology 2008;102(4):391‐401. [DOI] [PubMed] [Google Scholar]
- Peiffer JJ, Abbiss CR, Nosaka K, Peake JM, Laursen PB. Effect of cold water immersion after exercise in the heat on muscle function, body temperatures, and vessel diameter. Journal of Science and Medicine in Sport 2009;12(1):91‐6. [DOI] [PubMed] [Google Scholar]
Peiffer 2009b {published data only}
- Peiffer JJ, Abbiss CR, Watson G, Nosaka K, Laursen PB. Effect of cold water immersion duration on body temperature and muscle function. Journal of Sports Sciences 2009;27(10):987‐93. [DOI] [PubMed] [Google Scholar]
Peiffer 2010b {published data only}
- Peiffer JJ, Abbiss CR, Watson G, Nosaka K, Laursen PB. Effect of a 5‐min cold‐water immersion recovery on exercise performance in the heat. British Journal of Sports Medicine 2010;44(6):461‐5 [Epub 2008 Jun 6]. [DOI] [PubMed] [Google Scholar]
Peñailillo 2006 {published data only}
- Peñailillo L, Plaza P, Husak T, Reyes W, Gurovich A. Cryotherapy as control delayed onset muscle soreness treatment. Medicine and Science in Sports and Exercise 2006;38(5):S26. [Google Scholar]
Price 2004 {published data only}
- Price MJ, Mather MI. Comparison of lower‐ vs. upper‐body cooling during arm exercise in hot conditions. Aviation, Space, and Environmental Medicine 2004;75(3):220‐6. [PubMed] [Google Scholar]
Schniepp 2002 {published data only}
- Schniepp J, Campbell TS, Powell KL, Pincivero DM. The effects of cold‐water immersion on power output and heart rate in elite cyclists. Journal of Strength and Conditioning Research 2002;16(4):561‐6. [PubMed] [Google Scholar]
Sipaviciene 2007 {published data only}
- Sipaviciene S, Skurvydas A, Ramanauskiene I, Senikiene Z, Dumciene A. Cooling makes recovery faster after eccentric‐concentric than concentric exercise [Pasaldymas labiau pagreitina raumens atsigavima po ekscentrinio‐koncentrinio fizinio kruvio nei po koncentrinio]. Medicina (Kaunas) 2008;44(3):225‐31. [PubMed] [Google Scholar]
Taylor 2008 {published data only}
- Taylor NA, Caldwell JN, Heuvel AM, Patterson MJ. To cool, but not too cool: that is the question‐‐immersion cooling for hyperthermia. Medicine and Science in Sports and Exercise 2008;40(11):1962‐9. [DOI] [PubMed] [Google Scholar]
Tessitore 2007 {published data only}
- Tessitore A, Meeusen R, Cortis C, Capranica L. Effects of different recovery interventions on anaerobic performances following preseason soccer training. Journal of Strength and Conditioning Research 2007;21(3):745‐50. [DOI] [PubMed] [Google Scholar]
Udermann 1997 {published data only}
- Udermann BE, Graves JE, Reider LR, Meyer JM, Li YH, King GR, et al. Delayed onset muscle soreness in the lumbar extensor muscle. Medicine & Science in Sports & Exercise 1997;29(5):66. [Google Scholar]
Vaile 2007 {published data only}
- Vaile JM, Gill ND, Blazevich AJ. The effect of contrast water therapy on symptoms of delayed onset muscle soreness. Journal of Strength and Conditioning Research 2007;21(3):697‐702. [DOI] [PubMed] [Google Scholar]
Vaile 2008a {published data only}
- Vaile J, Halson S, Gill N, Dawson B. Effect of cold water immersion on repeat cycling performance and thermoregulation in the heat. Journal of Sports Sciences 2008;26(5):431‐40. [DOI] [PubMed] [Google Scholar]
Vaile 2008b {published data only}
- Vaile J, Halson S, Gill N, Dawson B. The effect of hydrotherapy on recovery from fatigue. International Journal of Sports Medicine 2008;29(7):539‐544. [DOI] [PubMed] [Google Scholar]
Verducci 2000 {published data only}
- Verducci FM. Interval cryotherapy decreases fatigue during repeated weight lifting. Journal of Athletic Training 2000;35(4):422‐6. [PMC free article] [PubMed] [Google Scholar]
Webborn 2005 {published data only}
- Webborn N, Price MJ, Castle PC, Goosey‐Tolfrey VL. Effects of two cooling strategies on thermoregulatory responses of tetraplegic athletes during repeated intermittent exercise in the heat. Journal of Applied Physiology 2005;98(6):2101‐7. [DOI] [PubMed] [Google Scholar]
Yamane 2006 {published data only}
- Yamane M, Teruya H, Nakano M, Ogai R, Ohnishi N, Kosaka M. Post‐exercise leg and forearm flexor muscle cooling in humans attenuates endurance and resistance training effects on muscle performance and on circulatory adaptation. European Journal of Applied Physiology 2006;96(5):572‐80. [DOI] [PubMed] [Google Scholar]
Yeargin 2006 {published data only}
- Yeargin SW, Casa DJ, McClung JM, Knight JC, Healey JC, Goss PJ, et al. Body cooling between two bouts of exercise in the heat enhances subsequent performance. Journal of Strength and Conditioning Research 2006;20(2):383‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ascensao 2011 {published data only}
- Ascensao A, Leite M, Rebelo AN, Magalhaes S, Magalhaes J. Effects of cold water immersion on the recovery of physical performance and muscle damage following a one‐off soccer match. Journal of Sports Sciences 2011;29(3):217‐25. [DOI] [PubMed] [Google Scholar]
Fowles 2003 {published data only (unpublished sought but not used)}
- Fowles JR, Boutilier G, Murphy RJ. Post‐exercise cooling has an effect on submaximal performance. Medicine and Science in Sports and Exercise 2003;35(5):S183. [Google Scholar]
Pournot 2011 {published data only}
- Pournot H, Bieuzen F, Duffield R, Lepretre PM, Cozzolino C, Hausswirth C. Short term effects of various water immersions on recovery from exhaustive intermittent exercise. European Journal of Applied Physiology 2011;111(7):1287‐95. [DOI] [PubMed] [Google Scholar]
Smith 2008 {published data only}
- Smith LC, Mroczkowski SA, Buser S, Bemis S, Otterstetter R. Effects Of thermotherapy and cryotherapy on muscle hypertrophy and muscle contraction force following resistance exercise. Medicine & Science in Sports & Exercise 2008;40(5):S258. [Google Scholar]
Stacey 2010 {published data only}
- Stacey DL, Gibala MJ, Martin Ginis KA, Timmons BW. Effects of recovery method after exercise on performance, immune changes, and psychological outcomes. Journal of Orthopaedic & Sports Physical Therapy 2010;40(10):656‐65. [DOI] [PubMed] [Google Scholar]
Vaile 2011 {published data only}
- Vaile J, O'Hagan C, Stefanovic B, Walker M, Gill N, Askew CD. Effect of cold water immersion on repeated cycling performance and limb blood flow. British Journal of Sports Medicine 2011;45(10):825‐9. [DOI] [PubMed] [Google Scholar]
- Vaile J, Stefanovic B, O'Hagan C, Walker M, Gill N, Askew C. Effect of cold water immersion on recovery and limb blood flow following high intensity cycling. Journal of Science and Medicine in Sport 2009;12S:Abstract 58. [Google Scholar]
Additional references
Banfi 2008
- Banfi G, Melegati G. Effect on sport hemolysis of cold water leg immersion in athletes after training sessions. Laboratory Hematology 2008;14(2):15‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barnett 2006
- Barnett A. Using recovery modalities between training sessions in elite athletes: does it help?. Sports Medicine 2006;36(9):781‐96. [DOI] [PubMed] [Google Scholar]
Bennett 2005
- Bennett MH, Best TM, Babul‐Wellar S, Taunton JE. Hyperbaric oxygen therapy for delayed onset muscle soreness and closed soft tissue injury. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004713.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bleakley 2004
- Bleakley CM, McDonough SM, MacAuley DC. The use of ice in the treatment of acute soft‐tissue injury: a systematic review of randomized controlled trials. American Journal of Sports Medicine 2004;32(1):251‐61. [DOI] [PubMed] [Google Scholar]
Bleakley 2010
- Bleakley CM, Davison GW. What is the biochemical and physiological rationale for using cold‐water immersion in sports recovery? A systematic review. British Journal of Sports Medicine 2010;44(3):179‐87. [DOI] [PubMed] [Google Scholar]
Chatzinikolaou 2010
- Chatzinikolaou A, Fatouros IG, Gourgoulis V, Avloniti A, Jamurtas AZ, Nikolaidis MG, Douroudos I, Michailidis Y, Beneka A, Malliou P, Tofas T, Georgiadis I, Mandalidis D, Taxildaris K. Time course of changes in performance and inflammatory responses after acute plyometric exercise. Journal of Strength and Conditioning Research 2010;24(5):1389‐98. [DOI] [PubMed] [Google Scholar]
Cheung 2003
- Cheung K, Hume P, Maxwell L. Delayed onset muscle soreness: treatment strategies and performance factors. Sports Medicine 2003;33(2):145‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cleak 1992
- Cleak MJ, Eston RG. Muscle soreness, swelling, stiffness, and strength loss after intense eccentric exercise. British Journal of Sports Medicine 1992;26(4):267‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane 2004
- Cochrane DJ. Alternating hot and cold water immersion for athlete recovery: A review. Physical Therapy in Sport 2004;5(1):26‐32. [EMBASE: 2004120600] [Google Scholar]
Deeks 2008
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Deeks 2008a
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Section 9.5.2. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic reviews of Interventions 5.0.2 (updated September 2008). Available from www.cochrane‐handbook.org.
Deeks 2008b
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Section 9.6.2. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic reviews of Interventions 5.0.2 (updated September 2008). Available from www.cochrane‐handbook.org.
Guyatt 2002
- Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR, Aaronson N. Methods to explain the clinical significance of health status measures. Mayo Clinic Proceedings 2002;77(4):371–83. [DOI] [PubMed] [Google Scholar]
Herbert 2011
- Herbert RD, Noronha M, Kamper SJ. Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD004577.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. Section 8.5. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 [updated September 2009]. Available from www.cochrane‐handbook.org.
Hing 2008
- Hing WA, White SG, Bouaaphone A, Lee P. Contrast therapy‐‐a systematic review. Physical Therapy in Sport 2008;9(3):148‐61. [DOI] [PubMed] [Google Scholar]
Knight 2000
- Knight KL, Brucker JB, Stoneman PD, Rubley MD. Muscle injury management with cryotherapy. Athletic Therapy Today 2000;5(4):26‐30. [EMBASE: 2000225657] [Google Scholar]
Kunz 2007
- Kunz R, Vist GE, Oxman AD. Randomisation to protect against selection bias in healthcare trials. Cochrane Database of Systematic Reviews 2007, Issue 2 10.1002/14651858.MR000012.pub3.. [DOI: 10.1002/14651858.MR000012.pub3] [DOI] [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. Box 6.4.d. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2 (updated September 2009). Available from www.cochrane‐handbook.org.
Low 2000
- Low J, Reed A. Electrotherapy explained: principles and practice. 3rd Edition. Oxford: Butterworth and Heinemann, 2000. [ISBN ‐ 0750641495] [Google Scholar]
McDermott 2009
- McDermott BP, Casa DJ, Ganio MS, Lopez RM, Yeargin SW, Armstrong LE, et al. Acute whole‐body cooling for exercise‐induced hyperthermia: a systematic review. Journal of Athletic Training 2009;44(1):84‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peiffer 2010
- Peiffer JJ, Abbiss CR, Watson G, Nosaka K, Laursen PB. Effect of cold water immersion on repeated 1‐km cycling performance in the heat. Journal of Science and Medicine in Sport 2010;13(1):112‐6. [DOI] [PubMed] [Google Scholar]
Pildal 2007
- Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomized trials. International Journal of Epidemiology 2007;36(4):847‐57. [DOI] [PubMed] [Google Scholar]
Proske 2001
- Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. Journal of Physiology 2001;537(Pt 2):333‐45. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowsell 2011
- Rowsell GJ, Coutts AJ, Reaburn P, Hill‐Haas S. Effect of post‐match cold‐water immersion on subsequent match running performance in junior soccer players during tournament play. Journal of Sports Sciences 2011;29(1):1‐6. [DOI] [PubMed] [Google Scholar]
Sellwood 2007b
- Sellwood KL, Brukner P, Williams D, Nicol A, Hinman R. Ice‐water immersion and delayed‐onset muscle soreness: a randomised controlled trial. British Journal of Sports Medicine 2007;41(6):392‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2005
- Smith JE. Cooling methods used in the treatment of exertional heat illness. British Journal of Sports Medicine 2005;39(8):503‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tashjian 2009
- Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. Journal of Shoulder and Elbow Surgery 2009;18(6):927‐32. [DOI] [PubMed] [Google Scholar]
Vaile 2008d
- Vaile J, Halson S, Gill N, Dawson B. Effect of cold water immersion on repeat cycling performance and thermoregulation in the heat. Journal of Sports Sciences 2008;26(5):431‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vaile 2008e
- Vaile J, Halson S, Gill N, Dawson B. Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. European Journal of Applied Physiology 2008;102(4):447‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Dam 2008
- Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ 2008;337:a1440. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilcock 2006
- Wilcock IM, Cronin JB, Hing WA. Physiological response to water immersion: a method for sport recovery?. Sports Medicine 2006;36(9):747‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


