Abstract
Background
Pulmonary arterial hypertension (PAH) is characterised by pulmonary vascular changes, leads to elevated pulmonary artery pressures, dyspnoea, a reduction in exercise tolerance, right heart failure, and ultimately death.
Prostacyclin analogue drugs mimic endogenous prostacyclin which leads to vasodilation, inhibition of platelet aggregation, and reversal of vascular remodelling. Prostacyclin's short half‐life theoretically enhances selectivity for the pulmonary vascular bed by direct (via central venous catheter) administration. Initial continuous infusion prostacyclins were efficacious, but use of intravenous access increases the risk of adverse events. Newer and safer subcutaneous, oral and inhaled preparations are now available, though possibly less potent.
Selexipag is an oral selective prostacyclin receptor (IP receptor) agonist that works similarly to prostacyclin, potentially more stable, with less complex administration and titration.
Objectives
To determine the efficacy and safety of prostacyclin, prostacyclin analogues or prostacyclin receptor agonists for PAH in adults and children.
Search methods
We performed searches on CENTRAL, MEDLINE, and Embase up to 16 September 2018. We handsearched review articles, clinical trial registries, and reference lists of retrieved articles.
Selection criteria
We included any randomised controlled trials (RCTs) which compared prostacyclin, prostacyclin analogues or prostacyclin receptor agonists to control (placebo, any other treatment or usual care) for at least six weeks.
Data collection and analysis
We used standard methods specified by Cochrane. Primary outcomes included change in World Health Organization (WHO) functional class, six‐minute walk distance (6MWD), and mortality.
Main results
Seventeen trials with 3765 mostly adult participants were included; median trial duration was 12 weeks. Fifteen trials used prostacyclin analogues: intravenous (N = 4); subcutaneous (N = 1); oral (N = 5); inhaled (N = 5); two used oral prostacyclin receptor agonists. Three intravenous and two inhaled trials were open‐label.
Participants using prostacyclin had 2.39 times greater odds of improving by at least one WHO functional class (95% confidence interval (CI) 1.72 to 3.32; 24 per 100 (95% CI 18.5 to 30.4) with prostacyclin compared to 12 per 100 with control; 8 trials, 1066 participants; moderate‐certainty evidence). Improvement occurred with intravenous (odds ratio (OR) 14.96, 95% CI 4.76 to 47.04), and inhaled (OR 2.94, 95% CI 1.53 to 5.66), but not with oral preparations. Participants using prostacyclin increased their 6MWD by 19.50 metres (95% CI 14.82 to 24.19; 13 trials, 2283 participants; low‐certainty evidence), which was clinically significant with intravenous (mean difference (MD) 91.76 metres; 95% CI 58.97 to 124.55), but not with non‐intravenous preparations (subcutaneous: MD 16.00 metres, 95% CI 7.38 to 24.62; oral: MD 14.76 metres, 95% CI 7.81 to 21.70; inhaled: MD 26.97 metres, 95% CI 17.21 to 36.73). Mortality was reduced in the intravenous (OR 0.29, 95% CI 0.12 to 0.69; risk of death 6 per 100 (95% CI 2.38 to 12.31) with prostacyclin compared to 17 per 100 with control; 4 trials, 255 participants), but not in the non‐intravenous studies (OR 0.82, 95% CI 0.48 to 1.40; risk of death 21 per 1000 (95% CI 12.00 to 34.20) with prostacyclin compared to 25 per 1000 with control; moderate‐certainty evidence; 12 trials, 2299 participants). We reduced the certainty of evidence due to few studies per subgroup and use of open‐label trials.
Prostacyclins improved cardiopulmonary haemodynamics (reduction in mean pulmonary artery pressure by 3.60 mmHg (95% CI ‐4.73 to ‐2.48); pulmonary vascular resistance by 2.81 WU (95% CI ‐3.80 to ‐1.82); right atrial pressure by 1.90 mmHg (95% CI ‐2.58 to ‐1.22), and increase in cardiac index by 0.31 L/min/m2 (95% CI 0.23 to 0.38); low‐certainty evidence), improved dyspnoea (low‐certainty evidence, and improved quality of life (moderate‐certainty evidence), when compared to control. When only subcutaneous/inhaled trials were included the effect was still significant, but the magnitude was smaller. There was no difference across oral trials.
Adverse events were increased in all prostacyclin preparations, including vasodilation (OR 5.03, 95% CI 3.84 to 6.58), headache (OR 3.16, 95% CI 2.62 to 3.80), jaw pain (OR 5.25, 95% CI 3.96 to 6.98), diarrhoea (OR 2.81, 95% CI 2.29 to 3.46), nausea/vomiting (OR 2.39, 95% CI 1.98 to 2.88), myalgias (OR 2.75, 95% CI 1.65 to 4.58), upper respiratory tract events (OR 1.61, 95% CI 1.22 to 2.13), extremity pain (OR 3.36, 95% CI 2.32 to 4.85), and infusion site reactions (OR 14.41, 95% CI 9.16 to 22.66). In the intravenous trials, there was a 12%‐25% risk of serious non‐fatal events including sepsis, haemorrhage, pneumothorax and pulmonary embolism.
Two trials (1199 participants) compared oral selexipag to placebo; no trials compared selexipag with prostacyclin. There was a small 12.62 metre improvement in 6MWD (95% CI 1.90 to 23.34; high‐certainty evidence), and weak evidence for haemodynamics. The effect was uncertain for WHO functional class. The risk of death with selexipag was five per 100 compared to three per 100 with placebo, though the CI crossed zero so the true effect is uncertain (risk difference (RD) 0.02 (95% CI ‐0.00 to 0.04). There was less clinical worsening with selexipag (OR 0.47, 95% CI 0.37 to 0.60), though more side effects, including vasodilation (OR 2.67, 95% CI 1.72 to 4.17), headache (OR 3.91, 95% CI 3.07 to 4.98), jaw pain (OR 5.33, 95% CI 3.64 to 7.81), diarrhoea (OR 3.11, 95% CI 2.39 to 4.05), nausea/vomiting (OR 2.92, 95% CI 2.29 to 3.73), pain in the extremities (OR 2.44, 95% CI 1.69 to 3.52), and myalgias (OR 3.05, 95% CI 2.02 to 4.58).
Authors' conclusions
This review demonstrates clinical and statistical benefit for intravenous prostacyclin (compared to control) with improved functional class, 6MWD, mortality, symptoms scores, and cardiopulmonary haemodynamics, but at a cost of adverse events. This may be due to a true effect, or may be overestimated due to the inclusion of small, short or open‐label studies. There was a statistical and small clinical benefit in function and haemodynamics for inhaled prostacyclin, but the effect is uncertain for mortality. The effect of oral prostacyclins are less certain. Selexipag demonstrated less clinical worsening without discernable impact on survival, increased adverse events; and the effect on other outcomes is less certain. Real‐world registry data may provide further information about clinical effect.
Plain language summary
Prostacyclin in pulmonary arterial hypertension
Review question
We wanted to review whether a group of drugs called prostacyclin analogues help people with pulmonary hypertension. Cochrane researchers collected and analysed all relevant studies to answer this question.
Why this review is important
Pulmonary hypertension can cause breathlessness, reduced exercise tolerance, reduced quality of life, hospitalisations, and early death. Prostacyclin analogues may improve blood circulation in the right heart and lungs. We wanted to make sure if these drugs are being used, there is evidence of benefit and little or no harm.
Main findings
We found and included 17 trials with 3765 people. Most of the studies were 12 weeks long. Some trials were as long as 52 weeks. Most trials involved adults. People who were given prostacyclin analogues were compared to people who were not given prostacyclin. People in four trials were given the drugs by a continuous drip (24 hours/day) into a vein (intravenous) and in one trial through continuous injection under the skin (subcutaneous). In five trials people inhaled the drugs through a nebuliser and in five trials they took tablets (oral). People in two studies took selexipag tablets. Selexipag is an agonist of the prostacyclin receptor and the trials in selexipag were analysed separately.
People who were given prostacyclins via intravenous drip showed improved survival (a lower chance of dying). They could also walk on average 92 metres further in six minutes than people not given the prostacyclin drip. They were also more likely to improve their functional class (what you can and cannot do on a daily basis). People with intravenous prostacyclins had better heart function on average than those who had no treatment.
Overall, the results were less clear for people given oral, inhaled or subcutaneous prostacyclins. It was not clear whether giving the drug in these ways led to improved survival. People who took inhaled (nebuliser) prostacyclins improved their functional class, walked on average 27 metres further in six minutes, and had better heart function. There was also some evidence that subcutaneous prostacyclins improved heart function. It was not clear if taking tablets improved functional class or heart function. People receiving this treatment only walked 15 metres further in six minutes than those not receiving prostacyclin tablets.
Whilst this review found evidence was best for prostacyclin via continuous drip, it may be inconvenient, and might increase risks such as intravenous line‐related infections. Furthermore almost all people taking recommended doses in any form have important drug‐related side effects (including flushing, headache, jaw pain, diarrhoea, pain in their extremities, upper respiratory tract side effects, nausea and vomiting).
People taking selexipag had less clinical worsening, and a small 13‐metre difference in their six‐minute walk test compared to people taking placebo. People who used selexipag were also more likely to have side effects including flushing, jaw pain, diarrhoea, nausea/vomiting, and pain in the muscles/extremities.
Limitations
There is moderate‐certainty evidence that prostacyclin helps people compared to those who do not use it. The benefit is best for those who receive the drug via a continuous drip, but the risks are higher. Also, on average, the studies only lasted three months (some up to 1 year), and this may not be enough time to see benefit or risks.
This review only looked at those with a diagnosis of pulmonary arterial hypertension, not those with pulmonary hypertension associated with left heart disease, lung disease, or pulmonary hypertension due to blood clots.
This review is current to September 2018.
Summary of findings
Background
Description of the condition
Pulmonary hypertension is defined as a mean pulmonary arterial pressure (mPAP) exceeding 25 mmHg measured by right heart catheterisation (Galiè 2016). More than 50 diseases across five main categories (World Health Organization (WHO) type 1 to 5) are reported as potential aetiologies (Simonneau 2013). Many cause progressive disease, with associated right ventricular strain, hypertrophy, remodelling within the pulmonary vasculature and premature death. In the later stages of the disease, cardiopulmonary dysfunction leads to burdensome symptoms, such as exercise intolerance, syncope, oedema and breathlessness. The development of several specific therapies for WHO Group 1 pulmonary arterial hypertension (PAH) has led to heightened interest in the condition. Unfortunately, most people presenting with PAH have progressed to advanced disease at the time of specialist referral (Humbert 2006; Thenappan 2007); and the true prevalence of pulmonary hypertension is likely under‐recognised (Galiè 2016). Modern therapies have reduced morbidity and improved survival (Thenappan 2007); however the risks and side effects warrant their careful selection. Prostaglandins have an unusual spectrum of side effects and almost all patients on an effective dose will have significant prostaglandin‐related side effects.
The WHO classification system for pulmonary hypertension is widely used, grouping disorders based on underlying mechanisms (Simonneau 2013). This provides a framework for treatment, as pathophysiology varies greatly between groups. Group 1 comprises PAH, formerly termed "primary pulmonary hypertension", which refers to precapillary flow obstruction, independent of venous thromboembolism or hypoxaemic lung disease (Badesch 2009). PAH is a rare disease, with an estimated prevalence of 10 to 52 cases per million (Ling 2012; Peacock 2007).
The gold standard diagnostic tool in pulmonary hypertension is right heart catheterisation, which determines a diagnosis of pulmonary hypertension, and further characterises the aetiology according to the WHO classification (Galiè 2016). PAH is determined as pulmonary hypertension (mean pulmonary arterial pressure (mPAP) equal to or higher than 25 mmHg) with a normal back pressure from the heart (a pulmonary arterial wedge pressure equal to or less than 15 mmHg) and a pulmonary vascular resistance (PVR) more than 3 Wood units measured during right heart catheterisation. A pulmonary arterial wedge pressure higher than 15 mmHg indicates contributing left heart dysfunction. Other baseline evaluation includes high‐resolution computed tomography (HRCT) and ventilation‒perfusion (VQ) scanning to rule out other causes (non‐WHO Group 1); and exercise testing such as six‐minute walk distance (6MWD) (Galiè 2016) for baseline evaluation and prognostication.
Beyond confirmation of the diagnosis, right heart catheterisation and other baseline tests assist to stratify risk of progression which assists in directing treatment. Goals of therapy are relief of symptoms, improved exercise capacity, improved quality of life, arresting progression and reducing mortality. People with PAH often respond to disease‐specific modifying therapies, including calcium channel blockers, prostacyclin analogues, endothelin receptor antagonists and phosphodiesterase‐5 inhibitors. In contrast, indications for advanced therapies in other groups of pulmonary hypertension are less clear cut and treatment of underlying conditions is first line (Galiè 2016).
Description of the intervention
Prostacyclin is endogenously synthesised by endothelial cells using the cyclo‐oxygenase arachidonic pathway. Prostacyclin exerts vasodilatory, antithrombotic and antiproliferative effects that are essential for endothelial function (Mitchell 2014). The principal target of prostacyclin is the IP G protein‐coupled receptor in the smooth muscle of arterioles. Its activation triggers intracellular cyclic adenosine monophosphate formation, activating protein kinase A, which mediates vasodilation of the pulmonary arteries, inhibition of platelet aggregation, and relaxation of the smooth muscle (Humbert 2015). Disequilibrium between vasodilating mediators, such as a reduction in the normal release of prostacyclin, and increased release of vasoconstricting mediators, such as thromboxane A2, plays a causative role in PAH (Christman 1992; Sitbon 2016). Currently there are three prostacyclin analogues available ‐ epoprostenol, iloprost and treprostinil. Selexipag is a selective IP prostacyclin receptor agonist that is structurally distinct from prostacyclin. It is rapidly hydrolysed to a long‐acting metabolite that binds to IP receptors, resulting in the same actions as prostacyclin ‐ vasodilation, inhibition of platelet aggregation, and anti‐inflammatory effects (Noel 2017).
How the intervention might work
Epoprostenol directly vasodilates the pulmonary and systemic arterial vasculature, and has been demonstrated in previous trials to reduce ventricular afterload, pulmonary vascular resistance (PVR) and platelet aggregation, and to increase cardiac output (Sitbon 2016).
The key attributes of synthetic prostacyclin agents are prostacyclin's short half‐life at room temperature (minutes) and that it mainly only exerts local effects (Mitchell 2014). The first synthetic agent (epoprostenol) demonstrated significant efficacy as a therapeutic agent in the improvement of haemodynamic parameters, exercise capacity, and mortality (Barst 1996). However it is not without drawbacks. Its short half‐life requires continuous intravenous infusion, via a central venous catheter and continuous pump, requiring central line placement, and potentially introducing the risk of central line‐associated blood stream infection (Kallen 2008). Initial preparations were required to be refrigerated or kept on ice; however newer preparations have a more stable half‐life of 24 hours (Sitbon 2012).
Iloprost is a prostacyclin analogue that is most frequently used via inhalation. It has a slightly longer half‐life of 20 to 30 minutes, but still requires 5 to 10 inhalation doses throughout the day. Treprostinil has a much more stable half‐life of four hours, and can be administrated at much lower infusion rates via a subcutaneous or intravenous pump (Tapson 2006). However, treprostinil is metabolised by cytochrome P450 (CYP)2C8 in the liver and its metabolites are renally excreted, so clearance may be affected by hepatic impairment. Cumulative effects of treprostinil can occur if used with antihypertensives or anticoagulants (Simonneau 2002).
Beraprost is also available as an orally active prostacyclin analogue, theorised to maintain a stable structure due to its cyclopenta benzofuranyl skeleton. It acts by binding to prostacyclin membrane receptors to inhibit the release of calcium, leading to relaxation of smooth muscle cells and vasodilation, and inhibiting platelet aggregation. Given three times a day, it has previously exhibited improved outcomes in those with intermittent claudication due to peripheral arterial disease (Melian 2002).
For all prostacyclin agents, dose titration is individualised according to the individual patient. A characteristic pattern of adverse effects, particularly systemic hypotension, but also including flushing, diarrhoea, and muscle pains (Barst 1996; Sitbon 2016), may limit dose escalation. Indeed the dose is often up‐titrated until side effects are evident. This makes patient and investigator concealment (blinding) somewhat problematic in clinical trials. The method of delivery and the drug itself are expensive. Furthermore, therapy must be continuous, as abrupt withdrawal may precipitate rebound pulmonary hypertension, which can be fatal.
Selexipag is an oral selective prostacyclin receptor (IP receptor) agonist that works similarly to prostacyclin. It is postulated that the density of prostacyclin receptors varies between patients, therefore requiring complex personally tailored dosing of prostacyclin analogues, however, clinical trials in selexipag indicates patients respond similarly to the low‐, medium‐ and high‐dose regiments, therefore it offers a potentially more stable drug, with less complex administration and titration (GRIPHON).
Why it is important to do this review
Evidence in the literature suggests that prostacyclin analogues are efficacious in the treatment of PAH; however the treatment may come with considerable risks and side effects. The purpose of this review is to summarise the available published data regarding the relative efficacy and safety of prostacyclin analogues, in particular on haemodynamic response, and on participant‐centred outcomes, such as exercise tolerance, adverse effects, and quality of life.
Unfortunately, patients with PAH usually have advanced disease at presentation. Early diagnosis and management of this progressive condition offers a greater scope to delay or prevent onset of end‐stage symptoms. Recognising the presence of pulmonary hypertension as well as the underlying cause allows early initiation of appropriate treatment and potentially avoidance of end‐stage disease states.
Objectives
To determine the efficacy and safety of prostacyclin, prostacyclin analogues or prostacyclin receptor agonists, compared to placebo or any other treatment, for pulmonary arterial hypertension (PAH) in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
We included any randomised controlled trials (RCTs) which compared prostacyclin or analogues to control (placebo, any other treatment or usual care) for at least six weeks. We defined 'randomised' as studies which are described by the author as 'randomised' anywhere in the manuscript. All defined trials, published or unpublished, in any language, were potentially eligible for inclusion.
Types of participants
We included any individual with a diagnosis of World Health Organization (WHO) Group 1 pulmonary hypertension, referred to as pulmonary arterial hypertension (PAH), as per the present definition of a mean pulmonary arterial pressure (mPAP) higher than 25 mmHg by right heart catheterisation (Galiè 2016). We did not include other WHO diagnostic groups (2 to 5) of pulmonary hypertension. We planned to specify subgroups of adults older than 18 years and a paediatric population younger than 18 years, however, no trials reported separate outcome data or individual patient data to make these subgroup comparisons.
Types of interventions
We included studies comparing any type of prostacyclin treatment by any route of administration with placebo or any other treatment for at least six weeks. This included, but was not limited to, prostaglandins, epoprostenol, iloprost, beraprost, treprostinil, prostacyclin receptor agonist and selexipag, via the intravenous, subcutaneous, inhaled, and oral route. We separated comparisons into prostacyclin versus control and selexipag versus control. We included studies with co‐interventions, provided they were not part of the randomised treatment, by any route of administration, with placebo or any other treatment used for pulmonary hypertension. Where multiple doses were used, we planned to perform subgroup analyses by dose, however, in the included studies, doses were titrated per individual participant. Where studies were too heterogeneous for meta‐analyses, or where only descriptive data were available, we described them in narrative form.
Types of outcome measures
Primary outcomes
Change in WHO or New York Heart Association (NYHA) functional class (Badesch 2009)
Six‐minute walk distance (6MWD) test (Badesch 2009)
Mortality
Secondary outcomes
Cardiopulmonary haemodynamics: including mean pulmonary artery pressure (mPAP), pulmonary vascular resistance (PVR), cardiac index, cardiac output, systemic arterial oxygen saturation and systemic oxygen transport
Exercise capacity tests other than 6MWD test
Symptom scales: Borg dyspnoea score (Badesch 2009), dyspnoea‐fatigue ratings (Badesch 2009)
Quality of life
Clinical worsening
Adverse events
Cost analysis
Reporting of one or more outcomes was not a criterion for inclusion of a study in the review. We only included trials which have treated participants for at least six weeks. We did not find any studies which reported multiple time points, nor did we find any studies which reported post‐intervention follow‐up separate to the initial trial results. We are aware that some included trials may use composite outcomes. Where these were presented, we re‐analysed data to report only outcomes specified above.
Search methods for identification of studies
Electronic searches
We identified studies from searches of the following databases up to 16 September 2018.
Cochrane Airways Register of Trials through the Cochrane Register of Studies (CRS Web).
Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies (CRS Web).
MEDLINE Ovid SP 1946 to 16 September 2018.
Embase Ovid SP 1974 to 16 September 2018.
In addition, we searched the CENTRAL database in the Cochrane Library for conference abstracts and grey literature. The database search strategies are listed in Appendix 1. We did not apply any restrictions for language, date or type of publication.
We also searched the following trials registries for additional trials for inclusion and for additional data for included trials.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.ClinicalTrials.gov).
World Health Organization (WHO) International Clinical Trials Registry Platform (apps.who.int/trialsearch).
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We handsearched reference lists of included studies, relevant chapters and review articles. We used Google to search for grey literature and conference abstracts. We planned to translate any relevant article into English for potential inclusion, however we did not identify any other language papers. Where data were missing, we checked on trial registries and attempted to contact the trial investigators. We searched for errata or retractions from included studies published in full text on PubMed and reported the date this was done within the review.
Data collection and analysis
Selection of studies
Two independent review authors (HB, HLY) independently screened all abstracts to determine if they met the accepted inclusion criteria using Covidence. We obtained full‐text publications for those papers which definitely or may meet inclusion criteria. Two independent review authors (HB, HLY) then reviewed all full‐text articles to determine eligibility, and recorded reasons for any that are ineligible. We resolved any concerns or disagreement through discussion with other review authors (AB, TW, MH). We included a PRISMA study flow diagram in the full review to document the screening process and included a 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
Two review authors (HB and HLY) independently extracted data from included studies, and where appropriate, pooled data in Cochrane’s statistical software Review Manager 5 (RevMan 5) (Review Manager 2014), for further analysis. Following, both review authors met to check consistency of data entered into RevMan 5 prior to meta‐analyses being performed. The Cochrane Airways group methodologist (Christopher Cates) assisted with generic inverse variance analysis. We planned to resolve disagreements by consensus or by involving a third review author (AB). We used a data collection form which was piloted for inclusion in the review, containing the following data.
Methods: study design, duration, study setting, date of study
Participants: number, mean age and age range, gender, inclusion and exclusion criteria, and differences in baseline characteristics
Intervention: type of prostacyclin analogue, dose, mode of administration, control drug, co‐interventions and exclusions
Outcomes: primary and secondary outcomes as specified, type of scale used, time points collected
'Risk of bias' summary
Other: funding for trial, any conflicts of interest for trial authors
Assessment of risk of bias in included studies
Two independent authors (HB, HLY) assessed the included studies for risk of bias using Cochrane's tool for assessment of risk of bias according to the following domains (Higgins 2011).
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We judged each potential source of bias as low, unclear risk (insufficient information to form a judgement), or high risk, and provided justification with evidence from each trial in the 'Risk of bias' table. When considering treatment effects, we took into account the risk of bias for the studies that contribute to that outcome. We provided a quote from the study report together with justification for our judgement in the 'Risk of bias' table.
Assessment of bias in conducting the systematic review
We conducted the review according to our previously published protocol and justified any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
Where possible, we pooled and presented results from dichotomous data as odds ratios (ORs). Where zero totals were obtained, we presented these data as risk differences (RDs). Where possible, we presented results from continuous variables using a fixed‐effect model and calculated the mean differences (MDs) or standardised mean differences (SMDs) where scales are combined, with the 95% confidence intervals (95% CIs). If data from rating scales are combined in a meta‐analysis, we ensured that they are entered with a consistent direction of effect (e.g. lower scores always indicate improvement). Where both change from baseline and endpoint scores were available for continuous data, we used change from baseline scores where possible. We only combined data reported at different time points if this is clinically appropriate. We described skewed data narratively (e.g. as medians and interquartile ranges for each group).
We used intention‐to‐treat or 'full analysis set' analyses where they are reported (i.e. those where data have been imputed for participants who were randomly assigned but did not complete the study) instead of 'completer' or 'per‐protocol' analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of children admitted to hospital, rather than number of admissions per child). However, where rate ratios are reported in a study, we analysed them on this basis. No cluster‐randomised trials were included, however if cluster‐randomised trials are included in future versions of the review, we will only use data which has been, or can be, adjusted to account for the clustering.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. Han 2017 to obtain individual data). Where this was not possible, and the missing data are thought to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
For pooled analyses, we quantified statistical heterogeneity using the I² statistic, which describes the percentage of total variation across trials due to heterogeneity rather than sampling error. Significant statistical heterogeneity was considered to be present if the I² is greater than 50%. Where significant heterogeneity was identified, we planned to explore possible causes using prespecified subgroup analyses.
Assessment of reporting biases
We were unable to pool more than 10 studies using the same intervention, so we did not explore further possible small‐study and publication biases as stated a priori.
Data synthesis
We performed pooled quantitative meta‐analysis where trials were considered clinically homogenous. We used a fixed‐effect model to synthesise and report mean difference (MD) and 95% CIs. We synthesised and report dichotomous and continuous data separately for each outcome.
Where there was substantial heterogeneity (> 50%), we also reported outcomes in the text, including the direction and size of the effect along with the strength of the evidence (risk of bias).
'Summary of findings' table
We created a 'Summary of findings' table using the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions along with GRADEPro GDT software (GRADEpro GDT; Higgins 2011). The outcomes included:
WHO functional class status;
mortality;
change in haemodynamics;
6MWD;
dyspnoea;
quality of life;
adverse events.
We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contribute data for the prespecified outcomes. We justified all decisions to downgrade the quality of studies using footnotes and made comments to aid the reader’s understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses comparing the different routes of administration, and presented these results under each outcome. We planned to compare different prostacyclin analogues, but assessed there were too few studies (one to two per type) to draw meaningful comparisons. We planned to compare children versus adults, but separate data were not available. We planned to compare the effect of WHO functional class at baseline, however almost all trials included functional class III/IV.
Sensitivity analysis
We included a fixed‐effect versus random‐effect sensitivity analysis in a tabular format.
We included open‐label versus blinded trials sensitivity analysis under each per‐protocol specified outcomes (functional class, 6MWD, mortality, and cardiopulmonary haemodynamics).
Results
Description of studies
Results of the search
We identified 3031 citations in the initial search as described in the methods, and after two review authors (HB and HLY) independently screened abstracts, we selected 74 articles for full‐text review. After further assessment, we included 17 trials with 3765 participants in the final meta‐analysis, which included 30 separate citations (see Figure 1). We also noted three ongoing studies (see Characteristics of ongoing studies). The search was run on 16 September 2018.
1.
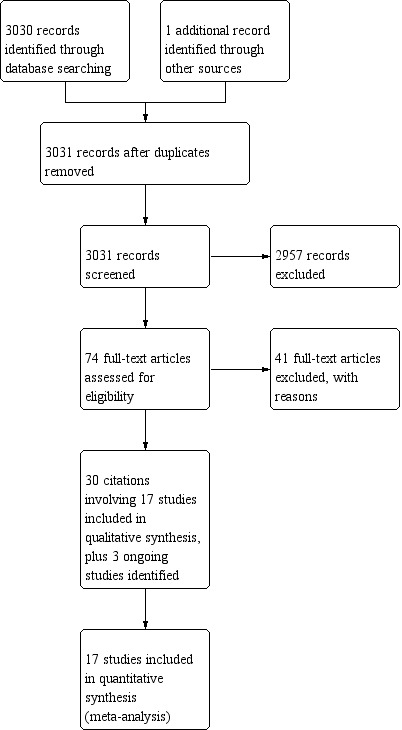
Study flow diagram
Included studies
We included 17 trials with 3765 participants in the final meta‐analysis (see Characteristics of included studies; Table 3). All included studies were randomised, parallel‐group trials involving people with World Health Organization (WHO) Group 1 pulmonary arterial hypertension (PAH) (confirmed on right heart catheterisation). Five studies were open‐label (Badesch 2000; Barst 1996; Han 2017; Olschewski 2010; Rubin 1990), where participants were randomised to prostacyclin or conventional treatment.
1. Summary of study characteristics.
| Study | N | Intervention | Comparator | Blinded | Duration |
| AIR | 203 | Inhaled iloprost | Placebo | Blinded | 12 weeks |
| ALPHABET | 130 | Oral beraprost | Placebo | Blinded | 12 weeks |
| Badesch 2000 | 111 | Intravenous epoprostenol | Usual treatment | Open‐label | 12 weeks |
| Barst 1996 | 81 | Intravenous epoprostenol | Conventional treatment | Open‐label | 12 weeks |
| Barst 2003 | 116 | Oral beraprost | Placebo | Blinded | 12 months |
| FREEDOM‐C | 349 | Oral treprostinil | Placebo | Blinded | 16 weeks |
| FREEDOM‐C2 | 310 | Oral treprostinil | Placebo | Blinded | 16 weeks |
| FREEDOM‐M | 349 | Oral treprostinil | Placebo | Blinded | 12 weeks |
| GRIPHON | 1156 | Selexipag | Placebo | Blinded | Median 63 weeks |
| Han 2017 | 27 | Inhaled iloprost | Other treatment* | Open‐label | 12 weeks |
| McLaughlin 2006 | 67 | Inhaled iloprost | Placebo | Blinded | 12 weeks |
| Olschewski 2010 | 63 | Inhaled iloprost | Placebo | Open‐label | 2 years |
| Rubin 1990 | 19 | Intravenous epoprostenol | Conventional treatment | Open‐label | 8 weeks |
| Simonneau 2002 | 470 | Subcutaneous treprostinil | Placebo | Blinded | 12 weeks |
| Simonneau 2012 | 43 | Selexipag | Placebo | Blinded | 17 weeks |
| TRIUMPH | 235 | Inhaled treprostinil | Placebo | Blinded | 12 weeks |
| TRUST | 44 | Intravenous treprostinil | Placebo | Blinded | 12 weeks |
N = number of participants
*Inhaled iloprost + bosentan versus inhaled iloprost alone versus bosentan alone
Fifteen trials compared a prostacyclin analogue with placebo/conventional treatment, and two trials compared selexipag (an oral selective IP prostacyclin receptor agonist) to placebo (GRIPHON; Simonneau 2012).
In those trials which studied prostacyclin, three used intravenous epoprostenol (Badesch 2000; Barst 1996; Rubin 1990), and one used intravenous treprostinil (TRUST). All of these studies recruited mostly or exclusively NYHA functional class III and IV. Three studies were open‐label (Badesch 2000; Barst 1996; Rubin 1990), and one was placebo‐controlled (TRUST). Badesch 2000 recruited people with scleroderma‐associated PAH, and all other trials recruited people with Group 1 PAH.
One trial used subcutaneous treprostinil compared to placebo (Simonneau 2002). Most (80%) participants were functional class III, and 10% were functional class II and 10% functional class IV.
Five trials used oral prostacyclin compared to placebo, including treprostinil (FREEDOM‐C; FREEDOM‐C2; FREEDOM‐M), and beraprost (ALPHABET; Barst 2003). In the FREEDOM studies, the participants were mostly functional class III, but in Barst 2003 50% were functional class II and 50% functional class III.
Five trials used inhaled preparations, including iloprost (AIR; Han 2017; McLaughlin 2006; Olschewski 2010), and treprostinil (TRIUMPH). Participants were all functional class III/IV.
Prostacyclin in any form is usually up‐titrated in a dose‐dependent manner, initially limited by side effects, but as the patient develops tolerance the dose is able to be increased. In most studies, both the intervention and control group were given opportunity to up‐titrate, and final doses in each group were provided.
Some trials enrolled participants already on PAH‐specific disease modifying therapy (PDE‐5 inhibitor or ERA) (AIR; FREEDOM‐C; FREEDOM‐C2; McLaughlin 2006; Simonneau 2002; Simonneau 2012; TRIUMPH), but some trials specifically excluded these participants and studied prostacyclin as initial therapy (FREEDOM‐M; GRIPHON).
Trial duration was a mean of 19 weeks (median 12 weeks), and most included an initial titration phase, prior to commencement.
Excluded studies
We excluded 41 studies for the following reasons: wrong study design (n = 24); wrong participant population (n = 2); duration of study did not meet prespecified criteria (n = 6); study was withdrawn before participants were enrolled (n = 4); wrong intervention (compared different doses or delivery devices) (n = 5); see Characteristics of excluded studies.
Risk of bias in included studies
We assessed risk of bias in the included studies using the Cochrane 'Risk of bias' assessment tool (Higgins 2011), including the domains of allocation, blinding, incomplete outcome data, and selective reporting. Please see Figure 2 and Figure 3 for a summary of the 'Risk of bias' findings.
2.
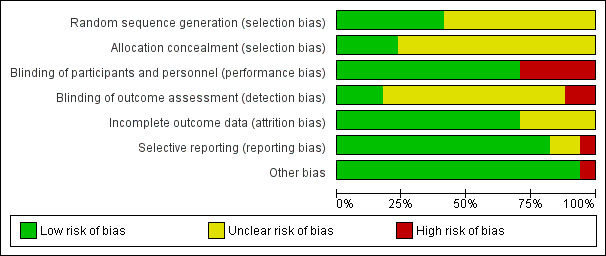
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
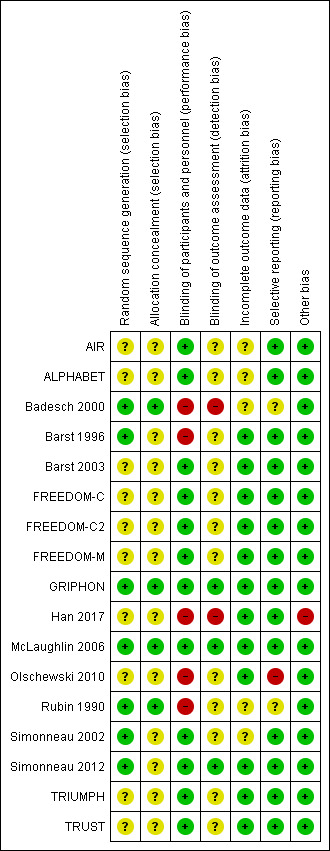
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Although all studies were reported as randomised, few studies reported methods of randomisation or allocation concealment. Badesch 2000, GRIPHON, McLaughlin 2006 and Rubin 1990 clearly reported both domains, and Barst 1996, Simonneau 2002 and Simonneau 2012 clearly reported methods of randomisation and we judged them to be at low risk of bias. All other studies were probably randomised appropriately, but methods were not clearly stated.
Blinding
Twelve studies were placebo‐controlled (judged to be at low risk of bias) and five studies were open‐label (Badesch 2000; Barst 1996; Han 2017; Olschewski 2010; Rubin 1990), with participants randomised to an intervention group or conventional treatment. We judged the latter to be at high risk of bias.
In those with a placebo arm, saline infusions or inhalational preparations were utilised. In TRUST, a central venous catheter was placed in participants from both arms of the study. Given this was an up‐titration study, most studies (except FREEDOM‐M and TRUST), reported the final cumulative prostacyclin and equivalent placebo doses, as a method to confirm blinding. We noted in FREEDOM‐C, FREEDOM‐C2 and Simonneau 2002, the placebo dose was twice as high as the prostacyclin cumulative dose.
Blinding of outcome assessment was only explicitly reported in three studies (GRIPHON; McLaughlin 2006; Simonneau 2012), which we judged to be at low risk of bias. Simonneau 2002 explicitly reported blinding for six‐minute walk distance (6MWD) only. Outcome assessment for other placebo‐controlled studies were probably blinded, but methods were not clearly stated, and so we assigned these studies an unclear risk in this domain.
Incomplete outcome data
AIR, ALPHABET, Badesch 2000, Rubin 1990, and Simonneau 2002 did not report dropouts or withdrawals, so we judged these to be at unclear risk of bias. The remaining studies were at low risk of bias.
Selective reporting
Rubin 1990 reported data as post‐treatment scores but reported confidence intervals (CIs) for the mean difference (MD). Badesch 2000 did not report CIs or error bars for some reported outcomes. Olschewski 2010 randomised participants to inhaled prostacyclin or conventional treatment for three months, at which point all participants were on prostacyclin, and then reported results at the end of two years. We assessed selective reporting bias as low risk for all other studies.
Other potential sources of bias
We assessed Han 2017 as a high risk of bias as analysis reported as standard deviation (SD) were re‐analysed using individual patient data as standard error. It is unclear if there are other methodological issues with this paper. No other issues were identified for the remaining studies.
Effects of interventions
Summary of findings for the main comparison. Prostacyclin compared to control for pulmonary arterial hypertension.
| Prostacyclin compared to control for pulmonary arterial hypertension | ||||||
| Patient or population: pulmonary arterial hypertension Setting: outpatients Intervention: prostacyclin Comparison: control (placebo or usual care) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Risk with control | Risk with prostacyclin | |||||
| Improvement in WHO functional class Mean follow‐up 16 weeks |
Study population | OR 2.39 (1.72 to 3.32) | 1066 (8 RCTs) | ⊕⊕⊕⊝ Moderate1 | ||
| 116 per 1000 | 239 per 1000 (185 to 304) | |||||
| 6MWD Mean follow‐up 15 weeks |
The mean 6MWD was 257 m* | MD 19.50 m higher (14.82 higher to 24.19 higher) | ‐ | 2283 (13 RCTs) | ⊕⊕⊝⊝ Low1,2 |
6MWD in PAH MCID is 41 m |
| Mortality Mean follow‐up 15 weeks |
Study population | OR 0.60 (0.38 to 0.94) | 2554 (15 RCTs) | ⊕⊕⊕⊝ Moderate1 | ||
| 39 per 1000 | 24 per 1000 (15 to 37) | |||||
| mPAP (the higher the mPAP, the worse the pulmonary hypertension) Mean follow‐up 11 weeks |
The mPAP ranged from 56 to 66 mmHg# | MD 3.60 mmHg lower (4.73 lower to 2.48 lower) | ‐ | 1132 (8 RCTs) | ⊕⊕⊝⊝ Low1,2 |
|
| PVR (the higher the PVR, the worse the pulmonary hypertension) Mean follow‐up 11 weeks |
The mean PVR ranged from 26 to 29 units/m2# | MD 2.81 WU lower (3.80 lower to 1.82 lower) | ‐ | 658 (7 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Cardiac index (the lower the cardiac index, the worse the pulmonary hypertension) Mean follow‐up 11 weeks |
The mean cardiac Index ranged from 2 to 2.4 L/min/m2# | MD 0.31 L/min/m 2 higher (0.23 higher to 0.38 higher) | ‐ | 868 (6 RCTs) | ⊕⊕⊝⊝ Low1,2 |
|
| RAP (the lower the RAP, the worse the pulmonary hypertension) Mean follow‐up 11 weeks |
The mean RAP ranged from 8 to 13 mmHg# | MD 1.90 mmHg lower (2.58 lower to 1.22 lower) | ‐ | 1060 (6 RCTs) | ⊕⊕⊕⊝ Moderate1 | The higher the RAP, the worse the pulmonary hypertension |
| Dyspnoea (lower scores indicates more severe breathlessness) Mean follow‐up 17 weeks |
‐ | SMD 0.21 lower (0.32 lower to 0.11 lower) | ‐ | 1521 (8 RCTs) | ⊕⊕⊝⊝ Low1,2 |
Using an illustrative SD, this converts to a difference of 0.64 units on the Borg scale. MCID in PAH is 0.9 units |
| Quality of life Mean follow‐up 12 weeks |
‐ | SMD 0.28 better (0.04 better to 0.42 better) | ‐ | 271 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Headache+ Mean follow‐up 12 weeks |
277 per 1000 | 529 per 1000 (95% CI 501 to 593) |
3.16 (2.62 to 3.80) | 2351 (12 RCTs) |
⊕⊕⊕⊝ Moderate2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: six‐minute walk distance; CI: confidence interval; MCID: minimum clinically important difference; MD: mean difference; OR: odds ratio; PAH: pulmonary arterial hypertension; mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; RAP: right atrial pressure; RCT: randomised controlled trials; SD: standard deviation; SMD: standardised mean difference; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded due to the risk of bias with open‐label studies. 2Downgraded due to imprecision owing to significantly high heterogeneity, although the direction of effect is consistent. *based on only one study which published placebo data; all other studies reported a mean difference between groups. #based on baseline data; all other studies reported a mean difference between groups. +This was chosen as the most commonly experienced adverse event.
Summary of findings 2. Selexipag compared to placebo for pulmonary arterial hypertension.
| Selexipag compared to placebo for pulmonary arterial hypertension | ||||||
| Patient or population: pulmonary arterial hypertension Setting: outpatients Intervention: selexipag Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Risk with placebo | Risk with selexipag | |||||
| Improvement in WHO functional class Mean follow‐up 17 weeks |
Study population | OR 1.61 (0.17 to 15.63) | 43 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | ||
| 100 per 1000 | 152 per 1000 (19 to 635) | |||||
| 6MWD Mean follow‐up 40 weeks |
The mean 6MWD ranged from 348 to 396 m | MD 12.62 m higher (1.90 higher to 23.34 higher) | ‐ | 1199 (2 RCTs) | ⊕⊕⊕⊕ High | 6MWD in PAH MCID is 41 m |
| Mortality Mean follow‐up 40 weeks |
Study population | Risk difference 0.02 (‐0.00 to 0.04) | 1199 (2 RCTs) | ⊕⊕⊕⊝ Moderate1 | ||
| 30 per 1000 | 48 per 1000 (27 to 84) | |||||
| mPAP the higher the mPAP, the worse the pulmonary hypertension) Mean follow‐up 17 weeks |
The mPAP was 60 mmHg | MD 7.4 mmHg lower (15.9 lower to 1.1 higher) | ‐ | 43 (1 RCT) | ⊕⊕⊕⊝ Moderate2 | |
| PVR (the higher the PVR, the worse the pulmonary hypertension) Mean follow‐up 17 weeks |
The mean PVR was 1687 dyn/sec/m2 | MD 33 dyn/sec/m2 lower (47 lower to 19 lower) | ‐ | 43 (1 RCT) | ⊕⊕⊕⊝ Moderate2 | |
| Cardiac index (the lower the cardiac index, the worse the pulmonary hypertension) Mean follow‐up 17 weeks |
The mean cardiac index was 2.3 L/min/m2 | MD 0.5 L/min/m2 higher (0.13 higher to 0.87 higher) | ‐ | 43 (1 RCT) | ⊕⊕⊕⊝ Moderate2 | |
| RAP (the lower the RAP, the worse the pulmonary hypertension) Mean follow‐up 17 weeks |
The mean RAP was 8.3 mmHg | MD 3.2 mmHg higher (0.8 higher to 5.6 higher) | ‐ | 43 (1 RCT) | ⊕⊕⊕⊝ Moderate2 | |
| Dyspnoea (lower scores indicates more severe breathlessness) Mean follow‐up 17 weeks |
‐ | MD 0.1 lower (1.4 lower to 1.2 higher) | ‐ | 43 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | MCID in PAH is 0.9 units |
| Headache+ Mean follow‐up 40 weeks |
Study population | 3.91 (3.07 to 4.98) | 1199 (2 RCTs) | ⊕⊕⊕⊕ High | ||
| 325 per 1000 | 653 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWD: six‐minute walk distance; CI: confidence interval; MCID: minimum clinically important difference; MD: mean difference; OR: odds ratio; PAH: pulmonary arterial hypertension; mPAP: mean pulmonary arterial pressure; PVR: pulmonary vascular resistance; RAP: right atrial pressure; RCT: randomised controlled trials; RR: risk ratio; SD: standard deviation; WHO: World Health Organization | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded due to imprecision with confidence intervals including no difference. 2Downgraded due to imprecision owing to small participant numbers in one trial. +This was chosen as the most commonly experienced adverse event.
Prostacyclin versus control
Change in World Health Organization (WHO) functional class
Those who were using prostacyclin were more likely to improve their WHO functional class (239 per 1000) compared to those who did not (116 per 1000); (odds ratio (OR) 2.39, 95% confidence interval (CI) 1.72 to 3.32; P < 0.00001; 8 trials, 1066 participants; Analysis 1.1). Using the Chi2 test for subgroup differences, there was a significant difference between route of administration (P = 0.0003), with the greatest effect seen in the intravenous prostacyclin arm.
1.1. Analysis.
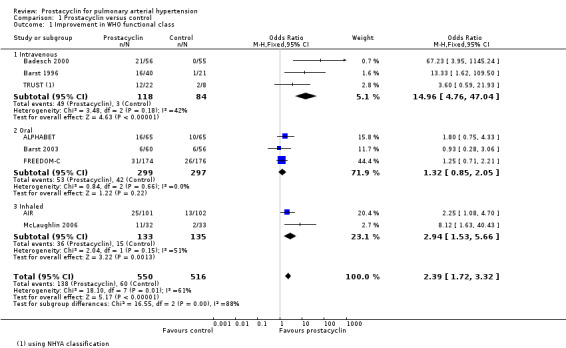
Comparison 1 Prostacyclin versus control, Outcome 1 Improvement in WHO functional class.
When excluding open‐label trials, there was still a significant difference, though the effect size was smaller: OR 2.39 (95% CI 1.72 to 3.32) for all trials compared to OR 1.77 (95% CI 1.24 to 2.52) when open‐label trials were excluded, and there was no significant difference between fixed‐ and random‐effects (see Table 4; Table 5). A post hoc sensitivity analysis was carried out whereby the TRUST trial was excluded due to its premature termination following safety concerns. The removal of this study had a minimal impact on the pooled effect estimates.
2. Sensitivity analysis: fixed‐ versus random‐effects.
| Outcome | Number of studies | Effect measure | Fixed‐effect size (95% CI) | Random‐effect size (95% CI) |
| Functional class ‐ improvement | 8 | OR | 2.39 (1.72 to 3.32) | 2.66 (1.37 to 5.19) |
| Functional class ‐ worsening | 5 | OR | 0.88 (0.57 to 1.37) | 0.88 (0.56 to 1.40) |
| Six‐minute walk distance | 13 | MD | 19.50 (14.82 to 24.19)* | 29.55 (18.63 to 40.48)* |
| Mortality | 15 | OR | 0.60 (0.38 to 0.94) | 0.68 (0.42 to 1.10) |
| mPAP | 8 | MD | ‐3.60 (‐4.73 to ‐2.48)* | ‐4.10 (‐6.22 to ‐1.99)* |
| PVR | 7 | MD | ‐2.81 (‐3.80 to ‐1.82)* | ‐2.40 (‐4.44 to ‐0.35)* |
| Cardiac index | 6 | MD | 0.31 (0.23 to 0.38)* | 0.34 (0.17 to 0.52)* |
| Cardiac output | 2 | MD | 0.57 (0.32 to 0.81) | 0.41 (‐0.34 to 1.15) |
| RAP | 6 | MD | ‐1.90 (‐2.58 to ‐1.22) | ‐1.90 (‐2.58 to ‐1.22) |
| Dyspnoea | 8 | SMD | ‐0.21 (‐0.32 to ‐0.11)* | ‐0.29 (‐0.50 to ‐0.08)* |
| Quality of life | 3 | SMD | 0.28 (0.04 to 0.52)* | 0.48 (‐0.11 to 1.08)* |
*High heterogeneity
Abbreviations: MD ‐ mean difference; SMD ‐ standardised mean difference; CI ‐ confidence interval; mPAP ‐ mean pulmonary arterial pressure; PVR ‐ pulmonary vascular resistance; RAP ‐ right atrial pressure
3. Sensitivity analysis: blinded versus open‐label studies.
| Outcome | All studies effect size (95% CI) | Blinded studies only effect size (95% CI) |
| Functional class ‐ improvement | 2.39 (1.72 to 3.32)+ | 1.77 (1.24 to 2.52)+ |
| Functional class ‐ worsening | 0.88 (0.57 to 1.37) | 0.85 (0.54 to 1.35) |
| Six‐minute walk test distance | 19.50 (14.82 to 24.19)+ | 17.55 (12.82 to 22.29)+ |
| Mortality | 0.60 (0.38 to 0.94)+ | 0.76 (0.45 to 1.29)* |
| PAP | ‐3.60 (‐4.73 to ‐2.48)+ | ‐2.58 (‐3.86 to ‐1.30)+ |
| PVR | ‐2.81 (‐3.80 to ‐1.82)+ | ‐1.32 (‐2.95 to 0.32)* |
| Cardiac index | 0.31 (0.23 to 0.38)+ | 0.18 (0.08 to 0.27)+ |
| Cardiac output | 0.57 (0.32 to 0.81)+ | 0.57 (0.32 to 0.81)+ |
| RAP | ‐1.90 (‐2.58 to ‐1.22)+ | ‐1.80 (‐2.55 to ‐1.06)+ |
| Dyspnoea | ‐0.21 (‐0.32 to ‐0.11)+ | ‐0.18 (‐0.29 to ‐0.08)+ |
| Quality of life | 0.28 (0.04 to 0.52)+ | 0.07 (‐0.22 to 0.36)* |
+Statistically significant; *no longer statistically significant
Abbreviations: CI ‐ confidence interval; PAP ‐ pulmonary arterial pressure; PVR ‐ pulmonary vascular resistance; RAP ‐ right atrial pressure
There was no difference in the proportion of those worsening across the two arms (OR 0.88, 95% CI 0.57 to 1.37; P = 0.7; 5 trials, 805 participants; Analysis 1.2), and no difference across subgroups.
1.2. Analysis.
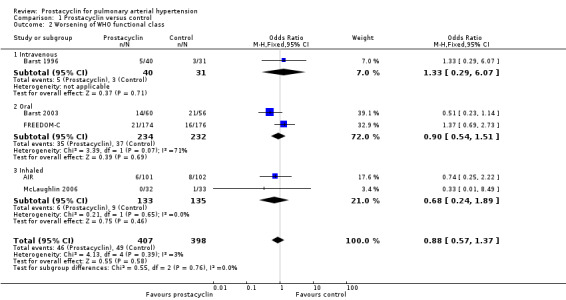
Comparison 1 Prostacyclin versus control, Outcome 2 Worsening of WHO functional class.
Six‐minute walk distance (6MWD)
There was a small, statistically significant improvement in 6MWD (mean difference (MD) 19.50 metres, 95% CI 14.82 to 24.19; P < 0.00001; 13 trials, 2283 participants; Analysis 1.3; Figure 4), though it did not meet the minimum clinically important threshold of 41 metres (Khair 2016). Although all modes of administration produced a significant improvement, there was a statistically significant difference across subgroups (P < 0.0001), with the greatest effect seen in the intravenous trials: MD 91.76 metres compared to 16.00 metres in the subcutaneous trial, 14.76 metres in the oral trials, and 26.97 metres in the inhaled trials.
1.3. Analysis.
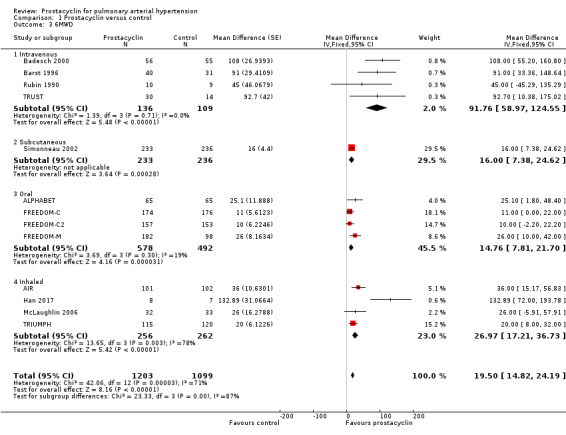
Comparison 1 Prostacyclin versus control, Outcome 3 6MWD.
4.
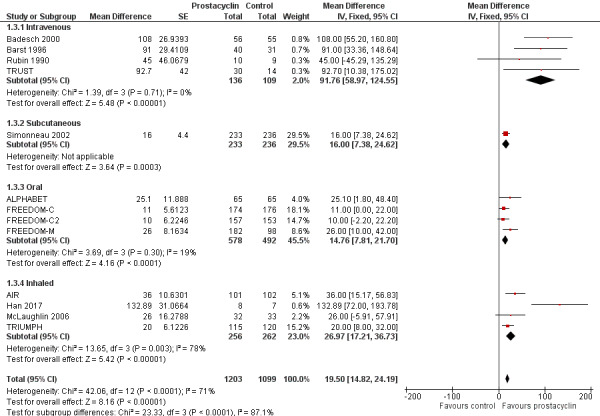
Forest plot of comparison: 1 Prostacyclin versus control, outcome: 1.3 6MWD.
When excluding open‐label trials, there was still a significant difference, though the effect size was slightly smaller (MD 19.50 metres, 95% CI 14.82 to 24.19) for all trials compared to MD 17.55 metres (95% CI 12.82 to 22.29) when open‐label trials were excluded, and there was no significant difference between fixed‐ and random‐effects (see Table 4; Table 5). Exclusion of the TRUST trial had a minimal impact on the pooled effect estimates.
Mortality
There was a significant difference in mortality overall (OR 0.60, 95% CI 0.38 to 0.94; P = 0.02; 15 trials, 2554 participants; Analysis 1.4; Figure 5), whereby the risk of death over 12 weeks was two per 100 participants in the prostacyclin group compared to four per 100 in the control group (95% CI 1.50 to 4.12). This effect was largely due to the intravenous trials, and when the intravenous trials were excluded, this effect was lost (OR 0.82, 95% CI 0.48 to 1.40; P = 0.46). However, most studies were only approximately 12 weeks duration and most were not powered to assess mortality.
1.4. Analysis.
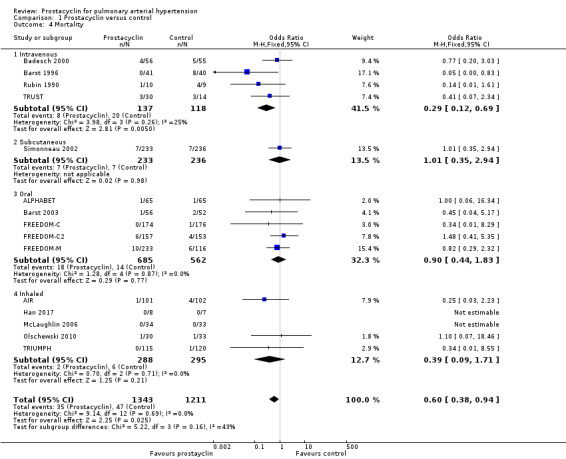
Comparison 1 Prostacyclin versus control, Outcome 4 Mortality.
5.
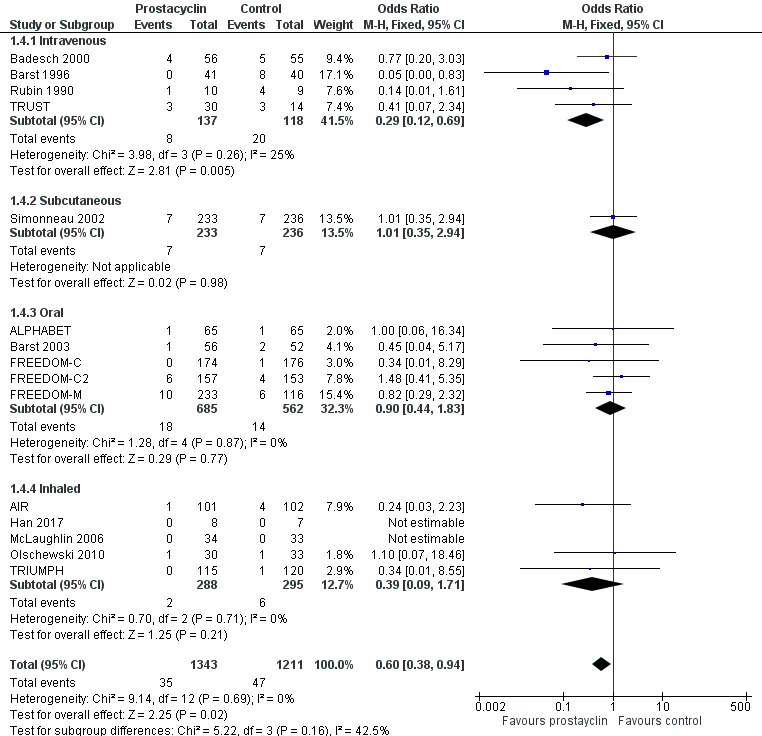
Forest plot of comparison: 1 Prostacyclin versus control, outcome: 1.4 Mortality.
When intravenous trials are analysed separately, the risk of death was 17 per 100 in the control group, compared to six (95% CI 2.38 to 12.31) per 100 for the prostacyclin group. There is, however, higher baseline mortality in these trials compared to the other included studies.
There was no significant difference between fixed‐ and random‐effects (see Table 4). When open‐label studies were excluded (which were almost all intravenous studies), the effect on mortality was lost (OR 0.76, 95% CI 0.45 to 1.29; P = 0.32) (see Table 5). However, it is less likely that the outcome of mortality would be affected by the degree of blinding of the studies. This was a post hoc sensitivity analysis whereby the TRUST trial was excluded due to its premature termination following safety concerns. The removal of this study had a minimal impact on the pooled effect estimates of mortality.
Cardiopulmonary haemodynamics
Only eight trials assessed change in haemodynamic parameters over the trial duration (AIR; ALPHABET; Badesch 2000; Barst 1996; Barst 2003; Han 2017; McLaughlin 2006; Simonneau 2002).
There was a significant improvement in mean pulmonary arterial pressure (mPAP) (MD ‐3.60 mmHg, 95% CI ‐4.73 to ‐2.48; P < 0.00001; Analysis 1.5); pulmonary vascular resistance (PVR) (MD ‐2.81 WU, 95% CI ‐3.80 to ‐1.82; P < 0.00001; Analysis 1.6), (Simonneau 2002 measured PVR as a geometric mean, so was not included in the meta‐analysis, but demonstrated a similar effect, whereby the change from baseline for treprostinil was ‐3.5 WU, standard error (SE) 0.6, and change from baseline for control was 1.2 WU, SE 0.06, P = 0.0001); cardiac index (MD 0.31 L/min/m2, 95% CI 0.23 to 0.38; P < 0.00001; Analysis 1.7); cardiac output (MD 0.57 L/min, 95% CI 0.32 to 0.81; P < 0.00001; Analysis 1.8); and right atrial pressure (RAP) (MD ‐1.90 mmHg, 95% CI ‐2.58 to ‐1.22; P < 0.00001; Analysis 1.9). There was a significant difference across route of administration subgroups for mPAP (P = 0.006), cardiac index (P < 0.0001), and cardiac output (P < 0.0001), however some of these differences may be accounted for by other differences between studies rather than route of administration.
1.5. Analysis.
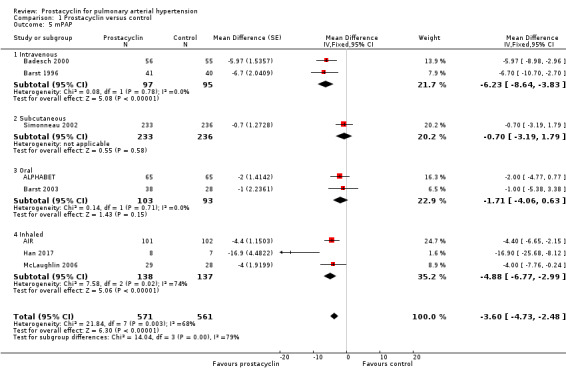
Comparison 1 Prostacyclin versus control, Outcome 5 mPAP.
1.6. Analysis.
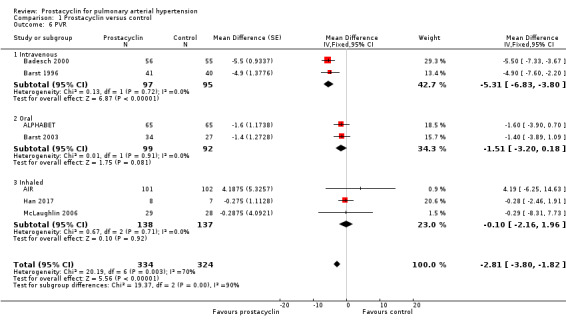
Comparison 1 Prostacyclin versus control, Outcome 6 PVR.
1.7. Analysis.
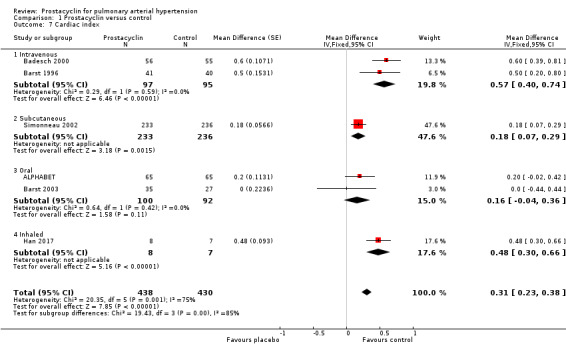
Comparison 1 Prostacyclin versus control, Outcome 7 Cardiac index.
1.8. Analysis.

Comparison 1 Prostacyclin versus control, Outcome 8 Cardiac output.
1.9. Analysis.
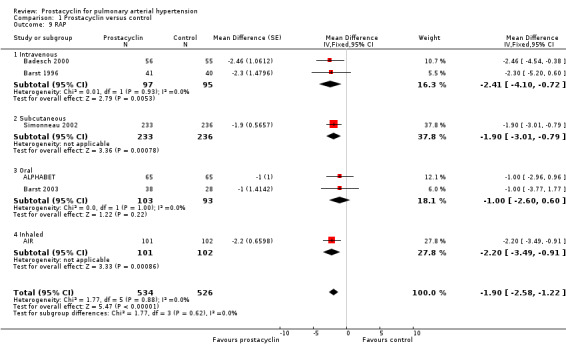
Comparison 1 Prostacyclin versus control, Outcome 9 RAP.
Comparing fixed‐effect to random‐effects, there was a difference in PVR and cardiac index (see Table 4). There was also substantial heterogeneity in these outcomes, suggesting that small‐study effects may be influencing the overall effect size. When open‐label studies were excluded, the effects were still significant (see Table 5).
Although no minimum clinically relevant data currently exists for pulmonary haemodynamics, the clinical impact may be contextualised by applying these MDs to the risk stratification data, which correlates with mortality. A RAP < 8 mm Hg is classified as low risk, and > 14 mmHg as high risk. The overall reduction in RAP in all prostacyclin preparations was ‐1.90 mmHg (95% CI ‐2.58 to ‐1.22), and the intravenous preparations demonstrated a reduction in RAP of ‐2.41 (95% CI ‐4.10 to ‐0.72) compared to control. A cardiac index > 2.5 L/min/m2 is classified as low risk and < 2.0 L/min/m2 as high risk. Overall all prostacyclin preparations improved cardiac index by 0.31 L/min/m2 (95% CI 0.23 to 0.38) and intravenous preparations by 0.57 L/min/m2 (95% CI 0.40 to 0.74).
Exercise capacity tests
Only Barst 2003 reported additional exercise capacity tests. Using cardiopulmonary exercise testing and measuring peak VO2 with cycle ergometry, there was a trend towards improvement in favour of beraprost (Hodges Lehmann estimate MD between groups at 12 months of 66 mL/min), though this did not reach statistical significance.
Symptom scales including dyspnoea and fatigue
Five studies (ALPHABET; FREEDOM‐C2; FREEDOM‐M; McLaughlin 2006; TRUST) assessed dyspnoea using the Borg dyspnoea scale, AIR used the Mahler Transition Dyspnoea Index, and Barst 1996 and Simonneau 2002 used the Dyspnoea Fatigue Rating. For all scales lower scores indicate more severe breathlessness (Badesch 2009). These results were pooled in a standardised mean difference (SMD) to account for the different scales used, and the direction of effect was imputed to be consistent across scales. There was a significant improvement in dyspnoea (SMD ‐0.21, 95% CI ‐0.32 to ‐0.11; P < 0.00001; Analysis 1.10). Significant heterogeneity was noted (I2 = 72%; P = 0.0007). Using the calculated SD from the largest study (Simonneau 2002), an illustrative Borg score of ‐0.64 was determined. This is less than the minimum clinically important difference of 0.9 (Khair 2016).
1.10. Analysis.
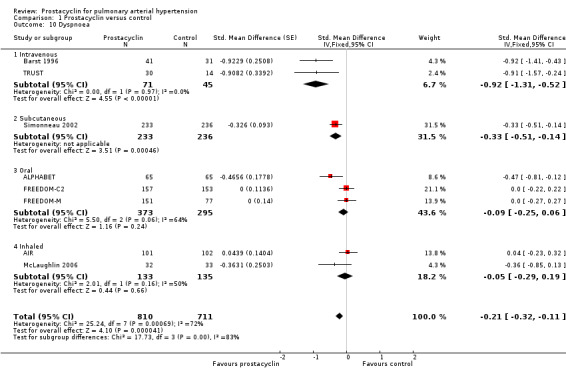
Comparison 1 Prostacyclin versus control, Outcome 10 Dyspnoea.
When open‐labelled studies were excluded, there was still a significant difference (SMD ‐0.18, 95% CI ‐0.29 to ‐0.08; P = 0.0007; 7 trials, 1449 participants).
Badesch 2000 provided dyspnoea data using the Borg dyspnoea scale and Dyspnoea Fatigue Rating, but did not provide CIs, so we were unable to combine these data into the meta‐analysis. The post‐treatment score for Borg at 12 weeks was 1 in the conventional group and ‐2 in the epoprostenol group, and the post‐treatment score for Dyspnoea Fatigue Rating at 12 weeks was ‐1 in the conventional group and 1 in the epoprostenol group (lower scores indicate more breathlessness in both scales).
Quality of life
Three studies provided quality of life data suitable for meta‐analyses: Barst 1996 using the Chronic Heart Failure Questionnaire (Mastery) (Guyatt 1989) (lower scores indicate worse quality of life), FREEDOM‐C2 used the Cambridge Pulmonary Hypertension Outcome Review (McKenna 2006) (lower scores indicate better quality of life), and Han 2017 used the Minnesota Living with Heart Failure Questionnaire (Cenedese 2006) (lower scores indicate better quality of life). These results were pooled in a SMD to account for the different scales used, and the direction of effect was imputed to be consistent across scales. When data were pooled, there was a significant difference in quality of life scores (SMD 0.28, 95% CI 0.04 to 0.52; P = 0.02; Analysis 1.11). There was significant heterogeneity across trials (I2 = 72%; P = 0.03). When open‐labelled studies were excluded, results were no longer significant (MD 0.07, 95% CI ‐0.22 to 0.36; P = 0.65), however this only included one trial with 187 participants.
1.11. Analysis.
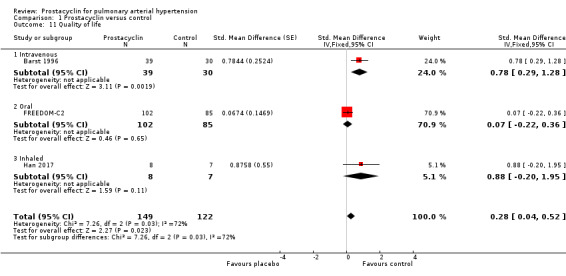
Comparison 1 Prostacyclin versus control, Outcome 11 Quality of life.
A further three studies provided descriptive data. Barst 2003 (using the Minnesota Living with Heart Failure Questionnaire) reported beraprost did not result in significant improvement relative to control in global, physical, or emotional indices of quality of life. TRIUMPH (using the Minnesota Living with Heart Failure Questionnaire) reported a between‐treatment median difference of 4 in the global score (P = 0.027) and 2 in the physical score (P = 0.037), for participants receiving inhaled treprostinil. Simonneau 2002 (also using the Minnesota Living with Heart Failure Questionnaire reported that participants treated with treprostinil experienced a significant improvement in their physical dimension score at Week 12 (P = 0.0064) with a trend toward improvement in the global dimension score (P = 0.17) as compared with the control group.
Clinical worsening
There was a significant difference in clinical worsening favouring prostacyclins (OR 0.67, 95% CI 0.48 to 0.92; P = 0.001; 12 trials, 2238 participants; Analysis 1.12). In the control group, seven out of 100 participants experienced clinical worsening, compared to five participants (95% CI 4.50 to 8.27) in the prostacyclin group. The definition of clinical worsening varied across studies (see Characteristics of included studies), but this did not affect heterogeneity of results.
1.12. Analysis.
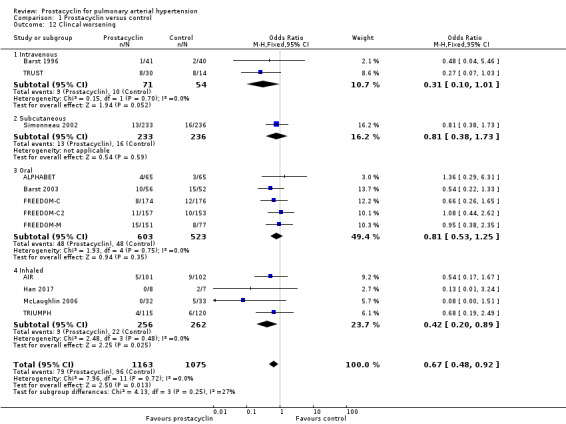
Comparison 1 Prostacyclin versus control, Outcome 12 Clincal worsening.
Adverse events
There was an increased risk of adverse events in the prostacyclin group including vasodilation (OR 5.03, 95% CI 3.84 to 6.58; P < 0.00001; 11 trials, 2277 participants; Analysis 1.15), headache (OR 3.16, 95% CI 2.62 to 3.80; P < 0.00001; 12 trials, 2351 participants; Analysis 1.16), jaw pain (OR 5.25, 95% CI 3.96 to 6.98; P < 0.00001; 10 trials, 2149 participants; Analysis 1.17), diarrhoea (OR 2.81, 95% CI 2.29 to 3.46; P < 0.00001; 10 trials, 2317 participants; Analysis 1.18), nausea or vomiting (OR 2.39, 95% CI 1.98 to 2.88; P < 0.00001; 11 trials, 2399 participants; Analysis 1.20), pain in the extremities (OR 3.36, 95% CI 2.32 to 4.85; P < 0.00001; 6 trials, 1236 participants; Analysis 1.22), myalgias (OR 2.75, 95% CI 1.65 to 4.58; P = 0.00001; 3 trials, 1009 participants; Analysis 1.23), upper respiratory tract events (OR 1.61, 95% CI 1.22 to 2.13; P = 0.0009; 7 trials, 1038 participants; Analysis 1.24), and infusion site reactions (OR 14.41, 95% CI 9.16 to 22.66; P < 0.00001; 2 trials, 580 participants; Analysis 1.26).
1.15. Analysis.
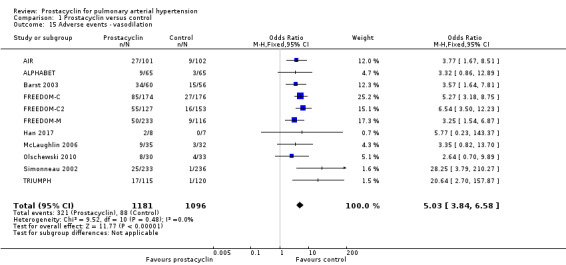
Comparison 1 Prostacyclin versus control, Outcome 15 Adverse events ‐ vasodilation.
1.16. Analysis.
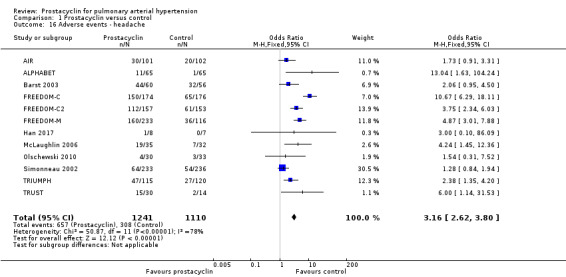
Comparison 1 Prostacyclin versus control, Outcome 16 Adverse events ‐ headache.
1.17. Analysis.
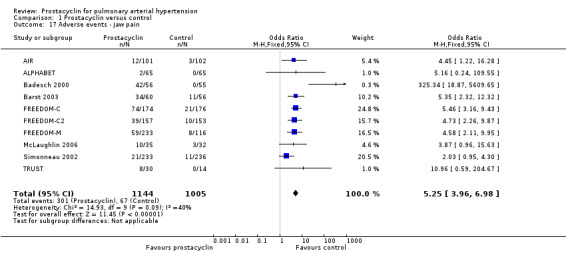
Comparison 1 Prostacyclin versus control, Outcome 17 Adverse events ‐ jaw pain.
1.18. Analysis.
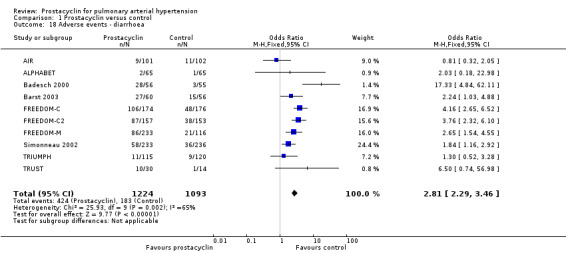
Comparison 1 Prostacyclin versus control, Outcome 18 Adverse events ‐ diarrhoea.
1.20. Analysis.
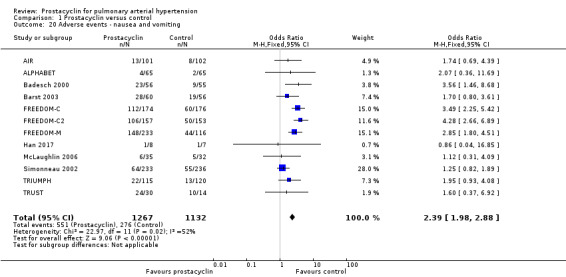
Comparison 1 Prostacyclin versus control, Outcome 20 Adverse events ‐ nausea and vomiting.
1.22. Analysis.
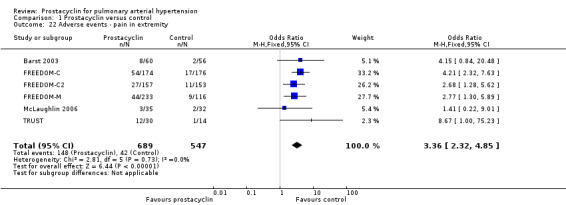
Comparison 1 Prostacyclin versus control, Outcome 22 Adverse events ‐ pain in extremity.
1.23. Analysis.
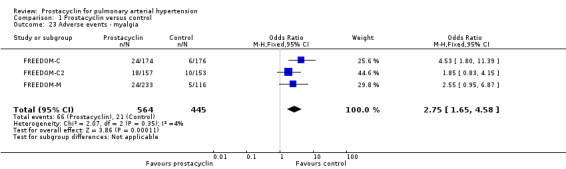
Comparison 1 Prostacyclin versus control, Outcome 23 Adverse events ‐ myalgia.
1.24. Analysis.
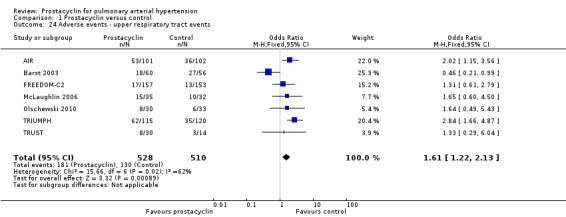
Comparison 1 Prostacyclin versus control, Outcome 24 Adverse events ‐ upper respiratory tract events.
1.26. Analysis.
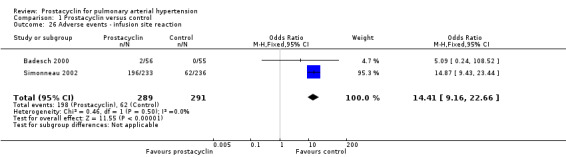
Comparison 1 Prostacyclin versus control, Outcome 26 Adverse events ‐ infusion site reaction.
There was no significant difference in the incidence of syncope (OR 0.77, 95% CI 0.42 to 1.42; P = 0.41; 4 trials, 560 participants; Analysis 1.13), dizziness (OR 1.09, 95% CI 0.84 to 1.42; P = 0.52; 7 trials, 1939 participants; Analysis 1.14), leg pain (OR 2.96, 95% CI 1.02 to 8.62; P = 0.05; 2 trials, 246 participants; Analysis 1.19), abdominal pain (OR 1.35, 95% CI 0.75 to 2.42; P = 0.32; 2 trials, 465 participants; Analysis 1.21), or peripheral oedema (OR 1.46, 95% CI 0.98 to 2.17; P = 0.06; 6 trials, 1228 participants; Analysis 1.25); see Table 6 for all adverse events and their corresponding risks.
1.13. Analysis.
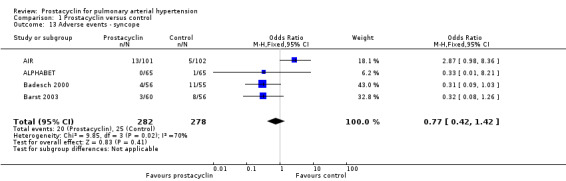
Comparison 1 Prostacyclin versus control, Outcome 13 Adverse events ‐ syncope.
1.14. Analysis.
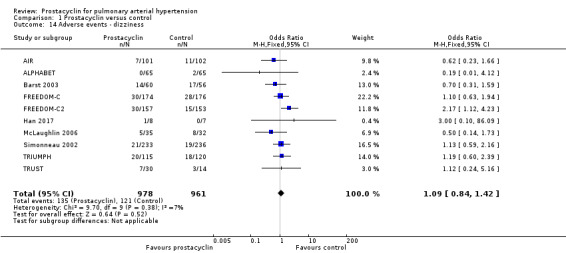
Comparison 1 Prostacyclin versus control, Outcome 14 Adverse events ‐ dizziness.
1.19. Analysis.
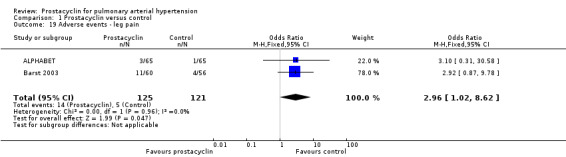
Comparison 1 Prostacyclin versus control, Outcome 19 Adverse events ‐ leg pain.
1.21. Analysis.
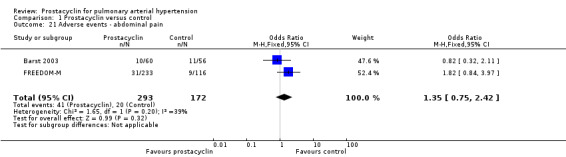
Comparison 1 Prostacyclin versus control, Outcome 21 Adverse events ‐ abdominal pain.
1.25. Analysis.

Comparison 1 Prostacyclin versus control, Outcome 25 Adverse events ‐ peripheral oedema.
4. Prostacyclin versus control: adverse events.
| Outcome | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | |
| Risk with placebo | Risk with selexipag | |||
| Syncope | 90 per 1000 | 71 per 1000 (40 to 123) |
OR 0.77 (0.42 to 1.42) | 560 (4) |
| Dizziness | 126 per 1000 | 136 per 1000 (108 to 170) |
OR 1.09 (0.84 to 1.42) | 1939 (10) |
| Vasodilation | 80 per 1000 | 305 per 1000 (251 to 365) |
OR 5.03 (3.84 to 6.58) | 2277 (11) |
| Headache | 227 per 1000 | 548 per 1000 (502 to 593) |
OR 3.16 (2.62 to 3.80) | 2351 (12) |
| Jaw pain | 67 per 1000 | 273 per 1000 (220 to 333) |
OR 5.25 (3.96 to 6.98) | 2149 (10) |
| Diarrhoea | 167 per 1000 | 361 per 1000 (315 to 410) |
OR 2.81 (2.29 to 3.46) | 2317 (10) |
| Leg pain | 41 per 1000 | 113 per 1000 (42 to 271) |
OR 2.96 (1.02 to 8.62) | 246 (2) |
| Nausea and vomiting | 244 per 1000 | 435 per 1000 (390 to 481) |
OR 2.39 (1.98 to 2.88) | 2399 (12) |
| Abdominal pain | 116 per 1000 | 151 per 1000 (90 to 242) |
OR 1.35 (0.75 to 2.42) | 465 (2) |
| Pain in extremities | 77 per 1000 | 218 per 1000 (162 to 287) |
OR 3.36 (2.32 to 4.85) | 1236 (6) |
| Myalgia | 47 per 1000 | 120 per 1000 (76 to 185) |
OR 2.75 (1.65 to 4.58) | 1009 (3) |
| Upper respiratory tract events | 255 per 1000 | 355 per 1000 (294 to 422) |
OR 1.61 (1.22 to 2.13) | 1038 (7) |
| Peripheral oedema | 75 per 1000 | 106 per 1000 (74 to 150) |
OR 1.46 (0.98 to 2.17) | 1228 (6) |
| Infusion site reactions | 213 per 1000 | 796 per 1000 (713 to 860) |
OR 14.41 (9.16 to 22.66) | 580 (2) |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio
In the intravenous studies, three of four trials adequately reported line‐related side effects. There was a 12% to 25% risk of serious non‐fatal events attributed to the catheter, including sepsis, haemorrhage, pneumothorax and pulmonary embolism (Barst 1996: 5/41; Badesch 2000: 8/56; TRUST: 11/44; Rubin 1990: not reported), and two participants in TRUST died due to catheter‐related events on control in the double‐blind phase. Pump failure resulting in a temporary discontinuation in drug delivery occurred on five occasions (total 10 participants) in Rubin 1990 and on 26 occasions (total 41 participants) in Barst 1996. (Badesch 2000 and TRUST not reported).
Cost analysis
No trials reported cost analysis.
Selexipag versus placebo
Two studies (1199 participants) compared selexipag (a selective IP prostacyclin receptor antagonist) with placebo (GRIPHON; Simonneau 2012).
Change in WHO functional class
There was no significant difference in the number of participants who improved (OR 1.61, 95% CI 0.17 to 15.63; P = 0.68; 1 trial, 43 participants; Analysis 2.1), but the CI is wide. One per 100 participants in the placebo group had an improvement compared to 15 per 100 (95% CI 1.90 to 63.50) participants in the selexipag group.
2.1. Analysis.
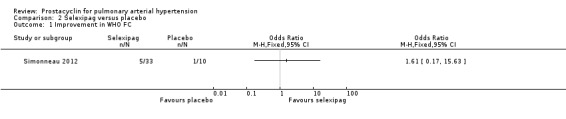
Comparison 2 Selexipag versus placebo, Outcome 1 Improvement in WHO FC.
There was a benefit of selexipag compared to placebo for worsening in WHO functional class (OR 0.79, 95% CI 0.60 to 1.04; P = 0.09; 2 trials, 1199 participants; Analysis 2.2), but the CI includes no difference. Twenty‐one participants per 100 in the prostacyclin group experienced worsening in WHO functional status compared to 25 per 100 (95% CI 17.48 to 26.13) in the placebo group.
2.2. Analysis.
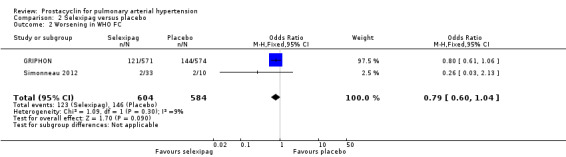
Comparison 2 Selexipag versus placebo, Outcome 2 Worsening in WHO FC.
Six‐minute walk distance (6MWD)
There was a small significant improvement in 6MWD (MD 12.62 metres, 95% CI 1.90 to 23.34; P = 0.02; 2 trials, 1199 participants; Analysis 2.3), though it did not meet the minimum clinically important threshold of 41 metres (Khair 2016).
2.3. Analysis.
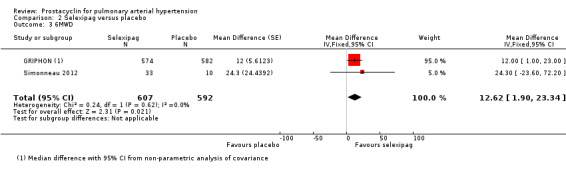
Comparison 2 Selexipag versus placebo, Outcome 3 6MWD.
Mortality
There was no statistically significant difference in mortality (risk difference (RD) 0.02 (95% CI ‐0.00 to 0.04); P = 0.13; 2 trials, 1159 participants; Analysis 2.4). Risk of death was increased as five per 100 participants in the selexipag group died compared to three per 100 participants in the placebo group, though the CI crossed zero, so the true effect is uncertain.
2.4. Analysis.
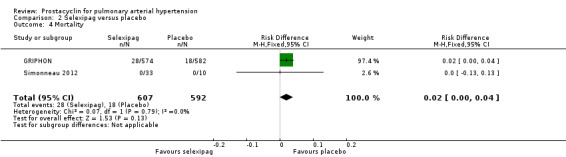
Comparison 2 Selexipag versus placebo, Outcome 4 Mortality.
Cardiopulmonary haemodynamics
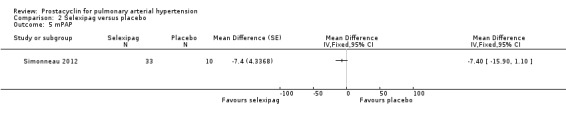
Only one trial assessed change in haemodynamic parameters (Simonneau 2012; 43 participants), and found an improvement in PVR (MD ‐33.00 dyn/s/cm‐5, 95% CI ‐47.00 to ‐19.00; P < 0.00001; Analysis 2.6), cardiac index (MD 0.50 L/min/m2, 95% CI 0.13 to 0.87; P = 0.008; Analysis 2.7), and RAP (MD 3.20 mmHg, 95% CI 0.80 to 5.60; P = 0.009; Analysis 2.8), but no significant difference in mPAP (MD ‐7.40 mmHg, 95% CI ‐15.90 to 1.10; Analysis 2.5).
2.6. Analysis.
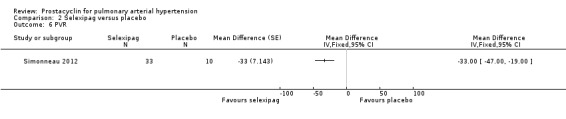
Comparison 2 Selexipag versus placebo, Outcome 6 PVR.
2.7. Analysis.

Comparison 2 Selexipag versus placebo, Outcome 7 Cardiac index.
2.8. Analysis.

Comparison 2 Selexipag versus placebo, Outcome 8 RAP.
2.5. Analysis.
Comparison 2 Selexipag versus placebo, Outcome 5 mPAP.
Exercise capacity tests
Neither study assessed exercise capacity tests.
Symptom scales including dyspnoea and fatigue
There was no significant difference in dyspnoea, as assessed with the Borg dyspnoea scale (MD ‐0.10, 95% CI ‐1.40 to 1.20; P = 0.88; 1 trial, 43 participants; Analysis 2.9) (lower scores indicate better control of dyspnoea; minimum clinically important difference in PAH is 0.9 units).
2.9. Analysis.
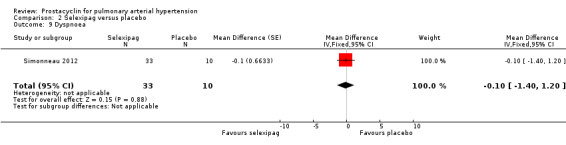
Comparison 2 Selexipag versus placebo, Outcome 9 Dyspnoea.
Quality of life
Neither study assessed quality of life.
Clinical worsening
Both studies (1199 participants) assessed clinical worsening. There was a significant difference in clinical worsening (OR 0.47, 95% CI 0.37 to 0.60; P < 0.00001; Analysis 2.10), favouring selexipag. In the placebo group, 38 out of 100 people experienced clinical worsening compared to 22 (95% CI 18 to 27) in the selexipag group.
2.10. Analysis.
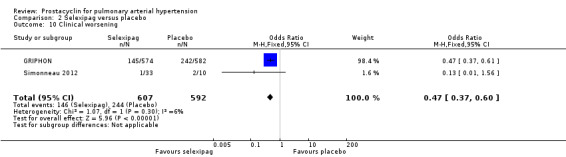
Comparison 2 Selexipag versus placebo, Outcome 10 Clinical worsening.
Adverse events
There was a significant increase in incidence of headache (OR 3.91, 95% CI 3.07 to 4.98; P < 0.00001; Analysis 2.12), vasodilation (OR 2.67, 95% CI 1.72 to 4.17; P < 0.0001; Analysis 2.13), jaw pain (OR 5.33, 95% CI 3.64 to 7.81; P < 0.00001; Analysis 2.14), diarrhoea (OR 3.11, 95% CI 2.39 to 4.05; P < 0.00001; Analysis 2.15), nausea and vomiting (OR 2.92, 95% CI 2.29 to 3.73; P < 0.00001; Analysis 2.16), pain in extremities (OR 2.44, 95% CI 1.69 to 3.52; P < 0.00001; Analysis 2.17), or myalgias (OR 3.05, 95% CI 2.02 to 4.58; P < 0.00001; Analysis 2.18). There was no difference in dizziness (OR 1.04, 95% CI 0.76 to 1.44; P = 0.79; Analysis 2.11), or upper respiratory tract infections (OR 0.99, 95% CI 0.78 to 1.26; P = 0.96; Analysis 2.19), see Table 7 for all adverse events and their corresponding risks.
2.12. Analysis.

Comparison 2 Selexipag versus placebo, Outcome 12 Adverse events ‐ headache.
2.13. Analysis.
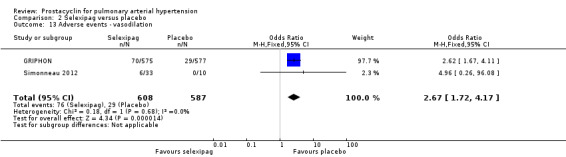
Comparison 2 Selexipag versus placebo, Outcome 13 Adverse events ‐ vasodilation.
2.14. Analysis.
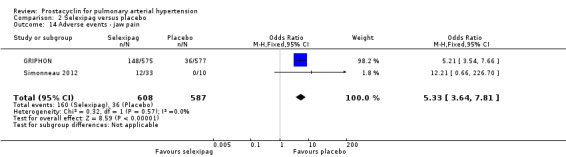
Comparison 2 Selexipag versus placebo, Outcome 14 Adverse events ‐ jaw pain.
2.15. Analysis.
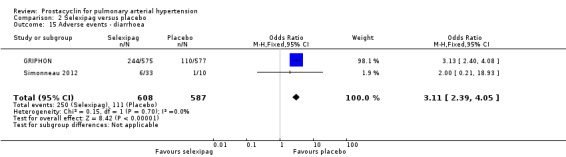
Comparison 2 Selexipag versus placebo, Outcome 15 Adverse events ‐ diarrhoea.
2.16. Analysis.
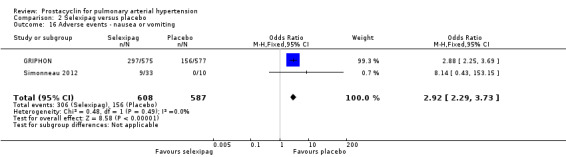
Comparison 2 Selexipag versus placebo, Outcome 16 Adverse events ‐ nausea or vomiting.
2.17. Analysis.
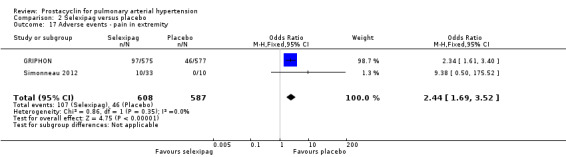
Comparison 2 Selexipag versus placebo, Outcome 17 Adverse events ‐ pain in extremity.
2.18. Analysis.
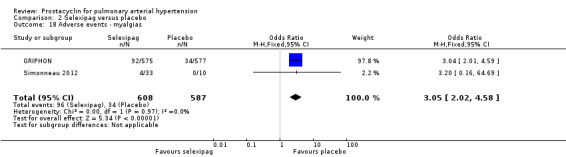
Comparison 2 Selexipag versus placebo, Outcome 18 Adverse events ‐ myalgias.
2.11. Analysis.
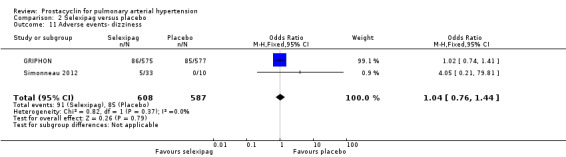
Comparison 2 Selexipag versus placebo, Outcome 11 Adverse events‐ dizziness.
2.19. Analysis.
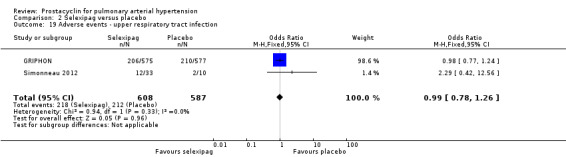
Comparison 2 Selexipag versus placebo, Outcome 19 Adverse events ‐ upper respiratory tract infection.
5. Selexipag versus placebo: adverse events.
| Outcome | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | |
| Risk with placebo | Risk with selexipag | |||
| Dizziness | 145 per 1000 | 150 per 1000 (114 to 196) | OR 1.04 (0.76 to 1.44) | 1195 (2) |
| Headache | 325 per 1000 | 653 per 1000 (597 to 706) | OR 3.91 (3.07 to 4.98) | 1195 (2) |
| Vasodilation | 49 per 1000 | 122 per 1000 (82 to 178) | OR 2.67 (1.72 to 4.17) | 1195 (2) |
| Jaw pain | 61 per 1000 | 258 per 1000 (192 to 338) | OR 5.33 (3.64 to 7.81) | 1195 (2) |
| Diarrhoea | 189 per 1000 | 420 per 1000 (358 to 486) | OR 3.11 (2.39 to 4.05) | 1195 (2) |
| Nausea or vomiting | 266 per 1000 | 514 per 1000 (453 to 574) | OR 2.92 (2.29 to 3.73) | 1195 (2) |
| Pain in extremity | 78 per 1000 | 172 per 1000 (126 to 230) | OR 2.44 (1.69 to 3.52) | 1195 (2) |
| Myalgias | 58 per 1000 | 158 per 1000 (110 to 220) | OR 3.05 (2.02 to 4.58) | 1195 (2) |
| Upper respiratory tract infection | 361 per 1000 | 359 per 1000 (306 to 416) | OR 0.99 (0.78 to 1.26) | 1195 (2) |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio
Cost analysis
No studies assessed cost analysis.
Discussion
Summary of main results
This review demonstrates clinical and statistical benefit for the use of intravenous prostacyclin compared to control in terms of improved functional class, six‐minute walk distance (6MWD), mortality, symptoms scores, and cardiopulmonary haemodynamics, but at a cost of increased risk of adverse events.
This review also demonstrates a statistical and small benefit for inhaled prostacyclin compared to placebo in terms of improvement in functional class, symptoms scores, and cardiopulmonary haemodynamics, a statistical benefit for 6MWD, but the effect is uncertain for mortality. The use of oral prostacyclin did not demonstrate a statistical or clinical benefit for improvement in functional class, symptoms scores, cardiopulmonary haemodynamics, or mortality.
In these trials, there was only demonstrably significant mortality benefit using intravenous preparations; but not in subcutaneous, oral or inhaled preparations. This may be due to a true effect, or the inclusion of unblinded trials using intravenous preparations that may have over estimated the result, the low participant numbers, and relatively short trial duration.
Selexipag is a selective IP prostacyclin receptor agonist that works similarly to prostacyclin, offering a potentially more stable drug, with oral administration and titration, with potentially similar efficacy. We assessed two trials comparing selexipag to placebo; no trials compared selexipag with prostacyclin. When compared to placebo in large, long‐term trials, selexipag had less clinical worsening, but increased adverse events; the effect on other clinical outcomes is less certain. The rate of death was increased in the selexipag group, though the confidence interval crossed zero, so the true effect is uncertain.
Overall completeness and applicability of evidence
In the included trials comparing prostacyclin with control, there was demonstrable mortality benefit using intravenous preparations; but not in subcutaneous, oral or inhaled preparations. The certainty of evidence for mortality benefit was reduced as three out of four of these included trials were open‐label. It is unclear in other studies if using non‐intravenous preparations did not confer a mortality benefit due to their short (median 12 weeks) duration, or that they were under‐powered to detect a mortality difference, or if this is a true signal of no benefit.
When prostacyclin was first developed for PAH, it was delivered intravenously due to the short half‐life and potent local effects of the drug. Given the risks of rebound effects if the continuous infusion was suddenly stopped, the drug was administered by a central venous catheter ‐ a direct and reliable access. Invasive central catheter placement is associated with increased risk of adverse events, including infection, bleeding, and damage to surrounding structures. Of concern, these included studies demonstrated a 12% to 25% risk of non‐fatal serious line‐related events and two line‐related deaths. Although efficacious, the decision to commence intravenous prostacyclin must be weighed against the increased risk of serious side effects. The heterogeneity of line‐related events between these included trials likely reflects real‐world heterogeneity between clinical centres, and catheter placement should only be considered in experienced centres.
The decision to commence continuous intravenous therapy should also be weighed by the patient's ability to reliably control the pump device; several participants in these studies were not randomised because of their inability to work the device. Particular consideration should be given to people with connective tissue disease‐related PAH, who may have reduced dexterity.
In recent years, research has been undertaken to develop a safer, more convenient preparation of prostacyclin ‐ including via the inhaled, subcutaneous and oral routes. One subcutaneous trial (Simonneau 2002), five oral trials (ALPHABET; Barst 2003; FREEDOM‐C; FREEDOM‐C2; FREEDOM‐M), and four inhaled trials (AIR; Han 2017; McLaughlin 2006; TRIUMPH), were included in this review. Subgroup analyses suggest that these preparations did not result in the same mortality benefit as intravenous preparations and the overall effect on 6MWD and improvement in World Health Organization (WHO) functional class was less, though it still provided some benefit. However, these trials were not powered for mortality and median duration was only 12 weeks. No randomised controlled trials (RCTs) have compared different preparations head to head. One small study compared inhaled to continuous infusion prostacyclin in 16 participants for 12 weeks (and 4 participants up to 1 year) (Pepke‐Zaba 2000). They reported no difference in effect between groups, though data were limited. Another retrospective study reported the safe transition from intravenous or subcutaneous to inhaled treprostinil, with no immediate significant change in function (Enderby 2014).
Certainty of the evidence
We included several open‐label studies, which reduced the certainty of the evidence. When these studies were excluded, the differences were still significant, and the direction of effect was still the same, but the magnitude of the effect was smaller. Using subgroup analyses to draw these conclusions, however, relies on a smaller sample size which reduced confidence in these conclusions.
We found the evidence for 6MWD, some haemodynamics, and quality of life scores to be of moderate or low certainty due to imprecision of results from significant heterogeneity. We found that the Chi² test for subgroup difference was also significant, indicating that this heterogeneity is in part explained by the difference between the different drugs used.
Although there was statistical significance in the difference in 6MWD with the use of prostacyclins, the overall effect did not meet the minimum clinically important threshold of 41 metres. In addition, although there was a statistically significant difference in the level of dyspnoea reduction, the illustrative Borg score did not meet the clinically important threshold of 0.9 units.
Potential biases in the review process
We conducted this review in accordance with established Cochrane standards. Two review authors independently screened search results and resolved discrepancies by discussion and consensus. We did not restrict the literature search by language. Publication bias is possible, whereby failure to identify unpublished negative trials could have led to an overestimation of effect.
Agreements and disagreements with other studies or reviews
The data from this review are limited by the short follow‐up duration. At least two further real‐world long‐term registry studies have assessed the longer‐term use of intravenous epoprostenol in PAH patients (McLaughlin 2002; Sitbon 2002). McLaughlin 2002 followed patients for at least three years, and found observed survival with epoprostenol therapy at one, two, and three years was 87.8%, 76.3%, and 62.8% respectively. Sitbon 2002 followed patients for five years and observed an overall survival rate at one, two, three, and five years of 85%, 70%, 63%, and 55%, respectively. These data are compared to historical cohort data with no therapy, where the expected survival was 58.9%, 46.3%, and 35.4% at one, two, and three years, respectively (D’Alonzo 1991). Our data for intravenous‐only studies (duration of 12 weeks only) found a survival rate of 94% for intravenous epoprostenol at 12 weeks compared to an 83% survival rate without intravenous epoprostenol. While there are limitations to observational registry data, it supports the findings in this review that intravenous prostacyclin analogues do suggest a mortality benefit.
The European Society of Cardiology (ESC) guidelines (Galiè 2016), and further registry studies (McLaughlin 2002; Sitbon 2002), have derived and validated clinical and investigational parameters to stratify risk of mortality in PAH patients. They found that WHO functional class, 6MWD, and pro‐brain natriuretic peptide (BNP), as well as cardiopulmonary parameters including right atrial pressure (RAP) and cardiac index, were the most reliable predictors of survival. Whilst this review had short duration of follow‐up, which limits the conclusions regarding overall mortality, it did find that use of prostacyclin significantly improved WHO functional class, 6MWD, RAP, and cardiac index. These effects were largest using intravenous prostacyclin, but changes were also present across other preparations.
Authors' conclusions
Implications for practice.
This review demonstrates clinical and statistical benefit for the use of prostacyclin compared to control in terms of improved functional class, six‐minute walk distance (6MWD), mortality, symptoms scores, and cardiopulmonary haemodynamics, (low‐ to moderate‐certainty evidence) but at a cost of increased risk of adverse events. There is a statistical benefit for the use of 6MWD, haemodynamics, and avoidance of clinical worsening using selexipag, however the clinical significance remains uncertain.
Implications for research.
In these trials, there was only mortality benefit using intravenous preparations; but not in subcutaneous, oral or inhaled preparations. This may be due to a true effect, however this effect may be overestimated due to the inclusion of small, short or open‐label studies. Larger trials or real‐world registry data examining the use of non‐intravenous preparations may provide further information about long‐term effect.
Acknowledgements
Rebecca Normansell was the Editor for this review and commented critically on the review. We thank Christopher Cates for his statistical input and Emma Dennett for her comments on this review.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
CENTRAL & Cochrane Airways Trials Register
#1 MESH DESCRIPTOR Hypertension, Pulmonary EXPLODE ALL
#2 MESH DESCRIPTOR Pulmonary Heart Disease EXPLODE ALL
#3 pulmonary* NEAR2 hypertensi*:ti,ab,kw
#4 #1 OR #2 OR #3
#5 MESH DESCRIPTOR Epoprostenol
#6 MESH DESCRIPTOR Iloprost
#7 epoprostenol OR iloprost OR beraprost OR treprostinil OR selexipag OR prostacyclin*
#8 #5 OR #6 OR #7
#9 #8 AND #4
MEDLINE (Ovid)
1. exp Hypertension, Pulmonary/
2. Pulmonary Heart Disease/
3. (pulmonary adj2 hypertensi$).tw.
4. or/1‐3
5. Epoprostenol/
6. Iloprost/
7. epoprostenol.tw.
8. iloprost.tw.
9. beraprost.tw.
10. treprostinil.tw.
11. selexipag.tw.
12. prostacyclin*.tw.
13. or/5‐12
14. 4 and 13
15. (controlled clinical trial or randomised controlled trial).pt.
16. (randomised or randomised).ab,ti.
17. placebo.ab,ti.
18. dt.fs.
19. randomly.ab,ti.
20. trial.ab,ti
21. groups.ab,ti.
22. or/15‐21
23. Animals/
24. Humans/
25. 23 not (23 and 24)
26. 22 not 25
27. 14 and 26
Embase (Ovid)
1. pulmonary hypertension/
2. (pulmonary$ adj2 hypertensi$).ti,ab.
3. 1 or 2
4. prostacyclin/
5. iloprost/
6. beraprost/
7. uniprost/
8. bosentan/
9. prostacyclin.ti,ab.
10. iloprost.ti,ab.
11. beraprost.ti,ab.
12. treprostinil.ti,ab.
13. epoprostenol.ti,ab.
14. selexipag.ti,ab.
15. or/4‐14
16. 3 and 15
17. Randomized Controlled Trial/
18. randomisation/
19. controlled clinical trial/
20. Double Blind Procedure/
21. Single Blind Procedure/
22. Crossover Procedure/
23. (clinica$ adj3 trial$).tw.
24. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (mask$ or blind$ or method$)).tw.
25. exp Placebo/
26. placebo$.ti,ab.
27. random$.ti,ab.
28. ((control$ or prospectiv$) adj3 (trial$ or method$ or stud$)).tw.
29. (crossover$ or cross‐over$).ti,ab.
30. or/17‐29
31. exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
32. human/ or normal human/ or human cell/
33. 31 and 32
34. 31 not 33
35. 30 not 34
36. 16 and 35
ClinicalTrials.gov
| Field | Search terms |
| Study type: | Interventional |
| Condition: | pulmonary hypertension |
| Interventions: | prostacylin OR epoprostenol OR iloprost OR beraprost OR treprostinil OR selexipag |
Data and analyses
Comparison 1. Prostacyclin versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in WHO functional class | 8 | 1066 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.72, 3.32] |
| 1.1 Intravenous | 3 | 202 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.96 [4.76, 47.04] |
| 1.2 Oral | 3 | 596 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.85, 2.05] |
| 1.3 Inhaled | 2 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.94 [1.53, 5.66] |
| 2 Worsening of WHO functional class | 5 | 805 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.57, 1.37] |
| 2.1 Intravenous | 1 | 71 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.29, 6.07] |
| 2.2 Oral | 2 | 466 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.54, 1.51] |
| 2.3 Inhaled | 2 | 268 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.24, 1.89] |
| 3 6MWD | 13 | 2302 | Mean Difference (Fixed, 95% CI) | 19.50 [14.82, 24.19] |
| 3.1 Intravenous | 4 | 245 | Mean Difference (Fixed, 95% CI) | 91.76 [58.97, 124.55] |
| 3.2 Subcutaneous | 1 | 469 | Mean Difference (Fixed, 95% CI) | 16.0 [7.38, 24.62] |
| 3.3 Oral | 4 | 1070 | Mean Difference (Fixed, 95% CI) | 14.76 [7.81, 21.70] |
| 3.4 Inhaled | 4 | 518 | Mean Difference (Fixed, 95% CI) | 26.97 [17.21, 36.73] |
| 4 Mortality | 15 | 2554 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.94] |
| 4.1 Intravenous | 4 | 255 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.69] |
| 4.2 Subcutaneous | 1 | 469 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.35, 2.94] |
| 4.3 Oral | 5 | 1247 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.44, 1.83] |
| 4.4 Inhaled | 5 | 583 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.09, 1.71] |
| 5 mPAP | 8 | 1132 | Mean Difference (Fixed, 95% CI) | ‐3.60 [‐4.73, ‐2.48] |
| 5.1 Intravenous | 2 | 192 | Mean Difference (Fixed, 95% CI) | ‐6.23 [‐8.64, ‐3.83] |
| 5.2 Subcutaneous | 1 | 469 | Mean Difference (Fixed, 95% CI) | ‐0.7 [‐3.19, 1.79] |
| 5.3 Oral | 2 | 196 | Mean Difference (Fixed, 95% CI) | ‐1.71 [‐4.06, 0.63] |
| 5.4 Inhaled | 3 | 275 | Mean Difference (Fixed, 95% CI) | ‐4.88 [‐6.77, ‐2.99] |
| 6 PVR | 7 | 658 | Mean Difference (Fixed, 95% CI) | ‐2.81 [‐3.80, ‐1.82] |
| 6.1 Intravenous | 2 | 192 | Mean Difference (Fixed, 95% CI) | ‐5.31 [‐6.83, ‐3.80] |
| 6.2 Oral | 2 | 191 | Mean Difference (Fixed, 95% CI) | ‐1.51 [‐3.20, 0.18] |
| 6.3 Inhaled | 3 | 275 | Mean Difference (Fixed, 95% CI) | ‐0.10 [‐2.16, 1.96] |
| 7 Cardiac index | 6 | 868 | Mean Difference (Fixed, 95% CI) | 0.31 [0.23, 0.38] |
| 7.1 Intravenous | 2 | 192 | Mean Difference (Fixed, 95% CI) | 0.57 [0.40, 0.74] |
| 7.2 Subcutaneous | 1 | 469 | Mean Difference (Fixed, 95% CI) | 0.18 [0.07, 0.29] |
| 7.3 Oral | 2 | 192 | Mean Difference (Fixed, 95% CI) | 0.16 [‐0.04, 0.36] |
| 7.4 Inhaled | 1 | 15 | Mean Difference (Fixed, 95% CI) | 0.48 [0.30, 0.66] |
| 8 Cardiac output | 2 | 260 | Mean Difference (Fixed, 95% CI) | 0.57 [0.32, 0.81] |
| 8.1 Inhaled | 2 | 260 | Mean Difference (Fixed, 95% CI) | 0.57 [0.32, 0.81] |
| 9 RAP | 6 | 1060 | Mean Difference (Fixed, 95% CI) | ‐1.90 [‐2.58, ‐1.22] |
| 9.1 Intravenous | 2 | 192 | Mean Difference (Fixed, 95% CI) | ‐2.41 [‐4.10, ‐0.72] |
| 9.2 Subcutaneous | 1 | 469 | Mean Difference (Fixed, 95% CI) | ‐1.90 [‐3.01, ‐0.79] |
| 9.3 Oral | 2 | 196 | Mean Difference (Fixed, 95% CI) | ‐1.0 [‐2.60, 0.60] |
| 9.4 Inhaled | 1 | 203 | Mean Difference (Fixed, 95% CI) | ‐2.2 [‐3.49, ‐0.91] |
| 10 Dyspnoea | 8 | 1521 | Std. Mean Difference (Fixed, 95% CI) | ‐0.21 [‐0.32, ‐0.11] |
| 10.1 Intravenous | 2 | 116 | Std. Mean Difference (Fixed, 95% CI) | ‐0.92 [‐1.31, ‐0.52] |
| 10.2 Subcutaneous | 1 | 469 | Std. Mean Difference (Fixed, 95% CI) | ‐0.33 [‐0.51, ‐0.14] |
| 10.3 Oral | 3 | 668 | Std. Mean Difference (Fixed, 95% CI) | ‐0.09 [‐0.25, 0.06] |
| 10.4 Inhaled | 2 | 268 | Std. Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.29, 0.19] |
| 11 Quality of life | 3 | 271 | Std. Mean Difference (Fixed, 95% CI) | 0.28 [0.04, 0.52] |
| 11.1 Intravenous | 1 | 69 | Std. Mean Difference (Fixed, 95% CI) | 0.78 [0.29, 1.28] |
| 11.2 Oral | 1 | 187 | Std. Mean Difference (Fixed, 95% CI) | 0.07 [‐0.22, 0.36] |
| 11.3 Inhaled | 1 | 15 | Std. Mean Difference (Fixed, 95% CI) | 0.88 [‐0.20, 1.95] |
| 12 Clincal worsening | 12 | 2238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.48, 0.92] |
| 12.1 Intravenous | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 1.01] |
| 12.2 Subcutaneous | 1 | 469 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.38, 1.73] |
| 12.3 Oral | 5 | 1126 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.25] |
| 12.4 Inhaled | 4 | 518 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.20, 0.89] |
| 13 Adverse events ‐ syncope | 4 | 560 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.42] |
| 14 Adverse events ‐ dizziness | 10 | 1939 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.84, 1.42] |
| 15 Adverse events ‐ vasodilation | 11 | 2277 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.03 [3.84, 6.58] |
| 16 Adverse events ‐ headache | 12 | 2351 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.16 [2.62, 3.80] |
| 17 Adverse events ‐ jaw pain | 10 | 2149 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.25 [3.96, 6.98] |
| 18 Adverse events ‐ diarrhoea | 10 | 2317 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [2.29, 3.46] |
| 19 Adverse events ‐ leg pain | 2 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.96 [1.02, 8.62] |
| 20 Adverse events ‐ nausea and vomiting | 12 | 2399 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.98, 2.88] |
| 21 Adverse events ‐ abdominal pain | 2 | 465 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.75, 2.42] |
| 22 Adverse events ‐ pain in extremity | 6 | 1236 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.36 [2.32, 4.85] |
| 23 Adverse events ‐ myalgia | 3 | 1009 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.65, 4.58] |
| 24 Adverse events ‐ upper respiratory tract events | 7 | 1038 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.22, 2.13] |
| 25 Adverse events ‐ peripheral oedema | 6 | 1228 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.98, 2.17] |
| 26 Adverse events ‐ infusion site reaction | 2 | 580 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.41 [9.16, 22.66] |
Comparison 2. Selexipag versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Improvement in WHO FC | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Worsening in WHO FC | 2 | 1188 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.60, 1.04] |
| 3 6MWD | 2 | 1199 | Mean Difference (Fixed, 95% CI) | 12.62 [1.90, 23.34] |
| 4 Mortality | 2 | 1199 | Risk Difference (M‐H, Fixed, 95% CI) | 0.02 [‐0.00, 0.04] |
| 5 mPAP | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 PVR | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Cardiac index | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8 RAP | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9 Dyspnoea | 1 | 43 | Mean Difference (Fixed, 95% CI) | ‐0.1 [‐1.40, 1.20] |
| 10 Clinical worsening | 2 | 1199 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.37, 0.60] |
| 11 Adverse events‐ dizziness | 2 | 1195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.76, 1.44] |
| 12 Adverse events ‐ headache | 2 | 1195 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.91 [3.07, 4.98] |
| 13 Adverse events ‐ vasodilation | 2 | 1195 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.72, 4.17] |
| 14 Adverse events ‐ jaw pain | 2 | 1195 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.33 [3.64, 7.81] |
| 15 Adverse events ‐ diarrhoea | 2 | 1195 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.11 [2.39, 4.05] |
| 16 Adverse events ‐ nausea or vomiting | 2 | 1195 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.92 [2.29, 3.73] |
| 17 Adverse events ‐ pain in extremity | 2 | 1195 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.69, 3.52] |
| 18 Adverse events ‐ myalgias | 2 | 1195 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.05 [2.02, 4.58] |
| 19 Adverse events ‐ upper respiratory tract infection | 2 | 1195 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.78, 1.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AIR.
| Methods | Randomised, blinded, parallel‐group placebo‐controlled trial | |
| Participants | People with Group 1 PAH (including primary or idiopathic), drug‐induced, or scleroderma‐associated PAH The inclusion criteria were a mean PAH greater than 30 mm Hg, 6MWD 50 metres to 500 metres, and a NYHA functional class of III or IV despite the use of standard conventional therapy (anticoagulants, diuretics, digitalis, calcium channel blockers, and supplemental oxygen). People who were taking investigational drugs, prostanoids, or beta blockers were excluded. Approximately 50% in each group were taking oral vasodilator therapy. n = 203 |
|
| Interventions | Inhaled iloprost at 2.5 μg to 5 μg per inhalation (90% of participants used 5 μg) six or nine times daily, with an overnight break (n = 101) compared to placebo using the same inhalation device with saline (n = 102) | |
| Outcomes | Change in WHO functional class, 6MWD, mortality, haemodynamic parameters, Mahler Transition Dyspnoea Index, clinical worsening (not explicitly stated), adverse events Outcomes were measured at 12 weeks |
|
| Notes | Industry sponsored | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper states that participants were randomly assigned after stratification according to NYHA functional class (III or IV) and type of pulmonary hypertension (primary or non‐primary) by an independent committee whose members were unaware of patients’ identities. Random sequence generation was probably done, however, methods of random sequence generation were not provided. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The same inhaler device was used in the intervention and placebo group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated whether the outcome assessors were blinded or who performed outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts or non‐completers were not reported. The study included data on participants who prematurely discontinued the study using a last‐observation‐carried‐forward analysis for the 6MWD test. Participants who died were assigned a value of 0 metres. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
ALPHABET.
| Methods | Randomised, blinded, parallel‐group placebo‐controlled trial | |
| Participants | Males or females over 8 years of age with PAH in NYHA functional classes II and III, including primary pulmonary hypertension, pulmonary hypertension associated with collagen vascular disease, congenital systemic‐to‐pulmonary shunts, portal hypertension and HIV infection; a baseline 6MWD between 50 metres and 500 metres, a mPAP > 25 mmHg and a pulmonary capillary wedge pressure < 15 mmHg were required for inclusion. Participants were excluded if they had received long‐term treatment with other prostacyclin analogues within one month of enrolment. Additional therapies including anticoagulants, diuretics, and calcium channel blockers were included; use of PDE‐5 inhibitors or ERAs were not reported. n = 130 |
|
| Interventions | Oral beraprost sodium (n = 65) compared to placebo (n = 65) In the first six weeks participants were up‐titrated with 20 μg or matching placebo four times a day for the first week, and the dose was increased by 20 μg or matching placebo four times a day each week. The maximal dose allowed in the study was 120 μg four times a day at week 6. At the end of 12 weeks, mean dose of drug was 80 + 35 μg four times a day (median 80 μg four times a day) in the beraprost sodium group, and the hypothetical dose in the placebo group was 111 + 22μg four times a day. |
|
| Outcomes | Change in WHO functional class, 6MWD, Borg dyspnoea score, clinical worsening (hospitalisation for worsening of symptoms related to pulmonary hypertension), haemodynamics, adverse events Outcomes were measured at 6 and 12 weeks |
|
| Notes | Industry funded: Sanofi‐Adventis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised, and probably done, but no methods of randomisation were detailed |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled. Given this was an up‐titration study, number of doses between intervention and placebo were outlined. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States how missing data were dealt with, but does not state how many participants were affected. In the event that no data were available at week 12 for the primary or secondary efficacy variable, the week 6 values or, if lacking, the baseline values were carried forward and used as values at week 12 (last observation carried forward). Two additional methods for missing data imputation were prospectively planned for the primary efficacy variable to ensure robustness of the results: the "left censored data" and "worst quartile" methods. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
Badesch 2000.
| Methods | Randomised, parallel‐group open‐label trial | |
| Participants | People with pulmonary hypertension secondary to the scleroderma spectrum of disease. 69% in each group were on oral vasodilator therapy n = 111 |
|
| Interventions | Intravenous epoprostenol plus usual treatment compared to usual treatment only Mean final dosing not given |
|
| Outcomes | 6MWD, NHYA functional class, Borg dyspnoea score, Dyspnoea Fatigue Rating, cardiopulmonary haemodynamics, adverse events, mortality Outcomes measured at 6 and 12 weeks |
|
| Notes | Industry funded: "The funding source for the study, Glaxo Wellcome, Inc. assisted in the collection, gathering, and analysis of data and was aware of the decision to submit the paper for publication". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators contacted a central randomisation centre to obtain treatment assignment, which was based on a stratified randomised block design. Assignments were stratified on the basis of vasodilator use at baseline (yes or no) and exercise capacity at baseline (50 metres to < 200 metres or ≥ 200 metres) and were randomised within blocks. |
| Allocation concealment (selection bias) | Low risk | Investigators contacted a central randomisation centre to obtain treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators were not blinded to treatment group assignment; however, independent blinded observers assessed the primary efficacy measure, exercise capacity. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | For outcomes of 6MWD, assessors were blinded. For mortality, this would not have had an impact on the interpretation of results. However for other outcomes, assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals and cross‐overs not reported |
| Selective reporting (reporting bias) | Unclear risk | Confidence intervals or error bars not reported for some outcomes |
| Other bias | Low risk | Other bias unlikely |
Barst 1996.
| Methods | Randomised, parallel‐group, open‐label trial | |
| Participants | Primary pulmonary hypertension who were NHYA III or IV despite optimal treatment (which included anticoagulants, oral vasodilators (68% in each group), diuretic agents, cardiac glycosides, and supplemental oxygen n = 81 |
|
| Interventions | Intravenous epoprostenol (flolan) versus conventional therapy mean max dose of epoprostenol was 9.2 + 0.5 ng/kg/min |
|
| Outcomes | 6MWD, cardiopulmonary haemodynamics, Chronic Heart Failure Questionnaire, Dyspnoea Fatigue Rating, clinical worsening (transplantation), adverse events, mortality Outcomes measured at 12 weeks |
|
| Notes | Partly industry funded and partly publicly funded ‐ "Supported in part by Burroughs Wellcome, Research Triangle Park, N.C. Dr.Rubin is the recipient of an academic award in vascular disease from the National Heart, Lung, and Blood Institute. Dr. Badesch is the recipient of a clinical investigator award from the National Institutes of Health". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated, adaptive randomisation was performed, with stratification according to the functional class, study centre, and baseline vasodilator use |
| Allocation concealment (selection bias) | Unclear risk | Not clear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts reported |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
Barst 2003.
| Methods | Randomised, blinded, parallel‐group placebo‐controlled trial | |
| Participants | Males or females over eight years of age with PAH in WHO functional classes II or III, including primary pulmonary hypertension, and PAH related to collagen vascular diseases or congenital systemic‐to‐pulmonary shunts, despite treatment with anticoagulant drugs, vasodilators (other than prostanoids or endothelin receptor antagonists), diuretics, cardiac glycosides, or supplemental oxygen. The inclusion criteria were a baseline peak oxygen consumption (VO2) during exercise between 8 mL/kg/min and 28 mL/kg/min determined during upright cycle ergometry (actual range for inclusion varied based on age and gender), a resting mPAP ≥ 25 mmHg, with mean pulmonary capillary wedge pressure of ≤ 15 mmHg and PVR > 3U. People were excluded if they had started or stopped any PAH therapy within one month before screening. n = 116 |
|
| Interventions | Oral beraprost (n = 60) versus placebo (n = 56) People received 20 μg of beraprost sodium or matching placebo four times daily with meals for the first two weeks, and the dose was increased by 20 μg or matching placebo four times daily with meals every two weeks, based on increasing signs, symptoms, and tolerability. The maximal dose allowed in the study was 200 μg four times daily. At the end of 3, 6, 9, and 12 months, the mean dose of drug was 71 + 3 μg (n = 60), 92 + 4 μg (n = 57), 98 + 6 μg (n = 49), and 107 + 7 μg (n = 8), respectively, four times daily in the beraprost group, and 83 + 4 μg (n = 53), 104 + 4μg (n = 47), 117 + 4 μg (n = 43), and 122 + 6 μg (n = 31), respectively, four times daily in the placebo group. |
|
| Outcomes | Change in WHO functional class, 6MWD, mortality, clinical worsening (described as disease progression), quality of life (though data not shown), adverse events Outcomes were evaluated at baseline and after 3, 6, 9, and 12 months of treatment with study drug |
|
| Notes | Industry funded: "This study was supported by United Therapeutics Corporation, Research Triangle Park, North Carolina" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised, and probably done, but methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled and as this was an up‐titration study, number of doses between intervention and placebo were outlined. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clearly reported: the study was prematurely terminated by the sponsor to accelerate assessment of the study results. Fifty‐six participants (93%) on beraprost and 52 participants (93%) on placebo were included in the 12‐month evaluation; and 116 participants (100%) were included in the 9‐month evaluation per protocol. |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
FREEDOM‐C.
| Methods | Randomised, blinded, parallel‐group placebo‐controlled trial | |
| Participants | People 12 to 70 years of age with symptomatic idiopathic PAH (including PAH associated with anorexigen/toxin use); familial PAH; or PAH associated with congenital heart disease (repaired congenital systemic‐to‐pulmonary shunts for 5 years), connective tissue disease, or HIV infection. The diagnosis of PAH required a mPAP ≤ 25 mmHg, pulmonary capillary wedge pressure or left ventricular end‐diastolic pressure ≤ 15 mmHg, PVR > 3 Wood units, and absence of unrepaired congenital heart disease, with a baseline 6MWD of 100 metres to 450 metres. All participants were required to be on an approved PDE‐5 inhibitor or ERA therapy, or combination thereof, for 90 days and at a stable dose for 30 days before study entry. n = 349 |
|
| Interventions | Oral treprostinil or placebo twice daily in combination with a PDE‐5 inhibitor and/or an ERA. At study initiation, participants were administered a 1 mg twice daily starting dose, with increases in 1 mg increments. Additional tablet doses of 0.5 mg and 0.25 mg were made available to participants at sequentially later times in the study. Participants for whom all doses were available received oral treprostinil at 0.5 mg twice daily and, if clinically tolerated, received dose increases by 0.5 mg increments every 3 days. Doses were increased up to a maximum of 16 mg twice daily over the 16 weeks, depending on adverse events and symptoms and signs of PAH, at the end of 16 weeks. At week 16, the mean dose of UT‐15C was 3.1 mg (SD 1.9), and the mean dose of placebo was 6.1 mg (SD 3.6). |
|
| Outcomes | 6MWD, time to clinical worsening (death; transplantation or atrial septostomy; or clinical deterioration, defined as hospitalisation related to PAH, 20% decrease in 6MWD from baseline and a decrease in
WHO functional class, or initiation of a new PAH therapy), Borg dyspnoea score, and Dyspnoea Fatigue Rating, adverse events Outcomes were measured at 16 weeks |
|
| Notes | Some raw data for outcomes were obtained on the NCT clinical trials registry Industry funded: "This study was funded by United Therapeutics Corporation" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised, and probably done, but methods not reported. Blocked and stratified by background therapy (PDE‐5 inhibitor alone, ERA alone, or PDE‐5 inhibitor 1 ERA) and baseline 6MWD (< 350 metres and > 350 metres) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled, and as this was an up‐titration study, numbers of tablets across the intervention and placebo groups were reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts, withdrawals, and cross‐over participants were reported with reasons supplied |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
FREEDOM‐C2.
| Methods | Randomised, blinded, parallel‐group placebo‐controlled trial | |
| Participants | People aged 18 to 75 years with idiopathic PAH (including PAH associated with appetite suppressant or toxin use), familial PAH, or PAH associated with congenital heart disease (repaired congenital systemic‐to‐pulmonary shunts for 5 years); connective tissue disease; or HIV. Diagnosis of PAH required a mPAP of ≤ 25 mmHg, pulmonary capillary wedge pressure or left ventricular end diastolic pressure of ≤ 15 mmHg, PVR of > 3 Wood units, and the absence of unrepaired congenital heart disease. Participants were required to have received ERA or PDE‐5 inhibitor therapy for 90 days with a stable dose for 30 days before baseline and throughout the duration of the study. Baseline 6MWD was required to be between 150 metres and 425 metres. n = 310 |
|
| Interventions | Oral treprostinil or placebo twice daily in combination with a PDE‐5 inhibitor and/or an ERA. The study drug was initiated at 0.25 mg twice daily (every 12 + 1 h), with dose escalation of an additional 0.25 mg twice daily every 3 days if clinically indicated. After the first 4 weeks, dose escalations could include increments of either 0.25 mg or 0.5 mg twice daily every 3 days. Mean treprostinil dose at 16 weeks: 3.1 + 1.9 mg twice daily (range: 0.25 mg to 10.5 mg) compared with placebo dose: 6.1 + 3.6 mg twice daily (range: 0.25 mg to 16.0 mg) |
|
| Outcomes | 6MWD, Borg dyspnoea score, NT‐proBNP level, WHO functional classification, Cambridge Pulmonary Hypertension Outcome Review, Clinical worsening defined as death, transplantation, or atrial septostomy; hospitalisation as a result of right‐side heart failure; a decrease in 6MWD of 20% from baseline (or too ill to walk) and the addition of an inhaled prostacyclin, ERA, or PDE‐5 inhibitor; or initiation of parenteral prostacyclin therapy for the treatment of PAH, adverse events Outcomes were measured at 16 weeks |
|
| Notes | This is a different cohort of participants from the FREEDOM‐M study, which examined monotherapy, and the FREEDOM C‐2 study, which uses treprostinil on a background of combination PDE5‐inhibitor and ERA, but due to the high dropout rate for side effects starting at 1 mg twice daily, this FREEDOM C‐2 study was created to commence treprostinil at 0.25 mg twice daily. Inudstry funded: "This study was funded by United Therapeutics Corporation" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised, and probably done, but methods not reported. Blocked and stratified by background therapy (PDE‐5 inhibitor alone, ERA alone, or PDE‐5 inhibitor 1 and ERA) and baseline 6MWD (< 350 metres and > 350 metres) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled, and as this was an up‐titration study, numbers of tablets across the intervention and placebo groups were reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts, withdrawals, and cross‐over participants were reported with reasons supplied |
| Selective reporting (reporting bias) | Low risk | It was stated that no secondary outcomes were significant, and no further data given, however these were reported on the clinical trials registry |
| Other bias | Low risk | Other bias unlikely |
FREEDOM‐M.
| Methods | Randomised, blinded, parallel‐group placebo‐controlled trial | |
| Participants | People aged 12 to 75 years of age with idiopathic or hereditary PAH (including PAH associated with appetite suppressant/toxin use), PAH associated with repaired congenital systemic‐to‐pulmonary shunts (repaired ≥ 5 years), or PAH associated with collagen vascular disease or HIV. Participants were ineligible if they had received ERA, PDE‐5I, or prostacyclin therapy within 30 days of baseline or if they had evidence of significant left‐sided heart disease or parenchymal lung disease. Baseline 6MWD was required to be between 100 metres and 450 metres. Participants were required to be optimally treated with conventional PAH therapies (e.g. anticoagulants, oral vasodilators, oxygen, digoxin) n = 349 |
|
| Interventions | Oral trepostinil compared to placebo Study drug was originally initiated at 1.0 mg twice daily; however, on the basis of tolerability issues identified in the FREEDOM‐C study, the study protocol was later amended to lower the starting dose to 0.5 mg twice daily and eventually 0.25 mg twice daily. Study drug was administered every 12 ± 2 hours with dose escalation of an additional 0.25 mg to 0.5 mg twice daily every 3 days and a maximum possible dose of 12 mg twice daily. |
|
| Outcomes | 6MWD, Borg dyspnoea score, clinical worsening, Dyspnoea Fatigue Rating, WHO functional class, and symptoms of PAH. Clinical worsening was defined as one of the following: cardiovascular death, transplantation, atrial septostomy, or clinical deterioration. Clinical deterioration was defined as the initiation of new, approved PAH‐specific therapy (ERA, PDE‐5I, or prostacyclin) plus either hospitalisation for decompensated PAH or a ≥ 20% decrease in 6MWD from baseline combined with worsening WHO functional class Outcomes were reported at 12 weeks |
|
| Notes | This studies a different cohort of participants than FREEDOM C or FREEDOM C‐2, which examined oral treprostinil with combination therapy Data were presented according to intention‐to‐treat and modified intention‐to‐treat. The primary analysis population or mean ITT population was the population of patients with access to the 0.25 mg strength tablet at the time of randomisation. The ITT population (as well as the safety population) was defined as all randomised participants. Industry based: "This study was funded by United Therapeutics Corporation" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised, and probably done, but methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled. Reported the average dose for treprostinil but not for placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts, withdrawals, and cross‐over participants were reported with reasons supplied |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
GRIPHON.
| Methods | Randomised, double‐blinded, parallel‐group placebo‐controlled trial, event driven | |
| Participants | Participants aged 18 to 75 years of age who had idiopathic or inheritable PAH or PAH associated with HIV infection, drug use or toxin exposure, connective tissue disease, or repaired congenital systemic‐to‐pulmonary shunts. Confirmation of the diagnosis by means of right heart catheterisation was required before screening. Participants were required to have a PVR of at least 5 Wood units (400 dyn·sec/cm−5) (which is higher than the usual stated criteria) and a 6MWD of 50 metres to 450 metres. Participants who were not receiving treatment for PAH and those who were receiving an ERA, a PDE‐5 inhibitor, or both at a dose that had been stable for at least 3 months were eligible for enrolment; participants who were receiving prostacyclin analogues were not eligible n = 1156 |
|
| Interventions | Oral selexipag versus placebo During the 12‐week dose adjustment phase, selexipag was initiated at a dose of 200 μg twice daily and was increased weekly in twice‐daily increments of 200 μg until unmanageable adverse effects associated with prostacyclin use, such as headache or jaw pain, developed. The dose was then decreased by 200 μg in both daily doses, and this reduced dose was considered to be the maximum tolerated dose for that participant. The maximum dose allowed was 1600 μg twice daily. |
|
| Outcomes | The primary endpoint in a time‐to‐event analysis was a composite of death or a complication related to PAH, whichever occurred first, up to the end of the treatment period. Complications related to PAH were disease progression or worsening of PAH that resulted in hospitalisation, initiation of parenteral prostanoid therapy or long‐term oxygen therapy, or the need for lung transplantation or balloon atrial septostomy as judged by the physician. Disease progression was defined as a decrease from baseline of at least 15% in the 6MWD (confirmed by means of a second test on a different day) accompanied by a worsening in WHO functional class (for those with WHO functional class II or III at baseline) or the need for additional treatment of PAH (for the patients with WHO functional class III or IV at baseline). Median time‐to‐event was 63 weeks Separate outcomes including 6MWD, mortality, absence of worsening in WHO functional class, and adverse events were also included |
|
| Notes | A very detailed protocol was supplied in the supplementary appendix Industry funded: "Funded by Actelion Pharmaceuticals" ClinicalTrials.gov number: NCT01106014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation and drug packaging was performed by an independent company. Patients were randomised to one of the treatment groups using a centralised randomisation system via the Interactive Web/Voice Response System (IWRS/IVRS). |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by an independent company, with placebo‐controlled drug packaging, so selection bias was unlikely |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled, states participants and investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled, states participants and investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts, withdrawals, and cross‐over participants were reported with reasons supplied |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported, and the protocol was supplied in a supplementary appendix |
| Other bias | Low risk | Other bias unlikely |
Han 2017.
| Methods | Randomised, multicentre, open‐label controlled trial | |
| Participants | Participants were between 15 and 80 years old, had WHO functional class III or IV with symptoms of PAH, and had been diagnosed with idiopathic PAH or chronic thromboembolism pulmonary hypertension, according to criteria in current guidelines. For each participant, mPAP was required to be ≥ 25 mmHg, and the pulmonary capillary wedge pressure was required to be ≤ 15 mmHg. Participants had not received previous treatment with an approved therapy for PAH before enrolment. Participants with acute pulmonary thromboembolism, left‐sided heart disease, pulmonary disease with FEV 1/FVC < 50% predicted or total lung capacity < 60%, renal insufficiency, chronic liver disease, or portal hypertension were excluded from the study. N = 27 total, with 14 included in our analysis |
|
| Interventions | Combination inhaled iloprost with bosentan, versus iloprost alone, versus bosentan alone, in three randomised arms Iloprost was administered at increasing doses to a target of 10 µg 4 to 6 times/day |
|
| Outcomes | The primary endpoint was change in peak 6MWD that were defined as within 10 to 60 min after iloprost inhalation at week 6 and 3 months Prespecified secondary efficacy endpoints included changes in haemodynamic variables that were measured by right heart catheterisation from baseline to 3 months after the initiation of treatment. Secondary efficacy endpoints also included changes in NT‐proBNP levels, WHO functional class, and Minnesota Living with Heart Failure Questionnaire scores from baseline to 6 weeks and 3 months after the initiation of treatment. Safety assessments included laboratory measurements and evaluation of adverse events |
|
| Notes | Data from combined bosentan and iloprost versus bosentan alone were used (N = 14). Individual patient data was kindly sent by authors and re‐analysed. Where SD was reported, it was likely actually standard error according to reanalysis and so was imputed into the meta‐analysis as such. This work was supported by National Natural Science Foundation Grants. The study drugs were provided by Actelion Pharmaceuticals Ltd (Allschwil, Switzerland) (bosentan) and Bayer (Leverkusen, Germany) (iloprost). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised, but methods of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Methods of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | High risk | Incorrectly reported as SD, not standard error, however individual patient data was provided for reanalysis |
McLaughlin 2006.
| Methods | Randomised, double‐blinded, parallel‐group placebo‐controlled trial | |
| Participants | People aged 10 to 80 years with symptomatic PAH receiving bosentan for 4 months or more, with a 6MWD of 100 metres to 425 metres, resting mPAP greater than 25 mmHg, pulmonary capillary wedge pressure less than 15 mmHg, and PVR of 240 dyn·s/cm‐5 or greater n = 67 |
|
| Interventions | Either iloprost inhalation (5 μg) or placebo inhaled six to nine times daily while awake using the Prodose AAD device, with existing therapy with oral bosentan (125 mg twice daily). The mean number of study drug inhalations per day was 5.6 in the iloprost group and 5.7 in the placebo group. The mean total daily dose of study drug was 26.8 μg (range: 2.5 to 32.4 μg) in the iloprost group and 27.8 μg (range: 11.6 to 33.3μg) in the placebo group | |
| Outcomes | 6MWD, performed pre‐inhalation at baseline, post‐inhalation at weeks 4 and 8, and at both time points at Week 12, with the two tests separated by at least 2 h and the temporal sequence randomised (i.e. whether pre‐ or post‐inhalation). NYHA functional class and post‐inhalation Borg dyspnoea score were also assessed at baseline and Weeks 4, 8, and 12. Haemodynamic parameters were measured by right‐heart catheterisation at baseline and week 12, both pre‐ and post‐inhalation (15 min). Clinical worsening (described as clinical deterioration) | |
| Notes | Industry funded: "Supported by CoTherix, Inc. (South San Francisco, CA)" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was communicated to sites using a blinded interactive voice response system, and was stratified and blocked according to etiology: idiopathic PAH or associated PAH; i.e. collagen vascular disease, repaired congenital heart disease, HIV infection, or anorexigen use) |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed outside the site and drug labelling was placebo‐controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled, and as this was an up‐titration study, numbers of tablets across the intervention and placebo groups were reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported as blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts, withdrawals, and cross‐over participants were reported with reasons supplied |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes and their measurements with respect to timing of inhalation were reported |
| Other bias | Low risk | Other bias unlikely |
Olschewski 2010.
| Methods | Randomised, parallel‐group, open‐label study | |
| Participants | People aged from 18 to 70 years with idiopathic or familial PAH, previously classified as primary pulmonary hypertension (idiopathic PAH group), or other forms of pulmonary hypertension, previously classified as secondary pulmonary hypertension, with a mPAP at rest of 40 mmHg. People on PDE‐5 inhibitors or ERAs were excluded This was a separately recruited group to the AIR study. n = 63 |
|
| Interventions | Inhaled iloprost 4 μg, 6 to 9 times daily, compared to conventional treatment The mean inhaled daily dose of iloprost divided into 6 to 9 doses was 25 μg at month 1 and 29 μg at year 2 |
|
| Outcomes | Adverse events, mortality | |
| Notes | The trial included three months with one group randomised to epoprostenol and one group randomised to conventional treatment; following, all participants were treated with epoprostenol. Outcomes were reported at the end of the study (at two years) so this data could not be used in the meta‐analysis. Adverse events could be extracted Industry funded: "This study was sponsored by Schering AG, Berlin, Germany" ClinicalTrials.gov Identifier: NCT00414687 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "randomised" but no details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No suggestion that outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts reported |
| Selective reporting (reporting bias) | High risk | Only outcomes at 2 years were reported |
| Other bias | Low risk | Other bias unlikely |
Rubin 1990.
| Methods | Randomised, parallel‐group open‐label trial | |
| Participants | Primary pulmonary hypertension, no vasodilators (unresponsive or unable to tolerate) n = 19 |
|
| Interventions | Intravenous epoprostenol (flolan) compared to conventional therapy | |
| Outcomes | Cardiopulmonary haemodynamics, 6MWD, adverse events, mortality Outcomes measured at 8 weeks |
|
| Notes | Funding source not stated but it is noted several of the authors are employed by Burroughs Wellcome Company. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned after the dose‐ranging study by calling a central telephone number. At that time the next available sequentially numbered sealed envelope for that patient's status was opened, and the patient allocated to conventional therapy or prostacyclin |
| Allocation concealment (selection bias) | Low risk | Sequentially sealed envelopes used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | Data not interpretable (presented as mean baseline and mean post‐treatment scores, with the confidence intervals of the mean differences) |
| Other bias | Low risk | Other bias unlikely |
Simonneau 2002.
| Methods | Randomised, double‐blinded, parallel‐group placebo‐controlled trial | |
| Participants | Inclusion criteria
Exclusion criteria
n = 470 |
|
| Interventions | Continuous subcutaneous infusion of treprostinil plus conventional therapy versus continuous infusion of placebo (vehicle solution without treprostinil) plus conventional therapy. Conventional therapy could include oral vasodilators, oral anticoagulants, diuretics, and/or digitalis. Chronic study drug infusion was initiated at the dose of 1.25 ng/kg/min. During the 12‐week study, doses were increased to a maximum dose at which pulmonary hypertension signs and symptoms were improved while achieving an acceptable side effect profile. At week 12, the maximum allowable dose was 22.5 ng/kg/min. By the end of the 12‐week study period, the mean dose of the study drug received was 9.3 ng/kg/min versus 19.1 ng/kg/min in the placebo group (P < 0.001). |
|
| Outcomes | 6MWD, signs or symptoms of PAH, lung transplantation, mortality, Dyspnoea Fatigue Rating, cardiopulmonary haemodynamics measured by right heart catheterisation, global, physical, and emotional quality of life using the Minnesota Living with Heart Failure Questionnaire, adverse events Outcomes were assessed at week 6 and 12 |
|
| Notes | Industry funded: "This study was supported by United Therapeutics Corporation, Research Triangle Park, North Carolina" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a permuted block design stratified on the basis of baseline exercise capacity and etiology of PAH. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled. As this was an up‐titration study, doses for both groups were reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was reported that for the 6MWD test was administered by a “blinded” tester not involved in the participant’s daily care and unaware of the participant’s treatment assignment, however other outcomes were not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts and withdrawals were not reported |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
Simonneau 2012.
| Methods | Randomised, double‐blinded, parallel‐group placebo‐controlled trial | |
| Participants | Male or female adults (over 18 years) with symptomatic PAH of idiopathic or hereditary origin, associated with connective tissue diseases (PAH‐connective tissue disease), corrected congenital heart disease (congenital systemic‐to‐pulmonary shunts surgically repaired > 5 yrs previously), or anorexigen use. Background targeted treatment with ERAs and/or PDE‐5 inhibitors was mandatory and participants had to have been on stable doses for > 12 weeks before screening. Participants were required to have a baseline PVR of > 400 dyn.s/cm‐5, and two 6MWD tests of 150 metres to 500 metres inclusive and within 15% of each other. Participants were excluded if they had clinically unstable right heart failure within the last 3 months (WHO functional class IV), had received or were scheduled to receive long‐term epoprostenol within 3 months of screening, had a ventilation perfusion lung scan or pulmonary angiography indicative of thromboembolic disease, had evidence of left‐sided heart disease, or had received any investigational drug within 30 days of screening. n = 43 |
|
| Interventions | Selexipag 200 mg twice daily or matching placebo on day 1, then up‐titrated to 400 mg twice daily on day 3, to 600 mg twice daily on day 7, and to 800 mg twice daily on day 21 according to side effects. Final dosage was required to be stable for > 4 weeks prior to evaluation at week 17. Participants on selexipag received treatment for a mean of 143.3 (SD 28.6) days (median 149.0 days; range 17 to 176 days), compared with 135.1 (SD 27.4) days (median 146.0 days; range 61 to 152 days) for participants on placebo. Among selexipag‐treated participants, 14 (42.4%) were on a final dosage of 800 mg twice daily, seven (21.2%) were on 600 mg twice daily, six (18.2%) were on 400 mg twice daily and four (12.1%) were on 200 mg twice daily. |
|
| Outcomes | Haemodynamic parameters measured on right heart catheterisation, 6MWD, aggravation of PAH (defined as death, transplantation, hospitalisation due to worsening PAH, or aggravation of PAH symptoms, i.e. a > 10% deterioration in 6MWD or the need for additional PAH‐specific therapies), Borg dyspnoea score, WHO functional class, and NT‐proBNP concentration Outcomes were measured at week 17 |
|
| Notes | Industry funded: by Actelion Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule 3:1 (selexipag:placebo) was computer generated by Penn Pharmaceutical Services Ltd |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled. As this was an up‐titration study, doses and duration for both groups were reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "As the study was blinded, investigators assessed the relationship between adverse events and study treatment before the treatment code was broken. After week 17 data were fixed and locked, participants eligible to enter the open‐label extension were not blinded. For participants who discontinued prematurely or otherwise did not enter the open‐label extension, study treatment remained blinded until all week 17 data were cleaned and reconciled" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were reported |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
TRIUMPH.
| Methods | Randomised, blinded, parallel‐group placebo‐controlled trial | |
| Participants | Adults aged 18 and 75 years with a confirmed diagnosis of idiopathic or familial PAH or PAH associated with collagen vascular disease, HIV infection, or anorexigen use. Participants were NYHA functional class III or IV severity with a baseline 6MWD between 200 metres and 450 metres and were receiving bosentan 125 mg daily or any prescribed dose of sildenafil, 20 mg three times daily, for at least 3 months before study
entry. n=235 |
|
| Interventions | Inhaled treprostinil sodium or placebo 4 times daily in combination with bosentan or sildenafil. At the discretion of the study investigator, participants initiated therapy at 3 breaths (18 μg)/inhalation. If clinically tolerated, the dosing was to be increased over the first 2 weeks to reach a maximum of 9 breaths (54 μg) at each of the 4 daily doses. The mean dose of study drug was 50 +10μg in the inhaled treprostinil group and 52 +7μg in the inhaled placebo group. |
|
| Outcomes | NYHA functional classification, 6MWD, Borg dyspnoea score (immediately after 6MWD) Minnesota Living with Heart Failure Questionnaire, NT‐pro BNP, adverse events, time to clinical worsening (defined as death, transplantation, hospital stay due to worsening PAH, or initiation of additional approved PAH‐specific therapy) Outcomes reported at 12 weeks |
|
| Notes | Industry funded: "This research was funded by United Therapeutics Corporation" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Called a randomised trial, but methods not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled, as this was an up‐titration study, final doses in the intervention and placebo group were reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | Other bias unlikely |
TRUST.
| Methods | Randomised, blinded, parallel‐group placebo‐controlled trial | |
| Participants | Adults aged 16 to 75 years with idiopathic PAH (sporadic or familial) or PAH associated with HIV or collagen vascular disease. Other entry criteria included: mPAP > 35 mm Hg; pulmonary capillary wedge pressure < 16 mmHg; PVR 5 mm Hg/litre/min; stable NYHA Functional Class III or IV symptoms on adjunctive therapies (anticoagulants, diuretics, digoxin, oxygen, vasodilators (27%)); and screening 6MWD of 50 to 325 meters. n = 44 Enrollment was closed after 45 of the planned 126 patients were randomised because of safety considerations, mostly in the treatment arm |
|
| Interventions | Intravenous treprostinil compared to placebo Treprostinil (1 mg/mL) or placebo was provided in blind‐labelled, multiple entry vials for infusion after dilution in sterile saline via and ambulatory pump (CADD pump). The initial study drug dose was 4 ng/kg/min treprostinil or an equivalent volume of diluted placebo; the range of doses at week 1 was 8 to 14 ng/kg/min (actively treated) and 6 to 10 ng/kg/ min (placebo treated). After the first week, dose increases up to 8 ng/kg/min weekly (or placebo equivalent) were allowed to a maximum of 100 ng/kg/min. |
|
| Outcomes | 6MWD, Borg dyspnoea score, Dysponea Fatigue Rating, clinical worsening (death, lung transplant, hospitalisation, unblinding for rescue or too‐ill‐to‐walk), change in NYHA functional class, adverse events, cytokine growth factors | |
| Notes | Industry funded: "United Therapeutics Corp. Research Triangle Park, NC" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants had central venous catheters placed, and placebo matched infusions were given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specifically stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals were reported |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported |
| Other bias | Low risk | Other bias unlikely |
Abbreviations 6MWD: six‐minute walk distance; CT: computed tomography; ITT: intention‐to‐treat analysis; ERA: endothelin receptor antagonist; HIV: human immunodeficiency virus; mITT: modified intention‐to‐treat analysis; mPAP: mean pulmonary arterial pressure; n: number of participants; NCT: National Clinical Trials; NT‐proBNP: plasma N‐terminal pro‐brain natriuretic peptide; NYHA: New York Heart Association; PAH: pulmonary arterial hypertension; PVR: pulmonary vascular resistance; SD: standard deviation; WHO: World Health Organization; PDE‐5 inhibitor: phosphodiesterase‐5 inhibitor; SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bruderer 2014 | Wrong patient population (includes healthy participants), and does not meet minimum required trial duration |
| Herald 2006 | Wrong study design ‐ letter to the editor with no new data |
| Higenbottam 1996 | Wrong study design ‐ letter to the editor with no new data |
| Higenbottam 1998 | Wrong patient population |
| Jing 2013 | Wrong study design ‐ not clearly a randomised controlled trial |
| Klings 2000 | Wrong study design ‐ letter to the editor with no new data |
| Kumar 2013 | Wrong study design ‐ does not compare to a control/placebo group |
| Kunieda 2013 | Wrong study design ‐ not randomised |
| Leuchte 2003 | Wrong study design ‐ case report |
| Matthes 2001 | Wrong study design ‐ review |
| NCT00709098 2008 | Wrong study design ‐ does not compare to a placebo/other intervention group |
| NCT00709956 2008 | Wrong timeframe ‐ too short |
| NCT00760916 2008 | Study was withdrawn before participants were enrolled |
| NCT00993408 2009 | Single test dose study |
| NCT01094067 2010 | Single test dose study |
| NCT01105091 2010 | Wrong timeframe ‐ only 28 days |
| NCT01302444 2011 | Wrong intervention ‐ all participants were on prostacyclin and study compared tadalafil to no tadalafil |
| NCT01393795 2011 | Wrong intervention ‐ does not compare prostacyclin drug |
| NCT01557647 2012 | Study was withdrawn before participants were enrolled ‐ no reasons were given |
| NCT01598441 2012 | Wrong timeframe ‐ only two days duration |
| NCT02032836 2014 | Wrong intervention ‐ compared different delivery devices |
| NCT02482402 2014 | Study was withdrawn due to lack of recruitment |
| NCT02893995 2016 | Wrong study design ‐ compared different rates of the same drug |
| NCT02999906 2016 | Study was withdrawn before enrolment due to "business reasons" |
| Olschewski 1998 | Wrong study design ‐ not clearly a randomised controlled trial and trial duration of only four weeks |
| Pepke‐Zaba 2000 | Wrong intervention ‐ compares different forms of prostacyclin |
| Preston 2015 | Wrong intervention |
| Robbins 2000 | Wrong study design ‐ not a randomised controlled trial |
| Rubenfire 2007 | Wrong study design ‐ only 14 days duration |
| Rubin 2005 | Wrong study design ‐ case report |
| Saba 2001 | Wrong study design ‐ letter to the editor with no new data |
| Saggar 2013 | Wrong study design ‐ does not compare to an intervention/placebo arm, only conventional treatment |
| Shah 2010 | Wrong study design ‐ no long‐term follow‐up |
| Voswinckel 2006 | Wrong study design ‐ no long‐term follow‐up |
| Voswinckel 2006a | Wrong study design ‐ letter to the editor with no new data |
| Wade 2007 | Wrong study design ‐ not clearly a randomised controlled trial |
| White 2013 | Wrong study design ‐ not a randomised controlled trial |
| White 2013a | Wrong study design ‐ not a randomised controlled trial |
| Wilkens 2001 | Wrong study design ‐ not a randomised controlled trial |
| Wilkens 2001a | Wrong timeframe ‐ short‐term duration |
| Zamanian 2013 | Wrong study design ‐ observational study |
Characteristics of ongoing studies [ordered by study ID]
NCT 2013.
| Trial name or title | Beraprost‐314d Added‐on to Tyvaso® (BEAT) |
| Methods | Randomised controlled trial |
| Participants | Participants with pulmonary arterial hypertension |
| Interventions | BPS‐314d‐MR (beraprost sodium) when added‐on to inhaled treprostinil (Tyvaso®) compared to placebo |
| Outcomes | Primary outcome: time to clinical worsening Secondary outcomes: 6‐minute walk distance, Borg dyspnoea score, WHO functional class, NT‐pro‐BNP levels Outcome measures will be assessed at 12 and 144 weeks |
| Starting date | July 26, 2013 |
| Contact information | Lung Biotechnology PBC |
| Notes | clinicaltrials.gov/show/NCT01908699 |
NCT 2015.
| Trial name or title | The efficacy and safety of initial triple versus initial dual oral combination therapy in patients with newly diagnosed pulmonary arterial hypertension (TRITON) |
| Methods | Randomised controlled trial |
| Participants | Newly diagnosed pulmonary arterial hypertension |
| Interventions | Triple oral combination treatment (macitentan, tadalafil, and selexipag) compared to dual oral combination treatment (macitentan, tadalafil, and placebo) |
| Outcomes | Pulmonary vascular resistance (no other outcomes listed) at 26 weeks |
| Starting date | September 23, 2015 |
| Contact information | loic.perchenet@actelion.com |
| Notes | clinicaltrials.gov/show/NCT02558231 |
TRACE 2018.
| Trial name or title | TRACE 2018 |
| Methods | Multicentre, double‐blind, randomised, exploratory Phase 4 study |
| Participants | Eligible PAH patients are 18‐75 years, in World Health Organization functional class (WHO FC) II or III, with a 6‐minute walk distance (6MWD) >= 100 m, and on stable treatment with an endothelin receptor antagonist alone or in combination with a phosphodiesterase‐5 inhibitor or soluble guanylate cyclase stimulator |
| Interventions | Randomises (1:1) 100 patients to receive selexipag or placebo for 24 weeks |
| Outcomes | Primary outcomes: daily life physical activity (DLPA), measured by the wrist‐worn accelerometer ActiGraph GT9X Link, day and night during a 14 day baseline period and throughout the 24‐week treatment period. Daily data upload on a provided smartphone allows wear‐time monitoring. The PAH‐SYMPACT questionnaire is the first PAH‐specific PRO instrument developed and validated according to the FDA guidance The primary endpoint is change from baseline to week 24 in actigraphy‐assessed DLPA, as measured by: daily time spent in non‐sedentary activity, total DLPA per day, total sleep time, wake time after sleep onset, number of awakenings and sleep efficiency. Secondary endpoints include change in score from baseline in cardiovascular symptoms, cardiopulmonary symptoms, physical impact and cognitive/emotional impact domains of PAH‐SYMPACT, change in WHO FC, 6MWD, Borg dyspnoea score and NT‐proBNP. Safety and tolerability of selexipag are monitored. |
| Starting date | Not stated |
| Contact information | I Preston, Tufts Medical Centre, Boston, MA, USA |
| Notes | adisinsight.springer.com/trials/700282735 |
Differences between protocol and review
We did not explicitly state we would present dichotomous data with zero totals as risk difference (RD).
In the protocol, we intended to assess changes in New York Heart Association (NYHA) functional class, however, the nomenclature for pulmonary arterial hypertension (PAH) patients is now World Health Organization (WHO) functional class. It should be noted however, that the NHYA and WHO classification system both classify patients in the same way.
Although we did not specify that we would include adverse events in the 'Summary of findings' table in the protocol, we added them to the review as they are clinically relevant and to comply with Cochrane standards.
We initially planned to only include single‐ or double‐blinded trials, however given the importance of unblinded trials, we included these in the final meta‐analysis and described similarities and differences between them.
We initially stated in our Objectives that we would assess the efficacy of prostacyclin, however as it was noted in peer review, our a priori outcomes also included safety, therefore we amended our objectives to include assessment of efficacy and safety.
We added 'clinical worsening' as an outcome.
Following peer review, we performed a post hoc sensitivity analysis on all relevant outcomes, whereby we excluded the TRUST trial due to its premature termination following safety concerns.
Contributions of authors
HB and TF drafted the protocol, and AB and TW provided comments and changes. HB and HLY screened abstracts and TW and AB provided a third opinion where required. HB and HLY extracted data. HB drafted the review and HLY, AB, MH, and TW provided revisions and comments.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor, Contact Editor): edited the review; advised on methodology, interpretation and content; approved the final review prior to publication. Chris Cates (Co‐ordinating Editor) checked the data entry prior to the full write up of the review. Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review. Emma Jackson (Assistant Managing Editor): conducted peer review, and edited the Plain Language Summary and reference sections of the protocol and the review. Elizabeth Stovold (Information Specialist): designed the search strategy, ran the searches and edited the search methods section.
Sources of support
Internal sources
The authors declare that no such funding was received for this systematic review, Other.
External sources
The authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
HB: has previously been awarded a travel scholarship that was determined by an independent abstract committee, funded by GSK. GSK had no involvement in the determination of recipients. The Cochrane funding arbiter was consulted and agreed it did not represent a conflict of interest as GSK had no role in the allocation of the travel scholarship.
HLY: none known
TF: none known
AB: none known
MH: has relationships with drug companies in the field of pulmonary hypertension including Actelion, Arena, Bayer, Eiger, GSK, and United Therapeutics. In addition to being investigator in trials involving these companies, relationships include consultancy service and membership of scientific advisory boards. MH has relationships with drug companies in the field of severe asthma outside the submitted work. These companies are Astrazeneca, GSK, MSD, Novartis, Roche/Genentech, Sanofi/Regereron, and Teva. In addition to being investigator in trials involving these companies, relationships include consultancy service and membership of scientific advisory boards.
TW: is a paid member of scientific advisory boards for Actelion Australia, GSK Australia and Bayer Australia. He has received travel support, consultancy payments and research/education grants from Actelion Australia.
New
References
References to studies included in this review
AIR {published data only}
- Ghofrani A. Positive outcome of the Aerosolized Iloprost Randomized study in primary and non‐primary pulmonary hypertension (AIR study). Journal fur Anasthesie und Intensivbehandlung 2002;9(2):41. [Google Scholar]
- Olschewski H, Galie N, Higenbottam T, Naeije R, Rubin LJ, Simonneau G, et al. Positive outcome of the aerosolized iloprost randomised trial for treatment of severe pulmonary hypertension (AIR study). American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A411. [Google Scholar]
- Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, et al. Inhaled iloprost for severe pulmonary hypertension. New England Journal of Medicine 2002;347(5):322‐9. [DOI] [PubMed] [Google Scholar]
ALPHABET {published data only}
- Galie N, Humbert M, Vachiery JL, Vizza CD, Kneussl M, Manes A, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double‐blind, placebo‐controlled trial. Journal of the American College of Cardiology 2002;39(9):1496‐502. [DOI] [PubMed] [Google Scholar]
- Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Effects of beraprost sodium, an oral prostacyclin analogue in patients with pulmonary arterial hypertension: a randomised, double‐blind, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;165(6):800‐4. [DOI] [PubMed] [Google Scholar]
Badesch 2000 {published data only}
- Badesch DB, Tapson VF, McGoon MD, Brundage BH, Rubin LJ, Wigley FM, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Annals of Internal Medicine 2000;132(6):425‐34. [DOI] [PubMed] [Google Scholar]
Barst 1996 {published data only}
- Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. New England Journal of Medicine 1996; Vol. 334:296‐301. [DOI] [PubMed]
- Hinderliter AL, Willis PW 4th, Barst RJ, Rich S, Rubin LJ, Badesch DB. Effects of long‐term infusion of prostacyclin (epoprostenol) on echocardiographic measures of right ventricular structure and function in primary pulmonary hypertension. Primary Pulmonary Hypertension Study Group. Circulation 1997;95(6):1479‐86. [DOI] [PubMed] [Google Scholar]
Barst 2003 {published data only}
- Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, et al. Beraprost Study Group. Beraprost therapy for pulmonary arterial hypertension. Journal of the American College of Cardiology 2003;41(12):2119‐25. [DOI] [PubMed] [Google Scholar]
FREEDOM‐C {published data only}
- Ruggiero RM, Bartolome S, Chin KM, Torres F. Combination therapy for pulmonary arterial hypertension with oral treprostinil: long‐term follow up [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183:A5906. [Google Scholar]
- Tapson VF, Torres F, Kermeen F, Keogh AM, Allen RP, Frantz RP, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM‐C study): a randomized controlled trial. Chest 2012;142:1383‐90. [DOI] [PubMed] [Google Scholar]
FREEDOM‐C2 {published data only}
- Tapson V, Jing ZC, Xu K, Pan L, Feldman J, Kiely D, et al. Freedom‐C2: efficacy and safety of oral treprostinil diethanolamine in combination with an era and/or a PDE5 inhibitor in patients with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2012; Vol. 185:A2493.
- Tapson VF, Jing ZC, Xu KF, Pan L, Feldman J, Kiely DG, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM‐C2 study): a randomized controlled trial. Chest 2013;144:952‐8. [DOI] [PubMed] [Google Scholar]
FREEDOM‐M {published data only}
- Jing ZC, Parikh K, Pulido T, Jerjes‐Sanchez C, White RJ, Allen R, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013;127:624‐33. [DOI] [PubMed] [Google Scholar]
- Rubin L, Parikh K, Pulido T, Jerjes‐Sanchez C, Allen R, White J, et al. FREEDOM‐M: efficacy and safety of oral treprostinil diethanolamine as monotherapy in patients with pulmonary arterial hypertension [Abstract]. Chest 2011;140(4):1044A. [Google Scholar]
GRIPHON {published data only}
- Channick R, Chin K, Scala L, Frey A, Preiss R, Gaine S, et al. Individualized dosing of selexipag based on tolerability in the GRIPHON study shows consistent efficacy and safety in patients with pulmonary arterial hypertension (PAH). Chest 2015;184:961A. [Google Scholar]
- Galie N, Channick R, Chin K, Frey A, Gaine S, Ghofrani A, et al. Effect of selexipag on morbidity/mortality in pulmonary arterial hypertension: Results of the griphon study. Journal of Heart and Lung Transplantation 2015;34:S163. [Google Scholar]
- Lang I, Gaine S, Galie N, Ghofrani HA, Brun FO, McLaughlin V, et al. Effect of selexipag on long‐term outcomes in patients with pulmonary arterial hypertension (PAH) receiving one, two or no PAH therapies at baseline: Results from the GRIPHON study. European Heart Journal 2015;36:381‐2. [Google Scholar]
- McLaughlin VV, Channick R, Chin K, Frey A, Gaine S, Ghofrani A, et al. Effect of selexipag on morbidity/mortality in pulmonary arterial hypertension: Results of the GRIPHON study. Journal of the American College of Cardiology 2015;65:A1538. [Google Scholar]
- Sitbon O, Channick R, Chin K, Frey A, Galie N, Ghofrani HA, et al. Effect of selexipag on longterm outcomes in key subgroups of patients with pulmonary arterial hypertension (PAH): GRIPHON study results. European Respiratory Journal 2015;46(Suppl 59):OA4995. [Google Scholar]
- Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galie N, et al. Selexipag for the treatment of pulmonary arterial hypertension. New England Journal of Medicine 2015;373(26):2522‐33. [DOI] [PubMed] [Google Scholar]
Han 2017 {published data only}
- Han X, Zhang Y, Dong L, Fang L, Chai Y, Niu M, et al. Treatment of pulmonary arterial hypertension using initial combination therapy of bosentan and iloprost. Respiratory Care 2017;62(4):489‐96. [DOI] [PubMed] [Google Scholar]
McLaughlin 2006 {published data only}
- McLaughlin VV, Oudiz R, Frost A, Tapson V, Murali S, Channick R, et al. A randomised, double‐blind, placebo‐controlled study of iloprost inhalation as add‐on therapy to bosentan in pulmonary arterial hypertensions (PAH). Chest 2005;128(4):160S. [Google Scholar]
- McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2006;174:1257‐63. [DOI] [PubMed] [Google Scholar]
Olschewski 2010 {published data only}
- Olschewski H, Hoeper MM, Behr J, Ewert R, Meyer A, Borst MM, et al. Long‐term therapy with inhaled iloprost in patients with pulmonary hypertension. Respiratory Medicine 2010;104(5):731‐40. [DOI] [PubMed] [Google Scholar]
Rubin 1990 {published data only}
- Rubin LJ, Mendoza J, Hood M, McGoon M, Barst R, Williams WB, et al. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Annals of Internal Medicine 1990;112(7):485‐91. [DOI] [PubMed] [Google Scholar]
Simonneau 2002 {published data only}
- Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double‐blind, randomized, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2002;165(6):800‐4. [DOI] [PubMed] [Google Scholar]
Simonneau 2012 {published data only}
- Simonneau G, Lang I, Torbicki A, Hoeper MM, Delcroix M, Karlocai K, et al. Efficacy, safety and tolerability of ACT‐293987, a novel oral, non‐prostanoid, prostaglandin I2 (IP) receptor agonist: results from a phase IIa study in pulmonary arterial hypertension (PAH). American Journal of Respiratory and Critical Care Medicine 2010;181:A2515. [Google Scholar]
- Simonneau G, Torbicki A, Hoeper MM, Delcroix M, Karlocai K, Galie N, et al. Selexipag: an oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. European Respiratory Journal 2012;40(4):874‐80. [DOI] [PubMed] [Google Scholar]
- Torbicki A, Lang I, Hoeper M, Delcroix M, Karlocai K, Galie N, et al. A new drug class for Pulmonary Arterial Hypertension (PAH): results from a phase II study of ACT 293987, an oral IP receptor agonist. European Society of Cardiology Congress; 2010 28 Aug ‐ 1 Sep; Stockholm. 2010:22.
TRIUMPH {published data only}
- McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. Journal of the American College of Cardiology 2010;4(55):1915‐22. [DOI] [PubMed] [Google Scholar]
TRUST {published data only}
- Hiremath J, Thanikachalam S, Parikh K, Shanmugasundaram S, Bangera S, Shapiro L, et al. Exercise improvement and plasma biomarker changes with intravenous treprostinil therapy for pulmonary arterial hypertension: a placebo‐controlled trial. Journal of Heart and Lung Transplantation 2010;29:137‐49. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bruderer 2014 {published data only}
- Bruderer S, Hurst N, Kaufmann P, Dingemanse J. Multiple‐dose up‐titration study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of selexipag, an orally available selective prostacyclin receptor agonist, in healthy subjects. Pharmacology 2014;94(3‐4):148‐56. [DOI] [PubMed] [Google Scholar]
Herald 2006 {published data only}
- Herald N, Laliberte K. Treprostinil for the treatment of pulmonary arterial hypertension in patients with New York Heart Association class II‐IV symptoms. Pharmacotherapy 2006;26(8):1203‐4. [DOI] [PubMed] [Google Scholar]
Higenbottam 1996 {published data only}
- Higenbottam T. Intravenous epoprostenol for primary pulmonary hypertension. New England Journal of Medicine 1996;334(22):1477‐8. [DOI] [PubMed] [Google Scholar]
Higenbottam 1998 {published data only}
- Higenbottam TW, Butt AY, Dinh‐Xaun AT, Takao M, Cremona G, Akamine S. Treatment of pulmonary hypertension with the continuous infusion of a prostacyclin analogue, iloprost. Heart 1998;79(2):175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jing 2013 {published data only}
- Jing ZC, Zhao QH, Jiang X, Peng FH, Wu WH, He J, et al. Addition of beraprost to existing sildenafil therapy in patients with pulmonary arterial hypertension: a randomized, open‐label study (Best study). American Journal of Respiratory and Critical Care Medicine 2013;187:A6066. [Google Scholar]
Klings 2000 {published data only}
- Klings ES, Farber HW. Epoprostenol for pulmonary hypertension in scleroderma. Annals of Internal Medicine 2000;133(2):158. [DOI] [PubMed] [Google Scholar]
Kumar 2013 {published data only}
- Kumar P, Arneson C, Laliberte K, Nelsen AC. Dose‐response relationship of oral treprostinil diolamine (TRE) in patients with pulmonary arterial hypertension (PAH). American Thoracic Society International Conference; 2013 May 17‐22; Philadelphia. 2013; Vol. 187:A3271.
Kunieda 2013 {published data only}
- Kunieda T. Clinical trial of long‐acting beraprost sodium in Japanese patients with pulmonary arterial hypertension. Therapeutic Research 2013;34(9):1203‐6. [Google Scholar]
Leuchte 2003 {published data only}
- Leuchte HH, Baumgartner RA, Behr J. Treatment of severe pulmonary hypertension with inhaled iloprost. Annals of Internal Medicine 2003;139(4):306. [DOI] [PubMed] [Google Scholar]
Matthes 2001 {published data only}
- Matthes J, Mathen F, Herzig S, Wassermann K. Therapy of severe pulmonary hypertension with prostacyclin and iloprost. Deutsche Medizinische Wochenschrift (1946) 2001;126(21):631‐7. [DOI] [PubMed] [Google Scholar]
NCT00709098 2008 {unpublished data only}
- NCT00709098. Safety study extension of iloprost power 15 in pulmonary arterial hypertension (PROWESS 15 Ext). clinicaltrials.gov/ct2/show/NCT00709098 (first received 3 July 2008).
NCT00709956 2008 {unpublished data only}
- NCT00709956. Iloprost power 15 in pulmonary arterial hypertension (PROWESS 15). clinicaltrials.gov/ct2/show/NCT00709956 (first received 3 July 2008).
NCT00760916 2008 {unpublished data only}
- NCT00760916. FREEDOM DR: oral treprostinil in combination with an endothelin receptor antagonist (ERA) and/or a phosphodiesterase‐5 (PDE‐5) inhibitor or as monotherapy for the treatment of pulmonary arterial hypertension (PAH). clinicaltrials.gov/ct2/show/NCT00760916 (first received 26 September 2008).
NCT00993408 2009 {unpublished data only}
- NCT00993408. Study of ACT‐293987 (NS‐304) in pulmonary arterial hypertension (PAH). clinicaltrials.gov/ct2/show/NCT00993408 (first received 12 October 2009).
NCT01094067 2010 {unpublished data only}
- NCT01094067. Tezosentan in patients with pulmonary arterial hypertension. clinicaltrials.gov/ct2/show/NCT01094067 (first received 26 March 2010).
NCT01105091 2010 {unpublished data only}
- NCT01105091. Epoprostenol for injection in pulmonary arterial hypertension (EPITOME‐1). clinicaltrials.gov/ct2/show/NCT01105091 (first received 16 April 2010).
NCT01302444 2011 {unpublished data only}
- NCT01302444. Treprostinil combined with tadalafil for pulmonary hypertension (T2). clinicaltrials.gov/ct2/show/NCT01302444 (first received 24 February 2011).
NCT01393795 2011 {unpublished data only}
- NCT01393795. Qutenza®‐Remodulin® in pulmonary arterial hypertension patients. clinicaltrials.gov/ct2/show/NCT01393795 (first received 13 July 2011).
NCT01557647 2012 {unpublished data only}
- NCT01557647. Safety and efficacy of inhaled treprostinil in patients with PAH. clinicaltrials.gov/ct2/show/NCT01557647 (first received 19 March 2012).
NCT01598441 2012 {unpublished data only}
- NCT01598441. Multicenter study of iloprost inhaled in pulmonary hypertension after repair of congenital heart diseases (CHD). clinicaltrials.gov/ct2/show/NCT01598441 (first received 15 May 2012).
NCT02032836 2014 {unpublished data only}
- NCT02032836. Comparative PK PD Study in PAH patients (Fox vs. I‐Neb). clinicaltrials.gov/ct2/show/NCT02032836 (first received 10 January 2014).
NCT02482402 2014 {unpublished data only}
- NCT02482402. Iloprost for bridging to heart transplantation in PH (BRIDGE). clinicaltrials.gov/ct2/show/NCT02482402 (first received 26 June 2015).
NCT02893995 2016 {unpublished data only}
- NCT02893995. Safety, tolerability, pharmacokinetics and efficacy of two different rates of subcutaneous remodulin® dose titration in pulmonary arterial hypertension. clinicaltrials.gov/ct2/show/NCT02893995 (first received 9 September 2016).
NCT02999906 2016 {unpublished data only}
- NCT02999906. Study to compare triple therapy (oral treprostinil, ambrisentan, and tadalafil) with dual therapy (ambrisentan, tadalafil, and placebo) in subjects with pulmonary arterial hypertension. clinicaltrials.gov/ct2/show/NCT02999906 (first received 21 December 2016).
Olschewski 1998 {published data only}
- Olschewski H, Walmrath D, Schermuly R, Ghofrani HA, Grimminger F, Seeger W. Prostacyclin and iloprost in aerosol form in severe pulmonary hypertension. Pneumologie 1998;52(1):3‐7. [PubMed] [Google Scholar]
Pepke‐Zaba 2000 {published data only}
- Pepke‐Zaba J, Parameshwar J, Eiskjaer H, Sharples L, Mainwood A, McNeil K. A comparison of three month's treatment with nebulised iloprost (Ilo) and continuous intravenous prostacyclin (PG1²) infusion for severe pulmonary hypertension (PH). European Respiratory Journal 2000;16(Suppl 31):395s. [Google Scholar]
- Pepke‐Zaba J, Parameshwar J, Eiskjaer H, Sharples L, Mainwood A, McNeil K. A comparison of three month's treatment with nebulised iloprost (Ilo) and continuous intravenous prostacyclin (PGI2) infusion for severe pulmonary hypertension (PH). American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A214. [Google Scholar]
Preston 2015 {published data only}
- Preston I, Hill N, Ghofrani HA, Hoeper M, Langleben D, Vizza CD, et al. Riociguat in combination with prostacyclin analogs for the treatment of pulmonary arterial hypertension (PAH): a subgroup analysis of the PATENT studies. Chest 2015;148:4. [Google Scholar]
Robbins 2000 {published data only}
- Robbins I, Gaine S, Schilz R, Tapson V, Rubin L, Loyd J. Epoprostenol for treatment of pulmonary hypertension in patients with systemic lupus erythematosus. Chest 2000;117(1):14‐8. [DOI] [PubMed] [Google Scholar]
Rubenfire 2007 {published data only}
- Rubenfire M, McLaughlin VV, Allen RP, Elliott G, Park MH, Wade M, et al. Transition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trial. [Erratum appears in Chest. 2007 Nov;132(5): 1721]. Chest 2007;132(3):757‐63. [DOI] [PubMed] [Google Scholar]
Rubin 2005 {published data only}
- Rubin L. Epoprostenol and nesiritide in pulmonary hypertension. Chest 2005;127(5):1870. [DOI] [PubMed] [Google Scholar]
Saba 2001 {published data only}
- Saba T, Peacock AJ. Long‐term treatment of pulmonary hypertension with aerosolized iloprost. European Respiratory Journal 2001;18(1):247. [DOI] [PubMed] [Google Scholar]
Saggar 2013 {published data only}
- Saggar R, White RJ, Rischard F, Mathier M, Elwing J, Allen RP, et al. Effect of oral treprostinil on hemodynamics during exercise in patients with pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine 2013;187:A3281. [Google Scholar]
Shah 2010 {published data only}
- Shah AM, Siddiqui F, Preston IR, Brennan J, Roberts K, Howard W, et al. Comparison of acute hemodynamic effects of inhaled nitric oxide (iNo) and inhaled epoprostenol (iEPO) in patients with pulmonary arterial hypertension (PAH) [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181:A3382. [Google Scholar]
Voswinckel 2006 {published data only}
- Voswinckel R, Enke B, Reichenberger F, Kohstall M, Kreckel A, Krick S, et al. Favorable effects of inhaled treprostinil in severe pulmonary hypertension: results from randomized controlled pilot studies. Journal of the American College of Cardiology 2006;48(8):1672‐81. [DOI] [PubMed] [Google Scholar]
Voswinckel 2006a {published data only}
- Voswinckel R, Ghofrani HA, Grimminger F, Seeger W, Olschewski H. Inhaled treprostinil [corrected] for treatment of chronic pulmonary arterial hypertension. [Erratum appears in Ann Intern Med. 2006 Jun 6;144(11):863]. Annals of Internal Medicine 2006;144(2):149‐50. [DOI] [PubMed] [Google Scholar]
Wade 2007 {published data only}
- Wade M. Intravenous treprostinil in pulmonary arterial hypertension: a controlled trial in India. Chest 2007;132(4):635b‐6. [DOI] [PubMed] [Google Scholar]
White 2013 {published data only}
- White RJ, Torres F, Allen R, Jerjes C, Pulido T, Yehle D, et al. Pharmacokinetics of oral treprostinil sustained release tablets during chronic administration to patients with pulmonary arterial hypertension. Journal of Cardiovascular Pharmacology 2013;61(6):474‐81. [DOI] [PubMed] [Google Scholar]
White 2013a {published data only}
- White RJ, Jing Z‐C, Parikh K, Bartolome S, Allen RP, Xu K‐F, et al. An open‐label extension trial of oral treprostinil in subjects with pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2013;187:A3270. [Google Scholar]
Wilkens 2001 {published data only}
- Wilkens H, Groeschel‐Guth A, Koening J, Forestier N, Boehm M, Sybrecht GW. Inhaled iloprost plus oral sildenafil in patients with pulmonary hypertension. European Respiratory Journal 2001;18(Suppl 33):324s. [DOI] [PubMed] [Google Scholar]
Wilkens 2001a {published data only}
- Wilkens H, Guth A, Konig J, Forestier N, Cremers B, Hennen B, et al. Effect of inhaled Iloprost plus oral sildenafil in patients with primary pulmonary hypertension. Circulation 2001;104(11):1218‐22. [DOI] [PubMed] [Google Scholar]
Zamanian 2013 {published data only}
- Zamanian RT, Scott V, Marlin C, Gomberg‐Maitland M, Preston IR, Cuttica MJ. CONFRONT. American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A3298. [Google Scholar]
References to ongoing studies
NCT 2013 {unpublished data only}
- NCT01908699. Beraprost‐314d added‐on to Tyvaso® (BEAT). clinicaltrials.gov/ct2/show/NCT01908699 (first received 26 July 2013).
NCT 2015 {published data only}
- NCT02558231. The efficacy and safety of initial triple versus initial dual oral combination therapy in patients with newly diagnosed pulmonary arterial hypertension. clinicaltrials.gov/ct2/show/NCT02558231 (first received 23 September 2015).
TRACE 2018 {published data only}
- Preston I, Frantz R, Hemnes A, Rosenkranz S, Skaara H, Pfister T, et al. Rationale and design of trace: a double‐blind, placebo‐controlled phase 4 study in pulmonary arterial hypertension to assess the effect of selexipag on physical activity, patient‐reported symptoms and their impact, in daily life. American Journal of Respiratory and Critical Care Medicine 2018;197:A5680. [Google Scholar]
Additional references
Badesch 2009
- Badesch DB. Diagnosis and assessment of pulmonary arterial hypertension. Journal of the American College of Cardiology 2009;54(Suppl 1):S55‐66. [DOI] [PubMed] [Google Scholar]
Cenedese 2006
- Cenedese E, Speich R, Dorschner L, Ulrich S, Maggiorini M, Jenni R, et al. Measurement of quality of life in pulmonary hypertension and its significance. European Respiratory Journal 2006;28(4):808‐15. [DOI] [PubMed] [Google Scholar]
Christman 1992
- Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. New England Journal of Medicine 1992;327(2):70‐5. [DOI] [PubMed] [Google Scholar]
D’Alonzo 1991
- D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Annals of Internal Medicine 1991;115:343–9. [DOI] [PubMed] [Google Scholar]
Enderby 2014
- Enderby CY, Soukup M, Al Omari M, Zeiger T, Burger C. Transition from intravenous or subcutaneous prostacyclin therapy to inhaled treprostinil inpatients with pulmonary arterial hypertension: a retrospective case series. Journal of Clinical Pharmacy and Therapeutics 2014;39(5):496‐500. [DOI] [PubMed] [Google Scholar]
Galiè 2016
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Revista Espanola de Cardiologia 2016;69(2):177. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 29 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 1989
- Guyatt GH, Nogradi S, Halcrow S, et al. Development and testing of a new measure of health status for clinical trials in heart failure. Journal of General Internal Medicine 1989;4:101‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Humbert 2006
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. American Journal of Respiratory and Critical Care Medicine 2006;173(9):1023‐30. [DOI] [PubMed] [Google Scholar]
Humbert 2015
- Humbert M, Ghofrani H. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 2015;71(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kallen 2008
- Kallen AJ, Lederman E, Balaji A, Trevino I, Petersen EE, Shoulson R, et al. Bloodstream infections in patients given treatment with intravenous prostanoids. Infection Control and Hospital Epidemiology 2008;29(4):342–9. [DOI] [PubMed] [Google Scholar]
Khair 2016
- Khair RM, Nwaneri C, Damico RL, Kolb T, Hassoun PM, Mathai SC. The minimal important difference in Borg dyspnea score in pulmonary arterial hypertension. Annals of the American Thoracic Society 2016;13(6):842‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ling 2012
- Ling Y, Johnson MK, Kiely DG. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. American Journal of Respiratory and Critical Care Medicine 2012;186(8):790‐6. [DOI] [PubMed] [Google Scholar]
McKenna 2006
- McKenna S, Doughty N, Meads DM, Doward LC, Pepke‐Zaba J. The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR): a measure of health‐related quality of life and quality of life for patients with pulmonary hypertension. Quality of Life Research 2006;15:13‐15. [DOI] [PubMed] [Google Scholar]
McLaughlin 2002
- McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation 2002;106:1477–82. [DOI] [PubMed] [Google Scholar]
Melian 2002
- Melian EB, Goa KL. Beraprost: a review of its pharmacology and therapeutic efficacy in the treatment of peripheral arterial disease and pulmonary arterial hypertension. Drugs 2002;62(1):107‐33. [DOI] [PubMed] [Google Scholar]
Mitchell 2014
- Mitchell JA, Ahmetaj‐Shala B, Kirkby N, Wright W, Mackenzie L, Reed D, et al. Role of prostacyclin in pulmonary hypertension. Global Cardiology Science and Practice 2014;2014(4):382‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel 2017
- Noel ZR, Kido K, Macaulay TE. Selexipag for the treatment of pulmonary arterial hypertension. American Journal of Health‐System Pharmacy 2017;74(15):1135‐41. [DOI] [PubMed] [Google Scholar]
Peacock 2007
- Peacock AJ, Murphy NF, McMurray JJ. An epidemiological study of pulmonary arterial hypertension. European Respiratory Journal 2007;30(1):104‐9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Simonneau 2013
- Simonneau G. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25):D34‐41. [DOI] [PubMed] [Google Scholar]
Sitbon 2002
- Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Hervé P, et al. Long‐term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. Journal of the American College of Cardiology 2002;40:780–8. [DOI] [PubMed] [Google Scholar]
Sitbon 2012
- Sitbon O, Delcroix M, Bergot E, Boonstra A, Escribano, Subias P, et al. EPITOME‐2, An open‐label study evaluating a new formulation of epoprostenol sodium in pulmonary arterial hypertension patients switched from Flolan. American Journal of Respiratory and Critical Care Medicine 2012;185:A2500. [Google Scholar]
Sitbon 2016
- Sitbon O, Vonk Noordegraaf A. Epoprostenol and pulmonary arterial hypertension: 20 years of clinical experience. European Respiratory Review 2017;26:160055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tapson 2006
- Tapson VF, McLaughlin VV, Gomberg‐Maitland M, Widlitz AC, Krichman AD, Laliberte KJ, et al. Delivery of intravenous treprostinil at low infusion rates using a miniaturized infusion pump in patients with pulmonary arterial hypertension. Journal of Vascular Access 2006;7(3):112‐7. [DOI] [PubMed] [Google Scholar]
Thenappan 2007
- Thenappan T, Shah SJ, Rich S, Gomberg‐Maitland M. A USA‐based registry for pulmonary arterial hypertension: 1982–2006. European Respiratory Journal 2007;30(6):1103–10. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Paramothayan 2005
- Paramothayan NS, Lasserson TJ, Wells A, Walters EH. Prostacyclin for pulmonary hypertension in adults. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002994.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


