Abstract
Background
Insomnia is a major public health issue affecting between 6% to 10% of the adult population in Western countries. Eszopiclone is a hypnotic drug belonging to a newer group of hypnotic agents, known as new generation hypnotics, which was marketed as being just as effective as benzodiazepines for this condition, while being safer and having a lower risk for abuse and dependence. It is the aim of the review to integrate evidence from randomised controlled trials and to draw conclusions on eszopiclone's efficacy and safety profile, while taking methodological features and bias risks into consideration.
Objectives
To assess the efficacy and safety of eszopiclone for the treatment of insomnia compared to placebo or active control.
Search methods
We searched the Cochrane Central Register of Controlled trials (CENTRAL), MEDLINE, Embase, PsycINFO, PSYNDEX and registry databases (WHO trials portal, ClinicalTrials.gov) with results incorporated from searches to 10 February 2016. To identify trials not registered in electronic databases, we contacted key informants and searched reference lists of identified studies. We ran an update search (21 February 2018) and have placed studies of interest in awaiting classification/ongoing studies. These will be incorporated into the next version of the review, as appropriate.
Selection criteria
Parallel group randomised controlled trials (RCTs) comparing eszopiclone with either placebo or active control were included in the review. Participants were adults with insomnia, as diagnosed with a standardised diagnostic system, including primary insomnia and comorbid insomnia.
Data collection and analysis
Two authors independently extracted outcome data; one reviewer assessed trial quality and the second author cross‐checked it.
Main results
A total of 14 RCTs, with 4732 participants, were included in this review covering short‐term (≤ 4 weeks; 6 studies), medium‐term (> 4 weeks ≤ 6 months; 6 studies) and long‐term treatment (> 6 months; 2 studies) with eszopiclone. Most RCTs included in the review included participants aged between 18 and 64 years, three RCTs only included elderly participants (64 to 85 years) and one RCT included participants with a broader age range (35 to 85 years). Seven studies considered primary insomnia; the remaining studies considered secondary insomnia comorbid with depression (2), generalised anxiety (1), back pain (1), Parkinson's disease (1), rheumatoid arthritis (1) and menopausal transition (1).
Meta‐analytic integrations of participant‐reported data on sleep efficacy outcomes demonstrated better results for eszopiclone compared to placebo: a 12‐minute decrease of sleep onset latency (mean difference (MD) ‐11.94 min, 95% confidence interval (CI) ‐16.03 to ‐7.86; 9 studies, 2890 participants, moderate quality evidence), a 17‐minute decrease of wake time after sleep onset (MD ‐17.02 min, 95% CI ‐24.89 to ‐9.15; 8 studies, 2295 participants, moderate quality evidence) and a 28‐minute increase of total sleep time (MD 27.70 min, 95% CI 20.30 to 35.09; 10 studies, 2965 participants, moderate quality evidence). There were no significant changes from baseline to the first three nights after drug discontinuation for sleep onset latency (MD 17.00 min, 95% CI ‐4.29 to 38.29; 1 study, 291 participants, low quality evidence) and wake time after sleep onset (MD ‐6.71 min, 95% CI ‐21.25 to 7.83; 1 study, 291 participants, low quality evidence). Adverse events during treatment that were documented more frequently under eszopiclone compared to placebo included unpleasant taste (risk difference (RD) 0.18, 95% CI 0.14 to 0.21; 9 studies, 3787 participants), dry mouth (RD 0.04, 95% CI 0.02 to 0.06; 6 studies, 2802 participants), somnolence (RD 0.04, 95% CI 0.02 to 0.06; 8 studies, 3532 participants) and dizziness (RD 0.03, 95% CI 0.01 to 0.05; 7 studies, 2933 participants). According to the GRADE criteria, evidence was rated as being of moderate quality for sleep efficacy outcomes and adverse events and of low quality for rebound effects and next‐day functioning.
Authors' conclusions
Eszopiclone appears to be an efficient drug with moderate effects on sleep onset and maintenance. There was no or little evidence of harm if taken as recommended. However, as certain patient subgroups were underrepresented in RCTs included in the review, findings might not have displayed the entire spectrum of possible adverse events. Further, increased caution is required in elderly individuals with cognitive and motor impairments and individuals who are at increased risk of using eszopiclone in a non‐recommended way.
Plain language summary
Eszopiclone (Lunesta) for sleep difficulty
Why is this review important?
Insomnia is the medical term for sleep difficulty covering trouble falling asleep, difficulties staying asleep, waking up too early or experiencing sleep as non‐restorative. Insomnia can be treated with different methods including behaviour modification, relaxation techniques, or sleeping medication. Eszopiclone (Lunesta) is a sleeping medication that belongs to a class of sleeping tablets known as non‐benzodiazepine hypnotics.
Who will be interested in this review?
People who are affected by insomnia, general practitioners, professionals working in health services, and addiction treatment and health policy makers.
What questions does this review aim to answer?
The review aimed to find out more about the wanted effects and unwanted effects of eszopiclone. Wanted effects included the immediate effects eszopiclone has on sleep; unwanted effects included side effects, effects on next‐day functioning, but also addictive properties of the drug.
Which studies were included in the review?
The review summarised findings from 14 clinical studies with 4732 people, either receiving eszopiclone or an identically‐appearing, but inert substance (placebo).
What does the evidence from the review tell us?
On average, people taking eszopiclone fell asleep 12 minutes faster than those taking placebo, were 17 minutes less awake during the night and had, in total, about half an hour more sleep than people in the placebo group. As side effects, eszopiclone can cause unpleasant taste, dizziness, dry mouth, and tiredness during the day. Clinical studies did not find evidence that eszopiclone was causing serious harm or withdrawal symptoms or whether it was addictive if it was stopped and not taken after several weeks or months of treatment. Nevertheless, as clinical studies included in the review did not cover certain groups (e.g. elderly people with cognitive or motor problems or certain conditions of medication intake), it is important for patients to consult their doctor who knows their medical history and condition.
What should happen next?
Future research needs to compare eszopiclone with other sleep medications to help physicians and patients decide which of the available treatment options to prefer. In addition, sleep medications that are also well tolerated by elderly individuals and individuals with alcohol or drug problems need to be identified.
Summary of findings
Summary of findings for the main comparison. Eszopiclone for insomnia.
| Eszopiclone versus placebo for insomnia | |||||
|
Patient or population: Patients with insomnia
Settings: Outpatient
Intervention: Eszopiclone Comparator: Placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Eszopiclone | ||||
|
Sleep onset latency
Participant reports. Scale from 30 to 540 minutes; fewer minutes equalled better outcome; CFB and double‐blind average values included |
The mean sleep onset latency in the control groups was 20 to 65.7 minutes | The mean sleep onset latency in the intervention groups was 11.94 minutes lower (16.03 to 7.86 lower) | 2890 (9 studies) | ⊕⊕⊕⊝ moderate1 | |
| Wake time after sleep onset Participant reports. Scale from: 30 to 540 minutes; fewer minutes equalled better outcome; CFB and double‐blind average values included | The mean wake time after sleep onset ranged across control groups from 46 to 78.1 minutes | The mean wake time after sleep onset in the intervention groups was 17.02 minutes lower (24.89 to 9.15 lower) | 2295 (8 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Rebound insomnia ‐ sleep onset latency Scale from: 0 to 540 minutes. Follow‐up: 3 days (14 days); fewer minutes equalled better outcome; CFB were calculated by subtracting the mean average of the first three nights of the placebo run‐out period from initial scores | The mean rebound insomnia ‐ sleep onset latency in the control group was ‐24.02 minutes | The mean rebound insomnia ‐ sleep onset latency in the intervention groups was 17 minutes higher (4.29 lower to 38.29 higher) | 291 (1 study) | ⊕⊕⊝⊝ low3 | |
| Rebound insomnia ‐ wake time after sleep onset Scale from: 0 to 540 minutes. Follow‐up: 3 days (14 days); fewer minutes equalled better outcome; CFB were calculated by subtracting the mean average of the first three nights of the placebo run‐out period from initial scores | The mean rebound insomnia ‐ sleep onset latency in the control group was ‐22.15 minutes | The mean rebound insomnia ‐ wake time after sleep onset in the intervention groups was 6.71 minutes lower (21.25 lower to 7.83 higher) | 291 (1 study) | ⊕⊕⊝⊝ low3 | |
| Total sleep time Participant reports. Scale from: 300 to 840; more minutes equalled better outcome; CFB and double‐blind average values included | The mean total sleep time ranged across control groups from 324.8 to 382.2 minutes | The mean total sleep time in the intervention groups was 27.70 minutes higher (20.30 to 35.09 higher) | 2935 (9 studies) | ⊕⊕⊕⊝ moderate1 | |
| Next‐day alertness Participant reports. Scale from: 0 to 10 points; higher scores equalled better outcome; CFB and double‐blind average values included | The mean next‐day alertness ranged across control groups from 5.7 to 7.3 on a 11‐point Likert Scale | The mean next‐day alertness in the intervention groups was 0.46 points higher (0.28 to 0.63 higher) | 2061 (8 studies) | ⊕⊕⊝⊝ low4 | |
| Serious adverse events (as defined in the primary study) Participant reports. Serious adverse events observed during double‐blind treatment period | Study population | 4289 (12 studies) | ⊕⊕⊕⊝ moderate1 | Risks were calculated from pooled risk differences | |
| 9 per 1000 | 9 per 1000 (‐1 to 19) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 We downgraded evidence for sleep efficacy outcomes and adverse events by one grade due to methodological limitations (omission of specific design features from trial reports; taste properties of eszopiclone potentially revealing the identity of medication). 2 Even though some inconsistency of results was shown for WASO, heterogeneity was mainly attributable to one trial (Scharf 2005), whose exclusion resulted into a I2 reduction from 55% to 6%. 3 We downgraded evidence for rebound insomnia outcomes by two grades as five RCTs (with a duration > two weeks) applied open‐label extensions, naturalistic follow‐ups or no follow‐up, which we did not consider appropriate to control bias effects
4 We downgraded evidence for next‐day alertness assessed through subjective measures by two grades as it was expected to be rather the objective than the subjective measures of next‐day functioning that might determine the risk of harm, including injuries and accidents
CFB: Change from baseline
Background
Description of the condition
Affecting between 6% to 10% of the adult population in Western countries, insomnia is not only a psychological burden to the individual affected by the condition, but also a major public health issue (Moloney 2011; Morin 2006; Morin 2012; Ohayon 2002; Ohayon 2009; Roth 2003). Complaints increase with age and are twice as prevalent in women than in men (Morin 2012).
The predominant symptom of insomnia is difficulty initiating sleep (sleep‐onset insomnia), maintaining sleep (sleep‐maintenance insomnia) or early morning awakening with inability to return to sleep (Riemann 2015). According to the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5) (American Psychiatric Association 2013), and the third edition of the International Classification of Sleep Disorders (ICSD‐3) (American Academy of Sleep Medicine 2014), dissatisfaction with sleep quantity or quality has to occur at least three nights per week over at least three months to be diagnosed as chronic insomnia. In addition, diagnosis of insomnia disorder requires that sleep problems occur despite adequate opportunity for sleep and cause at least one related daytime impairment, affecting social, occupational, or other important areas of functioning.
Insomnia criteria in the DSM‐5 (American Psychiatric Association 2013), and ICSD‐3 (American Academy of Sleep Medicine 2014) differ from previous classifications by considering frequency criteria and by increasing duration of the condition from one to three months. However, the most fundamental change to former definitions is that primary insomnia and secondary insomnia are not considered as different conditions anymore, but rather as a common category for insomnia disorder. Since causal attribution labels are removed now, insomnia disorder can be recognised as a condition requiring clinical attention irrespective of the presumed underlying causes (Morin 2012; Riemann 2014).
Various models of insomnia refer to a common framework proposed by Spielman 1991, distinguishing between predisposing, precipitating, and perpetuating factors. While predisposing factors, such as maladaptive coping stress strategies, cognitive‐emotional hyperarousal and older age, make individuals more vulnerable to sleeping problems (Fernández‐Mendoza 2010), increased life‐stress, irregular sleep habits, and poor sleep hygiene further precipitate their occurrence (Bastien 2004). If sleep is repeatedly disturbed, a further perpetuation of the problem results from the selective attention directed towards the inability to fall asleep, creating a vicious cycle that often leads to chronicity. The neurocognitive model of insomnia (Perlis 1997; Buysse 2011) emphasises the role of hyperarousal, including an increased level of somatic, cognitive and cortical activity, which is enforced through classical conditioning and which promotes abnormal levels of sensory and information processing, thought to render the insomniac individual especially vulnerable to perturbation by environmental or other stimuli (Riemann 2009). The inhibition model developed by Espie 2002, conceptualises insomnia as the failure to inhibit wakefulness rather than the inability to induce sleep and underscores the originally functional role of wakefulness in the presence of stressors. If a 'threat' is not eliminated, attention is increasingly focused on sleep and motivational processes, including the conscious intent to fall asleep. The attention‐intention‐effort pathway model (Espie 2006), explains how these processes interfere with the otherwise automatic response of inhibiting wakefulness. Current research outlines the interaction between genetics, personality, coping styles and sleep‐interfering processes like increased stress‐reactivity and hyperarousal (Harvey 2018; Palagini 2014). It is hypothesized that epigenetic mechanisms involved in both sleep regulation and brain‐stress response persist into adulthood through effects on brain plasticity (Palagini 2014).
Insomnia has traditionally been considered as a symptom of another disease, rather than a discrete disorder (Morin 2012). Even though insomnia is often associated with psychiatric and medical conditions that contribute to sleep disturbance in diverse ways (Katz 1998; Krystal 2012b), it is now conceptualised as a discrete disorder and as an independent risk factor for further medical and psychiatric problems (Morin 2012). Insomnia is known to increase the risk for depression and substance use disorders (Falcón 2009; Johnson 2001; Riemann 2007; Roane 2008), and coronary heart disease (e.g. Cappuccio 2011; Ferrie 2007; Li 2014; Parthasaraty 2015; Sofi 2014; Winkelman 2015; Xiao 2014). Besides causing psychological distress, insomnia leads to next‐day cognitive and psychomotor impairments (Fortier‐Brochu 2012; Shekleton 2010), irritability, and decreased job performance (Metlaine 2005), and has been shown to reduce life quality (Rosekind 2010; Zammit 1999), and longevity (Roth 2009a). Untreated insomnia does usually not remit with time (Angst 1989; Leshner 2005), underscoring the need for effective and safe treatment interventions.
Description of the intervention
Insomnia is still under‐recognised and often goes untreated (Morin 2012). Even though often recommended as first‐line treatments for chronic insomnia (Hajak 1997; Ramakrishnan 2007; van Straten 2018), non‐pharmacological treatment strategies, including cognitive behavioural therapy (CBT), are rarely used in clinical practice (Cape 2015). Benzodiazepine hypnotics are effective for short‐term treatment of insomnia (Buscemi 2007), but carry the risk of rebound insomnia, withdrawal symptoms, dependence (Ballenger 2000; Lader 1999; Royal College of Psychiatrists 1997), and next‐day hangover effects, responsible for traffic and machine operation accidents (Barbone 1998), self injuries and hip fractures, the latter commonly seen in elderly patients (Bolton 2008; Woolcott 2009).
In the 1980s and early 1990s, a new group of hypnotic agents, known as new generation hypnotics, non‐benzodiazepine hypnotics, benzodiazepine receptor agonists or 'z‐drugs', were introduced to the markets. Meanwhile, zopiclone, zolpidem, zaleplon and eszopiclone, four different non‐benzodiazepine hypnotic compounds, have been developed and introduced as insomnia therapies (Nutt 2010). Eszopiclone is a pyrrolo pyrazine derivative of the cyclopyrrolone class and the (S)‐enantiomer of racemic zopiclone. While racemic zopiclone was approved in 1986 for the European market and used as a hypnotic in many countries for more than two decades without being licensed in the USA, eszopiclone (Lunesta) received the US Food and Drug Administration (FDA) approval in December 2004. In the USA, eszopiclone is approved for short‐ or long‐term treatment of sleep onset and sleep maintenance insomnia in adults and is marketed in 1 mg, 2 mg and 3 mg film‐coated tablets. To date, eszopiclone is not available in Europe as the originator of Lunesta, Sepracor Pharmaceuticals Ltd, withdrew the application for a centralised marketing authorisation for Lunivia to the European Medicines Agency (EMEA) in 2009 (European Medicines Agency 2009).
As shown by animal studies, the (S)‐enantiomer of racemic zopiclone is the one of two stereoisomers that mainly mediates its hypnotic effects (Melton 2005; Hair 2008). Accordingly, recommended clinical dosages are about 50% lower for eszopiclone compared to racemic zopiclone (Greenblatt 2012). The recommended starting dose for eszopiclone was initially 2 mg in non‐elderly adults and 1 mg for elderly patients (Sepracor 2004 [pers comm]; Hair 2008) and lowered to 1 mg for men and women of all age groups by a current FDA safety alert (Food and Drug Administration 2015) due to the risk of next‐day impairments as shown in a randomised, double‐blind cross‐over study (Boyle 2012). The maximum recommended dose of eszopiclone is 3 mg in non‐elderly and 2 mg in elderly subjects (Lunesta 2004 [pers comm]). Eszopiclone is rapidly absorbed (maximum plasma concentration (Tmax ˜ one hour), has a relatively long elimination half‐life time (t1/2˜ six hours) compared to other non‐benzodiazepine hypnotic compounds, and was shown not to accumulate after multiple once‐daily administration (Hair 2008; Nutt 2010). Eszopiclone is known to cause a bitter or metallic taste, while there is no convincing explanation for this effect (Greenblatt 2012). The drug is classified as a Schedule IV controlled substance (Najib 2006).
How the intervention might work
Similar to benzodiazepines, non‐benzodiazepine hypnotics develop their sedative properties through activity at the gamma‐aminobutyric acid‐A (GABA‐A) receptor, whose endogenous ligand, GABA, is the major inhibitory neurotransmitter in the central nervous system, involved in anxiolysis, sedation, seizure suppression and muscle relaxation (Bateson 2004; Rudolph 2011). The GABA‐A receptor is composed of five protein subunits and at least 19 distinct subunit isoforms, mediating different behavioural and pharmacological responses (Dolder 2007; Drover 2004; Dündar 2004b; Sieghart 2006). Alpha 1 subunits of the GABA‐A receptor are thought to be mainly responsible for the mediation of sedative drug effects, alpha 2 and alpha 3 subunits for anxiolytic and antidepressant drug activities, and alpha 5 receptor subunits for cognitive effects including memory and learning (Lingford‐Hughes 2002; Nutt 2010). While benzodiazepines modulate different subunits of the GABA‐A receptor, the non‐benzodiazepine hypnotics, zaleplon and zolpidem, bind more selectively to the alpha 1‐containing receptor subtypes responsible for sedation (Monti 2007). Accordingly, non‐benzodiazepine hypnotics are assumed to produce an advantageous clinical profile compared to benzodiazepines, particularly with respect to residual effects, tolerance and dependence (Drover 2004; Follesa 2002). The cyclopyrrolone derivates, zopiclone and eszopiclone, are not receptor subtype‐specific, but zopiclone has shown to have high affinity binding sites in the cerebral cortex, hippocampus and cerebellum, and greater affinity for alpha 1 and alpha 2 subunits than benzodiazepines (Najib 2006). Like racemic (R,S) zopiclone, eszopiclone shows relatively high binding affinity for the alpha 1, but also for the 2 and 3 receptor subtype, which might indicate that eszopiclone has both hypnotic and anxiolytic effects (Greenblatt 2012; Nutt 2010).
Further differences in the clinical effects of non‐benzodiazepine hypnotics are assumed to be associated with their unique pharmacokinetic profiles, including the bioavailability of the drug, the volume of distribution and the elimination half‐life time (Drover 2000). Like zopiclone, eszopiclone has a longer half‐life time than the non‐benzodiazepine hypnotic compounds, zaleplon or zolpidem, and is thus expected to be particularly useful for the treatment of sleep‐maintenance insomnia (Drover 2000). The prolonged elimination time of eszopiclone may, on the other hand, increase the risk of next‐day impairments (Nutt 2010). Considering the rapid onset of action, eszopiclone appears to have an improved pharmacokinetic profile compared to racemic zopiclone, presumably due to the absence of the confounding effects of (R)‐zopiclone, resulting in a slightly faster onset of action and a reduced individual variability in response (Greenblatt 2012; Nutt 2010). Levels of (S)‐desmethylzopiclone, one of the active metabolites of eszopiclone and zopiclone, are lower than those seen after an equivalent effective dose of racemic zopiclone, suggesting a reduced risk of residual effects for the pure active enantiomer (Brunello 2008).
Why it is important to do this review
Use of hypnotic drugs increased over the past decades, with a striking rise in prescriptions for non‐benzodiazepine hypnotics (Bertisch 2014). Being marketed as just as effective as benzodiazepines, while being safer and having a lower risk for abuse and dependence, non‐benzodiazepine hypnotics have meanwhile replaced benzodiazepines as the most commonly prescribed hypnotic drugs and emerged as the first‐line drugs for insomnia treatment (Erman 2005; Hausken 2009; Hoffmann 2009; NHS Prescribing Service 2010; Siriwardena 2008).
At the same time, there is an increasing controversy about the safety profile of non‐benzodiazepine hypnotics (Cimolai 2007). Various reviews of preclinical and clinical evidence (Drover 2004; Dündar 2004a; Dündar 2004b; Montplaisir 2003; Zammit 2009), post‐marketing surveillance studies (Delahaye 1990; Jaffe 2004), and reviews of case study reports (Hajak 2003; Lader 1999; Soyka 2000) confirm the advantages of non‐benzodiazepine hypnotics in terms of next‐day impairments and their potential for abuse and dependence. Double‐blind studies examining the subjective effects of zolpidem in drug‐naive individuals (Licata 2008) and assessing polysomnographic withdrawal effects of zopiclone and zolpidem in healthy subjects (Vorderholzer 2001) have found a low risk of tolerance and dependency for these drugs, if taken in recommended doses. Further studies and reviews, likewise referring to patient surveys and pharmacovigilance data, rate the abuse liability of non‐benzodiazepine hypnotics as comparable to that of benzodiazepine hypnotics (e.g. Hoffmann 2009; Hoffmann 2014; Siriwardena 2008; Victorri‐Vigneau 2014). Parasomnia, amnesia, and hallucinations have been documented as adverse events of zolpidem (Ben‐Hamou 2011) and there is evidence that eszopiclone can cause euphoria and hallucination if taken in elevated doses (Scharf 2006; Monti 2007).
In addition, current evidence indicates that the risk of the non‐benzodiazepine hypnotic drugs in causing next‐day impairments might be higher than initially assumed (Gunja 2013). While a first randomised, double‐blind, placebo‐controlled, cross‐over trial did not indicate next‐day residual effects for 3 mg nighttime eszopiclone in young to middle‐aged individuals (Boyle 2008), a subsequent study applying a mild sleep restriction protocol (Boyle 2012) demonstrated next‐day impairments, giving reason for a FDA safety alert (see also Description of the intervention; Food and Drug Administration 2015). Retrospective analyses of medical care and health insurance data demonstrate an alerting risk for falls, injuries, and hip fractures for zolpidem (Finkle 2011; Wang 2001) and for non‐benzodiazepine hypnotic substances in general (Berry 2013; Diem 2014). Eszopiclone might also decrease immune function, as indicated by a meta‐analysis of data submitted to the FDA, which showed an increased risk of infections for eszopiclone (Joya 2009). Carcinogenicity and mutagenesis associated with eszopiclone have been discussed and require further monitoring (Strebbing 2005).
It is the aim of the review to integrate efficacy and safety data from randomised controlled trials (RCTs) on eszopiclone, to allow the drawing of conclusions on the drug`s efficacy and safety profile, while taking study quality and bias risks into consideration. This review forms part of a suite of four reviews on non‐benzodiazepine hypnotics for insomnia; the other three reviews will assess the effectiveness and safety of zolpidem (Rösner 2013a), zopiclone (Rösner 2013b) and zaleplon (Rösner 2013c).
Objectives
The objectives of the review are:
to determine the effectiveness of eszopiclone for insomnia treatment in comparison with placebo and active comparators;
to determine the safety profile of eszopiclone in comparison with placebo and active comparators; and
to compare eszopiclone with other non‐benzodiazepine hypnotics in terms of effectiveness and safety.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel group randomised controlled trials (RCTs), comparing eszopiclone with either placebo or active control in its efficacy to improve sleep and its risk of causing adverse events, withdrawal symptoms, or rebound insomnia. Run‐out phases were included if controlled with placebo. Cross‐over trials were not included in the review due to sleep stabilising effects that have been reported for eszopiclone after discontinuation of dosing (Zammit 2004), making it difficult to control carry‐over effects by wash‐out.
Types of participants
Adults aged 18 years and over with insomnia, as diagnosed using a standardised diagnostic system such as the DSM (American Psychiatric Association 2013), the ICD (World Health Organization 1992), or the ICSD (American Academy of Sleep Medicine 2014), were included irrespective of insomnia type (primary insomnia; insomnia associated with comorbid conditions; see Differences between protocol and review). We did not include healthy subjects from laboratory models of transient sleep as it is unclear whether these conditions are generalisable to clinical insomnia.
Types of interventions
Experimental intervention: eszopiclone as monotherapy.
Comparator interventions: placebo, other non‐benzodiazepine hypnotics, short‐/ intermediate‐/long‐acting benzodiazepines; other active controls that allowed double‐blind treatment. Any treatment setting (inpatient and outpatient) and any formulation were included.
Types of outcome measures
Outcomes of the review are a selection of outcomes considered in primary studies (see Characteristics of included studies). We selected the primary and secondary outcomes of the review with regard to the clinical relevance of outcome criteria and the avoidance of conceptual overlaps. All types of measurement including objective measures (e.g. polysomnography) and participant‐reported sleep measures, as well as different types of scores (change from baseline scores, double‐blind average scores), were considered. If both objective and subjective measures were provided in a study publication, subjective measures were included in the meta‐analysis and we examined the impact of measurement type with sensitivity analyses (see Sensitivity analysis). Change from baseline scores and double‐blind average scores were integrated in the same meta‐analyses, as outlined by Deeks 2011. For the assessment of adverse events (withdrawal symptoms and next‐day alertness), any types of outcome measures were included.
Timing of outcome assessment
Double‐blind average scores were based on the average of the results over the double‐blind treatment period. While change from baseline scores for efficacy outcomes were obtained by subtracting the measurement at the end of treatment from initial scores, change from baseline values for discontinuation outcomes (rebound insomnia) were calculated by subtracting the mean average of the first three nights of the placebo run‐out period from initial scores. Safety was assessed over the entire double‐blind treatment period, and discontinuation effects over the first three nights of the placebo run‐out period. Interventions up to four weeks were considered as short‐term treatments, interventions between four weeks and six months as medium‐term treatments, and interventions with a duration over six months as long‐term treatments.
Primary outcomes
Primary efficacy outcomes
Sleep onset latency (SOL)
Wake time after sleep onset (WASO)
Treatment effectiveness was assessed through two outcomes: 1. 'sleep onset latency' (SOL), defined as the length of time (in minutes) after lights‐out until sleep onset, and 2. 'wake time after sleep onset' (WASO), defined as the length of time (in minutes) of wakefulness after the onset of persistent sleep. The consideration of two effectiveness outcomes was reasoned by their conceptual distinctiveness, with SOL measuring a drug's impact on sleep onset, and WASO measuring the potential to improve sleep maintenance; the former reflecting its suitability for the treatment of sleep‐onset insomnia and the latter for sleep‐maintenance insomnia (see Description of the condition).
Primary discontinuation outcomes
Withdrawal symptoms
Rebound insomnia
Discontinuation effects were assessed through 1. withdrawal symptoms, defined as adverse symptoms that either a) appeared for the first time during the placebo run‐out period, or b) already appeared during treatment, but deteriorated during the placebo run‐out interval; and 2.rebound insomnia, defined as the temporary worsening of sleep during the placebo run‐out interval. Worsening of sleep was evaluated as mean change from baseline for the primary efficacy outcomes (SOL, WASO) during the first three days of the placebo run‐out period (Gillin 1989). The consideration of two variables for assessing effects of drug discontinuation was based on the fact that most studies provided data on either one or the other outcome.
Secondary outcomes
Total sleep time (TST)
Next‐day alertness
Adverse events (AEs)
Total sleep time (TST) was the total time (in minutes) a person spent sleeping during the in‐bed interval. Calculated as time in bed minus SOL and minus WASO (Schutte‐Rodin 2008),TST is a common outcome measure in insomnia treatment, reflecting both sleep onset and maintenance effects within a single variable (Goforth 2014). To avoid conceptual overlaps with the primary efficacy outcomes, TST was considered as a secondary outcome of the review. Next‐day alertness reflected the state of vigilance the day after hypnotics had been taken and was mainly assessed with an 11‐point‐Likert scale (0 to 10), with higher scores indicating improved function. Adverse events (AEs) were all types of unfavourable symptoms that occurred during the course of the study.
Hierarchy of outcome assessment
The study endpoints of the primary outcomes were considered as essential to determine efficacy and safety of eszopiclone, while secondary outcomes had only complementary value in the interpretation of results. Thereby, the primary efficacy outcomes, SOL and WASO, were considered as distinctive compounds and weighted equally in the determination of sleep efficacy. Discontinuation effects were considered as being present if either adverse events or indicators of sleep efficacy changed during the placebo run‐out period.
Search methods for identification of studies
Electronic searches
The Cochrane Common Mental Disorders Group's Trials Search Co‐ordinator (TSC) searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1950 onwards), Embase (1980 onwards), PsycINFO (1987 onwards) and PSYNDEX (in English and German) with a last update of the search on February 10, 2016 (Appendix 2). The WHO trials portal and ClinicalTrials.gov were searched to identify any ongoing or completed trials with unpublished results. Search strategies were developed by the TSC comprehensively to simultaneously address different non‐benzodiazepine hypnotic compounds (eszopiclone, zopiclone, zolpidem, zaleplon). The results of the search and reviews for zopiclone, zolpidem and zaleplon will be presented in Rösner 2013a, Rösner 2013b and Rösner 2013c, respectively.
In keeping with the MECIR conduct standard (C37 re‐running searches within 12 months of publication), we ran an update search on CENTRAL, CCMDCTR, MEDLINE, Embase, PsycINFO and the international trial registries (21 February 2018). We identified three new studies which we have placed in 'awaiting classification'/'ongoing studies', these will be incorporated in the next version of the review, as appropriate.
Searching other resources
We contacted key informants, experts, public sponsors, and drug manufacturers with the request to indicate further studies of potential relevance. For this purpose, we provided reference lists with identified studies and the criteria for inclusion and exclusion in the review. Finally, we handsearched the reference lists of included studies and current reviews to complete and to verify the preceding searches. All eligible studies identified with the search were included irrespective of language, publication type, or status.
Data collection and analysis
Selection of studies
We assessed the eligibility and relevance of trials on the basis of their abstracts retrieved from the electronic searches. For studies that appeared to meet the inclusion criteria according to the abstract information, we obtained full‐text versions for closer inspection. Two review authors assessed the relevance and eligibility of studies independently. The process of study identification and its results were outlined as a flow diagram according to the PRISMA statement (Moher 2009).
Data extraction and management
Two review authors extracted relevant outcome data independently into prespecified data extraction forms and compared data value by value. In case of disagreements, we have undertaken the following sequential procedures in descending order:
comparison of published and extracted information to identify transcription and comprehension errors;
explanation of the coding decisions by each review author, followed by consensus discussion and arbitration.
Finally, after comparisons and corrections were concluded, we entered data into the Review Manager software (Review Manager 2014). For meta‐analyses, we planned to compare eszopiclone individually with either placebo or active control (though we only found placebo‐controlled trials). It had been planned to group benzodiazapine‐active control drugs according to their duration of action into short‐acting (less than five hours), intermediate‐acting (five to 24 hours) and long‐acting (more than 24 hours) agents (Greenblatt 1981), potentially generating the following comparisons:
eszopiclone versus placebo:
eszopiclone versus other new generation hypnotics;
eszopiclone versus short‐acting benzodiazepines;
eszopiclone versus intermediate‐acting benzodiazepines;
eszopiclone versus long‐acting benzodiazepines;
eszopiclone versus other active controls (compounds to be specified at a later date).
Assessment of risk of bias in included studies
We assessed the risk of bias in accordance with The Cochrane Collaboration's 'Risk of bias' assessment tool (Higgins 2011). We considered the equivalence of baseline characteristics and the equivalence of treatment utilisation as further bias risks in the rating of the item 'free of other bias'. We judged the general susceptibility to bias effects in consideration of the objectivity of outcome information and rated this separately for measures of sleep and next‐day functioning. Two review authors independently assessed the risk of bias and divergent ratings were resolved by consensus discussion. The criteria considered as constitutive for the rating of bias risks are outlined in Appendix 1.
Measures of treatment effect
We measured treatment effects for continuous outcomes with the mean differences (MD), as these were measured on the same scale. As more commonly reported, we gave priority to final measurement scores compared to change‐from‐baseline scores, if both types of outcomes were provided in the trial publication. Nevertheless, we pooled change and final scores in meta‐analysis as outlined by Deeks 2011, using the (unstandardized) mean difference method in RevMan (Review Manager 2014). For subjective measures of next‐day functioning, higher scores indicate a more positive state; if provided differently in the primary study, scales were reversed in their polarity. Adverse events were assessed using risk difference (RD) as this measure can also be calculated in cases where there are no events in either group (Deeks 2011). We calculated all treatment effects together with 95% confidence intervals (CIs). A P value of 0.05 and below has been chosen to indicate statistical significance of effects. We planned to measure treatment effects for dichotomous effectiveness outcomes using risk ratio (RR) and ‘number needed to treat for an additional beneficial outcome’ (NNTB) or ‘number needed to treat for an additional harmful outcome’ (NNTH) for outcomes that reached statistical significance, but, beside adverse events, no additional dichotomous data were available. We did not provide NNTH for adverse events as these related to the number of events, not participants (see Unit of analysis issues). In the future, if we find any dichotomous data, we plan to calculate NNTB for effects on binary outcomes which reach statistical significance.
Unit of analysis issues
Only individually randomised trials with the individual participant constituting the unit of analysis were included in the review. In multi‐arm studies with different dose schedules, only the initially recommended dose group (2 mg in non‐elderly, 1 mg in elderly participants; see Types of interventions) was considered. Meta‐analyses of adverse events were based on number of events, which did not necessarily correspond with the number of participants (as one participant can theoretically report multiple adverse events). The latter did not apply to dropouts due to adverse events, where the number of participants matched exactly the number of dropout events.
Dealing with missing data
Outcome statistics were included in the review, as provided by the study publications, irrespective of how missing individuals were handled in the primary analysis. We imputed sample sizes for continuous outcomes which were not explicitly provided in the trial publication by the size of treatment‐received samples or, if not available, by the size of the randomised sample. Missing standard deviations were obtained from standard errors (SEs) or CIs for group means, missing SEs from standard deviations (SDs), CIs, or t values and P values. If only the medians were provided in the trial publications, outcome statistics were not included in the meta‐analyses, but information on the significance of effects (yes, no) was inserted into an overview table and described qualitatively in the discussion of results.
Assessment of heterogeneity
We quantified inconsistency across studies with the I2 statistic (Higgins 2003), using threshold values for substantial heterogeneity as outlined by Deeks 2011. Heterogeneity was assumed if the I2 value was above 75%. The Tau2 statistic was additionally considered to provide an estimate of between‐study variance (Rücker 2008), independent of the sample size. In cases of heterogeneity, we attempted to identify and explain the heterogeneity using subgroup analysis.
Assessment of reporting biases
If there are more than 10 included studies in future versions of this review, we will graphically illustrate the risk of publication bias with the funnel plot method (Egger 1997; Light 1984).
Data synthesis
For synthesising aggregate outcome measures, we used a random‐effects model (DerSimonian 1986), with study effects being weighted using the Mantel‐Haenszel approach (Mantel 1959).
Subgroup analysis and investigation of heterogeneity
Due to age‐related changes in the architecture of sleep and pharmacokinetic changes, elderly participants are repeatedly shown to respond differently to hypnotic drugs than younger people (Dolder 2007). In addition, there is evidence that treatment effects might depend on insomnia as a primary or secondary condition (Krystal 2012b; Wilson 2010). Thus, we conducted subgroup analyses limited to samples, a) of age groups over 65 years and b) of participants with insomnia associated with psychiatric and medical comorbidity to determine differential effectiveness of eszopiclone in participants with older age or with comorbid insomnia. To additionally investigate whether effects demonstrated in investigator‐initiated studies significantly differed from sponsor‐initiated studies as a result of funding bias (Lexchin 2003), we compared both groups of trials by subgroup analyses.
Sensitivity analysis
We conducted sensitivity analyses to determine the influence of the following variables on the primary effectiveness outcomes (SOL, WASO):
the method of sleep efficacy measurement by integrating effects measured with polysomnography;
the method of withdrawal assessment by integrating scores of the Benzodiazepine Withdrawal Symptom Questionnaire.
Summary of findings tables
Summary of findings tables were completed to summarise the best evidence for all relevant outcomes including SOL, WASO, withdrawal symptoms, rebound insomnia, TST, next‐day alertness and adverse events. The rating of single GRADE criteria for downgrading (risk of bias, inconsistency, indirectness, imprecision, publication bias) and upgrading quality of evidence (magnitude, dose‐response gradient, change of results by confounding) was reasoned and outlined in detail under Quality of the evidence. The GRADE assessment was performed by one author (SRO) and discussed with a second author (CE) in case of ambiguity.
Results
Description of studies
Results of the search
Search for studies
Results of the electronic biomedical database searches (to February 2016), simultaneously addressing different non‐benzodiazepine hypnotic compounds (eszopiclone, zopiclone, zolpidem, zaleplon) were screened in parallel (by SR, CE) and allocated to different reviews, as appropriate. Results of the search and reviews for zopiclone, zolpidem and zaleplon will be presented in Rösner 2013a, Rösner 2013b and Rösner 2013c, respectively. The steps of trial identification for eszopiclone and their results are illustrated in Figure 1 as a flow diagram, according to the PRISMA statement (Moher 2009).
1.
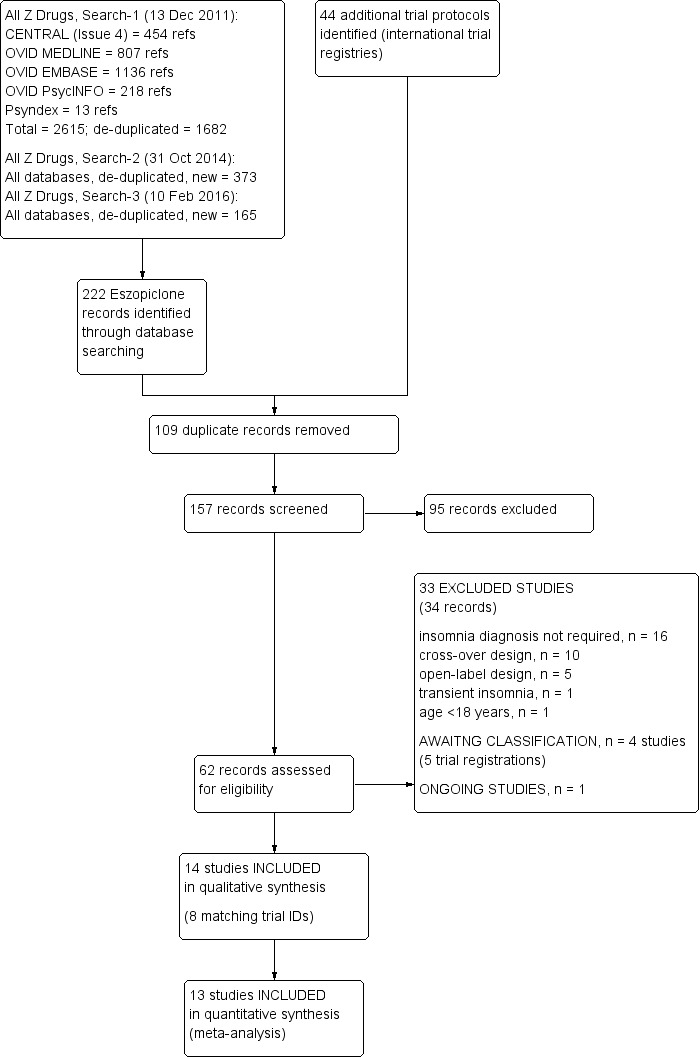
Study flow diagram (search results to Feb 2016)
Electronic searches for eszopiclone (only) yielded 222 potentially relevant journal references. Personal communications with investigators and sponsors did not yield further studies. From the 222 yielded references, 94 were recognised as duplicates and removed. For the remaining 128 references, abstracts were screened by two review authors independently (SR, CE and RW, CE). On the basis of information provided in the title and abstracts, a further 80 references were excluded, while for the remaining 48 records, full‐text articles were retrieved (where available). Inspection of the study reports led to the exclusion of a further 33 studies (34 reports) and reasons for the exclusions are outlined under Excluded studies.
The search of the international trial registries (to February 2016) identified an additional 44 trial protocols; 15 were excluded as irrelevant and, on closer inspection, 15 were duplicates as they had already been excluded as trial records retrieved from the bibliographic databases search, with the reasons for exclusions outlined under Excluded studies. Of the remaining 14 trial protocols, eight linked to full study reports already included in the review, four were added to 'awaiting classification', one was ongoing and there was one duplicate trial registration.
In keeping with the MECIR conduct standard (c37: re‐running searches within 12 months of publication), we ran an update search (21 February 2018) and identified four new studies, two studies (Baran 2017; Buxton 2017) placed in 'studies awaiting classification', one (NCT02456532) placed in ongoing studies and another (retrospectively) excluded (Uchimura 2012b). Thus, including the results of the update search, six studies were categorized as 'studies awaiting classification',two studies as ongoing and a total of 34 excluded studies. A journal article relating to a trial protocol awaiting classification (NCT01100164) was also identified at this time (Pinto 2016).
Finally, 14 RCTs were eligible for the review; of these, 13 RCTs provided data for meta‐analytic integrations of sleep efficacy or safety outcomes.
The PRISMA diagram includes details of the search results to 10 February 2016 only (Figure 1).
Acquisition of unreported outcomes
To obtain unreported outcome data for primary efficacy outcomes of the review, correspondence authors of primary studies were contacted by email and requested to provide unreported data. From 10 authors requested, six authors responded, referring to the drug manufacturer, Sunovion (www.sunovion.com/), which did not provide unreported data.
Included studies
Fourteen RCTs (Ancoli‐Israel 2010; Fava 2006; Goforth 2014; Krystal 2003; McCall 2006; McCall 2010a; Menza 2010; Pollack 2008; Roth 2009; Scharf 2005; Soares 2006; Spierings 2015; Walsh 2007; Zammit 2004), based on data from 4732 study participants, were included in the review. Table 2 provides an overview of all included studies. Detailed information on study designs, sample characteristics, interventions, and outcomes for each individual trial is presented in the Characteristics of included studies tables.
1. Overview of included studies.
| Author | N | Female (%) | Specific sample characteristics | Primary insomnia | Method | Duration (weeks) | Single‐blind run‐out (days) |
| Ancoli‐Israel 2010 | 388 | 63 | Elderly | X | Participant reports (diary) | 12 | 14 |
| Fava 2006 | 545 | 66.6 | Comorbid depression | — | Participant reports (IVRS) | 8 | 14 |
| Goforth 2014 | 58 | 63 | Comorbid back pain | — | Participant reports (diary) | 4 | — |
| Krystal 2003 | 788 | 63 | — | X | Participant reports (IVRS) | 24 | — (follow‐up) |
| McCall 2006 | 264 | 67.4 | Elderly | X | Participant reports (IVRS), PSG | 2 | — |
| McCall 2010a | 60 | 66.7 | Comorbid depression | — | Participant reports (diary); actigraphy | 8 | — (follow‐up) |
| Menza 2010 | 30 | 20 | Comorbid Parkinsons disease | — | Participant reports (diary) | 3 | — |
| Pollack 2008 | 595 | 66 | Comorbid anxiety disorder | — | Participant reports (diary) | 8 | 14 |
| Roth 2009 | 153 | 86.9 | Comorbid rheumatoid arthritis | — | Participant reports (IVRS) | 4 | 7 |
| Scharf 2005 | 231 | 57.8 | Elderly | X | Participant reports (IVRS) | 2 | — |
| Soares 2006 | 410 | 100 | Menopause | — | Participant reports (IVRS) | 4 | 7 |
| Spierings 2015 | 75 | 82.5 | Migraine | X | Participant reports (diary) | 6 | 2 (open label) |
| Walsh 2007 | 830 | 61 | — | X | Participant reports (IVRS) | 24 | 14 |
| Zammit 2004 | 305 | 65 | — | X | Participant reports (IVRS), PSG | 6 | 2 |
IVRS: interactive voice response system
N: number
PSG: polysomnography
Design and setting
All trials included in the review were based on a randomised controlled parallel group design. No trials with active controls (new‐generation hypnotics, short‐acting benzodiazepines, intermediate‐acting benzodiazepines, long‐acting benzodiazepines) were included, limiting comparisons to 'eszopiclone versus placebo'. Eszopiclone and placebo were provided as home treatment; three of the 14 RCTs (Fava 2006; McCall 2010a; Zammit 2004), additionally included overnight stays in the sleep laboratory. All studies but three Goforth 2014, McCall 2010a, Menza 2010 were based on multicentre designs, including 43 (Roth 2009), and up to 82 (Ancoli‐Israel 2010), participating study centres. Thirteen RCTs were undertaken in the United States, and one trial in Canada (Soares 2006) Follow‐up after drug discontinuation was considered in ten RCTs, of which seven applied single‐blind placebo run‐out periods (2 days Zammit 2004, 7 days Roth 2009; Soares 2006 and 14 days Ancoli‐Israel 2010; Fava 2006; Pollack 2008; Walsh 2007), two open‐label extensions (2 weeks Spierings 2015, 6 months Krystal 2003), and one trial with naturalistic follow‐up monthly by telephone for four months after randomised treatment (McCall 2010a). There was a single‐blind placebo run‐in period to establish baseline values for sleep and daytime functioning and to ensure compliance with the dosing regimen preceded treatment in some trials (Ancoli‐Israel 2010; Pollack 2008; Roth 2009; Soares 2006).
Sponsoring, initiation and publication
With the exception of two non‐profit funded RCTs (McCall 2010a; Pollack 2008), trials included in the review were financially supported by the pharmaceutical industry. Five of the 14 included RCTs were initiated by the investigator (Goforth 2014; Menza 2010; McCall 2010a; Pollack 2008; Spierings 2015), and the remaining nine RCTs by a sponsor. All trials were published as journal articles.
Sample size
Sample sizes varied from 30 (Menza 2010) to 830 participants (Walsh 2007), with most studies including between 150 to 400 participants (Ancoli‐Israel 2010; McCall 2006; Roth 2009; Scharf 2005; Zammit 2004).
Participants: age
In most RCTs included in the review, participants were recruited from young to middle‐aged groups (18 to 64 years), three RCTs defined older age (64 and 85 years) as a criterion of inclusion (Ancoli‐Israel 2010; McCall 2006; Scharf 2005), while in the trial of Menza 2010, a broader spectrum of age was considered (35 to 85 years). The mean age of participants varied between 40 and 50 years in most studies; in the studies with elderly participants (Ancoli‐Israel 2010; McCall 2006; Scharf 2005), mean age was 71.5 years, and in the trial with the broader age range (Menza 2010), mean age of participants was 56 years.
Participants: gender
RCTS were based on mixed‐gender samples, apart from one trial testing eszopiclone after menopausal transition (Soares 2006), thus exclusively including only female participants. In mixed‐gender samples, females constituted the majority of participants by representing 63% and 67% of the sample. A higher proportion of females was seen in the trials focusing on comorbid rheumatoid arthritis (86.9%; Roth 2009) and migraineurs (82.5%; Spierings 2015), and a lower proportion in the trial with comorbid Parkinson's disease (20%; Menza 2010).
Participants: insomnia diagnosis
Participants of included trials either met DSM‐5 or DSM‐4‐TR criteria for primary insomnia (Ancoli‐Israel 2010; Krystal 2003; McCall 2006; Scharf 2005; Spierings 2015; Walsh 2007; Zammit 2004), or DSM‐5 criteria for insomnia associated with a comorbid psychiatric or medical condition (Fava 2006; Goforth 2014; McCall 2010a; Menza 2010; Pollack 2008; Roth 2009; Soares 2006).
The study conducted by Spierings 2015, including participants with primary insomnia and suffering migraine, took an intermediate position between primary insomnia studies and comorbidity trials. Total sleep time (TST) was required to be lower than six hours in two studies (Ancoli‐Israel 2010; Soares 2006), or 6.5 hours (Fava 2006; Goforth 2014; Krystal 2003; McCall 2006; Menza 2010; Pollack 2008; Roth 2009; Scharf 2005; Spierings 2015; Walsh 2007; Zammit 2004), and wake time after sleep onset (WASO) had to be at least 30 minutes (Fava 2006; Goforth 2014; Krystal 2003; McCall 2006; McCall 2010a; Menza 2010; Pollack 2008; Scharf 2005; Walsh 2007; Zammit 2004), or 45 minutes (Ancoli‐Israel 2010; Roth 2009; Soares 2006). Insomnia symptoms had to occur at least three nights per week (Ancoli‐Israel 2010; Fava 2006; McCall 2010a; Menza 2010; Pollack 2008; Roth 2009; Scharf 2005; Spierings 2015), on a typical night (Goforth 2014; Walsh 2007), or each night during the last month (Krystal 2003; McCall 2006; Scharf 2005; Zammit 2004).
Participants: comorbidity
Comorbid conditions associated with insomnia included major depression (Fava 2006; McCall 2010a), general anxiety disorder (Pollack 2008), chronic low back pain (Goforth 2014), Parkinson's`s disease (Menza 2010), rheumatoid arthritis (Roth 2009), and complaints in the context of the menopausal transition (Soares 2006). Some trials demanded that comorbid symptoms must either have predated insomnia (Soares 2006; Roth 2009), or postdated insomnia less than four weeks (Goforth 2014) or 10 weeks (Fava 2006). In the remaining studies considering comorbidity, no temporal relationship between insomnia and comorbid disorders was required (McCall 2010a; Menza 2010; Pollack 2008). Individuals with another primary or secondary sleep disorder (e.g. sleep apnea, restless legs syndrome, periodic leg movement disorder) or with a known or suspected acute medical or psychiatric condition that impacted or was likely to impact sleep, were excluded.
A lifetime history of substance abuse or dependence was a criterion of exclusion in five of 14 RCTs (Krystal 2003; Pollack 2008; Soares 2006; Walsh 2007; Zammit 2004), while other studies excluded participants only if substance abuse or dependence occurred within the last 12 months (Goforth 2014), five years (Spierings 2015), or was present at screening (McCall 2010a). Some trials also defined positive urine screening for drugs or alcohol (Fava 2006), drinking more than two standard drinks per day (Krystal 2003; McCall 2006; Scharf 2005; Soares 2006; Spierings 2015; Zammit 2004) or more than 14 drinks per week (Krystal 2003; McCall 2006) as a criterion of exclusion. Even so, four RCTs (McCall 2006; Menza 2010; Roth 2009; Scharf 2005) did not mention substance use or substance use disorders as a criterion of exclusion.
Intervention: comparisons and doses
Twelve of the 14 RCTs used a two‐armed design, testing eszopiclone dosed as recommended (3 mg for non‐elderly, 2 mg for elderly participants) against placebo, and two RCTs (Scharf 2005; Zammit 2004) compared recommended doses of eszopiclone with lower dose groups (2 mg for non‐elderly in Zammit 2004; 1 mg for elderly participants in Scharf 2005) using a three‐armed design. From these trials, only the initially recommended dose groups (3 mg for non‐elderly, 2 mg for elderly participants) were included in the meta‐analytic integration. For home treatment, participants were instructed to take study medication at bedtime, in the sleep laboratory condition (McCall 2006; Zammit 2004), or a single bedtime dose was administered 30 minutes before lights out.
Intervention: comedication
Eszopiclone was coadministered with open‐label fluoxetine (starting dose 20 mg; dose range: 20 to 40 mg/day) in participants with coexisting major depressive disorder (Fava 2006; McCall 2010a), open‐label escitalopram (10 mg) in participants with comorbid generalised anxiety disorder (Pollack 2008), naproxen (50 mg open‐label; twice daily) and lansoprazole (15 mg open‐label; once daily) in participants with low back pain (Goforth 2014), open‐label hormones for menopause symptoms (Soares 2006) or open‐label disease‐modifying medications for Parkinson's`s disease (Menza 2010) and rheumatoid arthritis (Roth 2009).
Intervention: treatment duration
Single‐blind placebo run‐in periods were used to establish baseline values for sleep and daytime functioning and to ensure compliance with the dosing regimen in some trials (Ancoli‐Israel 2010; Pollack 2008; Roth 2009; Soares 2006). Duration of treatment with eszopiclone and placebo varied from two weeks (McCall 2006; Scharf 2005) to 24 weeks (Krystal 2003; Walsh 2007), including short‐term treatment of insomnia (≤ four weeks; Goforth 2014; McCall 2006; Menza 2010; Roth 2009; Scharf 2005; Soares 2006), medium‐term treatment (> four weeks ≤ six months; Ancoli‐Israel 2010; Fava 2006; McCall 2010a; Pollack 2008; Spierings 2015; Zammit 2004) and long‐term treatment (> six months; Krystal 2003; Walsh 2007). Length of follow‐up intervals was two days (Zammit 2004), one week (Roth 2009; Soares 2006) or two weeks (Ancoli‐Israel 2010; Fava 2006; Pollack 2008; Walsh 2007; Spierings 2015) for placebo run‐out periods, and four months (McCall 2010a) or six months (Krystal 2003) for open‐label or naturalistic follow‐up extensions.
Outcomes: sleep efficacy
Sleep efficacy outcomes (SOL, WASO, TST) constituted the primary efficacy endpoints in all but one trial focusing on health‐related quality of life (McCall 2010a). Besides latency to persistent sleep (LPS), WASO and TST, Zammit 2004 additionally analysed time and percentage of time spent in the different sleep stages. Sleep efficacy outcomes were either provided as mean change from baseline values (McCall 2006; Pollack 2008; Soares 2006; Scharf 2005) or mean values defined as the average over a defined time interval or the entire double‐blind treatment period (Ancoli‐Israel 2010; Goforth 2014; Krystal 2003; Menza 2010; Scharf 2005; Spierings 2015; Zammit 2004). Due to the skewed distribution of data, for some trials medians were exclusively (Fava 2006; Roth 2009; Walsh 2007) or additionally reported (Goforth 2014; Krystal 2003; McCall 2006; Pollack 2008; Scharf 2005; Soares 2006; Walsh 2007; Zammit 2004). The trial focusing on quality of life (McCall 2010a) reported ß‐, SE and t‐values for repeated measures mixed modelling. Sleep efficacy outcomes were assessed through participant self‐reports in all but three RCTs (McCall 2006; McCall 2010a; Zammit 2004) which included both participant‐reported and objective measures of sleep. Objective sleep measures were assessed via polysomnography (PSG) recording during overnight stays in the sleep laboratory (McCall 2006; Zammit 2004) and via actigraphy (McCall 2010a), where participants continuously wore an actigraph unit on their non‐dominant wrist for the duration of the study. Participant self‐reports on sleep were assessed with paper sleep diaries (Spierings 2015) or electronic sleep diaries (Ancoli‐Israel 2010; Pollack 2008; Menza 2010) completed in the morning or with the aid of an interactive voice response system (IVRS; Fava 2006; Krystal 2003; McCall 2006McCall 2010a; Menza 2010; Scharf 2005; Soares 2006; Walsh 2007; Zammit 2004) that had either to be called daily (McCall 2006McCall 2010a; Menza 2010; Scharf 2005; Soares 2006; Zammit 2004) or weekly (Fava 2006; Krystal 2003; Walsh 2007) in the morning to report the previous night’s sleep.
Outcomes: discontinuation effects
Rebound effects, assessed through change from baseline for sleep efficacy outcomes during placebo run‐out period, were provided for seven RCTs (Ancoli‐Israel 2010; Fava 2006; Pollack 2008; Roth 2009; Soares 2006; Walsh 2007; Zammit 2004). One RCT provided means for change from baseline for each single day during placebo run‐out (Ancoli‐Israel 2010), the remaining trials either reported median change values (Fava 2006; Pollack 2008; Roth 2009; Walsh 2007; Zammit 2004) or referred to the significance of effects without providing outcome statistics (Soares 2006). Prevalence of new or worsening of adverse events ( Pollack 2008; Roth 2009; Soares 2006) or central nervous system‐related adverse events (Ancoli‐Israel 2010; Fava 2006; Pollack 2008; Zammit 2004) reflecting withdrawal effects were provided by seven RCTs (Ancoli‐Israel 2010; Fava 2006; Krystal 2003; Pollack 2008; Roth 2009; Soares 2006; Zammit 2004). In two trials (Ancoli‐Israel 2010; Walsh 2007), withdrawal effects were evaluated with the Benzodiazepine Withdrawal Symptom Questionnaire (Tyrer 1990) administered following the discontinuation period.
Outcomes: next‐day functioning
Daytime functioning was recorded in the evening with electronic wake diaries (Ancoli‐Israel 2010; Pollack 2008; Menza 2010) or by evening calls to the interactive voice response system (Fava 2006; Krystal 2003; McCall 2006McCall 2010a; Menza 2010; Scharf 2005; Soares 2006; Walsh 2007; Zammit 2004). Next‐day functioning was rated on an 11‐point Likert scale (0 to 10), with higher scores indicating improved functioning (Ancoli‐Israel 2010; Krystal 2003; McCall 2006; Menza 2010; Roth 2009; Scharf 2005; Spierings 2015; Zammit 2004, in one RCT (Zammit 2004), next‐day residual effects were additionally evaluated with the Digit‐Symbol Substitution Test (DSST; Wechsler 1955).
Excluded studies
Thirty‐four studies were excluded on the basis of full‐text papers. Among reasons for exclusion, insomnia diagnosis not being mandatory for including subjects in the primary study (Attarian 2011; Demanuele 2014; Dimsdale 2011b; Eckert 2011; Lettieri 2008; NCT00460993; NCT00511134; NCT00616655; NCT00685269; NCT00811746; NCT00813735; NCT00826111; NCT01102270; NCT01641900; Tek 2014, Huang 2015) was most common (n = 16). Further reasons for exclusion were the use of a cross‐over design (Boyle 2008; Boyle 2012; Erman 2008; NCT00120250; NCT00368056; NCT00374192; NCT00900159; Pollack 2011; Rosenberg 2007; Uchimura 2012a; Uchimura 2012b), an open‐label design (Gross 2011; NCT00889200; NCT00900159; NCT01710631; Peng 2013), the inclusion of healthy subjects passing through a model of transient insomnia (Rosenberg 2005) and younger age participants (< 18 years) (Sangal 2014). Individual trials excluded from the review and the corresponding reasons for exclusion are outlined under Characteristics of excluded studies.
Ongoing studies
Searches in registry databases (WHO trials portal; ClinicalTrials.gov) (to February 2016) yielded one trial (Emiko 2015) in the stage of 'currently recruiting', which seemed to meet the inclusion criteria of the review. In this randomised study, conducted at the School of Medicine at Nihon University, efficacy and safety of eszopiclone was examined for the treatment of insomnia complicated with nocturnal awakenings. The other ongoing study, under consideration after the update search in February 2018, was (NCT02456532).
Studies awaiting classification
A total of six studies have been identified as 'awaiting classification'.
The search in February 2016 identified four RCTs (NCT00392041; NCT00435279; NCT00374556; Pinto 2016/NCT01100164) with a completed or unknown recruitment status in registry databases (WHO trials portal; ClinicalTrials.gov); no study publications were identified at this time. Accordingly, eligibility of the trials could not be conclusively assessed on the basis of published materials and requests to investigators. These studies included RCTs on eszopiclone in the treatment of insomnia associated with fibromyalgia (NCT00392041), with major depressive disorder (NCT00435279), with osteoarthritis (NCT00374556), and of primary insomnia according to DSM‐4 (Pinto 2016). Accordingly, the eligibility of the studies will be checked again in updates of the review.
The update search in February 2018 identified a further two studies awaiting classification (Baran 2017) and (Buxton 2017) (see Results of the search).
Risk of bias in included studies
For details of the risk of bias judgements for each study, see Characteristics of included studies. Graphical representations of the overall risk of bias in included studies are presented in Figure 2 and Figure 3.
2.
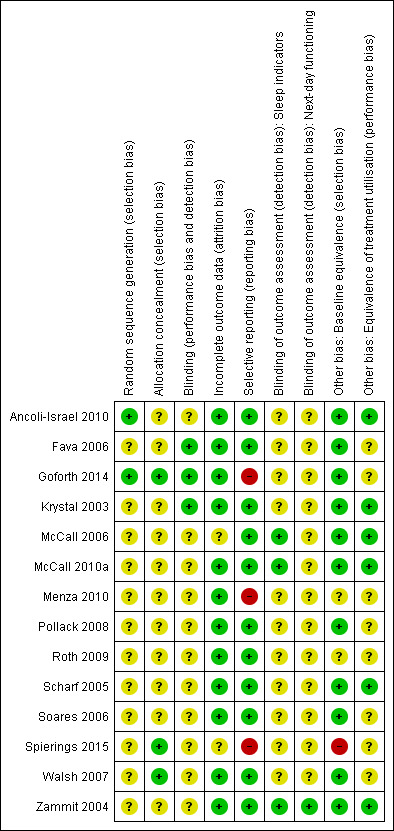
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
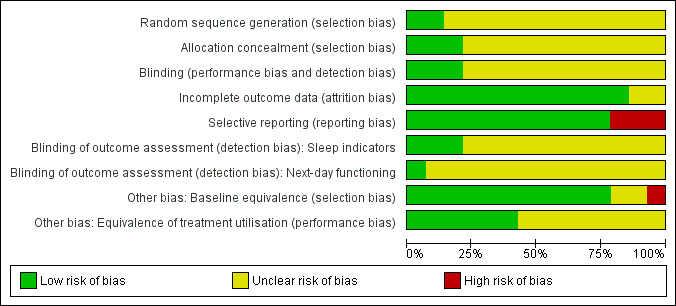
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
Methods used for sequence generation were specified in two RCTs (Ancoli‐Israel 2010; Goforth 2014), describing the generation of the random allocation schedule as being based on an internet randomisation system (Ancoli‐Israel 2010) and a computer‐driven pseudo‐random number generator (Goforth 2014). Accordingly, methods used for sequence generation were rated as being adequate in two RCTs (Ancoli‐Israel 2010; Goforth 2014) and unclear for the remaining 12 studies.
Allocation concealment
Randomisation was described as centralised and conducted by an independent support unit remote from participant recruitment centres in three RCTs (Goforth 2014; Spierings 2015; Walsh 2007). Goforth 2014 additionally reported that drug capsules were supplied by the sponsor in sequentially numbered pill containers and that the random allocation sequence was only provided to the investigators after all subjects had completed the study. The remaining 11 RCTs did not specify methods used for allocation concealment. Applying our criteria for adequate allocation concealment (centralised drug preparation performed remote from the participant recruitment), risk of bias in the randomisation process was rated as being low in three of 14 RCTs (Goforth 2014; Spierings 2015; Walsh 2007).
Baseline equivalence
Baseline equivalence for age, gender, and indicators of sleep initiation and maintenance are were confirmed in 10 of 14 RCTs (Ancoli‐Israel 2010; Fava 2006; Goforth 2014; Krystal 2003; McCall 2006; McCall 2010a; Pollack 2008; Scharf 2005; Soares 2006; Walsh 2007) and in one trial (Zammit 2004), gender differences between treatment groups were detected, but adequately controlled in the statistical analyses. In all RCTs including insomnia associated with comorbid conditions, baseline equivalence for comorbid symptoms was tested and confirmed (Fava 2006; Goforth 2014; McCall 2010a; Pollack 2008; Roth 2009; Soares 2006) or, if differences were shown (Menza 2010), these were controlled in the statistical analyses. All in all, three RCTs did not fulfil our criteria for baseline equivalence (baseline equivalence or control for age, gender, sleep initiation, sleep maintenance, and comorbidity), including two RCTs (Menza 2010; Roth 2009), which did either not provide information on gender or baseline sleep initiation and one RCT (Spierings 2015) identifying a significant group difference for sleep latency at baseline, which was not reported to be controlled.
Blinding
Blinding integrity was described as being tested and confirmed in one trial (Goforth 2014), and two trials (Fava 2006; Krystal 2003) tested adherence and treatment success of participants who perceived an unpleasant taste and found consistent results for the entire sample. Accordingly, the risk of unmasking blinding was rated as being low in three RCTs (Fava 2006; Goforth 2014; Krystal 2003), while for the remaining 11 RCTs, the risk was judged as being uncertain.
Incomplete outcome data
Two RCTs included in the review did not provide information on the principles of analysis used in the study (McCall 2006) or applied further criteria such as compliance at least five days per week for the first two weeks (Spierings 2015). For the remaining 12 of 14 RCTs, it was reported that statistical analyses were conducted according to the intention‐to‐treat principle, analysing all randomised participants (Krystal 2003; McCall 2010a; Menza 2010; Soares 2006) or those who have received at least one dose of treatment (treatment‐received analysis; Ancoli‐Israel 2010; Fava 2006; Goforth 2014; Pollack 2008; Roth 2009; Scharf 2005; Walsh 2007; Zammit 2004) in the group they had been allocated to by randomisation. When analysing the ITT (intention‐to‐treat) population comprising all randomised participants, the last‐observation‐carried‐forward (LOCF) technique was used to impute missing data in seven studies (Ancoli‐Israel 2010; Fava 2006; Goforth 2014; Krystal 2003; McCall 2010a; Pollack 2008; Walsh 2007). All in all, 12 of 14 RCTs (Krystal 2003; McCall 2010a; Menza 2010; Soares 2006; Ancoli‐Israel 2010; Fava 2006; Goforth 2014; Pollack 2008; Roth 2009; Scharf 2005; Walsh 2007; Zammit 2004) met our criteria for an adequate handling of incomplete outcome data.
Selective reporting
Outcomes listed in the methods section were adequately reported and properly interpreted in all but one trial (Spierings 2015), which mentioned TST during the run‐out period as a secondary outcome in the methods section, but did not provide results in the result section. All trials included in the review considered both indicators of sleep induction and sleep maintenance as primary or secondary endpoints. Nevertheless, outcome diversity was limited in two trials (Goforth 2014; Menza 2010), which had a study duration assumed to be sufficient to conclusively assess withdrawal and rebound insomnia, while these variables had not been assessed or at least reported as being assessed. Accordingly, 11 of 14 RCTs (Ancoli‐Israel 2010; Fava 2006; Krystal 2003; McCall 2006; McCall 2010a; Pollack 2008; Roth 2009; Scharf 2005; Soares 2006; Walsh 2007; Zammit 2004) fulfilled our criteria of adequate outcome reporting and outcome diversity.
Other potential sources of bias
Performance bias
Nine of 14 RCTs tested and confirmed the equivalence of medication compliance between groups (Ancoli‐Israel 2010; Fava 2006; Krystal 2003; McCall 2006; McCall 2010a; Scharf 2005; Soares 2006; Walsh 2007; Zammit 2004), while the remaining five RCTs (Goforth 2014; Menza 2010; Pollack 2008; Roth 2009; Spierings 2015) did not provide such information. With the exception of one trial (Goforth 2014), studies permitting comedication for comorbid conditions tested and confirmed the equivalence of medication for comorbid conditions between groups (Fava 2006; McCall 2010a; Menza 2010; Pollack 2008; Roth 2009; Soares 2006; Spierings 2015); in two of the comorbidity studies with the option of dose titration for antidepressive comedication (Fava 2006; McCall 2010a), differences in titrations were tested between groups. The use of further medication was allowed in some studies (Ancoli‐Israel 2010; Fava 2006; Menza 2010; Roth 2009; Scharf 2005; Walsh 2007); three of these (Ancoli‐Israel 2010; Roth 2009; Scharf 2005) compared the use of concomitant medication and confirmed the equivalence between groups. All trials including elderly participants (Ancoli‐Israel 2010; McCall 2006; Scharf 2005) compared daytime napping between groups to control for the occurrence of compensatory sleep. Applying all criteria for an equivalent treatment utilisation (equivalence of medication compliance, use of further medications and daytime napping) simultaneously, six of 14 RCTs (Ancoli‐Israel 2010; Krystal 2003; McCall 2006; McCall 2010a; Scharf 2005; Zammit 2004) were rated to have a low risk of performance bias.
General susceptibility to bias
Eleven of 14 RCTs assessed sleep efficacy outcomes exclusively on the basis of participant reports using an electronic sleep diary (Ancoli‐Israel 2010; Pollack 2008; Menza 2010), paper sleep diary (Spierings 2015), or interactive voice response system (IVRS; Krystal 2003; McCall 2006McCall 2010a; Menza 2010; Scharf 2005; Soares 2006; Walsh 2007; Zammit 2004), while three RCTs McCall 2006; McCall 2010a; Zammit 2004) combined self‐report measures with polysomnography (PSG) (McCall 2006; McCall 2010a; Zammit 2004) and actigraphy recording (McCall 2010a). Thus, susceptibility to bias effects for sleep outcomes was rated as being low for three of 14 RCTs (McCall 2006; McCall 2010a; Zammit 2004) and as being uncertain for the remaining 11 RCTs (Ancoli‐Israel 2010; Fava 2006; Goforth 2014; Krystal 2003; Menza 2010; Pollack 2008; Roth 2009; Scharf 2005; Soares 2006; Spierings 2015; Walsh 2007). With the exception of one RCT (Zammit 2004), which assessed next‐day functioning by self‐reports and the Digit‐Symbol Substitution Test (DSST), RCTs included in the review measured functioning during the next day on the basis of participant reports. Accordingly, susceptibility to bias effects for next‐day functioning was rated as being low in Zammit 2004 and as being uncertain in the remaining 13 RCTs (Ancoli‐Israel 2010; Fava 2006; Goforth 2014; Krystal 2003; McCall 2006; McCall 2010a; Menza 2010; Pollack 2008; Roth 2009; Scharf 2005; Soares 2006; Spierings 2015; Walsh 2007).
Publication bias
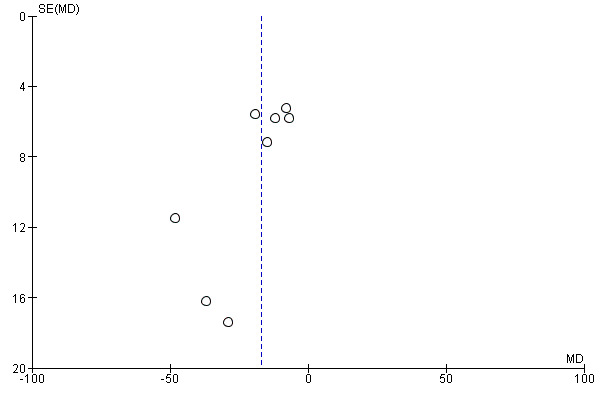
By plotting of the mean differences against their standard error for the primary efficacy outcomes SOL (Figure 4) and WASO (Figure 5), we did not identify asymmetry, but note that the interpretation of funnel plot graphs was impeded by the small number of included studies, limiting the conclusiveness of the funnel plot method.
4.
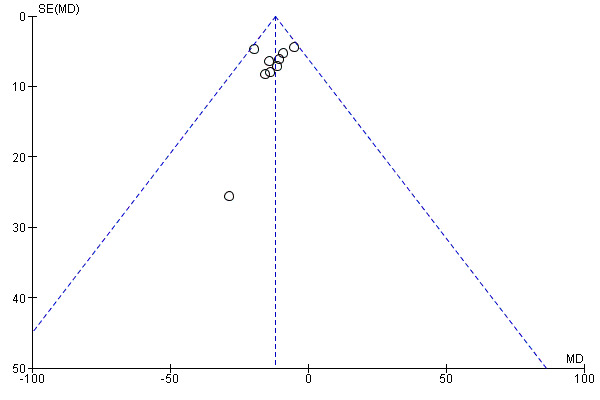
Funnel plot of comparison: 1 Eszopiclone versus placebo, outcome: 1.1 Sleep onset latency (SOL).
5.
Funnel plot of comparison: 1 Eszopiclone versus placebo, outcome: 1.2 Wake time after sleep onset (WASO).
Effects of interventions
See: Table 1
Comparison 1: Eszopiclone versus placebo
Of 14 RCTs included in the review, 13 RCTs contributed to the meta‐analysis. One RCT (McCall 2010a) provided statistics for repeated measures mixed model analyses, which could not be integrated into meta‐analyses. From the two RCTs using a three‐armed design (Scharf 2005; Zammit 2004), only the study arms with eszopiclone under recommended dosing (3 mg for non‐elderly, 2 mg for elderly participants) and placebo were included. Results for the primary and secondary outcomes of the review are described below and outlined for the most important findings in the Table 1.
Primary outcomes
1.1 Sleep onset latency (SOL)
Meta‐analyses of participant‐reported data show that eszopiclone significantly decreased length of time after lights‐out until sleep by approximately 12 minutes (Mean Difference (MD) ‐11.94 min, 95% confidence interval (CI) ‐16.03 to ‐7.86; participants = 2890; studies = 9; I2 = 0%; moderate quality evidence; Analysis 1.1) compared to placebo.
1.1. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 1 Sleep onset latency (SOL).
1.2 Wake time after sleep onset (WASO)
Compared to placebo, eszopiclone significantly reduced participant‐reported wake time after sleep onset by about 17 minutes (MD ‐17.02 min, 95% CI ‐24.89 to ‐9.15; participants = 2295; studies = 8; I2 = 55%; moderate quality evidence; Analysis 1.2).
1.2. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 2 Wake time after sleep onset (WASO).
1.3 Withdrawal symptoms
Following drug discontinuation during single‐blind placebo run‐out periods, a total of 22 new or deteriorated adverse events were documented from seven RCTs with 3125 participants (Analysis 1.3). The overall risk of being affected by withdrawal symptoms did not differ between the eszopiclone and the placebo group (RD 0.00, 95% CI ‐0.03 to 0.04; participants = 2103; studies = 5; I2 = 42%). Accidental injury, agitation, anxiety, back pain, dizziness, headache, nausea, pharyngitis, and pain were listed in more than one study, with headache being the most frequently reported symptom (RD 0.00, 95% CI ‐0.01 to 0.01; participants = 2237; studies = 6; I2 = 0%; Analysis 1.3). Nevertheless, for the 22 adverse events reported during the single‐blind placebo run‐out, the risk difference was not shown to significantly differ between groups.
1.3. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 3 Withdrawal symptoms.
1.4 Rebound insomnia
Mean change from baseline values for the primary efficacy outcomes SOL (MD 17.00 min, 95% CI ‐4.29 to 38.29; participants = 291; studies = 1; I2 and T2: not applicable; low quality evidence) and WASO (MD ‐6.71, 95% CI ‐21.25 to 7.83; participants = 291; studies = 1; I2 and T2: not applicable; low quality evidence) averaged over the first three nights of the single‐blind run‐out period did not indicate worsening of sleep after drug discontinuation (Analysis 1.4). Negative signs of change from baseline values, shown for each single night during the discontinuation period in the eszopiclone group as well as the placebo group (data not shown), indicated that hypnotic efficacy measures improved after treatment, irrespective of treatment condition.
1.4. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 4 Rebound insomnia.
Secondary outcomes
1.5 Total sleep time (TST)
Compared to placebo, eszopiclone significantly increased total sleep time by about 28 minutes (MD 27.70 min, 95% CI 20.30 to 35.09; participants = 2965; studies = 10; I2 = 39%; moderate quality evidence; Analysis 1.5).
1.5. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 5 Total sleep time (TST).
1.6 Next‐day alertness
Meta‐analytic results showed that next‐day alertness during double‐blind treatment was rated as being significantly higher in the eszopiclone than in the placebo group (MD 0.46, 95% CI 0.28 to 0.63; participants = 2061; studies = 8; I2 = 31%; low quality evidence; Analysis 1.6).
1.6. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 6 Next‐day alertness.
1.7 Adverse events
Compared to participants in the placebo group, participants treated with eszopiclone did not significantly differ in their risk of dropping out from treatment due to adverse events (RD 0.01, 95% CI ‐0.01 to 0.02; participants = 4007; studies = 11; I2 = 52%; moderate quality evidence; Analysis 1.7). Among a total of 34 adverse events, headache (n = 10), unpleasant taste (n = 9) and somnolence (n = 8) were most commonly reported, followed by accidental injury and dizziness (n = 7) as well as and back pain and dry mouth (each reported in six RCTs). Significant risk differences were demonstrated for unpleasant taste (RD 0.18, 95% CI 0.14 to 0.21; NNTH = 5.6, 95% CI 4.8 to 7.1; participants = 3787; studies = 9; I2 = 72%), dry mouth (RD 0.04, 95% CI 0.02 to 0.06; NNTH = 25, 95% CI 16.7 to 50.0; participants = 2802; studies = 6; I2 = 11%), somnolence (RD 0.04, 95% CI 0.02 to 0.06; NNTH = NNTH = 25, 95% CI 16.7 to 50.0; participants = 3532; studies = 8; I2 = 10%) and dizziness (RD 0.03, 95% CI 0.01 to 0.05; NNTH = 33.3, 95% CI 20.0 to 100.0; participants = 2933; studies = 7; I2 = 48%).
1.7. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 7 Adverse events.
Serious adverse events occurred in eight of 12 RCTs (Ancoli‐Israel 2010; Fava 2006; Krystal 2003; McCall 2006; Pollack 2008; Roth 2009; Scharf 2005; Walsh 2007). There was no significant difference between groups in the occurrence of serious adverse events (RD 0.00, 95% CI ‐0.01 to 0.01; participants = 4289; studies = 12; I2 = 0%; T2 = 0.00). Serious adverse events observed in the eszopiclone groups included suicide and death due to arteriosclerotic heart disease (Ancoli‐Israel 2010), anxiety and confusion (Fava 2006), gastrointestinal disorder (Krystal 2003), moderate to severe chest pain (Krystal 2003; Roth 2009; Scharf 2005), accidental injury due to a fall (McCall 2006), asthma, cholelithiasis, concussion with multiple fractures and loss of consciousness (Pollack 2008) and cerebrovascular accident (Walsh 2007). With the exception of one RCT, in which serious adverse events in 0.34% of participants (2/593) taking eszopiclone over the 6‐month treatment were considered to be “possibly related” to therapy (Krystal 2003), serious adverse events in further trials were not considered by the investigators to be treatment‐related. Also, where accidental injury due to a fall occurred two days after the end of treatment (McCall 2006) or due to slipping on a wet floor in the late afternoon (Pollack 2008), investigators classified these serious events as unrelated to treatment.
Subgroup analyses
Primary versus comorbid insomnia
Subgroup analyses for different types of insomnia showed significant effects for eszopiclone in samples with primary insomnia (SOL: MD ‐15.14 minutes, 95% CI ‐21.13 to ‐9.15; participants = 1803; studies = 5; I2 = 0%; WASO: MD ‐15.76 min, 95% CI ‐25.60 to ‐5.92; participants = 1803; studies = 5; I2 = 68%; TST: MD 30.04 min, 95% CI 19.09 to 40.98; participants = 1878; studies = 6; I2 = 52%) and comorbid insomnia as well (WASO: MD ‐21.20 min, 95% CI ‐40.76 to ‐1.65; participants = 462; studies = 2; I2 = 36%; TST: MD 23.37 min, 95% CI 12.61 to 34.12; participants = 462; studies = 2; I2 = 0%), except for SOL, which did not reach statistical significance in the comorbid insomnia sample (SOL: MD ‐8.10 min, 95% CI ‐17.77 to 1.57; participants = 462; studies = 2; I2 = 24%).
Sleep efficacy outcomes, separately analysed for primary insomnia and comorbid insomnia subgroups, are shown in Analysis 1.8 for SOL, Analysis 1.9 for WASO, and Analysis 1.10 for TST.
1.8. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 8 Subgroups: insomnia type ‐ SOL.
1.9. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 9 Subgroups: insomnia type ‐ WASO.
1.10. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 10 Subgroups: insomnia type ‐ TST.
Young to middle‐aged versus older age
Analyses for the subgroups of young to middle‐aged and older age individuals demonstrated significant effects in samples aged between 18 to 64 years (SOL: MD ‐13.08 minutes, 95% CI ‐19.15 to ‐7.00; participants = 2049; studies = 5, I2 = 25%; WASO: MD ‐12.20 minutes, 95% CI ‐19.02 to ‐5.37; participants = 1454; studies = 4; I2 = 5%; TST: MD 29.66 minutes, 95% CI 21.60 to 37.72; participants = 2124; studies = 6; I2 = 30%) and samples with an age over 64 years (SOL: MD ‐11.41 minutes, 95% CI ‐20.37 to ‐2.45; participants = 811; studies = 3; I2 = 0%; WASO: MD ‐22.16 minutes, 95% CI ‐40.70 to ‐3.63; participants = 811; studies = 3; I2 = 81%; TST: MD 27.01 minutes, 95% CI 11.83 to 42.18; participants = 811; studies = 3; I2 = 49%). Sleep efficacy outcomes separately analysed for different age subgroups are shown in Analysis 1.8 for SOL, Analysis 1.9 for WASO, and Analysis 1.10 for TST.
Next‐day alertness was significantly increased by 0.56 points on the 11‐point Likert scale in young to middle‐aged individuals (MD 0.56, 95% CI 0.37 to 0.75; participants = 1220; studies = 4; I2 = 0%) and 0.34 points in the elderly (MD 0.34, 95% CI 0.01 to 0.67; participants = 811; studies = 3; I2 = 58%). Analyses of serious adverse events, limited to the subgroup of elderly participants, did not show a difference between the eszopiclone and placebo condition (RD 0.00, 95% CI ‐0.01 to 0.02; participants = 804; studies = 3; I2 = 58%). Serious adverse events in the elderly included suicide and death due to arteriosclerotic heart disease (Ancoli‐Israel 2010), moderate to severe chest pain (Scharf 2005) and accidental injury due to a fall, which occurred two days after the end of treatment (McCall 2006) and was classified by the investigator as unrelated to treatment. Compared to participants in the placebo group, elderly participants treated with eszopiclone did not significantly differ in their risk of dropping out from treatment due to adverse events (RD ‐0.00, 95% CI ‐0.03 to 0.03; participants = 811; studies = 3; I2 = 30%). Among a total of 19 adverse events reported in participants aged over 64 years, significant risk differences were demonstrated for unpleasant taste (RD 0.11, 95% CI 0.08 to 0.15; participants = 811; studies = 3; I2 = 0%), dry mouth (RD 0.07, 95% CI 0.02 to 0.12; participants = 264; studies = 1), and dizziness (RD 0.03, 95% CI 0.01 to 0.06; participants = 652; studies = 2; I2 = 0%). For an overview of adverse events analyses in elderly subgroups, see Analysis 1.15.
1.15. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 15 Subgroups: older participants ‐ adverse events.
Study initiation
Subgroup analyses for type of study initiation showed significant effects in investigator‐initiated trials (SOL: MD ‐8.29 minutes, 95% CI ‐14.24 to ‐2.34; participants = 677; studies = 3; I2 = 0%) (WASO: MD ‐33.29 minutes, 95% CI ‐56.47 to ‐10.10; participants = 82; studies = 2; I2 = 0%) and sponsor‐initiated trials (SOL: MD ‐15.21 min, 95% CI ‐20.83 to ‐9.59; participants = 2213; studies = 6; I2 = 0%) (WASO: MD ‐15.31 min, 95% CI ‐23.50 to ‐7.11; participants = 2213; studies = 6; I2 = 61%) (TST: MD 28.40 min, 95% CI 19.60 to 37.21; participants = 2288; studies = 7; I2 = 48%), except for TST, which was not significant in investigator‐initiated trials (TST: MD 21.04 minutes, 95% CI ‐4.19 to 46.27; participants = 677; studies = 3; I2 = 41%).
Sleep efficacy outcomes, separately analysed for sponsor‐initiated versus investigator‐initiated, are shown in Analysis 1.16 for SOL, Analysis 1.17 for WASO and Analysis 1.18 for TST.
1.16. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 16 Subgroups: study initiation ‐ SOL.
1.17. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 17 Subgroups: study initiation ‐ WASO.
1.18. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 18 Subgroups: study initiation ‐ TST.
Sensitivity analyses
Assessment of sleep outcomes
Effect estimates based on objective assessment methods such as polysomnography and actigraphy were slightly lower in their magnitude (SOL: MD ‐15.50 min, 95% CI ‐19.89 to ‐11.11; participants = 468; studies = 2; WASO: MD ‐12.37 min, 95% CI ‐18.61 to ‐6.13; participants = 468; studies = 2; I2 = 0%) (TST: MD 28.60 min, 95% CI 18.14 to 39.06; participants = 264; studies = 1) than participant‐reported outcomes, while still reaching statistical significance. Accordingly, the demonstration of effects of eszopiclone on sleep efficacy outcomes did not seem to depend on type of measurement (Analysis 1.19).
1.19. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 19 Sensitivity: sleep assessment.
Assessment of withdrawal
Withdrawal assessed with the BWSQ scale (SMD ‐0.06, 95% CI ‐0.26 to 0.14; participants = 1218; studies = 2) did not indicate a significant difference between the eszopiclone and placebo group, confirming the findings on reported events (Analysis 1.20).
1.20. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 20 Sensitivity: withdrawal assessment.
Discussion
Summary of main results
A total of 14 RCTs with 4732 participants were included in this review. Most RCTs included in the review covered short‐term (≤ four weeks; six RCTs) and medium‐term treatment with eszopiclone (> four weeks ≤ six months; five RCTs), with three RCTs having a treatment duration of 12 months or more. Eszopiclone was provided in a dose of 3 mg for non‐elderly and 2 mg for elderly individuals.
Meta‐analyses of participant‐reported data on sleep efficacy outcomes demonstrated a 12‐minute decrease of SOL, a 17‐minute decrease of WASO and an approximate 28‐minute increase of TST for eszopiclone compared to placebo. There were no significant changes from baseline to the first night after drug discontinuation for SOL and WASO in the majority of trials and no significant differences between groups in the prevalence of new or worsening adverse events. Participant‐reported data also indicated that next‐day alertness significantly improved under eszopiclone compared to placebo, while adverse events, documented significantly more frequently under eszopiclone compared to placebo, included unpleasant taste, dry mouth, somnolence, and dizziness.
Subgroup analyses indicated that eszopiclone improved most sleep efficacy outcomes irrespective of insomnia type (primary and comorbid insomnia), age groups (young to middle‐aged and elderly individuals) and study initiation (investigator initiation and sponsor initiation). The statistical and qualitative integration of evidence from RCTS indicated moderate, but robust, therapeutic effects of eszopiclone on sleep efficacy outcomes. Nevertheless, safety should be determined on the base of individual risk patterns and monitored closely during treatment.
When counterbalancing risks against benefits, a half an hour increase of sleep time per night might not seem much at first glance. However, it represents a mean value averaged over nights of poor sleep and good nights` sleep, both contributing to the night‐to‐night variability of sleep in insomniacs (Valieres 2005). A limitation of eszopiclone intake to nights of poor sleep according to 'treatment as needed' can be expected to clearly exceed demonstrated effects. Intermittent dosing or 'treatment as needed' with eszopiclone might be an alternative to daily scheduled treatment and bring about advantages in terms of habituation and discontinuation effects that has to be tested in further trials.
Overall completeness and applicability of evidence
Through including insomnia as a primary or a comorbid condition, the review concerns a wide range of insomniac problems and approximates the distribution of insomniac conditions in the general population (Katz 1998). In addition, with a mean age between 40 and 50 years and the percentage of women varying between 63% and 67%, the distribution of age and gender in the primary studies corresponds with the larger insomnia population (Delahaye 1990; Zammit 2004). Contextual factors, such as length of treatment and comedications use, further contribute to the variety of treatment conditions. Nevertheless, due to reasons of safety and accessibility, certain subgroups of individuals might be underrepresented in clinical studies with eszopiclone. This concerns elderly participants with cognitive and psychomotor impairments, shown to have an increased risk for falls, serious injury, and hip fractures (Berry 2013) and individuals with substance use disorder, who might be at increased risk of using eszopiclone in an unrecommended way. Further limitations in the external validity might arise from the cultural context of clinical research. With the exception of one trial (Soares 2006), study sites were exclusively located in the United States. Even though post hoc analyses of studies with eszopiclone indicated the generalisability of findings across different ethnicities (McCall 2006), cultural differences in values, norms, and health‐related beliefs might play a role in treatment utilisation, length of use, dosing, and compliance. With placebo as a comparator, integrated evidence on eszopiclone is only applicable to therapeutic decisions that concern eszopiclone versus 'no treatment', while for recommendations concerning the relative efficacy and safety of eszopiclone compared to other available interventions, no direct evidence is available.
All in all, the nonrestrictive definition of inclusion criteria in terms of comorbidity, gender, age, and treatment conditions contributes to the external validity of the review and increases the applicability of findings to everyday clinical practice. Limitations in the variability of participant characteristics and treatment conditions originating from the cultural context, criteria of inclusion, differences in accessibility to clinical research and the monitoring of treatment implementation in clinical trials, have to be taken into consideration when determining the applicability of evidence. Thus, a weighting of risks and benefits for prescribing eszopiclone has always been made against the background of individual participant characteristics, particularly those associated with a patients' vulnerability to adverse events and those influencing medication‐taking behaviour. As placebo was the only comparator considered in RCTs with eszopiclone, available evidence did not allow conclusions on the superiority or inferiority of eszopiclone compared to alternative therapeutic options.
Quality of the evidence
Applying GRADE criteria for down‐ and upgrading the quality of evidence, we rated the overall quality as being moderate for sleep efficacy outcomes and adverse events and as being low for rebound effects and next‐day functioning. We downgraded quality to moderate because of threats to bias resulting from incomplete reporting of certain design features and unmatched taste of eszopiclone. Downgrading quality of evidence for rebound insomnia to a low grade was based on the poor study design of most studies for assessing discontinuation effects, while for next‐day functioning, the assumed inadequacy of subjective reporting for representing clinically relevant qualities of functioning caused downgrading of the evidence. Thus, due to methodological limitations, such as the open‐label design of run‐out intervals in some trials and a potential lack of sensitivity to subjective measures for detecting psychomotor and cognitive impairments, safety conclusions of the review concerning rebound insomnia and next‐day functioning might be of limited validity. Quality rating for each outcome is shown in the summary of findings table (Table 1), and the rating of single GRADE criteria for downgrading (risk of bias, inconsistency, indirectness, imprecision, publication bias) is outlined in detail in the following.
Risk of bias: efficacy outcomes
Various design characteristics of the RCTs included in the review ensured the methodological quality of evidence. Most studies (12 of 14 RCTs) stated that participants were randomly assigned to treatment groups to prevent selection bias and to ensure equivalence between groups at baseline, and treatment and placebo groups were compared for age, gender and for indicators of sleep initiation and sleep maintenance (11 of 14 RCTs). All participants, or at least those who have received at least one dose of treatment, were analysed in the group they had been allocated to by randomisation (12 of 14 RCTs) to avoid attrition bias, while the risk of performance bias was limited by ensuring the equivalence between groups in the use of concomitant medication for comorbid disorders (seven of eight RCTs), in the use of substances with a secondary effect on sleep (three of six RCTs) and in medication compliance (nine of 14 RCTs). In addition, all RCTs including elderly participants compared treatment groups for daytime napping to control the occurrence of compensatory sleep as a further risk of performance bias. Outcomes listed in the method sections were adequately reported and properly interpreted and all RCTs included in the review considered both indicators of sleep induction and sleep maintenance as endpoints of the statistical analyses at the same time.
Despite these measures, some uncertainties persisted. Since specific features of the study design were omitted from most trial reports, it remained unclear whether these had not been implemented or whether these had been implemented, but not reported. Frequently omitted information concerned the specification of methods used for sequence generation, allocation concealment, and blinding procedures. Unclear concealment, has repeatedly been shown to be associated with bias effects in various fields of clinical research (Huwiler‐Muntener 2002; Pildal 2007; Schulz 1995). In addition, subject‐specific threats to bias might have arisen from eszopiclone`s unpleasant taste, which was reported by a considerable proportion of participants treated with eszopiclone (see Table 3). Drug characteristics, revealing the identity of medication to participants or investigators and their impact on estimates of efficacy, have strikingly been shown in the example of antidepressants drugs (Moncrieff 2004). Even though perception of unpleasant taste has not been significantly associated with treatment adherence or success in selected studies (Fava 2006; Krystal 2003), the evidence removed all doubts. Accordingly, we downgraded the quality of evidence to a moderate degree for sleep efficacy outcomes and adverse events.
2. Rates of unpleasant taste.
| Author | ESZ (%) | PBO (%) |
| Ancoli‐Israel 2010 | 12.4 | 1.55 |
| Fava 2006 | 22.7 | 6.93 |
| Krystal 2003 | 26.1 | 5.64 |
| McCall 2006 | 12.5 | 0.00 |
| Pollack 2008 | 24.1 | 3.68 |
| Roth 2009 | 27.3 | 0.00 |
| Scharf 2005 | 11.4 | 1.25 |
| Soares 2006 | 17.4 | 0.48 |
| Walsh 2007 | 19.7 | 1.07 |
| Zammit 2004 | 33.3 | 3.03 |
ESZ: eszopiclone
PBO: placebo
Risk of bias: rebound effects
For rating the quality of evidence on discontinuation effects, methodological features of the interval subsequent to the randomised controlled treatment period were taken into consideration. Seven of the 12 RCTs exceeding the threshold duration of two weeks for assessing discontinuation effects applied a single‐blind placebo run‐out period to assess change from baseline for sleep efficacy outcomes, while the remaining five RCTs (with a duration > two weeks) applied open‐label extensions, naturalistic follow‐up, or no follow‐up. Considering the latter as not being appropriate for controlling bias effects, we downgraded the quality of evidence for rebound insomnia outcomes to a low degree.
Inconsistency
Even though some inconsistency of results has been shown for the primary efficacy outcome WASO, heterogeneity appeared to be mainly attributable to one trial (Scharf 2005), whose exclusion resulted into a I2 reduction from 55% to 6%. Additionally, considering the low to moderate heterogeneity of results shown for further outcomes of the review (SOL: 0%; serious adverse events: 0%; TST: 31%; next‐day alertness: 31%), consistency of results was considered as not being serious.
Indirectness
With placebo as a comparator, integrated evidence on eszopiclone was only applicable to therapeutic decisions that concerned eszopiclone versus 'no treatment', while for recommendations concerning the relative efficacy and safety of eszopiclone compared to other available interventions, no direct evidence was available. On the other hand, study samples, features of the therapeutic interventions (duration, dosing, etc.), and outcomes of the RCTs included in the review contributed to the directness of evidence in terms of population, intervention, comparator and efficacy outcomes, not raising considerable uncertainty about the applicability of the evidence to the relevant questions of daily practice. Thus, we did not rate limitations in directness of evidence as being serious for sleep efficacy outcomes and discontinuation effects. In contrast, we downgraded evidence due to indirectness for subjective next‐day functioning. In contrast to subjective efficacy outcomes, it was expected that the objective rather than the subjective measures of next‐day functioning might have determined the risk of harm, including injuries and accidents. Thus, questioning the clinical relevance of subjective next‐day functioning (compared to objective measures only applied in one RCT), we rated the directness of evidence for next‐day functioning as being limited and downgraded the evidence for next‐day functioning to low‐quality.
Imprecision
Eleven of 14 RCTs included in the review were multicentre trials based on large study samples that allowed precise estimations of eszopiclone`s efficacy and safety; thus we did not judge the quality of evidence to be lowered by imprecision.
Publication and funding bias
With the exception of two non‐profit funded RCTs (McCall 2010a; Pollack 2008), trials included in the review were financially supported by the pharmaceutical industry. As subgroup analyses according to 'sponsoring type' would not be conclusive, due to the imbalance of sample size between non‐profit sponsored (two RCTs) and industry‐sponsored (10 RCTs) subgroups, we formed subgroups according to the type of study initiation. Comparisons of effects from investigator‐ versus sponsor‐initiated trials did not demonstrate statistically significant differences (Analysis 1.16; Analysis 1.17; Analysis 1.18). Plotting of the mean differences against standard errors for SOL (Figure 4) and WASO (Figure 5) did not indicate asymmetry, but, due to the small number of included studies, the conclusiveness of the funnel plot method was limited (see Other potential sources of bias). Nevertheless, a suspicion of funding bias (Lexchin 2003) remained due to the overweight of industry‐sponsored trials.
Potential biases in the review process
Even though, according to the standards of the Cochrane Collaboration, various strategies have been implemented in the planning and conduction of the review to limit bias in the review process and to increase research transparency, a number of methodological decisions were left to the authors and are discussed for their impact on efficacy and safety conclusions of the review in the following comments.
We decided to exclude studies with a cross‐over design due to the fact that sleep stabilising effects of eszopiclone have been reported even after drug discontinuation (Zammit 2004), making it difficult to control carry‐over effects by wash‐out. We identified two studies with a cross‐over design fulfilling further inclusion criteria of our review (Erman 2008; Joffe 2010), both confirming the sleep‐promoting effects of eszopiclone. Accordingly, we assumed that our decision to include parallel group studies was unlikely to have affected the efficacy and safety conclusions of the review.
Treatment effectiveness was assessed through two outcomes: 1. 'sleep onset latency' (SOL) defined as the length of time (in minutes) after lights‐out until sleep onset, and 2. 'wake time after sleep onset' (WASO), defined as the length of time (in minutes) of wakefulness after the onset of persistent sleep. The consideration of two effectiveness outcomes was reasoned by their conceptual distinctiveness, with SOL measuring a drug's impact on sleep onset, and WASO measuring its potential to improve sleep maintenance. Thereby, the former reflected its suitability for the treatment of sleep‐onset insomnia and the latter for sleep‐maintenance insomnia (see Description of the condition). Even though assessed on the base of conceptual distinctive outcomes, sleep efficacy outcomes of the review might not have captured the full extent of insomnia. Nevertheless, to control for Type I error and to ensure clarity and comprehensibility of the review, outcomes were limited on the base of theoretical considerations.
A further important methodological decision concerned the 'type of participants' considered as eligible for the review. The original protocol had limited 'type of participants' to patients with primary insomnia. A careful weighing of the available evidence and a reconsideration of the referees` comments made us extend the inclusion criteria by including both primary and secondary insomnia. Our arguments for doing so are outlined in the section Differences between protocol and review. Primary and comorbid insomnia was considered as criteria for subgroup analyses (Analysis 1.8; Analysis 1.9; Analysis 1.10), allowing a statistical analysis of the impact that type of insomnia diagnosis had on efficacy outcomes of the review.
A limitation arose from the asymmetric distribution of primary sleep efficacy data and the provision of outcome statistics as medians in some studies, which had to be excluded from pooling of continuous data (Hozo 2005). The problem marginally concerned sleep efficacy outcomes, provided as means in the majority of studies (Ancoli‐Israel 2010; Goforth 2014; Krystal 2003; McCall 2010a; Menza 2010; Pollack 2008; Scharf 2005; Soares 2006; Spierings 2015; Zammit 2004), but affected rebound effects to a more considerable extent. Effect estimations for change from baseline during run‐out intervals were based on only one trial (Ancoli‐Israel 2010), while outcomes from the remaining trials were provided as medians (Fava 2006; Pollack 2008; Roth 2009; Walsh 2007) or p‐values (Soares 2006; Zammit 2004). Table 4 compared information of significance between studies providing means and medians. Only one trial (Roth 2009), not included in the statistical pooling of data, found a small, non‐significant increase in WASO on day one and three of the run‐out period and a significant decrease in TST on day one.
3. Overview of outcome: means and medians.
| Author | Outcome statistic | Sleep efficacy | Rebound insomnia | ||||
| SOL | WASO | TST | LPS | WASO | TST | ||
| Sign. | Sign. | Sign. | — | — | — | ||
| Ancoli‐Israel 2010 | Means | P < 0.001 | P < 0.001 | P < 0.001 | Ns | Ns | Ns |
| Fava 2006 | Medians | P < 0.001 | P < 0.001 | P = 0.004 | Ns | Ns | Ns |
| Goforth 2014 | Medians, means | P = 0.026 | P < 0.001 | P = 0.017 | — | — | — |
| Krystal 2003 | Medians, means | P < 0.001 | P = 0.032 | P < 0.001 | — | — | — |
| McCall 2006 | Medians, means | P < 0.001 | P = 0.022 | P < 0.001 | — | — | — |
| McCall 2010a | ß, SE | P = 0.008 | P = 0.16 | P = 0.04 | — | — | — |
| Menza 2010 | Means | Ns | P = 0.071. | Ns | — | — | — |
| Pollack 2008 | Medians, means* | P < 0.001* | P < 0.001 | P < 0.001* | Ns | Ns | Ns |
| Roth 2009 | Medians | P < 0.01 | P < 0.01 | P < 0.001 | Ns | Ns | P = 0.01 |
| Scharf 2005 | Medians, means | P = 0.003 | P < 0.04 | P < 0.001 | — | — | — |
| Soares 2006 | Medians, means | P < 0.001 | P < 0.001 | P < 0.01 | Ns | Ns | Ns |
| Spierings 2015 | Means | Nr | Nr | 0.33 | — | — | — |
| Walsh 2007 | Medians | P < 0.001 | P < 0.001 | P < 0.001 | Ns | Ns | Ns |
| Zammit 2004 | Medians, means | P < 0.001 | P = 0.02 | P < 0.001 | Ns | Ns | Ns |
LPS: latency to persistent sleep
Nr: not reported
Ns: not statistically significant (no quantitative information provided)
P: P value
SOL: sleep onset latency
TST: total sleep time
WASO: wake time after sleep onset
bold: not included in the meta‐analyses
A strength of the review might have come from the nonrestrictive definition of inclusion criteria and from analysing the impact of potentially effect‐determining factors on the basis of subgroup and sensitivity analyses. A further strength was owed to the support we received from the primary investigators and further experts (see Acknowledgements), who checked the completeness of our study search and who provided feedback on design characteristics and outcome statistics. Nevertheless, no unpublished trials and data could be included. This, together with the overweight of industry‐sponsored studies, clearly increased the risk of overestimating effects due to funding bias (Lexchin 2003).
Finally, to limit the influence of reviewers interests and expectations, all outcome statistics were extracted by at least two reviewers independently (SR & CE, CE & RW), and disagreements were resolved in consensus discussions between three reviewers (SR, CE, RW). To additionally prevent confirmation bias (Nickerson 1998), at least one author, who had not been involved in insomnia research before, participated in each review step.
Agreements and disagreements with other studies or reviews
Efficacy conclusions of the review largely agree with those of previous reviews and meta‐analyses (Buscemi 2007; Huedo‐Medina 2012; Sateia 2017), suggesting that eszopiclone is an effective therapeutic option in the treatment of insomnia. With a reduction of SOL by about 12 minutes, a decrease of WASO by about 17 minutes and an increase of TST by about 28 minutes, effects are comparable in their magnitude with those shwon by Sateia 2017 for SOL and TST in the 2 mg eszopiclone dosing group (SOL: MD = ‐17.78; 95% CI ‐28.52 to ‐7.04; TST: MD = 27.53; 95% CI 18.29 to 36.76) and for WASO in the 3 mg dosing group (WASO: MD = ‐14.49; 95% CI ‐17.68 to ‐11.69). In contrast to the review at hand, Sateia 2017 included cross‐over trials and provided separate analyses for 2 mg and 3 mg dosing groups. Furthermore, our review excluded medians from pooling of continuous data as suggest by Hozo 2005, which did not allow the inclusion of the largest trial (Walsh 2007) into meta‐analyses of primary sleep efficacy outcomes.
Adverse events identified in the review like unpleasant taste (RD 0.18, 95% CI 0.14 to 0.219), dry mouth (RD 0.04, 95% CI 0.02 to 0.06), somnolence (RD 0.04, 95% CI 0.02 to 0.06) and dizziness (RD 0.03, 95% CI 0.01 to 0.05) have been reported formerly (e.g. Hair 2008; Najib 2006) and are listed in the Lunesta package insert (Lunesta 2004 [pers comm]). In contrast to case‐control studies (Berry 2013; Diem 2014), subgroup analyses of adverse events in samples of the elderly did not identify an increased risk for serious adverse events like falls, injury, and hip fractures, which might be due to the more controlled conditions in clinical trials compared to everyday life. The exclusion of certain participant subgroups and the monitoring of treatment implementation in clinical research might also explain the absence of central nervous system (CNS) side effects of eszopiclone, such as amnesia or hallucinations, which have previously been reported in case studies for racemic zopiclone (Elko 1998; Toner 2000; Tsai 2003) and eszopiclone (Duggal 2007), occurring preferably if hypnotic drugs were taken in high doses or if combined with other psychoactive substances.
Meta‐analyses of participant‐reported next‐day alertness (MD = 0.46; 95% CI 0.28 to 0.63; 8 RCTs; 2061 participants) did not identify residual effects as currently shown in a randomised, double‐blind cross‐over study (Boyle 2012). Reasons for divergent results might be found from a lack of the sensitivity of subjective measures in displaying psychomotor and cognitive impairments or the application of sleep restriction protocols in the cross‐over study. Nevertheless, as a restricted sleep protocol might adequately represent 'likely scenarios in insomniacs' (Gunja 2013), significant weight might be given to the findings of the cross‐over study (Boyle 2012).
Finally, the review did not identify withdrawal symptoms and distinct rebound effects after eszopiclone was discontinued, supporting the conclusion that, if taken as recommended, eszopiclone has a low potential to cause dependence and withdrawal. Findings from randomised placebo‐controlled studies with racemic zopiclone, which failed to demonstrate polysomnographic withdrawal effects after a four‐week treatment in a recommended dose range (Vorderholzer 2001) and did not show abuse‐like effects in drug‐naive participants (Licata 2008), are consistent with our conclusion. Nevertheless, withdrawal symptoms, craving, and severe rebound insomnia associated with the high dose use of zopiclone in individuals with preexisting chemical abuse or psychiatric disorders, as documented in case reports (Cimolai 2007), suggests that safety conclusions might only be valid for the use of eszopiclone in the recommended dose range and without contraindicated substances.
Authors' conclusions
Implications for practice.
The review of 14 RCTs with 4732 participants showed significant effects of eszopiclone on primary and secondary sleep efficacy outcomes. Compared to placebo, eszopiclone was shown to reduce time to fall asleep by about 12 minutes and wake time after sleep onset by about 17 minutes, contributing to a more or less half an hour increase of total sleep time per night. Efficacy of eszopiclone on sleep has been shown to cover different age groups and insomnia types, including insomnia as a primary and comorbid condition. Evidence from two six‐month trials indicated that therapeutic benefits can be maintained over medium‐ to long‐term treatment periods. Participants taking eszopiclone may subjectively experience better functioning the next day than participants taking placebo, although the effect is likely to be small. Participants in the eszopiclone group reported more often unpleasant taste, dry mouth, somnolence, and dizziness. Discontinuation of eszopiclone after several weeks and months of treatment did not result in withdrawal symptoms, while rebound effects were occasionally reported, but not observed in the majority of RCTs.
However, these implications for practice should not be made without refering to potential limitations in the quality and generalisability of evidence. First of all, due to the open‐label design of run‐out intervals (see Quality of the evidence), the risk of rebound insomnia after the discontinuation of eszopiclone might be underestimated by some trials. In addition, the exclusion of certain participant groups, such as elderly participants with cognitive and psychomotor impairments or individuals with high dose or combined use from clinical trials, might limit the safety conclusions of the review (see Overall completeness and applicability of evidence).
The review suggests that in healthy individuals who use the drug as prescribed for a limited time, eszopiclone can be considered a safe and efficacious treatment for insomnia. Intermittent dosing or 'treatment as needed' might be an alternative to daily scheduled treatment, but the risk‐benefit profile has to assessed by future research (see Implications for research).
Implications for research.
The RCTs included in the review showed various methodological strengths, which might serve as standards for future research. Such standards include baseline comparisons in sleep indicators between study groups to prevent selection bias, and the monitoring of medication compliance and of concomitant drugs as a strategy to control the risk of performance bias. Comparisons between groups for daytime napping have been implemented in studies with elderly subjects only, but might be equally helpful to control compensatory sleep in all age group samples. The consideration of both types of outcomes, SOL and WASO, allows a simultaneous assessment of distinctive drug effects on sleep onset and sleep maintenance and the identification of potential shifts in the efficacy profile of a hypnotic drug.
At the same time, a stricter adherence to methodological standards of reporting, as outlined in the CONSORT statement (Schulz 2010), would help to remove prevailing doubts and uncertainties resulting from an incomplete description of methods used for sequence generation, allocation concealment, and blinding procedures. Moreover, eszopiclone`s specific taste properties, which can potentially reveal the identity of the medication to participants or investigators (see Moncrieff 2004), constitute the need to taste‐match placebo to the active comparator drug in future RCTs. Finally, single‐blind run‐out periods should be routinely applied after treatment discontinuation in hypnotic drug trials to allow a valid assessment of withdrawal and rebound effects, which in turn serve as indicators of chronic use and dependence (Vorderholzer 2001).
Despite the wide range of methods and conditions considered in RCTs, some issues were left for future research. One of these concerns the relative effectiveness and safety of eszopiclone compared to other hypnotic drugs. While findings from RCTs comparing eszopiclone with placebo are applicable to the question: 'Can this intervention work?' (Krishnan 2011), and the therapeutic decision whether to use eszopiclone or not, it is the evidence from active‐controlled trials that clinicians refer to when deciding which of the available treatment options to prefer.
A further unresolved problem concerns the identification of appropriate treatment strategies for participant subgroups with an increased vulnerability to adverse events. The exclusion of specific samples from RCTs, such as participants with substance use disorders, contrasts with the high occurrence of insomnia in these groups of individuals and the potential impact insomnia has on substance use. In participants with comorbid alcohol dependence ‐ a group of individuals known to often use alcohol for self‐medicating sleeping problems ‐ insomnia was not only shown to significantly determine the severity of alcohol problems, but also the risk of a relapse to drinking during alcohol recovery (Arnedt 2007; Brower 2001; Conroy 2006; Foster 1999; Kaplan 2014). The exclusion of these participant subgroups from RCTs, a decision justified from a safety perspective, might bear the risk of adhering to higher risk treatments (Brunette 2003) or to treatments with unclear indications and effectiveness (Friedmann 2003).
In conclusion, to ensure that efficacy research meets the needs of every day clinical practice, relevant samples (e.g. elderly with impairments, individuals with substance use disorders) and treatment conditions (e.g. as‐needed dosing regimen; Hajak 2002a; Hajak 2002b), flexible strategies have to be included in high quality research. Available RCTs that consider longer treatment durations point in the right direction, while the conclusiveness of results might be increased by elaborate study designs to assess withdrawal and rebound effects. Such studies would not only provide credible answers to urgent questions, but might also serve as further examples illustrating that clinical relevance does not necessarily preclude internal validity.
Acknowledgements
We thank the Federal Ministry of Education and Research (BMBF Germany) for the financial support (FKZ:01KG0727). The authors are pleased to acknowledge the team of the Cochrane Common Mental Disorders Group (CCMD) for their support in the preparation of this review and Yoon Loke (University of East Anglia, UK, Co‐Convenor of the Cochrane Adverse Effects Methods Group (CAEMG)), who provided feedback on the analyses of adverse events.
Furthermore, we give thanks to Hrayr Attarian, Department of Neurology, Circadian Rhythms and Sleep Research Lab, Chicago, Illinois, USA; Joel E. Dimsdale, University of California, San Diego, CA, USA; Todd A. Grinnell, Clinical Development and Medical Affairs, Sunovion Pharmaceuticals Inc, Marlborough, MA, USA; Tara Lauriat, Dept. of Psychiatry, Steward St, Elizabeth’s Medical Center, Boston, MA, USA; Egilius Spierings, Craniofacial Pain Center, Tufts University School of Dental Medicine, Boston, MA, USA; Rose Nourse, formerly Lehigh Center for Clinical Research, Allentown, PA, USA; Sergio Tufik, Cristina Jorge, Departamento de Psicobiologia, Universidade Federal de São Paulo, São Paulo, Brasil; Sinan Guloksuz, Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA; Michael T. Smith, John Hopkins University School of Medicine, Center for Sleep Related Symptom Science, Baltimore, MD, USA for the provision of information on their studies; and with special thanks to Hrayr Attarian (see above) for supplying unreported outcomes from his primary study.
We also thank R Bart Sangal, Clinical Neurophysiology Services, Sterling Heights, MI, USA; James K. Walsh, Sleep Medicine and Research Center, St. Luke's Hospital, Chesterfield, MO, USA; Gary Zammit, Cliniclabs, NY, USA; and Thomas Roth, Henry Ford Hospital, Detroit, MI, USA; for providing contact and further information.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Criteria for bias assessment
|
Item |
Judgement |
Description |
|
| Sequence generation (selection bias) | Is the method used for randomisation adequate? | Yes | The method used for sequence generation constitutes a random process, in which every study participant has an equal chance to be assigned to each of the treatment conditions (e.g. allocation by random number table, computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice) |
| No | The method used for sequence generation allows the prediction of assignments to treatment groups (e.g. allocation by date of birth, date of admission, hospital or clinic record number etc.) | ||
| Unclear | Insufficient information about the sequence generation process to permit judgement of 'Yes' or 'No' | ||
|
Allocation concealment (selection bias, detection bias) |
Was the treatment allocation concealed? | Yes |
At least one of the following measures was undertaken to ensure allocation concealment: ‐ randomisation or drug preparation were performed centralised and remote from the patient recruitment centres ‐ sequentially numbered, opaque, sealed envelopes (SNOSE) were used for enclosing assignments ‐ all of the drug containers were tamper‐proof, equal in weight and similar in appearance |
| No | Methods for allocation were used that allow unconcealment such as an open random allocation schedule, assignment envelopes without appropriate safeguards, alternation or rotation | ||
| Unclear | Insufficient information about measures to ensure allocation concealment to permit judgement of 'Yes' or 'No' | ||
| Blinding (detection bias, expectation bias) | Was knowledge of the allocated interventions adequately prevented during the study? | Yes |
At least one of the following measures were undertaken to ensure blinding: ‐ participants and research staff were explicitly mentioned to be included in the blinding procedures AND active medication and placebo were of identical appearance, odour* and taste* OR ‐ the influence of unpleasent tase perception on results or integrity of blinding was checked and confirmed at the end of the treatment. *Only required for drugs with a typical inherent taste or odour such as eszopiclone and valerian |
| No | Evidence that indicates an unmasking of blinding by either participants, treatment providers or research staff such as significant group differences in the perception of appearance, odour or taste or significant group differences in guessing the affiliation to treatments as demonstrated by inquiries on blinding integrity | ||
| Unclear | Insufficient information about blinding to permit judgement of 'Yes' or 'No' | ||
| Handling of incomplete outcome data (attrition bias) | Were incomplete outcome data adequately addressed? | Yes |
At least one of the following procedures were undertaken to ensure adequate incomplete outcome data handling: All randomised participants (intention‐to‐treat analysis) or those who have received at least one dose of treatment (treatment‐received analysis) were analysed in the group they had been allocated to by randomisation Dropouts were excluded from the analyses (available case analysis), but those who were excluded were shown not to differ from trial completers |
| No | Dropouts were excluded from the analyses (available case analysis) and shown to differ from trial completers | ||
| Unclear | Dropouts were excluded from the analyses (available case analysis) without testing if those who dropped out differ from trial completers OR Insufficient reporting of dropout rates or dropout handling to permit judgement of 'Yes' or 'No' |
||
| Selective reporting (Reporting Bias) | Are reports of the study free of suggestion of selective outcome reporting? | Yes | The reporting of outcomes in the trial publication fulfils both of the following criteria: ‐ all outcomes listed in the methods section of the publication were adequately reported in the results section ‐ the primary and secondary endpoints represent an adequate diversity of outcome criteria including at least one indicator of sleep induction, sleep maintenance, rebound insomnia* or withdrawal symptoms* *Only required in studies with treatment duration of 2 weeks or longer |
| No | One or more outcomes listed in the study protocol or the methods section of the publication were not adequately reported in the results section OR The outcomes of the study are of limited diversity |
||
| Unclear | Outcomes were not explicitly stated in the study protocol or the methods section of the trial publication | ||
| General susceptibility to bias effects: Blinding of outcome assessment (detection bias) ‐ sleep efficacy indicators | Are sleep outcomes determined in a way that prevents bias effects? | Yes | The assessment of quantitative sleep outcomes fulfils one of the following conditions: (1) the quantitative sleep outcomes of the study are exclusively based on objective measures (e.g. PSG, electroencephalogram (EEG)) (2) the quantitative sleep outcomes of the study are based on patient self‐reports and confirmed by objective measures, meaning that both types of measures come to consistent significance conclusions |
| No | Objective measures and participant self‐reports come to different significance conclusions | ||
| Unclear | No objective measures were considered in the study Objective measures and participant self‐reports were considered in the study, but it is unclear whether these come to consistent significance conclusions |
||
| General susceptibility to bias effects: Blinding of outcome assessment (detection bias): next‐day functioning | Are next‐day functioning outcomes determined in a way that prevents bias effects? | Yes | The assessment of next‐day functioning fulfils one of the following conditions: (1) indicators of next‐day functioning are exclusively based on objective measures (e.g. objective testes of psychomotor co‐ordination, attention, vigilance, reaction time) (2) next‐day functioning was assessed by subjective measures (visual analogue scale (VAS), Likert scale, etc.) and objective tests, both coming to consistent significance conclusions |
| No | Objective measures and participant self‐reports come to different significance conclusions | ||
| Unclear | No objective measures were considered in the study Objective measures and participant self‐reports were considered in the study, but it is unclear whether these come to consistent significance conclusions |
||
| Other bias: Baseline equivalence (selection bias) | Are groups equivalent at baseline? | Yes | The testing of baseline age, gender and sleep fulfils at least one of the following conditions: ‐ baseline equivalence between groups was confirmed for age, gender AND at least one indicator of sleep induction and sleep maintenance ‐ baseline differences between groups were demonstrated, but adequately controlled in the statistical analyses In RCTs on insomnia associated with a comorbid condition, baseline equivalence for comorbid symptoms were tested and confirmed OR controlled in the statistical analyses |
| No | Differences between groups in one or more relevant baseline characteristics became evident, but were not controlled in the statistical analyses | ||
| Unclear | Insufficient reporting of baseline equivalence or its testing to permit judgement of 'Yes' or 'No' | ||
|
Other bias: Equivalence of treatment utilisation (performance bias) |
Are groups equivalent in the utilisation of treatments? | Yes | Treatment utilisation fulfils at least one of the following conditions: ‐ equivalence of medication compliance ‐ equivalence of the use of further medications that might have a secondary effect on sleep ‐ equivalence of daytime napping (*in elderly samples only) ‐ differences in medication compliance* or the use of medications that might affect sleep were demonstrated, but adequately controlled in the statistical analyses |
| No | Differences between groups in compliance or the use of medication became evident and were not controlled in the statistical analyses | ||
| Unclear | Insufficient reporting of medication compliance or the concomitant use of medications to permit judgement of 'Yes' or 'No' |
Appendix 2. Search strategies: CENTRAL, MEDLINE, EMBASE, PsycINFO
The Cochrane Central Register of Controlled Trials (CENTRAL) was searched (all years to Febraury 10 2016) using the following terms :
#1. (generation NEAR hypnotic*) #2. (nonbenzodiazepin* or "non benzodiazepin*" or non‐benzodiazepin*) #3. (imidazopyridin* or cyclopyrrolon*) #4. (eszopiclon* or zaleplon or zolpidem or zopiclon*) #5. (z NEXT hypnotic*) or (z NEXT drug*) #6. (#1 or #2 or #3 or #4 or #5) #7. insomn* #8. sleep* #9. MeSH descriptor Sleep Initiation and Maintenance Disorders explode all trees #10. MeSH descriptor Sleep Disorders, this term only #11. MeSH descriptor Sleep, this term only #12. MeSH descriptor Sleep Stages, this term only #13. MeSH descriptor Wakefulness, this term only #14. (#7 or #8 or #9 or #10 or #11 or #12 or #13) #15 (#6 and #14)
OVID MEDLINE was searched (from 1950 to February 10 2016) using the following terms :
((new generation or third generation) adj3 hypnotic*).tw.
(nonbenzodiazepin* or non benzodiazepin*).tw.
(imidazopyridin* or cyclopyrrolon*).tw.
(eszopiclon* or zaleplon or zolpidem or zopiclon*).mp.
(z hypnotic* or z drug*).tw.
or/1‐5
insomnia*.tw.
insomn*.ot.
sleep*.tw.
exp Sleep Initiation and Maintenance Disorders/
Sleep Disorders/
Sleep/ or Sleep Stages/
Wakefulness/
or/7‐13
randomised controlled trial.pt.
controlled clinical trial.pt.
randomi#ed.ti,ab.
randomly.ab.
placebo.ab.
drug therapy.fs.
trial.ab.
groups.ab
(control$ adj3 (trial or study)).ab,ti.
((singl$ or doubl$ or tripl$ or trebl$) adj3 (blind$ or mask$ or dummy$)).mp.
(animals not (humans and animals)).sh.
or/15‐24
26 not 25
6 and 14 and 27
OVID EMBASE was searched (from 1980 to February 10 2016) using the following terms:
((new generation or third generation) adj3 hypnotic*).tw.
(nonbenzodiazepin* or non benzodiazepin*).tw.
(imidazopyridin* or cyclopyrrolon*).tw.
(eszopiclon* or zaleplon or zolpidem or zopiclon*).mp.
Eszopiclone/ or Zaleplon/ or Zolpidem/ or Zopiclone/
(z hypnotic* or z drug*).tw.
or/1‐6
insomnia*.tw.
insom*.ot.
exp Insomnia/
sleep*.tw.
Sleep/ or Sleep Induction/ or Sleep Pattern/ or Sleep Stage/ or Sleep Time/ or Sleep Waking Cycle/
Sleep Parameters/
Sleep Disorder/
Wakefulness/
or/8‐15
randomised controlled trial.de.
randomisation.de.
placebo.de.
placebo.ti,ab.
randomi#ed.ti,ab.
randomly.ab.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp.
factorial$.ti,ab.
allocat$.ti,ab.
assign$.ti,ab.
volunteer$.ti,ab.
crossover procedure.de.
(crossover$ or cross over$).ti,ab.
(quasi adj (experimental or random$)).mp.
(control$ adj3 (trial$ or study or studies or group$)).ti,ab.
((animal or nonhuman) not (human and (animal or nonhuman))).de.
or/17‐31
33 not 32
7 and 16 and 34
OVID PsycINFO was searched (all years to February 10 2016) using the following terms:
((new generation or third generation) adj3 hypnotic*).tw.
(nonbenzodiazepin* or non benzodiazepin*).tw.
(imidazopyridin* or cyclopyrrolon*).tw.
(eszopiclon* or zaleplon or zolpidem or zopiclon*).mp.
(z hypnotic* or z drug*).tw.
or/1‐6
insomnia*.tw.
insomn*.ot.
Insomnia/
sleep*.tw.
(insomnia or sleep).tm.
Sleep/ or Sleep Onset/ or Sleep Wake Cycle/
Sleepiness/
Sleep Disorders/
Wakefulness/
or/7‐15
treatment effectiveness evaluation.sh.
clinical trials.sh.
mental health program evaluation.sh.
placebo.sh.
placebo.ti,ab.
randomly.ab.
randomi#ed.ti,ab.
trial.ti,ab.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp.
(control$ adj3 (trial$ or study or studies or group$)).ti,ab.
factorial$.ti,ab.
allocat$.ti,ab.
assign$.ti,ab.
volunteer$.ti,ab.
(crossover$ or cross over$).ti,ab.
(quasi adj (experimental or random$)).mp.
"2000".md.
or/17‐33
(6 and 16 and 34)
Psyndex was searched for the initial Z‐Drug search in 2011, using the following terms:
(eszopiclon* or zaleplon or zolpidem or zopiclon*)
No unique studies were retrieved from this database, and as access was not always available, it was dropped from any further updates.
Update search (February 2018) ‐ Intervention only (results not yet incorporated) 1. CENTRAL via Cochrane Register of Studies (CRSO): eszopiclone or lunesta AND 31/01/2016 TO 21/02/2018:DL (n=18) 2 Cochrane Common Mental Disorders Specialised Register (CCMD‐CTR): eszopiclone or lunesta AND 31/01/2016 TO 21/02/2018:DL (n=0) 3. Ovid Cross‐Search Databases: PsycINFO <1806 to February Week 2 2018>, Embase <1974 to 2018 Week 08>, Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to 21‐02‐2018> Limited 2016 to date ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (eszopiclone or lunesta).af. 2 (2016* or 2017* or 2018*).yr,dc,dd,dp,dt,ed,ep. 3 (in‐process or in data review or publisher).st. 4 1 and (2 or 3) 5 remove duplicates from 4 (244) 4. PubMed not MEDLINE #1 (eszopiclone OR lunesta) #2 pubmednotmedline[sb] #3. (#1 and #2), n = 24 5 ClinicalTrials.gov, WHO ICTRP eszopiclone OR lunesta | First posted from 01/01/2016 to 02/21/2018 (n = 3) Total = 271 Deduplicated = 265 265 abstracts screened; 230 excluded; 35 records of potential interest identified at abstract screening stage (RCTs, Reviews, Guidelines); 3 new studies to assess.
Appendix 3. Abbreviations
IC Inclusion criteria
ALC Alcohol abuse
DRUG Drug abuse
BENZ Benzodiazepine use 14 days before treatment
DSM‐4‐TR Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision
DSST Digit‐Symbol Substitution Test
HSAD History of substance abuse or dependence
ICD‐10 International Classification of Mental and Behavioural Disorders
ICSD International Classification of Sleep Disorders, second edition
ITT Intention to treat
BZD benzodiazepine
FLX Fluoxetine
IVRS Interactive Voice Recording System
LS Likert Scale
MA meta‐analysis
OSRD Other primary or secondary sleep disorders than insomnia such as sleep apnea, restless legs syndrome, periodic leg movement
OCAS Other medical or psychiatric condition that impacts or is likely to impact sleep
PRO participant‐reported outcomes
PP Per Protocol
PSG polysomnography
PSY psychiatric disorders
SAD Sleep affecting disorder
SAM Sleep affecting medication
SAS
SDU standard drink unit
SE sleep efficiency
tmax time to maximum plasma concentration
TR Treatment‐received
Q‐LES‐Q Quality of Life Enjoyment and Satisfaction Questionnaire
Data and analyses
Comparison 1. Eszopiclone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sleep onset latency (SOL) | 9 | 2890 | Mean Difference (IV, Fixed, 95% CI) | ‐11.94 [‐16.03, ‐7.86] |
| 2 Wake time after sleep onset (WASO) | 8 | 2295 | Mean Difference (IV, Random, 95% CI) | ‐17.02 [‐24.89, ‐9.15] |
| 3 Withdrawal symptoms | 7 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Abdominal pain | 1 | 478 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.03] |
| 3.2 Accidental injury | 3 | 1068 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 3.3 Agitation | 2 | 701 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 3.4 Anxiety | 2 | 590 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 3.5 Arthritis | 1 | 153 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.01, 0.09] |
| 3.6 Backpain | 3 | 1058 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 3.7 Dizziness | 4 | 1291 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 3.8 Dysmenorrhea | 1 | 359 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 3.9 Headache | 6 | 2237 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 3.10 Hyperesthesia | 1 | 204 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 3.11 Infection | 1 | 478 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.00, 0.04] |
| 3.12 Insomnia | 1 | 386 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 3.13 Memory impairment | 1 | 386 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 3.14 Nausea | 2 | 590 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.03, 0.02] |
| 3.15 Neurosis | 1 | 204 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 3.16 Nightmares | 1 | 204 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 3.17 Paresthesia | 1 | 410 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 3.18 Pharyngitis | 2 | 878 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.02, 0.01] |
| 3.19 Photosensivity | 1 | 204 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 3.20 Pain | 4 | 1417 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 3.21 Pruritis | 1 | 410 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 3.22 Tremor | 1 | 153 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.07] |
| 3.23 Any | 5 | 2103 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.03, 0.04] |
| 4 Rebound insomnia | 1 | 873 | Mean Difference (IV, Random, 95% CI) | ‐1.76 [‐18.55, 15.04] |
| 4.1 Rebound ‐ SOL | 1 | 291 | Mean Difference (IV, Random, 95% CI) | 17.0 [‐4.29, 38.29] |
| 4.2 Rebound ‐ WASO | 1 | 291 | Mean Difference (IV, Random, 95% CI) | ‐6.71 [‐21.25, 7.83] |
| 4.3 Rebound ‐ TST | 1 | 291 | Mean Difference (IV, Random, 95% CI) | ‐14.30 [‐36.42, 7.82] |
| 5 Total sleep time (TST) | 10 | 2965 | Mean Difference (IV, Random, 95% CI) | 27.70 [20.30, 35.09] |
| 6 Next‐day alertness | 8 | 2061 | Mean Difference (IV, Random, 95% CI) | 0.46 [0.28, 0.63] |
| 7 Adverse events | 13 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Serious adverse events | 12 | 4289 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
| 7.2 Dropout | 11 | 4007 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 7.3 Chest pain | 1 | 58 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.14, 0.06] |
| 7.4 Accidental injury | 7 | 3374 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 7.5 Anxiety | 2 | 652 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.04] |
| 7.6 Abdominal pain | 2 | 1381 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.06, 0.05] |
| 7.7 Arthralgia | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 7.8 Asthenia | 3 | 1484 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.9 Backpain | 6 | 2647 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 7.10 Confusion | 1 | 153 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.07] |
| 7.11 Coughing | 1 | 153 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 7.12 Decreased libido | 1 | 593 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.00, 0.06] |
| 7.13 Diarrhea | 2 | 1381 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.04] |
| 7.14 Dry mouth | 6 | 2802 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.02, 0.06] |
| 7.15 Dizziness | 7 | 2933 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.01, 0.05] |
| 7.16 Dyspepsia | 5 | 2728 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.03] |
| 7.17 Edema | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 7.18 Fatigue | 1 | 593 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.01, 0.06] |
| 7.19 Hallucinations | 1 | 388 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 7.20 Headache | 10 | 4124 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 7.21 Infection | 3 | 2159 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.02, 0.10] |
| 7.22 Memory impairment | 2 | 652 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.03] |
| 7.23 Mood changes | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 7.24 Muscle pain | 1 | 828 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.00, 0.06] |
| 7.25 Nausea | 3 | 1924 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.06, 0.07] |
| 7.26 Nervousness | 5 | 1552 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 7.27 Nightmares | 2 | 357 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.02, 0.03] |
| 7.28 Pharyngitis | 6 | 3295 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.04] |
| 7.29 Pain | 5 | 2833 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 7.30 Poor concentration | 1 | 388 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 7.31 Respiratory infection | 3 | 1156 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.04] |
| 7.32 Rhinitis | 1 | 788 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.06] |
| 7.33 Sinusitis | 1 | 788 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.02] |
| 7.34 Skin rash | 2 | 1052 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.04] |
| 7.35 Somnolence | 8 | 3532 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [0.02, 0.06] |
| 7.36 Unpleasant taste | 9 | 3787 | Risk Difference (M‐H, Random, 95% CI) | 0.18 [0.14, 0.21] |
| 8 Subgroups: insomnia type ‐ SOL | 7 | 2265 | Mean Difference (IV, Random, 95% CI) | ‐12.25 [‐16.99, ‐7.50] |
| 8.1 Primary insomnia: SOL | 5 | 1803 | Mean Difference (IV, Random, 95% CI) | ‐15.14 [‐21.13, ‐9.15] |
| 8.2 Comorbid insomnia: SOL | 2 | 462 | Mean Difference (IV, Random, 95% CI) | ‐8.10 [‐17.77, 1.57] |
| 9 Subgroups: insomnia type ‐ WASO | 7 | 2265 | Mean Difference (IV, Random, 95% CI) | ‐16.55 [‐24.76, ‐8.35] |
| 9.1 Primary insomnia: WASO | 5 | 1803 | Mean Difference (IV, Random, 95% CI) | ‐15.76 [‐25.60, ‐5.92] |
| 9.2 Comorbid insomnia: WASO | 2 | 462 | Mean Difference (IV, Random, 95% CI) | ‐21.20 [‐40.76, ‐1.65] |
| 10 Subgroups: insomnia type ‐ TST | 8 | 2340 | Mean Difference (IV, Random, 95% CI) | 28.37 [20.16, 36.58] |
| 10.1 Primary insomnia: TST | 6 | 1878 | Mean Difference (IV, Random, 95% CI) | 30.04 [19.09, 40.98] |
| 10.2 Secondary insomnia: TST | 2 | 462 | Mean Difference (IV, Random, 95% CI) | 23.37 [12.61, 34.12] |
| 11 Subgrups: age groups ‐ SOL | 8 | 2860 | Mean Difference (IV, Random, 95% CI) | ‐12.48 [‐16.92, ‐8.04] |
| 11.1 Younger age ‐ SOL | 5 | 2049 | Mean Difference (IV, Random, 95% CI) | ‐13.08 [‐19.15, ‐7.00] |
| 11.2 Older age ‐ SOL | 3 | 811 | Mean Difference (IV, Random, 95% CI) | ‐11.41 [‐20.37, ‐2.45] |
| 12 Subgroups: age groups ‐ WASO | 7 | 2265 | Mean Difference (IV, Random, 95% CI) | ‐16.55 [‐24.76, ‐8.35] |
| 12.1 Younger age ‐ WASO | 4 | 1454 | Mean Difference (IV, Random, 95% CI) | ‐12.20 [‐19.02, ‐5.37] |
| 12.2 Older age ‐ WASO | 3 | 811 | Mean Difference (IV, Random, 95% CI) | ‐22.16 [‐40.70, ‐3.63] |
| 13 Subgroups: age groups ‐ TST | 9 | 2935 | Mean Difference (IV, Random, 95% CI) | 28.54 [21.81, 35.27] |
| 13.1 Younger age ‐ TST | 6 | 2124 | Mean Difference (IV, Random, 95% CI) | 29.66 [21.60, 37.72] |
| 13.2 Older age ‐ TST | 3 | 811 | Mean Difference (IV, Random, 95% CI) | 27.01 [11.83, 42.18] |
| 14 Subgroups: age groups ‐ alertness | 7 | 2031 | Mean Difference (IV, Random, 95% CI) | 0.44 [0.26, 0.63] |
| 14.1 Younger age: next‐day alertness | 4 | 1220 | Mean Difference (IV, Random, 95% CI) | 0.56 [0.37, 0.75] |
| 14.2 Older age: next‐day alertness | 3 | 811 | Mean Difference (IV, Random, 95% CI) | 0.34 [0.01, 0.67] |
| 15 Subgroups: older participants ‐ adverse events | 3 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Serious adverse events | 3 | 804 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
| 15.2 Dropout | 3 | 811 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.03, 0.03] |
| 15.3 Accidental injury | 2 | 652 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 15.4 Anxiety | 2 | 652 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [0.00, 0.04] |
| 15.5 Arthralgia | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 15.6 Backpain | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 15.7 Dry mouth | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.02, 0.12] |
| 15.8 Dizziness | 2 | 652 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [0.01, 0.06] |
| 15.9 Dyspepsia | 1 | 159 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.03] |
| 15.10 Edema | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.01, 0.05] |
| 15.11 Hallucinations | 1 | 388 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 15.12 Headache | 2 | 547 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.05, 0.07] |
| 15.13 Memory impairment | 2 | 652 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.03] |
| 15.14 Mood changes | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.04] |
| 15.15 Nervousness | 2 | 652 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.00, 0.03] |
| 15.16 Pharyngitis | 1 | 388 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.05, 0.04] |
| 15.17 Pain | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 15.18 Poor concentration | 1 | 388 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.01, 0.02] |
| 15.19 Skin rash | 1 | 264 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.04, 0.05] |
| 15.20 Somnolence | 2 | 423 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.03, 0.05] |
| 15.21 Unpleasant taste | 3 | 811 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.08, 0.15] |
| 16 Subgroups: study initiation ‐ SOL | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 16.1 Investigator‐initiated ‐ SOL | 3 | 677 | Mean Difference (IV, Random, 95% CI) | ‐8.29 [‐14.24, ‐2.34] |
| 16.2 Sponsor‐initiated ‐ SOL | 6 | 2213 | Mean Difference (IV, Random, 95% CI) | ‐15.21 [‐20.83, ‐9.59] |
| 17 Subgroups: study initiation ‐ WASO | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 Investigator‐initiated WASO | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐33.29 [‐56.47, ‐10.10] |
| 17.2 Sponsor‐initiated WASO | 6 | 2213 | Mean Difference (IV, Random, 95% CI) | ‐15.31 [‐23.50, ‐7.11] |
| 18 Subgroups: study initiation ‐ TST | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Investigator‐initiated TST | 3 | 677 | Mean Difference (IV, Random, 95% CI) | 21.04 [‐4.19, 46.27] |
| 18.2 Sponsor‐initiated TST | 7 | 2288 | Mean Difference (IV, Random, 95% CI) | 28.40 [19.60, 37.21] |
| 19 Sensitivity: sleep assessment | 2 | 1200 | Mean Difference (IV, Fixed, 95% CI) | ‐9.92 [‐13.32, ‐6.53] |
| 19.1 Sleep onset latency | 2 | 468 | Mean Difference (IV, Fixed, 95% CI) | ‐15.50 [‐19.89, ‐11.11] |
| 19.2 Wake time after sleep onset | 2 | 468 | Mean Difference (IV, Fixed, 95% CI) | ‐12.37 [‐18.61, ‐6.13] |
| 19.3 Total sleep time | 1 | 264 | Mean Difference (IV, Fixed, 95% CI) | 28.6 [18.14, 39.06] |
| 20 Sensitivity: withdrawal assessment | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only |
1.11. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 11 Subgrups: age groups ‐ SOL.
1.12. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 12 Subgroups: age groups ‐ WASO.
1.13. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 13 Subgroups: age groups ‐ TST.
1.14. Analysis.
Comparison 1 Eszopiclone versus placebo, Outcome 14 Subgroups: age groups ‐ alertness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ancoli‐Israel 2010.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT (treatment‐received analysis) Setting: outpatient Study sites: 82 Country: USA |
|
| Participants |
N = 388 Diagnosis: primary insomnia (DSM‐4‐TR) Sample: elderly participants Age: M = 72 years (SD = 5.1; range: 65 to 85 years) Gender: 63 % female Sleep‐related criteria of inclusion: TST≤ 6 hours, WASO ≥ 45 min (for ≥ 3 nights per week in the last month); further inclusion criteria: run‐in compliance ≥ 4 doses Exclusion criteria: OSRD, OCAS, SAM (e.g. antidepressants on a stable dose), HSAD (6 months before screening) |
|
| Interventions |
Experimental: ESZ (2 mg); n = 194 Control: PBO; n = 194 Treatment duration: 12 weeks Run‐out: 4 weeks (2 weeks single‐blind PBO; 2 weeks no drug period) Run‐in: 1 (+1) week single‐blind PBO for compliance assessment Dosing: nightly |
|
| Outcomes |
Primary outcomes: TST Secondary outcomes: SOL, WASO, ISI (Bastien 2001); next day functioning: alertness, ability to function, well‐being, ability to concentrate, rebound insomnia, withdrawal effects; adverse events; further outcomes: SF‐36 (Ware 1993); BWSQ (Tyrer 1990) |
|
| Financial support | Sepracor, Marlborough, Massachusetts; sponsored phase‐IV study | |
| Data assessment |
Timepoints for assessment: clinic visits at 3‐week intervals Quantitative sleep measures: daily, participant reports (electronic sleep/wake diary) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function) Rebound insomnia: mean SOL, TST and WASO change from baseline for each day of the two week run‐out period Withdrawal effects: Benzodiazepine Withdrawal Symptom Questionnaire (Tyrer 1990); new or worsening central nervous system or psychiatric adverse events that occurred during the follow‐up period Tolerance: reduction of treatment effects throughout the study Compliance: pill count |
|
| Treatment adherence |
Dropout: 24.2 % (ESZ); 23.7 % (PBO); group difference: ns Compliance: 97.3 % (ESZ); 97.6 % (PBO); group difference: ns |
|
| Notes | Declaration of interest: Trial investigators have served as consultants or advisory board members to Sepracor, have received research support from Sepracor or were Sepracor employees. Further publications: A further publication concerned the evaluation of predictors of response to eszopiclone (Marshall 2011a). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised with internet based randomisation system |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: no information on appearance provided; 12.4% in the ESZ group and 1.5% in the PBO group reported an unpleasant taste; testing of blinding integrity: explicitly stated that blinding integrity was not tested |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT (treatment‐received analysis); handling of missing data: LOCF |
| Selective reporting (reporting bias) | Low risk | Reporting: primary and secondary endpoints adequately listed, reported and interpreted; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia, withdrawal symptoms) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender:yes; sleep initiation: yes; sleep maintenance: yes |
| Other bias: Equivalence of treatment utilisation (performance bias) | Low risk | Compliance equivalence: yes; concomitant use of SAM: tested for various substances; no significant difference between groups; slightly more participants in the PBO group used analgesics, but the difference was not statistically significant; occurrence of compensatory sleep: a significantly greater decrease in naps per week was noted over the first three weeks of treatment with eszopiclone, but not at week 6, 9, or 12; napping endpoints were also analysed in ITT |
Fava 2006.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT (treatment‐received analysis) Setting: outpatient; sleep laboratory (night 1, 15, 29, 45, 46), Study sites: 67 Country: USA |
|
| Participants |
N = 545 Diagnosis: 1. insomnia (DSM‐4); 2. major depressive disorder (MDD; DSM‐4); insomnia symptoms must not have predated MDD > 10 weeks Sample: depressive comorbidity Age: 41.0 years (SD = 11.0; range: 21 to 64 years) Gender: 66.6% female Sleep‐related criteria of inclusion: SOL ≧ 30 min, TST ≤ 6.5 hours, WASO ≧ 45 min (for ≥ 3 nights per week in the last month) Exclusion criteria: OSRD, OCAS, SAM (chronic prescription medication on a stable dose, over‐the‐counter (OTC) medications not intended for soporific use allowed), HSAD (6 months before screening) or positive urine test at screening |
|
| Interventions |
Experimental: ESZ (3 mg); n = 270 Control: PBO; n = 275 Treatment duration: 8 weeks Run‐out: 2 weeks single‐blind PBO Dosing: nightly Comedication: fluoxetine (20 mg open‐label; with the option of dose titration to 40 mg) |
|
| Outcomes |
Primary outcomes: WASO Secondary outcomes: SOL, TST, HAM‐D‐17 (Maier 1985); next day functioning: daytime alertness; tolerance; rebound insomnia; withdrawal effects; adverse events; further outcomes: sleep quality, sleep depth, daytime functioning, physical well‐being |
|
| Financial support | Sepracor Inc. | |
| Data assessment |
Timepoints for assessment: weekly, Quantitative sleep measures: participant reports (IVRS, morning questionnaire) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function); DSST Rebound insomnia: SOL, WASO and TST median change from baseline for each night during 14 days run‐out period Withdrawal effects: new or worsening CNS and CNS‐related adverse events during ESZ run‐out period Tolerance: no information on tolerance assessment provided: compliance: pill count; adverse events: any event, regardless of its relation to the study drug, was recorded |
|
| Treatment adherence |
Dropout: 6.3 % (ESZ); 7.7 % (PBO); group difference: ns Compliance: 96 to 100%; no group specific values provided; presumably ns |
|
| Notes | Declaration of interest: Trial investigators have served as consultants or advisory board members to Sepracor, have received research support from Sepracor or were Sepracor employees. Further publications: Further publications of the study concerned the evaluation of discontinuation (Krystal 2007) and antidepressant effects (Fava 2011). The study was also included in a post hoc analysis (Krystal 2012b), comparing effect sizes of primary insomnia samples and medical and psychiatric comorbidity samples from 5 RCTs on eszopiclone (Fava 2006; Pollack 2008; Roth 2009; Soares 2006; Walsh 2007). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blinded: double‐blind; no further information provided; placebo appearance: no information on appearance provided; 22.7% in the ESZ group and 0.7% in the PBO group reported unpleasant taste; testing of blinding integrity: reanalysis of data in participants who did not experience unpleasant taste was consistent with the efficacy results of the entire sample |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT (treatment‐received analysis); handling of missing data: LOCF |
| Selective reporting (reporting bias) | Low risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia, withdrawal symptoms) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: yes; sleep initiation: yes; sleep maintenance: yes; no differences in depression as measured by the Hamilton Rating Scale for depression (HAM‐D‐17; Hamilton 1960 ) were found between groups |
| Other bias: Equivalence of treatment utilisation (performance bias) | Unclear risk | Compliance equivalence: yes; concomitant use of comorbidity medication: tested for fluoxetine dose titration from 20 mg to 40 mg, which significantly differed between the ESZ‐group (44.7%) and the PBO group (53.7%) at week 4, but not at the end; further medication (on a stable dose, not tested); concomitant use of further SAM: not reported |
Goforth 2014.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT (treatment‐received analysis) Setting: outpatient Study sites: 1 Country: USA |
|
| Participants |
N = 58 Diagnosis: 1. insomnia (DSM‐4‐TR); 2. chronic low back pain (LBP); insomnia did not predate LBP onset > 4 weeks Sample: participants with chronic low back pain (LBP) Age: M = 43.5 years (SD = 11.7; range: 65 to 85 years) Gender: 63 % female Sleep‐related criteria of inclusion: TST < 6.5 hours, SOL > 30 min (on a usual night in the last month) Exclusion criteria: OSRD, OCAS, SAM, HSAD (12 months before screening) |
|
| Interventions |
Experimental: ESZ (3 mg); n = 33 Control: PBO; n = 25 Treatment duration: 4 weeks Dosing: nightly Comedication: naproxen (50 mg open‐label; twice daily), lansoprazole (15 mg; once daily) |
|
| Outcomes |
Primary outcomes: TST Secondary outcomes: SOL, WASO, SE, ISI (Bastien 2001); further outcomes: Roland Morris Disability Questionnaire (RMDQ; Roland 1983); Hamilton Rating Scale for depression (Hamilton 1960) |
|
| Financial support | investigator‐initiated study; sponsored by Sunovion Coropration (then Sepracor Corporation) | |
| Data assessment |
Timepoints for assessment: week 1, 2 and 4 Quantitative sleep measures: daily, sleep diaries Compliance: pill count |
|
| Treatment adherence |
Dropout: 12.1 % (ESZ); 32.0 % (PBO); excluded from analyses: 3% (ESZ); 20% (PBO) Compliance: not reported |
|
| Notes | Declaration of interest: One trial investigator has received research support from Sunovion/Sepracor; the other authors have indicated no financial conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation schedule based on computer‐driven pseudo‐random number generator |
| Allocation concealment (selection bias) | Low risk | The sponsor supplied identical ESZ and PBO capsules and provided the investigator with sequentially numbered pill containers in order to implement the random allocation sequence. The random allocation sequence was only provided to the investigators after all subjects have completed the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blinded: double‐blind; all investigators and study personal were blinded; placebo appearance: identical to ESZ capsules; no information on unpleasant taste provided; testing of blinding integrity: all investigators and study personal were shown to remain blind to treatment assignment throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT (treatment‐received analysis); handling of missing data: LOCF |
| Selective reporting (reporting bias) | High risk | Reporting: primary and secondary endpoints were adequately listed, reported and interpreted; outcome diversity: limited; no data on rebound insomnia or withdrawal symptoms provided |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: yes; sleep initiation: yes; sleep maintenance: yes; no differences in pain and back pain severity were found between groups |
| Other bias: Equivalence of treatment utilisation (performance bias) | Unclear risk | Compliance equivalence: not reported;concomitant use of comorbidity medication: differences in use of analgesics between groups was not reported; concomitant use of SAM: not allowed (see criteria of exclusion) |
Krystal 2003.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT Setting: outpatient Study sites: 70 Country: USA |
|
| Participants |
N = 788 Diagnosis: primary insomnia (DSM‐4) Age: 44 years (SD = 11; range 21 to 65 years) Gender: 63% female Sleep‐related criteria of inclusion: SOL > 30 min, TST< 6.5 hours (each night in the last month) Exclusion criteria: OSRD, OCAS, SAM, HSAD (lifetime), ALC > SDU per day or > 14 SDU per week |
|
| Interventions |
Experimental: ESZ (3 mg); n = 595 Control: PBO; n = 196 Treatment duration: 6 months (randomised, double‐blind design) Treatment continuation: 6‐month open‐label with 3 mg ESZ after the randomised period (available to all participants) Dosing: nightly |
|
| Outcomes |
Primary outcomes: SOL Secondary outcomes: TST, WASO;next day functioning: ability to function, daytime alertness, physical well‐being, ability to concentrate; further outcomes: tolerance, withdrawal symptoms; adverse events |
|
| Financial support | Sepracor‐sponsored phase‐III study | |
| Data assessment |
Timepoints for sleep indicator assessment: weekly (value reported once a week for average nightly values during that week) Timepoints for safety and compliance assessment: monthly, termination visit 5 to 7 days after the last dose Quantitative sleep measures: participant reports (IVRS) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function) Rebound insomnia: not considered Withdrawal effects: new events following drug discontinuation Tolerance: reduction of treatment effects throughout the study Compliance: pill count |
|
| Treatment adherence |
Dropout: 39.5 % (ESZ); 43.4% (PBO); group difference: ns Compliance: 94.4% (ESZ); 90.6% (PBO); group difference: ns |
|
| Notes | Declaration of interest: Trial investigators have served as consultants or advisory board members to Sepracor, have received research support from Sepracor or were Sepracor employees. Further publications: Further publication of the study concerned the evaluation of the open‐label period (Roth 2005), cost‐effectiveness analyses (Botteman 2007; Snedecor 2009) and WASO‐subgroup effects (Krystal 2012a). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blinded: double‐blind; no further information provided; placebo appearance: no information on appearance provided; 26.1% in the ESZ group and 5.6% in the PBO group reported an unpleasant taste; testing of blinding integrity: a comparison between participants who perceived unpleasant taste and those who did not, did not indicate differences in adherence and treatment success. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT; handling of missing data: LOCF (sensitivity of results to handling of missing data methods (ITT, PP) was tested and shown to be robust) |
| Selective reporting (reporting bias) | Low risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: yes; sleep initiation: yes; sleep maintenance: yes |
| Other bias: Equivalence of treatment utilisation (performance bias) | Low risk | Compliance equivalence: yes; concomitant use of SAM: not allowed (see criteria of exclusion) |
McCall 2006.
| Methods |
Design: parallel group randomised trial Principle of analysis: not reported Setting: sleep laboratory (night 1, 2, 13, 14); outpatient (night 3 to 12) Study sites: 49 Country: USA |
|
| Participants |
N = 264 Diagnosis: primary insomnia (DSM‐4) Sample: elderly participants Age: 71.1 years (SD = 5.1; range: 64 to 84 years) Gender: 67.4% female Sleep‐related criteria of inclusion: SOL > 30 min, TST ≤ 6.5 hours (each night in the last month), PSG screening Exclusion criteria: OSRD, OCAS, SAM, ALC > 2 (14) SDU per day (week) |
|
| Interventions |
Experimental: ESZ (2 mg); n = 136 Control: PBO; n = 128 Treatment duration: 2 weeks Run‐out: not considered Dosing: nightly |
|
| Outcomes |
Primary outcomes: LPS, SE Secondary outcomes: WASO, NAW, WTPS, WTDS, CWT, ISI (Bastien 2001); next day functioning: morning sleepiness, QOS, depth of sleep, sense of well‐being; further outcomes: SF‐36 (Ware 1992); % stage 1 sleep; time spent in slow wave/REM sleep; adverse events |
|
| Financial support | Sepracor‐sponsored phase‐III study: it was reported that the sponsor did not place limitations on the data analyses, interpretation of results, and manuscript writing. | |
| Data assessment |
Timepoints for sleep indicator assessment: daily Timepoints for safety and compliance assessment: 4 visits Quantitative sleep measures: 1. PSG (night 1, 2, 13, 14); 2. participant reports (IVRS questionnaire) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function) Rebound insomnia: not considered Withdrawal effects: not considered Tolerance: compliance: dosing cards; adverse events: personal interviews and reports |
|
| Treatment adherence |
Dropout: 2.2% (ESZ); 4.6% (PBO); group differences: ns Compliance: 99.3% (ESZ); 99.2% (PBO); group difference: ns |
|
| Notes | Declaration of interest: Trial investigators have served as consultants or advisory board members to Sepracor, have received research support from Sepracor or were Sepracor employees. Further authors have indicated no financial conflicts of interest. Further publications:Erman 2004. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: no information on appearance provided; 12.5% in the ESZ group and 0% in the PBO group reported unpleasant taste; testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Principle of analysis: not reported |
| Selective reporting (reporting bias) | Low risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, next‐day functioning) *rebound insomnia not required because of 2‐week treatment duration |
| Blinding of outcome assessment (detection bias) Sleep indicators | Low risk | PSG assessments (4 nights) and participant reports (10 nights) came to consistent effectiveness conclusions except for NAW, which only reached statistical significance for participant‐reported data |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: yes; sleep initiation: yes; sleep maintenance: yes |
| Other bias: Equivalence of treatment utilisation (performance bias) | Low risk | Compliance equivalence: yes; concomitant use of SAM: not allowed (see criteria of exclusion); occurrence of compensatory sleep: daytime napping was nearly 50% in both groups: number and duration of naps was higher in the PBO than in the ESZ, but did not reach statistical significance (P = 0.07) |
McCall 2010a.
| Methods |
Design: parallel group randomised trial Principle of analysis: not reported Setting: outpatient Study sites: 1 Country: USA |
|
| Participants |
N = 60 Diagnosis: 1. insomnia (Research Dignostic Criteria; Edinger 2004)); 2. major depressive episode (DSM‐4); no temporal relationship between insomnia and major depressive episode required Sample: depressive comorbidity Age: 41.2 years (SD = 12.5; range: 18 to 70 years) Gender: 66.7% female Sleep‐related criteria of inclusion: SOL > 30 min; SE (sleep efficiency) < 85% at least 4 nights per week in the last month; PSG screening Exclusion criteria: OSRD, OCAS, SAM, absence of substance abuse (current) |
|
| Interventions |
Experimental: ESZ (3 mg); n = 30 Control: PBO; n = 30 Treatment duration: 8 weeks Run‐out: not considered (naturalistic follow‐up conducted monthly by telephone for 4 months after treatment) Dosing: nightly Comedication: fluoxetine (20 mg open‐label; with the option of dose titration to 40 mg) |
|
| Outcomes |
Primary outcomes: Health‐related quality of life (HRQOL) Secondary outcomes: SL, WASO, TST, number of naps, nap time, SE, NAW, ISI (Bastien 2001); next day functioning: daily living and role functional subscale of the BASIS‐32 (Eisen 1994) was considered as the primary outcome |
|
| Financial support | Not industry‐supported study. Funded by NIH MH70821; M01‐RR07122. Medications from Sepracor Inc, material support from Mini Mitter | |
| Data assessment |
Timepoints for sleep indicator assessment: daily Timepoints for safety and compliance assessment: 4 visits Quantitative sleep measures: 1. actigraphy (Mini Mitter Actiwatch); 2. sleep diary Next‐day functioning: HRQOL; BASIS‐32 (Eisen 1994) Rebound insomnia: not considered Withdrawal effects: not considered Tolerance: compliance: adverse events: open‐ended questions |
|
| Treatment adherence |
Dropout: 16.6% (ESZ); 13.3% (PBO); group differences: ns Compliance: not reported; group difference: ns |
|
| Notes | Declaration of interest: Trial investigators have participated in speaking engagement for Sepracor or have received research support from Sepracor, further authors have indicated no financial conflicts of interest. Further publications: Further publications assessed effects of eszopiclone on quality of life (McCall 2009), insomnia severity as an indicator of suicidal ideation (McCall 2010b), analyses of the placebo effect (McCall 2011a), and the comparison between actigraphic and diary sleep measurements (McCall 2011b; McCall 2012). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: no information on appearance provided; 46% in the ESZ group reported an unpleasant taste (placebo rate of unpleasant taste not provided); testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT; handling of missing data: information provided for naturalistic follow‐up, for which missing data appeared to be missing at random, so a mixed linear model was used to compare groups |
| Selective reporting (reporting bias) | Low risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Low risk | PSG was assessed at the beginning and the end of treatment, actigraphic monitoring throughout the entire duration of the study. Pre‐post PSG differences failed to reveal an advantage for ESZ for either latency to the first epoch and to persistent sleep; a comparison between actigraphic monitoring and sleep diaries indicated differences between objective and subjective sleep measurements in depressed insomniacs (McCall 2012); the results of this study found that, overall, actigraphic sleep measurements demonstrated significantly increased sleep time and decreased wake time in the laboratory relative to the home environment |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: yes; sleep initiation: yes; sleep maintenance: yes |
| Other bias: Equivalence of treatment utilisation (performance bias) | Low risk | Compliance equivalence: yes; concomitant use of comorbidity medication: tested for fluoxetine dose titration from 20 mg to 40 mg, which did not significantly differ between the ESZ and PBO group; concomitant use of SAM: not allowed (see criteria of exclusion) |
Menza 2010.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT Setting: outpatient Study sites: 5 Country: USA |
|
| Participants |
N = 30 Diagnosis: 1. insomnia; 2. Parkinson's disease; no temporal relationship between insomnia and parkinson's disease required Sample: Parkinson's`s disease comorbidity Age: 56 years (range: 35 to 85 years) Gender: 20% female Sleep‐related criteria of inclusion: SOL > 30 min, TST < 6.5 hours (in ≥ 3 from 7 nights in the last month), PSG screening Exclusion criteria: OSRD, OCAS (PSG), SAM (antidepressants on a stable dose, benzodiazepines taken during the day, Parkinson's medication (e.g. levodopa, COMT inhibitor, MAO inhibitor were allowed) |
|
| Interventions |
Experimental: ESZ (3/2 mg); n = 15 (dose stratified by age; 3 mg for age < 65 years; 2 mg for age ≥ 65 years) Control: PBO; n = 15 Treatment duration: 6 weeks Run‐out: not considered Dosing: nightly |
|
| Outcomes |
Primary outcomes: TST Secondary outcomes: WASO, NAW; next day functioning; next day alertness; adverse events; further outcomes: Parkinson's severity; QOL and motor functioning |
|
| Financial support | Investigator‐initiated study; not sponsored by Sepracor besides providing study medication | |
| Data assessment |
Timepoints for sleep indicator assessment: daily Timepoints for safety and compliance assessment: weeks 0, 2, 4 and 6 Quantitative sleep measures: sleep diary (National Sleep Foundation Sleep Diary) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function); QOL: assessed with the short version of the Parkinsons Disease Questionnaire (PDQ‐8; Jenkinson 1997) Rebound insomnia: not considered Tolerance: reduction of treatment effects throughout the study; compliance: not reported |
|
| Treatment adherence |
Dropout: 20% (ESZ); 53.3% (PBO); group differences: not reported Compliance: not reported |
|
| Notes | Declaration of interest: Some trial investigators have received research support from Sepracor; further authors have indicated no financial conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: equal appearance to eszopiclone pill; none of the participants reported an unpleasant taste (see discussion); testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT; handling of missing data: no information provided |
| Selective reporting (reporting bias) | High risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: limited; no data on rebound insomnia or withdrawal symptoms provided |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided (except: PSG baseline evaluation) |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Unclear risk | Baseline equivalence: age: not reported; gender: yes; sleep initiation: not reported; sleep maintenance: yes; baseline scores indicated a higher severity of Parkinson's disease in the eszopiclone group; covariate comprised participant age, gender, and the score at baseline of a Parkinson's disease rating scale |
| Other bias: Equivalence of treatment utilisation (performance bias) | Unclear risk | Compliance equivalence: not reported; concomitant use of comorbidity medication: equivalence of Parkinson's medication confirmed; concomitant use of SAM: concomitant medications were listed in the outcome section, but no group‐specific values were provided |
Pollack 2008.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT (treatment‐received analysis) Setting: outpatient Study sites: 69 Country: USA |
|
| Participants |
N = 595 Diagnosis: 1. insomnia (DSM‐4‐TR); 2. general anxiety disorder (GAD; DSM‐4‐TR); insomnia was defined as being related to GAD, while no requirement for a particular temporal relationship between the onset of GAD and insomnia was required Sample: general anxiety disorder comorbidity Age: 40.0 years (range: 18 to 64 years) Gender: 66% female Sleep‐related criteria of inclusion: SOL ≥ 30 min; TST ≤ 6.5 hours (for ≥ 3 nights per week in the last month); 70% compliance and diary entries in the run‐in period Exclusion criteria: OSRD, OCAS, SAM, HSAD |
|
| Interventions |
Experimental: ESZ (3 mg); n = 294 Control: PBO; n = 301 Treatment duration: 8 weeks Run‐in: 10‐day single‐blind PBO (compliance ensured) Run‐out: 2 weeks single‐blind PBO (rebound, withdrawal effects) Dosing: nightly Comedication: escitalopram (open label; 10 mg daily) |
|
| Outcomes |
Primary outcomes: SOL Secondary outcomes: TST, WASO, NAW, TST, QOS, DOS, ISI (Bastien 2001); next‐day functioning: ability to function, ability to concentrate, daytime alertness, physical well‐being; severity of insomnia; tolerance; rebound insomnia; withdrawal effects; adverse events; further outcomes: anxiety (Hamilton Anxiety Scale (HAM‐A; Hamilton 1959). |
|
| Financial support | Investigator‐initiated study, not sponsored by Sepracor | |
| Data assessment |
Timepoints for assessment: weeks 1, 2, 4, 6, 8 and 10 Quantitative sleep measures: daily participant reports (electronic diary) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function) Rebound insomnia: SOL, TST, WASO change to baseline for each day during the 2 week ESZ run‐out period Withdrawal effects: adverse events and central nervous events during run‐out period Tolerance: significance reduction of treatment effects throughout the study Compliance: pill count Adverse events: medical event calendar |
|
| Treatment adherence |
Dropout: 20.1% (ESZ); 21.3% (PBO); group differences: ns Lost to follow‐up: ESZ: 6%; PBO: 5.8%; group differences: ns Compliance: not reported (only participants who took at least 70% of the required doses in the single‐blind run‐in period were included in the randomisation) |
|
| Notes | Declaration of interest: Trial investigators have served as consultants or advisory board members to Sepracor, or have received research support from Sepracor. Further publications: The study was included in a post hoc analysis (Krystal 2012b), comparing effect sizes of primary insomnia samples and medical and psychiatric comorbidity samples from 5 RCTs on eszopiclone (Fava 2006; Pollack 2008; Roth 2009; Soares 2006; Walsh 2007). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: matched to ESZ; 24.1% in the ESZ group and 3.7% in the PBO group reported unpleasant taste; testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT (treatment‐received analysis); handling of missing data: LOCF |
| Selective reporting (reporting bias) | Low risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia, withdrawal symptoms) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: yes; sleep initiation: yes; sleep maintenance: yes; furthermore, no differences in depression as measured with the Hamilton Rating Scale for depression (HAM‐A; Hamilton 1959) were found between groups |
| Other bias: Equivalence of treatment utilisation (performance bias) | Unclear risk | Compliance equivalence: not reported; concomitant use of comorbidity medication: tested for escitalopram, which did not significantly differ between the ESZ‐group (45%) and the PBO group (41%); concomitant use of further SAM: not allowed (see criteria of inclusion) |
Roth 2009.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT (treatment‐received analysis) Setting: outpatient Study sites: 43 Country: USA |
|
| Participants |
N = 153 Diagnosis: 1. insomnia (DSM‐4); 2. rheumatoid arthritis; the diagnosis of rheumatoid arthritis must have predated the onset of insomnia symptoms Age: 52.1 years (SD = 8.8; range: 25 to 64 years) Gender: 86.9% female Sleep‐related criteria of inclusion: TST < 6.5 hours, WASO ≥ 45 min (for ≥ 3 nights per week in the last month) Exclusion criteria: OSRD, OCAS, SAM (SSRI on a stable dose, selected medications in the event of an exacerbation of rheumatoid arthritis symptoms were allowed) |
|
| Interventions |
Experimental: ESZ (3 mg); n = 77 Control: PBO; n = 76 Treatment duration: 4 weeks Run‐in: 3 to 7 days single‐blind PBO Run‐out: 2 weeks single‐blind PBO Dosing: nightly |
|
| Outcomes |
Primary outcomes: WASO Secondary outcomes: SOL,TST, QOS, DOS; next‐day functioning: ability to function, daytime alertness, physical well‐being, ability to concentrate; rebound insomnia: withdrawal symptoms; adverse events; further outcomes: QOL (SF‐36; Ware 1992); HAQDI |
|
| Financial support | Sepracor‐sponsored phase‐IIIb study | |
| Data assessment |
Timepoints for assessment: weeks 0, 2 and 4 Quantitative sleep measures: daily; participant reports (IVRS) Severity of insomnia: ISI Next‐day functioning: 11 point scale (0 to 10; higher scores indicating improved function) Rebound insomnia: SOL, TST, WASO change to baseline during ESZ run‐out period Withdrawal effects: new or worsening adverse events during ESZ run‐out period Compliance: not reported Quality of life: SF‐36 (Ware 1992) Time of assessment: morning administration; frequency of assessment: daily |
|
| Treatment adherence |
Dropout: 5.2 % (ESZ); 9.2 % (PBO); group difference: ns Compliance: not reported |
|
| Notes | Declaration of interest: Trial investigators have served as consultants to Sepracor, were Sepracor employees, or stock shareholders in Sepracor. One investigator reported no financial or other affiliations relevant to the subject of the study. Further publications: Schnitzer 2005 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: no information on appearance provided; 27.3% in the ESZ group and 0% in the PBO group reported unpleasant taste; testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT (treatment‐received analysis); handling of missing data: no imputation methods used for missing data |
| Selective reporting (reporting bias) | Low risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia, withdrawal symptoms) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Unclear risk | Baseline equivalence: age: yes; gender: yes; sleep initiation: not reported; sleep maintenance: not reported |
| Other bias: Equivalence of treatment utilisation (performance bias) | Unclear risk | Compliance equivalence: not reported;concomitant use of SAM and comorbidity medication: no group differences in dose increase or new prescription of either pain or disease‐modifying medications between groups observed |
Scharf 2005.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT (treatment‐received analysis) Setting: outpatient Study sites: multicentre (number not specified) Country: USA |
|
| Participants |
N = 231 Diagnosis: primary insomnia (DSM‐4) Sample: elderly participants Age: 72.3 years (SD = 5.1; range 64 to 85 years) Gender: 57.8% female Sleep‐related criteria of inclusion: SOL > 30 min, TST ≤ 6.5 hours (each night in the last month) Exclusion criteria: OSRD, OCAS, SAM, alcohol use was permitted but limited to 2 or fewer drinks per day |
|
| Interventions |
Experimental 1: ESZ (2 mg); n = 79 Experimental 2: ESZ (1 mg); n = 72 Control: PBO; n = 80 Treatment duration: 2 weeks Dosing: nightly * 1 mg dosing group was not included in the MA |
|
| Outcomes |
Primary outcome: SOL Secondary outcomes: TST, WASO, NAW, QOS, DOS; next‐day functioning: daytime alertness, daytime ability to function, sense of well‐being; number and duration of naps; adverse events; tolerance; QOL |
|
| Financial support | Sepracor‐sponsored phase‐III study | |
| Data assessment |
Timepoints for assessment: weekly Quantitative sleep measures: daily, participant reports (IVRS) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function) Rebound insomnia: not considered Withdrawal effects: not considered Tolerance: significance of effects in week 1 and 2 Compliance: pill count |
|
| Treatment adherence |
Dropout: 2.5 % (ESZ 2 mg); 6.3 % (PBO); group difference: ns Compliance: 98.7% (ESZ 2 mg); 98.2%; group difference: ns |
|
| Notes | Declaration of interest: Trial investigators have received research support from Sepracor or were Sepracor employees. Data were analysed by Sepracor Inc; the paper was written by the authors with the assistance of Sepracor Inc. Reporting was excellent. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: no information on appearance provided; 11.4% in the ESZ group and 1.3% in the PBO group reported unpleasant taste; testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT (treatment‐received analysis); handling of missing data: not reported |
| Selective reporting (reporting bias) | Low risk |
Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, next‐day functioning); *rebound insomnia not required because of 2‐week treatment duration |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: yes; sleep initiation: yes; sleep maintenance: yes |
| Other bias: Equivalence of treatment utilisation (performance bias) | Low risk | Compliance equivalence: yes; concomitant use of SAM: tested for various substances; no significant difference between groups; occurrence of compensatory sleep: participants in the eszopiclone group had significantly fewer daytime naps compared to PBO |
Soares 2006.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT Setting: outpatient Study sites: multicentre (number not specified) Country: Canada |
|
| Participants |
N = 410 Diagnosis: insomnia (DSM‐4) Sample: women in peri‐ or early postmenopausal stage; the onset of menopausal transition must have predated insomnia Age: 49.1 years (SD = 4.0; range 40 to 60 years) Gender: 100% female Sleep‐related criteria of inclusion: SOL > 45 min and TST ≤ 6 hours (at least 3 nights per week in the last month) Exclusion criteria: OSRD, OCAS, HSAD (lifetime), ALC > 2 (14) SDU per day (week); SAM (except hormone therapy on a stable dose allowed); PSY |
|
| Interventions |
Experimental: ESZ (3 mg); n = 201 Control: PBO; n = 209 Treatment duration: 4 weeks Run‐in: 3 to 7 day single‐blind PBO Run‐out: 1 week single‐blind PBO Dosing: nightly |
|
| Outcomes |
Primary outcome: SOL Secondary outcomes: TST, WASO, NAW, QOS; next‐day functioning: daytime alertness, daytime ability to function, daytime ability to concentrate, sense of well‐being; adverse events; rebound insomnia; withdrawal effects; further outcomes: menopause‐related symptoms, menopause‐specific quality of life |
|
| Financial support | Sepracor‐sponsored phase‐IIIb study | |
| Data assessment |
Timepoints for assessment: weekly Quantitative sleep measures: daily, participant reports (IVRS) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function) Rebound insomnia: SOL, TST and WASO change to baseline during run‐out period (data not shown) Withdrawal effects: adverse events during run‐out week Tolerance: significance of effects in week 1 and 2 Quality of life: Shehan Disbility Scale (menopause‐specific quality of life scale) |
|
| Treatment adherence |
Dropout: 11.9 % (ESZ); 12.9 % (PBO); group difference: ns Compliance: not reported; group difference: ns |
|
| Notes | Declaration of interest: Trial investigators have served as consultants to Sepracor, were Sepracor employees, or stock shareholders in Sepracor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: no information on appearance provided; 17.6% in the ESZ group and 0.5% in the PBO group reported unpleasant taste; testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT; handling of missing data: not reported |
| Selective reporting (reporting bias) | Low risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia, withdrawal symptoms) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: yes; sleep initiation: yes; sleep maintenance: yes; no differences in symptoms of menopause were found between groups |
| Other bias: Equivalence of treatment utilisation (performance bias) | Unclear risk | Compliance equivalence: yes; concomitant use of comorbidity medication: a subgroup analysis was prospectively analysed between baseline hormone users and non‐users; concomitant use of comorbidity medication: not allowed (see exclusion criteria) |
Spierings 2015.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT (modified) Setting: outpatient Study sites: 5 (investigator information) Country: USA |
|
| Participants |
N = 75 (randomised: N = 79) Diagnosis: 1. primary insomnia (DSM‐4); 2. migraine (IHS‐ICHD II) Sample: participants suffering migraine Age: 44.4 years (SD = 10.8; range: 18 to 64 years) Gender: 82.5% female Sleep‐related criteria of inclusion: TST ≤ 6.5 hours Exclusion criteria: SAM (with the exception of preventive migraine treatment with beta‐blockers or calcium‐entry blockers; treatment of insomnia for 2 weeks prior to screening), HSAD (last 5 years), ALC > 2 SDU per day on average |
|
| Interventions |
Experimental: ESZ (3 mg); n = 35 Control: PBO; n = 40 Treatment duration: 6 weeks Baseline‐period: 2 weeks Run‐out: 2 weeks open‐label run‐out period (information on blindness reported by the primary investigator) Dosing: nightly |
|
| Outcomes |
Primary outcomes: TST (averaged over 6 weeks) Secondary outcomes: TST (2‐week intervals; change from baseline), SOL, NAW, next‐day functioning: daytime alertness, functioning, fatigue; sleep quality; headache frequency, duration, intensity |
|
| Financial support | Investigator initiated trial; study was conducted with a grant from Sunovian Pharmaceuticals | |
| Data assessment |
Timepoints for assessment: weekly Quantitative sleep measures: paper sleep diary (every morning) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function) Rebound insomnia: TST change from baseline (not included in the meta‐analysis due to the open‐label design of the run‐out period) Withdrawal effects: not considered Tolerance: not considered;compliance: pill count; adverse events: not considered |
|
| Treatment adherence |
Dropout: not reported (4 randomised subjects, 3 taking medication for less than 2 weeks, 1 lost‐to‐follow‐up) were not included in the analyses Compliance: not reported |
|
| Notes | Declaration of interest: No conflict of interest reported; study was conducted with a grant from Sunovian Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | The sponsor provided blinded study‐drug kits, each containing three bottles with a 2‐week supply of tablets. The kits were packaged in blocks of four, each provided with a subject number, and were dispensed as much as possible in sequential order to ensure even distribution. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: eszopiclone and placebo tablets were identical‐looking, plain white tablets and contained all the ingredients of the verum other than eszopiclone; rates of unpleasant taste were not provided; testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Principle of analysis: ITT‐modified, including all subjects who took study medication at least 5 days per week for the first 2 weeks; handling of missing data: not reported |
| Selective reporting (reporting bias) | High risk | Reporting: TST during the run‐out period was mentioned as a secondary outcome in the methods section, but not reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | High risk | Baseline equivalence: age: yes; gender: yes; sleep maintenance: yes; sleep initiation: no (statistically significant difference for sleep latency, which was higher in the eszopiclone group compared to placebo); no differences in headache frequency, intensity, and duration were found between groups |
| Other bias: Equivalence of treatment utilisation (performance bias) | Unclear risk | Compliance equivalence: not reported;concomitant use of comorbidity medication: not reported;concomitant use of SAM: not allowed (see criteria of exclusion) |
Walsh 2007.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT (treatment‐received analysis) Setting: outpatient Study sites: 54 Country: USA |
|
| Participants |
N = 830 Diagnosis: primary insomnia (DSM‐4) Age: 46 years (SD = 11.8; range 21 to 64 years) Gender: 61% female Sleep‐related criteria of inclusion: SOL > 30 min and TST ≤ 6.5 hours (on a typical night in the last month) Exclusion criteria: OSRD, OCAS, HSAD (lifetime), SAM (exception: certain OTC medications and chronic prescription medication if taken on a stable dose allowed) |
|
| Interventions |
Experimental: ESZ (3 mg); n = 550 Control: PBO; n = 280 Treatment duration: 6 months Run‐out: 2 weeks single‐blind PBO Dosing: nightly |
|
| Outcomes |
Primary outcome: SOL Secondary outcomes: WASO, TST; next‐day functioning: daytime alertness; adverse events; rebound insomnia; withdrawal symptoms; further outcomes: quality of life; work limitations |
|
| Financial support | Sepracor‐sponsored phase‐IV study; it was reported that the sponsor placed no limitations on the data analyses, interpretation, and manuscript publication. | |
| Data assessment |
Timepoints for assessment: baseline visit, 6‐monthly visits, final visit Quantitative sleep measures: daily, participant reports (IVRS) Next‐day functioning: Likert scale (0 to 10; higher scores indicating improved function) Rebound insomnia: SOL, TST and WASO change from baseline during run‐out period Withdrawal: Benzodiazepine Withdrawal Symptom Questionnaire (Tyrer 1990) QOL: SF‐36 (Leger 2001); Work Limitations Questionnaire (Lerner 2001) Adverse events: COSTART dictionary (Version 5.0, 1995) Compliance: pill count |
|
| Treatment adherence |
Dropout: 37.1 % (ESZ); 52.1 % (PBO) Compliance: rates not reported; group difference: ns |
|
| Notes | Declaration of interest: Trial investigators have received research support from Sepracor or were Sepracor employees. Further publications: A further publication concerned the evaluation of predictors of response to eszopiclone (Marshall 2011b). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Drug preparation and randomisation were performed centralised by the sponsor |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind including participants, research and sponsor personnel; placebo appearance: ESZ and PBO were of identical appearance; 19.7% in the ESZ group and 1.1% in the PBO group reported unpleasant taste; testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT (treatment‐received analysis); handling of missing data: LOCF; dropouts were shown not to differ from trial completers in baseline characteristics, compliance rates and outcome measures |
| Selective reporting (reporting bias) | Low risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia, withdrawal symptoms) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Unclear risk | No objective measures of sleep provided |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Unclear risk | No objective measures of next‐day functioning provided |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: yes; sleep maintenance: yes; sleep inition: yes; duration of insomnia: yes |
| Other bias: Equivalence of treatment utilisation (performance bias) | Unclear risk | Compliance equivalence: yes; concomitant use of SAM medication: not reported |
Zammit 2004.
| Methods |
Design: parallel group randomised trial Principle of analysis: ITT (treatment‐received analysis) Setting: sleep laboratory (night 1, 15, 29, 45, 46), outpatient (41 nights) Study sites: multicentre (number not specified) Country: USA |
|
| Participants |
N = 308 Diagnosis: primary insomnia (DSM‐4) Age: 39.4 years (SD = 11.7; range 21 to 64 years Gender: 65% female Sleep‐related criteria of inclusion: SOL > 30 min and TST ≤ 6.5 hours (each night during the last month) Exclusion criteria: OSRD, OCAS, SAM, HSAD (lifetime), ALC > 2 SDU per day |
|
| Interventions |
Experimental 1: ESZ (3 mg); n = 105 Experimental 2: ESZ (2 mg)*; n = 104 Control: PBO; n = 99 Treatment duration: 6 weeks Run‐out: 2 days single‐blind PBO Dosing: nightly * 2 mg dosing group was not included in the MA |
|
| Outcomes |
Primary outcome: SOL, LPS Secondary outcomes: WASO, TST; next‐day functioning: daytime alertness; DSST; tolerance; rebound insomnia; withdrawal effects; adverse events; further outcomes: SE, stage 1, 3, 4 and REM sleep |
|
| Financial support | Sepracor‐sponsored phase‐III study | |
| Data assessment |
Timepoints for assessment: Quantitative sleep measures: 1. PSG (nights 1, 15, 29, 45, 46); 2. participant reports (IVRS), daily Next‐day functioning: 1. Likert scale (0 to 10; higher scores indicating improved function); 2. DSST Rebound insomnia: LPS, SE and WASO change from baseline for each of 2 nights during run‐out period Withdrawal: New CNS and CNS‐related adverse events in the week after treatment discontinuation Tolerance: PSG measures for LPS, SE and WASO on nights 1, 15, and 29; Adverse events: COSTART dictionary (Version 5.0, 1995) Compliance: pill count |
|
| Treatment adherence |
Dropout: 5% (no group‐specific values provided) Compliance: ESZ: 98.2%; PBO: 97.8%; group difference: ns |
|
| Notes | Declaration of interest: not provided;Further publications: A conference publication described effects of eszopiclone on stage 2 sleep (Zammit 2009) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants blinded: double‐blind; no further information provided; placebo appearance: no information on appearance provided; 33.3% in the ESZ group and 3% in the PBO group reported unpleasant taste; testing of blinding integrity: no information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Principle of analysis: ITT (treatment‐received analysis); handling of missing data: not reported |
| Selective reporting (reporting bias) | Low risk | Reporting: all primary and secondary endpoints listed in the methods section were adequately reported in the results section; outcome diversity: adequate (sleep induction, sleep maintenance, rebound insomnia, withdrawal symptoms) |
| Blinding of outcome assessment (detection bias) Sleep indicators | Low risk | PSG assessments (3 nights) and participant reports came to consistent effectiveness conclusions |
| Blinding of outcome assessment (detection bias) Next‐day functioning | Low risk | Digit‐Symbol Substitution Test (DSST) and participant reports of next‐day functioning came to consistent conclusions concerning next‐day impairments |
| Other bias: Baseline equivalence (selection bias) | Low risk | Baseline equivalence: age: yes; gender: no (65% female; ESZ > PBO); sleep inition: yes; sleep maintenance: yes; gender differences were included as covariates in the efficacy analysis mode |
| Other bias: Equivalence of treatment utilisation (performance bias) | Low risk | Compliance equivalence: yes; concomitant use of SAM medication: not allowed (see criteria of exclusion) |
ALC: alcohol
BASIS: Behaviour and Symptom Identification Scale
BWSQ: Benzodiazepine Withdrawal Symptom Questionnaire
CGI: Clinical Global Impression scale
CNS: central nervous system
COMT: Catechol‐O‐methyltransferase
COSTART: Coding Symbols for a Thesaurus of Adverse Reaction Terms
CWT: continous wavelet transform
DOS: duration of sleep
DSM: Diagnostic and Statistical Manual of Mental Disorders
DSST: digital symbol substitution test
ESZ: eszopiclone
GAD: generalised anxiety disorder
HAM: Hamilton Rating Scale for Depression
HAQDI: Health Assessment Questionnaire without Didability Index
HRQOL: Health‐Related Quality of Life
HSAD: hydrolase
ICHD: International Classification of Headache Disorders
IHS: idiopathic hyperinsomnia
ISI: Insomnia Severity Index
ITT: intention‐to‐treat
IVRS: interactive voice response system
LBP: low back pain
LOCF: last‐observation‐carried‐forward
LPS: latency to persistent sleep
M: Mean
MA: meta‐analysis
MAO: monoamine oxidase inhibitors
MDD: major depressive disorder
mg: milligram
min: minute
n: number
NAW: number of nighttime awakenings
NIH: National Institute of Health
ns: not significant
OCAS: oral contraceptive agents
OSRD: other primary or secondary sleep disorders than insomnia such as sleep apnea, restless legs syndrome, periodic leg movement
OTC: over the counter
PBO: placebo
PDQ: Parkinson's Disease Questionnaire
PP: Per Protocol
PSG: polysomnography
PSY: psychiatric disorders
QOL: quality of life
QOS: quality of sleep
RCT: randomised controlled trial
REM: rapid eye movement
RMDQ: Roland‐Morris Disability Questionnaire
SAM: sleep‐affecting medication
SD: sleep duration
SDU: standard drink units
SE: sleep efficiency
SF‐36: Short Form (36) Health Survey
SL: sleep latency
SOL: sleep onset latency
SSRI: selective serotonin reuptake inhibitors
TST: total sleep time
WASO: wake time after sleep onset
WTDS: wake time during sleep
WTPS: wake time before persistent sleep
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Attarian 2011 | Insomnia diagnosis was not required for inclusion |
| Boyle 2008 | Cross‐over design |
| Boyle 2012 | Cross‐over design; healthy volunteers |
| Demanuele 2014 | Insomnia diagnosis was not required for inclusion; patients with schizophrenia |
| Dimsdale 2011b | Insomnia diagnosis was not required for inclusion (according to investigator information); patients with mucositis associated with hematologic malignancies |
| Eckert 2011 | Insomnia diagnosis was not required for inclusion; patients with obstructive sleep apnoea (OSA) |
| Erman 2008 | Cross‐over design |
| Gross 2011 | Open‐label design; control: mindfulness meditation training |
| Huang 2015 | Insomnia diagnosis was not required for inclusion; patients with schizophrenia |
| Joffe 2010 | Cross‐over design |
| Lettieri 2008 | Insomnia diagnosis was not required for inclusion; patients with sleep‐disordered breathing (SDB) |
| NCT00120250 | Cross‐over design |
| NCT00368056 | Cross‐over design |
| NCT00374192 | Cross‐over design; participants were peri‐ and postmenopausal women |
| NCT00460993 | Insomnia diagnosis was not required for inclusion; nursing home patients with low sleep quality; no further information available |
| NCT00511134 | Insomnia diagnosis was not required for inclusion; smokers with sleep problems related to smoking cessation |
| NCT00616655 | Insomnia diagnosis was not required for inclusion; patients with generalised anxiety disorder |
| NCT00685269 | Insomnia diagnosis was not required for inclusion (according to investigator information); patients with obstructive sleep apnea syndrome (OSA) |
| NCT00811746 | Insomnia diagnosis was not required for inclusion; patients with obstructive sleep apnea syndrome (OSA) |
| NCT00813735 | Insomnia diagnosis was not required for inclusion; Insomnia Severity Index > 15 (according to register information) |
| NCT00826111 | Insomnia diagnosis was not required for inclusion (according to investigator information); participants had to score above a threshold score of the Insomnia Severity Index (Bastien 2001) |
| NCT00889200 | Open‐label design |
| NCT00900159 | Cross‐over design |
| NCT01102270 | Insomnia diagnosis was not required for inclusion; patients with obstructive sleep apnea syndrome (OSA) |
| NCT01641900 | Insomnia diagnosis was not required for inclusion; effects on brain activity during sleep and memory in patients with schizophrenia |
| NCT01710631 | Open‐label extension of a six‐months phase‐II study (Krystal 2003) |
| Peng 2013 | Open‐label; no placebo or active drug control group (control: eszopiclone without psychotherapy) |
| Pollack 2011 | Cross‐over design; insomnia diagnosis was not required for inclusion (sleep disturbance) |
| Rosenberg 2005 | Healthy subject model of transient insomnia |
| Rosenberg 2007 | Cross‐over design; insomnia diagnosis was not required for inclusion; patients with obstructive sleep apnea syndrome (OSA)) |
| Sangal 2014 | Sample: children and adolescents (< 18 years) |
| Tek 2014 | Insomnia diagnosis was not required for inclusion; patients with schizophrenia or schizoaffective disorder according to DSM‐4; sleep problems twice per week in the proceeding month, Insomnia Severity Index rating > 10 |
| Uchimura 2012a | Cross‐over design; no placebo or active drug control group (control: different dose groups (1 mg, 2 mg, 3 mg)) |
| Uchimura 2012b | Cross‐over design |
Characteristics of studies awaiting assessment [ordered by study ID]
Baran 2017.
| Methods | Polysomnography study on coordination of slow waves with sleep spindles in schizophrenia |
| Participants | Schizophrenia participants and matched healthy controls |
| Interventions | Eszopiclone 3 mg versus placebo |
| Outcomes | spindle‐coordination, sleep‐dependent memory consolidation |
| Notes | — |
Buxton 2017.
| Methods | Randomised, double‐blind, placebo‐controlled trial to test effects of pharmacological treatment on glucose metabolism in primary insomnia |
| Participants | Adult men and women meeting clinical criteria for primary insomnia |
| Interventions | Eszopiclone 3 mg versus placebo |
| Outcomes | Change in glucose metabolism |
| Notes | NCT00900159; status: completed; study publication available: Buxton 2017 |
NCT00374556.
| Methods | Insomnia and osteoarthritis study |
| Participants | Osteoarthritis Insomnia |
| Interventions | Eszopiclone 3 mg versus placebo |
| Outcomes | WASO, pain sensitivity |
| Notes |
NCT00374556; status: completed; no publications provided; study was terminated early due to recruitment issues (according to Sunovion) Principal Investigator: Michael T. Smith, Ph.D, Johns Hopkins University |
NCT00392041.
| Methods | Eszopiclone in the treatment of insomnia and fibromyalgia |
| Participants | ACR criteria for fibromyalgia; L Allen requested |
| Interventions | Eszopiclone 3 mg versus placebo |
| Outcomes | TST, WASO, sleep quality, fibromyalgia symptoms |
| Notes | NCT00392041; status: completed; no publications provided |
NCT00435279.
| Methods | Study of eszopiclone coadministered with venlafaxine in subjects with major depressive disorder and insomnia |
| Participants | Major depressive disorder |
| Interventions | Eszopiclone 3 mg versus placebo |
| Outcomes | Depression score |
| Notes | NCT00435279; status: completed; no publications provided; no further information available (Sunovion) |
Pinto 2016.
| Methods | A non‐inferiority study with two treatment arms, eszopiclone 3 mg versus zopiclone 7.5 mg, for the treatment of insomnia |
| Participants | Diagnosis of primary insomnia according to DSM‐4 |
| Interventions | Eszopiclone 3 mg; zopiclone 7.5 mg |
| Outcomes | LPS, WASO, NAW |
| Notes | NCT01100164; status: completed; study publication available: Pinto 2016 |
ACR: American College of Rheumatology
DSM: Diagnostic and Statistical Manual of Mental Disorders
LPS: Latency to persistent sleep
mg: milligram
NAW: number of nighttime awakenings
SE: sleep efficiency
SOL: sleep onset latency
TST: total sleep time
WASO: wakefulness after sleep onset
Characteristics of ongoing studies [ordered by study ID]
Emiko 2015.
| Trial name or title | Efficacy and safety of eszopiclone in the treatment of insomnia complicated with nocturnal awakenings associated with urination |
| Methods | Parallel group randomised controlled trial |
| Participants | Subjects with insomnia complicated with nocturnal awakenings associated with urination |
| Interventions | Eszopiclone, no treatment |
| Outcomes | Primary outcome: quality of life |
| Starting date | Date of first enrolment: 6/2016 |
| Contact information | matsui.tsuyoshi@nihon‐u.ac.jp |
| Notes | ICTRP: JPRN‐UMIN000013808 |
NCT02456532.
| Trial name or title | Safety and efficacy of chronic hypnotic use 2 |
| Methods | Parallel group randomised controlled trial |
| Participants | Subjects with chronic insomnia |
| Interventions | Eszopiclone, zolpidem, placebo |
| Outcomes | Primary outcomes: change in number capsules chosen, discontinuation difficulty |
| Starting date | 7/2015 |
| Contact information | Gail Koshorek, BS; gkoshor1@hfhs.org |
| Notes | Sponsor: Henry Ford Health System |
Differences between protocol and review
The original protocol limited 'type of participants' to patients with primary insomnia, which was based on the argument that therapeutic success in comorbid insomnia might depend on resolving the causative problem rather than on the efficacy of insomnia treatment. Neverthless, current developments in insomnia research, diagnosis, and treatment and a careful reappraisal of the current evidence, led us to include both primary and comorbid insomnia instead of limiting the review to primary insomnia only. To identify the impact that type of insomnia had on efficacy outcomes, we conducted subgroup analyses for participant groups with a) primary and b) comorbid insomnia. In detail, the following arguments were decisive for the change:
The utility of diagnostic distinctions between primary and secondary insomnia are questioned by DSM‐5 criteria for sleep‐wake disorders (American Psychiatric Association 2013), dropping the DSM‐5’s 'primary insomnia' diagnosis in favour of 'insomnia disorder' (Riemann 2014; Riemann 2015), reflecting a general move away from causal attributions and their relevance for insomnia treatment;
The high prevalence of comorbid insomnia within the insomniac population (Katz 1998; Krystal 2012b), but also in potentially eligible studies for the review, further underscored the clinical relevance of this participant subgroup. Accordingly, a limitation of the review to primary insomnia would have reduced the clinical relevance of its conclusions;
Definitions of primary and comorbid insomnia, based on different temporal relationships between insomnia and comorbid symptoms as applied in eligible studies for the review, further illustrated the difficulty of reliably distinguishing different types of insomnia from each other;
Even though larger benefits for eszopiclone are to be expected in participants with primary insomnia, treatment effects were also shown to reach statistical significance in samples with comorbid insomnia (Krystal 2012b; Wilson 2010).
In the protocol, not being aware of the problem, we missed specifying how of change from baseline scores and double‐blind average scores would be handled. In order to provide clarity to the reader, we described the handling of different score types in the methods section (see Types of outcome measures). Furthermore, when writing the protocol we were not conscious of the fact that meta‐analyses of adverse events would be based on number of events instead of participants. This has subsequently been added to Unit of analysis issues in the methods section. In addition, we added that 'temporary worsening' of sleep was evaluated as mean change from baseline for the primary efficacy outcomes (SOL, WASO) during the first three nights of the placebo run‐out period (see Types of outcome measures).
Against our original planning in the protocol, we omitted sensitivity analyses for low and high heterogeneity outcomes due to methodological limitations, like the dependence between the mode of analyses and results. Furthermore, diverging from the protocol, we examined differences in results between investigator‐ and industry‐initiated trials by subgroup analyses instead of sensitivity analyses as we became aware that sponsoring was not a result of methodological decision in the review process, but rather a criterion of the study itself.
We also think it should be briefly noted in the text of the review wherever a change has been made, and a link to the ‘Differences’ section provided.
Contributions of authors
Susanne Rösner:
protocol elaboration
study selection
data extraction
data management
analysis of data (MAL)
interpretation and discussion of results
writing of the review
securing funding
Christian Englbrecht:
study selection
data extraction
Renate Wehrle:
study selection
data extraction
Göran Hajak:
protocol elaboration
interpretation and discussion of results
Michael Soyka:
protocol elaboration
interpretation and discussion of results
securing funding
Sources of support
Internal sources
-
Ludwig Maximilian University of Munich, Germany.
Provision of infrastructure and related services
-
Forel Klinik, Switzerland.
Provision of infrastructure, related services and salary
External sources
-
Federal Ministry of Education and Research, Germany.
Financial support/salary
Declarations of interest
Rösner S: no conflict of interest known
Englbrecht C: no conflict of interest known
Wehrle R: no conflict of interest known
Hajak G: received speaker/consultancy/advisory board honoraria from Actelion, Astra‐Zeneca, Bristol‐Meyers Squibb, Boehringer Ingelheim, Cephalon, EuMeCom, GlaxoSmithKline, Janssen‐Cilag, Lilly, Lundbeck, Novartis, Organon, Pfizer, Sanofi‐Aventis, Servier, Takeda, Wyeth. Advisory Boards: Actelion, Astra‐Zeneca, Bristol‐Meyers Squibb, Janssen‐Cilag, Lilly, Lundbeck, Neurocrine, Organon, Pfizer, Sanofi‐Aventis, Sepracor, Servier, Takeda, Wyeth.
Industrie‐Drittmittel: Actelion, Affectis, Astra‐Zeneca, Boehringer Ingelheim, GlaxoSmithKline, Lundbeck, Novartis, Organon, Sanofi‐Aventis, Sepracor, Servier, Takeda.
Soyka M: received speaker/consultancy/advisory board honoraria from Lipha Pharmaceuticals, Forest Laboratories, Sanofi‐Aventis, Essex Pharma, Eli Lilly, Prempharm, Lundbeck, and AstraZeneca.
New
References
References to studies included in this review
Ancoli‐Israel 2010 {published data only}
- Ancoli‐Israel S, Krystal AD, McCall WV, Schaefer K, Wilson A, Claus R, et al. A 12‐week, randomised, double‐blind, placebo‐controlled study evaluating the effect of eszopiclone 2 mg on sleep/wake function in older adults with primary and comorbid insomnia. Sleep 2010;33(2):225‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fava 2006 {published data only}
- Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, et al. Eszopiclone co‐administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biological Psychiatry 2006;59(11):1052‐60. [DOI] [PubMed] [Google Scholar]
Goforth 2014 {published data only}
- Goforth HW, Preud'homme X, Krystal AD. A randomised, double‐blind, placebo‐controlled trial of eszopiclone for the treatment of insomnia in patients with chronic low back pain. Sleep 2014;37(6):1053‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krystal 2003 {published data only}
- Krystal AD, Walsh JK, Laska E, Caron J, Amato DA, Wessel TC, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomised, double‐blind, placebo‐controlled study in adults with chronic insomnia. Sleep 2003;26(7):793‐9. [DOI] [PubMed] [Google Scholar]
McCall 2006 {published data only}
- McCall WV, Erman M, Krystal AD, Rosenberg R, Scharf M, Zammit GK, et al. A polysomnography study of eszopiclone in elderly patients with insomnia. Current Medical Research and Opinion 2006;22(9):1633‐42. [DOI] [PubMed] [Google Scholar]
McCall 2010a {published data only}
- McCall WV, Blocker JN, D’Agostino RB Jr, Kimball J, Boggs N, Lasater B, et al. Treatment of insomnia in depressed insomniacs: effects on health‐related quality of life, objective and self reported sleep, and depression. Journal of Clinical Sleep Medicine 2010;6:322‐9. [PMC free article] [PubMed] [Google Scholar]
Menza 2010 {published data only}
- Menza M, Dobkin RD, Marin H, Gara M, Bienfait K, Dicke A, et al. Treatment of insomnia in Parkinson's disease: a controlled trial of eszopiclone and placebo. Movement Disorders 2010;25(11):1708‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollack 2008 {published data only}
- Pollack M, Kinrys G, Krystal A, McCall WV, Roth T, Schaefer K, et al. Eszopiclone co‐administered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Archives of General Psychiatry 2008;65(5):551‐62. [DOI] [PubMed] [Google Scholar]
Roth 2009 {published data only}
- Roth T, Price JM, Amato DA, Rubens RP, Roach JM, Schnitzer TJ. The effect of eszopiclone in patients with insomnia and coexisting rheumatoid arthritis: a pilot study. Primary Care Companion to the Journal of Clinical Psychiatry 2009;11(6):292‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scharf 2005 {published data only}
- Scharf M, Erman M, Rosenberg R, Seiden D, McCall WV, Amato D, et al. A 2‐week efficacy and safety study of eszopiclone in elderly patients with primary insomnia. Sleep 2005;28(6):720‐7. [DOI] [PubMed] [Google Scholar]
Soares 2006 {published data only}
- Soares CN, Joffe H, Rubens R, Caron J, Roth T, Cohen L. Eszopiclone in patients with insomnia during perimenopause and early postmenopause: a randomised controlled trial. Obstetrics & Gynecology 2006;108(6):1402‐10. [DOI] [PubMed] [Google Scholar]
Spierings 2015 {published data only}
- Spierings EL, McAllister PJ, Bilchik TR. Efficacy of treatment of insomnia in migraineurs with eszopiclone (Lunesta) and its effect on total sleep time, headache frequency, and daytime functioning: a randomised, double‐blind, placebo‐controlled, parallel‐group, pilot study. Cranio 2015;33(2):115‐21. [DOI] [PubMed] [Google Scholar]
Walsh 2007 {published data only}
- Walsh JK, Krystal AD, Amato DA, Rubens R, Caron J, Wessel TC, et al. Nightly treatment of primary insomnia with eszopiclone for six months: effect on sleep, quality of life, and work limitations. Sleep 2007;30(8):959‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zammit 2004 {published data only}
- Zammit GK, McNabb LJ, Caron J, Amato DA, Roth T. Efficacy and safety of eszopiclone across 6‐weeks of treatment for primary insomnia. Current Medical Research and Opinion 2004;20(12):1979‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Attarian 2011 {published data only}
- Attarian H, Applebee G, Applebee A, Wang B, Clark M, McCormick B, et al. Effect of eszopiclone on sleep disturbances and daytime fatigue in multiple sclerosis patients. International Journal of MS Care 2011;13(2):84‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2008 {published data only}
- Boyle J, Trick L, Johnsen S, Roach J, Rubens R. Next‐day cognition, psychomotor function, and driving‐related skills following nighttime administration of eszopiclone. Human Psychopharmacology 2008;23(5):385‐97. [DOI] [PubMed] [Google Scholar]
Boyle 2012 {published data only}
- Boyle J, Groeger JA, Paska W, Cooper JA, Rockett C, Jones S, et al. A method to assess the dissipation of residual hypnotics: eszopiclone versus zopiclone. Journal of Clinical Psychopharmacology 2012;32(5):704‐9. [DOI] [PubMed] [Google Scholar]
Demanuele 2014 {published data only}
- Demanuele C, Bartsch U, Wamsley EJ, Shinn AK, Goff DC, Jones MW. The effects of eszopiclone on slow wave modulation of sleep spindles in schizophrenia. 69th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry. New York, United States, 2014.
Dimsdale 2011b {published data only}
- Dimsdale JE, Ball ED, Carrier E, Wallace M, Holman P, Mulroney C. Effect of eszopiclone on sleep, fatigue, and pain in patients with mucositis associated with hematologic malignancies. Supportive Care in Cancer 2011;19:2015‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eckert 2011 {published data only}
- Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim‐Yeh S, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clinical Science 2011;120(12):505‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erman 2008 {published data only}
- Erman MK, Zammit G, Rubens R, Schaefer K, Wessel T, Amato D, et al. A polysomnographic placebo‐controlled evaluation of the efficacy and safety of eszopiclone relative to placebo and zolpidem in the treatment of primary insomnia. Journal of Clinical Sleep Medicine 2008;4(3):229‐34. [PMC free article] [PubMed] [Google Scholar]
Gross 2011 {published data only}
- Gross CR, Kreitzer MJ, Reilly‐Spong M, Wall M, Winbush NY, Patterson R, et al. Mindfulness‐based stress reduction versus pharmacotherapy for chronic primary insomnia: a randomized controlled clinical trial. Explore 2011;7(2):76‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2015 {published data only}
- Huang Y, Zheng Y. Sleep disorder of schizophrenia treated with shallow needling: a randomised controlled trial. Zhongguo Zhenjiu 2015;35(9):869‐73. [PubMed] [Google Scholar]
Joffe 2010 {published data only}
- Joffe H, Petrillo L, Viguera A, Koukopoulos A, Silver‐Heilman K, Farrell A, et al. Eszopiclone improves insomnia and depressive and anxious symptoms in perimenopausal and postmenopausal women with hot flashes: a randomised, double‐blinded, placebo‐controlled crossover trial. American Journal of Obstetrics & Gynecology 2010;202(2):171.e1‐171.e11. [DOI] [PubMed] [Google Scholar]
Lettieri 2008 {published data only}
- Lettieri CJ, Quast TN, Eliasson AH, Andrada T. Eszopiclone improves overnight polysomnography and continuous positive airway pressure titration: a prospective, randomized, placebo‐controlled trial. Sleep 2008;31(9):1310‐6. [PMC free article] [PubMed] [Google Scholar]
NCT00120250 {unpublished data only}
- NCT00120250. Eszopiclone for sleep disturbance and nightmares in post‐traumatic stress disorder. clinicaltrials.gov/show/NCT000120250 (first received 15 July 2005).
NCT00368056 {unpublished data only}
- NCT00368056. Effects of one evening dose of 3 mg eszopiclone on next day driving ability. clinicaltrials.gov/ct2/results?term=NCT00368056 (first received 24 August 2006).
NCT00374192 {unpublished data only}
- NCT00374192. The treatment of insomnia in symptomatic peri‐ and postmenopausal women. clinicaltrials.gov/ct2/show/NCT00374192 (first received 11 September 2006).
NCT00460993 {unpublished data only}
- NCT00460993. Efficacy & safety of eszopiclone (Lunesta) in nursing home patients. clinicaltrials.gov/show/NCT000460993 (first received 17 April 2007).
NCT00511134 {unpublished data only}
- NCT00511134. Study of lunesta versus placebo for sleep problems related to smoking cessation and zyban. clinicaltrials.gov/ct2/show/NCT00511134 (first received 3 August 2007).
NCT00616655 {published data only}
- NCT00616655. Generalized anxiety disorder proof of concept efficacy and safety study of SEP‐225441 (eszopiclone) In GAD subjects. clinicaltrials.gov/show/NCT000616655 (first received 15 February 2008).
NCT00685269 {unpublished data only}
- NCT00685269. Safety and efficacy of eszopiclone with mild to moderate obstructive sleep apnea syndrome (OSAS). clinicaltrials.gov/show/NCT00685269 (first received 28 May 2008).
NCT00811746 {unpublished data only}
- NCT00811746. Hypnotics to improve polysomnography yield: eszopiclone vs ramelteon?. clinicaltrials.gov/show/NCT00811746 (first received 19 December 2008).
NCT00813735 {unpublished data only}
- NCT00813735. Eszopiclone co‐administered with escitalopram for insomnia in elderly adults with major depressive disorder. clinicaltrials.gov/ct2/results?term=NCT00813735 (first received 23 December 2008).
NCT00826111 {unpublished data only}
- NCT00826111. The effects of eszopiclone and lexapro on prefrontal glutamate and GABA in depression with anxiety and insomnia. clinicaltrials.gov/show/NCT00826111 (first received 21 January 2009).
NCT00889200 {unpublished data only}
- NCT00889200. Eszopiclone treatment & cortisol responsitivity. clinicaltrials.gov/show/NCT00889200 (first received 28 April 2009).
NCT00900159 {unpublished data only}
- NCT00900159. Effects of daytime eszopiclone administration in shift workers. clinicaltrials.gov/show/NCT00900159 (first received 12 May 2009).
NCT01102270 {unpublished data only}
- NCT011022270. The effect of eszopiclone on the arousal threshold in sleep apnea syndrome. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2010‐ [cited 2015 April 15]. Available from: clinicaltrials.gov/show/NCT01102270 NLM Identifier: NCT01102270 (first received 13 April 2010).
NCT01641900 {unpublished data only}
- NCT01641900. Effects of eszopiclone on sleep and memory in schizophrenia. clinicaltrials.gov/show/NCT01641900 (first received 17 July 2012).
NCT01710631 {unpublished data only}
- NCT01710631. Twelve month study of the safety of eszopiclone in adult subjects with insomnia. clinicaltrials.gov/show/NCT01710631 (first received 19 October 2012).
Peng 2013 {published data only}
- Peng Y. Efficacy of eszopiclone combined with psychotherapy in the treatment of insomnia. Chinese Journal of New Drugs 2013;22:443‐6. [Google Scholar]
Pollack 2011 {published data only}
- Pollack MH, Hoge EA, Worthington JJ, Moshier SJ, Wechsler RS, Brandes M, et al. Eszopiclone for the treatment of posttraumatic stress disorder and associated insomnia: a randomised, double‐blind, placebo‐controlled trial. Journal of Clinical Psychiatry 2011;72(7):892‐7. [DOI] [PubMed] [Google Scholar]
Rosenberg 2005 {published data only}
- Rosenberg R, Caron J, Roth T, Amato D. An assessment of the efficacy and safety of eszopiclone in the treatment of transient insomnia in healthy adults. Sleep Medicine 2005;6(1):15‐22. [DOI] [PubMed] [Google Scholar]
Rosenberg 2007 {published data only}
- Rosenberg R, Roach JM, Scharf M, Amato DA. A pilot study evaluating acute use of eszopiclone in patients with mild to moderate obstructive sleep apnea syndrome. Sleep Medicine 2007;8(5):464‐70. [DOI] [PubMed] [Google Scholar]
Sangal 2014 {unpublished data only}
- Sangal RB, Blumer JL, Lankford DA, Grinnell TA, Huang H. Eszopiclone for insomnia associated with attention‐deficit/hyperactivity disorder. Pediatrics 2014;134(4):1095‐103. [DOI] [PubMed] [Google Scholar]
Tek 2014 {published data only}
- Tek C, Palmese LB, Krystal AD, DeGeorge PC, Reutenauer EL, Guloksuz S. The impact of eszopiclone insomnia treatment on cognition in schizophrenia: a double‐blind, randomized, placebo‐controlled trial. 69th Annual Scientific Convention and Meeting of the Society of Biological Psychiatry. New York, United States, 2014.
Uchimura 2012a {published data only}
- Inoue Y, Kamijo A, Nagai R. Patient background factors affecting the therapeutic outcomes in response to eszopiclone in adult patients with chronic insomnia: A post hoc analysis of a double‐blind phase III study in Japan. Journal of Clinical Sleep Medicine 2015;15(11):1171‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N, Kamijo A, Takase T. Effects of eszopiclone on safety, subjective measures of efficacy, and quality of life in elderly and non‐elderly Japanese patients with chronic insomnia, both with and without comorbid psychiatric disorders: a 24‐week, randomised, double‐blind study. Annals of General Psychiatry 2012;25(11):2‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uchimura 2012b {published data only}
- Uchimura N, Kamijo A, Kuwahara H, Uchiyama M, Shimizu T, Chiba S, Inoue Y. A randomized placebo‐controlled polysomnographic study of eszopiclone in Japanese patients with primary insomnia. Sleep Med 2012;13(10):1247‐53. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Baran 2017 {published data only}
- Baran B, Demanuele C, Vuper TC. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a double‐blind randomised trial. Sleep Conference: 31st Anniversary Meeting of the Associated Professional Sleep Societies, LLC, SLEEP. United States, 2017; Vol. 40:A415.
Buxton 2017 {published data only}
- Buxton OM, Pavlova MK, O'Connor SP, Wang W, Winkelman JW. Lack of change in glucose metabolism in eszopiclone‐treated primary insomnia patients. Nature and Science of Sleep 2017;9:187‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00374556 {published data only}
- NCT00374556. The efficacy of eszopiclone (Lunesta) for chronic insomnia associated with osteoarthritis. clinicaltrials.gov/ct2/show/NCT00374556 (first received 11 September 2006).
NCT00392041 {unpublished data only}
- NCT00392041. Eszopiclone in the treatment of insomnia and associated symptoms of fibromyalgia. clinicaltrials.gov/ct2/show/NCT00392041 (first received 25 October 2006).
NCT00435279 {unpublished data only}
- EUCTR2006‐001529‐24 [HU, AT, GB, FR]. Adults administered Venlafaxine and Eszopiclone Response to Treatment (AVERT): A 31‐Week, Efficacy, Safety and Tolerability Study of Eszopiclone 3 mg Co‐administered with Venlafaxine in Subjects with Major Depressive Disorder (MDD) and Co‐existing Insomnia. https://www.clinicaltrialsregister.eu/ 2006.
- NCT00435279. A 31‐week, efficacy, safety and tolerability study of eszopiclone 3 mg co‐administered with venlafaxine in subjects with major depressive disorder (MDD) and co‐existing insomnia. clinicaltrials.gov/ct2/show/NCT00435279 (first received 14 February 2007).
Pinto 2016 {published data only}
- NCT01100164. A Non‐inferiority Study With Two Treatment Arms Eszopiclone 3 mg Versus Zopiclone 7.5 mg ‐ for the Treatment of Insomnia (Eszo). https://clinicaltrials.gov/ct2/show/NCT01100164 2010.
- Pinto LR Jr, Bittencourt LR, Treptow EC, Braga LR, Tufik S. Eszopiclone versus zopiclone in the treatment of insomnia. Clinics (Sao Paulo) 2016; Vol. 71, issue 1:5‐9. [DOI] [PMC free article] [PubMed]
References to ongoing studies
Emiko 2015 {unpublished data only}
- Emiko H, Tsuyoshi M. Efficacy and safety of eszopiclone in the treatment of insomnia complicated with nocturnal awakenings associated with urination. upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000015966&language=E (accessed 16‐Feb‐2016).
NCT02456532 {unpublished data only}
- NCT02456532. Safety and efficacy of chronic hypnotic use 2 (CIS2). clinicaltrials.gov/ct2/show/NCT02456532 (first received 28 May 2015).
Additional references
American Academy of Sleep Medicine 2014
- American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual, ICSD‐3. 3nd. Chicago: American Academy of Sleep Medicine, 2014. [Google Scholar]
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM‐5. 5th Edition. Washington DC: American Psychiatric Publishing, 2013. [Google Scholar]
Angst 1989
- Angst J, Vollrath M, Koch R, Dobler‐Mikola A. The Zurich Study. VII. Insomnia: symptoms, classification and prevalence. European Archives of Psychiatry and Neurological Sciences 1989;238(5‐6):285‐93. [DOI] [PubMed] [Google Scholar]
Arnedt 2007
- Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. Journal of Addictive Diseases 2007;26(4):41‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballenger 2000
- Ballenger JC. Benzodiazepine receptor agonists and antagonists. In: Sadock VA, Sadock BJ, Kaplan HI editor(s). Kaplan & Sadock's Comprehensive Textbook of Psychiatry. 7th Edition. Philadelphia: Lippincott Williams & Wilkins, 2000. [Google Scholar]
Barbone 1998
- Barbone F, McMahon AD, Davey PG, Morris AD. Association of road‐traffic accidents with benzodiazepine use. Lancet 1998;352(9137):1331‐6. [DOI] [PubMed] [Google Scholar]
Bastien 2001
- Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Medicine 2001;2:297‐307. [DOI] [PubMed] [Google Scholar]
Bastien 2004
- Bastien CH, Vallières A, Morin CM. Precipitating factors of insomnia. Behavioral Sleep Medicine 2004;2(1):50‐62. [DOI] [PubMed] [Google Scholar]
Bateson 2004
- Bateson AN. The benzodiazepine site of the GABAA receptor: an old target with new potential?. Sleep Medicine 2004;5(1):9‐15. [DOI] [PubMed] [Google Scholar]
Ben‐Hamou 2011
- Ben‐Hamou M, Marshall NS, Grunstein RR, Saini B, Fois RA. Spontaneous adverse event reports associated with zolpidem in Australia 2001‐2008. Journal of Sleep Research 2011;20(4):559‐68. [DOI] [PubMed] [Google Scholar]
Berry 2013
- Berry SD, Lee Y, Cai S, Dore DD. Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents. JAMA Internal Medicine 2013;173(9):754‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bertisch 2014
- Bertisch SM, Herzig SJ, Winkelman JW, Buettner C. National use of prescription medications for insomnia: NHANES 1999‐2010. Sleep 2014;37(2):343‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolton 2008
- Bolton JM, Metge C, Lix L, Prior H, Sareen J, Leslie WD. Fracture risk from psychotropic medications: a population‐based analysis. Journal of Clinical Psychopharmacology 2008;28(4):384‐91. [DOI] [PubMed] [Google Scholar]
Botteman 2007
- Botteman MF, Ozminkowski RJ, Wang S, Pashos CL, Schaefer K, Foley DJ. Cost effectiveness of long‐term treatment with eszopiclone for primary insomnia in adults: a decision analytical model. CNS Drugs 2007;21(4):319‐34. [DOI] [PubMed] [Google Scholar]
Brower 2001
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self‐medication, and relapse to alcoholism. American Journal of Psychiatry 2001;158:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brunello 2008
- Brunello N, Bettica P, Amato D, Maier G, Nutt D. Pharmacokinetics of (S)‐zopiclone and (S)‐desmethylzopiclone following dosing with zopiclone and eszopiclone. Neuropsychopharmacology 2008;18:581. [Google Scholar]
Brunette 2003
- Brunette MF, Noordsy DL, Xie H, Drake RE. Benzodiazepine use and abuse among patients with severe mental illness and co‐occurring substance use disorders. Psychiatric Services 2003;54(10):1395‐401. [DOI] [PubMed] [Google Scholar]
Buscemi 2007
- Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta‐analysis of RCTs. Journal of General Internal Medicine 2007;22(9):1335‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buysse 2011
- Buysse DJ, Germain A, Hall M, Monk TH, Nofzinger EA. A neurobiological model of insomnia. Drug Discovery Today: Disease Models 2011;8(4):129‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cape 2015
- Cape J, Leibowitz J, Whittington C, Espie CA, Pilling S. Group cognitive behavioural treatment for insomnia in primary care: a randomised controlled trial. Psychological Medicine 2015;16:1‐11. [DOI] [PubMed] [Google Scholar]
Cappuccio 2011
- Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. European Heart Journal 2011;32(12):1484‐92. [DOI] [PubMed] [Google Scholar]
Cimolai 2007
- Cimolai, N. Zopiclone. Is it a pharmacologic agent for abuse?. Canadian Family Physician 2007;53:2124‐9. [PMC free article] [PubMed] [Google Scholar]
Conroy 2006
- Conroy DA, Todd Arnedt J, Brower KJ, Strobbe S, Consens F, Hoffmann R, et al. Perception of sleep in recovering alcohol‐dependent patients with insomnia: relationship with future drinking. Alcoholism: Clinical and Experimental Research 2006;30(12):1992‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Delahaye 1990
- Delahaye C, Ferrand B, Pieddeloup C, Musch B. Post marketing surveillance of zopiclone: interim analysis on the first 10,000 cases in a clinical study in general practice. International Clinical Psychopharmacology 1990;5 Suppl(2):131‐8. [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird NM. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Diem 2014
- Diem SJ, Ewing SK, Stone KL, Ancoli‐Israel S, Redline S, Ensrud KE, the Osteoporotic Fractures in Men (MrOS) Study Group. Use of non‐benzodiazepine sedative hypnotics and risk of falls in older men. Journal of Gerontology & Geriatric Research 2014;3(3):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolder 2007
- Dolder C, Nelson M, McKinsey J. Use of non‐benzodiazepine hypnotics in the elderly: are all agents the same?. CNS Drugs 2007;21(5):389‐405. [DOI] [PubMed] [Google Scholar]
Drover 2000
- Drover D, Lemmens H, Naidu S, Cevallos W, Darwish M, Stanski D. Pharmacokinetics, pharmacodynamics, and relative pharmacokinetic/pharmacodynamic profiles of zaleplon and zolpidem. Clinical Therapeutics 2000;22(12):1443‐61. [DOI] [PubMed] [Google Scholar]
Drover 2004
- Drover DR. Comparative pharmacokinetics and pharmacodynamics of short‐acting hypnosedatives: zaleplon, zolpidem and zopiclone. Clinical Pharmacokinetics 2004;43(4):227‐38. [DOI] [PubMed] [Google Scholar]
Duggal 2007
- Duggal HS. New‐onset transient hallucinations possibly due to eszopiclone: a case study. The Primary Care Companion to the Journal of Clinical Psychiatry 2007;9(6):468‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dündar 2004a
- Dündar Y, Dodd S, Strobl J, Boland A, Dickson R, Walley T. Comparative efficacy of newer hypnotic drugs for the short‐term management of insomnia: a systematic review and meta‐analysis. Human Psychopharmacology 2004;19(5):305‐22. [DOI] [PubMed] [Google Scholar]
Dündar 2004b
- Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al. Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation. Health Technology Assessment 2004;8(24):iii‐x, 1‐125. [DOI] [PubMed] [Google Scholar]
Edinger 2004
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. American Academy of Sleep Medicine Work Group. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep 2004;27(8):1567‐96. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisen 1994
- Eisen SV, Dill DL, Grob MC. Reliability and validity of a brief patient‐report instrument for psychiatric outcome evaluation. Hospital & Community Psychiatry 1994;45(3):242‐7. [DOI] [PubMed] [Google Scholar]
Elko 1998
- Elko CJ, Burgess JL, Robertson WO. Zolpidem‐associated hallucinations and serotonin reuptake inhibition: a possible interaction.. Journal of Toxicology: Clinical Toxicology 1998;36:195–203. [DOI] [PubMed] [Google Scholar]
Erman 2004
- Erman M, Rosenberg R, Caron Y. Polysomnographic and patient‐reported evaluation of the efficacy and safety of eszopiclone in elderly subjects with chronic insomnia. Sleep 2004;27 Suppl:257‐8. [Google Scholar]
Erman 2005
- Erman MK. Therapeutic options in the treatment of insomnia. Journal of Clinical Psychiatry 2005;66(9):18‐23. [PubMed] [Google Scholar]
Espie 2002
- Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annual Review of Psychology 2002;53:215‐43. [DOI] [PubMed] [Google Scholar]
Espie 2006
- Espie CA, Broomfield NM, MacMahon KM, Macphee LM, Taylor LM. The attention‐intention‐effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Medicine Reviews 2006;10(4):215‐45. [DOI] [PubMed] [Google Scholar]
European Medicines Agency 2009
- European Medicines Agency. Sepracor Pharmaceuticals Ltd withdraws its marketing authorisation application for Lunivia (eszopiclone). www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2009/11/news_detail_000083.jsp (accessed 16 February 2015).
Falcón 2009
- Falcón E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology 2009;56(1):91‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fava 2011
- Fava M, Schaefer K, Huang H, Wilson A, Iosifescu DV, Mischoulon D, et a. A post hoc analysis of the effect of nightly administration of eszopiclone and a selective serotonin reuptake inhibitor in patients with insomnia and anxious depression. Journal of Clinical Psychiatry 2011;72(4):473‐9. [DOI] [PubMed] [Google Scholar]
Fernández‐Mendoza 2010
- Fernández‐Mendoza J, Vela‐Bueno A, Vgontzas AN, Ramos‐Platón MJ, Olavarrieta‐Bernardino S, Bixler EO, et al. Cognitive‐emotional hyperarousal as a premorbid characteristic of individuals vulnerable to insomnia. Psychosomatic Medicine 2010;72(4):370‐403. [DOI] [PubMed] [Google Scholar]
Ferrie 2007
- Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep 2007;30(12):1659‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finkle 2011
- Finkle WD, Der JS, Greenland S, Adams JL, Ridgeway G, Blaschke T, et al. Risk of fractures requiring hospitalisation after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. Journal of the American Geriatrics Society 2011;59(10):1883‐90. [DOI] [PubMed] [Google Scholar]
Follesa 2002
- Follesa P, Mancuso L, Biggio F, Cagetti E, Franco M, Trapani G, et al. Changes in GABA(A) receptor gene expression induced by withdrawal of, but not by long‐term exposure to, zaleplon or zolpidem. Neuropharmacology 2002;42(2):191‐8. [DOI] [PubMed] [Google Scholar]
Food and Drug Administration 2015
- Food, Drug Administration. Eszopiclone containing sleep aids: drug safety communication ‐ can cause next‐day impairment. www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm397536.htm (accessed 12 March 2015).
Fortier‐Brochu 2012
- Fortier‐Brochu E, Beaulieu‐Bonneau S, Ivers H, Morin CM. Insomnia and daytime cognitive performance: a meta‐analysis. Sleep Medicine Reviews 2012;16(1):83‐94. [DOI] [PubMed] [Google Scholar]
Foster 1999
- Foster JH, Peters TJ. Impaired sleep in alcohol misusers and dependent alcoholics and the impact upon outcome. Alcoholism: Clinical and Experimental Research 1999;23(6):1044–51. [PubMed] [Google Scholar]
Friedmann 2003
- Friedmann PD, Herman DS, Freedman S, Lemon SC, Ramsey S, Stein MD. Treatment of sleep disturbance in alcohol recovery: a national survey of addiction medicine physicians. Journal of Addictive Diseases 2003;22(2):91‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillin 1989
- Gillin JC, Spinweber CL, Johnson LC. Rebound insomnia: a critical review. Journal of Clinical Psychopharmacology 1989;9(3):161‐72. [PubMed] [Google Scholar]
Greenblatt 1981
- Greenblatt DJ, Shader RI, Divoll M, Harmatz JS. Benzodiazepines. A summary of pharmacokinetic properties. British Journal of Clinical Pharmacology 1981;11(Suppl 1):11‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenblatt 2012
- Greenblatt DJ, Zammit GK. Pharmacokinetic evaluation of eszopiclone: clinical and therapeutic implications. Expert Opinion in Drug Metabolism and Toxicology 2012;8(12):1609‐18. [DOI] [PubMed] [Google Scholar]
Gunja 2013
- Gunja, N. In the Zzz zone: the effects of Z‐drugs on human performance and driving. Journal of Medical Toxicology 2013;9(2):163‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hair 2008
- Hair PI, McCormack PL, Curran MP. Eszopiclone: a review of its use in the treatment of insomnia. Drugs 2008;68(10):1415‐34. [DOI] [PubMed] [Google Scholar]
Hajak 1997
- Hajak G, Muller‐Popkes K, Riemann D, Mayer G, Lauer C. Psychological, psychotherapeutic and other non‐pharmacologic forms of therapy in treatment of insomnia. Fortschritte der Neurologie‐Psychiatrie 1997;65(3):133‐44. [DOI] [PubMed] [Google Scholar]
Hajak 2002a
- Hajak G, Bandelow B, Zulley J, Pittrow D. "As needed" pharmacotherapy combined with stimulus control treatment in chronic insomnia‐‐assessment of a novel intervention strategy in a primary care setting. Ann Clin Psychiatry 2002;14(1):1‐7. [PubMed] [Google Scholar]
Hajak 2002b
- Hajak, G. Zolpidem "as needed" versus continuous administration: Pan‐European study results. Sleep Med Rev 2002;6(Suppl 1):21‐8. [DOI] [PubMed] [Google Scholar]
Hajak 2003
- Hajak G, Müller WE, Wittchen HU, Pittrow D, Kirch W. Abuse and dependence potential for the non‐benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction 2003;98(10):1371‐8. [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol 1959;32(1):50‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamiltion, M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harvey 2018
- Harvey CJ, Gehrman P, Espie CA. Who is predisposed to insomnia: a review of familial aggregation, stress‐reactivity, personality and coping style. Sleep Med Rev 2014;18(3):237‐47. [DOI] [PubMed] [Google Scholar]
Hausken 2009
- Hausken AM, Furu K, Skurtveit S, Engeland A, Bramness JG. Starting insomnia treatment: the use of benzodiazepines versus z‐hypnotics. A prescription database study of predictors. European Journal of Clinical Pharmacology 2009;65(3):295‐301. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Chichester (UK), 2011:243‐96.
Hoffmann 2009
- Hoffmann F, Scharffetter W, Glaeske G. Use of zolpidem and zopiclone on private prescriptions between 1993 and 2007. Der Nervenarzt 2009;80(5):578‐83. [DOI] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann F, Glaeske G. Benzodiazepine hypnotics, zolpidem and zopiclone on private prescriptions: use between 1993 and 2012. Der Nervenarzt 2014;85(11):1402‐9. [DOI] [PubMed] [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;20(5):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huedo‐Medina 2012
- Huedo‐Medina TB, Kirsch I, Middlemass J, Klonizakis M, Siriwardena AN. Effectiveness of non‐benzodiazepine hypnotics in treatment of adult insomnia: meta‐analysis of data submitted to the Food and Drug Administration. British Medical Journal 2012;17(345):e8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huwiler‐Muntener 2002
- Huwiler‐Muntener K, Juni P, Junker C, Egger M. Quality of reporting of randomised trials as a measure of methodologic quality. Journal of the American Medical Association 2002;287(21):2801‐4. [DOI] [PubMed] [Google Scholar]
Jaffe 2004
- Jaffe JH1, Bloor R, Crome I, Carr M, Alam F, Simmons A, et al. A post‐marketing study of relative abuse liability of hypnotic sedative drugs. Addiction 2004;99(2):165‐73. [DOI] [PubMed] [Google Scholar]
Jenkinson 1997
- Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson's Disease Questionnaire (PDQ‐39): development and validation of a Parkinson's disease summary index score. Age and Ageing 1997;26(5):353‐7. [DOI] [PubMed] [Google Scholar]
Johnson 2001
- Johnson EO, Breslau N. Sleep problems and substance use in adolescence.. Drug and Alcohol Dependence 2001;64(1):1‐7. [DOI] [PubMed] [Google Scholar]
Joya 2009
- Joya FL, Kripke DF, Loving RT, Dawson A, Kline LE. Meta‐analyses of hypnotics and infections: eszopiclone, ramelteon, zaleplon, and zolpidem. Journal of Clinical Sleep Medicine 2009;5(4):377‐83. [PMC free article] [PubMed] [Google Scholar]
Kaplan 2014
- Kaplan KA, McQuaid J, Primich C, Rosenlicht N. An evidence‐based review of insomnia treatment in early recovery. Journal of Addiction Medicine 2014;8(6):389‐94. [DOI] [PubMed] [Google Scholar]
Katz 1998
- Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Archives of Internal Medicine 1998;158(10):1099–1107. [DOI] [PubMed] [Google Scholar]
Krishnan 2011
- Krishnan JA, Schatz M, Apter AJ. A call for action: comparative effectiveness research in asthma. Journal of Allergy and Clinical Immunology 2011;127(1):123‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krystal 2007
- Krystal A, Fava M, Rubens R, Wessel T, Caron J, Wilson P, et al. Evaluation of eszopiclone discontinuation after co‐therapy with fluoxetine for insomnia with coexisting depression. Journal of Clinical Sleep Medicine 2007;3(1):48‐55. [PubMed] [Google Scholar]
Krystal 2012a
- Krystal AD, Huang H, Zummo J, Grinnell T, Marshall RD. A WASO sub‐group analysis of a 6‐month study of eszopiclone 3 mg. Sleep Medicine 2012;13(6):691‐6. [DOI] [PubMed] [Google Scholar]
Krystal 2012b
- Krystal AD, McCall WV, Fava M, Joffe H, Soares CN, Huang H, et al. Eszopiclone treatment for insomnia: effect size comparisons in patients with primary insomnia and insomnia with medical and psychiatric comorbidity. The Primary Care Companion for CNS Disorders 2012;14(4):doi: 10.4088/PCC.11m01296. [DOI: 10.4088/PCC.11m01296] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lader 1999
- Lader MH. Limitations on the use of benzodiazepines in anxiety and insomnia: are they justified?. European Neuropsychopharmacology 1999;9(Suppl 6):399‐405. [DOI] [PubMed] [Google Scholar]
Leger 2001
- Leger D, Scheurmaier K, Philip P, Paillard M, Guilleminault C. SF‐36: evaluation of quality of life in severe and mild insomniac compared with good sleepers. Psychosomatic Medicine 2001;63(1):49‐55. [DOI] [PubMed] [Google Scholar]
Lerner 2001
- Lerner D, Amick BC 3rd, Rogers WH, Malspeis S, Bungay K, Cynn D. The Work Limitations Questionnaire. Medical Care 2001;39(1):72‐85. [DOI] [PubMed] [Google Scholar]
Leshner 2005
- Leshner A. NIH State‐of‐the‐Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH Consensus and State‐of‐the‐Science Statements. 2005; Vol. 22:1‐30. [PubMed]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. Brisitsh Medical Journal 2003;31(326):1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2014
- Li Y, Zhang X, Winkelman JW, Redline S, Hu FB, Stampfer M, et al. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation 2014;129(7):737‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Licata 2008
- Licata SC, Penetar DM, Dunlap S, Lukas SE. A therapeutic dose of zolpidem has limited abuse‐like effects in drug‐naïve females: a pilot study. European Journal of Pharmacology 2008;598(1‐3):64‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Light 1984
- Light RJ, Pillemer BD. The Science of Reviewing Research. Cambridge (MA): Harvard University Press, 1984. [Google Scholar]
Lingford‐Hughes 2002
- Lingford‐Hughes A, Hume SP, Feeney A, Hirani E, Osman S, Cunningham VJ, et al. Imaging the GABA‐benzodiazepine receptor subtype containing the alpha 5‐subunit in vivo with (11C)Ro15 4513 positron emission tomography. Journal of Cerebral Blood Flow and Metabolism 2002;22:878–88. [DOI] [PubMed] [Google Scholar]
Lunesta 2004 [pers comm]
- Lunesta® (eszopiclone) package insert. Sepracor Inc (Sunovion Pharmaceuticals): Marlborough (MA) 2004.
Maier 1985
- Maier W, Philipp M. Improving the assessment of severity of depressive states: a reduction of the Hamilton Depression Scale. Pharmacopsychiatry 1985;18:114‐5. [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective disease. Journal of the National Cancer Institute 1959;22:719–48. [PubMed] [Google Scholar]
Marshall 2011a
- Marshall RD, Grinnell T, Zummo J, Schweizer E. Treatment of primary insomnia in the elderly: predictors of response to eszopiclone. 25th Anniversary Meeting of the Associated Professional Sleep Societies, LLC, SLEEP; 2011; Minneapolis, United States. 2011.
Marshall 2011b
- Marshall RD, Grinnell T, Zummo J, Schweizer E. Treatment with eszopiclone in adults with primary insomnia: predictors of response. 25th Anniversary Meeting of the Associated Professional Sleep Societies; 2011; Minneapolis, United States. 2011.
McCall 2009
- McCall WV, D’Agostino RB, Kimball JN, Boggs N, Lasater B, Dunn A, et al. Eszopiclone at bedtime improves the quality of life in depressed insomniacs receiving fluoxetine. Sleep 2009;32:A348‐A349. [Google Scholar]
McCall 2010b
- McCall WV, Blocker JN, D'Agostino R Jr, Kimball J, Boggs N, Lasater B, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Medicine 2010;11(9):822‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCall 2011a
- McCall WV, D'Agostino R Jr, Rosenquist PB, Kimball J, Boggs N, Lasater B, et al. Dissection of the factors driving the placebo effect in hypnotic treatment of depressed insomniacs. Sleep Medicine 2011;12(6):557‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCall 2011b
- McCall WV, Blocker J, Dagostino JR, Wang J, Shannon W. Both actigraphy data and the intensity of insomnia complaints differ between suicidal and non‐suicidal depressed patients enrolled in a hypnotic clinical trial. Journal of Sleep Research 2011;20(Suppl s1):24. [Google Scholar]
McCall 2012
- McCall C, McCall WV. Objective vs. subjective measurements of sleep in depressed insomniacs: first night effect or reverse first night effect?. Journal of Clinical Sleep Medicine 2012;15(8 (1)):59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Melton 2005
- Melton ST, Wood JM, Kirkwood CK. Eszopiclone for insomnia. Annals of Pharmacotherapy 2005;39(10):1659‐66. [DOI] [PubMed] [Google Scholar]
Metlaine 2005
- Metlaine A, Leger D, Choudat D. Socioeconomic impact of insomnia in working populations. Industrial Health 2005;43(1):11‐9. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moloney 2011
- Moloney ME, Konrad TR, Zimmer CR. The medicalisation of sleeplessness: a public health concern. American Journal of Public Health 2011;101:1429–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moncrieff 2004
- Moncrieff J, Wessely S, Hardy R. Active placebos versus antidepressants for depression. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD003012.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monti 2007
- Monti JM, Pandi‐Perumal SR. Eszopiclone: its use in the treatment of insomnia. Neuropsychiatric Disease and Treatment 2007;3(4):441‐53. [PMC free article] [PubMed] [Google Scholar]
Montplaisir 2003
- Montplaisir J, Hawa R, Moller H, Morin C, Fortin M, Matte J, et al. Zopiclone and zaleplon vs benzodiazepines in the treatment of insomnia: Canadian consensus statement. Human Psychopharmacology 2003;18(1):29‐38. [DOI] [PubMed] [Google Scholar]
Morin 2006
- Morin CM, LeBlanc M, Daley M, Grégoire JP, Mérette C. Epidemiology of insomnia: prevalence, self‐help treatments, consultations, and determinants of help‐seeking behaviours. Sleep Medicine 2006;7:123‐30. [DOI] [PubMed] [Google Scholar]
Morin 2012
- Morin CM, Benca R. Chronic insomnia. Lancet 2012;24(379):1129‐41. [DOI] [PubMed] [Google Scholar]
Najib 2006
- Najib J. Eszopiclone, a nonbenzodiazepine sedative‐hypnotic agent for the treatment of transient and chronic insomnia. Clinical Therapeutics 2006;28(4):491‐516. [DOI] [PubMed] [Google Scholar]
NHS Prescribing Service 2010
- National Prescribing Service. Addressing hypnotic medicines use in primary care. www.nps.org.au/health_professionals/publications/nps_news/current/nps_news_67 2010.
Nickerson 1998
- Nickerson RS. Confirmation bias: a ubiquitous phenomenon in many guises. Review of General Psychology 1998;2(2):175–220. [Google Scholar]
Nutt 2010
- Nutt DJ, Stahl SM. Searching for perfect sleep: the continuing evolution of GABAA receptor modulators as hypnotics. Journal of Psychopharmacology 2010;24(11):1601‐12. [DOI] [PubMed] [Google Scholar]
Ohayon 2002
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews 2002;6(2):97‐111. [DOI] [PubMed] [Google Scholar]
Ohayon 2009
- Ohayon MM, Reynolds CF 3rd. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM‐IV and the International Classification of Sleep Disorders (ICSD). Sleep Medicine 2009;10(9):952‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palagini 2014
- Palagini L, Biber K, Riemann D. The genetics of insomnia‐‐evidence for epigenetic mechanisms?. Sleep Med Rev 2014;18(3):225‐35. [DOI] [PubMed] [Google Scholar]
Parthasaraty 2015
- Parthasarathy S, Vasquez MM, Halonen M, Bootzin R, Quan SF, Martinez FD, et al. Persistent insomnia is associated with mortality risk. American Journal of Medicine 2015;128(3):268‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perlis 1997
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. Journal of Sleep Research 1997;6:179‐88. [DOI] [PubMed] [Google Scholar]
Pildal 2007
- Pildal J, Hrobjartsson A, Jorgensen KJ, Hilden J, Altman DG, Gotzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomized trials. International Journal of Epidemiology 2007;36(4):847‐57. [DOI] [PubMed] [Google Scholar]
Ramakrishnan 2007
- Ramakrishnan K, Scheid DC. Treatment options for insomnia. American Family Physician 2007;76(4):517‐26. [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riemann 2007
- Riemann D. Insomnia and comorbid psychiatric disorders. Sleep Medicine 2007;8(4):15‐20. [DOI] [PubMed] [Google Scholar]
Riemann 2009
- Riemann D, Kloepfer C, Berger M. Functional and structural brain alterations in insomnia: implications for pathophysiology. European Journal of Neuroscience 2009;29(9):1754‐60. [DOI] [PubMed] [Google Scholar]
Riemann 2014
- Riemann D, Baglioni C, Feige B, Spiegelhalder K. Insomnia ‐ state of the science. Der Nervenarzt 2014;85(1):43‐9. [DOI] [PubMed] [Google Scholar]
Riemann 2015
- Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurolology 2015;14(5):547‐58. [DOI] [PubMed] [Google Scholar]
Roane 2008
- Roane BM, Taylor DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep 2008;31(10):1351‐6. [PMC free article] [PubMed] [Google Scholar]
Roland 1983
- Roland MO, Morris RW. A study of the natural history of back pain. Development of a reliable and sensitive measure of disability in low back pain. Spine 1983;8:141‐4. [DOI] [PubMed] [Google Scholar]
Rosekind 2010
- Rosekind MR, Gregory KB. Insomnia risks and costs: health, safety, and quality of life. American Journal of Managed Care 2010;16(8):617‐26. [PubMed] [Google Scholar]
Roth 2003
- Roth T, Roehrs T. Insomnia: epidemiology, characteristics, and consequences. Clinical Cornerstone 2003;5(3):5‐15. [DOI] [PubMed] [Google Scholar]
Roth 2005
- Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Medicine 2005;6(6):487‐95. [DOI] [PubMed] [Google Scholar]
Roth 2009a
- Roth T. Sleep duration, insomnia and longevity. Sleep Medicine 2009;10(10):1071‐2. [DOI] [PubMed] [Google Scholar]
Royal College of Psychiatrists 1997
- Royal College of Psychiatrists. Benzodiazepines: risks, benefits or dependence. A re‐evaluation. Council Report CR59; 1997; London 1997.
Rudolph 2011
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nature Reviews Drug Discovery 2011;10(9):685‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rösner 2013a
- Rösner S, Soyka M, Hajak G, Wehrle R. Zolpidem for insomnia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rösner 2013b
- Rösner S, Soyka M, Hajak G, Wehrle R. Zopiclone for insomnia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010701] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rösner 2013c
- Rösner S, Soyka M, Hajak G, Wehrle R. Zaleplon for insomnia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010702] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter JR, Schumacher M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Medical Research Methodology 2008;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sateia 2017
- Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017;13(2):307‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scharf 2006
- Scharf M. Eszopiclone for the treatment of insomnia. Expert Opinion in Pharmacotherapy 2006;7(3):345‐56. [DOI] [PubMed] [Google Scholar]
Schnitzer 2005
- Schnitzer T, Rubens R, Price. The effect of eszopiclone 3 mg compared with placebo in patients with rheumatoid arthritis and co‐existing insomnia. American College of Rheumatology Conference; 2005 November 12–17; 2005; San Diego (poster). 2005.
Schulz 1995
- Schulz KF. Subverting randomization in controlled trials. Journal of the American Medical Association 1995;274(18):1456‐8. [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLOS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schutte‐Rodin 2008
- Schutte‐Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of Clinical Sleep Medicine 2008;4(5):487‐504. [PMC free article] [PubMed] [Google Scholar]
Sepracor 2004 [pers comm]
- Prescribing information. Lunesta® (eszopiclone). Sepracor Inc; Marlborough MA.
Shekleton 2010
- Shekleton JA, Rogers NL, Rajaratnam SM. Searching for the daytime impairments of primary insomnia. Sleep Medicine Reviews 2010;14(1):47‐60. [DOI] [PubMed] [Google Scholar]
Sieghart 2006
- Sieghart W. Structure, pharmacology, and function of GABAA receptor subtypes. Advances in Pharmacology 2006;54:231‐63. [DOI] [PubMed] [Google Scholar]
Siriwardena 2008
- Siriwardena AN, Qureshi MZ, Dyas JV, Middleton H, Orner R. Magic bullets for insomnia? Patients' use and experiences of newer (Z drugs) versus older (benzodiazepine) hypnotics for sleep problems in primary care. British Journal of General Practice 2008;58(551):417‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snedecor 2009
- Snedecor SJ, Botteman MF, Bojke C, Schaefer K, Barry N, Pickard AS. Cost‐effectiveness of eszopiclone for the treatment of adults with primary chronic insomnia. Sleep 2009;32(6):817‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sofi 2014
- Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini G. Insomnia and risk of cardiovascular disease: a meta‐analysis. European Journal of Preventive Cardiology 2014;21(1):57‐64. [DOI] [PubMed] [Google Scholar]
Soyka 2000
- Soyka M, Bottlender R, Möller HJ. Epidemiological evidence for a low abuse potential of zolpidem. Pharmacopsychiatry 2000;33(4):138‐41. [DOI] [PubMed] [Google Scholar]
Spielman 1991
- Spielman AJ, Glovinsky PB. The varied nature of insomnia. In: Hauri P editor(s). Case Studies in Insomnia. New York: Plenum Press, 1991:1‐15. [Google Scholar]
Strebbing 2005
- Stebbing J, Waters L, Davies L, Mandalia S, Nelson M, Gazzard B, et al. Incidence of cancer in individuals receiving chronic zopiclone or eszopiclone requires prospective study. Journal of Clinical Oncology 2005;23(31):8134‐6. [DOI] [PubMed] [Google Scholar]
Toner 2000
- Toner LC, Tsambiras BM, Catalano G. Central nervous system side effects associated with zolpidem treatment. Clinical Neuropharmacology 2000;23:54‐8. [DOI] [PubMed] [Google Scholar]
Tsai 2003
- Tsai MJ, Huang YB, Wu PC. A novel clinical pattern of visual hallucination after zolpidem use. Journal of Toxicology: Clinical Toxicology 2003;41:869–72. [DOI] [PubMed] [Google Scholar]
Tyrer 1990
- Tyrer P, Murphy S, Riley P. The Benzodiazepine Withdrawal Symptom Questionnaire. Journal of Affective Disorders 1990;19:53‐61. [DOI] [PubMed] [Google Scholar]
Valieres 2005
- Vallières A, Ivers H, Bastien CH, Beaulieu‐Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. Journal of Sleep Research 2005;14(4):447‐53. [DOI] [PubMed] [Google Scholar]
van Straten 2018
- Straten A, Zweerde T, Kleiboer A, Cuijpers P, Morin CM, Lancee J. Cognitive and behavioral therapies in the treatment of insomnia: A meta‐analysis. Sleep Med Rev 2018;38:3‐16. [DOI] [PubMed] [Google Scholar]
Victorri‐Vigneau 2014
- Victorri‐Vigneau C, Gérardin M, Rousselet M, Guerlais M, Grall‐Bronnec M, Jolliet P. An update on zolpidem abuse and dependence. Journal of Addictive Diseases 2014;33(1):15‐22. [DOI] [PubMed] [Google Scholar]
Vorderholzer 2001
- Voderholzer U, Riemann D, Hornyak M, Backhaus J, Feige B, Berger M, et al. A double‐blind, randomised and placebo‐controlled study on the polysomnographic withdrawal effects of zopiclone, zolpidem and triazolam in healthy subjects. European Archives of Psychiatry and Clinical Neuroscience 2001;251(3):117‐23. [DOI] [PubMed] [Google Scholar]
Wang 2001
- Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Zolpidem use and hip fractures in older people. Journal of the American Geriatrics Society 2001;49.(12):1685‐90. [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Wechsler 1955
- Wechsler D. Manual for the Wechsler scale. New York, NY: Psychological Corporation, 1955. [Google Scholar]
Wilson 2010
- Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence‐based treatment of insomnia, parasomnias and circadian rhythm disorder. Journal of Psychopharmacology 2010;24(11):1577‐601. [DOI] [PubMed] [Google Scholar]
Winkelman 2015
- Winkelman JW. Insomnia [Insomnia Disorder]. N Engl J Med 2015;373(15):1437‐44. [DOI] [PubMed] [Google Scholar]
Woolcott 2009
- Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, et al. Meta‐analysis of the impact of 9 medication classes on falls in elderly persons. Archives of Internal Medicine 2009;169(21):1952‐60. [DOI] [PubMed] [Google Scholar]
World Health Organization 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders. Geneva: The World Health Organization, 1992. [Google Scholar]
Xiao 2014
- Xiao Q, Keadle SK, Hollenbeck AR, Matthews CE. Sleep duration and total and cause‐specific mortality in a large US cohort: interrelationships with physical activity, sedentary behavior, and body mass index. American Journal of Epidemiology 2014;180(10):997‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zammit 1999
- Zammit GK, Weiner J, Damato N, Sillup GP, McMillan CA. Quality of life in people with insomnia. Sleep 1999;22(2):379‐85. [PubMed] [Google Scholar]
Zammit 2009
- Zammit G. Comparative tolerability of newer agents for insomnia. Drug Safety 2009;32(9):735‐48. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Rösner 2013d
- Rösner S, Soyka M, Hajak G, Wehrle R, Englbrecht C. Eszopiclone for insomnia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010703] [DOI] [PMC free article] [PubMed] [Google Scholar]


