Abstract
Background
Fetal fibronectin (FFN) is an extracellular matrix glycoprotein localized at the maternal‐fetal interface of the amniotic membranes, between chorion and decidua, where it is concentrated in this area between decidua and trophoblast. In normal conditions, FFN is found at very low levels in cervico‐vaginal secretions. Levels greater than or equal to 50 ng/mL at or after 22 weeks have been associated with an increased risk of spontaneous preterm birth. In fact, FFN is one of the best predictors of preterm birth in all populations studied so far, and can help selecting which women are at significant risk for preterm birth.
Objectives
To assess the effectiveness of management based on knowledge of FFN testing results for preventing preterm birth.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (January 2008), MEDLINE (1966 to December 2007) and all references in identified articles.
Selection criteria
Randomized controlled trials of pregnant women between the gestational ages of 22 and 34 weeks screened with FFN for risk of preterm birth. Studies included are based exclusively on knowledge of FFN results versus no such knowledge, and we have excluded studies including women with only positive or only negative FFN results.
Data collection and analysis
All four authors assessed studies for inclusion and quality and extracted data.
Main results
We identified 13 trials, of which five were eligible for inclusion. The five included studies randomized 474 women, of which 235 were randomized to knowledge and 249 to no knowledge of FFN.
Preterm birth less than 37 weeks was significantly decreased with management based on knowledge of FFN results (15.6%) versus controls without such knowledge (28.6%; risk ratio 0.54; 95% confidence interval 0.34 to 0.87). All other outcomes for which there were available data (preterm birth at less than 34, 32, or 28 weeks; gestational age at delivery; birthweight less than 2500 grams; perinatal death; maternal hospitalization; tocolysis; steroids for fetal lung maturity; and time to evaluate) were similar in the two groups. No other maternal or neonatal outcome was available for meaningful analysis.
Authors' conclusions
Although FFN is commonly used in labor and delivery units to help in the management of women with symptoms of preterm labor, currently there is not sufficient evidence to recommend its use. Since this review found an association between knowledge of FFN results and a lower incidence of preterm birth before 37 weeks, further research should be encouraged.
Keywords: Female, Humans, Pregnancy, Biomarkers, Biomarkers/analysis, Fetus, Fibronectins, Fibronectins/analysis, Premature Birth, Premature Birth/prevention & control, Randomized Controlled Trials as Topic
Fetal fibronectin testing for reducing the risk of preterm birth
Preterm birth before 37 weeks is the main cause of death and sickness for newborn infants. While most women have preterm labor symptoms such as contractions before having a preterm birth, most such women with symptoms deliver at term (greater than or equal to 37 weeks). Fetal fibronectin is a test that can identify the women with symptoms of preterm labor most at risk for preterm birth by measuring the level in secretions from the vagina and/or cervix. This review of five controlled studies that randomised 474 pregnant women did not find enough evidence to support or refute the use of the fetal fibronectin test for the management of women with symptoms of preterm labor. Further research should be encouraged.
Background
Importance of preterm birth
Preterm birth is defined by the World Health Organization as birth between 20 and 36 6/7 weeks. Its incidence is about 5% to 8% in most developed and developing countries. This incidence is increasing in many countries, including developing countries. Preterm birth is the main cause of neonatal morbidity and mortalities in most countries, especially in developed countries. In the USA, 75% of perinatal mortality occurs in preterm babies; more than two‐thirds of perinatal mortality (60% of total) occurs in infants born at less than 32 week. Mortality and morbidities are inversely associated with gestational age at birth. Morbidities include respiratory distress syndrome (RDS), bronchopulmonary dysplasia, intraventricular haemorrhage (IVH), necrotizing enterocolitis, sepsis, retinopathy, etc. All members of a family in which a preterm birth occurs suffer greatly, in several aspects, including medically, socially, psychologically, and financially.
Interventions to reduce preterm birth
Despite extensive research efforts, the incidence of preterm birth is increasing in many countries. It has increased to 12.9% in 2006 in the USA (a greater than 20% increase in the last 10 years), representing more than 500,000 births in this nation alone. Preterm birth can be spontaneous, and follow preterm labor (50%), or preterm premature rupture of membranes (30%); or be iatrogenic (20%). The interventions that have been shown to effectively reduce the risk of preterm birth or improve outcomes for babies born preterm in asymptomatic women have been smoking cessation counselling for smokers (Lumley 2004); antibiotics for asymptomatic bacteriuria (Smaill 2001); intramuscular progesterone for women with prior preterm birth now carrying a singleton gestation (Dodd 2006); ultrasound‐indicated cerclage in women with both a prior preterm birth and shortening of cervical length less than 25 mm before 24 weeks in the current singleton pregnancy (Berghella 2005); and history‐indicated cerclage in women with three or more prior preterm births or second trimester losses (Drakeley 2003). In symptomatic women, corticosteroids (betamethasone 12 mg intramuscularly for 2 doses 24 hours apart between 24 and 33 6/7 weeks is preferred if available) given to mother prior to preterm birth are effective in preventing RDS, IVH, and neonatal mortality (Roberts 2006). Cyclo‐oxygenase inhibitors are the only class of primary tocolysis shown to decrease preterm birth before 37 weeks in women with preterm labor compared to placebo, but this effectiveness is based on less than 100 randomized women (King 2005).
Fetal fibronectin to predict and reduce preterm birth
Fetal fibronectin is an extracellular matrix glycoprotein. Fetal fibronectin in biologic fluids is produced by amniocytes and by cytotrophoblast. It is present throughout gestation in all pregnancies. It is not subject to genetic polymorphism. There are very high levels in amniotic fluid (100 µg/mL) in the second trimester, and 30 µg/mL at term. It is localized at the maternal‐fetal interface of the amniotic membranes, between chorion and decidua, where it is concentrated in this area between decidua and trophoblast. Here it acts as a 'glue' between the pregnancy and the uterus. Concentration of fetal fibronectin protein found in blood is 1/5 that found in amniotic fluid; it is not present in urine. In normal conditions, this glycoprotein remains in this area between chorion and decidua, and very low levels are found in cervico‐vaginal secretions after 22 weeks (less than 50 ng/mL). Levels above this value (greater than or equal to 50 ng/mL) at or after 22 weeks in the cervico‐vaginal secretions collected by a swab have been associated with an increased risk of spontaneous preterm birth. The fetal fibronectin test assesses risk of preterm labor and preterm birth by measuring amount of fetal fibronectin in cervicovaginal secretions. In fact, fetal fibronectin is one of the best predictors of preterm birth in all populations studied so far, including low‐ and high‐risk women without preterm labor, twins, and women in preterm labor (Leitich 1999). The overall sensitivity and specificity are 56% and 84% for preterm before 37 weeks, respectively, but vary according to gestational age at collection, population studied, prevalence of preterm birth, single versus multiple screening, etc (Leitich 1999). Its positive predictive value varies from about 9% to 46% depending on the incidence of preterm labor in the population studies (Leitich 1999). Even at 13 to 22 weeks, higher (using 90th percentile) fetal fibronectin levels are associated with a two‐ to three‐fold increase risk in subsequent spontaneous preterm labor.
Objectives
To assess the outcome of management based on knowledge of fetal fibronectin testing results for preventing preterm birth.
Methods
Criteria for considering studies for this review
Types of studies
Published and unpublished randomized and quasi‐randomized controlled trials.
Types of participants
Pregnant women between the gestational ages of 22 and 34 weeks screened with fetal fibronectin for risk of preterm birth.
Types of interventions
A screening test such as fetal fibronectin can only be considered effective if interventions based on fetal fibronectin screening results reduce the outcome of preterm birth. Interventions based on fetal fibronectin screening results can also be classified as:
interventions based on knowledge of fetal fibronectin results (e.g. fetal fibronectin is collected on all women, but women are randomized so that in 50% of them the result is available to them and the managing obstetrician, while in 50% the fetal fibronectin is blind to them and the managing obstetrician; or fetal fibronectin screening is done only on half of the women);
interventions based on positive fetal fibronectin;
interventions based on negative fetal fibronectin.
This review focuses exclusively on (1), i.e. interventions based on knowledge of fetal fibronectin results.
Types of outcome measures
Primary outcomes
(1) Preterm birth (less than 37 weeks)
Secondary outcomes
(2) Preterm birth less than 34 weeks (3) Preterm birth less than 32 weeks (4) Preterm birth less than 28 weeks (5) Gestational age at delivery (6) Birthweight less than 2500 grams (7) Perinatal death (fetal death and neonatal death) (8) Maternal hospitalisation (9) Tocolysis (10) Steroids for fetal lung maturity (11) Time to evaluate (time from arrival to hospital for evaluation of preterm labor to decision regarding admission, discharge, or extended monitoring) (12) Respiratory distress syndrome (13) Intraventricular haemorrhage (14) Necrotizing enterocolitis (15) Sepsis (16) Neonatal intensive care unit (NICU) admission (17) NICU days (18) Maternal well‐being (e.g. stress level, etc) (19) Economic analysis (cost effectiveness, cost utility)
We will report outcomes 1,2,3,4,5,8,9,10,11,18, and 19 with 'mothers' are the denominators. We will report outcomes 6,7,12,13,14,15,16, and 17 with 'fetuses/neonates' as denominator.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (January 2008).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched MEDLINE (January 1966 to December 2007) using the search strategy detailed in Appendix 1.
We reviewed the reference list of all articles, in particular trials and review articles. We contacted all researchers of included trials to provide actual databases and any pertinent further information. We contacted experts in the field for additional and ongoing trials.
We did not apply any language restrictions.
Data collection and analysis
Selection of studies
We assessed for inclusion all potential studies we identified as a result of the search strategy. Independently, all authors assessed studies for inclusion in the review using the inclusion criteria. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. All authors independently extracted the data using the agreed form. We resolved any disagreement through discussion. We used the Review Manager software (RevMan 2008) to double enter all the data or a subsample.
When information regarding any of the above is unclear, or to obtain additional data not published, we contacted authors of the original reports to provide further details.
Assessment of methodological quality of included studies
We assessed the validity of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005). Methods used for generation of the randomization sequence were described for each trial.
(1) Selection bias (allocation concealment)
We assigned a quality score for each trial, using the following criteria: (A) adequate concealment of allocation: such as telephone randomization, consecutively‐numbered, sealed opaque envelopes; (B) unclear whether adequate concealment of allocation: such as list or table used, sealed envelopes, or study does not report any concealment approach; (C) inadequate concealment of allocation: such as open list of random‐number tables, use of case record numbers, dates of birth or days of the week.
(2) Attrition bias (loss of participants, for example, withdrawals, dropouts, protocol deviations)
We assessed completeness to follow up using the following criteria: (A) less than 5% loss of participants; (B) 5% to 9.9% loss of participants; (C) 10% to 19.9% loss of participants; (D) more than 20% loss of participants.
(3) Performance bias (blinding of participants, researchers and outcome assessment)
We assessed blinding using the following criteria: (1) blinding of participants (yes/no/unclear); (2) blinding of caregiver (yes/no/unclear); (3) blinding of outcome assessment (yes/no/unclear).
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. If heterogeneity was found, we explored this by sensitivity analysis followed by random‐effects if required.
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We used the standardized mean difference to combine trials that measure the same outcome, but used different methods. If there was evidence of skew ness, we reported this.
Unit of analysis issues
Cluster‐randomized trials
We identified no cluster‐randomized trials.
Dealing with missing data
We analysed data on all participants with available data in the group to which they are allocated, regardless of whether or not they received the allocated intervention. If in the original reports participants were not analysed in the group to which they were randomized, and there was sufficient information in the trial report or in the databases submitted, we attempted to restore them to the correct group.
Assessment of heterogeneity
We applied tests of heterogeneity between trials, if appropriate, using the I2 statistic. If we identified high levels of heterogeneity among the trials (exceeding 50%), we explored it by prespecified subgroup analysis and performed sensitivity analysis. A random‐effects meta‐analysis was used as an overall summary if this was considered appropriate.
Subgroup analyses
We planned to conduct subgroup analyses classifying whole trials by interaction tests as described by Deeks 2001. We planned to carry out the following group analyses:
asymptomatic compared with signs and symptoms of preterm labor;
low‐risk singleton gestations compared with high‐risk (e.g. prior preterm labor) singleton gestations;
singleton pregnancies compared with multiple gestations;
timing of availability of results;
gestational age at collection of fetal fibronectin (22 to 23, 24 to 28, more than 28 weeks).
Sensitivity analyses
We carried out sensitivity analysis to explore the effect of trial quality. This involved analysis based on an A, B, C, or D rating of selection bias and attrition bias. We excluded studies of poor quality in the analysis (those rating B, C, or D) in order to assess for any substantive difference to the overall result.
We carried out sensitivity analysis to explore the effect of trial quality assessed by concealment of allocation, by excluding studies with clearly inadequate allocation of concealment (rated C).
If we included quasi‐randomized trials in the review, we planned to perform a sensitivity analysis by trial quality.
Results
Description of studies
Our search identified 13 trials, of which five were eligible for inclusion. We identified no quasi‐randomized trials. We excluded seven of the eight excluded studies because they only included women with positive or only with negative fetal fibronectin (FFN) results. The other excluded study (Kalchbrenner 1999) did not report data regarding the results of the FFN test, with no management reported based on FFN results.
The five included studies randomized 474 women, of which 235 were randomized to knowledge and 249 to no knowledge of FFN. Of the included studies, one study (Ness 2007) used knowledge of both FFN and transvaginal ultrasound cervical length for management interventions. We requested patient‐level databases from all authors, and obtained them from two trials (Ness 2007; Plaut 2003), and additional information (but not the database) from one other trial (Grobman 2004), despite multiple attempts with the other authors. Our analysis was based on singleton gestations, and this was possible for all included studies, except for Nguyen 2002 (unclear if twins included) and Lowe 2004 (eight twins included). We avoided repeated entries of the same patients, which was possible since we had patient‐level data for the one trial where this occurred (Plaut 2003).
Risk of bias in included studies
Risk of selection bias was not present in four of the five included studies. The fifth study (Nguyen 2002) did not describe allocation concealment (only the abstract of this study has been published).
All randomized patients were included in an intention‐to‐treat analysis in two trials (Grobman 2004; Ness 2007). This could not be assessed in one trial (Nguyen 2002), which was reported only as an abstract. Approximately 12% of randomized patients were excluded from analysis after randomization in the other two included trials (Lowe 2004; Plaut 2003). Attrition bias in terms of loss of data on outcomes analysed was 21% in Plaut 2003, and even higher for some outcomes in Ness 2007.
Performance bias was present in all trials, as participants and researchers were aware of the arm to which they were randomized, but this was inevitable.
Effects of interventions
Preterm birth before 37 weeks was significantly decreased with management based on knowledge of FFN results (15.6%) versus controls without such knowledge (28.6%; risk ratio (RR) 0.54; 95% confidence interval 0.34 to 0.87). All trials had RRs less than one, with no significant heterogeneity. All other outcomes for which there were available data (preterm birth less than 34, 32, or 28 weeks; gestational age at delivery (weeks); birthweight less than 2500 grams; perinatal death; maternal hospitalisation; tocolysis; steroids for fetal lung maturity; and time to evaluate) were similar in the two groups. No other maternal or neonatal outcome was available for meaningful analysis.
Subgroup analyses were not feasible, since all women included had signs and symptoms of preterm labor; low‐risk singleton gestations were not reported separately from high‐risk (e.g. prior preterm labor) singleton gestations; there were insufficient data for multiple gestations, timing of availability of results, and gestational age at collection of FFN to make meaningful comparisons.
Discussion
Knowledge of fetal fibronectin results in the management of women with symptoms of preterm labor is associated with a lower incidence of preterm birth before 37 weeks. As all our available outcomes were not affected, and no perinatal outcome other than perinatal death was reported, further research is necessary before fetal fibronectin testing can be recommended in this clinical scenario. Furthermore, it is still unclear which interventions are most beneficial once fetal fibronectin results are known. In fact, only one study (Ness 2007) reported a detailed protocol of management based on fetal fibronectin (and cervical length) results, and could be replicated in further research trials or clinical management. Our review did not include, by design, assessment of effectiveness of interventions based on positive fetal fibronectin testing, or negative fetal fibronectin testing. We identified no trials on women without signs or symptoms of labor.
Authors' conclusions
Although fetal fibronectin is marketed and commonly used in labor and delivery units to help in the management of women with symptoms of preterm labor, currently there is not sufficient evidence to recommend its use.
Since this review found an association between knowledge of fetal fibronectin results and a lower incidence of preterm birth before 37 weeks, further research should be encouraged, minimizing attrition bias. Future studies should include specific populations (e.g. singleton gestations with symptoms of preterm labor), a study group managed with a protocol based on the fetal fibronectin results, and report not only maternal but also significant perinatal outcomes. Cost‐effect analyses are also needed.
Acknowledgements
Sonja Henderson and Jolene Seibel‐Seamon, MD, for advice and support through the protocol and review process.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Other searches
#1 exp Obstetric labor, premature/ #2 Fibronectins/ #3 #1 and #2 #4 fetal adj3 fibronectin #5 #3 or #4
Data and analyses
Comparison 1.
Comparison 01 FFN knowledge versus no knowledge, Outcome 01 Preterm birth < 37 week
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 01 Preterm birth < 37 weeks | 3 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.34, 0.87] |
Analysis 1.1.
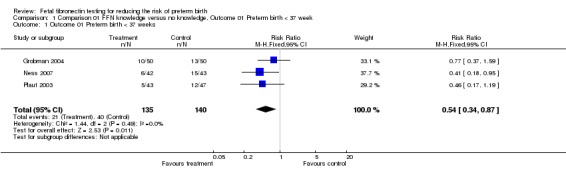
Comparison 1 Comparison 01 FFN knowledge versus no knowledge, Outcome 01 Preterm birth < 37 week, Outcome 1 Outcome 01 Preterm birth < 37 weeks.
Comparison 2.
Comparison 01 FFN knowledge versus no knowledge, Outcome 02 Preterm birth < 34 week
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 02 Preterm birth < 34 week | 3 | 281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.41, 2.47] |
Analysis 2.1.
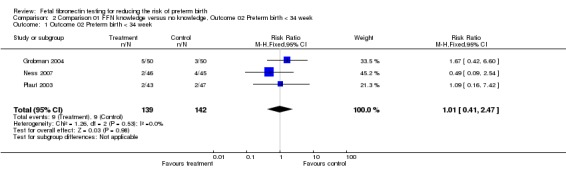
Comparison 2 Comparison 01 FFN knowledge versus no knowledge, Outcome 02 Preterm birth < 34 week, Outcome 1 Outcome 02 Preterm birth < 34 week.
Comparison 3.
Comparison 01 FFN knowledge versus no knowledge, Outcome 03 Preterm birth < 32 week
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 03 Preterm birth < 32 week | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.28, 2.58] |
Analysis 3.1.
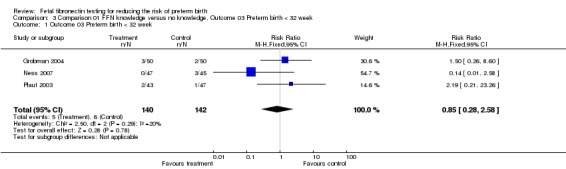
Comparison 3 Comparison 01 FFN knowledge versus no knowledge, Outcome 03 Preterm birth < 32 week, Outcome 1 Outcome 03 Preterm birth < 32 week.
Comparison 4.
Comparison 01 FFN knowledge versus no knowledge, Outcome 04 Preterm birth < 28 week
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 04 Preterm birth < 28 week | 3 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.82] |
Analysis 4.1.
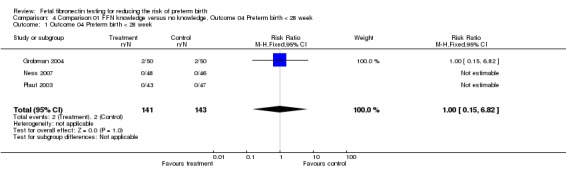
Comparison 4 Comparison 01 FFN knowledge versus no knowledge, Outcome 04 Preterm birth < 28 week, Outcome 1 Outcome 04 Preterm birth < 28 week.
Comparison 5.
Comparison 01 FFN knowledge versus no knowledge, Outcome 05 Gestational age at delivery (weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 05 Gestational age at delivery | 3 | 288 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.35, 0.93] |
Analysis 5.1.
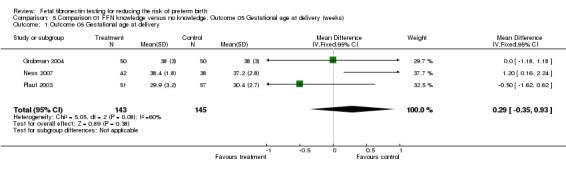
Comparison 5 Comparison 01 FFN knowledge versus no knowledge, Outcome 05 Gestational age at delivery (weeks), Outcome 1 Outcome 05 Gestational age at delivery.
Comparison 6.
Comparison 01 FFN knowledge versus no knowledge, Outcome 06 Birthweight 2500 grams
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 06 Birthweight < 2500 g | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.21, 2.44] |
Analysis 6.1.
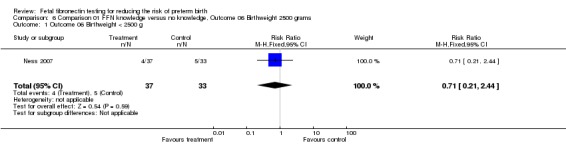
Comparison 6 Comparison 01 FFN knowledge versus no knowledge, Outcome 06 Birthweight 2500 grams, Outcome 1 Outcome 06 Birthweight < 2500 g.
Comparison 7.
Comparison 01 FFN knowledge versus no knowledge, Outcome 07 Perinatal death
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 07 Perinatal death | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 7.1.
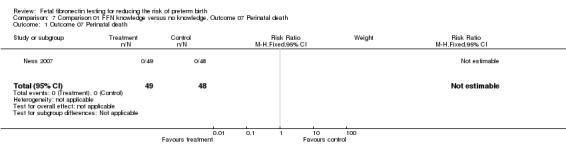
Comparison 7 Comparison 01 FFN knowledge versus no knowledge, Outcome 07 Perinatal death, Outcome 1 Outcome 07 Perinatal death.
Comparison 8.
Comparison 01 FFN knowledge versus no knowledge, Outcome 08 Maternal hospitalization
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 08 Maternal hospitalization | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.90, 2.07] |
Analysis 8.1.
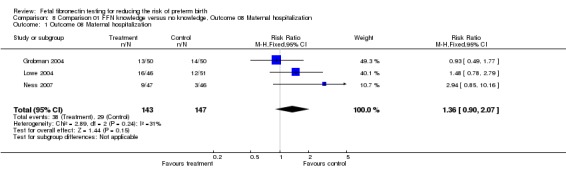
Comparison 8 Comparison 01 FFN knowledge versus no knowledge, Outcome 08 Maternal hospitalization, Outcome 1 Outcome 08 Maternal hospitalization.
Comparison 9.
Comparison 01 FFN knowledge versus no knowledge, Outcome 09 Tocolysis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 09 Tocolysis | 4 | 350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.30] |
Analysis 9.1.
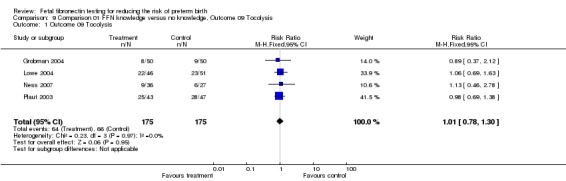
Comparison 9 Comparison 01 FFN knowledge versus no knowledge, Outcome 09 Tocolysis, Outcome 1 Outcome 09 Tocolysis.
Comparison 10.
Comparison 01 FFN knowledge versus no knowledge, Outcome 10 Steroids for fetal lung maturity
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 10 Steroids for Fetal Lung Maturity | 3 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.85, 1.79] |
Analysis 10.1.
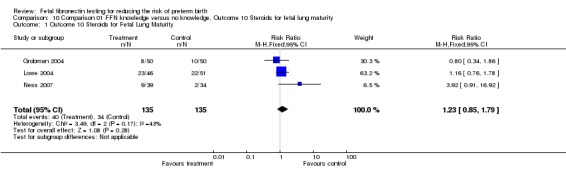
Comparison 10 Comparison 01 FFN knowledge versus no knowledge, Outcome 10 Steroids for fetal lung maturity, Outcome 1 Outcome 10 Steroids for Fetal Lung Maturity.
Comparison 11.
Comparison 01 FFN knowledge versus no knowledge, Outcome 11 Time to evaluate (hours)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Outcome 11 Time to evaluate | 3 | 234 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.39, 0.47] |
Analysis 11.1.
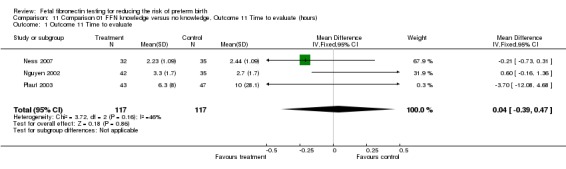
Comparison 11 Comparison 01 FFN knowledge versus no knowledge, Outcome 11 Time to evaluate (hours), Outcome 1 Outcome 11 Time to evaluate.
What's new
| Date | Event | Description |
|---|---|---|
| 16 December 2007 | Amended | Converted to new review format. |
Differences between protocol and review
None.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial. | |
| Participants | 24‐34 week singletons with > 6 contractions/hour. Twins excluded. Number of participants: 100 (50/50: knowledge/no knowledge). |
|
| Interventions | FFN knowledge or not. Time FFN results available: unknown. Protocol for FFN knowledge group: no. |
|
| Outcomes | Primary: total costs. | |
| Notes | Intention to treat; only singletons; no protocol. Positive FFN test in each group: 5 (10%) in knowledge, 3 (6%) in no knowledge group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Computer‐generated, opaque envelopes. |
| Methods | Randomized controlled trial. | |
| Participants | 23‐34 week singletons/twins with contractions +/‐ cervical change. Number of participants: 97 [89 singletons/8 twins] (46/51: knowledge/no knowledge). |
|
| Interventions | FFN knowledge or not. Time FFN results available: < 1 hour. Protocol for FFN knowledge group: no. |
|
| Outcomes | Primary: length of stay. | |
| Notes | 13/110 excluded post‐randomization: not intention to treat; included twins; no protocol. Positive FFN test in each group: 11 (24%) in knowledge group; FFN test not performed in no knowledge group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Computer‐generated, opaque envelopes, blocks of 10. |
| Methods | Randomized controlled trial. | |
| Participants | 24‐33 6/7 week singletons/twins with >= 6 contractions/hour or symptoms of PTL. Number of participants: 100 [97 singletons, 3 twins] (49/51: knowledge/no knowledge). |
|
| Interventions | FFN (and cervical length) knowledge versus not. Time FFN results available: < 1 hour. Protocol for FFN knowledge group: yes. |
|
| Outcomes | Primary: time from initial evaluation to discharge from triage area. | |
| Notes | Intention to treat; included twins; protocol. Positive FFN test in each group: 10 (20%) in knowledge, 10 (22%) in no knowledge group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Computer‐generated, opaque envelopes. |
| Methods | Randomized controlled trial. | |
| Participants | 24‐35 week (unclear if twins included) with symptoms of PTL. Number of participants: 77 (42/35: knowledge/no knowledge). |
|
| Interventions | FFN testing done (and knowledge available) versus not done (so knowledge not available). Time FFN results available: unknown. Protocol for FFN knowledge group: yes. |
|
| Outcomes | Primary: cost‐effectiveness. | |
| Notes | Abstract only; unclear if intention to treat, twins included, protocol, etc. Positive FFN test in each group: not available in knowledge group; FFN not performed in no knowledge group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | No description available (only abstract published). |
| Methods | Randomized controlled trial. | |
| Participants | 24‐34 6/7 week singletons/twins with 'symptoms of PTL'. Number of participants: 114 (unclear from text) randomized [96 singleton, 12 twins] (analysed: 51/57: knowledge/no knowledge). |
|
| Interventions | FFN knowledge or not. Time FFN results available: 1‐2 hours. Protocol for FFN knowledge group: no. |
|
| Outcomes | Primary: transport rates (not reported). | |
| Notes | 6/114 (?) excluded post‐randomization: not intention to treat; 8 patients entered in study twice!; stopped trial prematurely; sponsored by Adeza; included twins; no protocol. Positive FFN test in each group: 6 (12%) in knowledge, 6 (10.5%) in no knowledge group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Computerized opaque envelopes. |
FFN: fetal fibronectin PTL: preterm labor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2001 | Only women with positive FFN were included. |
| Bisits 2004 | Only women with positive FFN were included. They were randomized to tocolysis or not. |
| Elliott 2005 | Only women with negative FFN were included. |
| Goldenberg 2001 | Only women with positive FFN were included (secondary analysis of NICHD BV/TV study). |
| Hauth 2001 | Only women with positive FFN were included. |
| Kalchbrenner 1999 | This was a trial comparing speculum versus digital collection of FFN. There were no data regarding the results of the FFN test, and no intervention was reported based on FFN results. |
| Lachelin 2004 | Only women with positive FFN were included. This is an ongoing trial not yet published. |
| Shennan 2006 | Only women with positive FFN were included. |
FFN: fetal fibronectin
Contributions of authors
Jason K. Baxter, Edward Hayes, and John Visintine: 1. helped with the initial idea of a fetal fibronectin review; 2. met several times with the main author (Vincenzo Berghella (VB)) regarding all aspects of the development of the protocol and review; 3. contributed to the writing and the editing of the protocol and the review; 4. helped to revise and respond to the feedback received on the first draft of the protocol and the review. VB prepared the first draft and finalised the revised draft of the protocol and the review in response to feedback. VB is the guarantor of the review.
Declarations of interest
None known.
New
References
References to studies included in this review
- Grobman W, Welshman E, Calhoun E. Does fetal fibronectin use in the diagnosis of preterm labor affect physician behavior and health care costs [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S80. [DOI] [PubMed] [Google Scholar]; Grobman WA, Welshman EE, Calhoun EA. Does fetal fibronectin use in the diagnosis of preterm labor affect physician behavior and health care costs? A randomized trial. American Journal of Obstetrics and Gynecology 2004;191:235‐40. [DOI] [PubMed] [Google Scholar]
- Hansen W, Lowe MP, Zimmerman B. Effect of the fetal fibronectin assay on preterm labor management [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S136. [DOI] [PubMed] [Google Scholar]; Lowe MP, Zimmerman B, Hansen W. Prospective randomized trial of fetal fibronectin on preterm labor management in a tertiary care center. American Journal of Obstetrics and Gynecology 2004;190:358‐62. [DOI] [PubMed] [Google Scholar]
- Ness A, Visintine J, Ricci E, Berghella V. Does knowledge of cervical length and fetal fibronectin affect management of women with preterm labor? A randomized trial. American Journal of Obstetrics and Gynecology 2007;197(4):426.el‐426.e7. [DOI] [PubMed] [Google Scholar]; Ness A, Visintine J, Ricci E, Boyle K, Berghella V. Use of fetal fibronectin and transvaginal ultrasound cervical length to triage women with suspected preterm labor: a randomized trial. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S67. [DOI] [PubMed] [Google Scholar]
- Nguyen TCQ, Toy EC, Baker B. The cost‐effectiveness of fetal fibronectin testing in suspected preterm labor: a randomized trial. Obstetrics & Gynecology 2002;99(4 Suppl):97S. [Google Scholar]
- Plaut MM, Smith W, Kennedy K. Fetal fibronectin: the impact of a rapid test on the treatment of women with preterm labor symptoms. American Journal of Obstetrics and Gynecology 2003;188:1588‐95. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Andrews WW, Sibai BM, Thom EA, Dudley D, Ernest JM, McNellis D, et al. Randomized clinical trial of metronidazole plus erythromycin to prevent spontaneous preterm delivery in fetal fibronectin‐positive women. Obstetrics & Gynecology 2003;101(5 Pt 1):847‐55. [DOI] [PubMed] [Google Scholar]
- Bisits A, Madsen G, Knox M, Gill A, Smith R, Yeo G, et al. The randomized nitric oxide tocolysis trial (RNOTT) for the treatment of preterm labor. American Journal of Obstetrics and Gynecology 2004;19(3):683‐90. [DOI] [PubMed] [Google Scholar]
- Elliott JP, Miller HS, Coleman S, Rhea D, Abril D, Hallbauer K, et al. A randomized multicenter study to determine the efficacy of activity restriction for preterm labor management in patients testing negative for fetal fibronectin. Journal of Perinatology 2005;25(10):626‐30. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Klebanoff M, Carey JC, Macpherson C. Metronidazole treatment of women with a positive fetal fibronectin test result. American Journal of Obstetrics and Gynecology 2001;185(2):485‐6. [DOI] [PubMed] [Google Scholar]
- Hauth JC, Cliver S, Hodgkins P, Andrews WW, Schwebke JR, Hook EW, et al. Mid‐trimester metronidazole and azithromycin did not prevent preterm birth in women at increased risk: a double‐blind trial [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S86. [Google Scholar]
- Kalchbrenner M, Weisenborn B, Jacques D, Coleman S, Chyu J. A randomized comparison of specimen collection methods for the fetal fibronectin immunoassay [abstract]. Obstetrics & Gynecology 1999;93(4 Suppl):51S. [Google Scholar]
- Lachelin GCL. Randomised double blind placebo controlled trial of antimicrobial treatment in pregnant women at risk of preterm labour. http://controlled‐trials.com/mrct (accessed 15 September 2004).
- Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, Jones G, et al. A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET Study. BJOG: an international journal of obstetrics and gynaecology. 2006;113(1):65‐74. [DOI] [PubMed] [Google Scholar]
Additional references
- Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for a short cervix on ultrasonography: meta‐analysis of trials using individual patient‐level data. Obstetrics & Gynecology 2005;106:181‐9. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001. [Google Scholar]
- Dodd JM, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 1. [Art. No.: CD004947. DOI: 10.1002/14651858.CD004947.pub2] [DOI] [PubMed] [Google Scholar]
- Drakeley AJ, Roberts D, Alfirevic Z. Cervical stitch (cerclage) for preventing pregnancy loss in women. Cochrane Database of Systematic Reviews 2003, Issue 1. [Art. No.: CD003253. DOI: 10.1002/14651858.CD003253] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated March 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester, UK: John Wiley & Sons, Ltd2005. [DOI: 10.1002/14651858] [DOI]
- King J, Flenady V, Cole S, Thorton S. Cyclooxygenase (COX) inhibitors for treating preterm labour. Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD001992. DOI: 10.1002/14651858.CD001992.pub2] [DOI] [PubMed] [Google Scholar]
- Leitich H, Egarter C, Kaider A, Hohlagschwandtner M, Berghammer P, Husslein P. Cervico‐vaginal fetal fibronectin as a marker for preterm delivery: a meta‐analysis. American Journal of Obstetrics and Gynecology 1999;180(5):1169‐76. [DOI] [PubMed] [Google Scholar]
- Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk for preterm birth. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
- Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2001, Issue 2. [Art. No.: CD000490. DOI: 10.1002/14651858.CD000490] [DOI] [PubMed] [Google Scholar]


