Abstract
Background
Current guidelines for the treatment of acute ischemic stroke are mainly based on the time between symptom onset and initiation of treatment. This time is unknown in patients with wake-up stroke (WUS). We investigated clinical and multimodality CT imaging characteristics on admission in patients with WUS and in patients with a stroke with a known onset time.
Methods
All patients were selected from a large prospective cohort study (Dutch acute stroke study). WUS patients last seen well > 4.5 and ≤4.5 h were separately compared to patients with a known onset time ≤4.5 h. In addition, WUS patients with a proximal occlusion of the anterior circulation last seen well > 6 and ≤6 h were separately compared to patients with a known onset time ≤6 h and a proximal occlusion. National Institute of Health Stroke Score, age, gender, history of atrial fibrillation, non-contrast CT (NCCT) Alberta Stroke Program Early CT Score (ASPECTS), CT-perfusion abnormalities, proximal occlusions, and collateral filling on CT angiography were compared between groups using the Mann-Whitney U test and Fisher's exact test.
Results
WUS occurred in 149/1,393 (10.7%) patients. Admission clinical and imaging characteristics of WUS patients last seen well > 4.5 h (n = 81) were not different from WUS patients last seen well ≤4.5 h (n = 68). Although WUS patients last seen well > 4.5 h had a significantly lower NCCT ASPECTS than patients with a known time of stroke symptom onset of ≤4.5 h (n = 1,026), 85.2% had an NCCT ASPECTS > 7 and 75% had a combination of favorable ASPECTS > 7 and good collateral filling. There were no statistically significant differences between the admission clinical and imaging characteristics of WUS patients with proximal occlusions last seen well > 6 h (n = 23), last seen well ≤6 h (n = 40), and patients with a known time to stroke symptom onset ≤6 h (n = 399). Of all WUS patients with proximal occlusions last seen well > 6 h, only 4.3% had severe ischemia (ASPECTS < 5), 13 (56.5%) had ASPECTS > 7 and good collateral filling.
Conclusions
There are only minor differences between clinical and imaging characteristics of WUS patients and patients who arrive in the hospital within the time criteria for intravenous or endovascular treatment. Therefore, CT imaging may help to identify WUS patients who would benefit from treatment and rule out those patients with severe ischemia and poor collaterals.
Keywords: Acute stroke imaging, Acute ischemic stroke, Computed tomography, Wake-up stroke
Introduction
The current guideline to start treatment with intravenous tissue plasminogen activator (IV-rtPA) for acute ischemic stroke (AIS) patients is based on the time between symptom onset and initiation of treatment. Administration of IV-rtPA is recommended for patients with AIS presenting within 4.5 h after symptom onset [1]. In patients with a proximal occlusion of the intracranial carotid artery up to the M2 branch of the middle cerebral artery (MCA), endovascular treatment (EVT), such as a thrombectomy, has been shown beneficial up to 6 h after symptom onset. If there is moderate to good collateral filling and a favorable Alberta Stroke Program Early CT Score (ASPECTS), with a lower limit ranging from 5 to 7, EVT can be considered up to 12 h after symptom onset [2]. A recent trial has even shown that patients can be selected for EVT up to 24 h when using MR DWI or CT-perfusion (CTP) [3, 4].
Between 8 and 28% of patients with ischemic stroke notice the symptoms of their stroke when they wake (wake-up stroke [WUS]) [5, 6, 7, 8]. In these patients, the exact duration of the symptoms is not known and symptom onset is calculated from the moment when the patient was last seen well [5]. As a result, most WUS patients are not eligible for IV-rtPA or EVT [9]. However, if a patient would wake up because of the stroke, IV-rtPA or EVT might still be beneficial. Imaging may help to identify WUS patients who would still benefit from treatment and rule out patients with already severe ischemia.
The purpose of this study is to compare clinical and imaging characteristics on admission between WUS patients and patients who were either eligible for intravenous or endovascular stroke treatment.
Methods
Study Population
All patients were selected from a prospective multicenter observational cohort study in the Netherlands (the Dutch acute stroke study [DUST]) between May 2009 and August 2013 [10]. Patients, older than 18 years, with symptoms of AIS were included if they presented to the emergency department within 9 h after symptom onset and had a National Institute of Health Stroke Score (NIHSS) of at least 2, or 1 if an indication for IV-rtPA is present (e.g., isolated aphasia). Patients were excluded if they had an intracranial hemorrhage or another diagnosis that explained the symptoms on NCCT, known renal dysfunction, or contrast allergy. All patients underwent a non-contrast CT (NCCT), CTP and CT angiography (CTA) on admission [11]. The local medical ethics committees of the participating centers approved the study protocol. All patients or next of kin gave written informed consent, unless a patient died before consent could be obtained, in that case the need for consent was waived by the medical Ethics Committee.
For the current study, patients were excluded if it was unclear whether a patient awoke with stroke symptoms. Patients were also excluded if the NCCT or CTA could not be evaluated, since these are the modalities that are frequently used for EVT selection.
Patient Characteristics
Time since symptom onset was determined for all patients. For patients with WUS, the time since symptom onset was based on the time since the patient was last seen well.
In all patients, age, gender, previous history of atrial fibrillation, and NIHSS were recorded. After 3 months, the modified Rankin Scale was determined. A poor functional outcome was defined as a modified Rankin score of > 2 at 90 day follow-up.
Imaging Protocol
An NCCT and CTP of the brain, and CTA of the cervical and cerebral arteries, were performed at admission in all patients [10, 11]. Multidetector CT scanners, ranging from 40 to 320 detectors, were used for all imaging studies (Philips, Siemens, General Electric, Toshiba).
The NCCT was acquired with 120 kV, 300–375 mAs at a slice thickness of 0.625 mm and reconstructed at 5 mm.
The CTP was acquired with 80 kV and 150 mAs per rotation at a slice thickness of 0.625 mm and reconstructed at 5 mm. It involved successive gantry rotations in cine mode (every 2 s for 50 s and 6 additional rotations 30 s apart) during intravenous administration of 40 mL of nonionic contrast material followed by 40 mL of saline with a flow of 6 mL/s. The CTP coverage ranged from 40 mm to full brain coverage, including at least the ganglionic and supraganglionic regions to ensure inclusion of the ASPECTS levels [10, 11].
The CTA was acquired from the aortic arch to the vertex at using 50–70 mL of intravenous contrast followed by 40 mL of saline, with a flow of 6 mL/s.
Imaging Evaluation
On the NCCT, the ASPECTS score or pc-ASPECTS for posterior circulation strokes was determined [12, 13]. For the analyses, the pc-ASPECTS was dichotomized with a cutoff value of 7, which is known to be a discriminator of outcome for patients treated with intravenous thrombolysis [12], and with a cutoff value of 5, which has been used in one trial to select patients for EVT [14].
On the CTP (if available), the Cerebral Blood Volume and Mean Transit Time was automatically calculated using commercially available software for CTP (Extended Brilliance Workstation 4.5; Philips Healthcare) and in-house developed perfusion software applying a bSVD algorithm [15]. The Cerebral Blood Volume and Mean Transit Time parameter maps were visually classified using the ASPECTS/pc-ASPECTS score [16]. The penumbra and infarct core volume within the whole scan range were quantified with the in-house software using a Tmax > 6 s for the total ischemic area and a relative cerebral blood flow (CBF) threshold of 30% to differentiate penumbra from infarct [17, 18].
For all WUS patients and patients eligible for IV-rtPA, it was determined whether the penumbra size was > 20% of the ischemic area [19].
For all WUS patients and patients with a proximal occlusion of the anterior circulation eligible for EVT, it was additionally determined whether the mismatch between the total ischemic area and the infarct core was more than 1.2 [20] or more than 1.8 [3], and whether the infarct core volume was ≤50 mL [4]. Patients with no perfusion deficit were grouped in the favorable mismatch groups.
On the CTA, the intracranial thrombus location, the clot burden score (CBS), and collateral filling were determined. Proximal occlusions ranged from the intracranial internal carotid artery to the M2-branch of the MCA [2]. The CBS was assessed in the anterior circulation of the symptomatic hemisphere by subtracting occluded vessel segments from the maximum score of 10. For the analyses, the CBS was dichotomized at 6 [21].
Collateral filling was graded on 10 mm CTA MIP images: 0 = absent; 1 = < 50% filling; 2 = 50–< 100% filling; 3 = 100% filling [21]. These scores were dichotomized into 2 categories: poor collateral filling (0–1) and good collateral filling (2–3). Patients without an occlusion were considered to have good collateral filling.
All imaging data were analyzed by one of 3 observers (I.C.S., B.K.V., and J.W.D.), all with more than 5 years of experience in stroke imaging.
Statistical Analysis
WUS patients who were last seen well > 4.5 h were compared to WUS patients last seen well ≤4.5 h and patients with a known time of symptom onset ≤4.5 h, since this is the time limit for IV-rtPA.
Additionally, WUS patients with a proximal occlusion of the anterior circulation who were last seen well > 6 h were compared to WUS patients with a proximal occlusion last seen well ≤6 h and patients with a proximal occlusion and a known time of symptom onset ≤6 h, since this is a frequently used time limit for EVT.
Categorical variables were analyzed using Fisher's exact tests. The distribution of continuous variables was assessed with the Kolmogorov-Smirnov normality test. As all the continuous variables had a non-normal distribution, they were compared using the Mann-Whitney U test. Numbers and percentages for each variable were calculated. The median and interquartile range was noted for every continuous variable.
Results
Of the 1,393 patients in the DUST, 1,159 (83.2%) had a known time of stroke symptom onset, 149 (10.7%) had a WUS, and 85 patients (6.1%) had an unwitnessed daytime stroke. These 85 patients were excluded from further analyses. In addition, 20 patients with known time of stroke symptom onset were excluded because either the admission NCCT (n = 1) or CTA (n = 19) imaging data were insufficient. Of the remaining 1,139 patients with a known time of stroke symptom onset, 1,026 (90%) were imaged within 4.5 h and 399 patients (35%) who also had a proximal occlusion of the anterior circulation were imaged within 6 h after symptom onset. Of the 149 WUS patients, 81 were last seen well > 4.5 h. A proximal intracranial occlusion of the anterior circulation was seen in 63 (42%) WUS patients of whom 23 were last seen well >6 h.
Table 1 shows 2 comparisons: (1) the comparison between WUS patients imaged within 4.5 h after last seen well (n = 68) and WUS patients imaged > 4.5 h after last seen well (n = 81), and (2) the comparison between WUS patients imaged > 4.5 h after last seen well and patients imaged within 4.5 h after a known time of stroke symptom onset (n = 1,026).
Table 1.
Comparison between WUS and patients who had a CT-scan ≤4.5 h after known symptom onset
| ≤4.5 h WUS (n = 68) |
>4.5 h WUS (n = 81) | p value* | Known onset stroke with CT ≤4.5 h (n = 1,026) | p value** | |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 70 (61–78) | 65 (56–75) | 0.107 | 69 (58–78) | 0.046 |
| Gender, female,n (%) | 27 (39.7) | 37 (45.7) | 0.509 | 427 (41.6) | 0.485 |
| Stroke severity (NIHSS), median (IQR) | 7 (3–13) | 6 (3–11) | 0.610 | 6 (3–12) | 0.832 |
| AF in medical history,n (%) | 13 (19.1) | 13 (16.0) | 0.669 | 134 (13.1) | 0.495 |
| Minutes between last seen well and CT, median (IQR) | 135 (135–153) | 450 (315–450) | 0.000 | 99 (68–135) | 0.000 |
| IV-rtPA,n (%) | 33 (48.5) | 4 (4.9) | 0.000 | 750 (73.1) | 0.000 |
| EVT,n (%) | 3 (4.5) | 4 (4.9) | 1.000 | 67 (6.5) | 1.000 |
| Non-contrast CT findings | |||||
| NCCT ASPECTS, median (IQR) | 10 (10–10) | 10 (9–10) | 0.206 | 10 (10–10) | 0.001 |
| NCCT ASPECTS ≤7,n (%) | 9 (13.2) | 12 (14.8) | 0.818 | 86 (8.4) | 0.066 |
| NCCT ASPECTS ≤5,n (%) | 2 (2.9) | 5 (4.9) | 0.455 | 25 (2.4) | 0.062 |
| CT angiography findings | |||||
| Proximal occlusion,n (%) | 29 (42.6) | 34 (42.0) | 1.000 | 382 (37.2) | 0.406 |
| Poor collateral filling,n (%) | 8 (11.8) | 10 (12.3) | 1.000 | 135 (13.2) | 1.000 |
| CBS, median (IQR) | 10 (6–10) | 10 (8–10) | 0.982 | 10 (8–10) | 0.250 |
| Anterior circulation CBS >6,n (%) | 50 (73.5) | 64 (79.0) | 0.445 | 846 (82.5) | 0.450 |
| CT perfusion findings † | |||||
| CBV ASPECTS≤7, n (%) | 12 (19.4) | 22 (28.3) | 0.241 | 215 (22.3) | 0.261 |
| MTT ASPECTS≤7, n (%) | 24 (38.7) | 35 (45.5) | 0.491 | 383 (39.8) | 0.336 |
| Penumbra size within scan range, mL, median (IQR) | 18.8 (6.3–59.4) | 26.1 (10.7–51.2) | 0.612 | 23.4 (6.7–58.7) | 0.647 |
| Infarct core size within scan range, mL, median (IQR) | 26.2 (16.0–50.8) | 28.4 (16.7–65.9) | 0.623 | 25.9 (14.3–53.1) 0.363 | |
| Penumbra size >20% of ischemic area within scan range, n (%) | 56 (98) | 65 (98.5) | 1.000 | 805 (99.3) | 0.423 |
| Clinical outcome | |||||
| mRS >2 after 3 months,n (%) | 23 (33.8) | 32 (40.0) | 0.496 | 370 (36.6) | 0.549 |
p value, between WUS last seen well ≤4.5 and >4.5 h.
p value, between WUS last seen well >4.5 h and patients with a CT ≤4.5 h after known symptom onset.
Number of valid cases varies for the perfusion analyses.
WUS, wake-up stroke; NIHSS, National Institute of Health Stroke Scale of stroke severity; AF, atrial fibrillation; IV-rtPA, intravenous recombinant tissue plasminogen activator; EVT, endovascular treatment; NCCT, non-contrast CT; ASPECTS, Alberta Stroke Program Early CT Score; CBS, Clot Burden Score; CBV, cerebral blood volume; MTT, mean transit time; mRS, modified Rankin Score.
WUS Patients Imaged within 4.5 versus > 4.5 h after Last Seen Well
Except for the difference in IV rtPA use and the time since last seen well, there were no statistically significant differences in clinical and imaging characterization admission.
WUS Patients Imaged > 4.5 h after Last Seen Well versus Patients with Known Time of Stroke Onset < 4.5 h
The > 4.5 h WUS patients were younger (median age of 65 vs. 69, p = 0.046). The NCCT ASPECTS score was significantly lower (median ASPECTS of 10 vs. 10, p = 0.001). There was no significant difference in severe ischemic changes, with ASPECTS < 5 (4.9 vs. 2.4%, p = 0.062). Of all > 4.5 h WUS patients, 69 (85.2%) had NCCT ASPECTS > 7 and 61 (75%) had a combination of favorable ASPECTS > 7 and good collateral filling (> 50% MCA territory). Of the > 4.5 h WUS patients, nearly all (99%) had a penumbra size > 20% of the ischemic area within the scan range.
Table 2 shows 2 comparisons: (1) the comparison between WUS patients with a proximal occlusion of the anterior circulation imaged ≤6 h after last seen well (n = 40) and > 6 h (n = 23), and (2) the comparison between > 6 h WUS patients with a proximal occlusion of the anterior circulation and patients imaged within 6 h after a known time of stroke symptom onset with a proximal occlusion of the anterior circulation (n = 399).
Table 2.
Comparison between WUS with a proximal occlusion in the anterior circulation, and patients with a proximal occlusion of the anterior circulation and a CT ≤6 h after known symptom onset
| ≤6 h WUS with proximal occlusion (n = 40) | >6 h WUS with proximal occlusion (n = 23) | p value* | Known onset stroke with proximal occlusion and CT <6 h (n = 399) | p value ** | |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 65.4 (53–75) | 65 (53–72) | 0.648 | 69 (59–79) | 0.112 |
| Gender, female,n (%) | 1 (42.5) | 12 (52.2) | 0.600 | 176 (44.1) | 0.520 |
| Stroke severity (NIHSS), median (IQR) | 12 (9–18) | 12 (8–18) | 0.943 | 13 (7–17) | 0.996 |
| AF in medical history,n (%) | 10 (25) | 2 (8.7) | 0.183 | 61 (15.3) | 0.553 |
| Minutes between last seen well and CT, median (IQR) | 135 (135–297) | 450 (450–450) | 0.000 | 86 (61–135) | 0.000 |
| IV-rtPA,n (%) | 20 (50) | 1 (4.3) | 0.000 | 313 (78.4) | 0.000 |
| EVT,n (%) | 3 (7.5) | 2 (8.7) | 1.000 | 55 (13.8) | 1.000 |
| Non-contrast CT findings | |||||
| NCCT ASPECTS, median (IQR) | 9 (7–10) | 10 (7–10) | 0.807 | 10 (8–10) | 0.378 |
| NCCT ASPECTS ≤7,n (%) | 11 (27.5) | 7 (30.4) | 1.000 | 84 (21.2) | 0.302 |
| NCCT ASPECTS ≤5,n (%) | 5 (12.5) | 1 (4.3) | 0.402 | 24 (6.0) | 1.000 |
| CT angiography findings | |||||
| Poor collateral filling,n (%) | 12 (30) | 3 (13.0) | 0.218 | 116 (29.1) | 0.150 |
| CBS, median (IQR) | 6 (4–8) | 7 (5–8) | 0.612 | 7 (6–9) | 1.000 |
| Anterior circulation CBS >6,n (%) | 16 (40) | 12 (52.2) | 0.433 | 185 (53.6) | 0.517 |
| CT perfusion findings † | |||||
| CBV ASPECTS≤7, n (%) | 18 (48.6) | 14 (63.6) | 0.293 | 200 (53.9) | 0.510 |
| MTT ASPECTS≤7, n (%) | 30 (83.3) | 22 (100) | 0.073 | 336 (90.6) | 0.242 |
| Penumbra size within scan range, mL, median (IQR) | 56.8 (19.2–95.2) | 41.2 (26.3–71.8) | 0.595 | 55.2 (28.4–92.7) | 0.902 |
| Infarct core size within scan range, mL, median (IQR) | 44.4 (24.6–64.4) | 35.4 (25.4–74.5) | 0.891 | 42.5 (22.6–81.9) | 0.284 |
| Infarct core≤50 mL within scan range,n (%) | 20 (55.6) | 10 (55.6) | 1.000 | 178 (57.4) | 1.000 |
| Infarct core≤70 mL within scan range,n (%) | 28 (77.8) | 12 (66.7) | 0.512 | 216 (69.7) | 0.795 |
| Ischemic area/core mismatch >1.2 within scan range, n (%) | 24 (66.7) | 11 (61.1) | 0.766 | 237 (76.5) | 0.159 |
| Ischemic area/core mismatch >1.8 within scan range, n (%) | 13 (36.1) | 8 (44.4) | 0.569 | 158 (51.0) | 0.635 |
| Clinical outcome | |||||
| mRS after 3 months >2,n (%) | 22 (55.0) | 12 (52.2) | 1.000 | 207 (52.4) | 1.000 |
p value, between WUS with a proximal occlusion last seen well ≤6 and >6 h.
p value, between WUS with a proximal occlusion last seen well >6 h and patients with a proximal occlusion and a CT ≤6 h after known symptom onset.
Number of valid cases varies for the perfusion analyses.
WUS, wake-up stroke; NIHSS, National Institute of Health Stroke scale of stroke severity; AF, atrial fibrillation; IV-rtPA, intravenous recombinant tissue plasminogen activator; EVT, endovascular treatment; NCCT, non-contrast CT; ASPECTS, Alberta Stroke Program Early CT Score; CBS, Clot Burden Score; CBV, cerebral blood volume; MTT, mean transit time; mRS, modified Rankin Score.
There were no statistically significant differences in admission clinical and imaging characteristics of the 3 groups. Only 1 (4.3%) of the > 6 h WUS patients with a proximal occlusion had severe ischemia (ASPECTS < 5), and 13 (56.5%) had ASPECTS > 7 and good collateral filling. Sixty-one percent of the > 6 h WUS patients had a mismatch of more than 1.2 and 44% a mismatch of more than 1.8.
The differences in NCCT ASPECTS between WUS patients and patients with a known time of stroke symptom onset are illustrated in Figure 1.
Fig. 1.
Spread of pc-ASPECTS: (a) patients with symptom onset within 4.5 h before imaging and > 4.5 h WUS patients; (b) patients with symptom onset within 6 h before imaging and a proximal anterior circulation occlusion and > 6 h WUS patients with a proximal anterior circulation occlusion. ASPECTS, Alberta Stroke Program Early CT Score; WUS, wake-up stroke.
Figures 2 and 3 show examples of a WUS patient last seen well > 4.5 h with favorable imaging findings for treatment and a patient with known time to symptom onset ≤4.5 h with unfavorable imaging findings.
Fig. 2.
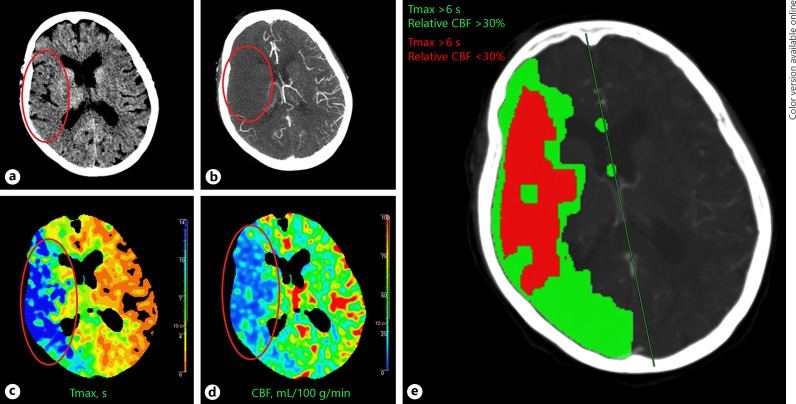
Fifty-one-year-old male patient WUS patient last seen well 7.5 h before imaging. a The NCCT showed some hypodensity in the left temporal lobe (red oval) with ASPECTS 9. b The CTA showed collateral filling of > 50% of the MCA flow territory (red oval) and an occlusion in the distal M1 segment (not shown). The CTP showed (c) a large area with an increased Tmax (red oval), and (d) a smaller area with decreased CBF (red oval). The CTP summary map (e) shows the area of Tmax > 6 s (green) and the area of > 30% relative CBF decrease (red). The patient was not treated with IV-rtPA but showed good recovery with a 90-day mRS of 2. WUS, wake-up stroke; NCCT, non-contrast CT; ASPECTS, Alberta Stroke Program Early CT Score; CTA, CT angiography; MCA, middle cerebral artery; CTP, CT-perfusion; IV-rtPA, intravenous tissue plasminogen activator; mRS, modified Rankin Scale.
Fig. 3.
Eighty-six-year-old female patient imaged within 4.5 h after symptom onset. a The NCCT showed a large hypodense area (red oval) in the right hemisphere with ASPECTS 1. b The CTA showed poor collateral filling (red oval) and a proximal occlusion in the distal ICA and M1 segment (different slice). The CTP showed (c) a large area with an increased Tmax (red oval), and (d) a large area with decreased CBF (red oval). The CTP summary map (e) shows the area of Tmax > 6 s (green) and the area of > 30% relative CBF decrease (red). The patient was treated with IV-rtPA and died within 90 days. NCCT, non-contrast CT; ASPECTS, Alberta Stroke Program Early CT Score; CTA, CT angiography; ICA, intracranial carotid artery; CTP, CT-perfusion; IV-rtPA, intravenous tissue plasminogen activator.
Discussion
In the DUST cohort, one in 10 patients woke up with symptoms of acute stroke. Almost half of the WUS patients woke up within the established treatment time window of IV-rtPA treatment and almost two thirds within a 6 h time window for EVT. Our results show that there is no difference in admission clinical and imaging characteristics between WUS patients last seen well within the time limit for IV-rtPA or EVT and WUS patients last seen well outside of this time window. Compared to patients with a known time of stroke symptom onset who were imaged within 4.5 h, WUS patients last seen well > 4.5 h had significantly more ischemic changes on NCCT (lower ASPECTS). However, the majority (75%) of WUS patients had a favorable ASPECTS and good collateral filling on CTA. This suggests that they may benefit from IV-rtPA treatment. No significant differences were seen when comparing WUS patients with a proximal occlusion of the anterior circulation last seen well > 6 h to patients with a proximal occlusion and CT imaging within 6 h after a known time of stroke symptom onset, that is, eligible for EVT. More than half (57%) of the WUS patients with a proximal occlusion who were last seen well > 6 h had favorable imaging characteristics, with only minor ischemic changes (ASPECTS > 7) and good collateral filling. Our findings suggest that CT imaging can be used to select WUS patients that are potential candidates for IV-rtPA or EVT.
In a previous study, no significant differences were shown in clinical features between patients with a WUS and patients with a known onset time of their stroke [5, 6, 9, 21, 22]. In another study, WUS patients were not at increased odds for unfavorable functionality or in-hospital mortality after adjustment for age, NIHSS score on admission, and IV-rtPA use [5]. In one study comparing patients imaged within 4.5 h after symptom onset in WUS patients, WUS patients had lower pc-ASPECTS scores comparable to our results [22]. Studies that compared WUS patients to patients with a known time to symptom onset of < 3 h showed no difference in ischemic changes on NCCT findings [9, 23]. The WUS patients underwent imaging at a mean of 7.5 h after last seen well. An additional comparison between WUS patients and patients with unknown onset time showed that WUS patients have significantly less ischemic changes than patients with an unknown time of stroke onset [23]. This difference suggests that the stroke in WUS patients has only just occurred and caused the patient to wake up. The time since symptom onset may therefore be highly overestimated in WUS patients when using the time since the patient was last seen well. Our findings support this hypothesis.
A recent study selected WUS patients with a small infarct core and a penumbra size > 20% of the ischemic area on CTP for IV-rtPA treatment [19]. The investigators compared safety of IV-rtPA therapy and clinical outcome between WUS patients and patients treated with IV-rtPA within 4.5 h of symptom onset [19]. There was no significant difference in functional outcome after 3 months or in incidence of intracranial hemorrhage between the 2 groups. Nearly all of our > 4.5 h WUS patients had a penumbra size > 20% (98.7% at the ASPECTS levels and 98.5% within the whole scan range) and therefore may have been eligible for IV-rtPA.
Several randomized trials have recently shown benefit of EVT in patients with a proximal occlusion (intracranial carotid artery-M2). Three of these trials used the ASPECTS as an additional inclusion criterion for treatment selection, with two of them including patients with an ASPECTS > 5 and one with ASPECTS > 6 [14, 24, 25]. Only the MR CLEAN trial did not use additional imaging criteria. A subgroup analysis of MR CLEAN showed that NCCT ASPECTS 5–7 patients can be safely treated with EVT [26]. According to these results, the vast majority of the WUS patients with a proximal anterior circulation occlusion in our study (96% with ASPECTS > 5) would have been eligible for EVT. Several trials used perfusion imaging as an imaging inclusion criterion [4, 20, 24]. Two studies included patients for EVT randomization if the mismatch ratio between critical hypoperfusion and the infarct core was more than 1.2 [4, 20] and another with a mismatch of more than 1.8 [3]. Of the > 6 h WUS patients with a proximal occlusion in our study, 61 and 44% fulfilled these criteria, and therefore would be eligible for EVT. The third study used an infarct core of < 50 mL on CTP as an inclusion criterion for patients with an M1 occlusion arriving within 24 h after symptom onset. In our population, 56% of WUS patients last seen well > 6 h had an infarct core volume < 50 mL. Our volume estimates were however limited to the scan range and may therefore be an underestimation.
Our data were collected before the general application of EVT. Surprisingly, EVT and clinical outcome were not different between WUS patients with a proximal occlusion of the anterior circulation and last seen well > 6 h and patients with a proximal occlusion and CT imaging within 6 h after known symptom onset. With current guidelines, many more patients with a proximal occlusion would have been treated with EVT within and beyond 6 h after symptom onset.
An important strength of our study is the size of this prospectively collected cohort. All patients underwent NCCT, CTA, and CTP at admission and structured clinical evaluation. Patients who were last seen well > 9 h were excluded. This may explain why we found a relatively low time between last seen well and CT for the WUS patients and a relatively low percentage of WUS patients compared to other cohorts [7]. However, the cohort most similar to ours also found similar results [23].
There are several limitations to the study that need to be taken into account. First, the comparisons in the study between WUS patients and patients with a known onset time of stroke symptoms was based on anamnestic determination of the time since last seen well. This may have introduced some error, as seen in the estimated times of the WUS patients in the 6–9 h group. Second, for some variables, the number of patients with a positive finding was low, especially in the analyses of patients with a proximal occlusion. This may also introduce a source of error. Third, a large part of the included patients did not show imaging abnormalities on admission. This could imply that TIA and stroke mimics were included. However, all patients included in the analysis had an NIHSS at baseline of ≥2 or 1 if an indication for IV-rtPA is present (e.g., isolated aphasia) [10]. They therefore had suspected stroke and an indication for IV-rtPA. Moreover, the detection of ischemia on CT perfusion is not 100%; small infarcts and infarcts outside the scan range can be missed [27]. Since this is true for the entire study population, it should not have influenced the analyses.
Conclusions
There were only small differences between clinical and imaging characteristics of WUS patients and patients with a known onset time of stroke symptoms. This supports the hypothesis that patients with WUS may wake up from their stroke symptoms. Most WUS patients have only limited ischemic findings (ASPECTS > 7) and good collaterals, which may make them eligible for IV-rtPA treatment. Most WUS patients with a proximal anterior circulation occlusion have good collateral filling and no severe ischemia (ASPECTS < 5) and may therefore be eligible for EVT.
Disclosure Statement
This study was supported by grants from the Netherlands Heart Foundation (grant numbers 2008 T034 and 2012 T061) and the Nuts Ohra Foundation (grant number 0903-012).
Acknowledgments
The DUST investigators are Majoie CB, Roos YB, Academic Medical Center, Amsterdam, The Netherlands; Duijm LE, Keizer K, Catharina Hospital, Eindhoven, The Netherlands; van der Lugt A, Dippel DW, Erasmus Medical Center, Rotterdam, The Netherlands; Droogh-de Greve KE, Bienfait HP, Gelre Hospitals, Apeldoorn, The Netherlands; van Walderveen MA, Wermer MJ, Leiden University Medical Center, Leiden, The Netherlands; Lycklama à Nijeholt GJ, Boiten J, Medical Center Haaglanden, The Hague, The Netherlands; Duyndam D, Kwa VI, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands; Meijer FJ, van Dijk EJ, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands; Kesselring FO, Hofmeijer J, Rijnstate Hospital, Arnhem, The Netherlands; Vos JA, Schonewille WJ, St. Antonius Hospital, Nieuwegein, The Netherlands; van Rooij WJ, de Kort PL, St. Elisabeth Hospital, Tilburg, The Netherlands; Pleiter CC, Bakker SL, St. Franciscus Hospital, Rotterdam, The Netherlands; Bot J, Visser MC, VU Medical Center, Amsterdam, The Netherlands; Velthuis BK, van der Schaaf IC, Dankbaar JW, Mali WP, van Seeters T, Horsch AD, Niesten JM, Biessels GJ, Kappelle LJ, Luitse MJ, van der Graaf Y, University Medical Center Utrecht, Utrecht, The Netherlands.
References
- 1.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316:1279–1288. doi: 10.1001/jama.2016.13647. [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 5.Denny MC, Boehme AK, Dorsey AM, George AJ, Yeh AD, Albright KC, et al. Wake-up strokes are similar to known-onset morning strokes in severity and outcome. J Neurol Neurol Disord. 2014;1 doi: 10.15744/2454-4981.1.102. pii: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackey J, Kleindorfer D, Sucharew H, Moomaw CJ, Kissela BM, Alwell K, et al. Population-based study of wake-up strokes. Neurology. 2011;76:1662–1667. doi: 10.1212/WNL.0b013e318219fb30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin MN, Barrett KM. What to do with wake-up stroke. Neurohospitalist. 2015;5:161–172. doi: 10.1177/1941874415576204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomalla G, Fiebach JB, Ostergaard L, Pedraza S, Thijs V, Nighoghossian N, et al. A multicenter, randomized, double-blind, placebo-controlled trial to test efficacy and safety of magnetic resonance imaging-based thrombolysis in wake-up stroke (WAKE-UP) Int J Stroke. 2014;9:829–836. doi: 10.1111/ijs.12011. [DOI] [PubMed] [Google Scholar]
- 9.Roveri L, La Gioia S, Ghidinelli C, Anzalone N, De Filippis C, Comi G. Wake-up stroke within 3 hours of symptom awareness: imaging and clinical features compared to standard recombinant tissue plasminogen activator treated stroke. J Stroke Cerebrovasc Dis. 2013;22:703–708. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 10.van Seeters T, Biessels GJ, Kappelle LJ, van der Schaaf IC, Dankbaar JW, Horsch AD, et al. The prognostic value of CT angiography and CT perfusion in acute ischemic stroke. Cerebrovasc Dis. 2015;40:258–269. doi: 10.1159/000441088. [DOI] [PubMed] [Google Scholar]
- 11.van Seeters T, Biessels GJ, van der Schaaf IC, Dankbaar JW, Horsch AD, Luitse MJ, et al. Prediction of outcome in patients with suspected acute ischaemic stroke with CT perfusion and CT angiography: the Dutch acute stroke trial (DUST) study protocol. BMC Neurol. 2014;14:37. doi: 10.1186/1471-2377-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 13.Puetz V, Sylaja PN, Coutts SB, Hill MD, Dzialowski I, Mueller P, et al. Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. 2008;39:2485–2490. doi: 10.1161/STROKEAHA.107.511162. [DOI] [PubMed] [Google Scholar]
- 14.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 15.Kudo K, Sasaki M, Ogasawara K, Terae S, Ehara S, Shirato H. Difference in tracer delay-induced effect among deconvolution algorithms in CT perfusion analysis: quantitative evaluation with digital phantoms. Radiology. 2009;251:241–249. doi: 10.1148/radiol.2511080983. [DOI] [PubMed] [Google Scholar]
- 16.van Seeters T, Biessels GJ, Niesten JM, van der Schaaf IC, Dankbaar JW, Horsch AD, et al. Reliability of visual assessment of non-contrast CT, CT angiography source images and CT perfusion in patients with suspected ischemic stroke. PLoS One. 2013;8:e75615. doi: 10.1371/journal.pone.0075615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–3440. doi: 10.1161/STROKEAHA.111.618355. [DOI] [PubMed] [Google Scholar]
- 18.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke. 2009;40:469–475. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morelli N, Rota E, Immovilli P, Cosottini M, Giorgi-Pierfranceschi M, Magnacavallo A, et al. Computed tomography perfusion-based thrombolysis in wake-up stroke. Intern Emerg Med. 2015;10:977–984. doi: 10.1007/s11739-015-1299-0. [DOI] [PubMed] [Google Scholar]
- 20.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 21.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–531. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costa R, Pinho J, Alves JN, Amorim JM, Ribeiro M, Ferreira C. Wake-up stroke and stroke within the therapeutic window for thrombolysis have similar clinical severity, imaging characteristics, and outcome. J Stroke Cerebrovasc Dis. 2016;25:511–514. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 23.Todo K, Moriwaki H, Saito K, Tanaka M, Oe H, Naritomi H. Early CT findings in unknown-onset and wake-up strokes. Cerebrovasc Dis. 2006;21:367–371. doi: 10.1159/000091545. [DOI] [PubMed] [Google Scholar]
- 24.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 25.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 26.Yoo AJ, Berkhemer OA, Fransen PS, van den Berg LA, Beumer D, Lingsma HF, et al. Effect of baseline Alberta Stroke Program Early CT Score on safety and efficacy of intra-arterial treatment: a subgroup analysis of a randomised phase 3 trial (MR CLEAN) Lancet Neurol. 2016;15:685–694. doi: 10.1016/S1474-4422(16)00124-1. [DOI] [PubMed] [Google Scholar]
- 27.Niesten JM, van der Schaaf IC, van der Graaf Y, Kappelle LJ, Biessels GJ, Horsch AD, et al. Predictive value of thrombus attenuation on thin-slice non-contrast CT for persistent occlusion after intravenous thrombolysis. Cerebrovasc Dis. 2014;37:116–122. doi: 10.1159/000357420. [DOI] [PubMed] [Google Scholar]