Abstract
Objective
Congenital hypothyroidism (CH) per se, when not treated or undertreated, may lead to severe behavioural problems (cretinism), whereas overtreatment of CH seems associated with attention problems.
Design and Methods
For 55 CH patients, prospectively followed from birth until 11 years, parents rated the Child Behaviour Checklist and teachers the Teacher's Report Form at children's ages 6 and 11 years. We related scores regarding Attention, Delinquency, and Aggression (ADA scores, indicative for attention deficit hyperactivity syndrome, ADHD), and scores regarding Withdrawn, Anxious, Social, and Thought problems (WAST scores, indicative for autism) to the occurrence of over- and undertreatment in five age periods. Over- and undertreatment were defined as free thyroxine (fT4) concentrations above/below the range of the patient's individual fT4 steady state concentration.
Results
ADA scores at 6 and 11 years for patients overtreated in the period 1–3 months postnatally were higher than those for patients who were not overtreated. Patients with severe CH undertreated in the period 3–6 months postnatally had higher WAST scores at 6 and 11 years than all other patients.
Conclusions
This is the first study suggesting that permanent ADHD as well as autism in CH patients at ages 6 and 11 years are the result of early overtreatment and undertreatment, respectively.
Keywords: Congenital hypothyroidism, Early overtreatment, Early undertreatment, ADHD, Autism, fT4 steady state concentrations
Introduction
Thyroid abnormalities have been associated with attention deficit hyperactivity disorder (ADHD) in various studies [1, 2, 3]. This association is most clearly seen in a study on thyroid hormone resistance [1], where the majority of the patients were found to have ADHD. In adolescents with congenital hypothyroidism (CH) the proportion of patients with ADHD was estimated at 30–40% [2]. In a group of fifty 10-year-old CH patients poor attention was seen after overtreatment in the first 6 months after birth [3]. Two other studies, however, failed to confirm poor impulse control and externalizing problems in CH patients. These patients were predominantly introvert [4, 5]. In an earlier study we demonstrated that severe maternal hypothyroxinaemia, in a period when the foetus is still largely dependent on maternal thyroxine (T4) supply, led to a 4-fold higher risk of autism in the off-spring than higher maternal T4 concentrations [6]. The exact mechanism underlying the presence of behavioural problems in CH is still unclear.
Rovet et al. [7] investigated temperamental behaviour in early-treated CH patients [7] and found that serum T4 concentrations of patients classified as “difficult” at the age of 6 months had been higher in the period 1–3 months postnatally than those of patients classified as “easy” [7]. This difference in temperament persisted until at least the age of 2 years.
In the study presented here we evaluated behaviour in CH children by means of questionnaires completed by parents and teachers at different children's ages. The results were related to overtreatment (OT) and undertreatment (UT). Assessment of OT and UT was based upon the individual free T4 (fT4) steady state concentrations (SSCs) [8]. We investigated whether behavioural problems in CH are associated with OT and UT, respectively, and if so, from which period these problems originated, how long they persisted, and whether they equally affected patients with severe and mild CH.
Methods
Subjects
The study group was part of a cohort of 61 children (22 male) with early-treated permanent CH described previously [8, 9, 10, 11, 12]. They were born in the Netherlands between February 1993 and July 1996. It was a nationwide study. All patients were under the care of their own local paediatrician. Inclusion and exclusion criteria were described earlier [9]. Included were patients with the diagnosis of permanent CH, on L-T4 therapy. Exclusion criteria were as follows: risk for abnormal psychomotor development for reasons other than CH (asphyxia, meningitis, chromosomal abnormalities, a syndrome with severe hypotonia, severe prematurity), having a mother with known thyroid abnormalities, and social reasons (e.g., children of refugees). Severity of CH was classified according to aetiology [13]. Severe CH was defined as total incapability to produce T4, and mild CH as partial incapability [7, 8].
Psychological Tests
At children's ages 6 and 11 years, parents rated the Child Behaviour Checklist (CBCL) [14] and teachers the Teacher's Report Form (TRF) [15]. CBCL and TRF are screening tests, both consisting of 113 questions, to be rated on a 3-point scale, and subdivided in 8 domains of behaviour: Socially withdrawn, Somatic complaints, Anxious/depressed, Social problems, Thought problems, Attention problems, Delinquency, and Aggression. The raw scores can be converted to age-standardized scores, T scores, that can be compared with scores obtained from normative samples of children within the same age range. A higher score means a greater number or intensity of behavioural problems. T scores below 65 are interpreted as normal, between 65–70 as borderline, and above 70 as clinical. Thyroid status around the test moments was checked. The aggregated score for the domains Attention problems, Delinquency, and Aggression, addressed as ADA score, was determined by taking the mean T score of these three domains. The aggregated score for the domains Socially withdrawn, Anxious/depressed, Social problems, and Thought problems, addressed as WAST score, was determined by taking the mean T score of these four domains. The control group at 6 years contained 36 children, and at 11 years 28 children. It comprised healthy, age-matched children, from regular primary schools in the Leiden-Rotterdam region.
Over- and Undertreatment
Over- and undertreatment status was based on the individual fT4 steady state concentration (fT4 SSC) [7], defined as that fT4 concentration at which it is constant, despite ongoing processes that might change it, such as medication, T4 clearance, and alterations in the equilibrium between the different deiodinase pathways [16]. The rationale of the SSC is that it is not possible to treat CH patients properly without knowing what euthyroidism means for the individual patient [11]. SSC provides fT4 concentrations to aim at, as well as individual ranges that indicate over- and undertreatment. In healthy, euthyroid subjects and subjects with stable thyroid disease individual SSCs represent the mean value of a series of determinations under stable and euthyroid conditions as much as possible [17, 18, 19]. For the determination of SSCs in CH patients, we assumed that most euthyroid samples would be present in samples with a thyroid stimulating hormone (TSH) between 0.5 and 10 mU/L and obtained at an age at which fT4 and TSH have stabilized after the onset of therapy, i.e., from 1.5 months up to 132 months after the start of therapy. Next, samples with fT4 concentrations outside the 95% CI were excluded (Table 1). This selection procedure yielded a mean of 21 samples per patient, out of the mean of 44 samples that were available per patient. As described previously, individual fT4 SSCs decrease slightly in the period 0–11 years and are largely independent of the L-T4 dosage [7]. In the initial period, i.e., from therapy start to 1.5 months later, values are somewhat higher than in the post-initial period without reaching significance: mean (SD) 22.5 (3.0) versus 21.7 (2.6) pmol/L, respectively (p = 0.07) (Table 1) [7]. Over- and undertreatment were defined as fT4 concentrations above +2 SD/below −2 SD of the individual fT4 SSC. Patients were considered as over-/undertreated (OT/UT) during a specific time period if in that period they had experienced one or more episodes of over- or undertreatment. The interval between 1 month after birth and 11 years of age was subdivided into five periods: 1–3 months, 3–6 months, 6–24 months, 2–6 years, and 6–11 years. Patients were classified per period as OT positive (OT+) or OT negative (OT–), and as UT positive (UT+) or UT negative (UT–).
Table 1.
fT4 and TSH SSC values of the 61 CH patients of the original study group [7]
| Age interval, months | Samples per patient,n | SSC | CV, % | |
|---|---|---|---|---|
| Post-initial fT 4 SSC, pmol/L | 1.5–132 | 21.0 | 21.7 (2.6) [16.6; 28.7] | 12.0 (3.9) |
| Initial fT 4 SSC, pmol/L | 0.23–1.5 | 4.6 | 22.5(3.0)[16.2; 27.3] | 13.2 (6.3) |
| Post-initial TSH SSC, mU/L | 1.5–132 | 21.0 | 3.3 (1.9) [0.9; 5.7] | 59.9 (13.6) |
SSC values are presented as mean (SD) [95% CI]. Age interval: age after onset of therapy. CV: mean coefficient of variation (CV) within the individual SSCs. Difference between initial SSCs and SSCs for interval 1.5–132 months:p = 0.07.
Statistical Analysis
Statistical analysis was done with SPSS version 24.0 (SPSS Inc. Chicago, IL, USA). Eight separate multiple regression procedures were used to compare the effect of OT/UT on the parental and teachers' ADA and WAST scores at children's ages 6 and 11 years. In this analysis the OT+/OT– and UT+/UT– treatment groups of the five age periods were the independent variables, and ADA and WAST scores at 6 and 11 years the dependent variables. Multiple regressions were applied to identify behavioural score differences between patients with severe and mild CH within the OT+, OT–, UT+, and UT– groups. ANOVAs were applied to compare fT4 concentrations (in pmol/L and SSC SDS) as well as TSH values of the OT+/OT– and UT+/UT– groups during the five age periods. CBCL and TRF scores of patients and controls were compared with Student t tests. Pearson correlations were performed to find potential associations between the scores at 6 and 11 years. For all tests a significance level of 0.05 was used.
Results
Subjects and Tests
The initial cohort consisted of 61 patients (22 male), that at 6 years of 46 patients (13 male), and that at 11 years of 55 patients (19 male). Of these 55 patients 8 were nonethnic Dutch, i.e., Moroccan or Turkish. According to aetiology, 23 cases of CH were classified as severe and 32 as mild, with mean (SD) pre-treatment fT4 concentrations of 2.6 (1.0) and 8.7 (3.8) pmol/L, respectively. In the severe group 19 patients had agenesis, and 4 had total dyshormonogenesis; in the mild group 28 patients had an ectopic gland, and 4 patients had partial dyshormonogenesis. Age at onset of treatment was 14.2 (4.6) days, and the initial L-T4 dose was 8.7 (2.2) µg/kg. Mean socio-economic status (SES) was 2.13 (0.75), on a 3-point scale. Follow-up data on fT4, TSH, and L-T4 dose were collected.
At 6 years 40 CBCL forms and 31 TRF forms were filled out; at 11 years 55 of both forms were filled. Three patients were formally diagnosed as having ADHD, 2 met the criteria for autism. Two ADHD patients were treated with methylphenidate after the age of 6 years (no medication was taken during testing). For one of those, TRF ADA scores from 6 to 11 years decreased from 74 to 63; for the other scores were comparable (69 and 71).
All but 6 patients had a normal thyroid status at testing. At both test moments 1 patient was undertreated and 2 were overtreated. Parents' and teachers' WAST scores of the 2 hypothyroid patients were below 60; teacher's ADA score for one of them was in the borderline range (69) at age 6 years and in the clinical range (71) at age 11 years. The 2 hyperthyroid patients had teachers' ADA scores of 58 and 64 at 6 years. At 11 years the latter score was 57. At age 11 years the 2 hyperthyroid patients had teachers' ADA scores of 51 and 69. The latter borderline score could not be compared with a previous score, because it was lacking.
Parental ADA and WAST scores at 6 and 11 years showed a good relationship (0.686 and 0.617, respectively, p < 0.001 for both). The same holds for the teachers' scores (0.818 and 0.575, respectively, p < 0.001), considering that the rating was done by two different teachers.
Relation of ADA and WAST Scores with Treatment
Table 2 shows the differences in behavioural scores (ADA and WAST) between the groups with and without overtreatment (OT+/OT–) and with and without undertreatment (UT+/UT–), respectively, for the periods 1–3, 3–6, and 6–24 months after birth.
Table 2.
Differences in ADA and WAST scores, rated by parents and teachers at children's ages 6 and 11 years, between treatment groups OT+ and OT– and between groups UT+ and UT–, for the periods 1–3, 3–6, and 6–24 months after birth
| Score | Treatmentgroups | Age period, months | 6 years |
11 years |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| parents |
teacher |
parents |
teacher |
|||||||||||
| patients,n | score difference | p * | patients,n | score difference | p * | patients,n | score difference | p * | patients,n | score difference | p * | |||
| ADA | OT+ vs. OT– | 1–3 | 24/16 | 1.94 (1.73) | 0.271 | 18/13 | 7.82 (2.63) | 0.007 | 31/24 | 4.21 (1.56) | 0.009 | 31/24 | 5.19 (1.58) | 0.008 |
| ADA | OT+ vs. OT– | 3–6 | 15/25 | 2.50(1.73) | 0.158 | 12/19 | –4.66 (2.63) | 0.089 | 18/37 | –4.20 (1.52) | 0.007 | 18/37 | 4.43 (1.78) | 0.016 |
| ADA | OT+ vs. OT– | 6–24 | 19/21 | 0.71 (1.66) | 0.674 | 17/14 | 0.08 (2.43) | 0.975 | 26/29 | 1.18(1.42) | 0.408 | 26/29 | 1.47(1.64) | 0.376 |
| WAST | UT+ vs. UT– | 1–3 | 8/32 | 1.74 (1.83) | 0.349 | 5/26 | –1.48 (2.67) | 0.583 | 10/45 | 3.25 (1.77) | 0.073 | 10/45 | 0.36 (1.80) | 0.845 |
| WAST | UT+ vs. UT– | 3–6 | 7/33 | 7.45 (1.94) | 0.001 | 5/26 | 4.18 (2.67) | 0.137 | 10/45 | 6.32 (1.94) | 0.002 | 10/45 | 6.25 (1.97) | 0.003 |
| WAST | UT+ vs. UT– | 6–24 | 23/17 | –0.16(1.54) | 0.917 | 13/18 | –1.57 (2.05) | 0.450 | 31/24 | –4.08 (1.48) | 0.008 | 31/24 | –3.92 (1.50) | 0.012 |
Score differences are presented as mean (SEM). Patients: number of patients per treatment group (OT+/OT– or UT+/UT–). OT+, overtreated in a specific period; OT–, not overtreated in that period; UT+, undertreated in a specific period; UT–, not undertreated in a that period.
Bold p values: significant score difference between OT+/OT– and UT+/UT– groups.
Apart from the parental ADA score at age 6 years, ADA scores were positively related to overtreatment in the period 1–3 months after birth (Table 2). Negative associations were established between overtreatment in the period 3–6 months and the ADA scores of both parents and teachers at age 11 years. No significant relation between ADA scores and undertreatment could be found in any of the periods. WAST scores at ages 6 and 11 years were positively related to undertreatment in the period 3–6 months (Table 2), with the exception of the teachers' WAST score at age 6, possibly due to the small number of undertreated patients in the UT+ group (n = 5). Undertreatment in the period 6–24 months was significantly negatively associated with WAST scores of parents and teachers at age 11 years (Table 2). No significant relation between overtreatment and WAST scores was found in any of the investigated periods. No significant associations were established for the two later periods, from 24 months to 11 years, either between ADA scores and overtreatment or between WAST scores and undertreatment. In all analyses ADA scores of the OT– groups and WAST scores of the UT– groups were non-significantly different from those of the control groups (p ≥ 0.084 for all).
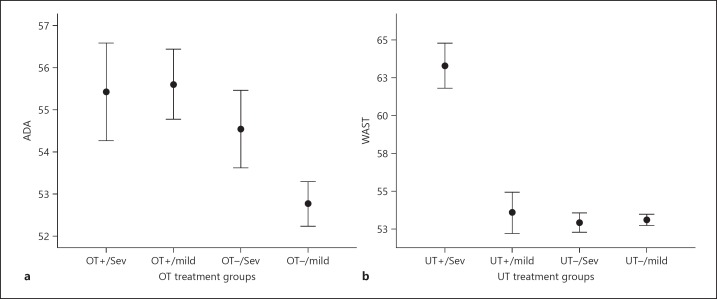
Figure 1 shows the combined mean behavioural scores of the ratings at 6 and 11 years by parents and teachers, for the period 1–3 months (ADA scores; Fig. 1a) and 3–6 months (WAST sores; Fig. 1b). Mean (± SEM) combined ADA scores of the OT+ and OT– groups differed significantly: 55.9 (0.7) (number of ratings, n = 104) vs. 52.8 (78) (n = 78; p < 0.001), as did the mean combined WAST scores of the UT+ and UT– groups: 58.1 (1.3) (n = 32) vs. 53.0 (0.3) (n = 149; p < 0.001).
Fig. 1.
a Mean combined ADA scores (± SEM), rated at children's ages 6 and 11 years by parents and teachers, of over- and not overtreated patients in the period 1–3 months, subdivided into treatment groups OT+/severe, OT+/mild, OT–/severe, and OT–/mild. Difference between ADA scores of OT+ and OT– patients: p < 0.001; difference between severe and mild congenital hypothyroidism (CH) patients in the OT+ group: not significant. b Mean WAST scores (± SEM), rated at ages 6 and 11 by parents and teachers, of under- and not undertreated patients in the period 3–6 months, subdivided into treatment groups UT+/severe, UT+/mild, UT–/severe, and UT–/mild. Difference between WAST scores of UT+ and UT– patients: p < 0.001; difference between severe and mild CH patients in the UT+ group: p < 0.001. OT, overtreatment; UT, undertreatment; Sev, severe.
Relation of ADA and WAST Scores with Type of CH
Table 3 shows the differences in mean ADA and WAST scores, rated by parents and teachers at children's ages 6 and 11 years, between severe and mild CH patients in the treatment groups OT+, OT–, UT+, and UT–, for the periods 1–3 months (ADA scores) and 3–6 months (WAST scores). There were 13 and 16 severe and mild cases, respectively, in the OT+ group, 10 and 16 cases in the OT– group, 5 and 5 cases in the UT+ group, and 18 and 27 cases in the UT– group. The ADA scores did not differ significantly between severe and mild patients in the OT+ or OT– groups (Fig. 1a). Highly significant differences were found, however, between the severe and mild patients in all UT+ groups (p < 0.001 for all) (Table 3; Fig. 1b). The risk of a WAST score > 60 was 33.3 (95% CI 10.3; 108.0)-fold higher for a patient with severe CH and undertreated in the period 3–6 months than for all other CH patients. Pre-treatment fT4 concentrations of patients with UT+ and severe CH were lower than those of patients with UT– and severe CH: −9.0 (1.3) vs. −7.2 (2.4) SSC SDS, respectively (p = 0.043). In the UT+ group, mean fT4 (in SSC SDS) and TSH concentrations of the severe and mild patients in the period 3–6 months were comparable: fT4 −1.17 (1.47) vs. −1.19 (1.01) SSC SDS, respectively (p = 0.986); TSH 16.1 (8.5) vs. 14.5 (9.3) mU/L, respectively (p = 0.773). The same holds for the mean duration of undertreatment: 1.61 (0.77) vs. 1.81 (0.43) months, respectively (p = 0.673).
Table 3.
Differences in ADA and WAST scores, rated by parents and teachers at 6 and 11 years, between severe and mild CH patients of treatment groups OT+, OT–, UT+, and UT–, for the period 1–3 months (ADA scores) and 3–6 months (WAST scores)
| Score | Age period, months | Treatment groups | 6 years |
11 years |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| parents |
teacher |
parents |
teacher |
|||||||
| score diff. S/M |
p * | score diff. S/M |
p * | score diff. S/M |
p * | score diff. S/M |
p * | |||
| ADA ADA |
1–3 1–3 |
OT+ OT– |
–1.8 (2.3) 0.6 (l.9) |
0.403 0.793 |
–1.2 (2.3) 4.3 (2.2) |
0.586 0.195 |
0.6 (2.1) 0.2 (1.9) |
0.818 0.891 |
0.54 (2.7) 2.7 (2.0) |
0.850 0.299 |
| WAST WAST |
3–6 3–6 |
UT+ UT– |
11.4 (2.3) 0.3 (1.3) |
<0.001 0.760 |
11.5 (3.1) –2.1 (1.5) |
0.001 0.549 |
7.5 (2.4) –0.3 (1.5) |
0.001 0.878 |
10.2 (2.7) 0.6 (1.3) |
<0.001 0.701 |
Score differences are presented as mean (SEM). Score diff. S/M: score difference between severe and mild CH within the indicated group.
Boldp values indicate significant results.
fT4 and TSH Concentrations
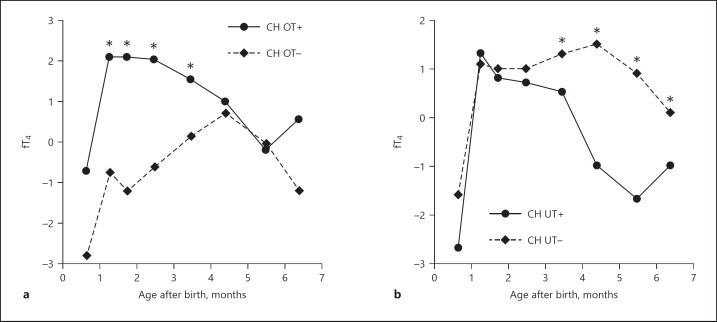
In the period 1–3 months postnatally fT4 concentrations (in pmol/L and in individual SSC SDS values) in the OT+ group were higher and TSH values were lower than those in the OT– group (p < 0.001 for all) (Table 4; Fig. 2a). Likewise, in the period 3–6 months fT4 concentrations in the UT+ group were lower and TSH values were higher than those in the UT– group (p < 0.001 for all) (Table 4; Fig. 2b).
Table 4.
fT4 and TSH concentrations of the OT+/OT– and UT+/UT- groups in age periods 1–3, 3–6, and 6–24 months
| Age period, months | Treatment groups | Patients,n | Samples,n | fT4,pmol/L | p* | fT4, SSC SDS | p* | TSH, mU/L | p* |
|---|---|---|---|---|---|---|---|---|---|
| 1–3 | OT+ | 29 | 139 | 25.8 (0.7) | <0.001 | 2.16 (0.27) | <0.001 | 7.6 (2.2) | <0.001 |
| OT– | 26 | 123 | 21.3 (0.6) | –0.59 (0.30) | 21.7 (3.2) | ||||
| 3–6 | OT+ | 18 | 59 | 27.6 (1.2) | <0.001 | 2.66 (0.43) | <0.001 | 3.1 (1.4) | 0.012 |
| OT– | 37 | 102 | 21.4 (0.7) | –0.10 (0.21) | 9.1 (1.5) | ||||
| 6–24 | OT+ | 26 | 249 | 22.9 (0.7) | 0.003 | 0.44 (0.18) | <0.001 | 5.4 (1.0) | 0.009 |
| OT– | 29 | 290 | 20.1 (0.6) | –0.87 (0.22) | 9.6 (1.2) | ||||
| 1–3 | UT+ | 10 | 51 | 19.0 (1.5) | <0.001 | –1.22 (0.73) | <0.001 | 24.4 (6.5) | <0.001 |
| UT– | 45 | 211 | 25.1 (0.8) | 1.53 (0.35) | 10.7 (2.2) | ||||
| 3–6 | UT+ | 10 | 35 | 19.0 (1.3) | <0.001 | –0.77 (0.62) | <0.001 | 14.1 (4.5) | <0.001 |
| UT– | 45 | 126 | 24.7 (0.9) | 1.27 (0.37) | 5.5 (1.3) | ||||
| 6–24 | UT+ | 31 | 296 | 20.1 (0.6) | <0.001 | –0.83 (0.20) | <0.001 | 8.9 (1.5) | <0.001 |
| UT– | 24 | 243 | 22.6 (0.7) | 0.53 (0.18) | 4.6 (1.2) |
fT 4 and TSH concentration values are presented as mean (SEM).
Bold p values: significant difference between OT+/OT– and UT+/UT–groups.
Fig. 2.
Mean fT4 concentrations (in SSC SDS) of the study group from initiation of therapy to 7 months. a OT+ and OT– patients of the period 1–3 months after birth. b UT+ and UT– patients of the period 3–6 months after birth. * p < 0.001 between OT+/OT– values and between UT+/UT– values. CH, congenital hypothyroidism; OT, overtreatment; UT, undertreatment.
Relation of ADA and WAST Scores with Other Variables
Regarding the period 1–3 months, patients in the OT+ and OT– groups did not differ in initial L-T4 dose: 9.2 (2.0) vs. 8.4 (2.0) µg/kg (p = 0.057), day of onset of treatment: 16.1 (6.2) vs. 13.4 (3.0) days (p = 0.109), L-T4 dose: 6.7 (1.7) vs. 6.7 (1.5) µg/kg (p = 0.858), or SES: 2.12 (0.73) vs. 2.04 (0.77) (p = 0.409). The groups differed significantly, however, in fT4 increase during the first week after therapy onset: 7.2 (4.0) vs. 4.3 (2.7) SSC SDS (p = 0.004) (Fig. 2a). A higher fT4 increase during the first week was associated with a higher teachers' ADA score at age 11 (p = 0.015). The duration of the overtreatment episodes in the period 1–3 months in the OT+ group: 0.85 (0.23) months, was not related to the ADA scores.
Regarding the period 3–6 months, the patients of the UT+ and UT– groups did not differ in initial L-T4 dose: 8.5 (1.9) vs. 8.6 (2.3) µg/kg (p = 0.857), day of onset of treatment: 12.4 (8.1) vs. 14.4 (4.8) days (p = 0.209), SES: 2.30 (0.68) vs. 2.09 (0.76) (p = 0.424), or fT4 increase during the first week: 12.5 (9.0) vs. 14.2 (9.9) pmol/L (p = 0.430). The UT+ and UT– groups differed, however, in L-T4 dose in the period 3–6 months: 4.6 (1.0) vs. 5.6 (1.4) µg/kg (p = 0.032) (Fig. 2b). The duration of the undertreatment episodes in the period 3–6 months in the UT+ group: 1.71 (0.59) months, was not related to the WAST scores (p = 0.308). The pre-treatment fT4 concentrations (in SSC SDS) of the UT+ patients with severe CH were lower than those of severe cases in the UT– group: −9.0 (1.4) vs. −7.2 (2.4) SSC SDS (p = 0.043).
Discussion
Our data suggest that CH patients who experienced one or more overtreatment episodes in the period 1–3 months postnatally may show behavioural problems at a later age, especially poor attention and externalizing behavioural problems. Severe CH patients who had been undertreated in the period 3–6 months postnatally more frequently showed behavioural problems like introversion, anxiety, social problems, and thought problems than mild CH patients and patients who were not undertreated. Problems were not present in all over- and undertreated patients, but if present at the age of 6 years seem to be sustained at the age of 11 years. Both over- and undertreated CH patients in the indicated periods presented with a specific pattern of CBCL and TRF elevations for the domains included in the ADA and WAST scores, resembling the patterns found for ADHD and autism spectrum disorders (ASD), respectively [20, 21, 22]. Overtreatment in the period 1–3 months equally affected patients with severe and mild CH – as was also found in another study [6] – suggesting a postnatal cause for the high ADA scores. Undertreatment in the period 3–6 months predominantly affected patients with severe CH, with a high risk for ASD-like problems, suggesting an additional factor, perhaps from prenatal origin, rendering patients with severe CH more vulnerable for postnatal hypothyroidism. The pre-treatment fT4 concentrations of the UT+ patients with severe CH were among the lowest values of all severe cases in our study, suggesting a severe intrauterine T4 deficiency. In an earlier study we found an association between early severe maternal hypothyroxinaemia and an increased risk for autism [21]. Another study demonstrated that very low T4 concentrations at birth (<P3) were related to a higher risk for ASD [23]. Other factors, like for instance a genetic factor, may of course also be involved in the ADA as well as WAST elevations.
Behavioural problems could only be related to over- and undertreatment in the early periods 1–3 and 3–6 months, respectively, suggesting that normal fT4 concentrations in these short time windows are essential for preventing behavioural problems at a later age. We assume that over- and undertreatment in these respective periods are causative factors for the high ADA and WAST scores, and that the negative associations between behavioural scores and over- and undertreatment in the subsequent periods, i.e., 3–6 and 6–24 months, respectively, are a consequence of the positive associations in the previous periods. A negative association in this respect means that the behavioural scores for the groups without over- and undertreatment were higher than those for the groups with over- and undertreatment. These negative associations were the result of the high behavioural scores for several patients who had been over- or undertreated in the periods 1–3 or 3–6 months, respectively, but not in the subsequent periods. The absence of over- or undertreatment in these subsequent periods is unlikely to be a causative factor for the high behavioural scores. If the absence of over- and undertreatment would be a causative factor, a substantial proportion of all healthy newborns with normal thyroid function without over- or undertreatment in these periods would show ADHD-like or ASD-like behaviour, which is obviously not the case. Furthermore, the conclusion that overtreatment in the period 1–3 months is a causative factor for high ADA scores, and that the absence of overtreatment in the period 3–6 months is not, is in line with previous studies [3, 6]. One of these [6] suggests that at least a considerable proportion of the “difficult” patients experienced overtreatment in the period 1–3 months, because at 3 years they showed more advanced motor development than did the “easy” patients. The other study [3] showed that poor attention in CH patients at the age of 10 years was predicted by the number of overtreatment episodes, defined as TSH < 0.05 mU/L, during the first 6 months. Interestingly, as in the present study, the highest mean fT4 concentrations in that study were found in the period 1–3 months after birth.
On the other hand, studies dating from the early years of the CH screening programmes failed to confirm poor impulse control and externalizing problems in CH patients [4, 5]. At 7.5 years these children showed normal impulse control and at 7.5 and 9.5 years were predominantly introvert, similar to our patients undertreated in the period 3–6 months. The findings could not be related to under- or overtreatment, because follow-up data of fT4 and TSH were not collected. Initiation of therapy was late (27 days) and the starting L-T4 dosage was low (6 µg/kg). Circumstantial evidence suggests that this regimen will only sporadically lead to overtreatment, but more frequently to undertreatment [24, 25, 26]. In the studies in question, also dating from the early years of CH screening, mean TSH was relatively high (> 10 mU/L) [23, 25] and mean T4 relatively low (130–145 nmol/L) during the first year [24, 25, 26]. This suggests that in the studies that found predominantly introvert behaviour [4, 5] only few of the patients had been overtreated in the period 1–3 months after birth, explaining the absence of poor impulse control and externalizing problems. Still, a substantial proportion of them may have been undertreated in the period 3–6 months, explaining the introvert behaviour, as also found in our study.
Behavioural problems in CH may be associated with thyroid status at the time of testing [27], but then are mostly transient. We believe that this phenomenon has had little impact in our study, because only few patients were dysthyroid at testing and their ADA and WAST scores were not consistently associated with over- or undertreatment.
We used the fT4 SSC method to assess under- and overtreatment [7, 11] because several overtreated patients could have been missed with the use of TSH age-specific reference ranges. In CH, especially during the first 24 months, high fT4 concentrations fail to suppress TSH concentrations to the low values found in healthy subjects [11], probably due to a resistance of the hypothalamic-pituitary-thyroid axis [28]. Moreover, the TSH response to increasing fT4 values is delayed and quantitatively less than in healthy individuals [11]. Still, overtreated patients may also be missed with the use of fT4 age-specific reference ranges, because the ranges are too wide and two fixed upper and lower limit values will never be able to properly distinguish between normal or abnormal in all CH patients, whatever values are chosen [11].
A drawback of our study is its relative small sample size. Still, the longitudinal design allowed obtaining CBCL as well as TRF ratings twice, which importantly strengthened the results.
Regarding the optimum treatment regimen, we recommend choosing an initial L-T4 dosage with which early overtreatment is avoided as much as possible. A limiting factor is that the individual fT4 SSC value, the main target of treatment, is not known at the beginning of therapy. We have designed an initial dosage scheme (Table 5) [11] that shows which fT4 concentrations might be expected in the first 4–17 days with a specific L-T4 dosage and considering the patient's minimal initial fT4 deficit. Minimal initial fT4 deficit was defined as the fT4 difference between the lower limit of the 95% CI of the fT4 SSCs of the total CH cohort, i.e., 16.6 pmol/L (1.28 ng/dL), and the individual pre-treatment fT4. Thereafter, fT4 and TSH should be checked frequently to avoid both over- and undertreatment.
Table 5.
fT4 concentrations of the first three follow-up samples, at 4.3, 9.2, and 17.0 days, by initial L-T4 dose and minimal initial fT4 deficit
| Initial L-T 4 dose, µg/kg | Minimal initial fT 4 deficit, pmol/L |
||
|---|---|---|---|
| <10 pmol/L | 10–15 pmol/L | >15 pmol/L | |
| 5.0 | –0.7 [−2.4; 1.1] | –2.3 [−3.9; −0.7] | –4.0 [−5.4; −2.5] |
| 7.5 | 1.0 [−0.7; 2.7] | 0.7 [−2.1; 0.8] | –2.3 [-3.7; −0.9] |
| 10.0 | 2.7 [1.2; 4.1] | 1.0 [−0.1; 2.0] | 0.7 [−1.7; 0.5] |
| 12.5 | 4.3 [l.9; 6.7] | 2.7 [0.3; 5.1] | 1.0 [−1.0; 3.0] |
| 15.0 | 5.9 [3.6; 8.4] | 4.3 [1.9; 6.7] | 2.7 [0.7; 4.7] |
fT 4 concentrations are presented as mean [95% CI]. Minimal initial fT 4 deficit: difference between −2 SD value of the 95% CI of the fT 4 SSC range of the whole cohort, i.e., 16.6 pmol/L (1.28 ng/dL), and individual pre-treatment fT 4. Fields with bold figures: increased risk of overtreatment, i.e., fT 4 concentration >+2 SDS, relative to the individual SSCs of the patients. Reprinted from Bongers et al. [11] with permission from the authors.
In conclusion, not only should the prevention of cognitive damage be considered a major aim of CH screening and treatment, but also the prevention of neuropsychological damage. Although clinically relevant behavioural problems were only found in a limited number of our patients, mainly due to the small sample size of our cohort, it seems relevant to screen all CH patients on behavioural problems. Our study is the first to show that both early over- and undertreatment may lead to permanent and distinct behavioural problems: early overtreatment to ADHD and early undertreatment to ASD. The clinical impact of our findings is obvious: adequate treatment may prevent serious behavioural complications. Future studies will have to confirm this.
Statement of Ethics
The study was approved by the privacy committee of the CH Screening Board and by the Erasmus MC ethics review board. Written informed consent was obtained from all parents.
Disclosure Statement
None of the authors have competing financial interests.
Funding Sources
This study was financially supported by unrestricted grants from the Theia Foundation, Jan Dekker & Ludgardine Bouwman Foundation, and Zilveren Kruis.
Author Contributions
Dr. de Muinck Keizer-Schrama and Dr. Bongers-Schokking conceived the study and were responsible for data collection and analysis. Prof. de Rijke was involved in the biochemical aspects of the study. Prof. Resing conducted the psychological testing and evaluation of the patients. Dr. Oostdijk was, as paediatric endocrinologist, involved in the treatment of the children. All members of the team participated in writing the paper.
Acknowledgements
We are indebted to our patients and their parents for their cooperation in this long study. We also want to thank the participating paediatricians for their willingness to make their patients files available to us.
References
- 1.Hauser P, Zametkin AJ, Martinez P, Vitiello B, Matochik JA, Mixson AJ, et al. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993 Apr;328((14)):997–1001. doi: 10.1056/NEJM199304083281403. [DOI] [PubMed] [Google Scholar]
- 2.Rovet JF. Congenital hypothyroidism: long-term outcome. Thyroid. 1999 Jul;9((7)):741–8. doi: 10.1089/thy.1999.9.741. [DOI] [PubMed] [Google Scholar]
- 3.Álvarez M, Iglesias Fernández C, Rodríguez Sánchez A, Dulín Lñiguez E, Rodríguez Arnao MD. Episodes of overtreatment during the first six months in children with congenital hypothyroidism and their relationships with sustained attention and inhibitory control at school age. Horm Res Paediatr. 2010;74((2)):114–20. doi: 10.1159/000313370. [DOI] [PubMed] [Google Scholar]
- 4.Kooistra L, Vulsma T, van der Meere J. An investigation of impulsivity in children with early-treated congenital hypothyroidism. Dev Neuropsychol. 2004;26((2)):595–610. doi: 10.1207/s15326942dn2602_4. [DOI] [PubMed] [Google Scholar]
- 5.Kooistra L, Stemerdink N, van der Meere J, Vulsma T, Kalverboer AF. Behavioural correlates of early-treated congenital hypothyroidism. Acta Paediatr. 2001 Oct;90((10)):1141–6. doi: 10.1080/080352501317061549. [DOI] [PubMed] [Google Scholar]
- 6.Román GC, Ghassabian A, Bongers-Schokking JJ, Jaddoe VW, Hofman A, de Rijke YB, et al. Association of gestational maternal hypothyroxinemia and increased autism risk. Ann Neurol. 2013 Nov;74((5)):733–42. doi: 10.1002/ana.23976. [DOI] [PubMed] [Google Scholar]
- 7.Rovet JF, Ehrlich RM, Sorbara DL. Effect of thyroid hormone level on temperament in infants with congenital hypothyroidism detected by screening of neonates. J Pediatr. 1989 Jan;114((1)):63–8. doi: 10.1016/s0022-3476(89)80602-x. [DOI] [PubMed] [Google Scholar]
- 8.Bongers-Schokking JJ, de Ridder MA, de Rijke YB, de Muinck Keizer-Schrama SM. Experience in treating congenital hypothyroidism: implications regarding free T4 and TSH steady state concentrations during optimal Levothyroxine treatment. Thyroid. 2013;23:160–5. doi: 10.1089/thy.2011.0262. [DOI] [PubMed] [Google Scholar]
- 9.Bongers-Schokking JJ, Resing WC, de Rijke YB, de Ridder MA, de Muinck Keizer-Schrama SM. Cognitive development in congenital hypothyroidism: is overtreatment a greater threat than undertreatment? J Clin Endocrinol Metab. 2013 Nov;98((11)):4499–506. doi: 10.1210/jc.2013-2175. [DOI] [PubMed] [Google Scholar]
- 10.Bongers-Schokking JJ, Koot HM, Wiersma D, Verkerk PH, de Muinck Keizer SM. Influence of timing and dose of thyroid hormone replacement on mental, psychomotor, and behavioral development in infants with congenital hypothyroidism. J Pediatr. 2000;136:292–7. doi: 10.1067/mpd.2000.103351. [DOI] [PubMed] [Google Scholar]
- 11.Bongers-Schokking JJ, de Muinck Keizer-Schrama SM. Influence of timing and dose of thyroid hormone replacement on mental, psychomotor, and behavioral development in children with congenital hypothyroidism. J Pediatr. 2005 Dec;147((6)):768–74. doi: 10.1016/j.jpeds.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Bongers-Schokking JJ, Resing WC, Oostdijk W, de Rijke YB, de Muinck Keizer-Schrama SM. Individualized treatment to optimize eventual cognitive outcome in congenital hypothyroidism. Pediatr Res. 2016 Dec;80((6)):816–23. doi: 10.1038/pr.2016.159. [DOI] [PubMed] [Google Scholar]
- 13.Léger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, et al. Butler; on behalf of ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE, and the Congenital Hypothyroidism Conference Group. Consensus guidelines on screening, diagnosis, and management of Congenital hypothyroidism. JCEM. 2014;99:363–84. doi: 10.1159/000358198. [DOI] [PubMed] [Google Scholar]
- 14.Achenbach TM. Burlington (VT): University of Vermont, Department of Psychiatry; 1991. Manual for the Child Behavior Checklist/4-18, YSR, and TRF profiles. [Google Scholar]
- 15.Achenbach TM. Burlington (VT): University of Vermont, Department of Psychiatry; 1991. Manual for the Teacher's Report Form and 1991 TRF profile. [Google Scholar]
- 16.Friedrichsen S, Christ S, Heuer H, Schäfer MK, Mansouri A, Bauer K, et al. Regulation of iodothyronine deiodinases in the Pax8-/- mouse model of congenital hypothyroidism. Endocrinology. 2003 Mar;144((3)):777–84. doi: 10.1210/en.2002-220715. [DOI] [PubMed] [Google Scholar]
- 17.Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab. 2002 Mar;87((3)):1068–72. doi: 10.1210/jcem.87.3.8165. [DOI] [PubMed] [Google Scholar]
- 18.Biersack HJ, Hartmann F, Rödel R, Reinhardt M. Long term changes in serum T4, T3, and TSH in benign thyroid disease: proof of a narrow individual variation. Nucl Med (Stuttg) 2004 Oct;43((5)):158–60. doi: 10.1267/nukl04050158. [DOI] [PubMed] [Google Scholar]
- 19.Karmisholt J, Andersen S, Laurberg P. Variation in thyroid function tests in patients with stable untreated subclinical hypothyroidism. Thyroid. 2008 Mar;18((3)):303–8. doi: 10.1089/thy.2007.0241. [DOI] [PubMed] [Google Scholar]
- 20.Warnick EM, Bracke MB, Kasl S. Screening efficiency of the CBCL and strengths and difficulties questionnaire: a systematic review. Child Adolesc Ment Health. 2007;13:140–7. doi: 10.1111/j.1475-3588.2007.00461.x. [DOI] [PubMed] [Google Scholar]
- 21.Rovet JF, Hepworth SL. Dissociating attention deficits in children with ADHD and congenital hypothyroidism using multiple CPTs. J Child Psychol Psychiatry. 2001 Nov;42((8)):1049–56. doi: 10.1111/1469-7610.00804. [DOI] [PubMed] [Google Scholar]
- 22.Mazefski CA, Anderson R, Connor CM, Minshew N. Child Behavior Checklist for school-aged children with autism: preliminary evidence of pattern suggesting the need of referral. J Psychopathol Behav Assess. 2011;3((1)):31–7. doi: 10.1007/s10862-010-9198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshiko S, Grether JK, Windham GC, Smith D, Fessel K. Are thyroid hormone concentrations at birth associated with subsequent autism diagnosis? Autism Res. 2011 Dec;4((6)):456–63. doi: 10.1002/aur.219. [DOI] [PubMed] [Google Scholar]
- 24.Hulse JA, Grant DB, Jackson D, Clayton BE. Growth, development, and reassessment of hypothyroid infants diagnosed by screening. Br Med J (Clin Res Ed) 1982 May;284((6327)):1435–7. doi: 10.1136/bmj.284.6327.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.New England Congenital Hypothyroidism Collaborative Characteristics of infantile hypothyroidism discovered on neonatal screening. J Pediatr. 1984 Apr;104((4)):539–44. doi: 10.1016/s0022-3476(84)80543-0. [DOI] [PubMed] [Google Scholar]
- 26.Bongers-Schokking JJ, Colon EJ, Mulder PG, Hoogland RA, de Groot CJ, Van den Brande JL. Influence of treatment on the maturation of the somesthetic pathway in infants with primary congenital hypothyroidism during the first year of life. Pediatr Res. 1993 Jul;34((1)):73–8. doi: 10.1203/00006450-199307000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Rovet JF. Congenital hypothyroidism: an analysis of persisting deficits and associated factors. Child Neuropsychol. 2002 Sep;8((3)):150–62. doi: 10.1076/chin.8.3.150.13501. [DOI] [PubMed] [Google Scholar]
- 28.Fisher DA, Schoen EJ, La Franchi S, Mandel SH, Nelson JC, Carlton EI, et al. The hypothalamic-pituitary-thyroid negative feedback control axis in children with treated congenital hypothyroidism. J Clin Endocrinol Metab. 2000 Aug;85((8)):2722–7. doi: 10.1210/jcem.85.8.6718. [DOI] [PubMed] [Google Scholar]