Abstract
Background
Intradialytic hypotension (IDH) is a common complication of haemodialysis (HD) and associated with adverse outcomes, especially when a nadir definition (systolic blood pressure <90 mm Hg) is used. The pathogenesis of IDH is directly linked to the discontinuous nature of the HD treatment, in combination with patient-related factors such as age, diabetes mellitus and cardiac failure.
Summary
Although the decline in blood volume due to removal of fluid by ultrafiltration is the prime mover, thermally induced reflex vasodilation compromises the haemodynamic response to hypovolemia. Recent studies have stressed the relevance of changes in tissue perfusion during HD, which may translate in long-term organ damage. Monitoring changes in tissue perfusion, for which emerging evidence becomes available, appears to have great promise in the fine-tuning of the dialysis procedure.
Key Messages
While it is unlikely that IDH can be completely prevented, reduction in inter-dialytic weight gain, prevention of an increase in core temperature by adjusting the dialysate temperature and more frequent or prolonged dialysis treatment remain cornerstones in providing a more comfortable and safe treatment.
Keywords: Dialysis, Prevention, New insights, Hypotension
Introduction
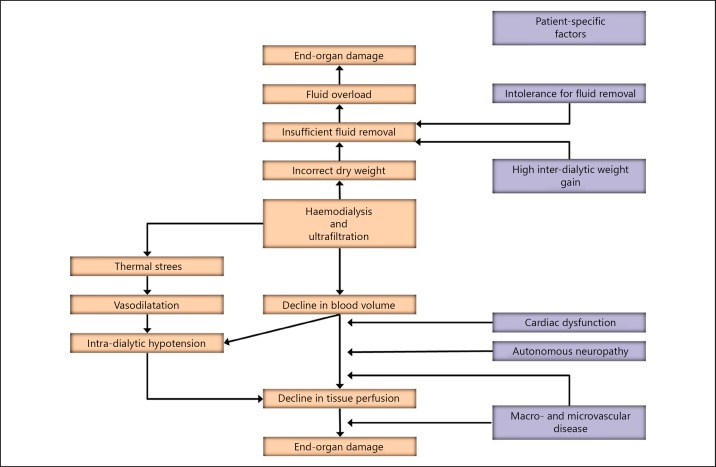
Intradialytic hypotension (IDH) is a frequent complication of haemodialysis (HD) sessions. Depending on the definitions, IDH is reported to occur between 5and 30% also based on different definitions in varying patient populations [1]. In a recent study assessing 44,801 treatments in 1,137 patients, in which IDH was defined as a decrease in systolic blood pressure (SBP) by more than 30 mm Hg to a level of less than 90 mm Hg, the incidence was 17.2% [2]. In a study in the HEMO population, the incidence of IDH was 9.6% when the Kidney Disease Outcomes Quality Initiative definition (decline in SBP >20 mm Hg including symptoms) was applied, and 11.3% when a nadir (SBP <90 mm Hg) was used [3]. Next to patient discomfort, IDH is also associated with adverse outcomes [2, 3]. Therefore, insight in its mechanisms and consequences of IDH has emerged. The aim of this paper is to project novel insights on the pathogenesis of IDH (Fig. 1) in the perspective of established literature.
Fig. 1.
Novel insights into the pathogenesis of intra-dialytic hypotension.
Pathophysiology of IDH: Patient-Related Factors
The discontinuous nature of HD, in which fluid that accumulates during one week is generally removed in 12 h, yields a significant burden on the cardiovascular system of the patient. Dialysis patients may be more sensitive to a decline in blood volume as compared to a healthy population, in whom a decline in SBP is usually associated with a fall in blood volume of 20% or more [4, 5]. Both patient- as well as treatment-related factors play a role in the pathogenesis of IDH. A recent multicentre study found an association between IDH with factors such as age, female gender, diabetes mellitus, Hispanic origin, longer dialysis vintage, higher body mass index and lower pre-dialytic SBP [2]. Also, the presence of cardiac dysfunction [6] and autonomous neuropathy [7] can increase the vulnerability for the haemodynamic effects of HD [1, 8].
Pathophysiology of IDH: Treatment-Related Factors
Next to unmodifiable patient-related factors, treatment-related factors also play a major role in the pathogenesis of IDH. The decline in blood volume, induced by ultrafiltration, is the prime mover. In a study in hypotension-prone patients, the mean decline in relative blood volume (RBV) before the occurrence of IDH was 12.3% [4], although changes in RBV may underestimate changes in absolute blood volume due to the refill of blood with a lower haematocrit from the microcirculation [9, 10]. While a high ultrafiltration rate is a major risk factor for IDH [2], its effect on changes in RBV is highly variable between patients, likely depending on the refill of plasma volume from the interstitial compartment [10, 11].
Ultrafiltration can lead to a decline in blood pressure because of a reduction in central blood volume and cardiac output [12]. In a recent study, we observed a fall in cardiac output of −1.4 ± 1.5 L/min during 4 h of HD with a decline in RBV of 8.1 ± 1.5%. [13]. However, even despite a fall in cardiac output, blood pressure can still be maintained if systemic vascular resistance rises appropriately. In previous years, we and others have extensively studied the relation between the extracorporeal energy balance and the haemodynamic response during dialysis [14, 15, 16]. In short, an increase in core temperature during conventionally used dialysate temperatures (such as 37–37.5°C) may lead to the redistribution of blood volume to the vasodilated skin vessels and counteracts the normal response to hypovolemia. Although the exact magnitude of this redistribution has not been precisely calculated, under severe heat stress skin blood flow can increase up to 7.5 till 8 L/min [17, 18]. In addition to this, dilation of the cutaneous veins can lead to the pooling of “unstressed” blood volume, impairing the redistribution to the central blood volume compartment [12, 19]. These observations have direct therapeutic consequences as the fall in RBV can be mitigated by longer dialysis sessions [13], thereby improving vascular reactivity and blood pressure response by reducing the temperature of the dialysate [16, 20].
A Fall in Blood Pressure during Dialysis Is Not Always Detrimental
Blood pressure usually falls during HD. In the HEMO cohort, a decline of SBP of 20 mm Hg or more was observed in 68% of treatments, and a decline in SBP of 30 mm Hg or more in 51%. Although both were associated with increased mortality in unadjusted analysis, this relation lost statistical significance after correction for confounders, unless accompanied by a nadir SBP of 90 mm Hg or lower [3]. In contrast, an increase in blood pressure during dialysis is also associated with increased mortality [21].
The same type of apparently paradoxical relations was observed for changes in RBV. Higher ultrafiltration rates, both at thresholds of 10 or 13 mL/kg/h, are associated with increased mortality; however, low declines in RBV are also associated with adverse outcomes. A decline in RBV slope below the median of 1.35%/h was associated with an increased risk in mortality [22], which is likely related to the expansion of the interstitial fluid compartment, leading to increased refill of plasma volume. Moreover, higher slopes of RBV decline were associated with an increase in arterial oxygenation, suggesting a beneficial effect on pulmonary oxygen exchange due to fluid removal [10]. Therefore, it might be suggested that higher ultrafiltration rates may be associated with increased mortality because of impaired tissue perfusion, and because of the accompanied patient characteristics, a low decline in blood volume or a rise in SBP may be associated with adverse outcomes because of concomitant fluid overload in these patients [22, 23]. In a recent study, we noticed that the prognostic value of changes in SBP was related to the pre-dialytic blood pressure. While in patients with low SBP a decline was associated with an adverse outcome, the opposite was observed in patients with high pre-dialytic SBP, which appears in line with the pathophysiologic considerations. Therefore, most likely not the fall in blood pressure per se is detrimental but the associated changes in tissue perfusion.
Effects of Dialysis on Tissue Perfusion
Tissue perfusion is expressed at blood flow rates per 100 mL of tissue and is expressed by the formula Q = P/R (Q = flow, P = pressure, R = resistance). Therefore, a decline in tissue perfusion may occur when a decline in pressure is not accompanied by regional autoregulatory vasodilation or when the decline in pressure is too high. Moreover, in case of proximal stenosis or in case of microcirculatory alterations, which have been described in cardiac tissue of uremic patients and animals [24], even a relatively small decline in systemic blood pressure might lead to an impaired tissue perfusion. Alternatively, for example, in case of left ventricular hypertrophy, the increased oxygen demand may make the cardiac tissue more vulnerable for a decline in blood pressure [25]. Also, even in non-uremic subjects, changes in tissue perfusion may already occur when cardiac output declines before a change in blood pressure is already apparent [5].
These factors may explain why even in the presence of relatively small changes in blood pressure, HD is associated with a reduction in tissue perfusion in vital organs, such as the heart and brain, which is of importance, given the relation between temporary perfusion deficits and persistent end-organ damage [26, 27, 28, 29, 30]. However, the risk of myocardial stunning appears to be even greater when HD is accompanied by a significant decline in blood pressure [31]. Moreover, the splanchnic region, and especially the gut mucosa, is especially sensitive for tissue hypoperfusion because of high α-adrenergic activity [5]. In 9 patients with acute renal failure, despite a stable blood pressure, cardiac output declined from 3.0 to 2.7 mL/min/m2 and was accompanied by a reduction in splanchnic but not femoral blood flow, as measured by dye dilution. These changes were also accompanied by an increase in tumor necrosis factor alfa [32], potentially indicating the translocation of bacterial fragments. As it is true for the blood pressure response, cooling or individualizing of dialysate has also been shown to reduce hypoperfusion-related complications such as cardiac stunning [33] and resulted in less white matter lesions in the long term [29]. In addition, more frequent HD also resulted in a reduction in myocardial stunning [33]. Recent developments, notably Sidestream Dark Field imaging, a microscopic technique using polarised light to visualise erythrocytes passing through sublingual capillaries, have enabled the direct visualization of the microcirculation during HD and observed a decline in perfused vessel density during HD. This was not related to changes in blood pressure, showing that microcirculatory changes can occur, which are unnoticed by changes in macrovascular parameters [34].
Changes in Perfusion Are Important, But How Can They Be Measured Routinely?
While studies on regional changes in tissue perfusion, or change in microcirculation during dialysis have yielded highly important results for pathophysiologic understanding of the haemodynamic effects of HD, they are unsuitable for routine clinical applications on a treatment-to-treatment basis. For this purpose, changes in central venous oxygen saturation (ScvO2), which can be assessed in patients treated with a central venous catheter, is an integrated parameter influenced by cardiac output, tissue oxygen delivery and oxygen extraction, predominantly of the upper body [35]. While ScvO2 above 70% is considered optimal, most HD patients already start the dialysis treatment with substantially lower values [35, 36]. ScvO2 is influenced by ultrafiltration rate [36], whereas the change may be different in patients prone to IDH. In a study in 11 hypotension-prone and 9 stable HD patients, ScvO2 dropped by 7.7% ± 1.7 in hypotension prone patients, in contrast to a rise of 1.0 ± 1.3% in the stable group. It has also been suggested that continuous assessment of arterial oxygen saturation (SaO2), in patients with arteriovenous fistulae, or ScvO2 could both be useful in the early prediction of IDH [37]. Future studies should be performed to investigate whether treatment guided by changes in ScvO2 would be of clinical benefit to the patient in preventing IDH. A drawback of ScvO2 is that it is only available for patients with a central venous catheter. Easily applicable, non-invasive techniques assessing changes in tissue perfusion would therefore be a great asset for the fine-tuning of the HD treatment. Near infrared spectroscopy (NIRS) could be an interesting technique for this purpose. NIRS operates by the same principle as pulse oximetry with the difference that while the former measures only oxyhaemoglobin, NIRS measures the difference between oxyhaemoglobin and deoxygenated haemoglobin [38]. Recently, this method was applied in intensive care unit patients with acute renal failure [39], but to the best of our knowledge not yet in the haemodynamic monitoring of chronic HD patients. A recent study used an older tool, transcutaneous pO2 (TcpO2) monitoring, in the assessment of changes in tissue perfusion in the lower limb. While critical ischemia, defined as TcpO2 <10 mm Hg, occurred only in patients with macrocirculatory abnormalities, 47% of the 50 patients experienced severe ischemia, defined as TcpO2 <30 mm Hg, during a conventional HD procedure. Overall, TcpO2 declined from 45.7 ± 15.5 vs. 33.5 ± 15.6 mm Hg. This tissue again shows that significant changes in tissue perfusion may accompany routine HD treatments [40].
Prevention
The fact that HD is an intermittent technique is makes it unlikely that IDH can be completely prevented. Therefore, it is of utmost importance to find an optimal balance between prevention of fluid overload on one hand and haemodynamic tolerance on the other, which can be very difficult to achieve using thrice weekly treatments. While prevention of interdialytic weight gain by sodium restriction is a cornerstone of treatment [41], the dilemma between too rapid fluid removal and persistent fluid overload may in some cases be solved only by increasing dialysis time and/or frequency [42]. As preventive methods have been discussed in detail both in older and more recent literature [8, 23], we will focus here on recently published trials. Antlanger et al. [43] randomized fluid overloaded patients, defined as an overhydration/extracellular water ratio above 15%, to a reduction in dry weight using conventional strategies, to ultrafiltration-dialysate conductivity controlled feedback modelling and to ultrafiltration-temperature controlled feedback. The latter strategy was associated with the lowest incidence of intradialytic morbid events (21%) as compared to conventional and ultrafiltration-conductivity feedback (34 and 39%) respectively. However, it also shows that dry weight reduction even in fluid overloaded patients may be associated with a significant percentage in intradialytic morbid events and should therefore be undertaken gradually. Also, online haemodiafiltration (HDF) was associated with a reduction in IDH [44], which is possibly related to its thermal effects [45]. However, the exact role of online HDF in the prevention of IDH has not been definitely established, as a recent study paradoxically observed an increase in IDH with online HDF as compared to high flux HD [46]. Until now, most solid evidence regarding modifiable effects of the HD treatment in the prevention of IDH appears to be obtained from studies addressing time, frequency of treatments and temperature of dialysate.
Conclusion
IDH, due to the discontinuous nature of the HD procedure is a common complication, which is associated with adverse outcomes. Increasing evidence suggests that not the decline in blood pressure per se, but rather associated changes in tissue perfusion are related to short- or long-term complications in vital organs such as heart, gut and brain. Therefore, routine online measurement of tissue perfusion would be a great asset in the prevention of dialysis-associated morbidities. At present, reducing interdialytic weight gain, extending dialysis time and/or frequency, and preventing thermally induced reflex vasodilation are functions that remain cornerstones in the prevention of IDH.
Disclosure Statement
There is nothing to disclosure.
References
- 1.Chou JA, Kalantar-Zadeh K, Mathew AT. A brief review of intradialytic hypotension with a focus on survival. Semin Dial. 2017;30:473–480. doi: 10.1111/sdi.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sands JJ, Usvyat LA, Sullivan T, Segal JH, Zabetakis P, Kotanko P, et al. Intradialytic hypotension: frequency, sources of variation and correlation with clinical outcome. Hemodial Int. 2014;18:415–422. doi: 10.1111/hdi.12138. [DOI] [PubMed] [Google Scholar]
- 3.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26:724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barth C, Boer W, Garzoni D, Kuenzi T, Ries W, Schaefer R, et al. Characteristics of hypotension-prone haemodialysis patients: is there a critical relative blood volume? Nephrol Dial Transplant. 2003;18:1353–1360. doi: 10.1093/ndt/gfg171. [DOI] [PubMed] [Google Scholar]
- 5.Kreimeier U. Pathophysiology of fluid imbalance. Crit Care. 2000;4((suppl 2)):S3–S7. doi: 10.1186/cc968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Sande FM, Mulder AW, Hoorntje SJ, Peels KH, van Kuijk WH, Kooman JP, et al. The hemodynamic effect of different ultrafiltration rates in patients with cardiac failure and patients without cardiac failure: comparison between isolated ultrafiltration and ultrafiltration with dialysis. Clin Nephrol. 1998;50:301–308. [PubMed] [Google Scholar]
- 7.Shafi T, Mullangi S, Jaar BG, Silber H. Autonomic dysfunction as a mechanism of intradialytic blood pressure instability. Semin Dial. 2017;30:537–544. doi: 10.1111/sdi.12635. [DOI] [PubMed] [Google Scholar]
- 8.Kooman J, Basci A, Pizzarelli F, Canaud B, Haage P, Fouque D, et al. EBPG guideline on haemodynamic instability. Nephrol Dial Transplant. 2007;22((suppl 2)):ii22–ii44. doi: 10.1093/ndt/gfm019. [DOI] [PubMed] [Google Scholar]
- 9.Mitra S, Chamney P, Greenwood R, Farrington K. The relationship between systemic and whole-body hematocrit is not constant during ultrafiltration on hemodialysis. J Am Soc Nephrol. 2004;15:463–469. doi: 10.1097/01.asn.0000108970.48370.33. [DOI] [PubMed] [Google Scholar]
- 10.Anand S, Sinha AD, Agarwal R. Determinants and short-term reproducibility of relative plasma volume slopes during hemodialysis. Clin J Am Soc Nephrol. 2012;7:1996–2001. doi: 10.2215/CJN.04190412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de los Reyes VA, Fuertinger DH, Kappel F, Meyring-Wosten A, Thijssen S, Kotanko P. A physiologically based model of vascular refilling during ultrafiltration in hemodialysis. J Theor Biol. 2016;390:146–155. doi: 10.1016/j.jtbi.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Beerenhout C, Dejagere T, van der Sande FM, Bekers O, Leunissen KM, Kooman JP. Haemodynamics and electrolyte balance: a comparison between on-line pre-dilution haemofiltration and haemodialysis. Nephrol Dial Transplant. 2004;19:2354–2359. doi: 10.1093/ndt/gfh315. [DOI] [PubMed] [Google Scholar]
- 13.Cornelis T, van der Sande FM, Eloot S, Cardinaels E, Bekers O, Damoiseaux J, et al. Acute hemodynamic response and uremic toxin removal in conventional and extended hemodialysis and hemodiafiltration: a randomized crossover study. Am J Kidney Dis. 2014;64:247–256. doi: 10.1053/j.ajkd.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 14.van der Sande FM, Wystrychowski G, Kooman JP, Rosales L, Raimann J, Kotanko P, et al. Control of core temperature and blood pressure stability during hemodialysis. Clin J Am Soc Nephrol. 2009;4:93–98. doi: 10.2215/CJN.01800408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneditz D. Temperature and thermal balance in hemodialysis. Semin Dial. 2001;14:357–364. doi: 10.1046/j.1525-139x.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- 16.van der Sande FM, Rosales LM, Brener Z, Kooman JP, Kuhlmann M, Handelman G, et al. Effect of ultrafiltration on thermal variables, skin temperature, skin blood flow, and energy expenditure during ultrapure hemodialysis. J Am Soc Nephrol. 2005;16:1824–1831. doi: 10.1681/ASN.2004080655. [DOI] [PubMed] [Google Scholar]
- 17.Santelli J, Sullivan JM, Czarnik A, Bedolla J. Heat illness in the emergency department: keeping your cool. Emerg Med Pract. 2014;16:1–21. quiz 21–22. [PubMed] [Google Scholar]
- 18.Rowell LB. Reflex control of the cutaneous vasculature. J Invest Dermatol. 1977;69:154–166. doi: 10.1111/1523-1747.ep12497938. [DOI] [PubMed] [Google Scholar]
- 19.Kooman JP, Gladziwa U, Bocker G, van Bortel LM, van Hooff JP, Leunissen KM. Role of the venous system in hemodynamics during ultrafiltration and bicarbonate dialysis. Kidney Int. 1992;42:718–726. doi: 10.1038/ki.1992.339. [DOI] [PubMed] [Google Scholar]
- 20.Selby NM, McIntyre CW. A systematic review of the clinical effects of reducing dialysate fluid temperature. Nephrol Dial Transplant. 2006;21:1883–1898. doi: 10.1093/ndt/gfl126. [DOI] [PubMed] [Google Scholar]
- 21.Inrig JK, Patel UD, Toto RD, Szczech LA. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis. 2009;54:881–890. doi: 10.1053/j.ajkd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal R. Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension. 2010;56:512–517. doi: 10.1161/HYPERTENSIONAHA.110.154815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assimon MM, Flythe JE. Intradialytic blood pressure abnormalities: the highs, the lows and all that lies between. Am J Nephrol. 2015;42:337–350. doi: 10.1159/000441982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amann K, Ritz E. Microvascular disease - the Cinderella of uraemic heart disease. Nephrol Dial Transplant. 2000;15:1493–1503. doi: 10.1093/ndt/15.10.1493. [DOI] [PubMed] [Google Scholar]
- 25.Kronenberg MW, Cohen GI, Leonen MF, Mladsi TA, Di Carli., MF Myocardial oxidative metabolic supply-demand relationships in patients with nonischemic dilated cardiomyopathy. J Nucl Cardiol. 2006;13:544–553. doi: 10.1016/j.nuclcard.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, de Jong PE, et al. Hemodialysis-induced regional left ventricular systolic dysfunction: prevalence, patient and dialysis treatment-related factors, and prognostic significance. Clin J Am Soc Nephrol. 2012;7:1615–1623. doi: 10.2215/CJN.00850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasselaar JJ, Slart RH, Knip M, Pruim J, Tio RA, McIntyre CW, et al. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant. 2009;24:604–610. doi: 10.1093/ndt/gfn501. [DOI] [PubMed] [Google Scholar]
- 28.McIntyre CW, Odudu A. Hemodialysis-associated cardiomyopathy: a newly defined disease entity. Semin Dial. 2014;27:87–97. doi: 10.1111/sdi.12197. [DOI] [PubMed] [Google Scholar]
- 29.Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol. 2015;26:957–965. doi: 10.1681/ASN.2013101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakob SM, Ruokonen E, Vuolteenaho O, Lampainen E, Takala J. Splanchnic perfusion during hemodialysis: evidence for marginal tissue perfusion. Crit Care Med. 2001;29:1393–1398. doi: 10.1097/00003246-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning) Clin J Am Soc Nephrol. 2011;6:1326–1332. doi: 10.2215/CJN.05200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meinders AJ, Nieuwenhuis L, Ince C, Bos WJ, Elbers PW. Haemodialysis impairs the human microcirculation independent from macrohemodynamic parameters. Blood Purif. 2015;40:38–44. doi: 10.1159/000380902. [DOI] [PubMed] [Google Scholar]
- 35.Campos I, Chan L, Zhang H, Deziel S, Vaughn C, Meyring-Wosten A, et al. Intradialytic hypoxemia in chronic hemodialysis patients. Blood Purif. 2016;41:177–187. doi: 10.1159/000441271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harrison LE, Selby NM, McIntyre CW. Central venous oxygen saturation: a potential new marker for circulatory stress in haemodialysis patients? Nephron Clin Pract. 2014;128:57–60. doi: 10.1159/000362557. [DOI] [PubMed] [Google Scholar]
- 37.Mancini E, Perazzini C, Gesualdo L, Aucella F, Limido A, Scolari F, et al. Intra-dialytic blood oxygen saturation (SO2): association with dialysis hypotension (the SOGLIA study) J Nephrol. 2016;30:811–819. doi: 10.1007/s40620-016-0346-x. [DOI] [PubMed] [Google Scholar]
- 38.Green MS, Sehgal S, Tariq R. Near-infrared spectroscopy: the new must have tool in the intensive care unit? Semin Cardiothorac Vasc Anesth. 2016;20:213–224. doi: 10.1177/1089253216644346. [DOI] [PubMed] [Google Scholar]
- 39.Pipili C, Vasileiadis I, Grapsa E, Tripodaki ES, Ioannidou S, Papastylianou A, et al. Microcirculatory alterations during continuous renal replacement therapy in ICU: a novel view on the ‘dialysis trauma' concept. Microvasc Res. 2016;103:14–18. doi: 10.1016/j.mvr.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Benhamou Y, Begarin L, David N, Cailleux N, Bessin C, Levesque H, et al. Detection of microcirculatory impairment by transcutaneous oxymetry monitoring during hemodialysis: an observational study. BMC Nephrol. 2014;15:4. doi: 10.1186/1471-2369-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ok E, Levin NW, Asci G, Chazot C, Toz H, Ozkahya M. Interplay of volume, blood pressure, organ ischemia, residual renal function, and diet: certainties and uncertainties with dialytic management. Semin Dial. 2017;30:420–429. doi: 10.1111/sdi.12612. [DOI] [PubMed] [Google Scholar]
- 42.Morfin JA, Fluck RJ, Weinhandl ED, Kansal S, McCullough PA, Komenda P. Intensive hemodialysis and treatment complications and tolerability. Am J Kidney Dis. 2016;68:S43–S50. doi: 10.1053/j.ajkd.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Antlanger M, Josten P, Kammer M, Exner I, Lorenz-Turnheim K, Eigner M, et al. Blood volume-monitored regulation of ultrafiltration to decrease the dry weight in fluid-overloaded hemodialysis patients: a randomized controlled trial. BMC Nephrol. 2017;18:238. doi: 10.1186/s12882-017-0639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maduell F, Moreso F, Pons M, Ramos R, Mora-Macia J, Carreras J, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–497. doi: 10.1681/ASN.2012080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinney JH, Oates T, Davenport A. Haemodiafiltration does not reduce the frequency of intradialytic hypotensive episodes when compared to cooled high-flux haemodialysis. Nephron Clin Pract. 2011;119:c138–c144. doi: 10.1159/000324428. [DOI] [PubMed] [Google Scholar]
- 46.Smith , JR, Zimmer N, Bell E, Francq BG, McConnachie A, Mactier R. A randomized, single-blind, crossover trial of recovery time in high-flux hemodialysis and hemodiafiltration. Am J Kidney Dis. 2017;69:762–770. doi: 10.1053/j.ajkd.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]