Abstract
Background
In many rural areas of tropical countries such as Indonesia, the prevalence of soil-transmitted helminths (STH) infections remains high. At the same time, the burden of allergic disorders in such rural areas is reported to be low and inversely associated with helminth infections. To reduce the morbidity and transmission of helminth infections, the world health organization recommends preventive treatment of school children by providing mass drug administration (MDA) with albendazole. Here, we had an opportunity to evaluate the prevalence of skin reactivity to allergens before and after albendazole treatment to get an indication of the possible impact of MDA on allergic sensitization.
Methods
A study was conducted among 150 school children living in an area endemic for STH infections. Before and 1 year after anthelminthic treatment with albendazole, stool samples were examined for the presence of STH eggs, skin prick tests (SPT) for cockroach and house dust mites were performed, blood eosinophilia was assessed, and total immunoglobulin E (IgE) and C-reactive protein (CRP) were measured in plasma.
Results
Anthelminthic treatment significantly reduced the prevalence of STH from 19.6 before treatment to 6% after treatment (p < 0.001). Levels of total IgE (estimate: 0.30; 95% CI 0.22–0.42, p < 0.0001), CRP (estimate: 0.60; 95% CI 0.42–0.86, p = 0.006), and eosinophil counts (estimate: 0.70; 95% CI 0.61–0.80, p < 0.001) decreased significantly. The prevalence of SPT positivity increased from 18.7 to 32.7%. Multivariate analysis adjusted for confounding factors showed an increased risk of being SPT positive to any allergen (OR 3.04; 95% CI 1.338–6.919, p = 0.008).
Conclusions
This study indicates that 1 year of MDA with albendazole was associated with a reduced prevalence of STH infections. This study shows that the prevalence of allergic sensitization increases after 1 year of albendazole treatment. Placebo-controlled and larger studies are needed to further substantiate a role of deworming treatment in an increased risk of allergic sensitization.
Keywords: Mass drug administration, Skin prick test, Soil-transmitted helminths, Allergens
Introduction
Allergic diseases are a major global health burden and their prevalence is increasing. Worldwide, 20–30% of the global population suffers from some form of allergy [1] with notably higher rates of such immune disorders in industrialized nations [2]. On a smaller scale, communities in low- and middle-income countries transitioning from rural to urban settings are also experiencing an increase in the frequency of allergic diseases [3].
According to the “hygiene hypothesis,” first coined by Strachan in 1989, the prevalence of allergic disease will increase if there is a decreased exposure to common infectious diseases in early life or a decreased diversity of infectious agents is encountered [4, 5, 6]. Epidemiological studies examining the relationships between helminth infections, which are highly prevalent in low-income countries, and allergic disorders have provided evidence for an inverse association between helminth infections and allergic disorders [3, 7, 8]. The strongest evidence for the association between helminth infections and allergy has been provided by small intervention studies that have shown increases in the prevalence or the risk of atopy after anthelmintic treatment of infected children [9, 10]. However, there are also studies showing no or a positive association between helminths and allergies, indicating that other factors, such as lifestyle, can play an essential role in the pathogenesis of allergies [11, 12, 13].
As in the case of allergic disorders, chronic helminth infections induce a strong T-helper 2 (Th2) immune response that leads to elevated levels of immunoglobulin E (IgE), eosinophils, basophils, and mast cells [8, 14]. However, unlike immune responses induced by allergens, helminth infections also lead to increased anti-inflammatory cytokines, such as interleukin (IL-10) and transforming growth factor-β [5, 16]. The anti-inflammatory environment induced by chronic helminth infections can spill over to third party antigens, thereby attenuating the responsiveness to “bystander antigens” including a decreased reactivity to allergens [17], and it thus results in a decreased prevalence or risk of atopy and allergic disorders.
Indonesia, a country in economic transition, is also experiencing a steady increase in the prevalence of allergic disorders [15, 18] in the face of a decreasing prevalence of helminth infections due to health education, changes in lifestyle, improvements in sanitation, and mass drug administration (MDA) [19]. As school-based MDA programs are increasingly advocated and implemented by the Indonesian government, there is a window of opportunity to investigate the effect of MDA with albendazole on the prevalence of SPT reactivity to allergens in school children in Indonesia.
Methods
Study Population and Design
This study was performed in an elementary school (SD Katolik Nangamboa) in the Nangapanda Village, Ende District, Flores Island, Indonesia. The study site was selected on the basis of a high prevalence of soil-transmitted helminths (STH) [20]. Data were collected in October 2015 and October 2016. Prior to this study, written informed consent was obtained from the parents/legal guardians of the children. Approval was obtained from the ethical committee of the Faculty of Medicine of the University of Indonesia (Jakarta, Indonesia; approval No. 194/PT2.FK/ETIK/2006 and 825/UN2.F1/ETIK/2016). In total, 150 children were included in this study.
Anthelminthic Treatment
As part of the school-based MDA program, school children were treated with a single dose (400 mg) of albendazole biannually in October 2015 and March 2016.
Data Collection
A standardized questionnaire was used to assess general population characteristics including date of birth and gender. The questionnaire was administered face-to-face by one researcher accompanied by a teacher.
Body height and weight were measured using a portable stadiometer (SECA model 213; Seca GmbH & Co., Hamburg, Germany) and a mobile flat scale (SECA model 876; Seca), respectively [21].
Infections with STH, Ascaris lumbricoides, Trichuris trichiura, and hookworm were examined via 2 Kato-Katz smears from 1 fresh stool sample [22].
Regarding allergy outcomes, SPT reactivity was examined against allergen extracts of cockroach (Blatella germanica) and 2 species of Dermatophagoides house dust mites (D. pteronyssinus and D. farinae; ALK-Abello BV, Almere, The Netherlands). Histamine chloride and allergen diluents were used as positive and negative controls, respectively. SPT was performed on the volar side of the lower arm and the results were measured after 15 min. SPT reactivity was considered positive if the longest diameter plus the perpendicular diameter of the wheal divided by 2 was at least 3 mm.
To assess eosinophil counts, a Giemsa-stained thin-blood smear was made from finger prick blood and eosinophil counts were expressed in percentages.
Levels of plasma C-reactive protein (CRP) were measured using commercial reagents (DuoSet ELISA, R&D Systems Europe, Ltd., Abingdon, UK), according to the manufacturers' protocol. In brief, mouse anti-human CRP capture antibody was coated overnight on MaxiSorp plates. Diluted capillary plasma (1: 10,000) was added to the wells and incubated at room temperature for 2 h, followed by a second biotinylated mouse anti-human CRP detection antibody. After washing, plates were incubated with streptavidin-HRP for 20 min. The colour was developed with tetramethylbenzidine and stopped with 10% H2SO4. A serial human CRP standard with a limit of detection of 15.6 pg/mL was included in each plate to calculate the CRP concentration of the samples. The results were expressed in milligrams per liter.
Total IgE levels were measured as previously described [23]. Briefly, MaxiSorp plates were coated overnight with rabbit anti-human IgE (Dako, Glostrup, Denmark). Plates were blocked with phosphate-buffered saline containing 5% bovine serum albumin (Albumin Fraction V; Boehringer, Mannheim, Germany). Capillary plasma was diluted (1: 100) in phosphate-buffered saline containing 0.05% Tween-20 and added to the wells. As a reference, the WHO standard of human serum IgE (NIBSC, Potters Bar, UK) was used, starting at a concentration of 90 IU/mL with a limit of detection of 0.04 IU/mL. Plates were incubated for 1 h and, after a washing step, IgE biotinylated goat anti-human IgE antibody (Vector Laboratories, Burlingame, CA, USA) was added and incubated for 1 h, followed by incubation with streptavidin alkaline phosphatase conjugate (Boehringer). The colour was developed by addition of para-nitrophenylphosphate (Boehringer) diluted in diethanolamine buffer, and optical density was measured at 405 nm. The results were expressed in International Units per milliliter.
Statistical Analysis
Descriptive data was analysed using IBM SPSS Statistics version 23 (IBM Inc., USA) and expressed as medians (IQR) or frequencies (% of collected data). Continuous data were log-transformed to obtain normally distributed variables. Changes in outcomes between 2 time points (before and after MDA) were assessed using linear mixed models with subject random effects. For continuous variables, parameter estimates (95% CI) were reported. The reported P values were obtained using a likelihood ratio test comparing the model with and without a time effect. For the binary outcomes, a logistic model was used with random subject effects. All models were fitted using the lme4 package (R software version 3.1.3) and the scripts are given in the online supplementary Methods (see www.karger.com/doi/10.1159/000490952 for all online suppl. material).
Results
Study Population
The baseline characteristics of the 150 participants are shown in Table 1. The age of the children ranged from 7 to 15 years, with a mean age of 10.1 years; 51.3% of the children were male. At baseline 19.6% of the children were infected with 1 or more helminth species; T. trichiura was the predominant species (11.2%), followed by A. lumbricoides (8.4%) and hookworm (4.9%). Among the 28 helminth-infected subjects, 7 (25%) had multiple helminth infections. The prevalence of SPT reactivity to any allergen at baseline was 18.7%, with 17.3% being positive to cockroach and only 2.7% being positive to any house dust mite (Table 1).
Table 1.
Characteristics of the study population at baseline
| Parameter | Value |
|---|---|
| Age, years | 10.1±1.5 |
| Sex | |
| Male | 77 (51.3) |
| Female | 73 (48.7) |
| STH infection (N = 143) | |
| Any STH | 28 (19.6) |
| A. lumbricoides | 12 (8.4) |
| T. trichiura | 16(11.2)) |
| Hookworm | 7 (4.9) |
| Skin prick reactivity | |
| Any allergen | 28 (18.7) |
| B. germanica (cockroach) | 26 (17.3) |
| House dust mite | 4 (2.7) |
| D. pteronyssinus | 4 (2.7) |
| D. farinae | 3 (2.0) |
Values are presented as numbers (%) or means ± SD. The total number of patients was 150 unless otherwise stated.
Prevalence of STH Infection after MDA
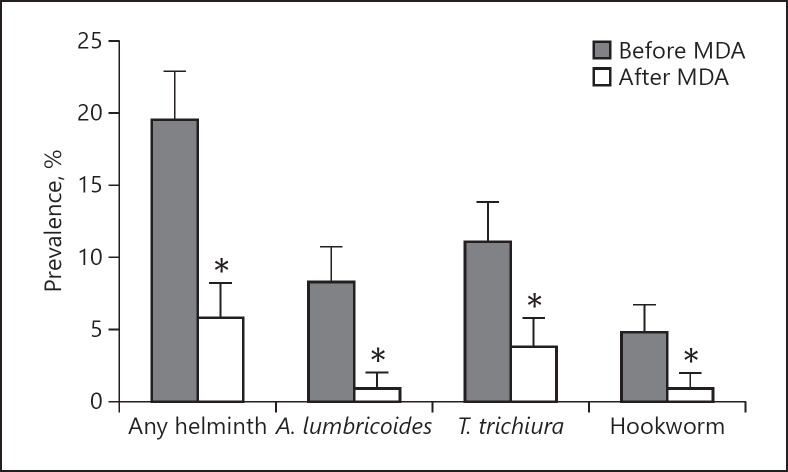
One year after MDA, 113 out of 150 children returned for follow-up and stool samples were available from 102 children. Following anthelminthic treatment, the prevalence of STH infections decreased significantly (Fig. 1). For any helminth, the prevalence decreased from 19.6 to 5.9%, for A. lumbricoides it decreased from 8.4 to 1.0%, for T. trichiura it decreased from 11.2 to 3.9%, and for hookworm it decreased from 4.9 to 1.0% (all p < 0.001).
Fig. 1.
Helminth prevalence before and after MDA with albendazole. Percentage of any helminth, A. lumbricoides, T. trichiura, and hookworm before and after MDA. MDA was associated with a significant decrease in the prevalence of any helminth, A. lumbricoides, T. trichiura, and hookworm. p values were calculated using a logistic model with subject random effects. * p < 0.001.
Changes in Immune Parameters following MDA
Changes in immune parameters following MDA were assessed using a linear mixed model adjusting for age and sex. The results in Table 2 show that all immune parameters measured in the total population, including total IgE (estimate: 0.30; 95% CI 0.22–0.42, p < 0.0001), eosinophils (estimate: 0.70; 95% CI 0.61–0.80, p < 0.001), and CRP (estimate: 0.60; 95% CI 0.42–0.86, p = 0.006) decreased significantly 1 year after MDA. Additionally, stratification analysis based on STH status at baseline showed that the changes in immune parameters over time did not differ between helminth-positive and helminth-negative subjects (online suppl. Table S1).
Table 2.
Changes in immune parameters following MDA in the total study population
| Parameter | Baseline | Follow-up | Estimate (95% CI) | p value |
|---|---|---|---|---|
| Total IgE, IU/mL | 667.87 (316.67–877.36) a | 109.10 (27.90–628.17) c | 0.30 (0.22–0.42) | <0.0001 |
| Eosinophil count, % | 8 (5–11) b | 5 (3–8) d | 0.70 (0.61–0.80) | <0.001 |
| CRP, mg/L | 1.71 (0.53–6.23) b | 0.95 (0.11–3.47) c | 0.60 (0.42–0.86) | 0.006 |
Values are presented as medians (IQR) unless otherwise stated. Estimates and 95% CI are based on generalized linear mixed models from log-transformed data. Values shown are back-transformed. p values were generated using a likelihood ratio test comparing models with and without a time effect. a n = 146. b n = 150. c n = 112. d n = 111.
SPT Reactivity to Allergens before and after MDA
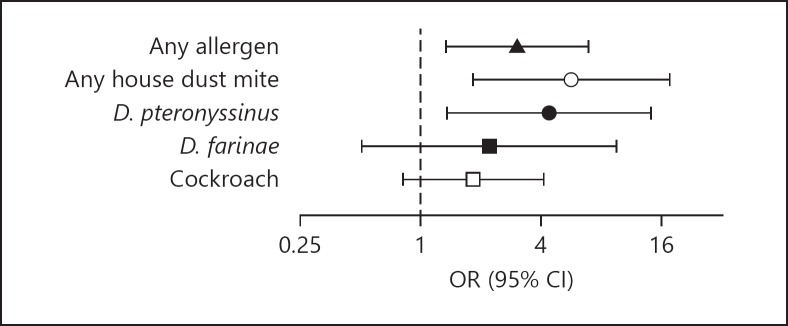
Following MDA, SPT reactivity to allergens was assessed in 113 children. The results show that the prevalence of SPT reactivity increased from 18.7 to 32.7% for any allergen, from 17.3 to 26.5% for cockroach, and from 2.7 to 14.2% for any house dust mite 1 year after MDA. When species of house dust mites are considered, the results show that the prevalence of SPT increased from 2.7 to 10.6% and from 2 to 4.4% for D. pteronyssinus and D. farinae, respectively. Multivariate analysis adjusting for age and sex showed that skin reactivity to any allergen (OR 3.04; 95% CI 1.338–6.919, p = 0.008) and to any house dust mite (OR 5.66; 95% CI 1.83–17.54, p = 0.003) but not to cockroach (OR 1.83; 95% CI 0.81–4.14, p = 0.15) (Fig. 2) increased after MDA. The increased risk of skin reactivity to house dust mite allergens after MDA is mainly due to the increased risk of reactivity to D. pteronyssinus (OR 4.38; 95% CI 1.36–14.14, p = 0.013) and not to D. farinae (estimate: 2.21; 95% CI 0.51–9.54, p = 0.29) (Fig. 2). Stratification analysis based on the STH status at baseline further showed that the prevalence of SPT reactivity increased from 20.9% (24/115) to 34.1% (29/85) and from 3.6% (1/28) to 25% (6/24) in helminth-negative and helminth-positive subjects, respectively. Whereas a borderline significant effect was seen in the helminth-positive subjects (OR 0.62; 95% CI 0.73–5.30, p = 0.05), in helminth-negative subjects MDA resulted in a significant increase in SPT reactivity (OR 2.41; 95% CI 1.06–5.51, p = 0.036).
Fig. 2.
Changes in SPT reactivity to allergens before and after MDA with albendazole. The risk of positive SPT reactivity following MDA is shown as OR with 95% CI calculated using a logistic model adjusted for age and sex. Of the 150 SPT-tested subjects before treatment, SPT reactivity to any allergen, any house dust mite, D. pteronyssinus, D. farinae, and cockroach was detected in 28 (18.7%), 4 (2.7%), 4 (2.7%), 3 (2%), and 26 (17.3%) subjects, respectively. After anthelminthic treatment, SPT performed in 113 subjects showed that the number of subjects positive to any allergen, any house dust mite, D. pteronyssinus, D. farinae, and cockroach was 37 (32.7%), 16 (14.2%), 12 (10.6%), 5 (4.4%), and 30 (26.5%), respectively.
Discussion
STH infections are among the most prevalent infections in the world. MDA programs have become the most commonly used national intervention strategy in helminth-endemic countries to achieve the goal of STH elimination [24]. For the deworming program in the Nangapanda community, the Indonesian government implements WHO guidelines [25] and provides albendazole treatment to school children biannually. The results of this study show that treatment with albendazole led to a significant reduction in the prevalence of A. lumbricoides, T. trichiura, and hookworm infections.
The decrease in helminth prevalence after MDA was accompanied by a decrease in total IgE levels and eosinophil counts. These findings are in agreement with our previous results which showed that albendazole treatment leads to a decrease in total IgE levels [27, 28, 29]. Likewise, a school-based cluster-randomized study in Ecuador observed a strong association between total IgE and STH infection but not with age, nutritional, or socioeconomic status, suggesting that STH infection is an important determinant of total IgE [30]. With respect to the number of STH infections, Tahapary et al. [26] observed that the effect of albendazole treatment on total IgE levels and eosinophil counts was more pronounced in subjects with multiple helminth infections compared to single or no infection. However, this was not observed in the current study, probably due to the lower STH prevalence and the small sample size.
The finding that the level of CRP, a marker of inflammation, decreased after albendazole treatment is in contrast with our previously reported study conducted in adults (age ≥ 16 years) in the same area, which showed that albendazole treatment had no effect on high-sensitivity CRP levels [26]. Similarly, a study in Tanzanian school children infected with hookworm or Schistosoma haematobium reported that albendazole or praziquantel treatment had no effect on CRP levels [31]. Furthermore, a study conducted in an area endemic for helminths and malaria in Tanzania observed a strong association between CRP levels and malaria but not with helminth infections [32]. Although no information regarding malaria status was collected in the current study, from our previous studies in the area it is known that this area is endemic for Plasmodium spp. [20]. Moreover, as we had no placebo arm to control for changes not related to MDA that occur in 1 year, we cannot rule out that changes in other exposures are associated with the change in CRP levels.
In the stratification analysis, we did not observe larger changes in immune parameters following MDA in helminth-positive compared to helminth-negative subjects, probably due to the small sample size. It should also be noted that the presence of STH infections was determined in a single stool sample by microscopy, which misses light infections and subsequently can misclassify STH status. Thus, deworming can have a much larger effect in the community than that which is detectable by assessing helminth using microscopy. Moreover, as this is not a placebo-controlled trial, the effects could be due to other environmental changes. Finally, there is also the possibility that albendazole affects macro- and microbiomes directly, which can affect the immune system [33, 34, 35].
In this study, the decrease in STH prevalence following MDA was accompanied by an increase in the prevalence of SPT reactivity, especially to the house dust mite D. pteronyssinus. This is in line with the notion that chronic helminth infection dampens excessive inflammatory responses through the induction of a suppressive immune environment [5]. Several other studies have shown a similar inverse relationship between STH infections and SPT reactivity. A study in Gabon found that SPT reactivity was significantly reduced in children infected with S. haematobium and/or filariae [36]. Similarly, a Venezuelan study in an area with a high STH infection prevalence found that a decrease in the prevalence of STH infections was accompanied by an increase in positive SPT reactivity to house dust mites [9]. Though not significant, our previous study conducted in children aged 5–19 years from neighbouring villages found an incremental increase in SPT positivity to allergens at 9 and 21 months after the initiation of treatment [20]. It has to be noted that the reduction in helminth prevalence was less substantial (50%) than that in the current study (70%). Interestingly, a study in Ecuador showed that albendazole treatment every 2 months over the course of 1 year, while reducing the prevalence of STH infection, did not result in an increased prevalence of atopy or allergic symptoms [30]. In the Ecuadorian study, albendazole treatment resulted in a 70.4% (from 69.3 to 20.5%) reduction in helminth prevalence, similar to the finding in this study. However, despite a similar extent of reduction, the actual helminth prevalence after treatment in the Ecuadorian study was 8.5 times (50.5 vs. 5.9%) higher compared to our study, which might explain the lack of effect of deworming on the prevalence of SPT reactivity in Ecuador.
The substantial decrease in the prevalence of helminth infections in our study, due to a biannual single-dose albendazole MDA program, is rather surprising given that an earlier study [26] in adults in the same area showed that 3 consecutive doses of albendazole at 3 monthly intervals are needed to achieve an 86.6% reduction in helminth prevalence. However, in the present study the baseline prevalence of helminth infections was much lower at 19.6% compared to the 41.7% reported in adults [26]. This indicates that biannual MDA might be sufficient in areas where there is a low prevalence of helminth infections.
The strength of the current study is its longitudinal design; however, we acknowledge the limitations which include its small sample size, no control group, no data on allergen exposure or allergic symptoms, and no data on coinfections.
In summary, the present study, carried out in school children in a rural area of Flores Island, Indonesia, showed that biannual MDA with albendazole resulted in a significant decrease in the prevalence of STH infections which was accompanied by an increase in the prevalence of SPT reactivity to 2 of the most important allergens. Further studies in larger populations and in other endemic areas are needed to confirm our results and further examine the effect of MDA with albendazole on the prevalence of allergic disorders.
Disclosure Statement
We declare that we have no conflict of interest.
Funding Sources
Leiden University Medical Center. The sponsor of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of this report.
Author Contributions
Conception and design of the experiments: R.R., M.Y., T.S., and E.S. Data collection and experimentation: S.L.S., S.K.L.H., S.A.V., R.C.T., M.V., I.I.V., K.C.S., J.K.G., A.C.P., K.A., A.W., C.P., Y.D., D.L.T., and E.S. Interpretation of data and statistical analysis: S.L.S., S.K.L.H., S.A.V., R.C.T., M.V., I.I.V., K.C.S., J.K.G., K.A., C.P., Y.D., L.A.L., D.L.T., and E.S. Writing of this paper: S.L.S., S.K.L.H., S.A.V., R.C.T., M.V., I.I.V., K.C.S., J.K.G., L.A.L., D.L.T., and E.S. The corresponding author had full access to all of the data in this study and takes final responsibility for the decision to submit it for publication.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgement
We gratefully acknowledge Eka Setya Mulyawan, Dewi Ramliani Yani, and Selly for their time and tremendous support in the field. Also, we wish to express our gratitude to Stella de Jong, Nienke de Vries, and Jilke Speulman for their contributions to collecting and processing of the data. Most of all we are grateful for the participation of the children, parents, and teachers of the SD Katolik Nangamboa Primary School in Nangapanda.
References
- 1.Pawanker RC, Holgate ST, Lockey RF, editors. 2011. World Allergy Organization (WAO) White Book on Allergy 2011-2020. [Google Scholar]
- 2.Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 2010 Feb;65((2)):152–67. doi: 10.1111/j.1398-9995.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- 3.Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy. 2005 Nov;60((11)):1357–60. doi: 10.1111/j.1398-9995.2005.00961.x. [DOI] [PubMed] [Google Scholar]
- 4.Santiago HC, Nutman TB. Human Helminths and Allergic Disease: The Hygiene Hypothesis and Beyond. Am J Trop Med Hyg. 2016 Oct;95((4)):746–53. doi: 10.4269/ajtmh.16-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitcharungsi R, Sirivichayakul C. Allergic diseases and helminth infections. Pathog Glob Health. 2013 Apr;107((3)):110–5. doi: 10.1179/2047773213Y.0000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989 Nov;299((6710)):1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper PJ, Chico ME, Rodrigues LC, Ordonez M, Strachan D, Griffin GE, et al. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003 May;111((5)):995–1000. doi: 10.1067/mai.2003.1348. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PJ, Chico ME, Amorim LD, Sandoval C, Vaca M, Strina A, et al. Effects of maternal geohelminth infections on allergy in early childhood. J Allergy Clin Immunol. 2016 Mar;137((3)):899–906.e2. doi: 10.1016/j.jaci.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch NR, Hagel I, Perez M, Di Prisco MC, Lopez R, Alvarez N. Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J Allergy Clin Immunol. 1993 Sep;92((3)):404–11. doi: 10.1016/0091-6749(93)90119-z. [DOI] [PubMed] [Google Scholar]
- 10.van den Biggelaar AH, Rodrigues LC, van Ree R, van der Zee JS, Hoeksma-Kruize YC, Souverijn JH, et al. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004 Mar;189((5)):892–900. doi: 10.1086/381767. [DOI] [PubMed] [Google Scholar]
- 11.Haileamlak A, Lewis SA, Britton J, Venn AJ, Woldemariam D, Hubbard R, et al. Validation of the International Study of Asthma and Allergies in Children (ISAAC) and U.K. criteria for atopic eczema in Ethiopian children. Br J Dermatol. 2005 Apr;152((4)):735–41. doi: 10.1111/j.1365-2133.2005.06511.x. [DOI] [PubMed] [Google Scholar]
- 12.Davey G, Berhane Y, Duncan P, Aref-Adib G, Britton J, Venn A. Use of acetaminophen and the risk of self-reported allergic symptoms and skin sensitization in Butajira, Ethiopia. J Allergy Clin Immunol. 2005 Oct;116((4)):863–8. doi: 10.1016/j.jaci.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Palmer LJ, Celedón JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002 Jun;165((11)):1489–93. doi: 10.1164/rccm.2107020. [DOI] [PubMed] [Google Scholar]
- 14.Stein M, Greenberg Z, Boaz M, Handzel ZT, Meshesha MK, Bentwich Z. The Role of Helminth Infection and Environment in the Development of Allergy: A Prospective Study of Newly-Arrived Ethiopian Immigrants in Israel. PLoS Negl Trop Dis. 2016 Jan;10((1)):e0004208. doi: 10.1371/journal.pntd.0004208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamid F, Wiria AE, Wammes LJ, Kaisar MM, Lell B, Ariawan I, et al. A longitudinal study of allergy and intestinal helminth infections in semi urban and rural areas of Flores, Indonesia (ImmunoSPIN Study) BMC Infect Dis. 2011 Apr;11((1)):83. doi: 10.1186/1471-2334-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010 Jan;10((1)):3–12. doi: 10.1007/s11882-009-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McSorley HJ, O'Gorman MT, Blair N, Sutherland TE, Filbey KJ, Maizels RM. Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur J Immunol. 2012 Oct;42((10)):2667–82. doi: 10.1002/eji.201142161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beasley R, The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. Lancet. 1998 Apr;351((9111)):1225–32. [PubMed] [Google Scholar]
- 19.World Bank and Health in Indonesia [cited 2017 11/09] Available from: http://www.worldbank.org/en/country/indonesia/brief/world-bank-and-health-in-indonesia. [Google Scholar]
- 20.Wiria AE, Hamid F, Wammes LJ, Kaisar MM, May L, Prasetyani MA, et al. The effect of three-monthly albendazole treatment on malarial parasitemia and allergy: a household-based cluster-randomized, double-blind, placebo-controlled trial. PLoS One. 2013;8((3)):e57899. doi: 10.1371/journal.pone.0057899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cogill B. Anthropometric Indicators Measurement Guide. 2003 [Available from: http://www.fantaproject.org/sites/default/files/resources/anthropometry-2003-ENG.pdf. [Google Scholar]
- 22.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972 Nov-Dec;14((6)):397–400. [PubMed] [Google Scholar]
- 23.Wiria AE, Prasetyani MA, Hamid F, Wammes LJ, Lell B, Ariawan I, et al. Does treatment of intestinal helminth infections influence malaria? Background and methodology of a longitudinal study of clinical, parasitological and immunological parameters in Nangapanda, Flores, Indonesia (ImmunoSPIN Study) BMC Infect Dis. 2010 Mar;10((1)):77. doi: 10.1186/1471-2334-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortu G, Assoum M, Wittmann U, Knowles S, Clements M, Ndayishimiye O, et al. The impact of an 8-year mass drug administration programme on prevalence, intensity and co-infections of soil-transmitted helminthiases in Burundi. Parasit Vectors. 2016 Sep;9((1)):513. doi: 10.1186/s13071-016-1794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. WHO Intestinal worms strategy [cited 2018 20/01]. Available from: http://www.who.int/intestinal_worms/strategy/en/
- 26.Tahapary DL, de Ruiter K, Martin I, Brienen EA, van Lieshout L, Cobbaert CM, et al. Effect of Anthelmintic Treatment on Insulin Resistance: A Cluster-Randomized, Placebo-Controlled Trial in Indonesia. Clin Infect Dis. 2017 Sep;65((5)):764–71. doi: 10.1093/cid/cix416. [DOI] [PubMed] [Google Scholar]
- 27.Knopp S, Mohammed KA, Speich B, Hattendorf J, Khamis IS, Khamis AN, et al. Albendazole and mebendazole administered alone or in combination with ivermectin against Trichuris trichiura: a randomized controlled trial. Clin Infect Dis. 2010 Dec;51((12)):1420–8. doi: 10.1086/657310. [DOI] [PubMed] [Google Scholar]
- 28.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008 Apr;299((16)):1937–48. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 29.de Ruiter K, Tahapary DL, Wammes LJ, Wiria AE, Hamid F, van Lieshout L, et al. The effect of three-monthly albendazole treatment on Th2 responses: differential effects on IgE and IL-5. Parasite Immunol. 2017 Jun;39((6)):e12428. doi: 10.1111/pim.12428. [DOI] [PubMed] [Google Scholar]
- 30.Cooper PJ, Chico ME, Vaca MG, Moncayo AL, Bland JM, Mafla E, et al. Effect of albendazole treatments on the prevalence of atopy in children living in communities endemic for geohelminth parasites: a cluster-randomised trial. Lancet. 2006 May;367((9522)):1598–603. doi: 10.1016/S0140-6736(06)68697-2. [DOI] [PubMed] [Google Scholar]
- 31.Bhargava A, Jukes M, Lambo J, Kihamia CM, Lorri W, Nokes C, et al. Anthelmintic treatment improves the hemoglobin and serum ferritin concentrations of Tanzanian schoolchildren. Food Nutr Bull. 2003 Dec;24((4)):332–42. doi: 10.1177/156482650302400403. [DOI] [PubMed] [Google Scholar]
- 32.Kung'u JK, Goodman D, Haji HJ, Ramsan M, Wright VJ, Bickle QD, et al. Early helminth infections are inversely related to anemia, malnutrition, and malaria and are not associated with inflammation in 6- to 23-month-old Zanzibari children. Am J Trop Med Hyg. 2009 Dec;81((6)):1062–70. doi: 10.4269/ajtmh.2009.09-0091. [DOI] [PubMed] [Google Scholar]
- 33.Sittipo P, Lobionda S, Lee YK, Maynard CL. Intestinal microbiota and the immune system in metabolic diseases. J Microbiol. 2018 Mar;56((3)):154–62. doi: 10.1007/s12275-018-7548-y. [DOI] [PubMed] [Google Scholar]
- 34.Flandroy L, Poutahidis T, Berg G, Clarke G, Dao MC, Decaestecker E, et al. The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci Total Environ. 2018 Jun;627:1018–38. doi: 10.1016/j.scitotenv.2018.01.288. [DOI] [PubMed] [Google Scholar]
- 35.Postler TS, Ghosh S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017 Jul;26((1)):110–30. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, et al. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000 Nov;356((9243)):1723–7. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data