Abstract
Alcohol use disorders (AUD) are a major contributor to the global burden of disease, and have huge societal impact. Some studies show that AUD patients carrying the G-allele of the OPRM1 variant c.118A>G respond better to naltrexone, resulting in reduced relapse rates compared to carriers of the AA genotype. Genotype-guided treatment allocation of these patients carrying a G-allele to naltrexone could potentially improve the treatment outcome. However, cost-effectiveness of this strategy should be investigated before considering clinical implementation. We, therefore, evaluated costs and Quality-Adjusted Life-Years (QALYs), using a modelling approach, from an European perspective, of genotype-guided treatment allocation (G-allele carriers receiving naltrexone; AA homozygotes acamprosate or naltrexone) compared to standard care (random treatment allocation to acamprosate or naltrexone), by using a Markov model. Genotype-guided treatment allocation resulted in incremental costs of EUR 66 (95% CI −28 to 149) and incremental effects of 0.005 QALYs (95% CI 0.000–0.011) per patient (incremental cost-effectiveness ratio of EUR 13,350 per QALY). Sensitivity analyses showed that the risk ratio to relapse after treatment allocation had the largest impact on the cost-effectiveness. Depending on the willingness to pay for a gain of one QALY, probabilities that the intervention is cost-effective varies between 6 and 79%. In conclusion, pharmacogenetic treatment allocation of AUD patients to naltrexone, based on OPRM1 genotype, can be a cost-effective strategy, and could have potential individual and societal benefits. However, more evidence on the impact of genotype-guided treatment allocation on relapse is needed to substantiate these conclusions, as there is contradictory evidence about the effectiveness of OPRM1 genotyping.
Keywords: Cost-effectiveness, Pharmacogenetics, Alcohol use disorder, Naltrexone, Acamprosate
Introduction
Alcohol use disorders (AUD) are major contributors to the global burden of disease, with a net contribution of 3.8% to all global deaths and 4.6% to all global disability-adjusted life years (Disability-Adjusted Life Years; a measure of the burden of disease) [1]. Worldwide, this corresponds to 2·2 million deaths and 70 billion Disability-Adjusted Life Years lost to AUD in a year [2]. This is mainly caused by alcohol-induced physical adversities, like liver disease, pancreatitis, several types of cancer, fetal alcohol syndrome, and neuropsychiatric disorders [3]. This burden of disease comes with substantial societal impact. In Europe, an estimated 14.6 million people are affected by AUD, corresponding to a total cost of about 62.3 billion Euros a year [4].
Evidence-based treatment of AUD includes psychological and pharmacological interventions [5, 6]. Naltrexone and acamprosate are the most effective pharmacological interventions currently available for AUD treatment [5, 7]. It has been shown that naltrexone significantly reduces the number of drinking days and the level of alcohol craving [8], with a number needed to treat to prevent a relapse into heavy drinking of 9 [9]. Comparably, the anti-craving drug, acamprosate, has been shown to reduce the risk of relapse into any drinking [10], with a number needed to treat to prevent a relapse of 8 [9]. In clinical practice, naltrexone or acamprosate are both considered first-choice pharmacological treatments, and are prescribed with similar frequencies [5, 6, 10, 11].
Despite their proven potential effectiveness, less than 20% of treated AUD patients receive anti-craving medication [12]. This might be due to the side effects of naltrexone and acamprosate, such as nausea, headache, dizziness, anxiety, and diarrhea [5, 8, 10, 13], and medication contraindications: kidney failure for both naltrexone and acamprosate; liver issues for naltrexone [13]. Hence, healthcare professionals are sometimes skeptical about the role of pharmacotherapy in the treatment of addictive behaviors [14]. Improving patient-treatment matching, by selecting those patients with higher chances of good response might be an important step to improve the implementation of pharmacotherapy and treatment outcome for AUD.
The genetic background of a patient is one of the factors underlying differential responses to pharmacological treatment (“pharmacogenetics”) [15, 16, 17]. Meta-analysis showed that a genetic variant (single nucleotide polymorphism) in the OPRM1 gene (rs1799971), resulting in a change of A to G at position 118, is associated with increased effectiveness of naltrexone. AUD carriers of a G-allele (15% of the Caucasian population [18, 19]) have 2 times lower relapse rates compared to persons with the AA genotype, when treated with naltrexone [15, 20, 21]. Prescription of naltrexone, instead of acamprosate, for all AUD carriers of a G-allele could improve the overall treatment effectiveness, by preventing more relapses and therefore reducing costs associated with AUD. However, a recent study did not support previous findings on OPRM1 genotype-guided treatment allocation in AUD patients [22].
Given the substantial economic costs associated with AUD and relapse, systematic screening for OPRM1 genotype could, however, be economically attractive, even when there is a small effect. However, it is unknown whether the potential cost-savings by potential optimizing treatment for part of the AUD population outweigh the screening costs of genetic testing of the whole AUD population. Such cost-effectiveness analyses of genotype-guided treatment allocation are currently lacking [23]. However, given the contradictory evidence concerning the increased effectiveness of naltrexone in OPRM1 G-allele carriers compared to A-allele homozygotes, it is highly relevant to evaluate at which threshold of increased effectiveness OPRM1 genotyping becomes cost-effective.
The aims of this study are to evaluate: (1) cost-effectiveness, by using a modelling approach based on existing data, of OPRM1 genotype-guided treatment allocation of naltrexone to G-allele carrying AUD patients, compared to random (non-genotype guided) treatment allocation to pharmacological treatment with naltrexone or acamprosate, and (2) at which threshold of added effectiveness OPRM1 genotype screening is cost-effective, from an European perspective.
Methods
Model Structure
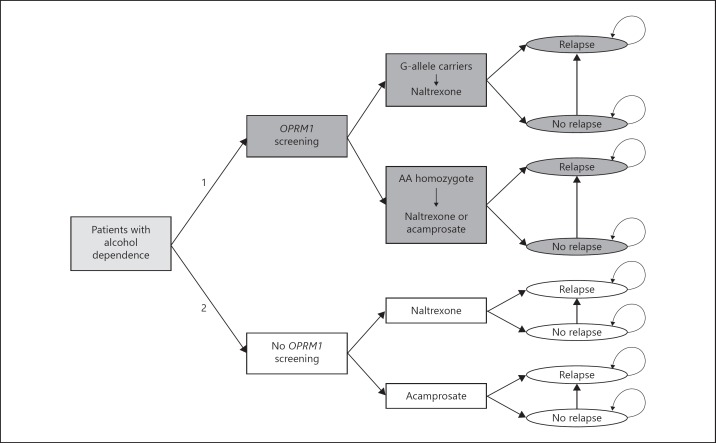
A Markov model was built to compare the 2 different treatment strategies (genotype-guided treatment allocation, versus random treatment). This Markov model is used to model different treatment options and outcomes over time by using the probabilities that these events or outcomes occur within a certain time range (Fig. 1 for an outline of the model) [24]. This analysis applied a societal perspective, taking all relevant societal costs into account. The 2 strategies were: (1) to screen all patients for the OPRM1 c.118A>G variant, which guided treatment allocation (G-allele carriers receiving naltrexone, AA homozygotes receiving random naltrexone or acamprosate (resembling current practice as much as possible)), and (2) to randomly assign treatment of naltrexone or acamprosate to all patients (non-genotype guided). After the patients received one of the treatment options depending on the strategy, they had a certain chance to relapse or not. The relapse state was an absorption state, indicating that a patient stayed in this health state for the rest of the time horizon. The cycle length in both strategies for the risk of relapse or not was 1 month, with a maximum time horizon of the analysis of 12 months. The analyses (including sensitivity analysis) were performed in Microsoft Office Excel 2007.
Fig. 1.
Structure of the Markov model. In this model, the strategy of OPRM1 screening was compared to no screening. In the screening strategy (1; in dark gray), alcohol-dependent patients with a G-allele received naltrexone, and AA homozygotes randomly received either acamprosate or naltrexone. In the no screening strategy (2; in white), treatment allocation was non-genotype guided, and therefore patients randomly received acamprosate or naltrexone. The risk of relapse and associated costs and effects were compared across strategies.
Parameters
The parameters used for the analyses are shown in Table 1. For each parameter, a summary value and uncertainty range were searched. The summary values were used in the base-case analysis, and the uncertainty ranges in the sensitivity analysis. The prevalence estimation of the G-allele and AA genotype was based on several population studies [18, 19]. The estimation of the risk of relapse after treatment with naltrexone or acamprosate was based on a recent network meta-analyses on the effectiveness of pharmacological interventions on AUD, performed as part of a National Institute of Health and Care and Excellence (NICE) guideline for AUD [25]. Both treatments are considered equally effective, as shown by the credible interval of the network meta-analyses. Therefore, the same relapse chance was considered for both drugs. The chance of no relapse was complementary to the chance of relapse, counting up to 100%.
Table 1.
Parameters used for cost-effectiveness analysis in the Markov model, with corresponding references
| Base-case | Uncertainty ranges | References | |
|---|---|---|---|
| Probability of G-allele carriers in Caucasians | 0.150 | 0.100–0.200 | [18, 19] |
| Probability of AA homozygous in Caucasians | 0.850 | 0.800–0.900 | [18, 19] |
| Relapse chance with acamprosate/naltrexone treatment | 0.132 | 0.040–0.479 | [25] |
| No relapse chance with acamprosate/naltrexone treatment | 0.868 | 0.521–0.960 | [25] |
| Risk ratio to relapse when G-allele carriers treated with naltrexone (screening strategy) | 0.508 | 0.273–0.943 | [15] |
| Utility of relapse | 0.540 | 0.390–0.670 | [25] |
| Utility of no relapse event | 0.860 | 0.830–0.890 | [25] |
| Costs of successful AUD treatment (no relapse) per month | EUR 82.50 | EUR 40.00–125.00 | [25] |
| Costs of failed AD treatment (relapse) per month | EUR 533 | EUR 333–583 | [28] |
| Costs of OPRM1 genotyping | EUR 150 | EUR 100–200 | Human Genetics Department, Radboudumc |
AUD, alcohol use disorders.
The risk of relapse in G-allele carriers when treated with naltrexone was based on recent meta-analysis of the effectiveness of naltrexone treatment in AUD G-allele carries and persons with the AA genotype [15]. This risk ratio was multiplied with the general relapse chance with acamprosate/naltrexone to determine the relapse chance in G-allele carriers treated with naltrexone.
The utility values of the events of “relapse” and “no relapse” were based on the same NICE guideline for AUD [25]. A utility (expression of the health-related quality of life) represents the preference for a certain health state, where 1 represents perfect health and 0 represents death, and is used to calculate Quality-Adjusted Life-Years (QALYs) (see Analysis) [24, 26]. The resources used in case of “no relapse” (including all healthcare costs) were also based on this guideline, and transformed to the Dutch situation by multiplying these resources with Dutch standard cost prices [27]. The costs of the event “relapse” was based on a recent report about Dutch addiction care (“Verslavingszorg in beeld – alcohol en drugs”[28]), and updated with price indices to 2015. These costs included all societal costs (including healthcare, non-healthcare, and indirect costs) associated with alcohol use [29]. The costs for genetic screening were based on current prices provided by the Department of Human Genetics of the Radboudumc Nijmegen. As costs of naltrexone and acamprosate are comparable, these were not included in the analysis.
Analysis
At first we did a base-case analysis, where the parameters, as presented in Table 1, were entered into the model. This base-case analysis was performed with the data of the meta-analyses. A Monte Carlo simulation with 5000 iterations was performed [24, 26, 30]. In these iterations, parameter uncertainty of all parameters was taken into account at the same time, by randomly choosing a value from their distribution based on the uncertainty ranges of Table 1. In economic evaluations, we use beta distributions for probability rates and utilities, as these values can vary between 0 and 1, lognormal distribution for risk ratios, as the confidence limits values are calculated on a log-scale, and gamma distributions for costs, as these values are always non-negative. QALYs were calculated by multiplying the utility value of the health state (“relapse” or “no relapse”) with the time spent in that health state [24]. Subsequently, for each iteration of the Monte Carlo simulation, an incremental cost-effectiveness ratio (ICER) was calculated, based on the comparison between screening and no screening. The results were plotted in a cost-effectiveness plane, and were used to draw a cost-effectiveness acceptability (CEA) curve, which shows the probability that screening is cost-effective, compared to no screening, for different willingness to pay (WTP) thresholds [24, 26]. These WTP thresholds indicate the maximum amount of money society is willing to pay to gain one unit of effect (i.e., QALYs). Subsequently, we did univariate sensitivity analyses to investigate the influence of variation per parameter within an uncertainty range. These analyses were done to show which parameter mostly determines cost-effectiveness. The results of these analyses were summarized in a Tornado diagram (Fig. 3), which shows the maximum impact of the extremes of the uncertainty ranges on the incremental net monetary benefit (iNMB) for each parameter. The iNMB shows the value of screening in monetary terms, compared to random treatment, by re-scaling the QALY gain into monetary value using the amount of money society is willing to pay for this unit of effect. This value is calculated as: iNMB = WTP * incremental QALYs-incremental costs. For these calculations, a WTP of EUR 80,000/QALY was considered, which is often considered as the maximum society is willing to pay to gain one QALY. A positive iNMB indicates that the intervention is cost-effective, and vice versa.
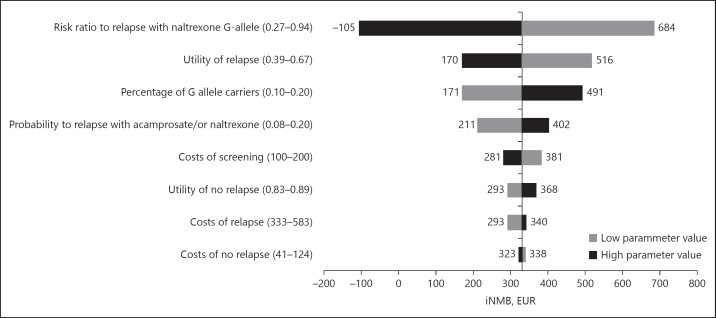
Fig. 3.
Tornado diagram, summarizing the results of the univariate sensitivity analyses. In these analyses, the presented parameter estimates vary within an uncertainty range (presented behind each parameter), to see the influence on the incremental Net Monetary Benefit (iNMB) for a Willingness To Pay (WTP) of EUR 80,000/QALY (vertical line representing the iNMB of the base-case analysis).
Eventually, we performed a threshold analyses for the risk of relapse in G-allele carriers using naltrexone, as the study of Oslin et al. [22] showed no-effect of OPRM1 genotyping. With this threshold analysis, the minimal added effectiveness of genotype-based treatment allocation at which screening is cost-effective was determined. This was calculated by varying the relative risk ratio for relapse in the genotype-based treatment allocation between 0 and 1 and then evaluate the ICER at each risk ratio.
Results
The results of the simulation of the base-case shows that treatment allocation of naltrexone versus acamprosate based on OPRM1 genotyping was more expensive with EUR 66.22 per patient (95% CI −28 to 149), but also more effective with 0.005 QALYs (95% CI 0.000–0.011) gained per patient, compared to non-genotype guided treatment allocation of AUD patients (Table 2). This results in an ICER of EUR 13,349.71 (95% CI 442,000 to dominant) per QALY.
Table 2.
Costs and effects of the strategies of screening and no screening
| Strategy | Cost per patient (95% CI) |
Incremental costs (95% CI) |
QALYs per patient (95% CI) |
Incremental QALY (95% CI) |
|---|---|---|---|---|
| OPRM1 screening | EUR 3,610.66 (EUR 1,166–5,681) | EUR 66.22 (–EUR 28 to 149) | 0.714 (0.574–0.862) | 0.005 (0.000–0.011) |
| No OPRM1 screening | EUR 3,544.44 (EUR 1,024–5,629) | 0.709 (0.567–0.862) |
Costs and QALY's are presented as means with 95% CI, resulting from Monte Carlo simulation with 5000 iterations. QALY, quality adjusted life year.
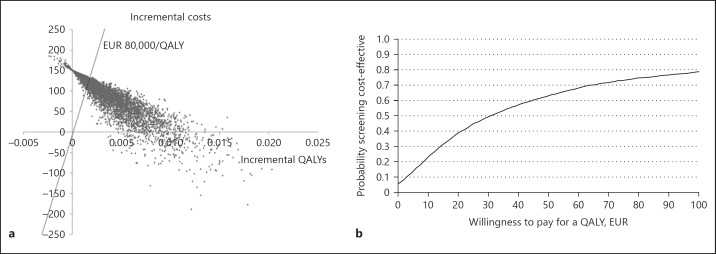
The results of the simulation are presented in a cost-effectiveness plane in Figure 2a, with corresponding CEA curve in Figure 2b. The curve shows the probability of cost-effectiveness for different WTP levels. At a WTP of EUR 0, screening has a probability of 6% being “dominant,” indicating that in 6% of the iterations screening resulted in increased effectiveness (increased QALYs) and cost savings, compared to no screening (south-east corner of the cost-effectiveness plane). With a WTP of EUR 80,000 per increased QALY, often considered as the maximum people are willing to pay for a QALY, screening for the genetic variant in OPRM1 and adjusting treatment accordingly has a probability of 75% of being the most cost-effective strategy, corresponding to 75% of the iterations lying below this threshold (represented by the line in Fig. 2a). It can also been seen in the CEA curve (Fig. 2b) that this probability slowly rises (up to maximum 79%) when the WTP becomes higher.
Fig. 2.
a, b Cost-effectiveness plane and cost-effectiveness acceptability (CEA) curve. Outcomes of the simulation presented in a cost-effectiveness plane, with corresponding CEA curve. This curve shows that the probability OPRM1-guided treatment allocation is cost-effective, compared to no screening for different willingness to pay (WTP) thresholds.
Univariate Sensitivity Analysis
The results of the univariate sensitivity analyses are presented as a Tornado diagram in Figure 3. This diagram shows the effect of changing a parameter (tested ranges are presented behind each parameter) on the iNMB. The risk ratio to relapse in G-allele carriers treated with naltrexone has the largest effect on the iNMB, ranging from not cost-effective at –EUR 105 to cost-effective at EUR 684, when changing this parameter to a lower or higher level than in the initial analysis. Furthermore, for all other parameter values the iNMB stays positive, ranging between about EUR 170 and 516. These positive iNMBs show that for each parameter value screening stays cost-effective.
Threshold Analysis
The results of the threshold analysis are presented in Figure 4. In this Figure, it can be seen that at a risk ratio ≤0.81 screening is cost-effective with a WTP of EUR 80,000/QALY. When the risk ratio is ≤0.25, screening becomes the “dominant” strategy (more QALY gain at lower costs) compared to no screening.
Fig. 4.
Diagram summarizing the results of the threshold analyses. This figure shows the impact of the risk to relapse in G-allele carriers using naltrexone on the incremental cost-effectiveness ratio (ICER), and at which risk ratio screening is cost-effective at a Willingness To Pay (WTP) of EUR 80,000/QALY. The solid line indicates the ICER for each value of the risk ratio.
Discussion
This is the first study investigating cost-effectiveness of OPRM1 genotype-guided treatment allocation of naltrexone in AUD patients. In our base-case analysis, assuming an effect of the OPRM1 genotype, we showed that OPRM1 screening can be cost-effective. However, these results need to be interpreted very carefully as recent evidence did not confirm prior data on OPRM1 pharmacogenetics in AUD [22]. On average, the genotype strategy is slightly more expensive, but also slightly more effective compared to no genotyping strategy. This results in an ICER of approximately EUR 13,350 per QALY, which is generally considered acceptable [31]. Depending on how much decision makers are willing to pay for a QALY, the probability that OPRM1 screening is the preferred strategy ranges between 6 and 79%. The univariate sensitivity analysis showed that the ICER is mostly determined by the risk of relapse in AUD G-allele carriers treated with naltrexone. Threshold analysis showed that when the risk ratio of relapse between naltrexone in G-allele carriers versus A-allele homozygotes was smaller than 0.82, screening was cost-effective at a WTP of EUR 80,000/QALY, and at a risk ratio smaller than 0.25 screening was the dominant strategy. When looking at the CI of the relative risk ratio for relapse in the G-allele carriers versus AA homozygotes in the meta-analysis of 0.273–0.943, this threshold seems reachable, but the study of Oslin et al. [22] could not confirm this. To give a definite answer on the cost-effectiveness of OPRM1 genotyping, more studies are needed to determine the effectiveness of naltrexone in AUD G-allele carriers compared to AA homozygotes.
The percentage of G-allele carriers in the population also had a major impact on the sensitivity analysis. Higher G-allele frequencies increase the cost-effectiveness of OPRM1-guided treatment allocation. In Asian populations, the percentage of G-allele carriers is much higher (about 60%) than in Caucasian (about 15%) and African populations (about 1%) [19, 32]. Cost-effectiveness of OPRM1 genotype-guided treatment allocation in AUD might therefore be more cost-effective in Asian regions, while less cost-effective in African regions, as compared to Caucasian populations [19, 33].
Finally, the cost of genotyping, which probably will decrease in the coming years, influenced cost-effectiveness [34, 35]. In our model, a price below EUR 129 per genetic screening, is associated with increased effectiveness at lower costs (“dominant”), indicating that OPRM1 genotype-guided treatment allocation would be the preferred strategy. Importantly, cost-effectiveness will also increase with increasing costs related to AUD and relapse. Though the costs of relapse have no major impact on the cost-effectiveness, our cost estimation of about EUR 6,402 per year is a conservative estimation of the costs [4, 36, 37]. Literature on AUD-related costs is, however, scarce and sometimes outdated. Future data on the costs of AUD might shed new light on cost-effectiveness of OPRM1 genotype-guided treatment allocation in AUD.
Economic evaluations, like the present cost-effectiveness analysis, are widely used to inform policy makers about which treatment innovations should be reimbursed or promoted [24, 26]. Such evaluations have become increasingly important, given the rising healthcare costs worldwide. Information about the potential benefits of an innovation for prevention, diagnosis or treatment at acceptable costs is critical to keep healthcare budgets sustainable. Cost-effectiveness studies of pharmacological interventions for AUD have shown that pharmacotherapy for AUD is highly cost-effective [38]. Adding pharmacogenetic treatment allocation to the treatment algorithm is associated with increased costs. In this study we showed under which conditions this intervention could be of potential societal value in the treatment of AUD patients. This evidence is of great value, as evidence surrounding the most important parameter, the increased effectiveness of naltrexone after pharmacogenetic matching is still contradictory. As AUD-related costs are estimated at billions of Euros worldwide, a relative simple intervention with even a small effect to improve pharmacological treatment outcome, like genetic screening, could prevent tremendous harm and societal costs [4].
Though the potential clinical utility of genetic screening seems promising, integration of genetic services into clinical practice is not fully supported by all healthcare professionals [34]. In a recent meta-analysis, four major barriers for implementation of genotype-guided treatment allocation were identified: lack of knowledge/skills, lack of infrastructure, ethical, legal and social issues, and lack of evidence. Barriers related to insufficient knowledge and skills are most frequently cited in literature [39]. This warrants education and training of physicians on genetics and genetic testing to develop pharmacogenetic competencies required in clinical practice. Furthermore, system-level barriers (lack of access to genetic services, time constraints) are noted as significant impediments to primary-care providers integrating genetics into their practice [39]. Development of easy accessible genetic services and coverage by health insurance could greatly contribute to the implementation of genotype-guided treatment allocation in clinical practice. Importantly, personalizing pharmacotherapy could impact the potential motivational aspects in AUD patients. This could increase pharmacotherapy coverage and treatment adherence in AUD patients, and therefore have a huge public health impact.
Finally, barriers related to scientific evidence of genetics services are identified [39]. The results of the cost-effectiveness analysis presented here do support the need for further development of strategies to implement genotype-guided treatment allocation for AUD patients. In future, recommendations on genotype-guided treatment allocation should be incorporated in treatment guidelines, including those on AUD. For example, OPRM1 genotype-guided treatment allocation is not mentioned in current guidelines [5, 6, 25, 40]. Cost-effectiveness analyses of pharmacogenetic strategies are therefore urgently needed, to further optimize clinical practice.
Moreover, several other potential candidate genes for genotype-guided treatment allocation in AUD have been suggested. For example, potential candidates possibly related to the effectiveness of acamprosate are the DRD2, GABRA6, GABRB2, and GATA4 genotypes [23, 41]. If patient-treatment matching could be further optimized by adding a list of genes predictive for treatment response to a range of medications, cost-effectiveness could increase even more. As such, future evidence on genotype-guided treatment allocation in AUD could further optimize the treatment of AUD patients.
The presented model has several assumptions that need to be taken into account when interpreting the results of this study. First, the risk-value used in the base-case scenario was based on a recent meta-analysis evaluating the efficacy of naltrexone in AUD G-allele carriers versus AA homozygotes [15]. Recent evidence did not confirm the conclusions of this meta-analysis [22]. Our findings provide an indication what added effectiveness in G-allele carriers minimally needs to be achieved to let OPRM1 screening be the preferable strategy in AUD treatment from a cost-effectiveness perspective.
Next, in our study both AA homozygotes and the non-genotyping arm received acamprosate in 50% of cases. There is currently no evidence that in AA homozygotes, acamprosate is actually better than naltrexone. Scientific evidence mainly focused on the association between naltrexone efficacy and the OPRM1 G-allele. In general, there appears to be no difference between acamprosate and naltrexone in controlling alcohol consumption, as shown by Jonas et al. [11] Studies on the efficacy of acamprosate in AA homozygotes are highly needed. To minimize the risk of overestimation of cost-effectiveness, we applied a conservative approach assuming similar effectiveness for naltrexone and acamprosate. We also wanted the control condition to resemble the current practice as much as possible, to show potential benefit compared to current practice. Therefore, we chose 50% exposure to acamprosate in the AA-homozygous group and in the non-genotyping arm.
In our model, treatment allocation is unchanged over the one-year iteration period. It has to be acknowledged that current treatment guidelines for AUD do not mention a strict evaluation window for (pharmacological) interventions, unlike for example for antidepressant medication [42]. Moreover, in clinical practice a relapse is not necessarily an indication that the medication is ineffective, or a common reason for medication switch. On the contrary, in case of relapse it cannot be ruled out that the medication provided is ineffective, and patients should better switch to other potentially more effective medications. If this were true, the current model might overestimate the cost-effectiveness of pharmacogenetic treatment allocation of naltrexone in AUD.
The estimated probabilities of relapse with acamprosate or naltrexone treatment should be interpreted carefully. We assumed that both drugs are equally effective. However, in clinical practice their effectiveness varies highly between patients and across studies [10, 11]. If acamprosate happens to be more effective than naltrexone, the current results overestimate the cost-effectiveness of OPRM1 genotype-guided treatment allocation, or vice versa. For our effectiveness parameters, we used a meta-analyses from a NICE guideline, which had 2 major limitations [25]. First, the time horizon of the model in this study was 12 months. The meta-analyses on the effectiveness of naltrexone and acamprosate in AUD included only studies with a time horizon of 3–6 months. Since in clinical practice naltrexone and acamprosate are commonly prescribed for more than 3–6 months, results were inferred to 1-year outcome [38]. The extrapolation of the data over a 12-month period, could have introduced uncertainty in our model. If the effectiveness of naltrexone or acamprosate declines after 3 or 6 months, this could have led to an overestimation of our results. Second, in all trials used in the meta-analyses, pharmacotherapy was used as an adjunct to psychological therapies. The authors of these analyses assumed that any differences in effect were related to pharmacotherapy, as opposed to the psychological therapies. This assumption could have also led to an overestimation of our results, as the effects of naltrexone or acamprosate could have been limited.
Also, some studies do suggest that acamprosate might be more effective in the long-term, compared to naltrexone [5]. In contrast, several other studies favor efficacy of naltrexone in the treatment of AUD [9, 10, 16, 43]. If the effectiveness of naltrexone indeed declines after 6 months, this could lead to an overestimation of the cost-effectiveness in our model. In addition, it has to be taken into account that European and US-based trials in AUD are often incomparable, given the common differences in patient characteristics in these studies, including differences in AUD severity and medication effectiveness [10, 44]. However, in our sensitivity analyses, we showed that the probability to relapse with one of the 2 drugs has limited effect on our results.
This study focused on the return to heavy drinking as definition of relapse, assuming that this is most relevant in terms of costs of relapse. It has been suggested that naltrexone is more effective in reducing the total amount of alcohol consumption, whereas acamprosate may be more effective in obtaining complete abstinence [11]. Results of the cost-effectiveness analysis do depend on the definition of relapse. For example, including any level of drinking as outcome (instead of heavy drinking) might increase or decrease the cost-effectiveness. For running cost-effectiveness analyses, the outcome measure should be comparable between both arms and should be translated into monetary costs. The only available cost estimates do not specify specific drinking levels. Therefore, we decided to compare relapse versus abstinence, though this does not fully match the current evidence on the efficacy of naltrexone versus acamprosate.
Acamprosate was chosen as an alternative for naltrexone in the pharmacological treatment of AUD. There are several other effective drugs available for AUD treatment, including for instance disulfiram, baclofen, and topiramate [13, 43]. Some studies show that disulfiram might be less effective, compared to naltrexone and acamprosate [11]. Evidence for baclofen and topiramate in AUD is still limited, with their use in AUD being mainly off-label [5, 38, 45]. Moreover, pharmacotherapy is often combined with psychosocial interventions, which might influence the cost-effectiveness. In future, it may be needed for additional treatment options in cost-effectiveness analysis as well.
In conclusion, our cost-effectiveness analysis showed that genotype-guided treatment allocation of naltrexone in patients with AUD can be a cost-effective strategy, compared to the random (non-genotype guided) allocation of acamprosate or naltrexone. However, uncertainty surrounds the evidence for pharmacogenetic treatment allocation of naltrexone in AUD, and supported by our sensitivity analyses, more studies on the effectiveness of naltrexone in G-allele carriers are needed to affirm the cost-effectiveness of genotype-guided treatment allocation in AUD. This intervention could, however, be of potential value in the treatment of AUD patients at acceptable costs, if evidence of increased effectiveness is confirmed and costs of genotyping decrease.
Disclosure Statement
The authors declare that there are no conflicts of interest to disclose.
References
- 1.WHO: Health Statistics and Information Systems - Metrics: Disability-Adjusted Life Year (DALY), 2016.
- 2.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 3.World Health., Organisation (WHO) Global Status Report on Alcohol and Health. 2014 [Google Scholar]
- 4.Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19:155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- 5.Trimbos Instituut. Multidisciplinaire Richtlijn Stoornissen in Het Gebruik Van Alcohol (in Dutch) 2009:2016. [Google Scholar]
- 6.Nederlands Huisartsen., Genootschap NHG-Standaard Problematisch Alcoholgebruik (in Dutch) 2016 [PubMed] [Google Scholar]
- 7.Roozen HG, de Waart R, van der Windt DA, van den Brink W, de Jong CA, Kerkhof AJ. A systematic review of the effectiveness of naltrexone in the maintenance treatment of opioid and alcohol dependence. Eur Neuropsychopharmacol. 2006;16:311–323. doi: 10.1016/j.euroneuro.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Srisurapanont M, Jarusuraisin N. Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2005;8:267–280. doi: 10.1017/S1461145704004997. [DOI] [PubMed] [Google Scholar]
- 9.Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donoghue K, Elzerbi C, Saunders R, Whittington C, Pilling S, Drummond C. The efficacy of acamprosate and naltrexone in the treatment of alcohol dependence, Europe versus the rest of the world: a meta-analysis. Addiction. 2015;110:920–930. doi: 10.1111/add.12875. [DOI] [PubMed] [Google Scholar]
- 11.Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- 12.de Graaf R, Ten Have M, van Dorsselaer., S De Psychische Gezondheid Van de Nederlandse Bevolking. NEMESIS-2: Opzet En Eerste Resultaten (in Dutch). [Google Scholar]
- 13.Zorginstituut Nederland. Farmacotherapeutisch Kompas. 2016 [Google Scholar]
- 14.Mark TL, Kranzler HR, Poole VH, Hagen CA, McLeod C, Crosse S. Barriers to the use of medications to treat alcoholism. Am J Addict. 2003;12:281–294. [PubMed] [Google Scholar]
- 15.Chamorro AJ, Marcos M, Miron-Canelo JA, Pastor I, Gonzalez-Sarmiento R, Laso FJ. Association of μ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addict Biol. 2012;17:505–512. doi: 10.1111/j.1369-1600.2012.00442.x. [DOI] [PubMed] [Google Scholar]
- 16.Rubio G, Ponce G, Rodriguez-Jimenez R, Jimenez-Arriero MA, Hoenicka J, Palomo T. Clinical predictors of response to naltrexone in alcoholic patients: who benefits most from treatment with naltrexone? Alcohol Alcohol. 2005;40:227–233. doi: 10.1093/alcalc/agh151. [DOI] [PubMed] [Google Scholar]
- 17.Monterosso , JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O'Brien CP, Volpicelli JR. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict. 2001;10:258–268. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- 18.Bergen AW, Kokoszka J, Peterson R, Long JC, Virkkunen M, Linnoila M, Goldman D. Mu opioid receptor gene variants: lack of association with alcohol dependence. Mol Psychiatry. 1997;2:490–494. doi: 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- 19.Gelernter J, Kranzler H, Cubells J. Genetics of two mu opioid receptor gene (OPRM1) exon I polymorphisms: population studies, and allele frequencies in alcohol- and drug-dependent subjects. Mol Psychiatry. 1999;4:476–483. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- 20.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- 22.Oslin DW, Leong SH, Lynch KG, Berrettini W, O'Brien CP, Gordon AJ, Rukstalis M. Naltrexone vs placebo for the treatment of alcohol dependence: a randomized clinical trial. JAMA Psychiatry. 2015;72:430–437. doi: 10.1001/jamapsychiatry.2014.3053. [DOI] [PubMed] [Google Scholar]
- 23.Schellekens A. Farmacogenetica en de behandeling van verslaving (in Dutch) Nederlands Tijdschrift Voor Geneeskunde. 2013;157:A5725. [PubMed] [Google Scholar]
- 24.Drummond M. Oxford Medical Publications; 2005. Methods for the Economic Evaluation of Health Care Programmes, ed 3; p. 379. [Google Scholar]
- 25.National Institute for Health and Clinical Excellence . Leicester (UK): 2011. Alcohol-Use Disorders: Diagnosis, Assessment and Management of Harmful Drinking and Alcohol Dependence. [PubMed] [Google Scholar]
- 26.Briggs AH, Claxton K, Sculpher MJ. Oxford Handbooks in Health Economic Evaluation. Oxford: Oxford University Press; 2006. Decision Modelling for Health Economic Evaluation. [Google Scholar]
- 27.Hakkaart-van Roijen L, van der Linden N, Bouwmans C, Kanters T, Swan Tan., S Kostenhandleiding: Methodologie van Kostenonderzoek En Referentieprijzen Voor Economische Evaluaties in de Gezondheidszorg (in Dutch) 2015 [Google Scholar]
- 28.Polman PI, Visser ECM. Verslavingszorg in Beeld - Alcohol En Drugs (in Dutch) 2014 [Google Scholar]
- 29.KPMG BEA. Hoofddorp: KPMG; 2002. Kosten en Baten van Alcoholzorg En - Preventie, Eindrapport (in Dutch) [Google Scholar]
- 30.Zorginstituut Nederland. Richtlijnen voor Farmaco-Economisch Onderzoek. 2006:22. [Google Scholar]
- 31.Cleemput I, Neyt M, Thiry N, De Laet C, Leys M. Brussels: Health Care Knowledge Centre (KCE); 2008. Threshold Values for Cost-Effectiveness in Health Care Health Technology Assessment (HTA) KCE reports 100C (D/2008/10.273/96) [Google Scholar]
- 32.Arias A, Feinn R, Kranzler HR. Association of an Asn40Asp (A118G) polymorphism in the mu-opioid receptor gene with substance dependence: a meta-analysis. Drug Alcohol Depend. 2006;83:262–268. doi: 10.1016/j.drugalcdep.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 33.Boyle P. OUP Oxford; 2013. Alcohol: Science, Policy and Public Health. [Google Scholar]
- 34.Swen JJ, Guchelaar HJ. Just how feasible is pharmacogenetic testing in the primary healthcare setting? Pharmacogenomics. 2012;13:507–509. doi: 10.2217/pgs.12.19. [DOI] [PubMed] [Google Scholar]
- 35.Veenstra DL. The value of routine pharmacogenomic screening-Are we there yet? A perspective on the costs and benefits of routine screening-shouldn't everyone have this done? Clin Pharmacol Ther. 2016;99:164–166. doi: 10.1002/cpt.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, Dodel R, Ekman M, Faravelli C, Fratiglioni L, Gannon B, Jones DH, Jennum P, Jordanova A, Jonsson L, Karampampa K, Knapp M, Kobelt G, Kurth T, Lieb R, Linde M, Ljungcrantz C, Maercker A, Melin B, Moscarelli M, Musayev A, Norwood F, Preisig M, Pugliatti M, Rehm J, Salvador-Carulla L, Schlehofer B, Simon R, Steinhausen HC, Stovner LJ, Vallat JM, Van den Bergh P, van Os J, Vos P, Xu W, Wittchen HU, Jonsson B, Olesen J;, CDBE2010Study Group Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 37.de Wit GA, van Gils PF, Over EAB, Suijkerbuijk AWM, Lokkerbol J, Smit F, Mosca I, Spit WJ. Social Cost-Benefit Analysis of Regulatory Policies to Reduce Alcohol Use in The Netherlands, Dutch National Institute for Public Health and the Environment (RIVM) 2016:148. [Google Scholar]
- 38.Suijkerbuijk AW, van Gils PF, Greeven PG, de Wit., GA De kosteneffectiviteit van interventies gericht op verslaving aan alcohol en drugs (in Dutch) Tijdschrift Psychiatrie. 2015;57:498–507. [PubMed] [Google Scholar]
- 39.Mikat-Stevens NA, Larson IA, Tarini BA. Primary-care providers' perceived barriers to integration of genetics services: a systematic review of the literature. Genet Med. 2015;17:169–176. doi: 10.1038/gim.2014.101. [DOI] [PubMed] [Google Scholar]
- 40.Kleber HD, Weiss RD, Anton RF, Jr, George TP, Greenfield SF, Kosten TR, O'Brien CP, Rounsaville BJ, Strain EC, Ziedonis DM, Hennessy G, Connery HS, McIntyre JS, Charles SC, Anzia DJ, Cook IA, Finnerty MT, Johnson BR, Nininger JE, Summergrad P, Woods SM, Yager J, Pyles R, Cross CD, Peele R, Shemo JP, Lurie L, Walker RD, Barnovitz MA, Gray SH, Saxena S, Tonnu T, Kunkle R, Albert AB, Fochtmann LJ, Hart C, Regier D;, Work Group on Substance Use Disorders; American Psychiatric Association; Steering Committee on Practice Guidelines Treatment of patients with substance use disorders, second edition. American Psychiatric Association. Am J Psychiatry. 2007;164:5–123. [PubMed] [Google Scholar]
- 41.Kiefer F, Witt SH, Frank J, Richter A, Treutlein J, Lemenager T, Nothen MM, Cichon S, Batra A, Berner M, Wodarz N, Zimmermann US, Spanagel R, Wiedemann K, Smolka MN, Heinz A, Rietschel M, Mann K. Involvement of the atrial natriuretic peptide transcription factor GATA4 in alcohol dependence, relapse risk and treatment response to acamprosate. Pharmacogenomics J. 2011;11:368–374. doi: 10.1038/tpj.2010.51. [DOI] [PubMed] [Google Scholar]
- 42.Hermens ML, Oud M, Sinnema H, Nauta MH, Stikkelbroek Y, van Duin D, Wensing M. The multidisciplinary depression guideline for children and adolescents: an implementation study. Eur Child Adolesc Psychiatry. 2015;24:1207–1218. doi: 10.1007/s00787-014-0670-4. [DOI] [PubMed] [Google Scholar]
- 43.Soyka M, Rosner S. Opioid antagonists for pharmacological treatment of alcohol dependence - a critical review. Curr Drug Abuse Rev. 2008;1:280–291. doi: 10.2174/1874473710801030280. [DOI] [PubMed] [Google Scholar]
- 44.Center for., Substance Abuse T SAMHSA/CSAT Treatment Improvement Protocols; Incorporating Alcohol Pharmacotherapies Into Medical Practice. Rockville (MD), Substance Abuse and Mental Health Services Administration (US) 2009 [PubMed] [Google Scholar]
- 45.Crowley P. Long-term drug treatment of patients with alcohol dependence. Aust Prescr. 2015;38:41–43. doi: 10.18773/austprescr.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]