Abstract
Transplantation of stem cells is a promising potential therapy for central nervous system disease and injury. The capacity for self-renewal, proliferation of progenitor cells, and multi-lineage potential underscores the need for controlling stem cell fate. Furthermore, transplantation within a hostile environment can lead to significant cell death and limited therapeutic potential. Tissue-engineered materials have been developed to both regulate stem cell fate, increase transplanted cell viability, and improve therapeutic outcomes. Traditionally, regulation of stem cell differentiation has been driven through soluble signals, such as growth factors. While these signals are important, insoluble factors from the local microenvironment or extracellular matrix (ECM) molecules also contribute to stem cell activity and fate. Understanding the microenvironment factors that influence stem cell fate, such as mechanical properties, topography, and presentation of specific ECM ligands, is necessary for designing improved biomaterials. Here we review some of the microenvironment factors that regulate stem cell fate and how they can be incorporated into biomaterials as part of potential CNS therapies.
1. Introduction
Stem cell based therapies have garnered much interest and shown significant potential when used in a multitude of tissues for injuries and diseases. These strategies typically aim to replenish lost or defective cells and rely on the successful differentiation of stem cells into mature fates. The delivery of viable stem cells is achieved through either direct injection or incorporation within a supportive biomaterial. For many applications, the cells are cultured within the biomaterial in vitro then the material is implanted. In other applications, the stem cells and biomaterial precursor solution are injected into the tissue then the biomaterial reacts to form the supportive matrix. The high proliferation rates, ability for self-renewal, and potential to replace lost cell types make stem cells a potentially promising cell source for CNS therapeutics. High proliferation rates allow for the rapid expansion of cells into clinically relevant numbers, however, transplantation of undifferentiated stem cells can lead to tumor formation. Thus, purification to remove or limit the presence of undifferentiated stem cells must occur prior to transplantation (Johnson et al. 2010).
During development, stem cells are exposed to soluble and insoluble factors that regulate their proliferation, differentiation, and migration. In the developing CNS, stem cells give rise to more fate-restricted, tissue-specific stem and progenitor cells fated to become neurons, oligodendrocytes, and astrocytes, termed neural stem/progenitor cells (NSPCs). The main NSPCs in the maturing and adult CNS are neuroepithelial and radial glia stem cells located within the subventricular zone of the lateral ventricle and hippocampal subgranular zone (Kriegstein and Alvarez-Buylla 2009; Ma et al. 2005), while stem cells in the adult spinal cord are thought to be restricted to ependymal cells lining the ventricles of the central canal (Johansson et al. 1999; Sabelstrom et al. 2014; Weiss et al. 1996). Fate-mapping studies suggest a heterogeneity to NSPCs that display a regional diversity for areas in the brain and spinal cord segments, making identification and characterization of endogenous NSPCs difficult (Sabelstrom et al. 2014; Ihrie et al. 2011; Bonaguidi et al. 2011).
CNS trauma drives proliferation of endogenous NSPCs; however, the response is not capable of restoring all lost specialized cell types, and the proliferative cells mainly contribute to reactive gliosis, driving scar formation that limits recovery (Buffo et al. 2008; Fitch and Silver 2008; Cregg et al. 2014; Sabelstrom et al. 2014). For this reason, CNS stem cell therapies typically utilize exogenous cell transplantation over targeting endogenous stem cells. Two main types of stem cells have been utilized in CNS injuries: mesenchymal stem cells (MSCs) and NSPCs. MSCs are easily isolated from bone marrow providing for an autologous therapy, but they are potentially limited to indirect trophic and immunomodulatory support (Hofstetter et al. 2002; Parr et al. 2007; Tetzlaff et al. 2011; Urdzikova et al. 2014; Iyer et al. 2017). This review will mainly focus on NSPCs due to their proven potential to differentiate into important CNS cell types including astrocytes, oligodendrocytes, and neurons.
Direct injection of NSPCs into the injured CNS provides trophic support and replaces lost cells but is limited by poor viability of transplanted cells due to the harsh in vivo environment (Pearse et al. 2007). Additionally, long-distance migration of transplanted cells away from the transplant site has been documented which could lead to ectopic colony formation that could affect healthy tissue (Guzman et al. 2007; Li et al. 2003; Pearse et al. 2007; Sharp et al. 2014; Steward et al. 2014). Thus, it is necessary to protect the transplanted cells in a supportive biomaterial or artificial extracellular matrix (ECM). The ECM microenvironment encompasses ~20% of the adult brain volume and is a heterogeneous mixture of soluble and insoluble factors that each contribute to cell survival and fate (Nicholson and Sykova 1998; Novak and Kaye 2000; Hubert et al. 2009). The ability to design artificial ECM allows for inclusion of specific cues that affect NSPC differentiation and fate to improve CNS therapies.
Classically, soluble factors, such as morphogens, cytokines, and growth factors, have been used for controlling cell differentiation in vitro. Inclusion of these soluble factors, which are often expensive, in biomaterials requires additional design criteria including maintenance of bioactivity of the molecule, sustained release over a desired time window, and temporal control over dosages, all of which are difficult to do in vivo. Designing the biomaterial to incorporate insoluble factors that control NSPC fate would not only promote cell survival but also may limit the need for soluble cues. Many of these factors reside in the ECM, therefore understanding the dynamic ECM microenvironment can aid in developing improved CNS therapeutics.
The ECM composition, including both soluble and insoluble factors termed the matrisome, is dependent upon many factors and varies during development, maturation, injury, and location. Two methodologies that can probe the matrisome on a global scale are proteomics and bioinformatics. Proteomics using mass spectrometry techniques aid in evaluating complex ECM derived from tissue samples and cell culture systems. Solubilization or enzymatic digestion of the ECM into fragments allows for MS analysis. The fragmented ECM can be further fractionated by multiple properties, such as hydrophobicity and charge, for tandem MS to provide structural information that can determine amino acid sequences and post-translational modifications within the complex ECM mixture (Byron et al. 2013, Naba et al. 2017). MS database search tools are used to identify theoretical mass spectra of known proteins to the mass spectra of ECM protein samples (Naba et al. 2017). In addition, bioinformatics can help identify ECM constituents through gene-centric lists with NCBI Entrez Gene database as a reference that encompasses both mouse and human genes (Naba et al. 2016). These gene databases can further become annotation tools for large data sets and make peptide ion identification easier.
However, the source of the ECM is important with respect to fetal compared to adult tissue sources. Numerous studies have explored the aspects of fetal ECM, such as umbilical cord ECM, to support a higher tissue remodeling response in host implantation with better modulation of macrophage phenotypes (Sicari et al. 2012). Human umbilical cord ECM-based hydrogels showed higher GAG composition that also supported higher differentiation of neural stem cells in vitro and short gelation time in a rat cortical ischemic lesion in vivo (Koci et al. 2017). The plasticity of fetal porcine brain ECM provides the developmental cues to differentiate progenitor cells into specific mature cell types and support neural circuit formation (Caprile et al. 2017). Furthermore, the combination of proteins in fetal ECM aided in producing dense axonal networks with higher calcium signaling and spiking activity in neural network activities (Sood et al. 2015). Using the bioinformatics and proteomics tools to probe the fetal ECM can aid in our understanding of the necessary developmental cues required for neural differentiation and regeneration.
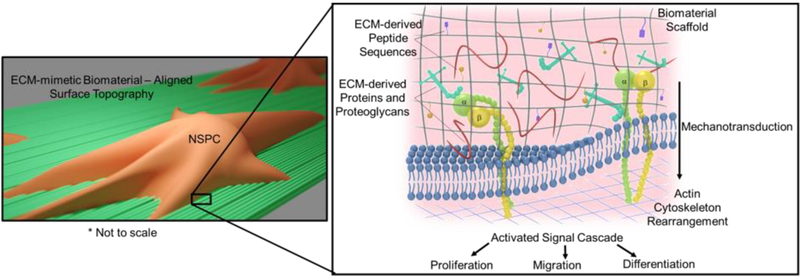
To this end, research using ECM-mimetic biomaterials have been engineered to manipulate NSPCs in vitro and in vivo. Particular focus has been given to the protein composition of the native ECM; however, the physical characteristics, such as topography and stiffness are also key factors in NSPC differentiation (Figure 1). It is important to note that many studies have utilized both soluble and insoluble factors to control NSPC differentiation and that a main benefit of many ECM-derived molecules is their ability to interact with soluble factors.
Figure 1:
Graphical representation of factors that can be incorporated into biomaterial design to control cell fate including surface topography, mechanotransduction, and ECM-derived peptides, proteins, and proteoglycans.
This review will focus on the current understanding of key microenvironment-specific ECM factors that influence NSPC fate and their incorporation into biomaterials for CNS therapies.
2. Biomechanical and material properties that can affect NSPC fate
The biomechanical properties of CNS tissue change throughout development, during maturation, and after injury and play a key role in NSPC differentiation (Clarke et al. 2009; Fiford and Bilston 2005; Cheng et al. 2008). Additionally, the CNS is heterogeneous with the grey matter having twice the stiffness of white matter, with this trend consistent across rodents and humans (Budday et al. 2017; Christ et al. 2010; Koser et al. 2015; Moeendarbary et al. 2017). Injury to the CNS drastically alters the mechanical properties of the tissue and may be a contributor to the lack of regeneration and recovery. In contrast to most other tissue injuries that result in fibrotic scar formation (Swift et al. 2013), CNS tissue softens at the injury site. Using atomic force microscopy indentation to measure elastic modulus the rat cortex showed a decrease in elastic modulus 8–10 days post-injury (acute stage) compared to uninjured or 3 weeks post-injury (sub-acute stage) in a stab injury model. Additionally in the same publication, crush injuries to the rat spinal cord at the C5 level led to similar decreases in elastic modulus compared uninured controls (Moeendarbary et al. 2017). These results show that CNS tissue softening after injury occurs in both the brain and spinal cord, and in both sharp and blunt injuries. In addition to mechanical properties, the ECM’s topography, alignment, and architecture also affect cell fate and activity. Understanding how these changes affect NSPC will provide important insights for designing biomaterials that could lead to better control over cell fate and improved therapeutic outcomes. For this section, we will provide a brief overview of the effects of ECM mechanical properties and topographic cues on NSPC fate.
2.1. Material stiffness affects NSPC fate
The material stiffness is known to affect cellular activities, such as migration, apoptosis, proliferation, and differentiation in multiple tissue types (Guilak et al. 2009). Dissociated rat NSPCs cultured on laminin-coated polyacrylamide gels with a stiffness of ~200 Pa (adult rat brain ~300 Pa) improved neuronal differentiation compared to stiffer gels (~9000 Pa), which generated more astrocytes. In the same article, similar results were found using fibrin hydrogels (Georges et al. 2006). Adult rat NSPCs were cultured on laminin-coated variable moduli interpenetrating polymer networks (vmIPNs) synthesized by polymerizing poly(ethylene glycol) within a polyacrylamide hydrogel. The stiffness or modulus of the vmIPNs could be adjusted from ~10–10,000 Pa. Softer vmIPNs (100–500 Pa) appeared to favor neurogenesis, and stiffer vmIPNs (1,000–10,000 Pa) promoted astrocyte differentiation. Interestingly, hydrogels with very soft moduli (~10 Pa, similar to the injured CNS) resulted in decreased cell spreading, proliferation, and differentiation (Saha et al. 2008).
Generally, a large range of moduli is reported for influencing cell differentiation because it is difficult to determine the exact modulus that drives cell fate decisions. NSPCs harvested from adult rat brains and cultured on methacrylamide chitosan biomaterials of variable moduli were capable of neuronal differentiation on soft substrates (<1,000 Pa) and oligodendrocyte differentiation on hard substrates (>7,000 Pa). Note that for these studies, oligodendrocyte maturation and myelination was improved on the soft substrates that favored neuronal differentiation (Leipzig and Shoichet 2009). Additionally, oligodendrocyte progenitors (cultured on polyacrylamide gels with moduli ~1,000 Pa) resulted in increased proliferation, migration, and differentiation into oligodendrocytes. The range of biomaterial moduli that appears to favor oligodendrocyte differentiation, (myelination) and neuronal differentiation mimics the range of stiffness of the white matter (between 500–2000 Pa) (Weickenmeier et al. 2016; Budday et al. 2015; Christ et al. 2010).
2.2. Mechanotransduction via focal adhesions and topography affect NSPC fate
The ECM plays a major role in dictating a tissue’s biomechanical properties and in mechanotransduction of signals to the cells through focal adhesion complexes that link their cytoskeletons to the ECM. Integrins, adaptors, and signaling proteins together form the focal adhesion protein complexes that connect the actomyosin cytoskeleton and the ECM (DuFort et al. 2011a). Focal adhesions can consist of more than 100 proteins and serve as channels for signal transduction in response to physical forces (DuFort et al. 2011b; Stukel and Willits 2016). The sequence of force transduction starts from integrin-ECM interactions and proceeds through adaptor proteins bound to actin. The forces are then transmitted from the actin filaments through the myosin head (Roca-Cusachs et al. 2012). The mechanism of mechanotransduction is not fully understood, but multiple downstream signaling pathways have been shown to be linked with mechanotransduction (Gattazzo et al. 2014). For example, the activation of focal adhesion kinase (FAK) by integrin binding regulates neural differentiation. Phosphorylation of FAK contributes to expression of the mature neuronal marker and dendritic protein, microtubule associated protein 2 (MAP2) (Teo et al. 2013). Focal adhesions also regulate cytoskeleton rearrangement and organization through the Rho/ROCK, Src family kinases, and ERK1/2 signaling pathways (McBeath et al. 2004; Roca-Cusachs et al. 2012; Mammoto et al. 2012). The inhibition of integrin β1 mediated-binding and the Rho-associated signaling pathway, a key pathway in mechanotransduction, reduced NSPC differentiation (Yang et al. 2014). Blocking the MEK-ERK signaling pathway with a small molecule inhibitor (U0126) decreased human NSPC adhesion, spreading, neurite outgrowth, and differentiation into neurons or glia (Yang et al. 2013).
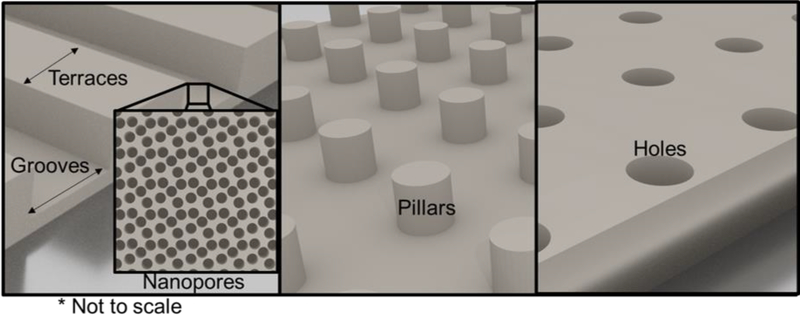
In addition to the biomechanical properties, the topography of the ECM substrate also affects cell fate. The ECM topography can affect cell shape by regulating cell adhesion and cytoskeleton arrangement, which applies mechanical forces to the cell that are then transduced into a chemical response. Cells sense topographic cues including 3D and 2D environments, substrate dimensions, and geometry at nano- and micro- scales (Guilak et al. 2009). Control of surface topography can be achieved using micro-patterned substrates, however terminology describing the type of patterning is not uniform throughout the field. For this review, a graphical depiction of types of patterning is displayed in Figure 2. A groove is defined as a cut or valley in the substrate and the terrace is the distance between grooves. Note that studies have also used topographies not centered on grooves and terraces but on holes, pillars, and other 3D shapes (Moe et al. 2012). Adult rat hippocampal NSPCs cultured on a laminin-modified polystyrene micropatterned substrate (13 μm terrace width, 16 μm groove width, 4 μm groove depth) exhibited over 75% alignment in the groove direction and increased expression of βIII-tubulin (neuronal differentiation marker) compared to a planar substrate (Recknor et al. 2006). This indicates that the 3D topography of the substrate promotes neuronal differentiation of NSPCs. In another study, NSPC differentiation on a micropatterned substrate (1.5 μm terrace width, 1.5 μm groove width, 625 nm groove depth) that had 10 nm nanopores on the surface of the grooves promoted neuronal differentiation and increased expression of focal adhesion proteins compared to a flat substrate (Yang et al. 2014).
Figure 2:
Graphical representation of common micropatterned surfaces with controllable terrace width, groove width and depth, pillar height and diameter, hole depth and diameter, and inclusion of nanopores.
Surface topography can also influence the mechanotransduction of physical properties to cellular activity through interactions with focal adhesions. Binding of focal adhesions induces cytoskeleton rearrangement and generates tension within the cytoskeleton, which is transduced to the cell nucleus and causes reorganization of the nucleus that alters gene expression and affects cell fate. A fibronectin-coated micropatterned substrate with smaller groove width (300 nm) enhanced focal adhesion formation of NSPCs and promoted differentiation toward neurons and astrocytes compared to a substrate with larger groove size (1500 nm). Image analysis indicated that smaller grooves may provide more contact points that facilitate NSPC focal adhesion formation, and western blot results revealed that phosphorylated FAK was upregulated in NSPCs cultured on substrates with smaller groove size (Yang et al. 2013). In addition, the MEK-ERK signaling pathway has been shown to be linked with mechanostransduction-induced NSPC differentiation (Yang et al. 2013). Vaysse et al. fabricated laminin-coated micropatterned substrates with 25 μm deep microchannels. Adult NSPC differentiation was evaluated on different terrace and groove widths (Béduer et al. 2012). Larger terrace and groove width (20/20 μm) promoted more neuronal differentiation of NSPCs compared to narrower substrates (5/5 μm). Individual neurons had more neurites and longer neurite length on the wider micropatterned substrate compared to the narrower ones. The direction of neurite extension was also affected by substrate topography, where neurites extended in all directions on flat substrates, while neurites cultured on aligned micropatterned substrates extended in the direction of alignment.
Electrospun fibers of variable fiber diameter and alignment also effect NSPC differentiation. When cultured on laminin-coated electrospun fibers, rat hippocampus-derived adult NSPCs showed a 40% increase in oligodendrocyte differentiation on ~300 nm fibers and a 20% increase in neuronal differentiation on 750 nm fibers compared to a laminin-coated tissue culture polystyrene surface. The NSPCs on 300 nm fibers also showed a stretched morphology that is similar to oligodendrocytes and extended in multiple directions to follow the underlying fibers (Christopherson et al. 2009). Adult NSPCs cultured on either aligned or random electrospun fibers showed greater neuronal differentiation and cell body elongation along the fiber axis of aligned substrates (Lim et al. 2010; Mahairaki et al. 2011). NSPCs induced with retinoic acid differentiated into a higher percentage of neurons on aligned fibers over random fibers or unpatterened surfaces. Interestingly, alignment led to decreased survival and attachment rates of differentiated oligodendrocytes compared to random fibers (Lim et al. 2010).
In addition to 3D/2D structure and substrate dimensions, the geometry of the substrate also affects cell fate. Moe et al. used multi-architecture arrays to introduce multiple, distinct topographies on a single substrate. Anisotropic grooves on the substrate (250 nm or 2 μm wide grooves) promoted neuronal differentiation of primary murine NSPCs, while an isotropic substrate with either holes (2 μm diameter) or pillars (1 μm diameter) promoted glial differentiation (Moe et al. 2012). Similar results were also reported by the same group on human embryonic stem cell-derived NSPCs where the anisotropic pattern promoted neuronal differentiation, and the isotropic pattern promoted glial differentiation (Ankam et al. 2013).
Tuning both stiffness and topography of the biomaterial should enable more control over NSPC differentiation. To this end, aligned fibrillar fibrin hydrogels were fabricated with a stiffness of ~1,000 Pa and promoted increased neuronal differentiation over randomly oriented fibers (Yao et al. 2016). When transplanted into a rat spinal cord dorsal hemisection, endogenous neural crest cells migrated along the aligned fibers, and axonal extension into the transplant occurred along the fibers to form axonal “cables”. Recent work evaluated the differentiation of fetal NSPCs on aligned polycaprolactone electrospun fiber mats with large (mimicking blood vessel topography, 10 μm) or small (mimicking radial glial process topography, 800 nm) fiber diameters with or without laminin (Czeisler et al. 2016). The smaller fiber diameters improved neuronal differentiation, axonal extension, and migration compared to the large fibers. These studies suggest that both alignment and surface topography of the ECM and biomaterial play roles in NSPC differentiation and presentation of bioactive cues.
3. Effect of ECM-derived constituents on NSPC fate and their use in biomaterials
The modulus, topography, and effect on cell shape appear to be key drivers of NSPC fate; however, important ECM-derived factors can be incorporated into biomaterials to provide additional regulatory cues. Discovery of these factors is typically achieved through studying the predominant ECM proteins in the developing, mature, and injured CNS. During development, NSPCs are seen migrating and differentiating through the CNS in heterogeneous environments, which makes the constituents of their microenvironment difficult to analyze. In the mature CNS, adult stem cells reside in unique specialized niches, termed fractones due to the fractal ultrastructure that is essential to regulating these cells. Research into fractone constituents will improve our understanding of what factors are necessary to maintain the stemness of cells and potentially what factors specify cell fates. Laminin, collagen IV, and heparan sulfate proteoglycans (HSPGs) are major constituents of fractones (Mercier 2016; Mercier et al. 2002) Table 1.
Table 1:
Summary of the major ECM components of the CNS.
| Location | ECM Component | Role |
|---|---|---|
| Major ECM constituents of the stem cell niche/fractone | Laminin | Cell adhesion, migration, guidance |
| Collagen | Cell adhesion, migration, guidance; structural integrity | |
| Heparin sulfate proteoglycans | Associate with growth factors to regulate differentiation | |
| Additional constituents of the CNS ECM | Fibronectin | Expression coincides with phases of neuronal differentiation during development; Aids cell adhesion, migration, and survival |
| Tenascin-C, Tenascin-R | TN-C: Cell adhesion; associate with growth factors to regulate differentiation TN-R: Associated with NSPC proliferation and neuronal differentiation |
|
| Chondroitin sulfate proteoglycans | Stabilize synaptic connections. Major component of the inhibitory glial scar formed by astrocytes after injury. | |
| Hyaluronic acid | Cell adhesion. Aids differentiation in the developing CNS. Supportive matrix for NSPC transplantation that also decreases immune response and reactive gliosis. |
3.1. Major ECM components of the stem cell niche/fractones
Laminin is a family of trimetric proteins containing α, β, and γ subunits with only certain subunits found in mammals and is a key component of basal lamina, a layer of ECM found in many tissues including the brain and blood vessels in the CNS (Nirwane and Yao 2018). Laminin-α1β1γ1, a commonly researched isoform also termed laminin-1 or laminin-111, is known to regulate axonal guidance, neuronal extension, and synaptic formation (Barros et al. 2011; Franco and Muller 2011; Nirwane and Yao 2018). Loss of laminin or laminin isoforms results in neurological impairment or death due to decreased neuronal survival, migration, and guidance (Coles et al. 2006; Franco and Muller 2011; Ichikawa-Tomikawa et al. 2012). Many laminin isoforms (α1-α5, β1-β4, and γ1-γ3) are present throughout the developing CNS - for a detailed review of laminin expression in the CNS refer to Nirwane and Yao, 2018.
Laminin coatings are commonly used to improve cell adhesion to substrates. Studies have shown laminin substrates and laminin-rich Matrigel® produced robust neuronal differentiation of human embryonic stem cells compared to fibronectin or collagen I substrates (Ma et al. 2008). This is possibly due to the interactions of laminin with integrin α6 and β1 subunits on cell surfaces, suggesting laminin as a key factor to induce neuronal over glial differentiation.
CNS injury increases basal membrane deposition, which largely consists of laminin, around the lesion sites, thus modulating binding of growth factors. Astrocytes interact with the basal membrane deposits and contribute to scar formation by contracting or constricting the scar around the lesion area (Stichel and Muller 1998). However, after injury, expression levels of laminin within the lesion area are significantly decreased, possibly contributing to the lack of axonal extension into and through the lesion (Moeendarbary et al. 2017).
Laminin and laminin-derived peptides were tethered to substrates at varying densities to determine the optimal level for neuronal differentiation (Yang et al. 2015; Dodla and Bellamkonda 2006). Note, a significant decrease in optimal concentration was measured when switching from tissue culture plastic substrates to 3D culture within poly(ethylene glycol) hydrogels (Yang et al. 2015). 3D culture of neurospheres from murine NSPC within methylcellulose scaffolds functionalized with the peptide IKVAV, derived from the laminin α1 subunit, upregulated expression of neuronal and oligodendrocyte precursor markers (Stabenfeldt et al. 2010). Self-assembled nanofiber networks formed from amphiphilic peptides designed to present the peptide IKVAV induced differentiation of NSPCs into neurons over oligodendrocytes and astrocytes, showing the potential for laminin α1 to drive neuronal differentiation (Mammadov et al. 2014; Silva et al. 2004).
Collagen is a major constituent of ECM throughout the body but is less prevalent in the CNS, limited largely to the meninges and basement membranes. There are twenty-nine types of collagens, with the possibility of various isoforms for each type and each having a distinct function. Thus, collagens represent a large, diverse family of extracellular proteins characterized by a triple helical quaternary structure. Collagen type I, IV, and XVIII may play a role in axonal guidance during neural development with collagen IX suggested as a repellant for growing axons due to its chondroitin sulfate glycosylation (Ring et al. 1996; Hubert et al. 2009; Klapka and Muller 2006; Leclere et al. 2007; Rautavuoma et al. 2004; Schneider and Granato 2006; Xiao and Baier 2007). Collagens and collagen-derived peptide sequences largely interact with cells through α1β1 and α2β1 integrins. Importantly, collagen I, IV, and XVIII have been found in fractones, the ECM microenvironment of the subventricular zone adult SC niche, where they may be involved in controlling neurogenesis (Mercier et al. 2002; Kerever et al. 2007; Mercier 2016). Incorporation of collagen I-derived peptide sequences aided neuronal differentiation from NSPCs (Cooke et al. 2010; Nakajima et al. 2007; O’Connor et al. 2001), whereas collagen IV reduced neuronal differentiation (Cooke et al. 2010). On the other hand, fibroblasts upregulate collagen I and IV following CNS injury, leading to deposition within the ECM and interaction with reactive astrocytes that promote glial scar formation (Hara et al. 2017; Liesi and Kauppila 2002; Neo and Tang 2017).
Collagens have long been used as biomaterials to support cell transplantation in the CNS and other tissue types. Primary NSPCs from rat neuroepithelium dispersed in type I collagen containing basic fibroblast growth factor (bFGF) provided cues for neuronal differentiation with functional receptors, indicating glutamatergic and GABAergic activity (Ma et al. 2004). Furthermore, these neurons were able to show recycling of synaptic vesicles, suggesting functional synaptic connections. Complexes of NSPCs and collagen I promoted neuronal differentiation and synapse formation when transplanted into rat brains under cerebral ischemia (Yu et al. 2010).
Incorporation of cell-adhesive collagen-derived peptide sequences into biomaterials enables interaction with integrins without the need for purified collagen. Human NSPCs adhered to the GFOGER peptide sequence, derived from collagen type 1, α1 chain, through the interaction with β1 integrin and differentiated into neurons and astrocytes (Que et al. 2018). Recently, collagen-based hydrogels were fabricated with mechanical properties tailored to mimic the developing spinal cord and incorporated with hyaluronic acid and laminin. These biomaterials demonstrated directed differentiation of NSPCs to oligodendrocytes in vitro and improved functional recovery when transplanted into a rat unilateral cervical contusion SCI model (Geissler et al. 2018; Hatami et al. 2009).
As mentioned earlier, an important benefit of many ECM-derived molecules is their interaction with growth factors. To this end, heparin sulfate proteoglycans (HSPGs) play a key role in NSPC differentiation through their association with FGF and other growth factors (Ford-Perriss et al. 2003). HSPGs are present in all tissues and consist of two main types: 1) perlecans that are deposited within the ECM, and 2) syndecans located on cell surfaces (Cui et al. 2013). Perlecan in the fractone aids in regulation of FGF-2 signaling, and perlecan deficiency blocks FGF-2 from playing a role in neurosphere formation (Kerever et al. 2007). Perlecan deficiency in telencephalons led to decreased neurogenesis in the neuroepithelium during development and lower amounts of neurons within the cortical plate and subplate of the neocortex (Giros et al. 2007). Syndecans are expressed by cells in the adult stem cell niche at times of high NSPC proliferation and migration (Ford-Perriss et al. 2003). Syndecan-1 and syndecan-4 localize in the stem cell niche in the ventricular zone of the brain during development (Ford-Perriss et al. 2003). Syndecan-3 is found on the surface of neurons during development and may promote neurite extension (Raulo et al. 2005). Therefore, understanding the role of perlecans and syndecans in the developing, mature, and injured CNS may aid in future biomaterial design.
The role of HSPGs in CNS injury is largely unknown apart from if the HSPGs have increased or decreased expression. After brain injury in adult mice, all syndecan forms were upregulated around necrotic tissue and mRNA expression levels peaked at day 7 (Iseki et al. 2002). After CNS injury, there are higher levels of sulfated glycosaminoglycan (GAG) side chains in the HSPGs, increasing the association of growth factors with the HSPGs (Properzi et al. 2008). For example, upregulation of syndecan-1 in astrocytes at the injury site may increase FGF concentrations and promote astrogliosis (Properzi et al. 2008). Incorporating HSPGs into ECM-mimetic material design to influence NSPC fate is a promising technique when combined with growth factors. Agarose hydrogels conjugated with a laminin α1-derived peptide sequence (RLQVQLSIR, shown to bind syndecans) aided HSPG-regulated NSPC adhesion and proliferation (Yamada et al. 2012). In vitro testing has shown that perlecan-coated plates and perlecan addition to the media increased NSPC attachment and proliferation while suppressing astrocyte proliferation (Nakamura et al. 2015). Thus, HSPGs in the form of perlecans and syndecans can aid in regulating differentiation through the association with growth factors; however, the effect on proliferation or cell fate may be caused more by the growth factor than HSPGs.
3.2. Additional major ECM constituents
Outside of the stem cell niche, the CNS ECM consists of a heterogeneous mixture of molecules that is largely maintained by astrocytes. Astrocytes are highly dynamic, heterogeneous throughout the CNS, and undergo reactive gliosis following injury. The ability to control astrocyte phenotype enables control over the ECM microenvironment or the production of either inhibitory or regenerative ECM. For example, incorporation of decellularized ECM derived from protoplasmic (grey matter) astrocyte phenotypes into hyaluronic acid hydrogels reduced the glial scar, improved axonal extension into the injured area, and had immunomodulatory effects in a rat spinal cord injury model (Thompson et al. 2018). The protoplasmic ECM was shown to have increased levels of axonal growth-promoting proteins including collagen 4α1, perlecan, and laminins (α1, α5, β1, and γ1) (Thompson et al. 2017).
Fibronectin, a glycoprotein, is transiently expressed in the developing spinal cord and coincides with phases of increased neuronal differentiation (Krolo et al. 1998). In the adult CNS, fibronectin is found in low levels but mainly deposited in the basement membrane (Lau et al. 2013). CNS injuries commonly lead to the formation of a cystic cavity surrounded by a glial scar; however, in some instances the injury area is dominated by fibrotic scars containing high amounts of fibronectin among other constituents (Cooper et al. 2018). Fibronectin is mostly associated with adhesion and motility for many cell types, including neural SCs, but has been shown to limit morphological differentiation of oligodendrocytes and remyelination (Lau et al. 2013; Siskova et al. 2006; Stoffels et al. 2013). Fibronectin dissolved in plasma can enter the CNS via disruption of the blood-brain barrier to promote migration and proliferation of oligodendrocyte precursors (Baron et al. 2002; Stoffels et al. 2015). Thus, fibronectin can be used to recruit and proliferate oligodendrocyte precursors but must be removed to allow maturation into myelin forming mature oligodendrocytes. Collagen-based scaffolds incorporated with fibronectin did improve survival and migration of transplanted NSPCs compared to cell transplants alone, but collagen scaffolds with laminin further improved cell survival and functional recovery in mice with traumatic brain injury (Tate et al. 2009). Most studies involving fibronectin incorporate the RGD/RGDS peptide sequence into biomaterials to enhance cell adhesion with limited focus on differentiation.
In addition to the major constituents of fractones, tenascin-C (TN-C) is an important glycoprotein involved in development of the CNS through regulation of cell adhesion and signaling (Faissner and Reinhard 2015; Garcion et al. 2004; Jones and Jones 2000). NSPCs in mammalian CNS are initially FGF-2 responsive but transition to EGF responsiveness that drives the production of neuronal over glial precursors (Garcion et al. 2004). TN-C aids the transition around embryonic day 13.5–15.5 from early neurogenic FGF-2 responsive cells to late gliogenic FGF-2 and EGF-responsive NSPCs in mice (Karus et al. 2011). Knockdown of EGF receptors results in a decrease in glial precursors and more production of neurons. Studies investigating loss of tenascin showed that FGF receptor 3-expressing immature astrocytes had delayed migration and increased proliferation of NSPCs suggesting a connection between TN-C and FGF/EGF regulation. Furthermore, TN-C deficiency resulted in a shift of the patterning genes Nkx6.1 and Nkx2.2 that correlated with increased GFAP-positive mature astrocytes. Thus, TN-C may contribute to proliferation and regulation of NSPCs and glial progenitors during development of the CNS (Karus et al. 2011).
TN-C is also present in the mature and injured CNS with adult TN-C deficient mice displaying a reduction in spontaneous recovery following spinal cord injury compared to wild-type mice. Additionally, overexpression of TN-C reduced the glial scar and lesion volume, and improved axonal sprouting and functional recovery (Chen et al. 2010). Berns et al. encapsulated neurospheres-derived cells in hydrogels containing aligned nanofibers of a self-assembled TN-C mimetic peptide and showed improved neurite growth possibly due to interaction with β1 integrin receptors (Berns et al. 2016). Exploration of the use of 2D surfaces and 3D hydrogel scaffolds with TN-C peptide epitopes showed that a 3D environment produced the extensive neurite growth and high neuronal marker expression of PC12 cells (Sever et al. 2018). The neuroactive peptides from TN-C can covalently modify the nanofibers to enhance adhesion of neurons (Ahmed et al. 2006). These TN-C peptide scaffolds highlight the potential of using ECM derived constituents to direct NSPC differentiation into neuronal fates upon transplantation into the injured CNS. In contrast, after adult traumatic brain injury in mice, TN-C expression is upregulated, localized over the lesion site, and associated with GFAP-expressing reactive astrocytes (Chen et al. 2010; Laywell et al. 1992). The role of TN-C and lack of regeneration or functional recovery following injury needs to be further investigated. Another member of the tenascin family, tenascin-R, is expressed solely in the CNS and has been linked to NSPC proliferation, neuronal differentiation, and generation of GABAergic neurons through β1 integrin interactions (Tsai et al. 2014; Hargus et al. 2008; Liao et al. 2008).
In development, chondroitin sulfate proteoglycans (CSPGs) modulate neural tissue morphology and function through their GAG side chains connected to a core protein. In the mature CNS, CSPGs maintain synaptic connections through the formation of perineuronal nets and are expressed by astrocytes throughout the brain and spinal cord. The molecules are also expressed by adult NSPCs and radial glia. CSPGs in the CNS are largely studied in the context of glial scar formation following injury. They are a key constituent that provides inhibitory chemical cues when deposited by reactive astrocytes around the injury site. Cleavage of the GAG side chains from the core protein of CSPGs via enzymatic treatment with chondroitinase ABC (chABC) improves axonal extension and cell migration into and through the injured area, with some studies showing improvements in functional outcomes (Hu et al. 2018; Bradbury et al. 2002; Bartus et al. 2014). The inhibitory CSPGs (aggrecan, brevican, neurocan, and phosphacan) are well-documented for their effects on cell migration and axonal growth, with limited study of their effects on NSPC fate (Siebert et al. 2014). Dissociated mouse NSPCs were formed into neurospheres with chABC in the media and resulted in astrogliosis over neurogenesis. Furthermore, chABC treatment affected NSPC differentiation in the neurospheres through regulation of FGF-2 (decreased neuronal differentiation) and EGF (decreased glial differentiation) (Sirko et al. 2010). Chondoitin sulfate GAG side chains (CS-GAGs) used without the core protein and modified with crosslinkable methacrylate functional groups were shown to retain incorporated FGF-2 for up to one week with minimal loss. Transplantation of encapsulated NSPCs within the FGF-2 loaded crosslinked CS-GAGs matrix showed maintenance of the undifferentiated state up to 4 weeks after injury in a rat model of traumatic brain injury (Betancur et al. 2017). This is attributed to the CS-GAGs’ ability to associate with the growth factor and limit diffusion of the protein out of the material. These studies suggest that NSPC differentiation is responsive to FGF-2 and EGF, which can be regulated by CS-GAGs. However, increased deposition of CSPGs by reactive astrocytes in the injured CNS causes a chemical barrier that limits axonal regeneration.
Hyaluronic acid (HA) is a major constituent of the CNS matrix. HA has domains that bind to TN-C and proteoglycans to form a HA-proteoglycan network that supports NSPCs (Ruoslahti 1996). HA is highly abundant during fetal brain development and surrounds NSPCs to aid differentiation in the spinal cord (Banerjee and Toole 1991; Meszar et al. 2008). Testing with monoclonal antibody for HA-binding protein found that HA was prevalent in the neuroectoderm during development and concentrated in the layer of neuroectoderm that contains dense neuronal processes (Banerjee and Toole 1991). Mouse embryonic NSPCs were cultured within methacrylate functionalized HA hydrogels that were crosslinked via photo-initiation. NSPCs encapsulated in soft hydrogels (compressive modulus ~3000 Pa) increased neuronal differentiation, while NSPCs encapsulated in stiffer hydrogels (compressive modulus ~5000 Pa) increased astrocyte differentiation (Seidlits et al. 2010). Encapsulation of human induced pluripotent-NSPCs as either single cell suspensions or cell aggregate spheroids within crosslinked HA hydrogels (compressive modulus of ~500 Pa) were capable of neuronal differentiation and neurite extension (Wu et al. 2017). HA has the beneficial roles of promoting axonal growth and reducing the effects of scarring from CNS injury. Hydrogels composed of high molecular-weight HA reduced the presence of immune cells and reactive astrocyte CSPG deposition at the lesion site in a rat dorsal hemisection spinal cord injury model (Khaing et al. 2011). Furthermore, HA hydrogels crosslinked through click chemistry have tunable moduli and shear thinning properties that allow for injection of a crosslinked material directly into the CNS lesion, making them an ideal biomaterial candidate for CNS therapies (Nimmo et al. 2011; Thompson et al. 2018; Owen et al. 2013; Smith et al. 2018).
4. Conclusion
This review focused largely on major constituents of the ECM microenvironment that affect NSPC differentiation. Here we reviewed both physical and chemical cues found in the ECM that drive NSPC differentiation and regulate cell fate. Physical properties, such as material stiffness and surface topography are important factors during development, maturation, and injury. Bioengineered materials can be tailored to provide specific mechanical and topographical signals, leading to increased differentiation of NSPCs into astrocytes, oligodendrocytes, or neurons. The biomaterials can also incorporate ECM-derived constituents that directly or indirectly influence NSPC differentiation. Major ECM components located in fractones, such as laminins, collagens, and HSPGs, aid in proliferation, migration, and differentiation through integrin-mediated binding or regulation of growth factors. Additional ECM constituents found throughout the CNS, including fibronectin, tenascin, and CSPGs, were also shown to affect NSPC differentiation.
Current work focuses on using proteomics and bioinformatics to compare the CNS matrisome differences during developmental stages, in various locations throughout the CNS, and after injury. This could lead to improved biomaterial designs that incorporate multiple factors at levels known to improve tissue regeneration. For example, it has been noted that ECM derived from fetal tissue may provide improved tissue regeneration compared to adult tissue (Ren et al. 2015).
It was previously mentioned that NSPCs are difficult to characterize due to the heterogeneous mixture of cells that is found within primary isolates that have regional diversity depending on the location in the brain and spinal cord from which they are obtained. This review has assumed the NSPCs used in the reported studies have the ability to differentiate into neural cell types including astrocytes, oligodendrocytes, and neurons. However, it should be noted that the NSPCs used different experimental studies are not, nor should they be expected to be, uniform and in many cases the NSPCs will contain other cell types that may influence the results. This heterogeneity could lead to research showing a specific ECM constituent promotes glial fates, while other research with the same ECM constituent promotes neuronal fates with the only difference in the experiments being the NSPCs used for the studies. Pre-sorting NSPCs prior to use can alleviate some heterogeneity from the cultures and allow for improved reproducibility. Determining and creating a universal classification system is difficult because many factors expressed by NSPCs are also in other cell types, meaning an array of markers must be used.
It is important to note that the use of potent soluble factors in vitro allows for exact temporal and dosage control of multiple factors to direct differentiation into specific neural cell types. These techniques have been fundamental to refining NSPC differentiation protocols to derive unique subtypes of neural cells. For example, NSPCs exposed to caudalizing agents (retinoic acid) will drive a spinal identity, then the cells can undergo dorsoventral patterning through exposure to cocktails of soluble factors (bone morphogenic proteins, sonic hedgehog, smoothened agonists, Notch inhibitors, FGF, Wnt) that can result in the generation of specific neural cell types (dorsal/ventral interneuron populations, motor neurons, glial restricted precursors, astrocytes). This level of control is not yet possible by solely relying on ECM-derived insoluble cues; however, the importance of the microenvironment is evident. Thus, it is important to consider the types of both soluble and insoluble factors used for development of CNS therapies as they play key roles in controlling NSPC fate and repopulation of desired cell types.
Highlights:
Neural stem cell fate is influenced by local microenvironment factors
Local microenvironment cues include mechanical and topographical signals
Presentation and abundance of specific microenvironment ligands affect cell fate
Knowing factors that affect neural stem cell fate will improve biomaterial design
Acknowledgements
This work was supported by NINDS R01 NS090617. The authors gratefully acknowledge editorial assistance from Jennifer Pardieck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed I, Liu HY, Mamiya PC, Ponery AS, Babu AN, Weik T, Schindler M, and Meiners S 2006. ‘Three-dimensional nanofibrillar surfaces covalently modified with tenascin-C-derived peptides enhance neuronal growth in vitro’, J Biomed Mater Res A, 76: 851–60. [DOI] [PubMed] [Google Scholar]
- Ankam S, Suryana M, Chan LY, Moe AA, Teo BK, Law JB, Sheetz MP, Low HY, and Yim EK 2013. ‘Substrate topography and size determine the fate of human embryonic stem cells to neuronal or glial lineage’, Acta Biomater, 9: 4535–45. [DOI] [PubMed] [Google Scholar]
- Banerjee SD, and Toole BP 1991. ‘Monoclonal antibody to chick embryo hyaluronan-binding protein: changes in distribution of binding protein during early brain development’, Dev Biol, 146: 186–97. [DOI] [PubMed] [Google Scholar]
- Baron W, Shattil SJ, and ffrench-Constant C 2002. ‘The oligodendrocyte precursor mitogen PDGF stimulates proliferation by activation of alpha(v)beta3 integrins’, EMBO J, 21: 1957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros CS, Franco SJ, and Muller U 2011. ‘Extracellular matrix: functions in the nervous system’, Cold Spring Harb Perspect Biol, 3: a005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yanez-Munoz RJ, Rogers JH, Schneider BL, Muir EM, and Bradbury EJ 2014. ‘Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury’, J Neurosci, 34: 4822–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béduer Amélie, Vieu Christophe, Arnauduc Florent, Sol Jean-Christophe, Loubinoux Isabelle, and Vaysse Laurence. 2012. ‘Engineering of adult human neural stem cells differentiation through surface micropatterning’, Biomaterials, 33: 504–14. [DOI] [PubMed] [Google Scholar]
- Berns EJ, Alvarez Z, Goldberger JE, Boekhoven J, Kessler JA, Kuhn HG, and Stupp SI 2016. ‘A tenascin-C mimetic peptide amphiphile nanofiber gel promotes neurite outgrowth and cell migration of neurosphere-derived cells’, Acta Biomater, 37: 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur MI, Mason HD, Alvarado-Velez M, Holmes PV, Bellamkonda RV, and Karumbaiah L 2017. ‘Chondroitin Sulfate Glycosaminoglycan Matrices Promote Neural Stem Cell Maintenance and Neuroprotection Post-Traumatic Brain Injury’, ACS Biomater Sci Eng, 3: 420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, and Song H 2011. ‘In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics’, Cell, 145: 1142–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, and McMahon SB 2002. ‘Chondroitinase ABC promotes functional recovery after spinal cord injury’, Nature, 416: 636–40. [DOI] [PubMed] [Google Scholar]
- Budday S, Nay R, de Rooij R, Steinmann P, Wyrobek T, Ovaert TC, and Kuhl E 2015. ‘Mechanical properties of gray and white matter brain tissue by indentation’, J Mech Behav Biomed Mater, 46: 318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budday S, Sommer G, Birkl C, Langkammer C, Haybaeck J, Kohnert J, Bauer M, Paulsen F, Steinmann P, Kuhl E, and Holzapfel GA 2017. ‘Mechanical characterization of human brain tissue’, Acta Biomater, 48: 319–40. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, and Gotz M 2008. ‘Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain’, Proc Natl Acad Sci U S A, 105: 3581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron A, Humphries JD, and Humphries JM 2013. ‘Defining the extracellular matrix using proteomics’, International Journal of Experimental Pathology, 94: 75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprile T, and Montecinos H 2017. ‘Analyzing the role of extracellular matrix during nervous system development to advance new regenerative strategies’, Neural regeneration research, 12(4): 566–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Joon Lee H, Jakovcevski I, Shah R, Bhagat N, Loers G, Liu HY, Meiners S, Taschenberger G, Kugler S, Irintchev A, and Schachner M 2010. ‘The extracellular matrix glycoprotein tenascin-C is beneficial for spinal cord regeneration’, Mol Ther, 18: 1769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Clarke EC, and Bilston LE 2008. ‘Rheological properties of the tissues of the central nervous system: a review’, Med Eng Phys, 30: 1318–37. [DOI] [PubMed] [Google Scholar]
- Christ AF, Franze K, Gautier H, Moshayedi P, Fawcett J, Franklin RJ, Karadottir RT, and Guck J 2010. ‘Mechanical difference between white and gray matter in the rat cerebellum measured by scanning force microscopy’, J Biomech, 43: 2986–92. [DOI] [PubMed] [Google Scholar]
- Christopherson GT, Song H, and Mao HQ 2009. ‘The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation’, Biomaterials, 30: 556–64. [DOI] [PubMed] [Google Scholar]
- Clarke EC, Cheng S, and Bilston LE 2009. ‘The mechanical properties of neonatal rat spinal cord in vitro, and comparisons with adult’, J Biomech, 42: 1397–402. [DOI] [PubMed] [Google Scholar]
- Coles EG, Gammill LS, Miner JH, and Bronner-Fraser M 2006. ‘Abnormalities in neural crest cell migration in laminin alpha5 mutant mice’, Dev Biol, 289: 218–28. [DOI] [PubMed] [Google Scholar]
- Cooke MJ, Zahir T, Phillips SR, Shah DS, Athey D, Lakey JH, Shoichet MS, and Przyborski SA 2010. ‘Neural differentiation regulated by biomimetic surfaces presenting motifs of extracellular matrix proteins’, J Biomed Mater Res A, 93: 824–32. [DOI] [PubMed] [Google Scholar]
- Cooper JG, Jeong SJ, McGuire TL, Sharma S, Wang W, Bhattacharyya S, Varga J, and Kessler JA 2018. ‘Fibronectin EDA forms the chronic fibrotic scar after contusive spinal cord injury’, Neurobiol Dis, 116: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, and Silver J 2014. ‘Functional regeneration beyond the glial scar’, Exp Neurol, 253: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H, Freeman C, Jacobson GA, and Small DH 2013. ‘Proteoglycans in the central nervous system: role in development, neural repair, and Alzheimer’s disease’, IUBMB Life, 65: 108–20. [DOI] [PubMed] [Google Scholar]
- Czeisler C, Short A, Nelson T, Gygli P, Ortiz C, Catacutan FP, Stocker B, Cronin J, Lannutti J, Winter J, and Otero JJ 2016. ‘Surface topography during neural stem cell differentiation regulates cell migration and cell morphology’, J Comp Neurol, 524: 3485–502. [DOI] [PubMed] [Google Scholar]
- Dodla MC, and Bellamkonda RV 2006. ‘Anisotropic scaffolds facilitate enhanced neurite extension in vitro’, J Biomed Mater Res A, 78: 213–21. [DOI] [PubMed] [Google Scholar]
- DuFort CC, Paszek MJ, and Weaver VM 2011a. ‘Balancing forces: architectural control of mechanotransduction’, Nat Rev Mol Cell Biol, 12: 308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuFort Christopher C., Paszek Matthew J., and Weaver Valerie M. 2011b. ‘Balancing forces: architectural control of mechanotransduction’, Nature reviews. Molecular cell biology, 12: 308–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner A, and Reinhard J 2015. ‘The extracellular matrix compartment of neural stem and glial progenitor cells’, Glia, 63: 1330–49. [DOI] [PubMed] [Google Scholar]
- Fiford RJ, and Bilston LE 2005. ‘The mechanical properties of rat spinal cord in vitro’, J Biomech, 38: 1509–15. [DOI] [PubMed] [Google Scholar]
- Fitch MT, and Silver J 2008. ‘CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure’, Exp Neurol, 209: 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford-Perriss M, Turner K, Guimond S, Apedaile A, Haubeck HD, Turnbull J, and Murphy M 2003. ‘Localisation of specific heparan sulfate proteoglycans during the proliferative phase of brain development’, Dev Dyn, 227: 170–84. [DOI] [PubMed] [Google Scholar]
- Franco SJ, and Muller U 2011. ‘Extracellular matrix functions during neuronal migration and lamination in the mammalian central nervous system’, Dev Neurobiol, 71: 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcion E, Halilagic A, Faissner A, and ffrench-Constant C 2004. ‘Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C’, Development, 131: 3423–32. [DOI] [PubMed] [Google Scholar]
- Gattazzo Francesca, Urciuolo Anna, and Bonaldo Paolo. 2014. ‘Extracellular matrix: a dynamic microenvironment for stem cell niche’, Biochim Biophys Acta, 1840: 2506–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler SA, Sabin AL, Besser RR, Gooden OM, Shirk BD, Nguyen QM, Khaing ZZ, and Schmidt CE 2018. ‘Biomimetic hydrogels direct spinal progenitor cell differentiation and promote functional recovery after spinal cord injury’, J Neural Eng, 15: 025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges PC, Miller WJ, Meaney DF, Sawyer ES, and Janmey PA 2006. ‘Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures’, Biophys J, 90: 3012–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros A, Morante J, Gil-Sanz C, Fairen A, and Costell M 2007. ‘Perlecan controls neurogenesis in the developing telencephalon’, BMC Dev Biol, 7: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak Farshid, Cohen Daniel M., Estes Bradley T., Gimble Jeffrey M., Liedtke Wolfgang, and Chen Christopher S. 2009. ‘Control of stem cell fate by physical interactions with the extracellular matrix’, Cell Stem Cell, 5: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman R, Uchida N, Bliss TM, He D, Christopherson KK, Stellwagen D, Capela A, Greve J, Malenka RC, Moseley ME, Palmer TD, and Steinberg GK 2007. ‘Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI’, Proc Natl Acad Sci U S A, 104: 10211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y, and Okada S 2017. ‘Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury’, Nat Med, 23: 818–28. [DOI] [PubMed] [Google Scholar]
- Hargus G, Cui Y, Schmid JS, Xu J, Glatzel M, Schachner M, and Bernreuther C 2008. ‘Tenascin-R promotes neuronal differentiation of embryonic stem cells and recruitment of host-derived neural precursor cells after excitotoxic lesion of the mouse striatum’, Stem Cells, 26: 1973–84. [DOI] [PubMed] [Google Scholar]
- Hatami M, Mehrjardi NZ, Kiani S, Hemmesi K, Azizi H, Shahverdi A, and Baharvand H 2009. ‘Human embryonic stem cell-derived neural precursor transplants in collagen scaffolds promote recovery in injured rat spinal cord’, Cytotherapy, 11: 618–30. [DOI] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, and Olson L 2002. ‘Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery’, Proc Natl Acad Sci U S A, 99: 2199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Granger N, Pai SB, Bellamkonda RV, and Jeffery ND 2018. ‘Therapeutic efficacy of microtube-embedded chondroitinase ABC in a canine clinical model of spinal cord injury’, Brain, 141: 1017–27. [DOI] [PubMed] [Google Scholar]
- Hubert T, Grimal S, Carroll P, and Fichard-Carroll A 2009. ‘Collagens in the developing and diseased nervous system’, Cell Mol Life Sci, 66: 1223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa-Tomikawa N, Ogawa J, Douet V, Xu Z, Kamikubo Y, Sakurai T, Kohsaka S, Chiba H, Hattori N, Yamada Y, and Arikawa-Hirasawa E 2012. ‘Laminin alpha1 is essential for mouse cerebellar development’, Matrix Biol, 31: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Shah JK, Harwell CC, Levine JH, Guinto CD, Lezameta M, Kriegstein AR, and Alvarez-Buylla A 2011. ‘Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity’, Neuron, 71: 250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki K, Hagino S, Mori T, Zhang Y, Yokoya S, Takaki H, Tase C, Murakawa M, and Wanaka A 2002. ‘Increased syndecan expression by pleiotrophin and FGF receptor-expressing astrocytes in injured brain tissue’, Glia, 39: 1–9. [DOI] [PubMed] [Google Scholar]
- Iyer NR, Wilems TS, and Sakiyama-Elbert SE 2017. ‘Stem cells for spinal cord injury: Strategies to inform differentiation and transplantation’, Biotechnol Bioeng, 114: 245–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, and Frisen J 1999. ‘Identification of a neural stem cell in the adult mammalian central nervous system’, Cell, 96: 25–34. [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Tatara A, McCreedy DA, Shiu A, and Sakiyama-Elbert SE 2010. ‘Tissue-engineered fibrin scaffolds containing neural progenitors enhance functional recovery in a subacute model of SCI’, Soft Matter, 6: 5127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones FS, and Jones PL 2000. ‘The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling’, Dev Dyn, 218: 235–59. [DOI] [PubMed] [Google Scholar]
- Karus M, Denecke B, ffrench-Constant C, Wiese S, and Faissner A 2011. ‘The extracellular matrix molecule tenascin C modulates expression levels and territories of key patterning genes during spinal cord astrocyte specification’, Development, 138: 5321–31. [DOI] [PubMed] [Google Scholar]
- Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT, and Mercier F 2007. ‘Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu’, Stem Cells, 25: 2146–57. [DOI] [PubMed] [Google Scholar]
- Khaing ZZ, Milman BD, Vanscoy JE, Seidlits SK, Grill RJ, and Schmidt CE 2011. ‘High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury’, J Neural Eng, 8: 046033. [DOI] [PubMed] [Google Scholar]
- Klapka N, and Muller HW 2006. ‘Collagen matrix in spinal cord injury’, J Neurotrauma, 23: 422–35. [DOI] [PubMed] [Google Scholar]
- Kočí Z, Výborný K, Dubišová J, Vacková I, Jäger A, Lunov O, Jirakova K, and Kubinová S 2017. ‘Extracellular matrix hydrogel derived from human umbilical cord as a scaffold for neural tissue repair and its comparison with extracellular matrix from porcine tissues’, Tissue Engineering Part C: Methods, 23(6): 333–45. [DOI] [PubMed] [Google Scholar]
- Koser DE, Moeendarbary E, Hanne J, Kuerten S, and Franze K 2015. ‘CNS cell distribution and axon orientation determine local spinal cord mechanical properties’, Biophys J, 108: 2137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, and Alvarez-Buylla A 2009. ‘The glial nature of embryonic and adult neural stem cells’, Annu Rev Neurosci, 32: 149–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolo M, Vilovic K, Sapunar D, Vrdoljak E, and Saraga-Babic M 1998. ‘Fibronectin expression in the developing human spinal cord, nerves, and ganglia’, Croat Med J, 39: 386–91. [PubMed] [Google Scholar]
- Lau LW, Cua R, Keough MB, Haylock-Jacobs S, and Yong VW 2013. ‘Pathophysiology of the brain extracellular matrix: a new target for remyelination’, Nat Rev Neurosci, 14: 722–9. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Dorries U, Bartsch U, Faissner A, Schachner M, and Steindler DA 1992. ‘Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury’, Proc Natl Acad Sci U S A, 89: 2634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclere PG, Norman E, Groutsi F, Coffin R, Mayer U, Pizzey J, and Tonge D 2007. ‘Impaired axonal regeneration by isolectin B4-binding dorsal root ganglion neurons in vitro’, J Neurosci, 27: 1190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipzig ND, and Shoichet MS 2009. ‘The effect of substrate stiffness on adult neural stem cell behavior’, Biomaterials, 30: 6867–78. [DOI] [PubMed] [Google Scholar]
- Li X, Dancausse H, Grijalva I, Oliveira M, and Levi AD 2003. ‘Labeling Schwann cells with CFSE-an in vitro and in vivo study’, J Neurosci Methods, 125: 83–91. [DOI] [PubMed] [Google Scholar]
- Liao H, Huang W, Schachner M, Guan Y, Guo J, Yan J, Qin J, Bai X, and Zhang L 2008. ‘Beta 1 integrin-mediated effects of tenascin-R domains EGFL and FN6–8 on neural stem/progenitor cell proliferation and differentiation in vitro’, J Biol Chem, 283: 27927–36. [DOI] [PubMed] [Google Scholar]
- Liesi P, and Kauppila T 2002. ‘Induction of type IV collagen and other basement-membrane-associated proteins after spinal cord injury of the adult rat may participate in formation of the glial scar’, Exp Neurol, 173: 31–45. [DOI] [PubMed] [Google Scholar]
- Lim Shawn H., Liu Xingyu Y., Song Hongjun, Yarema Kevin J., and Mao Hai-Quan. 2010. ‘The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells’, Biomaterials, 31: 9031–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Ming GL, and Song H 2005. ‘Glial influences on neural stem cell development: cellular niches for adult neurogenesis’, Curr Opin Neurobiol, 15: 514–20. [DOI] [PubMed] [Google Scholar]
- Ma W, Fitzgerald W, Liu QY, O’Shaughnessy TJ, Maric D, Lin HJ, Alkon DL, and Barker JL 2004. ‘CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels’, Exp Neurol, 190: 276–88. [DOI] [PubMed] [Google Scholar]
- Ma W, Tavakoli T, Derby E, Serebryakova Y, Rao MS, and Mattson MP 2008. ‘Cell-extracellular matrix interactions regulate neural differentiation of human embryonic stem cells’, BMC Dev Biol, 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahairaki Vasiliki, Lim Shawn H., Christopherson Gregory T., Xu Leyan, Nasonkin Igor, Yu Christopher, Mao Hai-Quan, and Koliatsos Vassilis E. 2011. ‘Nanofiber Matrices Promote the Neuronal Differentiation of Human Embryonic Stem Cell-Derived Neural Precursors In Vitro’, Tissue Engineering Part A, 17: 855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammadov B, Guler MO, and Tekinay AB 2014. ‘Extracellular matrix mimetic peptide scaffolds for neural stem cell culture and differentiation’, Methods Mol Biol, 1202: 131–48. [DOI] [PubMed] [Google Scholar]
- Mammoto A, Mammoto T, and Ingber DE 2012. ‘Mechanosensitive mechanisms in transcriptional regulation’, J Cell Sci, 125: 3061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, and Chen CS 2004. ‘Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment’, Dev Cell, 6: 483–95. [DOI] [PubMed] [Google Scholar]
- Mercier F 2016. ‘Fractones: extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease’, Cell Mol Life Sci, 73: 4661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, and Hatton GI 2002. ‘Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network’, J Comp Neurol, 451: 170–88. [DOI] [PubMed] [Google Scholar]
- Meszar Z, Felszeghy S, Veress G, Matesz K, Szekely G, and Modis L 2008. ‘Hyaluronan accumulates around differentiating neurons in spinal cord of chicken embryos’, Brain Res Bull, 75: 414–8. [DOI] [PubMed] [Google Scholar]
- Moe AA, Suryana M, Marcy G, Lim SK, Ankam S, Goh JZ, Jin J, Teo BK, Law JB, Low HY, Goh EL, Sheetz MP, and Yim EK 2012. ‘Microarray with micro- and nano-topographies enables identification of the optimal topography for directing the differentiation of primary murine neural progenitor cells’, Small, 8: 3050–61. [DOI] [PubMed] [Google Scholar]
- Moeendarbary E, Weber IP, Sheridan GK, Koser DE, Soleman S, Haenzi B, Bradbury EJ, Fawcett J, and Franze K 2017. ‘The soft mechanical signature of glial scars in the central nervous system’, Nat Commun, 8: 14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, and Hynes RO 2016. ‘The extracellular matrix: Tools and insights for the “omics” era’, Matrix Biology, 49: 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naba A, Pearce OM, Del Rosario A, Ma D, Ding H, Rajeeve V, Cutillas PR, Balkwill FR, and Hynes RO 2017. ‘Characterization of the extracellular matrix of normal and diseased tissues using proteomics’, Journal of Proteome Research, 16: 3083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Ishimuro T, Kato K, Ko IK, Hirata I, Arima Y, and Iwata H 2007. ‘Combinatorial protein display for the cell-based screening of biomaterials that direct neural stem cell differentiation’, Biomaterials, 28: 1048–60. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Nakamura F, and Fukunaga S 2015. ‘Diverse functions of perlecan in central nervous system cells in vitro’, Anim Sci J, 86: 904–11. [DOI] [PubMed] [Google Scholar]
- Neo SH, and Tang BL 2017. ‘Collagen 1 signaling at the central nervous system injury site and astrogliosis’, Neural Regen Res, 12: 1600–01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, and Sykova E 1998. ‘Extracellular space structure revealed by diffusion analysis’, Trends Neurosci, 21: 207–15. [DOI] [PubMed] [Google Scholar]
- Nimmo CM, Owen SC, and Shoichet MS 2011. ‘Diels-Alder Click cross-linked hyaluronic acid hydrogels for tissue engineering’, Biomacromolecules, 12: 824–30. [DOI] [PubMed] [Google Scholar]
- Nirwane A, and Yao Y 2018. ‘Laminins and their receptors in the CNS’, Biol Rev Camb Philos Soc. [DOI] [PubMed] [Google Scholar]
- Novak U, and Kaye AH 2000. ‘Extracellular matrix and the brain: components and function’, J Clin Neurosci, 7: 280–90. [DOI] [PubMed] [Google Scholar]
- O’Connor SM, Stenger DA, Shaffer KM, and Ma W 2001. ‘Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices’, Neurosci Lett, 304: 189–93. [DOI] [PubMed] [Google Scholar]
- Owen SC, Fisher SA, Tam RY, Nimmo CM, and Shoichet MS 2013. ‘Hyaluronic acid click hydrogels emulate the extracellular matrix’, Langmuir, 29: 7393–400. [DOI] [PubMed] [Google Scholar]
- Parr AM, Tator CH, and Keating A 2007. ‘Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury’, Bone Marrow Transplant, 40: 609–19. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Sanchez AR, Pereira FC, Andrade CM, Puzis R, Pressman Y, Golden K, Kitay BM, Blits B, Wood PM, and Bunge MB 2007. ‘Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery’, Glia, 55: 976–1000. [DOI] [PubMed] [Google Scholar]
- Properzi F, Lin R, Kwok J, Naidu M, van Kuppevelt TH, Ten Dam GB, Camargo LM, Raha-Chowdhury R, Furukawa Y, Mikami T, Sugahara K, and Fawcett JW 2008. ‘Heparan sulphate proteoglycans in glia and in the normal and injured CNS: expression of sulphotransferases and changes in sulphation’, Eur J Neurosci, 27: 593–604. [DOI] [PubMed] [Google Scholar]
- Que RA, Arulmoli J, Da Silva NA, Flanagan LA, and Wang SW 2018. ‘Recombinant collagen scaffolds as substrates for human neural stem/progenitor cells’, J Biomed Mater Res A, 106: 1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulo E, Tumova S, Pavlov I, Pekkanen M, Hienola A, Klankki E, Kalkkinen N, Taira T, Kilpelainen I, and Rauvala H 2005. ‘The two thrombospondin type I repeat domains of the heparin-binding growth-associated molecule bind to heparin/heparan sulfate and regulate neurite extension and plasticity in hippocampal neurons’, J Biol Chem, 280: 41576–83. [DOI] [PubMed] [Google Scholar]
- Rautavuoma K, Takaluoma K, Sormunen R, Myllyharju J, Kivirikko KI, and Soininen R 2004. ‘Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice’, Proc Natl Acad Sci U S A, 101: 14120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recknor JB, Sakaguchi DS, and Mallapragada SK 2006. ‘Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates’, Biomaterials, 27: 4098–108. [DOI] [PubMed] [Google Scholar]
- Ring C, Hassell J, and Halfter W 1996. ‘Expression pattern of collagen IX and potential role in the segmentation of the peripheral nervous system’, Dev Biol, 180: 41–53. [DOI] [PubMed] [Google Scholar]
- Roca-Cusachs P, Iskratsch T, and Sheetz MP 2012. ‘Finding the weakest link: exploring integrin-mediated mechanical molecular pathways’, J Cell Sci, 125: 3025–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E 1996. ‘Brain extracellular matrix’, Glycobiology, 6: 489–92. [DOI] [PubMed] [Google Scholar]
- Sabelstrom H, Stenudd M, and Frisen J 2014. ‘Neural stem cells in the adult spinal cord’, Exp Neurol, 260: 44–9. [DOI] [PubMed] [Google Scholar]
- Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, and Healy KE 2008. ‘Substrate modulus directs neural stem cell behavior’, Biophys J, 95: 4426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider VA, and Granato M 2006. ‘The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration’, Neuron, 50: 683–95. [DOI] [PubMed] [Google Scholar]
- Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, and Schmidt CE 2010. ‘The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation’, Biomaterials, 31: 3930–40. [DOI] [PubMed] [Google Scholar]
- Sever M, Gunay G, Guler MO, and Tekinay AB 2018. ‘Tenascin-C derived signaling induces neuronal differentiation in a three-dimensional peptide nanofiber gel’, Biomater Sci, 6: 1859–68. [DOI] [PubMed] [Google Scholar]
- Sharp KG, Yee KM, and Steward O 2014. ‘A re-assessment of long distance growth and connectivity of neural stem cells after severe spinal cord injury’, Exp Neurol, 257: 186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicari BM, Johnson SA, Siu BF, Crapo PM, Daly KA, Jiang H, Medberry CJ, Tottey S, Turner NJ, and Badylak SF 2012. ‘The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold’, Biomaterials, 33(22): 5524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert JR, Conta Steencken A, and Osterhout DJ 2014. ‘Chondroitin sulfate proteoglycans in the nervous system: inhibitors to repair’, Biomed Res Int, 2014: 845323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, and Stupp SI 2004. ‘Selective differentiation of neural progenitor cells by high-epitope density nanofibers’, Science, 303: 1352–5. [DOI] [PubMed] [Google Scholar]
- Sirko S, von Holst A, Weber A, Wizenmann A, Theocharidis U, Gotz M, and Faissner A 2010. ‘Chondroitin sulfates are required for fibroblast growth factor-2dependent proliferation and maintenance in neural stem cells and for epidermal growth factor-dependent migration of their progeny’, Stem Cells, 28: 775–87. [DOI] [PubMed] [Google Scholar]
- Siskova Z, Baron W, de Vries H, and Hoekstra D 2006. ‘Fibronectin impedes “myelin” sheet-directed flow in oligodendrocytes: a role for a beta 1 integrin-mediated PKC signaling pathway in vesicular trafficking’, Mol Cell Neurosci, 33: 150–9. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Taimoory SM, Tam RY, Baker AEG, Binth Mohammad N, Trant JF, and Shoichet MS 2018. ‘Diels-Alder Click-Cross-Linked Hydrogels with Increased Reactivity Enable 3D Cell Encapsulation’, Biomacromolecules, 19: 926–35. [DOI] [PubMed] [Google Scholar]
- Sood D, Chwalek K, Stuntz E, Pouli D, Du C, Tang-Schomer M, Georgakoudi I, Black LD, and Kaplan DL 2015. ‘Fetal brain extracellular matrix boosts neuronal network formation in 3D bioengineered model of cortical brain tissue’, ACS biomaterials science & engineering, 2(1): 131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenfeldt SE, Munglani G, Garcia AJ, and LaPlaca MC 2010. ‘Biomimetic microenvironment modulates neural stem cell survival, migration, and differentiation’, Tissue Eng Part A, 16: 3747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Sharp KG and Matsudaira YK 2014, Long-distance migration and colonization of transplanted neural stem cells, Cell 156, 385–387. [DOI] [PubMed] [Google Scholar]
- Stichel CC, and Muller HW 1998. ‘The CNS lesion scar: new vistas on an old regeneration barrier’, Cell Tissue Res, 294: 1–9. [DOI] [PubMed] [Google Scholar]
- Stoffels JM, de Jonge JC, Stancic M, Nomden A, van Strien ME, Ma D, Siskova Z, Maier O, Ffrench-Constant C, Franklin RJ, Hoekstra D, Zhao C, and Baron W 2013. ‘Fibronectin aggregation in multiple sclerosis lesions impairs remyelination’, Brain, 136: 116–31. [DOI] [PubMed] [Google Scholar]
- Stoffels JM, Hoekstra D, Franklin RJ, Baron W, and Zhao C 2015. ‘The EIIIA domain from astrocyte-derived fibronectin mediates proliferation of oligodendrocyte progenitor cells following CNS demyelination’, Glia, 63: 242–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukel JM, and Willits RK 2016. ‘Mechanotransduction of Neural Cells Through Cell-Substrate Interactions’, Tissue Eng Part B Rev, 22: 173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, and Discher DE 2013. ‘Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation’, Science, 341: 1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, and LaPlaca MC 2009. ‘Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain’, J Tissue Eng Regen Med, 3: 208–17. [DOI] [PubMed] [Google Scholar]
- Teo BK, Wong ST, Lim CK, Kung TY, Yap CH, Ramagopal Y, Romer LH, and Yim EK 2013. ‘Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase’, ACS Nano, 7: 4785–98. [DOI] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, and Kwon BK 2011. ‘A systematic review of cellular transplantation therapies for spinal cord injury’, J Neurotrauma, 28: 1611–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RE, Lake A, Kenny P, Saunders MN, Sakers K, Iyer NR, Dougherty JD, and Sakiyama-Elbert SE 2017. ‘Different Mixed Astrocyte Populations Derived from Embryonic Stem Cells Have Variable Neuronal Growth Support Capacities’, Stem Cells Dev, 26: 1597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RE, Pardieck J, Smith L, Kenny P, Crawford L, Shoichet M, and Sakiyama-Elbert S 2018. ‘Effect of hyaluronic acid hydrogels containing astrocyte-derived extracellular matrix and/or V2a interneurons on histologic outcomes following spinal cord injury’, Biomaterials, 162: 208–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HL, Chiu WT, Fang CL, Hwang SM, Renshaw PF, and Lai WF 2014. ‘Different forms of tenascin-C with tenascin-R regulate neural differentiation in bone marrow-derived human mesenchymal stem cells’, Tissue Eng Part A, 20: 1908–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdzikova LM, Ruzicka J, LaBagnara M, Karova K, Kubinova S, Jirakova K, Murali R, Sykova E, Jhanwar-Uniyal M, and Jendelova P 2014. ‘Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat’, Int J Mol Sci, 15: 11275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickenmeier J, de Rooij R, Budday S, Steinmann P, Ovaert TC, and Kuhl E 2016. ‘Brain stiffness increases with myelin content’, Acta Biomater, 42: 265–72. [DOI] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, and Reynolds BA 1996. ‘Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis’, J Neurosci, 16: 7599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Xu R, Duan B, and Jiang P 2017. ‘Three-Dimensional Hyaluronic Acid Hydrogel-Based Models for In Vitro Human iPSC-Derived NPC Culture and Differentiation’, J Mater Chem B, 5: 3870–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, and Baier H 2007. ‘Lamina-specific axonal projections in the zebrafish tectum require the type IV collagen Dragnet’, Nat Neurosci, 10: 1529–37. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Hozumi K, Aso A, Hotta A, Toma K, Katagiri F, Kikkawa Y, and Nomizu M 2012. ‘Laminin active peptide/agarose matrices as multifunctional biomaterials for tissue engineering’, Biomaterials, 33: 4118–25. [DOI] [PubMed] [Google Scholar]
- Yang K, Jung H, Lee HR, Lee JS, Kim SR, Song KY, Cheong E, Bang J, Im SG, and Cho SW 2014. ‘Multiscale, hierarchically patterned topography for directing human neural stem cells into functional neurons’, ACS Nano, 8: 7809–22. [DOI] [PubMed] [Google Scholar]
- Yang K, Jung K, Ko E, Kim J, Park KI, Kim J, and Cho SW 2013. ‘Nanotopographical manipulation of focal adhesion formation for enhanced differentiation of human neural stem cells’, ACS Appl Mater Interfaces, 5: 10529–40. [DOI] [PubMed] [Google Scholar]
- Yang YH, Khan Z, Ma C, Lim HJ, and Smith Callahan LA 2015. ‘Optimization of adhesive conditions for neural differentiation of murine embryonic stem cells using hydrogels functionalized with continuous Ile-Lys-Val-Ala-Val concentration gradients’, Acta Biomater, 21: 55–62. [DOI] [PubMed] [Google Scholar]
- Yao S, Liu X, Yu S, Wang X, Zhang S, Wu Q, Sun X, and Mao H 2016. ‘Co-effects of matrix low elasticity and aligned topography on stem cell neurogenic differentiation and rapid neurite outgrowth’, Nanoscale, 8: 10252–65. [DOI] [PubMed] [Google Scholar]
- Yu H, Cao B, Feng M, Zhou Q, Sun X, Wu S, Jin S, Liu H, and Lianhong J 2010. ‘Combinated transplantation of neural stem cells and collagen type I promote functional recovery after cerebral ischemia in rats’, Anat Rec (Hoboken), 293: 911–7. [DOI] [PubMed] [Google Scholar]