Abstract
Background
Ear wax (cerumen) is a normal bodily secretion that can become a problem when it obstructs the ear canal. Symptoms attributed to wax (such as deafness and pain) are among the commonest reasons for patients to present to primary care with ear trouble.
Wax is part of the ear's self‐cleaning mechanism and is usually naturally expelled from the ear canal without causing problems. When this mechanism fails, wax is retained in the canal and may become impacted; interventions to encourage its removal may then be needed. Application of ear drops is one of these methods. Liquids used to remove and soften wax are of several kinds: oil‐based compounds (e.g. olive or almond oil); water‐based compounds (e.g. sodium bicarbonate or water itself); a combination of the above or non‐water, non‐oil‐based solutions, such as carbamide peroxide (a hydrogen peroxide‐urea compound) and glycerol.
Objectives
To assess the effects of ear drops (or sprays) to remove or aid the removal of ear wax in adults and children.
Search methods
We searched the Cochrane ENT Trials Register; Cochrane Register of Studies; PubMed; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the most recent search was 23 March 2018.
Selection criteria
Randomised controlled trials (RCTs) in which a 'cerumenolytic' was compared with no treatment, water or saline, an alternative liquid treatment (oil or almond oil) or another 'cerumenolytic' in adults or children with obstructing or impacted ear wax.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. The primary outcomes were 1) the proportion of patients (or ears) with complete clearance of ear wax and 2) adverse effects (discomfort, irritation or pain). Secondary outcomes were: extent of wax clearance; proportion of people (or ears) with relief of symptoms due to wax; proportion of people (or ears) requiring further intervention to remove wax; success of mechanical removal of residual wax following treatment; any other adverse effects recorded and cost. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
Main results
We included 10 studies, with 623 participants (900 ears). Interventions included: oil‐based treatments (triethanolamine polypeptide, almond oil, benzocaine, chlorobutanol), water‐based treatments (docusate sodium, carbamide peroxide, phenazone, choline salicylate, urea peroxide, potassium carbonate), other active comparators (e.g. saline or water alone) and no treatment. Nine of the studies were more than 15 years old.
The overall risk of bias across the 10 included studies was low or unclear.
Primary outcome: proportion of patients (or ears) with complete clearance of ear wax
Six studies (360 participants; 491 ears) contributed quantitative data and were included in our meta‐analyses.
Active treatment versus no treatment
Only one study addressed this comparison. The proportion of ears with complete clearance of ear wax was higher in the active treatment group (22%) compared with the no treatment group (5%) after five days of treatment (risk ratio (RR) 4.09, 95% confidence interval (CI) 1.00 to 16.80); one study; 117 ears; NNTB = 8) (low‐quality evidence).
Active treatment versus water or saline
We found no evidence of a difference in the proportion of patients (or ears) with complete clearance of ear wax when the active treatment group was compared to the water or saline group (RR 1.47, 95% CI 0.79 to 2.75; three studies; 213 participants; 257 ears) (low‐quality evidence). Two studies applied drops for five days, but one study only applied the drops for 15 minutes. When we excluded this study in a sensitivity analysis it did not change the result.
Water or saline versus no treatment
This comparison was only addressed in the single study cited above (active versus no treatment) and there was no evidence of a difference in the proportion of ears with complete wax clearance when comparing water or saline with no treatment after five days of treatment (RR 4.00, 95% CI 0.91 to 17.62; one study; 76 ears) (low‐quality evidence).
Active treatment A versus active treatment B
Several single studies evaluated 'head‐to‐head' comparisons between two active treatments. We found no evidence to show that one was superior to any other.
Subgroup analysis of oil‐based active treatments versus non‐oil based active treatments
We found no evidence of a difference in this outcome when oil‐based treatments were compared with non‐oil‐based active treatments.
Primary outcome: adverse effects: discomfort, irritation or pain
Only seven studies planned to measure and did report this outcome. Only two (141 participants;176 ears) provided useable data. There was no evidence of a significant difference in the number of adverse effects between the types of ear drops in these two studies. We summarised the remaining five studies narratively. All events were mild and reported in fewer than 30 participants across the seven studies (low‐quality evidence).
Secondary outcomes
Three studies reported 'other' adverse effects (how many studies planned to report these is unclear). The available information was limited and included occasional reports of dizziness, unpleasant smell, tinnitus and hearing loss. No significant differences between groups were reported. There were no emergencies or serious adverse effects reported in any of the 10 studies.
There was very limited or no information available on our remaining secondary outcomes.
Authors' conclusions
Although a number of studies aimed to evaluate whether or not one type of cerumenolytic is more effective than another, there is no high‐quality evidence to allow a firm conclusion to be drawn and the answer remains uncertain.
A single study suggests that applying ear drops for five days may result in a greater likelihood of complete wax clearance than no treatment at all. However, we cannot conclude whether one type of active treatment is more effective than another and there was no evidence of a difference in efficacy between oil‐based and water‐based active treatments.
There is no evidence to show that using saline or water alone is better or worse than commercially produced cerumenolytics. Equally, there is also no evidence to show that using saline or water alone is better than no treatment.
Plain language summary
Ear drops for the removal of ear wax
Background
Build up of ear wax is common. It can be uncomfortable for the patient and can cause hearing problems. Ear drops have been studied as a potential tool to soften the wax, preventing the need for further treatment such as syringing. This review looks at which treatment (oil‐ and water‐based drops or sprays) can help resolve wax build up.
Study characteristics
In March 2018 we searched for clinical trials where ear drops were used to help soften and remove build up of ear wax in patients' ears. We found and included 10 studies with a total of 623 participants. However, only six of these studies provided data with which we could analyse our primary outcome, the proportion of patients with complete ear wax clearance. These six studies included a total of 360 participants, both children and adults (of all ages), with partial or full blockage of the external ear canal with ear wax.
Key results
The 10 included studies looked at either oil‐based drops (triethanolamine polypeptide, almond oil, benzocaine, chlorobutanol), water‐based drops (docusate sodium, carbamide peroxide, phenazone, choline salicylate, urea peroxide, potassium carbonate), saline (salty water) or water alone, or no treatment.
Only one study compared using drops with an active ingredient to not using drops at all. The drops may help increase the proportion of ears cleared of wax from 1 in 20 (if you do nothing) to about 1 in 5 (if you use drops).
We did not find any evidence that water‐based or oil‐based drops were any different to saline or water. However, we also did not find any evidence that water or saline were better than doing nothing.
Adverse (side) effects were not common. Fewer than 30 patients reported any adverse events when using the drops and these were mild (such as slight irritation or pain, or unpleasant smell). No serious side effects were reported by any participant.
Quality of the evidence
We rated the quality of the evidence from studies using four levels: very low, low, moderate or high quality. High‐quality evidence means that we are very confident in the results. Very low‐quality evidence means that we are very uncertain about the results. For wax clearance, we rated the quality of the evidence as low. For adverse effects we rated the quality of the evidence as low.
Conclusions
We have found that using ear drops when you have a partially or completely blocked ear canal may help to remove the ear wax in your ear. It is not clear whether one type of drop is any better than another, or whether drops containing active ingredients are any better than plain or salty water.
Summary of findings
Background
Description of the condition
Ear wax (cerumen) is a normal bodily secretion that becomes a problem when it obstructs the ear canal. People may seek medical attention when they have a sensation of blockage or hearing loss. These symptoms can also be caused by conditions other than wax. Nonetheless, symptoms attributed to wax are among the commonest reasons for patients to present to their primary care or general practitioner (GP) with ear trouble.
Wax obstruction is more common in men than in women and is particularly common in the elderly and people with intellectual impairment (Kumar Sinha 2008; Moore 2002; Roeser 1997; Roland 2008). Ear wax removal is the most common ENT procedure performed in primary care; it is performed four million times each year in the UK (Guest 2004).
Wax is part of the self‐cleaning mechanism of the ear and is usually naturally expelled from the ear canal without causing problems. However, when this mechanism fails, wax is retained in the canal and may become impacted. An occluding wax plug is not associated with poor personal hygiene. Wax may get impacted if people try to clean their ear with cotton buds or when they regularly put things in their ears (for example, ear bud‐type headphones and hearing aids).
The accumulation of wax may be associated with a sensation of blockage but, conversely, not all patients who feel their ears are blocked actually have a problem related to wax. Wax accumulation has several consequences: (a) it can interfere with the clinician's view of the tympanic membrane; (b) it can cause a conductive hearing loss and hence may interfere with the measurement of any underlying loss (not related to wax); (c) if in contact with the tympanic membrane it can cause discomfort and occasionally vertigo; and (d) it can contribute to infection (Keane 1995). Wax removal can help to solve these problems and avoid potential complications.
Once wax has accumulated enough to cause symptoms or prevent a clear view of the tympanic membrane, interventions to encourage its removal may be considered. There are several ways in which this may be done and the methods chosen vary around the world.
Self‐administered remedies include the use of drops to soften or disperse the wax. This may prevent the need for any further intervention. Alternatively, it may make the alternative, mechanical methods of wax removal easier and more effective.
Mechanical methods of wax removal are of two types: dry or wet. With the dry methods the wax is removed under direct vision or with a microscope, using an ear curette (a type of surgical instrument), hook or suction. With the wet cleansing methods, ear syringing or irrigation with body temperature water is used to 'wash out' the wax from the ear canal. There are advantages and disadvantages of each of these methods and not all methods are suitable for all patients. In particular, the mechanical methods described here are less often undertaken in children.
Description of the intervention
A variety of topical medications are available that can be applied directly into the ear canal with the aim of softening the wax to aid natural expulsion or mechanical removal. The word 'cerumenolytic' has been used to refer to compounds that lead to the disintegration of wax. These are typically administered in drop or spray form.
Liquids used to remove/soften wax are of several kinds:
Oil‐based compounds, which soften the wax by dissolution (for example, olive or almond oil).
Water‐based compounds, which improve water miscibility (for example, sodium bicarbonate) or water itself.
A combination of the above.
Non‐water, non‐oil‐based solutions, such as carbamide peroxide (a hydrogen peroxide‐urea compound) and glycerol.
How the intervention might work
The intended mode of action for these medications is to dissolve or soften the wax sufficiently to allow natural expulsion or to make mechanical removal easier.
Why it is important to do this review
Ear wax accumulation is common and may cause considerable problems. The widespread use of ear drops (with or without ear syringing or suction) suggests that many practitioners believe them to be effective (Burton 2009; Hand 2004), although a more recent systematic review has shown weak evidence for cerumenolytics alone in improving wax clearance compared to no treatment (Wright 2015). The Cochrane Review of ear drops for the removal of ear wax was last updated in 2009 (Burton 2009). It is important to replace this with an up‐to‐date review because it addresses a common clinical problem. This review aims to collate the currently available literature to inform the clinician on which type of cerumenolytic is most effective.
Objectives
To assess the effects of ear drops (or sprays) to remove or aid the removal of ear wax in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) including cluster‐randomised controlled trials and quasi‐randomised trials. We planned to only use the first phase of cross‐over trials.
We also included trials that randomised patients by ear as we believe it is possible to ensure that the effects of any intervention considered can be localised and the treatment of one ear will not have an effect on the opposite ear.
Types of participants
We included studies of adults (aged 18 years and over) and children (aged under 18 years) with ear wax requiring removal because (a) it is symptomatic or (b) it is preventing an adequate view of the ear drum.
We excluded studies where the majority of patients have wax that is being removed 'routinely' rather than for a specific reason.
Types of interventions
We included studies on all topical preparations regardless of dose, frequency or duration of use. Active preparations included:
commercially produced cerumenolytics;
hydrogen peroxide;
oil (olive or almond);
sodium bicarbonate or any other topical preparation;
water;
saline.
Active treatment preparation/trade names and active ingredients are shown in Table 4.
1. Active treatments: preparation names and active ingredients.
| Preparation name | Active ingredients | |
| Oil‐based | Cerumenex | Triethanolamine polypeptide |
| Earex | Arachis oil, almond oil, camphor oil | |
| Cerumol | Arachis oil, chlorobutanol hemihydrate | |
| CleanEars | Mineral oil, squalane, spiramint oil | |
| Almond oil | Almond oil | |
| Water‐based | Colace | Docusate sodium |
| Otocerol | Phenazone, sodium bicarbonate | |
| Sodium bicarbonate | Sodium bicarbonate solution | |
| Acetic acid | Aqueous acetic acid | |
|
Non‐water, non‐oil‐based |
Audax | Choline salicylate, glyceride |
| Auro | Carbamide hydroxide, anhydrous glycerin |
The main comparators were: water or saline or no treatment.
The main comparison pairs were:
any active treatment versus no treatment;
any active treatment other than water or saline versus water or saline;
water or saline versus no treatment.
Other possible comparison pairs included:
preparation A versus preparation B;
preparation A with duration of treatment X versus preparation A with duration of treatment Y.
Types of outcome measures
Outcomes had to be evaluated at the end of treatment or within a week thereof.
Primary outcomes
Proportion of patients (or ears) with complete clearance of ear wax, as determined by follow‐up otoscopy (clearance being complete without the need for additional treatment as determined by each study's clinical aims).
Adverse effects: discomfort, irritation or pain.
Secondary outcomes
Extent of wax clearance (difference between degree of obstruction before and after treatment), as determined by follow‐up otoscopy.
Proportion of people (or ears) with relief of symptoms due to wax.
Proportion of people (or ears) requiring further intervention to remove wax.
Success of mechanical removal of residual wax following treatment.
Any other adverse effects recorded in the study.
Any available data on cost of treatment.
We did not exclude studies solely on the basis that the data were not available relating to any of these outcomes. We recorded for each study the different clinical aims of removing ear wax depending on the clinical site at which each study was carried out.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 23 March 2018.
Electronic searches
The Information Specialist searched:
the Cochrane Register of Studies ENT Trials Register (searched 23 March 2018);
CENTRAL (via the Cochrane Register of Studies) (searched 23 March 2018);
PubMed (1946 to 23 March 2018);
Ovid EMBASE (1974 to 23 March 2018);
EBSCO CINAHL (1982 to 23 March 2018);
Ovid AMED (1985 to 23 March 2018);
Ovid CAB abstracts (1910 to 23 March 2018);
LILACS (search to 23 March 2018);
KoreaMed (search to 23 March 2018);
IndMed (search to 23 March 2018);
PakMediNet (search to 23 March 2018);
Web of Knowledge, Web of Science (1945 to 23 March 2018);
ClinicalTrials.gov (search via the Cochrane Register of Studies to 23 March 2018);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (searched 23 March 2018);
ISRCTN (searched 23 March 2018).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
Data collection and analysis
We have followed the methods outlined in our protocol (Burton 2016).
Selection of studies
Two authors (KA and TC) independently screened all the retrieved records based on the titles and abstracts to identify potentially relevant studies. Two authors then independently assessed the full texts of these studies. Any differences were resolved by discussion and consensus, with involvement of a third author where necessary.
Data extraction and management
Two authors (KA and TC) independently extracted the data from studies. We used a standardised data collection form. Where a study had more than one publication, we attempted to retrieve all publications to ensure complete extraction of data. If there were differences in the data extracted by different review authors, we resolved this situation by reference to the original publications and through discussion and consensus, involving a third author as necessary. Where data were missing or unclear, we contacted the original study authors for clarification.
We extracted the following key characteristics of each study: study design, setting, sample size, population, definition of outcomes and how these were collected. We collected baseline information on prognostic factors or effect modifiers, including duration of symptoms. We also extracted information on the rationale for and aims of wax clearance treatment.
For our specified outcomes, we extracted the findings of the studies on an available case analysis basis. That is, we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to the pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias (see below), we extracted the following summary statistics for each study and each outcome:
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we planned to extract the values for change from baseline.
For binary data: the number of participants experiencing an event and the number of patients assessed at the time point.
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggests parametric tests were appropriate, then we planned to treat the outcome measures as continuous data. Alternatively, if data were available, we planned to possibly convert into binary data.
We have specified the time point of interest for the outcomes in this review. We anticipated that some studies may report data at multiple time points, but we planned to only extract the data available from the latest time point within the interval between 'end of treatment' and one week post‐treatment.
Assessment of risk of bias in included studies
Two authors (KA and TC) independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions and we used the Cochrane 'Risk of bias' tool (Handbook 2011). With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessment;
incomplete outcome data;
selective reporting;
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients (ears) with complete clearance of ear) using risk ratios (RR) with 95% confidence intervals (CI).
For the key outcomes presented in the 'Summary of findings' table, we also expressed the results as absolute numbers, based on the pooled results and compared to the assumed risk. We also planned to calculate the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk will usually be either (a) the median of the risks of the control groups in the included studies (this being used to represent a 'medium‐risk population') or, alternatively, (b) the average risk of the control groups in the included studies, this being the 'study population' (Handbook 2011). If a large number of studies were available, and if it was appropriate, we also planned to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For outcomes measured on a continuous scale, we used the mean difference (MD) with a standard deviation (SD). We would have used a standardised mean difference (SMD) if different scales had been used to measure the same outcome and we would have provided a clinical interpretation of the SMD values.
Unit of analysis issues
The treatment options assessed in this review are administered topically to one or both ears. We expected the results to be reported as parallel‐group studies. We analysed the data based on randomisation with between‐patient or within‐patient controls. We followed the advice in the Cochrane Handbook for Systematic Reviews of Interventions when considering if, and how, to pool data from studies with between‐patient and within‐patient controls (Handbook 2011).
Dealing with missing data
Where data relating to an outcome of interest were not reported, but the methods of the study suggested that the outcome had been measured, we tried to contact study authors by email to obtain this information. We also planned to do this if some of the data required for meta‐analysis were unreported, unless the missing data were standard deviations. If standard deviation data were not available, we planned to approximate these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If it was impossible to estimate these, we planned to contact the study authors.
We did not plan to carry out any imputation other than for missing standard deviations. We extracted and analysed all data using the available case analysis method.
Assessment of heterogeneity
We assessed both clinical and statistical heterogeneity. Clinical heterogeneity may be present even in the absence of statistical heterogeneity. We examined the included studies for evidence of differences in the types of participants recruited, interventions, controls or outcomes measured.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P < 0.10) and the I² statistic. The latter calculates the percentage of variability that is not due to chance. I² values over 50% suggest substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed two aspects of reporting bias: between‐study publication bias and within‐study outcome reporting bias.
Publication bias (between‐study reporting bias)
Where sufficient studies (more than 10) were available for an outcome, we planned to use a funnel plot to assess publication bias. If we observed asymmetry, we planned to conduct a more formal investigation using the methods proposed by Egger 1997.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report with those listed in the study protocol whenever possible, or ‐ if the protocol was not available ‐ with those listed in the methods section. Where results were mentioned, but not reported in a way that allowed analysis, we sought further information from the study authors in order to try to reduce bias in the meta‐analysis. If further information was not available, this was reflected in a designation of 'high' risk of bias. Where there was insufficient information to judge the risk of bias, we noted this as an 'unclear' risk of bias (Handbook 2011).
Data synthesis
We used Review Manager 5.3 to carry out meta‐analyses (RevMan 2014). Where possible we analysed data to give a summary measure of effect. If no or minimal heterogeneity was seen, we used a fixed‐effect model for meta‐analysis to measure the effect. Where considerable heterogeneity was observed, we used a random‐effects model. We analysed data separately where combinations of interventions were presented.
For dichotomous data, we analysed treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data were from the same scale, we pooled mean values obtained at follow‐up (endpoint data) with change outcomes and reported this as a MD. We did not plan to pool endpoint and change data if the SMD had to be used as an effect measure.
When statistical heterogeneity is low, the differences in treatment effects seen when using methods based on a random‐effects versus a fixed‐effect model are trivial. When statistical heterogeneity was high we planned to use the random‐effects method as this provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We conducted some subgroup analyses even if statistical heterogeneity was not observed. These analyses were planned as the factors indicated are suspected to be potential effect modifiers. They included:
severity of wax occlusion of the ear canal: total obstruction versus partial;
alternative types of preparation: water‐based versus oil‐based.
When studies had a mixed group of patients – total obstruction/impaction versus partial ‐ we analysed the study as one of these subgroups (rather than as a mixed group) if more than 80% of patients belonged to one category.
In addition to the subgroups above, we planned to conduct the following subgroup analysis in the presence of statistical heterogeneity:
patient age (children versus adults).
Sensitivity analysis
We carried out sensitivity analyses to determine whether or not the findings were robust based on the decisions made in undertaking the review. We planned analyses for the following factors (where possible):
model chosen: fixed‐effect versus random‐effects;
risk of bias of included studies (excluding studies with high risk of bias);
methods of outcome measurement (evaluating the impact of including data where the validity of the measurement is unclear).
Studies at high risk of bias are defined as those that have a high risk of allocation or concealment bias (or both) and a high risk of attrition bias (overall loss to follow‐up of > 20% or differential follow‐up observed, or both).
GRADE and 'Summary of findings' table
We used the GRADE approach to rate the overall quality of evidence. We used the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: 'high', 'moderate', 'low' and 'very low'. A rating of 'high' quality of evidence implies that we were confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate. A rating of 'very low' quality implies that any estimate of effect obtained was very uncertain.
The GRADE approach rates evidence from RCTs that did not have any serious limitations as 'high quality'. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision;
publication bias.
We included 'Summary of findings' tables, constructed according to the recommendations described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), which present the primary outcomes 'proportion of patients (or ears) with complete clearance of ear wax' and 'adverse effects: discomfort, irritation or pain'.
Results
Description of studies
Results of the search
A PRISMA study flow diagram is provided in Figure 1.
1.
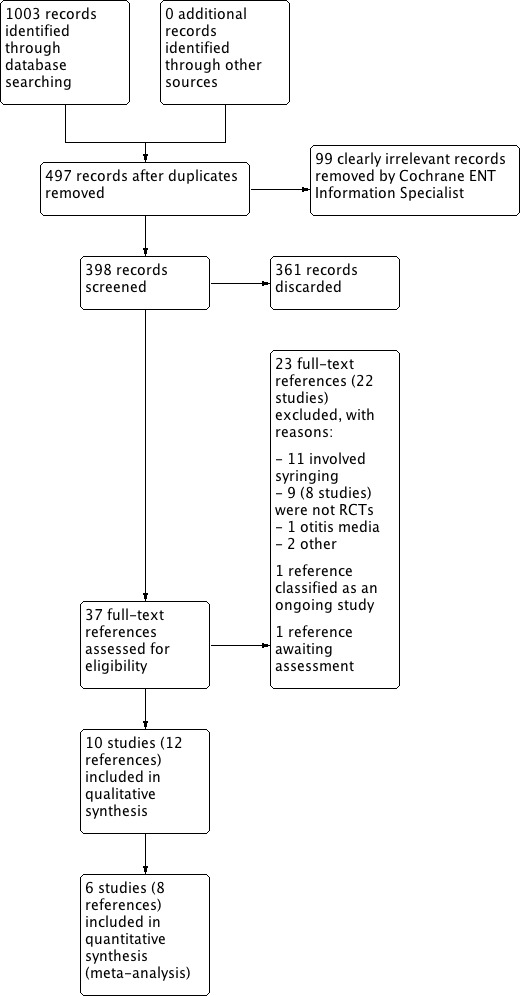
Study flow diagram ‐ PRISMA.
We conducted the latest full searches in March 2018. The searches retrieved a total of 1003 records. After duplicates and some clearly irrelevant results had been removed, we screened 398 titles and abstracts and subsequently discarded 361 references.
We assessed 37 full‐text reports for eligibility, from which we excluded 22 studies (23 references) with reasons (see Excluded studies). We identified one reference as an ongoing study (TCTR20160803001). One reference is awaiting assessment (Kriukov 2014).
We included 10 studies (12 references) in the review: Carr 2001; Dummer 1992; Jaffe 1978; Keane 1995; Lyndon 1992; Meehan 2002; Oron 2011; Singer 2000 (two references); Vanlierde 1991; Whatley 2003 (two references).
Included studies
Ten studies met the criteria for inclusion. See Characteristics of included studies for full details.
Design
Out of the 10 studies that met the inclusion criteria, six studies compared two parallel groups (active treatment A versus active treatment B) (Carr 2001; Dummer 1992; Jaffe 1978; Lyndon 1992; Singer 2000; Vanlierde 1991), three studies compared three parallel groups (active treatment A versus active treatment B versus placebo) (Meehan 2002; Whatley 2003) or (active treatment A versus active treatment B versus active treatment C) (Oron 2011), and one study compared four parallel groups (active treatment A versus active treatment B versus placebo versus no treatment) (Keane 1995).
Sample size
Sample sizes (completing the study) ranged from 35 participants (70 ears) (Lyndon 1992) to 97 participants (155 ears) (Keane 1995).
Setting
Three studies took place in general practices in the UK (Dummer 1992; Jaffe 1978; Lyndon 1992). Three studies took place in hospital emergency departments in the USA (Meehan 2002; Singer 2000; Whatley 2003). One study took place in a hospital in Ireland (Keane 1995). One study took place in a rehabilitation centre in Israel (Oron 2011). Two studies took place in primary care wards in unstated locations (Carr 2001; Vanlierde 1991).
Participants
Four studies investigated adults only (Dummer 1992; Lyndon 1992; Oron 2011; Vanlierde 1991). Two studies investigated children only (Meehan 2002; Whatley 2003). Three studies investigated both adults and children (Carr 2001; Jaffe 1978; Singer 2000). One study did not state the ages of the participants (Keane 1995).
Five studies had almost an equal number of male and female participants (Jaffe 1978; Lyndon 1992; Meehan 2002; Oron 2011; Whatley 2003). Two studies had two‐thirds male participants (Dummer 1992; Singer 2000). The remaining three studies did not state the gender ratios.
All 10 studies required participants to have sufficient amounts of occlusion of ear wax to require intervention.
Interventions
Treatment options varied between:
oil‐based treatments (triethanolamine polypeptide, arachis oil with almond oil and camphor oil, arachis oil with chlorobutanol hemihydrate mineral oil and squalane and spiramint oil, almond oil);
water‐based treatments (docusate sodium, phenazone with sodium bicarbonate, sodium bicarbonate solution, aqueous acetic acid);
non‐oil and non‐water‐based treatments (choline salicylate, glyceride, carbamide hydroxide, anhydrous glycerin, placebo (saline or water alone); and
no treatment.
The duration of treatment varied from 15 minutes (Meehan 2002; Singer 2000) to 14 days (Carr 2001).
All included studies investigated and reported the effects of ear drops as the only intervention prior to evaluation of the primary outcome (ear wax clearance). We came across several studies that had investigated the effects of ears drops PLUS an additional intervention (such as syringing), and in which the outcome assessed was the effect of the ear drops AND the additional intervention. These studies could not be included, as they did not represent the sole effect of a cerumenolytic on ear wax, so they are listed in the Characteristics of excluded studies table with reasons provided.
Outcomes
Primary outcomes
Of the 10 included studies, six (360 participants; 491 ears) reported our first primary outcome: proportion of patients (or ears) with complete clearance of ear wax. These six studies reported quantitative data and were included in our meta‐analyses (Keane 1995; Lyndon 1992; Meehan 2002; Oron 2011; Singer 2000; Whatley 2003).
Seven out of the 10 included studies planned to measure and did report our second primary outcome: adverse effects (discomfort, irritation or pain) (Jaffe 1978; Keane 1995; Lyndon 1992; Meehan 2002; Oron 2011; Singer 2000; Whatley 2003). Only two of these studies (141 participants; 176 ears) provided useable quantitative data that could be included in our meta‐analysis (Jaffe 1978; Lyndon 1992). We summarised the remaining five studies in a narrative synthesis.
Secondary outcomes
Our secondary outcomes were reported less frequently. Extent of wax clearance was reported by three studies (Keane 1995; Meehan 2002; Oron 2011). Proportion of people (or ears) with relief of symptoms due to wax was reported by only one study (Lyndon 1992). Proportion of people (or ears) requiring further intervention to remove wax was reported by two studies (Jaffe 1978; Lyndon 1992). Success of mechanical removal of residual wax following treatment was reported by two studies (Jaffe 1978; Lyndon 1992). 'Other' adverse effects were reported by three studies (Jaffe 1978; Lyndon 1992; Oron 2011).
Cost of treatment data were not reported by any studies.
Excluded studies
We excluded 22 studies (Amjad 1975; Baker 1969; Burgess 1966; Caballero 2009; Chaput de Saintonge 1973; Dubow 1959; Eekhof 2001; Fahmy 1982a; Fahmy 1982b; Fahmy 1982c; Fraser 1970; Fullington 2017; GPRG 1965; GPRG 1967; Hewitt 1970; Hinchcliffe 1955; Pavlidis 2005; Proudfoot 1968; Roland 2004; Sauris 2000; Soy 2015; Spiro 1997). See Characteristics of excluded studies for full details.
Eleven studies included irrigation or syringing around the same time as or shortly after the administration of ear drops, with outcomes measured after the combined intervention; the intervention was therefore not ear drops alone. Eight studies were not randomised. Two studies had inappropriate participants (company employees). One study included participants with otitis media.
Studies awaiting classification
One study (in Russian) is awaiting classification (Kriukov 2014).
Ongoing studies
One study remains classified as ongoing (TCTR20160803001). We have made attempts to obtain an update on its progress, with no success.
Risk of bias in included studies
See Figure 2 for a 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for a 'Risk of bias' summary (our judgements about each risk of bias item for each included study).
2.
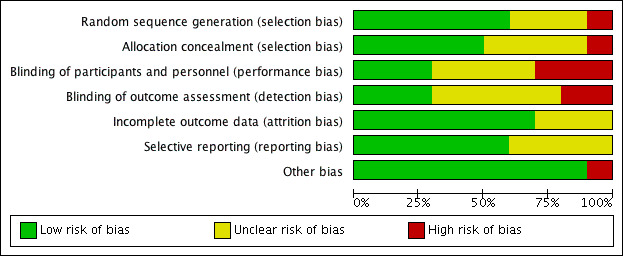
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
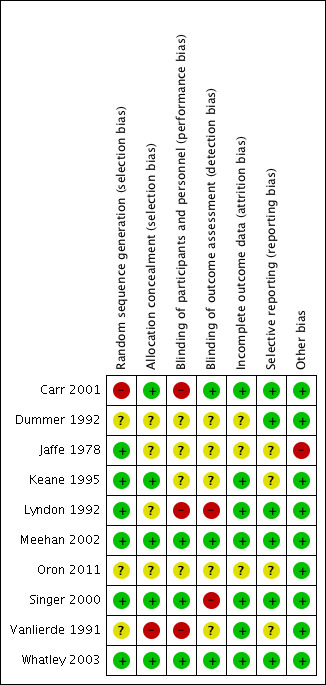
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Six studies adequately reported their methods of randomisation (Jaffe 1978; Keane 1995; Lyndon 1992; Meehan 2002; Singer 2000; Whatley 2003). For this we judged a low risk of selection bias.
Three studies were stated to be randomised but failed to provide details of adequate methods of randomisation (Dummer 1992; Oron 2011; Vanlierde 1991). For this we judged an unclear risk of selection bias.
One study described "randomisation by patient choice of one of two unlabelled bottles", failing to produce a truly random approach (Carr 2001). For this we judged a high risk of selection bias.
Allocation concealment
Five studies adequately reported their methods of allocation concealment (Carr 2001; Keane 1995; Meehan 2002; Singer 2000; Whatley 2003). For this we judged a low risk of selection bias.
Four studies failed to provide adequate description of allocation concealment (Dummer 1992; Jaffe 1978; Lyndon 1992; Oron 2011). For this we judged an unclear risk of selection bias.
One study failed to mention or describe any consideration of or approach to concealing the allocation of treatment to participants (Vanlierde 1991). For this we judged a high risk of selection bias.
Blinding
Blinding of participants and personnel
Three studies adequately described methods of blinding participants and personnel (Meehan 2002; Singer 2000; Whatley 2003). For this we judged a low risk of performance bias.
Four studies were stated to be double‐blind, but failed to provide adequate descriptions of blinding participants and personnel (Dummer 1992; Jaffe 1978; Keane 1995; Oron 2011). For this we judged an unclear risk of performance bias.
In three studies participants and personnel were clearly not blinded. In Carr 2001, "the odour of acetic acid was detected by participants". Lyndon 1992 was not blinded. In Vanlierde 1991, "intraobserver variability was negligible due to prior self standardisation". For this we judged a high risk of performance bias.
Blinding of outcome assessors
Three studies adequately described methods of blinding the outcome assessors (Carr 2001; Meehan 2002; Whatley 2003). For this we judged a low risk of detection bias.
Five studies were stated to be double‐blind, but failed to provide adequate descriptions of blinding the outcome assessors (Dummer 1992; Jaffe 1978; Keane 1995; Oron 2011; Vanlierde 1991). For this we judged an unclear risk of detection bias.
Two studies were not adequately blinded to the outcome assessors. Lyndon 1992 was not blinded. In Singer 2000, observer bias may have occurred as the solutions were different colours. For this we judged a high risk of detection bias.
Incomplete outcome data
The overall risk of attrition bias was low.
Seven studies adequately accounted for all participants from randomisation stage to follow‐up stage, including withdrawals (Carr 2001; Keane 1995; Lyndon 1992; Meehan 2002; Singer 2000; Vanlierde 1991; Whatley 2003). For this we judged a low risk of attrition bias.
Three studies failed to adequately account for all participants from the randomisation stage to follow‐up (Dummer 1992; Jaffe 1978; Oron 2011). The number of participants randomised and withdrawn was not clearly stated. For this we judged an unclear risk of attrition bias.
No studies displayed a high risk of attrition bias.
Selective reporting
Six studies adequately reported on all outcomes planned in the methods sections of their studies (Carr 2001; Dummer 1992; Lyndon 1992; Meehan 2002; Singer 2000; Whatley 2003). For this we judged a low risk of reporting bias.
Four studies failed to provide adequate information as the outcomes were not clearly defined in the methods (Jaffe 1978; Keane 1995; Oron 2011; Vanlierde 1991). For this we judged an unclear risk of reporting bias.
No studies displayed a high risk of reporting bias.
Other potential sources of bias
Overall, we felt that Jaffe 1978 displayed a high risk of bias, due to the lack of information provided for all domains.
We identified no other potential sources of bias in the included studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Ear drops (any active treatment) compared with no treatment for ear wax.
| Ear drops (any active treatment) compared with no treatment for ear wax | ||||||
|
Patient or population: adults and children with ear wax requiring removal Settings: primary care Intervention: any active treatment ear drops Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Any active treatment | |||||
| Proportion of patients (or ears) with complete clearance of ear wax | 5 per 100 | 22 per 100 | RR 4.09, 95% CI 1.00 to 16.80 | 73 participants; 117 ears (1 study) | ⊕⊕⊝⊝ Low1,2 | — |
| Adverse effects: discomfort, irritation or pain | This study reported "excellent" patient acceptability of ear drops, reporting no adverse effects of discomfort, irritation or pain in either group. | ⊕⊕⊝⊝ Low1,2 | — | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by one level for imprecision (one study with a large confidence interval).
2Downgraded by one level due to study limitations (unclear risk of bias).
Summary of findings 2. Ear drops (any active treatment) compared with ear drops (water or saline) for ear wax.
| Ear drops (any active treatment) compared with ear drops (water or saline) for ear wax | ||||||
|
Patient or population: adults and children with ear wax requiring removal Settings: primary care Intervention: ear drops (any active treatment) Comparison: water or saline drops | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Water or saline | Any active treatment | |||||
| Proportion of patients (or ears) with complete clearance of ear wax | 13 per 100 | 19 per 100 | RR 1.47, 95% CI 0.79 to 2.75 | 213 participants; 257 ears (3 studies) | ⊕⊕⊝⊝ Low1,2 | — |
| Adverse effects: discomfort, irritation or pain | Two studies reported no discomfort, irritation or pain adverse effects in either group. One study reported these adverse effects in 10 participants after irrigation but did not state in which treatment group they occurred. | ⊕⊕⊝⊝ Low1,2 | — | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by one level for imprecision (one study with a large confidence interval).
2Downgraded by one level due to study limitations (unclear risk of bias).
Summary of findings 3. Ear drops (water or saline) compared with no treatment for ear wax.
| Ear drops (water or saline) compared with no treatment for ear wax | ||||||
|
Patient or population: adults and children with ear wax requiring removal Settings: primary care Intervention: water or saline ear drops Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Water or saline | |||||
| Proportion of patients (or ears) with complete clearance of ear wax | 5 per 100 | 21 per 100 | RR 4.00, 95% CI 0.91 to 17.62 | 48 participants; 76 ears (1 study) |
⊕⊕⊝⊝ Low1,2 | — |
| Adverse effects: discomfort, irritation or pain | This study reported "excellent" patient acceptability of ear drops, reporting no adverse effects of discomfort, irritation or pain in either group. | ⊕⊕⊝⊝ Low1,2 | — | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by one level for imprecision (one study with a large confidence interval).
2Downgraded by one level due to study limitations (unclear risk of bias).
We included 10 studies, with a total of 623 participants (900 ears) who completed the study.
Of these 10 included studies, six studies (360 participants; 491 ears) reported our first primary outcome: proportion of patients (or ears) with complete clearance of ear wax. These six studies reported quantitative data and were included in our meta‐analyses (Keane 1995; Lyndon 1992; Meehan 2002; Oron 2011; Singer 2000; Whatley 2003).
Seven studies reported on our second primary outcome (adverse events: discomfort, irritation or pain) (Jaffe 1978; Keane 1995; Lyndon 1992; Meehan 2002; Oron 2011; Singer 2000; Whatley 2003). Of these, two studies (141 participants; 176 ears) contributed quantitative data and were included in the meta‐analysis (Jaffe 1978; Lyndon 1992). The remaining five studies were summarised in our narrative synthesis.
We found sufficient data to conduct meta‐analyses for five comparison pairs, including a prespecified subgroup analysis within our first comparison pair.
See Table 1 for the comparison of any active treatment versus no treatment.
See Table 2 for the comparison of any active treatment (other than water or saline) versus water or saline.
See Table 3 for the comparison of water or saline versus no treatment.
We did not create 'Summary of findings' tables for the remaining comparisons.
Main comparisons
Comparison 1: Active treatment versus no treatment
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
For the comparison of active treatment versus no treatment, only one study reported on the proportion of patients (or ears) with complete clearance of ear wax (Keane 1995). Keane 1995 compared arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) and sodium bicarbonate (NaHCO3 5 g, glycerol and purified water) (each four drops, twice daily for five days) with no treatment.
This study showed active treatment to be better than no treatment (risk ratio (RR) 4.09, 95% confidence interval (CI) 1.00 to 16.80; one study; 117 ears; number needed to treat to benefit (NNTB) = 8) (Analysis 1.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision (only one study, with wide confidence intervals) and once for limitations to study design (unclear risk of bias).
1.1. Analysis.
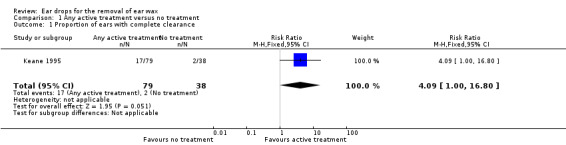
Comparison 1 Any active treatment versus no treatment, Outcome 1 Proportion of ears with complete clearance.
Primary outcome: Adverse effects (discomfort, irritation or pain)
Keane 1995 measured and reported "excellent" patient acceptability of the ear drops, reporting these adverse effects in 0 out of 49 participants (79 ears) in the active treatment group, and 0 out of 24 participants (38 ears) in the no treatment group. We rated the quality of evidence for this outcome as low, downgraded once for imprecision (only one study with small participant numbers) and once for limitations to study design (unclear risk of bias).
Secondary outcomes
Any other adverse effects
See the primary outcome 'Adverse effects (discomfort, irritation or pain)' for the only available information on this outcome (Keane 1995).
Other secondary outcomes
For this comparison no further information was available for our other secondary outcomes.
Comparison 2: Any active treatment (other than water or saline) versus water or saline
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
For the comparison of any active treatment versus water or saline, three studies reported the proportion of patients (or ears) with complete clearance of ear wax (Keane 1995; Meehan 2002; Whatley 2003). Keane 1995 compared arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) and sodium bicarbonate (NaHCO3 5 g, glycerol and purified water) with sterile water (each four drops, twice daily for five days). Meehan 2002 compared docusate sodium (Colace) and triethanolamine polypeptide (Cerumenex) with saline (each 1 mL, for 15 minutes). Whatley 2003 compared docusate sodium (Colace) and triethanolamine polypeptide (Cerumenex) with saline (five drops, twice daily for five days).
We found no evidence of a difference between active treatment drops and water or saline drops (RR 1.47, 95% CI 0.79 to 2.75; I2 = 0%; three studies; 213 participants; 257 ears) (Analysis 2.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision (only one study, with wide confidence intervals) and once for limitations to study design (unclear risk of bias).
2.1. Analysis.
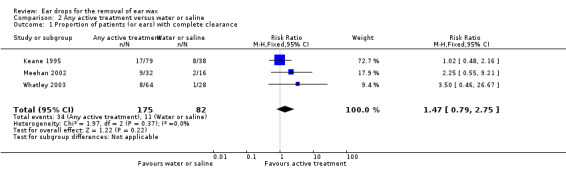
Comparison 2 Any active treatment versus water or saline, Outcome 1 Proportion of patients (or ears) with complete clearance.
Although we detected no statistical heterogeneity in this analysis, there is an important difference between the studies. Two studies administered drops for a period of five days (twice daily), whereas one administered drops for only 15 minutes (Meehan 2002). We undertook a sensitivity analysis, removing Meehan 2002 from this analysis. We still found no evidence of a difference between active treatment and water or saline drops (RR 1.30, 95% CI 0.65 to 2.62; I2 = 0%; two studies; 178 participants; 222 ears).
Primary outcome: Adverse effects: discomfort, irritation or pain
Keane 1995 measured and reported "excellent" patient acceptability from the ear drops, reporting these adverse effects in 0 out of 49 participants in the active treatment group, and 0 out of 24 participants in the water or saline group.
Meehan 2002 measured and reported adverse effects in 10 participants after irrigation but did not state which treatment group they belonged to. We therefore assumed that there were no adverse effects in the docusate sodium, triethanolamine polypeptide and saline groups before using any further intervention.
Whatley 2003 measured and reported adverse effects, with none of the participants reporting discomfort, irritation or pain.
We rated the quality of evidence for this outcome as low, downgraded once for imprecision (one out of three studies had wide confidence intervals) and once for limitations to study design (unclear risk of bias).
Secondary outcomes
Any other adverse effects
Whatley 2003 measured and reported adverse effects and noted one participant who reporting bleeding after irrigation but did not state which treatment group they belonged to. We therefore assumed that there were no other adverse effects in the docusate sodium, triethanolamine polypeptide and saline groups.
See the primary outcome 'Adverse effects (discomfort, irritation or pain)' for the only available information on this outcome (Keane 1995; Meehan 2002).
Other secondary outcomes
No further information was available on our remaining secondary outcomes.
Comparison 3: Water or saline versus no treatment
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
For the comparison of water or saline versus no treatment one study reported the proportion of patients (or ears) with complete clearance of ear wax (Keane 1995). Keane 1995 compared sterile water (four drops, twice daily for five days) with no treatment.
This study did not show a difference between water or saline and no treatment (RR 4.00, 95% CI 0.91 to 17.62; one study, 48 participants; 76 ears) (Analysis 3.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision (only one study, with wide confidence intervals) and once for limitations to study design (unclear risk of bias).
3.1. Analysis.
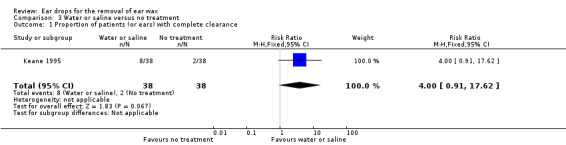
Comparison 3 Water or saline versus no treatment, Outcome 1 Proportion of patients (or ears) with complete clearance.
Primary outcome: Adverse effects: discomfort, irritation or pain
Keane 1995 measured and reported "excellent" patient acceptability of the ear drops, reporting adverse effects in 0 out of 24 participants (38 ears) in the water or saline group, and 0 out of 24 participants (38 ears) in the no treatment group.
We rated the quality of evidence for this outcome as low, downgraded once for imprecision (only one study, with wide confidence intervals) and once for limitations to study design (unclear risk of bias).
Secondary outcomes
Any other adverse effects
See the primary outcome 'Adverse effects (discomfort, irritation or pain)' for the only available information on this outcome (Keane 1995).
Other secondary outcomes
No further information was available on our remaining secondary outcomes.
Other comparisons of the type active treatment A versus active treatment B
Comparison 4: Active treatment A (phenazone and sodium carbonate (Otocerol)) versus active treatment B (arachis oil, chlorobutanol, para‐dichlorobenzene (Cerumol))
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
No data were available for this outcome.
Primary outcome: Adverse effects: discomfort, irritation or pain
One study compared phenazone and sodium carbonate (Otocerol) with arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) (each four drops at night, three applications in total) and reported adverse effects (Jaffe 1978).
In this study there was no evidence of a significant difference in adverse effects between Otocerol and Cerumol (RR 0.57, 95% CI 0.26 to 1.25; one study; 106 participants; 106 ears) (Analysis 4.1). Primary adverse effects (discomfort, irritation or pain) occurred in 8 out of 53 participants in the Otocerol group: discomfort (0); irritation (7); pain (1). These also occurred in 14 out of 53 participants in the Cerumol group: discomfort (0); irritation (12); pain (2). The study also measured and reported other adverse effects (see secondary outcome 'Any other adverse effects' below).
4.1. Analysis.
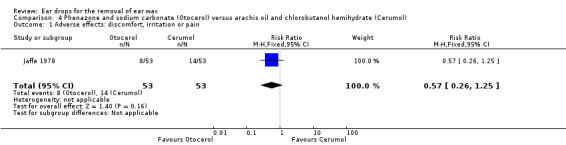
Comparison 4 Phenazone and sodium carbonate (Otocerol) versus arachis oil and chlorobutanol hemihydrate (Cerumol), Outcome 1 Adverse effects: discomfort, irritation or pain.
We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
Secondary outcomes
Any other adverse effects
Jaffe 1978 also measured and reported other adverse effects. One patient out of 53 in the Otocerol group complained of slight giddiness and one patient out of 53 in the Cerumol group complained of an unpleasant smell (106 participants; 106 ears).
Other secondary outcomes
No information was available on our remaining secondary outcomes.
Comparison 5: Active treatment A (docusate sodium (Colace)) versus active treatment B (triethanolamine polypeptide (Ceruminex))
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
Three studies compared docusate sodium (Colace) versus triethanolamine polypeptide (Ceruminex) and reported the proportion of patients (or ears) with complete clearance of ear wax (Meehan 2002; Singer 2000; Whatley 2003). Meehan 2002 compared docusate sodium (Colace) with triethanolamine polypeptide (Ceruminex) (each 1 mL, for 15 minutes). Singer 2000 compared docusate sodium (Colace) with triethanolamine polypeptide (Ceruminex) (1 mL, once, wait 15 minutes). Whatley 2003 compared docusate sodium (Colace) with triethanolamine polypeptide (Ceruminex) (five drops, twice daily for five days).
In a pooled analysis we found no evidence of a difference between the active treatments (RR 0.81, 95% CI 0.38 to 1.72; I2 = 44%; three studies; 146 participants; 146 ears) (Analysis 5.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
5.1. Analysis.
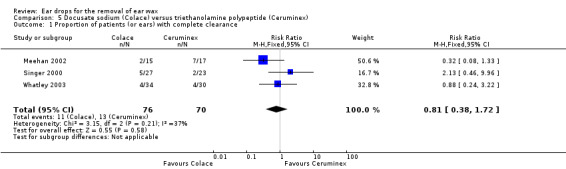
Comparison 5 Docusate sodium (Colace) versus triethanolamine polypeptide (Ceruminex), Outcome 1 Proportion of patients (or ears) with complete clearance.
Primary outcome: Adverse effects (discomfort, irritation or pain)
Singer 2000 measured and reported adverse events (0 out of 27 participants in the docusate sodium (Colace) group, and 0 out of 23 participants in the triethanolamine polypeptide (Ceruminex) group).
Whatley 2003 measured and reported adverse effects, noting bleeding in one participant after irrigation but it did not state which treatment group they belonged to. We therefore assumed that there were no primary outcome adverse effects in the docusate sodium (Colace), triethanolamine polypeptide (Ceruminex) and saline groups.
Secondary outcomes
Any other adverse effects
For Singer 2000 and Whatley 2003 see the primary outcome 'Adverse effects (discomfort, irritation or pain)' for the only available information on this outcome.
Other secondary outcomes
No further information was available on our remaining secondary outcomes.
Comparison 6: Active treatment A (arachis oil, chlorobutanol, para‐dichlorobenzene (Cerumol)) versus active treatment B (sodium bicarbonate)
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
One study compared arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) with sodium bicarbonate (NaHCO3 5 g, glycerol and purified water) (each four drops, twice daily for five days) and reported the proportion of patients (or ears) with complete clearance of ear wax (Keane 1995).
In this study there was no evidence of a significant difference between the active treatments (RR 1.10, 95% CI 0.47 to 2.55; one study; 49 participants; 79 ears) (Analysis 6.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
6.1. Analysis.
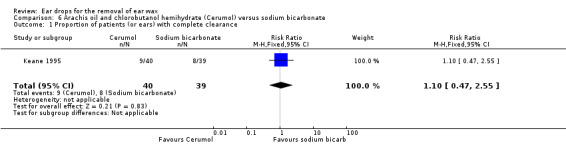
Comparison 6 Arachis oil and chlorobutanol hemihydrate (Cerumol) versus sodium bicarbonate, Outcome 1 Proportion of patients (or ears) with complete clearance.
Primary outcome: Adverse effects: discomfort, irritation or pain
Keane 1995 measured and reported "excellent" patient acceptability of the ear drops, reporting adverse effects in 0 out of 24 participants (40 ears) in the arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) group, and 0 out of 25 participants (39 ears) in the sodium bicarbonate group. We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
Secondary outcomes
Any other adverse effects
See the primary outcome 'Adverse effects (discomfort, irritation or pain)' for the only available information on this outcome.
Other secondary outcomes
No further information was available on our secondary outcomes.
Comparison 7: Active treatment A (choline salicylate, ethylene oxide‐polyoxypropylene glycol, glycol and glycerol (Audax)) versus active treatment B (arachis oil, almond oil and rectified camphor oil (Earex))
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
One study compared choline salicylate 20%, ethylene oxide‐polyoxypropylene glycol, glycol and glycerol (Audax) versus arachis oil, almond oil and rectified camphor oil (Earex). The amount of drops was unstated ‐ "fill the ear" at night and in the morning for four days. This study reported the proportion of patients (or ears) with complete clearance of ear wax (Lyndon 1992).
In this study there was no evidence of a significant difference between the active treatments (RR 1.40, 95% CI 0.57 to 3.44; one study; 35 participants; 70 ears) (Analysis 7.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
7.1. Analysis.
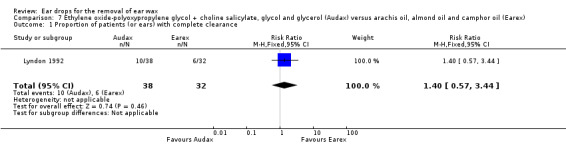
Comparison 7 Ethylene oxide‐polyoxypropylene glycol + choline salicylate, glycol and glycerol (Audax) versus arachis oil, almond oil and camphor oil (Earex), Outcome 1 Proportion of patients (or ears) with complete clearance.
Primary outcome: Adverse effects: discomfort, irritation or pain
One study compared choline salicylate 20%, ethylene oxide‐polyoxypropylene glycol, glycol and glycerol (Audax) versus arachis oil, almond oil and rectified camphor oil (Earex) and reported adverse effects (Lyndon 1992). In this study there was no evidence of a significant difference in adverse effects between the active treatments (RR 0.28, 95% CI 0.01 to 6.51; one study; 35 participants; 70 ears) (Analysis 7.2). Adverse effects included one report of slight irritation in the Earex group and no adverse effects in the Audax group. We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
7.2. Analysis.
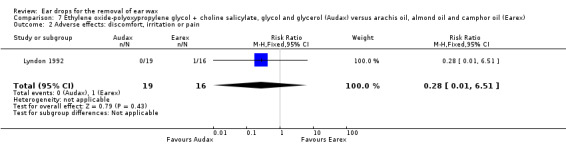
Comparison 7 Ethylene oxide‐polyoxypropylene glycol + choline salicylate, glycol and glycerol (Audax) versus arachis oil, almond oil and camphor oil (Earex), Outcome 2 Adverse effects: discomfort, irritation or pain.
Secondary outcomes
Proportion of people (or ears) with relief of symptoms due to wax
One study compared choline salicylate 20%, ethylene oxide‐polyoxypropylene glycol, glycol and glycerol (Audax) versus arachis oil, almond oil and rectified camphor oil (Earex) and reported the proportion of people (or ears) with complete symptom relief (Lyndon 1992). In this study there was no evidence of a significant difference between the active treatments (RR 1.35, 95% CI 0.55 to 3.31); one study; 35 participants; 70 ears) (Analysis 7.3). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
7.3. Analysis.
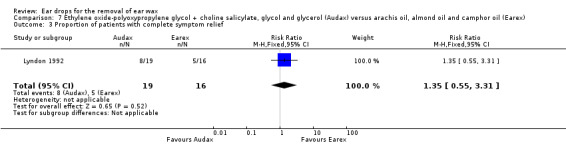
Comparison 7 Ethylene oxide‐polyoxypropylene glycol + choline salicylate, glycol and glycerol (Audax) versus arachis oil, almond oil and camphor oil (Earex), Outcome 3 Proportion of patients with complete symptom relief.
Proportion of people (or ears) requiring further intervention to remove wax
One study compared choline salicylate 20%, ethylene oxide‐polyoxypropylene glycol, glycol and glycerol (Audax) versus arachis oil, almond oil and rectified camphor oil (Earex) and reported the proportion of people (or ears) requiring further intervention to remove wax (Lyndon 1992). In this study there was no evidence of a significant difference between the active treatments (RR 0.72, 95% CI 0.53 to 0.97; 35 participants; 70 ears) (Analysis 7.4). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
7.4. Analysis.
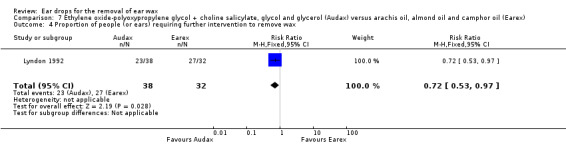
Comparison 7 Ethylene oxide‐polyoxypropylene glycol + choline salicylate, glycol and glycerol (Audax) versus arachis oil, almond oil and camphor oil (Earex), Outcome 4 Proportion of people (or ears) requiring further intervention to remove wax.
Success of mechanical removal of residual wax following treatment
Although some data were provided for this outcome by the study authors (Lyndon 1992), it was not possible to enter these data into an analysis (very low‐quality evidence).
Any other adverse effects
Lyndon 1992 measured and reported other adverse effects. These were reported in 0 out of 19 participants in the choline salicylate 20%, ethylene oxide‐polyoxypropylene glycol, glycol and glycerol (Audax) group, and 1 out of 17 participants in the arachis oil, almond oil and rectified camphor oil (Earex) group (complaint of an unpleasant smell).
Other secondary outcomes
No further information was available on our remaining secondary outcomes.
Comparison 8: Active treatment A (arachis oil, chlorobutanol and para‐dichlorobenzene (Cerumol)) versus active treatment B (squalane, spiramint oil and paraffin (CleanEars))
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
One study compared arachis oil, chlorobutanol and para‐dichlorobenzene (Cerumol) with squalane, spiramint oil and paraffin (CleanEars) (three drops, three times daily for one week) and reported the proportion of people (or ears) with complete clearance of ear wax (Oron 2011).
In this study there was no evidence of a significant difference between the active treatments (RR 0.71, 95% CI 0.39 to 1.30; one study; 26 participants; 52 ears) (Analysis 8.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
8.1. Analysis.
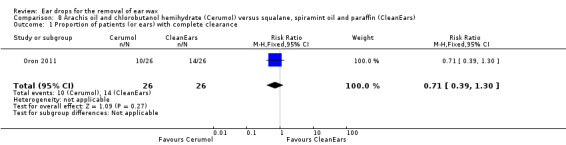
Comparison 8 Arachis oil and chlorobutanol hemihydrate (Cerumol) versus squalane, spiramint oil and paraffin (CleanEars), Outcome 1 Proportion of patients (or ears) with complete clearance.
Primary outcome: Adverse effects (discomfort, irritation or pain)
Oron 2011 measured adverse effects (see the secondary outcome 'Any other adverse effects' below), but did not report any of our primary adverse effects of interest: discomfort, irritation or pain. We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
Secondary outcomes
Extent of wax clearance
One study compared arachis oil, chlorobutanol and para‐dichlorobenzene (Cerumol) versus squalane, spiramint oil and paraffin (CleanEars) and reported the extent of wax clearance in people (or ears) (Oron 2011).
In this study there was no evidence of a significant difference between the active treatments (mean difference (MD) ‐0.84, 95% CI ‐1.78 to 0.10; one study; 26 participants; 52 ears) (Analysis 8.2). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
8.2. Analysis.
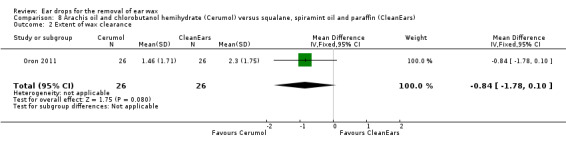
Comparison 8 Arachis oil and chlorobutanol hemihydrate (Cerumol) versus squalane, spiramint oil and paraffin (CleanEars), Outcome 2 Extent of wax clearance.
Any other adverse effects
Oron 2011 measured and reported two other adverse effects attributed to chlorobutanol, arachis oil and dichlorobenzene (Cerumol): smell (1) and itchiness (2).
Other secondary outcomes
No further information was available on our remaining secondary outcomes.
Comparison 9: Active treatment A (arachis oil, chlorobutanol and para‐dichlorobenzene (Cerumol)) versus active treatment B (carbamide peroxide and anhydrous glycerin (Auro))
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
One study compared arachis oil, chlorobutanol and para‐dichlorobenzene (Cerumol) versus carbamide peroxide and anhydrous glycerin (Auro) (three drops, three times daily for one week) and reported the proportion of people (or ears) with complete clearance of ear wax (Oron 2011).
In this study there was no evidence of a significant difference between active treatments (RR 0.92, 95% CI 0.47 to 1.82; one study; 25 participants; 50 ears) (Analysis 9.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
9.1. Analysis.
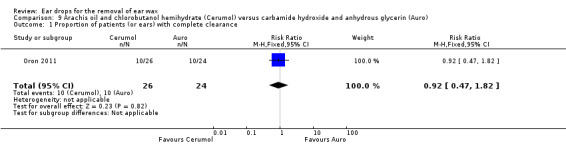
Comparison 9 Arachis oil and chlorobutanol hemihydrate (Cerumol) versus carbamide hydroxide and anhydrous glycerin (Auro), Outcome 1 Proportion of patients (or ears) with complete clearance.
Primary outcome: Adverse effects (discomfort, irritation or pain)
Oron 2011 measured adverse effects (see the secondary outcome 'Any other adverse effects' below), but did not report any of our primary adverse effects of interest: discomfort, irritation or pain. We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
Secondary outcomes
Extent of wax clearance
One study compared arachis oil, chlorobutanol and para‐dichlorobenzene (Cerumol) versus carbamide peroxide and anhydrous glycerin (Auro) and reported the extent of wax clearance in people (or ears) (Oron 2011).
In this study there was no evidence of a significant difference between active treatments (MD ‐0.46, 95% CI ‐1.28 to 0.36; one study; 25 participants; 50 ears) (Analysis 9.2). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
9.2. Analysis.
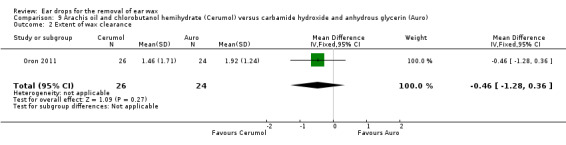
Comparison 9 Arachis oil and chlorobutanol hemihydrate (Cerumol) versus carbamide hydroxide and anhydrous glycerin (Auro), Outcome 2 Extent of wax clearance.
Any other adverse effects
Oron 2011 measured and reported two other adverse effects attributed to chlorobutanol, arachis oil and dichlorobenzene (Cerumol): smell (1) and itchiness (1).
Other secondary outcomes
No further information was available on our remaining secondary outcomes.
Comparison 10: Active treatment A (carbamide peroxide and anhydrous glycerin (Auro)) versus active treatment B (squalane, spiramint oil and paraffin (CleanEars))
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
One study compared carbamide peroxide and anhydrous glycerin (Auro) versus squalane, spiramint oil and paraffin (CleanEars) (three drops, three times daily for one week) and reported the proportion of people (or ears) with complete clearance of ear wax (Oron 2011).
In this study there was no evidence of a significant difference between active treatments (RR 0.77, 95% CI 0.43 to 1.40; one study; 25 participants; 50 ears) (Analysis 10.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
10.1. Analysis.
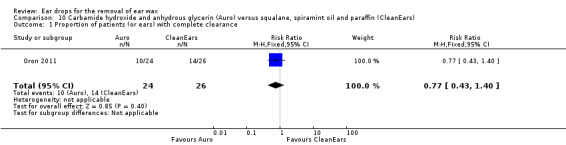
Comparison 10 Carbamide hydroxide and anhydrous glycerin (Auro) versus squalane, spiramint oil and paraffin (CleanEars), Outcome 1 Proportion of patients (or ears) with complete clearance.
Primary outcome: Adverse effects (discomfort, irritation or pain)
Oron 2011 measured adverse effects (see the secondary outcome 'Any other adverse effects' below), but did not report any of our primary adverse effects of interest: discomfort, irritation or pain. We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
Secondary outcomes
Extent of wax clearance
One study reported the extent of wax clearance in people (or ears) with carbamide peroxide and anhydrous glycerin (Auro) versus squalane, spiramint oil and paraffin (CleanEars) (Oron 2011). This study showed no significant effect of one active treatment over the other (MD ‐0.38, 95% CI ‐1.22 to 0.46; one study; 25 participants; 50 ears) (Analysis 10.2). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
10.2. Analysis.
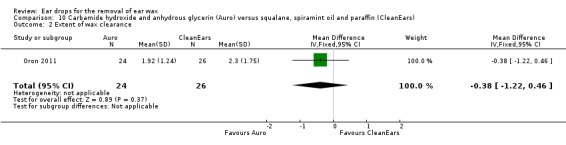
Comparison 10 Carbamide hydroxide and anhydrous glycerin (Auro) versus squalane, spiramint oil and paraffin (CleanEars), Outcome 2 Extent of wax clearance.
Any other adverse effects
Oron 2011 measured and reported other adverse effects, however they were not attributed to either carbamide peroxide and anhydrous glycerin (Auro) or squalane, spiramint oil and paraffin (CleanEars).
Other secondary outcomes
No further information was available on our remaining secondary outcomes.
Comparison 11: Active treatment A (arachis oil, chlorobutanol and para‐dichlorobenzene (Cerumol)) versus active treatment B (almond oil)
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax/Secondary outcome: Extent of wax clearance
No studies comparing arachis oil, chlorobutanol and para‐dichlorobenzene (Cerumol) versus almond oil reported the proportion of patients (or ears) with complete clearance. However, one study compared these two compounds (five drops given twice daily for five days) and reported the proportion of patients with partial clearance (Vanlierde 1991).
This study reported that seven out of 34 ears (21%) using almond oil achieved partial clearance, and 13 out of 35 ears (37%) using arachis oil, chlorobutanol and para‐dichlorobenzene (Cerumol) achieved partial clearance of ear wax. The number of participants was unstated. We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
Primary outcome: Adverse effects (discomfort, irritation or pain)
No further information was available for this primary outcome.
Secondary outcomes
Any other adverse effects
Vanlierde 1991 reported an adverse effect attributed to chlorobutanol, arachis oil and dichlorobenzene (Cerumol): otitis externa in one participant. The participant was withdrawn from the study due to this adverse effect.
Other secondary outcomes
No further information was available on our remaining secondary outcomes.
Comparison 12: Preparation A with duration of treatment X versus preparation A with duration of treatment Y
For this comparison no data were available nor any other information for any of our primary or secondary outcomes.
Subgroup analyses
Alternative types of preparation: oil‐based treatments versus non‐oil based treatments
Five studies compared an oil‐based treatment with a non‐oil‐based treatment (Jaffe 1978; Keane 1995; Meehan 2002; Singer 2000; Whatley 2003). The active ingredients, doses and regimens of the drops used in each study were as follows:
Jaffe 1978: arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) (OIL‐BASED, five drops at night) versus phenazone and sodium carbonate (Otocerol) (NON‐OIL‐BASED, four drops at night, three applications in total).
Keane 1995: arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) (OIL‐BASED) versus sodium bicarbonate (NaHCO3 5 g, glycerol and purified water) (NON‐OIL‐BASED) (four drops, twice daily for five days).
Meehan 2002: triethanolamine polypeptide (Cerumenex) (OIL‐BASED) versus docusate sodium (Colace) (NON‐OIL‐BASED) (1 mL, wait 15 minutes).
Singer 2000: triethanolamine polypeptide (Cerumenex) (OIL‐BASED) versus docusate sodium (Colace) (NON‐OIL‐BASED) (1 mL, once, wait 15 minutes).
Whatley 2003: triethanolamine polypeptide (Cerumenex) (OIL‐BASED) versus docusate sodium (Colace) (NON‐OIL‐BASED) (five drops, twice daily for five days).
Primary outcome: Proportion of patients (or ears) with complete clearance of ear wax
Four studies reported the proportion of people (or ears) with complete clearance (Keane 1995; Meehan 2002; Singer 2000; Whatley 2003). (See doses above).
We found no evidence of a significant difference in effect between oil‐based and non‐oil‐based active treatments (RR 0.85, 95% CI 0.48 to 1.49; I2= 16%; four studies; 195 participants; 225 ears) (Analysis 11.1). We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
11.1. Analysis.
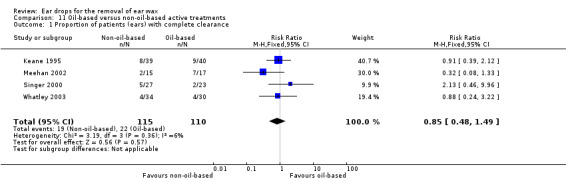
Comparison 11 Oil‐based versus non‐oil‐based active treatments, Outcome 1 Proportion of patients (ears) with complete clearance.
Primary outcome: Adverse effects: discomfort, irritation or pain
All five studies measured and reported adverse effects.
One study reported our primary outcome: adverse effects (discomfort, irritation or pain) (Jaffe 1978). (See doses above). These were as follows:
Discomfort: 0 out of 53 participants in the oil‐based group (arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol)), and 0 out of 53 participants in the non‐oil‐based group (phenazone and sodium carbonate (Otocerol)).
Irritation: 12 out of 53 participants in the oil‐based group (arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol)), and 7 out of 53 participants in the non‐oil‐based group (phenazone and sodium carbonate (Otocerol)).
Pain: 2 out of 53 participants in the oil‐based group (arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol)), and 1 out of 53 participants in the non‐oil‐based group (phenazone and sodium carbonate (Otocerol)).
We found no evidence of a significant difference between the oil‐based treatment (arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol)) and the non‐oil‐based treatment (phenazone and sodium carbonate (Otocerol)) (RR (non‐event) 1.15, 95% CI 0.95 to 1.41; one study; 106 participants; 106 ears) (Analysis 11.2).
11.2. Analysis.
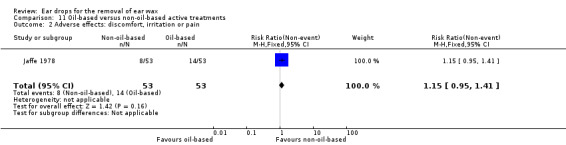
Comparison 11 Oil‐based versus non‐oil‐based active treatments, Outcome 2 Adverse effects: discomfort, irritation or pain.
The remaining four studies reported adverse events in general either as no adverse events occurring or as problems related to irrigation at later stages of the study (Keane 1995; Meehan 2002; Singer 2000; Whatley 2003), which is not applicable to this review. We can therefore assume that there were no adverse effects of discomfort, irritation or pain in these four studies.
We rated the quality of evidence for this outcome as low, downgraded once for imprecision and once for limitations to study design (unclear risk of bias).
Secondary outcomes
Any other adverse effects
Jaffe 1978 reported some other adverse effects (other than discomfort, irritation or pain). The authors reported:
slight dizziness: 0 out of 53 participants in the oil‐based group (arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol)), and 1 out of 53 participants in the non‐oil‐based group (phenazone and sodium carbonate (Otocerol));
unpleasant smell: 1 out of 53 participants in the oil‐based group (arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol)), and 0 out of 53 participants in the non‐oil‐based group (phenazone and sodium carbonate (Otocerol)).
The remaining four studies reported adverse events in general either as no adverse events occurring or as problems related to irrigation at later stages of the study (Keane 1995; Meehan 2002; Singer 2000; Whatley 2003), which is not applicable to this review. We can therefore assume that there were no other adverse effects in these four studies.
Other secondary outcomes
No further information was available on our remaining secondary outcomes.
Other subgroup analyses
Due to the absence of data, we were unable to conduct either of our other two planned subgroup analyses:
severity of wax occlusion of the ear canal (total obstruction versus partial); or
patient age (children versus adults).
Discussion
See Table 1; Table 2; Table 3.
Summary of main results
This review includes 10 studies comparing the effects of ear drops for the removal of ear wax. The drops were of a variety of different types: oil‐based treatments, water‐based treatments and others, and the studies looked at both active comparators such as saline or water alone and no treatment.
Only one study addressed this latter comparison and we concluded that drops, used for five days, when compared to no treatment, may improve the likelihood of the ears becoming completely clear of wax. However, the effect size may be negligible or large. Without any treatment, complete clearance will occur, spontaneously, in 5 per 100 patients on average. This may increase very little (6 per 100) or be as high as 84 per 100.
When comparing drops containing an active component with drops comprising only water or saline, we found no evidence of a difference in the proportion of patients (or ears) with complete clearance. Two of the three studies applied drops for five days, but one study only applied the drops for 15 minutes. When we excluded this study in a sensitivity analysis it did not change the result.
The comparison of water or saline versus no treatment was only addressed in a single study and there was no evidence of a difference. Whilst several single studies evaluated 'head‐to‐head' comparisons between two active treatments, we found no evidence to show that one was superior to any other. Nor did we find evidence of a difference between oil‐based and non‐oil‐based active treatments.
Only two studies provided useable data on discomfort, irritation or pain and there was no evidence of a significant difference in the number of adverse effects between the different types of ear drops. Overall, any reported events were mild and were reported in fewer than 30 participants across seven of the 10 included studies.
Overall completeness and applicability of evidence
This review identified only a small number of studies (10), with limited data from only six studies contributing to the analysis of our first primary outcome. Nine of the studies were more than 15 years old. This body of evidence is insufficient to address all of the objectives of the review. The studies that have been identified are applicable to day‐to‐day practice, but do not comprehensively cover all the different situations in which ear drops are used to remove wax, nor the different ways in which they are used. For example, whether drops are used for a period of a few minutes before another intervention, or over several days to facilitate or improve the success of such an intervention or obviate the need for it altogether.
Quality of the evidence
The overall risk of bias across the 10 included studies was low or unclear. Some of the 10 included studies clearly described randomisation methods and methods used to blind participants and personnel. All studies provided information about withdrawals and dropouts. Only some studies provided information regarding adverse events. The studies recruited participants with adequate baseline wax occlusion, but not all reported clinically useful outcome measures.
We rated the quality of evidence (GRADE rating) for our first primary outcome, proportion of patients (or ears) with complete clearance of ear wax, as low for all of the three main comparisons: active treatment versus no treatment, active treatment versus water or saline and water or saline versus no treatment. In each case we downgraded once for imprecision (one study with a large confidence interval) and once for limitations to study design (unclear study risk of bias). This means that our confidence in the effect estimate for the primary outcome is limited for all three comparisons and the true effect may be substantially different from the estimate of the effect.
We also rated the quality of evidence for our other primary outcome, adverse effects: discomfort, irritation or pain, aslow for all three main comparisons, meaning that we have limited confidence in the effect estimate and the true effect is likely to be substantially different from the estimate of effect.
In the light of the small number of studies addressing each comparison, and thelow quality of the evidence for the primary outcomes, we have concluded that the body of evidence does not allow robust conclusions to be drawn and the overall quality of the whole body of evidence is low.
Potential biases in the review process
We carried out extensive searches of the major databases using broad search criteria and we also searched clinical trial registries. It is unlikely that we have missed any significant randomised controlled trials. We are not aware of any potential limitations of the search process.
We made no departures from the protocol and followed all of our planned methods (Burton 2016). We made no post hoc decisions about the analysis or investigations for heterogeneity after having seen the data and there were no marginal decisions around the inclusion or exclusion of studies or data analysis that could have had an impact on our findings.
We do not believe that the review process itself was biased; if we are mistaken, we do not think that this will have had a major impact in a situation in which we already have reservations about the quality and quantity of the evidence and have emphasised the uncertainty we have in the results.
Agreements and disagreements with other studies or reviews
This review supersedes the Cochrane Review Burton 2009. Our overall findings are in line with the earlier review in that oil‐based or water‐based ear drops may assist in removing cerumen, but we have not shown that active drops are any better (or worse) than water or saline. Using any type of ear drops may be better than using no treatment, but the improvement may be marginal. This is also in line with previous literature demonstrating that softeners, including water or saline, have an effect in clearing ear wax compared to using no treatment at all (Clegg 2010; Hand 2004; Loveman 2011). These studies did not solely look at ear drops as the end treatment for ear wax clearance and also evaluated other methods including syringing to further clear the wax occlusion; nevertheless, using softeners as a precursor to irrigation was better than using none at all.
A March 2018 search for any more recent systematic reviews on this topic found no new published papers.
Authors' conclusions
Implications for practice.
Although a number of studies aimed to evaluate whether or not one type of cerumenolytic is more effective than another, there is no high‐quality evidence to allow a firm conclusion to be drawn and the answer remains uncertain.
A single study suggests that applying ear drops for five days may result in a greater likelihood of complete wax clearance when compared with no treatment at all. However, we cannot draw conclusions on whether one type of active treatment is more effective than another and there was no evidence of a difference in efficacy between oil‐based and water‐based active treatments. Little or no information was available to assess our remaining outcomes.
There is no evidence to show that using saline or water alone is better or worse than commercially produced cerumenolytics. Equally, there is also no evidence to show that using saline or water alone is better than no treatment.
Very few minor adverse effects and no major adverse effects were highlighted with any cerumenolytic treatment, suggesting that treatment with a cerumenolytic agent and/or water or saline is safe and well tolerated by patients. However, the data from the included studies are limited and we cannot exclude the possibility that rare adverse events can occur and that these have not been revealed in the small number of participants included in the trials. It seems likely, from first principles, that contact hypersensitivity might occur with some of the active components of those drops that contain them, whereas this will not occur with water and saline.
Implications for research.
Research into the optimal management of patients with symptomatic wax should consider the whole patient pathway, of which the use of ear drops is only one part. Study investigators may decide to distinguish people with impacted wax causing symptoms such as pain, from wax that may ‐ or may not ‐ be causing deafness. This last group includes patients presenting with possible hearing impairment and whose ears drums are obscured by wax; it is often unknown at that time whether or not there really is a significant hearing loss, and still less whether or not the visible wax is contributing to it. Another important group comprises those who do not appear to have any wax‐related symptoms but in whom the main aim of wax clearance is to ensure that the drum has been fully inspected.
There are several options or decision points along the patient pathway. In some clinical situations, there is very little time to wait for ear drops to work. In other circumstances, using a treatment for several days is a clear option. In some settings, ear syringing or micro‐suction are readily available, and the use of drops is seen as a prelude to one of these interventions.
Any further randomised trials should have higher methodological quality including best efforts to minimise bias.
Feedback
Comment, 15 August 2018
Summary
Congratulations on the publication of this important review. I was particularly impressed the main title did not contain medical jargon. As with all Cochrane reviews, the implications for research section called for better designed primary research because the studies identified in the review were all of low quality. Some reviews go into a lot more detail about the design of future research than your review did. I liked the fact that you noted that research into effective interventions for removal of earwax should focus on the specific outcomes needed in particular circumstances e.g. speed is of the essence if the removal is to facilitate inspection of the eardrum.
I would like to challenge the authors of this review, and indeed for all Cochrane reviews, to go a step further from the implications for research section and design the perfect trial to answer the review question. I have proposed to the Cochrane Editor‐in‐Chief recently that the publication of a simple primary research protocol should become a standard section of all Cochrane reviews. With the knowledge about the strengths and weaknesses of previous research gained through writing and updating a review, a structured template protocol for a future trial written using the SPIRIT reporting guidelines would be feasible in a relatively short time. I accept that it's useful to point out the flaws in previous research which limit confidence in existing evidence. All Cochrane reviews do this. It would be more constructive for Cochrane to use the considerable methodological expertise of its authors, and its commitment to patient and public involvement to help future researchers do better quality primary research.
The addition of this as standard would distinguish Cochrane from all other producers of systematic reviews. It would be more useful than a review update in two or more years time where the good quality research called for may, or more likely may not, have been carried out. You could also take this opportunity to prove Cochrane’s stated commitment to patient and public involvement by involving patients and the public in the design and development of a protocol, particularly in the choice of outcomes. Prerequisites for use of these protocols by research teams would be open access publication of the results, adherence to reporting guidelines such as CONSORT and GRIPP2 for reporting patient involvement, and data sharing. Research teams who are successful in applying for funding to use these protocols could be monitored to ensure adherence and to ensure the results are reported fully and transparently, and the data is made available This would ensure the risk of bias is low across the board. Easy access to the data would facilitate inclusion in the review updates.
Cochrane could establish a database of these protocols which would be an invaluable resource for those involved in prioritising funding for research. Cochrane protocols would provide funders the assurance they need that the taxpayers money they award will go to well‐designed research of clear relevance and use to patients, healthcare providers and policy makers. Funding Cochrane to produce protocols in addition to reviews would provide better value for money than systematic reviews of mainly poor research.
Reply
Thank you for the comment on our review. This is an interesting idea and we look forward to Cochrane exploring it further.
Contributors
Caroline Struthers, August 2018.
Martin Burton, October 2018.
What's new
| Date | Event | Description |
|---|---|---|
| 8 October 2018 | Amended | Comment on review added (Feedback). |
Notes
This review supersedes the out of date Cochrane Review 'Ear drops for the removal of ear wax' (Burton 2009).
Acknowledgements
We are grateful to Professor Kevin Munro for peer reviewing the draft of this review and to Andrew Rix for his helpful comments.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
| CENTRAL (via CRS) | EMBASE (Ovid) | PubMed |
| #1 MeSH descriptor: [Cerumen] explode all trees #2 (Cerumen* or cerumin* or earwax or (ear and wax) or (ears and wax) or (ear and impaction) or (ears and impaction) or (ear and impacted) or (ears and impacted)):ti,ab,kw #3#1 or #2 | 1 cerumen/ 2 cerumen impaction/ 3 exp ceruminolytic agent/ 4 (Cerumen* or cerumin* or earwax or (ear and wax) or (ears and wax) or (ear and impaction) or (ears and impaction) or (ear and impacted) or (ears and impacted)).tw. 5 1 or 2 or 3 or 4 | #1 "CERUMEN" [Mesh] OR CERUMEN* [tiab] OR CERUMIN* [tiab] OR earwax [tiab] OR (EAR [tiab] AND WAX* [tiab]) OR (EARs [tiab] AND wax* [tiab]) OR (EAR [tiab] AND impacted [tiab]) OR (EARs [tiab] AND impacted [tiab]) OR (EAR [tiab] AND impaction [tiab]) OR (EARs [tiab] AND impaction [tiab]) |
| Web of Science | CINAHL (EBSCO) | Trial Registries |
| #1 TOPICS: (Cerumen* OR cerumin* OR earwax OR (ear AND wax) OR (ears AND wax) OR (ear AND impaction) OR (ears AND impaction) OR (ear AND impacted) OR (ears AND impacted)) | S3 S1 OR S2 S2 TX (Cerumen* or cerumin* or earwax or (ear and wax) or (ears and wax) or (ear and impaction) or (ears and impaction) or (ear and impacted) or (ears and impacted)) S1 (MH "Cerumen") OR (MH "Cerumen Impaction") OR (MH "Ceruminolytic Agents") |
ICTRP Cerumen* OR cerumin* OR earwax OR ear AND wax OR ears AND wax OR ear AND impaction OR ears AND impaction OR ear AND impacted OR ears AND impacted ClinicalTrials.gov (via CRS) Cerumen OR cerumin OR earwax OR (ear AND wax) OR (ears AND wax) OR (ear AND impaction) OR (ears AND impaction) OR (ear AND impacted) OR (ears AND impacted) |
Data and analyses
Comparison 1. Any active treatment versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of ears with complete clearance | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.09 [1.00, 16.80] |
Comparison 2. Any active treatment versus water or saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients (or ears) with complete clearance | 3 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.79, 2.75] |
Comparison 3. Water or saline versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients (or ears) with complete clearance | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.91, 17.62] |
Comparison 4. Phenazone and sodium carbonate (Otocerol) versus arachis oil and chlorobutanol hemihydrate (Cerumol).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects: discomfort, irritation or pain | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.26, 1.25] |
Comparison 5. Docusate sodium (Colace) versus triethanolamine polypeptide (Ceruminex).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients (or ears) with complete clearance | 3 | 146 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.38, 1.72] |
Comparison 6. Arachis oil and chlorobutanol hemihydrate (Cerumol) versus sodium bicarbonate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients (or ears) with complete clearance | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.47, 2.55] |
Comparison 7. Ethylene oxide‐polyoxypropylene glycol + choline salicylate, glycol and glycerol (Audax) versus arachis oil, almond oil and camphor oil (Earex).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients (or ears) with complete clearance | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.57, 3.44] |
| 2 Adverse effects: discomfort, irritation or pain | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.01, 6.51] |
| 3 Proportion of patients with complete symptom relief | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.55, 3.31] |
| 4 Proportion of people (or ears) requiring further intervention to remove wax | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.97] |
Comparison 8. Arachis oil and chlorobutanol hemihydrate (Cerumol) versus squalane, spiramint oil and paraffin (CleanEars).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients (or ears) with complete clearance | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.39, 1.30] |
| 2 Extent of wax clearance | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.84 [‐1.78, 0.10] |
Comparison 9. Arachis oil and chlorobutanol hemihydrate (Cerumol) versus carbamide hydroxide and anhydrous glycerin (Auro).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients (or ears) with complete clearance | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.47, 1.82] |
| 2 Extent of wax clearance | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.28, 0.36] |
Comparison 10. Carbamide hydroxide and anhydrous glycerin (Auro) versus squalane, spiramint oil and paraffin (CleanEars).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients (or ears) with complete clearance | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.40] |
| 2 Extent of wax clearance | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐1.22, 0.46] |
Comparison 11. Oil‐based versus non‐oil‐based active treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of patients (ears) with complete clearance | 4 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.48, 1.49] |
| 2 Adverse effects: discomfort, irritation or pain | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.95, 1.41] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carr 2001.
| Methods | Double‐blind, 2‐arm, parallel‐group, randomised controlled trial, with active comparator (no placebo) and daily application for 2 weeks duration | |
| Participants |
Setting: single centre, primary care, Canada Sample size:
Participant baseline characteristics:
Inclusion criteria: patients of any age with occlusive cerumen in at least one ear Exclusion criteria: tympanic membrane perforation, ventilation tubes, mastoid cavity, otitis externa |
|
| Interventions |
Intervention group (n = 35 participants; 70 ears): 10% aqueous sodium bicarbonate, ≥ 4 drops, wait 5 minutes, on a daily basis, for 14 days Comparator group (n = 34 participants; 68 ears): 2.5% aqueous acetic acid, ≥ 4 drops, wait 5 minutes, on a daily basis, for 14 days Use of additional interventions: none |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Timing of measurements:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Participants lost to follow‐up: 2 participants withdrew | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: randomisation by patient choice of 1 of 2 unlabelled bottles |
| Allocation concealment (selection bias) | Low risk | Comment: bottles were prepared by pharmacy and were unmarked except for A or B; un‐blinding occurred only at end of study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the odour of acetic acid was detected by participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: examiners were blinded and were unable to detect the smell of acetic acid |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were accounted for; 2 participants withdrew, with reasons described |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes proposed in the methods were reported in the results |
| Other bias | Low risk | Comment: no further sources of bias identified |
Dummer 1992.
| Methods | Single‐blind, 2‐arm, parallel‐group, randomised controlled trial, with active comparator (no placebo) and unclear duration of treatment | |
| Participants |
Setting: single centre, general practice, Edinburgh, UK Sample size:
Participant baseline characteristics:
Inclusion criteria: adults presenting with impacted or hard wax Exclusion criteria: inflammation, dermatitis, eczema of external ear canal, tympanic membrane perforation, salicylate sensitivity, hearing aid use, frequent swimmers |
|
| Interventions |
Intervention group (n = 27 participants; 54 ears): choline salicylate and polyoxypropylene glycol condensate (Audax), dose not stated, drops applied 2 times per day for an average of 4 days (range 3 to 7 days) Comparator group (n = 23 participants; 46 ears): arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol), dose not stated, drops applied 2 times per day for an average of 4 days (range 3 to 7 days) Use of additional interventions: none |
|
| Outcomes |
Primary outcomes:
Other outcomes reported by the study:
Timing of measurements:
|
|
| Funding sources | “Study supported by Napp Laboratories Ltd” | |
| Declarations of interest | “Study supported by Napp Laboratories Ltd” | |
| Notes | Participants lost to follow‐up: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "patients were then allocated at random to receive either ... ear drops" Comment: no further details of randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided regarding blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: stated that assessors were blinded but no details of method given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 50 patients were treated; no mention of withdrawals in adverse events section |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes proposed in the methods were reported in the results |
| Other bias | Low risk | Comment: no further sources of bias identified |
Jaffe 1978.
| Methods | Double‐blind, 2‐arm, parallel‐group, randomised controlled trial, with active comparator (no placebo) and 3 applications in total (no time period) | |
| Participants |
Setting: 15 general practices, Bournemouth, UK Sample size:
Participant baseline characteristics:
Inclusion criteria: all patients presenting with wax in their ears for whom a cerumenolytic would normally be prescribed Exclusion criteria: severe infection, tympanic membrane perforation |
|
| Interventions |
Intervention group (n = 53 participants; 53 ears): phenazone and sodium carbonate (Otocerol) 4 drops at night, 3 applications in total Comparator group (n = 53 participants; 53 ears): arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) 5 drops at night, 3 applications in total Use of additional interventions: syringing after drops treatment if required |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes reported by the study:
Timing of measurements:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Participants lost to follow‐up: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated according to a previously determined scheme" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided regarding concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided regarding blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided regarding blinding of the outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the number randomised and withdrawn was not stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information; outcomes were not clearly defined in the methods |
| Other bias | High risk | Comment: overall high risk of bias for entire study, due to lack of information for all risk of bias domains |
Keane 1995.
| Methods | Double‐blind, 4‐arm, parallel‐group, randomised controlled trial, with active comparator and placebo, and 5 days duration of treatment | |
| Participants |
Setting: single centre, hospital inpatient unit, Dublin, Ireland Sample size:
Participant baseline characteristics:
Inclusion criteria: not stated Exclusion criteria: any ear canal or tympanic membrane pathology, patients already taking ear drops |
|
| Interventions |
Intervention group (n = 24 participants, 40 ears): arachis oil 57.3%, chlorobutanol 5%, para‐dichlorobenzene 2%) (Cerumol), 4 drops, twice daily, 5 days Intervention group 2 (n = 25 participants, 39 ears): sodium bicarbonate (NaHCO3 5 g, glycerol and purified water), 4 drops, twice daily, 5 days Comparator group 1 (n = 24 participants, 38 ears): sterile water, 4 drops, twice daily, 5 days Comparator group 2 (n = 24 participants, 38 ears): no treatment, 4 drops, twice daily, 5 days Use of additional interventions: syringing after ear drops if required |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Timing of measurements:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Participants lost to follow‐up: 16 (13 discharged from hospital, 3 died) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment was allocated in random order and the code was not broken until the trial was complete" and from correspondence: "randomised by the pharmacist" |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment was allocated in random order and the code was not broken until the trial was complete" and from correspondence: "randomised by the pharmacist" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Quote: "drops were administered by the nursing staff" Comment: insufficient information to confirm blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk |
Quote: "drops were administered by the nursing staff, each ear was re‐examined, by the same observer, and the auditory canal classified..." Comment: insufficient information to confirm blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were accounted for |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information; outcomes were not well described in methods |
| Other bias | Low risk | Comment: no further sources of bias identified |
Lyndon 1992.
| Methods | 2‐arm, parallel‐group, randomised controlled trial, with active comparator (no placebo) and 4 days duration of treatment | |
| Participants |
Setting: single centre, general practice, UK Sample size:
Participant baseline characteristics
Inclusion criteria: patients of either gender and 16 years or older, with symptoms of hardened wax in either or both ears requiring cerumenolytic treatment Exclusion criteria: inflammation, middle ear pathology, tympanic membrane perforation, middle ear infection requiring systemic antibiotics, salicylate sensitivity |
|
| Interventions |
Intervention group 1 (n = 19, 38 ears): choline salicylate 20%, ethylene oxide‐polyoxypropylene glycol, glycol and glycerol (Audax), unstated amount of drops ("fill the ear") at night and in morning for 4 days Intervention group 2 (n = 16, 34 ears): arachis oil, almond oil and rectified camphor oil (Earex), unstated amount of drops ("fill the ear") at night and in morning for 4 days Use of additional interventions: syringing on day 5 after 4 days of treatment with drops |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Timing of measurements:
|
|
| Funding sources | No information provided | |
| Declarations of interest | The senior author works for Napp industries who make Audax drops | |
| Notes | Participants lost to follow‐up: 1 did not return for second day of testing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Pre‐determined random allocation schedule" used |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided regarding concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were accounted for. One patient did not complete follow‐up, but no reasons were provided. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes proposed in the methods were reported in the results |
| Other bias | Low risk | Comment: no further sources of bias identified |
Meehan 2002.
| Methods | Double‐blind, 3‐arm, parallel‐group, randomised controlled trial, with active comparator and placebo, and 1 application for 15 minutes | |
| Participants |
Setting: university paediatric emergency department, USA Sample size:
Participant baseline characteristics:
Inclusion criteria: not stated Exclusion criteria: not stated |
|
| Interventions |
Intervention group 1 (n = 15 participants; 15 ears): docusate sodium (Colace) 1 mL, wait 15 minutes, irrigation, irrigation repeated once if required Intervention group 2 (n = 17 participants; 17 ears): triethanolamine polypeptide (Cerumenex) 1 mL, wait 15 minutes, irrigation, irrigation repeated once if required Comparator group (n = 16 participants; 16 ears): saline 1 mL, wait 15 minutes, irrigation, irrigation repeated once if required Use of additional interventions: irrigation ‐ after treatment with drops was measured |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Timing of measurements:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Participants lost to follow‐up: 8 patients withdrew | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: (from correspondence) "The assigning was done by the computer through its randomization program" |
| Allocation concealment (selection bias) | Low risk |
Quote: (from correspondence) "The allocation was done by patient number in the pharmacy" Quote: "The test substance was concealed in a coloured syringe" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: the test substance was concealed in a coloured syringe and administered by nurses not involved in assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: assessors were informed of the syringe number only and not the test substance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were accounted for |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes proposed in the methods were reported in the results |
| Other bias | Low risk | Comment: no further sources of bias identified |
Oron 2011.
| Methods | Double‐blind, 3‐arm, parallel‐group, randomised controlled trial, with active comparator (no placebo) and 1 week duration of treatment | |
| Participants |
Setting: single centre, elderly care inpatient rehabilitation unit, Israel Sample size:
Participant baseline characteristics:
Inclusion criteria: patients over 18 years with cerumen impaction who were co‐operative enough to tolerate examination Exclusion criteria: previous ear disease, ear treatment |
|
| Interventions |
Intervention group 1 (n = 12 participants, 24 ears): carbamide peroxide and anhydrous glycerin (Auro), 3 drops, 3 times daily, 1 week Intervention group 2 (n = 13 participants, 26 ears): chlorobutanol, arachis oil and dichlorobenzene (Cerumol), 3 drops, 3 times daily, 1 week Intervention group 3 (n = 13 participants, 26 ears): squalane, spiramint oil and paraffin (CleanEars), 3 drops, 3 times daily, 1 week Use of additional interventions: aural microsuction ‐ after treatment with drops was measured |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes reported by the study:
Timing of measurements:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Participants lost to follow‐up: 3 (1 discharged from the hospital, 1 discontinued treatment, 1 transferred to another facility) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "subjects were randomly assigned to be treated..." Comment: method of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided regarding concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk |
Quote: "The examining physician was blind to the chosen treatment" Comment: no information on whether participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information provided regarding blinding of assessment of outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 38 participants (76 ears) were randomised; withdrawals not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Comment: insufficient information; outcomes were not clearly defined in methods |
| Other bias | Low risk | Comment: no further sources of bias identified |
Singer 2000.
| Methods | Double‐blind, 2‐arm, parallel‐group, randomised controlled trial, with active comparator (no placebo) and 1 application for 15 minutes | |
| Participants |
Setting: single centre, university hospital emergency department, USA Sample size:
Participant baseline characteristics:
Inclusion criteria: 1 year or older, presenting to emergency department with a condition requiring visualisation of the tympanic membrane and wax present Exclusion criteria: tympanic membrane perforation, infection, uncooperative, allergies to ingredients |
|
| Interventions |
Intervention group 1 (n = 27 participants; 27 ears): docusate sodium (Colace), 1 mL, once, wait 15 minutes Intervention group 2 (n = 23 participants; 23 ears): triethanolamine polypeptide (Cerumenex), 1 mL, once, wait 15 minutes Use of additional interventions: syringing if required after treatment with drops was measured and assessed |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
Timing of measurements:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Participants lost to follow‐up: none | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assignments were generated by a computerised randomisation programme" |
| Allocation concealment (selection bias) | Low risk | Quote: "for each patient, the next in a series of opaque, consecutively numbered 2‐mL syringes were used. Syringes were prepared by hospital pharmacy personnel and not connected to the ED or enrolment process" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "for each patient, the next in a series of opaque, consecutively numbered 2‐mL syringes were used. Syringes were prepared by hospital pharmacy personnel and not connected to the ED or enrolment process" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: observer bias may have occurred as the solutions were different colours |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing data; no withdrawals mentioned |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes proposed in the methods were reported in the results |
| Other bias | Low risk | Comment: no further sources of bias identified |
Vanlierde 1991.
| Methods | Double‐blind, 2‐arm, parallel‐group, randomised controlled trial, with active comparator (no placebo) and 5 days duration of treatment | |
| Participants |
Setting: multicentre, 7 continuing care wards Sample size:
Participant baseline characteristics:
Inclusion criteria: stable population of geriatric patients, must have grade 3 or 4 on a wax occlusion scale of 0 to 4 (0 being no wax) Exclusion criteria: none mentioned |
|
| Interventions |
Intervention group 1 (n = unclear participants; 34 ears): almond oil, 5 drops, twice daily, for 5 days Control group 2 (n = unclear participants; 35 ears): chlorobutanol, arachis oil and dichlorobenzene (Cerumol), 5 drops, twice daily, for 5 days Use of additional interventions: unstated |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
Timing of measurements:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | One patient developed otitis externa after using chlorobutanol, arachis oil and dichlorobenzene (Cerumol) and was withdrawn from the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "in a randomised observer‐blind fashion" Comment: insufficient information regarding methods used |
| Allocation concealment (selection bias) | High risk | Comment: not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "intraobserver variability was negligible due to prior self standardisation" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "in a randomised observer‐blind fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were accounted for |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear distinction between planned methods and results reported |
| Other bias | Low risk | Comment: no further sources of bias identified |
Whatley 2003.
| Methods | Double‐blind, 3‐arm, parallel‐group, randomised controlled trial, with active comparator and placebo, and 5 days duration of treatment | |
| Participants |
Setting: tertiary care children's hospital emergency department and paediatric outpatients, USA Sample size:
Participant baseline characteristics:
Eligibility criteria: paediatric patients with complete or partial external ear obstruction with wax Exclusion criteria: otitis externa, ventilation tubes, tympanic membrane perforation, hearing loss, allergies, prior complications of ear irrigation |
|
| Interventions |
Intervention group 1 (n = 34 participants; 34 ears): docusate sodium (Colace), 5 drops, twice daily, 5 days Intervention group 2 (n = 30 participants; 30 ears): triethanolamine polypeptide (Ceruminex), 5 drops, twice daily, 5 days Comparator group (n = 28 participants; 28 ears): saline, 5 drops, twice daily, 5 days Use of additional interventions: irrigation after treatment with drops was measured |
|
| Outcomes |
Primary outcome:
Secondary outcomes:
Other outcomes reported by the study:
Timing of measurements:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Participants lost to follow‐up: 1 patient was withdrawn by investigators due to error (irrigation took place before drops placed) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computerised randomisation created list order; consecutive patients took next in line |
| Allocation concealment (selection bias) | Low risk | Quote: "(trial substances) were placed in consecutively numbered envelopes by hospital pharmacist" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double‐blinded study design; test substance instilled by nurse not involved in assessing outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double‐blinded study design; assessor not involved in administering test substance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants were accounted for. One patient was withdrawn due to error in protocol. |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes proposed in the methods were reported in the results |
| Other bias | Low risk | Comment: no further sources of bias identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amjad 1975 | Allocation: randomised, double‐blinded Participants: 80 people (80 ears), most with hard or impacted cerumen Interventions: triethanolamine polypeptide (Cerumenex) + syringing versus carbamide peroxide + syringing |
| Baker 1969 | Allocation: non‐randomised, not double‐blinded nor placebo‐controlled |
| Burgess 1966 | Allocation: randomised, double‐blinded Participants: 50 people (74 ears) with more than one‐half occlusion of an ear with wax Interventions: Dioctyl‐medo ear drops (5% dioctyl sodium sulphosuccinate in a maize oil base) + syringing versus maize oil + syringing |
| Caballero 2009 |
Allocation: randomised, single‐blind Participants: adults with symptomatic complete ear canal obstruction Interventions: chlorobutanol or potassium carbonate + syringing |
| Chaput de Saintonge 1973 | Allocation: randomised, double‐blinded Participants: 67 ears, unstated number of participants Interventions: triethanolamine polypeptide oleate condensate (Xerumenex) + syringing versus olive oil + syringing |
| Dubow 1959 |
Allocation: randomised, double‐blinded Participants: 60 children with at least one completely cerumen‐occluded ear canal Interventions: drops (peroxide, triethanolamine polypeptide (Cerumenex) or mineral oil) + syringing |
| Eekhof 2001 | Allocation: quasi‐randomised (alternation), not blinded Participants: 42 people with persistent ear wax Interventions: olive oil + syringing versus water + syringing |
| Fahmy 1982a | Allocation: not randomised |
| Fahmy 1982b | Allocation: not randomised |
| Fahmy 1982c | Allocation: not randomised |
| Fraser 1970 | Allocation: randomised, double‐blinded Participants: 62 geriatric patients with hard wax completely occluding the external auditory meatus of both ears Interventions: arachis oil 57.3%, turpentine oil 10%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) versus sodium bicarbonate; olive oil versus sodium bicarbonate; docusate sodium (Waxsol) versus sodium bicarbonate; triethanolamine polypeptide oleate 10% in propylene glycerol (Xerumenex) versus sodium bicarbonate; docusate sodium in corn oil (Dioctyl) versus sodium bicarbonate. All treatments included a series of syringing attempts. |
| Fullington 2017 | Allocation: not randomised |
| GPRG 1965 | Allocation: randomised, double‐blinded Participants: 150 people with hard or impacted cerumen Interventions: 5% dioctyl sodium sulphosuccinate in a maize oil base (Dioctyl‐medo ear drops) + syringing versus maize oil + syringing |
| GPRG 1967 |
Allocation: randomised, double‐blinded Participants: 107 people with hard or impacted cerumen Interventions: dioctyl sodium sulphosuccinate (Waxsol) versus arachis oil 57.3%, turpentine oil 10%, chlorobutanol 5%, para‐dichlorobenzene 2% (Cerumol) + syringing versus maize oil + syringing |
| Hewitt 1970 | Allocation: randomised, not blinded Participants: all 31 participants presented with ear pain and the majority with acute otitis media |
| Hinchcliffe 1955 | Allocation: not randomised |
| Pavlidis 2005 | Allocation: randomised, non‐blinded Participants: 39 ears (of 26 patients) "partially or completely occluded by ear wax" Interventions: warm tap water instilled into the ear as a softening agent 15 minutes before syringing versus syringing alone |
| Proudfoot 1968 | Allocation: not randomised |
| Roland 2004 |
Allocation: randomised Participants: company employees, inappropriate study population |
| Sauris 2000 | Allocation: randomised Participants: convenience sample of co‐operative ambulatory patients requiring removal of cerumen to visualise tympanic membrane Interventions: both treatment groups contained irrigation; not known whether timing and results separate from ear drops alone |
| Soy 2015 | Allocation: not randomised |
| Spiro 1997 | Allocation: quasi‐randomised (sequential), not blinded Participants: 302 people with hard or impacted cerumen Interventions: docusate sodium (Colace) + syringing versus mineral oil + syringing versus no treatment + syringing versus syringing + 50% vinegar/50% alcohol solution |
Characteristics of studies awaiting assessment [ordered by study ID]
Kriukov 2014.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Article in Russian |
Characteristics of ongoing studies [ordered by study ID]
TCTR20160803001.
| Trial name or title | 'Cerumenolytic efficacy of 2.5% sodium bicarbonate and docusate sodium in patient with impacted cerumen. A randomized, controlled trial' |
| Methods | Allocation: randomised controlled trial Control: active Design: 2 arms, parallel groups Blinding: double‐blind |
| Participants |
Setting: Otolaryngology Department, Srinagarind Hospital Baseline characteristics: (currently recruiting) Inclusion criteria: adult patients with impacted cerumen in outpatient section and mobile unit ear check up Exclusion criteria: ear infection |
| Interventions |
Group 1: intra‐aural instillation of 2.5% sodium bicarbonate ear drops for 15 minutes Group 2: intra‐aural instillation of docusate sodium ear drops for 15 minutes If unsuccessful clearance, repeated treatment for 15 minutes; physician will clean ear canal again |
| Outcomes |
Primary outcomes: Visualising the tympanic membrane Secondary outcomes: Side effects |
| Starting date | August 2016 |
| Contact information |
Contact for Public Query's Name: Chanticha Laohakittikul
Phone: 0841293438
Email: l.chanticha@gmail.com
Postal Address: 123 Mitraparp Road, Otolaryngology Department, Srinagarind Hospital
State/Province: Khon Kaen Postal Code: 40002 Country: Thailand |
| Notes | — |
Contributions of authors
Ksenia Aaron: critically revising the protocol for essential intellectual content; screening studies for eligibility; data extraction; meta‐analysis; writing full review.
Tess Cooper: critically revised the protocol for essential intellectual content; acting as third independent review author when screening studies for eligibility; data extraction; meta‐analysis; writing full review.
Laura Warner: assisting in critically revising the protocol; screening studies for eligibility; editing full review.
Martin Burton: critically revising the protocol for essential intellectual content; data extraction; meta‐analysis; writing and editing full review; senior author.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
Ksenia Aaron: none known.
Tess Cooper: none known.
Laura Warner: none known.
Martin Burton: Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
Edited (no change to conclusions)
References
References to studies included in this review
Carr 2001 {published data only}
- Carr MM, Smith RL. Ceruminolytic efficacy in adults versus children. Journal of Otolaryngology 2001;30(3):154‐6. [DOI] [PubMed] [Google Scholar]
Dummer 1992 {published data only}
- Dummer DS, Sutherland IA, Murray JA. A single‐blind, randomized study to compare the efficacy of two ear drop preparations (Audax® and Cerumol®) in the softening of ear wax. Current Medical Research Opinions 1992;13:26‐30. [DOI] [PubMed] [Google Scholar]
Jaffe 1978 {published data only}
- Jaffe G, Grimshaw J. A multicentric clinical trial comparing Otocerol® with Cerumol® as cerumenolytics. Journal of International Medical Research 1978;6:241‐4. [DOI] [PubMed] [Google Scholar]
Keane 1995 {published data only}
- Keane EM, Wilson H, McGrane D, Coakley D, Walsh JB. Use of solvents to disperse ear wax. British Journal of Clinical Practice 1995;49(2):71‐2. [PubMed] [Google Scholar]
Lyndon 1992 {published data only}
- Lyndon S, Roy P, Grillage MG, Miller AJ. A comparison of the efficacy of two ear drop preparations (Audax® and Earex®) in the softening and removal of impacted ear wax. Current Medical Research Opinions 1992;13:21‐5. [DOI] [PubMed] [Google Scholar]
Meehan 2002 {published data only}
- Meehan P, Isenhour JL, Reeves R, Wrenn K. Ceruminolysis in the pediatric patient: a prospective, double‐blinded, randomized controlled trial. Academic Emergency Medicine 2002;9(5):521‐2. [Google Scholar]
Oron 2011 {published data only}
- Oron Y, Zwecker‐Lazar I, Levy D, Kreitler S, Roth Y. Cerumen removal: comparison of cerumenolytic agents and effect on cognition among the elderly. Archives of Gerontology and Geriatrics 2011;52:228‐32. [DOI] [PubMed] [Google Scholar]
Singer 2000 {published data only}
- Robinson A. Docusate sodium with irrigation was better than triethanolamine polypeptide with irrigation for dissolving earwax. Evidence‐Based Nursing 2001;4(2):48. [Google Scholar]
- Singer AJ, Sauris E, Viccellio AW. Ceruminolytic effects of docusate sodium: a randomized, controlled trial. Annals of Emergency Medicine 2000;36(3):228‐32. [DOI] [PubMed] [Google Scholar]
Vanlierde 1991 {published data only}
- Vanlierde MJ, Murray JAM, Tse E. A study to look at the efficacy of almond oil and Cerumol ear drops in the removal of ear wax. SA Family Practice 1991;12(8):324‐6. [Google Scholar]
Whatley 2003 {published data only}
- Robbins B. Randomized clinical trial of docusate, triethanolamine polypeptide, and irrigation in cerumen removal in children. Journal of Pediatrics 2004;145(1):138‐9. [CENTRAL: CN‐00497483; CRS: 1342965] [DOI] [PubMed] [Google Scholar]
- Whatley VN, Dodds CL, Paul RI. Randomized clinical trial of docusate, triethanolamine polypeptide, and irrigation in cerumen removal in children. Archives of Pediatrics & Adolescent Medicine 2003;157(12):1177‐80. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amjad 1975 {published data only}
- Amjad AH, Scheer AA. Clinical evaluation of cerumenolytic agents. Eye, Ear, Nose and Throat Monthly 1975;54:74‐6. [PubMed] [Google Scholar]
Baker 1969 {published data only}
- Baker BS. A clinical trial of a ceruminolytic agent. Transactions of the Society for Occupational Medicine 1969;19:62‐3. [DOI] [PubMed] [Google Scholar]
Burgess 1966 {published data only}
- Burgess EH. A wetting agent to facilitate ear syringing. The Practitioner 1966;197(182):811‐2. [PubMed] [Google Scholar]
Caballero 2009 {published data only}
- Caballero M, Navarrete P, Prades E, Domencch J, Bernal‐Sprekelsen M. Randomized, placebo‐controlled evaluation of chlorobutanol potassium carbonate, and irrigation in cerumen removal. Annals of Otolaryngology 2009;118(8):552‐5. [DOI] [PubMed] [Google Scholar]
Chaput de Saintonge 1973 {published data only}
- Chaput de Saintonge DM, Johnstone CI. A clinical comparison of triethanolamine polypeptide oleate‐condensate ear drops with olive oil for the removal of impacted wax. British Journal of Clinical Practice 1973;27:454‐5. [PubMed] [Google Scholar]
Dubow 1959 {published data only}
- Dubow E. A simple method to assure proper pediatric ear examinations. Archives of Pediatrics 1959;76:360‐3. [PubMed] [Google Scholar]
Eekhof 2001 {published data only}
- Eekhof JA, Bock GH, Cessie S, Springer MP. A quasi‐randomised controlled trial of water as a quick softening agent of persistent earwax in general practice. British Journal of General Practice 2001;51(469):635‐7. [PMC free article] [PubMed] [Google Scholar]
Fahmy 1982a {published data only}
- Fahmy S, Whitefield M. Multicentre clinical trial of Exterol® as a cerumenolytic. British Journal of Clinical Practice 1982;36(5):197‐204. [PubMed] [Google Scholar]
Fahmy 1982b {published data only}
- Fahmy S, Whitefield M. Multicentre clinical trial of Exterol® as a cerumenolytic. British Journal of Clinical Practice 1982;36(5):197‐204. [PubMed] [Google Scholar]
Fahmy 1982c {published data only}
- Fahmy S, Whitefield M. Multicentre clinical trial of Exterol® as a cerumenolytic. British Journal of Clinical Practice 1982;36(5):197‐204. [PubMed] [Google Scholar]
Fraser 1970 {published data only}
- Fraser JG. The efficacy of wax solvents: in vitro studies and a clinical trial. Journal of Laryngology and Otology 1970;84:1055‐64. [DOI] [PubMed] [Google Scholar]
Fullington 2017 {published data only}
- Fullington D, Song J, Gilles A, Guo X, Hua W, Anderson CE, et al. Evaluation of the safety and efficacy of a novel product for the removal of impacted human cerumen. BMC Ear, Nose, and Throat Disorders 2017;17(5):5. [CRS: 6212552; DOI: 10.1186/s12901-017-0038-8; NCT02829294; PUBMED: 28588421] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02829294. Evaluation of a novel product for the removal of impacted human cerumen (earwax). https://clinicaltrials.gov/ct2/show/NCT02829294 (first received 8 July 2016). [CRS: 7836188; NCT02829294]
GPRG 1965 {published data only}
- General Practitioner Research Group. A wetting agent to facilitate ear syringing. The Practitioner 1965;195:810‐2. [PubMed] [Google Scholar]
GPRG 1967 {published data only}
- General Practitioner Research Group. Wax softening with a new preparation. The Practitioner 1967;199:359‐62. [PubMed] [Google Scholar]
Hewitt 1970 {published data only}
- Hewitt HR. Clinical evaluation of choline salicylate eardrops. The Practitioner 1970;204:438‐40. [PubMed] [Google Scholar]
Hinchcliffe 1955 {published data only}
- Hinchcliffe R. Effect of current cerumenolytics. British Medical Journal 1955;2:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pavlidis 2005 {published data only}
- Pavlidis C, Pickering JA. Water as a fast acting wax softening agent before ear syringing. Australian Family Physician 2005;34(4):303‐4. [PubMed] [Google Scholar]
Proudfoot 1968 {published data only}
- Proudfoot J. Clinical trial of a ceruminolytic agent in general practice. British Journal of Clinical Practice 1968;22(2):69‐70. [PubMed] [Google Scholar]
Roland 2004 {published data only}
- Roland PS, Eaton DA, Gross RD, Wall GM, Conroy PJ, Garadi R, et al. Randomized, placebo‐controlled evaluation of Cerumenex and Murine earwax removal products. Archives of Otolaryngology ‐ Head and Neck Surgery 2004;130(10):1175‐70. [DOI] [PubMed] [Google Scholar]
Sauris 2000 {published data only}
- Sauris E, Singer AJ, Viccellio AW. Ceruminolytic effects of Cerumenex and docusate sodium: a randomized controlled trial. Academic Emergency Medicine 2000;7(5):471. [DOI] [PubMed] [Google Scholar]
Soy 2015 {published data only}
- Soy FK, Ozbay C, Kulduk E, Dundar R, Yazici H, Sakarya EU. A new approach for cerumenolytic treatment in children: in vivo and in vitro study. International Journal of Pediatric Otorhinolaryngology 2015;79:1096‐100. [DOI] [PubMed] [Google Scholar]
Spiro 1997 {published data only}
- Spiro S. A cost‐effectiveness analysis of earwax softeners. Nurse Practitioner 1997;22(8):28‐31,166. [PubMed] [Google Scholar]
References to studies awaiting assessment
Kriukov 2014 {published data only}
- Kriukov AI, Kunel'skaia NL, Gurov AB, Izotova GN, Shestakova EI. Comparative effectiveness of preparations for cerumenolysis. Vestnik Otorinolaringologii 2014;4:63‐6. [CRS: 6745209; PUBMED: 25377683] [PubMed] [Google Scholar]
References to ongoing studies
TCTR20160803001 {published data only (unpublished sought but not used)}
- Ceruminolytic efficacy of 2.5% sodium bicarbonate and docusate sodium in patient with impacted cerumen. A randomized, controlled trial. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20160803001 (first received 15 July 2016). [TCTR20160803001]
Additional references
Clegg 2010
- Clegg AJ, Loveman E, Gospodarevskaya E, Harris P, Bird A, Bryant J, et al. The safety and effectiveness of different methods of earwax removal: a systematic review and economic evaluation. Health Technology Assessment 2010;14(28):1‐192. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guest 2004
- Guest JF, Greener MJ, Robinson AC, Smith AF. Impacted cerumen composition, production, epidemiology and management. QJM 2004;97(8):477‐88. [DOI] [PubMed] [Google Scholar]
Hand 2004
- Hand C, Harvey I. The effectiveness of topical preparations for the treatment of earwax: a systematic review. British Journal of General Practice 2004;54:862‐7. [PMC free article] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kumar Sinha 2008
- Kumar Sinha A, Montgomery JK, Herer GR, McPherson DL. Hearing screening outcomes for persons with intellectual disability: a preliminary report of findings from the 2005 Special Olympics World Winter Games. International Journal of Audiology 2008;47:399‐403. [DOI] [PubMed] [Google Scholar]
Loveman 2011
- Loveman E, Gospodarevskaya E, Clegg A, Bryant J, Harris P, Bird A, et al. Ear wax removal interventions: a systematic review and economic evaluation. British Journal of General Practice 2011;61(591):e680‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2002
- Moore AM, Voytas J, Kowalski D, Maddens M. Cerumen, hearing, and cognition in the elderly. Journal of the American Medical Directors Association 2002;3(3):136‐9. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roeser 1997
- Roeser RJ, Ballachanda BB. Physiology, pathophysiology, and anthropology/epidemiology of human earcanal secretions. Journal of the American Academy of Audiology 1997;8:391–400. [PubMed] [Google Scholar]
Roland 2008
- Roland PS, Smith TL, Schwartz SR, Rosenfeld RM, Ballachanda B, Earll JM, et al. Clinical practice guideline: cerumen impaction. Otolaryngology ‐ Head and Neck Surgery 2008;139(3 Suppl 2):S1‐21. [DOI] [PubMed] [Google Scholar]
Wright 2015
- Wright T. Ear wax. BMJ Clinical Evidence 2015. [PMC free article] [PubMed]
References to other published versions of this review
Burton 2003
- Burton MJ, Doree CJ. Ear drops for the removal of ear wax. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD004326] [DOI] [PubMed] [Google Scholar]
Burton 2009
- Burton MJ, Doree C. Ear drops for the removal of ear wax. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004326.pub2] [DOI] [PubMed] [Google Scholar]
Burton 2016
- Burton MJ, Aaron K, Warner L. Ear drops for the removal of ear wax. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012171] [DOI] [PMC free article] [PubMed] [Google Scholar]


