Abstract
Objective
Suicide is the second leading cause of death among adolescents; however, objective biomarkers of suicide risk are lacking. Aberrant self-face amygdala activity is associated with suicide ideation, and its connectivity with neural regions that enable self-processing (eg medial prefrontal cortex) may be a suicide risk factor.
Method
Adolescents (aged 11–17 years; N = 120) were sorted into four groups: healthy controls (HC), depressed individuals with low suicide ideation (LS), depressed individuals with high suicide ideation (HS), and depressed suicide attempters (SA). Youth completed an emotional (Happy, Sad, Neutral) self-face recognition task in the scanner. Bilateral amygdala task-dependent functional connectivity was determined with psychophysiological interaction analysis. Connectivity was compared across groups and within Self versus Other faces across emotions and hemispheres. Voxelwise results were thresholded (p < .005, uncorrected) and corrected for multiple comparisons (p < .05, familywise error).
Results
Both HS and SA displayed greater amygdala connectivity with the dorsolateral prefrontal cortex, dorsomedial prefrontal cortex, and precuneus, compared to LS, who, in turn, showed greater connectivity than HC. Greater left amygdala–rostral anterior cingulate cortex (rACC) connectivity was observed in SA compared to all other groups, whereas right amygdala–rACC connectivity was greater in HS versus LS and HC.
Conclusion
Greater connectivity between amygdala and other regions implicated in self-face processing differentiated suicide ideation and suicide attempt groups. A dose-dependent response showed that greater rACC–left amygdala connectivity during self-face processing was associated with a recent suicide attempt, but that a greater rACC–right amygdala connectivity was associated with suicide ideation.
Keywords: suicide attempt, adolescence, depression, amygdala, self-face
Suicide affects individuals of all age groups; however, growing rates in recent years have placed suicide as the second leading cause of death in adolescents and young adults.1 Youth diagnosed with depressive disorders are particularly at risk for displaying suicidal behaviors, such that among adolescents with suicide ideation (SI), a diagnosis of major depressive disorder (MDD) predicts the development of a suicide plan and subsequent transition to suicide attempt.2 Therefore, isolating the neural correlates of suicide attempt behavior in this population may assist future studies identifying predictors of suicide attempt, which is of paramount importance. Current assessments of suicide risk rely on self-reports, which are limited because of unreliable reporting of suicide behaviors and because they yield nonspecific markers common to many young individuals who would never attempt suicide.3 Objective biomarkers of suicide risk are needed to effectively prevent suicide in this vulnerable population.
Self-Processing in Depression and Suicide Risk
Negative self-schemas increase risk for suicide by influencing perception, encoding, retrieval and interpretation of negative self-stimuli.4,5 Indeed, depressed adolescents and adults with high SI are more likely to endorse negative self-appraisals,6–8 recall negative self-traits, and attend to negative emotional stimuli4,9 relative to healthy controls. Research in adults indicates that holding negative views about oneself can transform self-awareness into a painful experience that motivates suicidal thoughts and behaviors as a means of escaping self-awareness and the accompanying negative emotions.10 Individuals are motivated to reach a state in which their self-concepts match ideal self-guides11; however, believing that it is impossible to reach an ideal self predicts hopelessness, negative affect, and SI.12 Adolescents with a history of suicide attempt display greater discrepancies between perceptions of actual versus ideal self, compared to healthy and psychiatric controls,8 which increases SI by way of heightened negative affective states.12 Thus, depressed adolescents with high SI are more prone to hold stable negative self-concepts, which perpetuate suicidal thoughts.
Neural Correlates of Self-Processing in Depression
The medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), and amygdala differentiate adult individuals with depression from healthy controls during emotional self-processing13 and are key areas of dysfunction in depression.14 Compared to controls, adult patients with depression display hyperactivation of mPFC and ACC during negative self-processing,13 suggesting stronger self-identification with negative qualities. Moreover, depressed adolescents display significantly greater activation of the amygdala during emotional self-processing compared to controls.15 In fact, amygdala–mPFC functional connectivity during negative self-processing is increased in adult patients compared to controls.16,17 Therefore, the amygdala is most engaged in self-processing when the self-relevant content is emotionally salient, and may interact with the mPFC to support negative self-processing.
Self-Face Processing and Suicide Risk in Adolescent Depression
Research in adults indicates that face processing is typically biased toward positively valenced stimuli and toward Self versus Other faces18; however, self-schemas can influence self-face processing.19 Indeed, depressed youth with high SI have weaker activation of ACC during Self versus Other face recognition than healthy and low SI controls.20 More broadly, amygdala functional connectivity with the mPFC/ACC may be useful for predicting suicide risk. Compared to healthy controls, bipolar adolescents (42% euthymic, 23% depressed, and 35% elevated mood state at time of scan) with a history of suicide attempt displayed decreased functional connectivity between the amygdala and mPFC/ACC while viewing novel faces that was negatively correlated with SI and attempt lethality in the attempters only.21 Connectivity during emotional self-face recognition may be a unique risk factor for suicidal behavior in depressed youth, given the strong bias toward negative self-attributions observed in those with depression and high SI.22,23 Research examining amygdala functional connectivity during emotional self-face processing is needed in depressed adolescents with a history of suicide attempt to confirm whether it is a neural marker of suicidal behavior.
In addition to activating general face-processing brain regions—for example, the fusiform gyrus, superior temporal sulcus, and cuneus24—face perception activates limbic brain regions, including the amygdala.24 A right hemisphere bias in the activation of the amygdala25,26 and mPFC27 has been observed during facial processing. Moreover, studies have reported laterality effects of amygdala function during emotion processing, such that the left amygdala supports explicit evaluation of emotions, whereas the right amygdala supports implicit evaluation of emotions.28,29 Explicit evaluation of emotion enabled by the left amygdala refers to a slower, voluntary emotion appraisal process, whereas implicit evaluation refers to a faster, automatic emotional process that takes place without conscious awareness (eg, unmasked versus masked emotion processing, respectively).29 There is mixed evidence in adults that the right amygdala and its connectivity are implicated in pathophysiology of SI30 and suicide attempt30,31; however, there is also evidence of aberrant left amygdala functional connectivity in depressed adults with a history of suicide attempt.30 Thus, amygdala function is likely associated with SI regardless of hemisphere, at least in adults.
Current Study
The current study examined amygdala functional connectivity during an emotional self-face recognition task in depressed youth with varying degrees of SI, including those with a recent suicide attempt, and healthy controls. Because of the bias toward negative self-processing in patients with depression and high SI,6,8 we hypothesized the following: (1) groups with high SI would demonstrate greater amygdala connectivity with neural regions implicated in self and face processing; (2) compared to controls, youth with recent suicide attempt would show greater amygdala–mPFC/ACC connectivity while recognizing sad self-images; and (3) right amygdala connectivity with mPFC/ACC would differentiate youth with recent suicide attempt more than left amygdala connectivity during sad self-face recognition.
METHOD
Participants
Adolescents (N = 120) between the ages of 11 and 18 years were recruited from short-term psychiatric inpatient units, psychiatric clinics, and school mental health clinics, and through fliers and radio advertisements in Minneapolis, Minnesota and in Pittsburgh, Pennsylvania.
Study groups were based on depression and SI, which were determined following clinical evaluations using the Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS-PL).32 Based on this assessment, youth were classified as having or not having a current depressive disorder or not having such a disorder (healthy controls [HC]; n = 38; females = 19). Depressed adolescents were further characterized by suicidality (SI and suicide attempts), which was calculated by standardizing and averaging SI items from the K-SADS-PL and the Child Depression Rating Scale (CDRS).33 A z score corresponding to a median-split raw score of 3.20 (raw score range, 1–5) classified youth as having high or low SI. A subset of depressed youth who recently attempted suicide (within the past 2–3 months), regardless of current SI, corresponded to a separate group (see Table S1, available online, for details). The final depression groups included low SI (LS; n = 31; females = 17), high SI (HS; n = 27; females = 15), and recent suicide attempt (SA; n = 24; females = 14). Participant characterization and site differences can be found in Supplement 1, available online.
Procedure
Participants completed two visits. During the first visit, youth assent and parent consent were obtained; clinical assessments and questionnaires were administered; and participant photographs with happy, sad, and neutral facial expressions were taken for later use during neuroimaging (details in Supplemental 1, available online). To determine participant IQ and pubertal status, youth completed the Wechsler Abbreviated Scale of Intelligence34 and Pubertal Development Scale,35 respectively. Parents provided information about family structure, income, and child medication use. Depression severity was represented as total CDRS score (minus SI items). Total number of years depressed, length of current depressive episode, and number of depressive episodes were determined with the K-SADS-PL. During a second visit 1 to 2 weeks later, participants underwent neuroimaging scanning. Institutional Review Boards at University of Minneapolis and University of Pittsburgh approved all procedures.
Emotional Self-Other Morph-Query (ESOM-Q) Task
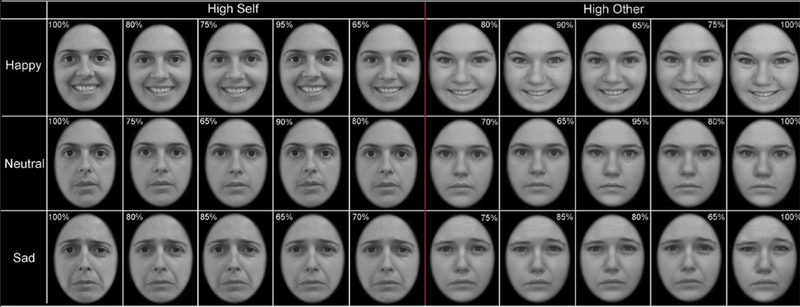
During neuroimaging, six blocks of faces (Happy-Self, Happy-Other, Sad-Self, Sad-Other, Neutral-Self and Neutral-Other; five counterbalanced task orders) were presented in one run (10:54 minutes) using E-Prime 2.0 (Psychology Research Tools, Pittsburgh, PA) (Figure 1; details in Supplemental 1, available online). Participants were instructed to identify whether the face in each photograph looked like them by pressing one of two buttons; recognition accuracy and reaction time (RT) were recorded for every trial and participant. This task has been published with an overlapping sample of the current study (n = 119; 99%). The present study includes a participant for whom data were recovered. The previous publication reported differences in task-related activation between healthy controls and depressed youth with high SI and low SI.20
FIGURE 1. Emotional Self-Other Morph-Query Task.
Note: Participants indicated via button box response whether a given face looked like them or not. They viewed images of faces with happy, sad, or neutral emotional expressions and with high or low percentage of self-features. The minimum percentage of self-features for self-blocks was 65% and the maximum percentage of self-features in other-blocks was 18%.
Magnetic Resonance Imaging Data Acquisition and Processing
Imaging data were collected using identical acquisition parameters on 3-Tesla Siemens Trio magnetic resonance imaging (MRI) scanners in Minneapolis (n = 1) and Pittsburgh (n = 1). Functional data were collected using a gradient echo planar imaging (EPI) sequence oblique to the axial plane (TR = 3,340 milliseconds, TE = 30 milliseconds; field of view [FOV] = 200 mm2, matrix = 80 mm2, flip angle = 90°; slice thickness = 2 mm, 60 slices). A high-resolution structural image was collected in the axial plane (TR = 2,100 milliseconds, TE = 3.31 milliseconds; TI = 1,050, flip angle = 8°, FOV = 256 × 200 mm, matrix = 256 × 200, slice thickness = 1 mm, 176 contiguous slices) during the same session for co-registration to functional data.
Preprocessing was performed with Statistical Parametric Mapping 12 (SPM12; http://fil.ion.ucl.ac.uk/spm) and included realignment, co-registration, segmentation of the structural image, spatial normalization into Montreal Neurological Institute (MNI) template space using the deformations from segmentation, and smoothing using a 7-mm full-width half-maximum Gaussian kernel. Functional volumes were examined for motion outliers (translation >2 mm and rotation >0.587°) and global signal intensities exceeding a value of >9, as determined with Artifact Detection Tools (ART) (http://web.mit.edu/swg/software.htm). Subject-level effects were estimated with the general linear model (GLM), with separate regressors for each condition (Happy-Self, Happy-Other, Sad-Self, Sad-Other, Neutral-Self and Neutral-Other) to estimate blood-oxygen-level–dependent (BOLD) signal. Volumes with high motion or elevated global intensities were censored from the first-level GLM analysis.
Group-Level Data Analysis
Demographic and Clinical Variables
Group differences were determined with univariate analysis of variance (ANOVA). Nominal variables were compared using χ2 analysis. Post hoc analyses were pursued, controlling for multiple comparisons using the Bonferroni procedure. All analyses were conducted in SPSS 24 (IBM Corp., Armonk, NY).
ESOM-Q Task Behavior
A repeated-measures ANOVA with two within-subject factors, namely, emotion (Happy, Sad and Neutral) and self (Self and Other), was used to compare mean accuracy across groups. Group differences in RT were determined with a separate repeated-measures ANOVA. Group differences in head motion during the scan (ie, number of data volumes censored with ART), were examined with univariate ANOVA. Depressive symptoms were correlated with RT during Happy-Self and Happy-Other conditions in SA youth (r2 = 0.54, p = .01 and r2 = 0.52, p = .02, respectively); therefore, depressive symptoms were included as a covariate. Greenhouse–Geisser correction was applied as appropriate, and significant effects were interrogated with post hoc analyses.
Functional Connectivity
Psychophysiological interaction (PPI) analyses were used to test task-dependent amygdala functional coupling with brain regions that responded differentially to task conditions.36 First, left and right amygdala seed regions were defined for each participant by creating two 7-mm (diameter) spheres centered around the peak activation coordinates within the boundaries of the left or right amygdala, as defined by the PickAtlas toolbox.37 This resulted in slightly different peak coordinates per participant that reflected the highest peak of amygdala activity for each individual. The peak coordinates of amygdala activation were statistically similar by group (F3, 104 = 1.79, p = .15), sex (F1, 104) = 3.81, p = .054), and scan site (F1, 104 = 0.001, p = .98). Peak coordinates and their distribution are reported in Table S2, available online. Second, for each participant, BOLD signal time courses were extracted from left and right seed regions for all conditions of interest and were convolved with the three contrasts of interest (Happy-Self versus Happy-Other; Sad-Self versus Sad-Other; Neutral-Self versus Neutral-Other), resulting in PPI activation maps. Third, the first-level PPI activation maps for the three contrasts were submitted to a second-level full factorial GLM with one between-group effect (four levels: SA, HS, LS, and HC) by two within-group effects: self-other within emotion contrasts (three levels: Happy-Self > Happy-Other, Sad-Self > Sad-Other, and Neutral-Self > Neutral-Other) and amygdala hemisphere (two levels: right and left). Nuisance regressors included variables that differed by groups (depression severity, family income, and IQ), as well as medication use and scan site. Parent marital status was not included, as it was significantly correlated with family income (Cramer V = 0.41, p < .001).
A combined voxel-height and cluster-extent threshold was calculated to control for type 1 error using Monte Carlo simulations in Analysis of Functional NeuroImages AFNI (v. 18.2.06).38 Using 3dClustSim, α= 0.05 was achieved via p < .005 for the principal GLM, such that a minimum of 138 voxels per cluster were required to reach significance. Smoothness estimates entered into 3dClustSim were averages of subject-level spatial autocorrelation function (acf) parameters based on individual subjects’ residuals from group-level models, as calculated by 3dFWHMx.
Significant main effects and interactions were interrogated with post hoc t tests in SPM12 (p < .005, uncorrected). Each pairwise comparison was masked with a binary mask of the significant cluster(s) from the respective main effects and interactions. Activity from each significant cluster was extracted using the SPM12 “eigenvariate” function to produce plots depicting the direction of effects.
Additional Analyses
To assist with interpretation of the results, ESOM-Q task activation was analyzed similar to Quevedo et al.,20 but using the current group designations. In addition, we conducted multiple regression analyses to explore dimensional associations of SI with amygdala functional connectivity during Self versus Other face recognition. See Supplement 1, available online, for the results.
RESULTS
Demographic and Clinical Characteristics
Diagnostic groups did not differ based on most demographic variables; however, the HC group had higher IQ than the depression groups, which did not differ from each other. More youth in the HC group had parents with higher income and who were married than did the depressed groups. Depression severity was higher in SA and HS, compared to LS and HC; LS also reported higher depression severity than HC. SA and HS endorsed higher SI, compared to LS and HC, but did not differ from each other; SI was also higher in LS versus HC. Diagnosis differed across depression groups, such that more youth in SA and HS had MDD diagnoses than LS. By contrast, more youth in LS had a diagnosis of depressive disorder not otherwise specified than HS, which, in turn, had had more youth with this diagnosis than SA (Table 1).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Healthy Control (HC) | Low Suicide Ideation (LS) | High Suicide Ideation (HS) | Suicide Attempt (SA) | Statistica | |
|---|---|---|---|---|---|
| n | 38 | 31 | 27 | 24 | |
| Age (mean ± SD) | 14.46 ± 1.52 | 14.87 ± 1.75 | 15.04 ± 1.68 | 14.61 ± 1.57 | F3, 116 = 0.79 |
| Puberty (mean ± SD) | 2.90 ± 0.61 | 3.02 ± 0.57 | 3.19 ± 0.46 | 3.13 ± 0.46 | F3, 116 = 1.80 |
| IQ (mean ± SD)b | 117.66 ± 11.46 | 104.00 ± 14.44 | 109.30 ± 12.56 | 107.17 ± 8.89 | F3, 116 = 8.06*** |
| Sex (% Female) | 50 | 55 | 56 | 58 | χ2(3) = 7.20 |
| Ethnicity (% white) | 76 | 55 | 59 | 58 | χ2(18) = 26.96 |
| Family Income (%) | χ2(27) = 47.20** | ||||
| <$15,000 | 3 | 3 | 19 | 21 | HC < HS = SA |
| $15–25,000 | 3 | 13 | 7 | 21 | HC < SA |
| $25–35,000 | 5 | 16 | 4 | 8 | |
| $35–50,000 | 8 | 19 | 15 | 17 | |
| $50–75,000 | 13 | 23 | 15 | 0 | LS = HS > SA |
| $75–100,000 | 24 | 10 | 7 | 21 | |
| $100–125,000 | 32 | 6 | 19 | 8 | HC > LS = SA |
| $125–150,000 | 3 | 10 | 0 | 0 | |
| $150–175,000 | 5 | 0 | 11 | 4 | |
| >$200,000 | 5 | 0 | 4 | 0 | |
| Parent Marital Status (%) | χ2(12) = 24.77* | ||||
| Married | 82 | 65 | 63 | 33 | HC = LS = HS > SA |
| Living with Partner | 5 | 13 | 4 | 13 | |
| Separated/Divorced | 8 | 19 | 19 | 17 | |
| Single/Never Married | 5 | 0 | 15 | 29 | HC = LS < SA LS < HS |
| Widowed | 0 | 3 | 0 | 4 | |
| Depression Severity (mean ± SD)c | 19.08 ± 5.52 | 53.87 ± 12.81 | 63.78 ± 13.56 | 63.21 ± 11.78 | F3, 116 = 124.01*** |
| Suicide Ideation (mean ± SD)d | 1.05 ± 0.19 | 1.91 ± 0.84 | 4.02 ± 0.47 | 3.63 ± 1.42 | F3, 116 = 94.96*** |
| Depression Diagnosis (%) | χ2(4) = 9.77* | ||||
| Major Depressive Disorder | N/A | 48 | 78 | 79 | LS < HS = SA |
| Dysthymia | N/A | 10 | 0 | 8 | |
| Depressive Disorder–NOS | N/A | 42 | 22 | 13 | LS > HS > SA |
| Comorbid Anxiety (%) Medication (%) | N/A | 65 | 85 | 58 | χ2(2) = 4.91 |
| Antidepressants | N/A | 32 | 48 | 54 | χ2(2) = 2.93 |
| Any Psychotropic | N/A | 35 | 59 | 63 | χ2(2) = 3.81 |
| Length of Current Episode in Years (mean ± SD) | N/A | 2.18 ± 1.74 | 2.19 ± 1.96 | 3.29 ± 2.11 | F2, 79 = 2.82 |
| Total Years Depressed (mean ± SD) | N/A | 2.81 ± 2.29 | 3.37 ± 2.06 | 3.83 ± 2.32 | F2, 79 = 1.47 |
| Total Depressive Episodes (mean ± SD) | N/A | 1.29 ± 0.78 | 1.56 ± 0.85 | 1.29 ± 0.62 | F2, 79 = 1.09 |
Note: Boldface indicates statistical significance. NOS = not otherwise specified.
Post hoc testing was corrected for multiple comparisons using the Bonferroni procedure.
HC > LS = HS = SA post hoc effects (p < .05).
HC < LS < HS = SA post hoc effects (p < .05); Childhood Depression Rating Scale total score minus suicide ideation items.
HC < LS < HS = SA post hoc effects (p < .05); suicide ideation average comprised of Child Depression Rating Scale (CDRS) suicide items and Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) suicide items. A score of 1 on the K-SADS indicates no significant suicide ideation, whereas CDRS scores ranging from 2 to 3 indicate low and fleeting thoughts of suicide. Three HC participants reported average suicide ideation values >1; however, none exceeded a score of 2, indicating that youth in the HC group did not display significant suicide ideation.
p < .05
p < .01
p < .001.
ESOM-Q Task Behavior
Statistics for results that did not reach significance are reported in Supplement 1, available online.
Accuracy
A main effect of emotion (F1.75, 194.25 = 14.01, p < .001) and an emotion-by-self interaction (F1.67, 185.48 = 7.67, p = .001) were found. Recognition accuracy was higher for Happy compared to Neutral (p < .001, Bonferroni) and Sad (p < .001, Bonferroni) faces; however, accuracy did not differ between Neutral and Sad faces (p = 1.00, Bonferroni). The self-by-emotion interaction was explained by higher accuracy for Happy Self versus Neutral Self faces across groups (p = .009, Bonferroni).
Reaction Time
A main effect of self was found (F1, 106 = 11.63, p = .001), with faster RT during Other versus Self conditions across emotions and groups.
Functional Connectivity
Head motion did not differ as a function of group (F3,104 = 1.56, p = .20), sex (F1,104 = 0.40, p = .53), or scan site (F1, 104 = 2.67, p = .11). Statistics for results that did not reach significance or that do not directly address the study hypotheses (ie, main effect of emotion with greater amygdala to IPL connectivity for sad and neutral versus happy faces; Figure S1, available online) are reported in Supplement 1, available online.
Main Effect of Group
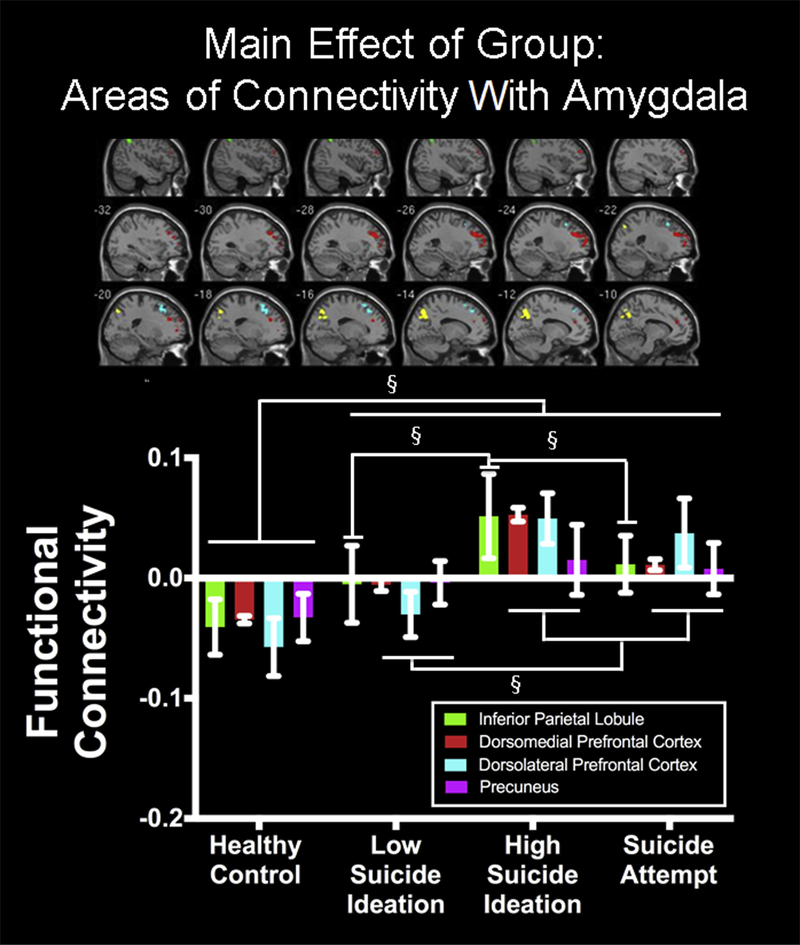
Amygdala connectivity varied as a function of group (F3,691 = 4.32, p < .005) (Table 2). Compared to HC, depression groups had higher connectivity between the amygdala and left inferior parietal lobule (IPL; LS: t691 = 4.13; HS: t691 = 4.85; SA: t691 = 4.08), dorsolateral prefrontal cortex (dlPFC)/dorsal anterior cingulate cortex (dACC; LS: t691 = 4.28; HS: t691 = 4.60; SA: t691 = 4.41), dorsomedial prefrontal cortex (dmPFC; LS: t691 = 3.61; HS: t691 = 4.08; SA: t691 = 4.47), and precuneus/ cuneus (LS: t691 = 3.95; HS: t691 = 4.32; SA: t691 = 4.44), as indicated by post hoc t testing. Post hoc t tests also indicated that SA and HS showed greater amygdala connectivity with dlPFC/dACC (HS: t691 = 3.92; SA: t691 = 3.45), dmPFC (HS: t691 = 4.08; SA: t691 = 3.57), and precuneus/cuneus (HS: t691 = 3.60; SA: t691 = 3.18) than LS. Finally, HS showed greater amygdala connectivity with IPL, compared to LS (t691 = 2.79) and SA (t691 = 2.77) (Figure 2; Table S3, available online). These effects were not influenced by head motion, as no significant correlations were found (all r2 < 0.11, p > .05) between connectivity and motion parameters.
Table 2.
Areas of Coupling With Amygdala During Self-Versus Other-Face Processing
| Whole-Brain Results | Voxels | Hemisphere | MNI Coordinates (X, Y, Z) | Z | ||
|---|---|---|---|---|---|---|
| Main Effect of Group Inferior Parietal Lobule, BA 40 | 203 | Left | −46 | −50 | 48 | 3.96 |
| Dorsolateral Prefrontal/Dorsal Anterior Cingulate Cortex, BA 10/9/32 | 655 | Left | −26 | 36 | 30 | 3.86 |
| Dorsomedial Prefrontal Cortex, BA 8 | 170 | Left | −16 | 20 | 58 | 3.71 |
| Precuneus/Cuneus, BA 7 | 316 | Left and right | −14 | −64 | 34 | 3.61 |
| Interaction of Amygdala Hemisphere and Diagnostic Group Rostral Anterior Cingulate Cortex, BA 10/24/32 | 235 | Left and right | −2 | 36 | 2 | 4.01 |
Note: BA = Brodmann Area; MNI = Montreal Neurological Institute.
FIGURE 2. Amygdala Functional Connectivity During Self Versus Other Processing (Mean ± Standard Error).
Note: Group differences were observed in amygdala functional connectivity with inferior parietal lobule (IPL), dorsomedial prefrontal cortex (dmPFC), dorsolateral prefrontal cortex (dlPFC)/dorsal anterior cingulate cortex (dACC), and precuneus/cuneus. Please note color figures are available online.
§p < .005.
Group-by-Hemisphere
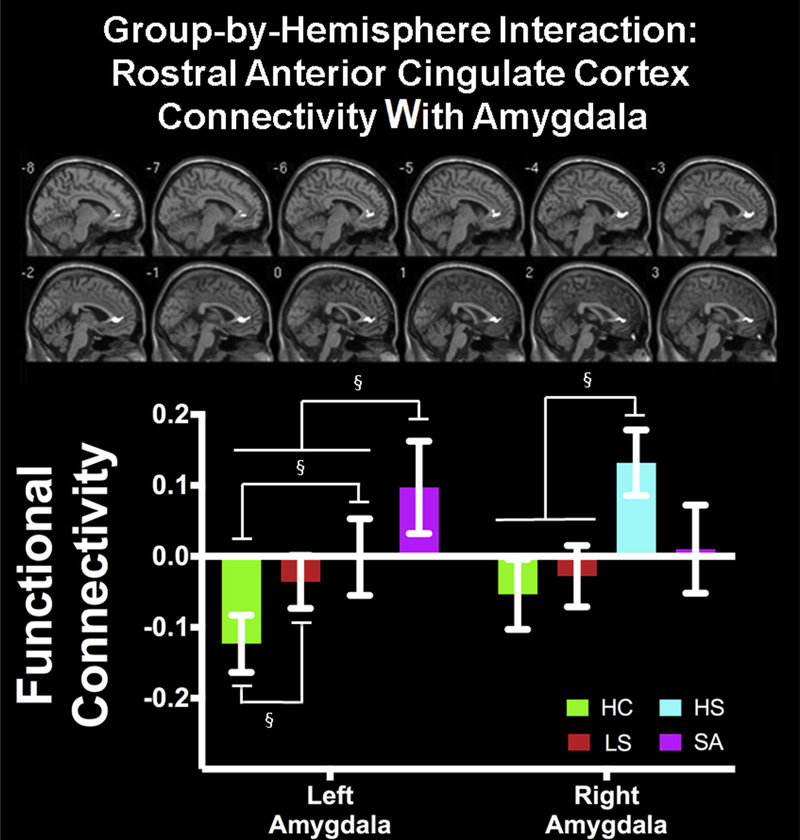
Connectivity between amygdala and bilateral rostral ACC (rACC) varied as a function of group and amygdala hemisphere (F3,691 = 4.32, p < .005) (Table 2). Post hoc t tests comparing groups within hemisphere revealed that HC showed the lowest left amygdala–rACC connectivity, compared to depression groups (HC < LS: t397 = 4.30; HC < HS: t373 = 3.52: HC < SA, t355 = 3.08). Moreover, SA showed greater left amygdala–rACC connectivity than all other groups (SA > HC: t355 = 3.08; SA > LS: t313 = 2.93; SA > HS: t289 = 3.05). By contrast, HS showed greater right amygdala–rACC connectivity compared to LS (t331 = 3.80) and HC (t373 = 3.46) (Figure 3; Table S3, available online). All other pairwise comparisons were not significant. These effects were not influenced by motion, as connectivity values were not associated with motion parameters (r2 < 0.16, p > .05).
FIGURE 3. Amygdala Functional Connectivity With Rostral Anterior Cingulate Cortex Varied as a Function of Group and Hemisphere (Mean ± Standard Error).
Note: HC = healthy control; HS = high suicide ideation; LS = low suicide ideation; SA = depressed suicide attempt. Please note color figures are available online.
§p < .005.
DISCUSSION
Depression and suicide ideation (SI) were related to altered amygdala functional connectivity with brain regions involved in self and face processing, with important distinctions between degrees of SI and suicide attempt evident in amygdala–rACC connectivity, regardless of emotion condition. Specifically, SA youth differed from HS due to greater left amygdala–rACC connectivity, suggesting that inefficient explicit or conscious emotion evaluation elicited by self-face processing is linked to suicide attempt in depressed youth. These findings suggest that depressed youth who endorse high SI might be at risk for suicide attempt if confronting self-related information elicits emotional responses that are inefficiently regulated.
Self-Face Processing Differs Based on Degree of Suicide Ideation
Our first hypothesis, namely, that depressed youth with high SI (ie, SA and HS) would show greater amygdala connectivity with self and face-processing brain regions, was supported. Compared to HC, all depressed groups displayed greater amygdala connectivity with brain regions implicated in self and face processing—namely, the IPL, dlPFC/dACC, dmPFC, and precuneus/cuneus.24 However, SA and HS showed a greater effect compared to LS, suggesting that recognizing self-faces might be more salient for depressed youth who report high SI. These findings are consistent with a previous study of the same task and participants who reported greater activation of the cuneus in HS versus LS and HC and in dlPFC in HS and LS versus HC, across self and emotion conditions.20 The lack of group-by-emotion interaction in the present and previous study20 indicates that facial emotions do not alter saliency of self-face processing for depressed groups with high SI. Moreover, analysis of the dimensional associations with SI (Supplement 1, available online) indicated that greater SI was associated with greater amygdala connectivity with cortical areas (cuneus, precuneus, and ACC/dlPFC) during Self versus Other face recognition. Thus, greater amygdala connectivity with face-processing brain regions may reflect greater sensitivity or cognitive effort in response to viewing one’s face, particularly in individuals with high SI. Moreover, there was a dose-dependent response, such that the greatest amygdala–cortical connectivity was observed in depressed youth with high SI, followed by those with low SI and finally healthy controls, who reported no clinically meaningful SI.
Notably, the majority of the identified brain regions in the current study are also important for emotion regulation—the IPL, dlPFC/dACC, and dmPFC39—raising the possibility that recognizing self-faces triggered a compensatory emotion regulation response in depressed youth with high SI. Although the ESOM-Q task does not measure emotion regulation explicitly, it is possible that recognizing self-faces triggered implicit regulation of attention if viewing one’s face elicited an emotional response. Indeed, research in depressed adults indicates that dmPFC, dlPFC, and dACC are hyperactive during implicit attention regulation of emotional stimuli.39 Moreover, adolescents who endorse SI have been shown to activate dlPFC more than youth without SI while regulating emotional responses.40 Thus, greater connectivity between amygdala and these cortical regions may reflect a neural compensatory response whereby regulation of amygdala by dmPFC, dlPFC, dACC, and IPL corresponds to implicit regulation of emotional responses elicited by recognizing self-faces. This mechanism has not been studied in depressed adolescents with high SI; therefore, this interpretation remains speculative.
Laterality Effects of Amygdala Functional Connectivity During Self-Face Processing
In healthy adults, left amygdala activation is associated with explicit evaluation of emotion, specifically when induced by viewing novel faces expressing sadness, whereas the right amygdala appears to be important for implicit evaluation of emotion.28,29 Contrary to our hypothesis, neither right nor left amygdala–rACC connectivity during recognition of self-faces differed based on group and emotion; however, a significant group-by-hemisphere interaction emerged in rACC that differentiated SA. Specifically, left amygdala–rACC functional connectivity was greatest in SA, followed by the other depression groups, who in turn had greater connectivity than HC. Right amygdala–rACC connectivity also differed based on group, such that HS, but not SA, showed greater functional connectivity compared to LS and HC. SI was statistically similar in the HS and SA groups; therefore, right amygdala–ACC functional connectivity may be linked with SI per se, whereas left amygdala–ACC connectivity may be a biomarker of risk for suicide attempt (or a consequence of previous suicide attempt).
The significant difference in left amygdala–rACC connectivity between SA and HS is mirrored by group differences in task activation during Self versus Other face processing (Table S4, Figures S2, S3, available online). Previously published work using the same task and sample found blunted activation of rACC during Self versus Other face recognition in HS compared to LS and HC.20 Replicating this work, SA showed blunted activity in a more ventral region of rACC during Self versus Other face recognition compared to HS and HC, who exhibited no significant differences in rACC activity between Self versus Other conditions, whereas LS showed more rACC activation during Self versus Other face recognition (Figure S3, available online).20 These results suggest that SA differed from HS youth in both rACC activity and left amygdala–rACC connectivity during self-face recognition (Figure S4, available online). Rostral ACC is part of the rostral–ventral affective division of the ACC that supports the detection of emotional and motivational information and the regulation of emotional responses.41 Thus, blunted rACC activity during Self versus Other face processing in SA might reflect ineffective regulatory capacity of the emotional aspects of self-face processing. Therefore, greater left amygdala–rACC functional connectivity may represent a compensatory response to weakened regulatory capacity in SA. By contrast, given the role of the right amygdala in implicit evaluation of emotion,28,29 its connectivity with rACC may represent a greater emotional response to viewing oneself in youth with greater SI, irrespective of depression diagnosis and suicide attempt history. In support of this interpretation, we found that right but not left amygdala–ACC functional connectivity was positively associated with SI across the full sample (Table S5, Figures S5, S6, available online). However, emotion regulation was not directly measured in this study; therefore, the above interpretations are speculative. In sum, rACC connectivity with both left and right amygdala differentiated depressed groups with high SI; however, only left amygdala–rACC functional connectivity during self-face processing distinguished SA youth.
This is the first study to measure neural functional connectivity during emotional self-face recognition in depressed adolescents with varying degrees of SI and histories of suicide attempt, which is critical given that previous suicide attempt is predictive of future attempts.2 A strength of this study is its relatively large sample size with thorough clinical assessment that yielded well-characterized groups. Finally, careful consideration of potential confounding variables, such as data collection site, depression severity, medication use, IQ, and family income resulted in connectivity analyses that controlled for these variables.
Limitations of this study should also be considered. First, despite statistically controlling for sites, we cannot definitively rule out an effect of location. However, data acquisition parameters were identical and head motion during scanning did not differ based on location, bolstering our confidence that imaging data were not biased by scan site. Similarly, although we controlled for medication status, we cannot confirm that it did not influence our outcomes. However, our finding differentiating SA from HS was not confounded by medication status, as depression groups did not differ from one another in this respect. Second, a potential limitation of our analytic approach is the definition of seeds as peak activation in the left and right amygdala unique to each participant.20 This approach might have resulted in seed regions that included adjacent structures if a given peak was close to the boundaries of the amygdala. However, this approach was implemented to ensure that voxels with the most robust and representative amygdala activation were included in the seed regions of a given individual. This potential limitation is outweighed by research showing that standardization of seed regions leads to inaccurate calculations of functional connectivity, at least under conditions of rest.42 Third, the morphed facial images may have influenced face recognition performance. Despite showing higher recognition accuracy of happy faces, in contrast to prior studies, participants were slower to respond to Self versus Other faces.19 This may indicate that morphed self and other faces required additional time for processing. Indeed, the rationale for using morphed faces was to increase attentional demands, produce varied stimuli, and prevent habituation, given that the amygdala habituates to emotional stimuli over time.29 Finally, given the cross-sectional design of this study, we cannot establish a causal relationship between functional connectivity and depression or suicide attempts. Prospective longitudinal studies need to confirm the direction of effects presented here.
In this study, depressed adolescents with high SI displayed greater functional connectivity between the amygdala and cortical regions that enable face processing, self-processing, and emotion regulation during recognition of self-faces, indicating that self-face stimuli were perceived as more salient and/or required greater cognitive effort to process among depressed youth who endorse high SI. Left amygdala–rACC connectivity during self-face processing may have the greatest potential for predicting suicide attempt among depressed youth with high SI. Prospective studies must confirm whether these neural mechanisms predict suicide attempts.
Supplementary Material
Acknowledgments
Funding awarded to Dr. Quevedo from the National Institute of Mental Health (NIMH; MH092601), the Brain and Behavior Research Foundation (NARSAD Young Investigator Award), and the University of Minnesota Clinical and Translational Science Institute supported data collection and analysis and manuscript preparation. Additional funding from NIMH (MH018951) to Dr. Alarcon supported data analysis and manuscript preparation. Funding sources were not involved in the design of this study, collection, analysis and interpretation of data, or manuscript preparation.
Footnotes
Disclosure: Drs. Alarcon, Forbes, Quevedo, Mr. Sauder, and Ms. Teoh report no biomedical financial interests or potential conflicts of interest.
Portions of this data were published as an abstract and poster at the 56th Annual Meeting of the Society for Psychophysiological Research, Minneapolis, MN, September 21-25, 2016.
Contributor Information
Gabriela Alarcón, University of Pittsburgh, PA..
Mitchell Sauder, University of Minnesota, Minneapolis, MN..
Jia Yuan Teoh, University of Minnesota, Minneapolis, MN..
Erika E. Forbes, University of Pittsburgh, PA..
Karina Quevedo, University of Minnesota, Minneapolis, MN..
REFERENCES
- 1.Thompson MP, Swartout K. Epidemiology of suicide attempts among youth transitioning to adulthood. J Youth Adolesc. 2018;47:807–817. [DOI] [PubMed] [Google Scholar]
- 2.Nock MK, Green JG, Hwang I, et al. Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2013;70:300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nock MK, Park JM, Finn CT, Deliberto TL, Dour HJ, Banaji MR. Measuring the suicidal mind: implicit cognition predicts suicidal behavior. Psychol Sci. 2010;21:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke TA, Hamilton JL, Ammerman BA, Stange JP, Alloy LB. Suicide risk characteristics among aborted, interrupted, and actual suicide attempters. Psychiatry Res. 2016;242:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wenzel A, Beck AT. A cognitive model of suicidal behavior: theory and treatment. Appl Prev Psychol. 2008;12:189–201. [Google Scholar]
- 6.Brunstein Klomek A, Zalsman G, Apter A, et al. Self-object differentiation in suicidal adolescents. Compr Psychiatry. 2007;48:8–13. [DOI] [PubMed] [Google Scholar]
- 7.Tang J, Wu S, Miao D. Experimental test of escape theory: accessibility to implicit suicidal mind. Suicide Life Threat Behav. 2013;43:347–355. [DOI] [PubMed] [Google Scholar]
- 8.Orbach I, Mikulincer M, Stein D, Cohen O. Self-representation of suicidal adolescents. J Abnorm Psychol. 1998;107:435–439. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg MS, Beck AT. Depression versus anxiety: a test of the content-specificity hypothesis. J Abnorm Psychol. 1989;98:9–13. [DOI] [PubMed] [Google Scholar]
- 10.Baumeister RF. Suicide as escape from self. Psychol Rev. 1990;97:90–113. [DOI] [PubMed] [Google Scholar]
- 11.Higgins ET. Self-discrepancy: a theory relating self and affect. Psychol Rev. 1987;94: 319–340. [PubMed] [Google Scholar]
- 12.Cornette MM, Strauman TJ, Abramson LY, Busch AM. Self-discrepancy and suicidal ideation. Cogn Emot. 2009;23:504–527. [Google Scholar]
- 13.Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. 2013;7:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P. Medial prefrontal cortex and the self in major depression. J Affect Disord. 2012;136:e1–e11. [DOI] [PubMed] [Google Scholar]
- 15.Quevedo K, Martin J, Scott H, Smyda G, Pfeifer JH. The neurobiology of self-knowledge in depressed and self-injurious youth. Psychiatry Res. 2016;254:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimura S, Okamoto Y, Onoda K, et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J Affect Disord. 2010;122:76–85. [DOI] [PubMed] [Google Scholar]
- 17.Kessler H, Taubner S, Buchheim A, et al. Individualized and clinically derived stimuli activate limbic structures in depression: an fMRI study. PLoS One. 2011;6: e15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu CP, Di X, Eickhoff SB, et al. Distinct and common aspects of physical and psychological self-representation in the brain: a meta-analysis of self-bias in facial and self-referential judgements. Neurosci Biobehav Rev. 2016;61:197–207. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, Han S. Why we respond faster to the self than to others? An implicit positive association theory of self-advantage during implicit face recognition. J Exp Psychol Hum Percept Perform. 2010;36:619–633. [DOI] [PubMed] [Google Scholar]
- 20.Quevedo K, Ng R, Scott H, et al. The neurobiology of self-face recognition in depressed adolescents with low or high suicidality. J Abnorm Psychol. 2016;125: 1185–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston JA, Wang F, Liu J, et al. Multimodal neuroimaging of frontolimbic structure and function associated with suicide attempts in adolescents and young adults with bipolar disorder. Am J Psychiatry. 2017:appiajp201615050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alloy LB, Abramson LY, Safford SM, Gibb BE. The Cognitive Vulnerability to Depression (CVD) Project: Current Findings and Future Directions. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2006. [Google Scholar]
- 23.Beck AT. A systematic investigation of depression. Compr Psychiatry. 1961;2:163–170. [DOI] [PubMed] [Google Scholar]
- 24.Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Cortical mechanisms of visual self-recognition. Neuroimage. 2005;24:143–149. [DOI] [PubMed] [Google Scholar]
- 25.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. [DOI] [PubMed] [Google Scholar]
- 26.Herrington JD, Taylor JM, Grupe DW, Curby KM, Schultz RT. Bidirectional communication between amygdala and fusiform gyrus during facial recognition. Neuroimage. 2011;56:2348–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sugiura M Three faces of self-face recognition: potential for a multi-dimensional diagnostic tool. Neurosci Res. 2015;90:56–64. [DOI] [PubMed] [Google Scholar]
- 28.Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58:57–70. [DOI] [PubMed] [Google Scholar]
- 29.McMenamin BW, Marsolek CJ. Can theories of visual representation help to explain asymmetries in amygdala function? Cogn Affect Behav Neurosci. 2013;13: 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang SG, Na KS, Choi JW, Kim JH, Son YD, Lee YJ. Resting-state functional connectivity of the amygdala in suicide attempters with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2017;77:222–227. [DOI] [PubMed] [Google Scholar]
- 31.Monkul ES, Hatch JP, Nicoletti MA, et al. Fronto-limbic brain structures in suicidal and non-suicidal female patients with major depressive disorder. Mol Psychiatry. 2007;12: 360–366. [DOI] [PubMed] [Google Scholar]
- 32.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Aged ChildrenPresent and Lifetime (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 33.Poznanski EO, Cook SC, Carroll BJ. A depression rating scale for children. Pediatrics. 1979;64:442–450. [PubMed] [Google Scholar]
- 34.Wechsler D Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: American Psychological Association; 1999. [Google Scholar]
- 35.Petersen AC, Crockett L, Tobin-Richards M, Boxer A. Measuring Pubertal Status: Reliability and Validity of a Self-Report Measure. University Park, PA: Pennsylvania State University; 1985. [Google Scholar]
- 36.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. [DOI] [PubMed] [Google Scholar]
- 37.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 38.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 39.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhe HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013;37: 2529–2553. [DOI] [PubMed] [Google Scholar]
- 40.Miller AB, McLaughlin KA, Busso DS, Brueck S, Peverill M, Sheridan MA. Neural correlates of emotion regulation and adolescent suicidal ideation. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. [DOI] [PubMed] [Google Scholar]
- 42.Sohn WS, Yoo K, Lee YB, Seo SW, Na DL, Jeong Y. Influence of ROI selection on resting state functional connectivity: an individualized approach for resting state fMRI analysis. Front Neurosci. 2015;9:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.