Hypoxia and anoxia are pathophysiologic characteristics of most solid tumors (1, 2). For nearly 150 years, nonpathogenic, anaerobic bacteria that preferentially localize and proliferate in the hypoxic regions of tumors have been investigated as treatments for experimental and human tumors with mixed success (Table 1). In recent years, there has been a renewed interest in using these bacteria as innovative delivery vehicles for gene therapy (Table 1). Now, as described in this issue of PNAS, Vogelstein and coworkers (11) have created a new strain of anaerobic bacteria, devoid of its toxic genes, that leads to dramatic and prolonged regression of subcutaneous tumors when systematically administered with conventional drugs. This strategy, referred to as combination bacteriolytic therapy (COBALT), adds a new weapon in the war against cancer. However, there are still obstacles that need to be overcome before it can be used safely in the clinic.
Ironically, a tumor's metabolically compromised microenvironment provides a haven for a number of anaerobic bacteria.
Table 1.
Examples of bacteriolytic therapy of tumors in vivo
| Organism | Species | Ref. | Year | Model | Animal | Combination | Length | Strategy | Results |
|---|---|---|---|---|---|---|---|---|---|
| Clostridium | histolyticum | 3 | 1947 | Sarcoma | Mouse | 66 d | Diminish toxicity of histolyticum with antitoxin and penicillin | Temporary regression and prolonged survival | |
| (obligatory anaerobe) | tetani | 4 | 1955 | Carcinoma, hepatoma | Mouse | 2 d | Localization of obligatory anaerobic C. tetani to hypoxic and necrotic regions in tumors | Rapid death of tumor bearing mice | |
| butyricum (M-55) | 5 | 1964 | Ehrlich carcinomas | Mouse | Short- until death | Identification of most effective of 14 different Clostridium species | Regression but eventual animal death | ||
| butyricum (M-55) | 6 | 1964 | Carcinoma, melanoma | Mouse, Rat | Heavy metal-iron dextran | <12 d | Heavy metals increase tumor lysis | Increased growth delay, eventual animal death | |
| acetobutylicum butyricum pectinovorum tyrobutyricum | 7 | 1964 | Sarcoma, melanoma, renal adenocarcinoma | Mouse Hamster | 5-FU, Tetramin, E-39, Mitomycin C | 3 mo | Use combination of Clostridium and various chemotherapeutics to kill viable rim | Clostridium regressed tumors but did not affect small tumors or metastases. All combinations significantly increased regression. Within 3 months all animals died. | |
| butyricum | 8 | 1967 | Human | 13 mo | Clostridium-induced lysis | Three cases: failure to lyse, lysis with death, lysis with survival | |||
| beijerinckii (acetobutylicum) | 9 | 1997 | EMT6 | Mouse | Clostridium delivery of nitroreductase to activate prodrug CB 1954 | Nitroreductase activity detected in tumor lysate | |||
| acetobutylicum | 10 | 2001 | Rhabdomyo-sarcoma | Rat | Clostridium delivery of enzyme to convert 5-FC to 5-FU | Cytosine deaminase activity detected in tumor lysate | |||
| novyi | 11 | 2001 | B16 melanoma, HCT116 colon carcinoma | Mouse | Cytotoxic and antivascular Chemo-therapeutics | 3 mo | Use combination chemo- and anti-vascular therapy with bacteriolytic therapy to shrink tumor masses | Complete cure in ∼50% of mice, complicated by death of ∼15–45% of mice | |
| Bifidobacterium (obligatory anaerobe) | infantis | 12 | 1978 | Meth-A sarcoma | Mouse | 30 d | Targeted immunomodulation | Tumor regression | |
| bifidum | 13 | 1980 | Fibrosarcoma | Mouse | 90 d | B. bifidum to identify tumors | B. bifidum localizes to tumors and is nontoxic | ||
| longum | 14 | 2000 | B16–F10 melanoma | Mouse | 7 d | Gene delivery using engineered Bifidobacterium | Engineered bacteria found only in tumors | ||
| longum | 15 | 2001 | DMBA-induced mammary carcinoma | Rat | 7 d | Test delivery to spontaneous tumors | Engineered B. longum also targets spontaneous tumors | ||
| Salmonella (facultative anaerobe) | typhimurium | 16 | 1999 | B16–F10 melanoma | Mouse | 40 d | Attenuate toxicity of Salmonella and retain tumor targeting | Significant delay in tumor growth | |
| typhimurium | 17 | 2000 | B16–F10 melanoma | Mouse Monkey | 30 d | Attenuated Salmonella target tumor over other organs | Accumulate in tumors >1,000-fold | ||
| Corynebacterium (obligatory anaerobe) | parvum | 18 | 1990 | Breast cancer | Human | Melphalan, 5-FU | 8 yrs | Investigate benefit of nonspecific immuno-stimulating agents | No significant benefit observed |
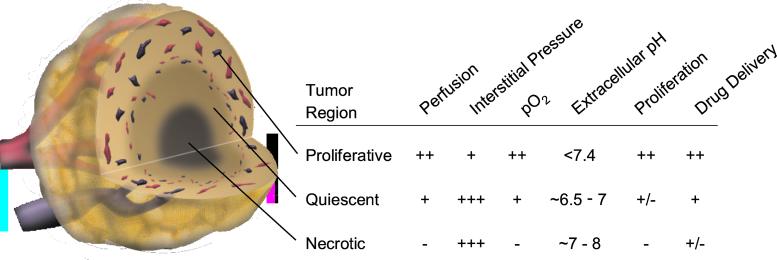
In tumors, blood vessels are structurally and functionally abnormal, resulting in temporally and spatially heterogeneous blood flow (19, 20). This heterogeneity hinders the delivery of blood-borne therapeutics to all cancer cells and leads to acutely and/or chronically hypoxic and acidic regions in tumors (Fig. 1). These conditions reduce the effectiveness of radiation and some chemotherapeutic agents and select for cancer cells that are more aggressive, metastatic, and resistant to various therapies (2, 21).
Figure 1.
Schematic of three microenvironmental regions in a centrally necrotic tumor. A spontaneous tumor may consist of many such necrotic foci. Decreasing magnitude of various physiological parameters is indicated as +++, ++, +, +/−, and −.
Ironically, a tumor's metabolically compromised microenvironment provides a haven for a number of anaerobic bacteria. And indeed, over the past 50 years, several strains of facultative and obligate anaerobic bacteria have been shown to localize and cause lysis in transplanted tumors in animals (Table 1). These initial animal studies were so encouraging that clinical trials using Clostridium began in the 1960s (8). Unfortunately, the results were not as impressive as anticipated and the trials were discontinued.
So why is there a resurgence of interest in using bacteria to treat solid tumors? To answer this question we need to examine the criteria for an ideal anticancer bacterium.
They should be: (i) nontoxic to the host; (ii) only able to replicate within the tumor; (iii) motile and able to disperse evenly throughout a tumor (including hypoxic and necrotic regions); (iv) slowly and completely eliminated from the host; (v) nonimmunogenic; and (vi) able to cause lysis of tumor cells by direct competition for nutrients, localized production of cytotoxins, or production of therapeutic amplifiers.
In the last decade, significant progress has been made on each of these fronts. Multiple approaches have been used to remove the toxin genes of bacteria (16, 17). For instance, Dang et al. (11) used heat shock to eliminate the lethal toxin genes from Clostridium novyi, located within a phage episome. Modern molecular approaches might be used once genome sequences of various strains of bacteria become available (22, 23). Of course, the use of naturally nonpathogenic bacteria (e.g., Clostridium oncolyticum) might avoid the toxicity problem altogether. Additionally, techniques developed to transfer genetic material into bacteria other than Escherichia coli, for example the anaerobic bacteria Clostridium acetobutylicum (24) and Bifidobacterium longum (25), have the potential to modulate the toxicity, motility, and protein expression of therapeutic bacteria.
Currently there are no rapid, reliable, and inexpensive methods to screen for an ideal bacterium. Dang et al. (11) screened 26 strains of bacteria for their ability to spread evenly throughout poorly vascularized regions of tumors. The selected bacteria were seen growing throughout the enlarged necrotic regions of tumors after systemic injection of spores. Apparently, the bacteria were destroying the viable cells at the interface of the necrotic region, and using the degradation products as nutrients. However, this treatment did not eradicate the tumor completely, leaving a ring of viable cells at the tumor periphery. To kill cells in the viable ring, Dang et al. chose to combine the bacteriolytic therapy with low molecular weight conventional chemotherapy (mitomycin C and cytoxan). Their rationale was that the bacteria would lyse the tumors from the inside out, and low molecular weight chemotherapeutic agents would attack cancer cells in the well-perfused, non-necrotic region, a concept used since 1964 (7) (Table 1).
To enhance the effect of chemotherapeutics (mitomycin C and cytoxan) and bacteria, Dang et al. used dolastatin (D-10), an antivascular agent. To our knowledge, this is the first time antivascular therapy has been combined with bacteriolytic therapy. The benefit of this addition to COBALT, as described by Dang et al., is that vascular stasis increases the extent of hypoxia thereby increasing the size of the region affected by C. novyi. It appears that this combination is the predominant reason for the effectiveness of COBALT. A problem with the low molecular weight chemotherapeutics is that they are rapidly cleared from perfused regions (i.e., the viable ring) (26). The additional benefit of including antivascular agents that lead to vascular shutdown is that they can trap extravasated molecules in tumors (27), thereby enhancing exposure to therapeutic agents in combination therapy.
Indeed, COBALT therapy did produce impressive results. Dang et al. treated two different tumor lines grown subcutaneously in mice and observed regression in most tumors and complete cure in a considerable proportion of mice that survived. Whether similar cure rates can be achieved with COBALT in orthotopic and spontaneous tumors needs to be examined.
Besides COBALT, there are several other strategies that amplify bacteriolytic therapy. One of these is to engineer bacteria to produce inflammatory cytokines (e.g., tumor necrosis factor α) that increase the sensitivity of tumors to radiation therapy and/or evoke a host immune response (28). Another approach is bacteria-directed enzyme prodrug therapy (BDEPT), a variation of antibody-directed enzyme prodrug therapy (ADEPT). In this approach, targeting bacteria are engineered to produce enzymes that can activate prodrugs within the tumor (9, 29). Another possibility is to place the genes of prodrug-activating enzymes under the control of radiation-inducible promoters to provide spatial and temporal control, thus enabling selective killing of tumor cells while sparing normal cells (28, 30).
So what are the potential problems with bacteriolytic therapy? First, there is the immediate problem encountered by Dang et al.: toxicity. Even after removing the toxin genes, COBALT therapy led to ∼15–45% mortality in mice. Whether this is caused by the so-called tumor lysis syndrome (31) or the efflux of toxic bacterial products is not known. Identification of the toxins released by rapidly lysing tumors or by large colonies of Clostridium contained within tumors is essential for alleviating the toxicity. Toxins expressed by the bacteria may be identified after complete sequencing of the respective genomes. Well-known strategies then can be used to tackle specific toxins. On the other hand, alleviating the toxicity from low molecular weight byproducts of dying cells will require careful control of the rate of tumor lysis.
Once the issues of systemic toxicity and incomplete tumor lysis are addressed, there are other potential pitfalls that may impede the success of COBALT therapy in the clinic. The most significant of these is acquired drug resistance, which lowers the effectiveness of the standard chemotherapeutics used in COBALT after repeated treatment. Even new drugs such as Gleevec are facing this age-old problem (32). However, antiangiogenic and antivascular agents may be less susceptible to this type of resistance (21, 33). Combined bacteriolytic antiangiogenesis therapy (COMBAT) may, thus, overcome or circumvent the problem of drug resistance.
A third and more difficult problem is that of treating small non-necrotic metastases of large primary tumors. The current strategy is to treat metastases as early as possible with conventional chemotherapeutics before the onset of physiological and/or multidrug resistance. COBALT would require one to wait until the metastases develop hypoxic/necrotic regions. Because metastasis is the major cause of mortality from cancer (34), we wonder whether it would be possible to engineer bacteria that can localize in small orthotopic tumors and their spontaneous metastases that do not contain large hypoxic regions? Such bacteria would not only facilitate treatment of metastases but also their early detection by using molecular imaging techniques.
Acknowledgments
We thank Drs. Brenda Fenton and Martin Brown for helpful discussions.
Footnotes
See companion article on page 15155.
References
- 1.Helmlinger G, Yuan F, Dellian M, Jain R K. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 2.Brown J M, Giaccia A J. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 3.Parker R C, Plummer H C, Siebenmann C O, Chapman M G. Proc Soc Exp Biol Med. 1947;66:461–467. doi: 10.3181/00379727-66-16124. [DOI] [PubMed] [Google Scholar]
- 4.Malmgren R A, Flanigan C C. Cancer Res. 1955;15:473–478. [PubMed] [Google Scholar]
- 5.Möse J R, Möse G. Cancer Res. 1964;24:212–216. [PubMed] [Google Scholar]
- 6.Gericke D, Engelbart K. Cancer Res. 1964;24:217–221. [PubMed] [Google Scholar]
- 7.Thiele E H, Arison R N, Boxer G E. Cancer Res. 1964;24:222–233. [PubMed] [Google Scholar]
- 8.Carey R W, Holland J F, Whang H Y, Neter E, Bryant B. Eur J Cancer. 1967;3:37–46. [Google Scholar]
- 9.Lemmon M J, van Zijl P, Fox M E, Mauchline M L, Giaccia A J, Minton N P, Brown J M. Gene Ther. 1997;4:791–796. doi: 10.1038/sj.gt.3300468. [DOI] [PubMed] [Google Scholar]
- 10.Theys J, Landuyt W, Nuyts S, Van Mellaert L, van Oosterom A, Lambin P, Anne J. Cancer Gene Ther. 2001;8:294–297. doi: 10.1038/sj.cgt.7700303. [DOI] [PubMed] [Google Scholar]
- 11.Dang L H, Bettegowda C, Huso D L, Kinzler K W, Vogelstein B. Proc Natl Acad Sci USA. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. . (First Published November 27, 2001; 10.1073/pnas.251543698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohwi Y, Imai K, Tamura Z, Hashimoto Y. Gann. 1978;69:613–618. [PubMed] [Google Scholar]
- 13.Kimura N T, Taniguchi S, Aoki K, Baba T. Cancer Res. 1980;40:2061–2068. [PubMed] [Google Scholar]
- 14.Yazawa K, Fujimori M, Amano J, Kano Y, Taniguchi S. Cancer Gene Ther. 2000;7:269–274. doi: 10.1038/sj.cgt.7700122. [DOI] [PubMed] [Google Scholar]
- 15.Yazawa K, Fujimori M, Nakamura T, Sasaki T, Amano J, Kano Y, Taniguchi S. Breast Cancer Res Treat. 2001;66:165–170. doi: 10.1023/a:1010644217648. [DOI] [PubMed] [Google Scholar]
- 16.Low K B, Ittensohn M, Le T, Platt J, Sodi S, Amoss M, Ash O, Carmichael E, Chakraborty A, Fischer J, et al. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 17.Clairmont C, Lee K C, Pike J, Ittensohn M, Low K B, Pawelek J, Bermudes D, Brecher S M, Margitich D, Turnier J, et al. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 18.Fisher B, Brown A, Wolmark N, Fisher E R, Redmond C, Wickerham D L, Margolese R, Dimitrov N, Pilch Y, Glass A, et al. Cancer. 1990;66:220–227. doi: 10.1002/1097-0142(19900715)66:2<220::aid-cncr2820660205>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Jain R K. Cancer Res. 1988;48:2641–2658. [PubMed] [Google Scholar]
- 20.Jain R K. Nat Med. 1998;4:655–657. doi: 10.1038/nm0698-655. [DOI] [PubMed] [Google Scholar]
- 21.Carmeliet P, Jain R K. Nature (London) 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 22.McClelland M, Sanderson K E, Spieth J, Clifton S W, Latreille P, Courtney L, Porwollik S, Ali J, Dante M, Du F, et al. Nature (London) 2001;413:852–856. doi: 10.1038/35101614. [DOI] [PubMed] [Google Scholar]
- 23.Parkhill J, Dougan G, James K D, Thomson N R, Pickard D, Wain J, Churcher C, Mungall K L, Bentley S D, Holden M T, et al. Nature (London) 2001;413:848–852. doi: 10.1038/35101607. [DOI] [PubMed] [Google Scholar]
- 24.Oultram J D, Peck H, Brehm J K, Thompson D E, Swinfield T J, Minton N P. Mol Gen Genet. 1988;214:177–179. doi: 10.1007/BF00340200. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura H, Takeuchi A, Kano Y. Biosci Biotechnol Biochem. 1997;61:1211–1212. doi: 10.1271/bbb.61.1211. [DOI] [PubMed] [Google Scholar]
- 26.Jain R K, Baxter L T. Cancer Res. 1988;48:7022–7032. [PubMed] [Google Scholar]
- 27.Pedley R B, Hill S A, Boxer G M, Flynn A A, Boden R, Watson R, Dearling J, Chaplin D J, Begent R H. Cancer Res. 2001;61:4716–4722. [PubMed] [Google Scholar]
- 28.Nuyts S, Van Mellaert L, Theys J, Landuyt W, Bosmans E, Anne J, Lambin P. Gene Ther. 2001;8:1197–1201. doi: 10.1038/sj.gt.3301499. [DOI] [PubMed] [Google Scholar]
- 29.Fox M E, Lemmon M J, Mauchline M L, Davis T O, Giaccia A J, Minton N P, Brown J M. Gene Ther. 1996;3:173–178. [PubMed] [Google Scholar]
- 30.Nuyts S, Van Mellaert L, Theys J, Landuyt W, Lambin P, Anne J. Radiat Res. 2001;155:716–723. doi: 10.1667/0033-7587(2001)155[0716:tuorib]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Altman A. Semin Oncol. 2001;28:3–8. doi: 10.1016/s0093-7754(01)90254-4. [DOI] [PubMed] [Google Scholar]
- 32.McCormick F. Nature (London) 2001;412:281–282. doi: 10.1038/35085665. [DOI] [PubMed] [Google Scholar]
- 33.Folkman J. In: Harrison's Textbook of Internal Medicine. Braunwald E, Fauci A S, Kasper D L, Hauser S L, Longo D L, Jameson J L, editors. New York: McGraw–Hill; 2001. pp. 517–530. [Google Scholar]
- 34.Fidler I J, Singh R K, Yoneda J, Kumar R, Xu L, Dong Z, Bielenberg D R, McCarty M, Ellis L M. Cancer J Sci Am. 2000;6:S225–S236. [PubMed] [Google Scholar]