Abstract
Background
The aim of this study was to assess the hypoglycemic effect of Cyclocarya paliurus extract (CPE) on diabetes mellitus (DM) mice.
Material/Methods
A DM mouse model was established to test FBG, TC, and TG. The DM mice were divided into 3 groups: a DM group, a DM+CPE (0.5 g/Kg) group, and a DM+CPE (1.0 g/Kg) group. The FBG and body weight were measured. The glucose tolerance ability was determined by OGTT test. FINS was measured to calculate ISI and IRI. Serum MDA, SOD, and GSH-Px levels were detected. NIT-1 cells were cultured in vitro and divided into 4 groups: a control group, a STZ group, a STZ+CPE (80 μg/mL) group, and a STZ+CPE (160 μg/mL) group. Cell apoptosis and ROS content were assessed by flow cytometry. Cell proliferation was detected by EdU staining.
Results
Compared with the control group, FBG, TC, and TG were significantly increased in the DM group. CPE gavage obviously reduced FBG level, increased body weight, enhanced glucose tolerance, elevated FINS level and ISI, and reduced IRI, all in a dose-dependent manner. CPE gavage reduced serum MDA content and increased SOD and GSH-Px enzyme activities in DM mice. STZ markedly enhanced ROS production, induced apoptosis, and inhibited proliferation in NIT-1 cells. CPE treatment clearly reduced ROS production and apoptosis, enhanced cell proliferation, and alleviated STZ damage to NIT-1 cells.
Conclusions
CPE has the effects of decreasing blood glucose and insulin resistance, and enhancing glucose tolerance in DM mice, which may be related to its effects of reducing oxidation and reduced apoptosis, and relieving STZ in pancreatic beta cell injury.
MeSH Keywords: Apoptosis, Diabetes Complications, Hypoglycemic Agents
Background
Cyclocarya paliurus (CP) is a plant belonging to the genus Chrysanthemum of the walnut family. It exists only in China and is mainly distributed in Jiangxi, Zhejiang, Hubei, Anhui, Hunan, Fujian, Sichuan, and Guizhou provinces. It is one of the national key protected endangered plants in China [1–3]. It was found that CP contains a variety of biological active ingredients, such as polysaccharides, flavonoids, saponins, and triterpenes [3–5], and thus has the pharmacological effects of hypoglycemia [6], lowering blood pressure [7], and hypolipidemia [8–11]. In addition, previous studies showed that C. paliurus aqueous extract inhibited α-glucosidase activity and decreased blood glucose level in diabetic mice [12]. Moreover, antihyperglycemic effect of the ethanol and aqueous extracts of C. paliurus has also been assessed in high fat diet- and streptozotocin-induced diabetic rats [11]. However, the underlying mechanisms by which CP exerts its effect on type 2 diabetes remains poorly understood.
Through establishing a mouse model of type 2 diabetes (diabetes mellitus, DM), the present study investigated the effect of CP on body weight, blood glucose, glucose tolerance, and insulin sensitivity in type 2 DM mice. Moreover, we used islet beta cells to explore the effect of CP on β cell proliferation, apoptosis, and insulin secretion, aiming to provide a theoretical basis of the action of CP in reducing blood glucose.
Material and Methods
Main reagents and materials
C57BL/6J mice (males and females, 5 weeks old, 16–18 g) were purchased from Guangzhou Cypress Biotech Co. The mouse islet β cell line NIT-1 was purchased from Wuhan Procell. DMEM medium, FBS, penicillin-streptomycin, and trypsin were purchased from Gibco (USA). Streptozocin (STZ)-citrate buffer was purchased from Sigma. Lipid peroxidation product malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) were purchased from Nanjing Jiancheng Bioengineering Institute. The 7180 automatic biochemical analyzer was purchased from Hitachi (Japan). Gallios flow cytometry equipment was purchased from Beckman Coulter (USA). Annexin V-FITC/PI apoptosis detection kits were purchased from Beyotime (Jiangsu, China). Mice were used for all experiments, and all procedures were approved by the Animal Ethics Committee of our hospital.
Extraction of CPE
CPE was purchased from Jiujiang Huahan Biotechnology Co. The dry leaves (10 kg) were extracted using 75% ethanol (EtOH). After the solvent was evaporated under reduced pressure, the residue was suspended in water (10 L) and partitioned with petroleum ether, chloroform, and ethyl acetate (EtOAc), successively. The aqueous layer was evaporated under reduced pressure, followed by column chromatography on macroporous adsorption resin (HP-20), and continuously eluted with H2O (20 L) and 30% EtOH (20 L). The ethanolic part represented CPE (147 g). The concentration of chlorogenic acid, cryptochlorogenic acid, and quercetin-3-O-β-D-glucuronide in CPE were determined to be 1.83%, 1.02%, and 29.12%, respectively, using high-performance liquid chromatography (HPLC) analysis.
Type 2 DM mouse model establishment
The mice in the model group were fed with HFSD for 2 months. After eating for 12 h, they were given an intraperitoneal injection of 1% STZ-citrate buffer at a dose of 40 mg/kg. After that, the HFSD was continued for 2 weeks to establish the model. After fasting for 12 h, blood was collected from the tail to determine fasting blood glucose (FBG). FBG >11.1 mmol/L was considered as successful modeling. The mice in the control group were treated by conventional dietary feeding and intraperitoneal injection of citrate buffer (without STZ) at 2 months after feeding, and then fed a regular diet for 2 weeks.
FBG, total cholesterol (TC), and triglyceride (TG) were tested using a 7180 automatic biochemical analyzer to determine the mouse blood glucose and lipid metabolism. The body weights of the 2 groups were measured and compared.
DM model grouping and CPE treatment
G power analysis showed 30 mice were required in this study to reach statistical significance based on alpha (0.05) and power (0.80). The 30 DM model mice were randomly and equally divided into 3 groups: a DM group given a corresponding volume of normal saline (0.5 mL/Kg) gavage once a day for 4 weeks; a DM+CPE (0.5 g/Kg) group intragastrically administered with 0.5 g/Kg CPE once a day for 4 weeks; and a DM+CPE (1.0 g/Kg) group intragastrically administered 1.0 g/Kg CPE once a day for 4 weeks. At the end of 1, 2, and 4 weeks after the administration, the blood was collected from the tail vein to measure FBG. The body weights of the mice were measured and recorded.
Oral glucose tolerance test (OGTT)
DM mice were fasted for 12 h after the last administration, and the FBG was measured as the basal blood glucose value for 0 min. Then, the mice received gavage with 2 g/Kg of glucose solution, and FBG was measured after 30 min, 60 min, and 120 min. The area under the curve (AUC) was calculated.
Insulin level, insulin sensitivity index, and resistance index measurements
Tail venous blood was collected from the mice after administration, and the serum was separated. The fasting insulin level (FINS, μIU/mL) was measured using the kit and the insulin sensitivity index (ISI) was calculated. ISI=Ln[(FBG×FINS)−1], insulin resistance index (IRI)=(FBG×FINS)/22.5.
Serum oxidative stress and antioxidant markers detection
Tail venous blood was collected from the mice after administration, and the serum was separated. MDA content was tested by UV spectrophotometry to assess the level of oxidative stress. SOD and GSH-Px activities were tested to assess the level of anti-oxidative stress.
NIT-1 cell culture and grouping
NIT-1 cells were cultured in DMEM medium containing 10% FBS and 1% penicillin-streptomycin at 37°C and 5% CO2. The cells were subcultured at a ratio of 1: 4.
NIT-1 cells were divided into 4 groups: a control group treated with the same volume of citrate buffer (without STZ), a STZ group treated with 20 μM of STZ, a STZ + CPE (80 μg/mL) group treated with 80 μg/mL of CPE on the basis of STZ treatment, and a STZ+CPE (160 μg/mL) group treated with 160 μg/mL of CPE on the basis of STZ treatment. After 48 h, the cells were tested by flow cytometry to determine ROS content and apoptosis.
Cell apoptosis
The cells were digested by trypsin and washed by PBS. After being resuspended in 100 μL binding buffer, and we added 10 μL Annexin V-FITC and 5 μL PI in the dark at room temperature for 15 min. Next, we added 400 μL binding buffer and performed Gallios flow cytometry.
ROS content detection
The cells of each treatment group were collected by trypsin digestion and incubated with 1 μM DCFH-DA dye at 37°C for 20 min in the dark. After being washed with PBS and resuspended in 400 μL PBS, flow cytometry was performed to determine ROS content.
EdU staining detection of cell proliferation
NIT-1 cells were resuspended in DMEM containing 10% FBS and incubated at 10 μM EdU for 120 min. Next, the cells were continuously cultured for 48 h as described above. Then, the cells were trypsinized and centrifuged in PBS containing 1% BSA. After fixing for 15 min, the cells were washed by PBS containing 1% BSA. The cells were further incubated for 15 min at room temperature and then we added 500 μL EdU reaction solution at room temperature in the dark for 30 min. Finally, the cells were resuspended in 500 μL washing reagent and flow cytometry was used to detect EdU content.
Caspase-3 activity detection
According to the instructions of the kit, different concentrations of pNA standards were prepared and absorbance was measured at 405 nm. The standard curve was drawn according to the concentration and absorbance values. NIT-1 cells were treated with lysate at 4°C for 10 min. After centrifuging at 10 000 g and at 4°C for 10 min, the supernatant was used to test the enzyme activity of Caspase 3. The relative enzyme activity unit was calculated based on experimental group A405/control group A405×100%.
Statistical analysis
All data analyses were performed on SPSS18.0 software. The measurement data are presented as mean ± standard deviation and compared by t test or Mann-Whitney U test. Multiple comparisons were performed using Bonferroni method after ANOVA. P<0.05 was considered as statistical significance.
Results
Blood glucose and lipid increased, while body weight was reduced in the DM mouse model
A total of 15 of the 20 mice were successfully modeled (success rate 75%). It was shown that the FBG level in the DM model group was significantly higher than that in the control group. The blood lipid test results revealed that compared with control, the TC and TG contents in the DM model group were markedly increased. It was found that the body weight in the DM model group was obviously lower than that in the control group (Table 1).
Table 1.
Blood glucose and lipid detection.
| Index | Control group (n=10) | DM group (n=15) |
|---|---|---|
| FBG (mmol/L) | 5.17±0.83 | 23.22±2.36* |
| TG (mmol/L) | 0.93±0.08 | 2.42±0.21* |
| TC (mmol/L) | 1.17±0.13 | 3.36±0.29* |
| Weight (g) | 27.53±2.17 | 20.26±1.67* |
P<0.05, compared with control.
CPE treatment reduced blood glucose and increased body weight
The DM mice were divided into 3 groups: a DM group, a low-dose CPE group, and a high-dose CPE group. FBG in mice with DM continued to be at a high level. Administration of CPE by gavage significantly reduced FBG in a dose-dependent manner. The weight gain of mice in the DM model group was relatively slow, whereas CPE gavage obviously increased body weight in a dose-dependent manner (Table 2).
Table 2.
Blood glucose and body weight detection.
| Index | Group | Before treatment | 1 week after treatment | 2 weeks after treatment | 4 weeks after treatment |
|---|---|---|---|---|---|
| Control | 5.17±0.83 | 5.53±0.93 | 6.01±0.96 | 5.97±0.63 | |
| FBG (mmol/L) | DM (n=5) | 22.16±1.81a | 21.53±1.92a | 20.26±2.04a | 21.89±1.96a |
| DM+CPE (0.5 g/Kg) (n=5) | 21.66±2.05 | 20.13±1.87 | 17.89±1.54ab | 12.29±1.33ab | |
| DM+CPE (1.0 g/Kg) (n=5) | 22.57±2.13 | 19.76±1.83 | 14.52±1.49abc | 9.64±0.85abc | |
| Control | 27.53±2.17 | 28.21±1.99 | 28.59±2.06 | 28.19±2.01 | |
| Body weight (g) | DM (n=5) | 20.26±1.67 | 21.34±2.23 | 22.15±1.93a | 23.24±2.02a |
| DM+CPE (0.5 g/Kg) (n=5) | 19.97±2.03 | 23.51±2.17 | 24.53±2.02ab | 25.71±2.06ab | |
| DM+CPE (1.0 g/Kg) (n=5) | 21.05±2.22 | 24.17±2.28ab | 25.35±2.18ab | 28.31±2.13abc |
P<0.05, compared with control group;
P<0.05, compared with DM group;
P<0.05, compared with DM+CPE (0.5 g/Kg) group.
CPE treatment enhanced glucose tolerance and alleviated insulin resistance
OGTT testing showed that the blood glucose level of DM mice increased significantly after 0.5 h of glucose administration, and reached a peak after 60 min. After 120 min, blood glucose remained at a relatively high level (Table 3). The basal blood glucose level in the low-dose CPE group was obviously lower than that in the DM group, and the blood glucose level after glucose administration was elevated, but the increase was markedly less than that in the DM group, and the effect was dose-dependent (Table 3). After 60 min of glucose administration, the blood glucose level of the high-dose CPE group was clearly lower than that of the low-dose CPE group, which was significantly lower than that of the DM group. After 120 min of glucose administration, the blood glucose levels of the high- and low-dose CPE groups were reduced to the basal level, whereas the blood glucose levels in the DM group were still high (Table 3).
Table 3.
OGTT and AUC detection.
| Index | Group | Before treatment | 1 week after treatment | 2 weeks after treatment | 4 weeks after treatment |
|---|---|---|---|---|---|
| Control | 5.39±0.47 | 5.65±0.51 | 6.01±0.62 | 5.89±0.52 | |
| OGTT (mmol/L) | DM (n=5) | 18.79±1.83a | 22.27±2.11a | 26.39±2.41a | 22.63±2.32a |
| DM+CPE (0.5 g/Kg) (n=5) | 13.32±1.57ab | 16.47±1.23ab | 18.52±1.87ab | 14.11±1.18ab | |
| DM+CPE (1.0 g/Kg) (n=5) | 10.33±1.08abc | 14.37±1.29abc | 15.62±1.53abc | 10.58±1.56abc | |
| Control | 18.65±2.06 | ||||
| AUC (mmol·h/L) | DM (n=5) | 46.33±5.17a | |||
| DM+CPE (0.5 g/Kg) (n=5) | 38.67±2.89ab | ||||
| DM+CPE (1.0 g/Kg) (n=5) | 31.28±2.43abc |
P<0.05, compared with control group;
P<0.05, compared with DM group;
P<0.05, compared with DM+CPE (0.5 g/Kg) group.
After 4 weeks of administration, the FINS content of the low-dose CPE group was significantly lower than that of the DM group, and the effect was dose-dependent. It was demonstrated that the ISI of the DM group was obviously lower than that of the CPE group, and the effect was dose-dependent. We found that the IRI in the DM group was higher than in the CPE group, the effect was dose-dependent (Table 4).
Table 4.
FINS, ISI, and IRI detection.
| Group | FINS (μIU/mL) | ISI | IRI |
|---|---|---|---|
| Control | 9.65±0.81 | −1.68±0.21 | 2.15±0.11 |
| DM (n=5) | 25.33±2.57a | −6.19±0.57a | 21.72±2.15a |
| DM+CPE (0.5 g/Kg) (n=5) | 21.61±2.11ab | −5.48±0.42ab | 10.71±1.22ab |
| DM+CPE (1.0 g/Kg) (n=5) | 16.97±1.88abc | −4.99±0.3abc | 6.54±0.72abc |
P<0.05, compared with control group;
P<0.05, compared with DM group;
P<0.05, compared with DM+CPE (0.5 g/Kg) group.
CPE decreased serum MDA content and enhanced SOD activity in DM mouse
Compared with the DM group, the MDA content in the CPE group was significantly reduced in a dose-dependent manner. Compared with the DM group, the SOD activity in the CPE group was obviously higher, and the effect was dose-dependent. Compared with the DM group, the GSH-Px activity in the CPE group was markedly increased in a dose-dependent manner (Table 5).
Table 5.
MDA, SOD, and GSH-Px detection.
| Group | MDA (μM) | SOD (mU/mL) | GSH-Px (mU/mL) |
|---|---|---|---|
| Control | 6.51±0.55 | 198.56±10.63 | 95.11±7.20 |
| DM (n=5) | 32.77±3.36a | 62.63±5.18a | 18.87±1.97a |
| DM+CPE (0.5 g/Kg) (n=5) | 25.21±2.62ab | 81.69±7.27ab | 26.52±2.79ab |
| DM+CPE (1.0 g/Kg) (n=5) | 19.86±2.06abc | 103.71±9.52abc | 34.81±3.76abc |
P<0.05, compared with control group;
P<0.05, compared with DM group;
P<0.05, compared with DM+CPE (0.5 g/Kg) group.
CPE inhibited ROS production and apoptosis in NIT-1 cells
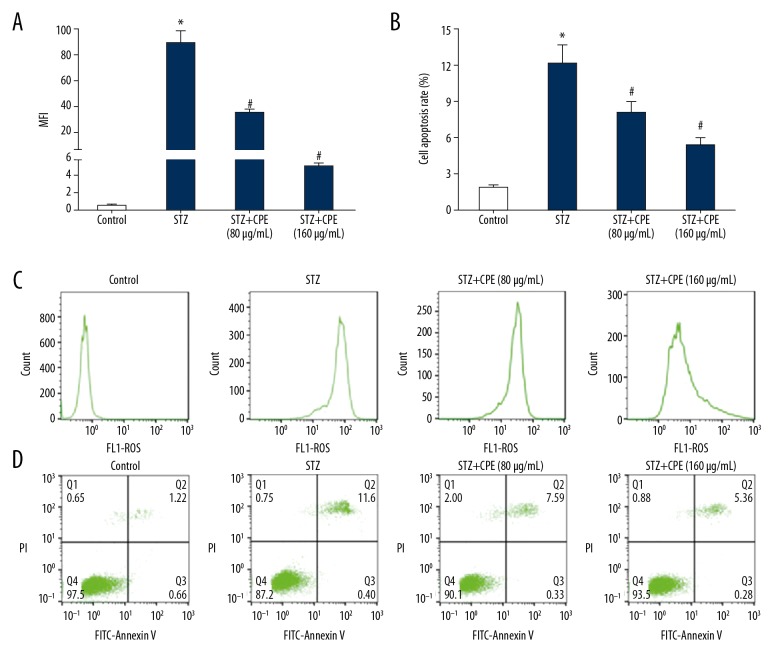
Flow cytometry demonstrated that compared with the control group, the ROS content in the NIT-1 cells of STZ group was significantly increased, and it was obviously reduced by the CPE treatment in a dose-dependent manner (Figure 1A, 1C). Flow cytometry showed that compared with the control group, NIT-1 cells apoptosis was markedly enhanced in the STZ group. CPE treatment clearly attenuated the apoptosis rate of NIT-1 cells in a dose-dependent manner (Figure 1B, 1D).
Figure 1.
CPE inhibited ROS production and NIT-1 cell apoptosis. (A) ROS content comparison; (B) cell apoptotic rate comparison; (C) flow cytometry detection of ROS content; (D) flow cytometry detection of cell apoptosis; * P<0.05, compared with control; # P<0.05, compared with STZ group.
CPE alleviated the proliferation inhibitory effect of STZ on NIT-1 cells
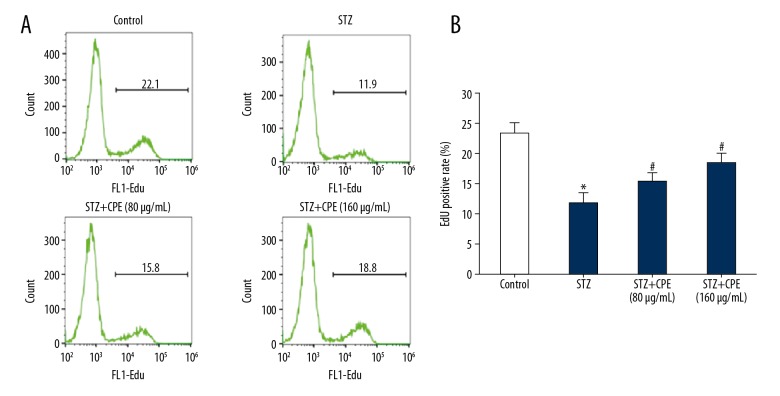
EdU staining revealed that compared with the control group, the EdU-positive rate of NIT-1 cells in the STZ-treated group was significantly reduced. CPE treatment clearly attenuated the inhibitory effect of STZ on proliferation of NIT-1 cells and the effect was dose-dependent (Figure 2A, 2B).
Figure 2.
CPE alleviated the proliferation inhibitory effect of STZ on NIT-1 cells. (A) Flow cytometry detection of cell proliferation; (B) EdU-positive rate comparison. * P<0.05, compared with control; # P<0.05, compared with STZ group.
Discussion
Due to population aging and dietary changes, the incidence of DM is increasing. T2DM accounts for more than 90% of DM, and it is currently the most common metabolic syndrome in the world [13,14]. DM treatment commonly includes oral hypoglycemic drugs, insulin injections, dietary control, and increasing physical activity [15–17]. However, adverse effects such as drug resistance, insulin resistance, leukopenia, hemolytic anemia, hypoglycemia, and even coma are major difficulties.
CP is sweet and moist, and is a cooling thirst quencher. It is mainly used as a sweet tea for the prevention and treatment of diabetes, hypertension, coronary heart disease, and other diseases. However, the exact mechanism by which CP exerts its effect on diabetes remains poorly understood. In the present study, through establishing a T2DM mouse model, we investigated the effect of CPE on DM mice, trying to provide a theoretical basis for DM treatment and prevention.
STZ, a glucosamine-nitrosourea, is a DNA alkylating agent that can enter cells through the GLUT2 glucose transport protein and selectively destroy islets in various species of animals [18,19]. In this study, a T2DM mouse model was constructed by administering HFSD and STZ to destroy pancreatic islet β cells [20,21]. Our results showed that compared with the control group, the levels of FBG, TC, and TG were significantly increased and the body weight was obviously reduced in the model group, indicating that the model was established successfully. Blood glucose monitoring revealed that the blood glucose level of the mice remained at a high level in the DM group treated by solvent gavage. However, CPE intervention clearly reduced FBG and increased body weight in a dose-dependent manner, consistent with a previous study showing that treatment with CPAE (one of the main active components of CP) reduced food intake, body weight, organ weight, fat mass, and body mass index (BMI), as well as decreasing the levels of fasting serum glucose, fasting serum insulin, and serum-free fatty acids in SHR/cp rats [22]. Four weeks after the administration, the blood glucose of the high-dose CPE group significantly decreased. OGTT testing demonstrated that CPE gavage treatment significantly increased glucose tolerance, leading to a significantly lower blood glucose ascensional range after oral glucose administration in mice. The insulin level and sensitivity analysis exhibited that CPE has the effect of elevating insulin levels, increasing insulin sensitivity, and attenuating insulin resistance in DM mice. Yoshitomi et al. [23] showed that CPE treatment can upregulate the expression of glucose transporter 4 (Glut4) in the muscle cell membrane, increase glucose uptake by muscle cells, and promote glycogen synthesis. In DM mice, CPE treatment obviously reduced the blood glucose level and enhance the glucose tolerance of mice. Wang et al. [11] reported that CPE treatment apparently decreased blood glucose levels and enhanced glucose tolerance in T2DM rats. Liu et al. [6] found that CPE can significantly reduce blood glucose levels in T2DM mice. Jiang et al. [24] induced mouse insulin resistance by stimulating macrophage culture medium, and observed that CPE markedly attenuated insulin resistance, increased glycogen synthesis in liver and muscle tissue, reduced blood glucose levels, elevated carbohydrate tolerance, and alleviated renal damage in DM rats. Xiao et al. [25] observed that CPE treatment significantly reduced the apoptosis of pancreatic tissue, decreased blood glucose levels, and enhanced glucose tolerance in DM mice induced by STZ. Jiang et al. [24] revealed that in a model of mouse insulin resistance stimulated by activated macrophage medium, CPE treatment inhibited the serine phosphorylation of insulin receptor substrate 1 (IRS-1), enhanced the tyrosine phosphorylation level of IRS-1, activated the downstream phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT/PKB) pathway activity, increased sensitivity to insulin signaling, and reduced insulin resistance. Consistent with the findings mentioned above, our study showed that CPE has an obvious hypoglycemic effect.
MDA is a lipid peroxidation product that can reflect the degree of lipid peroxidation [26]. SOD is the most important antioxidant enzyme in scavenging free radicals in organisms and is widely found in various organisms. It can scavenge oxygen free radicals and protect cells from oxidative damage [27,28]. Glutathione (GSH) is a tripeptide consisting of cysteine, glycine, and glutamic acid, with the main role of removing excess hydrogen peroxide and oxygen free radicals from the body. GSH-Px can catalyze the decomposition of hydrogen peroxide into water and oxidized GSH, thereby reducing the accumulation of hydrogen peroxide in the body, decreasing oxidative stress levels, inhibiting oxidative stress damage, and maintaining the structural and functional integrity of proteins and cells [29–31]. In the present study, we showed that CPE gavage treatment can significantly reduce the serum MDA content, decrease the level of oxidative stress, and enhance the activity of antioxidant enzymes SOD and GSH-Px in DM mice, consistent with previous studies showing that CPE treatment obviously reduced the serum MDA content and increased SOD and GSH-Px enzyme activities in T2DM rats [11]. In addition, our results also agree with a previous study revealing that CPE apparently reduced MDA content and enhanced SOD and GSH-Px enzyme activities in mice fed a high-glucose diet [10].
At present, there are few studies on the effects of CPE on pancreatic beta cells cultured in vitro. In the present study, we found that STZ significantly increased the production of ROS, induced apoptosis, and inhibited cell proliferation in NIT-1 cells. CPE obviously reduced the production of ROS and cell apoptosis, promoted cell proliferation, and alleviated the damage of STZ to NIT-1 cells. These results show that CPE reduces apoptosis and STZ damage to NIT-1 cells through anti-oxidation. However, the effect of CPE on pancreatic beta cells in mice is still unclear, and further studies are needed.
Conclusions
CPE has the effects of decreased blood glucose and insulin resistance and enhancing glucose tolerance in DM mice, which may be through anti-oxidation, anti-apoptosis, and amelioration of STZ-induced pancreatic beta cell injury.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Wang YR, Cui BS, Han SW, Li S. New dammarane triterpenoid saponins from the leaves of Cyclocarya paliurus. J Asian Nat Prod Res. 2018 doi: 10.1080/10286020.2018.1457653. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Xie J, Wang W, Dong C, et al. Protective effect of flavonoids from Cyclocarya paliurus leaves against carbon tetrachloride-induced acute liver injury in mice. Food Chem Toxicol. 2018;119:392–99. doi: 10.1016/j.fct.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Hu J, Xie J, et al. Isolation, structure, and bioactivities of polysaccharides from Cyclocarya paliurus (Batal.) Iljinskaja. Ann NY Acad Sci. 2017;1398(1):20–29. doi: 10.1111/nyas.13357. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Zhang MQ, Li XL, et al. Study on chemical constituents of Cyclocarya paliurus. J Asian Nat Prod Res. 2014;16(2):206–9. doi: 10.1080/10286020.2013.825254. [DOI] [PubMed] [Google Scholar]
- 5.Xie JH, Dong CJ, Nie SP, et al. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015;186:97–105. doi: 10.1016/j.foodchem.2014.06.106. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Cao Y, Fang S, et al. Antidiabetic effect of Cyclocarya paliurus leaves depends on the contents of antihyperglycemic flavonoids and antihyperlipidemic triterpenoids. Molecules. 2018;23(5) doi: 10.3390/molecules23051042. pii: E1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie JH, Xie MY, Shen MY, et al. Optimisation of microwave-assisted extraction of polysaccharides from Cyclocarya paliurus (Batal.) Iljinskaja using response surface methodology. J Sci Food Agric. 2010;90(8):1353–60. doi: 10.1002/jsfa.3935. [DOI] [PubMed] [Google Scholar]
- 8.Jiang C, Wang Q, Wei Y, et al. Cholesterol-lowering effects and potential mechanisms of different polar extracts from Cyclocarya paliurus leave in hyperlipidemic mice. J Ethnopharmacol. 2015;176:17–26. doi: 10.1016/j.jep.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Yao X, Lin Z, Jiang C, et al. Cyclocarya paliurus prevents high fat diet induced hyperlipidemia and obesity in Sprague-Dawley rats. Can J Physiol Pharmacol. 2015;93(8):677–86. doi: 10.1139/cjpp-2014-0477. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Jiang C, Yao N, et al. Antihyperlipidemic effect of Cyclocarya paliurus (Batal.) Iljinskaja extract and inhibition of apolipoprotein B48 overproduction in hyperlipidemic mice. J Ethnopharmacol. 2015;166:286–96. doi: 10.1016/j.jep.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Jiang C, Fang S, et al. Antihyperglycemic, antihyperlipidemic and antioxidant effects of ethanol and aqueous extracts of Cyclocarya paliurus leaves in type 2 diabetic rats. J Ethnopharmacol. 2013;150(3):1119–27. doi: 10.1016/j.jep.2013.10.040. [DOI] [PubMed] [Google Scholar]
- 12.Kurihara H, Fukami H, Kusumoto A, et al. Hypoglycemic action of Cyclocarya paliurus (Batal.) Iljinskaja in normal and diabetic mice. Biosci Biotechnol Biochem. 2003;67(4):877–80. doi: 10.1271/bbb.67.877. [DOI] [PubMed] [Google Scholar]
- 13.Bastawrous A, Mathenge W, Wing K, et al. The incidence of diabetes mellitus and diabetic retinopathy in a population-based cohort study of people age 50 years and over in Nakuru, Kenya. BMC Endocr Disord. 2017;17(1):19. doi: 10.1186/s12902-017-0170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strauss MB, Moon H, La S, et al. The incidence of confounding factors in patients with diabetes mellitus hospitalized for diabetic foot ulcers. Wounds. 2016;28(8):287–94. [PubMed] [Google Scholar]
- 15.Jonnalagadda VG, Char HP, Samudrala PK. Prediabetes, diabetes mellitus, and anti-diabetic treatment: Is anyone still healthy? Diabetes Metab Res Rev. 2018;34(5):e3009. doi: 10.1002/dmrr.3009. [DOI] [PubMed] [Google Scholar]
- 16.Hall KD, Chung ST. Low-carbohydrate diets for the treatment of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2018;21(4):308–12. doi: 10.1097/MCO.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 17.Samocha-Bonet D, Debs S, Greenfield JR. Prevention and treatment of type 2 diabetes: A pathophysiological-based approach. Trends Endocrinol Metab. 2018;29(6):370–79. doi: 10.1016/j.tem.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Gundala NKV, Naidu VGM, Das UN. Amelioration of streptozotocin-induced type 2 diabetes mellitus in Wistar rats by arachidonic acid. Biochem Biophys Res Commun. 2018;496(1):105–13. doi: 10.1016/j.bbrc.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Skovso S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig. 2014;5(4):349–58. doi: 10.1111/jdi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan M, Jiang H, Zhang Y, et al. Liraglutide enhances autophagy and promotes pancreatic beta cell proliferation to ameliorate type 2 diabetes in high-fat-fed and streptozotocin-treated mice. Med Sci Monit. 2018;24:2310–16. doi: 10.12659/MSM.906286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Y, Zhao X, Xin J, et al. Coumarins improved type 2 diabetes induced by high-fat diet and streptozotocin in mice via antioxidation. Can J Physiol Pharmacol. 2018;96(8):765–71. doi: 10.1139/cjpp-2017-0612. [DOI] [PubMed] [Google Scholar]
- 22.Xu G, Yoshitomi H, Sun W, et al. Cyclocarya paliurus (Batal.) Ijinskaja aqueous extract (CPAE) ameliorates obesity by improving insulin signaling in the hypothalamus of a metabolic syndrome rat model. Evid Based Complement Alternat Med. 2017;2017 doi: 10.1155/2017/4602153. 4602153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshitomi H, Tsuru R, Li L, et al. Cyclocarya paliurus extract activates insulin signaling via Sirtuin1 in C2C12 myotubes and decreases blood glucose level in mice with impaired insulin secretion. PLoS One. 2017;12(8):e0183988. doi: 10.1371/journal.pone.0183988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang C, Yao N, Wang Q, et al. Cyclocarya paliurus extract modulates adipokine expression and improves insulin sensitivity by inhibition of inflammation in mice. J Ethnopharmacol. 2014;153(2):344–51. doi: 10.1016/j.jep.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Xiao HT, Wen B, Ning ZW, et al. Cyclocarya paliurus tea leaves enhances pancreatic beta cell preservation through inhibition of apoptosis. Sci Rep. 2017;7(1):9155. doi: 10.1038/s41598-017-09641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arslan M, Ipekci SH, Kebapcilar L, et al. Effect of aerobic exercise training on MDA and TNF-alpha levels in patients with type 2 diabetes mellitus. Int Sch Res Notices. 2014;2014 doi: 10.1155/2014/820387. 820387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coudriet GM, Delmastro-Greenwood MM, Previte DM, et al. Treatment with a catalytic superoxide dismutase (SOD) mimetic improves liver steatosis, insulin sensitivity, and inflammation in obesity-induced type 2 diabetes. Antioxidants (Basel) 2017;6(4) doi: 10.3390/antiox6040085. pii: E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung H, Kim YY, Kim B, et al. Improving glycemic control in model mice with type 2 diabetes by increasing superoxide dismutase (SOD) activity using silk fibroin hydrolysate (SFH) Biochem Biophys Res Commun. 2017;493(1):115–19. doi: 10.1016/j.bbrc.2017.09.066. [DOI] [PubMed] [Google Scholar]
- 29.Yucel C, Karatoprak GS, Aktas Y. Nanoliposomal resveratrol as a novel approach to treatment of diabetes mellitus. J Nanoscience Nanotechnol. 2018;18(6):3856–64. doi: 10.1166/jnn.2018.15247. [DOI] [PubMed] [Google Scholar]
- 30.Niu J, Xu G, Jiang S, et al. L In vitro antioxidant activities and anti-diabetic effect of a polysaccharide from Schisandra sphenanthera in rats with type 2 diabetes. Int J Biol Macromol. 2017;94(Pt A):154–60. doi: 10.1016/j.ijbiomac.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Gillani SW, Azeem E, Siddiqui A, et al. Oxidative stress correlates (OSC) in diabetes mellitus patients. Curr Diabetes Rev. 2016;12(3):279–84. doi: 10.2174/1573399811666150520094631. [DOI] [PubMed] [Google Scholar]