Abstract
Purpose:
Cancer treatment-related heart failure (HF) is an emerging health concern, as the number of survivors is increasing rapidly, and cardiac health issues are a leading cause of mortality in this population. While there is general evidence for the efficacy of exercise rehabilitation interventions, more research is needed on exercise rehabilitation interventions for patients specifically with treatment-induced HF, and if such interventions are safe and well-accepted. This study provides feasibility and health outcomes of a pilot exercise intervention for cancer survivors with chemotherapy-induced HF.
Methods:
Twenty-five participants were randomized to a clinic-based exercise intervention or a wait-list control group, or alternatively allowed to enroll in a home-based exercise intervention if they declined the randomized study. For purposes of analysis, both types of exercise programs were combined into a single intervention group. Repeated measures ANOVA was conducted assessing for significant time and treatment group main effects separately, and time-by-treatment group interaction effects.
Results:
Significant improvements in VO2max were observed in the intervention group. Intervention satisfaction and adherence were high for both clinic and home-based interventions, with no reported serious adverse events. Enrollment was initially low for the clinic-based intervention, necessitating the addition of the home-based program as an intervention alternative.
Conclusions:
Results suggest exercise rehabilitation interventions are feasible in terms of safety, retention, and satisfaction, and have the potential to improve VO2max. To maximize adherence and benefits while minimizing participant burden, an ideal intervention may incorporate elements of both clinic-based supervised exercise sessions and a home-based program.
Keywords: Exercise, heart failure, cancer, intervention, feasibility
Condensed abstract:
General evidence for efficacy of exercise rehabilitation interventions exists, but more research is needed on intervention feasibility and efficacy for those with treatment-induced heart failure. This study demonstrates that an exercise program has the potential to improve VO2max, along with being safe and well-accepted by this cancer survivor population.
Although more cancer patients now survive the disease, the cost can include lasting effects of treatment, such as chemotherapy-induced cardiovascular toxicity leading to adverse effects such as arrhythmias, cardiomyopathy, endothelial dysfunction, and heart failure (HF).1 Chemotherapy agents like anthracyclines (a class of highly effective cytotoxic agents for hematopoietic and solid tumors) have been significantly associated with increased risk of HF and cardiomyopathy.2,3 Studies also show this association with targeted therapies including trastuzumab and bevacizumab, while newer antiangiogenic agents (bevacizumab, sunitinib, sorafenib) have also been found to lead to cardiovascular toxicity and hypertension, which are contributing factors to HF.4,5,6
These effects are often clinically silent until their severity causes symptoms and mandates treatment, often years after chemotherapy. For anthracyclines, over half of patients treated show effects of cardiac dysfunction up to 20 y post-treatment, while anthracyclines combined with trastuzumab have been linked to cardiac complications in 27% of patients up to 51 mo if receiving combined therapy.7 A study of leukemia survivors treated with anthracyclines reported left ventricular fractional shortening and systolic dysfunction up to 12 y post-diagnosis.8
RCTs have demonstrated the potential for improvement in cardiovascular health in non-cancer patients with HF through exercise rehabilitation.9,10,11 Meta-analyses of these programs have demonstrated positive effects on cardiac functioning, physical performance, and quality of life.12,13,14,15,16 Cochrane Reviews reported those receiving exercise showed a 27% reduction in all-cause mortality and a 31% reduction of total cardiac-specific related mortality compared to those not receiving exercise.17,18 Results from Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION), a large-scale RCT, showed participants receiving exercise had significantly lower rates of all-cause mortality and hospitalization, cardiovascular mortality, and HF hospitalization compared to standard care.19 Despite potential benefits, there is a lack of studies demonstrating positive effects of exercise for adult patients with chemotherapy-related HF, and it is unclear if these interventions can be delivered with the same efficacy and positive effects as in non-survivor populations.20
This study provides feasibility (recruitment, adherence, safety, satisfaction) and health outcomes of an exercise intervention for patients with chemotherapy-induced HF.
METHODS
We recruited participants for randomization to a control or a 16-wk clinic-based exercise intervention. After initial low-enrollment, a home-based exercise condition was added as an alternative only to those declining to participate after offered the original study of randomization to the control or clinic-based intervention (Figure 1). Due to limited sample size, for the purposes of analysis, the clinic and home-based groups were combined into a single intervention comparison group.
Figure 1.
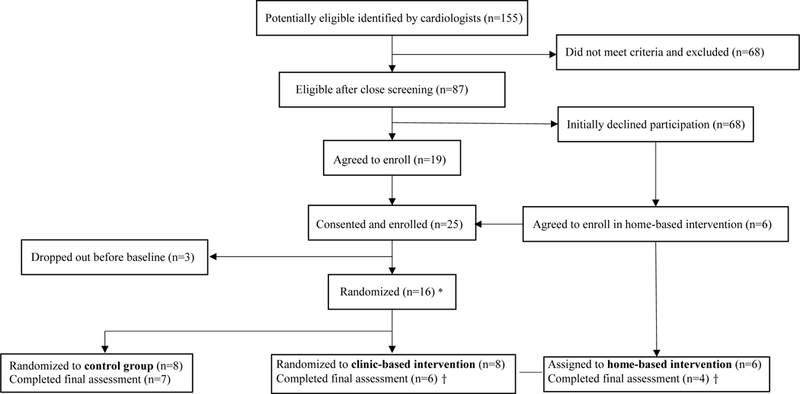
Study flow diagram
* Only participants agreeing to enroll after first being approached with the randomized study to either a control or clinic-based exercise group were randomized. Participants in the home-based group were directly assigned to this group without being randomized, as they were individuals who refused the randomized study initially, but were offered the chance to still participate by being allowed to directly enroll in a home-based program.
† For purposes of analyses, the clinic-based and home-based group were combined into a single intervention comparison group.
Participant Eligibility
Patients seen by the MD Anderson Cancer Center (MDACC) cardiology service for chemotherapy-related cardiomyopathy and HF were recruited. Eligibility criteria included: (1) HF diagnosis with New York Heart Association (NYHA) class I, II, or III functional classification; (2) previous treatment with potentially cardio-toxic anticancer agents contributing to HF; (3) living in the Houston area; (4) cancer survivor with no evidence of disease; and (5) completed treatment, or on long-term adjuvant/maintenance chemotherapy only. Therefore, patients could be survivors with no evidence of disease, or evidence of disease but in stable long-term maintenance. Patients were treated with standard-of-care maximally tolerated medical therapy of ACE inhibitors and beta-blockers and when necessary, diuretics, based on the American College of Cardiology (ACC) guidelines for management of chronic HF.21
Patients were excluded if: (1) remained in NYHA class IV HF despite optimal guideline directed medical therapy; (2) had health problems or current treatments making exercise unsafe; (3) were unable to provide informed consent.
Exercise Intervention
The exercise program was based on HF-ACTION and consisted of supervised 30-min exercise sessions 3 times/wk for 16 wk.19 The initial session was conducted by cardiologists and subsequent sessions were supervised by an exercise physiologist. Exercise training was done on a Cybex recumbent exercise bike. Sessions focused on improving exercise duration, intensity, and tolerance (working up to 30 min of continued activity at intensity level of 50% heart rate reserve). Starting intensity was prescribed and monitored using the Borg “6–20” RPE (rating of perceived exertion) scale.22 During initial sessions, participants were instructed to cycle at an RPE of 12 (between “fairly light” and “somewhat hard”). The progression plan involved increasing exercise duration and then intensity incrementally as tolerance improved. Participants were monitored with ECGs during sessions. Intervention development details have been previously reported.19,23
After 78% of eligible candidates declined potential randomization to a clinic-based program, a 12-wk home-based intervention was developed for reduced participant burden and potentially greater feasibility. The home-based program involved an initial supervised exercise session before starting home-based exercise to establish appropriate exercise intensity/duration. The exercise physiologist instructed participants how to assess/monitor intensity by going through an aerobic exercise session. Additionally, participants were trained to use the RPE method, and given a logbook to record exercise and intensity, as well as a pedometer to measure daily steps. Participants were then prescribed an aerobic exercise and walking program. Each participant was telephoned 1/wk to report progress, monitor for adverse events, and set exercise goals for the upcoming week.
Measures/Instruments
Feasibility measures included recruitment rates, adherence to sessions, retention, adverse events, and patient satisfaction questions. Participant satisfaction was assessed at 16-wk follow-up using items measuring difficulty attending sessions (1=not at all difficult to 5=very difficult), intervention satisfaction (1=satisfied to 5=not at all satisfied), and likelihood of recommending the intervention (1=likely to 5=not at all likely).
Outcomes of VO2max, left ventricular ejection fraction (LVEF), HF symptom severity, physical/role functioning, and physical activity were collected at baseline and 16-wk follow-up. VO2max was obtained through respiratory gas exchange analysis using cycle ergometry and LVEF was determined through echocardiograms. The biplane area-length method was utilized to calculate LVEF. Echocardiography and ergometry procedures have been previously reported.23 HF symptom severity and burden were assessed through the MD Anderson Symptom Inventory Heart-Failure (MDASI-HF)24, physical/role functioning were assessed by the Medical Outcomes Study Short Form-36 (SF-36)25, and the Community Health Activities Model Program for Seniors (CHAMPS)26 questionnaire assessed physical activity.
Data Analysis
For recruitment, percentages against total eligible at each recruitment step were calculated, while retention percentage was obtained similarly. Adherence was determined through proportion of total planned sessions attended. Repeated measures analysis of variance (ANOVA), were conducted to assess intervention effects on cardiovascular health, symptoms, QOL, and physical activity27. Models included main effects of time and intervention group, and also a time-by-group interaction, which tested the effects of the intervention on outcomes. A significant time-by-group interaction indicates the intervention has an effect. Additionally, differences in baseline characteristics between groups are controlled for, as both changes between groups and changes within groups are accounted for. We also calculated standardized mean group differences (Cohen’s d) in the change from baseline to follow-up for each outcome. This metric of effect size can be interpreted as a standardized measure of intervention effects, which can be communicated across different studies. Also, effect sizes can help determine sample size in follow-up studies.
RESULTS
A total of 155 potentially eligible were identified in the 36-mo recruitment period. After closer screening by cardiologists, 87 (56%) were eligible, of whom 25 (29%) consented and were enrolled. Among the enrolled, 3 (12%) dropped out before baseline, leaving 22 participants. Characteristics for the 22 participants who completed at least the baseline are summarized in Table 1. Of these 22, 17 (77%) remained and completed follow-up; 2 of the 8 participants in the randomized intervention arm and 1 of the 8 control participants dropped out before final assessment. Six participants were enrolled in the non-randomized home-based condition, and 4 completed participation (Figure 1).
Table 1.
Participant characteristics.
| Intervention | Control | |||
|---|---|---|---|---|
| Characteristic | Clinic-based n = 8 |
Home-based n = 6 |
n = 8 | |
| Gender | Male | 0 (0) | 1 (17) | 2 (25) |
| Female | 8 (100) | 5 (83) | 6 (75) | |
| Race | Black/non-Hispanic | 3 (38) | 3 (50) | 2 (25) |
| White/non-Hispanic | 4 (50) | 1 (17) | 4 (50) | |
| Asian/non-Hispanic | 0 (0) | 1 (17) | 0 (0) | |
| Hispanic | 1 (12) | 1 (17) | 2 (25) | |
| Marital Status | Single | 1 (12) | 1 (17) | 1 (12) |
| Married | 5 (62) | 4 (67) | 4 (50) | |
| Divorced | 2 (25) | 1 (17) | 1 (12) | |
| Separated | 0 (0) | 0 (0) | 2 (25) | |
| Cancer Site | Breast | 5 (62) | 4 (67) | 5 (62) |
| Sarcoma hip/thigh | 1 (12) | 0 (0) | 0 (0) | |
| Lymphoma | 0 (0) | 2 (33) | 1 (12) | |
| Multiple myeloma | 1 (12) | 0 (0) | 0 (0) | |
| Osteosarcoma | 0 (0) | 0 (0) | 0 (0) | |
| Hodgkin’s disease | 0 (0) | 0 (0) | 1 (12) | |
| Leukemia | 1 (12) | 0 (0) | 0 (0) | |
| Tongue | 0 (0) | 0 (0) | 1 (12) | |
| Age (y) | 28 – 76 | 56.1 (10.5) | 51.2 (9.5) | 55.2 (13.5) |
| BMI (kg/m2) | 21.4 – 57.4 | 31.1 (11.4) | 30.6 (5.6) | 30.2 (5.7) |
Abbreviation: BMI, body mass index.
Data are presented as n (%) or range, mean ± SD.
Regarding feasibility, a low proportion of participants were willing to attend an in-clinic exercise intervention. Out of participants recruited in the greater Houston area, over 1/3 eligible but declining participation cited travel (>20 miles) as a major barrier. For the clinic-based group, adherence was assessed through attendance to exercise sessions (# of sessions attended/# of sessions planned), where mean completion was 73% (completing ≥ 8 out of 11 sessions), and all but 1 participant completed ≥2/3 of their 48 in-clinic sessions. For the home-based group, adherence was assessed through proportion of counseling calls completed and activity in the walking program; mean percent of total counseling sessions completed was 84% (completing ≥ 9 out of 11 sessions) and 75% of participants walked ≥ 10,000 steps/d.
For satisfaction items, mean score for difficulty attending sessions was 1.87, 1.00 for overall intervention satisfaction, and 1.13 for likelihood of recommending the intervention. Only 1 participant reported that the intervention was “difficult”.
Regarding adverse events, 2 documented cases were related to exercise. In 1 case, premature ventricular contractions were observed during exercise, however, this participant was cleared to continue after full re-evaluation. In the second case, a participant exhibited increased fatigue with minimal exertion during exercise. After examination by the cardiologist, this participant was cleared to continue and did not demonstrate elevated fatigue subsequently.
Statistically significant changes for the time main effect for both intervention and control groups were observed for QOL physical functioning (P =.001) and role functioning (P =.0279) (Table 2). In addition, a statistically significant time-by-group interaction effect (difference in change between groups over time taking into account baseline measurements) was observed for VO2max, (P =.042) (Table 2). There were no statistically significant differences in LVEF, symptom scores (MDASI-HF), or physical activity. Observed standardized mean differences between intervention and control groups (Cohen’s d) in the change from baseline to follow-up ranged from large (MDASI-HF Cardiac Health: .83), to small (VO2max: .28; LVEF(%): .40; MDASI-HF Symptom Burden: .05; SF-36 Physical Functioning: .34; SF-36 Role Functioning: .01; CHAMPS Total hours: .45; CHAMPS High intensity hours: .13).
Table 2.
Repeated measures ANOVA for intervention group†, time, and interaction effects
| Group (n) | Baseline | Follow-Up | Group P Value | Time P Value | Group by Time P-Value | |
|---|---|---|---|---|---|---|
| Cardiac Health | ||||||
| VO2max (mL/kg/min) | Control (7) | 14.8 (2.0) 9.4–23.2 | 14.1 (2.0) 8.4–22.8 | 0.62 | 0.98 | 0.042* |
| Intervention (8) | 13.5 (1.9) 6.9–17.5 | 14.3 (2.0) 8.4–29.5 | ||||
| LVEF (%) | Control (6) | 53.3 (3.6) 42.0–62.0 | 52.8 (3.0) 40.0–62.0 | 0.17 | 0.62 | 0.46 |
| Intervention (7) | 45.0 (3.6) 32.0–58.0 | 48.0 (3.0) 40.0–58.0 | ||||
| MDASI-HF | ||||||
| Symptom severity | Control (6) | 30.7 (3.5) 14.0–41.0 | 33.3 (2.8) 27.0–39.0 | 0.41 | 0.18 | 0.97 |
| Intervention (10) | 27.2 (2.7) 18.0–40.0 | 30.2 (2.2) 16.0–45.0 | ||||
| Symptom burden | Control (6) | 13.3 (1.9) 4.0–18.0 | 13.8 (1.4) 11.0–16.0 | 0.87 | 0.66 | 0.91 |
| Intervention (10) | 13.1 (1.5) 7.0–18.0 | 13.4 (1.1) 7.00–18.0 | ||||
| Cardiac health | Control (6) | 5.00 (1.5) 0.0–12.0 | 2.83 (1.4) 0.00–6.00 | 0.22 | 0.30 | 0.053 |
| Intervention (10) | 1.40 (1.1) 0.0–4.00 | 2.10 (1.01) 0.00–11.0 | ||||
| SF-36 | ||||||
| Physical functioning | Control (8) | 37.0 (8.5) 10.0–65.0 | 53.8 (9.8) 25.0–100 | 0.17 | 0.001* | 0.15 |
| Intervention (10) | 58.5 (7.6) 20.0–100 | 66.5 (8.8) 30.0–95.0 | ||||
| Role functioning | Control (8) | 35.9 (8.4) 0.00–68.0 | 49.2 (10.7) 12.5–100 | 0.09 | 0.03* | 0.99 |
| Intervention (10) | 57.5 (7.5) 12.5–100 | 70.6 (9.6) 12.5–100 | ||||
| CHAMPS | ||||||
| Total hours | Control (8) | 13.7 (3.3) 2.2–35.3 | 11.4 (2.3) 2.80–21.5 | 0.25 | 0.82 | 0.40 |
| Intervention (10) | 8.03 (3.0) 0.5–21.0 | 9.38 (2.1) 3.00–21.3 | ||||
| High intensity hours | Control (8) | 4.25 (1.6) 0.0–9.75 | 4.72 (1.8) 0.00–14.0 | 0.92 | 0.60 | 0.83 |
| Intervention (10) | 3.75 (1.5) 0.0–15.5 | 4.85 (1.6) 0.00–14.5 |
P < .05
For purposes of analysis, the clinic-based exercise condition was combined with the home-based exercise condition to form a single intervention comparison group.
Data are presented as mean ± SE, range
DISCUSSION
This study demonstrates the potential of an exercise intervention to improve cardiorespiratory health (through change in the surrogate endpoint of VO2max) in patients with treatment-induced HF. Additionally, the study informs feasibility of clinic and home-based interventions that may be useful in developing future programs.
Among participants attending the clinic-based program, adherence and retention were high, indicating the exercise program may be feasible for a subset of participants. Given greater patient burden for the clinic-based intervention, but also observed long-term adherence issues with home-based programs in the literature (e.g. the HF-ACTION home component), an optimal intervention would likely involve a combination of these, for example, supervised sessions offered at locations close to the participant’s home like community centers.28 This reflects current recommendations for incorporating cardiac rehabilitation into oncology programs for patients who have completed treatment and no longer frequent clinical settings.29
Despite limited appeal during recruitment, satisfaction of those in the exercise intervention was high, and serious adverse events were not observed, which converges with evidence that exercise is safe for HF patients (although one study observed increased risk of clinical events in a cancer population).19,28
Several limitations are noted, inherent to the exploratory nature of the study. These include a small sample size, lack of random assignment to the home-based condition influencing internal validity, absence of long-term follow-up, and combination of the home-based and clinic-based groups for analysis, ergo, results should be interpreted considering limitations. Recruiting only through the cardiology clinic was likely a limiting factor for enrollment, and may be improved through recruiting more broadly (e.g., additionally through oncologists) and using a searchable EMR. Significant improvements were observed in QOL functioning (SF-36), however, this may be attributable to participants in both conditions receiving normal follow-up care or natural improvement in function over time.
VO2max improvements indicate that an exercise program shows potential in improving cardiorespiratory fitness, while feasibility results inform implementation for future intervention studies. Subsequent research with a larger population could further investigate these changes and if they can be sustained.
Acknowledgments
Funding Sources: National Institutes of Health (NIH), National Cancer Institute (NCI), Patient-Reported Outcomes, Survey & Population Research (PROSPR) Shared Resource at the University of Texas MD Anderson Cancer Center
This study was supported by the University of Texas School of Public Health Cancer Education and Career Development Program through NCI Grant R25 CA57712, University of Texas MD Anderson Cancer Prevention Research training grants R25 CA056452 and R25Ca057730, NCI Grant R21 CA135016, the Assessment, Intervention and Measurement (AIM) Shared Resource through NCI Grant P30 CA16672, and the Center for Energy Balance in Cancer Prevention and Survivorship of the Duncan Family Institute for Cancer Prevention and Risk Assessment at the University of Texas MD Anderson Cancer Center.
Footnotes
Conflicts of Interest: All authors declare no conflict of interest.
Clinical Trial Registration - URL: https://clinicaltrials.gov. Unique identifier: NCT00633633
REFERENCES
- 1.Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016; 66: 309–325. [DOI] [PubMed] [Google Scholar]
- 2.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005; 23:8597–605. [DOI] [PubMed] [Google Scholar]
- 3.Pinder MC, Duan Z, Goodwin JS, Hortobagyi GN, Giordano SH. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007; 25:3808–15. [DOI] [PubMed] [Google Scholar]
- 4.Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005; 23:7685–96. [DOI] [PubMed] [Google Scholar]
- 5.Jones RL, Ewer MS. Cardiac and cardiovascular toxicity of nonanthracycline anticancer drugs. Expert Rev Anticancer Ther. 2006; 6:1249–69. [DOI] [PubMed] [Google Scholar]
- 6.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nature Rev Cancer. 2007; 7:332–44. [DOI] [PubMed] [Google Scholar]
- 7.Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. 2013; 49:2900–9. [DOI] [PubMed] [Google Scholar]
- 8.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005; 23:2629–36. [DOI] [PubMed] [Google Scholar]
- 9.Giannuzzi P, Temporelli PL, Corrà U, Tavazzi L, ELVD-CHF Study Group. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure results of the exercise in left ventricular dysfunction and chronic heart failure (ELVD-CHF) trial. Circulation. 2003; 108:554–9. [DOI] [PubMed] [Google Scholar]
- 10.Wisløff U, Støylen A, Loennechen JP, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients a randomized study. Circulation. 2007; 115:3086–94. [DOI] [PubMed] [Google Scholar]
- 11.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999; 99:1173–82. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Amer J Med. 2004; 116:682–92. [DOI] [PubMed] [Google Scholar]
- 13.Collaborative E Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004; 328:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011; 20:123–33. [DOI] [PubMed] [Google Scholar]
- 15.Lin X, Zhang X, Guo J, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta‐analysis of randomized controlled trials. JAHA. 2015; 4:e002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey A, Parashar A, Kumbhani DJ, et al. Exercise training in patients with heart failure and preserved ejection fraction meta-analysis of randomized control trials. Circ Heart Fail. 2015; 8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: Cochrane systematic review and meta‐analysis. Eur J Heart Fail. 2010; 12:706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001; 1: CD001800. [DOI] [PubMed] [Google Scholar]
- 19.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009; 301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamo CE, Bloom MW, Cardinale D, et al. Cancer therapy–related cardiac dysfunction and heart failure Part 2: Prevention, treatment, guidelines, and future directions. Circ Heart Fail. 2016; 9:e002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: Executive summary: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure) developed in collaboration with the International Society for Heart and Lung Transplantation endorsed by the Heart Failure Society of America. JACC. 2001; 38:2101–13. [DOI] [PubMed] [Google Scholar]
- 22.Borg G Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 23.Hughes DC, Lenihan DJ, Harrison CA, Basen-Engquist KM. Exercise intervention for cancer survivors with heart failure. J Ex Sci Fit. 2011; 9:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadol A, Mendoza T, Gning I, et al. Psychometric testing of the MDASI-HF: a symptom assessment instrument for patients with cancer and concurrent heart failure. J Card Fail. 2008; 14:497–507. [DOI] [PubMed] [Google Scholar]
- 25.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992; 305:160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hekler EB, Buman MP, Haskell WL, et al. Reliability and validity of CHAMPS self-reported sedentary-to-vigorous intensity physical activity in older adults. J Phys Act Health. 2012; 9:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberfeld D, Franke T. Evaluating the robustness of repeated measures analyses: The case of small sample sizes and nonnormal data. Behavior Res Methods. 2013; 45:792–812. [DOI] [PubMed] [Google Scholar]
- 28.Jones LW, Douglas PS, Khouri MG, et al. Safety and efficacy of aerobic training in patients with cancer who have heart failure: an analysis of the HF-ACTION randomized trial. J Clin Oncol. 2014; 32:2496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dittus KL, Lakoski SG, Savage PD, Kokinda N, Toth M, Stevens D, Woods K, O’Brien P, Ades PA. Exercise-based oncology rehabilitation: leveraging the cardiac rehabilitation model. J Cardiopulm Rehab Prev. 2015; 35:130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]


