Summary
Aims
Autism spectrum disorder (ASD) is a condition defined by social communication deficits and repetitive restrictive behaviors. Association of the fatty acid amide palmitoylethanolamide (PEA) with the flavonoid luteolin displays neuroprotective and antiinflammatory actions in different models of central nervous system pathologies. We hypothesized that association of PEA with luteolin might have therapeutic utility in ASD, and we employed a well‐recognized autism animal model, namely sodium valproate administration, to evaluate cognitive and motor deficits.
Methods
Two sets of experiments were conducted. In the first, we investigated the effect of association of ultramicronized PEA with luteolin, co‐ultramicronized PEA‐LUT® (co‐ultraPEA‐LUT®) in a murine model of autistic behaviors, while in the second, the effect of co‐ultraPEA‐LUT® in a patient affected by ASD was examined.
Results
Co‐ultraPEA‐LUT® treatment ameliorated social and nonsocial behaviors in valproic acid‐induced autistic mice and improved clinical picture with reduction in stereotypes in a 10‐year‐old male child.
Conclusion
These data suggest that ASD symptomatology may be improved by agents documented to control activation of mast cells and microglia. Co‐ultraPEA‐LUT® might be a valid and safe therapy for the symptoms of ASD alone or in combination with other used drugs.
Keywords: Autism, Inflammation, Luteolin, Palmitoylethanolamide
Introduction
The term autism refers to a wide range of disease entities defined by the acronym ASD (fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5)). All share common clinical features constituting a peculiar symptom triad that is the basis for diagnosis or suspicion of the pathology. The diagnostic criteria for ASD as they appear in DSM‐5 are as follows:
deficits in social interaction and communication across various contexts;
restricted, repetitive patterns of behavior, interests, or activities;
symptoms must subsist in the first developmental period;
symptoms cause clinically impairment in social, occupational, or other important areas of current functioning;
these disturbances are not better explained by intellectual disability (intellectual developmental disorder) or global developmental delay.
Autism usually develops within the first 3 years of life. Although historical data indicate a median worldwide prevalence of autism as 0.62–0.70% 1, 2, estimates of 1–2% have emerged from the latest large‐scale surveys 3, 4, 5, 6, 7. A similar prevalence has been reported for adults 8. Approximately 45% of autistic individuals have intellectual disability 2. More men than women are affected by ASD 8, with these large‐scale population‐based studies showing that 2–3 times more males are affected, irrespective of intellectual disability 3, 5, 8. There are no imaging tools or laboratory determinants for the disease, and diagnosis is made by means of questionnaires for parents, the most widely used being the Childhood Autism Rating Scale, the Autism Diagnostic Interview‐Revised, the Autism Diagnostic Observation Schedule‐Generic, and the Autism Treatment Evaluation Checklist (ATEC). Although autism was first described some 70 years ago 9, the syndrome's cause(s) remains unknown. Comorbidity is common (>70% have concurrent medical, developmental or psychiatric conditions) 10, 11, and autism is associated with other diseases—in particular fragile X syndrome and tuberous sclerosis. Scientific interest in autism has grown in recent years due to the marked rise in reported cases 1. We appreciate that brain inflammation may be a key element in the pathogenesis of neuropsychiatric disorders 12, 13 including a significant proportion of ASD subjects 14, 15, 16. Food intolerance and eczema are present in ASD children 16, 17. Mast cells are implicated in allergic and inflammatory reactions and activated in autism 18, 19. ASD incidence is claimed to be 10‐fold higher in children with mastocytosis 20. There is interest in a role of the endocannabinoid (EC) system in neurological disorders. While data suggest a key role for the EC system in social and emotional processing 21, there are few studies dealing with this system in autism. Preclinical researches have shown that social play behavior enhances levels of the N‐acylethanolamine and endocannabinoid anandamide in amygdala, nucleus accumbens 22, and striatum 23. The EC system modulates physiological processes such as axonal growth, guidance during development 24, adult neurogenesis, and behavior 25. However, the role of these N‐acylethanolamines on social and emotional behavior remains to be been investigated.
N‐Palmitoylethanolamine (PEA) behaves as a lipid signaling molecule with antiinflammatory activity in preclinical models of acute and inflammatory pain and central nervous system injury and is efficacious for pain relief in man 26, 27. Luteolin, a flavonoid belonging to the flavone family, possesses neuroprotective actions 28, 29. It has been suggested that dietary flavonoids possess therapeutic potential in disorders especially where the monoaminergic system is involved. Luteolin has antioxidant 30, anticancer 31 memory‐improving 32, and anxiolytic activities 33. Chronic administration of co‐ultramicronized PEA+luteolin in mice reversed the behavioral dysfunction of chronic corticosterone exposure 34. Importantly, a dietary supplement containing luteolin improved ASD behavioral symptoms in autistic children 35, 36. There is no therapy for ASD and current medications address specific secondary behavioral symptoms. Based on the recognized neuroprotective properties of PEA and luteolin, we decided to investigate the effects of co‐ultramicronized PEA+luteolin (co‐ultraPEA‐LUT®) in the valproic acid (VPA) murine model of ASD 37 and in a patient with ASD.
Materials and Methods
ASD In Vivo Models
University of Messina Animal Care Review Board approved this research. Mice, housed in a controlled environment, were supplied with standard water and rodent chow. Animal care was in compliance with Italian (D.M. 116192), Europe (O.J. of E.C. L 358/1 12/18/1986), and USA (Animal Welfare Assurance No A5594‐01, US Department of Health and Human Services, Washington, D.C USA) regulations. C57/BL6 male mice were injected subcutaneously with saline or sodium valproate (VPA, 400 mg/kg) on postnatal day 14 (P14).
Two sets of experiments were carried out (Figure S1):
-
1
I:
Sham+Vehicle:
Sham+co‐ultraPEA‐LUT® (1 mg/kg, orally by gavage, from P15 for 2 weeks).
VPA+Vehicle;
VPA+co‐ultraPEA‐LUT® (1 mg/kg, orally by gavage from P15 for 2 weeks).
For each group (N = 30), 10 animals were allocated for behavior, immunohistochemistry, and Western blot.
-
2
II:
Sham+Vehicle;
Sham+co‐ultraPEA‐LUT® (1 mg/kg, orally by gavage from P15 for 3 months for neurogenesis studies).
VPA+Vehicle (n = 30);
VPA+co‐ultraPEA‐LUT® (1 mg/kg, orally by gavage from P15 for 3 months for neurogenesis studies).
Co‐ultraPEALut was prepared in vehicle (1.5% (w/v) carboxymethylcellulose in saline). The dose, administration route, and vehicle were based on previous study 38.
For each group N = 30.
Behavior
Behavioral tasks comprised social interaction test and elevated plus maze (EPM) on P30‐P40 and P120. Co‐ultraPEA‐LUT® was administered from P15 to the beginning of the behavioral and inflammatory studies.
Social Interaction Test
The test was carried out in a novel three‐chamber apparatus 39. (Supporting Information).
EPM
The animal was placed on the pivotal platform facing an open arm 40 (Supporting Information).
Western Blot for Bax, Bcl‐2, Inducible Nitric Oxide Synthase (iNOS), IκBα, Nuclear Factor‐κB (NF‐kB), and Glial Fibrillary Acidic Protein (GFAP)
Brain tissue was subjected to denaturating SDS gel electrophoresis followed by electroblotting and incubation with anti‐Bax, anti‐Bcl‐2, anti‐iNOS, anti‐NF‐κB p65, anti‐IκBα antibody, anti‐GFAP antibodies (Supplementary Information).
Immunohistochemical Localization of Chymase, Tryptase, Tumor Necrosis Factor Alpha (TNF‐α), and Interleukin‐1beta (IL‐1β)
Sections were incubated overnight at RT with antichymase, antitryptase, anti‐TNF‐α, and anti‐IL‐1β antibodies (Supporting Information).
Bromodeoxyuridine (BrdU) and Doublecortin (DCX) Immunohistochemistry
A stereological procedure was used to quantify BrdU‐ and DCX‐positive cells 41 (Supporting Information).
Golgi Impregnation
Golgi impregnation was performed according to the manufacturer's instructions (FD NeuroTechnologies, Ellicott City, MD, USA) 42 (Supporting Information).
Patient History
The participating physician who brought to our attention the patient for this study is part of our group's collaborative clinical network. This male child was born at term with natural spontaneous delivery [39 weeks + 6 days with an Apgar index between 8 and 10] and was found to have Tetralogy of Fallot, which was corrected surgically at the age of 3 months without complications. As is typical with autism, physical, and mental development appeared normal during the first few years of life. At the age of 28 months febrile episodes occurred, which were treated with antibiotics and nonsteroidal antiinflammatory drugs. In April 2005, the parents reported three febrile episodes in succession characterized by inflammation of the upper respiratory tract; these were treated with oral antibiotics and nonsteroidal antiinflammatory drugs and with paracetamol to control hyperpyrexia. At this time, over the course of 30 days, behavioral changes suddenly emerged: the child began to show rejection behavior of strangers with weeping and escape responses toward other children of same age; hyperactivity with constant running; changed eating behavior with refusal of food and limiting the choice only to 3–4 kind of aliments; up to 7–8 bowel movements per day; loss of acquired verbal skills, avoidance of gazing at people, and appearance of motor stereotypes. Approximately 30 days after onset of the first episode of fever, the child was admitted to the day hospital at the Pediatric Neuropsychiatry Clinic of Saint Gerardo Hospital—Monza, where a diagnosis of “delay of verbalization and socialization” with suspected autism was made. The child lives with his family in a provincial town, far from sources of industrial pollution. The mother (36 years old at time of childbirth) did not take any medications during pregnancy with the exception of supplementation with folic acid, iron, calcium, and magnesium; amniocentesis was performed at week 16 without complications and the remainder of the pregnancy followed a regular course. A genetics analysis of the patient was not performed, although there was no family history of autism.
Protocol with Co‐UltraPEA‐LUT®
The child was 10 years old when this study began. After diagnosis of ASD the child underwent 6 months of psychotherapy with his parents, without benefit. Psychomotor and speech therapy produced some benefit on behavior. A gluten‐free/casein‐free diet and vitamin supplements led to a rapid improvement in sleep–wake behavior and eye contact. However, vitamin supplements in particular, at doses far exceeding their daily recommended allowance, were suspended almost immediately due to worsening of hyperactivity and stereotypical behaviors. From time to time, the patient underwent homotoxicological‐homeopathic therapy with favorable results, especially with regard to mercury detoxification and treatment with probiotics.
Given the involvement of brain inflammation in the development of ASD, as well as the presence of activated mast cells 19, we undertook a therapy with the antiinflammatory and mast cell modulating agent co‐ultraPEA‐LUT®, at a dose of 700 mg+70 mg b.i.d. (Glialia® microgranules, Epitech Group SpA, Italy) for 1 year. During the study period (May 2012–2013), the child continued the gluten‐ and casein‐free diet. The patient was evaluated before treatment, after ~5 months and ~12 months using the ATEC questionnaire (http://www.autism.com/index.php/ind_atec_report) and by a quarterly questionnaire administered to the child's teachers for the assessment of motor stereotypic behaviors (http://www.stress-cocchi.net/Autism10-it.htm). The ATEC questionnaire is sensitive enough to measure changes in the child's condition and consists of four subgroup scales: Scale I. Speech/Language/Communication (14 items—scores can differ from 0 to 28), Scale II. Sociability (20 items—scores can differ from 0 to 40), Scale III. Sensory/Cognitive Awareness (18 items—scores can differ from 0 to 36), and Scale IV. Health/Physical Behavior (25 items—scores can differ from 0 to 75). The four subgroup scores can be utilized to calculate a total score (total scores can range from 0 to 180). The scores are estimated according to the reply and corresponding subscale. The higher the subscale and total score, the more impaired is the patient. The lower the subscale and total score, the less impaired the patient. The stereotyping behavior was evaluated, before, during, and after 12 months by means of a five‐step scale of intensity as follows:
0 = symptom or behavior not present; 1 = rarely present and not linked to particular situations; 2 = present only in precise moments (e.g., before sleeping); 3 = present if the child is not actively involved in the environmental situation; 4 = very present and difficult to interrupt.
In addition to cognitive, behavioral, and motor symptoms, the child experienced an elevated frequency of night‐time enuresis, independent of time of year, general conditions or diet. This problem is often present in autistic children over five years of age 43.
Parental consent was obtained for the study.
Statistics
Preclinical Data
Values in the figures are expressed as mean ± standard error of mean (SEM) of N observations. For in vivo studies, N = number of animals.
Statistically differences in behavioral score were analyzed by one‐way ANOVA, followed by Newman–Keuls multiple comparison test. ANOVA and post hoc Tukey tests were performed in immunohistochemistry studies with Bonferroni correction for multiple comparisons. All other results were tested by one‐way ANOVA followed by a Bonferroni post hoc test for multiple comparisons. A P‐value of <0.05 was considered significant.
Results
Preclinical Data
Behavioral effects of 2 weeks and 3 months of co‐ultraPEA‐LUT® in the ASD mouse
Social behavior represents a primary deficit in ASD. Analysis of behavior during the acclimatization period of mice to the novel three‐chamber sociability area prior to the introduction of an unfamiliar con‐specific mouse revealed that postnatal exposure to VPA did not modify time spent or locomotor activity in either side of the arena (data not shown). Following the introduction of the unfamiliar mouse and novel object (empty wire container) into the three‐chamber test arena, 2 weeks and 3 months analysis showed that time in the chamber with unfamiliar mouse was less in VPA‐exposed mice when compared to control (Figure 1A,B).
Figure 1.
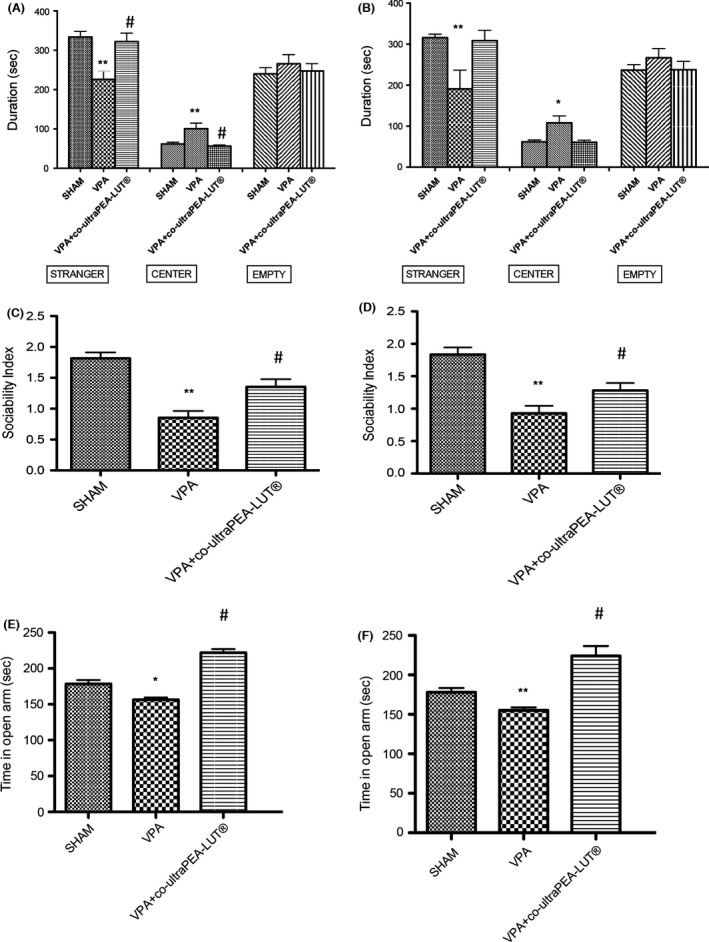
Behavioral effects of a 2 week and 3 months of co‐ultramicronized PEA‐LUT® treatment in VPA‐treated mice. Two weeks (A) and 3 months (B) co‐ultramicronized PEA‐LUT® administration restored the stay duration in stranger side (A,B). Duration in central area was significantly increased by VPA exposure but not by co‐ultramicronized PEA‐LUT® (A,B). Duration in empty side was not modified by VPA exposure or co‐ultramicronized PEA‐LUT® (A,b). Sociability Index (SI) was significantly decreased in VPA‐treated mice. Two weeks and 3 months of co‐ultramicronized PEA‐LUT® administration significantly restored this reduction (Fig. C,D). Effects of (E) 2 weeks and (F) 3 months of co‐ultramicronized PEA‐LUT® treatment on anxiety‐like behaviors in the elevated plus maze. Anxiety‐like behavior is expressed as mean of time spent in open arm, considering only the animals that have four paws in the open arm. Data are mean ± SEM (N = 10 mice for each group) (*P < 0.05 vs. sham; **P < 0.01 vs. sham; # P < 0.05 vs. VPA‐treated group).
Two weeks and 3 months of co‐ultraPEA‐LUT® re‐established stay duration in stranger side (Figure 1A,B). Duration in the central area was increased by VPA exposure but not by co‐ultraPEA‐LUT® (Figure 1A,B). Duration in the empty side was not modified by VPA exposure or co‐ultraPEA‐LUT® (Figure 1A,B). Analysis of SI displayed a significant decrease by VPA exposure. Two weeks and 3 months of co‐ultraPEA‐LUT® significantly restored this reduction (Figure 1C,D). In the EPM, co‐ultraPEA‐LUT® (2 weeks and 3 months) increased time spent in the open arm (Figure 1D,E).
Effects of Co‐ultraPEA‐LUT® on Inflammatory Protein Expression in the ASD Mouse
Western blot analysis of hippocampal and cerebellar tissue was employed to evaluate the cellular mechanism by which co‐ultraPEA‐LUT® might attenuate social and depressive behavior. iNOS and GFAP levels (Figures 2A–D) were significantly expressed in hippocampus and cerebellum of VPA mice. Co‐ultraPEA‐LUT® restored iNOS and GFAP expression to levels comparable to those of controls (Figures 2A–D). IκB‐α levels were reduced in brain tissue from VPA mice as compared to sham animals, whereas in co‐ultraPEA‐LUT®‐treated mice (Figure 2E,F), a significant increase in IκB‐α levels was detected. NF‐κB p65 levels in cerebral tissue from co‐ultraPEA‐LUT® treated mice displayed a significant decrease in expression when compared to VPA mice (Figure 2G,H).
Figure 2.
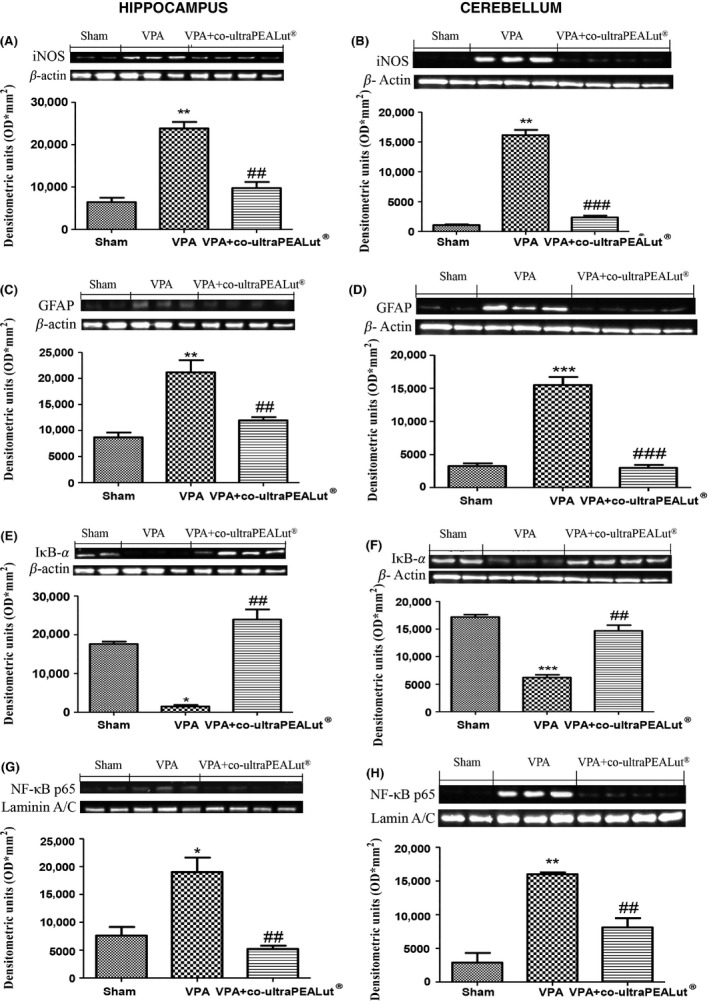
Effect of co‐ultramicronized PEA‐LUT® administration on inflammatory markers in VPA‐treated mice. (A) A significant increase in iNOS (A,B) and GFAP (C,D) expression, assayed by Western blot analysis, was detected in brain tissue from VPA‐treated mice. Co‐ultramicronized PEA‐LUT® treated mice show reduced iNOS (A,B) and GFAP expression (C,D) in both hippocampus and cerebellum. (B) IkB‐a levels were reduced substantially in hippocampus and cerebellum of VPA‐treated mice (E,F). Co‐ultramicronized PEA‐LUT® treatment prevented the VPA‐induced IkB‐a degradation (E,F). NF‐kB p65 levels in VPA‐treated mice brain nuclear fractions were increased significantly compared with co‐ultramicronized PEA‐LUT® treated mice (G,H). The figures are representative of at least three separate experiments. Data are means ± SEM (N = 10 mice for each group). *P < 0.05 versus sham; **P < 0.01; ***P < 0.001 versus sham; ## P < 0.01; ### P < 0.001 versus VPA‐treated group.
Effects of Co‐ultraPEA‐LUT® on TNF‐α, IL‐1β, Chymase, and Tryptase in ASD Mouse
Brain sections from VPA‐treated mice exhibited positive staining for TNF‐α (Figure 3A,B; see densitometry analysis G) and IL‐1β (Figure 3D,E; see densitometry analysis in G), whereas in brain sections from co‐ultraPEA‐LUT® expression of these cytokines was significantly attenuated (Figure 3C–F; see densitometry analysis in G).
Figure 3.
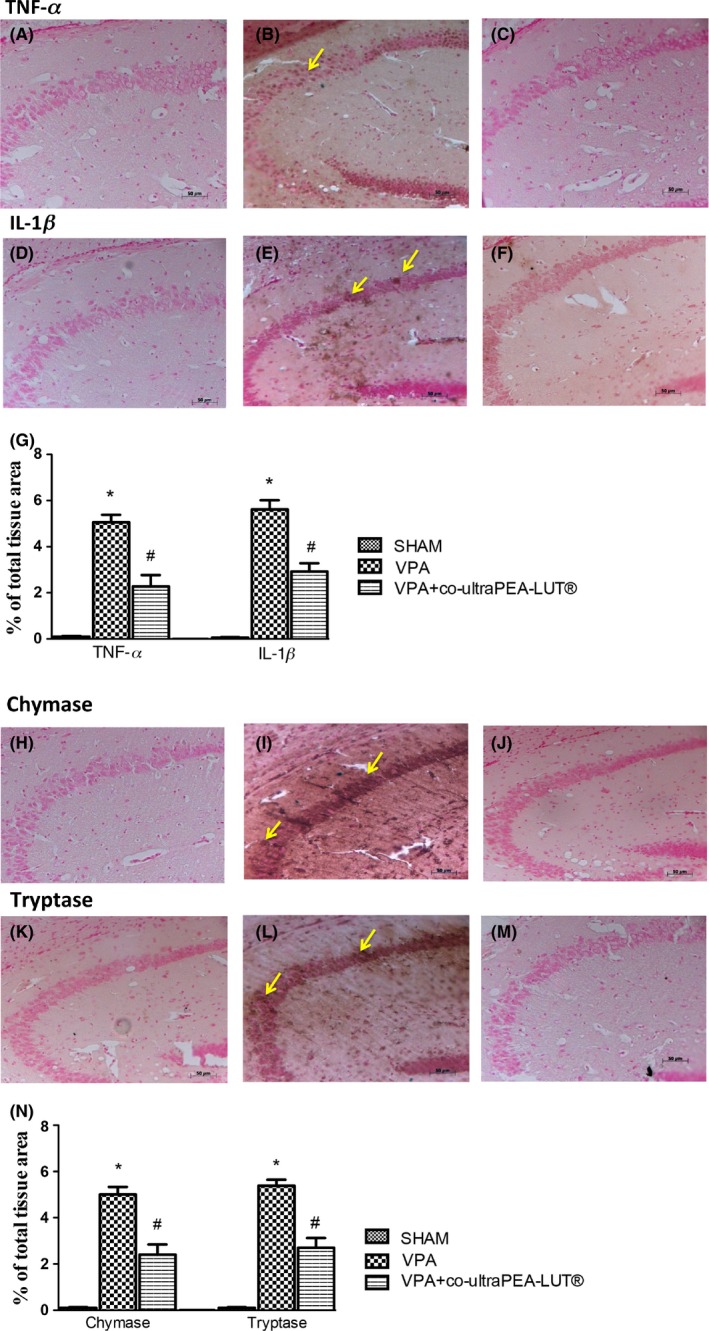
Effect of co‐ultramicronized PEA‐LUT® on TNF‐α, IL‐1β, and chymase and tryptase expression in VPA‐treated mice. Brain sections from VPA‐treated mice exhibited positive staining for TNF‐α (A–C) and IL‐1β (D–F). Co‐ultramicronized PEA‐LUT® treatment significantly reduced staining for both TNF‐α and IL‐1β (C,F). The graphs are representative of at least three separate experiments. Data are means ± SEM from N = 10 mice for each group. (*P < 0.05 vs. sham; # P < 0.05 vs. VPA‐treated group). Arrows indicate zone analyzed. A substantial increase in expression of the serine peptidases chymase and tryptase was found mainly confined in mast cells in brain tissues collected from VPA‐treated mice (I,L). Chymase and tryptase levels were attenuated in co‐ultramicronized PEA‐LUT® treated mice (J,M). This figure is representative of at least three separate experiments. Data are means ± SEM from N = 10 mice for each group (*P < 0.05 vs. sham; # P < 0.05 vs. VPA‐treated group). Arrows indicate zone analyzed.
To test whether co‐ultraPEA‐LUT® may change and direct the inflammatory response via the regulation of mast cell‐derived serine peptidases, we tested by immunohistochemistry the expression of chymase and tryptase in the brain. There was no staining in brain tissues from sham mice. A substantial increase in chymase (Figure 3H,I; see densitometry analysis in N) and tryptase expression (Figure 3K,L; see densitometry analysis in N) was found localized to mast cells in hippocampus and cerebellum of VPA mice. Expression of chymase and tryptase was attenuated with co‐ultraPEA‐LUT® (Figure 3J–M; see densitometry analysis in N).
Effect of Co‐ultraPEA‐LUT® on Apoptosis in the ASD Mouse
The appearance of effectors of canonical mitochondrial apoptosis in brain, such as pro‐apoptotic (Bax) and antiapoptotic (Bcl‐2) proteins, was investigated by Western blot in hippocampus and cerebellum. Co‐ultraPEA‐LUT® significantly reduced VPA‐induced Bax expression (see densitometry analysis, Figure 4A,B) in both areas. On the other hand, Bcl‐2 expression was significantly reduced in the VPA‐treated group and co‐ultraPEA‐LUT® reduced this inhibition (see densitometry analysis, Figure 4C,D) in hippocampus and cerebellum.
Figure 4.
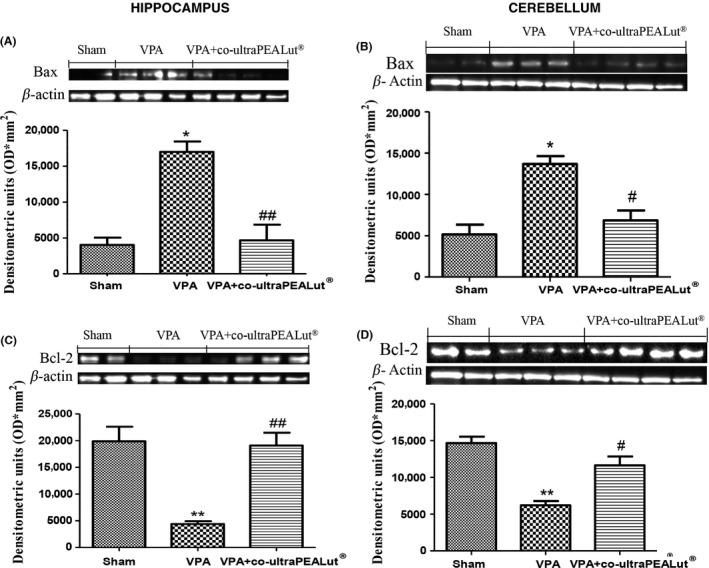
Effects of co‐ultramicronized PEA‐LUT® treatment on Bax and Bcl‐2 expression in VPA‐treated mice. (A) Co‐ultramicronized PEA‐LUT® treatment reduced levels of Bax in both hippocampus and cerebellum of VPA‐treated mice. β‐actin was used as internal control. (B) Co‐ultramicronized PEA‐LUT® administration increased Bcl‐2 levels in both hippocampus and cerebellum of VPA‐treated mice. A representative blot of lysates acquired from each group is displayed, and densitometric analysis of all animals is indicated (N = 10 mice from each group) (*P < 0.05; **P < 0.01 vs. sham; ## P < 0.05; # P < 0.05 vs. VPA‐treated group).
Co‐ultraPEA‐LUT® Favors Adult Hippocampal Neurogenesis and Synaptic Plasticity in the ASD Mouse
To investigate the potential cellular mechanisms underlying the behavioral effects of co‐ultraPEA‐LUT®, we studied changes in adult hippocampal cell proliferation and synaptic plasticity after 3 months of treatment. BrdU was given 2 h before sacrifice on the last day of treatment to tag proliferating neural progenitors. Number and proliferation of immature neurons were evaluated using DCX and BrdU immunohistochemistry, according to Wang et al. 44. VPA exposure decreased the number of BrdU‐positive cells analyzed in the dentate gyrus of the mouse hippocampus (Figure 5A; see images 7B–D), which was reversed by 3 months of co‐ultraPEA‐LUT® (Figure 5A; see images 7B–D). VPA exposure reduced DCX‐positive cells in the dentate gyrus of the mouse hippocampus (Figure 5E; see images, F–H) which was overcome by 3 months of co‐ultraPEA‐LUT® (Figure 5E; see images F–H). Tracing of the apical dendrites of granule cells in dentate gyrus revealed a significant reduction in development of dendritic spines in VPA‐treated mice that was reverted by co‐ultraPEA‐LUT® (Figure 5I; see images, J–L).
Figure 5.
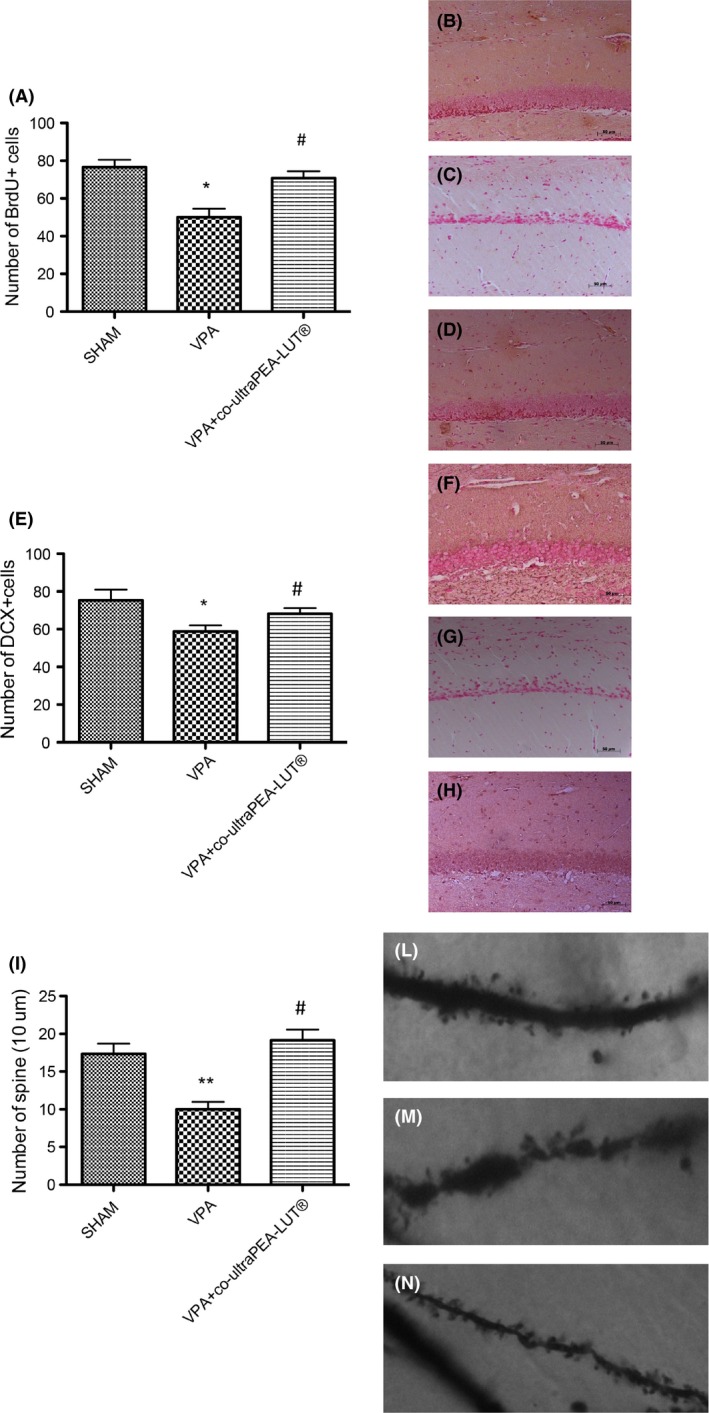
Cell proliferation, dendritic maturation, and synaptic plasticity in the dentate gyrus of mouse hippocampus: effect of VPA treatment and co‐ultramicronized PEA‐LUT®. (A) BrdU (150 mg/kg, intraperitoneally) was given 2 h before sacrifice to examine the effects of 3 months treatment with co‐ultramicronized PEA‐LUT®. The number of BrdU+ cells increased after co‐ultramicronized PEA‐LUT® treatment. (B) Control (C), VPA‐treated (D), VPA + co‐ultramicronized PEA‐LUT® treatment. (B–E) Effects of co‐ultramicronized PEA‐LUT® administration on total number of DCX‐positive cells. The number of DCX‐positive cells is increased after co‐ultramicronized PEA‐LUT® treatment. (F) DCX of controls, (G) DCX of VPA‐treated mice, (H) DCX of co‐ultramicronized PEA‐LUT® treatment. (I) Effects of co‐ultramicronized PEA‐LUT® administration on number of spines in the dentate gyrus; this number increased with co‐ultramicronized PEA‐LUT® treatment. (J–L) representative images of spine density. Values are mean ± SEM (N = 10 mice per each group). (*P < 0.05; **P < 0.01 vs. sham; # P < 0.05 vs. VPA‐treated group).
Clinical data
Clinical Assessment of an Autistic Patient Treated with Co‐ultraPEA‐LUT®
Co‐ultraPEA‐LUT® treatment was well tolerated by the patient. Evaluation using the ATEC test revealed a decrease of scores (both total score and subgroup scores), indicative of an improved behavioral outcome of about 23%. The most evident effect over time appeared in the sociability subgroup, where the score decreased from 24 to 13 (Table 1). Co‐ultraPEA‐LUT® reduced most indices of hyperactivity, as demonstrated by reduction in motor stereotypies which from the very beginning were present only rarely or at precise moments (Table 2).
Table 1.
ATEC scores in response to PEA‐LUT® treatment in a 10‐year‐old child with ASD
| Autism treatment evaluation checklist | Time (months) | ||
|---|---|---|---|
| 0 | 5 | 12 | |
| I. Speech | 24 | 23 | 21 |
| II. Sociability | 24 | 22 | 13 |
| III. Sensory/Cognitive | 31 | 29 | 26 |
| IV. Health/Physical/Behavior | 24 | 22 | 19 |
| Total | 103 | 96 | 79 |
Table 2.
Motor stereotypy changes in response to PEA‐LUT® treatment in a 10‐year‐old child with ASD
| Motor stereotypies | Time (months) | |||
|---|---|---|---|---|
| 0 | 4 | 8 | 12 | |
| Rocking | 3 | 3 | 2 | 1 |
| Head banging | 0 | 0 | 0 | 0 |
| Flapping tremor | 4 | 3 | 2 | 2 |
| Fingers movements | 4 | 3 | 2 | 3 |
| Walking on Tip‐Toes | 0 | 0 | 0 | 0 |
| Skill stereotypies (e.g., quickly turning a knife on a table) | 1 | 2 | 3 | 3 |
| Vocal stereotypy (unmotivated screaming, frog noise, grunts) | 4 | 3 | 2 | 1 |
Clinical improvement, as highlighted by the questionnaire results, coincided with significant progress in cognitive behavior as observed by parents and teachers. The patient is, in fact, now more able to understand simple commands and execute them with ease; eye contact is much improved and the child's behavior far more affectionate. Unfortunately, no significant progress in speech profile has been observed until now.
Daily control of enuresis, evaluated over a period of 4 months before treatment start showed this incontinence to be present in 91% of the nights. A similar follow‐up carried out after 7 months from treatment start demonstrated a slight decrease equal to 11.7% of nights with enuresis (enuresis was present 79.3% of the nights). The final follow‐up made after another 7 months revealed a remarkable decrease equal to 88.6% of the nights with enuresis (only 2.4% of the nights with enuresis) (Table 3).
Table 3.
Enuresis reduction in response to PEA‐LUT® treatment in a 10‐year‐old child with ASD
| Observation Period 4 months before each follow‐up | |||
|---|---|---|---|
| Before treatment start | 7 months after treatment start | 14 months after treatment start | |
| Nights with enuresis | 91% | 79.3% | 2.4% |
Discussion
ASD is a set of disparate neurodevelopmental conditions, characterized by early‐onset impairment in social cognition and social perception, executive dysfunction, atypical perceptual, and information processing problems in social communication and unusually limited, repetitive behavior, and interests. It is usually considered to be present from birth but, in fact, up to 40% of those diagnosed with autism have apparently normal development through the age of 18–30 months but then experience a period of regression where controlled skills fail to mature along a normal trajectory. Pharmacological approaches have focused on controlling symptoms, rather than the underlying pathogenesis. Using the VPA animal model of ASD, we show, for the first time, that a formulation of co‐ultraPEA‐LUT® is capable of ameliorating social and nonsocial behaviors in VPA‐induced autistic‐like mice. Co‐ultraPEA‐LUT® not only reduced the expression of pro‐inflammatory markers such as NF‐κB, GFAP, and nitrotyrosine, but modulated apoptosis in hippocampus and cerebellum, and increased neurogenesis and neuroplasticity in the hippocampus of VPA‐treated mice. Co‐ultraPEA‐LUT® improved the clinical picture in an autistic child in terms scoring by the Autism Treatment Evaluation Checklist and a reduction in motor stereotypes. These data point to the potential translatability of co‐ultraPEA‐LUT® treatment in autism.
VPA is primarily used for the treatment of epilepsy, major depressive disorder, and bipolar disorder. In utero exposure of VPA in humans produces symptoms similar to autism, such as deficits in language and communication, stereotypic behavior, hyperexcitability, and delays in behavioral development 45. These clinical analogies have led Rodier and colleagues 46 to suggest that prenatal VPA exposure in rodents might provide a useful animal model of autism. Pre‐ and early postnatal exposure to VPA led to neurodevelopmental deficits analogous to the motor and cognitive alterations seen in humans with autism 47. Critical cerebellar‐mediated behaviors first appeared on P14 47. Previous studies reported that injection of <500 mg/kg VPA in animals was sufficient to induce a severe autism‐like state without death 48. In our investigation, a single 400 mg/kg VPA exposure on P14 induced motor and cognitive deficits that mimicked autistic regression. P14 approximately coincides to the third trimester in human development 49; a time frame over which hippocampal and cerebellar granule cells undergo migration and differentiation 49. This therapeutic strategy for autism has changed little over the years, with major efforts aimed at selecting active ingredients so as to control hyperactivity and the repetitive stereotypical movements. The most widely used drug classes are antipsychotics and psychostimulants 50. Selective serotonin reuptake inhibitors have seen a discrete level of acceptance for patients with a mild form of the disorder as well as anxiolytics. Exclusion of gluten and casein from the diet can also improve symptoms of autism 51 suggesting a possible metabolic component in this disease.
Co‐ultraPEA‐LUT® appears to possess anxiolytic and antidepressant‐like activity in different experimental models 34 supporting the hypothesis that immune modulation might result in behavioral improvement without altering motor activity and body weight. A link between PEA and mood disorders is suggested by evidence that acute psychosocial stress increases PEA serum concentrations which correlate with circulating cortisol levels already 30 seconds following end of stress 34. Plasma levels of PEA are increased in patients with posttraumatic stress disorder 52. PEA is proposed to play a key role in maintaining cellular homeostasis against external stressors provoking, for example, neuroimmune dysregulation 53, and its alteration in mood disorders might be the body's attempt to restore homeostasis. In this research, co‐ultraPEA‐LUT® counteracted autistic‐like behavioral changes, including reduced sociability and increased anxiety‐related behavior in EPM. Utilizing BrdU and DCX immunohistochemistry, the fall of neurogenesis observed in VPA‐treated mice was partially restored by co‐ultraPEA‐LUT®. Quantitative analysis of dentate gyrus granule cell spine density revealed changes with co‐ultraPEA‐LUT®, suggesting that spine and dendritic morphology may be sensitive to this treatment. This formulation promotes neurogenesis and neuroplasticity in a mouse model of a depression‐like state 34. Precursor cells, located in the neurogenic areas of adult brain and local progenitors, play an important role in CNS injury 54.
Autism symptomatology could result from a chronic subclinical inflammation involving the gut and central nervous system 55. Increases in pro‐inflammatory cytokines and elevated immune response have been found in the brain of autistic patients 54. Molecules able to counteract inflammatory and neuroinflammatory processes might thus be of therapeutic utility in reducing the symptoms in ASD. Because the VPA mouse model of ASD used here is characterized by reduced social behavior and raised expression of neuroinflammatory markers in the brain, we treated VPA mice with co‐ultraPEA‐LUT®, which has documented antiinflammatory and neuroprotective effects mediated by downmodulation of mast cell and microglial activation 56, given the purported involvement of these nonneuronal cells in autism 20. Our findings demonstrate the effect of co‐ultraPEA‐LUT® on VPA‐induced neuroinflammation in brain by immunohistochemical and Western blot, revealing the ability of this compound to counteract VPA‐induced astroglial activation, as demonstrated by the downregulation of GFAP immunoreactivity along with hypertrophic astrocytes. Under physiological conditions in the central nervous system, inflammation contributes to repair of damaged tissues after injury 57. However, excessive inflammation damages surrounding healthy tissue. In particular, microglia release pro‐inflammatory and cytotoxic factors, including IL‐1β, IL‐6, TNF‐α, nitric oxide, and reactive oxygen species 58. In our study, co‐ultraPEA‐LUT® modulated TNF‐α and IL‐1β immunoreactivity.
In the described case report, chronic co‐ultraPEA‐LUT® reduced behavioral alterations of a child with ASD. Our observations are consistent with the results obtained after treatment with ultramicronized PEA, administered alone, in two children suffering from autism spectrum disorder 59. It is important to point out that children with autism experience a higher incidence of anxiety, particularly social anxiety, than children with other developmental disorders or with a normal development 60. Anxiety in children with ASD is related to sensory over‐responsivity and gastrointestinal problems, suggesting that these pathological states represent interconnected phenomena, sharing common underlying mechanisms mediated by mast cell and microglia activation. Our data suggest that ASD symptomatology may be improved by agents documented to control activation of mast cells and microglia. These results, while pointing to co‐ultraPEA‐LUT® as a valid and safe treatment in the autism symptoms—alone or in combination with other drugs—clearly need to be confirmed in a larger number of cases. The proof‐of‐principle study presented here encourages further investigations.
Conflict of Interests
Rosalia Crupi affirms that she has no conflict of interest. Daniela Impellizzeri affirms that she has no conflict of interest. Giuseppe Bruschetta affirms that he has no conflict of interest. Marika Cordaro affirms that she has no conflict of interest. Rosalba Siracusa affirms that she has no conflict of interest. Emanuela Esposito affirms that she has no conflict of interest. Bartolomeo Bertolino affirms that he has no conflict of interest. Salvatore Cuzzocrea is co‐inventor on patent WO2013121449 A8 (Epitech Group Srl) which treats with methods and compositions for the modulation of amidases capable of hydrolyzing N‐acylethanolamines employable in the treatment of inflammatory diseases. This invention is wholly unrelated to the present study. Moreover, Prof. Cuzzocrea is also, with Epitech Group, a co‐inventor on the following patent: EP 2 821 083; MI2014 A001495; 102015000067344 that are however unrelated to the study.
Supporting information
Figure S1 Schematic representation depicting the experimental design.
Data S1. Supporting informations.
Acknowledgments
The authors would like to thank Maria Antonietta Medici for excellent technical assistance, Mr. Francesco Soraci for secretarial and administrative assistance, and Miss Valentina Malvagni for editorial support with the manuscript. This study was supported by PON01‐02512 grant.
The first two authors contributed equally to this study.
References
- 1. Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res 2012;5:160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res 2009;65:591–598. [DOI] [PubMed] [Google Scholar]
- 3. Kim YS, Leventhal BL, Koh YJ, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry 2011;168:904–912. [DOI] [PubMed] [Google Scholar]
- 4. Baron‐Cohen S, Scott FJ, Allison C, et al. Prevalence of autism‐spectrum conditions: UK school‐based population study. Br J Psychiatry 2009;194:500–509. [DOI] [PubMed] [Google Scholar]
- 5. Idring S, Rai D, Dal H, et al. Autism spectrum disorders in the Stockholm Youth Cohort: Design, prevalence and validity. PLoS ONE 2012;7:e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Russell G, Rodgers LR, Ukoumunne OC, Ford T. Prevalence of parent‐reported ASD and ADHD in the UK: Findings from the Millennium Cohort Study. J Autism Dev Disord 2014;44:31–40. [DOI] [PubMed] [Google Scholar]
- 7. Saemundsen E, Magnusson P, Georgsdottir I, Egilsson E, Rafnsson V. Prevalence of autism spectrum disorders in an Icelandic birth cohort. BMJ Open 2013;3: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brugha TS, McManus S, Bankart J, et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry 2011;68:459–465. [DOI] [PubMed] [Google Scholar]
- 9. Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr 1968;35:100–136. [PubMed] [Google Scholar]
- 10. Hofvander B, Delorme R, Chaste P, et al. Psychiatric and psychosocial problems in adults with normal‐intelligence autism spectrum disorders. BMC Psychiatry 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lugnegard T, Hallerback MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011;32:1910–1917. [DOI] [PubMed] [Google Scholar]
- 12. Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflammation 2013;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Mol Cell Neurosci 2013;53:52–62. [DOI] [PubMed] [Google Scholar]
- 14. El‐Ansary A, Al‐Ayadhi L. Neuroinflammation in autism spectrum disorders. J Neuroinflammation 2012;9:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Theoharides TC, Asadi S, Patel AB. Focal brain inflammation and autism. J Neuroinflammation 2013;10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McDougle CJ, Carlezon WA Jr. Neuroinflammation and autism: Toward mechanisms and treatments. Neuropsychopharmacology 2013;38:241–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jyonouchi H. Autism spectrum disorders and allergy: Observation from a pediatric allergy/immunology clinic. Expert Rev Clin Immunol 2010;6:397–411. [DOI] [PubMed] [Google Scholar]
- 18. Theoharides TC, Doyle R, Francis K, Conti P, Kalogeromitros D. Novel therapeutic targets for autism. Trends Pharmacol Sci 2008;29:375–382. [DOI] [PubMed] [Google Scholar]
- 19. Theoharides TC, Angelidou A, Alysandratos KD, et al. Mast cell activation and autism. Biochim Biophys Acta 2012;1822:34–41. [DOI] [PubMed] [Google Scholar]
- 20. Theoharides TC. Autism spectrum disorders and mastocytosis. Int J Immunopathol Pharmacol 2009;22:859–865. [DOI] [PubMed] [Google Scholar]
- 21. Lutz B. Endocannabinoid signals in the control of emotion. Curr Opin Pharmacol 2009;9:46–52. [DOI] [PubMed] [Google Scholar]
- 22. Trezza V, Damsteegt R, Manduca A, et al. Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. J Neurosci 2012;32:14899–14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marco EM, Rapino C, Caprioli A, Borsini F, Maccarrone M, Laviola G. Social encounter with a novel partner in adolescent rats: Activation of the central endocannabinoid system. Behav Brain Res 2011;220:140–145. [DOI] [PubMed] [Google Scholar]
- 24. Berghuis P, Rajnicek AM, Morozov YM, et al. Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science 2007;316:1212–1216. [DOI] [PubMed] [Google Scholar]
- 25. Goncalves MB, Suetterlin P, Yip P, et al. A diacylglycerol lipase‐CB2 cannabinoid pathway regulates adult subventricular zone neurogenesis in an age‐dependent manner. Mol Cell Neurosci 2008;38:526–536. [DOI] [PubMed] [Google Scholar]
- 26. Skaper SD, Facci L, Fusco M, et al. Palmitoylethanolamide, a naturally occurring disease‐modifying agent in neuropathic pain. Inflammopharmacology 2014;22:79–94. [DOI] [PubMed] [Google Scholar]
- 27. Mattace Raso G, Russo R, Calignano A, Meli R. Palmitoylethanolamide in CNS health and disease. Pharmacol Res 2014;86:32–41. [DOI] [PubMed] [Google Scholar]
- 28. Guo DJ, Li F, Yu PH, Chan SW. Neuroprotective effects of luteolin against apoptosis induced by 6‐hydroxydopamine on rat pheochromocytoma PC12 cells. Pharm Biol 2013;51:190–196. [DOI] [PubMed] [Google Scholar]
- 29. Guerra‐Araiza C, Alvarez‐Mejia AL, Sanchez‐Torres S, et al. Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic Res 2013;47:451–462. [DOI] [PubMed] [Google Scholar]
- 30. Shimoi K, Masuda S, Furugori M, Esaki S, Kinae N. Radioprotective effect of antioxidative flavonoids in gamma‐ray irradiated mice. Carcinogenesis 1994;15:2669–2672. [DOI] [PubMed] [Google Scholar]
- 31. Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets 2008;8:634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu B, Li XX, He GR, et al. Luteolin promotes long‐term potentiation and improves cognitive functions in chronic cerebral hypoperfused rats. Eur J Pharmacol 2010;627:99–105. [DOI] [PubMed] [Google Scholar]
- 33. Coleta M, Campos MG, Cotrim MD, Lima TC, Cunha AP. Assessment of luteolin (3′,4′,5,7‐tetrahydroxyflavone) neuropharmacological activity. Behav Brain Res 2008;189:75–82. [DOI] [PubMed] [Google Scholar]
- 34. Crupi R, Paterniti I, Ahmad A, Campolo M, Esposito E, Cuzzocrea S. Effects of palmitoylethanolamide and luteolin in an animal model of anxiety/depression. CNS Neurol Disord Drug Targets 2013;12:989–1001. [DOI] [PubMed] [Google Scholar]
- 35. Taliou A, Zintzaras E, Lykouras L, Francis K. An open‐label pilot study of a formulation containing the anti‐inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther 2013;35:592–602. [DOI] [PubMed] [Google Scholar]
- 36. Theoharides TC, Asadi S, Panagiotidou S. A case series of a luteolin formulation (NeuroProtek(R)) in children with autism spectrum disorders. Int J Immunopathol Pharmacol 2012;25:317–323. [DOI] [PubMed] [Google Scholar]
- 37. Mabunga DF, Gonzales EL, Kim JW, Kim KC, Shin CY. Exploring the validity of valproic acid animal model of autism. Exp Neurobiol 2015;24:285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Impellizzeri D, Bruschetta G, Cordaro M, et al. Micronized/ultramicronized palmitoylethanolamide displays superior oral efficacy compared to nonmicronized palmitoylethanolamide in a rat model of inflammatory pain. J Neuroinflammation 2014;11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crawley JN. Designing mouse behavioral tasks relevant to autistic‐like behaviors. Ment Retard Dev Disabil Res Rev 2004;10:248–258. [DOI] [PubMed] [Google Scholar]
- 40. Kim KC, Kim P, Go HS, et al. Male‐specific alteration in excitatory post‐synaptic development and social interaction in pre‐natal valproic acid exposure model of autism spectrum disorder. J Neurochem 2013;124:832–843. [DOI] [PubMed] [Google Scholar]
- 41. Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 2000;20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Velazquez‐Zamora DA, Gonzalez‐Ramirez MM, Beas‐Zarate C, Gonzalez‐Burgos I. Egocentric working memory impairment and dendritic spine plastic changes in prefrontal neurons after NMDA receptor blockade in rats. Brain Res 2011;1402:101–108. [DOI] [PubMed] [Google Scholar]
- 43. Bolte S, Feineis‐Matthews S, Leber S, Dierks T, Hubl D, Poustka F. The development and evaluation of a computer‐based program to test and to teach the recognition of facial affect. Int J Circumpolar Health 2002;61:61–68. [DOI] [PubMed] [Google Scholar]
- 44. Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult‐born hippocampal granule cells. J Neurosci 2008;28:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH. Fetal valproate syndrome and autism: Additional evidence of an association. Dev Med Child Neurol 2001;43:202–206. [PubMed] [Google Scholar]
- 46. Rodier PM, Ingram JL, Tisdale B, Croog VJ. Linking etiologies in humans and animal models: Studies of autism. Reprod Toxicol 1997;11:417–422. [DOI] [PubMed] [Google Scholar]
- 47. Wagner GC, Reuhl KR, Cheh M, McRae P, Halladay AK. A new neurobehavioral model of autism in mice: Pre‐ and postnatal exposure to sodium valproate. J Autism Dev Disord 2006;36:779–793. [DOI] [PubMed] [Google Scholar]
- 48. Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology 2008;33:901–912. [DOI] [PubMed] [Google Scholar]
- 49. Rice D, Barone S Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect 2000;108:511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murray ML, Hsia Y, Glaser K, et al. Pharmacological treatments prescribed to people with autism spectrum disorder (ASD) in primary health care. Psychopharmacology 2014;231:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Millward C, Ferriter M, Calver S, Connell‐Jones G. Gluten‐ and casein‐free diets for autistic spectrum disorder. Cochrane Database Syst Rev 2008;2:CD003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dlugos A, Childs E, Stuhr KL, Hillard CJ, de Wit H. Acute stress increases circulating anandamide and other N‐acylethanolamines in healthy humans. Neuropsychopharmacology 2012;37:2416–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Skaper SD, Facci L. Mast cell‐glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos Trans R Soc Lond B Biol Sci 2012;367:3312–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Butti E, Bacigaluppi M, Rossi S, et al. Subventricular zone neural progenitors protect striatal neurons from glutamatergic excitotoxicity. Brain 2012;135(Pt 11):3320–3335. [DOI] [PubMed] [Google Scholar]
- 55. Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, Everall IP. Abnormal microglial‐neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res 2012;1456:72–81. [DOI] [PubMed] [Google Scholar]
- 56. Siracusa R, Paterniti I, Impellizzeri D, et al. The association of palmitoylethanolamide with luteolin decreases neuroinflammation and stimulates autophagy in Parkinson's disease model. CNS Neurol Disord Drug Targets 2015;14:1350–1365. [DOI] [PubMed] [Google Scholar]
- 57. Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience 2007;147:867–883. [DOI] [PubMed] [Google Scholar]
- 58. Aloisi F. Immune function of microglia. Glia 2001;36:165–179. [DOI] [PubMed] [Google Scholar]
- 59. Antonucci N, Cirillo A, Siniscalco D. Beneficial effects of palmitoylethanolamide on expressive language, cognition, and behaviors in autism: A report of two cases. Case Rep Psychiatry 2015;2015:325061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Solorzano C, Zhu C, Battista N, et al. Selective N‐acylethanolamine‐hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc Natl Acad Sci U S A 2009;106:20966–20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Schematic representation depicting the experimental design.
Data S1. Supporting informations.


