Summary
Background and Aims
EGFRvIII is the most prevalent glioblastoma mutation, occurring in more than 25% of glioblastomas. EGFRvIII cells release microvesicles that contain proteins, miRNAs, and mRNAs that enhance the growth and survival of surrounding tumor cells. However, little is known about the maturation process and regulatory mechanisms of secreted vesicles in EGFRvIII cells.
Methods
Signal peptide peptidase (SPP) provides a fascinating mechanism for protein cleavage and subsequent dislocation in the endoplasmic reticulum transmembrane domain.
Results
In this study, we reported that SPP facilitates the secretion of cytokines in vitro and promotes tumor progression in mice. Human cytokine antibody arrays revealed that EGFRvIII secreted higher levels of cytokines, but these levels were significantly reduced following SPP knockdown, suggesting that cytokines in EGFRvIII secretion profiles play important roles in GBM development. Identical results were confirmed in intracellular maturation tracking of TGF‐β1 in mouse serum. Clinically, analyses of GBM patient data from the database revealed that HM13 expression was closely related to patient prognosis and survival, suggesting an influence by the secreted vesicles of EGFRvIII tumor cells.
Conclusions
Collectively, our study identifies that SPP affects EGFRvIII secretion profiles and thus promotes tumor progression, providing further understanding of the formation of secreted vesicles and driving role of EGFRvIII in GBM.
Keywords: SPP, EGFRvIII, Secreted vesicles, TGF‐β1, Glioblastoma
Introduction
Gene amplification and DNA rearrangement are frequently observed in GBM 1. In particular, nearly 60% of GBMs are accompanied by epidermal growth factor receptor (EGFR) amplification 2, 3, and approximately one‐third of GBM contains the EGFRvIII mutation, which is the most common EGFR mutation in GBM 4. Due to the genomic deletion of exons 2–7 3, EGFRvIII has a greatly truncated extracellular domain 5 and therefore is constitutively activated, promoting tumor progression by inducing oncogenic transformation of adjacent cells through paracrine paths 6 as well as through the secretion of microvesicles derived from EGFRvIII tumor cells 7. However, the molecular mechanisms of secreted vesicles in EGFRvIII cells have largely remained a mystery, and it is therefore important to elucidate how secreted vesicles in EGFRvIII cells are regulated, with the goal of finding new points of intervention.
The signal peptide peptidase (SPP), encoded by HM13, is a member of the intramembrane cleaving proteases involved in leader peptide remnant release from the endoplasmic reticulum membrane 8. There is increasing evidence that SPP plays important roles in cell signaling 9, surveillance 10, regulation 11, cell intracellular communication 12, and cancer 13, 14. Here, we show that SPP contributes to GBM progression by affecting EGFRvIII secretion profiles. Experimentally, in mouse blood, there was significantly decreased expression of TGF‐β1 in SPP knockdown groups. Coupled with the results of cytokine assays and TGF‐β1 tracing in vitro, this study shows that the signal peptide peptidase may be a critical trigger point in regulating EGFRVIII secretion profiles for GBM.
Materials and Methods
Human Specimens and Cell Culture
Human tumor samples were obtained from patients undergoing surgical resection at TianTan Hospital (Beijing, China). All patients provided written informed consent, and all human experiments were approved by the Ethics Committee of TianTan Hospital. Human GBM cancer cell lines (U87, LN229, and U251) were purchased from the American Type Culture Collection. U87‐EGFRvIII (a GBM cell line carrying mutant EGFR) was a kind gift from Harbin Medical University (Professor. Ren Huan). U87‐EGFRvIII cells were maintained with G418 (Sigma, St. Louis, MO, USA) at a concentration of 400 mg/mL. All cells were incubated at 37°C with 5% CO2.
Cytokine Array
The profiles of cytokines secreted by GBM cancer cells were detected in the culture supernatants using a Human Cytokine Array (RayBiotech, Guangzhou, China) according to the manufacturer's instructions. The cytokines with distinct differences in expression were screened out.
In Vitro Assays
For siRNA transfection, GBM cancer cells were plated at 2 × 105 cells/mL in serum‐free media and transfected with siRNA duplexes using Lipofectamine 3000 Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. The siRNA sequences: 5′‐TGCCTGAAACAATCACCAGC‐3′ (siRNA‐1), 5′‐ GTCCATGTATTTCTTCGTGC‐3′ (siRNA‐2), and 5′‐ GCAGGAGGTTGATGTACTCC‐3′ (siRNA‐3).
For lentivirus preparation and in vitro infection, lentiviral vectors expressing nonsense control (Lenti‐NC) and Lenti‐siHM13 (5′‐TGCCTGAAACAATCACCAGC‐3′) were all generated by GenePharma (Shanghai, China). Cell infections were carried out according to GenePharma's recommendations.
For real‐time PCR assay, real‐time PCR (RT‐PCR) was performed according to the manufacturers’ instructions as previously described 15.
For ELISA, TGF‐β1 and TGF‐α ELISA kits were purchased from R&D Systems and used according to the manufacturer's instructions, and the samples were subsequently analyzed by a Microplate Reader at 450 nm.
In Vivo Assays
For the orthotopic nude mouse model, 4‐week‐old BALB/c‐A nude mice were purchased from Chinese Academy of Medical Science (Beijing, China) Animal Center and housed in pathogen‐free conditions. The intracranial tumor models were established as previously described 16. Briefly, 5 × 105 GBM cells infected with Lenti‐siHM13 were stereotactically injected into mouse brains using cranial guide screws. After survival observation, mice were sacrificed, and the brains were extracted for hematoxylin–eosin (H&E) and immunohistochemical (IHC) staining.
For immunohistochemical assays, tumor samples embedded in paraffin were sectioned at a thickness of 3 μm. Antigen retrieval was conducted using a pressure cooker for 25 min in 0.01 M citrate buffer. Tissue slices were incubated with antibodies overnight at 4°C. Cells were incubated with DAB for immunostaining for approximately 1 min according to the manufacturer's instructions and then counterstained with hematoxylin and viewed under a microscope at 400× magnification for evaluation.
Immunofluorescence and Microscopy
Immunofluorescence staining was performed using primary antibodies against smad2, p‐smad2, smad3, and p‐smad3 (1:100 dilutions, Cell Signaling Technology, Danvers, MA, USA), followed by 1 h of incubation with Alexa Fluor 488 donkey anti‐mouse IgG (H+L) and Alexa Fluor 594 goat anti‐rabbit IgG (H+L) (Molecular Probes, Life Technology, Eugene, OR, USA) at room temperature. All fluorescent fields were visualized using an FV‐1200 laser scanning confocal microscope.
Data Analysis
All experimental data are expressed as the mean ± SD. The t‐test was used for comparison between two groups, and one‐way ANOVA was used to determine significance among multiple groups. Statistical significance was determined at *P < 0.05, **P < 0.01 or ***P < 0.001.
Results
HM13 is Upregulated in High‐Grade Gliomas
We analyzed glioma samples from patients with different grades in the CGGA cohort. Among the 301 samples, 173 were diagnosed as low‐grade gliomas (WHO grade II and grade III), and 128 were diagnosed as high‐grade gliomas (WHO grade IV). The expression levels of HM13 are increased in high‐grade gliomas (HGG) compared to low‐grade gliomas (LGG). To avoid deviation resulting from monocentric data, we also analyzed different grades samples from TCGA, REMBRANDT, and GSE16011 (Table 1). We confirmed that HM13 was significantly overexpressed in HGG samples compared to LGG samples (Figure 1A). To determine whether HM13 expression levels determine the clinical progression or outcome of glioma patients, we examined the overall survival rates of the glioma patients (samples from CGGA, REMBRANDT, and GSE16011) using Kaplan–Maier analysis and log‐rank comparison. The results showed that high HM13 expression was associated with a decreased survival period in patients (Figure 1B). Furthermore, we analyzed the HM13 expression in EGFRvIII and non‐EGFRvIII patients in the CGGA database. The results showed that HM13 was overexpressed in EGFRvIII samples compared to non‐EGFRvIII samples (Figure 1C).
Table 1.
Cox regression analysis for the survival of patients in multiple datasets
| Univariate cox model | Multivariate cox model | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| CGGA | ||||||
| Age at diagnosis | 1.014 | 0.994–1.035 | 0.169 | |||
| Gender | 1.171 | 0.720–1.902 | 0.525 | |||
| IDH status | 0.54 | 0.254–1.145 | 0.108 | |||
| HM13 Expression | 1.812 | 1.131–2.904 | 0.013 | |||
| Rembrandt | ||||||
| Gender | 1.027 | 0.710–1.486 | 0.899 | |||
| HM13 Expression | 1.412 | 1.048–1.902 | 0.024 | |||
| GSE16011 | ||||||
| Age at diagnosis | 2.442 | 1.731–3.445 | 3.70E‐07 | 2.321 | 1.533–3.515 | 6.95E‐05 |
| Gender | 1.012 | 0.715–1.433 | 0.944 | |||
| IDH status | 0.581 | 0.368–0.917 | 0.02 | 0.823 | 0.503–1.346 | 0.438 |
| HM13 Expression | 1.469 | 1.056–2.042 | 0.022 | 1.359 | 0.921–2.004 | 0.122 |
| TCGA | ||||||
| Age at diagnosis | 1.021 | 1.009–1.033 | 0.001 | 1.021 | 1.010–1.033 | 3.58E‐04 |
| Gender | 0.853 | 0.657–1.109 | 0.235 | |||
| IDH status | 0.598 | 0.478–0.749 | 7.85E‐06 | 0.65 | 0.514–0.821 | 3.06E‐04 |
| HM13 Expression | 1.974 | 1.366–2.852 | 2.95E‐04 | 1.889 | 1.297–2.750 | 0.001 |
Figure 1.
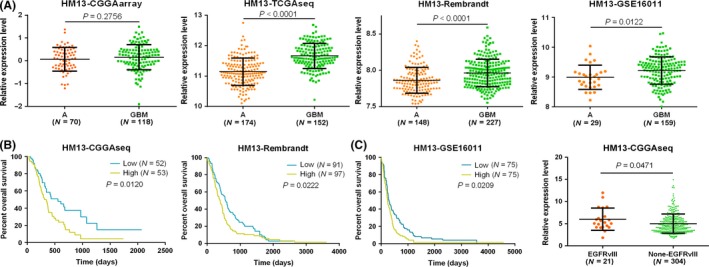
HM13 mRNA expression is increased in GBM and EGFRvIII cases, and high expression of HM13 is associated with poor prognoses in gliomas. (A) The expression levels of HM13 were analyzed in glioma tissues of the CGGA, TCGA, REMBRANDT, and GSE16011 glioma datasets. (B) Kaplan–Meier survival curve analysis of the CGGA, GSE16011, and REMBRANDT glioma datasets indicated that patients with lower HM13 expression showed prolonged survival compared to patients with high levels. (C) HM13 mRNA expression is increased in EGFRvIII cases than in non‐EGFRvIII cases.
HM13 Expression is Associated with Tumor Grade in Human Glioma Specimens
Our results showed that HM13 was closely related to tumor grade and patient survival in glioma patients. To further explore the role of the HM13 gene in gliomas, we first measured the expression of HM13 protein in 63 glioma specimens of different grades. Western blot and immunohistochemistry results showed that the protein expression of HM13 was dramatically upregulated with increased grade (Figure 2A,B). Additionally, the mRNA expression of HM13 was assayed by qPCR. According to the results of the experiment, the expression of HM13 mRNA was increased in HGG specimens compared to lower‐grade gliomas, but this difference was not statistically significant between grade II and grade III (Figure 2C).
Figure 2.
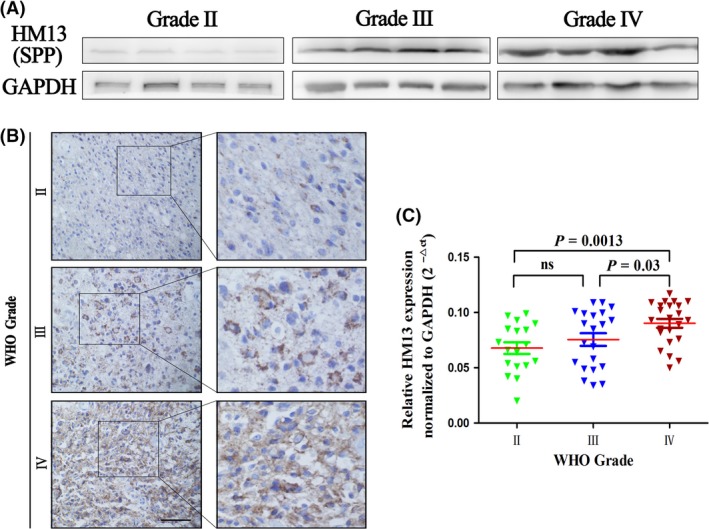
The expression of SPP (HM13) is increased in high‐grade glioma. (A) Western blot analysis was used to detect the expression of SPP in different grade glioma samples. GAPDH was used as a loading control. (B) Immunohistochemistry analysis was used to examine the expression of SPP in different grade glioma samples. (C) Compared with glioma tissues of WHO grade II (n = 20) and grade III (n = 22), GBM (n = 21) showed higher HM13 expression as determined by qPCR. ns, no significant difference.
Signal Peptide Peptidase (HM 13) Knockdown Abrogates the Cytokines Production from EGFRvIII Cancer Cells
To identify the cytokines secreted by EGFRvIII cancer cells that enhance tumorigenic ability, the cytokine profiles of cell supernatants from U87, U87EGFRvIII, and U87EGFRvIII infected with Lenti‐siHM13 were analyzed using a Human Cytokine Antibody Array from RayBiotech. We first selected the most effective small interfering RNA for knocking down HM13 using the transfection reagent Lipofectamine 3000 (Figure 3A). The expression of four cytokines (TGF‐β1, TGF‐α, BMP‐7, and insulin) was significantly increased in the supernatant of U87EGFRvIII cells compared with U87 cells and decreased dramatically when HM13 was knocked down (Figure 3B). ELISA assays further confirmed the increase in expression of the four cytokines in the supernatant of U87EGFRvIII cells and subsequent reduction when treated with Lenti‐siHM13 (data not shown). Furthermore, the expression of other cytokines substantially increased (Figure 3C) in the supernatant of U87EGFRvIII cells compared with U87 cells and decreased sharply (Figure 3D) after treatment with Lenti‐siHM13, indicating that there are distinct differences between U87EGFRvIII and U87 secretion profiles and that SPP plays an important role in regulating EGFRvIII cancer cell secretion profiles.
Figure 3.
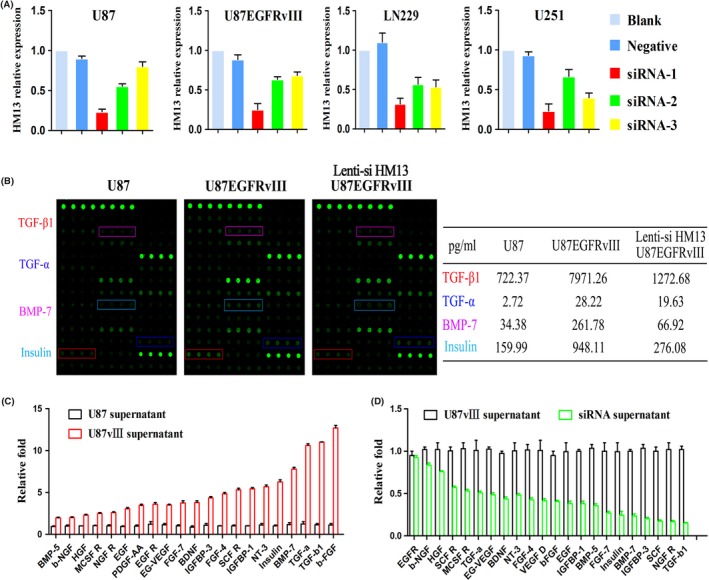
HM13 knockdown affects EGFRvIII secretion profiles in glioblastoma. (A) HM13 relative expression in different GBM cell lines when treated with siRNAs. (B) Cytokine array of the supernatant of U87, U87EGFRvIII, and siRNA‐treated U87EGFRvIII. A table summarizing the relative signal intensity of indicated cytokines is presented on the right. (C) The relative expression of U87EGFRvIII supernatant cytokines compared with U87. (D) The relative expression of U87EGFRvIII supernatant cytokines compared with siRNA‐treated U87EGFRvIII.
HM13 Knockdown Regulates TGF‐β1 Secretion in EGFRvIII Cancer Cells and Affects Surrounding Cell Biological Behaviors through the TGF‐β Pathway In Vitro
We have demonstrated that HM13 knockdown affects cytokines in EGFRvIII secretion profiles, which is often associated with tumor biological behaviors; thus, we performed a reporter assay to evaluate the TGF‐β pathway activity when cells were cultured with the supernatants of differently treated U87‐EGFRvIII cells (Figure 4A). We collected the supernatants from U87‐EGFRvIII cancer cells treated with Lenti‐NC or Lenti‐siHM13, added the supernatant samples to U87 cells and then extracted the nuclei, cytoplasm, and total proteins of the cells for Western blot (Figure 4B). We also performed immunofluorescent staining on these cells (Figure 4C). Both results indicated that when cultured with supernatant derived from U87EGFRvIII cancer cells infected by Lenti‐siHM13, cells showed a clear decrease in nuclear localization of p‐smad2 and p‐smad3, which are essential for TGF‐β pathway activation.
Figure 4.
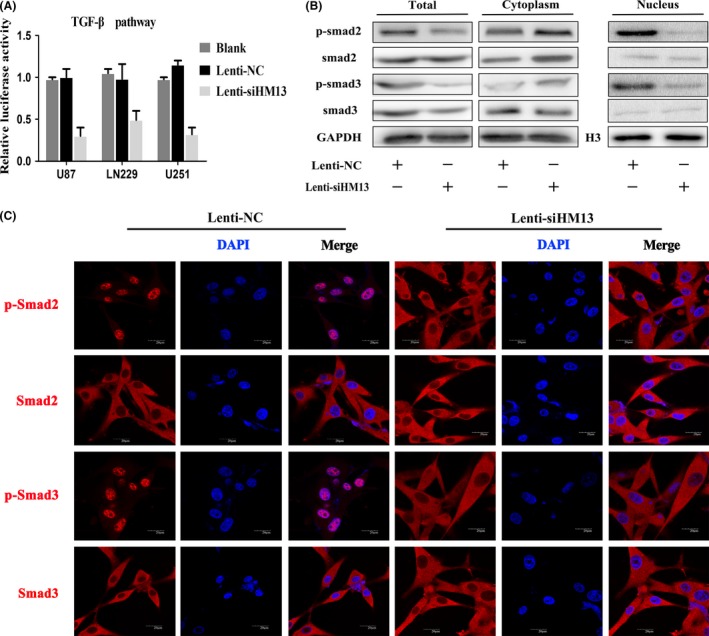
The supernatant of U87EGFRvIII when HM13 knockdown regulated the activity of the TGF‐β signaling pathway in vitro. (A) Luciferase reporter assay indicated that the supernatant of U87EGFRvIII when HM13 was knocked down inhibited TGF‐β transcriptional activity in different GBM cells (P < 0.01). (B) Western blot results showed the total, cytoplasm, and nuclear expression of smad2, p‐smad2, smad3, and p‐smad3 in U87 cells when cultured with supernatant of U87EGFRvIII infected with Lenti‐NC or Lenti‐siHM13. GAPDH or H3 was used as a loading control. (C) Confocal microscopy showed results consistent with Western blot. Compared with Lenti‐siHM13 cultured cells, Lenti‐NC‐treated cells exhibited higher p‐smad2 and p‐smad3 expression in the nucleus (magnification: 1000×).
To further validate these findings, we designed a pre‐TGF‐β1 lentivirus with red fluorescent protein for tracking the transportation process of TGF‐β1 (Figure 5C). We found that EGFRvIII cells, when treated with Lenti‐siHM13, exhibited an obvious accumulation of pre‐TGF‐β1 and a clear decrease in expression of polymerase I and transcript release factor (PTRF) (Figure 5D), suggesting that SPP knockdown greatly influenced the maturation of secreted proteins in EGFRvIII cancer cells and subsequently affected the biological behaviors of surrounding cells.
Figure 5.

The supernatant of U87EGFRvIII when HM13 was knocked down inhibits invasion of GBM cells via the suppression of TGF‐β1 secretion. (A) Representative images and histograms (B) of the Transwell assay, which was used to examine the invasive ability of U87, LN229, and U251 cell lines after culture with the supernatant of U87EGFRvIII infected with Lenti‐siHM13, TGF‐β1 neutralizing antibody, or Lenti‐NC. (C) The flowchart of the TGF‐β1 synthesis and transportation process in cells. (D) The pre‐TGF‐β1 and PTRF expression at different time points and subcellular locations were confirmed by confocal microscopy (magnification: 1000×).
To determine whether our findings are relevant to biological behaviors, we collected the supernatant of U87EGFRvIII cells treated with Lenti‐siHM13, TGF‐β1 neutralizing antibody, and Lenti‐NC and then cultured different glioma cell lines with these supernatant samples. Transwell (Figure 5A,B) results showed that cells cultured using the supernatant of U87EGFRvIII treated with Lenti‐siHM13 had a significantly reduced invasiveness ability.
HM13 Knockdown in U87EGFRvIII Cancer Cells Improved Mice Survival and Inhibited Orthotopic Tumor Growth
To further confirm the stimulative role of HM13 and explore the therapeutic potential of depleting HM13, we established orthotopic mouse models using U87EGFRvIII cancer cells infected with Lenti‐siHM13 or Lenti‐NC. Engraftment of the treated U87EGFRvIII cancer cells into the mice resulted in completely different survival times (Figure 6A) and tumor volumes (Figure 6C). These results indicated that groups treated with Lenti‐siHM13 had a longer survival time and smaller tumor volume. Furthermore, no metastatic foci were observed compared with Lenti‐NC groups.
Figure 6.
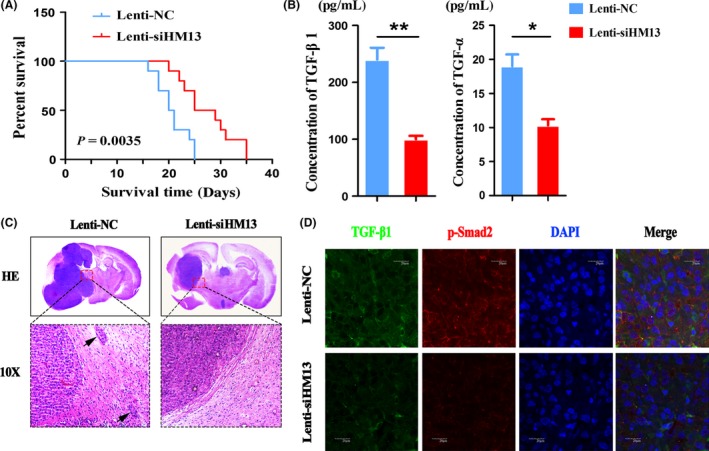
Lenti‐siHM13 inhibited tumorigenesis and invasiveness in a U87EGFRvIII orthotopic model in vivo. (A) Overall animal survival was assessed by Kaplan–Meier survival curves in the two groups. (B) Detection of TGF‐β1 and TGF‐α by ELISA in blood derived from mice bearing tumors. (C) The tissue slices from representative tumors in each group were stained with hematoxylin–eosin–saffron; the small black arrow indicates tumor metastasis. (D) TGF‐β1 and p‐smad2 expression were confirmed using confocal microscopy (1000×). The scale bar corresponds to 20 μm. Error bars represent the means ± SD obtained from three independent experiments.
To further verify the effect of HM13 knockdown on EGFRvIII secretion profiles, we collected mice blood from the two groups and detected the concentration of cytokines (TGF‐β1 and TGF‐α) using ELISA. Consistent with the findings in vitro, the two cytokines exhibited a statistically significant reduction in expression compared with the control animals (Figure 6B).
Furthermore, confocal microscopy showed the decreased expression of TGF‐β1 and p‐smad2 in animal tissue sections when treated with Lenti‐siHM13 (Figure 6D). Altogether, these mouse experiments demonstrate that HM13 knockdown inhibits tumor progression in vivo.
Discussion
Approximately 3–5% of all membrane proteins are tail‐anchored proteins 17, and signal peptide peptidase (SPP), which catalyzes the intramembrane cleavage of signal sequence stubs left in the ER membrane after signal peptidase (SP)–mediated processing of the precursor protein, plays an important role in the maturation of these proteins 18, 19. Previously, we had identified that specifically inhibiting the activity of signal peptidase could suppress tumor growth in an EGFRvIII/wt heterogenic model by abrogating the secretion of progrowth factors from EGFRvIII cells 20. Here, we analyzed the relative gene expression and survival period from TCGA, CGGA, REMBRANDT, and GSE16011 databases, knocked down HM13 gene, and established an intracranial model in mice to investigate the effect of HM13 on EGFRvIII secretion profiles and tumor progression (Figure 7). HM13 was required for dislocation of TGF‐β1 from the endoplasmic reticulum, which is consistent with the findings of Hidde L. Ploegh et al. 8. Therefore, our results suggest that signal peptide peptidase (HM13) may be a potential diagnostic biomarker and therapeutic target for glioblastoma.
Figure 7.
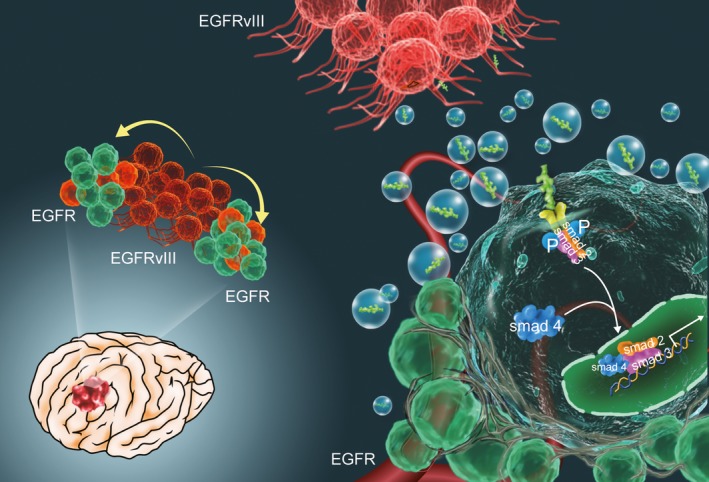
The mechanism diagram of secreted cytokines from EGFRvIII cells. EGFRvIII cells in GBM promote tumorigenesis and tumor metastasis via secreted proteins, which have been involved in biological behavior changes of surrounding cells by regulating the TGF‐β signaling pathway.
It has been shown that secretory vesicles can transfer some of their contents to other cell types 21, 22, 23, act to promote tumor growth and invasiveness, and suppress the immune response 24, 25, 26, 27. SPP is implicated in the clearance of signal peptides of secreted proteins as well as misfolded membrane proteins 28, 29. In our experiments, we tracked the TGF‐β1 expression at different time points and under different treatment conditions. The results showed that the expression of TGF‐β1 varied with time and knockdown treatment. Interestingly, the expression of PTRF was also changed, and presumably, this phenomenon is related to RNA interference. Furthermore, when HM13 was knocked down, the activity of the TGF‐β signaling pathway was obviously affected, as was the biological behavior of tumor cells, especially concerning cell invasive behavior. Therefore, we considered that signal peptide peptidase could facilitate the growth of surrounding tumor cells by affecting the expression of secretory proteins and promoting the progression of the entire tumor.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1. HM13 relative expression is associated to the subtype of samples from the CGGA, Rambrandt, GSE16011 and TCGA datasets using the PAM analysis.
Figure S2. HM13 knockdown decreased the cell proliferation capacity and viability.
Figure S3. HM13 knockdown abrogated the TGF‐β1 and TGF‐α secretion in patient‐derived neurospheres.
Figure S4. The expression of TGF‐β1, p‐smad2 and smad2 in mice tissue sections by confocal microscopy.
Video S1. Animation diagram showed the transportation of secreted protein in ER.
Acknowledgments
This work was supported by the National High Technology Research and Development Program 863 (NO. 2014AA021102), the National Key Research and Development Program (NO. 2016YFC0902502). The authors thank Wang qixue and Wang yunfei (Tianjin Medical University General Hospital) for their excellent technical assistance in the confocal microscopy imaging assay.
The first two authors contributed equally to this work.
References
- 1. Wong AJ, Bigner SH, Bigner DD, et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci USA 1987;84:6899–6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell 2013;155:462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furnari FB, Cloughesy TF, Cavenee WK, Mischel PS. Heterogeneity of epidermal growth factor receptor signalling networks in glioblastoma. Nat Rev Cancer 2015;15:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res 2005;11:1462–1466. [DOI] [PubMed] [Google Scholar]
- 5. Wikstrand CJ, Reist CJ, Archer GE, Zalutsky MR, Bigner DD. The class III variant of the epidermal growth factor receptor (EGFRvIII): Characterization and utilization as an immunotherapeutic target. J Neurovirol 1998;4:148–158. [DOI] [PubMed] [Google Scholar]
- 6. Inda MM, Bonavia R, Mukasa A, et al. Tumor heterogeneity is an active process maintained by a mutant EGFR‐induced cytokine circuit in glioblastoma. Genes Dev 2010;24:1731–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al‐Nedawi K, Meehan B, Micallef J, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 2008;10:619–624. [DOI] [PubMed] [Google Scholar]
- 8. Loureiro J, Lilley BN, Spooner E, et al. Signal peptide peptidase is required for dislocation from the endoplasmic reticulum. Nature 2006;441:894–897. [DOI] [PubMed] [Google Scholar]
- 9. Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: A control mechanism conserved from bacteria to humans. Cell 2000;100:391–398. [DOI] [PubMed] [Google Scholar]
- 10. Lemberg MK, Bland FA, Weihofen A, Braud VM, Martoglio B. Intramembrane proteolysis of signal peptides: An essential step in the generation of HLA‐E epitopes. J Immunol 2001;167:6441–6446. [DOI] [PubMed] [Google Scholar]
- 11. Mumm JS, Kopan R. Notch signaling: From the outside in. Dev Biol 2000;228:151–165. [DOI] [PubMed] [Google Scholar]
- 12. Urban S, Lee JR, Freeman M. Drosophila rhomboid‐1 defines a family of putative intramembrane serine proteases. Cell 2001;107:173–182. [DOI] [PubMed] [Google Scholar]
- 13. Sun Y, Lowther W, Kato K, et al. Notch4 intracellular domain binding to Smad3 and inhibition of the TGF‐beta signaling. Oncogene 2005;24:5365–5374. [DOI] [PubMed] [Google Scholar]
- 14. van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma‐secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005;435:959–963. [DOI] [PubMed] [Google Scholar]
- 15. Wang Q, Zhang J, Liu Y, et al. A novel cell cycle‐associated lncRNA, HOXA11‐AS, is transcribed from the 5‐prime end of the HOXA transcript and is a biomarker of progression in glioma. Cancer Lett 2016;373:251–259. [DOI] [PubMed] [Google Scholar]
- 16. Shi Z, Zhang J, Qian X, et al. AC1MMYR2, an inhibitor of dicer‐mediated biogenesis of Oncomir miR‐21, reverses epithelial‐mesenchymal transition and suppresses tumor growth and progression. Cancer Res 2013;73:5519–5531. [DOI] [PubMed] [Google Scholar]
- 17. Kalbfleisch T, Cambon A, Wattenberg BW. A bioinformatics approach to identifying tail‐anchored proteins in the human genome. Traffic 2007;8:1687–1694. [DOI] [PubMed] [Google Scholar]
- 18. Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B. Identification of signal peptide peptidase, a presenilin‐type aspartic protease. Science 2002;296:2215–2218. [DOI] [PubMed] [Google Scholar]
- 19. Martoglio B. Intramembrane proteolysis and post‐targeting functions of signal peptides. Biochem Soc Trans 2003;31:1243–1247. [DOI] [PubMed] [Google Scholar]
- 20. Wei JW, Cui JQ, Zhou X, et al. F25P preproinsulin abrogates the secretion of pro‐growth factors from EGFRvIII cells and suppresses tumor growth in an EGFRvIII/wt heterogenic model. Cancer Lett 2016;380:1–9. [DOI] [PubMed] [Google Scholar]
- 21. Mack M, Kleinschmidt A, Bruhl H, et al. Transfer of the chemokine receptor CCR5 between cells by membrane‐derived microparticles: A mechanism for cellular human immunodeficiency virus 1 infection. Nat Med 2000;6:769–775. [DOI] [PubMed] [Google Scholar]
- 22. Valadi H, Ekstrom K, Bossios A, et al. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654–659. [DOI] [PubMed] [Google Scholar]
- 23. Baj‐Krzyworzeka M, Szatanek R, Weglarczyk K, et al. Tumour‐derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol Immunother 2006;55:808–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wieckowski E, Whiteside TL. Human tumor‐derived vs dendritic cell‐derived exosomes have distinct biologic roles and molecular profiles. Immunol Res 2006;36:247–254. [DOI] [PubMed] [Google Scholar]
- 25. Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor‐derived exosomes selectively impair lymphocyte responses to interleukin‐2. Cancer Res 2007;67:7458–7466. [DOI] [PubMed] [Google Scholar]
- 26. Ginestra A, La Placa MD, Saladino F, et al. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res 1998;18:3433–3437. [PubMed] [Google Scholar]
- 27. Liu C, Yu S, Zinn K, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol 2006;176:1375–1385. [DOI] [PubMed] [Google Scholar]
- 28. Weihofen A, Lemberg MK, Ploegh HL, Bogyo M, Martoglio B. Release of signal peptide fragments into the cytosol requires cleavage in the transmembrane region by a protease activity that is specifically blocked by a novel cysteine protease inhibitor. J Biol Chem 2000;275:30951–30956. [DOI] [PubMed] [Google Scholar]
- 29. Crawshaw SG, Martoglio B, Meacock SL, High S. A misassembled transmembrane domain of a polytopic protein associates with signal peptide peptidase. Biochem J 2004;384:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. HM13 relative expression is associated to the subtype of samples from the CGGA, Rambrandt, GSE16011 and TCGA datasets using the PAM analysis.
Figure S2. HM13 knockdown decreased the cell proliferation capacity and viability.
Figure S3. HM13 knockdown abrogated the TGF‐β1 and TGF‐α secretion in patient‐derived neurospheres.
Figure S4. The expression of TGF‐β1, p‐smad2 and smad2 in mice tissue sections by confocal microscopy.
Video S1. Animation diagram showed the transportation of secreted protein in ER.


