Summary
Introduction
Apolipoprotein E4 (APOE4) is a major genetic risk factor for late‐onset sporadic Alzheimer disease. Emerging evidence demonstrates a hippocampus‐associated learning and memory deficit in aged APOE4 human carriers and also in aged mice carrying human APOE4 gene. This suggests that either exogenous APOE4 or endogenous APOE4 alters the cognitive profile and hippocampal structure and function. However, little is known regarding how Apoe4 modulates hippocampal dendritic morphology, synaptic function, and neural network activity in young mice.
Aim
In this study, we compared hippocampal dendritic and spine morphology and synaptic function of young (4 months) mice with transgenic expression of the human APOE4 and APOE3 genes.
Methods
Hippocampal dendritic and spine morphology and synaptic function were assessed by neuronal imaging and electrophysiological approaches.
Results
Morphology results showed that shortened dendritic length and reduced spine density occurred at hippocampal CA1 neurons in Apoe4 mice compared to Apoe3 mice. Electrophysiological results demonstrated that in the hippocampal CA3‐CA1 synapses of young Apoe4 mice, basic synaptic transmission, and paired‐pulse facilitation were enhanced but long‐term potentiation and carbachol‐induced hippocampal theta oscillations were impaired compared to young Apoe3 mice. However, both Apoe genotypes responded similarly to persistent stimulations (4, 10, and 40 Hz for 4 seconds).
Conclusion
Our results suggest significant alterations in hippocampal dendritic structure and synaptic function in Apoe4 mice, even at an early age.
Keywords: apolipoprotein E4, dendrites, hippocampus, synaptic plasticity, theta oscillations
1. INTRODUCTION
Alzheimer disease (AD) is a neurodegenerative disorder characterized by progressive learning and memory deficits. Amyloid plaques, neurofibrillary tangles, and neocortical synaptic loss contribute to AD pathogenesis.1, 2, 3, 4, 5, 6 The advent of molecular imaging techniques and biomarker studies has demonstrated that in AD patients, pathologic changes occur a couple of decades earlier than the onset of clinical symptoms.7, 8, 9, 10, 11, 12, 13 It is well accepted that synaptic dysfunction and loss are early changes in AD pathogenesis.14, 15, 16 The synaptic abnormalities are particularly pronounced in the hippocampus, which underlies cognitive deficits in the early stage of developing AD.
Of the three alleles of human apolipoprotein E (APOE2, 3, and 4), APOE4 is the major genetic risk factor for late‐onset sporadic AD.17, 18, 19, 20, 21, 22 Accumulating evidence demonstrates that APOE4 is associated with increased deposition of amyloid‐β,23, 24 hyperphosphorylation of tau,25, 26 altered synaptic morphology,27, 28, 29 impaired neuronal plasticity,30, 31, 32, 33 and declining memory.34, 35 Either exogenous APOE4 or endogenous APOE4 alters the cognitive profile and hippocampal structures and functions, which underlies synaptic impairment and learning and memory deficits in AD. In a large study of 815 subjects with normal cognitive status (317 APOE4 carriers and 498 noncarriers), APOE4 carriers showed a longitudinal decline in memory that began before the age of 60 years, and they had greater acceleration in cognitive decline than noncarriers.36 In another study of healthy infant carriers and noncarriers of APOE4, infant APOE4 carriers had lower white matter myelin and gray matter volume in brain areas that are vulnerable in AD, such as the precuneus, posterior/middle cingulate, and lateral temporal. This indicates the impact of APOE4 at the earliest stage of brain development.37 APOE4 likely affects hippocampal structure and function.33 Both hippocampal synaptic morphology and hippocampus‐associated learning behavior alterations have been found in young (4‐month‐old) Apoe4 transgenic mice (Apoe4 mice).38, 39 Interestingly, these synaptic pathologic changes are associated with an accumulation of amyloid β42 and hyperphosphorylated tau and reduced levels of vesicular glutamate transporters in hippocampal neurons. Electrophysiological studies demonstrated the impairment of hippocampal synaptic plasticity (long‐term potentiation, LTP) in aged Apoe4 mice using in vitro hippocampal slices40 and in wild‐type mice with local injection of Apoe4.41 However, the alterations in synaptic functions in young Apoe mice remain unclear. Both enhancements42 and no change32 of hippocampal synaptic LTP have been reported in young Apoe4 mice compared to Apoe3 mice. Although 4‐month‐old Apoe4 mice have shown hippocampal synaptic morphological changes and hippocampus‐associated learning behavioral decline,38, 39, 43, 44 the hippocampal synaptic function in these young Apoe4 mice has not been studied. In the present study, we profiled hippocampal dendritic and spine morphology in Apoe4 and Apoe3 mice; evaluated the roles of Apoe4 in hippocampal synaptic function including synaptic transmission, paired‐pulse facilitation (PPF), and LTP; examined hippocampal theta oscillations and synaptic responses to sustained stimuli with different frequencies using 4‐month‐old Apoe4 mice; and compared these results to age‐matched Apoe3 mice. Our results demonstrate the alterations in hippocampal synaptic morphology, function, and neuronal network synchronization in young Apoe4 mice.
2. METHODS
All experimental protocols were approved by and performed in accordance with guidelines set by the animal care and use and ethical committees at the Barrow Neurological Institute, Hebei Medical University (Shijiazhuang, Hebei, China) and Shantou University Medical College (Shantou, Guangdong, China).
2.1. Hippocampal slice preparation
Hippocampal slices were prepared from 4‐ and 12‐month‐old male mice. Apoe4 mice were generated as described previously.45 Apoe3 mice were used as controls. Briefly, Apoe transgenic mice were generated using a microinjection of allele‐specific human E3 or E4 genomic fragments to establish founders. The founders were then bred to Apoe knockout mice lacking a functional mouse Apoe protein. These transgenic lines transcribe and express appropriate human APOE3 and APOE4 proteins in brain, liver, and other tissues without contamination of the endogenous mouse Apoe gene. Under isoflurane anesthesia, mice were sacrificed in accordance with Barrow Neurological Institute's Institutional Animal Welfare Committee guidelines, as described previously.46, 47, 48 Brain tissue was quickly removed and bathed in cold (4°C) artificial cerebrospinal fluid (ACSF) containing (in m mol L−1): NaCl, 119; KCl, 2.5; NaHCO3, 26; MgSO2, 1.3; NaH2PO4, 1.0; CaCl2, 2.5; and glucose, 11. The ACSF was continuously bubbled with 95% O2‐5% CO2 (carbogen). Several coronal sections (400 μm thick) containing the dorsal hippocampus were cut using a vibratome (Vibratome 1000 Plus, The Vibratome Company, St. Louis, MO, USA) and incubated at room temperature (21±1°C) for at least 60 minutes prior to recording. Thereafter, one slice was transferred to a liquid‐air interface chamber (Warner Instrument, LLC, Hamden, CT, USA) and suspended on nylon net in a bath of continuously dripping oxygenated ACSF (2.0 to 2.5 mL/min). Humidified carbogen was passed along the upper surface of the slice, and bath temperature was regulated by a feedback circuit accurate to 31 ± 0.5°C. Baseline temperature was 30±1°C.
2.2. Electrophysiological recordings
Standard field potential recordings were performed on the hippocampal CA1 region using borosilicate glass micropipettes pulled to a tip diameter of about 1 μm and filled with 2 mol L−1 NaCl.46, 47, 48 To record synaptic potentials, a recording electrode was placed at the CA1 apical dendrite region. Stimulus intensity was set based upon input‐output relationships and was 50% of the maximal response. For testing PPF, two stimuli with 50% of the maximal intensity were given at 15‐, 50‐, 100‐, and 400‐ms intervals. For recording LTP, stable baseline synaptic potentials (50% of the maximal intensity) were recorded for 20 minutes, and then a theta‐burst tetanic stimulation that contained 15 burst trains at 5 Hz was delivered (each train contained five pulses at 100 Hz). Thereafter, the baseline intensity‐evoked field excitatory postsynaptic potentials (fEPSPs) were recorded for 60 minutes with 0.33 Hz. A custom bipolar platinum wire electrode (0.08 mm diameter) was placed at the Schaffer collateral pathway, and stimulation was delivered using a Model 2100 A‐M Systems Isolated Pulse Stimulator (Carlsborg, WA, USA). All evoked responses were recorded using an Axoclamp‐2B amplifier, and data acquisition was controlled by pClamp 10.2 software (Molecular Devices, Sunnyvale, CA, USA).
2.3. CA1 neuron morphology quantification
In experiments where quantification of CA1 neuronal morphology was desired, we included 0.25% biocytin in a K+‐based internal electrode solution and dialyzed the neurons in acute slices for 10 minutes (500 pA current injection). The slices were then fixed in 4% paraformaldehyde overnight, permeabilized with 0.2% Triton X‐100, followed by a 48‐hours incubation with avidin‐Alexa Fluor 488 (Invitrogen, Carlsbad, CA, USA). The dendritic arbor was traced into a three‐dimensional structure using Neurolucida (MicroBrightField, Williston, VT, USA), as described previously.49 Neurons were coded for blinded analysis. Dendritic measures including branch number, pattern, and total dendritic length were analyzed using Neurolucida Explorer. In addition, Sholl analyses50 were performed in these reconstructed neurons to extract morphometric parameters on regional alterations in the dendritic arbor structure, including dendritic length, branching complexity, and branch point locations within a series of concentric circles (20 μm radius increment from soma) on the entire dendritic arbors. At least three mice were reconstructed for each genotype group.
For dendritic spine analysis, we used Imaris software (version 8.02, 64 bit; Bitplane) on collected confocal Z‐stack images.49 We selected secondary apical dendritic branches that were located at 100‐200 μm from the soma for analysis. At least two dendritic segments were quantified per neuron. Image Z‐stacks were acquired using a Zeiss confocal microscope (LSM 710, with 63× NA 1.4 oil‐immersion objective, 0.2 μm Z resolution). The confocal files were imported into Imaris software, and the Filament Tracer module was used for three‐dimensional rendering of each dendritic segment. Reconstructed spines were subsequently edited manually to exclude spines that were misidentified. Spine parameters, including density, head size, and head volume, were quantified. At least 250 spines from three mice were constructed for each group.
2.4. Data acquisition and analysis
Electrophysiological data were acquired by pClamp 10.2 via an Axon Digidata 1550A (Molecular Devices) interface board set to a sampling frequency of 10 kHz, filtered in Clampfit 10.2 using an eight‐pole Bessel filter and a 1‐kHz low‐pass filter, and stored on hard media for subsequent off‐line analysis. Statistical comparisons of results were performed using Origin 8.0 (Microcal Software, Northampton, MA, USA). The Student t test, one‐way or two‐way (Sholl analyses of dendritic length and intersection numbers) analysis of variance was used when data passed normality tests. For dendritic spine volume tests, a nonparametric Mann‐Whitney U test was used. Statistical difference was assessed, and P<.05 was set as the level of significance.
3. RESULTS
3.1. Hippocampal neuronal dendritic and spine comparisons between young Apoe3 and Apoe4 mice
To compare the dendritic length and spine density of hippocampal CA1 neurons, we added biocytin into the recording solution and dialyzed the CA1 pyramidal neuron under the patch‐clamp whole‐cell recording in current‐clamp mode. We then reconstructed these labeled neurons for quantitative analysis (Figure 1A,B). As shown in Figure 2A,B, the dendritic length and the numbers of intersections of the dendritic arbor in Apoe4‐mouse CA1 neurons compared to those from Apoe3‐mouse neurons) were significantly reduced (genotype effects for dendritic length: F(1,187) = 20.54, P<.001; number of intersections: F(1,160) = 9.49, P=.002). To analyze hippocampal CA1 neuron spine morphology, we used Imaris reconstruction from confocal Z‐stack images (see Figure 1). Quantitative analysis revealed that Apoe4 neurons exhibited significantly reduced spine head volume compared to Apoe3 neurons (Figure 2C, P<.01, Mann‐Whitney U test). Spine density in Apoe4 neurons (n=7) was also significantly lower than in Apoe3 neurons (n=8, Figure 2D; 10.3 ± 0.33 spines per 10 μm dendrite in Apoe4 neurons compared with 13.2 ± 0.9 in Apoe3 neurons; P<0.01, unpaired t test). These morphology results indicate that hippocampal CA1 neurons in young Apoe4 mice have reduced dendritic length, spine size, and density compared with the Apoe3 controls. Considering that spine size and geometry are highly correlated with glutamatergic synapse function,51 these results suggest potentially altered hippocampal synaptic function in young Apoe4 mice.
Figure 1.
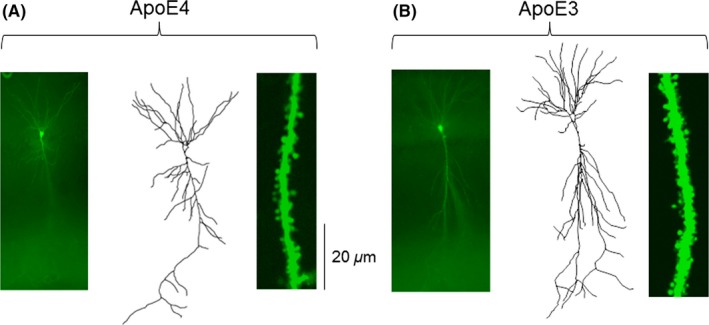
Comparison of dendritic length and spine density between Apoe4 (A) and Apoe3 (B) transgenic mice. Photomicrographs of biocytin‐stained hippocampal CA1 pyramidal neurons are shown in the left panels, representative dendritic arbor reconstructions using Neurolucida are shown in the middle panels, and dendritic segments from which dendritic spine density was calculated are shown in the right panels
Figure 2.
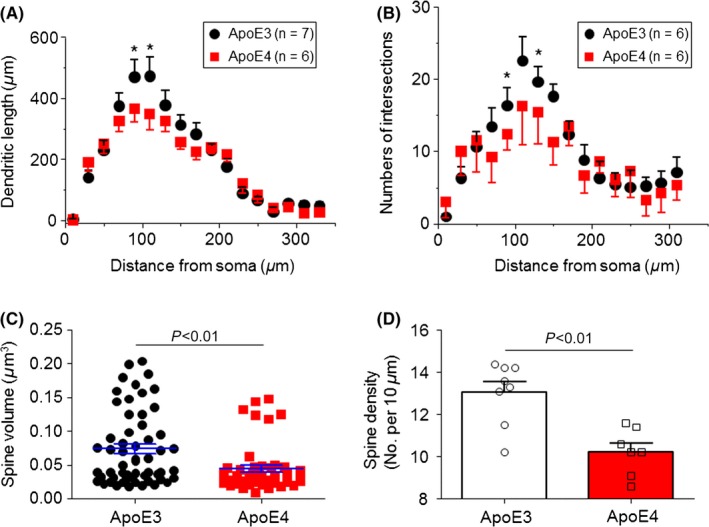
Quantitative analysis of dendritic complexity and spine size and density in Apoe3 and Apoe4 mice. Sholl analysis of hippocampal CA1 neuron dendritic complexity shows a significant reduction in dendritic length (A) and Intersection numbers (B) (*P<.05, t test). Further analysis shows a significant reduction in spine volume (C) and density (D) in Apoe4 compared to Apoe3 mice
3.2. Input‐output relationships in hippocampal slices prepared from young Apoe4 and Apoe3 mice
We next compared input‐output relationship curves between hippocampal slices prepared from 4‐month‐old Apoe4 and Apoe3 mice. Repetitive stimulation (0.33 Hz) of Schaffer collaterals evoked fEPSPs in the hippocampal CA1 region (Figure 3A). In both Apoe3 and Apoe4 groups, hippocampal slices were stimulated with different intensities (from 2 to 10 V at intervals of 33 seconds with the stimulation pulse duration of 0.1 ms), and these stimuli induced increases in the slope of the fEPSPs, which were plotted against stimulation intensities to form an input‐output relationship curve (Figure 3B). Because the input‐output curves between 4 and 10 V were linear, we fitted each individual slice (between 4 and 10 V) using a linear equation and compared the linear slopes of input‐output relationships between the Apoe3 and Apoe4 groups. As shown in Figure 3C, the linear slope for the Apoe3 group was 0.49 ± 0.04 mV/ms, while that for the Apoe4 group was 0.89 ± 0.08 mV/ms. Statistical analyses revealed a significant difference between these two groups (P<.0001, unpaired t test). These results suggest that compared to Apoe3 control mice, Apoe4 transgenic mouse hippocampal CA1 neurons exhibit an enhanced excitability.
Figure 3.
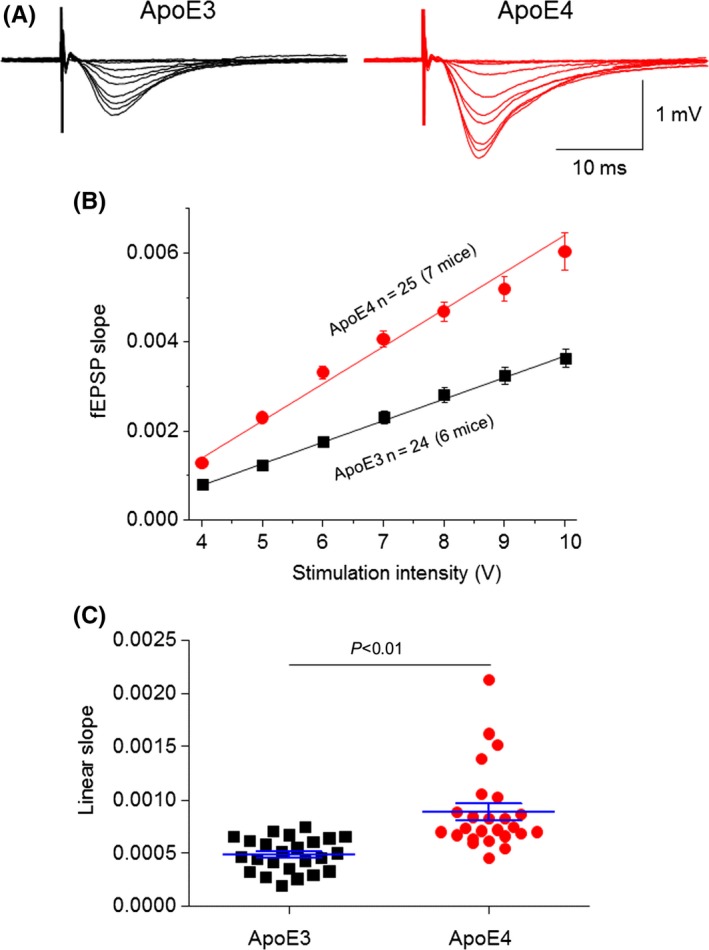
Comparison of input‐output relationship curves between Apoe4 and Apoe3 TG mice. (A) Representative traces of different stimulation intensity‐induced fEPSPs in a hippocampal CA1 slice prepared from Apoe3 (left) and Apoe4 (right). (B) Superimposition of input‐output relationship curves fitted by linear equation shows linear slopes of Apoe3 (black, n=24 slices from six mice) and Apoe4 (red, n=25 from seven mice) groups (stimuli range, 4‐10 V). (C) Summary of individual linear slopes from Apoe3 (black) and Apoe4 (red) groups. Statistical analysis (unpaired Student t test) indicates the difference is significant (P<.001)
3.3. Paired‐pulse facilitation in hippocampal slices prepared from young Apoe4 and Apoe3 mice
To measure synaptic short‐term plasticity, we used a PPF protocol in hippocampal slices from 4‐month‐old Apoe4 and Apoe3 mice. It has been reported that stimulus‐evoked response to a second stimulation is potentiated if it is delivered within 200 ms of the first stimulus.52 In these experiments, we measured PPF using interpulse intervals of 15, 50, 100, and 400 ms. Figure 4A shows the typical traces of PPF with a 50‐ms interpulse interval in Apoe3 and Apoe4 hippocampal slices. Statistical analysis from 54 total slices (30 from Apoe4 mice and 24 from Apoe3 mice) demonstrated a significant enhancement of the ratio of P2/P1 at all tested P1 and P2 intervals in Apoe4 mice compared to Apoe3 mice (Figure 4B), suggesting an enhanced hippocampal synaptic short‐term plasticity in young Apoe4 mice.
Figure 4.
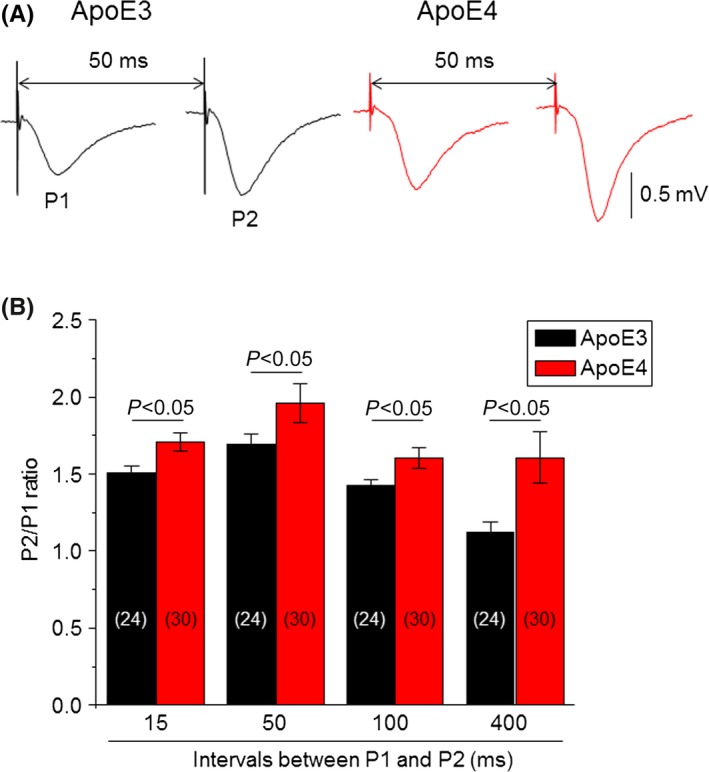
Comparison of paired‐pulse facilitation between Apoe4 and Apoe3 transgenic mice. (A) Typical traces of fEPSP responses evoked by two stimuli at an interval of 50 ms from Apoe3 (black) and Apoe4 (red) mice. (B) Bar graph summarizing the P2/P1 ratios evoked by different paired‐pulse stimulation intervals (15, 50, 100, and 400 ms) from Apoe3 (black, n=24 from six mice) and Apoe4 (red, n=30 from seven mice). The vertical bars represent ±SE. The number inside each column indicates the number of slices tested. Statistical analysis using unpaired Student t test between Apoe3 and Apoe4 mice in different paired‐pulse interval groups shows P <.05
3.4. Long‐term potentiation in hippocampal slices prepared from young Apoe4 and Apoe3 mice
To compare hippocampal CA1 synaptic LTP between Apoe4 and Apoe3 mice, we measured the theta‐burst stimuli‐induced LTP in the hippocampal Schaffer collateral‐CA1 pathway. The theta‐burst stimulation contained five trains with intervals of 10 ms (100 Hz), 10 times at 100‐ms intervals. For LTP recordings, baseline fEPSPs (50% of maximal stimuli with 0.33 Hz) were recorded for 20‐30 minutess. If the variation in the baseline was within ±10%, theta‐burst tetanic stimuli were delivered. Thereafter, baseline stimulus‐induced fEPSPs were recorded for 60 minutes (Figure 5A). Plot recording time to normalized fEPSP slopes (baseline level as 1) from pooled data showed an impaired LTP induction (after theta‐burst stimulation 0‐10 minutes) and maintenance (after theta‐burst stimulation 50‐60 minutes) in the Apoe4 group compared to Apoe3 mice (Figure 5B). Statistical analysis showed that the mean LTP induction in the Apoe4 group was 1.38 ± 0.02 and 1.52 ± 0.02 in the Apoe3 group (P<.001, unpaired t test); mean LTP maintenance in the Apoe4 group was 1.31 ± 0.01, and in the Apoe3 group, it was 1.47 ± 0.01 (P<.001, unpaired t test) (Figure 5C). These results suggest that a reduced LTP occurs in 4‐month‐old Apoe4 mouse hippocampus at the beginning of induction and is maintained afterward.
Figure 5.
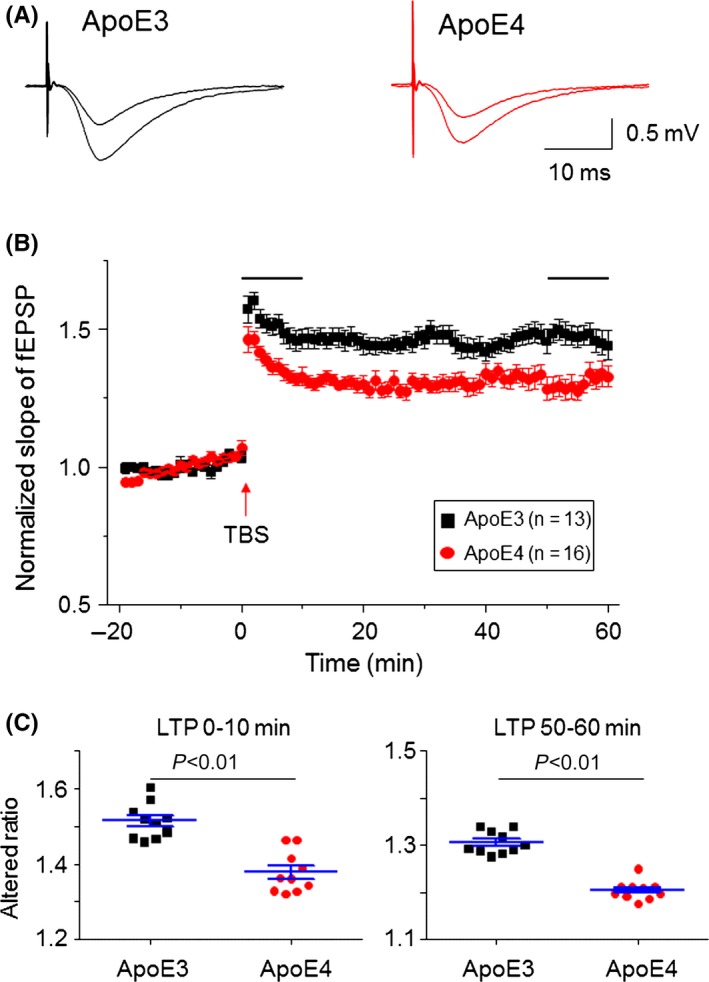
Comparison of hippocampal CA3‐CA1 synaptic LTP between Apoe4 and Apoe3 transgenic mice. (A) Typical fEPSPs recorded before and after theta‐burst tetanic stimulation from Apoe3 and Apoe4 mice. (B) Summary of hippocampal CA1 LTP from Apoe3 (black, n=13 slices from six mice) and Apoe4 (red, n=16 slices from seven mice). Theta‐burst stimulations are indicated by horizontal lines above the fEPSP responses. (C) Comparison between Apoe3 and Apoe4 groups for LTP induction (after tetanic stimulation 1‐10 min) and maintenance (after tetanic stimulation 50‐60 min) demonstrate the impaired LTP induction and maintenance in Apoe4 mice compared to Apoe3 mice. TBS, theta‐burst stimulation
3.5. Comparisons of synaptic function between young and aged Apoe4 mice
Data above clearly demonstrated synaptic abnormalities in 4‐month‐old Apoe mice as indicated by the enhanced basic synaptic transmission and PPF but impaired LTP induction and maintenance. We thus compared these synaptic functions between young (4‐month‐old) and aged (12‐month‐old) Apoe4 mice. Compared to young Apoe4 mice, aged Apoe4 mice showed a reduced basic synaptic transmission (Figure 6A). In 20 hippocampal slices from six aged mice, the mean linear slope between 4 and 10 V‐induced responses was 0.49 ± 0.04, while in 25 hippocampal slices from seven young mice tested, this slope was 0.89 ± 0.07 (P<.001, unpaired t test, Figure 6B). Aged Apoe4 mice also exhibited a reduced PPF compared to young Apoe4 mice tested at all four P1 and P2 intervals (Figure 6C). For LTP, although aged Apoe4 mice did not show a difference in mean LTP induction (LTP 0‐10 minutes) compared to young Apoe4 mice (aged Apoe4 = 1.34 ± 0.04 vs young Apoe4 = 1.38 ± 0.02, P>.05, Figure 6D), the LTP maintenance (after LTP 50‐60 minutes) was significantly reduced (aged Apoe4 = 1.20 ± 0.006 vs young Apoe4 = 1.31 ± 0.007, P<.0001, Figure 6E). Collectively, these results suggest that the Apoe4 genotype affects hippocampal synaptic transmission, PPF, and LTP in an age‐dependent manner.
Figure 6.
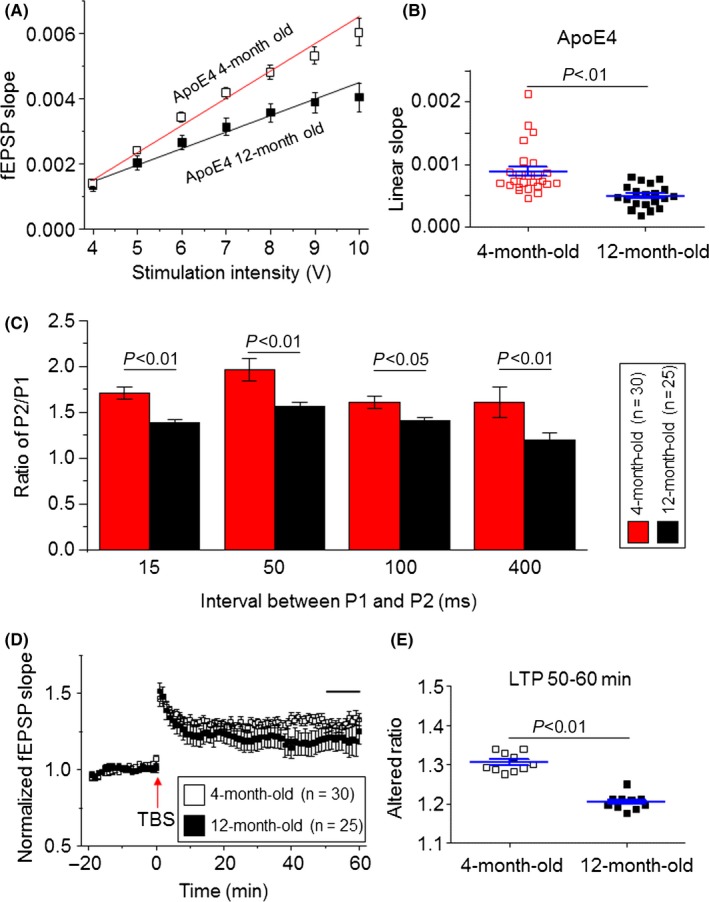
Comparison of synaptic functions between 4‐ and 12‐month‐old Apoe4 mice. (A) Representative input‐output relationship curves from 4‐month‐old (open squares, n=25 from seven mice, the same data from Figure 3B) and 12‐month‐old Apoe4 (filled squares, n=20 slices from seven mice) groups. (B) Statistical comparison of linear slopes of each linear fit individual input‐output relationship curve from 4‐month‐old (open squares) and 12‐month‐old (filled squares) Apoe4 groups (P<.01). (C) Bar chart shows comparison of P2/P1 ratios at different stimulation intervals between 4‐month‐old (white, n=30 slices from 14 mice) and 12‐month‐old (black, n=25 slices from seven mice) Apoe4 mice. (D) Comparison of hippocampal CA1 LTP between 4‐ (n=30 from 14 mice) and 12‐month‐old (n=20 slices from six mice) Apoe4 mice. (E) Statistical comparison of LTP maintenance (after tetanic stimulation 50‐60 min) between 4‐ and 12‐month‐old Apoe4 mice (P<.01)
3.6. Theta oscillations in hippocampal slices prepared from young Apoe4 and Apoe3 mice
Hippocampal theta oscillations represent the “online” state of the hippocampus and this rhythm is believed to be critical for temporal coding/decoding of active neuronal ensembles and the modification of synaptic weights.53 In these experiments, we compared carbachol (CCh)‐induced theta oscillations between young Apoe4 and Apoe3 mice using a field potential recording method, described in our previous report.22, 48 Continuous bath perfusion of 50‐μmol L−1 CCh to hippocampal slices induced impaired theta oscillations in Apoe4 slices compared to Apoe3 hippocampal slices (Figure 7A,B). To analyze these theta oscillations, we measured the theta‐burst event‐initiation time; total theta‐burst event numbers during a 20‐ minutes recording; and each theta‐burst duration, frequency, and amplitude. As shown in Figure 7C, Apoe4 mice were slower in initiation and had fewer burst events than Apoe3 mice.
Figure 7.
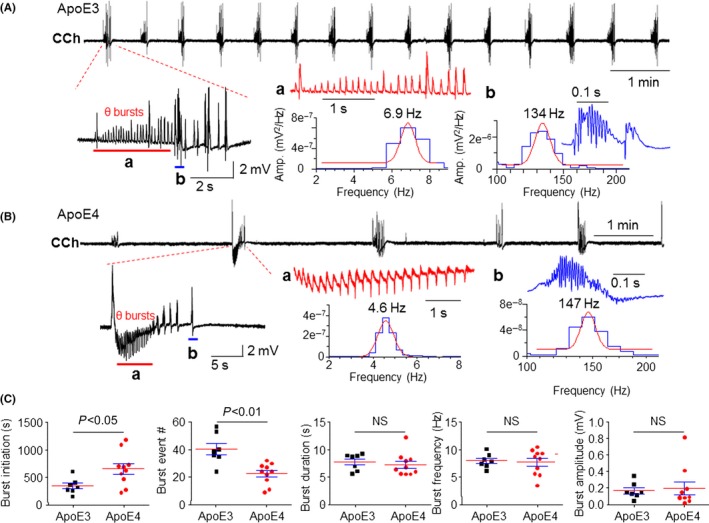
Comparison of CCh‐induced theta oscillations in hippocampal slices prepared from Apoe4 and Apoe3 mice. Typical traces of bath perfusion of 50 μmol L−1 CCh‐induced theta oscillations recorded in hippocampal CA1 area from Apoe3 (A) and Apoe4 mice (B) represent repetitive burst events during a 10‐min recording period. The expanded timescales on the left show two components of bursts, theta rhythm (indicated by red bar) and lower frequency spikes (blue bar). The further expanded timescales on the right show theta oscillations (red, 4‐9 Hz) and high‐frequency oscillations (blue, >100 Hz). (C) Statistical analysis shows that Apoe4 mice had significant impairment of CCh‐induced theta oscillations including a prolonged initiation time of burst events (P<.05) and reduced burst event numbers (P<.01). NS, not significant
3.7. Hippocampal synaptic vesicle recycling in young Apoe4 and Apoe3 mice
It has been reported that Apoe4 mice showed impaired presynaptic vesicle recycling.32 To compare hippocampal synaptic vesicle recycling in young Apoe4 and Apoe3 mice, we applied sustained electrical stimulations at the Schaffer collaterals pathway (5, 10, or 40 Hz for 4 seconds) similar to previously reported methods.54 Figures 8A‐C represent typical traces of 4, 10, and 40 Hz stimulation‐induced fEPSP responses, respectively, in an Apoe4 slice. A comparison of the ratio of last and first stimulation‐induced fEPSPs (Figure 8D) and the ratio of maximal and first peak responses (Figure 8E) demonstrated no significant difference between Apoe4 (n=10) and Apoe3 (n=8) mice in three stimulatory parameters (5, 10, and 40 Hz). These results showed no significant changes in hippocampal CA1 vesicle recycling in young Apoe4 mice.
Figure 8.
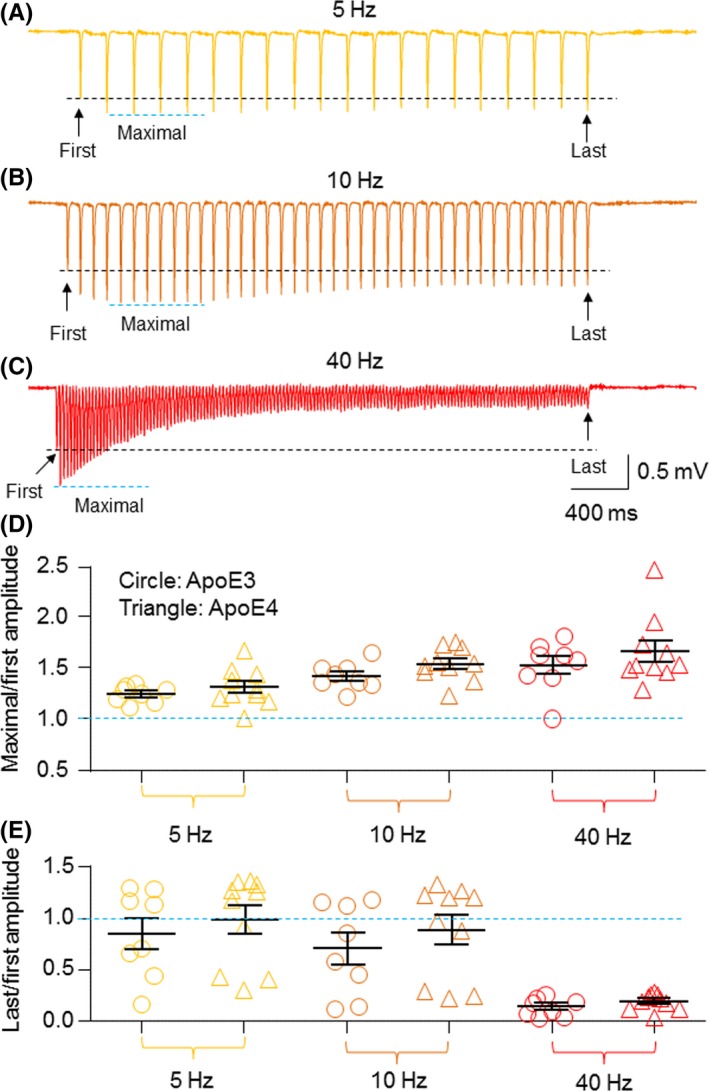
Comparison of fEPSP evoked by sustained stimulations (5, 10, and 40 Hz for 4 s) between Apoe4 and Apoe3 transgenic mice. Evoked fEPSPs in a hippocampal slice from 4‐month‐old Apoe4 mice were elicited by a sustained stimulation with 50% maximal stimulation intensity at 5 Hz (A), 10 Hz (B), or 40 Hz (C) for 4 s. (D) Graph summarizes the ratios of maximal fEPSP slope/first fEPSP slope by 5, 10, or 40 Hz stimulations from the Apoe3 and Apoe4 groups. (E) Graph summarizes the ratios of last fEPSP slope/first fEPSP slope by 5, 10, or 40 Hz stimulations from the Apoe3 and Apoe4 groups
4. DISCUSSION
The major findings of this study are that synaptic abnormalities in the hippocampus occur as early as 4 months in Apoe4 mice. Morphologically, hippocampal pyramidal neurons had reduced dendritic length and branch numbers, as well as decreased spine density in these young Apoe4 mice compared to Apoe3 mice. Functionally, these neurons demonstrated enhanced CA3‐CA1 basal synaptic transmission, enhanced PPF, but reduced LTP induction and maintenance. In addition, young Apoe4 mice also showed impaired carbachol‐induced hippocampal theta oscillations without changes in synaptic vesicle recycling. Taken together, our study provides direct experimental evidence that young Apoe4 mice exhibit morphologically and functionally impaired synapses in hippocampal CA1 neurons. This will help us to understand the impact of Apoe4 in synaptic function, in animal learning behaviors, as well as in learning and memory of young adults.
4.1. Young Apoe4 mice show abnormalities in hippocampal CA1 neuronal dendritic morphology and spine density
Previous studies have demonstrated a morphological alteration in the hippocampus in Apoe4 mice, including reduced levels of the presynaptic glutamatergic transporter, VGluT, in CA3, CA1, and DG hippocampal neurons of 4‐month‐old Apoe4 mice compared to Apoe3 mice.38 However, the corresponding inhibitory GABAergic nerve terminals were not affected by the Apoe genotype,38 although a significant change of GABAergic terminals occurred in aged Apoe4 mice.55 These findings suggest that the alterations in glutamatergic synaptic morphology and function are early pathological markers in Apoe4 mice. In addition, decreased levels of cortical, but not hippocampal, neuronal spines53; postsynaptic glutamate receptors32; and the scaffold protein PSD‐9529 have been reported in Apoe4 mice. These data suggest that the Apoe4 genotype affects neuronal dendritic morphology and synaptic spine density.43 In the present study, we demonstrated that, compared to 4‐month‐old Apoe3 mice, hippocampal CA1 pyramidal neurons from age‐matched Apoe4 mice showed significant reductions in dendritic length and spine density. Our results are consistent with previous reports that young (4‐month‐old) Apoe4 mice show significant changes in synaptic morphology and glutamate transporter.38 However, our results are different from the report of Dumanis et al. 53 in which there were no significant alterations in hippocampal dendritic spine density between 1‐, 3‐ and 12‐month‐old Apoe4 mice using Golgi staining. The difference in findings may be explained by the variation in experimental approach as Golgi staining tends to underestimate spine density.56, 57 Using Imaris reconstruction following confocal Z‐stack, we were able to quantify accurately the dendritic spines on the Z plane, which is not possible in the Golgi‐stained sections. Our approach (biocytin injection followed by avidin‐Alexa 488) also minimizes the tissue shrinkage that is inevitable in Golgi stain section. Therefore, the spine density using these two approaches may not be readily comparable. Collectively, we demonstrated abnormalities of dendritic morphology and spine density in young Apoe4 mice, which suggests an important basis for hippocampal synaptic functional deficits or even animal behavioral alterations.
4.2. Young Apoe4 mice show hippocampal synaptic functional abnormalities
We systemically examined the synaptic function of the hippocampus in young Apoe4 mice using electrophysiological recordings of brain slices, and compared these to the age‐matched Apoe3 mice because Apoe3 mice suffered the same transgenic process and showed no synaptic abnormality compared to wild type mice (Fig. S1). We choose mice of this age because hippocampal synaptic deficits have already been reported in aged Apoe4 mice.40 Previous studies in 4‐month‐old Apoe4 mice have found the reduced hippocampal presynaptic glutamatergic vesicles (VGlut1) and postsynaptic synaptic protein (PD95), as well as animal learning behaviors.38, 39 However, in addition to these protein expression and behavioral changes, it remains unclear whether hippocampal synaptic function is altered. Thus, our study sought to fill this knowledge gap. As expected, we found a significant reduction in theta‐burst tetanic stimulation‐induced LTP in CA3‐CA1 synapses. The mechanisms underlying these synaptic alterations are still unclear. Based on our data and previous reports, the Apoe gene affects both presynaptic and postsynaptic functions. Presynaptically, Apoe4 mice exhibit the reduced glutamatergic vesicles (VGlut1)38 and glutamate release probability (shown enhanced PPF ratio in Figure 4). Postsynaptically, Apoe4 mice show the reduced postsynaptic synaptic protein (PD95)39 and hippocampal dendritic morphology abnormalities (Figures 1, 2) and LTP deficit (Figure 5). These lines of evidence suggest a net deficit in synaptic transmission of Apoe4 hippocampal CA3‐CA1. It is intriguing to observe an enhanced hippocampal neuronal excitability (left shift of I‐O curve) in 4‐month‐old Apoe4 mice compared to age‐matched Apoe3 mice. We postulate that this change may reflect a functional compensation at an early stage of brain development in Apoe4 mice to overcome the synaptic and dendritic morphological abnormality and presynaptic glutamatergic vesicle deficit.38 Our findings are both consistent with and different from previous reports for various potential reasons. First is the mouse age. It has been well accepted that the APOE4 gene affects cognitive function and learning and memory in an age‐dependent manner.58, 59, 60 For example, different changes in hippocampal LTP in different ages of Apoe4 mice have been noted: in 2‐month‐old mice, hippocampal LTP was enhanced42 or had no change,32 but in 24‐ to 27‐month‐old mice, hippocampal LTP was impaired.40 We showed here that in 4‐month‐old Apoe4 mice, hippocampal LTP induction and maintenance were already reduced compared to 4‐month‐old Apoe3 mice. The finding of lower hippocampal synaptic LTP in 4‐month‐old ApoE4 mice is well supported by previous reports that 4‐month‐old Apoe4 mice showed reduced levels of the presynaptic glutamatergic vesicular transporter (VGluT)38 and impaired hippocampus‐related learning and memory tasks.39 The second reason for the difference between our results and other published studies is due to the method of LTP induction. In 2‐month‐old Apoe4 mice, hippocampal CA1 LTP was induced by high‐frequency tetanic stimulation (100 Hz for 1 seconds)42 When using similarly aged (2‐month‐old) Apoe4 mice, theta‐burst tetanic stimulation‐induced LTP showed no change between Apoe4 and Apoe3 mice.32 The third reason is related to the area of brain investigated. Using high‐frequency tetanic stimulation in young Apoe4 mice, hippocampal CA1 LTP was enhanced in CA3‐CA1 area42 while hippocampal dentate gyrus LTP was reduced compared to age‐matched Apoe3 mice.61 Collectively, these data suggest that there are several factors such as animal ages, LTP induction protocols, and brain areas that studies contributing to hippocampal synaptic plasticity in Apoe4 mice. Thus, caution is needed when analyzing and interpreting these data.
4.3. Young Apoe4 mice exhibit impaired hippocampal theta oscillations
The key role of the hippocampus for intact episodic memory has been firmly established by neuropsychological studies, animal models, computational models, and human neuroimaging.62 The hippocampus has three types of rhythmic activity: theta (4‐12 Hz), beta (13‐30 Hz), and gamma (30‐80 Hz). These activities reflect different states of the brain and behaviors.63 The theta oscillations represent the “online” state of the hippocampus. The extracellular currents underlying theta waves are generated mainly by the entorhinal input, CA3 (Schaffer) collaterals, and voltage‐dependent Ca2+ currents in pyramidal cell dendrites. The rhythm is believed to be critical for temporal coding/decoding of active neuronal ensembles and the modification of synaptic weights.64 Hippocampal neuronal network oscillations are associated with attention, learning, and memory and are impaired in disease conditions such as AD. Recently, aged Apoe4 mice were reported to have fewer slow‐wave responses than Apoe3 mice and significantly reduced slow gamma oscillations during sharp‐wave ripples, which critically contributes to Apoe4‐mediated learning and memory impairments.60 Furthermore, a significant reduction in hippocampal dentate gyrus GABAergic interneurons has been found in aged Apoe4 mice. Because the GABAergic neurons were not abnormal in young (3‐4 months old) Apoe4 mice,38 we did not examine hippocampal gamma oscillations in young Apoe4 mice. Instead, we focused on hippocampal cholinergic theta oscillations in young Apoe4 mice. We found significant alterations in CCh (50 μmol L−1)‐induced theta oscillation initial time and burst event numbers in young Apoe transgenic mice compared to the age‐matched Apoe3 transgenic mice. Collectively, these data provide the first evidence of possible neuronal mechanisms of learning behavioral deficits in 4‐month‐old Apoe4 mice.
5. CONCLUSION
Synaptic morphology and function abnormalities in the hippocampus occur as early as 4 months in Apoe4 mice. In addition, young Apoe4 mice also exhibit impaired carbachol‐induced hippocampal theta oscillations without changes in synaptic vesicle recycling. Therefore, this study provides direct experimental evidence that will help us to understand the impact of Apoe4 in synaptic function, in animal learning behaviors, as well as in learning and memory of young adults.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This study was funded by the Arizona Alzheimer's Disease Consortium (JS), Barrow Neurological Foundation (JW), the National Science Foundation of China (81671050 to JS), and the Program for High‐level Talent Fund of Hebei Province of China (No. A201401041 to GS).
Sun G‐Z, He Y‐C, Ma X‐K, et al. Hippocampal synaptic and neural network deficits in young mice carrying the human APOE4 gene. CNS Neurosci Ther. 2017;23:748–758. 10.1111/cns.12720
Jiong Shi and Jie Wu played an equal role as senior authors in this article.
REFERENCES
- 1. Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985; 82:4245‐4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tesseur I, Zou K, Esposito L, et al. Deficiency in neuronal TGF‐beta signaling promotes neurodegeneration and Alzheimer's pathology. J Clin Invest. 2006;116:3060‐3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bloom GS. Amyloid‐beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71:505‐508. [DOI] [PubMed] [Google Scholar]
- 4. Crimins JL, Pooler A, Polydoro M, Luebke JI, Spires‐Jones TL. The intersection of amyloid beta and tau in glutamatergic synaptic dysfunction and collapse in Alzheimer's disease. Ageing Res Rev. 2013;12:757‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer's disease and its models. Neuroscience. 2013;251:51‐65. [DOI] [PubMed] [Google Scholar]
- 6. Ingelsson M, Fukumoto H, Newell KL, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925‐931. [DOI] [PubMed] [Google Scholar]
- 7. Braak H, Braak E. Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol. 1991;82:239‐259. [DOI] [PubMed] [Google Scholar]
- 8. Fjell AM, Walhovd KB. Neuroimaging results impose new views on Alzheimer's disease–the role of amyloid revised. Mol Neurobiol. 2012;45:153‐172. [DOI] [PubMed] [Google Scholar]
- 9. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rusinek H, De Santi S, Frid D, et al. Regional brain atrophy rate predicts future cognitive decline: 6‐year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691‐696. [DOI] [PubMed] [Google Scholar]
- 11. Carmichael O, Schwarz C, Drucker D, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol. 2010;67:1370‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mosconi L, Berti V, Glodzik L, Pupi A, De Santi S, de Leon MJ. Pre‐clinical detection of Alzheimer's disease using FDG‐PET, with or without amyloid imaging. J Alzheimers Dis. 2010;20:843‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frisoni GB, Giannakopoulos P. The specificity of amyloid imaging in the diagnosis of neurodegenerative diseases. Neurobiol Aging. 2012;33:1021‐1022. [DOI] [PubMed] [Google Scholar]
- 14. Briggs CA, Chakroborty S, Stutzmann GE. Emerging pathways driving early synaptic pathology in Alzheimer's disease. Biochem Biophys Res Commun. 2017;483:988‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masliah E. Mechanisms of synaptic pathology in Alzheimer's disease. J Neural Transm Suppl. 1998;53:147‐158. [DOI] [PubMed] [Google Scholar]
- 16. Conforti L, Adalbert R, Coleman MP. Neuronal death: where does the end begin? Trends Neurosci. 2007;30:159‐166. [DOI] [PubMed] [Google Scholar]
- 17. Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late‐onset familial and sporadic Alzheimer's disease. Neurology. 1993;43:1467‐1472. [DOI] [PubMed] [Google Scholar]
- 18. Saunders AM, Schmader K, Breitner JC, et al. Apolipoprotein E epsilon 4 allele distributions in late‐onset Alzheimer's disease and in other amyloid‐forming diseases. Lancet. 1993;342:710‐711. [DOI] [PubMed] [Google Scholar]
- 19. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921‐923. [DOI] [PubMed] [Google Scholar]
- 20. Roses AD. Apolipoprotein E and Alzheimer's disease. A rapidly expanding field with medical and epidemiological consequences. Ann N Y Acad Sci. 1996;802:50‐57. [DOI] [PubMed] [Google Scholar]
- 21. Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta‐peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late‐onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649‐9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high‐avidity binding to beta‐amyloid and increased frequency of type 4 allele in late‐onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977‐1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strittmatter WJ, Saunders AM, Goedert M, et al. Isoform‐specific interactions of apolipoprotein E with microtubule‐associated protein tau: implications for Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:11183‐11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sunderland T, Mirza N, Putnam KT, et al. Cerebrospinal fluid beta‐amyloid1‐42 and tau in control subjects at risk for Alzheimer's disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56:670‐676. [DOI] [PubMed] [Google Scholar]
- 27. Nwabuisi‐Heath E, Rebeck GW, Ladu MJ, Yu C. ApoE4 delays dendritic spine formation during neuron development and accelerates loss of mature spines in vitro. ASN Neuro. 2014;6:e00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dumanis SB, DiBattista AM, Miessau M, Moussa CE, Rebeck GW. APOE genotype affects the pre‐synaptic compartment of glutamatergic nerve terminals. J Neurochem. 2013;124:4‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhu Y, Nwabuisi‐Heath E, Dumanis SB, et al. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia. 2012;60:559‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arendt T, Schindler C, Bruckner MK, et al. Plastic neuronal remodeling is impaired in patients with Alzheimer's disease carrying apolipoprotein epsilon 4 allele. J Neurosci. 1997;17:516‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. White F, Nicoll JA, Roses AD, Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiol Dis. 2001;8:611‐625. [DOI] [PubMed] [Google Scholar]
- 32. Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci USA. 2010;107:12011‐12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim J, Yoon H, Basak J, Kim J. Apolipoprotein E in synaptic plasticity and Alzheimer's disease: potential cellular and molecular mechanisms. Mol Cells. 2014;37:767‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid‐beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2009;106:6820‐6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heise V, Filippini N, Ebmeier KP, Mackay CE. The APOE varepsilon4 allele modulates brain white matter integrity in healthy adults. Mol Psychiatry. 2011;16:908‐916. [DOI] [PubMed] [Google Scholar]
- 36. Caselli RJ, Dueck AC, Osborne D, et al. Longitudinal modeling of age‐related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dean DC 3rd, Jerskey BA, Chen K, et al. Brain differences in infants at differential genetic risk for late‐onset Alzheimer disease: a cross‐sectional imaging study. JAMA Neurol. 2014;71:11‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liraz O, Boehm‐Cagan A, Michaelson DM. ApoE4 induces Abeta42, tau, and neuronal pathology in the hippocampus of young targeted replacement apoE4 mice. Mol Neurodegener. 2013;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salomon‐Zimri S, Boehm‐Cagan A, Liraz O, Michaelson DM. Hippocampus‐related cognitive impairments in young apoE4 targeted replacement mice. Neurodegener Dis. 2014;13:86‐92. [DOI] [PubMed] [Google Scholar]
- 40. Yun SH, Park KA, Kwon S, et al. Estradiol enhances long term potentiation in hippocampal slices from aged apoE4‐TR mice. Hippocampus. 2007;17:1153‐1157. [DOI] [PubMed] [Google Scholar]
- 41. Qiao F, Gao XP, Yuan L, Cai HY, Qi JS. Apolipoprotein E4 impairs in vivo hippocampal long‐term synaptic plasticity by reducing the phosphorylation of CaMKIIalpha and CREB. J Alzheimers Dis. 2014;41:1165‐1176. [DOI] [PubMed] [Google Scholar]
- 42. Kitamura HW, Hamanaka H, Watanabe M, et al. Age‐dependent enhancement of hippocampal long‐term potentiation in knock‐in mice expressing human apolipoprotein E4 instead of mouse apolipoprotein E. Neurosci Lett. 2004;369:173‐178. [DOI] [PubMed] [Google Scholar]
- 43. Ji Y, Gong Y, Gan W, Beach T, Holtzman DM, Wisniewski T. Apolipoprotein E isoform‐specific regulation of dendritic spine morphology in apolipoprotein E transgenic mice and Alzheimer's disease patients. Neuroscience. 2003;122:305‐315. [DOI] [PubMed] [Google Scholar]
- 44. Hartman RE, Wozniak DF, Nardi A, Olney JW, Sartorius L, Holtzman DM. Behavioral phenotyping of GFAP‐apoE3 and ‐apoE4 transgenic mice: apoE4 mice show profound working memory impairments in the absence of Alzheimer's‐like neuropathology. Exp Neurol. 2001;170:326‐344. [DOI] [PubMed] [Google Scholar]
- 45. Knouff C, Hinsdale ME, Mezdour H, et al. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103:1579‐1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu J, Fisher RS. Hyperthermic spreading depressions in the immature rat hippocampal slice. J Neurophysiol. 2000;84:1355‐1360. [DOI] [PubMed] [Google Scholar]
- 47. Song C, Murray TA, Kimura R, et al. Role of alpha7‐nicotinic acetylcholine receptors in tetanic stimulation‐induced gamma oscillations in rat hippocampal slices. Neuropharmacology. 2005;48:869‐880. [DOI] [PubMed] [Google Scholar]
- 48. Kimura R, Ma LY, Wu C, et al. Acute exposure to the mitochondrial complex I toxin rotenone impairs synaptic long‐term potentiation in rat hippocampal slices. CNS Neurosci Ther. 2012;18:641‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qiu S, Lu Z, Levitt P. MET receptor tyrosine kinase controls dendritic complexity, spine morphogenesis, and glutamatergic synapse maturation in the hippocampus. J Neurosci. 2014;34:16166‐16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387‐406. [PMC free article] [PubMed] [Google Scholar]
- 51. Lippman J, Dunaevsky A. Dendritic spine morphogenesis and plasticity. J Neurobiol. 2005;64:47‐57. [DOI] [PubMed] [Google Scholar]
- 52. Zucker RS, Regehr WG. Short‐term synaptic plasticity. Annu Rev Physiol. 2002;64:355‐405. [DOI] [PubMed] [Google Scholar]
- 53. Dumanis SB, Tesoriero JA, Babus LW, et al. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29:15317‐15322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Qiu X, Zhu Q, Sun J. Quantitative analysis of vesicle recycling at the calyx of Held synapse. Proc Natl Acad Sci USA. 2015;112:4779‐4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Andrews‐Zwilling Y, Bien‐Ly N, Xu Q, et al. Apolipoprotein E4 causes age‐ and Tau‐dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30:13707‐13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shen H, Sesack SR, Toda S, Kalivas PW. Automated quantification of dendritic spine density and spine head diameter in medium spiny neurons of the nucleus accumbens. Brain Struct Funct. 2008;213:149‐157. [DOI] [PubMed] [Google Scholar]
- 57. Asadi M, Rokni‐Yazdi H, Salehinia F, Allameh FS. Metastatic renal cell carcinoma initially presented with an intramedullary spinal cord lesion: a case report. Cases J. 2009;2:7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang X, Holland D, Dale AM, Miller MI. Alzheimer's Disease Neuroimaging I . APOE affects the volume and shape of the amygdala and the hippocampus in mild cognitive impairment and Alzheimer's Disease: age matters. J Alzheimers Dis. 2015;47:645‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thai C, Lim YY, Villemagne VL, et al. Amyloid‐related memory decline in preclinical Alzheimer's disease is dependent on apoe epsilon4 and is detectable over 18‐months. PLoS ONE. 2015;10:e0139082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gillespie AK, Jones EA, Lin YH, et al. Apolipoprotein e4 causes age‐dependent disruption of slow gamma oscillations during hippocampal sharp‐wave ripples. Neuron. 2016;90:740‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Trommer BL, Shah C, Yun SH, et al. ApoE isoform affects LTP in human targeted replacement mice. NeuroReport. 2004;15:2655‐2658. [DOI] [PubMed] [Google Scholar]
- 62. Sauvage MM, Fortin NJ, Owens CB, Yonelinas AP, Eichenbaum H. Recognition memory: opposite effects of hippocampal damage on recollection and familiarity. Nat Neurosci. 2008;11:16‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325‐340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


