Summary
Background
Exposure to pharmacological concentration of inhaled anesthetics such as isoflurane can cause short‐ or long‐term cognitive impairments in preclinical studies. The selective antagonists of the histamine H3 receptors are considered as a promising group of novel therapeutic agents for the treatment of cognitive disorders. In this study, we investigated whether ciproxifan, a nonimidazole antagonist of H3 histamine receptors, could overcome the functional and electrophysiological sequela associated with isoflurane anesthesia.
Methods
Adult male Sprague Dawley rats were exposed to 1.4% isoflurane or vehicle gas for 2 h. The memory tests (novel object recognition and passive avoidance) as well as in vivo hippocampal excitatory synaptic potentials were recorded 24 h postanesthesia. Locomotor activity, anxiety, and nociception 24 h after isoflurane were also examined. The drugs (ciproxifan 3 mg/kg or saline) were intraperitoneally injected 30 min prior to the behavioral tests or long‐term potentiation induction.
Results
Animals that were previously (24 h) exposed to 1.4% isoflurane for 2 h displayed no preference for novel objects and had impaired retention of a passive avoidance response at 1 h after sample phase. Treating isoflurane‐exposed rats with ciproxifan significantly improved the memory performance, as evidenced by an increased discrimination ratio in objects recognition and prolonged retention time in passive avoidance test. Accordingly, hippocampus long‐term potentiation was reduced in animals that received isoflurane, while administration of ciproxifan completely abolished the effect of isoflurane exposure on synaptic plasticity. Neither isoflurane nor ciproxifan altered motor performance, anxiety, and nociceptive responses.
Conclusion
These results suggest that H3R in the CNS, probably in the hippocampus, may serve as therapeutic target for improvement of anesthesia‐associated cognitive deficits.
Keywords: Ciproxifan, Hippocampus, Isoflurane, Memory, Synaptic plasticity
Implication Statement.
Systemic administration of ciproxifan, a histamine H3 receptor antagonist/inverse agonist, attenuated memory deficit and hippocampal synaptic plasticity impairment following isoflurane exposure. This finding suggests that H3R may serve as a therapeutic target against anesthesia‐associated cognitive deficits.
Introduction
Early postoperative cognitive dysfunction, confusion, and delirium are well‐known complications after major surgery 1, 2. General anesthesia has often been mentioned as a possible cause of these problems, because it affects brain function at multiple levels—from single neurons to neural networks. Although the recovery of consciousness from general anesthesia is usually rapid, several studies in humans and rodents indicate that the amnesic effects of general anesthetics such as isoflurane may persist beyond the time of anesthesia, leading to impairment of cognitive functions 1, 2, 3, 4, 5, 6, 7. The cellular changes contributing to these effects have yet to be fully understood. Consequently, progress toward developing pharmacotherapeutic agents that could ameliorate anesthetic‐induced cognitive deficits has been limited.
Histamine and its receptors in the brain are associated with the regulation of basic homeostatic and higher functions, including arousal, cognition, circadian, and feeding rhythms 8. Among the four identified histamine receptors, H3 receptors are predominantly expressed in the central nervous system, act as autoreceptors as well as heteroreceptors, and control presynaptic release of histamine and other neurotransmitters, including acetylcholine, serotonin, noradrenaline, and dopamine 8, 9, 10. Modulation of the H3R is expected to affect vigilance and sleep–wake regulation 11. In recent years, selective antagonists of the H3R are considered as a promising group of new potential drugs for the treatment of cognitive deficits such as Alzheimer's disease, attention deficit hyperactivity disorder, and schizophrenia 12.
Long‐term potentiation (LTP), a cellular model of activity‐dependent synaptic plasticity, is believed to be the major cellular mechanism that underlies learning and memory 13. Several studies have suggested that the memory impairments of general anesthetics are, in part, linked to physiological alterations that diminish the enhancement of synaptic neurotransmission in the hippocampus in vitro 14, 15, 16, 17. Histamine, on the other hand, has been shown to increase or even evoke LTP of synaptic transmission 8, 18. Antagonists of H3R have procognitive actions and therefore enhance the potentiation of synaptic transmission 19.
Using a mouse model, we previously showed that a robust deficit in short‐term object recognition memory occurred 1 day after exposure to isoflurane (1.3%) for 2 h 20. In this study, we asked the question as to whether ciproxifan 21, a well‐characterized nonimidazole antagonist of H3 histamine receptors, could overcome the functional and electrophysiological sequela associated with isoflurane anesthesia. We first assessed the effect of ciproxifan on memory deficit following exposure to isoflurane in behavioral paradigms including the novel object recognition and passive avoidance test. Next, we examined whether H3 receptor inhibition could rescue impairment of synaptic plasticity in the hippocampal CA1 region in vivo of isoflurane‐treated rats.
Materials and Methods
Animals
Experiments were performed on adult male Sprague Dawley rats (235–280 g). All animals were given water and regular rat chow ad libitum and housed under climate‐controlled conditions with a 12‐hour light/dark cycle (lights on at 7:00 am). All procedures were approved by the Institutional Animal Care and Use Committee.
Novel Object Recognition Test
The novel object recognition was conducted as previously reported to evaluate nonspatial hippocampal‐mediated memory 22. The apparatus consists of an open field (40 × 80 cm2) with two adjacently located imaginary circular zones. The zones are equally spaced in the center of the square and designated as “old object” and “novel object” zones. The old object used was square‐shaped, whereas the novel object was L‐shaped and assembled by building blocks from Lego toys, which were clearly distinct in shape and color. Two 5‐minute trials were performed. The first (training phase) trial was performed with two identical objects in both zones and the second (testing phase) trial with one old object and one novel object present in the respective zones of the open field. There was an intertrial interval of 1 h, during which the animals were returned to their home cages. A video camera was mounted on the ceiling above the chamber and connected to a monitor in an adjoining room. Object exploration was defined as sniffing or touching the object with the nose and/or forepaws. The time spent with each object was recorded by two trained investigators, uninformed of drug treatment, whose inter‐rater reliability was within 1 second of one another. Because rats inherently prefer to explore novel objects, a preference for the novel object indicates intact memory for the familiar object. The “discrimination index” for the second trial was calculated as: discrimination index = time spent in novel object zone/ (time spent in old object zone + time spent in novel object zone).
Passive Avoidance Test
The passive avoidance test was used to evaluate nonspatial, hippocampus‐mediated contextual memory, and fear‐related amygdala‐dependent emotional memory, as previously described 23. The one‐trial step‐through passive avoidance apparatus consisted of two adjoining compartments, one lighted and one darkened, divided by a guillotine door. The floor of each compartment consists of steel rods that deliver an electric foot shock to the rats. In the training phase of the passive avoidance test, a rat was placed in the lighted compartment, and when the rat entered the dark compartment, the guillotine door was closed manually by the experimenter and an electric foot shock of 0.5 mA for 3 seconds was delivered. The rats were confined in the dark compartment for 20 seconds after the electric shock and were then removed from the apparatus and returned to their home cages. In the testing phase performed 1 h later, the rats were tested for the retention of the passive avoidance response by placing them in the lighted compartment and recording the latency to enter into the previously shocked dark compartment. No shock was given during the test phase. If the rat did not enter into the dark compartment within 5 min, it was assigned a latency of 300 seconds.
Locomotor Activity
Spontaneous locomotor activity was tested using the open‐field method. The animals were individually placed in a corner facing the wall of the open‐field chamber (50 × 50 × 40 cm) and allowed to freely explore the chamber for 10 min. Total moving distance and mean speed were measured by computer‐based Any‐Maze automated video tracking system (Stoelting Co.)
Elevated Plus Maze Task
Elevated plus maze task (EPM) is one of the most widely used assessments to evaluate anxiety‐like behavior in rodents. The EPM apparatus comprised two open arms and two closed arms (50 × 10 cm), which extended from a common central platform (10 × 10 cm). Each arm was attached to a sturdy leg and elevated 50 cm from the ground. The animal was placed in the center of the maze and allowed to explore freely for 5 min. The time spent in the center and in the open and closed arms was measured.
Tail Flick
The nociceptive threshold was taken as the reaction time to remove the tail from a source of noxious heat stimulus using a hot water bath (49°C). The tip of the tail was inserted to approximately 1 cm depth into a bath of water. Latency for the rat to remove its tail from the water was recorded and used as an indication of nociception.
In Vivo Electrophysiology
In vivo electrophysiology in freely moving animals was performed as described previously 24. Briefly, the Sprague Dawley rats were anesthetized with sodium pentobarbital 60 mg/kg intraperitoneally and stereotaxically implanted with electrodes in the hippocampus. A pair of recording electrodes was implanted to straddle the hippocampal cell layer of CA1 (in mm: P4.6 and L2.8), and stimulating electrodes were placed in CA1 stratum oriens on the same side anterior to the recording electrodes (in mm: P3.2 and L1.7). Photoisolated current stimulus pulses (0.2 ms duration) were delivered cathodally to one stimulating electrode, and the evoked potentials were recorded from another pair of electrodes. The animals were allowed to recover from surgery for 14 days before experiments were conducted.
The evoked basal‐dendritic field excitatory postsynaptic potentials (fEPSPs) were recorded during awake immobility, and LTP was induced during behavioral exploration. After recording baseline fEPSPs for 1 h, a high‐frequency stimulus train (tetanus) was used for LTP induction. The train consisted of 100 pulses at 200 Hz (5‐ms interpulse interval), with a stimulus intensity of 0.8–1.5× the fEPSP threshold. fEPSPs were recorded with test pulses of 1.5× threshold intensity. The maximal slope of the negative fEPSP, recorded in stratum oriens, was measured during the falling phase (over 2 ms interval). The last six recordings before the tetanus were averaged to determine the baseline value, which was normalized to 100%. The enhanced synaptic plasticity was recorded at “fixed” times of 5, 10, 15, 20, 30, 60, 90, 120, 150, and 180 min after the tetanus. For each experiment, the response after tetanus was normalized to the baseline average. During baseline and at 1 and 2 h after tetanus, four different stimulus intensities (1×, 1.2×, 2×, and 4× fEPSP threshold) were used to generate input–output curves.
Experimental Design and Drug Treatment
Experiments were conducted between 9:00 and 16:00 h during the day. Animals were randomly divided into four experimental groups as follows: (1) vehicle gas plus saline injection (naïve–saline), (2) isoflurane plus saline injection (isoflurane–saline), (3) vehicle gas plus ciproxifan injection (naïve–ciproxifan), and (4) isoflurane plus ciproxifan injection (isoflurane–ciproxifan). Animals were exposed to 1.4% isoflurane or vehicle gas for 2 h, and the behavioral tests as well as electrophysiological recordings were performed 24 h postanesthesia. Ciproxifan hydrochloride (Sigma, Shanghai, China) was used in the experiments and administered at the dose of 3 mg/kg body weight i.p. in a volume of 1 mL of sterile saline 30 min prior to the training phase of behavioral tests or LTP induction. Control rats received 1 mL of sterile saline as a vehicle. Animals were used only once for the novel object recognition and passive avoidance tests, respectively. However, in the study of electrophysiological recordings, each rat was used in paired ciproxifan and saline treatments, in a random order with 7 days between experiments. Additional groups of rats were used to evaluate locomotor activity, anxiety level, and pain perception in the order of open‐field, EPM task and tail‐flick test, respectively.
Rats randomized to the anesthesia groups received 1.4% isoflurane in 30% oxygen for 2 h in a Plexiglas anesthetizing chamber, whereas the vehicle gas groups received 30% oxygen without any inhalational anesthetic for 2 h. The 1.4% concentration was chosen because it represents one minimum alveolar concentration (the concentration at which 50% of animals do not have a motor response to painful stimuli) of isoflurane in rats. The isoflurane concentration, oxygen, and carbon dioxide levels in the chamber were continuously monitored with an agent gas monitor, and the temperature of the Plexiglas anesthetizing chamber was controlled with a heating pad to maintain rectal temperature at 37°C ± 0.5°C. To determine adequacy of ventilation and blood pressure, a separate set of rats (n = 6) were implanted with a femoral arterial catheter for continuous measurement of mean arterial blood pressure (MAP) and heart rate. Arterial blood gases were measured before and at the end of the two‐hour anesthetic exposure.
Statistical Analysis
All data are expressed as mean ± SEM. The data were tested for normality before parametric analysis. If a measurement variable did not fit a normal distribution in different groups, a data transformation was performed followed by parametric analysis. In the novel object recognition test, differences in the mean exploration time spent with the two objects were compared using a Student's t‐test, and comparison of the discrimination index between groups was performed using one‐way ANOVA followed by the Student–Newman–Keuls test. Statistical differences in the passive avoidance test among groups were analyzed using the one‐way ANOVA, followed by post hoc Student–Newman–Keuls test. Statistical differences in the open‐field, EPM, and tail‐flick tests among groups were analyzed using the one‐way ANOVA. Electrophysiological data were analyzed by a two‐way repeated‐measures ANOVA. The data were analyzed using GraphPad Prism version 4.0 (GraphPad Software, San Diego, CA, USA). P < 0.05 was considered to be significant.
Results
Ciproxifan Ameliorates Object Recognition Memory Deficits After Isoflurane
Hippocampus‐mediated nonspatial learning and memory following isoflurane anesthesia was assessed by the novel object recognition test. As shown in Figure 1A, during the training phase, animals from all four groups showed an equal preference to the identical objects. During the choice phase performed 1 h after training, rats in the vehicle gas plus saline injection group (naïve–saline) spent more time than chance with the novel object (n = 12 per group, t = 8.133, df = 11, P < 0.0001; Figure 1B), indicating intact memory, whereas the rats exposed to isoflurane only (isoflurane–saline) spent similar times with the novel and familiar objects, exhibiting memory deficits (t = 1.932, df = 11, P = 0.0795, Figure 1B). Treating isoflurane‐exposed rats with ciproxifan (isoflurane–ciproxifan) increased preference to the novel object (n = 12 per group, t = 6.178, df = 11, P < 0.0001, Figure 1B), indicating that treatment restored recognition memory in isoflurane–ciproxifan injection rats. Furthermore, one‐way ANOVA analysis showed a significant effect of treatment on discrimination ratio (n = 12 per group, F = 6.264; P = 0.0012). Specifically, post hoc Student–Newman–Keuls analysis revealed that the isoflurane–ciproxifan injection group had a significant higher discrimination ratio when compared with the isoflurane–saline injection group (P < 0.01). Significant differences were also observed between naïve–saline injection and isoflurane–saline injection groups (P < 0.01). Without exposure to isoflurane, ciproxifan injection alone did not improve object preference, as compared to saline injection.
Figure 1.
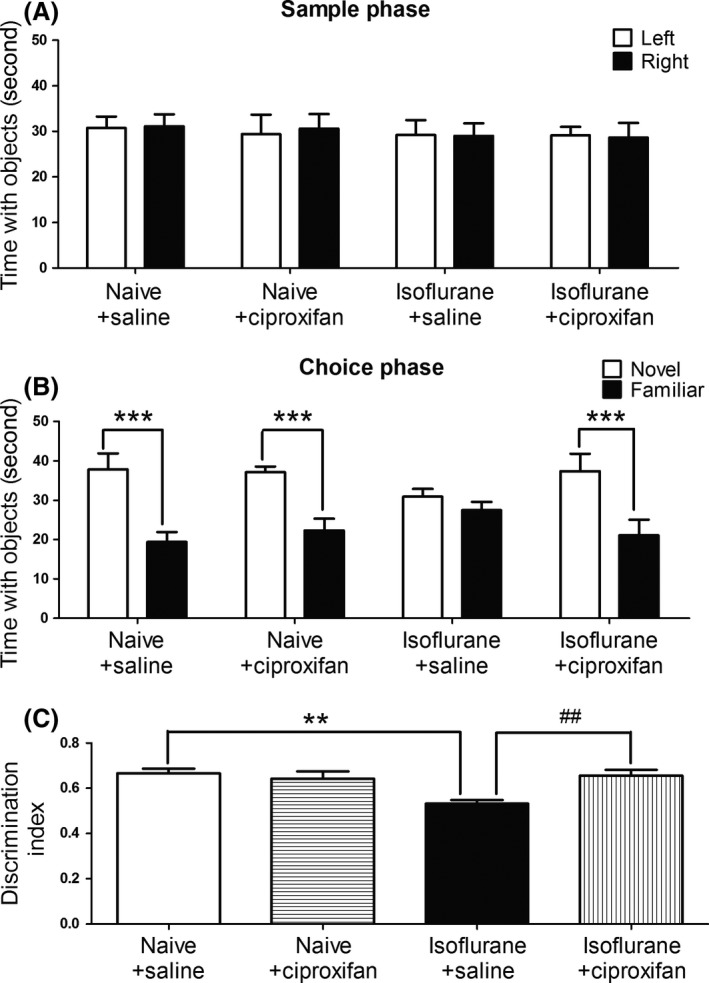
Ciproxifan ameliorates object recognition memory deficits after isoflurane. Animals were exposed to 1.4% isoflurane or vehicle gas for 2 h, and the novel object recognition test was performed 24 h postanesthesia. The drugs (ciproxifan 3 mg/kg or saline) were intraperitoneally injected in a volume of 1 mL 30 min prior to the training phase. (A) During the training phase, two identical objects were placed in two defined sites of the chamber. Animals from all four groups showed an equal preference to identical objects. (B) During the test phase performed 1 h after the training phase, one of the objects was replaced by a new object with a different shape. Time spent with the novel and familiar objects during test phase was recorded. (C) Discrimination ratio from the test phase for each treatment group. Data are expressed as mean ± SEM. (**P < 0.01, ***P < 0.001 and ##P < 0.01, respectively, n = 12 per group).
Ciproxifan Ameliorates Passive Avoidance Memory Deficits After Isoflurane
As a second memory test, we determined isoflurane‐induced cognitive deficits using a passive avoidance test. As shown in Figure 2A, there were no significant differences in the latency to enter the dark compartment in the training phase among the four groups of animals. One hour after training (Figure 2B), a significant effect of treatment on transfer times to the dark compartment was observed (n = 12 per group, F = 4.358; P = 0.0090). The retention time in the isoflurane–saline group (161.4 ± 33.3 seconds) was significantly shorter than that of the control naïve‐saline injection group (244.6 ± 24.88 seconds, P < 0.05). There was no significant difference between treatment with ciproxifan (270.1 ± 9.794 seconds, P > 0.05) and the naive group. In contrast, treatment with ciproxifan (257.0 ± 19.49 seconds, P < 0.05) showed significant improvement of the isoflurane‐induced cognitive deficit in this task.
Figure 2.
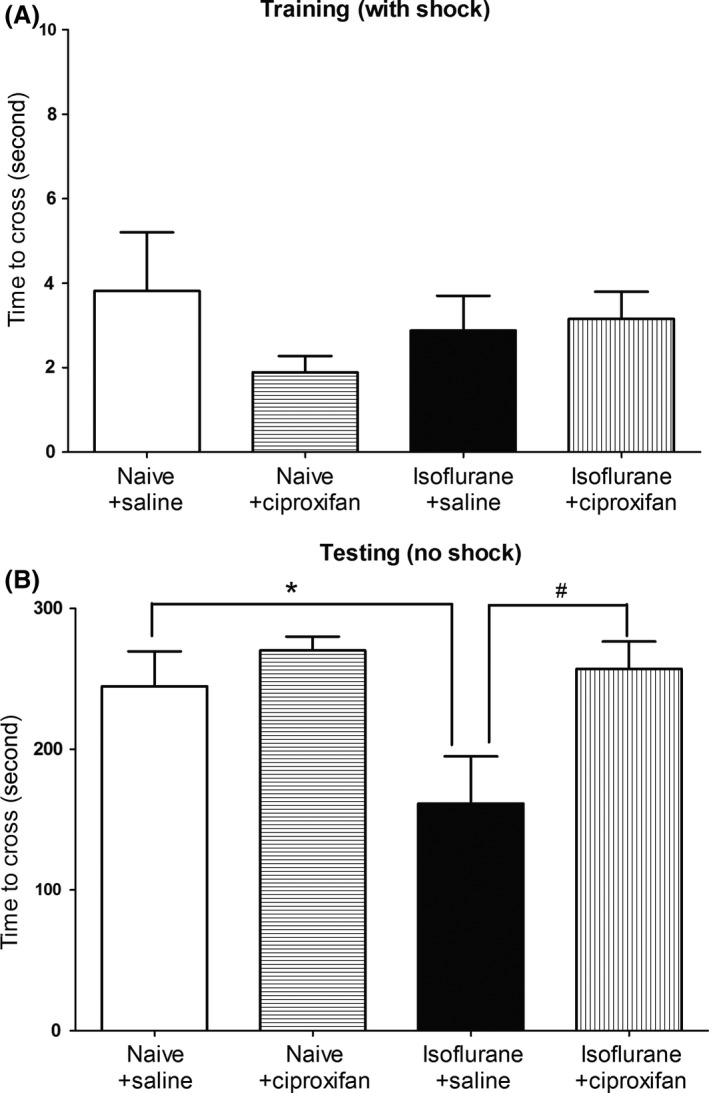
Ciproxifan ameliorates passive avoidance memory deficits after isoflurane. Animals were exposed to 1.4% isoflurane or vehicle gas for 2 h, and the passive avoidance test was performed 24 h postanesthesia. The drugs (ciproxifan 3 mg/kg or saline) were intraperitoneally injected in a volume of 1 mL 30 min prior to the training phase. (A) The rats were placed in a transfer chamber, and a foot shock was delivered upon entry into the dark compartment from the bright compartment. The rats from each group showed similar transfer times. (B) An hour later, the rats were placed in the transfer chamber again and the transfer time was recorded without foot shock. Data are expressed as mean ± SEM. (*P < 0.05 and #P < 0.05, respectively, n = 12 per group).
Neither Isoflurane Nor Ciproxifan Alters Motor Performance, Anxiety, and Nociceptive Responses
Locomotor activity was studied with open‐field test 24 h after exposure to isoflurane or vehicle gas. As shown in Figure S1A, animals in the groups of naïve–saline (5.37 ± 0.25 m), isoflurane–saline (5.74 ± 0.37 m), naïve–ciproxifan (5.15 ± 0.35 m), and isoflurane–ciproxifan(5.63 ± 0.35 m) displayed similar distance traveled during the 10 min of testing. There were no significant locomotor impairments in each of the groups when compared to the naïve–saline group (n = 12 per group, F = 0.6283, P = 0.60). No differences in mean speed were also observed among the four groups (n = 12 per group, F = 0.5532, P = 0.65, Figure S1B).
In the EPM task (Figure S1C–E), the proportion of time spent in open arms was not different among the four groups (n = 12 per group, F = 0.7067, P = 0.55): naïve–saline (34.08 ± 1.06%), isoflurane–saline (33.11 ± 0.66%), naïve–ciproxifan (32.47 ± 0.89%), and isoflurane–ciproxifan (33.86 ± 0.85%). The time spent exploring the closed arms and the central area of the maze was not statistically different among the four groups, suggesting that isoflurane/ciproxifan treatment did not alter anxiety measures.
Altered pain perception following anesthesia could also affect nonassociative fear learning. Therefore, nociception was studied using the tail‐flick assay 24 h after exposure to isoflurane or vehicle gas. No differences were detected in the latency to tail flick (n = 12 per group, F = 0.1075, P = 0.9553, Figure S1F), suggesting that all groups of animals perceived the same foot shock stimulus strength.
Neither Isoflurane Nor Ciproxifan Alters Baseline Input/Output Responses
Figure 3 depicts input/output curves for fEPSPs slope in control and isoflurane‐exposed rats treated with either saline or 3 mg/kg of ciproxifan. The fEPSPs evoked by stimuli ranging from one to four times threshold intensity collected before LTP induction were not affected by isoflurane exposure. Two‐way repeated‐measures ANOVA confirmed a lack of significant difference between the fEPSPs slope in naïve–saline group and that of isoflurane–saline group (F (1,30) = 0.81; P = 0.3902), and there was no significant group × time interaction (F (3,30) =1.71; P = 0.1856). Furthermore, ciproxifan treatment did not affect input/output curves compared with those of saline‐treated rats. Two‐way repeated‐measures ANOVA confirmed a lack of significant difference between the fEPSPs slope in naïve–saline group and that of naïve–ciproxifan group (F (1,30) = 0.01; P = 0.9176), and there was no significant group × time interaction (F (3,30) = 0.42; P = 0.7430). Thus, the ionotropic glutamate synaptic transmission was not altered by isoflurane anesthesia and/or ciproxifan treatment.
Figure 3.
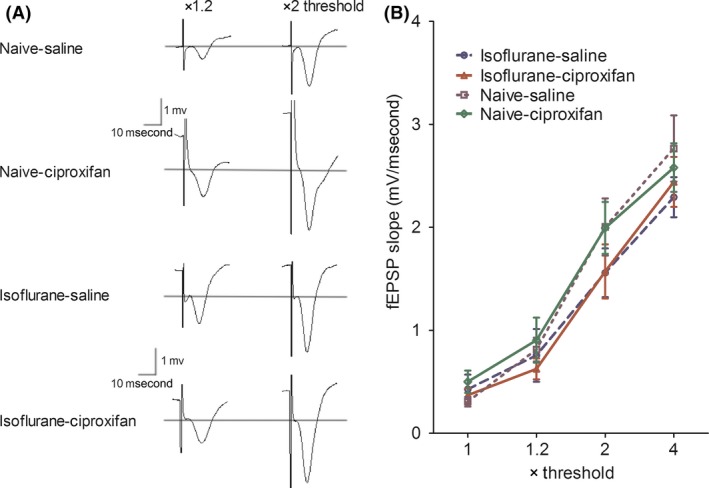
Isoflurane and ciproxifan do not alter hippocampal fEPSPs input/output responses. (A) Representative fEPSP traces at the basal‐dendritic electrode with stimulus intensities 1.2× and 2× test pulse. (B) Normalized basal‐dendritic fEPSPs responses in isoflurane/vehicle gas‐exposed rats in the presence or absence of ciproxifan. Data are expressed as mean ± SEM. A two‐way repeated‐measures ANOVA revealed no significant effect of isoflurane versus vehicle gas, and no significant effect of ciproxifan versus saline for input/output responses, n = 6 per group.
Ciproxifan Rescues Hippocampal Synaptic Plasticity After Isoflurane
Hippocampal CA1 basal‐dendritic LTP was quantified by an increase in fEPSPs slope from the baseline. As shown in Figure 4, in isoflurane‐free rats, ciproxifan did not enhance fEPSP slope compared with saline‐treated controls (Figure 4A, B). Two‐way repeated‐measures ANOVA confirmed a lack of significant difference between the LTP induced in naïve–ciproxifan and that induced in naïve–saline (F (1,160) = 0.05; P = 0.8161), and there was no significant treatment × time interaction (F (15,160) = 0.23; P = 0.999). Basal‐dendritic LTP in animals that received isoflurane was smaller than that of naïve rats. Repeated‐measures ANOVA showed a significant effect of isoflurane (F (1,160) = 17.11; P < 0.0001), a significant time effect (F (15,160) = 17.76; P < 0.0001), but no significant treatment × time interaction (F (15,160) = 0.87; P = 0.5992). When administered 30 min before tetanic stimulation, ciproxifan did not enhance the baseline fEPSPs slope compared with saline‐treated controls. However, the fEPSP responses after tetanic stimulation, in ciproxifan‐treated isoflurane rats, were significantly greater than those in saline‐treated isoflurane rats (Figure 4C, D). Repeated‐measures ANOVA showed a significant effect of ciproxifan (F (1,160) = 18.21; P < 0.0001), a significant time effect (F (15,160) = 25.27; P < 0.0001), but no significant treatment × time interaction (F (15,160) = 1.02; P = 0.4352).
Figure 4.
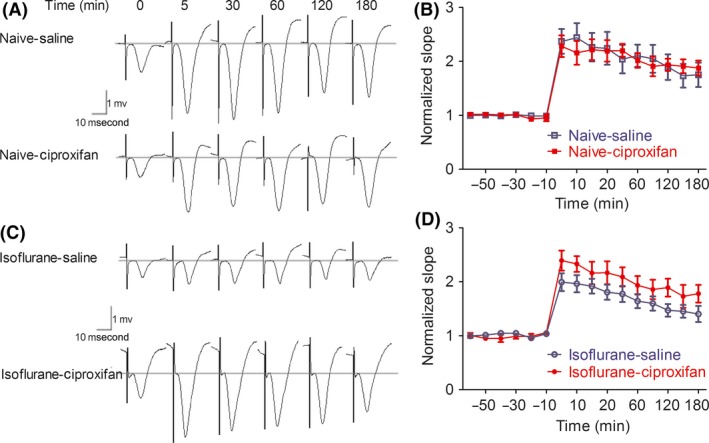
Ciproxifan rescues hippocampal synaptic plasticity after isoflurane. (A, C), fEPSP traces at the basal‐dendritic electrode of representative rats at 0 (baseline), 5, 30, 60, 120, and 180 min after tetanus, with tetanus given 30 min after i.p administration of either saline or ciproxifan in vehicle gas (A) and isoflurane (C)‐exposed rats. (B) Normalized fEPSP slopes with LTP induced after vehicle gas exposure and administration of either saline or ciproxifan 30 min before tetanus. (D) Normalized fEPSP slopes with LTP induced after isoflurane exposure and administration of either saline or ciproxifan 30 min before tetanus. Data are expressed as mean ± SEM. A two‐way repeated‐measures ANOVA revealed a significant effect of isoflurane versus vehicle gas for LTP induction, and a significant effect of ciproxifan versus saline for LTP induction in isoflurane‐exposed rats, n = 6 per group.
Isoflurane Anesthesia Does Not Change Physiological Parameters
As hypotension, hypercarbia, and hypoxia are potential causes of cognitive deficit, we analyzed the blood gas levels before and at the end of isoflurane exposure in a separate set of rats (n = 6), which revealed normal oxygenation (0 h/2 h: 132 ± 3.2 mmHg/136 ± 2.1 mmHg), adequate PaCO2 values (0 h/2 h: 40 ± 1.32 mmHg/43.2 ± 1.22 mmHg), and no acidosis (0 h/2 h: pH: 7.36 ± 0.00/7.35 ± 0.02). MAP and heart rate were also within the physiological range and remained stable over the course of the anesthesia (Table 1).
Table 1.
Effect of isoflurane on physiological parameter
| 0 h | 2 h | P value | |
|---|---|---|---|
| pH | 7.36 ± 0.02 | 7.35 ± 0.02 | >0.05 |
| PaO2 | 132 ± 3.2 | 136 ± 2.1 | >0.05 |
| PaCO2 | 40.0 ± 1.32 | 43.2 ± 1.22 | >0.05 |
| HCO3− | 23.2 ± 0.79 | 22.7 ± 0.61 | >0.05 |
| MAP | 105 ± 2 | 100 ± 2 | >0.05 |
| HR | 334 ± 7.3 | 327 ± 8.5 | >0.05 |
Values are mean ± SEM. There were no group differences in all measures (n = 6 per group).
Discussion
We demonstrated that ciproxifan is capable of reducing memory deficits caused by isoflurane anesthesia and attenuating isoflurane‐induced suppression of hippocampal LTP induction in vivo. To the best of our knowledge, this is the first preclinical work that supports the potential use of a H3R antagonist for treatment of general anesthesia‐associated cognitive disorders.
We performed behavioral tests including the novel object recognition and passive avoidance to assess the cognitive outcomes of rats following isoflurane anesthesia. Each task has a different role in investigating effects on memory. Novel object recognition is known to evaluate the function of nonspatial hippocampal memory 25. The passive avoidance is related to the amygdala–hippocampus complex, which involves both contextual memory and amygdala‐dependent emotional memory 26. In the present study, animals that were previously (24 h) exposed to 1.4% isoflurane for 2 h displayed no preference for the novel object and had impaired retention of a passive avoidance response at 1 h after sample phase. This is unlikely to be a consequence of physiological changes associated with general anesthesia, because blood pressure and arterial blood gases remained within the physiological range during anesthesia. It should also be noted that memory was the only remarkable deficit 24 h after exposure to isoflurane as no obvious impairment in motor, anxiety, and sensory function had been identified.
A number of preclinical studies indicate that exposure to volatile anesthetics, such as isoflurane, cause temporary or sustained impairments in learning and memory. In support with our finding, several studies reported that exposure of 2‐ to 4‐month‐old mice to 1–1.3% isoflurane impaired the memory performance when they were tested within 7 days after isoflurane exposure 7, 27, 28. Similarly, isoflurane (1 minimum alveolar concentration, 4 h) impaired retention memory for Morris water maze 1 week after exposure in young adult rats and resulted in a delayed although weak impairment at 4 weeks in middle‐aged rats 29. In contrast to these findings, another recent study demonstrated that two‐month‐old rats did not have impairment in spatial reference learning and fear conditioning test but had improved long‐term memory at 4 months after isoflurane anesthesia 30. The discrepancies of these findings remain unclear but may be related to animal species and strains, developmental stage, anesthetic agent, dosage, and duration of exposures.
Isoflurane exposure‐induced memory dysfunction was overcome by treatment with ciproxifan. The histamine H3 receptor antagonists have been found to improve performance in a variety of rodent models of memory impairment. Ciproxifan is able to restore the cognitive performance impaired by scopolamine and prolonged administration of corticosterone 31, 32. Single administration of ciproxifan potently prevents the deleterious effects of chronic restraint stress on cognitive functions tested with Morris water maze or Barnes maze 32. Furthermore, ciproxifan effectively improves the performance in the water maze and the object recognition test in Alzheimer's APPTg2576 mice 33. The procognitive effect of ciproxifan involves enhanced release of neurotransmitters of such as histamine, acetylcholine, dopamine, and glutamate 34, 35, 36. At the molecular level, the sustained increase in α5GABA(A)R activity contributes for impaired memory performance and synaptic plasticity in the hippocampus 17. α5GABAA receptor‐selective inverse agonist L‐655,708 effectively prevents memory deficit after anesthesia 27. Histamine has been shown to act through allosteric sites on GABAA receptor channels, depending upon subunit composition 37, 38. However, evidence for histamine‐GABA cross‐talk in native neuronal contexts remains to be elucidated 37.
Both novel object recognition and passive avoidance are sensitive to impairments in hippocampal function 39, 40. We therefore used an in vivo paradigm of LTP to investigate the putative hippocampal site of action for the cognitive effects observed with isoflurane exposure. Our finding demonstrated that hippocampal synaptic plasticity in freely moving rats is impaired after isoflurane anesthesia and that the LTP deficit can be ameliorated by treatment with ciproxifan. Moreover, ciproxifan at the 3 mg/kg dose rescued synaptic potentiation without increasing baseline input/output responses, suggesting that the histamine H3 receptor system affects synaptic potentiation at doses that do not affect baseline neurotransmission. It is of interest to note that LTP was not enhanced by the administration of ciproxifan in control rats, possibly because LTP induced during behavioral exploration had already maximized the degree of synaptic potentiation 24, 41. This also supports the finding that behavioral performance on the tasks could not be improved any further when ciproxifan was given to animals without isoflurane exposure.
Histaminergic neurons, located exclusively in the tuberomammillary nucleus of the posterior hypothalamus, are involved in the wake–sleep regulation. They enhance arousal via diffuse projections to the cerebral cortex 8. There is growing evidence that the neural histamine system is influenced by many general anesthetic agents 42, 43, 44. Inhalation of halothane dose dependently reduces endogenous histamine levels from the anterior hypothalamic area of rats 42. Depletion of brain histamine content by selective lesions of the histaminergic tuberomammillary nucleus facilitates anesthesia maintenance and delays anesthesia emergence by isoflurane 43. Moreover, exogenous administration of histaminergic agents modulates the depth of anesthesia and emergence time 44.
Memory impairment was found 24 h after isoflurane exposure in the present study. The 24‐hour time interval from anesthetic discontinuation is sufficient to avoid the confounding effects of residual anesthetic on neurobehavioral performance 27, 45. Moreover, it has been demonstrated that exposure to isoflurane does not alter subsequent levels of wakefulness 46. Thus, our finding suggests that H3R may serve as therapeutic target for improvement of anesthesia‐associated cognitive deficits. However, it cannot exclude the possibility that ciproxifan enhances cognition by improving vigilance. Indeed, recent evidence suggests that ciproxifan enhances vigilance and wake 11, 47, 48, 49. The arousal effect mainly depends on the activation of histaminergic systems through H1R 47, 48. A functional imaging study demonstrated that fronto‐cortical areas involved in higher cognitive function, and the network of pro‐arousing areas can be activated simultaneously by cognitive enhancing reagent 50. Further investigations are also required to determine the effect of ciproxifan on cortical activation, as well as acquisition, consolidation and retrieval of memory processes following anesthesia specifically.
The results of this study must be viewed in light of potential limitations. First, ciproxifan amelioration of memory impairment does not mean that the underlying mechanism of isoflurane‐induced memory dysfunction is mediated through the central histamine system per se. The inhibition of presynaptic H3 receptors by ciproxifan leads to the release of neurotransmitters including histamine, acetylcholine, noradrenaline, dopamine, and serotonin 34, 35, 36. The extent to which each of these putative mechanisms of ciproxifan action may have contributed to prolonged synaptic potentiation and memory enhancing needs further investigation. Direct measuring‐related neurotransmitter levels would help solving this question. Second, only two hippocampus‐related memory tasks and electrophysiological changes were investigated in this study. In addition to its high expression in the hippocampus, H3 receptors are also widely distributed in brain regions including the cerebral cortex, basal ganglia, globus pallidus, and hypothalamus that are implicated in learning and memory processes 8. Future research may reveal whether the effects found here can be generalized to nonhippocampal‐dependent cognitive functions. Third, this study was performed in young adult animals, which cannot be translated to postoperative cognitive impairments seen in elderly humans in clinical situations. Further investigations with evaluating the effect of ciproxifan in animal model of postoperative cognitive dysfunction will be of more clinical significance.
In summary, we have observed that systemic administration of ciproxifan attenuated memory deficit and hippocampal synaptic plasticity impairment following isoflurane exposure. It is worth noting that in March 2016, the European Commission granted a marketing authorization for pitolisant (as the first representative of the H3R inverse agonists) for the treatment of narcolepsy 51. The waking action of Pitolisant in experimental animals and healthy human volunteers is associated with a shift of the power distribution pattern of the cortical EEG in favor of the high‐frequency rhythms, which are known to accompany cognitive activities such as attention or learning 52. A future investigation evaluating the efficacy of pitolisant in improving arousal and cognition after general anesthesia may be of greater clinical significance.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1. Isoflurane and ciproxifan do not affect motor function, anxiety, and nociception in rats.
Acknowledgment
We would like to express great thanks to Dr. Stan Leung for critical reading of the manuscript and for his important comments and suggestions. This study was supported by Nature Science Foundation of China Grant number 81271205, 81301103 Guangdong Natural Science Foundation 2015A030313781, and Health and Family Planning Commission of Shenzhen Municipality Research Grant number 201501025.
References
- 1. Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth 2009;103(Suppl. 1):i41–i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bryson GL, Wyand A. Evidence‐based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anaesth 2006;53:669–677. [DOI] [PubMed] [Google Scholar]
- 3. Liang G, Ward C, Peng J, et al. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology 2010;112:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology 2011;61:1354–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao L, Li L, Lin D, et al. Isoflurane induces learning impairment that is mediated by interleukin 1beta in rodents. PLoS ONE 2012;7:e51431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Xu Z, Wang H, et al. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 2012;71:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zurek AA, Bridgwater EM, Orser BA. Inhibition of alpha5 gamma‐Aminobutyric acid type A receptors restores recognition memory after general anesthesia. Anesth Analg 2012;114:845–855. [DOI] [PubMed] [Google Scholar]
- 8. Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev 2008;88:1183–1241. [DOI] [PubMed] [Google Scholar]
- 9. Arrang JM, Garbarg M, Schwartz JC. Autoinhibition of histamine synthesis mediated by presynaptic H3‐receptors. Neuroscience 1987;23:149–157. [DOI] [PubMed] [Google Scholar]
- 10. Arrang JM, Garbarg M, Schwartz JC. Auto‐inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 1983;302:832–837. [DOI] [PubMed] [Google Scholar]
- 11. Gondard E, Anaclet C, Akaoka H, et al. Enhanced histaminergic neurotransmission and sleep‐wake alterations, a study in histamine H3‐receptor knock‐out mice. Neuropsychopharmacology 2013;38:1015–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esbenshade TA, Browman KE, Bitner RS, et al. The histamine H3 receptor: an attractive target for the treatment of cognitive disorders. Br J Pharmacol 2008;154:1166–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frey U, Morris RG. Synaptic tagging and long‐term potentiation. Nature 1997;385:533–536. [DOI] [PubMed] [Google Scholar]
- 14. Simon W, Hapfelmeier G, Kochs E, et al. Isoflurane blocks synaptic plasticity in the mouse hippocampus. Anesthesiology 2001;94:1058–1065. [DOI] [PubMed] [Google Scholar]
- 15. Haseneder R, Kratzer S, von Meyer L, et al. Isoflurane and sevoflurane dose‐dependently impair hippocampal long‐term potentiation. Eur J Pharmacol 2009;623:47–51. [DOI] [PubMed] [Google Scholar]
- 16. Ballesteros KA, Sikorski A, Orfila JE, et al. Effects of inhaled anesthetic isoflurane on long‐term potentiation of CA3 pyramidal cell afferents in vivo. Int J Gen Med 2012;5:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zurek AA, Yu J, Wang DS, et al. Sustained increase in alpha5GABAA receptor function impairs memory after anesthesia. J Clin Invest 2014;124:5437–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown RE, Fedorov NB, Haas HL, et al. Histaminergic modulation of synaptic plasticity in area CA1 of rat hippocampal slices. Neuropharmacology 1995;34:181–190. [DOI] [PubMed] [Google Scholar]
- 19. Panula P, Chazot PL, Cowart M, et al. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine receptors. Pharmacol Rev 2015;67:601–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding F, Zheng L, Liu M, et al. Ciproxifan, an H3 receptor antagonist, improves short‐term recognition memory impaired by isoflurane anesthesia. J Anesth 2016;30:684–690. [DOI] [PubMed] [Google Scholar]
- 21. Ligneau X, Lin J, Vanni‐Mercier G, et al. Neurochemical and behavioral effects of ciproxifan, a potent histamine H3‐receptor antagonist. J Pharmacol Exp Ther 1998;287:658–666. [PubMed] [Google Scholar]
- 22. Luo T, Wu J, Kabadi SV, et al. Propofol limits microglial activation after experimental brain trauma through inhibition of nicotinamide adenine dinucleotide phosphate oxidase. Anesthesiology 2013;119:1370–1388. [DOI] [PubMed] [Google Scholar]
- 23. Wu J, Stoica BA, Luo T, et al. Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment. Involvement of cell cycle activation. Cell Cycle 2014;13:2446–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo T, Leung LS. Endogenous histamine facilitates long‐term potentiation in the hippocampus during walking. J Neurosci 2010;30:7845–7852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 2012;13:93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu J, Zhao Z, Sabirzhanov B, et al. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci 2014;34:10989–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saab BJ, Maclean AJ, Kanisek M, et al. Short‐term memory impairment after isoflurane in mice is prevented by the alpha5 gamma‐aminobutyric acid type A receptor inverse agonist L‐655,708. Anesthesiology 2010;113:1061–1071. [DOI] [PubMed] [Google Scholar]
- 28. Valentim AM, Di Giminiani P, Ribeiro PO, et al. Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology 2010;113:1099–1108. [DOI] [PubMed] [Google Scholar]
- 29. Callaway JK, Jones NC, Royse CF. Isoflurane induces cognitive deficits in the Morris water maze task in rats. Eur J Anaesthesiol 2012;29:239–245. [DOI] [PubMed] [Google Scholar]
- 30. Stratmann G, Sall JW, May LD, et al. Isoflurane differentially affects neurogenesis and long‐term neurocognitive function in 60‐day‐old and 7‐day‐old rats. Anesthesiology 2009;110:834–848. [DOI] [PubMed] [Google Scholar]
- 31. Pascoli V, Boer‐Saccomani C, Hermant JF. H3 receptor antagonists reverse delay‐dependent deficits in novel object discrimination by enhancing retrieval. Psychopharmacology 2009;202:141–152. [DOI] [PubMed] [Google Scholar]
- 32. Trofimiuk E, Braszko JJ. Single dose of H3 receptor antagonist–ciproxifan–abolishes negative effects of chronic stress on cognitive processes in rats. Psychopharmacology 2014;231:209–219. [DOI] [PubMed] [Google Scholar]
- 33. Bardgett ME, Davis NN, Schultheis PJ, et al. Ciproxifan, an H3 receptor antagonist, alleviates hyperactivity and cognitive deficits in the APP Tg2576 mouse model of Alzheimer's disease. Neurobiol Learn Mem 2011;95:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bacciottini L, Passani MB, Giovannelli L, et al. Endogenous histamine in the medial septum‐diagonal band complex increases the release of acetylcholine from the hippocampus: a dual‐probe microdialysis study in the freely moving rat. Eur J Neurosci 2002;15: 1669–1680. [DOI] [PubMed] [Google Scholar]
- 35. Fox GB, Esbenshade TA, Pan JB, et al. Pharmacological properties of ABT‐239 [4‐(2‐{2‐[(2R)‐2‐Methylpyrrolidinyl]ethyl}‐benzofuran‐5‐yl)benzonitrile]: II. Neurophysiological characterization and broad preclinical efficacy in cognition and schizophrenia of a potent and selective histamine H3 receptor antagonist. J Pharmacol Exp Ther 2005;313:176–190. [DOI] [PubMed] [Google Scholar]
- 36. Bardgett ME, Points M, Kleier J, et al. The H3 antagonist, ciproxifan, alleviates the memory impairment but enhances the motor effects of MK‐801 (dizocilpine) in rats. Neuropharmacology 2010;59:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bianchi MT, Clark AG, Fisher JL. The wake‐promoting transmitter histamine preferentially enhances α‐4 subunit‐containing GABAA receptors. Neuropharmacology 2011;61:747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saras A, Gisselmann G, Vogt‐Eisele AK, et al. Histamine action on vertebrate GABAA receptors: direct channel gating and potentiation of GABA responses. J Biol Chem 2008;283:10470–10475. [DOI] [PubMed] [Google Scholar]
- 39. Stubley‐Weatherly L, Harding JW, Wright JW. Effects of discrete kainic acid‐induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res 1996;716:29–38. [DOI] [PubMed] [Google Scholar]
- 40. Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA 2004;101:14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leung LS, Shen B, Rajakumar N, et al. Cholinergic activity enhances hippocampal long‐term potentiation in CA1 during walking in rats. J Neurosci 2003;23:9297–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mammoto T, Yamamoto Y, Kagawa K, et al. Interactions between neuronal histamine and halothane anesthesia in rats. J Neurochem 1997;69:406–411. [DOI] [PubMed] [Google Scholar]
- 43. Luo T, Leung LS. Involvement of tuberomamillary histaminergic neurons in isoflurane anesthesia. Anesthesiology 2011;115:36–43. [DOI] [PubMed] [Google Scholar]
- 44. Luo T, Leung LS. Basal forebrain histaminergic transmission modulates electroencephalographic activity and emergence from isoflurane anesthesia. Anesthesiology 2009;111:725–733. [DOI] [PubMed] [Google Scholar]
- 45. Bekker A, Shah R, Quartermain D, et al. Isoflurane preserves spatial working memory in adult mice after moderate hypoxia. Anesth Analg 2006;102:1134–1138. [DOI] [PubMed] [Google Scholar]
- 46. Pick J, Chen Y, Moore JT, et al. Rapid eye movement sleep debt accrues in mice exposed to volatile anesthetics. Anesthesiology 2011;115:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parmentier R, Zhao Y, Perier M, et al. Role of histamine H1‐receptor on behavioral states and wake maintenance during deficiency of a brain activating system: a study using a knockout mouse model. Neuropharmacology 2016;106:20–34. [DOI] [PubMed] [Google Scholar]
- 48. Parmentier R, Anaclet C, Guhennec C, et al. The brain H3‐receptor as a novel therapeutic target for vigilance and sleep‐wake disorders. Biochem Pharmacol 2007;73:1157–1171. [DOI] [PubMed] [Google Scholar]
- 49. Parmentier R, Ohtsu H, Djebbara‐Hannas Z, et al. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock‐out mice: evidence for the role of brain histamine in behavioral and sleep‐wake control. J Neurosci 2002;22:7695–7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gozzi A, Colavito V, Seke Etet PF, et al. Modulation of fronto‐cortical activity by modafinil: a functional imaging and fos study in the rat. Neuropsychopharmacology 2012;37:822–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Syed YY. Pitolisant: first global approval. Drugs 2016;76:1313–1318. [DOI] [PubMed] [Google Scholar]
- 52. Schwartz JC. The histamine H3 receptor: from discovery to clinical trials with pitolisant. Br J Pharmacol 2011;163:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Isoflurane and ciproxifan do not affect motor function, anxiety, and nociception in rats.


