Summary
Aims
To evaluate the effectiveness of repetitive transcranial magnetic stimulation (rTMS) on motor recovery after stroke using a prospective, double‐blind, randomized, sham‐controlled study.
Methods
Patients with unilateral subcortical infarction in the middle cerebral artery territory within 1 week after onset were enrolled. The patients were randomly divided into an rTMS treatment group and a sham group. We performed high‐frequency rTMS or sham rTMS on the two groups. Motor functional scores were assessed pre‐ and post‐rTMS/sham rTMS and at 1 month, 3 months, 6 months, and 1 year after stroke onset. The scores included the National Institutes of Health Stroke Scale (NIHSS), Barthel Index (BI), Fugl‐Meyer Assessment Upper Limb/Lower Limb (FMA‐UL/LL), modified Rank Score (mRS), and the resting motor threshold (RMT) of the hemiplegic limb.
Results
At baseline, no significant differences were found between the two groups for motor functional scores. On the second day after rTMS treatment, score improvements of the NIHSS, BI, FMA‐UL in the real treatment group were more significant than those in the sham group. In addition, similar results were obtained at 1 month. However, at 3 months, 6 months, and 1 year after onset, no significant differences in improvement were observed between the two groups, except for the FMA‐UL score improvement.
Conclusion
rTMS facilitates motor recovery of acute stroke patients, and the effect can last to 1 month, except the function improvement on upper extremities could last for 1 year. A single course of rTMS in the acute stage may induce the improvement of upper extremities function lasted for 1 year.
Keywords: acute stroke, barthel Index, fugl‐meyer assessment upper limb/lower limb, modified Rank Score, national institutes of health stroke scale, repetitive transcranial magnetic stimulation
1. INTRODUCTION
Many methods have been applied to facilitate recovery after stroke. However, improvement in rehabilitation strategies is necessary because many patients remain disabled after treatment.1, 2 Traditional methods, such as physical therapy and occupational therapy, are time‐consuming and labor‐intensive. Repetitive transcranial magnetic stimulation (rTMS) has been increasingly used for the treatment of depression, motor dysfunction after stroke, aphasia, and mental disorders since 2000.3, 4, 5, 6, 7, 8, 9 It is particularly popular in research studies on functional recovery and brain reorganization after stroke. Currently, the internationally recognized strategy is high‐frequency (≥5 Hz) rTMS applied to the ipsilesional primary motor cortex (M1) to facilitate its reperfusion and reorganization.10 It has been proven to play a positive role in functional rehabilitation after stroke, and brain reorganization has been noted on functional magnetic resonance imaging (fMRI).9, 11 However, low‐frequency rTMS applied to the contralesional M1 may also facilitate recovery as it weakens the contralesional hemisphere's inhibitory effect on the ipsilesional hemisphere.12 Some studies have used measurements, including clinical assessment, fMRI, motor evoked potentials (MEPs), and central excitatory time, to compare the two types of rTMS and have found no significant differences.13, 14 Many studies have demonstrated the positive role of rTMS in functional improvement, although these studies have varied in observation time and stages of stroke.15, 16 However, the onset and maintenance time of rTMS remain uncertain.
Our study was a randomized, double‐blind, and sham‐controlled study of rTMS in acute ischemic stroke patients. We evaluated participants pre‐ and post‐rTMS/sham rTMS and at 1 month, 3 months, 6 months, and 1 year after onset to investigate whether high‐frequency rTMS over the ipsilesional M1 could facilitate functional improvement, and we examined how long this effect could be maintained.
2. MATERIALS AND METHODS
2.1. Clinical data
We recruited acute ischemic stroke patients from January 2013 to January 2016. Only patients with unilateral subcortical lesions in the middle cerebral artery territory as detected using diffusion weighted imaging (DWI) within 1 week after onset were enrolled in our study. The exclusion criteria were as follows: (i) a history of stroke or cerebral small vessel disease, (ii) cognitive impairment (Mini‐Mental State Examination score ≤24); (iii) a history of serious lung and heart diseases, liver and renal failure diseases or malignant tumors; and (iv) any MRI contraindications. The study protocol and consent forms were reviewed and approved by the ethics committee of the hospital, and full written consent was obtained from all participants. The study was registered under the Clinical Trials Registry Number NCT03163758 (http://register.clinicaltrials.gov. Title:Cerebral Reorganization of Stoke Patients after Repetitive Transcranial Magnetic Stimulation by Neuroimaging Analysis).
All patients were admitted to the hospital after enrollment. The patients were treated with a series of standardized therapies, including antiplatelet drugs and motor rehabilitative training (beginning on the second day after assessment and performed by the same rehabilitation physician). Routine examinations, such as routine blood, liver and renal function tests and electrocardiograms, were performed for every patient. An electroencephalogram was also performed before rTMS or sham rTMS.
2.2. Grouping and evaluation
The patients were divided into a real rTMS treatment group and a sham group in a random and double‐blinded manner. A random number was generated by a computer, and the processing method was placed into a sealed envelope. A nurse who was not involved in the clinical evaluation was responsible for issuing and registering the number. The functional scores of the patients were independently assessed by an experienced neurologist at each follow‐up time point. The staff members who implemented rTMS were not involved in the clinical assessment, and the rehabilitation physician was not aware of the patient groupings.
The scoring methods included the following: (i) National Institutes of Health Stroke Scale (NIHSS), (ii) Barthel Index (BI), (iii) Fugl‐Meyer Assessment Upper Limb/Lower Limb (FMA‐UL/LL), (iv) modified Rank Score (mRS), and (v) the resting motor threshold (MT) of the hemiplegic upper limbs. The former 3 scores were the primary endpoints, and the latter 2 scores were the secondary endpoints. We evaluated each patient at 6 time points, including grouping time, the second day after treatment, 1 month after onset, 3 month after onset, 6 month after onset, and 1 year after onset.
2.3. rTMS
The MT(motor threshold) of the ipsilesional and contralesional abductor digiti minimi muscles were determined for every patient before rTMS or sham rTMS to evaluate motor function for both cerebral hemispheres. The RMT was defined as the lowest intensity capable of eliciting at least 5 MEPs of 50 μV peak‐to‐peak amplitude in 10 consecutive stimulations when single‐pulse TMS was delivered to the contralateral cortex. If the minimum MEP amplitude could not be detected, then it was recorded as 100%. MTs for bilateral cerebral hemisphere were recorded.
All patients received consecutive 10‐day rTMS or sham rTMS. We used a Medtronic MagPro type magnetic stimulation device (Medtronic, Minneapolis, MN, USA) and a figure‐eight coil (MC‐B70, Medtronic). Regarding the safety threshold suggested by the International Federation of Clinical Neurophysiology (IFCN) and related studies,17 our protocol used 5 Hz rTMS applied to the ipsilesional M1 with a stimulation intensity set at 120% of the MT data of the contralateral side M1 because the MTs on lesion side were changed because of motor function destroyed since stroke. The treatment involved 50 trains of 20 pulses with 2‐second intertrain intervals daily. In the rTMS treatment group, coils were placed tangent to the scalp, while in the sham group, coils were placed perpendicular to the scalp. The patient wore a 10‐20 system EEG cap for scalp location and earplugs to protect their hearing.
2.4. Statistical analyses
Statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Measurement data are described using means and standard deviations and were compared using the Mann‐Whitney U test. Enumeration data were compared using the Chi‐square test. A P value < 0.05 means significant difference.
3. RESULTS
3.1. Baseline clinical data
Fifty‐two patients were recruited in our study and 42 were randomly divided into an rTMS treatment group and a sham group; each group consisted of 21 patients. However, 8 patients in the treatment group and 7 patients were lost to follow‐up due to personal reasons and 13 patients in real rTMS group for analyzed and 14 patients in sham rTMS group for analyzed (Figure 1). None of the patients complained of discomfort after rTMS or sham rTMS. In total, 30 males and 12 females with first‐onset acute ischemic stroke lesions detected by DWI participated in the study. Twenty‐three and 19 infarctions were located in the left and right hemisphere, respectively. The baseline assessment time ranged from 1 to 14 days (average 4.6 ± 3.7 days) after stroke onset. For all patients, the NIHSS score at baseline was 6.6 ± 2.1, the BI was 62.7 ± 8.3, the mRS was 3.8 ± 0.9, the FMA‐UL was 38.0 ± 8.3, the FMA‐LL was 21.5 ± 8.3, and the paralyzed upper limb MT was 59.1 ± 18.6.
Figure 1.
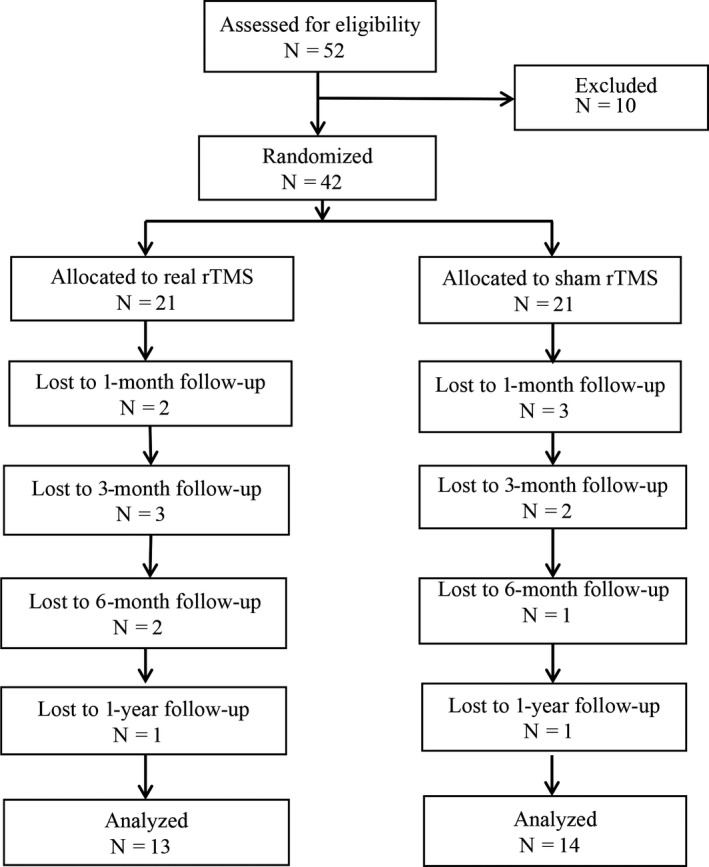
Consolidated standards of reporting trials flow diagram. rTMS, repetitive transcranial magnetic stimulation
No significant differences in age, gender, study entry time, and lesion side were observed between the rTMS treatment group and the sham group. The baseline functional assessment did not differ between the two groups (Table 1).
Table 1.
Demographic and clinical data of patients onset
| Real rTMS (N = 21) | Sham rTMS (N = 21) | P | Total | |
|---|---|---|---|---|
| Age | 59.7 ± 6.8 | 57.4 ± 14.0 | 0.528 | 58.5 ± 11.1 |
| Gender (M:F) | 16:5 | 14:7 | 0.496 | 30:12 |
| Days after onset | 3.8 ± 3.4 | 4.8 ± 4.1 | 0.427 | 4.6 ± 3.7 |
| Lesion side (L:R) | 11:10 | 12:9 | 0.757 | 23:19 |
| NIHSS | 6.9 ± 2.7 | 6.2 ± 1.2 | 0.269 | 6.6 ± 2.1 |
| BI | 59.3 ± 10.5 | 65.3 ± 8.9 | 0.572 | 62.7 ± 8.3 |
| mRS | 3.6 ± 0.5 | 3.4 ± 0.5 | 0.138 | 3.5 ± 0.5 |
| FMA‐UL | 37.4 ± 9.8 | 40.9 ± 8.9 | 0.231 | 39.1 ± 9.5 |
| FMA‐LL | 24.2 ± 3.5 | 25.6 ± 2.7 | 0.159 | 24.9 ± 3.2 |
| MT on lesion side | 52.9 ± 13.1% | 48.1 ± 15.8% | 0.135 | 50.5 ± 10.3 |
NIHSS, National Institutes of Health Stroke Scale; BI, Barthel Index; mRS, modified Rank Score; FMA‐UL, Fugl‐Meyer Assessment of Upper Limbs; FMA‐LL, Fugl‐Meyer Assessment of Lower Limbs; RMT, resting motor threshold of hemiplegic upper limbs.
3.2. Clinical data post‐rTMS/sham rTMS
No significant differences were observed in the first analyzed (the second day after treatment) motor function after treatment including NIHSS, BI, mRS, FMA‐UL, FMA‐LL, and the paralyzed upper limb MT score between real and sham groups. However, score improvement(the difference between scores after treatment and onset scores)in these functional scores pre‐ and post‐treatment, such as the NIHSS (P = .032), BI (P = .047), FMA‐UL (P = .037), but not FMA‐LL (P = .952) (Table 2).
Table 2.
Changes in the clinical assessment during follow‐up
| NIHSS | BI | mRS | FMA‐UL | FMA‐LL | MT | ||
|---|---|---|---|---|---|---|---|
| The second day after rTMS treatment | |||||||
| Score | Real | 4.0 ± 1.6 | 79.5 ± 9.1 | 3.2 ± 0.5 | 45.9 ± 9.1 | 28.4 ± 3.8 | 51.2 ± 10.9 |
| Sham | 4.1 ± 1.2 | 77.8 ± 9.0 | 3.0 ± 0.4 | 47.7 ± 8.1 | 30.4 ± 4.4 | 46.4 ± 5.1 | |
| P | 0.746 | 0.554 | 0.366 | 0.522 | 0.122 | 0.078 | |
| Score improvement | Real | 3.0 ± 1.5 | 16.7 ± 6.2 | 0.4 ± 0.6 | 8.6 ± 2.9 | 4.3 ± 2.0 | 1.7 ± 2.8 |
| Sham | 2.1 ± 0.9 | 11.9 ± 7.5 | 0.3 ± 0.6 | 6.7 ± 2.4 | 4.4 ± 2.9 | 1.4 ± 2.3 | |
| P | 0.035a | 0.030a | 0.556 | 0.033a | 0.952 | 0.770 | |
| 1 month after onset | |||||||
| Score | Real | 3.3 ± 1.4 | 85.0 ± 7.9 | 3.1 ± 0.5 | 51.1 ± 6.8 | 29.9 ± 2.4 | 51.1 ± 11.0 |
| Sham | 3.4 ± 1.1 | 84.4 ± 7.9 | 2.9 ± 0.4 | 53.8 ± 6.0 | 29.2 ± 1.8 | 45.6 ± 5.1 | |
| P | 0.660 | 0.839 | 0.593 | 0.206 | 0.353 | 0.063 | |
| Score improvement | Real | 3.8 ± 1.8 | 22.6 ± 6.5 | 0.5 ± 0.7 | 14.9 ± 4.4 | 5.7 ± 2.5 | 2.4 ± 3.5 |
| Sham | 2.7 ± 0.6 | 17.8 ± 6.9 | 0.4 ± 0.5 | 11.1 ± 3.8 | 4.2 ± 2.6 | 2.2 ± 2.6 | |
| P | 0.025a | 0.032a | 0.605 | 0.007a | 0.065 | 0.886 | |
| 3 months after onset | |||||||
| Score | Real | 2.6 ± 0.9 | 90.6 ± 6.0 | 2.9 ± 0.6 | 57.5 ± 3.7 | 30.9 ± 2.1 | 50.3 ± 11.8 |
| Sham | 2.6 ± 0.9 | 91.8 ± 6.0 | 2.9 ± 0.4 | 59.4 ± 3.6 | 31.0 ± 1.6 | 45.0 ± 5.2 | |
| P | 0.850 | 0.561 | 0.872 | 0.154 | 0.930 | 0.108 | |
| Score improvement | Real | 4.2 ± 1.3 | 28.4 ± 7.2 | 1.2 ± 1.5 | 20.4 ± 7.6 | 7.1 ± 3.7 | 3.4 ± 3.5 |
| Sham | 3.7 ± 0.8 | 25.9 ± 4.5 | 0.5 ± 0.6 | 15.2 ± 3.5 | 5.8 ± 2.6 | 2.8 ± 3.6 | |
| P | 0.397 | 0.251 | 0.141 | 0.017a | 0.230 | 0.625 | |
| 6 months after onset | |||||||
| Score | Real | 2.2 ± 0.9 | 94.3 ± 7.0 | 2.5 ± 0.7 | 59.2 ± 1.8 | 31.9 ± 2.4 | 48.6 ± 12.9 |
| Sham | 2.0 ± 0.7 | 94.3 ± 6.2 | 2.5 ± 0.4 | 60.8 ± 2.3 | 31.2 ± 1.3 | 43.0 ± 4.9 | |
| P | 0.490 | 0.985 | 0.991 | 0.063 | 0.362 | 0.132 | |
| Score improvement | Real | 4.7 ± 1.5 | 32.9 ± 6.7 | 1.8 ± 1.7 | 22.2 ± 7.9 | 7.3 ± 2.3 | 5.7 ± 3.8 |
| Sham | 4.2 ± 1.0 | 28.7 ± 5.8 | 0.9 ± 0.7 | 14.9 ± 3.2 | 5.7 ± 2.3 | 5.0 ± 4.6 | |
| P | 0.284 | 0.083 | 0.076 | 0.009a | 0.060 | 0.656 | |
| 1 year after onset | |||||||
| Score | Real | 1.5 ± 0.8 | 96.9 ± 4.8 | 1.9 ± 0.8 | 60.0 ± 2.1 | 31.9 ± 2.6 | 48.8 ± 13.4 |
| Sham | 1.1 ± 0.4 | 98.2 ± 4.2 | 1.8 ± 0.6 | 61.5 ± 1.8 | 31.9 ± 0.9 | 43.0 ± 4.6 | |
| P | 0.133 | 0.464 | 0.612 | 0.054 | 0.978 | 0.124 | |
| Score improvement | Real | 5.4 ± 1.5 | 36.1 ± 7.4 | 2.4 ± 1.9 | 22.4 ± 8.3 | 7.9 ± 2.9 | 6.1 ± 3.6 |
| Sham | 4.9 ± 1.2 | 31.4 ± 6.3 | 1.6 ± 0.9 | 15.2 ± 2.7 | 6.5 ± 2.3 | 4.3 ± 3.9 | |
| P | 0.308 | 0.086 | 0.154 | 0.006a | 0.201 | 0.207 | |
significantly different, P < .05; NIHSS, National Institutes of Health Stroke Scale; BI, Barthel Index; mRS, modified Rank Score; FMA‐UL, Fugl‐Meyer Assessment of Upper Limbs; FMA‐LL, Fugl‐Meyer Assessment of Lower Limbs; RMT, resting motor threshold of hemiplegic upper limbs.
3.3. Clinical data at 1 month, 3 months, 6 months, and 1 year after onset
There were still significant differences in changes in functional scores, including the NIHSS, BI, and FMA‐UL, which were observed between the rTMS treatment group and the sham group at 1 month after stroke onset. At 3 months after onset, only changes in the FMA‐UL differed between the two groups. And the improvements of FMA‐UL score were observed till 6 months and 1 year since stroke onset (Table 2, Figure 2).
Figure 2.
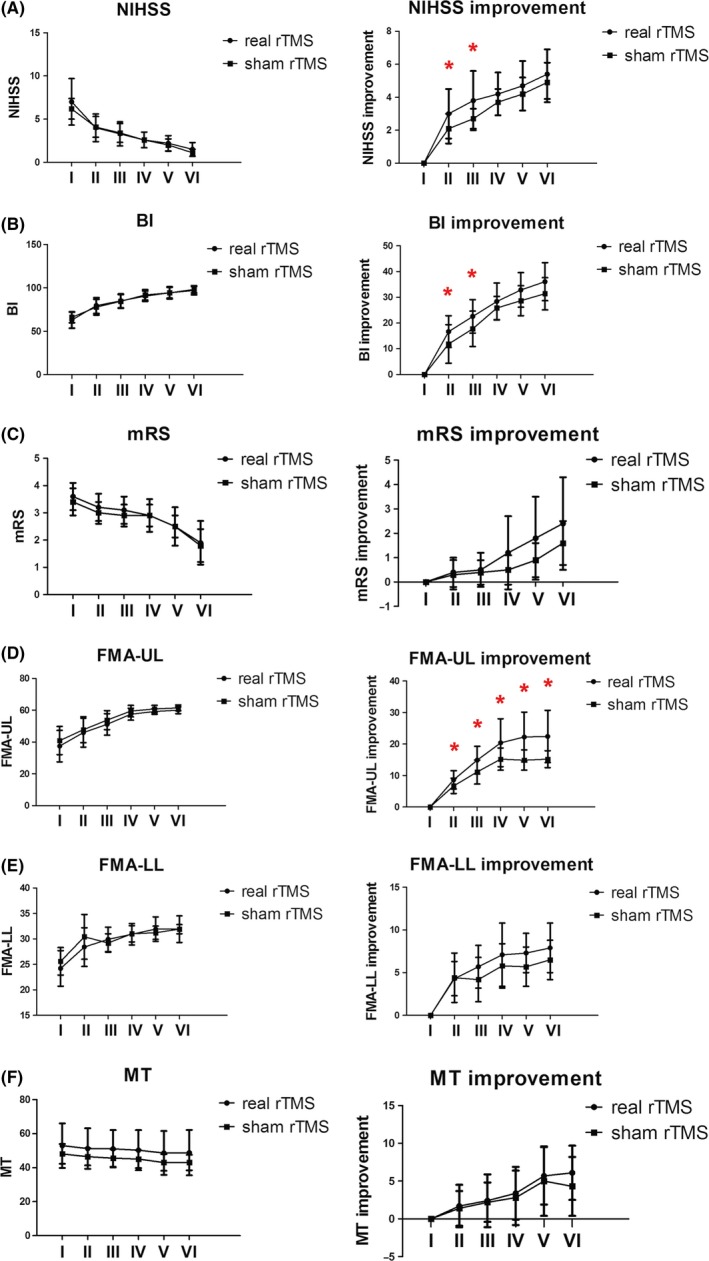
Changes in the NIHSS, BI, mRS, FMA‐UL, FMA‐LL, and MT scores at 6 time points for the rTMS real and sham treatment groups. I:onset;II:the second day after treatment; III: 1 month after onset; IV: 3 months after onset; V: 6 months after onset; VI: 1 year after onset. (A) NIHSS score and score improvement between real and sham rTMS. (B) BI score and score improvement between real and sham rTMS. (C) mRS score and score improvement between real and sham rTMS. (D) FMA‐UL score and score improvement between real and sham rTMS. (E) FMA‐LL score and score improvement between real and sham rTMS. (F) MT score and score improvement between real and sham rTMS. *significantly different, P < .05
4. DISCUSSION
Noninvasive brain stimulation, such as rTMS, could not only modulate cortical excitability but also change the function of the subcortex and spinal cord. From 1998 to 2012, approximately 1400 articles were published reporting the effectiveness of noninvasive brain stimulation, among which 141 articles described the use of rTMS in motor recovery after stroke.18 These research studies included high‐frequency rTMS applied over the ipsilesional hemisphere to increase its excitability, low‐frequency rTMS applied over the contralesional hemisphere to decrease its inhibitory connections with the lesioned cortex, and both types of rTMS combined, with or without traditional rehabilitation. Clinical measurements, such as motor functional scores, muscle force, and appreciation of daily living, were used to assess the paralyzed limb. fMRI and electrophysiology were also performed to evaluate the potential effect of rTMS.
The effectiveness of high‐frequency rTMS on motor recovery after acute stroke has been demonstrated by many randomized trials.19 rTMS can induce neuroplasticity of the ipsilesional M1, strengthen connections between cortical neurons, and briefly increase cerebral blood flow, thereby improving function of the corticospinal tract and facilitating motor rehabilitation. Our study found that compared to the sham group, 5‐Hz rTMS applied over the ipsilesional M1 of acute stroke patients could induce more prominent improvement in motor functional scores, such as the NIHSS, BI, FMA‐UL. Moreover, our previous study using fMRI found increased functional connectivity between bilateral hemispheres after rTMS treatment as Li et al reported in another article.9 We demonstrated that rTMS could also promote motor recovery by strengthening the positive role of contralesional mirror regions.
The NIHSS, BI, and FMA are widely used to assess motor function and quality of living and have been shown to exhibit great reliability. Using these three scores, our study found that improvement was more prominent in the rTMS treatment group than in the sham group at 1 month after onset. This result was very promising as it provided a rationale for the effectiveness of rTMS in stroke recovery in the acute stage.
How long will this effect last? The need for such information has been recently highlighted. A longitudinal study would be advantageous to follow‐up patients and to determine the answer. Our results showed that at 3 months after onset, no significant differences were observed in the clinical assessment, other than the FMA‐UL, between the rTMS treatment group and the sham group and the improvement could last till 6 months and 1 year after onset. This results concord with Khedr and colleagues who found that rTMS treatment, either at 3 Hz or 10 Hz, produced greater improvement than sham, and the effect lasted for 1 year.20 However, Du and colleagues found that the clinical effects of rTMS, as assessed by the NIHSS, BI, and FMA‐LL, persisted at least 3 months after 3 Hz or 1 Hz stimulation. However, no significant improvement in FMA‐UL scores was found in the 3‐Hz group.21 In our study, no significant difference was found in FMA‐LL score or score difference between the two groups at every time after onset. This discrepancy may be due to the different methods used in these studies and the different score assessment since the functions for leg were simple. The FMA score of assessment for upper extremities is more complicated and the differences could be observed more detailed. This finding also implied that the duration of the rTMS effect may vary within a large group of people. Theoretically, the stimulation effect was maintained for a short time 22, 23 in largely population. In our study, the rTMS effect continued until 1 month after onset, while after 3 months till 1 year, FMA‐UL score differences were found between the rTMS treatment group and the sham group. This finding motivated further studies aimed to achieve a better outcome by repeating the rTMS treatment. However, the patients in our research study who experienced great improvement in motor function had clinical scores that were very close to normal values; therefore, it was not necessary to repeat the rTMS treatment. However, these findings may prompt the use of multiple courses of rTMS in the treatment of patients with severe dysfunction after stroke.
There were some limitations in our study. First, our sample size was relatively small due to the strict patient inclusion criteria and the long‐term follow‐up evaluation. Second, we recruited patients with mild dysfunction for safety purposes, but mild stroke patients may have spontaneous recovery, which may represent potential confounders in the study.
5. CONCLUSION
rTMS treatment facilitated motor recovery of acute stroke patients, and the effect lasted until 1 months after onset. As a single course of rTMS in the acute stage may not induce significant improvement in functional assessment beyond 3 months after onset, rTMS could be repeated when treating patients with severe dysfunction after stroke.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by funds obtained from the National Natural Science Foundation of China (81271545), and Youth Foundation of Peking Union Medical College Hospital (2010104).
Guan Y‐Z, Li J, Zhang X‐W, et al. Effectiveness of repetitive transcranial magnetic stimulation (rTMS) after acute stroke: A one‐year longitudinal randomized trial. CNS Neurosci Ther. 2017;23:940–946. 10.1111/cns.12762
The first two authors contributed equally to this work.
Contributor Information
Li‐Ying Cui, Email: pumchcuily@sina.com.
Wei‐Hong Zhang, Email: zhangweihong@pumch.cn.
REFERENCES
- 1. Mayo NE, Wood‐Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil. 1999;21:258‐268. [DOI] [PubMed] [Google Scholar]
- 2. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990‐2010: findings from the global burden of disease study 2010. Lancet. 2014;383:245‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ziemann U. TMS induced plasticity in human cortex. Rev Neurosci. 2004;15:253‐266. [DOI] [PubMed] [Google Scholar]
- 4. Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112:1367‐1377. [DOI] [PubMed] [Google Scholar]
- 5. Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466‐468. [DOI] [PubMed] [Google Scholar]
- 6. Berlim MT, Van Den Eynde F. Repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex for treating posttraumatic stress disorder: an exploratory meta‐analysis of randomized, double‐blind and sham‐controlled trials. Can J Psychiatry. 2014;59:487‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGirr A, Van den Eynde F, Tovar‐Perdomo S, Fleck MP, Berlim MT. Effectiveness and acceptability of accelerated repetitive transcranial magnetic stimulation (rTMS) for treatment‐resistant major depressive disorder: an open label trial. J Affect Disord. 2015;173:216‐220. [DOI] [PubMed] [Google Scholar]
- 8. Hasan A, Guse B, Cordes J, et al. Cognitive effects of high‐frequency rTMS in schizophrenia patients with predominant negative symptoms: results from a multicenter randomized sham‐controlled trial. Schizophr Bull. 2016;42:608‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li J, Zhang XW, Zuo ZT, et al. Cerebral functional reorganization in ischemic stroke after repetitive transcranial magnetic stimulation: an fMRI study. CNS Neurosci Ther. 2016;22:952‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ameli M, Grefkes C, Kemper F, et al. Differential effects of high‐frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66:298‐309. [DOI] [PubMed] [Google Scholar]
- 11. Kakuda W, Abo M, Kaito N, Watanabe M, Senoo A. Functional MRI‐based therapeutic rTMS strategy for aphasic stroke patients: a case series pilot study. Int J Neurosci. 2010;120:60‐66. [DOI] [PubMed] [Google Scholar]
- 12. Pollak TA, Nicholson TR, Edwards MJ, David AS. A systematic review of transcranial magnetic stimulation in the treatment of functional (conversion) neurological symptoms. J Neurol Neurosurg Psychiatry. 2014;85:191‐197. [DOI] [PubMed] [Google Scholar]
- 13. Kim C, Choi HE, Jung H, Lee BJ, Lee KH, Lim YJ. Comparison of the effects of 1 Hz and 20 Hz rTMS on motor recovery in subacute stroke patients. Ann Rehabil Med. 2014;38:585‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang W, Liu TT, Song XB, et al. Comparison of different stimulation parameters of repetitive transcranial magnetic stimulation for unilateral spatial neglect in stroke patients. J Neurol Sci. 2015;359:219‐225. [DOI] [PubMed] [Google Scholar]
- 15. Cassidy JM, Chu H, Anderson DC, et al. A comparison of primed low‐frequency repetitive transcranial magnetic stimulation treatments in chronic stroke. Brain Stimul. 2015;8:1074‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Etoh S, Noma T, Takiyoshi Y, et al. Effects of repetitive facilitative exercise with neuromuscular electrical stimulation, vibratory stimulation and repetitive transcranial magnetic stimulation of the hemiplegic hand in chronic stroke patients. Int J Neurosci. 2016;126:1007‐1012. [DOI] [PubMed] [Google Scholar]
- 17. Rossi S, Hallett M, Rossini PM, Pascual‐Leone A. Safety of TMS consensus group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008‐2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klomjai W, Lackmy‐Vallee A, Roche N, Pradat‐Diehl P, Marchand‐Pauvert V, Katz R. Repetitive transcranial magnetic stimulation and transcranial direct current stimulation in motor rehabilitation after stroke: an update. Ann Phys Rehabil Med. 2015;58:220‐224. [DOI] [PubMed] [Google Scholar]
- 19. Khedr EM, Fetoh NA. Short‐ and long‐term effect of rTMS on motor function recovery after ischemic stroke. Restor Neurol Neurosci. 2010;28:545‐559. [DOI] [PubMed] [Google Scholar]
- 20. Khedr EM, Etraby AE, Hemeda M, Nasef AM, Razek AA. Long‐term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010;121:30‐37. [DOI] [PubMed] [Google Scholar]
- 21. Du J, Tian L, Liu W, et al. Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: a randomized controlled trial. Eur J Neurol. 2016;23(1666–1672):21. [DOI] [PubMed] [Google Scholar]
- 22. Vlachos A, Muller‐Dahlhaus F, Rosskopp J, Lenz M, Ziemann U, Deller T. Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures. J Neurosci. 2012;32:17514‐17523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lenz M, Galanis C, Muller‐Dahlhaus F, et al. Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat Commun. 2016;7:10020. [DOI] [PMC free article] [PubMed] [Google Scholar]


