Summary
Aims
Fatigue is a common burdensome problem in patients with Parkinson's disease (PD), but its pathophysiological mechanisms are poorly understood. This study aimed at investigating the neural substrates of fatigue in patients with PD.
Methods
A total of 17 PD patients with fatigue, 32 PD patients without fatigue, and 25 matched healthy controls were recruited. The 9‐item fatigue severity scale (FSS) was used for fatigue screening and severity rating. Resting‐state functional magnetic resonance imaging (RS‐fMRI) data were obtained from all subjects. Amplitude of low‐frequency fluctuations (ALFF) was used to measure regional brain activity, and functional connectivity (FC) was applied to investigate functional connectivity at a network level.
Results
PD‐related fatigue was associated with ALFF changes in right middle frontal gyrus within the attention network and in left insula as well as right midcingulate cortex within the salience network. FC analysis revealed that above three regions showing ALFF differences had altered functional connectivity mainly in the temporal, parietal, and motor cortices.
Conclusion
Our findings do reveal that abnormal regional brain activity within attention and salience network and altered FC of above abnormal regions are involved in neural mechanism of fatigue in patients with PD.
Keywords: Amplitude of low‐frequency fluctuations, Fatigue, Functional connectivity, Parkinson's disease
Introduction
Fatigue is one of the most prominent nonmotor manifestations of Parkinson disease (PD), defined as difficulty in initiating or sustaining voluntary activities 1, 2. Approximately 33–70% of patients with PD have significant fatigue during the disease course 3, 4, which severely worsens patients' daily activities and quality of life 5, 6, 7. Despite this, fatigue in patients with PD is still poorly understood. Factors including the presence of cognitive impairment, depression, excessive daytime sleepiness (EDS), and apathy had been recognized to be associated with fatigue in patients with PD 3, 8, 9, 10, 11, 12, while it still remains controversial whether the disease duration, motor severity, motor subtype, somatic affective symptoms, or antiparkinsonian medication are related to fatigue in patients with PD 4, 9, 11, 13. Obviously, factors mentioned above cannot fully explain the high prevalence of fatigue in PD, as patients free from these disturbances also suffer from fatigue 2, 14. Therefore, to further investigate the neural mechanisms of fatigue in PD could have great significance.
To date, resting‐state functional magnetic resonance imaging (RS‐fMRI), demonstrating spontaneous neuronal activity in a resting state 15, has been widely used in investigating the neural mechanisms of nonmotor symptoms in PD and fatigue in other neurological diseases 16, 17. Here, it should be noted that although fatigue is associated with many pathophysiological states of PD, the fatigue‐related brain regions are relatively constant. So it is meaningful to investigate the brain activity of fatigue in “a static state” by RS‐fMRI. In this regard, we use RS‐fMRI by applying amplitude of low‐frequency fluctuations (ALFF) and functional connectivity (FC) algorithms to explore the neural substrates of fatigue in patients with PD.
ALFF, measuring the spontaneous amplitude of low‐frequency (0.01–0.08 Hz) blood‐oxygen‐level‐dependent (BOLD) signal has been widely applied to detect regional neural activity, while FC can be used to investigate the functional relationship between two regions at a network level by reflecting the temporal BOLD signals of two different regions 18. Based on this, we combined both ALFF and FC methods to detect abnormal local activity and network‐level alterations in PD patients with fatigue.
Materials and Methods
Participants
Forty‐nine right‐handed patients with idiopathic PD diagnosed with the criteria of UK Parkinson's Disease Society Brain Bank for PD 19 and 25 healthy controls (HCs) were enrolled in this study. Patients were consecutively recruited from the outpatient clinic of our hospital. The exclusion criteria were as follows: (1) diagnosis uncertain for PD or parkinsonian plus syndromes, (2) diagnosis of some severe neurological and psychiatric diseases, (3) a contraindication for MRI scan or resistance to dopaminergic drugs, (4) with antidepressant or with treatment of some medications that has fatigue as side effect or with some diseases that can lead to the onset of fatigue, (5) the following exclusion criteria were also adopted to exclude potential confounding factors: significant cognitive dysfunction (Mini‐Mental State Examination (MMSE) scores < 24), moderate or severe depression symptoms (the 17‐item Hamilton Depression Rating Scale (HDRS‐17) > 14), excessive daytime sleepiness (Epworth sleepiness scale (ESS) > 10) and apathy (Apathy scale (AS) > 14). Similar exclusion criteria were used for the HCs patients. In addition, the clinical and MRI examinations were carried out after more than 12 h withdrawal of antiparkinsonian medications to alleviate the pharmacological effects on neural activity. This study was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University, and informed consent was obtained from all participants before beginning the experiment.
Clinical Assessment
Patients were divided into two groups according to the presence (n = 17) and absence (n = 32) of fatigue. The presence and severity of fatigue were defined by the fatigue severity scale (FSS). The FSS, containing 9 items related to both mental fatigue and subjective physical fatigue, was “recommended” for fatigue screening and fatigue severity rating. Besides, it has been widely used in PD on account of being reliable, valid, and sensitive to detect symptoms of fatigue 20. PD patients with an average FSS score >4.0 were enrolled in fatigue (PD‐F) group, while the remaining patients were classified in nonfatigue (PD‐NF) group. Disease stage and severity were evaluated by the Hoehn and Yahr (H &Y) scale and the motor component of Unified Parkinson's Disease Rating Scale (UPDRS‐III), respectively. The levodopa equivalent daily dose (LEDD) for each patient was calculated according to established methods 21.
Image Acquisition
MRI data were acquired using a Siemens 3.0‐Tesla signal scanner (Siemens Medical Solutions, Erlangen, Germany). Foam padding and earplugs were used to limit head motion and reduce scanner noise. Participants were instructed to remain as still as possible, close their eyes, remain awake, and manage not to think of anything. High‐resolution brain structural images were obtained using T1‐weighted, sagittal 3D magnetization‐prepared rapid gradient echo (MPRAGE) sequences with parameters as followed: repetition time (TR) = 1900 ms, echo time (TE) = 2.95 ms, flip angle (FA) = 9°, slice thickness = 1 mm, slices = 160, field of view (FOV) = 230 × 230 mm2, matrix size = 256 × 256, and voxel size = 1 × 1 × 1 mm3. Functional images were collected using an echo‐planar imaging (EPI) sequence (TR = 2000 ms, TE = 21 ms, FA = 90°, FOV = 256 × 256 mm2, in‐plane matrix = 64 × 64, slices = 35, slice thickness = 3 mm, no slice gap, voxel size = 3 × 3 × 3 mm3, total 4 volumes = 240) on each subject.
Data Preprocessing
Functional images were preprocessed with Statistical Parametric Mapping software (SPM8; www.fil.ion.ucl.ac.uk/spm) and Data Processing Assistant for R‐fMRI (DPARSF, http://www.restfmri.net/forum/DPARSF) toolkits 22. The first 10 volumes were discarded for scanner calibration and participants' adaptation to the scanning environment. The remaining 230 volumes were corrected for the acquisition time delay between slices and for the head motion. Patients with head motions exceeding 2.0 mm of translation or 2.0 degrees of rotation throughout the course of the scan were excluded from the study. Next, 3D T1‐weighted imagings were coregistered to functional imagings and segmented into white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF), followed by nonlinear deformation into the Montreal Neurological Institute (MNI) space. The functional images were resliced at a resolution of 3 × 3×3 mm3 and spatially smoothed with a Gaussian kernel (full width at half‐maximum = 6 × 6×6 mm3). Finally, band‐pass filtering (0.01 < f < 0.08 Hz) was performed and linear trend was removed.
ALFF Analysis
ALFF analysis was performed using the Resting‐State f‐MRI Data Analysis Toolkit (http://restfmri.net/forum/REST) 23. In summary, for a given voxel, fast fourier transformation was used to convert the time course to frequency domain. The mean square root, being computed and averaged throughout 0.01–0.08 Hz at each voxel, was regarded as the ALFF. In addition, each individual ALFF map was divided by the global mean ALFF within the mask for standardization purposes. Finally, all ALFF maps were spatially smoothed with a 6 mm full width at half‐maximum Gaussian kernel.
FC Analysis
Regions both showing significant ALFF differences between PD‐F and PD‐NF groups and correlated with FSS scores were finally defined as regions of interest (ROIs). And then, these ROIs were chosen as the seeds for FC analysis. Next, correlation analysis was performed between the seed and the whole brain in a voxel‐wise manner. Finally, an entire brain z‐value map was created after normalizing these FC values calculated from the correlation analysis by Fisher r‐to‐z transformation.
Statistical Analysis
Demographic and clinical characteristics of all subjects in different groups were compared by the one‐way ANOVA and post hoc t‐test, chi‐square test, independent samples t‐test, or Mann–Whitney U‐test, as appropriate. Statistical analyses were performed with SPSS 20.0 statistical analysis software (SPSS Inc. Chicago, IL, USA). Significance threshold was set to P = 0.05.
An analysis of covariance (ANCOVA) was performed on the ALFF maps with gray matter volume (GMV), age and gender as variables to identify brain regions with significant differences among the three groups (PD‐F, PD‐NF, and HCs groups; voxel‐level P < 0.01, cluster size > 2295 mm3/85 voxels, corresponding to a corrected P < 0.05 as determined by AlphaSim correction). Then, these areas were extracted as a mask. To examine between‐group ALFF difference, a two‐sample post hoc t‐test of the ALFF maps within this extracted mask was performed also with GMV, age, and gender as variables between each pair of the three groups (PD‐F vs. PD‐NF, PD‐F vs. HCs, PD‐NF vs. HCs). The clusters exhibiting significant differences (voxel‐level P < 0.01, cluster size > 216 mm3/8 voxels, corresponding to a corrected P < 0.05 as determined by AlphaSim correction) in ALFF between PD‐F and PD‐NF group were extracted. The average ALFF values of these clusters were computed. To determine the associations between regions showing ALFF differences between PD‐F and PD‐NF groups and fatigue severity, the Pearson correlation between mean ALFF values and FSS scores was examined using SPSS 20.0 software.
ANCOVA (voxel‐level P < 0.01, cluster size > 2295 mm3/85 voxels, corresponding to a corrected P < 0.05 as determined by AlphaSim correction) and post hoc tests were used to examine between‐group differences in FC, also with GMV, gender, and age as covariates. For each ROI, the clusters that showed significant differences in FC between PD‐F and PD‐NF groups were extracted. (voxel‐level P < 0.01, cluster size > 378, 243, 270 mm3/14, 9, 10 voxels, corresponding to a corrected P < 0.05 as determined by AlphaSim correction) (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf).
Results
Demographic and Clinical Characteristics
Table 1 provides clinical data and demographics for the PD‐F, PD‐NF, and HCs subject groups. There were no significant differences among the three groups in age, education, and gender (P > 0.05). And no significant differences were found in symptom‐onset side, disease duration, UPDRS‐III, H&Y, LEDD, MMSE, HDRS, ESS, and AS scores (P > 0.05) between PD‐F and PD‐NF groups. Differences in FSS scores were significant between PD‐F versus to PD‐NF (P < 0.001), PD‐NF versus to HCs (P < 0.001), and PD‐NF versus to HCs (P = 0.001).
Table 1.
Demographic and clinical characteristics of all subjects
| Groups | HCs (N = 25) Mean ± SD | PD‐F (N = 17) Mean ± SD | PD‐NF (N = 32) Mean ± SD | P‐value |
|---|---|---|---|---|
| Age, years | 64.6 ± 4.49 | 69.0 ± 8.34 | 65.0 ± 8.38 | 0.086 |
| Education, years | 10.7 ± 2.94 | 11.9 ± 3.08 | 11.4 ± 3.46 | 0.422 |
| Gender, female/male | 13/12 | 8/9 | 10/22 | 0.256 |
| Symptom‐onset side (R/L) | NA | 7/10 | 15/17 | 0.936 |
| Disease duration, years | NA | 3.72 ± 2.51 | 4.04 ± 3.98 | 0.737 |
| UPDRS‐III | NA | 26.9 ± 9.19 | 21.6 ± 9.99 | 0.055 |
| H & Y | NA | 2.43 ± 0.600 | 2.18 ± 0.670 | 0.175 |
| LEDD, mg/day | NA | 440 ± 290 | 326 ± 256 | 0.134 |
| MMSE | NA | 28.0 ± 1.94 | 28.3 ± 1.76 | 0.541 |
| HDRS | NA | 6.93 ± 2.95 | 5.09 ± 3.49 | 0.093 |
| ESS | NA | 4.52 ± 3.56 | 3.79 ± 2.53 | 0.364 |
| AS | NA | 12.5 ± 5.30 | 11.0 ± 4.60 | 0.378 |
| FSS/9 | 1.65 ± 0.69 | 5.36 ± 0.550 | 2.32 ± 0.870 | <0.001*** |
HCs, healthy controls; PD‐F, Parkinson's disease with fatigue; PD‐NF, Parkinson's disease without fatigue; UPDRS, Unified Parkinson's disease rating scale; H&Y, Hoehn and Yahr stage; LEDD, Levodopa equivalent daily dose; MMSE, Mini‐Mental State Examination; HDRS, Hamilton Depression Rating Scale; ESS, Epworth sleepiness scale; AS, apathy scale; FSS, fatigue severity scale; NA, not applicable. P < 0.05 was considered significant. ***Means significant difference between each pair of three groups (PD‐F vs. PD‐NF, PD‐F vs. HCs, PD‐NF vs. HCs).
ALFF
An ANCOVA revealed significant ALFF differences among the PD‐F, PD‐NF, and HCs groups in following regions: left insula, left supramarginal gyrus, left angular, bilateral middle frontal gyrus (MFG), left middle occipital gyrus, and right middle cingulate cortex (MCC). Next, two‐sample post hoc t‐test was performed to detect ALFF differences between each pair of the HCs, PD‐F, and PD‐NF groups. Compared with PD‐NF group, PD‐F group showed increased ALFF in left insula and right MCC and decreased ALFF in right MFG (Table 2 and Figure 1A). Compared with HCs group, PD‐F group displayed increased ALFF in bilateral MFG, left angular, left insula, and left supramarginal gyrus (Table 2 and Figure 1B). ALFF in bilateral MFG, left angular, and left middle occipital gyrus was significantly increased in the PD‐NF patients compared with HCs (Table 2 and Figure 1C).
Table 2.
ALFF differences between PD‐F, PD‐NF, and HCs groups
| Brain region (ALL) | Coordinates MNI | Clusters size (mm3) | T value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| PD‐F versus PD‐NF | |||||
| Insula_L | −38 | 17 | 1 | 22 | 3.06 |
| Cingulum_Mid_R | 13 | −33 | 45 | 18 | 3.26 |
| Frontal_Mid_R | 46 | 32 | 35 | 17 | −2.61 |
| PD‐F versus HCs | |||||
| Frontal_Mid_L | −24 | 39 | 15 | 61 | 3.92 |
| Frontal_Mid_R | 30 | 48 | 9 | 204 | 6.24 |
| Supramarginal_L | −60 | −33 | 36 | 44 | 4.75 |
| Angular_L | −36 | −63 | 48 | 86 | 4.19 |
| Insula_L | −38 | 18 | 1 | 31 | 4.12 |
| PD‐NF versus HCs | |||||
| Frontal_Mid_R | 30 | 49 | 8 | 223 | 4.66 |
| Frontal_Mid_L | −30 | 51 | 15 | 46 | 3.31 |
| Angular_L | −39 | −75 | 46 | 89 | 3.92 |
| Occipital_Mid_L | −40 | −76 | 36 | 36 | 3.92 |
PD‐F, Parkinson's disease with fatigue; PD‐NF, Parkinson's disease without fatigue; HC, healthy control; AAL, anatomical automatic labeling; R, right; L, left; Sup, superior; Inf, inferior; Mid, middle; MNI, Montreal Neurological Institute; all the coordinates are denoted by MNI space coordinates (P < 0.05, AlphaSim corrected). T‐score denotes the statistic value of post hoc two‐sample t‐test after analysis of covariance between the three groups.
Figure 1.
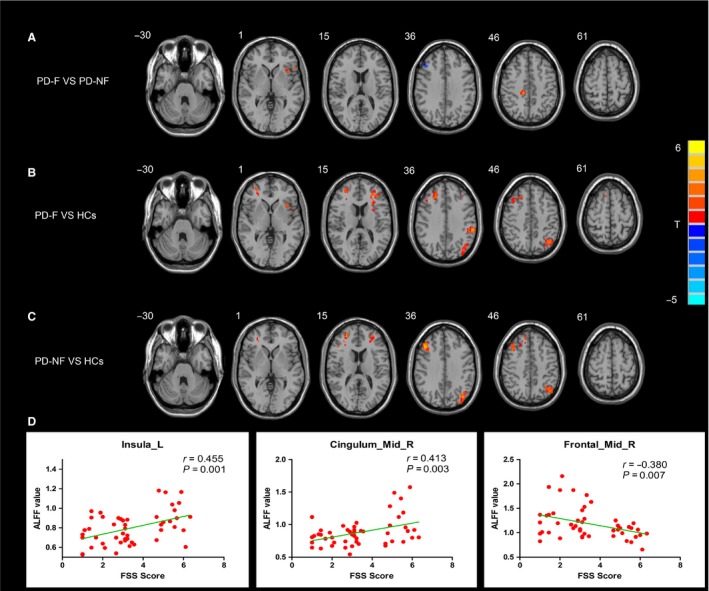
Significant differences in ALFF among three groups: PD‐F, PD‐NF, and HCs. (A) differences between PD‐F and PD‐NF; (B) differences between PD‐F and HCs; (C) differences between PD‐NF and HCs. Results are displayed at p < 0.05 corrected by AlphaSim. (D) the significant correlations between ALFF value and FSS score in left insula, right MCC and right MFG in PD patients. ALFF, amplitude of low‐frequency fluctuations; PD‐F, Parkinson's disease patients with fatigue; PD‐NF, Parkinson's disease patients without fatigue; HCs, healthy controls; MCC, midcingulate cortex; MFG, middle frontal gyrus; FSS, fatigue severity score.
FC
Based on the ALFF findings, correlations between FSS scores and ALFF values of brain regions showing significant differences between PD‐F and PD‐NF groups were examined for all patients with PD. The FSS scores were positively correlated with left insula (r = 0.455, P = 0.001) and right MCC (r = 0.413, P = 0.003) and negatively correlated with right MFG (r = −0.380, P = 0.007; Figure 1D). Then, we defined above three fatigue‐related regions as ROIs for FC analysis. Likewise, ANCOVA for each ROI was performed to determine differences in the brain networks of three groups, and post hoc tests were then performed to explore significant differences between PD‐F and PD‐NF groups. Compared with PD‐NF group, in PD‐F group, the left insula showed significant increased FC with right precentral gyrus, right inferior frontal gyrus, right superior and middle temporal gyrus, and right supplementary motor area (SMA), and no decreased FC was found in PD‐F group (Table 3 and Figure 2A). Also, in PD‐F group, right MCC showed significant increased FC with right precentral gyrus, right superior temporal gyrus, right SMA, and left paracentral lobule (Table 3 and Figure 2B). In addition, for PD‐F patients, the right MFG had significant increased FC with right middle temporal gyrus and had significant decreased FC with the left superior parietal gyrus and left precentral gyrus (Table 3 and Figure 2C).
Table 3.
FC differences between PD‐F and PD‐NF groups
| Brain region (ALL) | Coordinates MNI | Clusters Size (mm3) | Z‐value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Insula_L | |||||
| Precentral_R | 27 | −24 | 72 | 107 | 3.25 |
| Temporal_Sup_R | 57 | −23 | 12 | 43 | 3.15 |
| Temporal_Mid_R | 62 | −48 | 12 | 42 | 4.24 |
| Supp_Motor_Area_R | 6 | 12 | 51 | 27 | 3.42 |
| Frontal_Inf_R | 36 | 27 | 9 | 76 | 3.40 |
| Cingulum_Mid_R | |||||
| Paracentral_Lobule_L | −8 | −31 | 58 | 66 | 3.80 |
| Precentral_R | 22 | −21 | 75 | 52 | 3.48 |
| Temporal_Sup_R | 45 | −12 | −12 | 49 | 4.61 |
| Supp_Motor_Area_R | 5 | 2 | 68 | 33 | 3.55 |
| Frontal_Mid_R | |||||
| Temporal_Mid_R | 45 | −42 | 6 | 91 | −5.48 |
| Parietal_Sup_L | −22 | −56 | 59 | 46 | 3.13 |
| Precentral_L | −35 | 4 | 39 | 45 | 3.72 |
PD‐F, Parkinson's disease with fatigue; PD‐NF, Parkinson's disease without fatigue; HC, healthy control; AAL, anatomical automatic labeling; R, right; L, left; Sup, superior; Inf, inferior; Mid, middle; MNI, Montreal Neurological Institute; all the coordinates are denoted by MNI space coordinates (P < 0.05, AlphaSim corrected). Z‐score denotes the statistic value of post hoc two‐sample t‐test after analysis of covariance between the three groups.
Figure 2.
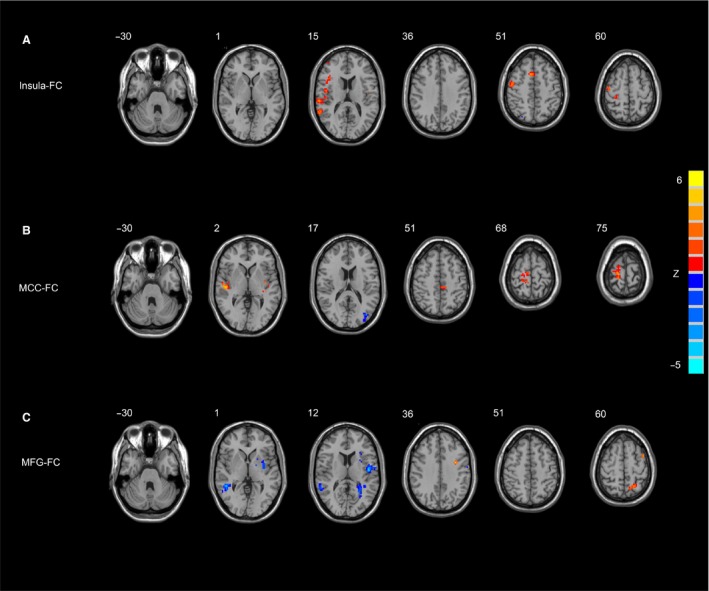
Significant differences in the FC of each ROI between PD‐F and PD‐NF group. (A) the left insula FC between. PD‐F and PD‐NF group; (B) the right MCC FC between PD‐F and PD‐NF group; (C) the right MFG FC between PD‐F and PD‐NF group. Results are displayed at p < 0.05 corrected by AlphaSim. PD‐F, Parkinson's disease patients with fatigue; PD‐NF, Parkinson's disease patients without fatigue; FC, functional connectivity; MCC, midcingulate cortex; MFG, middle frontal gyrus.
Discussion
The main focus of this study was to investigate abnormal neural activity and functional networks that associated with fatigue in patients with PD. Using RS‐fMRI, our study pointed to a correlation between fatigue severity and increased activity of left insula and right MCC, both of which are part of the salience network (SN), a network that is contributed to undergirding perception of the negative information 24. Based on this, we hypothesized the increased activity of limbic system regions caused a negative emotional experience and then resulted in the excessive self‐generated feeling of fatigue in PD. In fact, this was supported by the pathophysiological model of fatigue proposed by Chaudhuri et al. 1. In this model, pathological fatigue was regarded as an amplified sense of physiological fatigue that could be induced by some variables. In accordance with our results, a recent neuroimaging investigation suggested that fatigue in patients with PD was linked to both serotonergic dysfunction of the cingulate gyrus, amygdala, basal ganglia, and 18F‐dopa uptake reduction in the insula and caudate 25. Consequently, the MCC and insula may be blame to the generation of fatigue in patients with PD.
Increased FC of left insula and right MCC with motor cortices including the right precentral cortex and right SMA was observed in this study as well. This is in keeping with recent RS‐fMRI study that displayed a stronger FC between the subcortical structures and the motor network in patients with fatigued multiple sclerosis (MS) 26. Besides, Finke et al. also reported a positive relation between severity of fatigue and FC of basal ganglia with motor cortex in patients with fatigued MS 27. Perhaps, this provided an explanation that enhancing input signal to the motor areas could play a role of sustaining motor commands with increasing perception of fatigue.
In the present study, we also exhibited that reduced activity of right MFG was engaged in the neural bases of fatigue in PD, which was partially consistent with previous studies. For instance, in MS, involvement of MFG in fatigue pathophysiology had been demonstrated by a PET study 28. Meanwhile, in PD patients with fatigue, a SPECT study also elucidated that fatigue severity might be associated with frontal hypofusion 29. Applying FC method, we also found altered FC between right MFG and regions including the right middle temporal gyrus, left superior parietal gyrus, and left precentral gyrus.
Notably, the right MFG together with middle temporal gyrus is included in the ventral attention network (VAN), as well as the superior parietal gyrus is within the dorsal attention network (DAN) 30. DAN, acting in a goal‐directed manner, is involved in accomplishing endogenous goals and preparing to make a motor response 31, 32. Conversely, VAN, working in a stimulus‐driven manner, is considered to divert attention to an exogenous stimulus by sending signals to DAN to interrupt ongoing processing 33. Coincidentally, a recent study of a patient with right MFG resection clarified that right MFG was a crucial node of attentional circuits, and it controlled over both ventral and dorsal attention networks 34, 35. What's more, some evidence has already illuminated that attention deficit may be related to the presence of fatigue 36, 37, 38. For example, Jau‐Shin Lou et al. 36 reported PD patients with fatigue had abnormal attention network compared with controls. Moreover, Qi Wu et al. 37 revealed fatigue in ankylosing spondylitis patients were correlated with the dorsal and ventral attention networks. In this study, the results of decreased ALFF in right MFG and altered FC between right MFG and right middle temporal gyrus and left superior parietal gyrus further suggested that impaired attention network was likely involved in fatigue of PD.
On the contrary, a recent RS‐fMRI study, using independent component analysis (ICA) to explore FC differences within the resting‐state networks (RSN), demonstrated that the decreased SMA connectivity within the SMN contributed to the presence of fatigue, while the increased connectivity in the prefrontal and posterior cingulate cortices within the DMN played a compensatory role in the neural mechanism of fatigue 39. This contrary result may stem from differences in methodology (i.e. fatigue assessment, statistical analysis, imaging technique) and from that all their patients were in the early disease stage.
Here, it was worthy to note that two groups between PD‐F and PD‐NF in our study were well matched for age, gender, education, disease duration, LEDD, disease stage, the side of onset, and motor severity. Besides, all PD patients with common confounders of fatigue including dementia, overt depression, EDS, and apathy were excluded. Therefore, the differential RS‐fMRI findings observed in our study should be specific for fatigue in patients with PD. Moreover, the difference between right and left cerebral involvement should be not from the lateralities of onset but possibly from the different function of hemispheres, as each hemisphere is naturally differentiated no matter from the aspects of anatomy or function 40.
However, we also realized that there were some limitations of our study. First, the limited cognitive evaluation, especially attention evaluation, should be performed in the present study. Second, given a cross‐sectional design, results from this study could not be applied to infer the longitudinal impact of fatigue on neural pattern in PD. Finally, based on prior studies, patients with PD should have dysfunction in the basal ganglia by the nature of the disease, while no positive result had been observed in the basal ganglia in our study. This conflict may be related to the relatively small sample size or different algorithms of methodology in this observation. Therefore, future studies are deserved to further clarify the key role of basal ganglia in PD patients with fatigue.
In conclusion, we found that fatigue in PD is associated with altered attention network (involved in attention modulation) as well as SN (involved in negative priming). And altered FC between above brain regions may provide further evidence for demonstrating the role of regional brain areas involved in neural mechanism of fatigue in PD. Combining ALFF and FC methods, our study may offer new insights into the neural basis of PD‐related fatigue.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work was supported by The National Natural Science Foundation of China (No. 81671258), The Science and Technology Project of Jiangsu Provincial Commission of Health and Family Planning (No. H201602), The Natural science foundation of Jiangsu Province (No. BK20141494), The Project Funded by The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and The Science and Technology Project of Jiangsu Bureau of Traditional Chinese Medicine (No. YB2015163).
The first two authors contributed equally to this work.
References
- 1. Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet 2004;363:978–988. [DOI] [PubMed] [Google Scholar]
- 2. Friedman J, Friedman H. Fatigue in Parkinson's disease. Neurology 1993;43:2016–2018. [DOI] [PubMed] [Google Scholar]
- 3. Friedman JH, Abrantes A, Sweet LH. Fatigue in Parkinson's disease. Expert Opin Pharmacother 2011;12:1999–2007. [DOI] [PubMed] [Google Scholar]
- 4. Stocchi F, Abbruzzese G, Ceravolo R, et al. Prevalence of fatigue in Parkinson disease and its clinical correlates. Neurology 2014;83:215–220. [DOI] [PubMed] [Google Scholar]
- 5. Qin Z, Zhang L, Sun F, et al. Health related quality of life in early Parkinson's disease: Impact of motor and non‐motor symptoms, results from Chinese levodopa exposed cohort. Parkinsonism Relat Disord 2009;15:767–771. [DOI] [PubMed] [Google Scholar]
- 6. Rahman S, Griffin HJ, Quinn NP, Jahanshahi M. Quality of life in Parkinson's disease: The relative importance of the symptoms. Mov Disord 2008;23:1428–1434. [DOI] [PubMed] [Google Scholar]
- 7. Garber CE, Friedman JH. Effects of fatigue on physical activity and function in patients with Parkinson's disease. Neurology 2003;60:1119–1124. [DOI] [PubMed] [Google Scholar]
- 8. Skorvanek M, Gdovinova Z, Rosenberger J, et al. The associations between fatigue, apathy, and depression in Parkinson's disease. Acta Neurol Scand 2015;131:80–87. [DOI] [PubMed] [Google Scholar]
- 9. Chou KL, Kotagal V, Bohnen NI. Neuroimaging and clinical predictors of fatigue in Parkinson disease. Parkinsonism Relat Disord 2016;23:45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagell P, Brundin L. Towards an understanding of fatigue in Parkinson disease. J Neurol Neurosurg Psychiatry 2009;80:489–492. [DOI] [PubMed] [Google Scholar]
- 11. Schifitto G, Friedman J, Oakes D, et al. Fatigue in levodopa‐naive subjects with Parkinson disease. Neurology 2008;71:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castrioto A, Thobois S, Carnicella S, Maillet A, Krack P. Emotional manifestations of PD: Neurobiological basis. Mov Disord 2016;31:1103–1113. [DOI] [PubMed] [Google Scholar]
- 13. Berardelli A, Conte A, Fabbrini G, et al. Pathophysiology of pain and fatigue in Parkinson's disease. Parkinsonism Relat Disord 2012;18:S226–S228. [DOI] [PubMed] [Google Scholar]
- 14. Lou JS, Kearns G, Oken B, Sexton G, Nutt J. Exacerbated physical fatigue and mental fatigue in Parkinson's disease. Mov Disord 2001;16:190–196. [DOI] [PubMed] [Google Scholar]
- 15. Lang S, Duncan N, Northoff G. Resting‐state functional magnetic resonance imaging: Review of neurosurgical applications. Neurosurgery 2014;74:453–464. [DOI] [PubMed] [Google Scholar]
- 16. Wen MC, Chan LL, Tan LC, Tan EK. Depression, anxiety, and apathy in Parkinson's disease: Insights from neuroimaging studies. Eur J Neurol 2016;23:1001–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeLuca J, Genova HM, Capili EJ, Wylie GR. Functional neuroimaging of fatigue. Phys Med Rehabil Clin N Am 2009;20:325–337. [DOI] [PubMed] [Google Scholar]
- 18. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8:700–711. [DOI] [PubMed] [Google Scholar]
- 19. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedman JH, Alves G, Hagell P, et al. Fatigue rating scales critique and recommendations by the Movement Disorders Society task force on rating scales for Parkinson's disease. Mov Disord 2010;25:805–822. [DOI] [PubMed] [Google Scholar]
- 21. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 22. Chao‐Gan Y, Yu‐Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting‐State fMRI. Front Syst Neurosci 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song XW, Dong ZY, Long XY, et al. REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS ONE 2011;6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ. Fatigue in Parkinson's disease is linked to striatal and limbic serotonergic dysfunction. Brain 2010;133:3434–3443. [DOI] [PubMed] [Google Scholar]
- 26. Dogonowski AM, Siebner HR, Sorensen PS, et al. Expanded functional coupling of subcortical nuclei with the motor resting‐state network in multiple sclerosis. Mult Scler 2013;19:559–566. [DOI] [PubMed] [Google Scholar]
- 27. Finke C, Schlichting J, Papazoglou S, et al. Altered basal ganglia functional connectivity in multiple sclerosis patients with fatigue. Mult Scler 2015;21:925–934. [DOI] [PubMed] [Google Scholar]
- 28. Filippi M, Rocca MA, Colombo B, et al. Functional magnetic resonance imaging correlates of fatigue in multiple sclerosis. NeuroImage 2002;15:559–567. [DOI] [PubMed] [Google Scholar]
- 29. Abe K, Takanashi M, Yanagihara T. Fatigue in patients with Parkinson's disease. Behav Neurol 2000;12:103–106. [DOI] [PubMed] [Google Scholar]
- 30. Corbetta M, Shulman GL. Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 2002;3:201–215. [DOI] [PubMed] [Google Scholar]
- 31. Indovina I, Macaluso E. Dissociation of stimulus relevance and saliency factors during shifts of visuospatial attention. Cereb Cortex 2007;17:1701–1711. [DOI] [PubMed] [Google Scholar]
- 32. Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: The distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci 2001;13:698–710. [DOI] [PubMed] [Google Scholar]
- 33. Chica AB, Bartolomeo P, Lupianez J. Two cognitive and neural systems for endogenous and exogenous spatial attention. Behav Brain Res 2013;237:107–123. [DOI] [PubMed] [Google Scholar]
- 34. Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron 2008;58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 2006;103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lou J‐S. Physical and mental fatigue in Parkinson's disease. Drugs Aging 2009;26:195–208. [DOI] [PubMed] [Google Scholar]
- 37. Wu Q, Inman RD, Davis KD. Fatigue in ankylosing spondylitis is associated with the brain networks of sensory salience and attention. Arthritis Rheumatol 2014;66:295–303. [DOI] [PubMed] [Google Scholar]
- 38. Conte A, Voti PL, Pontecorvo S, et al. Attention‐related changes in short‐term cortical plasticity help to explain fatigue in multiple sclerosis. Mult Scler 2015;22:1359–1366. [DOI] [PubMed] [Google Scholar]
- 39. Tessitore A, Giordano A, De Micco R, et al. Functional connectivity underpinnings of fatigue in “Drug‐Naïve” patients with Parkinson's disease. Mov Disord 2016;31:1497–1505. [DOI] [PubMed] [Google Scholar]
- 40. Wexler BE. Cerebral laterality and psychiatry: A review of the literature. Am J Psychiatry 1980;137:279–291. [DOI] [PubMed] [Google Scholar]


