Summary
Aims
Insular cortex (IC) is involved in processing the information of pain. The aim of this study was to investigate roles and mechanisms of P2X7 receptors (P2X7Rs) in IC in development of visceral hypersensitivity of adult rats with neonatal maternal deprivation (NMD).
Methods
Visceral hypersensitivity was quantified by abdominal withdrawal reflex threshold to colorectal distension (CRD). Expression of P2X7Rs was determined by qPCR and Western blot. Synaptic transmission in IC was recorded by patch‐clamp recording.
Results
The expression of P2X7Rs and glutamatergic neurotransmission in IC was significantly increased in NMD rats when compared with age‐matched controls. Application of BzATP (P2X7R agonist) enhanced the frequency of spontaneous excitatory postsynaptic currents (sEPSC) and miniature excitatory postsynaptic currents (mEPSC) in IC slices of control rats. Application of BBG (P2X7R antagonist) suppressed the frequencies of sEPSC and mEPSC in IC slices of NMD rats. Microinjection of BzATP into right IC significantly decreased CRD threshold in control rats while microinjection of BBG or A438079 into right IC greatly increased CRD threshold in NMD rats.
Conclusion
Data suggested that the enhanced activities of P2X7Rs in IC, likely through a presynaptic mechanism, contributed to visceral hypersensitivity of adult rats with NMD.
Keywords: Insular cortex, Neonatal maternal deprivation, Purinergic P2X receptors, Visceral pain
Introduction
The insular cortex (IC), an important component of the limbic system, is involved in processing the sense of taste, emotion, and perception of innocuous warm and cold 1, 2, 3. Recent studies have shown that painful stimuli activate IC and that direct stimulation of IC evokes painful sensations 4, 5. There is a correlation between IC and phantom limb pain 6. IC plays roles in modulation of both affective and sensory components of pain 7, 8, 9. Although hypersensitivity in patients with irritable bowel syndrome (IBS) is accompanied by increased activities in IC and ACC 10, whether IC is involved in chronic visceral hypersensitivity (CVH) of adult rats with neonatal maternal deprivation (NMD) is unknown.
ATP as an energetic substance for cellular metabolism can be released into extracellular space and plays an important role in mediating fast excitatory neurotransmission 11 as well as calcium waves between astrocytic glial cells 12. ATP has also been considered to be a neuromodulator 13, 14. The P2X receptors for ATP are ligand‐gated nonspecific cation channels 11. Activation of P2X receptors could directly or indirectly increase synaptic transmission 11. Among the subtypes of P2X receptors, P2X7 receptors (P2X7Rs) are widely found in central nervous system (CNS) such as cerebral cortex 15, 16, 17. It is usually activated by a high concentration of ATP (>100 μM) in vivo 18. The activation of P2X7Rs is involved in the regulation of calcium‐dependent and calcium‐independent release of glutamate 19. Compared with other P2X receptors, P2X7Rs were more commonly seen in the presynaptic sites of CNS including the spinal cord, medulla oblongata and nodose ganglia 20, and excitatory presynaptic terminals in forebrain regions 21. However, the expression and distribution of P2X7Rs in IC are not clear. Neither is the physiological function of P2X7Rs in the IC in the development of CVH.
In this study, we assumed that P2X7Rs in IC were involved in regulation of visceral hypersensitivity of NMD rats. Integrative approaches, including whole cell patch clamping, Western blotting, qPCR, immunohistochemistry, and behavioral techniques, were used to prove the hypothesis.
Materials and Methods
NMD and Measurement of Chronic Visceral Hyperalgesia (CVH)
Handling of the animals was approved by the Institutional Animal Care and Use Committee at Soochow University and was strictly in accordance with the guidelines of the International Association for the Study of Pain. Pups of male Sprague Dawley rats for NMD group were separated from the maternity cages and placed in isolated cages with an electric blanket to keep them warm (32°C) for 3 h daily from postnatal days 2–15. After the separation period, pups were returned to their maternity cages. Pups for control group (CON) were not exposed to handling. CVH induction and assessment were performed according to the protocol described previously 22, 23. Experiments were performed in these rats at the age of 6–7 weeks.
Drug Administration
For behavioral experiments, BzATP (P2X7R agonist), brilliant blue G (BBG) (P2X7R antagonist), and A438079 (P2X7R antagonist) were diluted by normal saline (NS) (NaCl, wt/v 0.9%). The drugs, NS, or vehicle for A438079 (NS with DMSO) were stereotactically injected into the unilateral IC of rats. It is reported that there is a stronger functional link between right insula and autonomic regions, whereas left anterior regions do not show connectivity with the thalamus at all; there is a more prominent sympathetic role of the right insula 24. Because NMD mimics the effects of early life stress on the development of emotional and social behaviors, we mainly explored the role of the right IC in the development of visceral hypersensitivity of NMD rats by microinjecting drugs into right IC. A stainless guide cannula with 24 gauge was fixed unilaterally on the right hemisphere of the skull aiming at IC (coordinates with respect to Bregma: AP 0.5 mm, ML 5.6 mm, DV 7.0 mm, angle 0) using dental cement. The drugs, NS, or vehicle (1 μL) were injected into right IC by a needle head through the guide cannula and reached a final depth of 7.5 mm below dura. The way of microinjection was done as described previously 23. CRD threshold was assessed before drug microinjection and tested once every 5 min after microinjection until the effect of drug totally disappeared. The drug concentrations used in the study were based on our preliminary data and reports from other groups 19, 20, 25, 26.
Western Blotting
The Western blotting process was done as described in our previous reports 23. The membrane carried P2X7Rs (~90 KD) was incubated with anti‐P2X7Rs antibody (1:500, APR004, Alomone Labs, Jerusalem, Israel), and the membrane carried GAPDH (~37KD) was incubated with anti‐GAPDH antibody (1:200, sc‐25778, Santa Cruz, Dallas, TX, USA). Band intensities were measured using ImageJ software. P2X7Rs protein expression was normalized to GAPDH.
Real‐Time qPCR for P2X7R mRNA
Total RNA was extracted from IC of both hemispheres from control and NMD rats with TRIzol (15596026, Ambion, Shanghai, China). cDNA was synthesized from total RNA using an Reverse transcription kit (AE301‐03, Transgen Biotech, Beijing, China) following the supplier's instructions. The sequences of the primer pairs for p2x7r used in quantitative polymerase chain reaction are as follows: (F) 5′‐CGGCACCATCAAGTGGATCTT‐3′ and (R) 5′‐CTGCAACGCCTTTGACCTTG‐3′; the sequence of the primer pairs for gapdh (as an internal control) used in quantitative polymerase chain reaction is as follows: (F) 5′ ‐TGGAGTCTACTGGCGTCTT‐3′ and (R) 5′‐TGTCATATTTCTCGTGGTTCA‐3′. Control reactions were performed without cDNA templates.
Slice Preparation and Whole Cell Patch‐Clamp Recordings in the IC
Rats of both control and NMD group (100–130 g, 6–7 weeks of age) were anesthetized with 4% chloral hydrate. Transverse brain slices of the IC (400 μm) were cut using standard methods 23, 27 with oxygenated (95% O2, 5% CO2) solution (in mM): 93 NMDG, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 12 NAC, 10 MgSO4, 0.5 CaCl2, and 25 glucose, around 32°C. Ten minutes after cutting, slices were transferred into oxygenated holding solution (in mM): 94 NaCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 2 MgSO4, 2 CaCl2, 12 NAC, and 25 glucose, under room temperature. After recovery for at least 1 h, the slices were perfused with ACSF (in mM): 124 NaCl, 2.5 KCl, 1.2 NaH2PO4, 24 NaHCO3, 5 HEPES, 12.5 glucose, 2 MgSO4 and 2 CaCl2. The recording chamber was on the stage of a BX51WI microscope (Olympus, Shinjuku‐ku, Tokyo, Japan) equipped with infrared differential interference contrast optics for visualizing whole cell patch‐clamp recordings. Excitatory postsynaptic currents (EPSCs) and action potentials (APs) were recorded from IC with a Digidata 1440A interface, MultiClamp 700B amplifier, and pClamp10 software (Axon Instruments, Sunnyvale, CA, USA). The internal solution of the electrodes (4–8 MΩ tip resistance) contained the following (in mM): 133 K‐gluconate, 8 NaCl, 0.6 EGTA, 10 HEPES, 2 Mg‐ATP, and 0.3 Na‐GTP. The cell type was distinguished under current clamp mode according to the electrophysiological characteristics described by Washburn and Moises in response to intracellular injection of a depolarizing current (100–300 pA, step 50 pA, duration 1000 ms) 28. Only the data of excitatory neurons were used for further analyses of sEPSCs. The mEPSCs were recorded in the presence of TTX (1 μM) in the extracellular solution. Series resistance (<20 MΩ) was checked throughout the experiment to ensure high‐quality recordings. The membrane potential was held at −70 mV throughout the experiment for EPSC recording. Data were sampled and filtered at 10 kHz.
BzATP (an agonist of P2X7Rs) and BBG (an antagonist of P2X7Rs) were bought from Sigma (St. Louis, MO, USA). A438079, an antagonist of P2X7Rs, was bought from Tocris (Bristol, UK). All drugs were dissolved in ACSF on the day of experiment and added by perfusion.
Histology and Immunofluorescence Study
At the end of the experiment, the site of microinjection was verified following Nissl staining method described previously 23. The thickness of coronal slices was 30 μm. After staining, the images were analyzed by comparing them to an anatomical atlas. If a site was outside the IC, data of that rat were excluded and not been analyzed. For immunofluorescence, 15‐μm frozen sections of slices were simultaneously incubated with synaptophysin (1:100, ab8049, Abcam, Cambridge, UK), P2X7R (1:100, ab93354, Abcam), and IBA‐1 (1:1000, 019‐19741, Wako, Tokyo, Japan) antibodies for overnight at 4°C and then incubated with secondary antibody with Alexa Fluor 488 and 555 (1:500, Life Technologies Inc., Shanghai, China) for 2 h at room temperature. Negative controls were performed without the primary antibody.
Data Analyses
A fixed length of traces (3 min) of EPSCs was analyzed using MiniAnalysis program 6.0.3 (Synaptosoft, Decatur, GA, USA). Normality was checked for all data before comparisons. Data were analyzed using two‐sample t‐test, Mann–Whitney U‐test, paired‐sample t‐test, paired‐sample Wilcoxon signed‐rank test, one‐way repeated‐measures ANOVA followed by Tukey's post hoc test, Dunn's post hoc test following Friedman ANOVA with Origin 8 (Origin Lab Inc., Northampton, MA, USA), and Kolmogorov–Smirnov test, as appropriate. All values were shown as mean ± SEM; error bars in the figures stand for SEM. P < 0.05 was considered significant.
Results
Enhanced Expression of P2X7 Receptors in IC of NMD Rats
As CVH was induced by NMD at the age 6–7 weeks 22, the expression of P2X7Rs was checked in IC at the same time period after NMD. There was a significant upregulation of P2X7Rs in IC of both hemispheres at protein level as well as at mRNA level when compared with controls (Figure 1A,B, ***P < 0.001, *P < 0.05, n = 4 for each group, two‐tailed two‐sample t‐test). However, protein level of P2X1Rs showed a significant upregulation only in left but not right hemisphere of IC in NMD rats compared with controls. In contrast, P2X3Rs showed a significant upregulation only in right but not left side of IC in NMD rats compared with controls (Figure 1C,D, *P < 0.05, n = 4 for each group, two‐tailed two‐sample t‐test). As P2X7Rs were upregulated in both sides of IC, we therefore focused on the roles of P2X7Rs in the present study.
Figure 1.
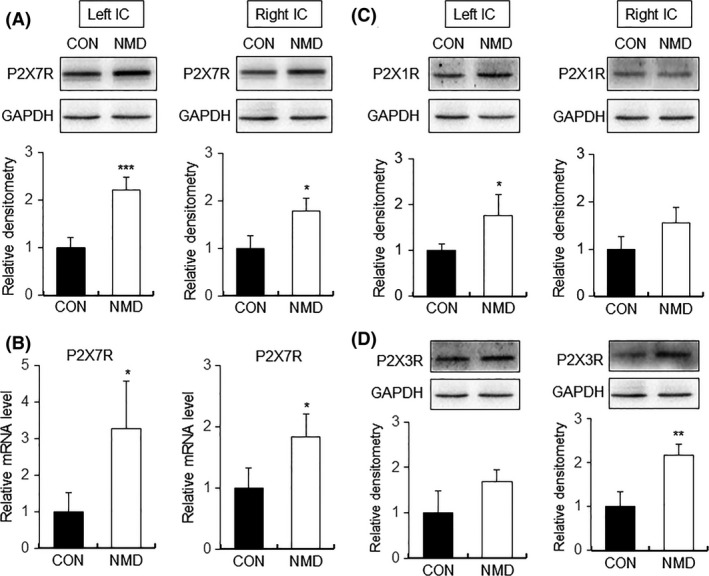
Upregulation of P2X7Rs expression in IC of NMD rats. (A) Expression of P2X7Rs at protein level was significantly increased in IC of both hemispheres of NMD rats when compared with age‐matched control rats (CON). (B) mRNA level of P2X7Rs was markedly enhanced in IC of both hemispheres of NMD group. (C) Protein level of P2X1Rs was significantly increased in left but not right hemisphere of IC of NMD rats compared with control rats. (D) Protein level of P2X3Rs was obviously increased in right but not left side of IC of NMD rats compared with control rats. n = 4 rats for each group, *P < 0.05, **P < 0.01, ***P < 0.001 versus CON.
Hyperexcitation of IC Neurons in NMD Rats
The firing frequency of excitatory neurons of IC slice was next examined for control (CON) and NMD rats. The neurons (n = 17) from NMD rats had a significant higher action potential firing rate than those (n = 15) from control rats at 6–7 weeks of age, under current clamp mode in response to intracellular depolarizing current stimulation at 100 pA (Figure 2A; *P < 0.05 vs. CON), Mann–Whitney U‐test for each current density), indicating the hyperexcitation of IC excitatory neurons of NMD group. However, membrane input resistance, resting membrane potential (RMP), and action potential (AP) threshold were not significantly altered after NMD when compared with controls (Figure 2B–D).
Figure 2.
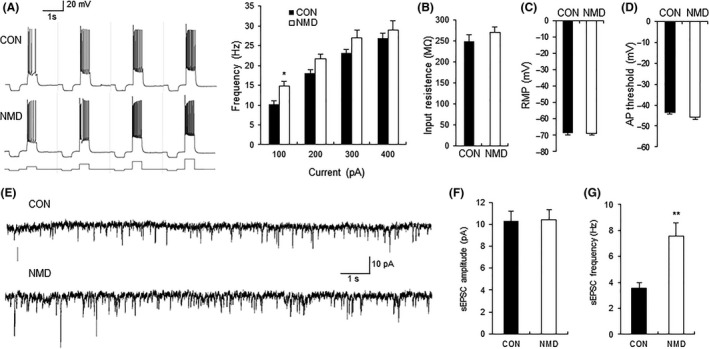
Comparison of membrane properties and sEPSCs of pyramidal neurons between control and NMD group. (A) Representative traces of action potential of control (CON) and NMD groups evoked by 100‐, 200‐, 300‐, and 400‐pA current stimulation. Bar plot illustrating higher firing frequency of neurons in NMD rats than those in control rats in response to different intensity of injected currents. (B) Membrane input resistance, (C) resting membrane potential (RMP), and (D) action potential (AP) threshold were not altered after NMD. n = 15 cells for control group and n = 17 for NMD group, *P < 0.05 versus CON. (E) Recordings illustrating sEPSCs of typical neurons in IC of CON (top) and NMD (bottom) rats. (F) No change in peak amplitude of sEPSC after NMD. (G) Increase in sEPSC frequency in NMD group. n = 18 cells for control group, n = 9 cells for NMD group, **P < 0.01 versus CON.
Increase in Glutamatergic Synaptic Activity of IC Neurons in NMD Rats
The spontaneous excitatory postsynaptic currents (sEPSCs) of IC neurons were compared between control and NMD group. The representative traces from two typical neurons of IC slices control and NMD rats illustrated an increase in frequency, without obvious change in amplitude of sEPSCs (Figure 2E). The average results in Figure 2F,G also suggested a significant increase in frequency of sEPSCs in IC of NMD rats (n = 18 for control, n = 9 for NMD, **P < 0.01, Mann–Whitney U‐test).
BzATP Enhanced Glutamatergic Synaptic Activity in IC of Control Groups Through a Presynaptic Mechanism
Activation of P2X7Rs by BzATP, a very potent agonist of P2X7Rs 20, should increase the glutamatergic synaptic activity of IC in control slices. We next showed that BzATP at 30 μM was effective in increasing sEPSCs in seven of seven excitatory neurons tested. The representative current trace (Figure 3A) demonstrated a shortening of interevent intervals with the presence of BzATP in a typical excitatory neuron. It is also shown in the figures of cumulative fraction of peak amplitude and interevent intervals (Figure 3B,C, left). Normalized mean values for amplitude and frequency are shown in Figure 3B,C (right). The results suggested that there was a significant increase in average frequency of sEPSCs (Fig. 3C, right, n = 7, *P < 0.05, paired‐sample Wilcoxon signed‐rank test), without change in its amplitude (Figure 3B, right). In all cells, the sEPSCs were completely blocked by CNQX (10 μM), suggesting that sEPSCs were mediated by non‐NMDA receptors (data not shown).
Figure 3.
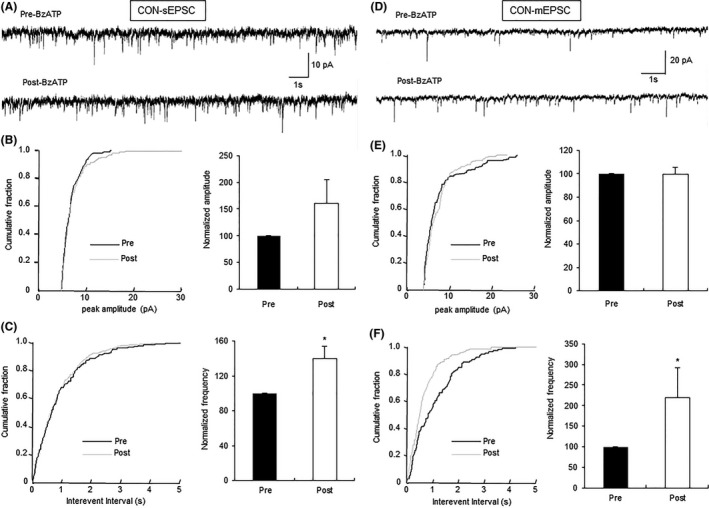
Enhancement of glutamatergic synaptic activity of neurons by BzATP in control rats. (A) Representative traces illustrating sEPSCs of an IC neuron of control rats before and after the addition of BzATP (30 μM). (B) Cumulative fraction of peak amplitude of sEPSCs in an IC pyramidal neuron under the pre‐ and postdrug conditions (left); bar plot showing no significant change in sEPSC peak amplitude by BzATP (right). (C) Cumulative fraction of interevent intervals of sEPSCs under the pre‐ and postdrug conditions (left); bar plot showing the significant increase in sEPSC frequency by BzATP (right). n = 7 cells, *P < 0.05 versus pre. (D) Representative traces illustrating mEPSCs of an IC neuron before and after the addition of BzATP (30 μM); TTX (1 μM) was used to block action potentials. (E) Cumulative fraction of peak amplitude of mEPSCs in one IC pyramidal neuron under the predrug and postdrug conditions (left); bar plot showing no significant change in mEPSC peak amplitude by BzATP (right). (F) Cumulative fraction of interevent intervals of mEPSCs under the predrug and postdrug conditions (left); bar plot showing that mEPSC frequency was markedly increased by BzATP treatment (right). Peak amplitude and frequency of BzATP‐treated neurons was normalized by that from the same neuron before BzATP treatment. n = 6 cells, *P < 0.05 versus Pre.
To assess whether BzATP acts on presynaptic or postsynaptic sites in the IC, the amplitude and frequency of mEPSCs were measured in the absence and presence of BzATP in brain slices of control rats. It was proved by Kolmogorov–Smirnov test that BzATP (30 μM) was effective in affecting mEPSCs in six of seven neurons tested. The typical current trace of a single neuron demonstrated an increase in frequency without change in mEPSCs amplitude after the addition of BzATP in IC neurons (Figure 3D). The average results are shown in Figure 3E,F (right, n = 6, *P < 0.05, paired‐sample Wilcoxon signed‐rank test). The present result of mEPSCs suggested the presynaptic activation of P2X7Rs by BzATP in control slices. As it is reported that the activation of P2X7Rs increases the release of presynaptic glutamate 20, we checked this conclusion in this study. No sEPSC was recorded in the presence of CNQX (10 μM) and D‐APV (30 μM) with or without BzATP (Figure S1A), which is consistent with the previous report 20. As BzATP has been reported to act also on P2X1 and P2X3 receptors 29, we applied BzATP (30 μM) after the application of A438079, a specific antagonist of P2X7Rs, on control slices. No significant excitatory effect on sEPSC of IC neurons was observed for BzATP with the presence of A438079 (Figure S1B–D).
Expression of P2X7Rs in IC
The distribution of P2X7Rs in IC was determined by immunofluorescence study. The anatomy on the brain marked by nucleus marker DAPI and the brain atlas pointed the site of IC (Figure 4A,B). P2X7Rs were also colocalized with NeuN, a marker of neurons (Figure 4C). As shown in Figure 4D, many P2X7Rs were colocalized with synaptophysin, a marker of presynaptic sites 30. The result is consistent with the presynaptic mechanism of P2X7Rs as suggested by our electrophysiological studies. Only very few P2X7Rs colocalized with IBA‐1 (a marker of microglia cells) and GFAP (a marker of astrocyte cells), as shown in Figure 4E,F.
Figure 4.
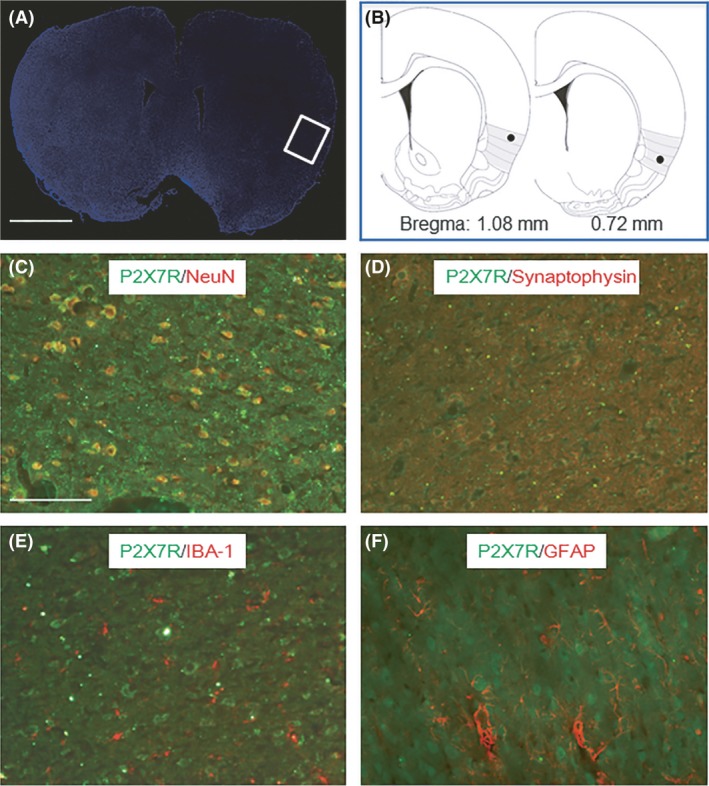
Colocalization of P2X7Rs and IC cells. (A) The anatomy on the brain dyed by nucleus marker DAPI, and IC part is pointed by white box. (B) Brain atlas shows the site of IC. Black spots represent the microinjection position. (C, D, E, F) P2X7Rs are marked by green dots. (C) Neurons were marked by NeuN in red dots. (D) Presynaptic sites were marked by synaptophysin in red dots. (E) Microglia cells were marked by IBA‐1 in red dots. (F) Astrocyte cells were marked by GFAP in red dots. Scale bar in Figure A and C equals 2 mm and 100 μm, respectively.
BBG Reduced Glutamatergic Synaptic Activity of IC Neurons in NMD Group Through a Presynaptic Mechanism
Brilliant blue G (BBG), an antagonist of P2X7Rs, was used in this study to affect sEPSCs of excitatory neurons of IC slices in NMD rats. The typical current traces (Figure 5A), the cumulative fraction of peak amplitude and interevent intervals for a representative neuron (Figure 5B,C, left), and normalized mean values for amplitude and frequency (Figure 5B,C, right, n = 6, *P < 0.05, paired‐sample Wilcoxon signed‐rank test) all suggested that blockade of P2X7Rs decreased the frequency of sEPSCs of IC neurons in NMD rats, without change in amplitude (Figure 5B, right). To further assess whether BBG acts on presynaptic or postsynaptic sites in the IC, the frequency and amplitude of mEPSCs were measured in the absence and presence of BBG in IC slices of NMD rats. The typical current traces (Figure 5D), the cumulative fraction of peak amplitude and interevent intervals for a representative neuron (Figure 5E,F, left), and normalized mean values for amplitude and frequency (Figure 5E,F, right, n = 6, *P < 0.05, paired‐sample Wilcoxon signed‐rank test) all suggested that the blocking site of P2X7Rs by BBG is most likely presynaptic, which is consistent with the finding that the site of activation of P2X7Rs in IC slices was presynaptic in control rats.
Figure 5.
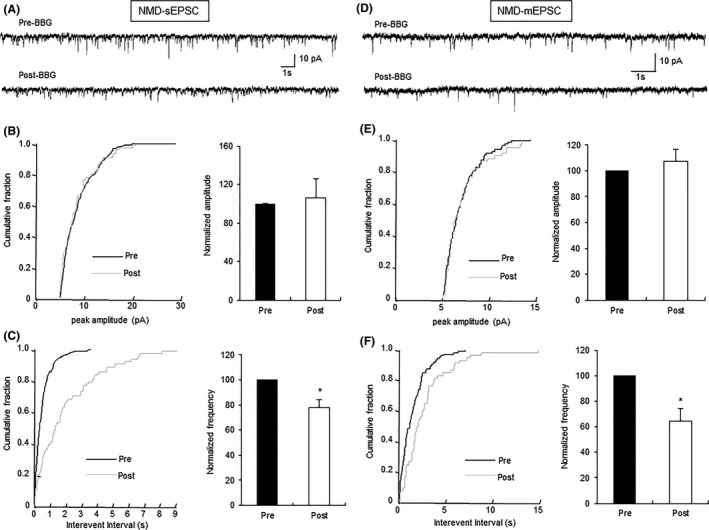
Decrease in glutamatergic synaptic activity of IC neurons by BBG in NMD rats. (A) Representative traces illustrating sEPSCs of an IC neuron before and after the addition of BBG (1 μM). (B) Cumulative fraction of peak amplitude of sEPSCs in an IC pyramidal neuron under the pre‐ and postdrug conditions (left); bar plot showing no significant change in sEPSC peak amplitude by BBG (right). (C) Cumulative fraction of interevent intervals of sEPSCs under the pre‐ and postdrug conditions (left); bar plot showing the significant decrease in sEPSC frequency by BBG (right). n = 6 cells, *P < 0.05 versus pre. (D) Representative traces illustrating mEPSCs of an IC neuron before and after the addition of BBG (1 μM); TTX (1 μM) was used to block action potentials. (E) Cumulative fraction of peak amplitude of mEPSCs in one IC pyramidal neuron under the pre‐ and postdrug conditions (left); bar plot showing no significant change in mEPSC peak amplitude by BBG (right). (F) Cumulative fraction of interevent intervals of mEPSCs under the pre‐ and postdrug conditions (left); bar plot showing that mEPSC frequency was remarkably decreased by BBG treatment (right). Peak amplitude and frequency of BBG‐treated neurons were normalized by those from the same neuron before BBG treatment. n = 6 cells, *P < 0.05 versus pre.
BzATP Induced Visceral Hypersensitivity in Control Rats and BBG or A438079 Reversed Visceral Hypersensitivity in NMD Rats
We then microinjected BzATP into right hemisphere of IC to examine the effect of BzATP in control rat. Microinjection of BzATP led to a large decrease in the CRD threshold in control rats. The microinjection sites are shown in Figures 6A and 4B. The significant hyperalgesia effect was observed at 30 μM BzATP (Figure 6B, n = 6 for each group, *P < 0.05 vs. NS, Tukey's post hoc test following one‐way repeated‐measures ANOVA) and lasted for 15 min (Figure 6C, n = 7 for each group, ***P < 0.001 vs. NS, Dunn's post hoc test following Friedman ANOVA), which was consistent with previous reports 31. We next examined the effect of blockade of P2X7Rs on visceral hypersensitivity of NMD rats. Injection of BBG (0.1 or 1 μM) significantly increased CRD threshold in NMD rats with a bigger effect at 1 μM when tested 5 min after BBG injection (Figure 6D, n = 6 for each group, *P < 0.05 vs. NS, **P < 0.01 vs. NS, Tukey's post hoc test following one‐way repeated‐measures ANOVA). The analgesia effect of BBG at dose of 1 μM disappeared when tested 15 min postinjection (Figure 6E, n = 6 for each group, ***P < 0.001, **P < 0.01 vs. NS, Tukey's post hoc test following one‐way repeated‐measures ANOVA). The effect of A438079, another highly selective antagonist of P2X7Rs 32, on CRD threshold in NMD rats was also determined. A438079 (500 or 1 mM) significantly increased CRD threshold (Figure 6G, n = 5 for each group, *P < 0.05 for 500 nM, **P < 0.01 for 1 mM, paired‐sample t‐test) when tested 5 min postinjection. The analgesia effect of A438079 (1 mM) existed at 5 min postinjection (Figure 6H, n = 5 for each group, **P < 0.01 vs. vehicle, Tukey's post hoc test following one‐way repeated‐measures ANOVA). A438079 (1 mM) did not alter CRD threshold in age‐matched healthy control rats (Figure 6F, n = 6 for each group).
Figure 6.
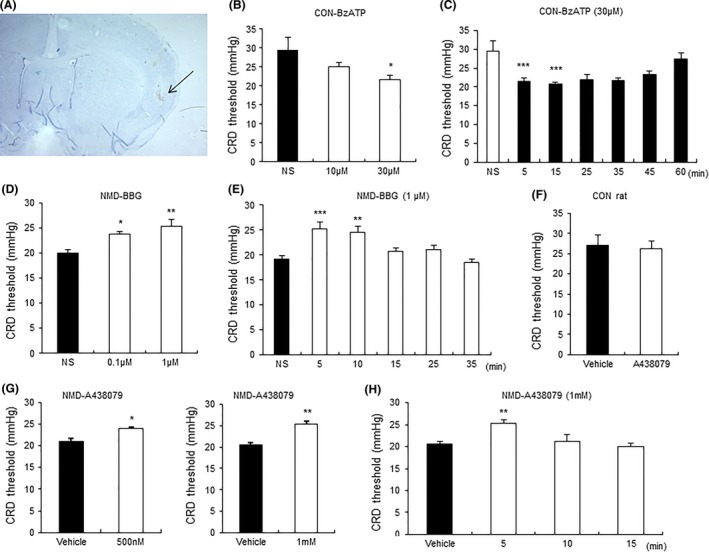
Modulation of CRD threshold by microinjection of agonist and antagonists of P2X7R into IC. (A) A representative picture of actual track of microinjection in the IC of right hemisphere as pointed out by the black arrow. (B) Bar plot illustrating a dose effect of BzATP. There is a significant decrease in CRD threshold in control rats by 30 μM BzATP when tested 5 min after microinjection. n = 6 rats for each group, *P < 0.05 versus normal saline (NS). (C) Bar plot illustrating the time course of the effect of BzATP (30 μM). BzATP significantly decreased CRD threshold when tested at 5 and 15 min postinjection. n = 7 rats for each group, ***P < 0.001 versus NS for each time point. (D) Bar plot showing the dose effect of BBG. There is a significant increase in CRD threshold in NMD rats by BBG (0.1 and 1 μM) when tested 5 min after microinjection, with bigger effect at 1 μM. n = 6 rats for each group, *P < 0.05, **P < 0.01 versus NS. (E) Bar plot showing the time course of the effect of BBG (1 μM). There is a significant increase in CRD threshold in NMD rats when tested at 5 and 10 min after microinjection. n = 6 rats for each group, **P < 0.01, ***P < 0.001 versus NS. (F) Bar plots showing no change in CRD threshold of control rats by A438079 (1 mM). n = 6 rats for each group. (G) Bar plots showing an increase in CRD threshold in NMD rats by both 500 nM and 1 mM A438079 when tested 5 min after microinjection. n = 5 rats for each group, *P < 0.05, **P < 0.01 versus vehicle. (H) Bar plot showing the time course of the effect of A438079 (1 mM). There is a significant increase in CRD threshold in NMD rats when tested at 5 min after microinjection. n = 5 rats for each group, **P < 0.01 versus vehicle.
Discussion
IC is involved in peripheral nerve ligation‐induced neuropathic pain 33, CFA‐induced hyperalgesia 34, phantom limb pain of human 6, but not in acute pain 35. In the present study, although the expression of P2X7Rs upregulated in both hemispheres of IC, microinjection of P2X7R agonist into right IC was efficient to induce visceral hypersensitivity in control rats and microinjection of P2X7R antagonist reversed CVH in NMD rats. These findings suggested that P2X7Rs in right IC plays an important role in the development of visceral pain induced by NMD, as being consistent with the more strong structural connections to autonomic control areas of right IC 24. P2X7R was also reported to play roles in the development of inflammatory pain 31, neuropathic hyperalgesia 36, and IBS 37. In addition to roles of P2X7R in IC, the increased expression of P2X7Rs in dorsal root ganglion enhanced visceral pain, just like the increased expression of P2X4 and P2X6 receptors 38. However, this study does not exclude the roles of the other subtypes of purinergic receptors in the development and maintenance of visceral hypersensitivity of NMD rats.
Under normal conditions, the expression of P2X7Rs in neurons, astrocytes, glial cells, and nonactivated microglia is low 31. However, in the present study the expression of P2X7Rs was significantly increased in IC of NMD rats although very few P2X7Rs were expressed on microglial cells and astrocytes in the IC. In the present study, there is an increase in frequency of mEPSCs by BzATP, without significant alteration in amplitude of mEPSCs. The result suggested the presynaptic action of P2X7Rs. Furthermore, NMD only increased the frequency of action potentials without changing the resting membrane potential, action potential threshold, and input resistance of IC neurons. This result could further support the presynaptic mechanism. In addition, P2X7Rs colocalized with synaptophysin and NeuN in IC. As P2X7R is a ligand‐gated nonselective cation channel and no EPSC was evoked by BzATP in the presence of CNQX and D‐APV, it is therefore reasonable to hypothesize that the P2X7R was not functionally expressed in the cell body but was functionally expressed in presynaptic part, which is consistent with the electrophysiological data.
It is reported that peripheral nerve ligation of mice enhanced AMPA receptor‐mediated excitatory synaptic transmission in the IC 39. This effect relies on the GluR1 subunit of AMPA receptor on synapses 40, and probably by inhibiting endocytosis at the cell membrane or increasing exocytosis of GluR1 at external sites of synapses to improve the AMPA receptor‐mediated synaptic transmission 41. However, this seems not to be the case in IC of NMD rats as there was only an increase in frequency without change in amplitude of sEPSC in NMD group when compared with control group.
Besides P2X7R, several other modulators might contribute to the overexcitation of IC in NMD rats. These modulators include PKMζ, GluR2 5, phosphorylated ERK‐1/2, pCREB, c‐Fos, GABA, dopamine 34, P2X7R‐mediated d‐serine release 42, opioids 43. P2X7Rs are reported to play an upstream transductional role in the development of neuropathic and inflammatory pain via the regulation of IL‐1β production 31. The mRNA level of IL‐1β was significantly increased in IC of both hemispheres in NMD rats (data not shown), suggesting that IL‐1β may also contribute to visceral hypersensitivity of NMD rats. There is also a growing body of evidence showed that P2X7Rs can attribute to inflammation in lung, heart, and islet transplantation and that oATP (P2X7R inhibitor) could reverse the effect 44, 45, 46. In addition, stress mediators and the gut microbiota can interact through complementary or opposing factors to influence visceral sensitivity 47. Investigation of roles of inflammation and immune system is definitely needed.
In addition to IC, there are several other parts of central nervous system taking part in modulation of visceral pain. It is proved that the overexcitation of basolateral amygdala contributed to the visceral hypersensitivity of NMD rats 23. The amygdala, particularly the basal complex, and the nucleus accumbens are important targets of IC efferent fibers 43. The overexcitation of amygdala may be resulted from the overexcitation of IC in NMD rats. IC also modulates pain through affecting middle cingulate cortex, periaqueductal gray, locus coeruleus, the nucleus accumbens, or raphe nucleus 43. The spinal cord, the first relay center for nociceptive processing, is worthy of exploring pain mechanism and effects of antinociceptive drugs 48. The detailed neural circuitry among these areas definitely needs to be further investigated.
Conclusion
In conclusion, the present study provided evidence that upregulation of P2X7Rs in IC increased glutamate neurotransmission and sensitized the IC neurons through a presynaptic mechanism, which resulted in the visceral hypersensitivity of NMD rats. Modulation of IC in right hemisphere by drugs could modulate pain processing.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Figure S1. BzATP increased sEPSC of IC through release of glutamate in control group.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (31300909 to YX, 81230024 and 81471137 to GYX) and from the Priority Academic Program Development of Jiangsu Higher Education Institutions of China. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The first two authors contributed equally to this work.
Contributor Information
Ying Xiao, Email: et790906@hotmail.com.
Guang‐Yin Xu, Email: guangyinxu@suda.edu.cn.
References
- 1. Gauriau C, Bernard JF. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci 2004;24:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benison AM, Chumachenko S, Harrison JA, et al. Caudal granular insular cortex is sufficient and necessary for the long‐term maintenance of allodynic behavior in the rat attributable to mononeuropathy. J Neurosci 2011;31:6317–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baier B, Eulenburg PZ, Geber C, et al. Insula and sensory insular cortex and somatosensory control in patients with insular stroke. Eur J Pain 2014;18:1385–1393. [DOI] [PubMed] [Google Scholar]
- 4. Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: A single‐trial fMRI study. Pain 2007;128:20–30. [DOI] [PubMed] [Google Scholar]
- 5. Han J, Kwon M, Cha M, et al. Plasticity‐related PKMζ Signaling in the insular cortex is involved in the modulation of neuropathic pain after nerve injury. Neural Plast 2015;2015:601767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu MG, Zhuo M. Loss of long‐term depression in the insular cortex after tail amputation in adult mice. Mol Pain 2014;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanamori T, Kunitake T, Kato K, et al. Responses of neurons in the insular cortex to gustatory, visceral, and nociceptive stimuli in rats. J Neurophysiol 1998;79:2535–2545. [DOI] [PubMed] [Google Scholar]
- 8. Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655–666. [DOI] [PubMed] [Google Scholar]
- 9. Lu C, Yang T, Zhao H, et al. Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci Bull 2016;32:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morgan V, Pickens D, Gautam S, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005;54:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nedergaard MLCM. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signalling 2009;5:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guthrie PB, Knappenberger J, Segal M, et al. ATP released from astrocytes mediates glial calcium waves. J Neurosci 1999;19:520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kennedy C, Saville VL, Burnstock G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur J Pharmacol 1986;122:291–300. [DOI] [PubMed] [Google Scholar]
- 14. Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci 1999;2:241–245. [DOI] [PubMed] [Google Scholar]
- 15. León D, Sánchez‐Nogueiro J, Marín‐García P, et al. Glutamate release and synapsin‐I phosphorylation induced by P2X 7 receptors activation in cerebellar granule neurons. Neurochem Int 2008;52:1148–1159. [DOI] [PubMed] [Google Scholar]
- 16. Yu Y, Ugawa S, Ueda T, et al. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res 2008;1194:45–55. [DOI] [PubMed] [Google Scholar]
- 17. Li N, Zhang P, Qiao M, et al. The effects of early life lead exposure on the expression of P2X7 receptor and synaptophysin in the hippocampus of mouse pups. J Trace Elem Med Biol 2015;30:124–128. [DOI] [PubMed] [Google Scholar]
- 18. North RA. Molecular physiology of P2X receptors. Physiol Rev 2002;82:1013–1067. [DOI] [PubMed] [Google Scholar]
- 19. Marcoli M, Cervetto C, Paluzzi P, et al. P2X7 pre‐synaptic receptors in adult rat cerebrocortical nerve terminals: A role in ATP‐induced glutamate release. J Neurochem 2008;105:2330–2342. [DOI] [PubMed] [Google Scholar]
- 20. Deuchars SA, Atkinson L, Brooke RE, et al. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci 2001;21:7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Armstrong JNMB. P2X7‐receptor activation depresses synaptic transmission at hippocampal mossy fiber‐CA3 synapses. Soc Neurosci Abstr 2000;26:884. [Google Scholar]
- 22. Hu S, Xiao Y, Zhu L, et al. Neonatal maternal deprivation sensitizes voltage‐gated sodium channel currents in colon‐specific dorsal root ganglion neurons in rats. Am J Physiol Gastrointest Liver Physiol 2013;304:G311–G321. [DOI] [PubMed] [Google Scholar]
- 23. Xiao Y, Chen X, Zhang PA, et al. TRPV1‐mediated presynaptic transmission in basolateral amygdala contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation. Sci Rep 2016;6:29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Macey PM, Wu P, Kumar R, et al. Differential responses of the insular cortex gyri to autonomic challenges. Auton Neurosci 2012;168:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Browne LE, North RA. P2X receptor intermediate activation states have altered nucleotide selectivity. J Neurosci 2013;33:14801–14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guo J, Fu X, Cui X, et al. Contributions of purinergic P2X3 receptors within the midbrain periaqueductal gray to diabetes‐induced neuropathic pain. J Physiol Sci 2015;65:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ting JT, Daigle TL, Chen Q, et al. Acute brain slice methods for adult and aging animals: application of targeted patch clamp analysis and optogenetics. Methods Mol Biol 2014;1183:221–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci 1992;12:4066–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bianchi BR, Lynch KJ, Touma E, et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol 1999;376:127–138. [DOI] [PubMed] [Google Scholar]
- 30. Okabe S, Miwa A, Okado H. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. J Neurosci 2001;21:6105–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuan YH, Shyu BC. Nociceptive transmission and modulation via P2X receptors in central pain syndrome. Mol Brain 2016;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leichsenring A, Riedel T, Qin Y, et al. Anoxic depolarization of hippocampal astrocytes: Possible modulation by P2X7 receptors. Neurochem Int 2013;62:15–22. [DOI] [PubMed] [Google Scholar]
- 33. Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog Neurobiol 2011;93:385–404. [DOI] [PubMed] [Google Scholar]
- 34. Peltz E, Seifert F, DeCol R, et al. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. NeuroImage 2011;54:1324–1335. [DOI] [PubMed] [Google Scholar]
- 35. Coffeen U, Ortega‐Legaspi JM, Lopez‐Munoz FJ, et al. Insular cortex lesion diminishes neuropathic and inflammatory pain‐like behaviours. Eur J Pain 2011;15:132–138. [DOI] [PubMed] [Google Scholar]
- 36. Kobayashi K, Takahashi E, Miyagawa Y, et al. Induction of the P2X7 receptor in spinal microglia in a neuropathic pain model. Neurosci Lett 2011;504:57–61. [DOI] [PubMed] [Google Scholar]
- 37. Keating C, Pelegrin P, Martinez CM, et al. P2X7 receptor‐dependent intestinal afferent hypersensitivity in a mouse model of postinfectious irritable bowel syndrome. J Immunol 2011;187:1467–1474. [DOI] [PubMed] [Google Scholar]
- 38. Chen L, Liu YW, Yue K, et al. Differential expression of ATP‐gated P2X receptors in DRG between chronic neuropathic pain and visceralgia rat models. Purinergic Signalling 2016;12:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qiu S, Zhang M, Liu Y, et al. GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. J Neurosci 2014;34:13505–13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kam AY, Liao D, Loh HH, et al. Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin‐dependent dephosphorylation of GluR1 subunits. J Neurosci 2010;30:15304–15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oh MC, Derkach VA, Guire ES, et al. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long‐term potentiation. J Biol Chem 2006;281:752–758. [DOI] [PubMed] [Google Scholar]
- 42. Pan HC, Chou YC, Sun SH. P2X7 R‐mediated Ca(2+) ‐independent d‐serine release via pannexin‐1 of the P2X7 R‐pannexin‐1 complex in astrocytes. Glia 2015;63:877–893. [DOI] [PubMed] [Google Scholar]
- 43. Jasmin L, Burkey AR, Granato A, et al. Rostral agranular insular cortex and pain areas of the central nervous system: A tract‐tracing study in the rat. J Comp Neurol 2004;468:425–440. [DOI] [PubMed] [Google Scholar]
- 44. Andrea Vergani CF, D'Addio F, Tezza S, et al. Effect of the purinergic inhibitor oxidized ATP in a model of islet allograft rejection. Diabetes 2013;62:1665–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Andrea Vergani ST, D'Addio F, Fotino C, et al. Long‐term heart transplant survival by targeting the ionotropic purinergic receptor P2X7. Circulation 2013;127:463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu K, Vergani A, Zhao P, et al. Inhibition of the purinergic pathway prolongs mouse lung allograft survival. Am J Respir Cell Mol Biol 2014;51:300–310. [DOI] [PubMed] [Google Scholar]
- 47. Moloney RD, Johnson AC, O'Mahony SM, et al. Stress and the microbiota‐gut‐brain axis in visceral pain: Relevance to irritable bowel syndrome. CNS Neurosci Ther 2016;22:102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rivera‐Arconada I, Roza C, Lopez‐Garcia JA. Spinal reflexes and windup in vitro: Effects of analgesics and anesthetics. CNS Neurosci Ther 2016;22:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. BzATP increased sEPSC of IC through release of glutamate in control group.


