Summary
Aims
Olig2 is one of the most critical factors during CNS development, which belongs to b‐HLH transcription factor family. Previous reports have shown that Olig2 regulates the remyelination processes in CNS demyelination diseases models. However, the role of Olig2 in contusion spinal cord injury (SCI) and the possible therapeutic effects remain obscure. This study aims to investigate the effects of overexpression Olig2 by lentivirus on adult spinal cord injury rats.
Methods
Lenti‐Olig2 expression and control Lenti‐eGFP vectors were prepared, and virus in a total of 5 μL (108 TU/mL) was locally injected into the injured spinal cord 1.5 mm rostral and caudal near the epicenter. Immunostaining, Western blot, electron microscopy, and CatWalk analyzes were employed to investigate the effects of Olig2 on spinal cord tissue repair and functional recovery.
Results
Injection of Lenti‐Olig2 significantly increased the number of oligodendrocytes lineage cells and enhanced myelination after SCI. More importantly, the introduction of Olig2 greatly improved hindlimb locomotor performances. Other oligodendrocyte‐related transcription factors, which were downregulated or upregulated after injury, were reversed by Olig2 induction.
Conclusions
Our findings provided the evidence that overexpression Olig2 promotes myelination and locomotor recovery of contusion SCI, which gives us more understanding of Olig2 on spinal cord injury treatment.
Keywords: lentivirus, myelin, Olig2, oligodendrocytes, spinal cord injury
1. Introduction
As one of the most devastating traumatic events, spinal cord injury (SCI) impacts a patient's physical, psychological, and social well‐being and poses a heavy financial burden on health care systems.1 There are two basic common pathological outcomes in various kinds of SCI, axonal rupture, and demyelination.2, 3 Demyelination, which caused by death or insufficient proliferation of the myelin oligodendrocytes (OLs), has been considered as a therapeutic point for SCI both in bench and the bedside.3, 4 Several studies have shown that endogenous oligodendrocyte precursor cells (OPCs) exist wildly of the CNS including the spinal cord, but the extent of spontaneous remyelination is limited.5 Therefore, promoting the maturation of these progenitors to myelin cells is a potential way to cure SCI.
Transcription factors of the basic helix‐loop‐helix (b‐HLH) family play an important role in the CNS development by controlling differentiation and maturation of oligodendrocytes, motor neurons, and astrocytes.6, 7 It has been reported previously that Olig2, member of the b‐HLH family, regulates the pattern formation and generation of motor neurons and oligodendrocyte lineage cells.8, 9 Furthermore, studies have demonstrated that Olig2 participant in not only the OLs development stages but also in repair processes such as remyelination for multiple sclerosis (MS) models.7, 10, 11, 12
Due to the critical function of Olig2, overexpression it in progenitor cells locally could be a possible method to enhance the oligodendrogenesis and finally remyelination in SCI. However, the exact role of Olig2 in CNS injuries remains controversial according to recent studies.13, 14 A team from Korea point out overexpression Olig2 by retroviral vector‐induced tumor formation in the injured spinal cord.15 On the contrary, overexpression Olig2 in embryonic stem cells (ESCs) and transplantation these cells to rat spinal cord improved functional recovery of irradiation injury with no sigh of tumorigenesis.10
Genetically derived from HIVs, lentiviruses offered unique advantages as gene‐delivery vehicles, such as broad tissue tropisms of in vivo applications and sustained target gene expression after transduction.16, 17, 18 Not only could lentivirus infect dividing cells like retrovirus does, but also they infect nondividing cells, for example, the late stage progenitors of oligodendrocytes.18 And, the modified production system makes lentiviruses potentially safer in integration site profile.18 Studies on other models have shown the lower oncogenic potential of lentiviral vector than retroviral ones.19, 20
Therefore, we upregulated Olig2 by lentiviral vectors locally injection to investigate the effects of Olig2 on oligodendrogenesis and myelin formation, and finally to determine the locomotor recovery following spinal cord contusion injury.
2. Methods
2.1. Olig2‐Lentvirus preparation
The Olig2 cDNA (NM_016967) plasma was a gift from Prof. Richard Lu (Cincinnati, USA). The Olig2 coding sequence was inserted into the pGV208 (Ubi‐MCS‐EGFP) plasmid (Shanghai Gene Genechem Co., LTD) to produce Lenti‐Olig2‐EGFP vector (Lenti‐Olig2). The lentiviral vector only expressing EGFP (Lenti‐EGFP) was generated as a negative control. The lentiviral titer was determined as 2E + 8 TU/mL. Polybrene (Sigma‐Aldrich, St. Louis, MO, USA) of 5 μg/mL was added in the virus solution when injecting. The structure of the vector and the infection property of the lentivirus in 293T cells were shown in Fig. S1.
2.2. Animals and spinal cord injury
Adult Sprague Dawley (SD) female rats, weighing 220~250 g were used in this study. All animal procedures were approved by the Institutional Animal Care and Ethics Committee of the Third Military Medical University, and animal care was performed by the guidelines of the committee.
Spinal cord contusion injury model was prepared according to the descriptions of our previous study with little modification.21, 22 Briefly, rats were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg/kg; Shanghai Pharmaceutical Factory). The spinal cord was exposed with a laminectomy at the T9~T11 level of the spine without disrupting the dura mater. After rats were fixed on the weight‐drop device (a kindly gift from Wise Young, Hong Kong University), the T10 spinal cords were then impacted by a 10 g weight dropped at the height of 15 mm (impacting force: 150 kdyn). Sham group animals only accepted a laminectomy operation without virus injection.
Thirty min after injury, a total of 5 μL virus (Lenti‐Olig2 or Lenti‐eGFP) solutions were injected at two different locations with 2.5 μL each (1.5 mm caudal and 1.5 mm rostral from lesion epicenter) with a depth of 1.0 mm using a Hamilton syringe. The rate of injection was 1 μL/min, and the pipette was left in place for at least 5 minutes to prevent leakage.15
Immediately after the operation and for an additional day, rats received Ringer's solution for hydration (5 mL, s.c.) and buprenorphine (0.3 mg/kg) to alleviate pain. Bladders were expressed three times daily until the rats reached spontaneous urination. Antibiotics (Penicillin; 80000 u, i.m.) were given to treat/prevent bladder infection.
2.3. Behavior assessment
The hindlimb function recovery was assessed by open‐field test, CatWalk analysis as reported previously.21, 23 All the tests were performed at the same time of each day and were graded by the blinded observers.
The standardized Basso, Beattie, and Bresnahan (BBB) score was measured by the Open‐field test for evaluating the movement, weight support, and coordination function of rats in a diameter plastic wading pool. This was observed by two raters (blinded to treatment) for 5 minutes to assess the performances. The assessment was processed before the operation and at days 1, 3, 7, and every week postinjury.
Locomotor gait dynamics of the animals were quantitatively assessed by CatWalk automated gait analysis system (Noldus Information Technology, the Netherlands). Rats were pretrained to across the glass runway (130×8 cm) for 5 days. After injury, animal performances were tested every other week. At least three uninterrupted crossings were effectively obtained for each rat. Footprints of every animal were manually marked and CatWalk software (Version 10.5) automatically calculated the gait parameters. The stride length, base of support, Max Contact At (%), and the print area parameters were considered as the markers of hindlimb function after spinal cord injury. All the parameters were shown as the average of left and right hindlimb.
2.4. Evoked potential examination
The evoked potential examinations were carried out before and injury and at days 7 and 42 postinjury as previously described.21, 24 After anesthetized, the periosteum of the posterior skull was exposed followed by a drilling hole (Bregma: 2 mm posterior, 1 mm lateral). The motion evoked potential (MEP) and somatosensory evoked potential (SEP) were tested using electronic instrument (Powerlab/16SP ADInstruments, Australia). Stimulus parameters were carried out as before. The stimulating electrode was placed on one side of sciatic nerve, the recording electrode on the surface of the other side of cortical somatosensory cortex area, the negative electrode on the lateral scalp, with the reference electrode in the paravertebral muscle when recording SEP. As for MEP, the stimulus electrode and recording electrode were exchanged, and the stimulus cathode (silver disk) was placed below the hard palate.
2.5. Tissue processing, electron microscopy, and immunofluorescent staining
After perfused with PBS and 4% paraformaldehyde, the harvested spinal cord was fixed with 4% paraformaldehyde overnight at 4°C and dehydrated gradually with 0.2 M PB containing sucrose (15%~25%). Serial tissue sections with thickness of 20 μm were prepared from frozen tissues and stored at −80°C until use.
HE staining was performed for assessment of the tissue sparing as previously described.22, 24 Axial sections peripheral to the lesion epicenter were collected systematically. Lesion epicenter and sections located equally distance rostral and caudal to epicenter were chosen to represent the whole lesion. Images were obtained by Nikon camera and calculated by blinded observers using Image J program.
2.6. Electron microscopy
Animals in each group were deeply anesthetized and perfused intracardially with ice‐cold PBS 7 weeks after injury. This was followed by solution of 4% paraformaldehyde mixed with 2.5% glutaraldehyde in 0.1M PB. The dorsal funiculus of the spinal cords was removed and then postfixed at least 1 day for the next procession as previously reported.25 Images with a magnification of 5000 were obtained using a transmission electron microscopy (TEM; Hitachi‐ 7500, Hitachi Ltd., Tokyo, Japan). Only representative images were shown in this article.
To measure the G ratio, three images of every animal and in a total of 50 myelinated axons in each group were measured using the MIAS image analysis system by two blinded researchers.26
2.7. Immunofluorescent staining
Immunocytochemistry was performed on 20 μm thick spinal cord coronal cryostat sections. Primary antibodies: AntiGFP (1:1000, Abcam, USA); antiGFP (1:500, Abcam, USA); antiOlig2 (1:200, Abcam, USA); antiA2B5 (1:1000, Abcam, USA); antiCC1 (1:1000, Millipore, USA); antiKi67 (1:500, Cell Signalling, USA); antiGFAP (1:400, Sigma, USA); antiβ‐tublinIII (1:800, Sigma, USA); and antiMBP (1:500, Sigma, USA) were used. All secondary antibodies (TRICT and FITC labeled secondary antibodies from goat were purchased from Zhongshan (China) Biotechnology. DAPI (10 μg/mL: Sigma, USA) were used for labeling the nuclear.
The number of Olig2, Ki67, NG2, A2B5, CC1, MBP‐positive, or negative GFP cells at different time points postinjury was obtained in the sections adjacent to those used for GFP cell counting. Three sagittal/coronal sections were serially selected in the medial part of each spinal cord from the animals (n=5 for each group). Two images were obtained per section from each spinal cord by Leica SP‐2 confocal microscope (Germany).
2.8. Western blot analysis
The spinal cord segments of 5 mm long containing the epicenter were quickly dissected and homogenized for protein extraction. After centrifuging, protein concentrations of the supernatant were determined using BCA protein assay kit (Beyotime Biotechnology, China). Equivalent amount of protein samples was loaded on SDS‐ polyacrylamide gels and transferred to PVDF membranes (Millipore, USA). The blots were blocked and subsequently probed with anti‐Olig2 (1:2000, Abcam, USA), antiMBP (1:1000, Sigma, USA),antiSox10 (1:1000, Millipore, USA),antiNkx2.2 (1:3000, Developmental Studies Hybridoma Bank, Iowa City, IA),antiOlig1 (1:1500, Abcam, USA),antiId2 (1:1000, Abcam, USA), and β‐actin (1:5000, Zhongshan Bio.) overnight. Secondary horseradish peroxidase‐conjugated antibodies (1:5000, Zhongshan Biotechnology, China) were used and finally, blots were detected with enhanced chemiluminescence detection reagents (Thermo Fisher, USA). β‐actin was recognized as the internal control in parallel running. Bio‐Rad mage Lab Software Version 5.2.1 was used for optical density quantify. Separate experiments were conducted for three times.
2.9. Statistical analysis
Differences between experimental groups were evaluated by two‐tailed unpaired Student's t test or one‐way ANOVA followed by a Tukey's post hoc analysis; repeated measures two‐way ANOVA was used to compare matched data at multiple time points, and Bonferroni's post hoc analysis was used to compare means at each time point unless otherwise stated. All data are presented as mean±SEM unless specified, and statistical analysis was performed using GraphPad Prism version 6.0 for Mac, GraphPad Software, San Diego California USA. P<.05 was considered to be statistically significant.
3. Results
3.1. The expression of Olig2 downregulated after SCI
We first checked the expression level of Olig2 at different time points following spinal cord injury. We found that Olig2 increased shortly after SCI at 1 and 3 days postinjury, both P<.05, compared with normal control (Figure 1A,B). However, the expression level decreased back to normal 7 days later.
Figure 1.
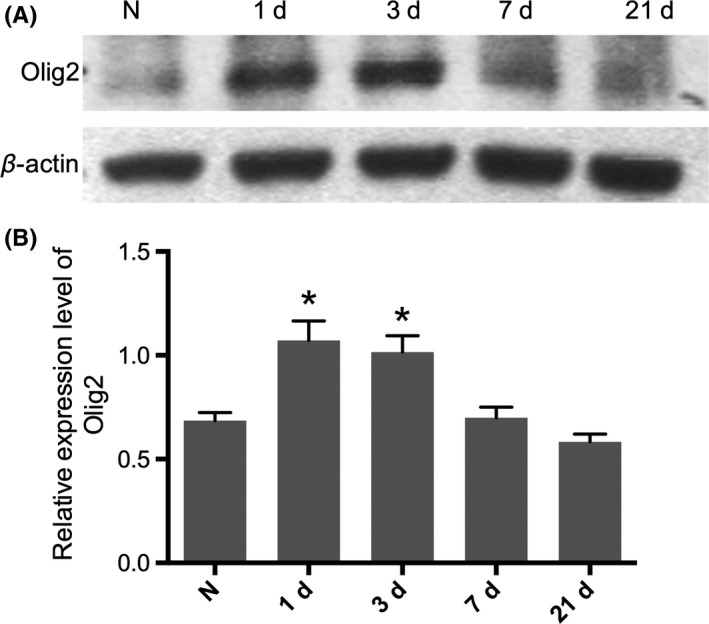
Relative expression level of Olig2 fluctuated after contusion spinal cord injury. A, The protein level of Olig2 upregulated in the early stage (1d, 3d) following SCI, but it is back to a normal after that (7d, 21d). B, Densitometry analyzes showed a higher expression of Olig2 shortly after injury but did not sustain. Data represent mean±SEM (n=3 in each group). *P<.05 compared with intact animal, One‐way ANOVA followed by Tukey's multiple comparison tests
3.2. The expression of injected lentivirus in injured spinal cord
Microscopy images showed that the successful expression of injected lentivirus in both groups at 7 days after injury, and the eGFP cells were mainly distributed on the dorsal column round about the epicenter of the lesion site (Figure 2A). No obvious differences were observed of the relative eGFP fluorescent intensity between groups, which means that both lentiviruses had approximately the same infection efficiency. However, the numbers of eGFP/Olig2 double positive cells were much more in the Lenti‐Olig2 group at each time point, all P<.05 (Figure 2B,C). In addition, the total amount of Olig2 protein level in the injured spinal cord segment were significantly increased in the Lenti‐Olig2 group compared to the other two groups 7 days after injection, both P<.05 (Figure 2D,E).
Figure 2.
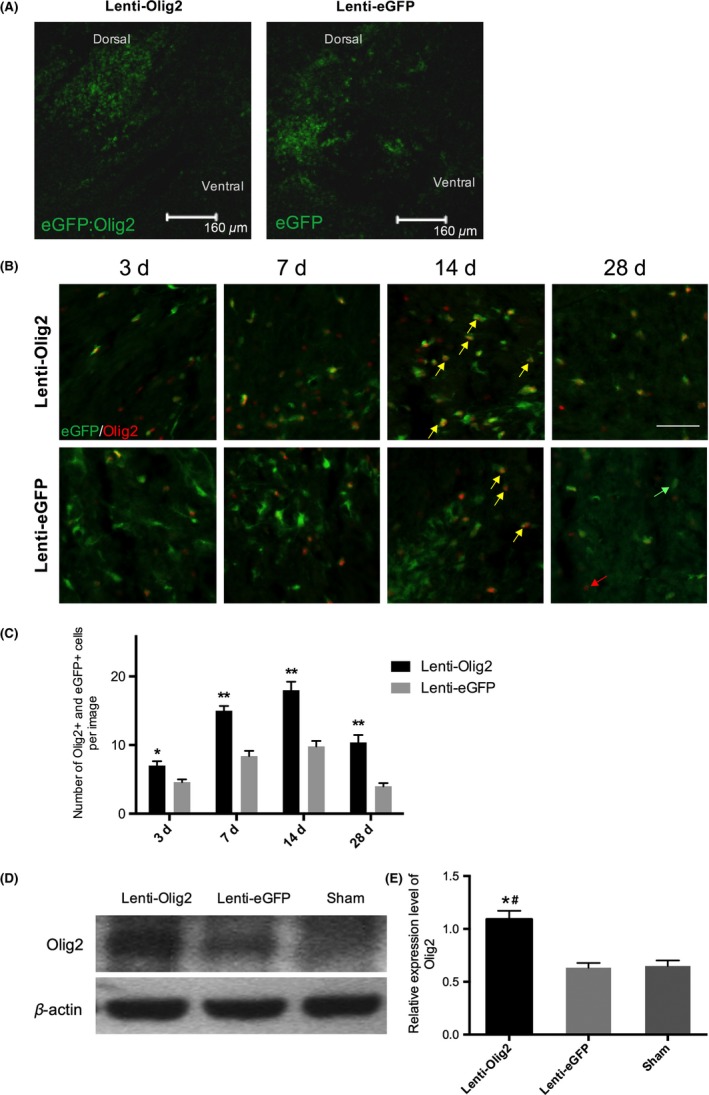
Olig2 upregulated by lentivirus injection. A, Representative images of sagittal spinal cord sections following virus transplantation. Note that the eGFP‐positive cells were distributed round about the lesion site in both groups 7‐day postinjection. Bar=160 μm. (B, C) Images of Olig2 and eGFP staining of both groups at different time points after injury. Most of the GFP cells in the Lenti‐Olig2 group also express Olig2 and the numbers of double positive cells (yellow arrows) were much more in compared with eGFP control. Red arrows: Olig2 single‐positive; Green arrows: eGFP single‐positive. Bar=40 μm. Data presented as mean±SEM (n=5 per group). *P<.05, ** P<.01 vs eGFP group by t tests and Bonferroni's post hoc analysis. (D, E) Western blot and quantitative analysis of Olig2 protein level after SCI in different groups. Values were showed as mean±SEM (n=5 per group). *P<.05 compared with Lenti‐eGFP; # P<.05 compared with Sham
3.3. Enhanced cellular proliferation after Olig2 overexpression
We then detected the proliferation ability of infected cells in the spinal cord. Immunofluorescence staining against the proliferation marker Ki67, we compared the percentage of Ki67 positive cells in the total population eGPF cells. And we found out that animals treated with Lenti‐Olig2 had a significantly higher proportion of Ki67 cell in compared with animals in the Lenti‐eGFP control group, at 68.4±4.0% and 32.1±1.9% separately, P<.01 (Figure 3A,B).
Figure 3.
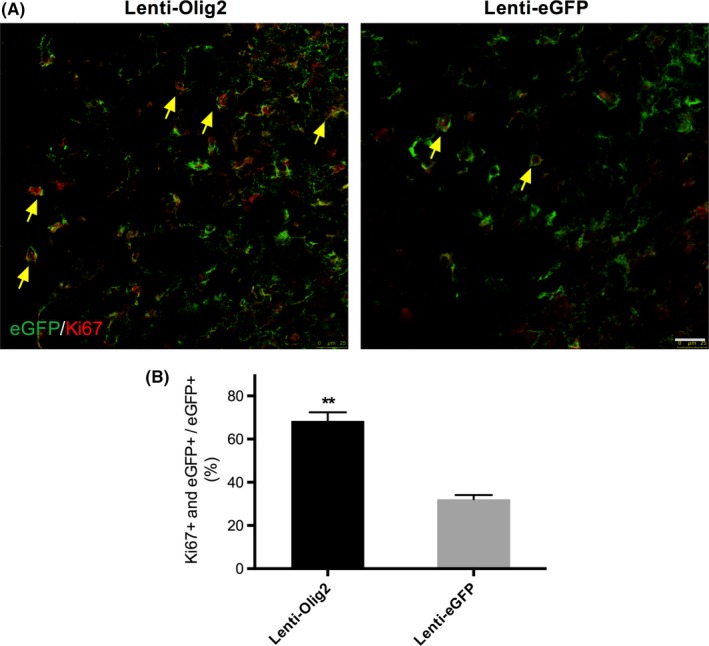
Upregulation of Olig2 enhanced cellular proliferation. A, Representative confocal images of Ki67 and eGFP in both groups 7‐day postinjection. Note that there were much more double positive cells in Lenti‐Olig2 group (yellow arrows). Bar=25 μm. B, Quantification analysis of the proportion of Ki67‐positive cells. Data represent mean±SEM (n=5 per group). **P<.01 compared with Lenti‐eGFP group by Mann‐Whitney U test
3.4. Promoted oligodendrocyte lineage cells differentiation by overexpression Olig2
We then determined the effects of overexpression Olig2 on oligodendrocyte lineage cells differentiation by immunostaining. The proportion of A2B5 positive eGFP cells were increased greatly by Olig2 7‐day postinjury, at 78.5±3.6% and 39.2±2.0, respectively, P<.01 (Figure 4A,B). Similar trends were observed in NG2 cells, with 68.8±4.9 and 42.5±3.4% in each group, P<.01(Figure 4C,D). Although it has been pointed out that A2B5 and NG2 also participate astrocytes differentiation,27 very seldom GFAP‐positive eGFP cells were observed (Figure 5A,B). Furthermore, we counted the number of matured oligodendrocytes using CC1 (APC) 14‐day postinjury. Very few of CC1‐positive eGFP cells were seen in the GFP control group with an average number of 1.1±0.2 per image. Meanwhile, overexpression Olig2 produced significantly more CC1‐positive eGFP cells, with the number of 5.4±0.3 per image, P<.01 (Figure 4E,F). Together, these results indicated that the introduction of Olig2 along is effective to promote proliferating glial progenitor cells into the OL lineage, even the matured stage.
Figure 4.
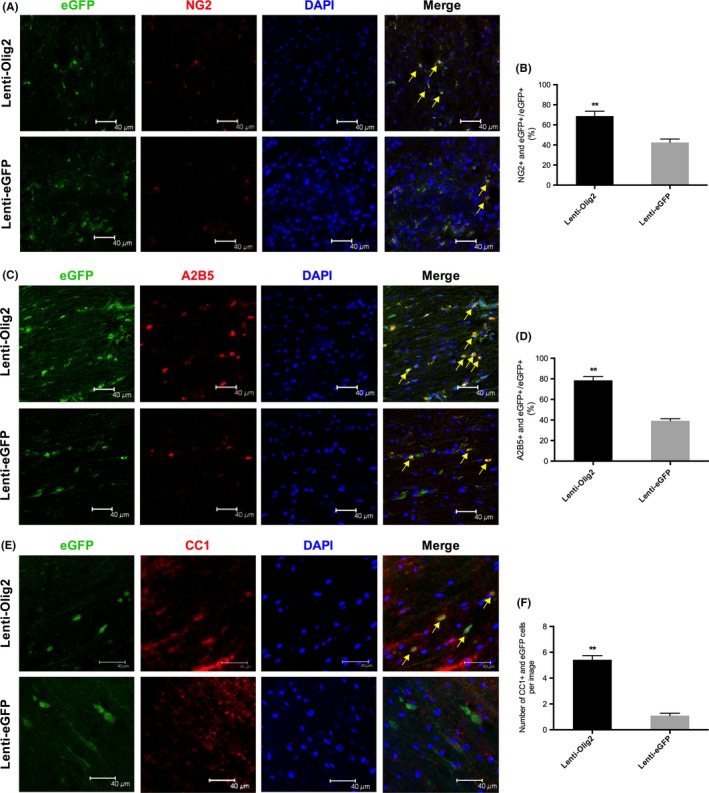
Differentiation of oligodendrocyte lineage cells after local injection of lentivirus. (A, C, E) Representative images of different stage oligodendrocyte makers in eGFP‐positive cells. A2B5 and NG2 indicating oligodendrocyte progenitor cell (OPC) marker were detected 7‐day postinjury. Matured oligodendrocyte maker CC1 was stained 14‐day post‐injury. Note that the number of double positive cells (yellow arrows) in Lenti‐Olig2 group was much more than the eGFP control group. Bar=40 μm. (B, D, F) Quantitative analysis of the percentage or the number of double positive cells in both groups. Values are presented as mean±SEM (n=5 for each group). **P<.01 compared with Lenti‐eGFP group
Figure 5.
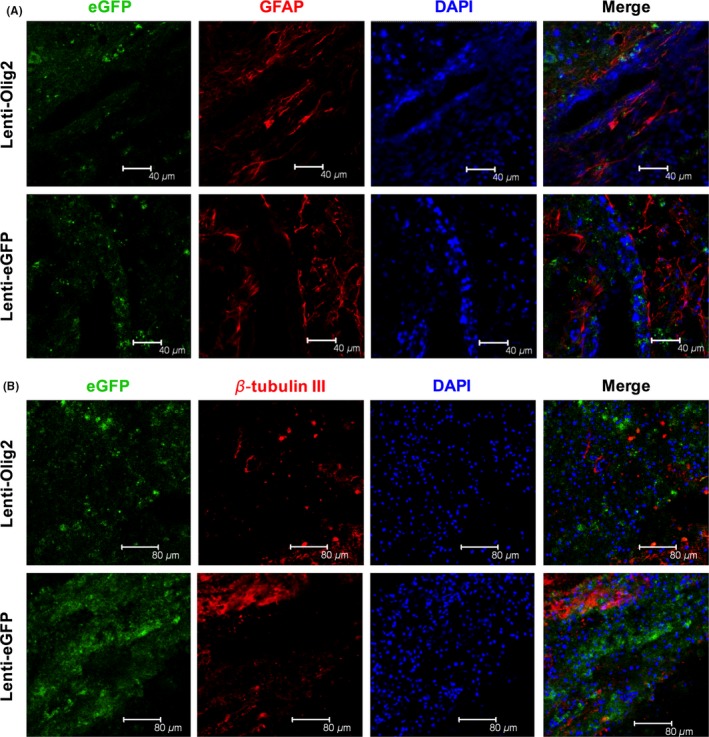
Astrocytes and neurons were not affected after local injection of lentivirus. (A, B) Representative images of specific cell type makers in eGFP‐positive cells. GFAP indicates astrocytes and β‐tubulin III is for neurons. Note that very few of double positive cells were observed in both groups. Bar=40 μm
3.5. No influence of overexpressing Olig2 on astrocytes and neurons after SCI
We further verified whether Olig2 overexpression would influence the differentiation and proliferation of astrocytes and neurons. The number of GFAP or β‐tubulin III‐positive eGFP cells were quite few in both groups 14 days after injury (Figure 5A,B; Fig. S2). This finding indicates that Olig2 has a relatively limited influence of astrocytes and neurons in adult spinal cord.
3.6. New myelination and enhanced tissue protection after upregulating Olig2 locally
We next examined whether those proliferating oligodendrocyte progenitors can myelinate and rescue the demyelination after contusion injury of the spinal cord. In the Olig2 overexpression group, MBP‐positive eGFP cells were observed more frequently than that of the GFP control. Some of the double positive cells exhibited the typical structure of the myelin sheath (Figure 6A). Western blot analysis was carried out to confirm furtherly the production of myelinating oligodendrocytes (Figure 6B). Relative expression level of MBP was greatly enhanced by Lenti‐Olig2 injection compared to eGFP control but did not reach the level in the sham group, both P<.05 (Figure 6C). Furthermore, less myelin debris and unmyelinated axons were observed in the Lenti‐Olig2 group (Fig. S3).
Figure 6.
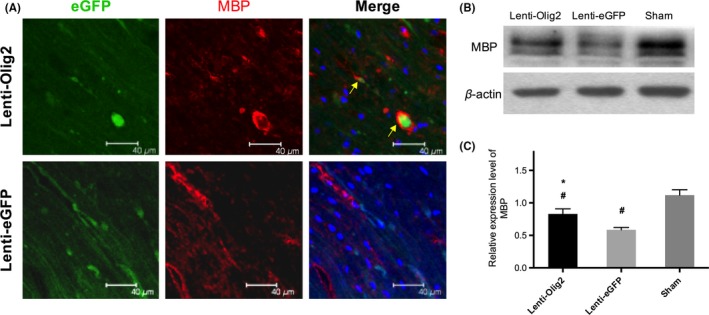
Myelin formation by eGFP‐positive oligodendrocytes 42 d after injury. (A) Representative immunofluorescence images of MBP expressed in eGFP cells (arrows). Bar=40 μm (B, C) Western blot and quantitative analysis of MBP expression level after SCI in different groups. Values were showed as mean±SEM (n=5 for each group). *P<.05 compared with Lenti‐eGFP; # P<.05 compared with Sham
Previous studies pointed out upregulating Olig2 alone would induce tumor formation in the spinal cord. However, we did not observe any tumor‐like structure even 7 weeks later by HE staining. Instead, the overexpression Olig2 reduced the tissue damage, as there was larger lesion area in the eGFP group (Fig. S4). Ultrastructural results showed that myelin sheaths became disordered, thickened, and even broke down after injury. And in the control group axonal degeneration and myelin sheaths edematous were more frequently observed. In contrast to that, the swelling degree and axonal degenerative degree were milder in the Olig2 group. By analyzing the G ratio of 50 axons in each group, we found that overexpression Olig2 lead to a higher myelin sheath thickness, P<.05 (Figure 7). Taking together, our results suggested that Olig2 was able to not only promote the myelination but also reduce the myelin tissue damage after SCI.
Figure 7.
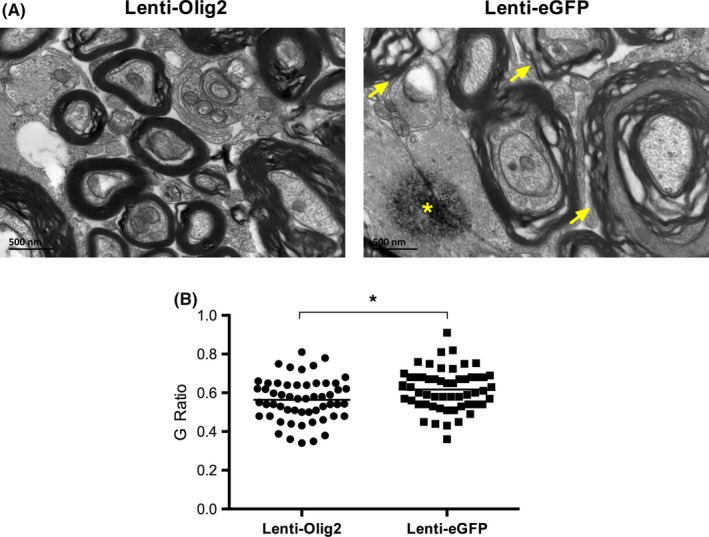
Ultrastructure of myelinated axons at the posterior funiculus in different groups. A, The myelin sheaths in the Lenti‐Olig2 group were more compacted and less swollen in compared with Lenti‐eGFP group. Note that degenerated axons (asterisk) and edema, disordered myelin sheaths (arrows) were frequently observed in GFP control group. Bar=500 nm. B, Quantitative analysis of G ratio in both groups. Data presented as individual values and mean. A total of 50 myelinated axons in each group were analyzed. * P<.05 compared with Lenti‐eGFP
3.7. Recovery of the electrophysiology
To evaluate the axonal conduction in SCI rats, MEP and SEP were recorded at various time points (Figure 8A), and the latency and amplitude of N1 in both groups were analyzed. The latency of MEP and SEP was greatly prolonged, while the amplitude was apparently dropped in both groups 7‐day postinjury. The latency of MEP and SEP decreased close to baseline at 42 days later, but no significant differences been found between groups, with 11.47±0.16 ms and 12.46±0.22 ms for MEP and 22.09±0.36 ms, and 24.86±0.47 ms for SEP, respectively, all P>.05 (Figure 8B,C). However, the amplitude of MEP and SEP in Lenti‐Olig2 group increased significantly compared with the Lenti‐eGFP at the same time point, at 31.82±0.63 μV and 26.34±0.30 μV for MEP, and 31.41±0.54 μV and 24.40±0.34 μV for SEP, respectively, all P<.05 (Figure 8D,E). These results demonstrated that enhanced myelination by Olig2‐induced recovery of the axonal conduction after SCI.
Figure 8.
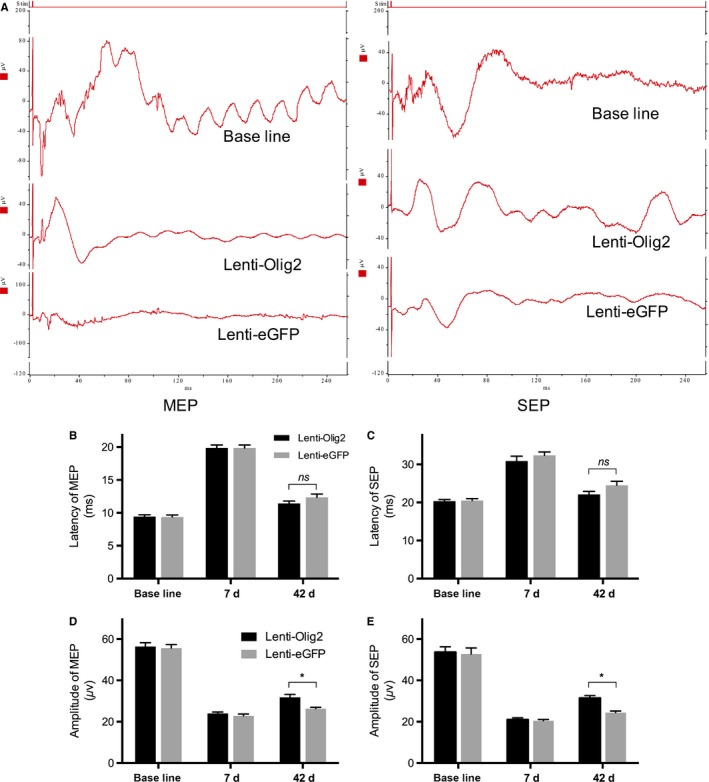
Electrophysiological recordings of the motor and cortical somatosensory evoked potential. A, Graphs of motor evoked potential (MEP) and somatosensory evoked potential (SEP) in both groups. Stim indicates stimulation. (B, C) Quantitative analysis of N1 latency (ms) at baseline, 7 d, and 42 d post‐injury. Data presented as mean±SEM (n=5 per group). No differences were found between groups at each time point by multiple t test. (D, E) Quantitative analysis of N1 amplitude (μV) at baseline, 7 d, and 42 d post‐injury. Data presented as mean±SEM (n=5 per group). *P<.05 vs eGFP group
3.8. Improved hindlimb functional recovery by local injection of the Lenti‐Olig2
To assess the functional recovery of the rats after SCI, BBB score, and CatWalk gait analysis were employed. All animals were completely paralyzed immediately after contusion, and the BBB score in both groups dropped to zero at day 1. However, rats in the Lenti‐Olig2 group showed a gradual improvement, which is better than animals in the Lenti‐eGFP group, significant differences were found at 21, 28, 35 days postinjury, all P<.05 (Figure 9A). Gait analysis confirmed the improvement of hindlimb functions in the Lenti‐Olig2 group. Overexpression Olig2 induced longer and effective hind paw supporting of the body, and the walking pattern in Lenti‐Olig2 group is quite close to the sham group (Figure 9B; Video [Link], [Link], [Link]). After SCI, rats walked with a shorter stride length and wider base of support for their hindlimbs than animals in the sham group. And the injury resulted in a decrease in hind paw print area as well as the proportion of the max contacting time (max contact at (%)). Statistical analysis found out animals that overexpression Olig2 exhibited a better recovery of all the parameters discussed above (P<.05 vs. eGFP group), but none of these reached the normal level (P<.05 vs. sham) (Figure 9C‐F). Therefore, these data provide proof of the functional restoration effects of Olig2 lentivirus.
Figure 9.
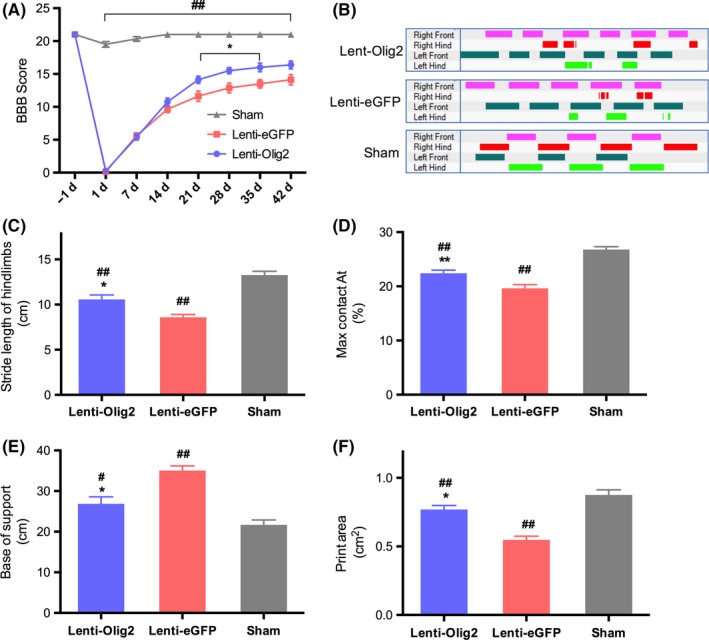
Locomotor function assessment after SCI by BBB scoring and CatWalk gait analysis. (A) Comparison of BBB score in different groups. Data presented as mean±SEM (n=8 per group). *P<.05 vs Lenti‐eGFP group; ## P<.01 vs Sham group by repeated measures two‐way ANOVA followed post hoc Tukey's test. (B) Representative limbs' supporting timing view of CatWalk gait analysis in three groups. (C~F) Statistical analysis of hindlimb Stride Length (cm), Max Contact at (%), Base of Support (cm), and Pint Area (cm2) at 42‐day postinjury. Values were shown as mean±SEM (n=8 in each group). *P<.05, ** P<.01 compared with Lenti‐eGFP group; # P<.05, ## P<.01 compared with Sham
3.9. Changes in the transcription factors following lentivirus injection
To further characterize the possible mechanisms underlying, we finally examined changes in the expression of other oligodendrocyte transcription factors such as Nkx2.2, Olig1, and Sox10, which have been considered as the coactivators during oligodendrocytes development at different stages.7 Seven days postinjury, the relative expression level of Nkx2.2, Olig1, and Sox10 was obviously upregulated by Olig2 overexpression compared to Lenti‐eGFP and sham group, all P<.05 (Figure 10A‐D). Meanwhile, the expression of Id2, which is a negative regulator of oligodendrocytes differentiation, was significantly inhibited by Lenti‐Olig2 injection, P<.05 (Figure 10E). Collectively, these data suggested that Olig2 might play the similar regulating role of related OLs transcription factors in myelination processes even after adult spinal cord injury.
Figure 10.
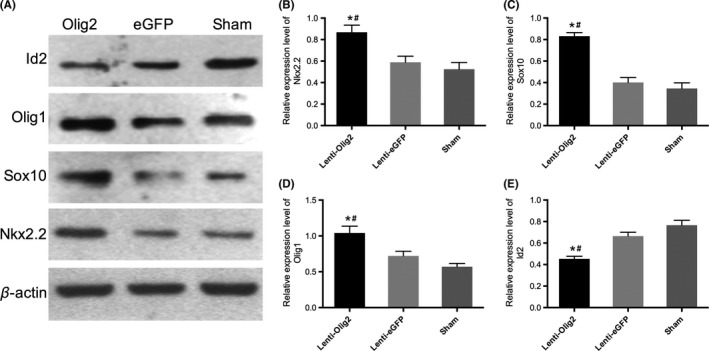
Expression level of related transcription factors in three groups. (A) Representative Western blot images of Nkx2.2, Sox10, Olig1, and Id2 7‐day postinjury. (B~E) Quantitative analysis of relative expression level for each factor. Data presented as mean±SEM (n=3 in each group). *P<.05 compared with Lenti‐eGFP group; # P<.05 compared with Sham
4. Discussion
In the current study, we demonstrated that local injection of Lenti‐Olig2 significantly increased the number of oligodendrocytes lineage cells and enhanced myelination after SCI in adult rats. More importantly, the induction of Olig2 significantly improved hindlimb locomotor performances. The oligodendrocytes related transcription factors, which were obviously downregulated or upregulated after injury, were reversed by Olig2 induction. Our findings provided the evidence that promoting myelination by overexpression Olig2 might be a therapeutic point, which worth to be consideration for SCI in the future.
Olig2 plays a central role in controlling the development of both motor neurons and oligodendrocytes indicating its strong correlations with various CNS disorders such as demyelination, injury, and even gliomas.7, 28 Fate mapping and tracking assays showed that most Olig2 progenitors proliferation and differentiation into OLs more efficiently in demyelination mice models.29 And gain‐of‐function studies further confirmed that upregulation Olig2 in stem/progenitor cells leads to matured OLs and myelination in vivo and in vitro.30, 31, 32 However, how Olig2 influences CNS injuries remain controversial. For example, Olig2 progenitors switch from gliogenic phenotype to functional motor neurons after SCI in zebrafish.33 Contrarily, Olig2 inhibits the neurogenesis in response to brain injury, which was observed by Buffo et al.13 However, most studies suggested that Olig2 induces neurological recovery is mainly associated with oligodendrogenesis.10, 14 Our results lead to the similar conclusion that Olig2 triggered most of the infected progenitor cells proliferation and development into OLs. Meanwhile, Olig2 overexpression might be able to reduce the tissue damage after injury, because there were smaller lesion size and less degenerated myelin and axons in the Lenti‐Olig2 group.
It is noticeable that some of the OPC makers we used are also for other glial lineage progenitors (eg, A2B5 for astrocytes),34 but we did not observe any eGFP‐positive astrocytes or neurons in both groups, which suggests that introduction Olig2 barely influence other lineage cells. Another study also showed that deletion of Olig2 in NG2 progenitor cells causes severe reduction in the number of oligodendrocytes, and loss of myelin.13 Therefore, upregulating Olig2 in NG2 or A2B5‐positive progenitors can induce the differentiation of OLs even in adult injured spinal cord.
It has been well demonstrated that Olig2 regulates the proliferation of neural stem/progenitor cells.35, 36 However, the enhanced proliferation by Olig2 may induce potential problems (eg, CNS tumors).37 Ligon and his colleagues found that Olig2 is universally expressed in diffuse gliomas, which is thought to arise from progenitor/stem cells.37 And RNAi of Olig2 decreases the spreading ability of oligodendrogliomas in a platelet‐derived growth factor (PDGF) derived human cell line.38 The alarming results from Hyuk and colleagues, who observed glioma formation after transinfection Olig2 alone in SCI rats, which could be prevented by simultaneous overexpression of Olig1.15 However, we did not observe any tumor formation in the present study up to 7‐week postinjury. Besides, the expression of Olig1 was upregulated by introduction Olig2 alone. Moreover, our results were supported by several other similar studies. Hu et al. transplanted NSCs, which was infected by Lenti‐Olig2, into the injured spinal cord and they found increased OL‐remyelinated axons with no tumor formed.14 Following transplantation of Olig2 overexpression OPCs into the spinal cord that underwent irradiation injury, Sun et al. also found no tumor‐like mass in the injury lesion and the grafted OPCs differentiated to myelinating OLs.10 On the other hand, as lentivirus vectors have an evolved packaging system to reduce the possibility of replication‐competent production, they are more effective and safer.18 Using a tumor‐prone mouse model, Montini and colleagues revealed that the prototypical lentiviral vectors have lower oncogenic potential than conventional retroviral vectors.19 Furtherly, the insertion pattern of lentiviral vectors was found to be approximately 3‐fold lower than that mediated by retroviral vectors to trigger the transformation of primary hematopoietic cells.20 Collectively, it is suggested that overexpressing Olig2 locally by lentivirus delivery systems is worthy of consideration in promoting myelin repair in CNS injuries.
The importance of remyelination during SCI repair might be various between species. Jurate once reported that chronic demyelination is not existed in SCI mouse.39 Comparatively, studies on rats and humans pointed the opposite condition. Minodora et al. documented the extent of demyelination and remyelination up to 450 days following contusive SCI, and the results demonstrated that SCI rats are accompanied by chronic progressive demyelination.3 Moreover, axonal demyelination can be detected in patient with SCI as well.4 Our study supplied as another evidence that enhancing remyelination promotes the SCI recovery. Not only the axonal conduction was elevated but also the better hindlimb locomotor capacity was detected.
We and other group have previously reviewed that the development of OLs was under control by several factors.7, 40, 41 The most potential ones belong to the b‐HLH family such as Nkx2.2, Id2, Olig2, and Sox10. Among which, Nkx2.2, Olig1, and Sox10 play as coactivators with Olig2 in promoting during OLs differentiation and maturation, while Id2 plays as the inhibitor in the early stage of OLs differentiation decision.42, 43, 44, 45 The current study showed that these coactivators including Olig2 were all down regulated after injury and the inhibitor Id2 kept a relative higher level in sham and eGFP control group. However, these expression trends were all reversed by Olig2 virus injection 7 days after injury, which might serve as the underlying molecular mechanism.
In conclusion, our data suggest that the injection of Lenti‐Olig2 enhanced functional recovery after spinal cord contusion injury by promoting myelination. This study provided evidence that Olig2 might be a potential target for therapeutic intervention in SCI and other CNS diseases.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Acknowledgements
We thank Prof. Qing Richard Lu in the University of Cincinnati for his kindly donate of the Olig2 cDNA plasma. This work was supported by grants from the National Natural Science Foundation of China (No.81071599),Chongqing National Natural Science Fund (CSTC, No.2012jjA10058) and Scientific and Technological Research Program of Chongqing Municipal Education Commission(No. KJ1500237). The author gratefully acknowledges financial support from China Scholarship Council. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Tan B‐T, Jiang L, Liu L, et al. Local injection of Lenti‐Olig2 at lesion site promotes functional recovery of spinal cord injury in rats. CNS Neurosci Ther. 2017;23:475–487. 10.1111/cns.12694
Contributor Information
Le‐Hua Yu, Email: yulehuadoc@aliyun.com.
Yuan Liu, Email: yliu312@sina.com.
References
- 1. Singh A, Tetreault L, Kalsi‐Ryan S, et al. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006;23:345‐359. [DOI] [PubMed] [Google Scholar]
- 3. Papastefanaki F, Matsas R. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia. 2015;63:1101‐1125. [DOI] [PubMed] [Google Scholar]
- 4. Guest J, Hiester E, Bunge R. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384‐393. [DOI] [PubMed] [Google Scholar]
- 5. Karimi‐Abdolrezaee S, Eftekharpour E, Wang J, et al. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377‐3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou Q, Wang S, Anderson DJ. Identification of a novel family of oligodendrocyte lineage‐specific basic helix–loop–helix transcription factors. Neuron. 2000;25:331‐343. [DOI] [PubMed] [Google Scholar]
- 7. Tan B, Yu J, Yin Y, et al. The Olig family affects central nervous system development and disease. Neural Regen Res. 2014;9:329‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu QR, Park JK, Noll E, et al. Oligodendrocyte lineage genes (OLIG) as molecular markers for human glial brain tumors. Proc Natl Acad Sci U S A. 2001;98:10851‐10856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61‐73. [DOI] [PubMed] [Google Scholar]
- 10. Sun Y, Xu CC, Li J, et al. Transplantation of oligodendrocyte precursor cells improves locomotion deficits in rats with spinal cord irradiation injury. PLoS ONE. 2013;8:e57534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salinas TL, Gudi V, Kucman V, et al. Oligodendroglial markers in the cuprizone model of CNS de‐and remyelination. Histol Histopathol. 2015;30:1455‐1464. [DOI] [PubMed] [Google Scholar]
- 12. Tomas‐Roig J, Wirths O, Salinas‐Riester G, Havemann‐Reinecke U. The Cannabinoid CB1/CB2 Agonist WIN55212.2 Promotes Oligodendrocyte Differentiation In Vitro and Neuroprotection During the Cuprizone‐Induced Central Nervous System Demyelination. CNS Neurosci Ther. 2016;22:387‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buffo A, Vosko MR, Ertürk D, et al. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A. 2005;102:18183‐18188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu J‐G, Shen L, Wang R, et al. Effects of Olig2‐overexpressing neural stem cells and myelin basic protein‐activated T cells on recovery from spinal cord injury. Neurotherapeutics. 2012;9:422‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim HM, Hwang DH, Choi JY, et al. Differential and cooperative actions of Olig1 and Olig2 transcription factors on immature proliferating cells after contusive spinal cord injury. Glia. 2011;59:1094‐1106. [DOI] [PubMed] [Google Scholar]
- 16. Connolly JB. Lentiviruses in gene therapy clinical research. Gene Ther. 2002;9:1730‐1734. [DOI] [PubMed] [Google Scholar]
- 17. Simonato M, Bennett J, Boulis NM, et al. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9:277‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sakuma T, Barry MA, Ikeda Y. Lentiviral vectors: basic to translational. Biochem J. 2012;443:603‐618. [DOI] [PubMed] [Google Scholar]
- 19. Montini E, Cesana D, Schmidt M, et al. Hematopoietic stem cell gene transfer in a tumor‐prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687‐696. [DOI] [PubMed] [Google Scholar]
- 20. Modlich U, Navarro S, Zychlinski D, et al. Insertional transformation of hematopoietic cells by self‐inactivating lentiviral and gammaretroviral vectors. Mol Ther. 2009;17:1919‐1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hou T, Wu Y, Wang L, et al. Cellular prostheses fabricated with motor neurons seeded in self‐assembling peptide promotes partial functional recovery after spinal cord injury in rats. Tissue Eng Part A. 2012;18:974‐985. [DOI] [PubMed] [Google Scholar]
- 22. Liu Y, Tan B, Wang L, et al. Endogenous neural stem cells in central canal of adult rats acquired limited ability to differentiate into neurons following mild spinal cord injury. Int J Clin Exp Pathol. 2015;8:3835‐3842. [PMC free article] [PubMed] [Google Scholar]
- 23. Chiang C‐Y, Sheu M‐L, Cheng F‐C, et al. Comprehensive analysis of neurobehavior associated with histomorphological alterations in a chronic constrictive nerve injury model through use of the CatWalk XT system: laboratory investigation. J Neurosurg. 2014;120:250‐262. [DOI] [PubMed] [Google Scholar]
- 24. Wang YT, Lu XM, Zhu F, et al. Ameliorative Effects of p75NTR‐ED‐Fc on Axonal Regeneration and Functional Recovery in Spinal Cord‐Injured Rats. Mol Neurobiol. 2015;52:1821‐1834. [DOI] [PubMed] [Google Scholar]
- 25. Liu L, Zhang W, Wang L, et al. Curcumin prevents cerebral ischemia reperfusion injury via increase of mitochondrial biogenesis. Neurochem Res. 2014;39:1322‐1331. [DOI] [PubMed] [Google Scholar]
- 26. Michailov GV, Sereda MW, Brinkmann BG, et al. Axonal neuregulin‐1 regulates myelin sheath thickness. Science. 2004;304:700‐703. [DOI] [PubMed] [Google Scholar]
- 27. Sypecka J, Sarnowska A, Gadomska‐Szabłowska I, et al. Differentiation of glia‐committed NG2 cells: the role of factors released from hippocampus and spinal cord. Acta Neurobiol Exp (Wars). 2013;73:116‐129. [DOI] [PubMed] [Google Scholar]
- 28. Chakrabarti L, Best TK, Cramer NP, et al. Olig1 and Olig2 triplication causes developmental brain defects in Down syndrome. Nat Neurosci. 2010;13:927‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Islam MS, Tatsumi K, Okuda H, et al. Olig2‐expressing progenitor cells preferentially differentiate into oligodendrocytes in cuprizone‐induced demyelinated lesions. Neurochem Int. 2009;54:192‐198. [DOI] [PubMed] [Google Scholar]
- 30. Maire CL, Wegener A, Kerninon C, Nait Oumesmar B. Gain‐of‐Function of Olig Transcription Factors Enhances Oligodendrogenesis and Myelination. Stem Cells 2010;28:1611‐1622. [DOI] [PubMed] [Google Scholar]
- 31. Yao R, Wang B, Ren C, et al. Olig2 overexpression accelerates the differentiation of mouse embryonic stem cells into oligodendrocyte progenitor cells in vitro. Dev Growth Differ. 2014;56:511‐517. [DOI] [PubMed] [Google Scholar]
- 32. Wegener A, Deboux C, Bachelin C, et al. Gain of Olig2 function in oligodendrocyte progenitors promotes remyelination. Brain. 2015;138:120‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reimer MM, Sörensen I, Kuscha V, et al. Motor neuron regeneration in adult zebrafish. J Neurosci. 2008;28:8510‐8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller RH, Raff MC. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci. 1984;4:585‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dimou L, Simon C, Kirchhoff F, et al. Progeny of Olig2‐expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434‐10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun Y, Meijer DH, Alberta JA, et al. Phosphorylation state of Olig2 regulates proliferation of neural progenitors. Neuron. 2011;69:906‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ligon KL, Huillard E, Mehta S, et al. Olig2‐regulated lineage‐restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Appolloni I, Calzolari F, Barilari M, et al. Antagonistic modulation of gliomagenesis by Pax6 and Olig2 in PDGF‐induced oligodendroglioma. Int J Cancer. 2012;131:E1078‐E1087. [DOI] [PubMed] [Google Scholar]
- 39. Lasiene J, Shupe L, Perlmutter S, Horner P. No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. J Neurosci. 2008;28:3887‐3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meijer DH, Kane MF, Mehta S, et al. Separated at birth? The functional and molecular divergence of OLIG1 and OLIG2. Nat Rev Neurosci. 2012;13:819‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tan B, Luo Z, Yue Y, et al. Effects of FTY720 (Fingolimod) on Proliferation, Differentiation, and Migration of Brain‐Derived Neural Stem Cells. Stem Cells Int. 2016;2016:9671732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang S, Sdrulla A, Johnson JE, et al. A role for the helix‐loop‐helix protein Id2 in the control of oligodendrocyte development. Neuron. 2001;29:603‐614. [DOI] [PubMed] [Google Scholar]
- 43. Nieto M, Schuurmans C, Britz O, Guillemot F. Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron. 2001;29:401‐413. [DOI] [PubMed] [Google Scholar]
- 44. Chen XS, Zhang YH, Cai QY, Yao ZX. ID2: a negative transcription factor regulating oligodendroglia differentiation. J Neurosci Res. 2012;90:925‐932. [DOI] [PubMed] [Google Scholar]
- 45. Imayoshi I, Kageyama R. bHLH factors in self‐renewal, multipotency, and fate choice of neural progenitor cells. Neuron. 2014;82:9‐23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


