Summary
Introduction
Sigma‐1 receptors (Sig‐1Rs) are unique endoplasmic reticulum proteins that have been implicated in both neurodegenerative and ischemic diseases, such as Alzheimer's disease and stroke. Accumulating evidence has suggested that Sig‐1R plays a role in neuroprotection and axon outgrowth. The underlying mechanisms of Sig‐1R‐mediated neuroprotection have been well elucidated. However, the mechanisms underlying the effects of Sig‐1R on axon outgrowth are not fully understood.
Methods
To clarify this issue, we utilized immunofluorescence to compare the axon lengths of cultured naïve hippocampal neurons before and after the application of the Sig‐1R agonist, SA4503. Then, electrophysiology and immunofluorescence were used to examine voltage‐gated calcium ion channel (VGCCs) currents in the cell membranes and growth cones.
Results
We found that Sig‐1R activation dramatically enhanced the axonal length of the naïve hippocampal neurons. Application of the Sig‐1R antagonist NE100 and gene knockdown techniques both demonstrated the effects of Sig‐1R. The growth‐promoting effect of SA4503 was accompanied by the inhibition of voltage‐gated Ca2+ influx and was recapitulated by incubating the neurons with the L‐type, N‐type, and P/Q‐type VGCC blockers, nimodipine, MVIIA and ω‐agatoxin IVA, respectively. This effect was unrelated to glial cells. The application of SA4503 transformed the growth cone morphologies from complicated to simple, which favored axon outgrowth.
Conclusion
Sig‐1R activation can enhance axon outgrowth and may have a substantial influence on neurogenesis and neurodegenerative diseases.
Keywords: axon outgrowth, growth cone, hippocampal neurons, sigma‐1 receptor, voltage‐gated calcium ions channels
1. INTRODUCTION
Sigma receptors consist of two subtypes, Sigma‐1 and Sigma‐2 receptors (Sig‐1R and Sig‐2R).1 Sig‐2R has not yet been cloned due to insufficient affinity. Sig‐1R was first cloned from guinea pig liver2 and was originally identified as one of the subtypes of opioid receptors, but further studies identified it as a novel ligand‐operated molecular chaperone.3 Sig‐1R is expressed in various types of peripheral and central organs, including liver, kidney, heart, and brain. Among these organs, Sig‐1R expression is highest in the brain4 and is especially concentrated in specific areas involved in memory, emotion and sensory and motor functions, such as the hypothalamus, olfactory bulb, several cortical layers, and the hippocampus. Sig‐1R is an endoplasmic reticulum chaperone that is implicated in neuronal survival, protection, axon outgrowth, and neurodegenerative disorders.5, 6 The molecular mechanisms underlying the neuroprotective effect of Sig‐1R remain uncharacterized. The neuroprotective effects of Sig‐1R include attenuation of hypoxia/hypoglycemia‐mediated neurotoxicity,7 preservation of antiapoptotic protein by regulating Bcl‐2 expression8 and reduction in intracellular Ca2+. Although some data have demonstrated that Sig‐1R is associated with axon elongation, the exact mechanism is not well characterized.
Axons are structurally and functionally distinct protrusions of neurons that modulate neurotransmitter release and neural function. Axonal malfunction can contribute to neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease, stroke, and multiple sclerosis.9 A large body of evidence has indicated that axonal growth is closely related to cytosolic Ca2+ levels. One theory suggests that Ca2+ serves as a negative regulator of process extension. For example, reductions in cytosolic Ca2+ have been found to promote axon outgrowth.10 On the other hand, when Ca2+ is elevated in growth cones, they typically slow down, stop or retract in a Ca2+‐dependent manner.11, 12 Some studies have proposed that the calcium transients in neurons are primarily mediated primarily by L‐type VGCCs, and silencing them with channel blockers can promote axon outgrowth.13 Other researchers have demonstrated that incubating superior mesenteric ganglia neurons with Interleukin‐17 significantly potentiated neurite length by inhibiting N‐type VGCCs because the addition of an N‐type VGCC blocker, ω‐conotoxin GVIA, completely mimicked this effect.14 Many studies have previously reported that Sig‐1R ligands regulate the activity of VGCCs. For example, Sig‐1R ligands have been suggested to block multiple subtypes of VGCC currents in primary hippocampal neurons.15 In addition, sigma receptors have been demonstrated to inhibit L‐, N‐, P/Q‐, and R‐type VGCC subtypes present in neurons of both autonomic ganglia with high efficacy.16 Recently, another research group suggested that Sig‐1R stimulation attenuates calcium influx through activated L‐type VGCCs in purified retinal ganglion cells.17
Therefore, we hypothesize that Sig‐1R enhances axon outgrowth by regulating VGCCs. Here, we used immunostaining and electrophysiological methods to demonstrate that treatment with SA4503, a selective Sig‐1R agonist, promotes axonal outgrowth in cultured hippocampal neurons by blocking VGCCs, primarily L‐type, N‐type, and P/Q‐type VGCCs.
2. MATERIALS AND METHODS
All experimental procedures were approved by the Committee of Animal Use for Research and Education of the Laboratory Animals Center of Beijing Institute of Pharmacology and Toxicology (Beijing, China) and consistent with the ethical guidelines recommended by the International Association for the Study of Pain in conscious animals. Efforts were made to minimize the animals' suffering.
2.1. Preparation of hippocampal neurons and lentivirus transduction
Primary neuronal cultures were prepared as previous report.18 Simply speaking, hippocampal explants isolated from postnatal 6 hours SD rats were digested with 0.25% trypsin for 30 minutes at 37°C, followed by trituration with pipette in plating medium (DMEM with 10% fetal bovine serum plus 10% horse serum). Dissociated neurons were plated onto 24‐well plates coated with poly‐D‐lysine or 6‐well plates (Corning, USA) at a density of 1 × 105 cells per well. After culturing for 3 hours, media were changed to neurobasal medium (Gibco, Grand Island, NY, USA) supplemented with 2% B27, and different concentrations of SA4503 and (or) sigma‐1R antagonist NE100 were added simultaneously. Sig‐1R‐specific or scrambled small interfering RNA oligonucleotides sequences were 5′‐ggcuugagcucaccaccua‐3′19 and 5′‐uagcgacuaaacacaucaauu‐3′, respectively. They were packaged into lentiviral vectors (Genomeditech, Shanghai, China). Transduction of neurons was performed with lentiviral vectors (MOI: 20) for an additional 48 hours both for immunofluorescence and Western blot as soon as they attached to the dishes.
2.2. Immunofluorescence
Neurons grown on coverslip were washed with PBS three times. They were fixed with 4% paraformaldehyde for 20 minutes, which were subsequently washed with PBS (3 × 5 minutes). Thereafter, they were permeabilized and blocked with 0.3% Triton X‐100 plus 3% BSA for 40 minutes at room temperature. And then, neurons were incubated with primary mouse monoclonal anti‐βIII tubulin antibody (1:100, Millipore) and (or) rhodamine phalloidin (1:250, Invitrogen) overnight at 4°C and washed with PBS (3 × 5 minutes). Goat anti‐mouse FITC conjugated secondary antibody (1:200) was put on for 1 hour at room temperature followed by repeated washing in PBS (3 × 5 minutes). Finally, the cells were mounted on slides, and the stained sections were observed with Olympus fluorescent microscope (Olympus BX51, Tokyo, Japan).
2.3. Protein preparations and Western blot
Neurons were collected and lysed in RIPA lysis buffer. Protein concentration was determined by Bio‐Rad Protein Assay (Pierce™ BCA Protein Assay Kit; Thermo, Rockford, IL, USA). Lysates (10 μg) were resolved by denaturing 12% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes and then blocked with 5% (w/v) skim milk in TBST (20 mmol/L Tris‐HCl (pH 7.4), 0.15 mol/L NaCl, 0.05% Tween 20) for 1 hour at room temperature. The blocked membranes were subsequently probed with antisigma‐1R (1:200, Proteintech) or β‐actin (1:1000; Invitrogen) antibodies at 4°C overnight. After the membrane had been washed three times with TBST, it was incubated for 1 hour at room temperature with IRDye 800CW goat anti‐rabbit secondary antibody (1:15 000). After the membrane had been washed with TBST, bands of protein on the membrane were visualized using the enhanced chemiluminescence detection (Li‐Con, Odyssey, Hongkong, China). Densitometry of imaged bands was performed in ImageJ software (Bethesda, MD, USA).
2.4. Assessment of neurite outgrowth
Primary cultured hippocampal neurons were prepared as above. After treatment with different concentrations of SA4503 for 48 or 72 hours, axons were assessed. Neurons were labeled by primary anti‐βIII tubulin antibody and an FITC conjugated secondary antibody. Axon outgrowth was quantified using the ImageJ software.20 Cells with neuritis shorter than the diameter of their somas were excluded from the analysis.
2.5. Patch‐clamp recording
Rats (15 days old) of either sex were anesthetized with pentobarbital (50 mg/kg, i.p.) and decapitated. The brain was quickly removed, and the hippocampus was transferred into a chilled oxygenated cutting solution containing (in mmol/L) 234 sucrose, 11 glucose, 24 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl2, and gassed with 95% O2/ 5% CO2 at 4°C. Hipocampal slices (300 μm) were cut using a vibratome (MA752, Campden Company, USA) and then incubated in standard artificial cerebrospinal fluid (ACSF) bubbled with 95% O2/5% CO2. Brain slices were placed into warm (32 ± 0.5°C) bubbled cutting solution for 30 minutes and then transferred to room temperature (22‐24°C) for an additional 30 minutes before being placed into a submersion recording chamber containing ACSF. The whole‐cell patch‐clamp recordings were performed at room temperature. Currents were measured with an Axopatch‐200B (Molecular Device, Sunnyvale, CA, USA) and recorded with p500 and over Hospital Bed Capacity lamp 10.1 software (Molecular Device). The output was digitized with a Digidata 1440 converter (Molecular Device). Patch pipettes were made by a two‐step vertical puller (Model P‐97, Sutter Instrument) from borosilicate glass and had resistances between 5 and 8 MΩ after perfusion of internal solution through the pipette. Brain slices were placed in a recording chamber and visualized with an infrared video microscopy (ECLIPSE FN1; Nikon, Tokyo, Japan). Experiments were performed at a holding potential of −70 mV for VGCCs. After gigaohm seal formation and membrane disruption, the whole‐cell capacitance was canceled, and series resistance was compensated for (>80%). Data were low‐pass‐filtered at 2 kHz, sampled at 10 kHz, and acquired with the pulse protocol. The liquid junction potential between internal and external solutions was −5 mV on average and was used to correct for the recorded membrane potential.21 The pipette solution was composed of the following (in mM): 80 Cs‐gluconate, 30 CsCl, 40 HEPES, 10 TEA‐Cl, 5 EGTA, 12 Na2 phosphocreatine, 1 MgCl2, 2 Mg‐ATP, and 0.5 Na‐GTP (adjusted to pH 7.3 with CsOH). VGCCs were recorded in an external solution that contained (mmol/L): 105 NaCl, 20 tetraethylammonium chloride (TEA‐Cl), 2 CaCl2, 6 MgCl2, 2.5 KCl, 26 NaHCO3, 10 glucose, 3 myo‐inositol, 2 sodium pyruvate, 0.5 ascorbic acid, 1.25 NaH2PO4, and 0.0005 TTX (pH 7.4, when saturated with 95% O2 /5% CO2).22
2.6. Data analysis
The data were analyzed using the pCLAMP 10.0 software (Molecular Device) and Origin 8.0 (Microcal Software, USA) software programs. All data were presented as mean ± SEM. Statistical significance was assessed using Prism5.0 software. The Student's t test analysis was used to assess the differences between the means of two groups. One‐way ANOVA followed by Dunnett's posttesting was performed to assess differences among more than two groups. Two‐way ANOVA Bonferroni's posttest was used to evaluate time‐dependence relationship of SA4503 treatment. P value < 0.05 was considered to be significant.
3. RESULTS
3.1. Sig‐1R agonists evoke axon elongation in a dose‐ and time‐dependent manner
To investigate the in vitro effects of the Sig‐1R agonist SA4503 on the growth of neurites in hippocampal neurons, we first bath applied SA4503 to dissociated neuronal cultures as soon as the neurons attached for 3 hours. The hippocampal neurons were cultured for an additional 48 hours in either the presence or absence of SA4503 and then immunostained with an anti‐β III tubulin antibody. After exposing the cultures to SA4503 at different concentrations (1‐20 μmol/L), we measured the treated neurite lengths and compared them with the control neurites, which were exposed to vehicle. After 48 hours of treatment, we found that SA4503 significantly increased neurite lengths in a dose‐dependent manner (Figure 1A, P < 0.01 in Dunnett's test). The control neurites averaged 128 μm, whereas the neurites exposed to SA4503 averaged 202 μm and reached lengths of >250 μm (Figure 1A). As the 5, 10, and 20 μmol/L doses of SA4503 did not induce significantly different effects on neurite lengths (P > 0.05), 10 μmol/L SA4503 was selected for use in the subsequent experiments. Next, to determine whether the neurites enhanced by SA4503 were axons, an anti‐Tau‐1 antibody (an axon‐specific marker) and an anti‐MAP2 antibody (a dendritic‐specific marker) were utilized to immunostain the hippocampal neurons (Figure 1B). As we expected, the neurites elongated by SA4503 were definitely axons. SA4503 induced hippocampal neuron axon outgrowth in a time‐dependent manner. There was no difference between the SA4503 group and the control group after 24 hours. However, axon length was notably enhanced after 48 and 72 hours (Figure 1C, P < 0.01 in two‐way ANOVA). The mean length of the SA4503‐treated axons increased by 300 μm after 72 hours, while the mean length of the control neurons remained at only 175 μm after 72 hours. In addition, incubation with SA4503 for 72 hours did not have any effect on neuronal survival, which was indicated by a PI test (data not shown). In conclusion, hippocampal axon outgrowth was enhanced by SA4503 in a dose‐ and time‐dependent manner, which validated previous reports.19
Figure 1.
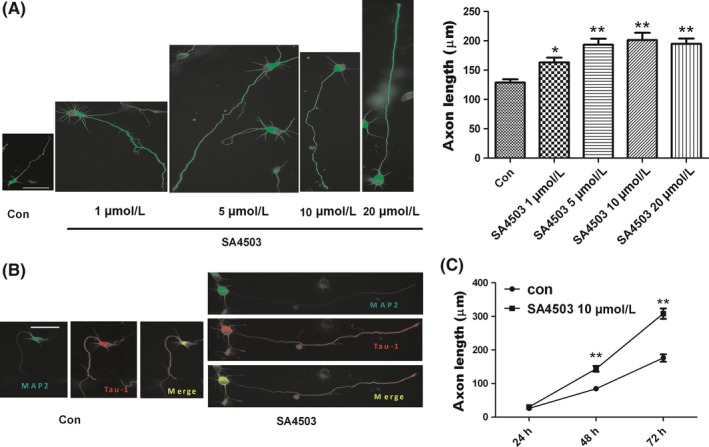
Sig‐1R agonist SA4503 enhances axon outgrowth of cultured hippocampal neurons. (A) Cultured hippocampal neurons are treated with vehicle (control) and with SA4503 (1‐20 μmol/L) for 48 hours. The axon is immunostained with anti‐β III tubulin antibody (left panel). The right panel is statistics from the left. Data are means ± SEM, *P < 0.05 and **P < 0.01 vs control group, Dunnett's test. (B) Neurons are treated with vehicle (control, left panel) and with 10 μmol/L SA4503 (right panel) followed by double immunostaining, anti‐MAP2 antibody (green) and anti‐Tau‐1 antibody (red), respectively. The yellow one is merged with green and red. (C) Effects of vehicle (control, circles) and 10 μmol/L SA4503 (squares) treatment on axon outgrowth in hippocampal cultures. Data are means ± SEM from three separate experiments including 3‐4 wells performed on different time points (**P < 0.01, two‐way ANOVA with Bonferroni's posttest). The bar represents 50 μm
3.2. Sig‐1R agonists increase axon outgrowth via sig‐1R
In addition to SA4503, another Sig‐1R‐specific agonist, Pre084, was used to examine the influence of Sig‐1R on axon length. We observed that 10 μmol/L Pre084 also notably enhanced axon length, as shown in Figure 2A. These results demonstrate that Sig‐1R agonists can induce the axon outgrowth of cultured hippocampal neurons. To test whether Sig‐1R participated in the effect on axon elongation, the dissociated hippocampal neurons were incubated with the Sig‐1R‐specific antagonist, NE‐100, 30 minutes before the SA4503 treatment, which lasted for an additional 48 hours. We observed that the axon elongation effect was abrogated when the neurons were cultured with both NE‐100 and SA4503 (Figure 2B). Treatment with NE‐100 alone did not cause any significant change in axon length when compared to that of the control cells. A lentiviral vector expressing shRNA targeting Sig‐1R significantly decreased Sig‐1R protein levels to about 78% compared to a scrambled shRNA sequence (Figure 2C). Previous research has shown that Sig‐1R knockdown causes the degeneration of hippocampal neurons.23 We obtained the same results in this study. We observed that Sig‐1R knockdown in primary hippocampal neurons caused decreased survival and counteracted the axon outgrowth enhanced by SA4503. Thus, these results provide strong evidence that Sig‐1R activation promotes axon outgrowth in primary cultured hippocampal neurons.
Figure 2.
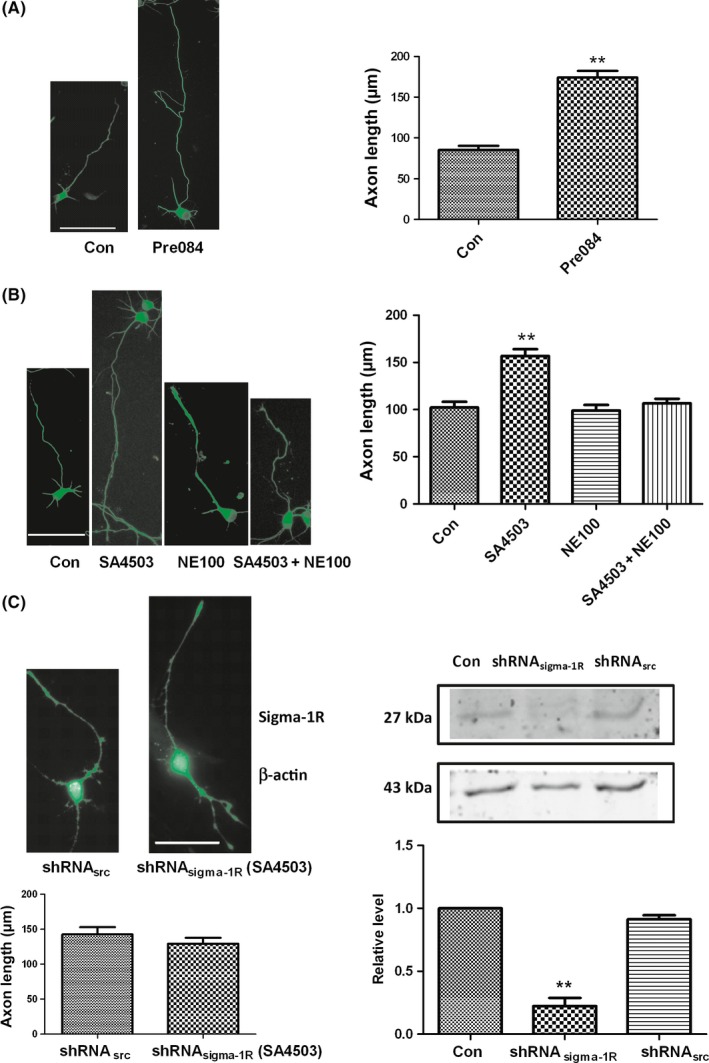
Sig‐1R plays an important role in enhancing the axon lengths. (A) The axon outgrowth is also increased by another Sig‐1R agonist Pre084 (10 μmol/L). The axon is immunostained with anti‐β III tubulin antibody (left panel). The right panel is statistics from the left. (B) Sig‐1R specific antagonist NE‐100 blocks the enhancing action induced by SA4503 when NE100 incubation 30 min earlier than SA4503. The right panel is statistics from the left. (C) The right panel shows that knockdown of the Sig‐1R by shRNA (shRNA sigma‐1R) apparently decreases receptor protein level on Western blots from lentivirus transfected neurons compared with negative control and positive control (scrambled sequence, shRNA src) (n = 3). The left panel shows that axon outgrowth is not enhanced by 10 μmol/L SA4503 in the shRNA sigma‐1R treatment of primary hippocampal cultures compared with shRNA sigma‐1R treatment cultures. Data are means ± SEM, separate experiments including 3‐4 wells, n ≥ 30 neurons, **P < 0.01 vs control group, Student's t test or Dunnett's test. The bar represents 50 μm
3.3. Enhanced axon outgrowth does not depend on glial cells
To test whether the glial cells present in the hippocampal neuronal cultures may mediate the effect of SA4503 on hippocampal neurite outgrowth, the cultures were simultaneously incubated with 5 μmol/L cytosine arabinoside (Ara‐C) and SA4503. There was no difference between the cultures cotreated with SA4503 and Ara‐C and the cultures treated with SA4503 at 24 hours as shown in Figure 3. However, at 48 and 72 hours, the SA4503 and Ara‐C cotreatment significantly increased axon length, but this effect was not different from that of the SA3405 treatment alone. Approximately 10%‐20% of the cells in the primary culture were glia, as evidenced by immunoreactivity with an anti‐GFAP antibody. In the presence of Ara‐C, the primary culture was in a poor condition due to the loss of nutritional support from glia. Despite the loss of glia, there were still elongated axons present in the hippocampal neuron cultures. Therefore, we concluded that the axon growth enhanced by SA4503 did not depend on glial cells.
Figure 3.
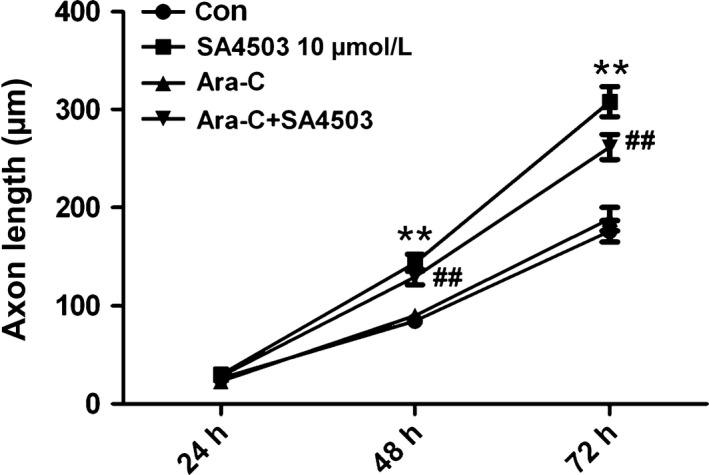
The effects of SA4503 do not depend on glial cells. The glial cell population was depleted with Ara‐C (5 μmol/L), SA4503 significantly enhanced the axon lengths after 48 and 72 hours. Data are means ± SEM, separate experiments including 2 wells, n ≥ 15 neurons, **P < 0.01 SA4503 vs control, ##P < 0.01 Ara‐C + SA4503 vs control, two‐way ANOVA with Bonferroni's posttest
3.4. Inhibiting VGCCs causes enhanced axon length
Calcium has been shown to be an important regulator of axon outgrowth.24, 25 Axon extension occurs within optimal levels of intracellular calcium but slows or ceases when calcium is above or below these levels, which are called set points.26 Previous studies have shown that activating Sig‐1R can block or enhance VGCCs under different experimental conditions, such as neuron types and experimental methods. In this paper, we proposed that SA4503 may increase axon length via blocking VGCCs. Hence, we first used electrophysiological methods to examine this hypothesis. It is difficult to record currents in immature neurons due to the fragile cell membranes. Thus, hippocampal brain slices from 13DIV rats were used to record calcium channel currents that mimicked those of the young cultured hippocampal neurons. We observed that 10 μmol/L SA4503 reversibly blocked 30% of the whole‐cell Ca2+ currents as shown in Figure 4B.
Figure 4.
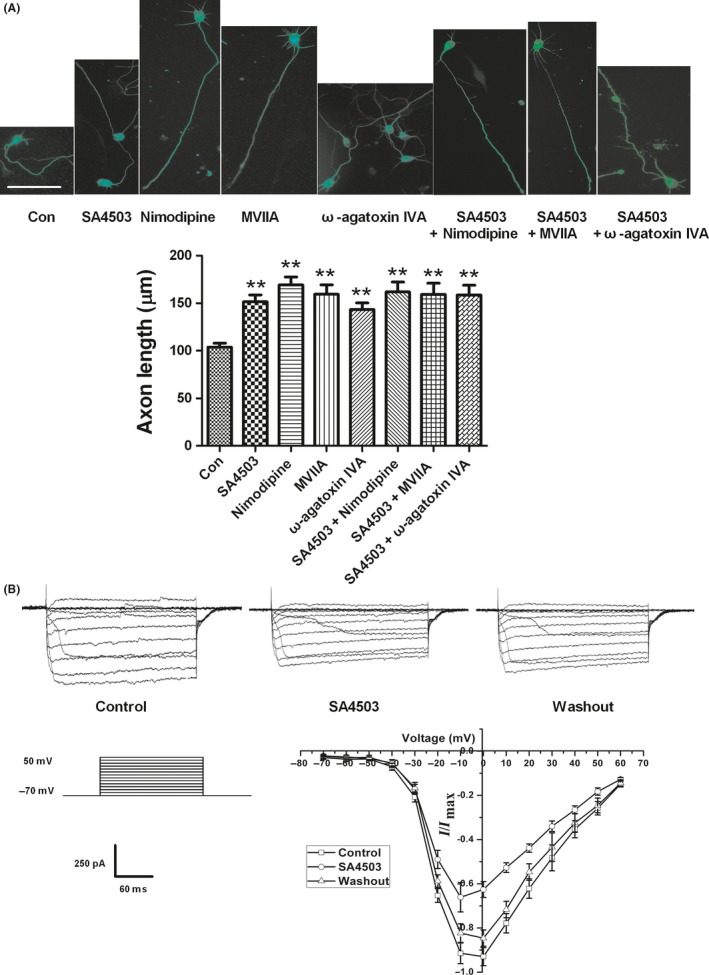
Application of VGCCs antagonists induces axon outgrowth and SA4503 blocks VGCCs expressed on hippocampal neurons. (A) Representative images of hippocampal neurons applied by different treat groups (top). The bottom is statistic result. Data are means ± SEM, separate experiments including 3‐4 wells, n ≥ 30 neurons, **P < 0.01 vs control group, Dunnett's posttest. The bar represents 50 μm. (B) SA4503 inhibits VGCCs expressed on hippocampal brain slice reversibly using whole‐cell patch‐clamp method. Left, the protocol to elicit VGCCs starting from a holding potential of −70 mV to the voltage of +50 mV in increments of +10 mV. Top, representative recordings of the VGCCs elicited by a series of voltage steps and inverse inhibition by SA4503. Right, Current/voltage (I/V) curve of VGCCs inhibition by SA4503. Each point is normalized to the maximal VGCCs (n = 5)
If Ca2+ current inhibition is involved in the axon outgrowth induced by SA4503, then the effects of SA4503 on axon outgrowth should be replicated by calcium channel blockers. Previous studies have demonstrated that blocking L‐type and N‐type calcium channels on developing neurons can increase axon length.13, 14 Consequently, we added specific L‐type and N‐type VGCC blockers, 10 μmol/L nimodipine and 3 μmol/L MVIIA, respectively, to the culture after 2 hours once the neurons were attached. After 48 hours, treatment with both nimodipine and MVIIA resulted in longer axons than treatment with vehicle (control), and the treatment with nimodipine and MVIIA was resulted in the same effect that was induced by SA4503 (Figure 4B,C). In addition, we tested the effect of the specific P/Q‐type VGCC antagonist, ω‐agatoxin IVA. To our surprise, 0.5 μmol/L ω‐agatoxin IVA notably increased axon length. Our experiments suggested that blocking L‐type, N‐type, and P/Q‐type VGCCs, which are expressed on young cultured hippocampal neurons,27 can induce increased axon length. Then, we tested the add‐on effect of SA4503 on the outgrowth‐promoting effect of nimodipine, MVIIA, or GIVA. SA4503 did not further potentiate axon outgrowth in the presence of these Ca2+ blockers (Figure 4A). We confirmed our speculation that SA4503 enhanced axon outgrowth by blocking the VGCCs expressed on neurons.
3.5. SA4503 changes growth cone morphologies
Growth cones located at the tip of developing or regenerated axons regulate neuronal axon elongation, retraction, and turning. Hippocampal growth cone morphologies are well correlated closely with their rates of extension. Small, simple, bullet‐shaped growth cones usually extend rapidly, whereas large and complex growth cones with expanded lamellipodia and numerous filopodia pause for many hours. Consequently, we used immunofluorescence methods to detect the effects of SA4503 on growth cone morphologies. As shown in Figure 5, growth cones were double immunostained with a primary phalloidin antibody (F‐actin, red) and an anti‐β III tubulin antibody (tubulin, green). We found that the control cultures treated with vehicle had many large growth cones and fewer long axons as mentioned above. In the 50 examined growth cones, approximately 38% exhibited simple morphologies. In contrast, after SA4503 application for 48 hours after plating, the neurons exhibited long axons with mostly simple growth cones. Approximately 73% of the 45 examined growth cones at the tips of long axons were small and simple. The small and simple cytoskeletal structures of growth cones usually included bundles of microtubules (MTs), loss of actin arc structure and reduction in F‐actin levels. MT extension into the growth cone periphery is an essential process for axon growth.28 We observed that, in approximately half of the hippocampal neuronal growth cones, the MTs spread out as they entered the growth cone, whereas in the other half, the MTs remained bundled (Figure 5A,D). In contrast, after treatment with SA4503 for 48 hours, the ratio of bundled MTs that were in tight arrays in growth cones increased to 80%. In the control growth cones, actin arc structures in the transitional zone actively inhibit MT extension. However, after application of SA4503, the actin arcs were reduced, which allowed the MTs to extend into the growth cone periphery as shown in Figure 5. Phalloidin is a specific label for F‐actin (red). We found that, compared to vehicle treatment, SA4503 treatment markedly reduced F‐actin content in the growth cones. In conclusion, these results demonstrated that the Sig‐1R agonist SA4503 likely increases axon outgrowth by transforming growth cone morphologies from complex to simple.
Figure 5.
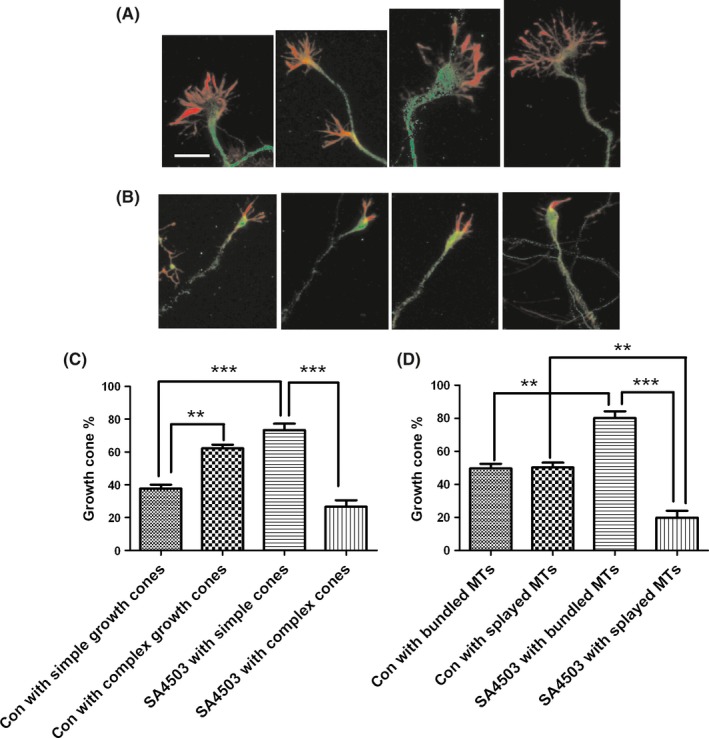
SA4503 activation induces change in growth cone cytoskeletal structures. The cultured naive hippocampal neurons in the presence of vehicle (A) or SA4503 (B) were stained with phalloidin and anti‐β III tubulin antibodies. Note that the actin arc structure is very obvious in the first, third, and last picture (exception for the second one) of A group. The arc structure is lost by application of SA4503 (B). F‐actin content (red) of SA4503 treatment (B) is much less than vehicle treatment (A). Representative images (A and B) and quantification of growth cone morphology (C) and MT structures (D) are shown. Data are means ± SEM, separate experiments including 3‐4 wells, n ≥ 40 neurons, **P < 0.01 and ***P < 0.001 vs control group, Dunnett's posttest. The bar represents 20 μm
4. DISCUSSION
Sig‐1R has been reported to play a role in neurodegenerative disorders, including AD, Parkinson's disease and ischemic diseases, such as stroke. As a molecular chaperone, Sig‐1R participates in many physiological and pathological processes. Previous studies have reported that several versatile actions of Sig‐1R may affect cellular survival as mentioned above.29, 30, 31 Although Sig‐1R is known to promote neurite elongation, the underlying molecular mechanisms are not completely understood. Kimura et al20 demonstrated that Sig‐1R activation promotes neurite elongation through activation of tropomyosin receptor kinase (Trk) in cerebellar granule neurons. Tsai et al31 demonstrated that Sig‐1Rs regulate the turnover of the short‐lived cyclin‐dependent kinase (cdk) activator p35 via myristic acid and thus play important roles in the maintenance of axon elongation in the brain. In the present study, we suggested that Sig‐1R increases axon outgrowth through regulation of VGCC activity in cultured naïve hippocampal neurons. Three pieces of evidence supported our opinion. First, Sig‐1R activation blocks VGCC currents; moreover, bath application of VGCC antagonists completely imitated the axon elongation caused by the Sig‐1R agonist SA4503. Second, the growth cone cytoskeletal structure changes induced by SA4503 were the same as those induced by VGCC antagonists.13 Third, SA4503 no longer potentiated axon outgrowth in the presence of VGCC blockers.
Different types of VGCCs are expressed in cultured hippocampal neurons, including L‐type, N‐type, and P/Q‐type, which contribute to ~32%, ~38%, and ~21%, respectively, of the highly expressed VGCCs.32 Pravettoni et al27 discovered that L‐type and N‐type VGCCs have distinct patterns of expression and distribution and play different functional roles during hippocampal neuron differentiation. L‐type channels are involved in the early stages of neurite outgrowth, whereas at later stages (3‐4 DIV), axonal growth is primarily associated with N‐type channels. This finding is consistent with our result showing that blocking L‐type and N‐type VGCCs enhances axon outgrowth during the first 72 hours once the neurons were attached to the petri dish. In addition, in our experiment, we discovered that inhibiting P/Q‐type VGCCs also increases axon length. To the best of our knowledge, this finding has not been reported by other laboratories. Application of specific L‐type, N‐type, and P/Q‐type VGCC inhibitors, nimodipine, MVIIA, and ω‐agatoxin IVA, respectively, produced similar axon lengths. Although nimodipine treatment appears to induce slightly longer axon lengths than those of MVIIA and ω‐agatoxin IVA treatments (Figure 4), there were no significant differences among the treatments. Moreover, L‐type, N‐type, and P/Q‐type VGCCs that occupy similar proportions of neurons are the main calcium channel types in hippocampal neurons. In addition, 10 μmol/L SA4503 blocked approximately 30% of the whole Ca2+ currents in neurons as shown in Figure 4C. Thus, SA4503 may inhibit several types of calcium channels, because its effect on axon length resembled the effects of nimodipine, MVIIA, and ω‐agatoxin IVA. Some studies have already reported that Sig‐1R activation may block L‐type and N‐type VGCCs.15 However, whether Sig‐1R activation can block P/Q‐type VGCCs needs to be addressed with further studies.
We demonstrated that Sig‐1R activation promotes axon outgrowth that is accompanied by morphological changes in growth cones. Hayashi et al33 suggested that Sig‐1R may cause structural alterations in NG‐108 cells by affecting the dynamics and rearrangement of cytoskeletal proteins. In the current study, Sig‐1R was first observed to change the growth cone morphology of primary cultured hippocampal neurons from big and complex to small and simple. Changes in the growth cone cytoskeleton have a direct impact on the rate of axon extension. Tang et al13 demonstrated that silencing L‐type voltage‐gated calcium channels always produced small and simple growth cones that promoted axon outgrowth. Sig‐1R activation induced by SA4503 achieves the same results. However, questions remain concerning the molecular mechanisms involved in this process. For example, Ca2+ channel blocking is an upstream effect, and the downstream signal transduction pathway is unknown. Additionally, the key treatment dose that leads to cytoskeletal protein rearrangement needs to be identified. Alternatively, in addition to the Ca2+ channel expressed on plasma membrane, Sig‐1R may also play a role in the regulation of ER‐mitochondrion Ca2+ signaling. These lingering questions could be the focus of a follow‐up study. Nevertheless, our results showed that Sig‐1R activation can drive axon elongation by blocking VGCCs to alter the growth cone cytoskeletal structure.
In conclusion, the Sig‐1R agonist SA4503 promotes axon outgrowth. In addition, this effect disappeared with the addition of the Sig‐1R antagonist NE100 or downregulation of Sig‐1R protein. The observed effect on axon length is independent of glia but closely related to VGCCs. Inhibiting VGCCs caused growth cone structure remodeling from complex to simple that led to axon elongation. Axon outgrowth induced by Sig‐1R may shape new synapses and build new neural circuits, which could help restore neurological function lost due to neurodegenerative diseases.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
We are grateful for support from The National Nature Science Foundation of China (No. 31400917).
Li D, Zhang S‐Z, Yao Y‐H, et al. Sigma‐1 receptor agonist increases axon outgrowth of hippocampal neurons via voltage‐gated calcium ions channels. CNS Neurosci Ther. 2017;23:930–939. 10.1111/cns.12768
The first two authors contributed equally to this work.
REFERENCES
- 1. Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994;268:9‐18. [DOI] [PubMed] [Google Scholar]
- 2. Hanner M, Moebius FF, Flandorfer A, et al. Purification, molecular cloning, and expression of the mammalian sigma1‐binding site. Proc Natl Acad Sci USA. 1996;93:8072‐8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaupel DB. Naltrexone fails to antagonize the sigma effects of PCP and SKF 10,047 in the dog. Eur J Pharmacol. 1983;92:269‐274. [DOI] [PubMed] [Google Scholar]
- 4. Langa F, Codony X, Tovar V, et al. Generation and phenotypic analysis of sigma receptor type I (sigma 1) knockout mice. Eur J Neurosci. 2003;18:2188‐2196. [DOI] [PubMed] [Google Scholar]
- 5. Feher A, Juhasz A, Laszlo A, et al. Association between a variant of the sigma‐1 receptor gene and Alzheimer's disease. Neurosci Lett. 2012;517:136‐139. [DOI] [PubMed] [Google Scholar]
- 6. Tsai SY, Pokrass MJ, Klauer NR, De Credico NE, Su TP. Sigma‐1 receptor chaperones in neurodegenerative and psychiatric disorders. Expert Opin Ther Targets. 2014;18:1461‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lockhart BP, Soulard P, Benicourt C, Privat A, Junien JL. Distinct neuroprotective profiles for sigma ligands against N‐methyl‐D‐aspartate (NMDA), and hypoxia‐mediated neurotoxicity in neuronal culture toxicity studies. Brain Res. 1995;675:110‐120. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Shi Y, Qiao L, et al. Sigma‐1 receptor agonists provide neuroprotection against gp120 via a change in bcl‐2 expression in mouse neuronal cultures. Brain Res. 2012;1431:13‐22. [DOI] [PubMed] [Google Scholar]
- 9. Lin L, Lesnick TG, Maraganore DM, Isacson O. Axon guidance and synaptic maintenance: preclinical markers for neurodegenerative disease and therapeutics. Trends Neurosci. 2009;32:142‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bixby JL, Spitzer NC. Early differentiation of vertebrate spinal neurons in the absence of voltage‐dependent Ca2 + and Na+ influx. Dev Biol. 1984;106:89‐96. [DOI] [PubMed] [Google Scholar]
- 11. Catsicas M, Allcorn S, Mobbs P. Early activation of Ca(2 + )‐permeable AMPA receptors reduces neurite outgrowth in embryonic chick retinal neurons. J Neurobiol. 2001;49:200‐211. [DOI] [PubMed] [Google Scholar]
- 12. Lankford KL, Letourneau PC. Evidence that calcium may control neurite outgrowth by regulating the stability of actin filaments. J Cell Biol. 1989;109:1229‐1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang F, Dent EW, Kalil K. Spontaneous calcium transients in developing cortical neurons regulate axon outgrowth. J Neurosci. 2003;23:927‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chisholm SP, Cervi AL, Nagpal S, Lomax AE. Interleukin‐17A increases neurite outgrowth from adult postganglionic sympathetic neurons. J Neurosci. 2012;32:1146‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Church J, Fletcher EJ. Blockade by sigma site ligands of high voltage‐activated Ca2 + channels in rat and mouse cultured hippocampal pyramidal neurones. Br J Pharmacol. 1995;116:2801‐2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang H, Cuevas J. Sigma receptors inhibit high‐voltage‐activated calcium channels in rat sympathetic and parasympathetic neurons. J Neurophysiol. 2002;87:2867‐2879. [DOI] [PubMed] [Google Scholar]
- 17. Mueller BH 2nd, Park Y, Daudt DR 3rd, et al. Sigma‐1 receptor stimulation attenuates calcium influx through activated L‐type Voltage Gated Calcium Channels in purified retinal ganglion cells. Exp Eye Res. 2013;107:21‐31. [DOI] [PubMed] [Google Scholar]
- 18. Ruscher K, Freyer D, Karsch M, et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J Neurosci. 2002;22:10291‐10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruscher K, Shamloo M, Rickhag M, et al. The sigma‐1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011;134:732‐746. [DOI] [PubMed] [Google Scholar]
- 20. Kimura Y, Fujita Y, Shibata K, Mori M, Yamashita T. Sigma‐1 receptor enhances neurite elongation of cerebellar granule neurons via TrkB signaling. PLoS ONE. 2013;8:e75760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang J, Liu X, Pan M, et al. Blockade of Nav1.8 currents in nociceptive trigeminal neurons contributes to anti‐trigeminovascular nociceptive effect of amitriptyline. Neuromolecular Med. 2014;16:308‐321. [DOI] [PubMed] [Google Scholar]
- 22. Miki T, Hirai H, Takahashi T. Activity‐dependent neurotrophin signaling underlies developmental switch of Ca2 + channel subtypes mediating neurotransmitter release. J Neurosci. 2013;33:18755‐18763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hedskog L, Pinho CM, Filadi R, et al. Modulation of the endoplasmic reticulum‐mitochondria interface in Alzheimer's disease and related models. Proc Natl Acad Sci USA. 2013;110:7916‐7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomez TM, Spitzer NC. Regulation of growth cone behavior by calcium: new dynamics to earlier perspectives. J Neurobiol. 2000;44:174‐183. [PubMed] [Google Scholar]
- 25. Spitzer C, Effler K, Freyberger HJ. Posttraumatic stress disorder, dissociation and self‐destructive behavior in borderline patients. Z Psychosom Med Psychother. 2000;46:273‐285. [PubMed] [Google Scholar]
- 26. Kater SB, Mills LR. Regulation of growth cone behavior by calcium. J Neurosci. 1991;11:891‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pravettoni E, Bacci A, Coco S, Forbicini P, Matteoli M, Verderio C. Different localizations and functions of L‐type and N‐type calcium channels during development of hippocampal neurons. Dev Biol. 2000;227:581‐594. [DOI] [PubMed] [Google Scholar]
- 28. Hur EM, Yang IH, Kim DH, et al. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc Natl Acad Sci USA. 2011;108:5057‐5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hayashi T, Su TP. Sigma‐1 receptor chaperones at the ER‐mitochondrion interface regulate Ca(2 + ) signaling and cell survival. Cell. 2007;131:596‐610. [DOI] [PubMed] [Google Scholar]
- 30. Tsai SY, Pokrass MJ, Klauer NR, Nohara H, Su TP. Sigma‐1 receptor regulates Tau phosphorylation and axon extension by shaping p35 turnover via myristic acid. Proc Natl Acad Sci USA. 2015;112:6742‐6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsai SY, Hayashi T, Harvey BK, et al. Sigma‐1 receptors regulate hippocampal dendritic spine formation via a free radical‐sensitive mechanism involving Rac1xGTP pathway. Proc Natl Acad Sci USA. 2009;106:22468‐22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wen L, Yang S, Qiao H, et al. SO‐3, a new O‐superfamily conopeptide derived from Conus striatus, selectively inhibits N‐type calcium currents in cultured hippocampal neurons. Br J Pharmacol. 2005;145:728‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayashi T, Su TP. Regulating ankyrin dynamics: roles of sigma‐1 receptors. Proc Natl Acad Sci USA. 2001;98:491‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]


